Highlights
-
•
Pediatric low-grade glioma (pLGG) is a MAPK pathway-altered disease with novel therapeutic options.
-
•
Clinical trials are vital in translation of targeted agents to influence patient care.
-
•
Targeted agents have unknown long-term toxicities and unclear impact on pLGG natural history.
-
•
Challenges include rapid clinical trial translation, tumor heterogeneity and need to harmonize molecular profiling techniques.
Keywords: MAPK, BRAF, Targeted therapy, mTOR, pLGG
Abbreviations: BRAF, B-Raf proto-oncogene; CNS, central nervous system; CPK, creatine phosphokinase; DNA, deoxyribonucleic acid; DNET, dysembryoplastic neuroepithelial tumors; FISH, fluorescent in situ hybridisation; FGFR, fibroblast growth factor receptor; GNT, glioneuronal tumor; IHC, immunohistochemistry; JPA, juvenile pilocytic astrocytoma; MAPK, mitogen activated protein kinase; MTOR, mammalian target of rapamycin; NF1, neurofibromatosis type 1; OS, overall survival; PFS, progression-free survival; pLGG, pediatric low-grade glioma; PLNTY, polymorphous neuroepithelial tumour of the young; PNOC, Pediatric Neuro-Oncology Consortium; RAPNO, Response Assessment in Pediatric Neuro-Oncology; RTK, receptor tyrosine kinases; TPCV, thioguanine, procarbazine, CCNU and vincristine; TSC, tuberous sclerosis complex; WES, whole exome sequencing; WHO, world health organisation
Abstract
pLGGs are a group of tumors for which the era of molecular diagnostics has truly shifted treatment paradigms and patient care. The discovery that this group of tumors is driven by single-gene alterations/fusions in the MAPK pathway has resulted in relatively rapid translation into targeted therapy options for patients with this often chronic disease. This translation has been facilitated through efforts of multiple collaboratives and consortia and has led to the development of clinical trials testing the role of targeted therapies in pLGG. Although these developments represent promise, many questions remain regarding these therapies including their long-term toxicities and their potential effects on the natural history of pLGG.
Introduction
Pediatric low-grade gliomas (pLGGs) are the most common brain tumor in children and represent roughly one-third of all pediatric central nervous system (CNS) tumors [1], [2], [3]. Recent scientific advances have drastically altered our understanding of the biological underpinnings of pLGGs. This improved understanding of the molecular alterations in pLGG, most commonly in the mitogen-activated protein kinase (MAPK) pathway, has translated to a shift in the therapeutic paradigm for children with pLGGs that now integrates novel targeted therapies in the upfront treatment setting. Despite these exciting developments, translating these novel therapeutic options to meaningful treatment in patients requires thoughtful and well-designed clinical trials. Moreover, novel targeted therapies present unique challenges in patient care including new toxicity profiles and potential impact on the natural history of this disease. Ultimately, pLGG continues to represent a chronic disease for many children and as such, the long-term morbidity of these tumors and the therapies utilized remain of paramount importance (Fig. 1). pLGGs are molecularly and clinically distinct from their adult counterparts. Survival outcomes for patients with pLGG are excellent, with a 10-year overall survival (OS) of 96% for pilocytic astrocytoma and 85% for other LGGs [4]. Unlike adult low grade gliomas, which are predominantly hemispheric, pLGGs have a predilection for the posterior fossa and optic pathway [5], [6], [7]. Adult patients with LGGs have a high risk of transformation to high grade gliomas and carry an inferior progression free survival (PFS) and OS to their pediatric counterparts [5,7,8]. The majority of pLGGs do not transform over time, and appear to quiesce on transition to adulthood [6,7]. This has been substantiated by studies of long-term survivors of pLGGs showing that after reaching adulthood, survivors of pLGG had a 30-year OS of 93% with mortality uncommonly attributed to pLGG recurrence [6]. Indeed in a second study, progression of the pLGGs themselves was also found to be uncommon in adult survivors of pLGGs [7].
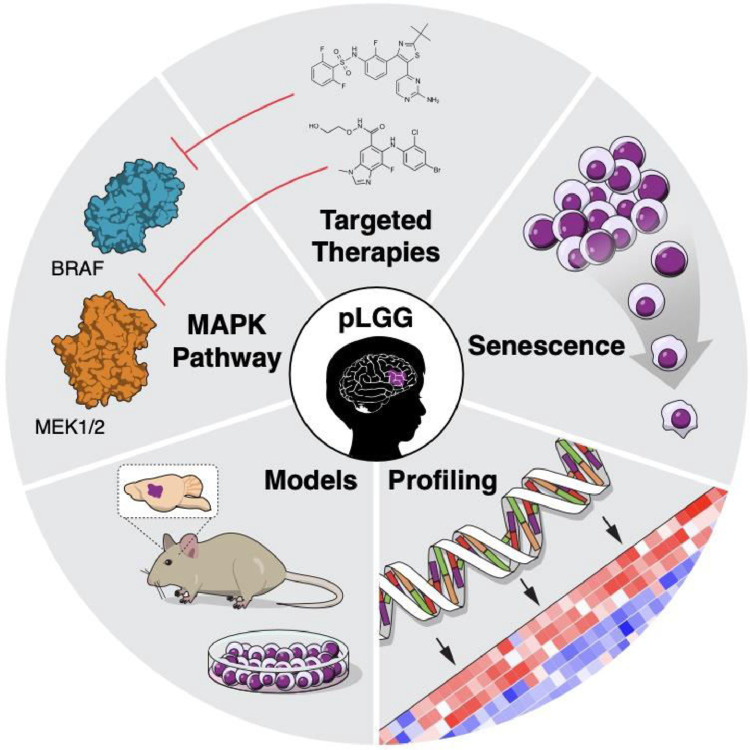
Fig. 1.
Considerations in treatment of pLGGs. A representative schematic showing important considerations in pLGG treatment, including understanding the MAPK pathway that altered in most pLGGs and can be targeted using small molecular inhibitors; pre-clinical in vitro and in vivo models; molecular profiling of tumors; and natural history of senescence.
Despite these favorable survival outcomes, the PFS for patients with pLGG varies significantly based on whether complete surgical resection is faesible. 10-year PFS exceeds 85% in tumours that have had a gross total resection (GTR) and can be below 50% in patients with residual disease [9]. Furthermore, many patients with pLGG suffer from significant morbidity related to tumors in anatomically-eloquent locations and the sequelae of therapy. Morbidity in patients with pLGG can manifest as abnormal vision, endocrinopathies, motor or sensory abnormalities, vasculopathies and neuropsychological sequelae amongst others [6,8,10,11].
Histology
pLGGs were an understudied tumor group for decades, in significant part due to the lack of models that adequately recapitulate this disease as well as limited molecular tools to better characterize these tumors [12]. With the introduction of next generation sequencing technologies, the molecular drivers of pLGGs were identified and relatively rapidly translated into targeted therapeutic options for patients with tumors harboring these mutations. It is increasingly evident that pLGGs represent a complex heterogenous disease with multiple distinct histopathologic entities. Landmark genetic profiling has identified that the majority of these diseases are driven by single-gene alterations in the MAPK pathway [13], [14], [15].
