Abstract
Extracellular vesicles (EVs) are a collective term for nanoscale or microscale vesicles secreted by cells that play important biological roles. Mesenchymal stem cells are a class of cells with the potential for self-healing and multidirectional differentiation. In recent years, numerous studies have shown that EVs, especially those secreted by mesenchymal stem cells, can promote the repair and regeneration of various tissues and, thus, have significant potential in regenerative medicine. However, due to the rapid clearance capacity of the circulatory system, EVs are barely able to act persistently at specific sites for repair of target tissues. Hydrogels have good biocompatibility and loose and porous structural properties that allow them to serve as EV carriers, thereby prolonging the retention in certain specific areas and slowing the release of EVs. When EVs are needed to function at specific sites, the EV-loaded hydrogels can stand as an excellent approach. In this review, we first introduce the sources, roles, and extraction and characterization methods of EVs and describe their current application status. We then review the different types of hydrogels and discuss factors influencing their abilities to carry and release EVs. We summarize several strategies for loading EVs into hydrogels and characterizing EV-loaded hydrogels. Furthermore, we discuss application strategies for EV-loaded hydrogels and review their specific applications in tissue regeneration and repair. This article concludes with a summary of the current state of research on EV-loaded hydrogels and an outlook on future research directions, which we hope will provide promising ideas for researchers.
Keywords: Extracellular vesicle, Hydrogel, Tissue repair, Regeneration, Exosome
Abbreviations: AD/CS/RSF, alginate-dopamine chondroitin sulfate and regenerated silk fibroin; ADSC, Adipose derived mesenchymal stem cells; ADSC-EVs, adipose mesenchymal stem cells derived EVs; ADSC-Exos, adipose mesenchymal stem cells derived exosomes; ATRP, Atom transfer radical polymerization; BCA, bicinchoninic acid; BMSC, Bone marrow mesenchymal stem cells; BMSC-EVs, bone marrow mesenchymal stem cells derived EVs; BMSC-Exos, bone marrow mesenchymal stem cells derived exosomes; CGC, chitosan-gelatin-chondroitin sulfate; CL, chitosan lactate; CNS, central nervous system; CPCs, cardiac progenitor cells; CS-g-PEG, chitosan-g-PEG; dECM, decellularized ECM; DPSC-Exos, dental pulp stem cells derived exosomes; ECM, extracellular matrix; EGF, epidermal growth factor; EVMs, extracellular vesicles mimetics; EVs, Extracellular vesicles; Exos, Exosomes; FEEs, functionally engineered EVs; FGF, fibroblast growth factor; GelMA, Gelatin methacryloyl; HA, Hyaluronic acid; HAMA, Hyaluronic acid methacryloyl; HG, nano-hydroxyapatite-gelatin; HIF-1 α, hypoxia-inducible factor-1 α; hiPS-MSC-Exos, human induced pluripotent stem cell-MSC-derived exosomes; HS-HA, hypoxia-sensitive hyaluronic acid; HUVEC, human umbilical vein endothelial cell; iPS-CPCs, pluripotent stem cell-derived cardiac progenitors; LAP, Lithium Phenyl (2,4,6-trimethylbenzoyl) phosphinate; LSCM, laser scanning confocal microscopy; MC-CHO, Aldehyde methylcellulose; MMP, matrix metalloproteinase; MNs, microneedles; MSCs, mesenchymal stem cells; MSC-EVs, mesenchymal stem cells derived EVs; MSC-Exos, mesenchymal stem cells derived exosomes; nHP, nanohydroxyapatite/poly-ε-caprolactone; NPCs, neural progenitor cells; NTA, nanoparticle tracking analysis; OHA, oxidized hyaluronic acid; OSA, oxidized sodium alginate; PDA, Polydopamine; PDLLA, poly(D l-lactic acid); PDNPs-PELA, Polydopamine nanoparticles incorporated poly (ethylene glycol)-poly(ε-cap-rolactone-co-lactide); PEG, Polyethylene glycol; PF-127, Pluronic F-127; PHEMA, phenoxyethyl methacrylate; PIC, photo-induced imine crosslinking; PKA, protein kinase A system; PLA, Poly lactic acid; PLGA, polylactic acid-hydroxy acetic acid copolymer; PLLA, poly(l-lactic acid); PPy, polypyrrole; PVA, polyvinyl alcohol; RDRP, Reversible deactivation radical polymerization; SCI, spinal cord injury; SEM, Scanning electron microscopy; sEVs, small extracellular vesicles; SF, Silk fibroin; SPT, single-particle tracking; TEM, transmission electron microscopy; UMSC, umbilical cord mesenchymal stem cells; UMSC-EVs, umbilical cord mesenchymal stem cells derived EVs; UMSC-Exos, umbilical cord mesenchymal stem cells derived exosomes; UV, ultraviolet; VEGF, vascular endothelial growth factor; VEGF-R, vascular endothelial growth factor receptor; WB, western blotting; β-TCP, β-Tricalcium Phosphate; 4-arm-PEG-MAL, four-armed polyethylene glycol (PEG) functionalized with maleimide group
Graphical abstract
1. Introduction
Tissue repair and regeneration have long been challenging for researchers to address, as the ability of human tissues to regenerate is often quite limited. Even skin tissues, which have good regenerative capacity, struggle to regenerate in pathological conditions; for example, severe refractory diabetic ulcers can put patients at risk of amputation [1,2]. For quite some time, researchers considered terminally differentiated somatic cells, such as cardiomyocytes and neuronal cells, nonrenewable due to a dramatic decrease in their ability to proliferate and differentiate in adults [3,4]. In the past, various types of tissue injuries or defects were treated primarily by pharmacological or surgical means, often with limited success. Certain tissue defects require surgical treatment and autologous tissue transplantation may lead to secondary damage at the donor site, while transplantation of prostheses or stents is associated with complications such as immune rejection and aseptic inflammation to varying degrees.
With the development of regenerative medicine, the emergence of stem cell therapy has given new hope to the repair and regeneration of tissues that were previously thought to lack these abilities [5,6]. Mesenchymal stem cells (MSCs) are pluripotent stem cells with multi-directional differentiation potential and self-renewal ability and have the potential to regenerate into various tissues, organs and cells [7]. MSC-related clinical research has been conducted extensively, and many significant breakthroughs have been achieved [8,9]. In addition to being used to restore hematopoietic function and treat autoimmune diseases, MSCs are also widely used to repair various types of tissue damage, such as in skin, bone and cartilage, heart and nerves [8,[10], [11], [12], [13]]. However, as stem cell research has intensified, researchers have discovered that stem cell therapies are a double-edged sword: Although stem cells can promote tissue repair, their indefinable, multidirectional differentiation potential makes them potentially tumorigenic and poses a major ethical problem [14,15].
Extracellular vesicles (EVs), which are nanoscale vesicles generated by paracellular secretion, have been a hot research topic in the field of regenerative medicine for several years. EVs have several advantages over stem cells due to their low antigenicity and relative controllability as cellular substructures [6]. Notably, MSC-derived EVs (MSC-EVs) are believed to have similar biological functions as MSCs themselves [16]. MSC-EVs are considered to be the most promising cell-free therapeutic strategy for tissue repair and regeneration [[17], [18], [19]]; however, they are limited to some extent by the difficulties of achieving sustained slow release of MSC-EVs at specific sites due to body fluid flow as well as circulatory rapid clearance [5,18].
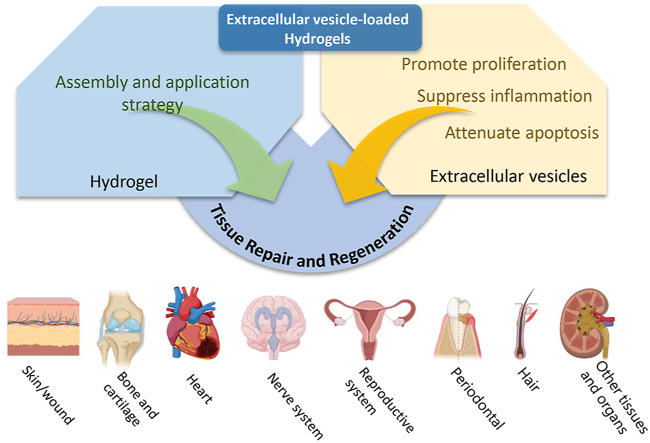
With the development of tissue engineering and biomaterials, researchers have found that combining EVs with biomaterials can compensate for the deficiencies of EVs in specific applications in tissue repair. As a traditional and classical biomaterial, hydrogels have been playing a prominent role in the field of tissue repair and reconstruction. Although hydrogels themselves are relatively devoid of bioactivity, the use of hydrogels loaded with EVs can enhance EVs stability and it helps to deliver EVs to the defect site for sustained in situ release [20,21]. A large number of studies have indicated that EV-loaded hydrogels have great potential for applications in tissue repair and regeneration (Fig. 1) [19,[22], [23], [24], [25], [26], [27]]. It can be used in almost all types of tissue damage, including skin [19], bone and cartilage [25,28], heart [27], central nervous system [26] and reproductive organs [29]. In this paper, we introduce the sources, roles, and extraction and characterization methods of EVs and summarize their current application status. We then review the most common polymers used in hydrogels and discuss factors that affect the loading and release of EVs. We summarize several strategies for loading EVs into hydrogels and describe methods for characterizing EV-loaded hydrogels. Additionally, we discuss application strategies for EV-loaded hydrogels in tissue regeneration and repair. Finally, we conclude with an overview of the current state of research on EV-loaded hydrogels and an outlook on future research directions to provide research ideas for scholars.
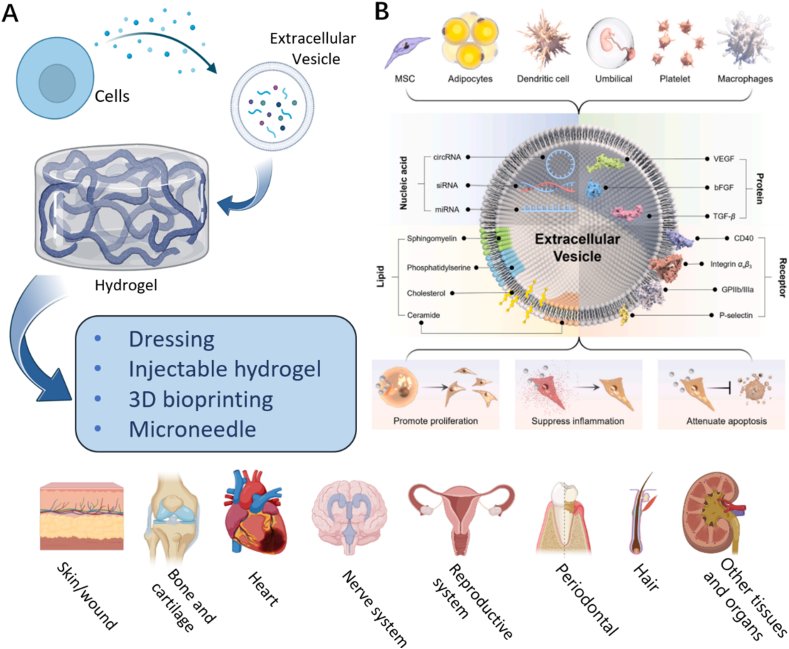
Fig. 1.
Schematic illustrations of Extracellular vesicles loaded hydrogels for tissue repair and regeneration. (A) Created with BioRender.com. (Agreement number: WX24RSIUWC).(B) The origin, contents and biological functions of extracellular vesicles. Adapted reprinted with permission from Ref. [30] (reference number: 221,031–022,389).
2. Extracellular vesicles
2.1. Definition of EVs
EVs are secreted nanoscale vesicles widely found in extracellular fluids, such as plasma, serum, urine, cerebrospinal fluid, and milk. In recent years, EVs have even been extracted from plants and honey [5,31,32]. As cellular substructures with low antigenicity, EVs include a large number of proteins, nucleic acids, growth factors, and other contents with a wide range of biological activities, and thus have important research applications in tissue repair and regeneration [6,18]. In addition, due to their structural properties, EVs can be used as a system for drug or nucleic acid delivery [33].
There are various subtypes of EVs such as endosome-origin “exosomes” and plasma membrane-derived “ectosomes” (microparticles/microvesicles) or apoptotic bodies derived from the apoptotic process [34]. Exosomes are considered to play a major function and have been investigated in many studies [35,36]. However, according to expert consensus from the International Society for Extracellular Vesicles (ISEV) in 2018 (Minimal information for studies of extracellular vesicles 2018, MISEV2018), it is not yet possible to extract purified exosomes with current extraction and characterization techniques; therefore, it is more appropriate to use EVs directly or use small EVs (sEVs) to mimic this subtype [37,38]. Current studies have not been able to clarify the functions possessed by each EV subtype. In other words, specific characterization of each subtype is not yet possible [34]. However, what is certain is that EVs obtained from different cell sources or different cell pretreatment methods (such as hypoxia) have differences in biological activity [39,40]. Pomatto et al. [40] specifically evaluated adipose mesenchymal stem cell-EVs (ADSC-EVs) and bone marrow mesenchymal stem cell-EVs (BMSC-EVs) and found differences in their contents. Between them, ADSC-EVs had more proangiogenic related substances. Therefore, a full elaboration of various factors such as cell source, advance processing method, size, and surface markers of EVs is necessary for the scientific and reproducible nature of the study findings.
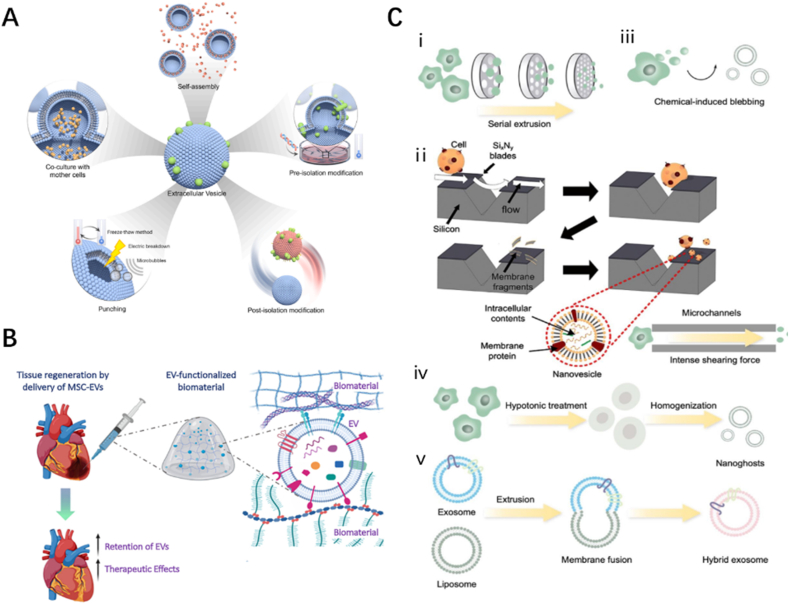
Currently, the process for isolating EVs is complex and achieves low yield, limiting the development of EVs for clinical application. Scholars have attempted to address this problem from three different perspectives. The first method involves pretreating cells by physicochemical means or creating engineered EVs to obtain more, or more biologically active, EVs (Fig. 2A) [41]. For example, Han et al. [39] found that EVs from hypoxia-treated ADSCs enhanced angiogenesis activity. The second method involves loading EVs into biomaterials to prolong the retention rate of EVs at target sites and improve their therapeutic efficacy (Fig. 2B) [42]. EVs combined with scaffolds are considered to be important players in the field of bone regeneration [43]. The third method involves the use of extracellular vesicles mimetics (EVMs). Unlike true EVs, which are secreted by cells, EVMs are obtained by physically or chemically splitting cells into small nano-vesicles (Fig. 2C). EVM preparation methods (See REVIEW [44] for details) can achieve yields hundreds of times higher than those of EVs while retaining similar bioactive functions [44]. EVM can be broadly classified into three different principles, which can be chosen according to different needs: direct cell preparation, preparation after removal of cytoplasm and nucleus, or fusion with cell-secreted EVs using liposomes [44]. Different preparation methods affect the surface markers and cargoes of EVM, depending on which of its properties the investigators want to exploit, bioactivity or drug-carrying capacity [44].
Fig. 2.
Breakthrough points in the application of extracellular vesicle research
(A) Strategies to enhance the bioactivity of EVs and construct engineered EVs. Adapted reprinted with permission from Ref. [30] (Reference number: 221,031–022,389). (B) EVs loaded with biomaterials. Adapted reprinted with permission from Ref. [22], Copyright © 2022, John Wiley and Sons GmbH (License number:5,440,800,437,958). (C) Preparation strategies for EVMs. Adapted reprinted with permission from Ref. [44], Copyright © 2022 Wiley-VCH GmbH (License number:5,440,800,929,863).
2.2. Mesenchymal stem cell-derived EVs
MSCs are currently a major focus of regenerative medicine research and paracrine signaling is considered a key aspect of their function. MSC-EVs are involved in promoting tissue repair and regeneration [18]. The proteins, mRNAs, microRNAs, long non-coding RNAs, and lipid components encapsulated in MSC-EVs activate relevant signaling pathways by acting directly or indirectly on target cells, which in turn exert reparative and regenerative effects [45,46]. Most biomaterial-loaded EVs are MSC-EVs, with ADSC-EVs, BMSC-EVs, and umbilical cord mesenchymal stem cells-EVs (UMSC-EVs) being the most common types. It has been well documented that MSC-EVs can promote epithelial cell proliferation and differentiation, induce angiogenesis, and promote nerve, bone, cartilage, and cardiac repair, among other functions [17,18,[47], [48], [49], [50]]. In addition, MSC-EVs can suppress inflammation and attenuate apoptosis [30]. Most notably, MSC-EVs loaded into hydrogels exhibit enhanced repair capacity and pro-regenerative effects when used for tissue repair and regeneration [42,48].
2.3. Separation of EVs
According to a survey conducted by ISEV at the end of 2015, ultracentrifugation is still the most common way to extract EVs [51]. This method obtains the required components mainly based on the difference in size and density of the components in the original solution [35,52]. However, it should be noted that the presence of fetal bovine serum in the cell culture supernatant may lead to contamination of EVs and affect their purity when ultracentrifugation is used [53]. The use of serum-free mediums may lead to changes in cell status, which in turn may affect the quality of secreted EVs, making it necessary to select a special serum (such as EVs/exosome-depleted fetal bovine serum) for EV isolation [53]. This technology is also time consuming and carries high costs. Currently, several other methods are being used to isolate and purify EVs, such as immunoaffinity enrichment, ultrafiltration, and size exclusion chromatography [51,54]. Although many new extraction techniques have been developed in recent years, their use has been limited by several disadvantages, such as low efficiency, high cost, and poor specificity [51,54]. Nonetheless, the combination of multiple extraction methods facilitates the isolation of good quality EVs. In addition, for different sample types, especially complex samples, a combination of density gradient centrifugation, filtration, and size-exclusion chromatography is often required [51]. Separation and purification techniques for EVs include a number of modern techniques, as detailed in REVIEW [35] and REVIEW [54].
2.4. Enhancement of EV production
The difficulty in achieving mass production is the most important factor limiting the development of EVs. Taking cell culture supernatant as an example, only 1 μg of EVs (quantified as protein) may be harvested from 1 ml of culture supernatant [55]. Tens to hundreds of milligrams of EVs (quantified as protein) may be needed to conduct a series of animal experiments [55]. In addition to optimizing purification methods or developing related technologies, the simplest and most straightforward path is to increase the cell culture area. For example, HYPERflasks or roller bottles can enhance the cell culture area and have been used to produce monoclonal antibodies and vaccines on a large scale [56,57]. In recent years, 3D cell culture methods have been a topic of interest for researchers [56]. Unlike traditional 2D culture, 3D cell culture is considered to be more compatible with the microenvironment of cell growth in the human body. In addition to the possibility of improving the acquisition of EVs with 3D culture, it has been shown that EVs in 3D culture have higher activity [58]. Yan et al. [58] showed that EVs from UMSC-based 3D cell culture have a higher osteochondral regeneration activity than those of 2D cell culture. Not only can 3D culture of cells be realized; bioreactors have the potential to simulate more realistic cell growth environments by employing a certain magnetic or force field, which in turn have greater potential [56]. De Almeida Fuzeta et al. [59] used a microcarrier-based bioreactor to increase the yield of EVs from three MSCs (BMSC, ADSC, UMSC) 3 to 6 times. Certain cell stimulation modalities (e.g., physically triggered [60], hypoxic [61] etc.) have also been shown to stimulate cells to release more EVs in the same cell culture area. Achieving large scale production of EVs is a difficult barrier to overcome in the EVs field (See REVIEW [56] for details on the large-scale production of EVs).
2.5. Characterization of EVs
In 2018, in response to the problem of irregularities in EVs research, the many experts in the ISEV joined together to develop MISEV2018 (an update from the MISEV2014). In order to make EV research scientifically rigorous and enhance the reproducibility of results, the consensus proposed a minimum amount of information that needed to be disclosed for EV research reporting and conducted a review of the current status of the field [37]. The consensus points out that EV studies needed to disclose, among others, the species, cell types, sample types, and experimental conditions used in the study. With regards to EV characterization, positive and negative markers, electron micrographs, and particle size analysis needed to be included. Also, studies involving EVs from MSCs required identification of the MSCs used as well as tri-lineage induced differentiation.
The three most widely used methods in the characterization of EVs are western blotting (WB), single-particle tracking (SPT), and electron microscopy. Among the SPT techniques, nanoparticle tracking analysis (NTA) is the most widely used approach [51]. In MISEV2018, it is noted that there are three classes of EV markers that can be characterized using WB or flow cytometry: transmembrane or GPI-anchored extracellular proteins associated with the plasma membrane and/or endosomes (e.g., CD63, CD81, CD82, CD47, etc.), cytosolic proteins recovered from EVs (e.g., TSG101, HSPA8, HSPA1A, etc.), and isolated major non-EVs fractions (e.g., lipoproteins, ApoA1/2, ApoB, albumin, etc.) [37]. Scanning electron microscopy (SEM) or transmission electron microscopy (TEM) images allow visualization of the size and bilayer lipid structure of individual EVs. In contrast, NTA can be used to quantify a large number of EVs and analyze the particle size distribution of the extracted samples, which are essential for characterizing EVs.
2.6. Enhancement of EV activity
As EVs are often difficult to obtain in large quantities due to limitations in extraction protocols, enhancing the activity of EVs through pretreatment methods can make up for low yields. It has been well established that EVs obtained by pretreating cells with physicochemical factors (e.g., hypoxia, iron nanoparticles, etc.) have stronger biological activity [39,62]. Hypoxia-treated ADSC-EVs were found to have higher pro-angiogenic activity due to an increase in the expression of vascular endothelial growth factor (VEGF), vascular endothelial growth factor receptor (VEGF-R), epidermal growth factor (EGF) and fibroblast growth factor (FGF). Additionally, increased miR-31 and let-7 expression in hypoxia-treated ADSC-EVs resulted in the activation of the protein kinase A system (PKA) signaling pathway in endothelial cells, thus inducing the expression of endogenous VEGF and VEGF-R and leading to enhanced angiogenesis [39,63,64]. The role of hypoxic ADSC-EVs in cartilage regeneration has also been evaluated; upregulation of chondrocyte-related gene expression in hypoxic ADSC-EVs was found to induce more cartilage matrix and proteoglycan production, and this study is expected to play an important role in cartilage tissue engineering [65]. Hypoxia is only one of the more classic pretreatment strategies, but there are many others, such as the addition of lipopolysaccharides [66], hydrogen peroxide [66], hydrogen peroxide [67], atorvastatin [68], and pioglitazone [69]. EV activity can also be enhanced by engineering or genetic strategies, including surface modifications, genetic modification, and epigenetic reprogramming [41,70,71]. In addition, as excellent natural carriers, EVs have been loaded with nucleic acids, growth factors, natural drugs, and other molecules to enhance their biological activity [72].
2.7. Administration of EVs
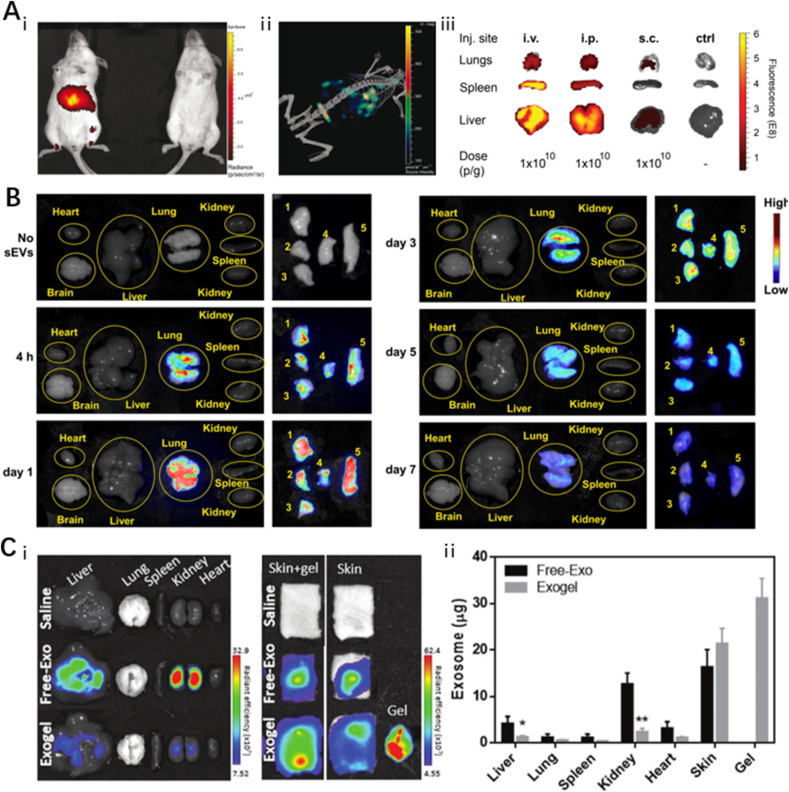
Currently, there are two methods of EV administration: systemic administration and topical application. Systemic administration is often achieved by intravenous or intraperitoneal injection. It has been shown that EVs administered systemically are rapidly cleared in the blood circulation and captured by macrophages in the reticuloendothelial system, eventually accumulating in the liver, spleen, lungs, and gastrointestinal organs [73]. In particular, Wiklander et al. [74] evaluated the effect of different administration modes on the biodistribution of EVs and found that subcutaneously administered EVs were less enriched in the liver and spleen compared to those administered by intravenous and intraperitoneal injections (Fig. 3A). The administration mode of EVs depends greatly on the clinical problem to be solved. Some relatively novel administration modalities have been proposed in recent years and are expected to play an important role in related disease areas. Betzer et al. [75] found that transnasal administration resulted in a large degree of enrichment of EVs in the brain. Han et al. [76] innovated the nebulized inhalation of EVs and found that they are almost exclusively enriched in the lungs and not in other non-target organs, within 7 days (Fig. 3B).
