Abstract
Enteropathogenic Escherichia coli (EPEC), like many other gram-negative pathogens, encodes a type III secretion apparatus dedicated to the release of virulence-associated proteins. One such protein, Tir, is translocated into host cells, where it is modified by the addition of phosphate groups, resulting in a number of species with distinct molecular mass. One phosphorylation event, on tyrosine residue 474 of Tir, does not contribute to shifts in molecular mass but is essential for its actin-nucleating function. The role of the nonphosphotyrosine related modifications is unknown. In this paper, we demonstrate, using three different approaches, that Tir does not encode sufficient information to facilitate its complete modification when introduced into host cells in EPEC-independent mechanisms. Each system revealed that Tir is a substrate for a host kinase whose action results in its partial modification to a form similar to one evident in EPEC-infected host cells. Further Tir modification could not be induced by infecting cells with EPEC, suggesting that Tir must be coexpressed with other EPEC factors to enable its full modification within host cells. One approach used Yersinia spp. to deliver Tir into host cells, and this system revealed that Tir secretion and translocation can occur in the absence of the Tir chaperone molecule, CesT (formerly known as OrfU). CesT was found to be an efficiency factor which was not required, unlike in EPEC, for Tir stability, indicating that it may function to guide Tir to the translocation apparatus or maintain it in a secretion-competent form.
Enteropathogenic Escherichia coli (EPEC) is a member of the attaching and effacing family of pathogens which infect a wide range of species, including humans (EPEC and enterohemorrhagic E. coli) rabbits, pigs, dogs, calves, and mice (9). Attachment of these pathogens to gut epithelia leads to the loss (effacement) of surrounding microvilli and the production of host cytoskeleton-rich pedestal-like structures beneath the adherent bacteria, processes correlated with disease. It is now apparent that these pathogens share a homologous DNA region, called LEE (for locus of enterocyte effacement), that encodes a type III secretion apparatus, secreted substrates, chaperone molecules, and the intimin outer membrane protein (1, 11, 12, 19). One of these type III secreted proteins, Tir (EspE in enterohemorrhagic E. coli), is translocated into the host cell (7, 23). This process is dependent on three secreted proteins, EspA, EspB, and EspD, whose primary function is to generate a translocon enabling the transfer of effector molecules, such as Tir, into host cells (16, 23, 28, 29, 41).
Tir translocation into host cells is also dependent on the LEE cesD and cesT (formerly known as orfU) genes, which encode chaperone molecules for EspB-EspD and Tir, respectively (1, 11, 40). Following its delivery into the host cell, Tir undergoes a series of phosphate-related modifications, resulting in changes in its apparent molecular mass (21, 23). Phosphorylation of Tir on residue 474 (a tyrosine) is essential for its actin-nucleating activity but does not alter its apparent molecular mass (21). The role of the non-phosphotyrosine-related modifications is unknown, though it is thought that the shifts in apparent molecular mass reflect conformational changes facilitating Tir insertion into the plasma membrane and subsequent tyrosine phosphorylation (21). Tir adopts a hairpin loop structure within the host plasma membrane, with both the N and C termini of Tir exposed to the cytoplasm (6, 15, 21). The intimin binding domain was localized to a 53-amino-acid region within the putative extracellular loop (21), which structural studies have confirmed and defined further (4, 34).
Many gram-negative pathogens employ type III secretion systems to secrete virulence-associated proteins, some of which are translocated directly into the target host cell (18). Yersinia spp. use this system to inject effector molecules into host cells, where they inhibit the uptake of Yersinia by professional phagocytes, and are cytotoxic to cultured epithelial cells (5, 32). The major Yersinia effector molecules are YopH (a tyrosine phosphatase), YopE and YopT (cytotoxic factors), YpkA-YopO (a threonine-serine kinase), YopP-YopJ (involved in apoptosis), and YopM (a protein with homology to thrombin binding protein) (reviewed by Cornelis et al. [5]). Although most type III secreted proteins share little or no homology, examples of heterologous secretion between type III secretion-containing organisms have been reported (31).