The recently published 2021 World Health Organization (WHO) Classification of Tumors of the CNS has reflected the focus on the integration of molecular and histopathological characteristics to facilitate a more accurate diagnosis [16]. The updated WHO classification describes three families of tumors that encompass pLGG and glioneuronal tumors (GNTs): “Pediatric type diffuse low grade gliomas,” “Circumscribed astrocytic gliomas,” and “Glioneuronal and neuronal tumor” [16,17]. Within this new classifier are poignant examples of LGGs which are now defined by their driver molecular alterations rather than by histopathological features alone. For example, “Diffuse astrocytoma, MYB- or MYBL1-altered” is a newly defined subset of pLGGs that do not harbor the classic histopathological features of angiocentric glioma, but display structural variants and recurrent amplifications in MYB and MYBL1, including fusions [16,17]. This distinction is critical, as this group of tumors was often previously defined as “Diffuse astrocytoma, IDH wildtype” using the WHO 2016 classification of CNS tumors. “Diffuse astrocytoma, IDH wildtype” is a cohort that predominantly encompasses tumors that are high-grade gliomas with an aggressive disease course and the inclusion of the typically indolent MYB/MYBl1 altered diffuse glioma in this group was erroneous (Table 1) [17].
Table 1.
WHO 2021 classification for pLGG/low-grade GNTs.
| Pediatric-type diffuse low-grade gliomas | 1. Diffuse astrocytoma, MYB- or MYBL1-altered 2. Angiocentric glioma 3. Polymorphous low-grade neuroepithelial tumor of the young (PLNTY) 4. Diffuse low-grade glioma, MAPK pathway-altered |
| Circumscribed astrocytic gliomas | 1. Pilocytic astrocytoma 2. Pleomorphic xanthoastrocytoma (PXA) 3. Subependymal giant cell astrocytoma (SEGA) 4. Choroid glioma |
| Glioneuronal and neuronal tumors | 1. Ganglioglioma 2. Desmoplastic infantile ganglioglioma/ desmoplastic infantile astrocytoma 3. Dysembryoplastic neuroepithelial tumor 4. Diffuse glioneuronal tumor with oligodendroglioma-like features and nuclear clusters 5. Rosette-forming glioneuronal tumor 6. Papillary glioneuronal tumor 7. Myxoid glioneuronal tumor 8. Diffuse leptomeningeal glioneuronal tumor (DLGNT) 9. Gangliocytoma 10. Multinodular and vacuolating neuronal tumor 11. Dysplastic cerebellar gangliocytoma (Lhermitte-Duclos disease) 12. Central neurocytoma 13. Extraventricular neurocytoma 14. Cerebellar liponeurocytoma |
Tumor biology
Studies have found that the majority of pLGGs harbor distinct driver alterations that typically result in activation of the MAPK pathway and downstream activation of the mammalian target of rapamycin (mTOR) pathway [13], [14], [15]. The MAPK pathway plays a central role in cellular signaling via receptor tyrosine kinases (RTKs) to downstream metabolic and transcriptional effectors. [18]. The B-Raf proto-oncogene (BRAF) is the gene most commonly altered in pLGG with two gene alterations predominating above all others. KIAA1549-BRAF fusion, which results in activation of BRAF kinase and ultimately MAPK activation, is the single most common molecular alteration in pLGG and is particularly prominent in patients with juvenile pilocytic astrocytoma (JPA) [13,19,20]. BRAF V600E activating mutations, resulting in valine to glutamine substitutions, are present in a smaller cohort of patients with notable enrichment in patients with ganglioglioma (18-45%) and pleomorphic xanthoastrocytoma (over 60%) [21,22].
Although alterations in the MAPK pathway are frequently somatic, MAPK activation can also occur as a result of genetic syndromes predisposing to pLGG including neurofibromatosis type 1 (NF1) and tuberous sclerosis complex (TSC) [23,24]. Patients with NF1 are more likely to be diagnosed with pilocytic astrocytoma and diffuse pLGG. Their disease course, therapeutic considerations, and treatment response are unique compared to sporadically occurring pLGG [23,25,26].
Next-generation sequencing of pLGG has resulted in identification of novel driver alterations with notable associations with certain tumor types. Abnormalities in fibroblast growth factor receptor (FGFR) genes including mutations and rearrangements are enriched in patients with dysembryoplastic neuroepithelial tumors (DNET) [27,28]. FGFR fusions have also been reported in the newly defined entity polymorphous neuroepithelial tumor of the young (PLNTY) [14,29]. The MYB (and related MYBL1) family of transcription factors is enriched in diffuse astrocytomas and MYB-QKI fusions define angiocentric gliomas [15,30]. Less commonly, alterations in NTRK, KRAS and IDH1 are observed in pLGG [14,30]. The role of IDH1 mutations in the natural history of pLGGs remains unclear. A recent case series found that patients with IDH1-mutant pLGGs had excellent short-term survival, but 5-year progression-free survival (PFS) was 42.9%, with glioma-related mortality after 10 years [31].
Therapeutic considerations
Treatment options for patients with pLGGs have evolved significantly over the last five decades. Historically, the mainstay of pLGG therapy was surgical resection and adjuvant radiation therapy until the advent of chemotherapeutic approaches for pLGGs in the 1980s [10,32]. Over the last decade, the introduction of genetically-defined targeted therapies has provided further treatment options, but whether these targeted therapies improve the overall long-term outcome for children with pLGGs has yet to be determined.
One of the remaining constants in the treatment of pLGG is the paramount role of complete surgical resection whenever safely feasible and the impact of surgical resection on delaying the need for further therapy or avoiding adjuvant therapy entirely [33]. The role of surgical biopsy in the treatment algorithm for patients with optic pathway glioma (OPG) has changed since the identification of genomic drivers of this disease. OPG comprise 40% of pLGG and were historically treated based on classical radiological and clinical features due to their location in anatomically eloquent locations and the perceived risk of biopsy [33,34]. In the current molecularly driven era, surgical biopsy of OPGs is now being considered more frequently in management planning, especially in patients with sporadic pLGG (without NF1), for whom validated targeted therapeutic options may exist including as part of a clinical trial [33].
Chemotherapeutic approaches for the treatment of patients with pLGG have been extremely well established in multiple prospective clinical trials with over 2000 pediatric patients with pLGG treated on these studies, with the goal of achieving disease control while minimizing long-term disease- and treatment-related morbidities [32,[35], [36], [37], [38], [39], [40], [41], [42]. These approaches have been studied in both sporadic pLGG and in children with NF1-associated pLGG and are of particular importance in patients with OPG for whom surgical resection and radiation therapy are associated with significant morbidity. Internationally, the most commonly utilized chemotherapy protocols for the treatment of patients with newly diagnosed pLGG are vincristine and carboplatin, monotherapy with vinblastine, or a combination of thioguanine, procarbazine, CCNU and vincristine (TPCV) [32,36,42]. The 5-year PFS for sporadic pLGG patients treated on these studies has been very similar (ranging from 35-45%) despite the differing toxicity profiles of the regimes [32,36,39,42]. The 5-year PFS is generally higher in patients with NF1 pLGG (60-70%) across chemotherapy protocols [35,37]. However, across both sporadic and NF1 associated pLGGs, a significant number of patients will progress despite chemotherapy and this group is of paramount importance in considering the long-term morbidity of their tumors and therapies.