Fig. 3.
Effect of different drug delivery strategies on the biodistribution of EVs
(A)Biodistribution of EVs by different injection methods. i. v.: Intravenous injection; i. p.: Intraperitoneal Injections; s. c.: Subcutaneous Injections. Adapted reprinted with permission from Ref. [74], based on Creative Commons Attribution-NonCommercial 4.0 International Public License (CC BY-NC 4.0) © 2015 Oscar P. B. Wiklander et al. (B) Biodistribution of EVs for inhalation drug delivery, EVs were observed in the lungs only during a period of up to seven days. Adapted reprinted with permission from Ref. [76], Copyright @ 2022 Elsevier B.V. All rights reserved. (License number: 5,440,810,081,513). (C) Biodistribution of topically administered EVs. Adapted reprinted from with permission [77], Copyright @ 2022 The Authors, Small published by Wiley-VCH GmbH (License number:5,440,810,773,416).
In comparison with systemic administration, local application of EVs reduces their removal by the circulatory system to some extent and also reduces the enrichment of EVs in non-target organs. Topical application can be achieved by local injection or direct coverage of the trauma site; however, due to the complexity of the trauma environment, EVs are often easily degraded and rendered inactive [49]. Biodegradable, sparse, and porous hydrogels can be used to carry EVs and prevent premature clearance. Even when only a relatively small amount of EVs is loaded, hydrogel carriers can facilitate the production and maintenance of the desired therapeutic effect over time [42,78]. Wang et al. [72] compared the bioremoval rates of EVs applied directly to local wounds against those loaded with hydrogel. The results showed that, with the same dose, the directly applied EVs were almost completely removed within four days, while the hydrogel-loaded EVs were still uniformly retained on day four. Kwak et al. [77] used PEG-based hydrogels loaded with EVs for wound and found that EVs in the hydrogel group were barely enriched to the liver and kidneys and acted mainly on the skin compared to direct application of EVs (Fig. 3C). Also, hydrogels can be injected locally into the target organ or prepared as microneedle patches for topical application to achieve a more localized and targeted delivery of EVs [79].
3. Hydrogels
3.1. Definition of hydrogels
Hydrogels are extremely hydrophilic three-dimensional network gels formed by the physical or chemical crosslinking of hydrophilic polymers and have been studied for more than 100 years [80]. Hydrogels can be divided into two main categories: natural and synthetic. Single-component hydrogels generally have their corresponding drawbacks, and stronger physical or chemical properties can be obtained by combining different raw materials. In addition, hydrogels can be produced in different sizes (microgels, nanogels, etc.). With advancements in technology, stimulus-responsive hydrogels, self-healing injectable hydrogels, strongly adhesive hydrogels, and even conductive hydrogels for nerve injury repair have entered the crosshairs of researchers. However, the lack of biological activity has been a drawback of hydrogels as biomaterials. Researchers have tried to enhance the function of hydrogels loading them with various drugs and bioactive factors. For example, Ahmadi et al. [81] showed that chitosan hydrogels alone do not have pro-angiogenic activity but can facilitate pro-angiogenic effects when loaded with MSCs. The development of hydrogels with higher bioactivity and functionality is of great importance and is expected to bring new breakthroughs in the field of tissue repair and regeneration.
3.2. Hydrogel materials
3.2.1. Natural polymers
Natural hydrogels are the earliest type of hydrogel to study because the raw materials are natural polymer substances from living organisms, such as polysaccharides (e.g., hyaluronic acid, sodium alginate, chitosan, agarose) and proteins (e.g., collagen, tropocollagen, gelatin, silk fibroin). Natural hydrogels have good biocompatibility and are considered to have extracellular matrix (ECM)-like functions that facilitate cell growth and differentiation. Natural polymer hydrogels are typically the types of hydrogels widely used for EV delivery; however, due to their poor stability, mechanical properties, and tissue adhesion properties, natural hydrogels often need to be crosslinked with other polymers.
3.2.1.1. Polysaccharides
Hyaluronic acid (HA) is a linear nonsulfated glycosaminoglycan. It is a major component of the ECM and is found in almost all body tissues and fluids [82]. As a major component of cartilage tissue, HA exhibits high biocompatibility in this context, and thus, HA hydrogels play an important role in cartilage tissue engineering [83]. During cartilage formation, HA induces the differentiation of MSCs into chondrocytes to maintain the chondrocyte phenotype and increase the deposition of ECM in cartilage. In addition, HA is widely present in connective tissues, and studies have indicated that HA hydrogels can provide sites for cell proliferation and differentiation; therefore, HA is widely used in tissue repair and reconstruction applications [84,85]. Burak Derkus [86] used HA hydrogels loaded with human cardiomyocyte-derived EVs and finds that they can induce the expression of heart-related genes in MSC.
Chitosan is a common ingredient used in the preparation of natural hydrogels [87]. Chitosan is hydrophilic, biocompatible, and biodegradable by lysozyme, acid, and colonic bacteria in the human body [87]. Chitosan can be distinguished from other polysaccharides because it has its own positively charged amine group [88]. This enables it to be used as a good drug delivery system because the surface charge allows it to influence the loading and release of certain drugs. The negatively charged phospholipid membrane of EVs can react with the positive charge on chitosan; therefore, chitosan has considerable potential for use in the delivery of EVs [19]. In addition, as it has its own amine group, chitosan can react with other polysaccharides with aldehyde groups via Schiff base reaction and is a good material for the preparation of self-healing hydrogels [89]. However, because chitosan is soluble in acidic solutions, it must be modified to improve its water solubility at physiological pH.
Alginate is a natural marine polysaccharide, of which the most common extract obtained from seaweed is sodium alginate [90]. Alginate is an abundant and readily available biopolymer with remarkable biocompatibility, good porosity, strong water retention capacity, and adjustable viscosity, making it a particularly suitable material for biomedical applications [91,92]. Sodium alginate has significant pH sensitivity and can rapidly form gels under extremely mild conditions through ion exchange reactions with cations [92]. It is expected to be used as a PH-responsive hydrogel for intelligent drug release in some specific contexts [92]. However, ion exchange processes, or ion loss, may lead to uncontrolled dissolution of the alginate polymer network, a drawback that somewhat limits its application [93].
3.2.1.2. Proteins
Collagen, the most abundant protein in the ECM, has a triple helix structure that provides great tensile strength. Due to the abundance of collagen in the natural ECM, collagen-based hydrogels are becoming increasingly popular as tissue engineering scaffolds. Among the different collagens, type I collagen is by far the most prevalent form and is popular in tissue engineering due to its ease of extraction and suitability for a variety of applications [94]. In addition, the structure of collagen allows crosslinking to form a 3D porous, fibrous mesh structure, which facilitates the loading of EVs. One study used collagen hydrogel as a carrier for Apis mellifera royal jelly EVs and found that it effectively released them continuously over a period of 7 days [31], supporting the use of collagen hydrogels for slow-release drug delivery. The authors also found that the concentration of collagen determined the release patterns of EVs. The concentration of 2 mg/ml is more favorable for the release of EVs compared to 3 mg/ml of collagen.
Gelatin is a partially hydrolyzed derivative of collagen, and its properties are highly dependent on the processing, molecular weight, and isoelectric point of collagen [95]. In contrast, gelatin is more biocompatible and has better thermal stability than collagen. In addition, several methods to enhance properties of gelatin, such as hybridization with other polymers, crosslinking strategies, and chemical modifications, are currently being assessed [[96], [97], [98]]; these are likely to broaden the scope of gelatin hydrogel applications. In 2000, Bulcke et al. synthesized a representative hydrogel formulation, commonly known as gelatin methacryloyl (GelMA), which is a composite enhanced version of gelatin hydrogel [98]. GelMA, obtained by combining methacrylic anhydride with gelatin, is a photosensitive biohydrogel material that has excellent biocompatibility and can be cured by ultraviolet (UV) or visible light to form a three-dimensional structure with strength suitable for supporting cell growth and differentiation [99]. In particular, Born et al. [97] evaluated the feasibility of GelMA hydrogels loaded with MSC-EVs for 3D bioprinting. The results showed that experiencing 3D bioprinting and light curing did not affect the activity of EVs, and indicated that 7% GelMA may be a more appropriate bioink concentration.
Silk fibroin (SF), derived from natural silk, is a natural polymeric protein polymer that is widely used in biomanufacturing. SF has high tensile biomechanical strength and is highly biocompatible and biodegradable [100]. Because of its intrinsic tendency to form regular β-sheet stacks, SF biopolymers can be processed into purely physically crosslinked hydrogels without chemical crosslinking agents. Cryo-self-assembled SF sponges have been developed as biodegradable platforms for the enzyme-responsive delivery of EVs [101]. Fibroin chains can self-assemble into silk I structures in ice-cold temperatures conditions when annealed above the glass transition temperature; in this context, EV release is enzyme-responsive, with rates primarily determined by the rate of enzymatic degradation of the scaffolds. During phase separation, the EVs gradually become tightly wrapped by the filamentous chains, making the release of EVs completely dependent on enzymatic scaffold degradation. During this process, the sustained release of EVs promotes cell migration and inward growth of myofibroblasts. In this way, the degradation-driven sustained release of EVs from SF sponges can be used for long-term tissue repair and regeneration, providing a new direction for EV-loaded hydrogel research [101].
Polydopamine (PDA) is a biopolymer produced by the oxidative polymerization of dopamine [102]. PDA can be produced simply and inexpensively without toxic solvents and, therefore, has low cytotoxicity and good biocompatibility (>80%). Furthermore, PDA has been shown to enhance cell adhesion and proliferation [102]. In addition, PDA is uniquely suited to biomedical applications by virtue of its hydrophilicity and its ability to functionalize different substrates [102]. A PDA-based mussel-like highly adhesive EV-loaded hydrogel has been shown to be important in the repair of cartilage [103]. Because the general hydrogel has poor adhesion on moist cartilage tissue.
3.2.2. Synthetic polymers
Synthetic hydrogels are made by crosslinking synthetic hydrophilic polymers, commonly polyethylene glycol (PEG), polyacrylic acid and its derivatives, polylactic acid-hydroxy acetic acid copolymer (PLGA), polyvinyl alcohol (PVA), and phenoxyethyl methacrylate (PHEMA). Compared with natural polymers, synthetic polymers have specific molecular weights, basic structural units, and can be pre-designed to obtain desired properties, including specific porosity, degradation times, as well as mechanical properties [104].
PEG, an adduct of polyethylene oxide and water, is a widely used artificial polymer with good biocompatibility [105,106]. Chemically crosslinked PEG hydrogels can be formed via light/UV-induced or radiation-induced radical polymerization, and end chains can be modified with different chemical groups [105]. PEG can also be used to generate physical crosslinked networks from various motifs, thus making the hydrogel reversible and responsive to stimulation [107]. Moreover, studies have confirmed that the mechanical properties and chemical composition of PEG can be fine-tuned, allowing easy control of the scaffold structure, making PEG an adaptable material for biomedical applications [106]. However, PEG hydrogels are biologically inert, have low antimicrobial properties, and exhibit more severe swelling characteristics, and thus requiring the addition of other polymers to overcome these disadvantages [108]. Kwak et al. [77] used degradable PEG hydrogels to encapsulate EVs, the release time of EVs could be adjusted to 5–26 days by adjusting the cross-link density and sealing properties. Mol et al. [109] constructed a supramolecular hydrogel by linking two ureido-pyrimi-dinone (UPy) units to the main chain of PEG. The results show that EVs can be released over a period of up to 2.5 weeks, and the authors also demonstrate that the released EVs remain cellularly active. The molecular weight of the PEG block within it may result in different kinetics of release. With a PEG block of 10 kg mol−1 (UPy10 k) showed a steadier release than UPy20 k.
3.2.3. Other polymers
Poly lactic acid (PLA) is obtained via the ring-opening polymerization of propyl cross-ester. PLA is characterized by high thermal stability, good cytocompatibility, and non-toxic degradation products. PLA exists in different forms, including poly (l-lactic acid) (PLLA) and poly (D, l-lactic acid) (PDLLA), and its degradation rate can be optimized by adjusting the L/D ratio [110]. PLGA, which is produced via the combination of polyglycolic acid and PLA, is an FDA approved polymer with good biocompatibility and biodegradability and tunable mechanical properties. In addition, depending on the choice of propylene cross ester monomer, PLLA and PDLLA can generate stereoisomers that alter the hardness of the hydrogels encapsulating MSCs [111]. These polymers are hydrophobic and do not have functional side groups for chemical crosslinking; when copolymerized with hydrophilic polymers, such as PEG, their physical properties are altered. The biphasic copolymers form micelles, which can be adjusted by changing the molecular weight of PLA and PEG [112,113]. The synthesis of PEG and PLA triblock copolymers (PEG-PLA-PEG or PLA-PEG-PLA) opened the door for PLA as a material for hydrogel synthesis [114]. One limitation of PLA-based hydrogels is that they are crosslinked primarily through physical interactions, which renders them less stable for use as an implant or scaffold. Future research on PLA-based hydrogel systems should include the development of injectable implants that can stay in the body for at least 1 year to increase their utility in the clinic [114]. Swanson et al. designed PLGA-PEG-PLGA terpolymer microspheres for encapsulating MSC-EVs and combined them with PLLA scaffolds, which can provide a good biomechanical structure and show great value in bone injury repair [115].
Self-assembling peptides (SAPs) is a nano-biomaterial composed of natural amino acids that can spontaneously form hydrogels in aqueous solutions under physiological conditions [116]. Because of its nanomaterial properties, it has been widely used for tissue engineering as well as drug delivery [116,117]. The amino acid groups on the SAPs can be functionally modified, which in turn can adjust the release rate of the loaded drug in the SAPs [117]. Zhou et al. [23] designed a Matrix metalloproteinases-2 (MMP2)-sensitive SAP hydrogel for the repair of renal injury by loading BMSC-EVs. EVs were rapidly cleared within 24 h when applied directly to the body, while EVs were still observed at 72 h by SAP hydrogel loading.
DNA molecules have specific recognition, self-assembly, and sequence programming abilities, and have become an excellent material for the construction of micro- and nanostructures. Back in 2006, Soong Ho Um et al. prepared pure DNA hydrogels under physiological conditions using ligases on branching DNA, opening the door to DNA hydrogel research [118]. There is no doubt that the introduction of DNA into hydrogels contributes to the programmability of the material. DNA hydrogels have received much attention because of their good biocompatibility and biodegradability, and the presence of designable stimulus response units has led to their wide use in the field of tissue engineering and regenerative medicine [119,120]. Reversible deactivation radical polymerization (RDRP) can achieve polymerization in a physiological environment, generating good porosity for controlled drug loading and release [121,122]. Atom transfer radical polymerization (ATRP) is a powerful and versatile RDRP process, which can even be used for nucleic acid molecules such as DNA, RNA, sugars or proteins [[121], [122], [123]]. Yerneni et al. [124] designed an ATRP-based hydrogel (using cholesterol-modified DNA tethers, the lipid membrane of exosomes was functionalized with initiators to graft polymers in the presence of additional initiators and crosslinker using photoinduced ATRP) for the controlled release of EVs and found that the release time of EVs was significantly prolonged up to one month. This hydrogel delivery system showed great potential.
The ECM is the most important component of the tissue microenvironment as it is rich in a variety of biomolecules and provides the most basic platform for cell growth and information exchange [125]. Decellularized ECM (dECM) is thought to preserve bioactive components while possessing excellent biocompatibility, making it an ideal material for the preparation of hydrogels. As a tissue-derived material, dECM hydrogels maintain the original microenvironment of tissues, have strong biocompatibility, and can induce the migratory differentiation of cells; thus, dECM hydrogels are expected to play an important role in the future of tissue repair and regeneration therapies [126]. In addition, as a complex mixture of proteins, ECM hydrogels contain sufficient binding sites to facilitate binding to EVs, leading to the exertion of stronger synergistic effects. Faust et al. [127] found that bladder ECM hydrogels increased the survival of primary hippocampal neurons and promoted neuronal synapse growth, with the matrix-bound vesicles in them playing an important role. In particular, Hernandez et al. [126] evaluated the effect of ECM hydrogels of different tissue sources on the encapsulation and release of EVs. Interestingly, the encapsulation efficiency and release time of EVs from ECM of different tissue sources differed, with lung ECM having a longer encapsulation time of EVs compared to muscle and heart. Tissue specificity affects the pore size, biomechanics and non-covalent interactions of ECM hydrogels. In the future, EV-loaded ECM hydrogels may become a hot topic for research on tissue repair and regeneration.
In general, natural polymers have good biocompatibility and promote cell adhesion and proliferation; however, they tend to be limited by low mechanical strength, shrinkage, handling difficulties, and, in some cases, high preparation costs [85,91,128]. While synthetic polymers have good mechanical properties, they lack the biosignature present in natural polymers [112]. Therefore, in order to obtain better mechanical strength and maintain good biocompatibility, hybrid hydrogels composed of natural and synthetic materials are attracting increasing interest [22,129]. With their structural similarity to natural ECM, adjustable viscoelastic and mechanical properties, high hydrogel content, and oxygen and essential nutrient permeability, hybrid hydrogels have emerged as promising candidates for use as tissue engineering scaffolds [111].
3.3. Types of hydrogels
3.3.1. Microgels and nanogels
Microgels (Hydrogel microspheres) and nanogels are particulate hydrogels with sizes in the micrometer and nanometer ranges, respectively [130]. Unlike macroscopic hydrogels, microgels and nanogels are much smaller than the inner diameter of a syringe needle and can be directly injected. In addition, their relative surface area is larger, allowing for easy natural clearance and an enhanced ability to penetrate tissue barriers. Different sizes of hydrogels are suitable for different routes of administration [131]. For example, microgels smaller than 5 μm in diameter are used for oral or pulmonary administration but are generally considered unsuitable for intravascular injection due to their faster circulating clearance rate. Nanogels 10–100 nm in size are suitable for systemic administration because they can leave small blood vessels through open windows in the endothelial lining, allowing extravasation into the tissue (See REVIEW [131] for details).
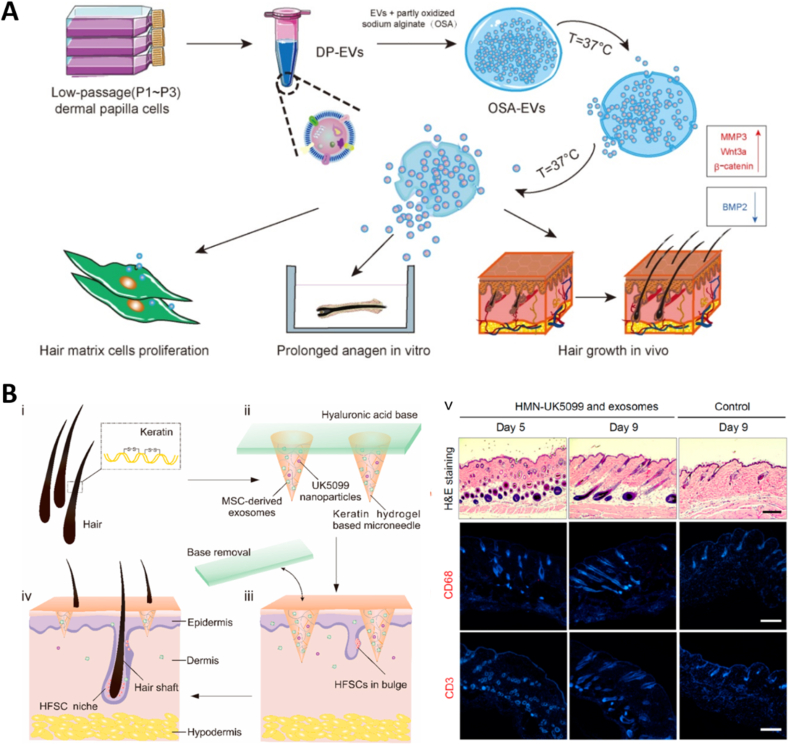
Microgels (Hydrogel microspheres) can be used to load cells, drugs, EVs, etc., and have been widely used in biomedical fields [[132], [133], [134], [135], [136], [137]]. Gan et al. [138] first encapsulated MSC-EVs in alginate hydrogel microspheres, and the hydrogel microspheres provided a good protective barrier to maintain the activity of MSC-EVs. To avoid damage to the MSC-EVs microspheres from the acidic environment of the stomach, the authors' team also wrapped gelatin around the outside of the microspheres. The composite MSC-EVs microsphere structure was used in a mouse model of inflammatory bowel disease and showed better therapeutic effects. Hydrogel microspheres loaded with EVs have also been used in hair regeneration [139] and in the repair of post-infarct heart tissue [140]. Microgels are advantageous as carriers for EVs delivery, but there are fewer studies in this area. Oral EV-loaded microgel are expected to be used for the repair of gastrointestinal tissues; intrapulmonary delivery is expected to apply EV-loaded microgel to the repair of lung tissues. It should be particularly emphasized that the nanogels are similar in size to EVs and whether they can be applied for loading of EVs or as its delivery system has not been reported.
3.3.2. Self-healing hydrogels
Self-healing hydrogels show great potential in 3D printing and drug delivery [141,142]. In recent years, they have also been widely used in the field of tissue regeneration [141]. Self-repairing hydrogels are usually prepared based on the principle of non-covalent interactions or dynamic covalent bonding. Their self-healing ability allows these hydrogels to adapt to defective tissues and organs, thus enabling their protection [141]. In addition, the self-healing hydrogel has injectable properties; it temporarily becomes fluid under high shear conditions and returns to gel form after the stress disappears [142,143]. Importantly, the self-healing hydrogel is physically stable in situ, which allows for a more durable protection of the encapsulated drug and, thus, enables their slow release [142]. The shear-thinning behavior is the result of physical crosslinking. In contrast to covalent bonding, physical crosslinking results from dynamic competition between pro-assembly forces (e.g., hydrophobic interactions, electrostatic interactions, and hydrogen bonding) and anti-assembly forces (e.g., solventization and electrostatic repulsion) and is reversible [143]. Dynamic covalent bonding, on the other hand, relies on the Diels-Alder reaction, Schiff base reaction, and thiol disulfide exchange reaction, among others [89,141]. The design of self-healing hydrogels can also be based on a combination of different interactions, such as cross-linking individual networks based on different types of interactions to prepare double-cross-linked hydrogels or combining two separate cross-linked networks to prepare double-networked hydrogels [142].
3.3.3. Stimulus-responsive hydrogels
Complex tissue-specific physiology from the nano-to macro-scale, combined with dynamic biophysical and biochemical stimuli, has inspired research scholars to explore the design of complex hydrogel and nanoparticle systems with stimulus-responsive properties. The combination of hydrogels and nanoparticles expands the scope of their respective applications in the biomedical field. Moreover, by simply and flexibly selecting different classes of nanomaterials and hydrogels or modulating nanoparticle-hydrogel physicochemical interactions, it is possible to obtain properties that greatly exceed those of conventional hydrogels. We can classify stimulus-responsive hydrogels according to the type of stimulus, which may be internal (i.e., features of normal or diseased/injured tissues) or external (i.e., heat, light, mechanical force, magnetic fields, or ultrasound) (see REVIEW [144] for details).
By responding to internal stimuli from the body, hydrogels can be designed as an intelligent delivery device that can release loads of bioactive material at specific sites. For example, HA-based hydrogel platforms containing self-assembled nanovesicles loaded with human recombinant insulin and glucose oxidase have been produced [145]. Specifically, in the hyperglycemic state, the local hypoxic microenvironment caused by the enzymatic oxidation of glucose promotes the reduction of hypoxia-sensitive hyaluronic acid (HS-HA), which rapidly triggers the dissociation of the vesicles and the release of the insulin therein. This layered design responds effectively to the hyperglycemic state by increasing insulin release, and insulin release decreases dynamically as basal glucose levels are restored. Owing to this self-regulatory feedback mechanism, the hybrid platform has exhibited superior therapeutic performance in a mouse model of type I diabetes compared to free insulin administration.
