Abstract
Neuroinflammation has become a well-accepted pathologic hallmark of Parkinson’s disease (PD). However, it remains unclear whether inflammation, triggered by α-syn aggregation and/or degeneration, contributes to the progression of the disease. Studies examining neuroinflammation in PD are unable to distinguish between Lewy body-associated inflammation and degeneration-associated inflammation, as both pathologies are present simultaneously. Intrastriatal and intranigral injections of alpha-synuclein (α-syn) preformed fibrils (PFFs) results in two distinct pathologic phases: Phase 1: The accumulation and peak formation of α-syn inclusions in nigrostriatal system and, Phase 2: Protracted dopaminergic neuron degeneration. In this review we summarize the current understanding of neuroinflammation in the α-syn PFF model, leveraging the distinct Phase 1 aggregation phase and Phase 2 degeneration phase to guide our interpretations. Studies consistently demonstrate an association between pathologic α-syn aggregation in the substantia nigra (SN) and activation of the innate immune system. Further, major histocompatibility complex-II (MHC-II) antigen presentation is proportionate to inclusion load. The α-syn aggregation phase is also associated with peripheral and adaptive immune cell infiltration to the SN. These findings suggest that α-syn like aggregates are immunogenic and thus have the potential to contribute to the degenerative process. Studies examining neuroinflammation during the neurodegenerative phase reveal elevated innate, adaptive, and peripheral immune cell markers, however limitations of single time point experimental design hinder interpretations as to whether this neuroinflammation preceded, or was triggered by, nigral degeneration. Longitudinal studies across both the aggregation and degeneration phases of the model suggest that microglial activation (MHC-II) is greater in magnitude during the aggregation phase that precedes degeneration. Overall, the consistency between neuroinflammatory markers in the parkinsonian brain and in the α-syn PFF model, combined with the distinct aggregation and degenerative phases, establishes the utility of this model platform to yield insights into pathologic events that contribute to neuroinflammation and disease progression in PD.
Keywords: Parkinson’s disease, aggregation, inclusions, synucleinopathy, microglia, astrocytes, neuroinflammation
Introduction:
Parkinson’s disease (PD) is the second most common neurodegenerative disorder in the United States (US), with motor symptoms that include tremor at rest, bradykinesia, rigidity, and postural instability. Nearly one million people in the US are living with PD and 60,000 people are diagnosed each year, with these numbers expected to rise to 1.2 million by 2030 (Marras et al., 2018). The yearly economic burden of PD is an estimated $51.9 billion (Yang et al., 2020). The hallmark pathology of PD is characterized by the presence of proteinaceous intraneuronal inclusions (Lewy bodies) and degeneration of dopaminergic neurons of the substantia nigra (SN) with axonopathy of striatal dopaminergic terminals preceding the degeneration of the soma (Kordower et al., 2013). In addition, loss of neurons in the pedunculopontine nucleus, locus coeruleus, dorsal motor nucleus of the vagus, and the nucleus basalis of Meynert is observed (Giguère et al., 2018) . Further, positron emission tomography (PET) imaging in early PD subjects or asymptomatic carriers of leucine-rich repeat kinase 2 (LRRK2) exhibit normal DA synthesis and storage, however DA turnover is increased and dopamine transporter (DAT) and vesicular monoamine transporter type 2 (VMAT2) are reduced (Adams et al., 2005; Sossi et al., 2002, 2010).
Lewy bodies are primarily composed of the misfolded, phosphorylated protein alpha-synuclein (α-syn). α-syn is abundantly expressed in neurons where it exists normally in its monomeric, soluble form, however this inherently disordered protein has a propensity to misfold into secondary and tertiary structures (Bisi et al., 2021). Misfolded a-syn exposes serine 129, allowing for its phosphorylation, however it is unclear whether this facilitates or inhibits aggregation (Fujiwara et al., 2002; Paleologou et al., 2008; Tenreiro et al., 2014). Within the parkinsonian brain, Lewy bodies are widespread in numerous regions including areas in which neurodegeneration is observed, such as the SN pars compacta (SNpc).
Neuroinflammation in PD
Neuroinflammation is increasingly appreciated as being associated with PD. Research examining microglial reactivity in PD has focused on markers of microglial activation and measurements of microglial-associated inflammatory cytokines in PD brain, cerebrospinal fluid (CSF) and blood. Increases in cells immunoreactive for ionized calcium binding adaptor molecule 1 (Iba1), a macrophage marker which is highly expressed in microglia, are observed in the PD SN, as are increases in CD68 positive ameboid microglia (Croisier et al., 2005; Doorn et al., 2014). Further, increased expression of human leukocyte antigen related-D (HLA-DR, human analog for major histocompatibility complex-II, MHC-II) is observed in the PD SN and striatum (Imamura et al., 2003; McGeer et al., 1993; Mcgeer et al., 1988a, 1988b). HLA-DR is a cell surface protein found on antigen presenting cells (e.g. microglia) that interact with T-cells and the adaptive immune system (Schetters et al., 2018). Increased risk of PD is associated with HLA-DR variants that confer overactivity (Kannarkat et al., 2015). The SN and striatum of PD brains possess elevated cytokines produced by microglia and immune cells, including interleukin-6 (IL-6), interIeukin-1-beta I (IL-1ß), interleukin-2 (IL-2), interleukin-4 (IL-4) and tumor necrosis factor-alpha (TNF-α) (Mogi et al., 1994a, 1994b; Nagatsu et al., 2000). Furthermore, PD CSF contains elevated levels of IL-1ß, IL-6 and transforming growth factor-beta 1 (TGF-ß1) (Chen et al., 2018). Analysis of blood levels in PD subjects reveals increased IL-6, TNF-α, IL-1ß, IL-2, interleukin-10 (IL-10), C reactive protein and regulated upon activation, normal T cell expressed and presumably secreted (RANTES) (Qin et al., 2016). Positron emission tomography (PET) imaging of microglial activation reveals widespread neuroinflammation not only in the SN, but in brain regions associated with further PD progression (Gerhard et al., 2006; Ouchi et al., 2005). While most studies examining innate immune activation in the PD brain have focused on the response of microglia, astrocytes are, in fact, the most abundant glial cell type of the brain (Matejuk & Ransohoff, 2020). Microglia can convert astrocytes to a toxic proinflammatory A1 astrocyte and A1 astrocytes have been observed in the PD SN (Liddelow et al., 2017). Additionally, genetic forms of PD have been shown to involve genes expressed by astrocytes with implications in astrocyte biology (e.g. GBA, LRRK2, PINK1) (Booth et al., 2017). Collectively, the evidence of heightened innate immune activity in the parkinsonian brain is abundant.
Whereas the aforementioned studies have focused on understanding the response of the innate immune system in PD, the observed increase in MHC-II, neuronal MHC-I expression (Cebrián et al., 2014) as well as the presence of immunoglobulin G on dopaminergic neurons in PD brains (Orr et al., 2005) suggests an additional role for the adaptive immune system in PD. In 2009, Brochard et al. reported T-cell infiltration (Both CD4+ and CD8+) in postmortem PD brains. Additionally, T cells from PD subjects, and not control subjects, recognize different forms of α-syn peptides (Lindestam Arlehamn et al., 2020; Sulzer et al., 2017). Ongoing investigations continue to characterize the response of the innate immune system in PD.
Lewy Bodies as a Potential Immunogenic Signal
The overwhelming majority of studies characterizing neuroinflammation in PD are unable to distinguish between Lewy body-associated inflammation and degeneration-associated inflammation, as both pathologies are present simultaneously. It is widely accepted that the formation of Lewy bodies precedes neurodegeneration, and that the formation of Lewy bodies is ultimately associated with pathogenic consequences (Braak et al., 2003; Miller et al., 2021a). Some studies provide evidence to suggest immunogenicity of Lewy bodies specifically, prior to degeneration; specifically, MHC-II class receptor expression (HLA-DQA1, HLA-DRA, HLA-DRB1) is upregulated in premotor PD SN (Braak stage 1–2) compared with controls, but not in other stages of the pathological progression (Dijkstra et al., 2015). Further, studies that examine the relationship between abundance of Lewy body pathology and neuroinflammation report a positive association. MHC-II immunoreactivity is observed coincident with Lewy body pathology in PD (Imamura et al., 2003; McGeer et al., 1993; McGeer et al., 1988a, 1988b; Rostami et al., 2020) with a significant correlation observed between the level of MHC-II expression and Lewy body deposition (Croisier et al., 2005; Rostami et al., 2020). Understanding whether Lewy bodies initiate specific immunogenic signals that contribute to neurodegeneration will be required to develop effective neuroprotective strategies in PD.