Molecularly targeted therapies for patients with pLGG have heralded great promise for the pediatric neuro-oncology community. Targeting the hyperactivation of the Ras-MAPK pathway has been the predominant focus of these endeavors and RAF inhibitors and MEK inhibitors are now either FDA approved or in clinical trials for patients with pLGG [43], [44], [45], [46]. FDA approval of BRAF inhibitors, vemurafenib and dabrafenib, as well as MEK inhibitors, trametinib and selumetinib, have stemmed from their promising preclinical and clinical activity in advanced melanoma and non-small cell lung cancer [43,44]. Importantly, first-generation Type 1 BRAF inhibitors are contraindicated in BRAF-rearranged pLGGs as they cause paradoxical activation of MAPK signaling through increased RAF dimerization[47]. This highlights the need for an in-depth understanding of tumor biology, the requirement to carefully identify genetic drivers of individual pLGGs, and the need to determine targets of specific tyrosine kinase inhibitors so as to not recapitulate the experience with the kinase inhibitor, sorafenib, that resulted in paradoxical tumor growth in BRAF-altered tumors [48]. Current studies are also investigating the role of mTOR inhibitors and receptor kinase inhibitors, both as monotherapy and in combination with other therapies [49]. Further, novel considerations in this domain include the role of pan-RAF inhibitors to facilitate therapy for patients with pLGG with varying MAPK pathway alterations [50].
Radiation therapy has been historically used in the treatment of unresectable, progressive pLGG both upfront and in the setting of relapse; however, radiation is no longer considered standard of care in the vast majority of patients. Although the 5-year PFS and OS for patients with treated with radiation is excellent (71-90% and 92-100% respectively), the long-term side effects can be severe in a cohort of patients who typically survive into adulthood [10,51,52]. These include increased risk of second malignancies, cognitive and growth deficits, and endocrine and vascular complications [10,51,52]. In recent decades, advancements in radiation planning and delivery, including the advent of proton radiotherapy, have maintained excellent tumor control, while reducing toxicity that was observed in the 2D era of radiotherapy [10,51,53,54].
Molecularly-targeted clinical trials in pLGG
The development of molecularly-stratified clinical trials in pLGG is undoubtedly a paradigm shift in pediatric neuro-oncology and an exciting development for patients with pLGG. Studies driven by clinical trial consortia have had sentinel roles in incorporating tumor biology and targeted therapies into clinical trials (Fig. 2) [45,46,55]. These consortia developed some of the first biologically-driven trials in pLGG and the mandated tissue diagnosis and analysis, at a time when this was truly considered experimental rather than standard of care. The Pacific Pediatric Neuro-Oncology Consortium (PNOC) has had a clear focus on developing molecularly-targeted trials for pLGG from the conception of the consortium. PNOC001, a phase II study of the mTOR pathway inhibitor, everolimus, for recurrent or progressive pLGG, was one of the first pLGG studies to mandate tissue diagnosis [56]. Subsequently, PNOC014 was the first study to evaluate the safety of a Pan-RAF inhibitor in pediatric patients with LGG and the exciting signal seen in this small cohort of patients has translated into the rapid development and opening of PNOC026/Day101-001, a phase II study evaluating the Oral Pan-Raf inhibitor (Day101) in patients with BRAF-altered recurrent or progressive pLGG (Table 2) [57].
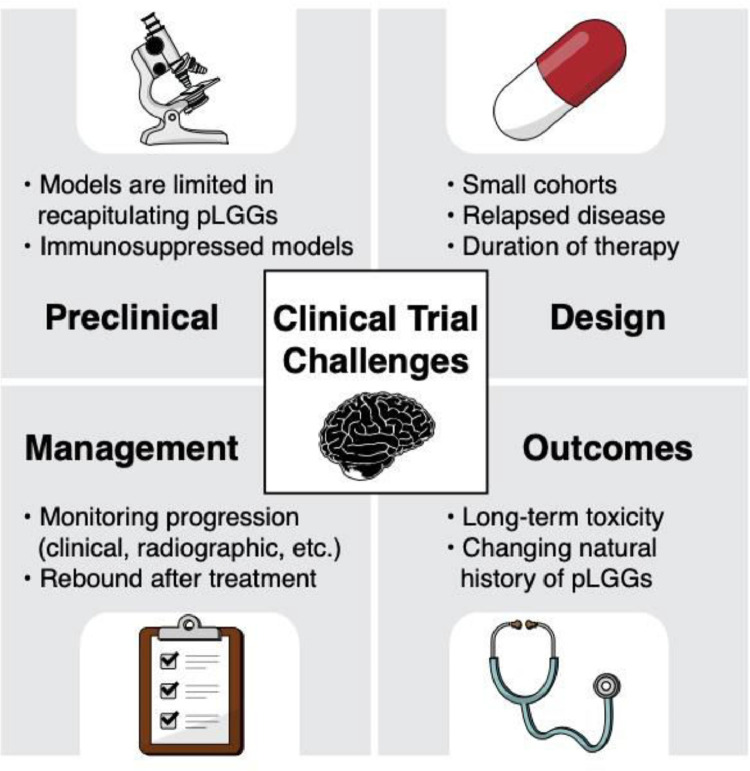
Fig. 2.
Clinical trial challenges in pLGG. A representative schematic showing the challenges for clinical trials for pediatric patients with LGG, including limitations involving preclinical research, clinical trial design, management of patients while on a clinical trial, and long-term outcomes.
Table 2.
List of completed and ongoing consortium clinical trials for pLGG using targeted therapy.