3.3.4. Conductive hydrogels
Bioelectrical signals are vital in regulating cellular behaviors that can promote cytokine secretion and improve the microenvironment of damaged tissue. Therefore, in regenerative medicine research, conductive nanomaterials (e.g., graphene, carbon nanotubes) and conductive polymers (e.g., polyaniline, polypyrrole) are often incorporated into hydrogel networks to construct composite conductive hydrogels [146]. Conductive hydrogels can be well adapted to the electrophysiological properties of nerve and heart tissues and can enhance intercellular information exchange, thereby contributing to nerve injury repair and myocardial repair after cardiac infarction [26,147]. Fan et al. [26] designed a conductive hydrogel loaded with BMSC-EVs for spinal cord injury (SCI) repair, which inhibited inflammation and promoted the regeneration of neurons and myelin-associated axons, highlighting the therapeutic potential of conductive hydrogels.
4. Underlying mechanisms affecting the loading and release of EVs
Hydrogels are three-dimensional, polymeric, hydrophilic reticulation structures with high water absorption capacity, and their physicochemical properties can be adjusted by changing their constituent components. Thus, we can customize hydrogels to achieve the desired load of EVs. The influence of hydrogel physicochemical properties on the loading and release of EVs has been extensively investigated in the literature [131,[148], [149], [150], [151]]. Modulating the porosity, swelling rate, surface charge, and degradation rate of hydrogels are all methods for adjusting the loading and release of EVs.
The loose and porous three-dimensional mesh structure of hydrogels is the structural basis for EVs loading. Mesh size depends on the polymer and crosslinker concentrations, as well as external stimuli, such as temperature and pH [131]. Large pores allow EVs to be loaded into hydrogels easily but will cause EVs to be released in a cascading manner, which often does not effectively prolong the retention time of EVs. Very small pores in the mesh make EV release dependent upon the swelling of the hydrogel that occurs during degradation [148]. Similar, certain stimulus-responsive hydrogels also use swelling to achieve drug release by exploiting the swelling reaction of hydrogels at different pH, temperatures, and glucose concentrations as a way to achieve drug release in specific situations [[149], [150], [151]].
Suitable porosity is the key for achieving effective loading and slow release of EVs. When the mesh size is close to the drug size (rmesh/rdrug ≈ 1), the effect of spatial site resistance on drug diffusion gradually increases and polymer chains exert significant frictional resistance to the diffusing drug, resulting in slow or delayed drug release [152,153]. Interestingly, different hydrogel particle shapes also affect the loading and release of EVs. Nikravesh et al. [154] compared anisotropic sheet particles formed using the shear technique to spherical particles fabricated using the vibration technique. In the study, EVs were loaded onto two separate alginate microgel systems, and the authors observed a significant increase in the amount of EVs released from the shear-formed microgel suspensions compared to the spherical particles.
Moreover, the interaction between the negatively charged phospholipid membranes of EVs and the charged residues of the glycocalyx, which can react with the charge carried by the biomaterial in an attractive or repulsive manner, is another factor affecting the loading of hydrogels [19]. Cationic delivery systems for chitosan-based hydrogels, for example, can adsorb EVs via electrostatic forces and prolong the loading time. In addition, adhesion molecules, such as α integrins, expressed on the surface of EVs allow EVs to adhere to ECM matrix components (type I collagen, fibronectin, and other derivative adhesion peptides), and this interaction can be used to control the release of EVs from hydrogels [155]. Using osteoinductive functionally engineered EVs (FEEs) derived from MSCs, Huang et al. [155] experimentally found that FEEs could bind to collagen mimetic peptides (DGEA, GFPGER) and fibronectin mimetic peptides (RGD). The binding of FEEs to the mimetic peptides resulted in the extended retention of FEE in hydrogels for up to 7 days, twofold longer than that observed in controls lacking mimetic peptides. Ma et al. [156] designed a fusion peptide to allow the binding of EVs to peptide sequences, thereby enhancing the ability of hydrogels to load EVs. The fusion peptide was designed by linking the collagen-binding domains of type I/III collagen to the exosome capture peptide CP05 (CRHSQMTVTSRL) either directly or via a rigid linker (EAAAK). According to the literature [157], the peptide sequence CP05 specifically recognizes and captures EVs tagged with CD63. In vitro experiments demonstrated that the fusion peptide had a positive effect in promoting osteogenic differentiation of EVs in BMSCs. In addition, the adhesive peptides, PPFLMLLKGSTR and RGD, which can bind to integrins on the surfaces of EVs, have been used to design and construct peptide-modified hydrogels to improve the time and efficiency of EV loading and their biological activity [158,159]. In addition, Man et al. [160] analyzed the ability of mixed hydrogels with different ratios of chitosan and type I collagen to load EVs. The results showed that EV release was strongly correlated with collagen concentration (R2 > 0.94), while CD63 enzyme-linked immunosorbent assay (ELISA) results revealed a significant increase in EV release from chitosan-containing gels (p ≤ 0.001). Compared to pure collagen gels, pure chitosan hydrogels significantly improved the compressive modulus (2.48-fold) and osteogenic differentiation capacity (3.07-fold) while reducing the number of gels (2.09-fold) [160].
The incorporation of nanoclay has also emerged in recent years as a way to modulate the drug release pattern of hydrogels [161]. Laponite is a promising nanomaterial that has been investigated to enhance the capabilities of hydrogels [162]. Several studies have reported that the addition of Laponite can enhance the retention rate of EVs in hydrogels [162,163]. The increased retention of EVs by Laponite may be due to nanoclay-protein electrostatic interactions that promote the immobilization of these EVs within the hydrogel [163]. The reduction of hydrogel porosity after Laponite addition is also considered to be a reason for enhancing the retention rate of EVs [164]. Laponite can also enhance the biomechanical strength of hydrogels and induce osteogenic differentiation of stem cells, thus showing great promise in bone regeneration [162,163].
The release kinetics of EV-loaded hydrogels may play an important role in influencing tissue repair and regeneration. Antunes et al. [165] specifically evaluated the delivery kinetics of sEVs isolated from human umbilical cord blood mononuclear cells that affect skin tissue repair and regeneration. Their results showed that a single high dose (2 μg) of sEVs promotes wound healing, whereas a single low dose (0.4 μg) does little to promote it. Interestingly, however, multiple very low doses of sEVs (0.02 μg, 2 applications per day for 10 days) showed the strongest pro-wound healing effect. Tissue repair and regeneration are complex processes involving the intervention of multiple biomolecules at different times [165]. EVs contain a variety of biologically active substances that may play different roles at different stages; hence, prolonging the release of EVs allows for stronger biological effects [165,166]. Taking wound healing as an example, the healing process of a wound can be roughly divided into three phases, the inflammatory phase (This stage lasts until about 48 h after injury), the cell proliferation phase (2–10 days), and the tissue remodeling phase (1–12months) [1]. If applied directly without hydrogel loading, EVs may be removed before they can play a role in promoting cell proliferation and tissue repair. For the preparation of targeted EV-loaded hydrogels, the physicochemical properties of the hydrogels themselves as well as the correlations between the constituent components and EV loading and release dynamics must be considered. We summarize some of the factors that can affect the duration of EVs release in Table 1. For the sake of unnecessary misinformation, it is important to state that the release time of EVs is influenced by a combination of factors and the table is for reference only.
Table 1.
Summary factors influencing the release pattern of EVs for EV-loaded hydrogels.
| Hydrogel components | Sources of EVs | Size of EVs | Sustained release time of EVsa | Factors influencing the release pattern of EVs | References |
|---|---|---|---|---|---|
| Type I collagen | Apis mellifera royal jelly | <150 nm | 7 days | 1 mg/ml: the release rate is highest on the day 3 and continues to decrease thereafter; 2 mg/ml: sustained high release rate over seven days; 3 mg/ml: sustained low release rate over seven days. |
[31] |
| GelMA + LAP | BMSC | 30–250 nm (mean = 130 ± 51 nm) | 14 days | 7% GelMA+0.1% LAP: shows an early burst release; 7% GelMA+0.2% LAP: more prolonged over the first 3 days. In both cases, the release was essentially complete by 14 days. |
[97] |
| HA-tyramine (HA-Tyr) | human cardiomyocyte | 50 nm | 5 days | 3% HA-Tyr: retained almost half of EVs in the first 2 days, while the cumulative release was found until nearly 5 days. | [86] |
| Self-assembling peptides (SAPs) | BMSC | 120.1 ± 55.4 nm | 168 h | The release rate of EVs was dose-dependently accelerated in the presence of MMP2 compared to the blank group. | [23] |
| Atom transfer radical polymerization (ATRP) | nonactivated macrophages J774A.1 |
30–150 nm | up to one month | The monomer-to-crosslinker ratio (molar ratio) and the crosslinking density of the polymer networks both affect the release of EVs. | [124] |
| Ureido-pyrimidinone (UPy) supramolecular (PEG coupled to two UPy units) |
CPCs |
100 nm | up to 2.5 weeks | The molecular weight of the PEG block within it may result in different kinetics of release. With a PEG block of 10 kg mol−1 (UPy10 k) showed a steadier release than UPy20 k. | [109] |
| 8-arm PEG Tren-SG (8P-TS) | M2 macrophages | 32.67–122.4 nm | 5–26 days | The release time of EVs can be regulated from 5 to 26 days by controlling the crosslinking density and tightness of hydrogel. | [77] |
| porcine-derived decellularized ECM | CPCs | / | 7 days | Different tissue sources of ECM can affect the release patterns of EVs. | [126] |
| PF-127 | ADSC | 30–100 nm | 96 h | PF-127 concentration affects the release of EVs. | [167] |
| 4-arm-PEG-MAL | ADSC | / | 20 days | The degradation rate of hydrogels can be controlled to regulate the release of EVs. | [168] |
| Chitosan-Collagen | BMSC | 131.3 ± 11.4 nm | 7 days | The release of EVs from these hydrogel formulations was dependent on chitosan/collagen ratios. | [160] |
Abbreviations: BMSC: Bone marrow mesenchymal stem cells; MSC: mesenchymal stem cells; CPCs: cardiac progenitor cells. PF-127: Pluronic F-127; ADSC: Adipose derived mesenchymal stem cells; PEG: Polyethylene glycol; HA: Hyaluronic acid; MSC: mesenchymal stem cells; GelMA: Gelatin methacryloyl; ECM: extracellular matrix; 4-arm-PEG-MAL: four-armed polyethylene glycol (PEG) functionalized with maleimide group.
Notes: The EVs release time here is for reference only and is actually influenced by various factors.
5. EV-loaded hydrogel preparations and application methods
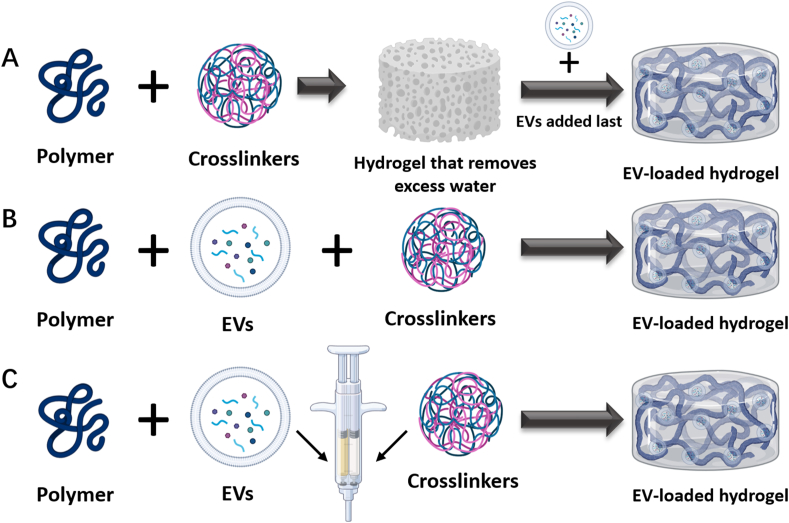
5.1. Loading strategies
5.1.1. ″Breathing method"
Due to the high-water retention and adsorption properties of hydrogels, after removing excess water from a swollen hydrogel with solvent, the voids in the hydrogel are exposed. Extracted EVs can then be directly added to the hydrogel to obtain an EV-loaded hydrogel [152] (Fig. 4A). This method requires large hydrogel porosity, as it is difficult to load EVs into the hydrogel when the EV particle size is larger than the pores of the hydrogel. However, when the pore size of the hydrogel is too large, the release of EVs by pouring is likely to occur [152].
Fig. 4.
Loading strategies for EV-loaded Hydrogels
(A)“Breathing method”. (B)Mix and cross-link. (C)In situ gel formation. Created with BioRender.com. (Agreemnt number: ZW24RSLYA2).
5.1.2. Mix and crosslink
EVs can also be mixed directly with the hydrogel precursor solution and then crosslinked into the hydrogel by adding a crosslinking agent or using a physical crosslinking method (Fig. 4B). For example, Qin et al. [169] mixed BMSC-EVs with thionylated HA, gelatin, and heparin, and then used polyethylene glycol diacrylate (PEGDA) as a gelling agent for gel formation. This method can also involve adding polymers that can self-assemble directly to the EV suspension. For example, when two self-assembling polymers, HA-AD and HA-CD, were dissolved in a suspension of EVs, and they rapidly assembled to form a hydrogel encapsulating the EVs [170].
5.1.3. In situ gel formation
In situ gel formation can be achieved by mixing EVs and polymers and injecting them together with a crosslinking agent into the target site using a double-lumen syringe [171] (Fig. 4C). This method carries additional risk as it requires consideration of potential toxic reactions of the crosslinking agent in vivo; therefore, is important to select non-toxic or low-toxic crosslinking agents [172].
5.2. Application strategies
5.2.1. Hydrogel dressings
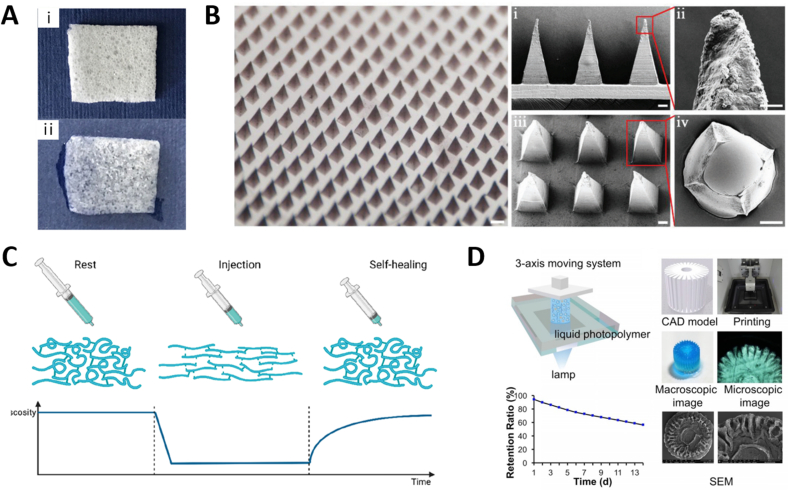
Hydrogels are considered an ideal skin substitute and wound dressing due to their ability to fight infection, absorb trauma exudate, maintain water balance and gas exchange, and encapsulate, protect and deliver bioactive molecules [173] (Fig. 5A). Trauma dressings are a common form of wound protection used to protect damaged tissue from environmental contaminants and bacterial infections. Ideally, dressings also actively support the healing process by creating an optimal wound environment and promoting wound closure [174]. Hydrogel dressings have desirable wound dressing properties due to their porous structure, viscoelasticity, and water content. Zhao et al. [175] developed GelMA hydrogels as wound dressings by incorporating human umbilical vein endothelial cell-derived EVs (HUVEC-EVs), and applied them to a full skin wound. Both in vivo and in vitro experiments demonstrated that the GelMA hydrogel dressing not only helped repair the wounded tissue but also achieved sustained release of the loaded HUVEC-EVs.
Fig. 5.
Application strategies for EV-loaded Hydrogels
(A) Morphology of dry hydrogel sponge and wet hydrogel sponge dressings. Adapted reprinted with permission from Ref. [176], based on Creative Commons Attribution License (CC BY), Copyright © 2017 Shi, Qian, Liu, Sun, Wang, Liu, Xu and Guo.(B) Optical image and SEM images of EV-loaded microneedles patch. Adapted reprinted with permission from Ref. [62], based on CC BY License, Copyright © 2022 The Authors. Advanced Science published by Wiley-VCH GmbH.(C) Schematic behavior of a self-healing injectable hydrogel. Adapted reprinted from Ref. [141], based on CC BY License. (D) 3D printing of radially oriented EV-loaded hydrogel bioink. Adapted reprinted with permission from Ref. [177], based on CC BY-NC License.
5.2.2. Microneedles
Because the skin is a natural barrier, traditional hydrogels often only achieve protection of the trauma surface and slow-release delivery of drug activity, with limited delivery to deeper tissues (Fig. 5B). One method for overcoming this limitation involves the use of microneedles (MNs) [178]. The materials commonly used in the preparation of MNs include gelatin, PLGA, PVA, and chitosan. In addition to conventional drug delivery, researchers have also delivered EVs and EVMs using MNs [62,79]. The structural properties of the soluble shell and core of MNs facilitate the deep, slow-release, phased delivery of bioactive cargo, which synergistically promotes wound healing [62]. The general process for the preparation of EV-loaded MNs is similar to the direct loading method discussed in section 5.1.2 in which EVs are mixed with a hydrogel precursor solution. After mixing, the EVs are placed into MN molds and subjected to optical or chemical crosslinking, followed by freeze-drying to obtain hydrogel microneedles encapsulated with EVs [79]. In contrast, the hierarchical construction method for the preparation of loaded EV-MNs involves first encapsulating the drug in the outer layer of the needle tip as described above, freeze-drying, encapsulating the hydrogel containing EVs in the inner core, and freeze-drying again to complete the construction of composite MNs [62].
5.2.3. Topical injection
Injectable hydrogels are among the most used hydrogels in the field of tissue repair and regeneration and can be applied not only to surface wounds but also to deep tissues and organs [141]. Active ingredients, such as drugs, growth factors, and cells, can be loaded into hydrogels and injected directly into the damaged area on demand; this allows for efficient repair while lessening the burden on patients by reducing the need for tedious surgical procedures. Local injection of EV-loaded hydrogels into the injured tissue is the most commonly used method of administration, resulting in the sustained local release of EVs to promote the repair and regeneration of the injured tissue [141]. Injectable hydrogels are low-viscosity fluids in a sol state that exhibit shear dilution properties prior to administration. After the sol is injected into the target site, it undergoes in situ gelation via a chemical or physical crosslinking reaction [35] (Fig. 5C). In situ gelation can be achieved by directly injecting EVs, polymers, and crosslinking agents together or by modulating ionic concentrations, temperatures, and pH responses in a manner that allows gelation under physiological conditions. For example, Pape et al. [179] designed a pH-responsive injectable hydrogel that can be loaded with drugs and injected into the heart. When injected into the porcine heart in vivo and ex vivo, pH changes caused gelation, enhanced retention time of the loaded drug, and achieved slow release of the drug locally. Cao et al. [180] used an injectable hydrogel loaded with EVs from human urine-derived stem cells (USC-EVs) for intrathecal injection, which promoted angiogenesis and SCI repair.
5.2.4. 3D bioprinting with bioinks
3D bioprinting using bioinks is a method for constructing hierarchically complex and customizable geometries by generating 3D digital models using computer-aided design software (Fig. 5D). Bioink, an ink that can be used in 3D printers and of which hydrogel is a common component. Hydrogels have excellent rheological properties, and 3D bioprinting technology can print scaffolds with fine-tuned structure, porosity, and mechanical properties that can effectively load cells and EVs [181]. Born et al. [97] used GelMA hydrogel bioinks containing MSC-EVs for 3D bioprinting. The results showed that MSC-EVs maintained their biological activity after 3D printing and photo-crosslinking. The data also showed that the burst release of EVs could be reduced by optimizing the concentration of crosslinker, while the hydrogel porosity and meshwork could be altered by altering GelMA synthesis and crosslinking parameters, which in turn significantly affected the release of EVs.
5.3. Characterization of EV-loaded hydrogels
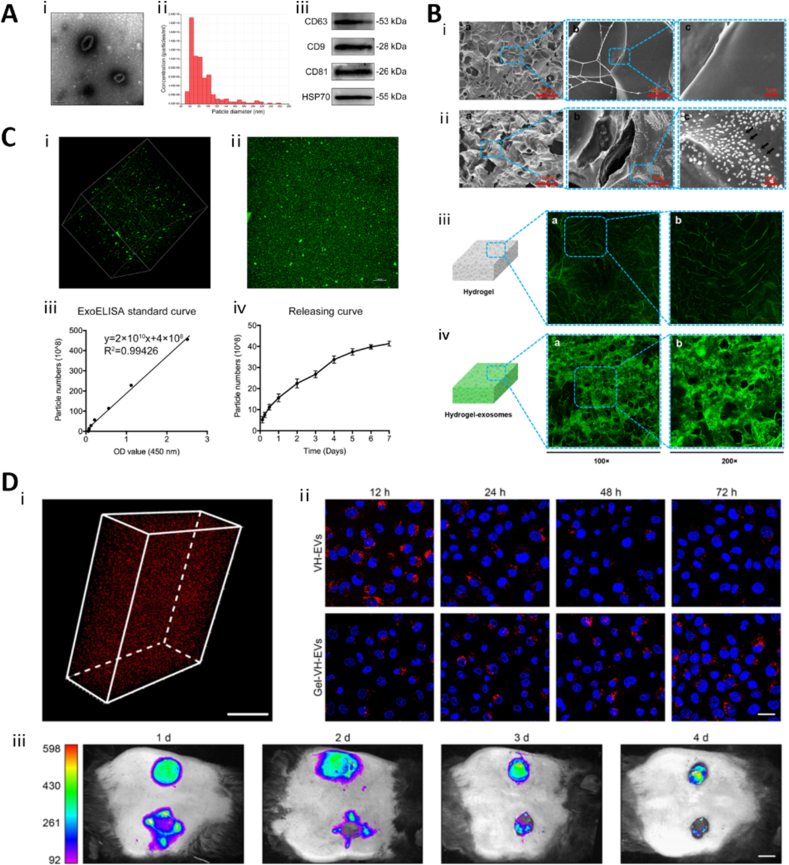
After EV-loaded hydrogels are prepared, it is imperative that they be characterized in detail. Unlike general bioactive substances or drugs, EVs are vesicular structures with bilayer phospholipid membranes, and their intact encapsulation in hydrogels (rather than fragmented incorporation) is a prerequisite for research and clinical applications. Before loading EVs into a hydrogel, the EVs must first be characterized using electron micrographs, particle size analysis, and WB (Fig. 6A). Other methods to characterize them include flow cytometry, proteomics, and ELISA [51]. The sparse and porous microstructure of the hydrogel and the distribution of EVs therein can be observed directly using SEM; however, due to the small particle size of EVs, observation of the specific distribution of EVs in the pore structure of hydrogels using SEM is often challenging [176]. In low-resolution image, we can observe the loose and porous structure of the hydrogel, but the distribution of EVs cannot be observed well and give the impression of coarseness and grittiness only. In the high-resolution images, we can clearly observe the EVs particles, but the structure of the hydrogel cannot be seen (Fig. 6B). The use of fluorescently labeled EVs can facilitate the observation of EV distribution within a hydrogel via laser scanning confocal microscopy (LSCM). It can be clearly observed that in the hydrogel loaded with fluorescently labeled EVs, the fluorescence signal is uniformly enhanced. This suggests that the EVs are uniformly distributed within the loose and porous structure of the hydrogel (Fig. 6B).
Fig. 6.
Characterization of EV-loaded hydrogels. (A) Characterization of EVs. Adapted reprinted with permission from Ref. [175], Copyright @ 2020, Springer Nature B.V. (License number: 5,441,160,193,635). (B) Detection of exosomes on the hydrogel sponge. (ⅰ & ⅱ) SEM images of hydrogel surface. The black arrows show the exosomes; (ⅲ & ⅳ) LSCM images of the hydrogel sponge. Adapted reprinted with permission from Ref. [176], based on CC BY License, Copyright © 2017 Shi, Qian, Liu, Sun, Wang, Liu, Xu and Guo.(C) Controlled release of EVs in GelMA hydrogels. (ⅰ) 3D image of PKH67-labeled EVs incorporated in GelMA; (ⅱ) Overlapping image; (ⅲ) ELISA standard curve; (ⅳ) EVs releasing curve. Adapted reprinted with permission from Ref. [175], Copyright @ 2020, Springer Nature B.V. (License number: 5,441,160,193,635). (D) (ⅰ) 3D reconstruction image of GelMA hydrogels with PKH26 labeled EVs; (ⅱ) Representative confocal images of cells that were co-cultured with PKH26 labeled EVs (red). The nuclei were stained by DAPI (blue). (ⅲ) Representative in vivo imaging picture of retention of PKH26 labeled EVs after applied on wound 1, 2, 3, 4 days. Adapted reprinted from Ref. [72], with permission from Elsevier, Copyright© 2022 Acta Materialia Inc (License number: 5,419,380,313,323). . (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fluorescent labels commonly used for EVs are PKH26 [72], PKH67 [175], DiO [176], and Dil [168] (Fig. 6C and D). PKH26 is a highly fluorescent lipophilic long-chain carbocyanine dye that has been applied to the study of EVs and their functions [182]. Phagocytosis of EVs by cells can even be monitored after fluorescent labeling of EVs using PKH26 (Fig. 6D). One particular point to note, however, is that there exists a potential drawback when using lipophilic fluorescent markers to track EVs; the half-life of the markers is much longer than that of the EVs [74]. This may result in the study of the biodistribution of EVs being conducted with longer half-life fluorescent dyes rather than EVs themselves. In addition, amphiphilic NIR-fluorescent probe [183] and nanogold [75], among others, have been applied to tracing EVs. To achieve higher resolution real-time EV tracer imaging in vivo, Zhao et al. [184] designed ultrasmall magnetically engineered Ag2Se quantum dots, which showed good results. The selection of appropriate markers to accurately reflect the biodistribution of extracellular vesicles in vivo is one of the more important aspects in the development of EV-related drugs. In particular, Lázaro-Ibáñez et al. [185] compared the advantages and disadvantages of various fluorescent, bioluminescent, and radioactive tracers in the in vivo tracing of EVs.