Leveraging the α-syn Preformed Fibril Model to Understand Neuroinflammation in PD
Limitations of Preclinical PD Models
Animal models can serve as platforms to investigate the role of neuroinflammation in PD. Many studies have investigated the neuroinflammatory consequences of nigral degeneration and/or α-syn aggregation using neurotoxicant models (Cicchetti et al., 2002; Forno et al., 1993; Koprich et al., 2008; Kurkowska-Jastrze et al., 1999); transgenic overexpression models of human wild type or mutated asyn (A53T, A30P) (Gao et al., 2011; Gomez-Isla et al., 2003); and/or viral vector mediated α-syn overexpression models (Koprich et al., 2008; Sanchez-Guajardo et al., 2010). However, while these models are useful, they are limited in their ability to capture a prolonged interval of α-syn aggregation that culminates in dopaminergic neuron loss. Neurotoxicant models result in nigrostriatal neurodegeneration with little to no α-syn pathology. Transgenic α-syn overexpression models, despite widespread α-syn pathology, rarely show significant degeneration. A viral vector based α-syn overexpression approach produces robust neuroinflammation prior to degeneration (Fischer et al., 2016; Sanchez-Guajardo et al., 2010), but the accelerated aggregation and toxicity observed in this model confounds examination of distinct disease stages. Further, the contribution to the inflammatory response of supraphysiological α-syn levels, or the species difference of the α-syn overexpressed, is unclear. Importantly, in idiopathic PD total α-syn levels are not increased (Su et al., 2017; Zhou et al., 2011), rather phosphorylation of α-syn, and the ratio of soluble to insoluble α-syn, increases (Zhou et al., 2011). As such, models based on the overexpression of α-syn are not likely to accurately recapitulate the pathological state of idiopathic PD. Indeed, this raises the possibility that supraphysiological α-syn expression may drive pathophysiological mechanisms that are not relevant to idiopathic PD. A model in which α-syn inclusions are triggered to form within the context of normal α-syn expression levels likely represents a more faithful animal model of idiopathic PD.
The α-syn Preformed Fibril Model
In the α-syn preformed fibril (PFF) model, widespread α-syn aggregation can be triggered to form within the context of normal levels of endogenous α-syn. This phenomenon was first developed in vitro using primary neuronal cultures (Volpicelli-Daley et al., 2011), in which recombinant α-syn fibrils are introduced to cell culture and taken up by neurons, which leads to the templating of the endogenous α-syn into accumulation of insoluble α-syn aggregates comprised of phosphorylated α-syn (Luk et al., 2009; Volpicelli-Daley et al., 2011, 2014) followed by neuronal dysfunction and degeneration. This toxicity is not due to the initial PFFs per se, but instead can be directly tied to the recruitment of endogenous α-syn into inclusions, as evidenced by the fact that PFFs do not induce toxicity when applied to α-syn−/− neurons (Volpicelli-Daley et al., 2011). Similarly, intraparenchymal injections of α-syn PFFs into mice, rats, marmosets and monkeys results in the templating, accumulation, and phosphorylation of endogenous α-syn (Abdelmotilib et al., 2017; Chu et al., 2019; Creed & Goldberg, 2020; Duffy et al., 2018; Henderson et al., 2019; Luk et al., 2012a, 2012b; Negrini et al., 2022; Patterson et al., 2019; Paumier et al., 2015; Shimozawa et al., 2017; Stoyka et al., 2020; Thakur et al., 2017). The observed α-syn inclusions share multiple features with Lewy bodies, including immunoreactivity for α-syn phosphorylated at serine 129 (pSyn), p62, and ubiquitin, and are also thioflavin-S positive and proteinase-K resistant (Duffy et al., 2018; Patterson et al., 2019; Paumier et al., 2015).
pSyn immunoreactivity labels multiple stages of a-syn aggregation, ranging from oligomers to fibrils (Duffy et al., 2018; Patterson et al., 2019). It is unclear what role, if any, early forms of pathological a-syn (dimers, trimers, oligomers) (Bengoa-Vergniory et al., 2017; Garcia et al., 2022; Wan & Chung, 2012; Winner et al., 2011) or end stage Lewy body-like inclusions directly play in toxicity and immunogenicity. In addition, the process of Lewy body-like inclusion formation may interfere with multiple cellular functions (Mahul-Mellier et al., 2020) and/or the depletion of monomeric α-syn (Kanaan & Manfredsson, 2012) may also have detrimental consequences. The presence of pSyn inclusions is associated with all of these potential contributors to pathogenesis.
The pattern of pSyn accumulation in the PFF model following intracerebral injection appears dictated by the connectivity of the injection site. For example, following intrastriatal PFF injection aggregates are observed in neurons located in brain regions with abundant innervation to the striatum (Wall et al., 2013) including multiple cortical regions, SN, ventral pallidum and amygdala with pSyn eventually accumulating within striatal neurons surrounding the injection site (Duffy et al., 2018; Guo et al., 2020; Luk et al., 2012a, 2012b; Patterson et al., 2019; Paumier et al., 2015; Thomsen et al., 2021)
The experimental parameters that determine the magnitude of α-syn inclusion formation in the α-syn PFF model can be grouped into four main categories: species of fibrils used; quantity of fibrils injected; amount of endogenous α-syn; and post injection interval. Regarding fibril species, comparisons of seeding efficiencies of different fibril types have been investigated in both rats and mice (Luk et al., 2016; Howe et al., 2021). Injections into WT mice of either mouse PFFs (mPFFs) or human PFFs (huPFFs) demonstrate that mPFFs possess a higher seeding efficiency, leading to more rapid peak aggregation accumulation than that observed following huPFF injection (Luk et al., 2016; Masuda-Suzukake et al., 2013; Sorrentino et al., 2017). Studies in rats comparing injections of mPFFs to rat PFFs (rPFFs) reveal superior seeding efficiency using mPFFs (Howe et al., 2021). Furthermore, seeding efficiency can also be influenced by the type of synuclein strain used (Peelaerts et al., 2015; Peng et al., 2018; Rey et al., 2016; Van der Perren et al., 2020). For the purposes of this paper we will focus on studies that utilize recombinant mouse, rat, and/or human fibrils.
Regarding levels of endogenous α-syn, it is well-established that the presence of endogenous α-syn is required for templating PFFs after internalization by neurons (Luk et al., 2012a, 2012b; Volpicelli-Daley et al., 2011). Further, the relative levels of intraneuronal α-syn also can influence the magnitude/rate of α-syn inclusion formation. Courte et al. (2020) demonstrated in vitro that neuronal cell types with lower α-syn expression levels (striatum) showed less efficient inclusion formation compared to neuronal cell types with higher levels of α-syn expression (cortex, hippocampus), despite an equivalent uptake of PFFs. In transgenic mice that exhibit α-syn overexpression (M20, M83, and A30P), α-syn inclusion formation is also more efficient compared with wild type (WT) mice (Gentzel et al., 2021; Sorrentino et al., 2017). Regarding the quantity of PFFs, it has been shown in rats that an increase in the quantity of mPFFs injected (8 μg versus 16 μg) results in increased α-syn inclusion formation as well as an increase in the subsequent magnitude of nigral degeneration (Patterson et al., 2019).
All the aforementioned factors contribute to the time course and magnitude of α-syn inclusion formation following injection of PFFs, resulting in a pathological cascade of events that can be understood in two main phases: Phase 1: The accumulation and peak formation of α-syn inclusions in nigrostriatal system and, Phase 2: Protracted dopaminergic neuron degeneration. In Phase 1, in WT mice or rats, intrastriatal or intranigral injection of mPFFs has an observed peak of α-syn aggregation in the SN at 1-3 months post injection (Duffy et al., 2018; Harms et al., 2017; Izco et al., 2021; Patterson et al., 2019) whereas injection of huPFFs into the striatum of WT mice delays this peak to approximately 4-6 months post injection (Earls et al., 2019; Sorrentino et al., 2017). The use of transgenic mice with higher levels of α-syn expression offsets the liability of using less efficient huPFFs, allowing for peak nigral α-syn inclusion formation at approximately 1-2 months post injection (Earls et al., 2020; Gentzel et al., 2021; Luk et al., 2012a). Importantly, α-syn inclusion formation in striatal neurons, despite direct injections to this location, is significantly delayed following injections of PFFs into WT rats and mice, likely due to lower levels of endogenous α-syn in this cell population (Courte et al., 2020). In WT mice and rats robust intraneuronal pSyn inclusion formation is not observed in the striatum until 5-6 months following intrastriatal mPFF injection, and pSyn observed in the striatum at earlier time points is generally localized to terminals of inclusion-bearing neurons of the SN and cortex (Duffy et al., 2018; Luk et al., 2012b; Patterson et al., 2019).
pSyn inclusions ultimately lead to neuronal dysfunction and degeneration (Osterberg et al., 2015; Volpicelli-Daley et al., 2011). In long term in vivo studies, data strongly suggest that the SN neurons that form pathological α-syn inclusions during Phase 1 are the same SN neurons that ultimately degenerate in Phase 2; for example, in WT mice and rats injected intrastriatally with mPFFs, the peak pSyn inclusion formation observed in the SN at two months is followed by a progressive reduction of nigral α-syn inclusions, in parallel to the loss of tyrosine hydroxylase (TH) and dopamine transporter (DAT) expression over the course of a six-month post injection interval (Duffy et al., 2018; Luk et al., 2012a; Patterson et al., 2019; Paumier et al., 2015). However, overt neuronal death (in contrast to loss of dopaminergic phenotype) is not observed until 5-6 months (Duffy et al., 2018; Luk et al., 2012a; Patterson et al., 2019; Thomsen et al., 2021) corresponding to the time point when the lowest numbers of pSyn containing nigral neurons are observed. Collectively, this pattern of observations suggests that in WT rats and mice nigral neurons survive with α-syn inclusions for a 3-month interval (Phase 1) after which undefined pathological sequelae lead to neuronal dysfunction and ultimately degeneration (Phase 2). Furthermore, the pattern of striatal terminal degeneration in the PFF model shares similarities with that observed in early-stage PD subjects (Adams et al., 2005; Sossi et al., 2002, 2010). Specifically, PET imaging in mPFF injected rats demonstrates that DA synthesis and storage is relatively preserved in the ipsilateral striatum at early time points, whereas loss of dopamine transporter (DAT) occurs earlier and progresses over time (Sossi et al., 2022). Reduction in vesicular monoamine transporter type 2 (VMAT2) is also observed in the ipsilateral striatum prior to overt nigral degeneration (Thomsen et al., 2021). Overall, the pattern of nigrostriatal degeneration observed following intrastriatal PFF injection to rats and mice is one of early nigrostriatal terminal dysfunction and loss of dopaminergic phenotype (Phase 1) followed by overt nigral degeneration (Phase 2) with the magnitude of these events dependent upon the efficiency of initial α-syn inclusion formation.