| Consortium | Phase; NCT # |
Targeted therapy; type of pLGG | Status | Design/Primary Objective(s) | Results |
|---|---|---|---|---|---|
| COG ACNS1831 | III; NCT03871257 |
Selumetinib vs. Carboplatin/ Vincristine for newly diagnosed NF1-associated pLGG |
Ongoing | RCT; primary objectives are to characterize event-free survival and determine number of participants with visual acuity improvement | - |
| COG ACNS1833 | III; NCT04166409 |
Selumetinib vs. Carboplatin/ Vincristine for newly diagnosed non-NF1 pLGG |
Ongoing | RCT; primary objective is to characterize event-free survival | - |
| COG ACNS1931 | III; NCT04576117 |
selumetinib vs. selumetinib/vinblastine for relapsed pLGG | Ongoing | RCT; primary objectives are to determine MTD/RP2D and event-free survival | |
| NFCTC RAD001 [49] | II; NCT01158651 | Everolimus for relapsed NF1-associated pLGG | Completed | One-stage design; primary objective is to assess best response of progressive LGG in previously treated individuals with NF1. | 23 pts (median age 9.4 y); 1 pt removed from study due to development of MPNST. 15/22 (68%) pts had response (1 CR, 2 PR, 12 SD); 10/15 had no progression after median follow-up of 33 months. All pts were alive. |
| NFCTC MEK162 |
I/II; NCT02285439 | MEK162 for pLGG and other Ras/Raf/MAP pathway activated tumors | Ongoing | One-stage design; primary objective of phase I are to determine MTD, and of phase II: to determine the response rate | - |
| PBTC-029B [45] | I/II; NCT01089101 |
Selumetinib for relapsed pLGG | Completed | One-stage design; primary objectives of phase I are RP2D and MTD, and of phase II is objective response (complete response + partial response) rate sustained for 8 weeks | 25 pts; 6 pts w/ PR, 14 pts w/ SD, 5 pts w/ PD; Median treatment courses = 26; 2-y PFS 78% |
| PBTC-055 | I/II; NCT04201457 |
Dabrafenib, trametinib, hydroxychloroquine for relapsed BRAF-mutant pLGG after prior therapy with a RAF and/or MEK inhibitor | Ongoing | One-stage design; primary objectives of phase I are RP2D and MTD, and of phase II is sustained objective response rate defined as “better response” than the best response on prior RAF and/or MEK inhibitor | - |
| PNOC001 [56] | II; NCT01734512 |
Everolimus for relapsed pLGG | Completed | Two-stage design; primary objective is to characterize PFS at 6 Months | 65 pts (median age 9 y); PFS at 6 months 63%; 1 CR, 1 PR, 33 SD, 17 PD |
| PNOC002 [46] | I/II; NCT01748149 | Vemurafenib; relapsed BRAFV600E-mutant pLGG | Completed | One-stage design; primary objectives are to determine the RP2D and DLTs, and characterize objective response rates | I: 19 pts, RP2D 550 mg/m2 twice daily after DLT criteria adjustment for rash; 1 CR, 5 PR, 13 SD |
| PNOC014 [57] | I/II; NCT03429803 | Tovorafenib/DAY101 (TAK-580/MLN2480) for relapsed RAS/RAF/MEK/ERK pathway activated pLGG | Ongoing | One stage design; primary objectives are to determine MTD and RP2D | 9 pts treated at 280, 350, and 420 mg/m2. No DLTs. One patient with grade 3 CPK elevation. Best response: 2 CR, 2 PR, 3 SD, 2 PD with median time to response of 10.5 weeks |
| PNOC021 | I; NCT04485559 |
Trametinib/ Everolimus for relapsed pLGG |
Ongoing | One-stage design; primary objectives are to estimate RP2D, define DLTs, and characterize pharmacokinetic profile of trametinib and everolimus in combination. | - |
| PNOC026 | II; NCT04775485 |
Tovorafenib/DAY101 (TAK-580/MLN2480) for BRAF-altered relapsed pLGG | Ongoing | One-stage design; primary objectives are to define overall response rate by RANO and RECIST v1.1 criteria and characterize safety and tolerability | - |
| POETIC [58] | II; NCT00782626 |
Everolimus for relapsed pLGG | Completed | One-stage design; primary objective is to determine if treatment demonstrated a response rate ≥25% | 23 pts (median age 9.2 y); By week 48, response rate of 52% - 2 pts w/ PR, 10 w/ SD; median FU 1.8 years, 2-y PFS 39%, 2-y OS 93% |
CR, complete response; DLT, dose-limiting toxicity; FU, follow-up; m, months; MPNST, malignant peripheral nerve sheath tumor; MTD, maximum tolerated dose; NFCTC, Neurofibromatosis Clinical Trials Consortium; OS, overall survival; PBTC, Pediatric Brain Tumor Consortium; PFS, progression-free survival; PNOC, Pacific Pediatric Neuro-Oncology Consortium; POETIC, Pediatric Oncology Experimental Therapeutics Investigators' Consortium; PR, partial response; RCT, randomized controlled trial; RP2D, recommended phase 2 dose; SD, stable disease; w/, with; y, years
Multiple agents that target the MAPK pathway have been studied in early phase clinical trials or are currently being studied in pLGG. The most studied targeted agents in this group of patients are MEK inhibitors and BRAF inhibitors (Fig. 1). A phase II study of the selective MEK-1/2 inhibitor, selumetinib, in children with relapsed and refractory pLGG remains the largest published study of MEK inhibitors in pLGG and showed impressive results in patients with sporadic OPG and hypothalamic LGG with a 24% partial response rate and 56% of patients with prolonged stable disease [45]. Moreover, selumetinib has recently shown very promising activity in the setting of NF1-induced plexiform neurofibromas, which further broadens the clinical role of MEK inhibitors in pediatric oncology[59,60]. Other MEK inhibitors in active study include trametinib, binimetinib and cobemitinib [61], [62], [63], [64]. Some prospective studies of MEK inhibition alone in pLGG show that a subset of patients can develop rapid progression after withdrawal of the MEK inhibitor, suggesting the possibility of tumor rebound [55,65]. The Children's Oncology Group is currently evaluating the role of MEK inhibition as first line treatment for children with pLGGs, and at recurrence, either as single agent or in combination with the chemotherapy agent, vinblastine.
Experience with BRAF inhibitors is also growing rapidly due to developmental of clinical trials through consortia or independently conceived by pharmaceutical companies that have been able to develop clinical trial designs that more rapidly answer the desired questions with relatively small cohorts of patients [46,66,67]. Dabrafenib and vemurafenib have shown excellent responses in patients with BRAF V600E mutant pLGG in early phase clinical trials. A recent update on the phase II trial of dabrafenib plus trametinib compared to standard of care chemotherapy (carboplatin/vincristine) in the treatment of BRAF V600E-mutant pLGG presented by Bouffet et al. at the American Society of Clinical Oncology (ASCO) Annual scientific Meeting showed an excellent overall response rate (ORR) of the combination of dabrafenib and trametinib (47%) compared to carboplatin plus vincristine (11%) [68]. Studies such as these are the next frontier of targeted therapy studies in pLGG as they compare targeted therapies to the historical control of standard-of-care chemotherapy. The Children's Oncology Group clinical trial comparing the MEK inhibitor, selumetinib, to conventional chemotherapy (carboplatin/vincristine) will further seek to ascertain whether targeted therapy can show equivalence or even superiority to standard-of-care.
The development of combinatorial targeted therapies are an area of ongoing investigation in pLGG. It remains unclear which patients with unresectable pLGG would benefit from combination therapy versus single agent targeted therapy. PNOC021 is the first pLGG trial to combine a MEK inhibitor (trametinib) and mTOR inhibitor (everolimus) and will look to answer the question as to whether a safe MTD can be obtained for this combination.
Targeted therapies harbor unique toxicity profiles that are distinct from conventional chemotherapies used in pLGG management. Conventional chemotherapeutic approaches used to treat pLGG are associated with side effects including myelosuppression, alopecia and less frequently ototoxicity (carboplatin) and decreased fertility potential (procarbazine) [32,36]. Conversely, the side effects of MEK and BRAF inhibitors include skin toxicities, creatine phosphokinase (CPK) elevation, cardiac sequelae, and ocular toxicities [55,66,69]. The use of MEK inhibitors in the treatment of NF1-associated plexiform neurofibromas has further informed the management of toxicities in patients with pLGG as these studies have a longer history and have also aimed to capture late effects of MEK inhibitors [70]. Although the management and surveillance of the toxicities of targeted therapies has improved as clinicians have increased experience with the agents, there remains unanswered questions about the long-term impact of these agents on growth and development, including neuropsychological outcomes in children [71,72]. Furthermore, follow-up remains short for many studies of targeted therapies, including BRAF, MEK, and mTOR inhibitors, in pediatric patients [46,55,73]. Thus, studies to characterize the long-term effects of these therapies are needed. PNOC has created a dedicated long-term follow-up study to systematically capture potentially novel late effects of targeted therapies and this will be vital in the surveillance and management of children treated with targeted therapies into the future.