The release of EVs from hydrogels is often characterized using a micro bicinchoninic acid (BCA) assay, ELISA, or fluorescent labeling. The BCA assay can be used to determine the percentage of released EVs by mixing the EVs with the hydrogel, placing the mixture in the upper chamber of a 24-well transwell plate, and measuring the protein concentration in the lower chamber at different time points [167]. Similarly, a dynamic dialysis method can be used to detect the release of EVs by placing the EV-loaded hydrogel in a dialysis bag and placing the bag in a constant-temperature phosphate buffered saline (PBS) solution. The protein concentration in the PBS solution can then be measured periodically using the BCA method to calculate the rate of EV release [186]. However, it is important to note that BCA cannot detect specific proteins, which can easily cause interference when using certain protein-based hydrogels loaded with EVs. In comparison, quantifying the release profile of EVs be quantified using ELISA is a better choice (Fig. 6C) [175]. EV transmembrane proteins, such as CD63, CD81, and CD9, are suitable markers for EV detection [51]. Additionally, as mentioned above, PKH26 fluorescent labels have been used to quantify EV release. By placing a hydrogel loaded with PKH26-labeled EVs in a 24-well plate filled with PBS, the EV release rate can be calculated by measuring PKH26 fluorescence in the supernatant over time [72]. In in vivo experiments, hydrogels loaded with PKH26-labeled EVs were applied to traumatic wound surfaces and the release rate was calculated by measuring the fluorescence intensity of PKH26 in tissues at different time periods [72,167]. Likewise, experiments in which EVs were labeled with DiI showed that DiI-labeled EVs were gradually released from hydrogels with increasing incubation time (Fig. 6D) [170].
In addition to the quantitative characterization of the EVs released from the hydrogel, it is also very important to characterize them qualitatively, i.e. to prove that they still have an intact structure or are still biologically active [77,162]. The most straightforward method is to observe the integrity of the EVs by electron microscope or label the EVs with fluorescence and observe using fluorescent microscope [77,162]. Released EVs can also be characterized using flow cytometry or particle size analyzers [77]. A better approach is to indirectly demonstrate the biological activity of the released EVs [77]. The EV-loaded hydrogels are incubated in cell culture medium and later compared with normal cell culture medium to observe the proliferative capacity of cells, and endothelial cells are also often verified using migration assays and tube formation assays [31,77]. The co-culture system is also a good option, where the hydrogel is placed in the upper chamber of the transwell and the lower chamber is inoculated with relevant cells to evaluate its biological activity [162].
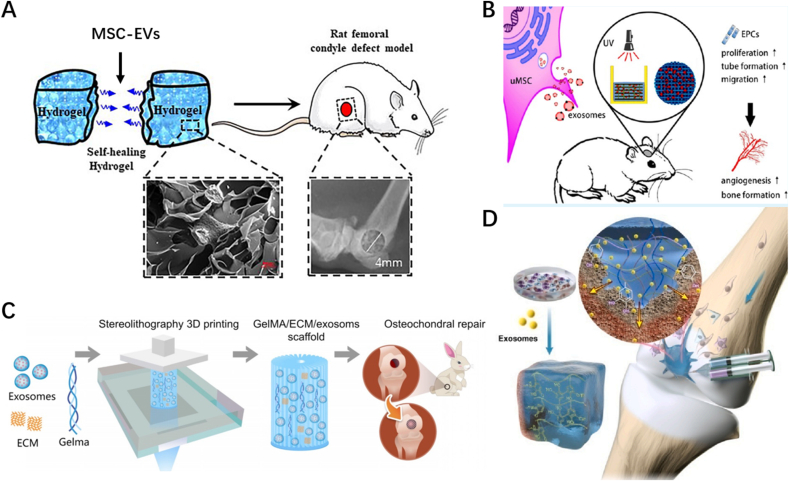
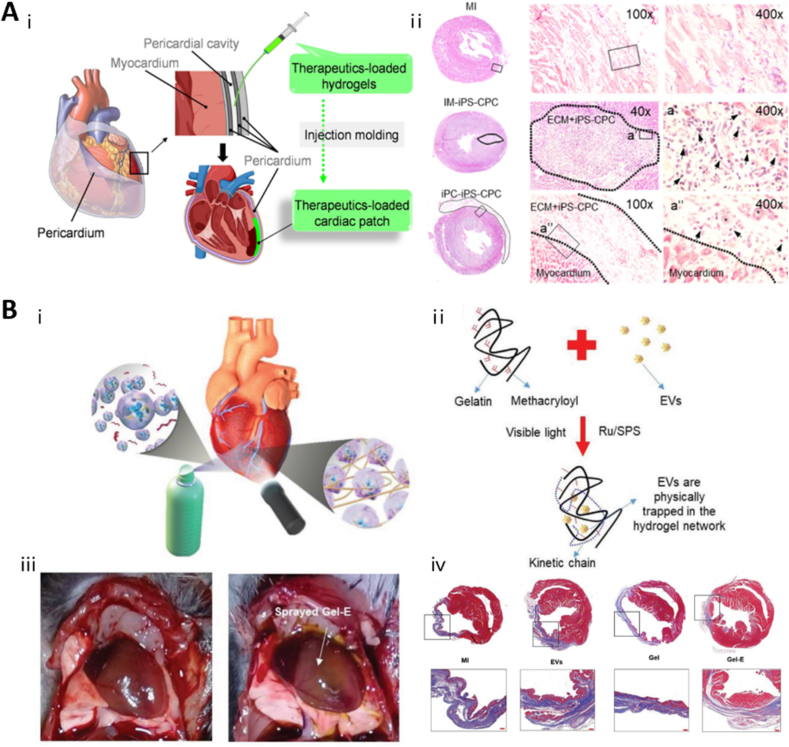
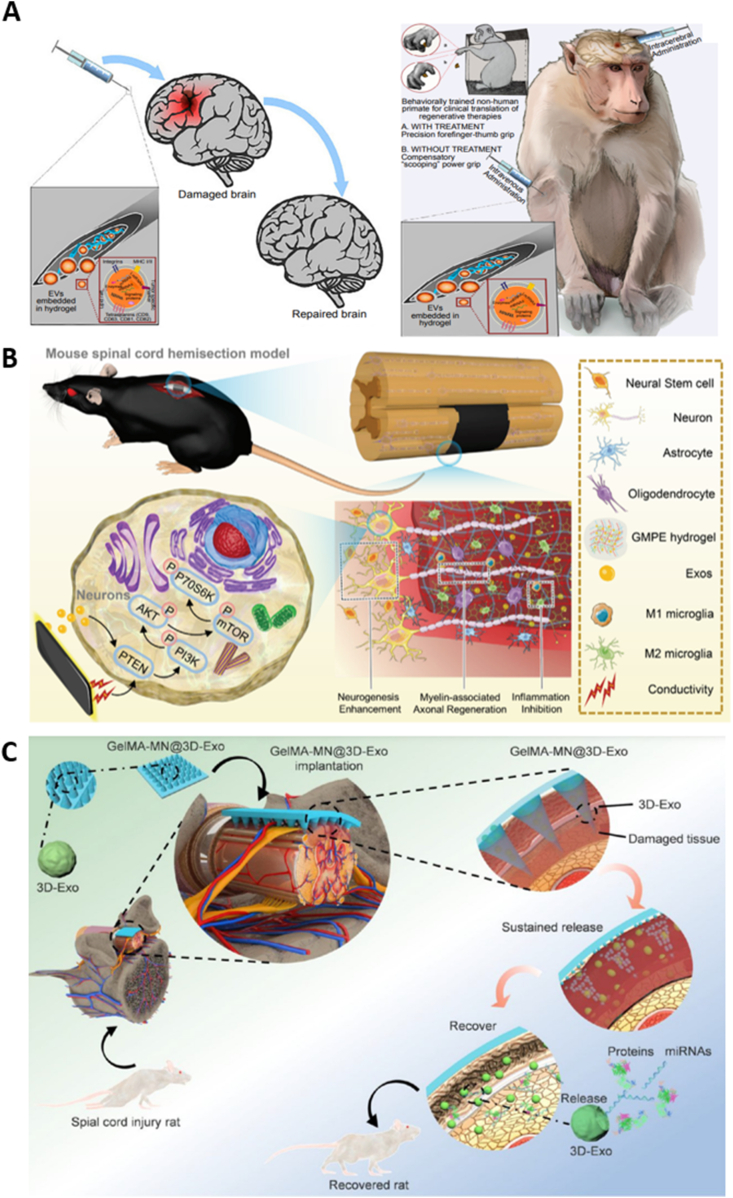
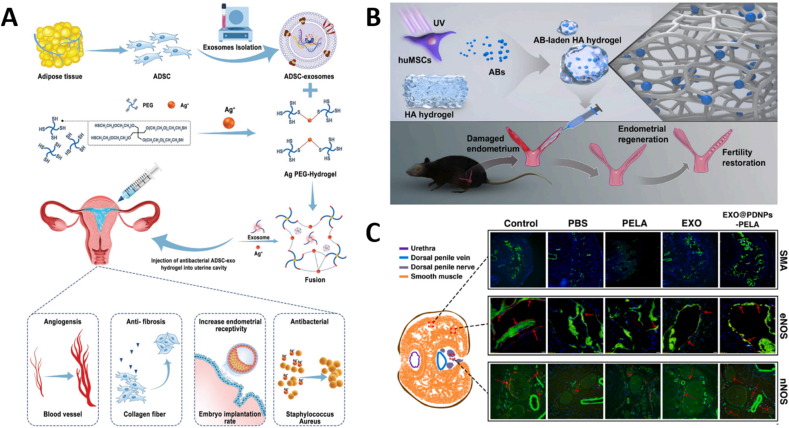
6. Tissue-specific applications of EV-loaded hydrogels
6.1. Skin/wound healing
Wound healing is an important and complex process in living organisms. Local infection, oxidative stress, inflammatory responses, and impaired angiogenesis are factors that contribute to the difficulty in healing chronic wounds [1,187]. EVs have been extensively studied for the cell-free treatment of wounds and can significantly promote wound angiogenesis, collagen deposition, and fibroblast proliferation [188,189]. Local injections of EVs often lead to premature degradation of EVs due to the harsh environment of the trauma surface, making it difficult to sustain their effects. As is clear by now, hydrogels can protect EVs from the trauma environment and greatly enhance their stability and release time [19].
Pluronic F-127 (PF-127) is a synthetic biocompatible hydrogel that has been approved by the FDA for use in humans. Zhou et al. [167] combined PF-127 hydrogel with human ADSC-Exosomes (hADSC-Exos) and found that it prolonged the release of hADSC-Exos up to 72 h, leading to the enhancement of cell proliferation, angiogenesis, collagen remodeling, and re-epithelialization of the wound site and ultimately accelerating the wound healing process. In addition, the self-healing hydrogel provided durable protection against bacterial or foreign body irritation, maintained the biological activity of the EVs, and significantly promoted wound repair. Wang et al. [172] constructed a multifunctional FHE(F127/OHA-EPL)@exo hydrogel consisting of PF-127, oxidized hyaluronic acid (OHA), and Poly-ε-l-lysine (EPL). The reversible Schiff base reaction between OHA and EPL makes the hydrogel self-healing, and the inclusion of PF-127 renders the hydrogel thermally responsive. While scar-free healing has been a major challenge in wound healing treatment, Yang et al. [170] loaded MSC-EVs into shear-thinned HA hydrogels and found that they promoted the conversion from M1-type to M2-type macrophages, inhibited fibroblast activation, and prevented scar tissue formation.
Diabetic wounds are difficult to treat due to hyperglycemia-induced peripheral vascular neuropathy, oxidative stress, and ease of infection, and patients may face a risk of amputation as a result [2]. To address this challenge, Wang et al. [72] pretreated EVs and loaded them with VH298 to enhance pro-angiogenic activity. VH298 is a stabilizer of hypoxia-inducible factor-1 α (HIF-1 α) designed by Ciulli et al. [190]. The enhanced VH298-EVs were mixed with GelMA hydrogel and applied to diabetic wounds, leading to significant wound healing by promoting HIF-1α-mediated angiogenesis. Moreover, a stimulus-responsive hydrogel targeting matrix metalloproteinase (MMP) was developed by gelating a maleimide group-functionalized four-armed PEG, a substrate peptide of matrix metalloproteinase, a sulfhydryl-functionalized PEG chain, and ADSC-Exos mixed at room temperature. This hydrogel significantly inhibited the oxidative stress response at the trabecular surface, and ADSC-Exos were released in response to MMP degradation, eventually leading to improved cellular function and wound healing [168]. Highly effective self-healing hydrogels in synergy with EVs have also shown a strong effect in promoting healing of diabetic wounds [191]. Wang et al. [191] prepared a highly efficient natural polymer-based self-healing hydrogel based on the principle of Schiff base, which can continuously release MSC-EVs and significantly promote the healing of diabetic wounds. Active ingredients in Chinese medicine have also been shown to act synergistically with EVs. Xu et al. [192] loaded both Curcuma zedoaria polysaccharide and platelet-rich plasma EVs into hydrogels, which could act synergistically and promote recovery of diabetic wounds better than the two alone. In addition, to address oxidative stress and hypoxia in diabetic wounds, Xiong et al. [193] designed a multifunctional self-healing HA hydrogel loaded with exosomes derived from M2-type macrophages, fibroblast growth factor, and an MnO2 nanoenzyme. The MnO2 nanoenzyme scavenges ROS from the wound and releases oxygen, while M2-Exos synergized with fibroblast growth factor to significantly promote trabecular angiogenesis and epithelialization (Fig. 7A).
Fig. 7.
Application of EV-loaded hydrogels in wound healing
(A) Construction and application of the multifunctional self-healing HA hydrogel loaded with exosomes. Adapted reprinted with permission from Ref. [193], based on CC BY-NC 4.0 license, Copyright © 2021 The Authors. Small published by Wiley-VCH GmbH.(B) Schematic illustrations of a composite core-shell structured MN patch for diabetic wound healing. Adapted reprinted with permission from Ref. [62], based on CC BY License, Copyright © 2022 The Authors. Advanced Science published by Wiley-VCH GmbH.
As a minimally invasive tool, MNs have received increasing attention in recent years. Constructing hydrogel microneedle patches for trauma treatment can deliver drugs to deeper subcutaneous tissues and improve drug delivery efficiency compared to hydrogels alone. Yuan et al. [79] constructed a hydrogel MN patch to achieve transdermal and controlled release of exosomes and tazarotene. The team prepared MNs using GelMA and PEGDA, loaded exosomes into the MNs, and grafted β-CD-AOI2 to carry tazarotene. The unique physical properties of the MN patch allowed it to bind tightly to the trauma surface and enable deep drug delivery. The results of the study showed that GelMA/PEGDA MNs effectively penetrated the skin and stably released drugs and exosomes to efficiently promote wound healing. Moreover, Ma et al. [62] constructed a composite core-shell structured MN patch consisting of an exo-HA methacrylate (HAMA) shell containing PDA and an inner EVM core containing iron nano, with a layered hierarchy that enabled phased cargo release, providing a novel construction idea (Fig. 7B).
6.2. Bone
Repairing bone defects has long been a focus of orthopedic research, and autologous bone grafting remains the current treatment of choice. With the development of bone tissue engineering strategies, tissue-engineered bone is expected to become the mainstream method of bone repair in the future. A growing number of studies have shown that EV-loaded hydrogels or scaffolds, alone or in combination, can significantly promote local bone regeneration [24,25,50]. The mechanism by which MSC-EVs promote bone repair is attributed to their pro-angiogenic effects as well as the upregulation of osteogenic gene expression and induction of osteoblast proliferation and differentiation [50,169]. Hydrogels can fill irregular bone defects while releasing EVs locally, thereby promoting in situ bone tissue regeneration. Zhang et al. [194]used a commercial hydrogel (HyStem-HP hydrogel, Catalog: GS315, Glycosan Biosystems, USA) loaded with UMSC-EVs for a femoral fracture model in rats, and topical application of EV-loaded hydrogel after intramedullary nail fixation promoted fracture healing. Man et al. [160] developed an ECM-mimetic hydrogel composed of chitosan and type I collagen that effectively carried BMSC-EVs and promoted proliferation and osteogenic differentiation into BMSCs, while significantly promoting ECM ossification of BMSCs. While Fan et al. chose EVMs as cargo, their preparation of EVM-loaded chitosan hydrogels similarly showed excellent pro-osteogenic effects [195]. Topical application of the EVM-loaded chitosan hydrogels to calvarial defects demonstrated robust bone regeneration.
Unfortunately, the mechanical properties, stability, and cell adhesion of conventional hydrogels are usually weak due to their fragile network structure, which hinders their application as bone structural materials [88]. To overcome this limitation, hydrogels are usually modified by chemical, physical, and biological methods to produce high-performance hydrogels with enhanced properties [196]. Wu et al. [197] used a chitosan/β-glycerophosphate hydrogel loaded with BMSC-EVs to repair cranial defects in rats, and the results indicated good biocompatibility.
The addition of inorganic substances with mechanical strength to conventional hydrogels can compensate for the relatively poor mechanical properties of hydrogels to some extent. In a study by Hu et al. [163], the addition of laponite nanoclay to GelMA hydrogels improved the mechanical and biological properties of the hydrogels and allowed for the sustained release of EVs in the presence of hydrogel degradation. In addition, Wang et al. [198] synthesized a novel coral hydroxyapatite/filamentous protein/glycolic chitosan/biofunctionalized PEG self-healing hydrogel and evaluated its therapeutic effect as a human umbilical cord MSC-derived exosome (UMSC-Exo) carrier for femoral condyle defects in rats. (Fig. 8A). This comprehensive hydrogel not only had good biocompatibility but also exhibited high mechanical strength, and the self-healing properties allowed it to tightly bind to the bone while the EV component exerted pro-bone regenerative effects.
Fig. 8.
Application of EV-loaded hydrogels in Bone and cartilage
(A) Schematic illustration of UMSC-EV-loaded Self-healing hydrogel for testing in rats with induced femoral condyle defect. Adapted reprinted with permission from Ref. [198], based on CC BY license, Copyright © 2020 Wang, Wang, Zhou, Sun, Zhu, Duan, Chen, Guo, Zhang and Guo.(B) Encapsulated UMSC-Exo in hyaluronic acid hydrogel and combined them with customized nanohydroxyapatite/poly-ε-caprolactone scaffolds to repair cranial defects in rats. Adapted reprinted with permission from Ref. [199], Copyright © 2021 American Chemical Society.(C) The use of 3D printed ECM/GelMA/EV scaffolds in the repair of bone cartilage defects. Adapted reprinted with permission from Ref. [177], based on CC BY-NC License. (D) An injectable Mussel-Inspired highly adhesive hydrogel loaded EVs to facilitate cartilage defect regeneration. Adapted reprinted with permission from Ref. [103], Copyright @ 2021 Elsevier Ltd. All rights reserved (License number: 5,441,190,459,082).
It is important to note that in some cases, hydrogels alone do not meet the high biomechanical needs of a specific site [50]. When applied to severe, large defects or a poor biomechanical environment, the mechanical properties of hydrogels are still not sufficient for effective injury repair, and bioactive scaffolds based on other biomaterials remain an important part of bone injury repair [50]. Bone tissue engineering scaffolds are not only bioresorbable and biodegradable but also provide temporary mechanical support for bone at the implantation site [110]. In addition, the scaffold has a highly porous and 3D structure that mimics the porosity and interconnectivity of native bone [110]. This specific structure promotes cell attachment and proliferation, providing space for new tissue growth and vascularization. Zhang et al. [200] first demonstrated that human induced pluripotent stem cell-MSC-derived exosomes (hiPS-MSC-Exos) can be used with a β-tricalcium phosphate (β-TCP) scaffold; the combination significantly promoted osteogenesis in a rat cranial defect model. Exosomes released from hiPS-MSC-Exos/β-TCP scaffolds internalized into human bone MSCs and stimulated the recruitment, proliferation, and differentiation of endogenous BMSCs. In addition, Yue et al. [201] developed an exosome-functionalized PLGA/metal-organic backbone scaffold with a unique nanostructure, which achieved controlled release of exosomes, thereby effectively promoting osteogenesis and angiogenesis and alleviating inflammation. The scaffold showed satisfactory repair results in rat calvarial defect model.
Biological scaffolds with good mechanical and stability properties can fill critical bone defects, while hydrophilic hydrogels can effectively fill complex and irregular bone defects due to their gel-like properties; combining the two can help to better achieve bone tissue repair and reconstruction [50]. A recent study showed that the addition of a novel injectable hydrogel, PEG maleate, to a citrate-based β-TCP scaffold can effectively enhance its composite strength and osteoinductive properties [202]. The authors then evaluated their bone regenerative efficiency in a rat posterolateral spinal fusion model, the results show that it can promote the fusion of the spine and also has some mechanical support strength. However, the authors also suggested that it provides only limited mechanical support. In addition, to enhance the sustained release of exosomes, Swanson et al. [115] developed a PLGA-PEG-PLGA microsphere as a release system by filling exosome-loaded microspheres with PLLA scaffold; the activity of the exosomes was found to be unimpaired and the repair of cranial defects in rats was significantly improved. Wang et al. [199] encapsulated UMSC-Exo in hyaluronic acid hydrogel and combined them with customized nanohydroxyapatite/poly-ε-caprolactone scaffolds to repair cranial defects in rats (Fig. 8B). Biological scaffolds combined with EV-loaded hydrogels have significant potential in repairing large bone defects. The effective combination of hydrogel, scaffold, and EVs can maximize their respective strengths and minimize their weaknesses and is expected to be an important future research direction for bone defect repair and bone tissue engineering.
6.3. Cartilage
Cartilage repair is a major clinical challenge due to a lack of self-healing capacity. Stem cell-derived EVs hold significant potential for cartilage regeneration, and Zhang et al. [203] demonstrated that MSC-EVs can promote cartilage repair by enhancing cell proliferation, cell migration, and matrix synthesis, and by inhibiting apoptosis and modulating immune responses. The high-water content and swelling properties of hydrogels make them a suitable scaffold for filling cartilage defects [204].
GelMA hydrogels have received increasing attention in tissue engineering in recent years due to their injectability and UV crosslinking properties. Guan et al. [205] introduced aldehyde-functionalized chondroitin sulfate (OCS) into GelMA hydrogels loaded with BMSC-Exos to form GMOCS-Exo hydrogels. The results showed that GMOCS-Exo hydrogels significantly promoted the synthesis of ECM via the release of OCS, while the BMSC-Exos therein further promoted chondrocyte anabolism by inhibiting inflammation, ultimately repairing cartilage damage through ECM remodeling. In addition, Chen et al. [177] reported the use of 3D printed ECM/GelMA/EV scaffolds in the repair of bone cartilage defects, which significantly promoted cartilage regeneration (Fig. 8C).
Recently, researchers have developed a photo-induced imine crosslinking (PIC) hydrogel containing the o-nitrobenzyl derivative of HA (HA-NB). HA-NB generates an aldehyde group under light irradiation, which reacts with amino groups distributed on other partial polymers (e.g., chitosan, gelatin) to form a hydrogel in situ on the tissue surface, allowing seamless attachment and integration of the hydrogel with the tissue [129,206]. The remarkable maneuverability, biocompatibility, tissue adhesion, and integration capabilities of PIC hydrogels make them well suited as an MSC-EVs scaffold for repairing cartilage defects. Indeed, the scaffold was found to retain MSC-EVs in vitro and positively regulated chondrocytes and BMSCs. Furthermore, the scaffold integrated with the natural cartilage matrix to promote cell deposition at the site of cartilage defects, thereby facilitating repair [28].
Because joint sites connect bone and cartilage, simultaneous defects in both the bone and cartilage can occur at these locations, and single-component hydrogels often cannot meet the needs of both tissues. Nikhil et al. [207] designed a novel bilayer gel comprising a chitosan-gelatin-chondroitin sulfate upper gel for articular cartilage and a nanohydroxyapatite-chondroitin lower gel for subchondral bone, to mimic the different mechanical and biological properties of bone and cartilage. The authors evaluated the capacity of EVs from chondrocytes to promote cartilage regeneration and proposed that the combination of the bilayer gel structure with chondrocyte-derived EVs could be used to repair osteochondral structures [207]. In addition, to address the poor adhesion of hydrogels to articular cartilage surfaces, Zhang et al. [103] designed an injectable, mussel-like, high-adhesion hydrogel with high binding strength to wet surfaces using a crosslinked network of sodium alginate dopamine, chondroitin sulfate, and regenerating filamentous protein (Fig. 8D). The hydrogel was loaded with MSC-Exos and accelerated the regeneration of in situ cartilage defects and ECM remodeling following injection into cartilage injury sites, providing a promising and minimally invasive treatment strategy for joint injuries [103].