It should be noted that in some studies in which mPFFs are unilaterally injected into the striatum of WT rats, degeneration of both the ipsilateral and contralateral SN can be observed (Duffy et al., 2018; Patterson et al., 2019; Paumier et al., 2015), although not consistently (Sossi et al., 2022; Thomsen et al., 2021). When contralateral SN degeneration does occur it is of a lesser magnitude than the ipsilateral SN degeneration, and is independent of pSyn inclusion formation which remains exclusively ipsilateral to the injected striatal hemisphere (Duffy et al., 2018; Patterson et al., 2019; Paumier et al., 2015). Loss of contralateral nigral TH immunoreactivity and ultimately neuronal loss proceeds on a delayed time course relative to the ipsilateral degeneration, suggesting that an imbalance in striatal DA may participate in this phenomenon (Patterson et al., 2019). We speculate that the contralateral intact striatum may increase DA synthesis/release in response to loss of striatal DA in the PFF injected hemisphere (Paumier et al., 2015), perhaps resulting in toxic levels of reactive oxygen species that impact the survival of contralateral nigral neurons. Ultimately, the specific PFF model parameters eliciting bilateral degeneration and the mechanism of this phenomenon remain to be determined.
An appreciation of the timing of the pathological events, and the factors that contribute to them, provides a framework to interpret results of studies examining neuroinflammation in the α-syn PFF model. Specifically, to characterize α-syn inclusion-associated immunogenicity it is necessary to know whether significant inclusion formation is present in the region examined at the time of analyses (Phase 1). Conversely, immunogenic signals that are associated with nigral degeneration can be appreciated by examining nigrostriatal tissue at later intervals (Phase 2), ideally with parallel analyses that confirm neurodegeneration. In the following section we summarize the current understanding of neuroinflammatory observations in the α-syn PFF model, leveraging the distinct Phase 1 aggregation phase and Phase 2 degeneration phase to guide our interpretations.
α-syn Inclusion Associated Neuroinflammation
Considerations of Experimental Parameters
Intraparenchymal injections of α-syn PFFs result in a relatively synchronous wave of inclusion formation and accumulation in the SNpc (Phase 1) followed by dopaminergic phenotype loss and nigrostriatal degeneration (Phase 2). For the purposes of this review, we will define the Phase 1 aggregation as the interval during which abundant intraneuronal pSyn immunoreactive inclusions form and accumulate in the SNpc. Of note, studies have examined the neuroinflammatory response to PFF injections, in mice and rats, within 1-3 days following injection, prior to the formation of pSyn inclusions, and have observed increases in Iba1 and MHC-II immunoreactivity (Harms et al., 2017; Karampetsou et al., 2017). This preaggregation phase represents an acute inflammatory response to the PFFs themselves, and not an inflammatory response to inclusion-bearing neurons. In support of this, in vitro studies using microglial cell lines (Sarkar et al., 2020) or isolated primary microglia (Harm et al., 2013; Hoenen et al., 2016) demonstrate an inflammatory response to the addition of α-syn PFFs, in the absence of inclusion formation. Further, when examining neuroinflammatory responses in the PFF model careful comparisons, ideally to monomer-injected, or vehicle-injected controls, are necessary to differentiate between the effect of inclusions and the effect of surgical injection. Although inflammation associated with surgical injection is expected to be particularly pronounced within days of PFF injection at the injection site (e.g. analysis of striatum following intrastriatal PFF injection), we have observed a significant neuroinflammatory response in the distal SN for up to 1 month following control intrastriatal injections in rats (Duffy et al., 2018). Further, analysis of neuroinflammation in the striatum in Phase 1, in both rats and mice, is less than ideal for identification of α-syn inclusion-associated neuroinflammation since pSyn accumulation at this phase is limited to the terminals of inclusion-bearing neurons in the SN and cortex (Duffy et al., 2018; Luk et al., 2012a; Patterson et al., 2019). Inflammasome activation has been identified in the striatum one month following intrastriatal injections of mPFFs in WT mice (Gordon et al., 2018), however the inclusion burden in the striatum was likely not significant at this time point. To identify the strongest evidence of neuroinflammation associated with the accumulation of pathological α-syn inclusions specifically, we highlight and summarize the results from studies examining neuroinflammation in the SN in which the experimental design facilitates interpretations in light of these potentially confounding factors.
Nigral α-syn Inclusions Are Associated with Increased Innate Immune System Markers
Multiple studies have revealed that the presence of pathological α-syn inclusions in the SNpc is associated with changes in the innate immune system, as assessed by immunohistological markers of microglial and/or astrocytic activation and increased expression of inflammatory cytokines. Changes in microglial shape, size and an increase in number have been reported in the SN of α-syn overexpressing mice, WT mice, and WT rats, in studies in which mouse or human α-syn PFF experimental conditions generated pSyn pathology in the SNpc (Duffy et al., 2018; Earls et al., 2019, 2020; Garcia et al., 2022; Gentzel et al., 2021; Guo et al.,2020; Izco et al., 2021). Similarly, studies in WT rats (Duffy et al., 2018; Harms et al., 2017; Miller et al., 2021b; Thomsen et al., 2021) and one study in WT mice (Earls et al., 2019a) reveal increased MHC-II expression in the SN in association with robust nigral pSyn pathology. Of note, when longer experimental intervals allow for analysis of the relationship between nigral pSyn burden and number of MHC-II presenting cells in the SNpc of rats, a strong positive correlation is observed (Duffy et al., 2018).
Although the main focus to date has been to examine microglial alterations in association with the formation of intraneuronal nigral inclusions, it is possible that microglia directly contribute to α-syn aggregation or spread. Microglia experimentally induced to form pSyn inclusions are capable of releasing α-syn-containing exosomes that, in turn, can induce the formation of pSyn inclusions in neurons both in vitro and in vivo (Guo et al., 2020). Further, microglial/macrophage-derived exosomes derived from PD patient CSF contain α-syn oligomers and can similarly induce aggregation of α-syn in neuronal cultures (Guo et al., 2020). Further study will be required to determine whether microglia significantly impact the accumulation of pathological α-syn in the brain.
Analysis of the astrocytic inflammatory response to nigral pSyn pathology resulting from mouse and human PFF injections to WT mice and WT rats provides evidence of increased astrocytic branching and glial fibrillary acidic protein (GFAP) immunoreactivity (Earls et al., 2019; Garcia et al., 2022; Izco et al., 2021; Miller et al., 2021a), with a positive association observed between pSyn and GFAP immunoreactivity (Miller et al., 2021b). Beyond immunohistochemical approaches, nigral pSyn pathology also is associated with increased mRNA expression of the proinflammatory cytokines TNF-α and IL-1ß (Izco et al., 2021) in studies using mPFF injections into WT mice. Additionally, transcriptomic analysis in WT mice following a mPFF striatal injection also has shown an enrichment of innate inflammatory pathways in response to α-syn aggregation (e.g. cytokine regulation, production and secretion, regulation of ROS production, and regulation of phagocytosis and endocytosis) (Garcia et al., 2022).
Nigral α-syn Inclusions are Associated with Peripheral and Adaptive Cell Infiltration
Although fewer studies have examined peripheral and adaptive immune involvement in the α-syn PFF model, three reports provide evidence of infiltration of lymphocytes and macrophages in WT mice and rats, and natural killer (NK) cells in α-syn overexpressing mice during the pathological α-syn aggregation phase. Peripheral cell infiltration, comprised of increased percentages of B lymphocytes (CD19+), T-helper lymphocytes (CD4+), T-cytotoxic lymphocytes (CD8+), activated myeloid cells (CD11b+/CD45 high) and natural killer cells (TCRß -/MK1.1+), is observed in the CNS parenchyma during the pSyn aggregation phase following intrastriatal hPFF injections to WT mice, as well as a decrease in the percentage of resting myeloid cells (CD11b+/CD45 low) (Earls et al., 2019). The infiltration of monocytes/macrophages (CD11b+/CD45 high and CD163+) and T-helper lymphocytes (CD4+), within pSyn inclusion-bearing nigral tissue specifically, has also been observed following intranigral injection of mPFFs into rats (Harms et al., 2017). In α-syn overexpressing mice given an intrastriatal injection of hPFFs , pSyn accumulation in the SNpc was accompanied by a 5-fold increase in infiltration of natural killer (NK) cells (Earls et al., 2020), a type of cytotoxic lymphocyte that can inhibit proinflammatory microglia (Earls & Lee, 2020). Additionally, transcriptomic analysis in the mouse striatum following a mPFF striatal injection also has shown an enrichment of peripheral inflammatory response in response to α-syn aggregation (e.g. Ptprc (CD45) and CD4 antigen) (Garcia et al., 2022). Collectively, these studies implicate multiple peripheral and adaptive immune cell types associated with the accumulation of pathological α-syn aggregates following PFF injection.