A challenge in the development novel clinical trials for patients with unresectable pLGG is the chronic nature of the disease for many patients and therefore, deciding what parameters constitute a need for commencing or changing treatment. Furthermore, comparing efficacy of molecularly targeted therapies to outcomes from previous therapies is vital. Radiological progression has historically been the main determinant for commencing therapy and yet, until recently, there was no consensus on the optimal imaging sequences for detecting disease progression [74]. The Response Assessment in Pediatric Neuro-Oncology (RAPNO) working group has outlined consensus recommendations for response-assessment in LGGs to facilitate a standardized approach to this common clinical problem (Fig. 2) [74].
Despite this, defining radiological progression for clinical trials in lesions that are often poorly defined and amorphous in nature remains a significant clinical challenge. Given that many tumors are located near critical organs, such as the optic pathway, small increases in tumor growth may lead to patients changing therapy even if it does not meet the criteria for progression based on consensus guidelines. Furthermore, the ability to determine outcomes remains challenging as radiographic response to tumor therapy does not always correlate with functional outcomes, especially visual outcomes in OPG [75] pLGG clinical trials highlight the importance of incorporating additional outcome measures, particularly functional outcomes such as vision, endocrine outcomes and neuropsychological sequelae into clinical trial designs.
Personalized medicine approaches to treating patients with pLGG using targeted therapeutic options will continue to grow in the coming decades. Despite this, questions remain regarding the optimal duration of treatment with these agents due to the established concerns about tumor rebound and whether combinatorial approaches will circumvent tumor resistance and rebound [76]. Furthermore, there are concerns that these targeted therapies may impacting the natural history of pLGG with the potential that patients with this disease are requiring treatment for longer. Of some concern is the potential impact of targeted therapies on cellular senescence that is typical of patients with pLGG as they transition into adulthood. Lastly, the long-term toxicities of many targeted therapies are still unknown, and this may present the field of pediatric neuro-oncology with novel challenges in the future.
Other challenges in pLGG
Despite the unparalleled insights into the biology underpinning pLGG, significant clinical heterogeneity remains evident between tumors that share molecular profiles. For example, BRAF V600E mutations are seen across tumor types from ganglioglioma to PXA to dysembryoplastic neuroepithelial tumors (DNETs) and even high-grade gliomas [16]. It is therefore inherent that these tumors have different prognoses and as such, integrative diagnoses that incorporate both molecular profiles and histological features are required.
Numerous well-established techniques exist to identify the genetic alterations within pLGGs. Despite this, there is no ‘gold-standard’ approach or algorithm to ascertain which molecular tests should be used for each patient to maximize yield and minimize cost [77]. Importantly, pLGGs are most frequently driven by single gene rearrangements or fusions which are often not detected by whole exome sequencing (WES). As such, specific assays to detect intronic rearrangements are the likely the ideal tools to identify driver alterations in this disease and this may require a combination of targeted sequencing panels and more universally accessible investigations such as SNP arrays, NanoString, Droplet digital PCR or even immunohistochemistry (IHC) and fluorescent in situ hybridization (FISH) [77], [78], [79]. Of significant interest is the role of DNA methylation profiling in pLGG, which has been shown to effectively identify subtype of pLGGs and other CNS tumors [80]. DNA methylation profiling in pLGG may be both a useful diagnostic tool and an adjunct to other molecular testing techniques in this patient group. However, access and cost remain challenges for the field of pediatric neuro-oncology, in particular in developing counties.
Conclusion
In the era of molecular testing of pediatric brain tumors, significant advancements have been made in our understanding of the driver genetic alterations underpinning tumorigenesis in pLGG. These advancements have translated into targeted therapy options in clinical trials for many patients with pLGGs. Although these successes represent a promising paradigm shift in a disease group with few novel therapeutic options in decades past, there remains numerous challenges for the field moving forward.
Author contribution
All authors wrote and edited the manuscript. Figs. 1 and 2 were generated by Mr. Eric Smith, working in consultation with the authors.
Declaration of Competing Interest
PB receives grant funding from Novartis Institute of Biomedical Research for unrelated work, has received grant funding from Deerfield Therapeutics for unrelated work and has served on a paid SAB advisory panel for QED Therapeutics.
Acknowledgements
We acknowledge and thank Mr. Eric Smith for his assistance in generating Figs. 1 and 2.
References
- 1.Farwell J.R., Dohrmann G.J., Flannery J.T. Central nervous system tumors in children. Cancer. 1977;40(6):3123–3132. doi: 10.1002/1097-0142(197712)40:6<3123::aid-cncr2820400656>3.0.co;2-6. Dec. [DOI] [PubMed] [Google Scholar]
- 2.Ostrom Q.T., Cioffi G., Waite K., Kruchko C., Barnholtz-Sloan J.S. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2014-2018. Neuro. Oncol. 2021;23(12 Suppl 2):iii1–iii105. doi: 10.1093/neuonc/noab200. Oct 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ostrom Q.T., Patil N., Cioffi G., Waite K., Kruchko C., Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2013-2017. Neuro. Oncol. 2020;22(12 Suppl 2):iv1–iv96. doi: 10.1093/neuonc/noaa200. Oct 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ostrom Q.T., de Blank P.M., Kruchko C., et al. Alex's lemonade stand foundation infant and childhood primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro. Oncol. 2015;16(Suppl 10):x1–x36. doi: 10.1093/neuonc/nou327. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oberheim Bush N.A., Chang S. Treatment strategies for low-grade glioma in adults. J. Oncol. Pract. 2016;12(12):1235–1241. doi: 10.1200/JOP.2016.018622. Dec. [DOI] [PubMed] [Google Scholar]