6.4. Heart
Although the development of reperfusion therapy has significantly reduced the mortality rate of patients with acute myocardial infarction, the irreversible myocardial damage and cardiac remodeling that occur following myocardial infarction can result in chronic heart failure [208]. Because adult cardiomyocytes are terminally differentiated and have a very low proliferation rate, the cardiac regenerative capacity is extremely limited. Stem cell therapy is considered a promising strategy for improving myocardial function after infarction, and stem cell-derived EVs have been shown to resist cardiomyocyte apoptosis, promote angiogenesis, reduce scar tissue formation, reduce infarct size, and reverse inflammation-induced injury [209,210]. However, intravenous EVs do not achieve targeted effects and accumulate in other organs [73]. Further, because of the high hemodynamics of the cardiac site, locally injected EVs are undetectable in the myocardium after only 3 h [211]. Therefore, EV-loaded hydrogels have become an option for cell-free therapy in the heart. Some research teams have utilized conventional hydrogels, such as HA and sodium alginate hydrogels, loaded with endothelial progenitor cell-derived EVs or other types of MSC-EVs for the treatment of post-infarction hearts, and all have reported positive therapeutic results with significant improvements in cardiac function [212,213].
In the study by Pape et al. [179] discussed in section 5.2.2, the pH-responsive injectable hydrogel easily passed through the catheter at the in vitro pH, but gelled when exposed to the in vivo pH of the porcine heart, thereby enhancing the retention time of the loaded drug and achieving local slow release. In another study, Waters et al. developed an injectable biocompatible hydrogel consisting of gelatin combined with Laponite to form a shear-thinned nanocomposite hydrogel that delivered EVs secreted by ADSCs to the peri-infarct myocardium [214]. The results demonstrated that, in the post-infarct heart after injection, capillary density significantly increased, scar area decreased, and cardiac function improved. In addition, Han et al. designed the first peptide-based self-assembled hydrogel loaded with UMSC-EVs for post-infarction treatment [48]. They incorporated cardiac protective peptides (GHRPS, His-DTrp-Ala-Trp-DPhe-Lys-NH2) and matrix metalloprotease-2 (MMP-2) degradable sequence Gly-Thr-Ala-Gly-Leu-Ile-Gly-Gln (GTAGLIGQ) into the PA and generated PA-GHRPS [48]. The PA-GHRPS peptide protected cardiomyocytes from hydrogen peroxide damage and the NapFF peptide enhanced the adhesion of PA-GHRPS to tissues; the two peptides were mixed to form a peptide-based self-assembled hydrogel that stably and released UMSC-EVs, thereby improving cardiac function after myocardial infarction [48]. Cardiac electrophysiology is an important factor affecting cardiac function, and the difference in electrical conductivity between the post-infarct myocardium and the surrounding normal myocardium affects, to some extent, the ability of the heart to contract synchronously, leading to arrhythmias [215]. To address this problem, Zou et al. [216] designed a conductive hydrogel system that significantly improved intercellular interactions, promoted cell proliferation and angiogenesis, and had a significant therapeutic effect on myocardial infarction, providing a promising treatment for injured myocardial tissue.
Paracardial injection of EV-loaded hydrogels into the infarcted myocardium ensures that the EVs are applied locally and reliably at the site of injury, but myocardial injections are associated with greater risk due to the influence of the beating heart. Therefore, Zhu et al. proposed a method of pericardial cavity injection that uses the pericardial cavity as a natural “mold” to prolong the duration of action of the drug. The method involves forming a structure in situ that matches the shape of the heart after intrapericardial injection of biocompatible hydrogels (Fig. 9A) [217]. The authors demonstrated that pericardial injection of EV-loaded hydrogels was a safe and effective method to attenuate post-infarct cardiac remodeling and improve cardiac function in rodent models of myocardial infarction [217]. An injection-free delivery method was also proposed by the team, in which an open-chest, direct-view cardiac spraying technique was used [27] (Fig. 9B). Although this method avoided the secondary damage associated with intramyocardial injections, the risks associated with open-chest surgery are clearly greater. The team also suggested the possibility of percutaneous catheter pericardial puncture spraying as an alternative method; however, this is essentially an intrapericardial cavity injection and further studies are needed to determine whether photo-crosslinking for glue formation will cause secondary injury to the already injured myocardium.
Fig. 9.
Application of EV-loaded hydrogels in heart
(A) (ⅰ) Schematic illustration of pericardial cavity injection and in situ formation of cardiac patch; (ⅱ) H&E staining showing the formation of a cardiac patch 7 days after pericardial cavity injection. Adapted reprinted with permission from Ref. [217], based on CC BY License. (B) (ⅰ) Schematic of injection-free delivery of EVs and (ⅱ) gelation mechanism of GelMA; (ⅲ) In situ crosslinking of EVs-loaded GelMA. (ⅳ) H&E staining. Adapted reprinted with permission from Ref. [27], Copyright @ 2021 Wiley-VCH GmbH (License number: 5,441,200,952,149).
In conclusion, EV-loaded hydrogels for intrapericardial lumen injection may be a breakthrough technology in the treatment of myocardial infarction as they are less invasive, relatively safe, and easy to use. Hydrogel delivery system design must consider the shear-thinning capacity of the hydrogel as percutaneous catheter puncture intervention requires the use of a long and thin catheter. In addition, self-healing and electrical conductivity properties are also of great importance in the context of cardiac therapies.
6.5. Nerves
Damage to the nervous system caused by trauma or disease is a difficult clinical problem. Whether adult neuronal cells can renew and regenerate after injury is a current point of debate in the academic community, and achieving neuronal cell regeneration has been a focus of researchers for some time [3]. A seminal study in 2020 showed that the corticospinal tract (CST), when stimulated by transplanted spinal cord-derived neural progenitor cells (NPCs), can regenerate new axons after SCI in adult mice [218]. To date, stem cell studies have provided the most promising breakthroughs for CST repair, and extracellular vesicles secreted by stem cells are the key to cell-free therapy. Tsintou et al. [219] proposed the hypothesis that EV-loaded hydrogels would play an important role in central nerve injury (See REVIEW [219] for details). (Fig. 10A). Faust et al. [127] investigated the role of different sources of ECM hydrogels in SCI repair and found that all ECM hydrogels have the ability to promote neuronal cell survival and promote neurite growth. The authors further hypothesized that EVs derived from ECM are essential to the success of the hydrogels. Many studies have shown that MSC-EVs are involved in SCI. Cheng et al. [220] used GelMA hydrogel as a delivery system for BMSC-Exos for SCI repair and showed that the use of GelMA hydrogel enhanced the retention of BMSC-Exos and promoted neuronal differentiation and extension. In addition, EV-loaded GelMA hydrogel promoted neurogenesis and attenuated glial scar formation in damaged nerve lesions. Furthermore, Liu et al. [221] specifically evaluated the role of photo-crosslinked HAMA hydrogel stiffness in the regeneration of injured nerves and found that soft hydrogels loaded with exosomes facilitated better repair of injured peripheral nerves than did their stiff counterparts. Soft hydrogels promote nerve repair by inhibiting macrophage infiltration and downregulating the expression of pro-inflammatory factors IL-1β and TNF-α through the rapid release of exosomes. In addition to suppressing the inflammatory response, there is increasing evidence that promoting spinal cord angiogenesis can also promote injury repair [222,223]. Cao et al. [180] used hydrogel loaded with UMSC-Exos for intrathecal injection and found that it promoted angiogenesis and SCI repair. Similarly, Mu et al. [223] loaded hypoxia-stimulated exosomes into a modified HA hydrogel that was subsequently transplanted into injured spinal cord sites for pro-angiogenic therapy. A significant increase in hypoxia-inducible factor 1-α content in the hypoxia-treated exosomes resulted in the overexpression of vascular endothelial growth factor in the endothelial cells surrounding the graft. Both in vitro and in vivo experiments showed significant angiogenesis and recovery of neurological function after injury. Li et al. [158] constructed similarly modified HA hydrogels loaded with MSC-EVs and showed that the microenvironment of the SCI area was improved, resulting in reduced oxidative stress and local inflammation and effectively promoting spinal cord regeneration and hindlimb motor functional recovery in rats.
Fig. 10.
Application of EV-loaded hydrogels in nervous system
(A) Experimental hypothesis about a combinatorial hydrogel/EVs therapeutic approach. Adapted reprinted with permission from Ref. [219], Copyright @ 2021 Neural Regeneration Research (Billing Account Number: 3002152861). (B) The mechanism of Exosomes-loaded conductive hydrogels hydrogel promoting SCI repair. Adapted reprinted with permission from Ref. [26], based on CC BY License, Copyright © 2022 The Authors. Advanced Science published by Wiley-VCH GmbH.(C) GelMA hydrogel MN patch for application to the damaged spinal cord site. Adapted reprinted with permission from Ref. [224], based on CC BY License, Copyright @ 2022 American Chemical Society.
Conductive hydrogels are ideal materials for accelerating SCI repair because they can match the electrophysiological and mechanical properties of neural tissues. Fan et al. [26] designed a conductive hydrogel loaded with BMSC-EVs for SCI repair using a hydrogel network of UV crosslinked gelatin methacrylate units (Fig. 10B). The hydrogel was sequentially immersed into solutions containing monomer polypyrrole (PPy) and tannic acid and ammonium persulfate to achieve in situ polymerization and crosslinking of the conductive PPy chains. The conductive hydrogels were loaded with BMSC-Exos, which inhibited inflammation, enhanced the recruitment of NSCs, and promoted the regeneration of neurons and myelin-associated axons after SCI.
In contrast to local injection, Han et al. [224] constructed a GelMA hydrogel MN patch for application to the damaged spinal cord site. The MNs were loaded with 3D culture-acquired MSC-Exos, which had higher dryness and contained more anti-inflammatory and anti-apoptotic factors. The exosome-loaded GelMA hydrogel MNs showed extremely high exosome retention and sustained release in rats with SCI, which significantly promoted the functional recovery (Fig. 10C).
EV-loaded hydrogels have been shown to promote spinal cord angiogenesis, inhibit fibrotic scar formation, enhance NSC recruitment and differentiation, inhibit inflammation, reduce oxidative stress, and promote neuronal cell regeneration, and thus have a promising future in nerve injury repair [26,158,180,225]. Low-rigidity hydrogels with conductive properties have great potential as they can be specifically adapted for neuroelectrophysiology and biomechanics [26]. However, it is important to note that the swelling properties of hydrogels require special attention when applied to the central nervous system (CNS); because CNS injuries are often accompanied by tissue edema, the high swelling properties of hydrogels can lead to increased intracranial pressure and consequent secondary injury after hydrogel injection [226].
6.6. Reproduction system
MSC-EVs also play important roles in reproductive medicine. Studies have been performed in which EV-loaded hydrogels were used to improve the endometrial environment, treat uterine adhesions, promote vaginal re-epithelialization, and treat male erectile dysfunction [227,228]. Lin et al. [227] crosslinked four-armed PEG molecules with Ag+, relying on Ag+-S dynamic coordination, to produce an injectable hydrogel loaded with ADSC-Exos for local injection into the endometrium (Fig. 11A). The results showed increased endometrial angiogenesis and reduced local tissue fibrosis. The hydrogel significantly improved the endometrial environment and increased the pregnancy rate of treated mice. In addition, Xin et al. [29] used HA hydrogels loaded with apoptotic bodies from UMSCs, and the authors found that apoptotic bodies induced macrophage immunomodulation, cell proliferation, and angiogenesis in vitro. In a mouse model of acute endometrial injury, implantation of apoptotic body-loaded HA hydrogels increased endometrial thickness and the number of endometrial glands, reduced fibrosis, and promoted endometrial receptivity and fertility recovery [29] (Fig. 11B). Xu et al. [229] designed photo-triggered imine crosslinked hydrogels containing sEVs from human urinary-derived stem cells for the treatment of vaginal mucosal defects and observed epithelial formation and angiogenesis in the vaginal mucosal of rabbits.
Fig. 11.
Application of EV-loaded hydrogels in reproductive system
(A) Schematic overview of the development of an ADSC-Exos loaded hydrogel for endometrial regeneration. Adapted reprinted with permission from Ref. [227], Copyright © 2021 Wiley-VCH GmbH (License number: 5,441,710,907,704). (B) Schematic illustration of the design and application of the apoptotic body-loaded HA hydrogel. Adapted reprinted with permission from Ref. [29], based on CC BY-NC License. (C) Corpus cavernosum intratunical injection of ADSC-exosomes loaded polydopamine thermosensitive hydrogel for erectile dysfunction treatment. Adapted reprinted with permission from Ref. [230], based on CC BY-NC License.
To treat erectile dysfunction, Liang et al. [230] prepared PEG-Polycaprolactone (PCL) hermosensitive hydrogels containing PDA nanoparticles for intraluminal administration of ADSC-EVs via simple in situ polymerization (Fig. 11C). The hydrogel provided sustained release of EVs, leading to endothelial cell and neuronal healing and increases intraluminal pressure in animal experiments, thus restoring erectile function. In particular, due to the good photoacoustic properties of the PDA nanoparticles in the thermosensitive gel, the hydrogel was precisely delivered into the white membrane under the guidance of real-time acoustic imaging. MSC-EV-loaded hydrogels are expected to facilitate the development of new treatment options for improving fertility and treating sexual dysfunction.
6.7. Periodontal tissue
Periodontitis is a chronic infectious disease that leads to attachment loss (deletion) and progressive resorption of alveolar bone. Periodontitis is especially devastating in patients with severe disease, for whom it is clinically difficult to achieve complete restoration of periodontal tissue structure and function [231]. MSC-EVs can promote periodontal regeneration [232]. Liu et al. [232] demonstrated that BMSC-EVs may be involved in the OPG-RANKLRANK signaling pathway and participate in regulating osteoclast function, macrophage polarization, and inflammatory immune responses; thus, BMSC-EV-loaded hydrogels can significantly promote periodontal tissue regeneration in the treatment of periodontitis. Shen et al. [233] used chitosan hydrogel loaded with DPSC-Exos (dental pulp stem cells derived exosomes) for the treatment of periodontitis and showed that DPSC-Exos modulated macrophage phenotypes. Furthermore, the hydrogels accelerated the healing of alveolar bone and periodontal epithelium in mice with periodontitis, while inhibiting the development of periodontitis. In addition, Shi et al. [234] found that DPSC-Exos pretreated with lipopolysaccharides were better able to promote periodontal regeneration.
6.8. Hair
Hair loss is a problem faced by more than half of the global population. First-line drugs, such as minoxidil and finasteride, have significant side effects, and hair transplantation has certain drawbacks [235,236]. There are problems of low survival rate of the transplanted hairs and damage to the donor site. Because of their ability to induce proliferation and regeneration in a variety of contexts, MSC-EVs are the most promising cell-free treatment strategy for hair regrowth. Chen et al. [139] wrapped human dermal papilla cell-derived EVs in a partial sodium oxalate (OSA) hydrogel for local injection, and in vivo and in vitro experiments revealed that OSA-EVs significantly promoted hair stromal cell proliferation, prolonged the primordial phase of cultured human hairs, and accelerated the regeneration of dorsal hairs in mice after hair removal. These effects may be due to the upregulation of hair growth-promoting signaling molecules, such as Wnt3a and β-catenin, and the downregulation of the inhibitory molecule BMP2 (Fig. 12A).
Fig. 12.
Application of EV-loaded hydrogels in hair regeneration
(A) Schematic of the preparation and functional mechanism of OSA-EVs nanospheres. Adapted reprinted with permission from Ref. [139], based on CC BY-NC License. (B) Schematic of hair loss therapy through a detachable microneedle patch system. H&E staining of the treated mice skin at day 5 and 9 postinsertion of the HMN patch and immunofluorescence staining against surface markers of macrophage (CD68) and lymphocyte infiltration (CD3) at day 5 and 9. Adapted reprinted with permission from Ref. [237], based on CC BY License, Copyright @ 2022 American Chemical Society.
Because MN systems allow for deeper drug delivery and the ability to design structures with multiple functions, several research teams have designed EV-loaded hydrogel MN patches for hair regeneration [237,238]. Yang et al. [237] prepared keratin hydrogel microneedles loaded with MSC-EVs and the small molecule drug UK5099 using keratin, a major component of hair. The experimental results demonstrated that the device improved drug delivery efficiency and significantly promoted skin pigmentation and hair regeneration in mice, with enhanced therapeutic effects compared to topical application or subcutaneous injection methods (Fig. 12B).
Alternatively, Shi et al. [238] prepared detachable MN patches consisting of chitosan lactate (CL) and ADSC-Exos for hair regeneration. After insertion of the MN into the skin, the HA substrate was rapidly dissolved and the expandable polyvinyl alcohol tip was retained. The ADSC-Exos continuously released from the needle tip was endocytosed by dermal papilla cells to promote cell proliferation by activating the Wnt signaling pathway, while l-lactic acid released from CL promoted cell growth by activating lactate dehydrogenase. CL and ADSC-Exos can synergistically regulate hair follicle circulation and promote hair regeneration. In animal studies, self-administered MN patches promoted hair regeneration more significantly and less frequently over 7 days compared to topical minoxidil application. In conclusion, the transdermal administration of drug-free MN patched is a simple, safe, and highly effective hair loss treatment strategy with great potential for clinical application.
6.9. Other tissues
MSC-EVs have bene applied to several other tissues. Zhang et al. [159] prepared RGD hydrogels loaded with MSC-EVs for kidney injury repair; the RGD peptide bound to integrins on the surfaces of MSC-EVs, improving the hydrogel load capacity. The RGD-EV-loaded hydrogel was used in a mouse model of acute kidney injury and significantly improved kidney function, reduced histopathological damage, decreased tubular damage, and promoted cell proliferation in the early stages of the disease. Similarly, MSC-EV-loaded hydrogels may be a promising strategy for the treatment of chronic liver failure. Mardpour et al. [148] mixed clickable PEG macromolecules with MSC-EVs to form EV-loaded PEG hydrogels through a rapid, biocompatible click reaction. When injected intraperitoneally into a rat model of chronic liver failure, the gradual degradation of the hydrogel allowed for the slow release of EVs over 1 month, leading to improved anti-fibrotic, anti-apoptotic, and regenerative effects. In addition, Tang et al. [239] used temperature-sensitive chitosan hydrogels loaded with MSC-EVs in the cornea and found that they effectively promoted the repair of damaged corneal epithelium and stroma, downregulated the mRNA expression of the three most abundant collagen proteins (type I α1, V α1, and V α2) in the corneal stroma, and reduced scar formation. Silva et al. [240] loaded ADSC-EVs into a thermo-responsive PF-127 gel that was injected locally in a porcine fistula model at 4 °C and gelated at body temperature to retain EVs throughout the fistula tract; all esophageal fistulas in animals receiving the EV-loaded hydrogel were repaired, whereas esophageal fistulas remained in the control group.
At the end, we had summarized the information on the use of EV-loaded hydrogels in various tissues repair and regeneration and provided a brief overview of their key findings in Table 2.
Table 2.
Tissue-specific applications of EV-loaded hydrogels.
| Tissue/organ to be regenerated | Hydrogel | Sources of EVs | Application strategies | Key findings | Reference |
|---|---|---|---|---|---|
| Skin/Wound healing | PF-127 | ADSC | Topical administration | PF-127 hydrogel-loaded EVs can effectively promote wound healing. | [167] |
| MMP-PEG smart hydrogel | ADSC | Topical administration | It can target the MMP2 response and significantly promote the recovery of diabetic wounds. | [168] | |
| HAMA Microneedle | MSC-EVM | Microneedle (MN) | MN patches containing EVs show the ability to deliver deep into the skin. | [62] | |
| Chitosan/Silk hydrogel sponge | gingival MSC | Wound Dressing | Demonstrated the feasibility of hydrogel sponge dressings loaded with EVs for use on wound. | [176] | |
| HA@MnO2/FGF-2 hydrogel | M2 macrophages | Topical administration | Simultaneous loading of nanoenzymes and bioactive factors as well as EVs provides a multifunctional effect, improving all aspects of wound healing. | [193] | |
| Chitosan/Silk hydrogel sponge | platelet-rich plasma | Wound Dressing | Combined application of active ingredients in Chinese medicine with EVs shows great potential. | [192] | |
| MC-CHO and CS-g-PEG | MSC | Topical administration | Self-healing hydrogels can synergize with EVs to promote wound healing. | [191] | |
| Bone and cartilage | HyStem-HP hydrogel (Catalog: GS315, Glycosan Biosystems) | UCSC | Topical administration | EV-loaded hydrogel can promote repair from fractures. | [194] |
| GelMA/Laponite | BMSC | in vitro only | Nanoclay combined with hydrogel can induce stem cell bone differentiation, showing great promise in bone repair. | [162] | |
| PEG/MC/β-TCP | BMSC | Topical administration | The composite hydrogel improves the local microenvironment and exhibits better mechanical strength. | [202] | |
| PLGA-PEG-PLGA microsphere/PLLA scaffold | dental pulp stem cells DPSC | Scaffold | Polymeric microspheres loaded with EVs combined with scaffolds can be used for the repair of skull defects. | [115] | |
| HA/nHP scaffolds | UMSC | Scaffold | EV-loaded hydrogel to encapsulate the nanoceramic scaffold can be used to repair large bone defects. | [199] | |
| CGC/HG | chondrocytes | Scaffold | Double-layered composite scaffold structure loaded with EVs holds promise for articular cartilage repair. | [207] | |
| AD/CS/RSF | BMSC | Topical administration | Mussel-like high adhesion hydrogel loaded EVs allows better adhesion to cartilage tissue for better repair. | [103] | |
| Heart | Gelatin and Laponite | ADSC | Topical administration | Intramyocardial injection of EV-loaded hydrogel can contribute to the recovery of the infarcted area. | [214] |
| HA Conductive Hydrogel | UMSC | Topical administration | EV- loaded conductive hydrogels can be adapted to the bioelectricity of the heart and are more promising | [216] | |
| ECM hydrogel | iPS-CPCs | Injection into the pericardial cavity | Intrapericardial injection of EV-loaded hydrogel is minimally invasive and effective. | [217] | |
| GelMA | MSC | Spray drug administration | The authors propose an injection-free delivery of EV-loaded hydrogel to the heart. | [27] | |
| Nerves | GelMA | BMSC | Topical administration | EV-loaded injectable hydrogel for minimally invasive treatment of spinal cord injury. |
[220] |
| HA/GelMA | MSC | Topical administration | Low-stiffness EV-loaded hydrogels showed better repair of injured peripheral nerves. | [221] | |
| Electroconductive hydrogels | BMSC | Topical administration | EV-loaded electroconductive hydrogels match the electrical and mechanical properties of neural tissue and promote tissue repair after spinal cord Injury. |
[26] | |
| GelMA Microneedle | MSC (3D culture) | Microneedle | The authors proposed a controlled 3D-EV-loaded hydrogel microneedle array patch to achieve spinal cord injury repair in situ. | [224] | |
| Reproductive System | Ag-PEG | ADSC | Topical administration | EV-loaded hydrogel improves the intrauterine environment and promotes fertility recovery. | [227] |
| PDNPs-PELA thermosensitive hydrogels | ADSC | Topical administration | EV-loaded hydrogel promotes the repair of penile corpus cavernosum tissue and the recovery of erectile function. | [230] | |
| Periodontal | Gelatin/Laponite | BMSC | Topical administration | EV-loaded hydrogel promotes periodontal tissue repair and regeneration. | [232] |
| Hair | OSA hydrogels | Dermal papilla cell | Topical administration | EV-loaded hydrogel accelerates the regrowth of hair and can be used to treat alopecia. | [139] |
| CL microneedle | ADSC | Microneedle | Such transdermally administrated microneedle patches provide a simple, safe and efficient strategy for hair regeneration. | [238] | |
| Liver | PEG | MSC | Intraperitoneal injection | EV-loaded hydrogel based on click reaction has potential for systemic drug delivery. | [148] |
Abbreviations: PF-127: Pluronic F-127; ADSC: Adipose derived mesenchymal stem cells; MMP: matrix metalloproteinase; PEG: Polyethylene glycol; HAMA: Hyaluronic acid Methacryloyl; BMSC: Bone marrow mesenchymal stem cells; MSC: mesenchymal stem cells; HA: Hyaluronic Acid; MC-CHO: Aldehyde methylcellulose; CS-g-PEG: chitosan-g-PEG; GelMA: Gelatin methacryloyl; LAP: Lithium Phenyl (2,4,6-trimethylbenzoyl) phosphinate; β-TCP: β-Tricalcium Phosphate; PLGA: poly (lactic-co-glycolic acid; PLLA: poly(l-lactic acid); nHP: nanohydroxyapatite/poly-ε-caprolactone; CGC: chitosan-gelatin-chondroitin sulfate; HG: nano-hydroxyapatite-gelatin; AD/CS/RSF: alginate-dopamine, chondroitin sulfate, and regenerated silk fibroin; UMSC: umbilical cord mesenchymal stem cells; ECM: extracellular matrix; iPS-CPCs: pluripotent stem cell-derived cardiac progenitors; PDNPs-PELA: Polydopamine nanoparticles incorporated poly (ethylene glycol)-poly(ε-cap-rolactone-co-lactide); OSA: oxidized sodium alginate; CL: chitosan lactate.
7. Conclusions and outlook
As a cellular substructure, EVs have low immunogenicity, good biocompatibility and almost no biosafety concerns. Although almost all cells secrete EVs, MSC-EVs continue to be the most researched in the field of regenerative medicine. The study of MSC-EVs presents new opportunities and challenges for the development of regenerative medicine. As we have discussed, MSC-EVs have been widely demonstrated to promote regeneration and repair in almost all tissues, including adult terminally differentiated neuronal cells and cardiomyocytes. Although some mechanisms of action have been elucidated, more and deeper mechanisms remain unclear. The prevailing view is that the contents encapsulated in MSC-EVs, including a large number of proteins, nucleic acid molecules, and biological factors, are key to their action. MSC-EVs from different sources differ from each other; for example, studies at the multi-omics level have reported that ADSC-EVs contain more proteins and miRNAs highly correlated with angiogenesis compared to BMSC-EVs. Some scholars have focused specifically on EVs secreted by adult cells, which can exert useful biological functions; however, adult cells are often more difficult to obtain and their translation to the clinic is challenging.