Conclusions: α-syn Inclusion Associated Neuroinflammation
Numerous studies consistently demonstrate that pathological α-syn aggregation in the SN is associated with activation of the innate immune system, supported by findings of multiple markers of microgliosis and astrogliosis. Although the present summary focuses on PFF-induced pSyn accumulation in the SN, support for the relationship between α-syn aggregation, microgliosis and astrogliosis also comes from studies in which mouse and human PFFs are used to induce pSyn aggregates in other brain regions, including the olfactory bulb and hippocampus (Luk, et al., 2012; Rey et al., 2016; Sacino et al., 2014; Uemura et al., 2021). Strong evidence also demonstrates that pSyn accumulation is associated with MHC-II immunoreactivity, which is proportionate to inclusion load. Our evolving understanding of the neuroinflammatory response to α-syn aggregation in the SN also suggests a role for the involvement of peripheral and adaptive immune cell infiltration. Importantly, the defined stages of the α-syn PFF model, with prolonged aggregation preceding degeneration, provides evidence that intraneuronal, Lewy-body like aggregates are immunogenic, and that α-syn inclusion associated neuroinflammation may contribute to the degenerative process.
Nigral Degeneration-Associated Neuroinflammation
Considerations of Experimental Parameters
Following the α-syn inclusion formation and accumulation observed in the SNpc in Phase 1, protracted dopaminergic phenotype loss and nigrostriatal degeneration occurs during Phase 2 over the course of several months. For the purposes of this review, we will define the Phase 2 as the interval during which pSyn immunoreactive inclusions are decreasing as nigral dopamine neurons degenerate, confirmed histologically using stereologic assessments of nigral TH immunoreactive neurons, ideally including additional stereological assessments to confirm overt neurodegeneration (Duffy et al., 2018; Ma et al., 2021; Patterson et al., 2019; Paumier et al., 2015; Thomsen et al., 2021). In some cases, neurodegeneration has been presumed in the studies detailed below based on predictions informed by the specific experimental parameters used and the timing of the assessments.
Nigral degeneration Increases Innate Immune System Markers
Intrastriatal mPFF injections into WT mice results in increased Iba1 immunoreactivity and Iba1 immunoreactive microglia at a time when nigral dopamine neurons have undergone significant degeneration (Yun et al., 2018). Under identical nigral degeneration conditions, mRNA expression levels of TNF-α, interleukin-1 alpha (IL-1 α) and complement component 1 Q subcomponent alpha (C1qa) are elevated in the SN compared with controls (Yun et al., 2018). Three studies to date, conducted using either intrastriatal or intranigral injections of mPFFs into WT rats, have examined the longitudinal microglial response in the SN during both the aggregation and dopamine neuron degeneration phases of the model (Duffy et al., 2018; Harms et al., 2017; Thomsen et al., 2021). Results of all three are in agreement: that inclusion-triggered nigral degeneration observed 5-6 months following PFF injection is associated with increased MHC-II immunoreactivity compared with control rats (Duffy et al., 2018; Harms et al., 2017; Thomsen et al., 2021), with some studies indicating that the magnitude of MHC-II immunoreactivity observed during the degenerative phase is considerably less than that observed previously during the aggregation phase (Duffy et al., 2018; Thomsen et al., 2021). Markers indicative of nucleotide-binding oligomerization domain, leucine-rich repeat-containing protein 3 (NLRP3) inflammasome activation, associated with a proinflammatory microglial response, are significantly increased following nigral degeneration (Gordon et al., 2018). Specifically, protein levels of NLRP3 and apoptosis-associated speck-like protein containing a CARD (ASC) are significantly increased in the SN 8 months after intrastriatal mPFF injections into mice (Gordon et al., 2018).
The response of astrocytes also has been investigated during the nigral degeneration phase of the PFF model, specifically examining components of the complement system, associated with both A1 proinflammatory astrocytes and activated microglia. Components of the complement system label neurons, tagging them for phagocytosis (Liddelow et al., 2017; Zamanian et al., 2012). In WT mice injected with intrastriatal mPFF, GFAP protein and complement component 3d (C3d) are increased in the SN in association with significant nigral degeneration, as is the percentage of GFAP+ astrocytes expressing C3d and many elevated transcripts associated with the A1 astrocytic phenotype (Yun et al., 2018). Further evidence of a role for the complement system during the degenerative phase of the SN following intrastriatal mPFF injections in mice comes from a comprehensive analysis of the proteome (Ma et al., 2021).
Nigral Degeneration is Associated with Peripheral Cell Infiltration
To our knowledge, only one study to date reports on peripheral immune cell markers in the SN during the degenerative phase induced by PFF injections. Six months following mPFF intranigral injections in rats, the infiltration of monocytes/macrophages (CD163+) is observed, indicating the earlier increase first observed during the aggregation phase was sustained long term (Harms et al., 2017).
Conclusions: Nigral Degeneration Associated Neuroinflammation
Studies demonstrate that the nigral degenerative phase induced in the α-syn PFF model is associated with activation of the innate immune system supported by findings of multiple markers of microgliosis and astrogliosis. However, studies in which a single time point during the degenerative phase is examined cannot rule out if neuroinflammation preceded, or was triggered by, nigral degeneration. Interestingly, the few reports that compare the neuroinflammatory response in the SN across both the aggregation and degeneration phases suggest that microglial activation, as assessed by MHC-II immunoreactivity, is greater in magnitude in the aggregation phase preceding degeneration. Relatively little is known about involvement of the peripheral and adaptive immune system during the degenerative phase of the α-syn PFF model.
Overall Conclusions and Future Directions
The α-syn PFF model provides an experimental platform that allows for investigation of the neuroinflammatory consequences of the progression of pathological α-syn to neurodegeneration ( Figure 1 ). The concordance between the neuroinflammatory events documented in the parkinsonian brain and those observed in the α-syn PFF model (microgliosis, association of MHC-II immunoreactivity with α-syn inclusions, cytokine expression, astrogliosis, and T-cell infiltration) suggests that this model platform can prove useful for design of anti-inflammatory neurotherapeutics. However, there is much that we still need to learn about neuroinflammation in this model and whether it fully recapitulates neuroinflammation in PD. For example, proinflammatory cytokines and chemokines are elevated in the blood (IL-6, TNF-α, IL-1ß, IL-2, IL-10, C reactive protein, RANTES) and CSF (IL-1ß, IL-6, TGF-ß1) (Chen et al., 2018; Qin et al., 2016) of PD subjects. It is presently unknown whether a similar elevation is observed in the α-syn PFF model. The observation that any of these factors are increased during the α-syn aggregation phase may support their use as a biomarker in prodromal PD. Further, whereas CD68, MHC-II, and C3d expression has been observed in the parkinsonian SN (Croisier et al., 2005; Doorn et al., 2014; Garcia et al., 2022; Liddelow et al., 2017; Rostami et al., 2020), our overall understanding of the microglial and astroglial phenotype in response to α-syn inclusions and/or degeneration is in its infancy. The α-syn PFF model could be harnessed to fully characterize the neuroinflammatory response of microglia and astrocytes and, if ultimately validated in PD brains, this approach may yield insight into the design of novel targeted therapeutics.
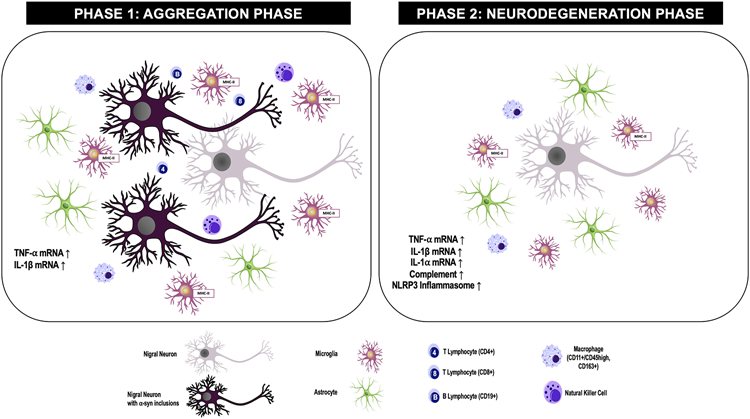
Figure 1: Inflammation markers identified in the substantia nigra during the aggregation or degeneration phases of the alpha-synuclein (α-syn) preformed fibril (PFF) model.
Aggregation Phase: α-syn Inclusions: pSyn accumulates and the number of pSyn immunoreactive neurons peaks. DA Neurons: No significant loss of DA neurons is observed. Microglia: Microglial immunoreactivity (Iba1), microglial soma size and MHC-II immunoreactivity is significantly elevated relative to control animals without pSyn inclusions. A positive association between MHC-II+ microglia and number of pSyn+ neurons is observed. Astrocytes: Astrocytic immunoreactivity (GFAP), length and branching of processes increases relative to control animals without pSyn inclusions. A positive association between GFAP immunoreactivity and number of pSyn+ neurons is observed. Natural Killer (NK) Cells: Infiltrating NK cells are increased relative to control animals without pSyn inclusions. Macrophages: Infiltrating macrophages (CD11b+/CD45+, CD163+) are increased relative to control animals without pSyn inclusions. T Lymphocytes: CD4+ and CD8+ T Lymphocytes are increased relative to control animals without pSyn inclusions. B lymphocytes: B Lymphocytes are increased relative to control animals without pSyn inclusions. Cytokines: TNF-α and IL-1ß mRNA is increased relative to control animals without pSyn inclusions. NLRP3 Inflammasome and Complement: Unknown.