- 6.Bandopadhayay P., Bergthold G., London W.B., et al. Long-term outcome of 4,040 children diagnosed with pediatric low-grade gliomas: an analysis of the Surveillance Epidemiology and End Results (SEER) database. Pediatr. Blood. Cancer. 2014;61(7):1173–1179. doi: 10.1002/pbc.24958. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krishnatry R., Zhukova N., Guerreiro Stucklin A.S., et al. Clinical and treatment factors determining long-term outcomes for adult survivors of childhood low-grade glioma: a population-based study. Cancer. 2016;122(8):1261–1269. doi: 10.1002/cncr.29907. Apr 15. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong G.T., Liu Q., Yasui Y., et al. Long-term outcomes among adult survivors of childhood central nervous system malignancies in the Childhood Cancer Survivor Study. J. Natl. Cancer Inst. 2009;101(13):946–958. doi: 10.1093/jnci/djp148. Jul 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wisoff J.H., Sanford R.A., Heier L.A., et al. Primary neurosurgery for pediatric low-grade gliomas: a prospective multi-institutional study from the Children's Oncology Group. Neurosurgery. 2011;68(6):1548–1554. doi: 10.1227/NEU.0b013e318214a66e. Jundiscussion 1554-5. [DOI] [PubMed] [Google Scholar]
- 10.Merchant T.E., Conklin H.M., Wu S., Lustig R.H., Xiong X. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: prospective evaluation of cognitive, endocrine, and hearing deficits. J. Clin. Oncol. 2009;27(22):3691–3697. doi: 10.1200/JCO.2008.21.2738. Aug 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merchant T.E., Kun L.E., Wu S., Xiong X., Sanford R.A., Boop FA. Phase II trial of conformal radiation therapy for pediatric low-grade glioma. J. Clin. Oncol. 2009;27(22):3598–3604. doi: 10.1200/JCO.2008.20.9494. Aug 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aldape K., Brindle K.M., Chesler L., et al. Challenges to curing primary brain tumours. Nat. Rev. Clin. Oncol. 2019;16(8):509–520. doi: 10.1038/s41571-019-0177-5. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J., Wu G., Miller C.P., et al. Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat. Genet. 2013;45(6):602–612. doi: 10.1038/ng.2611. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones D.T., Hutter B., Jager N., et al. Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nat. Genet. 2013;45(8):927–932. doi: 10.1038/ng.2682. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bandopadhayay P., Ramkissoon L.A., Jain P., et al. MYB-QKI rearrangements in angiocentric glioma drive tumorigenicity through a tripartite mechanism. Nat. Genet. 2016;48(3):273–282. doi: 10.1038/ng.3500. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Louis D.N., Perry A., Wesseling P., et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro. Oncol. 2021;23(8):1231–1251. doi: 10.1093/neuonc/noab106. Aug 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bale T.A., Rosenblum M.K. The 2021 WHO classification of tumors of the central nervous system: an update on pediatric low-grade gliomas and glioneuronal tumors. Brain Pathol. 2022:e13060. doi: 10.1111/bpa.13060. Feb 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qi M., Elion E.A. MAP kinase pathways. J. Cell Sci. 2005;118(Pt 16):3569–3572. doi: 10.1242/jcs.02470. Aug 15. [DOI] [PubMed] [Google Scholar]
- 19.Jones D.T., Kocialkowski S., Liu L., et al. Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res. 2008;68(21):8673–8677. doi: 10.1158/0008-5472.CAN-08-2097. Nov 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sievert A.J., Jackson E.M., Gai X., et al. Duplication of 7q34 in pediatric low-grade astrocytomas detected by high-density single-nucleotide polymorphism-based genotype arrays results in a novel BRAF fusion gene. Brain Pathol. 2009;19(3):449–458. doi: 10.1111/j.1750-3639.2008.00225.x. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schindler G., Capper D., Meyer J., et al. Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol. 2011;121(3):397–405. doi: 10.1007/s00401-011-0802-6. Mar. [DOI] [PubMed] [Google Scholar]
- 22.Dias-Santagata D., Lam Q., Vernovsky K., et al. BRAF V600E mutations are common in pleomorphic xanthoastrocytoma: diagnostic and therapeutic implications. PLoS One. 2011;6(3):e17948. doi: 10.1371/journal.pone.0017948. Mar 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khatua S., Gutmann D.H., Packer RJ. Neurofibromatosis type 1 and optic pathway glioma: molecular interplay and therapeutic insights. Pediatr. Blood. Cancer. 2018;65(3) doi: 10.1002/pbc.26838. Mar. [DOI] [PubMed] [Google Scholar]
- 24.Bongaarts A., Giannikou K., Reinten R.J., et al. Subependymal giant cell astrocytomas in Tuberous Sclerosis Complex have consistent TSC1/TSC2 biallelic inactivation, and no BRAF mutations. Oncotarget. 2017;8(56):95516–95529. doi: 10.18632/oncotarget.20764. Nov 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brems H., Beert E., de Ravel T., Legius E. Mechanisms in the pathogenesis of malignant tumours in neurofibromatosis type 1. Lancet Oncol. 2009;10(5):508–515. doi: 10.1016/S1470-2045(09)70033-6. May. [DOI] [PubMed] [Google Scholar]
- 26.Sellmer L., Farschtschi S., Marangoni M., et al. Non-optic glioma in adults and children with neurofibromatosis 1. Orphanet. J. Rare. Dis. 2017;12(1):34. doi: 10.1186/s13023-017-0588-2. Feb 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turner N., Grose R. Fibroblast growth factor signalling: from development to cancer. Nat. Rev. Cancer. 2010;10(2):116–129. doi: 10.1038/nrc2780. Feb. [DOI] [PubMed] [Google Scholar]
- 28.Rivera B., Gayden T., Carrot-Zhang J., et al. Germline and somatic FGFR1 abnormalities in dysembryoplastic neuroepithelial tumors. Acta Neuropathol. 2016;131(6):847–863. doi: 10.1007/s00401-016-1549-x. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huse J.T., Snuderl M., Jones D.T., et al. Polymorphous low-grade neuroepithelial tumor of the young (PLNTY): an epileptogenic neoplasm with oligodendroglioma-like components, aberrant CD34 expression, and genetic alterations involving the MAP kinase pathway. Acta Neuropathol. 2017;133(3):417–429. doi: 10.1007/s00401-016-1639-9. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qaddoumi I., Orisme W., Wen J., et al. Genetic alterations in uncommon low-grade neuroepithelial tumors: BRAF, FGFR1, and MYB mutations occur at high frequency and align with morphology. Acta Neuropathol. 2016;131(6):833–845. doi: 10.1007/s00401-016-1539-z. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeo K.K., Alexandrescu S., Cotter J.A., et al. Multi-institutional study of the frequency, genomic landscape and outcome of IDH-mutant glioma in paediatrics. Neuro. Oncol. 2022 doi: 10.1093/neuonc/noac132. May 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Packer R.J., Ater J., Allen J., et al. Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J. Neurosurg. 1997;86(5):747–754. doi: 10.3171/jns.1997.86.5.0747. May. [DOI] [PubMed] [Google Scholar]