Although EVs have many advantages, their use in the clinic is limited by their tendency to rapidly degrade and be cleared in the circulation, both of which make sustained, targeted therapy difficult. Fortunately, the excellent biocompatibility and physicochemical properties of hydrogels make them good carriers for EVs. Hydrogels can protect EVs from the harsh in vivo environments, especially those associated with trauma, and can facilitate their slow release to a variety of tissues. Multifunctional and intelligent EV-loaded hydrogels are currently a hot research topic, and hydrogel MN patches loaded with EVs are also being actively investigated. EV-loaded hydrogels have been applied to the bone, cartilage, heart, nervous system, reproductive system, oral cavity, hair, liver, kidney, esophagus, and other tissues and organs, highlighting their great potential for research and clinical applications in nearly all human tissues and organs.
There are still several challenges facing the use of EV-loaded hydrogels in the clinic. For example, the poor mechanical properties of hydrogels limit their applications in the field of bone and cartilage tissue repair. Such limitations can be remedied to some degree through the modification of natural polymers, introduction of synthetic polymers, mixing of inorganic materials, and addition of scaffold materials; however, there is still a long way to go before widespread adoption in clinical settings. In addition, the low yield of EVs makes it difficult to achieve factory-scale mass production. Breakthroughs in EV extraction technologies are a prerequisite for establishing EV-loaded hydrogel-based therapeutic strategies. Related developments in bioreactors, 3D cell culture, and specialized sera and reagents for the extraction of EVs will facilitate the development of EV-loaded hydrogels. The storage problem of EV-loaded hydrogels has received little attention from researchers, although it has shown great potential in the laboratory. EVs need to be stored at −80° to maintain their biological activity, and repeated freeze-thawing can lead to reduced activity or inactivation. Current studies have largely defaulted to ready-to-use EV-loaded hydrogels, with no reports on how they can be stored and how long their effectiveness can be maintained. The production, storage, transportation, and economic costs are all issues that need to be considered for clinical translation. It is worth mentioning that the development of hydrogel manufacturing technology will also provide more options for EV-loaded hydrogels: microfluidic-based hydrogel microsphere preparation technology, microneedle patch fabrication, hydrogel-based 3D bioprinting technology, etc.
In summary, EV-loaded hydrogels are a promising tissue repair and regeneration strategy that has been shown to work in almost all human tissues and organs. With the continued development of biomaterials science, EV-loaded hydrogels will open a new chapter in the field of human tissue repair and regenerative medicine.
Funding
This study was funded by the Natural Science Foundation of Hunan Province, China (2021JJ30928) and Hunan Provincial Health Commission Scientific Research Project, China (C202304106744).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
No data was used for the research described in the article.
References
- 1.Gurtner G.C., Werner S., Barrandon Y., Longaker M.T. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong D.G., Boulton A.J.M., Bus S.A. Diabetic foot ulcers and their recurrence. N. Engl. J. Med. 2017;376:2367–2375. doi: 10.1056/NEJMra1615439. [DOI] [PubMed] [Google Scholar]
- 3.Sorrells S.F., Paredes M.F., Cebrian-Silla A., Sandoval K., Qi D., Kelley K.W., James D., Mayer S., Chang J., Auguste K.I., Chang E.F., Gutierrez A.J., Kriegstein A.R., Mathern G.W., Oldham M.C., Huang E.J., Garcia-Verdugo J.M., Yang Z., Alvarez-Buylla A. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature. 2018;555:377–381. doi: 10.1038/nature25975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uygur A., Lee Richard T. Mechanisms of cardiac regeneration. Dev. Cell. 2016;36:362–374. doi: 10.1016/j.devcel.2016.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeng Y., Qiu Y., Jiang W., Shen J., Yao X., He X., Li L., Fu B., Liu X. Biological features of extracellular vesicles and challenges. Front. Cell Dev. Biol. 2022;10 doi: 10.3389/fcell.2022.816698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kou M., Huang L., Yang J., Chiang Z., Chen S., Liu J., Guo L., Zhang X., Zhou X., Xu X., Yan X., Wang Y., Zhang J., Xu A., Tse H.F., Lian Q. Mesenchymal stem cell-derived extracellular vesicles for immunomodulation and regeneration: a next generation therapeutic tool? Cell Death Dis. 2022;13:580. doi: 10.1038/s41419-022-05034-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y., Fang J., Liu B., Shao C., Shi Y. Reciprocal regulation of mesenchymal stem cells and immune responses. Cell Stem Cell. 2022;29:1515–1530. doi: 10.1016/j.stem.2022.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Pischiutta F., Caruso E., Lugo A., Cavaleiro H., Stocchetti N., Citerio G., Salgado A., Gallus S., Zanier E.R. Systematic review and meta-analysis of preclinical studies testing mesenchymal stromal cells for traumatic brain injury. NPJ Regen Med. 2021;6:71. doi: 10.1038/s41536-021-00182-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y., Zhang J., Yi H., Zheng J., Cai J., Chen W., Lu T., Chen L., Du C., Liu J., Yao J., Zhao H., Wang G., Fu B., Zhang T., Zhang J., Wang G., Li H., Xiang A.P., Chen G., Yi S., Zhang Q., Yang Y. A novel MSC-based immune induction strategy for ABO-incompatible liver transplantation: a phase I/II randomized, open-label, controlled trial. Stem Cell Res. Ther. 2021;12:244. doi: 10.1186/s13287-021-02246-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodríguez-Pallares J., García-Garrote M., Parga J.A., Labandeira-García J.L. Combined cell-based therapy strategies for the treatment of Parkinson's disease: focus on mesenchymal stromal cells. Neural. Regen. Res. 2022;18:478–484. doi: 10.4103/1673-5374.350193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pang Q.M., Deng K.Q., Zhang M., Wu X.C., Yang R.L., Fu S.P., Lin F.Q., Zhang Q., Ao J., Zhang T. Multiple strategies enhance the efficacy of MSCs transplantation for spinal cord injury. Biomed. Pharmacother. 2022;157 doi: 10.1016/j.biopha.2022.114011. [DOI] [PubMed] [Google Scholar]
- 12.Li A., Guo F., Pan Q., Chen S., Chen J., Liu H.F., Pan Q. Mesenchymal stem cell therapy: hope for patients with systemic lupus erythematosus. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.728190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luchetti F., Carloni S., Nasoni M.G., Reiter R.J., Balduini W. Melatonin, tunneling nanotubes, mesenchymal cells, and tissue regeneration. Neural. Regen. Res. 2022;18:760–762. doi: 10.4103/1673-5374.353480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caplan H., Olson S.D., Kumar A., George M., Prabhakara K.S., Wenzel P., Bedi S., Toledano-Furman N.E., Triolo F., Kamhieh-Milz J., Moll G., Cox C.S., Jr. Mesenchymal stromal cell therapeutic delivery: translational challenges to clinical application. Front. Immunol. 2019;10:1645. doi: 10.3389/fimmu.2019.01645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou T., Yuan Z., Weng J., Pei D., Du X., He C., Lai P. Challenges and advances in clinical applications of mesenchymal stromal cells. J. Hematol. Oncol. 2021;14:24. doi: 10.1186/s13045-021-01037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keshtkar S., Azarpira N., Ghahremani M.H. Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem Cell Res. Ther. 2018;9:63. doi: 10.1186/s13287-018-0791-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J.J., Hosseini-Beheshti E., Grau G.E., Zreiqat H., Little C.B. Stem cell-derived extracellular vesicles for treating joint injury and osteoarthritis. Nanomaterials. 2019:9. doi: 10.3390/nano9020261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagelkerke A., Ojansivu M., van der Koog L., Whittaker T.E., Cunnane E.M., Silva A.M., Dekker N., Stevens M.M. Extracellular vesicles for tissue repair and regeneration: evidence, challenges and opportunities. Adv. Drug Deliv. Rev. 2021;175 doi: 10.1016/j.addr.2021.04.013. [DOI] [PubMed] [Google Scholar]
- 19.Safari B., Aghazadeh M., Davaran S., Roshangar L. Exosome-loaded hydrogels: a new cell-free therapeutic approach for skin regeneration. Eur. J. Pharm. Biopharm. 2022;171:50–59. doi: 10.1016/j.ejpb.2021.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Riau A.K., Ong H.S., Yam G.H.F., Mehta J.S. Sustained delivery system for stem cell-derived exosomes. Front. Pharmacol. 2019;10:1368. doi: 10.3389/fphar.2019.01368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie Y., Guan Q., Guo J., Chen Y., Yin Y., Han X. Hydrogels for exosome delivery in biomedical applications. Gels. 2022:8. doi: 10.3390/gels8060328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brennan M.A., Layrolle P., Mooney D.J. Biomaterials functionalized with MSC secreted extracellular vesicles and soluble factors for tissue regeneration. Adv. Funct. Mater. 2020;30 doi: 10.1002/adfm.201909125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou Y., Liu S., Zhao M., Wang C., Li L., Yuan Y., Li L., Liao G., Bresette W., Zhang J., Chen Y., Cheng J., Lu Y., Liu J. Injectable extracellular vesicle-released self-assembling peptide nanofiber hydrogel as an enhanced cell-free therapy for tissue regeneration. J. Contr. Release. 2019;316:93–104. doi: 10.1016/j.jconrel.2019.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Pishavar E., Luo H., Naserifar M., Hashemi M., Toosi S., Atala A., Ramakrishna S., Behravan J. Advanced hydrogels as exosome delivery systems for osteogenic differentiation of MSCs: application in bone regeneration. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22126203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun J., Yin Z., Wang X., Su J. Exosome-laden hydrogels: a novel cell-free strategy for in-situ bone tissue regeneration. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.866208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan L., Liu C., Chen X., Zheng L., Zou Y., Wen H., Guan P., Lu F., Luo Y., Tan G., Yu P., Chen D., Deng C., Sun Y., Zhou L., Ning C. Exosomes-loaded electroconductive hydrogel synergistically promotes tissue repair after spinal cord injury via immunoregulation and enhancement of myelinated axon growth. Adv. Sci. 2022;9 doi: 10.1002/advs.202105586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang J., Cui X., Zhang Z., Xu Y., Guo J., Soliman B.G., Lu Y., Qin Z., Wang Q., Zhang H., Lim K.S., Woodfield T.B.F., Zhang J. Injection-free delivery of MSC-derived extracellular vesicles for myocardial infarction therapeutics. Adv.x Healthc. Mater. 2022;11 doi: 10.1002/adhm.202100312. [DOI] [PubMed] [Google Scholar]
- 28.Liu X., Yang Y., Li Y., Niu X., Zhao B., Wang Y., Bao C., Xie Z., Lin Q., Zhu L. Integration of stem cell-derived exosomes with in situ hydrogel glue as a promising tissue patch for articular cartilage regeneration. Nanoscale. 2017;9:4430–4438. doi: 10.1039/c7nr00352h. [DOI] [PubMed] [Google Scholar]
- 29.Xin L., Wei C., Tong X., Dai Y., Huang D., Chen J., Ma L., Zhang S. In situ delivery of apoptotic bodies derived from mesenchymal stem cells via a hyaluronic acid hydrogel: a therapy for intrauterine adhesions. Bioact. Mater. 2022;12:107–119. doi: 10.1016/j.bioactmat.2021.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D. Zheng, H. Ruan, W. Chen, Y. Zhang, W. Cui, H. Chen, H. Shen, Advances in extracellular vesicle functionalization strategies for tissue regeneration, Bioact. Mater. (2022) Available online 11 August 2022. [DOI] [PMC free article] [PubMed]
- 31.Ramirez O.J., Alvarez S., Contreras-Kallens P., Barrera N.P., Aguayo S., Schuh C. Type I collagen hydrogels as a delivery matrix for royal jelly derived extracellular vesicles. Drug Deliv. 2020;27:1308–1318. doi: 10.1080/10717544.2020.1818880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu X.H., Yuan T.J., Dad H.A., Shi M.Y., Huang Y.Y., Jiang Z.H., Peng L.H. Plant exosomes as novel nanoplatforms for MicroRNA transfer stimulate neural differentiation of stem cells in vitro and in vivo. Nano Lett. 2021;21:8151–8159. doi: 10.1021/acs.nanolett.1c02530. [DOI] [PubMed] [Google Scholar]
- 33.Shao J., Zaro J., Shen Y. Advances in exosome-based drug delivery and tumor targeting: from tissue distribution to intracellular fate. Int. J. Nanomed. 2020;15:9355–9371. doi: 10.2147/IJN.S281890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soekmadji C., Li B., Huang Y., Wang H., An T., Liu C., Pan W., Chen J., Cheung L., Falcon-Perez J.M., Gho Y.S., Holthofer H.B., Le M.T.N., Marcilla A., O'Driscoll L., Shekari F., Shen T.L., Torrecilhas A.C., Yan X., Yang F., Yin H., Xiao Y., Zhao Z., Zou X., Wang Q., Zheng L. The future of Extracellular Vesicles as Theranostics - an ISEV meeting report. J. Extracell. Vesicles. 2020;9 doi: 10.1080/20013078.2020.1809766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y., Bi J., Huang J., Tang Y., Du S., Li P. Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int. J. Nanomed. 2020;15:6917–6934. doi: 10.2147/IJN.S264498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kimiz-Gebologlu I., Oncel S.S. Exosomes: large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J. Contr. Release. 2022;347:533–543. doi: 10.1016/j.jconrel.2022.05.027. [DOI] [PubMed] [Google Scholar]
- 37.Lai J.J., Chau Z.L., Chen S.Y., Hill J.J., Korpany K.V., Liang N.W., Lin L.H., Lin Y.H., Liu J.K., Liu Y.C., Lunde R., Shen W.T. Exosome processing and characterization approaches for research and technology development. Adv. Sci. 2022;9 doi: 10.1002/advs.202103222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gould S.J., Raposo G. As we wait: coping with an imperfect nomenclature for extracellular vesicles. J. Extracell. Vesicles. 2013;2 doi: 10.3402/jev.v2i0.20389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han Y., Ren J., Bai Y., Pei X., Han Y. Exosomes from hypoxia-treated human adipose-derived mesenchymal stem cells enhance angiogenesis through VEGF/VEGF-R. Int. J. Biochem. Cell Biol. 2019;109:59–68. doi: 10.1016/j.biocel.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 40.Pomatto M., Gai C., Negro F., Cedrino M., Grange C., Ceccotti E., Togliatto G., Collino F., Tapparo M., Figliolini F., Lopatina T., Brizzi M.F., Camussi G. Differential therapeutic effect of extracellular vesicles derived by bone marrow and adipose mesenchymal stem cells on wound healing of diabetic ulcers and correlation to their cargoes. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22083851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watson D.C., Bayik D., Srivatsan A., Bergamaschi C., Valentin A., Niu G., Bear J., Monninger M., Sun M., Morales-Kastresana A., Jones J.C., Felber B.K., Chen X., Gursel I., Pavlakis G.N. Efficient production and enhanced tumor delivery of engineered extracellular vesicles. Biomaterials. 2016;105:195–205. doi: 10.1016/j.biomaterials.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murali V.P., Holmes C.A. Biomaterial-based extracellular vesicle delivery for therapeutic applications. Acta Biomater. 2021;124:88–107. doi: 10.1016/j.actbio.2021.01.010. [DOI] [PubMed] [Google Scholar]
- 43.Re F., Gabusi E., Manferdini C., Russo D., Lisignoli G. Bone regeneration improves with mesenchymal stem cell derived extracellular vesicles (EVs) combined with scaffolds: a systematic review. Biology. 2021;10(7):579. doi: 10.3390/biology10070579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Du Y., Wang H., Yang Y., Zhang J., Huang Y., Fan S., Gu C., Shangguan L., Lin X. Extracellular vesicle mimetics: preparation from top-down approaches and biological functions. Adv.x Healthc. Mater. 2022;11(19) doi: 10.1002/adhm.202200142. [DOI] [PubMed] [Google Scholar]
- 45.Fatima F., Ekstrom K., Nazarenko I., Maugeri M., Valadi H., Hill A.F., Camussi G., Nawaz M. Non-coding RNAs in mesenchymal stem cell-derived extracellular vesicles: deciphering regulatory roles in stem cell potency, inflammatory resolve, and tissue regeneration. Front. Genet. 2017;8:161. doi: 10.3389/fgene.2017.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wong D.E., Banyard D.A., Santos P.J.F., Sayadi L.R., Evans G.R.D., Widgerow A.D. Adipose-derived stem cell extracellular vesicles: a systematic review( ) J. Plast. Reconstr. Aesthetic Surg. 2019;72:1207–1218. doi: 10.1016/j.bjps.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 47.Qin Y., Sun R., Wu C., Wang L., Zhang C. Exosome: a novel approach to stimulate bone regeneration through regulation of osteogenesis and angiogenesis. Int. J. Mol. Sci. 2016;17(5):712. doi: 10.3390/ijms17050712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han C., Zhou J., Liang C., Liu B., Pan X., Zhang Y., Wang Y., Yan B., Xie W., Liu F., Yu X.Y., Li Y. Human umbilical cord mesenchymal stem cell derived exosomes encapsulated in functional peptide hydrogels promote cardiac repair. Biomater. Sci. 2019;7:2920–2933. doi: 10.1039/c9bm00101h. [DOI] [PubMed] [Google Scholar]
- 49.Li D., Wu N. Mechanism and application of exosomes in the wound healing process in diabetes mellitus. Diabetes Res. Clin. Pract. 2022;187 doi: 10.1016/j.diabres.2022.109882. [DOI] [PubMed] [Google Scholar]
- 50.Wang D., Cao H., Hua W., Gao L., Yuan Y., Zhou X., Zeng Z. Mesenchymal stem cell-derived extracellular vesicles for bone defect repair. Membranes. 2022;12(7):716. doi: 10.3390/membranes12070716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gardiner C., Di Vizio D., Sahoo S., Thery C., Witwer K.W., Wauben M., Hill A.F. Techniques used for the isolation and characterization of extracellular vesicles: results of a worldwide survey. J. Extracell. Vesicles. 2016;5 doi: 10.3402/jev.v5.32945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu Q., Fu S., Xiao H., Du J., Cheng F., Wan S., Zhu H., Li D., Peng F., Ding X., Wang L. Advances in extracellular vesicle nanotechnology for precision theranostics. Adv. Sci. 2022 doi: 10.1002/advs.202204814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim J.Y., Rhim W.K., Yoo Y.I., Kim D.S., Ko K.W., Heo Y., Park C.G., Han D.K. Defined MSC exosome with high yield and purity to improve regenerative activity. J. Tissue Eng. 2021;12 doi: 10.1177/20417314211008626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liangsupree T., Multia E., Riekkola M.L. Modern isolation and separation techniques for extracellular vesicles. J. Chromatogr. A. 2021;1636 doi: 10.1016/j.chroma.2020.461773. [DOI] [PubMed] [Google Scholar]
- 55.Gurunathan S., Kang M.H., Jeyaraj M., Qasim M., Kim J.H. 2019. Review of the Isolation, Characterization, Biological Function, and Multifarious Therapeutic Approaches of Exosomes; p. 8. Cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grangier A., Branchu J., Volatron J., Piffoux M., Gazeau F., Wilhelm C., Silva A.K.A. Technological advances towards extracellular vesicles mass production. Adv. Drug Deliv. Rev. 2021;176 doi: 10.1016/j.addr.2021.113843. [DOI] [PubMed] [Google Scholar]
- 57.Gronemeyer P., Ditz R., Strube J. Trends in upstream and downstream process development for antibody manufacturing. Bioengineering. 2014;1:188–212. doi: 10.3390/bioengineering1040188. [DOI] [PubMed] [Google Scholar]
- 58.Yan L., Wu X. Exosomes produced from 3D cultures of umbilical cord mesenchymal stem cells in a hollow-fiber bioreactor show improved osteochondral regeneration activity. Cell Biol. Toxicol. 2020;36:165–178. doi: 10.1007/s10565-019-09504-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Almeida Fuzeta M., Bernardes N., Oliveira F.D., Costa A.C., Fernandes-Platzgummer A., Farinha J.P., Rodrigues C.A.V., Jung S., Tseng R.J., Milligan W., Lee B., Castanho M., Gaspar D., Cabral J.M.S., da Silva C.L. Scalable production of human mesenchymal stromal cell-derived extracellular vesicles under serum-/xeno-free conditions in a microcarrier-based bioreactor culture system. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.553444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Piffoux M., Nicolas-Boluda A., Mulens-Arias V., Richard S., Rahmi G., Gazeau F., Wilhelm C., Silva A.K.A. Extracellular vesicles for personalized medicine: the input of physically triggered production, loading and theranostic properties. Adv. Drug Deliv. Rev. 2019;138:247–258. doi: 10.1016/j.addr.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 61.King H.W., Michael M.Z., Gleadle J.M. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012;12:421. doi: 10.1186/1471-2407-12-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ma W., Zhang X., Liu Y., Fan L., Gan J., Liu W., Zhao Y., Sun L. Polydopamine decorated microneedles with Fe-MSC-Derived nanovesicles encapsulation for wound healing. Adv. Sci. 2022;9 doi: 10.1002/advs.202103317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kang T., Jones T.M., Naddell C., Bacanamwo M., Calvert J.W., Thompson W.E., Bond V.C., Chen Y.E., Liu D. Adipose-derived stem cells induce angiogenesis via microvesicle transport of miRNA-31. Stem Cells Transl. Med. 2016;5:440–450. doi: 10.5966/sctm.2015-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xue C., Shen Y., Li X., Li B., Zhao S., Gu J., Chen Y., Ma B., Wei J., Han Q., Zhao R.C. Exosomes derived from hypoxia-treated human adipose mesenchymal stem cells enhance angiogenesis through the PKA signaling pathway. Stem Cell. Dev. 2018;27:456–465. doi: 10.1089/scd.2017.0296. [DOI] [PubMed] [Google Scholar]
- 65.Xue K., Jiang Y., Zhang X., Wu J., Qi L., Liu K. Hypoxic ADSCs-derived EVs promote the proliferation and chondrogenic differentiation of cartilage stem/progenitor cells. Adipocyte. 2021;10:322–337. doi: 10.1080/21623945.2021.1945210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu S.C., Kuo P.J., Rau C.S., Huang L.H., Lin C.W., Wu Y.C., Wu C.J., Tsai C.W., Hsieh T.M., Liu H.T., Huang C.Y., Hsieh C.H. Increased angiogenesis by exosomes secreted by adipose-derived stem cells upon lipopolysaccharide stimulation. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22168877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bai Y., Han Y.D., Yan X.L., Ren J., Zeng Q., Li X.D., Pei X.T., Han Y. Adipose mesenchymal stem cell-derived exosomes stimulated by hydrogen peroxide enhanced skin flap recovery in ischemia-reperfusion injury. Biochem. Biophys. Res. Commun. 2018;500:310–317. doi: 10.1016/j.bbrc.2018.04.065. [DOI] [PubMed] [Google Scholar]
- 68.Yu M., Liu W., Li J., Lu J., Lu H., Jia W., Liu F. Exosomes derived from atorvastatin-pretreated MSC accelerate diabetic wound repair by enhancing angiogenesis via AKT/eNOS pathway. Stem Cell Res. Ther. 2020;11:350. doi: 10.1186/s13287-020-01824-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hu Y., Tao R., Chen L., Xiong Y., Xue H., Hu L., Yan C., Xie X., Lin Z., Panayi A.C., Mi B., Liu G. Exosomes derived from pioglitazone-pretreated MSCs accelerate diabetic wound healing through enhancing angiogenesis. J. Nanobiotechnol. 2021;19:150. doi: 10.1186/s12951-021-00894-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li L., Wu P., Qian H., Xu W., Shi H., Jiang J. Tailored extracellular vesicles: novel tool for tissue regeneration. Stem Cell. Int. 2022;2022 doi: 10.1155/2022/7695078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pan Z., Sun W., Chen Y., Tang H., Lin W., Chen J., Chen C. Extracellular vesicles in tissue engineering: biology and engineered strategy. Adv.x Healthc. Mater. 2022;11 doi: 10.1002/adhm.202201384. [DOI] [PubMed] [Google Scholar]