Degeneration Phase: α-syn Inclusions: Neurons in which pSyn inclusion previously formed have degenerated, pSyn inclusions are nearly absent. Nigral Dopamine Neurons: Significant loss of DA neurons has occurred. Microglia: MHC-II+ positive microglia are increased relative to controls without degeneration but decreased relative to the number of MHC-II+ microglia observed during the aggregation phase. NLRP3 Inflammasome: NLRP3 inflammasome activation (NLRP3, ASC protein) is increased relative to control animals without degeneration. Astrocytes: Astrocytic immunoreactivity (GFAP) is increased relative to control animals without degeneration. Complement: Complement component 3d (C3d), GFAP+/C3d+ astrocytes and transcripts associated with the A1 astrocytic phenotype are increased relative to control animals without degeneration. Natural Killer Cells: Not observed in the degeneration phase. Macrophages: Infiltrating macrophages (CD163+) are increased relative to control animals without degeneration. Cytokines: TNF-α, IL-1ß and IL-1 α mRNA is increased relative to control animals without degeneration. T and B Lymphocytes: Unknown.
pSyn = α-syn phosphorylated at serine 129; DA = dopamine; Iba1 = ionized calcium binding adaptor molecule 1; MHC-II = major histocompatibility complex-II; GFAP = glial fibrillary acidic protein; NLRP3 = nucleotide-binding oligomerization domain, leucine-rich repeat-containing protein 3; ASC = apoptosis-associated speck-like protein containing a CARD; TNF-α = tumor necrosis factor-alpha; IL-1ß = interleukin 1-beta; IL-1 α = interleukin 1-alpha
Regarding the role of the adaptive and peripheral immune system, in general, relatively little is known about the time course and pattern of its involvement across the progressive stages of pathology in PD. Although infiltration by T and B lymphocytes, macrophages and natural killer cells has been identified during the α-syn aggregation phase of the PFF model (Earls et al., 2019, 2020; Harms et al., 2017), it remains unclear whether all these cell types persist and if the magnitude of their involvement is altered during the degenerative phase. Additional investigation of the temporal pattern of adaptive and peripheral immune cell infiltration using longitudinal analysis in the PFF model is warranted. Another area worthy of investigation is whether cytotoxic T cells isolated from brains of PFF model animals are able to recognize different epitopes of pathological α-syn when presented by MHCII, as has been shown with cytotoxic T cells isolated from PD brains (Sulzer et al., 2017).
Neuroinflammation has become a well-accepted pathological hallmark of PD. However, whether inflammation triggered by either α-syn aggregation and/or degeneration contributes to the progression of the disease remains one of the most critical questions facing the field. By leveraging the relatively synchronous wave of α-syn aggregation followed by the relatively synchronous wave of degeneration of the SNpc of the α-syn PFF model, numerous studies have identified a significant and early neuroinflammatory response of the innate, adaptive, and peripheral immune systems that is associated with the formation of pathological α-syn aggregates in the SN. These findings put the neuroinflammation suspect at the scene of the crime, prior to degeneration. Further investigation will be required ultimately to reveal how, and if, this early inflammation participates in PD progression.
Highlights:
Lewy bodies, degeneration, or both, may contribute to neuroinflammation in PD.
The α-syn PFF model produces distinct phases of aggregation and degeneration.
This model can be leveraged to understand specific contributors to neuroinflammation.
PFF model aggregates may be immunogenic and thus may contribute to degeneration.
Similar neuroinflammatory markers are observed in both PD and in the PFF model.
Acknowledgments:
We are grateful to Christopher J. Kemp for his assistance in the preparation of this manuscript.
Funding Sources:
Supported by NS099416 and NS111333 from the National Institute of Neurological Disorders and Stroke and T32GM092715 from the National Institute of General Medical Sciences.
References:
- Abdelmotilib H, Maltbie T, Delic V, Liu Z, Hu X, Fraser KB, Moehle MS, Stoyka L, Anabtawi N, Krendelchtchikova V, Volpicelli-Daley LA, & West A (2017). α-Synuclein fibril-induced inclusion spread in rats and mice correlates with dopaminergic Neurodegeneration. Neurobiology of Disease, 105, 84–98. 10.1016/j.nbd.2017.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JR, van Netten H, Schulzer M, Mak E, Mckenzie J, Strongosky A, Sossi V, Ruth TJ, Lee CS, Farrer M, Gasser T, Uitti RJ, Calne DB, Wszolek ZK, & Stoessl AJ (2005). PET in LRRK2 mutations: Comparison to sporadic Parkinson’s disease and evidence for presymptomatic compensation. Brain, 128(12), 2777–2785. 10.1093/brain/awh607 [DOI] [PubMed] [Google Scholar]
- Bengoa-Vergniory N, Roberts RF, Wade-Martins R, & Alegre-Abarrategui J (2017). Alpha-synuclein oligomers: a new hope. In Acta Neuropathologica (Vol. 134, Issue 6, pp. 819–838). Springer Verlag. 10.1007/s00401-017-1755-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisi N, Feni L, Peqini K, Pérez-Peña H, Ongeri S, Pieraccini S, & Pellegrino S (2021). α-Synuclein: An All-Inclusive Trip Around its Structure, Influencing Factors and Applied Techniques. In Frontiers in Chemistry (Vol. 9). Frontiers Media S.A. 10.3389/fchem.2021.666585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth HDE, Hirst WD, & Wade-Martins R (2017). The Role of Astrocyte Dysfunction in Parkinson’s Disease Pathogenesis. Trends in Neurosciences, 40(6), 358–370. 10.1016/j.tins.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Tredici K. del, Rüb U, de Vos RAI, Jansen Steur ENH, & Braak E (2003). Staging of brain pathology related to sporadic Parkinson’s disease. In Neurobiology of Aging (Vol. 24). [DOI] [PubMed] [Google Scholar]
- Cebrián C, Loike JD, & Sulzer D (2014). Neuronal mhc-i expression and its implications in synaptic function, Axonal regeneration and parkinson’s and other brain diseases. Frontiers in Neuroanatomy, 8(OCT), 1–9. 10.3389/fnana.2014.00114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Hu Y, Cao Z, Liu Q, & Cheng Y (2018). Cerebrospinal fluid inflammatory cytokine aberrations in Alzheimer’s disease, Parkinson’s disease and amyotrophic lateral sclerosis: A systematic review and meta-analysis. In Frontiers in Immunology (Vol. 9, Issue SEP). Frontiers Media S.A. 10.3389/fimmu.2018.02122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y, Muller S, Tavares A, Barret O, Alagille D, Seibyl John, Tamagnan G, Marek K, Luk KC, Trojanowski JQ, Lee VMY, & Kordower JH (2019). Intrastriatal alpha-synuclein fibrils in monkeys: spreading, imaging and neuropathological changes. Brain, 142, 3565–3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti F, Brownell AL, Williams K, Chen YI, Livni E, & Isacson O (2002). Neuroinflammation of the nigrostriatal pathway during progressive 6-OHDA dopamine degeneration in rats monitored by immunohistochemistry and PET imaging. European Journal of Neuroscience, 15(6), 991–998. 10.1046/j.1460-9568.2002.01938.x [DOI] [PubMed] [Google Scholar]
- Courte J, Bousset L, Boxberg Y. von, Villard C, Melki R, & Peyrin JM (2020). The expression level of alpha-synuclein in different neuronal populations is the primary determinant of its prion-like seeding. Scientific Reports, 10(1). 10.1038/s41598-020-61757-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed RB, & Goldberg MS (2020). Enhanced Susceptibility of PINK1 Knockout Rats to α-Synuclein Fibrils. Neuroscience, 437, 64–75. 10.1016/j.neuroscience.2020.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croisier E, Moran LB, Dexter DT, Pearce RKB, & Graeber MB (2005). Microglial inflammation in the parkinsonian substantia nigra: Relationship to alpha-synuclein deposition. Journal of Neuroinflammation, 2. 10.1186/1742-2094-2-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra AA, Ingrassia A, de Menezes RX, van Kesteren RE, Rozemuller AJM, Heutink P, & van de Berg WDJ (2015). Evidence for immune response, axonal dysfunction and reduced endocytosis in the substantia nigra in early stage Parkinson’s disease. PLoS ONE, 10(6). 10.1371/journal.pone.0128651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doorn KJ, Moors T, Drukarch B, Dj Van De Berg W, Lucassen PJ, & van Dam A-M (2014). Microglial phenotypes and toll-like receptor 2 in the substantia nigra and hippocampus of incidental Lewy body disease cases and Parkinson’s disease patients. http://www.actaneurocomms.org/content/2/1/90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy MF, Collier TJ, Patterson JR, Kemp CJ, Luk KC, Tansey MG, Paumier KL, Kanaan NM, Fischer LD, Polinski NK, Barth OL, Howe JW, Vaikath NN, Majbour NK, El-Agnaf OMA, & Sortwell CE (2018). Lewy body-like alpha-synuclein inclusions trigger reactive microgliosis prior to nigral degeneration. Journal of Neuroinflammation, 15(1). 