- 33.Walker D.A., Liu J., Kieran M., et al. A multi-disciplinary consensus statement concerning surgical approaches to low-grade, high-grade astrocytomas and diffuse intrinsic pontine gliomas in childhood (CPN Paris 2011) using the Delphi method. Neuro. Oncol. 2013;15(4):462–468. doi: 10.1093/neuonc/nos330. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samples D.C., Mulcahy Levy J.M., Hankinson T.C. Neurosurgery for optic pathway glioma: optimizing multidisciplinary management. Front. Surg. 2022;9 doi: 10.3389/fsurg.2022.884250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ater J.L., Xia C., Mazewski C.M., et al. Nonrandomized comparison of neurofibromatosis type 1 and non-neurofibromatosis type 1 children who received carboplatin and vincristine for progressive low-grade glioma: a report from the Children's Oncology Group. Cancer. 2016;122(12):1928–1936. doi: 10.1002/cncr.29987. Jun 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ater J.L., Zhou T., Holmes E., et al. Randomized study of two chemotherapy regimens for treatment of low-grade glioma in young children: a report from the Children's Oncology Group. J. Clin. Oncol. 2012;30(21):2641–2647. doi: 10.1200/JCO.2011.36.6054. Jul 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gnekow A.K., Falkenstein F., von Hornstein S., et al. Long-term follow-up of the multicenter, multidisciplinary treatment study HIT-LGG-1996 for low-grade glioma in children and adolescents of the German Speaking Society of Pediatric Oncology and Hematology. Neuro. Oncol. 2012;14(10):1265–1284. doi: 10.1093/neuonc/nos202. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gnekow A.K., Kortmann R.D., Pietsch T., Emser A. Low grade chiasmatic-hypothalamic glioma-carboplatin and vincristin chemotherapy effectively defers radiotherapy within a comprehensive treatment strategy – report from the multicenter treatment study for children and adolescents with a low grade glioma – HIT-LGG 1996 – of the Society of Pediatric Oncology and Hematology (GPOH) Klin. Padiatr. 2004;216(6):331–342. doi: 10.1055/s-2004-832355. Nov-Dec. [DOI] [PubMed] [Google Scholar]
- 39.Laithier V., Grill J., Le Deley M.C., et al. Progression-free survival in children with optic pathway tumors: dependence on age and the quality of the response to chemotherapy–results of the first French prospective study for the French Society of Pediatric Oncology. J. Clin. Oncol. 2003;21(24):4572–4578. doi: 10.1200/JCO.2003.03.043. Dec 15. [DOI] [PubMed] [Google Scholar]
- 40.Mahoney D.H., Jr., Cohen M.E., Friedman H.S., et al. Carboplatin is effective therapy for young children with progressive optic pathway tumors: a Pediatric Oncology Group phase II study. Neuro. Oncol. 2000;2(4):213–220. doi: 10.1093/neuonc/2.4.213. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gnekow A.K., Walker D.A., Kandels D., et al. A European randomised controlled trial of the addition of etoposide to standard vincristine and carboplatin induction as part of an 18-month treatment programme for childhood (</=16 years) low grade glioma - A final report. Eur. J. Cancer. 2017;81:206–225. doi: 10.1016/j.ejca.2017.04.019. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lassaletta A., Scheinemann K., Zelcer S.M., et al. Phase II weekly vinblastine for chemotherapy-naive children with progressive low-grade glioma: a canadian pediatric brain tumor consortium study. J. Clin. Oncol. 2016;34(29):3537–3543. doi: 10.1200/JCO.2016.68.1585. Oct 10. [DOI] [PubMed] [Google Scholar]
- 43.Kakadia S., Yarlagadda N., Awad R., et al. Mechanisms of resistance to BRAF and MEK inhibitors and clinical update of US Food and Drug Administration-approved targeted therapy in advanced melanoma. Onco Targets Ther. 2018;11:7095. doi: 10.2147/OTT.S182721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao Y., Adjei AA. The clinical development of MEK inhibitors. Nat. Rev. Clin. Oncol. 2014;11(7):385–400. doi: 10.1038/nrclinonc.2014.83. Jul. [DOI] [PubMed] [Google Scholar]
- 45.Fangusaro J., Onar-Thomas A., Poussaint T.Y., et al. A phase II trial of selumetinib in children with recurrent optic pathway and hypothalamic low-grade glioma without NF1: a Pediatric Brain Tumor Consortium study. Neuro. Oncol. 2021;23(10):1777–1788. doi: 10.1093/neuonc/noab047. Oct 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nicolaides T., Nazemi K.J., Crawford J., et al. Phase I study of vemurafenib in children with recurrent or progressive BRAF(V600E) mutant brain tumors: Pacific Pediatric Neuro-Oncology Consortium study (PNOC-002) Oncotarget. 2020;11(21):1942–1952. doi: 10.18632/oncotarget.27600. May 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sievert A.J., Lang S.S., Boucher K.L., et al. Paradoxical activation and RAF inhibitor resistance of BRAF protein kinase fusions characterizing pediatric astrocytomas. Proc. Natl. Acad. Sci. U. S. A. 2013;110(15):5957–5962. doi: 10.1073/pnas.1219232110. Apr 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karajannis M.A., Legault G., Fisher M.J., et al. Phase II study of sorafenib in children with recurrent or progressive low-grade astrocytomas. Neuro. Oncol. 2014;16(10):1408–1416. doi: 10.1093/neuonc/nou059. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ullrich N.J., Prabhu S.P., Reddy A.T., et al. A phase II study of continuous oral mTOR inhibitor everolimus for recurrent, radiographic-progressive neurofibromatosis type 1-associated pediatric low-grade glioma: a Neurofibromatosis Clinical Trials Consortium study. Neuro. Oncol. 2020;22(10):1527–1535. doi: 10.1093/neuonc/noaa071. Oct 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun Y., Alberta J.A., Pilarz C., et al. A brain-penetrant RAF dimer antagonist for the noncanonical BRAF oncoprotein of pediatric low-grade astrocytomas. Neuro. Oncol. 2017;19(6):774–785. doi: 10.1093/neuonc/now261. Jun 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Greenberger B.A., Pulsifer M.B., Ebb D.H., et al. Clinical outcomes and late endocrine, neurocognitive, and visual profiles of proton radiation for pediatric low-grade gliomas. Int. J. Radiat. Oncol. Biol. Phys. 2014;89(5):1060–1068. doi: 10.1016/j.ijrobp.2014.04.053. Aug 1. [DOI] [PubMed] [Google Scholar]
- 52.Cherlow J.M., Shaw D.W.W., Margraf L.R., et al. Conformal radiation therapy for pediatric patients with low-grade glioma: results from the children's oncology group phase 2 study ACNS0221. Int. J. Radiat. Oncol. Biol. Phys. 2019;103(4):861–868. doi: 10.1016/j.ijrobp.2018.11.004. Mar 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Willard V.W., Conklin H.M., Wu S., Merchant TE. Prospective longitudinal evaluation of emotional and behavioral functioning in pediatric patients with low-grade glioma treated with conformal radiation therapy. J. Neurooncol. 2015;122(1):161–168. doi: 10.1007/s11060-014-1696-7. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bitterman D.S., MacDonald S.M., Yock T.I., et al. Revisiting the role of radiation therapy for pediatric low-grade glioma. J. Clin. Oncol. 2019;37(35):3335–3339. doi: 10.1200/JCO.19.01270. Dec 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Banerjee A., Jakacki R.I., Onar-Thomas A., et al. A phase I trial of the MEK inhibitor selumetinib (AZD6244) in pediatric patients with recurrent or refractory low-grade glioma: a Pediatric Brain Tumor Consortium (PBTC) study. Neuro. Oncol. 2017;19(8):1135–1144. doi: 10.1093/neuonc/now282. Aug 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mueller S., Aboian M., Nazemi K., et al. LGG-53. PNOC001 ( NCT01734512): a phase ii study of everolimus for recurrent or progressive pediatric low-grade gliomas (pLGG) Neuro. Oncol. 2020;22(Suppl 3):iii376. Dec. [Google Scholar]