- 72.Wang Y., Cao Z., Wei Q., Ma K., Hu W., Huang Q., Su J., Li H., Zhang C., Fu X. VH298-loaded extracellular vesicles released from gelatin methacryloyl hydrogel facilitate diabetic wound healing by HIF-1alpha-mediated enhancement of angiogenesis. Acta Biomater. 2022;147:342–355. doi: 10.1016/j.actbio.2022.05.018. [DOI] [PubMed] [Google Scholar]
- 73.Smyth T., Kullberg M., Malik N., Smith-Jones P., Graner M.W., Anchordoquy T.J. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J. Contr. Release. 2015;199:145–155. doi: 10.1016/j.jconrel.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wiklander O.P., Nordin J.Z., O'Loughlin A., Gustafsson Y., Corso G., Mager I., Vader P., Lee Y., Sork H., Seow Y., Heldring N., Alvarez-Erviti L., Smith C.I., Le Blanc K., Macchiarini P., Jungebluth P., Wood M.J., Andaloussi S.E. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J. Extracell. Vesicles. 2015;4 doi: 10.3402/jev.v4.26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Betzer O., Perets N., Angel A., Motiei M., Sadan T., Yadid G., Offen D., Popovtzer R. Vivo neuroimaging of exosomes using gold nanoparticles. ACS Nano. 2017;11:10883–10893. doi: 10.1021/acsnano.7b04495. [DOI] [PubMed] [Google Scholar]
- 76.Han Y., Zhu Y., Youngblood H.A., Almuntashiri S., Jones T.W., Wang X., Liu Y., Somanath P.R., Zhang D. Nebulization of extracellular vesicles: a promising small RNA delivery approach for lung diseases. J. Contr. Release. 2022;352:556–569. doi: 10.1016/j.jconrel.2022.10.052. [DOI] [PubMed] [Google Scholar]
- 77.Kwak G., Cheng J., Kim H., Song S., Lee S.J., Yang Y., Jeong J.H., Lee J.E., Messersmith P.B., Kim S.H. Sustained exosome-guided macrophage polarization using hydrolytically degradable PEG hydrogels for cutaneous wound healing: identification of key proteins and MiRNAs, and sustained release formulation. Small. 2022;18 doi: 10.1002/smll.202200060. [DOI] [PubMed] [Google Scholar]
- 78.Liu B., Lee B.W., Nakanishi K., Villasante A., Williamson R., Metz J., Kim J., Kanai M., Bi L., Brown K., Di Paolo G., Homma S., Sims P.A., Topkara V.K., Vunjak-Novakovic G. Cardiac recovery via extended cell-free delivery of extracellular vesicles secreted by cardiomyocytes derived from induced pluripotent stem cells. Nat Biomed Eng. 2018;2:293–303. doi: 10.1038/s41551-018-0229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yuan M., Liu K., Jiang T., Li S., Chen J., Wu Z., Li W., Tan R., Wei W., Yang X., Dai H., Chen Z. GelMA/PEGDA microneedles patch loaded with HUVECs-derived exosomes and Tazarotene promote diabetic wound healing. J. Nanobiotechnol. 2022;20:147. doi: 10.1186/s12951-022-01354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wichterle O., Lím D. Hydrophilic gels for biological use. Nature. 1960;185:117–118. [Google Scholar]
- 81.Ahmadi R., Burns A.J., de Bruijn J.D. Chitosan-based hydrogels do not induce angiogenesis. J. Tissue Eng. Regen. Med. 2010;4:309–315. doi: 10.1002/term.247. [DOI] [PubMed] [Google Scholar]
- 82.Yasin A., Ren Y., Li J., Sheng Y., Cao C., Zhang K. Advances in hyaluronic acid for biomedical applications. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.910290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ngadimin K.D., Stokes A., Gentile P., Ferreira A.M. Biomimetic hydrogels designed for cartilage tissue engineering. Biomater. Sci. 2021;9:4246–4259. doi: 10.1039/d0bm01852j. [DOI] [PubMed] [Google Scholar]
- 84.Nelson D.W., Gilbert R.J. Extracellular matrix-mimetic hydrogels for treating neural tissue injury: a focus on fibrin, hyaluronic acid, and elastin-like polypeptide hydrogels. Adv. Healthc. Mater. 2021;10 doi: 10.1002/adhm.202101329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alven S., Aderibigbe B.A. Hyaluronic acid-based scaffolds as potential bioactive wound dressings. Polymers. 2021;13 doi: 10.3390/polym13132102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Derkus B. Human cardiomyocyte-derived exosomes induce cardiac gene expressions in mesenchymal stromal cells within 3D hyaluronic acid hydrogels and in dose-dependent manner. J. Mater. Sci. Mater. Med. 2021;32:2. doi: 10.1007/s10856-020-06474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tm M.W., Lau W.M., Khutoryanskiy V.V. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers. 2018:10. doi: 10.3390/polym10030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Do N.H.N., Truong Q.T., Le P.K., Ha A.C. Recent developments in chitosan hydrogels carrying natural bioactive compounds. Carbohydr. Polym. 2022;294 doi: 10.1016/j.carbpol.2022.119726. [DOI] [PubMed] [Google Scholar]
- 89.Mo C., Xiang L., Chen Y. Advances in injectable and self-healing polysaccharide hydrogel based on the Schiff base reaction. Macromol. Rapid Commun. 2021;42 doi: 10.1002/marc.202100025. [DOI] [PubMed] [Google Scholar]
- 90.Zhang H., Cheng J., Ao Q. Preparation of alginate-based biomaterials and their applications in biomedicine. Mar. Drugs. 2021;19 doi: 10.3390/md19050264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu W., Madry H., Cucchiarini M. Application of alginate hydrogels for next-generation articular cartilage regeneration. Int. J. Mol. Sci. 2022;23 doi: 10.3390/ijms23031147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Maity C., Das N. Alginate-based smart materials and their application: recent advances and perspectives. Top. Curr. Chem. 2021;380:3. doi: 10.1007/s41061-021-00360-8. [DOI] [PubMed] [Google Scholar]
- 93.Kharkar P.M., Kiick K.L., Kloxin A.M. Designing degradable hydrogels for orthogonal control of cell microenvironments. Chem. Soc. Rev. 2013;42:7335–7372. doi: 10.1039/c3cs60040h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Antoine E.E., Vlachos P.P., Rylander M.N. Review of collagen I hydrogels for bioengineered tissue microenvironments: characterization of mechanics, structure, and transport. Tissue Eng. B Rev. 2014;20:683–696. doi: 10.1089/ten.teb.2014.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Salahuddin B., Wang S., Sangian D., Aziz S., Gu Q. Hybrid gelatin hydrogels in nanomedicine applications. ACS Appl. Bio Mater. 2021;4:2886–2906. doi: 10.1021/acsabm.0c01630. [DOI] [PubMed] [Google Scholar]
- 96.Mahmood A., Patel D., Hickson B., DesRochers J., Hu X. Recent progress in biopolymer-based hydrogel materials for biomedical applications. Int. J. Mol. Sci. 2022;23 doi: 10.3390/ijms23031415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Born L.J., McLoughlin S.T., Dutta D., Mahadik B., Jia X., Fisher J.P., Jay S.M. Sustained released of bioactive mesenchymal stromal cell-derived extracellular vesicles from 3D-printed gelatin methacrylate hydrogels. J. Biomed. Mater. Res. 2022;110:1190–1198. doi: 10.1002/jbm.a.37362. [DOI] [PubMed] [Google Scholar]
- 98.Van Den Bulcke A.I., Bogdanov B., De Rooze N., Schacht E.H., Cornelissen M., Berghmans H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules. 2000;1:31–38. doi: 10.1021/bm990017d. [DOI] [PubMed] [Google Scholar]
- 99.Kurian A.G., Singh R.K., Patel K.D., Lee J.H., Kim H.W. Multifunctional GelMA platforms with nanomaterials for advanced tissue therapeutics. Bioact. Mater. 2022;8:267–295. doi: 10.1016/j.bioactmat.2021.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lujerdean C., Baci G.M., Cucu A.A., Dezmirean D.S. The contribution of silk fibroin in biomedical engineering. Insects. 2022;13 doi: 10.3390/insects13030286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sun M., Li Q., Yu H., Cheng J., Wu N., Shi W., Zhao F., Shao Z., Meng Q., Chen H., Hu X., Ao Y. Cryo-self-assembled silk fibroin sponge as a biodegradable platform for enzyme-responsive delivery of exosomes. Bioact. Mater. 2022;8:505–514. doi: 10.1016/j.bioactmat.2021.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li H., Yin D., Li W., Tang Q., Zou L., Peng Q. vol. 199. 2021. (Polydopamine-based Nanomaterials and Their Potentials in Advanced Drug Delivery and Therapy). [DOI] [PubMed] [Google Scholar]
- 103.Zhang F.X., Liu P., Ding W., Meng Q.B., Su D.H., Zhang Q.C., Lian R.X., Yu B.Q., Zhao M.D., Dong J., Li Y.L., Jiang L.B. Injectable Mussel-Inspired highly adhesive hydrogel with exosomes for endogenous cell recruitment and cartilage defect regeneration. Biomaterials. 2021;278 doi: 10.1016/j.biomaterials.2021.121169. [DOI] [PubMed] [Google Scholar]
- 104.Sanchez-Cid P., Jimenez-Rosado M., Romero A., Perez-Puyana V. Novel trends in hydrogel development for biomedical applications: a review. Polymers. 2022;14 doi: 10.3390/polym14153023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sabel-Grau T., Tyushina A., Babalik C., Lensen M.C. UV-VIS curable PEG hydrogels for biomedical applications with multifunctionality. Gels. 2022:8. doi: 10.3390/gels8030164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lin C.C., Anseth K.S. PEG hydrogels for the controlled release of biomolecules in regenerative medicine. Pharm. Res. (N. Y.) 2009;26:631–643. doi: 10.1007/s11095-008-9801-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dong R., Pang Y., Su Y., Zhu X. Supramolecular hydrogels: synthesis, properties and their biomedical applications. Biomater. Sci. 2015;3:937–954. doi: 10.1039/c4bm00448e. [DOI] [PubMed] [Google Scholar]
- 108.Stocke N.A., Zhang X., Hilt J.Z., DeRouchey J.E. Transport in PEG-based hydrogels: role of water content at synthesis and crosslinker molecular weight. Macromol. Chem. Phys. 2017;218 [Google Scholar]
- 109.Mol E.A., Lei Z., Roefs M.T., Bakker M.H., Goumans M.J., Doevendans P.A., Dankers P.Y.W., Vader P., Sluijter J.P.G. Injectable supramolecular ureidopyrimidinone hydrogels provide sustained release of extracellular vesicle therapeutics. Adv.x Healthc. Mater. 2019;8 doi: 10.1002/adhm.201900847. [DOI] [PubMed] [Google Scholar]
- 110.Collins M.N., Ren G., Young K., Pina S., Reis R.L., Oliveira J.M. Scaffold fabrication technologies and structure/function properties in bone tissue engineering. Adv. Funct. Mater. 2021;31 [Google Scholar]
- 111.Sun A.X., Lin H., Fritch M.R., Shen H., Alexander P.G., DeHart M., Tuan R.S. Chondrogenesis of human bone marrow mesenchymal stem cells in 3-dimensional, photocrosslinked hydrogel constructs: effect of cell seeding density and material stiffness. Acta Biomater. 2017;58:302–311. doi: 10.1016/j.actbio.2017.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bakaic E., Smeets N.M.B., Hoare T. Injectable hydrogels based on poly(ethylene glycol) and derivatives as functional biomaterials. RSC Adv. 2015;5:35469–35486. [Google Scholar]
- 113.Kutikov A.B., Song J. Biodegradable PEG-based amphiphilic block copolymers for tissue engineering applications. ACS Biomater. Sci. Eng. 2015;1:463–480. doi: 10.1021/acsbiomaterials.5b00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Basu A., Kunduru K.R., Doppalapudi S., Domb A.J., Khan W. Poly(lactic acid) based hydrogels. Adv. Drug Deliv. Rev. 2016;107:192–205. doi: 10.1016/j.addr.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 115.Swanson W.B., Zhang Z., Xiu K., Gong T., Eberle M., Wang Z., Ma P.X. Scaffolds with controlled release of pro-mineralization exosomes to promote craniofacial bone healing without cell transplantation. Acta Biomater. 2020;118:215–232. doi: 10.1016/j.actbio.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.La Manna S., Di Natale C., Onesto V., Marasco D. Self-assembling peptides: from design to biomedical applications. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms222312662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu J., Zhang L., Yang Z., Zhao X. Controlled release of paclitaxel from a self-assembling peptide hydrogel formed in situ and antitumor study in vitro. Int. J. Nanomed. 2011;6:2143–2153. doi: 10.2147/IJN.S24038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Um S.H., Lee J.B., Park N., Kwon S.Y., Umbach C.C., Luo D. Enzyme-catalysed Assembly of DNA Hydrogel. Nat Mater. 2006;5(10):797–801. doi: 10.1038/nmat1741. [DOI] [PubMed] [Google Scholar]
- 119.Chen C., Zhou J., Chen J., Liu H. Macromol Biosci; 2022. Advances in DNA Supramolecular Hydrogels for Tissue Engineering. [DOI] [PubMed] [Google Scholar]
- 120.Wang Z., Chen R., Yang S., Li S., Gao Z. Design and application of stimuli-responsive DNA hydrogels: a review. Mater. Today Bio. 2022;16 doi: 10.1016/j.mtbio.2022.100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Enciso A.E., Fu L., Russell A.J., Matyjaszewski K. A breathing atom-transfer radical polymerization: fully oxygen-tolerant polymerization inspired by aerobic respiration of cells. Angew Chem. Int. Ed. Engl. 2018;57:933–936. doi: 10.1002/anie.201711105. [DOI] [PubMed] [Google Scholar]
- 122.Matyjaszewski K. Advanced materials by atom transfer radical polymerization. Adv. Mater. 2018;30 doi: 10.1002/adma.201706441. [DOI] [PubMed] [Google Scholar]
- 123.Siegwart D.J., Oh J.K., Matyjaszewski K. ATRP in the design of functional materials for biomedical applications. Prog. Polym. Sci. 2012;37:18–37. doi: 10.1016/j.progpolymsci.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yerneni S.S., Lathwal S., Cuthbert J., Kapil K., Szczepaniak G., Jeong J., Das S.R., Campbell P.G., Matyjaszewski K. Controlled release of exosomes using atom transfer radical polymerization-based hydrogels. Biomacromolecules. 2022;23:1713–1722. doi: 10.1021/acs.biomac.1c01636. [DOI] [PubMed] [Google Scholar]
- 125.Brown M., Li J., Moraes C., Tabrizian M., Li-Jessen N.Y.K. Decellularized extracellular matrix: new promising and challenging biomaterials for regenerative medicine. Biomaterials. 2022;289 doi: 10.1016/j.biomaterials.2022.121786. [DOI] [PubMed] [Google Scholar]
- 126.Hernandez M.J., Gaetani R., Pieters V.M., Ng N.W., Chang A.E., Martin T.R., van Ingen E., Mol E.A., Sluijter J.P.G., Christman K.L. Decellularized extracellular matrix hydrogels as a delivery platform for MicroRNA and extracellular vesicle therapeutics. Adv. Ther. 2018;1 doi: 10.1002/adtp.201800032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Faust A., Kandakatla A., van der Merwe Y., Ren T., Huleihel L., Hussey G., Naranjo J.D., Johnson S., Badylak S., Steketee M. Urinary bladder extracellular matrix hydrogels and matrix-bound vesicles differentially regulate central nervous system neuron viability and axon growth and branching. J. Biomater. Appl. 2017;31:1277–1295. doi: 10.1177/0885328217698062. [DOI] [PubMed] [Google Scholar]
- 128.Katyal P., Mahmoudinobar F., Montclare J.K. Recent trends in peptide and protein-based hydrogels. Curr. Opin. Struct. Biol. 2020;63:97–105. doi: 10.1016/j.sbi.2020.04.007. [DOI] [PubMed] [Google Scholar]
- 129.Zhang J., Yang Y., Chen Y., Liu X., Guo S., Zhu L., Wang Y. An in situ phototriggered-imine-crosslink composite hydrogel for bone defect repair. J. Mater. Chem. B. 2016;4:973–981. doi: 10.1039/c5tb02377g. [DOI] [PubMed] [Google Scholar]
- 130.Oh J.K., Drumright R., Siegwart D.J., Matyjaszewski K. The development of microgels/nanogels for drug delivery applications. Prog. Polym. Sci. 2008;33:448–477. [Google Scholar]
- 131.Li J., Mooney D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016;1 doi: 10.1038/natrevmats.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yang L., Wang X., Yu Y., Shang L., Xu W., Zhao Y. Nano Research; 2022. Bio-inspired Dual-Adhesive Particles from Microfluidic Electrospray for Bone Regeneration. [Google Scholar]
- 133.Yang L., Sun L., Zhang H., Bian F., Zhao Y. Ice-inspired lubricated drug delivery particles from microfluidic electrospray for osteoarthritis treatment. ACS Nano. 2021;15:20600–20606. doi: 10.1021/acsnano.1c09325. [DOI] [PubMed] [Google Scholar]
- 134.Yang L., Liu Y., Sun L., Zhao C., Chen G., Zhao Y. Biomass microcapsules with stem cell encapsulation for bone repair. Nano-Micro Lett. 2021;14:4. doi: 10.1007/s40820-021-00747-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lei L., Zhu Y., Qin X., Chai S., Liu G., Su W., Lv Q., Li D. Magnetic biohybrid microspheres for protein purification and chronic wound healing in diabetic mice. Chem. Eng. J. 2021;425 [Google Scholar]
- 136.Lei L., Wang X., Zhu Y., Su W., Lv Q., Li D. Antimicrobial hydrogel microspheres for protein capture and wound healing. Mater. Des. 2022:215. [Google Scholar]
- 137.Lei L., Lv Q., Jin Y., An H., Shi Z., Hu G., Yang Y., Wang X., Yang L. Angiogenic microspheres for the treatment of a thin endometrium. ACS Biomater. Sci. Eng. 2021;7:4914–4920. doi: 10.1021/acsbiomaterials.1c00615. [DOI] [PubMed] [Google Scholar]
- 138.Gan J., Sun L., Chen G., Ma W., Zhao Y., Sun L. Mesenchymal stem cell exosomes encapsulated oral microcapsules for acute colitis treatment. Adv.x Healthc. Mater. 2022;11 doi: 10.1002/adhm.202201105. [DOI] [PubMed] [Google Scholar]
- 139.Chen Y., Huang J., Chen R., Yang L., Wang J., Liu B., Du L., Yi Y., Jia J., Xu Y., Chen Q., Ngondi D.G., Miao Y., Hu Z. Sustained release of dermal papilla-derived extracellular vesicles from injectable microgel promotes hair growth. Theranostics. 2020;10:1454–1478. doi: 10.7150/thno.39566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cheng P., Cheng L., Han H., Li J., Ma C., Huang H., Zhou J., Feng J., Huang Y., Lv Y., Huang H., Wang Y., Hou L., Chen Y., Li G. A pH/H(2) O(2)/MMP9 time-response gel system with sparc(high) tregs derived extracellular vesicles promote recovery after acute myocardial infarction. Adv.x Healthc. Mater. 2022;11 doi: 10.1002/adhm.202200971. [DOI] [PubMed] [Google Scholar]
- 141.P. Bertsch, M. Diba, D.J. Mooney, S.C.G. Leeuwenburgh, Self-healing injectable hydrogels for tissue regeneration, Chem. Rev. (2022).[published online ahead of print, 2022 Aug 5]. [DOI] [PMC free article] [PubMed]
- 142.Mondal P., Chatterjee K. Injectable and self-healing double network polysaccharide hydrogel as a minimally-invasive delivery platform. Carbohydr. Polym. 2022;291 doi: 10.1016/j.carbpol.2022.119585. [DOI] [PubMed] [Google Scholar]
- 143.Guvendiren M., Lu H.D., Burdick J.A. Shear-thinning hydrogels for biomedical applications. Soft Matter. 2012;8:260–272. [Google Scholar]
- 144.Lavrador P., Esteves M.R., Gaspar V.M., Mano J.F. Stimuli-responsive nanocomposite hydrogels for biomedical applications. Adv. Funct. Mater. 2020;31 [Google Scholar]
- 145.Yu J., Zhang Y., Ye Y., DiSanto R., Sun W., Ranson D., Ligler F.S., Buse J.B., Gu Z. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc. Natl. Acad. Sci. U. S. A. 2015;112:8260–8265. doi: 10.1073/pnas.1505405112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Walker B.W., Lara R.P., Mogadam E., Yu C.H., Kimball W., Annabi N. Rational design of microfabricated electroconductive hydrogels for biomedical applications. Prog. Polym. Sci. 2019;92:135–157. doi: 10.1016/j.progpolymsci.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Alamdari S.G., Alibakhshi A., de la Guardia M., Baradaran B., Mohammadzadeh R., Amini M., Kesharwani P., Mokhtarzadeh A., Oroojalian F., Sahebkar A. Conductive and semiconductive nanocomposite-based hydrogels for cardiac tissue engineering. Adv.x Healthc. Mater. 2022;11 doi: 10.1002/adhm.202200526. [DOI] [PubMed] [Google Scholar]
- 148.Mardpour S., Ghanian M.H., Sadeghi-Abandansari H., Mardpour S., Nazari A., Shekari F., Baharvand H. Hydrogel-mediated sustained systemic delivery of mesenchymal stem cell-derived extracellular vesicles improves hepatic regeneration in chronic liver failure. ACS Appl. Mater. Interfaces. 2019;11:37421–37433. doi: 10.1021/acsami.9b10126. [DOI] [PubMed] [Google Scholar]
- 149.Shirakura T., Kelson T.J., Ray A., Malyarenko A.E., Kopelman R. Hydrogel nanoparticles with thermally controlled drug release. ACS Macro Lett. 2014;3:602–606. doi: 10.1021/mz500231e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Obaidat A.A., Park K. Characterization of protein release through glucose-sensitive hydrogel membranes. Biomaterials. 1997;18:801–806. doi: 10.1016/s0142-9612(96)00198-6. [DOI] [PubMed] [Google Scholar]
- 151.Zhang S., Bellinger A.M., Glettig D.L., Barman R., Lee Y.A., Zhu J., Cleveland C., Montgomery V.A., Gu L., Nash L.D., Maitland D.J., Langer R., Traverso G. A pH-responsive supramolecular polymer gel as an enteric elastomer for use in gastric devices. Nat. Mater. 2015;14:1065–1071. doi: 10.1038/nmat4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Thomas V., Yallapu M.M., Sreedhar B., Bajpai S.K. Breathing-in/breathing-out approach to preparing nanosilver-loaded hydrogels: highly efficient antibacterial nanocomposites. J. Appl. Polym. Sci. 2008;111:934–944. [Google Scholar]
- 153.Amsden B. Solute diffusion within hydrogels. Mechanisms and models. Macromolecules. 1998;31:8382–8395. [Google Scholar]
- 154.Nikravesh N., Davies O.G., Azoidis I., Moakes R.J.A., Marani L., Turner M., Kearney C.J., Eisenstein N.M., Grover L.M., Cox S.C. Physical structuring of injectable polymeric systems to controllably deliver nanosized extracellular vesicles. Adv.x Healthc. Mater. 2019;8 doi: 10.1002/adhm.201801604. [DOI] [PubMed] [Google Scholar]
- 155.Huang C.C., Kang M., Shirazi S., Lu Y., Cooper L.F., Gajendrareddy P., Ravindran S. 3D Encapsulation and tethering of functionally engineered extracellular vesicles to hydrogels. Acta Biomater. 2021;126:199–210. doi: 10.1016/j.actbio.2021.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ma S., Wu J., Hu H., Mu Y., Zhang L., Zhao Y., Bian X., Jing W., Wei P., Zhao B., Deng J., Liu Z. Novel fusion peptides deliver exosomes to modify injectable thermo-sensitive hydrogels for bone regeneration. Mater. Today Bio. 2022;13 doi: 10.1016/j.mtbio.2021.100195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gao X., Ran N., Dong X., Zuo B., Yang R., Zhou Q., Moulton H.M., Seow Y., Yin H. Anchor peptide captures, targets, and loads exosomes of diverse origins for diagnostics and therapy. Sci. Transl. Med. 2018;10 doi: 10.1126/scitranslmed.aat0195. [DOI] [PubMed] [Google Scholar]
- 158.Li L., Zhang Y., Mu J., Chen J., Zhang C., Cao H., Gao J. Transplantation of human mesenchymal stem-cell-derived exosomes immobilized in an adhesive hydrogel for effective treatment of spinal cord injury. Nano Lett. 2020;20:4298–4305. doi: 10.1021/acs.nanolett.0c00929. [DOI] [PubMed] [Google Scholar]
- 159.Zhang C., Shang Y., Chen X., Midgley A.C., Wang Z., Zhu D., Wu J., Chen P., Wu L., Wang X., Zhang K., Wang H., Kong D., Yang Z., Li Z., Chen X. Supramolecular nanofibers containing arginine-glycine-aspartate (RGD) peptides boost therapeutic efficacy of extracellular vesicles in kidney repair. ACS Nano. 2020;14:12133–12147. doi: 10.1021/acsnano.0c05681. [DOI] [PubMed] [Google Scholar]
- 160.Man K., Brunet M.Y., Federici A.S., Hoey D.A., Cox S.C. An ECM-mimetic hydrogel to promote the therapeutic efficacy of osteoblast-derived extracellular vesicles for bone regeneration. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.829969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dawson J.I., Kanczler J.M., Yang X.B., Attard G.S., Oreffo R.O. Clay gels for the delivery of regenerative microenvironments. Adv. Mater. 2011;23:3304–3308. doi: 10.1002/adma.201100968. [DOI] [PubMed] [Google Scholar]