10.1186/s12974-018-1171-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earls RH, & Lee JK (2020). The role of natural killer cells in Parkinson’s disease. In Experimental and Molecular Medicine (Vol. 52, Issue 9, pp. 1517–1525). Springer Nature. 10.1038/s12276-020-00505-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earls RH, Menees KB, Chung J, Barber J, Gutekunst CA, Hazim MG, & Lee JK (2019). Intrastriatal injection of preformed alpha-synuclein fibrils alters central and peripheral immune cell profiles in non-transgenic mice. Journal of Neuroinflammation, 16(1). 10.1186/s12974-019-1636-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earls RH, Menees KB, Chung J, Gutekunst CA, Lee HJ, Hazim MG, Rada B, Wood LB, & Lee JK (2020). NK cells clear α-synuclein and the depletion of NK cells exacerbates synuclein pathology in a mouse model of α-synucleinopathy. Proceedings of the National Academy of Sciences of the United States of America, 117(3), 1762–1771. 10.1073/pnas.1909110117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer DL, Gombash SE, Kemp CJ, Manfredsson FP, Polinski NK, Duffy MF, & Sortwell CE (2016). Viral vector-based modeling of neurodegenerative disorders: Parkinson’s disease. In Methods in Molecular Biology (Vol. 1382, pp. 367–382). Humana Press Inc. 10.1007/978-1-4939-3271-9_26 [DOI] [PubMed] [Google Scholar]
- Forno L, DeLannet L, Irwin I, & Langston JW (1993). Similarities and differences between MPTP-induced parkinsonsim and Parkinson’s disease. Neuropathologic considerations. Adv Neuro, 60, 600–608. [PubMed] [Google Scholar]
- Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, & Iwatsubo T (2002). α-synuclein is phosphorylated in synucleinopathy lesions. Nature Cell Biology, 4(2), 160–164. 10.1038/ncb748 [DOI] [PubMed] [Google Scholar]
- Gao HM, Zhang F, Zhou H, Kam W, Wilson B, & Hong JS (2011). Neuroinflammation and α-synuclein dysfunction potentiate each other, driving chronic progression of neurodegeneration in a mouse model of Parkinson’s disease. Environmental Health Perspectives, 119(6), 807–814. 10.1289/ehp.1003013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia P, Jürgens-Wemheuer W, Uriarte Huarte O, Michelucci A, Masuch A, Brioschi S, Weihofen A, Koncina E, Coowar D, Heurtaux T, Glaab E, Balling R, Sousa C, Kaoma T, Nicot N, Pfander T, Schulz-Schaeffer W, Allouche A, Fischer N, … Buttini M (2022). Neurodegeneration and neuroinflammation are linked, but independent of alpha-synuclein inclusions, in a seeding/spreading mouse model of Parkinson’s disease. GLIA, 70(5), 935–960. 10.1002/glia.24149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentzel RC, Toolan D, Jinn S, Schachter JB, Ma L, Kahle PJ, Smith SM, & Marcus JN (2021). Intracranial administration of alpha-synuclein fibrils in A30P-synuclein transgenic mice causes robust synucleinopathy and microglial induction. Neurobiology of Aging, 106, 12–25. 10.1016/j.neurobiolaging.2021.05.012 [DOI] [PubMed] [Google Scholar]
- Gerhard A, Pavese N, Hotton G, Turkheimer F, Es M, Hammers A, Eggert K, Oertel W, Banati RB, & Brooks DJ (2006). In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiology of Disease, 21(2), 404–412. 10.1016/j.nbd.2005.08.002 [DOI] [PubMed] [Google Scholar]
- Giguère N, Nanni SB, & Trudeau LE (2018). On cell loss and selective vulnerability of neuronal populations in Parkinson’s disease. In Frontiers in Neurology (Vol. 9, Issue JUN). Frontiers Media S.A. 10.3389/fneur.2018.00455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Isla T, Irizarry MC, Mariash A, Cheung B, Soto O, Schrump S, Sondel J, Kotilinek L, Day J, Schwarzschild MA, Cha J-HJ, Newell K, Miller DW, Uéda K, Young AB, Hyman BT, & Ashe KH (2003). Motor dysfunction and gliosis with preserved dopaminergic markers in human-synuclein A30P transgenic mice. In Neurobiology of Aging (Vol. 24). [DOI] [PubMed] [Google Scholar]
- Gordon R, Albornoz EA, Christie DC, Langley MR, Kumar V, Mantovani S, Robertson AAB, Butler MS, Rowe DB, O’Neill LA, Kanthasamy AG, Schroder K, Cooper MA, & Woodruff TM (2018). Inflammasome inhibition prevents -synuclein pathology and dopaminergic neurodegeneration in mice. Science Translational Medicine, 10(465). 10.1126/scitranslmed.aah4066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Wang J, Zhao Y, Feng Y, Han S, Dong Q, Cui M, & Tieu K (2020). Microglial exosomes facilitate a-synuclein transmission in Parkinson’s disease. Brain, 143(5), 1476–1497. 10.1093/brain/awaa090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harm AS, Cao S, Rowse AL, Thome AD, Li X, Mangieri LR, Cron RQ, Shacka JJ, Raman C, & Standaert DG (2013). MHCII is required for α-Synuclein-induced activation of microglia, CD4 T cell proliferation, and dopaminergic neurodegeneration. Journal of Neuroscience, 33(23), 9592–9600. 10.1523/JNEUROSCI.5610-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms AS, Delic V, Thome AD, Bryant N, Liu Z, Chandra S, Jurkuvenaite A, & West AB (2017). α-Synuclein fibrils recruit peripheral immune cells in the rat brain prior to neurodegeneration. Acta Neuropathologica Communications, 5(1), 85. 10.1186/s40478-017-0494-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson MX, Cornblath EJ, Darwich A, Zhang B, Brown H, Gathagan RJ, Sandler RM, Bassett DS, Trojanowski JQ, & Lee VMY (2019). Spread of α-synuclein pathology through the brain connectome is modulated by selective vulnerability and predicted by network analysis. Nature Neuroscience, 22(8), 1248–1257. 10.1038/s41593-019-0457-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenen C, Gustin A, Birck C, Kirchmeyer M, Beaume N, Felten P, Grandbarbe L, Heuschling P, & Heurtaux T (2016). Alpha-synuclein proteins promote pro-inflammatory cascades in microglia: Stronger effects of the a53t mutant. PLoS ONE, 11(9). 10.1371/journal.pone.0162717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe JW, Sortwell CE, Duffy MF, Kemp CJ, Russell CP, Kubik M, Patel P, Luk KC, El-Agnaf OMA, & Patterson JR (2021). Preformed fibrils generated from mouse alpha-synuclein produce more inclusion pathology in rats than fibrils generated from rat alpha-synuclein. Parkinsonism and Related Disorders, 89(June), 41–47. 10.1016/j.parkreldis.2021.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura K, Hishikawa N, Sawada M, Nagatsu T, Yoshida M, & Hashizume Y (2003). Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson’s disease brains. Acta Neuropathologica, 106(6), 518–526. 10.1007/s00401-003-0766-2 [DOI] [PubMed] [Google Scholar]
- Izco M, Blesa J, Verona G, Cooper JM, & Alvarez-Erviti L (2021). Glial activation precedes alpha-synuclein pathology in a mouse model of Parkinson’s disease. Neuroscience Research, 170, 330–340. 10.1016/j.neures.2020.11.004 [DOI] [PubMed] [Google Scholar]
- Kanaan NM, & Manfredsson FP (2012). Loss of functional alpha-synuclein: A toxic event in Parkinson’s disease? In Journal of Parkinson’s Disease (Vol. 2, Issue 4, pp. 249–267). 10.3233/JPD-012138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannarkat GT, Cook DA, Lee JK, Chang J, Chung J, Sandy E, Paul KC, Ritz B, Bronstein J, Factor SA, Boss JM, & Tansey MG (2015). Common genetic variant association with altered HLA expression, synergy with pyrethroid exposure, and risk for Parkinson’s disease: An observational and case-control study. Parkinson’s Disease, 1(March). 10.1038/npjparkd.2015.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karampetsou M, Ardah MT, Semitekolou M, Polissidis A, Samiotaki M, Kalomoiri M, Majbour N, Xanthou G, El-Agnaf OMA, & Vekrellis K (2017). Phosphorylated exogenous alpha-synuclein fibrils exacerbate pathology and induce neuronal dysfunction in mice. Scientific Reports, 7(1), 1–18. 10.1038/s41598-017-15813-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprich JB, Reske-Nielsen C, Mithal P, & Isacson O (2008a). Neuroinflammation mediated by IL-1β increases susceptibility of dopamine neurons to degeneration in an animal model of Parkinson’s disease. Journal of Neuroinflammation, 5, 1–12. 10.1186/1742-2094-5-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower JH, Olanow CW, Dodiya HB, Chu Y, Beach TG, Adler CH, Halliday GM, & Bartus RT (2013). Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain, 136(8), 2419–2431. 10.1093/brain/awt192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurkowska-Jastrze I, Wronska A, Wronska W, Kohutnicka M, Członkowski A, & Członkowska A (1999). The Inflammatory Reaction Following 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine Intoxication in Mouse. http://www.idealibrary.com [DOI] [PubMed] [Google Scholar]
- Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, … Barres BA (2017). Neurotoxic reactive astrocytes are induced by activated microglia. Nature, 541(7638), 481–487. 10.1038/nature21029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow SA, Marsh SE, & Stevens B (2020). Microglia and Astrocytes in Disease: Dynamic Duo or Partners in Crime? Trends in Immunology, 41(9), 820–835. 10.1016/j.it.2020.07.006 [DOI] [PubMed] [Google Scholar]