- 57.Wright K., Krzykwa E., Greenspan L., et al. CTNI-19. phase I trial of day101 in pediatric patients with radiographically recurrent or progressive low-grade glioma (LGG) Neuro-oncol. 2020;22(Supplement_2) doi: 10.1093/neuonc/noaa215.186. ii46-ii46. [DOI] [Google Scholar]
- 58.Wright K.D., Yao X., London W.B., et al. A POETIC Phase II study of continuous oral everolimus in recurrent, radiographically progressive pediatric low-grade glioma. Pediatr. Blood. Cancer. 2021;68(2):e28787. doi: 10.1002/pbc.28787. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gross A.M., Wolters P.L., Dombi E., et al. Selumetinib in children with inoperable plexiform neurofibromas. N. Engl. J. Med. 2020;382(15):1430–1442. doi: 10.1056/NEJMoa1912735. Apr 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gross A.M., Glassberg B., Wolters P.L., et al. Selumetinib in children with neurofibromatosis Type 1 and asymptomatic inoperable plexiform neurofibroma at risk for developing tumor-related morbidity. Neuro Oncol. 2022 doi: 10.1093/neuonc/noac109. Apr 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Woodfield S.E., Zhang L., Scorsone K.A., Liu Y., Zage PE. Binimetinib inhibits MEK and is effective against neuroblastoma tumor cells with low NF1 expression. BMC Cancer. 2016;16:172. doi: 10.1186/s12885-016-2199-z. Mar 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perreault S., Larouche V., Tabori U., et al. A phase 2 study of trametinib for patients with pediatric glioma or plexiform neurofibroma with refractory tumor and activation of the MAPK/ERK pathway: TRAM-01. BMC Cancer. 2019;19(1):1250. doi: 10.1186/s12885-019-6442-2. Dec 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Manoharan N., Choi J., Chordas C., et al. Trametinib for the treatment of recurrent/progressive pediatric low-grade glioma. J. Neurooncol. 2020;149(2):253–262. doi: 10.1007/s11060-020-03592-8. Sep. [DOI] [PubMed] [Google Scholar]
- 64.Lassaletta A., Guerreiro Stucklin A., Ramaswamy V., et al. Profound clinical and radiological response to BRAF inhibition in a 2-month-old diencephalic child with hypothalamic/chiasmatic glioma. Pediatr. Blood. Cancer. 2016;63(11):2038–2041. doi: 10.1002/pbc.26086. Nov. [DOI] [PubMed] [Google Scholar]
- 65.Selt F., van Tilburg C.M., Bison B., et al. Response to trametinib treatment in progressive pediatric low-grade glioma patients. J. Neurooncol. 2020;149(3):499–510. doi: 10.1007/s11060-020-03640-3. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kieran M.W., Geoerger B., Dunkel I.J., et al. A phase I and pharmacokinetic study of oral dabrafenib in children and adolescent patients with recurrent or refractory Braf V600 mutation-positive solid tumors. Clin. Cancer Res. 2019;25(24):7294–7302. doi: 10.1158/1078-0432.CCR-17-3572. Dec 15. [DOI] [PubMed] [Google Scholar]
- 67.Hargrave D.R., Bouffet E., Tabori U., et al. Efficacy and safety of dabrafenib in pediatric patients with BRAF V600 mutation-positive relapsed or refractory low-grade glioma: results from a phase I/IIa study. Clin. Cancer Res. 2019;25(24):7303–7311. doi: 10.1158/1078-0432.CCR-19-2177. Dec 15. [DOI] [PubMed] [Google Scholar]
- 68.Bouffet E., Hansford J., Garré M.L., et al. Primary analysis of a phase II trial of dabrafenib plus trametinib (dab + tram) in BRAF V600–mutant pediatric low-grade glioma (pLGG) J. Clin. Oncol. 2022;40(17_suppl) doi: 10.1200/JCO.2022.40.17_suppl.LBA2002. LBA2002-LBA2002. [DOI] [Google Scholar]
- 69.Egan G., Hamilton J., McKeown T., et al. Trametinib toxicities in patients with low-grade gliomas and diabetes insipidus: related findings? J. Pediatr. Hematol. Oncol. 2020;42(4):e248–e250. doi: 10.1097/MPH.0000000000001427. May. [DOI] [PubMed] [Google Scholar]
- 70.Dombi E., Baldwin A., Marcus L.J., et al. Activity of selumetinib in neurofibromatosis type 1-related plexiform neurofibromas. N. Engl. J. Med. 2016;375(26):2550–2560. doi: 10.1056/NEJMoa1605943. Dec 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peterson R.K., McKeown T., Tabori U., Bartels U., Bouffet E., Janzen L. Neuropsychological impact of trametinib in pediatric low-grade glioma: a case series. Pediatr. Blood. Cancer. 2020;67(12):e28690. doi: 10.1002/pbc.28690. Dec. [DOI] [PubMed] [Google Scholar]
- 72.Walsh K.S., Wolters P.L., Widemann B.C., et al. Impact of MEK inhibitor therapy on neurocognitive functioning in NF1. Neurol Genet. 2021;7(5):e616. doi: 10.1212/NXG.0000000000000616. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Krueger D.A., Care M.M., Agricola K., Tudor C., Mays M., Franz DN. Everolimus long-term safety and efficacy in subependymal giant cell astrocytoma. Neurology. 2013;80(6):574–580. doi: 10.1212/WNL.0b013e3182815428. Feb 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fangusaro J., Witt O., Hernaiz Driever P., et al. Response assessment in paediatric low-grade glioma: recommendations from the response assessment in pediatric neuro-oncology (RAPNO) working group. Lancet Oncol. 2020;21(6):e305–e316. doi: 10.1016/S1470-2045(20)30064-4. Jun. [DOI] [PubMed] [Google Scholar]
- 75.Fisher M.J., Loguidice M., Gutmann D.H., et al. Visual outcomes in children with neurofibromatosis type 1-associated optic pathway glioma following chemotherapy: a multicenter retrospective analysis. Neuro. Oncol. 2012;14(6) doi: 10.1093/neuonc/nos076. Jun790-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jain P., Silva A., Han H.J., et al. Overcoming resistance to single-agent therapy for oncogenic BRAF gene fusions via combinatorial targeting of MAPK and PI3K/mTOR signaling pathways. Oncotarget. 2017;8(49):84697–84713. doi: 10.18632/oncotarget.20949. Oct 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ryall S., Tabori U., Hawkins C. Pediatric low-grade glioma in the era of molecular diagnostics. Acta Neuropathol. Commun. 2020;8(1):30. doi: 10.1186/s40478-020-00902-z. Mar 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ryall S., Arnoldo A., Krishnatry R., et al. Multiplex detection of pediatric low-grade glioma signature fusion transcripts and duplications using the nanostring ncounter system. J. Neuropathol. Exp. Neurol. 2017;76(7):562–570. doi: 10.1093/jnen/nlx042. Jul 1. [DOI] [PubMed] [Google Scholar]
- 79.Kline C.N., Joseph N.M., Grenert J.P., et al. Targeted next-generation sequencing of pediatric neuro-oncology patients improves diagnosis, identifies pathogenic germline mutations, and directs targeted therapy. Neuro. Oncol. 2017;19(5):699–709. doi: 10.1093/neuonc/now254. May 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Capper D., Jones D.T.W., Sill M., et al. DNA methylation-based classification of central nervous system tumours. Nature. 2018;555(7697):469–474. doi: 10.1038/nature26000. Mar 22. [DOI] [PMC free article] [PubMed] [Google Scholar]