- 162.Man K., Barroso I.A., Brunet M.Y., Peacock B., Federici A.S., Hoey D.A., Cox S.C. Controlled release of epigenetically-enhanced extracellular vesicles from a GelMA/nanoclay composite hydrogel to promote bone repair. Int. J. Mol. Sci. 2022;23 doi: 10.3390/ijms23020832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hu H., Dong L., Bu Z., Shen Y., Luo J., Zhang H., Zhao S., Lv F., Liu Z. miR-23a-3p-abundant small extracellular vesicles released from Gelma/nanoclay hydrogel for cartilage regeneration. J. Extracell. Vesicles. 2020;9 doi: 10.1080/20013078.2020.1778883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Paul A., Manoharan V., Krafft D., Assmann A., Uquillas J.A., Shin S.R., Hasan A., Hussain M.A., Memic A., Gaharwar A.K., Khademhosseini A. Nanoengineered biomimetic hydrogels for guiding human stem cell osteogenesis in three dimensional microenvironments. J. Mater. Chem. B. 2016;4:3544–3554. doi: 10.1039/C5TB02745D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Henriques-Antunes H., Cardoso R.M.S., Zonari A., Correia J., Leal E.C., Jimenez-Balsa A., Lino M.M., Barradas A., Kostic I., Gomes C., Karp J.M., Carvalho E., Ferreira L. The kinetics of small extracellular vesicle delivery impacts skin tissue regeneration. ACS Nano. 2019;13:8694–8707. doi: 10.1021/acsnano.9b00376. [DOI] [PubMed] [Google Scholar]
- 166.Eming S.A., Martin P., Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci. Transl. Med. 2014;6 doi: 10.1126/scitranslmed.3009337. 265sr266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhou Y., Zhang X.L., Lu S.T., Zhang N.Y., Zhang H.J., Zhang J., Zhang J. Human adipose-derived mesenchymal stem cells-derived exosomes encapsulated in pluronic F127 hydrogel promote wound healing and regeneration. Stem Cell Res. Ther. 2022;13:407. doi: 10.1186/s13287-022-02980-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jiang T., Liu S., Wu Z., Li Q., Ren S., Chen J., Xu X., Wang C., Lu C., Yang X., Chen Z. ADSC-exo@MMP-PEG smart hydrogel promotes diabetic wound healing by optimizing cellular functions and relieving oxidative stress. Mater. Today Bio. 2022;16 doi: 10.1016/j.mtbio.2022.100365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Qin Y., Wang L., Gao Z., Chen G., Zhang C. Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo. Sci. Rep. 2016;6 doi: 10.1038/srep21961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yang S., Jiang H., Qian M., Ji G., Wei Y., He J., Tian H., Zhao Q. MSC-derived sEV-loaded hyaluronan hydrogel promotes scarless skin healing by immunomodulation in a large skin wound model. Biomed. Mater. 2022;17 doi: 10.1088/1748-605X/ac68bc. [DOI] [PubMed] [Google Scholar]
- 171.Khayambashi P., Iyer J., Pillai S., Upadhyay A., Zhang Y., Tran S.D. Hydrogel encapsulation of mesenchymal stem cells and their derived exosomes for tissue engineering. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22020684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wang C., Wang M., Xu T., Zhang X., Lin C., Gao W., Xu H., Lei B., Mao C. Engineering bioactive self-healing antibacterial exosomes hydrogel for promoting chronic diabetic wound healing and complete skin regeneration. Theranostics. 2019;9:65–76. doi: 10.7150/thno.29766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Brumberg V., Astrelina T., Malivanova T., Samoilov A. Modern wound dressings: hydrogel dressings. Biomedicines. 2021;9 doi: 10.3390/biomedicines9091235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Vowden K., Vowden P. Wound dressings: principles and practice. Surgery. 2017;35:489–494. [Google Scholar]
- 175.Zhao D., Yu Z., Li Y., Wang Y., Li Q., Han D. GelMA combined with sustained release of HUVECs derived exosomes for promoting cutaneous wound healing and facilitating skin regeneration. J. Mol. Histol. 2020;51:251–263. doi: 10.1007/s10735-020-09877-6. [DOI] [PubMed] [Google Scholar]
- 176.Shi Q., Qian Z., Liu D., Sun J., Wang X., Liu H., Xu J., Guo X. GMSC-derived exosomes combined with a chitosan/silk hydrogel sponge accelerates wound healing in a diabetic rat skin defect model. Front. Physiol. 2017;8:904. doi: 10.3389/fphys.2017.00904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chen P., Zheng L., Wang Y., Tao M., Xie Z., Xia C., Gu C., Chen J., Qiu P., Mei S., Ning L., Shi Y., Fang C., Fan S., Lin X. Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics. 2019;9:2439–2459. doi: 10.7150/thno.31017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Guan G., Zhang Q., Jiang Z., Liu J., Wan J., Jin P., Lv Q. Multifunctional silk fibroin methacryloyl microneedle for diabetic wound healing. Small. 2022 doi: 10.1002/smll.202203064. [DOI] [PubMed] [Google Scholar]
- 179.Pape A.C., Bakker M.H., Tseng C.C., Bastings M.M., Koudstaal S., Agostoni P., Chamuleau S.A., Dankers P.Y. An injectable and drug-loaded supramolecular hydrogel for local catheter injection into the pig heart. JoVE. 2015 doi: 10.3791/52450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cao Y., Xu Y., Chen C., Xie H., Lu H., Hu J. Local delivery of USC-derived exosomes harboring ANGPTL3 enhances spinal cord functional recovery after injury by promoting angiogenesis. Stem Cell Res. Ther. 2021;12:20. doi: 10.1186/s13287-020-02078-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Halevi O., Chen J., Thangavel G., Morris S.A., Ben Uliel T., Tischler Y.R., Lee P.S., Magdassi S. Synthesis through 3D printing: formation of 3D coordination polymers. RSC Adv. 2020;10:14812–14817. doi: 10.1039/d0ra01887b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Puzar Dominkus P., Stenovec M., Sitar S., Lasic E., Zorec R., Plemenitas A., Zagar E., Kreft M., Lenassi M. PKH26 labeling of extracellular vesicles: characterization and cellular internalization of contaminating PKH26 nanoparticles. Biochim. Biophys. Acta Biomembr. 2018;1860:1350–1361. doi: 10.1016/j.bbamem.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 183.Monopoli M.P., Zendrini A., Wu D., Cheung S., Sampedro G., Ffrench B., Nolan J., Piskareva O., Stalings R.L., Ducoli S., Bergese P., O'Shea D.F. Endogenous exosome labelling with an amphiphilic NIR-fluorescent probe. Chem. Commun. 2018;54:7219–7222. doi: 10.1039/c8cc02135j. [DOI] [PubMed] [Google Scholar]
- 184.Zhao J.Y., Chen G., Gu Y.P., Cui R., Zhang Z.L., Yu Z.L., Tang B., Zhao Y.F., Pang D.W. Ultrasmall magnetically engineered Ag2Se quantum dots for instant efficient labeling and whole-body high-resolution multimodal real-time tracking of cell-derived microvesicles. J. Am. Chem. Soc. 2016;138:1893–1903. doi: 10.1021/jacs.5b10340. [DOI] [PubMed] [Google Scholar]
- 185.Lazaro-Ibanez E., Faruqu F.N., Saleh A.F., Silva A.M., Tzu-Wen Wang J., Rak J., Al-Jamal K.T., Dekker N. Selection of fluorescent, bioluminescent, and radioactive tracers to accurately reflect extracellular vesicle biodistribution in vivo. ACS Nano. 2021;15:3212–3227. doi: 10.1021/acsnano.0c09873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Li Q., Gong S., Yao W., Yang Z., Wang R., Yu Z., Wei M. Exosome loaded genipin crosslinked hydrogel facilitates full thickness cutaneous wound healing in rat animal model. Drug Deliv. 2021;28:884–893. doi: 10.1080/10717544.2021.1912210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Broughton G., 2nd, Janis J.E., Attinger C.E. Wound healing: an overview. Plast. Reconstr. Surg. 2006;117:1e. doi: 10.1097/01.prs.0000222562.60260.f9. S-32e-S. [DOI] [PubMed] [Google Scholar]
- 188.An Y., Lin S., Tan X., Zhu S., Nie F., Zhen Y., Gu L., Zhang C., Wang B., Wei W., Li D., Wu J. Exosomes from adipose-derived stem cells and application to skin wound healing. Cell Prolif. 2021;54 doi: 10.1111/cpr.12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.M.D. Hade, C.N. Suire, J. Mossell, Z. Suo, Extracellular vesicles: emerging frontiers in wound healing, Med. Res. Rev. (2022); 42(6):2102-2125. [DOI] [PubMed]
- 190.Frost J., Galdeano C., Soares P., Gadd M.S., Grzes K.M., Ellis L., Epemolu O., Shimamura S., Bantscheff M., Grandi P., Read K.D., Cantrell D.A., Rocha S., Ciulli A. Potent and selective chemical probe of hypoxic signalling downstream of HIF-alpha hydroxylation via VHL inhibition. Nat. Commun. 2016;7 doi: 10.1038/ncomms13312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wang C., Liang C., Wang R., Yao X., Guo P., Yuan W., Liu Y., Song Y., Li Z., Xie X. The fabrication of a highly efficient self-healing hydrogel from natural biopolymers loaded with exosomes for the synergistic promotion of severe wound healing. Biomater. Sci. 2019;8:313–324. doi: 10.1039/c9bm01207a. [DOI] [PubMed] [Google Scholar]
- 192.Xu N., Wang L., Guan J., Tang C., He N., Zhang W., Fu S. Wound healing effects of a Curcuma zedoaria polysaccharide with platelet-rich plasma exosomes assembled on chitosan/silk hydrogel sponge in a diabetic rat model. Int. J. Biol. Macromol. 2018;117:102–107. doi: 10.1016/j.ijbiomac.2018.05.066. [DOI] [PubMed] [Google Scholar]
- 193.Xiong Y., Chen L., Liu P., Yu T., Lin C., Yan C., Hu Y., Zhou W., Sun Y., Panayi A.C., Cao F., Xue H., Hu L., Lin Z., Xie X., Xiao X., Feng Q., Mi B., Liu G. All-in-One: multifunctional hydrogel accelerates oxidative diabetic wound healing through timed-release of exosome and fibroblast growth factor. Small. 2022;18 doi: 10.1002/smll.202104229. [DOI] [PubMed] [Google Scholar]
- 194.Zhang Y., Hao Z., Wang P., Xia Y., Wu J., Xia D., Fang S., Xu S. Exosomes from human umbilical cord mesenchymal stem cells enhance fracture healing through HIF-1alpha-mediated promotion of angiogenesis in a rat model of stabilized fracture. Cell Prolif. 2019;52 doi: 10.1111/cpr.12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Fan J., Lee C.S., Kim S., Chen C., Aghaloo T., Lee M. Generation of small RNA-modulated exosome mimetics for bone regeneration. ACS Nano. 2020;14:11973–11984. doi: 10.1021/acsnano.0c05122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhao Y., Zhu Z.S., Guan J., Wu S.J. Processing, mechanical properties and bio-applications of silk fibroin-based high-strength hydrogels. Acta Biomater. 2021;125:57–71. doi: 10.1016/j.actbio.2021.02.018. [DOI] [PubMed] [Google Scholar]
- 197.Wu D., Qin H., Wang Z., Yu M., Liu Z., Peng H., Liang L., Zhang C., Wei X. Bone mesenchymal stem cell-derived sEV-encapsulated thermosensitive hydrogels accelerate osteogenesis and angiogenesis by release of exosomal miR-21. Front. Bioeng. Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.829136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wang L., Wang J., Zhou X., Sun J., Zhu B., Duan C., Chen P., Guo X., Zhang T., Guo H. A new self-healing hydrogel containing hucMSC-derived exosomes promotes bone regeneration. Front. Bioeng. Biotechnol. 2020;8 doi: 10.3389/fbioe.2020.564731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zhang Y., Xie Y., Hao Z., Zhou P., Wang P., Fang S., Li L., Xu S., Xia Y. Umbilical mesenchymal stem cell-derived exosome-encapsulated hydrogels accelerate bone repair by enhancing angiogenesis. ACS Appl. Mater. Interfaces. 2021;13:18472–18487. doi: 10.1021/acsami.0c22671. [DOI] [PubMed] [Google Scholar]
- 200.Zhang J., Liu X., Li H., Chen C., Hu B., Niu X., Li Q., Zhao B., Xie Z., Wang Y. Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway. Stem Cell Res. Ther. 2016;7:136. doi: 10.1186/s13287-016-0391-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kang Y., Xu C., Meng L., Dong X., Qi M., Jiang D. Exosome-functionalized magnesium-organic framework-based scaffolds with osteogenic, angiogenic and anti-inflammatory properties for accelerated bone regeneration. Bioact. Mater. 2022;18:26–41. doi: 10.1016/j.bioactmat.2022.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zhang B., Huang J., Liu J., Lin F., Ding Z., Xu J. Injectable composite hydrogel promotes osteogenesis and angiogenesis in spinal fusion by optimizing the bone marrow mesenchymal stem cell microenvironment and exosomes secretion. Mater. Sci. Eng. C Mater. Biol. Appl. 2021;123 doi: 10.1016/j.msec.2020.111782. [DOI] [PubMed] [Google Scholar]
- 203.Zhang S., Chuah S.J., Lai R.C., Hui J.H.P., Lim S.K., Toh W.S. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 2018;156:16–27. doi: 10.1016/j.biomaterials.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 204.Vilela C.A., Correia C., Oliveira J.M., Sousa R.A., Espregueira-Mendes J., Reis R.L. Cartilage repair using hydrogels: a critical review of in vivo experimental designs. ACS Biomater. Sci. Eng. 2015;1:726–739. doi: 10.1021/acsbiomaterials.5b00245. [DOI] [PubMed] [Google Scholar]
- 205.Guan P., Liu C., Xie D., Mao S., Ji Y., Lin Y., Chen Z., Wang Q., Fan L., Sun Y. Exosome-loaded extracellular matrix-mimic hydrogel with anti-inflammatory property Facilitates/promotes growth plate injury repair. Bioact. Mater. 2022;10:145–158. doi: 10.1016/j.bioactmat.2021.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yang Y., Zhang J., Liu Z., Lin Q., Liu X., Bao C., Wang Y., Zhu L. Tissue-integratable and biocompatible photogelation by the imine crosslinking reaction. Adv. Mater. 2016;28:2724–2730. doi: 10.1002/adma.201505336. [DOI] [PubMed] [Google Scholar]
- 207.Nikhil A., Kumar A. Evaluating potential of tissue-engineered cryogels and chondrocyte derived exosomes in articular cartilage repair. Biotechnol. Bioeng. 2022;119:605–625. doi: 10.1002/bit.27982. [DOI] [PubMed] [Google Scholar]
- 208.Sutton M.G., Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101:2981–2988. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 209.Khan M., Nickoloff E., Abramova T., Johnson J., Verma S.K., Krishnamurthy P., Mackie A.R., Vaughan E., Garikipati V.N., Benedict C., Ramirez V., Lambers E., Ito A., Gao E., Misener S., Luongo T., Elrod J., Qin G., Houser S.R., Koch W.J., Kishore R. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ. Res. 2015;117:52–64. doi: 10.1161/CIRCRESAHA.117.305990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Huang K., Hu S., Cheng K. A new era of cardiac cell therapy: opportunities and challenges. Adv.x Healthc. Mater. 2019;8 doi: 10.1002/adhm.201801011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Gallet R., Dawkins J., Valle J., Simsolo E., de Couto G., Middleton R., Tseliou E., Luthringer D., Kreke M., Smith R.R., Marban L., Ghaleh B., Marban E. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur. Heart J. 2017;38:201–211. doi: 10.1093/eurheartj/ehw240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Chen C.W., Wang L.L., Zaman S., Gordon J., Arisi M.F., Venkataraman C.M., Chung J.J., Hung G., Gaffey A.C., Spruce L.A., Fazelinia H., Gorman R.C., Seeholzer S.H., Burdick J.A., Atluri P. Sustained release of endothelial progenitor cell-derived extracellular vesicles from shear-thinning hydrogels improves angiogenesis and promotes function after myocardial infarction. Cardiovasc. Res. 2018;114:1029–1040. doi: 10.1093/cvr/cvy067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lv K., Li Q., Zhang L., Wang Y., Zhong Z., Zhao J., Lin X., Wang J., Zhu K., Xiao C., Ke C., Zhong S., Wu X., Chen J., Yu H., Zhu W., Li X., Wang B., Tang R., Wang J., Huang J., Hu X. Incorporation of small extracellular vesicles in sodium alginate hydrogel as a novel therapeutic strategy for myocardial infarction. Theranostics. 2019;9:7403–7416. doi: 10.7150/thno.32637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Waters R., Alam P., Pacelli S., Chakravarti A.R., Ahmed R.P.H., Paul A. Stem cell-inspired secretome-rich injectable hydrogel to repair injured cardiac tissue. Acta Biomater. 2018;69:95–106. doi: 10.1016/j.actbio.2017.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Mihic A., Cui Z., Wu J., Vlacic G., Miyagi Y., Li S.H., Lu S., Sung H.W., Weisel R.D., Li R.K. A conductive polymer hydrogel supports cell electrical signaling and improves cardiac function after implantation into myocardial infarct. Circulation. 2015;132:772–784. doi: 10.1161/CIRCULATIONAHA.114.014937. [DOI] [PubMed] [Google Scholar]
- 216.Zou Y., Li L., Li Y., Chen S., Xie X., Jin X., Wang X., Ma C., Fan G., Wang W. Restoring cardiac functions after myocardial infarction-ischemia/reperfusion via an exosome anchoring conductive hydrogel. ACS Appl. Mater. Interfaces. 2021;13:56892–56908. doi: 10.1021/acsami.1c16481. [DOI] [PubMed] [Google Scholar]
- 217.Zhu D., Li Z., Huang K., Caranasos T.G., Rossi J.S., Cheng K. Minimally invasive delivery of therapeutic agents by hydrogel injection into the pericardial cavity for cardiac repair. Nat. Commun. 2021;12:1412. doi: 10.1038/s41467-021-21682-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Poplawski G.H.D., Kawaguchi R., Van Niekerk E., Lu P., Mehta N., Canete P., Lie R., Dragatsis I., Meves J.M., Zheng B., Coppola G., Tuszynski M.H. Injured adult neurons regress to an embryonic transcriptional growth state. Nature. 2020;581:77–82. doi: 10.1038/s41586-020-2200-5. [DOI] [PubMed] [Google Scholar]
- 219.Tsintou M., Dalamagkas K., Moore T.L., Rathi Y., Kubicki M., Rosene D.L., Makris N. The use of hydrogel-delivered extracellular vesicles in recovery of motor function in stroke: a testable experimental hypothesis for clinical translation including behavioral and neuroimaging assessment approaches. Neural. Regen. Res. 2021;16:605–613. doi: 10.4103/1673-5374.295269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Cheng J., Chen Z., Liu C., Zhong M., Wang S., Sun Y., Wen H., Shu T. Bone mesenchymal stem cell-derived exosome-loaded injectable hydrogel for minimally invasive treatment of spinal cord injury. Nanomedicine. 2021;16:1567–1579. doi: 10.2217/nnm-2021-0025. [DOI] [PubMed] [Google Scholar]
- 221.Liu Z., Tong H., Li J., Wang L., Fan X., Song H., Yang M., Wang H., Jiang X., Zhou X., Yuan H., Wang Y. Low-stiffness hydrogels promote peripheral nerve regeneration through the rapid release of exosomes. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.922570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Luo Z., Peng W., Xu Y., Xie Y., Liu Y., Lu H., Cao Y., Hu J. Exosomal OTULIN from M2 macrophages promotes the recovery of spinal cord injuries via stimulating Wnt/beta-catenin pathway-mediated vascular regeneration. Acta Biomater. 2021;136:519–532. doi: 10.1016/j.actbio.2021.09.026. [DOI] [PubMed] [Google Scholar]
- 223.Mu J., Li L., Wu J., Huang T., Zhang Y., Cao J., Ma T., Chen J., Zhang C., Zhang X., Lu T., Kong X., Sun J., Gao J. Hypoxia-stimulated mesenchymal stem cell-derived exosomes loaded by adhesive hydrogel for effective angiogenic treatment of spinal cord injury. Biomater. Sci. 2022;10:1803–1811. doi: 10.1039/d1bm01722e. [DOI] [PubMed] [Google Scholar]
- 224.Han M., Yang H., Lu X., Li Y., Liu Z., Li F., Shang Z., Wang X., Li X., Li J., Liu H., Xin T. Three-dimensional-cultured MSC-derived exosome-hydrogel hybrid microneedle array patch for spinal cord repair. Nano Lett. 2022;22:6391–6401. doi: 10.1021/acs.nanolett.2c02259. [DOI] [PubMed] [Google Scholar]
- 225.Wang C., Wang M., Xia K., Wang J., Cheng F., Shi K., Ying L., Yu C., Xu H., Xiao S., Liang C., Li F., Lei B., Chen Q. A bioactive injectable self-healing anti-inflammatory hydrogel with ultralong extracellular vesicles release synergistically enhances motor functional recovery of spinal cord injury. Bioact. Mater. 2021;6:2523–2534. doi: 10.1016/j.bioactmat.2021.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Chen Y., Lin J., Yan W. A prosperous application of hydrogels with extracellular vesicles release for traumatic brain injury. Front. Neurol. 2022;13 doi: 10.3389/fneur.2022.908468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Lin J., Wang Z., Huang J., Tang S., Saiding Q., Zhu Q., Cui W. Microenvironment-protected exosome-hydrogel for facilitating endometrial regeneration, fertility restoration, and live birth of offspring. Small. 2021;17 doi: 10.1002/smll.202007235. [DOI] [PubMed] [Google Scholar]
- 228.Wang X., Wu D., Li W., Yang L. Emerging biomaterials for reproductive medicine. Eng. Regener. 2021;2:230–245. [Google Scholar]
- 229.Xu Y., Qiu Y., Lin Q., Huang C., Li J., Chen L., Xue Z., Wu Q., Wang Y. miR-126-3p-loaded small extracellular vesicles secreted by urine-derived stem cells released from a phototriggered imine crosslink hydrogel could enhance vaginal epithelization after vaginoplasty. Stem Cell Res. Ther. 2022;13:331. doi: 10.1186/s13287-022-03003-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Liang L., Shen Y., Dong Z., Gu X. Photoacoustic image-guided corpus cavernosum intratunical injection of adipose stem cell-derived exosomes loaded polydopamine thermosensitive hydrogel for erectile dysfunction treatment. Bioact. Mater. 2022;9:147–156. doi: 10.1016/j.bioactmat.2021.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Page R.C., Kornman K.S. The pathogenesis of human periodontitis: an introduction. Periodontol. 2000;14(1997):9–11. doi: 10.1111/j.1600-0757.1997.tb00189.x. [DOI] [PubMed] [Google Scholar]
- 232.Liu L., Guo S., Shi W., Liu Q., Huo F., Wu Y., Tian W. Bone marrow mesenchymal stem cell-derived small extracellular vesicles promote periodontal regeneration. Tissue Eng. 2021;27:962–976. doi: 10.1089/ten.TEA.2020.0141. [DOI] [PubMed] [Google Scholar]
- 233.Shen Z., Kuang S., Zhang Y., Yang M., Qin W., Shi X., Lin Z. Chitosan hydrogel incorporated with dental pulp stem cell-derived exosomes alleviates periodontitis in mice via a macrophage-dependent mechanism. Bioact. Mater. 2020;5:1113–1126. doi: 10.1016/j.bioactmat.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Shi W., Guo S., Liu L., Liu Q., Huo F., Ding Y., Tian W. Small extracellular vesicles from lipopolysaccharide-preconditioned dental follicle cells promote periodontal regeneration in an inflammatory microenvironment. ACS Biomater. Sci. Eng. 2020;6:5797–5810. doi: 10.1021/acsbiomaterials.0c00882. [DOI] [PubMed] [Google Scholar]
- 235.Rogers N.E., Avram M.R. Medical treatments for male and female pattern hair loss. J. Am. Acad. Dermatol. 2008;59:547–566. doi: 10.1016/j.jaad.2008.07.001. quiz 567-548. [DOI] [PubMed] [Google Scholar]
- 236.Perper M., Aldahan A.S., Fayne R.A., Emerson C.P., Nouri K. Efficacy of fractional lasers in treating alopecia: a literature review. Laser Med. Sci. 2017;32:1919–1925. doi: 10.1007/s10103-017-2306-7. [DOI] [PubMed] [Google Scholar]
- 237.Yang G., Chen Q., Wen D., Chen Z., Wang J., Chen G., Wang Z., Zhang X., Zhang Y., Hu Q., Zhang L., Gu Z. A therapeutic microneedle patch made from hair-derived keratin for promoting hair regrowth. ACS Nano. 2019;13:4354–4360. doi: 10.1021/acsnano.8b09573. [DOI] [PubMed] [Google Scholar]
- 238.Shi Y., Zhao J., Li H., Yu M., Zhang W., Qin D., Qiu K., Chen X., Kong M. A drug-free, hair follicle cycling regulatable, separable, antibacterial microneedle patch for hair regeneration therapy. Adv.x Healthc. Mater. 2022 doi: 10.1002/adhm.202200908. [DOI] [PubMed] [Google Scholar]
- 239.Tang Q., Lu B., He J., Chen X., Fu Q., Han H., Luo C., Yin H., Qin Z., Lyu D., Zhang L., Zhou M., Yao K. Exosomes-loaded thermosensitive hydrogels for corneal epithelium and stroma regeneration. Biomaterials. 2022;280 doi: 10.1016/j.biomaterials.2021.121320. [DOI] [PubMed] [Google Scholar]
- 240.Silva A.K.A., Perretta S., Perrod G., Pidial L., Lindner V., Carn F., Lemieux S., Alloyeau D., Boucenna I., Menasche P., Dallemagne B., Gazeau F., Wilhelm C., Cellier C., Clement O., Rahmi G. Thermoresponsive gel embedded with adipose stem-cell-derived extracellular vesicles promotes esophageal fistula healing in a thermo-actuated delivery strategy. ACS Nano. 2018;12:9800–9814. doi: 10.1021/acsnano.8b00117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.