- Lindestam Arlehamn CS, Dhanwani R, Pham J, Kuan R, Frazier A, Rezende Dutra J, Phillips E, Mallal S, Roederer M, Marder KS, Amara AW, Standaert DG, Goldman JG, Litvan I, Peters B, Sulzer D, & Sette A (2020). α-Synuclein-specific T cell reactivity is associated with preclinical and early Parkinson’s disease. Nature Communications, 11(1). 10.1038/s41467-020-15626-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Covell DJ, Kehm VM, Zhang B, Song IY, Byrne MD, Pitkin RM, Decker SC, Trojanowski JQ, & Lee VMY (2016). Molecular and Biological Compatibility with Host Alpha-Synuclein Influences Fibril Pathogenicity. Cell Reports, 16(12), 3373–3387. 10.1016/j.celrep.2016.08.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, Trojanowski JQ, & Lee VMY (2012a). Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science, 338(6109), 949–953. 10.1126/science.1227157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Kehm VM, Zhang B, O’Brien P, Trojanowski JQ, & Lee VMY (2012b). Intracerebral inoculation of pathological α-synuclein initiates a rapidly progressive neurodegenerative α-synucleinopathy in mice. Journal of Experimental Medicine, 209(5), 975–988. 10.1084/jem.20112457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Song C, Stieber A, Branch JR, Brunden KR, Trojanowski JQ, & M-Y Lee V (2009). Exogenous-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. In PNAS (Vol. 106, pp. 20051–20056). www.pnas.org/cgi/content/full/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma SX, Seo BA, Kim D, Xiong Y, Kwon SH, Brahmachari S, Kim S, Kam TI, Nirujogi RS, Kwon SH, Dawson VL, Dawson TM, Pandey A, Na CH, & Ko HS (2021). Complement and Coagulation Cascades are Potentially Involved in Dopaminergic Neurodegeneration in α-Synuclein-Based Mouse Models of Parkinson’s Disease. Journal of Proteome Research, 20(7), 3428–3443. 10.1021/acs.jproteome.0c01002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahul-Mellier A-L, Burtscher J, Maharjan N, Weerens L, Croisier M, Kuttler F, Leleu M, Knott GW, & Lashuel HA (n.d.). The process of Lewy body formation, rather than simply α-synuclein fibrillization, is one of the major drivers of neurodegeneration. 10.6084/m9.figshare.11842389.v2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marras C, Beck JC, Bower JH, Roberts E, Ritz B, Ross GW, Abbott RD, Savica R, van den Eeden SK, Willis AW, & Tanner C (2018). Prevalence of Parkinson’s disease across North America. Npj Parkinson’s Disease, 4(1). 10.1038/s41531-018-0058-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda-Suzukake M, Nonaka T, Hosokawa M, Oikawa T, Arai T, Akiyama H, Mann DMA, & Hasegawa M (2013). Prion-like spreading of pathological α-synuclein in brain. Brain, 136(4), 1128–1138. 10.1093/brain/awt037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matejuk A, & Ransohoff RM (2020). Crosstalk Between Astrocytes and Microglia: An Overview. Frontiers in Immunology, 11(July), 1–11. 10.3389/fimmu.2020.01416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, Kawamata T, Walker DG, Akiyama H, Tooyama I, & EDITH Mc GEER AG (1993). Microglia in Degenerative Neurological Disease. In GLIA (Vol. 7). [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, & McGeer EG (1988a). Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology, 38(8), 1285–1285. 10.1212/WNL.38.8.1285 [DOI] [PubMed] [Google Scholar]
- Mcgeer PL, Itagaki S, & Mcgeer EG (1988b). Acta Heuropathologica Expression of the histocompatibility glycoprotein HLA-DR in neurological disease*. In Acta Neuropathol (Vol. 76). [DOI] [PubMed] [Google Scholar]
- Miklossy J, Doudet DD, Schwab C, Yu S, McGeer EG, & McGeer PL (2006). Role of ICAM-1 in persisting inflammation in Parkinson disease and MPTP monkeys. Experimental Neurology, 197(2), 275–283. 10.1016/j.expneurol.2005.10.034 [DOI] [PubMed] [Google Scholar]
- Miller KM, Mercado NM, & Sortwell CE (2021a). Synucleinopathy-associated pathogenesis in Parkinson’s disease and the potential for brain-derived neurotrophic factor. In npj Parkinson’s Disease (Vol. 7, Issue 1). Nature Research. 10.1038/s41531-021-00179-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KM, Patterson JR, Kochmanski J, Kemp CJ, Stoll AC, Onyekpe CU, Cole-Strauss A, Steece-Collier K, Howe JW, Luk KC, & Sortwell CE (2021b). Striatal afferent bdnf is disrupted by synucleinopathy and partially restored by stn dbs. Journal of Neuroscience, 41(9), 2039–2052. 10.1523/JNEUROSCI.1952-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogi M, Harada M, Kondo T, Riederer P, Inagaki H, Minami M, & Nagatsu E’ T (1994a). Interleukin-lfl, interleukin-6, epidermal growth factor and transforming growth factor-are elevated in the brain from parkinsonian patients. In Neuroscience Letters (Vol. 180). [DOI] [PubMed] [Google Scholar]
- Mogi M, Harada M, Riederer P, Narabayashi H, Fujita K, & Nagatsu D’ T (1994b). Tumor necrosis factor-(TNF-) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neuroscience Letters, 208–210. [DOI] [PubMed] [Google Scholar]
- Nagatsu T, Mogf M, Ichmose H, & Togari A (2000). Changes in cytokines and neurotrophins in Parkinson’s disease. In Advances in Research on Neurodegeneration (pp. 277–290). [DOI] [PubMed] [Google Scholar]
- Negrini M, Tomasello G, Davidsson M, Fenyi A, Adant C, Hauser S, Espa E, Gubinelli F, Manfredsson FP, Melki R, & Heuer A (2022). Sequential or Simultaneous Injection of Preformed Fibrils and AAV Overexpression of Alpha-Synuclein Are Equipotent in Producing Relevant Pathology and Behavioral Deficits. Journal of Parkinson’s Disease, 1–21. 10.3233/jpd-212555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr CF, Rowe DB, Mizuno Y, Mori H, & Halliday GM (2005). A possible role for humoral immunity in the pathogenesis of Parkinson’s disease. Brain, 128(11), 2665–2674. 10.1093/brain/awh625 [DOI] [PubMed] [Google Scholar]
- Osterberg VR, Spinelli KJ, Weston LJ, Luk KC, Woltjer RL, & Unni VK (2015). Progressive Aggregation of Alpha-Synuclein and Selective Degeneration of Lewy Inclusion-Bearing Neurons in a Mouse Model of Parkinsonism. Cell Reports, 10(8), 1252–1260. 10.1016/j.celrep.2015.01.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi Y, Yoshikawa E, Sekine Y, Futatsubashi M, Kanno T, Ogusu T, & Torizuka T (2005). Microglial activation and dopamine terminal loss in early Parkinson’s disease. Annals of Neurology, 57(2), 168–175. 10.1002/ana.20338 [DOI] [PubMed] [Google Scholar]
- Paleologou KE, Schmid AW, Rospigliosi CC, Kim HY, Lamberto GR, Fredenburg RA, Lansbury PT, Fernandez CO, Eliezer D, Zweckstetter M, & Lashuel HA (2008). Phosphorylation at Ser-129 but not the phosphomimics S129E/D inhibits the fibrillation of α-synuclein. Journal of Biological Chemistry, 283(24), 16895–16905. 10.1074/jbc.M800747200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson JR, Duffy MF, Kemp CJ, Howe JW, Collier TJ, Stoll AC, Miller KM, Patel P, Levine N, Moore DJ, Luk KC, Fleming SM, Kanaan NM, Paumier KL, El-Agnaf OMA, & Sortwell CE (2019). Time course and magnitude of alpha-synuclein inclusion formation and nigrostriatal degeneration in the rat model of synucleinopathy triggered by intrastriatal α-synuclein preformed fibrils. Neurobiology of Disease, 130. 10.1016/j.nbd.2019.104525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paumier KL, Luk KC, Manfredsson FP, Kanaan NM, Lipton JW, Collier TJ, Steece-Collier K, Kemp CJ, Celano S, Schulz E, Sandoval IM, Fleming S, Dirr E, Polinski NK, Trojanowski JQ, Lee VM, & Sortwell CE (2015). Intrastriatal injection of pre-formed mouse α-synuclein fibrils into rats triggers α-synuclein pathology and bilateral nigrostriatal degeneration. Neurobiology of Disease, 82, 185–199. 10.1016/j.nbd.2015.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelaerts W, Bousset L, van der Perren A, Moskalyuk A, Pulizzi R, Giugliano M, van den Haute C, Melki R, & Baekelandt V (2015). α-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature, 522(7556), 340–344. 10.1038/nature14547 [DOI] [PubMed] [Google Scholar]
- Peng C, Gathagan RJ, Covell DJ, Medellin C, Stieber A, Robinson JL, Zhang B, Pitkin RM, Olufemi MF, Luk KC, Trojanowski JQ, & Lee VMY (2018). Cellular milieu imparts distinct pathological α-synuclein strains in α-synucleinopathies. Nature, 557(7706), 558–563. 10.1038/s41586-018-0104-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin XY, Zhang SP, Cao C, Loh YP, & Cheng Y (2016). Aberrations in peripheral inflammatory cytokine levels in Parkinson disease: A systematic review and meta-analysis. JAMA Neurology, 73(11), 1316–1324. 10.1001/jamaneurol.2016.2742 [DOI] [PubMed] [Google Scholar]
- Rey NL, Steiner JA, Maroof N, Luk KC, Madaj Z, Trojanowski JQ, Lee VMY, & Brundin P (2016). Widespread transneuronal propagation of α-synucleinopathy triggered in olfactory bulb mimics prodromal Parkinson’s disease. Journal of Experimental Medicine, 213(9), 1759–1778. 10.1084/jem.20160368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostami J, Fotaki G, Sirois J, Mzezewa R, Bergström J, Essand M, Healy L, & Erlandsson A (2020). Astrocytes have the capacity to act as antigen-presenting cells in the Parkinson’s disease brain. Journal of Neuroinflammation, 17(1). 10.1186/s12974-020-01776-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacino AN, Brooks M, Shaw G, Golde TE, Giasson BI, McKinney AB, Thomas MA, Sacino AN, Brooks M, Golde TE, Giasson BI, Golde TE, Giasson BI, Shaw G, & Shaw G (2014). Brain injection of α-Synuclein induces multiple proteinopathies, gliosis, and a neuronal injury marker. Journal of Neuroscience, 34(37), 12368–12378. 10.1523/JNEUROSCI.2102-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Guajardo V, Febbraro F, Kirik D, & Romero-Ramos M (2010). Microglia acquire distinct activation profiles depending on the degree of α-synuclein neuropathology in a rAAV based model of Parkinson’s disease. PLoS ONE, 5(1). 10.1371/journal.pone.0008784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Dammer EB, Malovic E, Olsen AL, Raza SA, Gao T, Xiao H, Oliver DL, Duong D, Joers V, Seyfried N, Huang M, Kukar T, Tansey MG, Kanthasamy AG, & Rangaraju S (2020). Molecular Signatures of Neuroinflammation Induced by αSynuclein Aggregates in Microglial Cells. Frontiers in Immunology, 11. 10.3389/fimmu.2020.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schetters STT, Gomez-Nicola D, Garcia-Vallejo JJ, & van Kooyk Y (2018). Neuroinflammation: Microglia and T cells get ready to tango. Frontiers in Immunology, 8(JAN). 10.3389/fimmu.2017.01905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimozawa A, Ono M, Takahara D, Tarutani A, Imura S, Masuda-Suzukake M, Higuchi M, Yanai K, Hisanaga SI, & Hasegawa M (2017). Propagation of pathological α-synuclein in marmoset brain. Acta Neuropathologica Communications, 5(1), 12. 10.1186/s40478-017-0413-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino ZA, Brooks MMT, Hudson V, Rutherford NJ, Golde TE, Giasson BI, & Chakrabarty P (2017a). Intrastriatal injection of α-synuclein can lead to widespread synucleinopathy independent of neuroanatomic connectivity. Molecular Neurodegeneration, 12(1), 1–16. 10.1186/s13024-017-0182-z Neurodegeneration, 12(1). 10.1186/s13024-017-0182-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sossi V, de la Fuente-Fernández R, Holden JE, Doudet DJ, McKenzie J, Stoessl AJ, & Ruth TJ (2002). Increase in Dopamine Turnover Occurs Early in Parkinson’s Disease: Evidence From a New Modeling Approach to PET 18F-Fluorodopa Data. [DOI] [PubMed] [Google Scholar]
- Sossi V, de La Fuente-Fernández R, Nandhagopal R, Schulzer M, McKenzie J, Ruth TJ, Aasly JO, Farrer MJ, Wszolek ZK, & Stoessl JA (2010). Dopamine turnover increases in asymptomatic LRRK2 mutations carriers. Movement Disorders, 25(16), 2717–2723. 10.1002/mds.23356 [DOI] [PubMed] [Google Scholar]
- Sossi V, Patterson J, McCormick S, Kemp C, Miller K, Stoll A, Kuhn N, Kubik M, Kochmanski J, Luk K, & Sortwell C (2022). Dopaminergic PET imaging in the alpha-synuclein PFF model reveals similarities to early Parkinson’s disease. Movement Disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoyka LE, Arrant AE, Thrasher DR, Russell DL, Freire J, Mahoney CL, Narayanan A, Dib AG, Standaert DG, & Volpicelli-Daley LA (2020). Behavioral defects associated with amygdala and cortical dysfunction in mice with seeded α-synuclein inclusions. Neurobiology of Disease, 134. 10.1016/j.nbd.2019.104708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Fischer DL, Li X, Bankiewicz K, Sortwell CE, & Federoff HJ (2017). Alpha-Synuclein mRNA Is Not Increased in Sporadic PD and Alpha-Synuclein Accumulation Does Not Block GDNF Signaling in Parkinson’s Disease and Disease Models. In Molecular Therapy (Vol. 25, Issue 10, pp. 2231–2235). Cell Press. 10.1016/j.ymthe.2017.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Alcalay RN, Garretti F, Cote L, Kanter E, Agin-Liebes J, Liong C, McMurtrey C, Hildebrand WH, Mao X, Dawson VL, Dawson TM, Oseroff C, Pham J, Sidney J, Dillon MB, Carpenter C, Weiskopf D, Phillips E, … Sette A (2017). T cells from patients with Parkinson’s disease recognize α-synuclein peptides. Nature, 546(7660), 656–661. 10.1038/nature22815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenreiro S, Eckermann K, & Outeiro TF (2014). Protein phosphorylation in neurodegeneration: Friend or foe? In Frontiers in Molecular Neuroscience (Vol. 7, Issue MAY). Frontiers Research Foundation. 10.3389/fnmol.2014.00042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur P, Breger LS, Lundblad M, Wan OW, Mattsson B, Luk KC, Lee VMY, Trojanowski JQ, & Björklund A (2017). Modeling Parkinson’s disease pathology by combination of fibril seeds and α-synuclein overexpression in the rat brain. Proceedings of the National Academy of Sciences of the United States of America, 114(39), E8284–E8293. 10.1073/pnas.1710442114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen MB, Ferreira SA, Schacht AC, Jacobsen J, Simonsen M, Betzer C, Jensen PH, Brooks DJ, Landau AM, & Romero-Ramos M (2021). PET imaging reveals early and progressive dopaminergic deficits after intra-striatal injection of preformed alpha-synuclein fibrils in rats. Neurobiology of Disease, 149, 105229. 10.1016/j.nbd.2020.105229 [DOI] [PubMed] [Google Scholar]
- Uemura N, Ueda J, Yoshihara T, Ikuno M, Uemura MT, Yamakado H, Asano M, Trojanowski JQ, & Takahashi R (2021). α-Synuclein Spread from Olfactory Bulb Causes Hyposmia, Anxiety, and Memory Loss in BAC-SNCA Mice. Movement Disorders, 201870008(January), 1–13. 10.1002/mds.28512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Perren A, Gelders G, Fenyi A, Bousset L, Brito F, Peelaerts W, van den Haute C, Gentleman S, Melki R, & Baekelandt V (2020). The structural differences between patient-derived α-synuclein strains dictate characteristics of Parkinson’s disease, multiple system atrophy and dementia with Lewy bodies. Acta Neuropathologica, 139(6), 977–1000. 10.1007/s00401-020-02157-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli-Daley LA, Luk KC, & Lee VMY (2014). Addition of exogenous α-synuclein preformed fibrils to primary neuronal cultures to seed recruitment of endogenous α-synuclein to Lewy body and Lewy neurite-like aggregates. Nature Protocols, 9(9), 2135–2146. 10.1038/nprot.2014.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, Meaney DF, Trojanowski JQ, & Lee VMY (2011). Exogenous α-Synuclein Fibrils Induce Lewy Body Pathology Leading to Synaptic Dysfunction and Neuron Death. Neuron, 72(1), 57–71. 10.1016/j.neuron.2011.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall NR, DeLaParra M, Callaway EM, & Kreitzer AC (2013). Differential innervation of direct- and indirect-pathway striatal projection neurons. Neuron, 79(2), 347–360. 10.1016/j.neuron.2013.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan OW, & Chung KKK (2012). The role of alpha-synuclein oligomerization and aggregation in cellular and animal models of Parkinson’s disease. PLoS ONE, 7(6). 10.1371/journal.pone.0038545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winner B, Jappelli R, Maji SK, Desplats PA, Boyer L, Aigner S, Hetzer C, Loher T, Vilar M, Campioni S, Tzitzilonis C, Soragni A, Jessberger S, Mira H, Consiglio A, Pham E, Masliah E, Gage FH, & Riek R (2011). In vivo demonstration that α-synuclein oligomers are toxic. Proceedings of the National Academy of Sciences of the United States of America, 108(10), 4194–4199. 10.1073/pnas.1100976108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Hamilton JL, Kopil C, Beck JC, Tanner CM, Albin RL, Ray Dorsey E, Dahodwala N, Cintina I, Hogan P, & Thompson T (2020). Current and projected future economic burden of Parkinson’s disease in the U.S. Npj Parkinson’s Disease, 6(1). 10.1038/s41531-020-0117-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun SP, Kam TI, Panicker N, Kim S, Oh Y, Park JS, Kwon SH, Park YJ, Karuppagounder SS, Park H, Kim S, Oh N, Kim NA, Lee S, Brahmachari S, Mao X, Lee JH, Kumar M, An D, … Ko HS (2018). Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nature Medicine, 24(7), 931–938. 10.1038/s41591-018-0051-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, & Barres BA (2012). Genomic analysis of reactive astrogliosis. Journal of Neuroscience, 32(18), 6391–6410. 10.1523/JNEUROSCI.6221-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Broe M, Huang Y, Anderson JP, Gai WP, Milward EA, Porritt M, Howells D, Hughes AJ, Wang X, & Halliday GM (2011). Changes in the solubility and phosphorylation of α-synuclein over the course of Parkinson’s disease. Acta Neuropathologica, 121(6), 695–704. 10.1007/s00401-011-0815-1 [DOI] [PubMed] [Google Scholar]