In this study, we set out to address whether the Tir molecule, in the absence of other EPEC factors, encodes sufficient information to enable its full modification within host cells. Three different approaches were used, involving either (i) expression of Tir in host cells, (ii) introduction of purified Tir into a host permeabilized system, or (iii) delivering Tir into host cells via the Yersinia type III secretion apparatus. Although each system revealed that Tir could not direct its full modification, they demonstrated that Tir was a substrate for a host kinase, leading to its partial modification and generating a Tir species similar to one detected in EPEC-infected host cells. Further modification could not be induced by infecting the host cells with EPEC strains, indicating that other EPEC factors need to be coexpressed with Tir to enable its full modification within host cells. The Yersinia delivery system also demonstrated a CesT-independent Tir translocation process and that CesT can act at a level other than that of mediating Tir stability.
MATERIALS AND METHODS
Bacterial strains.
Strains used in this study were E. coli BL21(DE3) (39); EPEC E2348/69 (0127:H6) Δtir, espA, and cfm-14 strains (8, 23, 27, 33); and Yersinia pseudotuberculosis YPIII and multiply yop-defective strain pIB29MEKA (14). Y. pseudotuberculosis pIB29MEKA strains were selected with chloramphenicol (final concentration, 50 μg/ml) and kanamycin (final concentration, 25 μg/ml). Strains containing pBluescript (pSK; Stratagene)- and pcDNA3 (mammalian expression vector; Invitrogen)-based plasmids were selected with carbenicillin (final concentration 100 μg/ml), and pET (Novagen)-based plasmids were selected with kanamycin (final concentration, 25 μg/ml).
Cell lines and transfection conditions.
HeLa (human epitheloid cervical carcinoma; ATCC CCL2) and HEK293 (human embryonic kidney; ATCC CRL-1573) cells were grown at 37°C in 5% CO2 in Dulbecco's minimal Eagles medium supplemented with 10% (vol/vol) fetal calf serum (GIBCO BRL). HEK293 cells were transfected using the Geneporter transfection reagent (GTS Inc.) per the manufacturer's recommendations.
Plasmids.
The construction of plasmids carrying orf19, tir, and cesT (pSK-tir) and orf19, tirHSV, and cesT (pSK-HSV3) has been described previously (21). pSK-HSV2A (orf19 tirHSV) and pSK-HSV2B (tirHSV cesT) were generated from pSK-HSV3 by digesting with SalI and BamHI, respectively (to drop out fragments encoding cesT and the 5′ end of orf19, respectively), and vector fragments were religated. pSK-HSV1 (tirHSV) has both the BamHI and SalI fragments deleted (see Fig. 4). The vector encoding tirHSVHis (TirHH) was generated by inserting the BamHI/NheI fragment of pSK-HSV3 (3′ of orf19 to within the herpes simplex virus [HSV] tag of tirHSV) into the same sites of pET-27b (Novagen). tirHSV was cloned into pcDNA3 on a BamHI/SalI fragment (3′ of orf19 and tirHSV) into the BamHI/XhoI sites of pcDNA3.
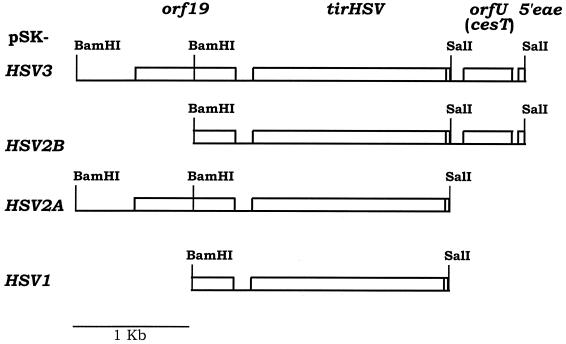
FIG. 4.
Schematic representation of plasmids carrying the EPEC tir region. The name of the plasmid is shown at left next to a schematic representation of the LEE region cloned into pBluescript (pSK). orf19 encodes a protein which is translocated into host cells, where it appears to interfere with mitochondrial function (26), while orfU (renamed cesT) encodes a Tir chaperone molecule (1, 11). tirHSV is a 3′ in-frame fusion of tir with the sequence encoding an HSV epitope. This tag does not interfere with Tir delivery or modification or actin-nucleating function following transfer into host cells by EPEC (21). eae encodes the intimin protein, with only a small portion of it present in these constructs. Restriction enzyme sites used in the generation of these constructs are also shown.
TirHH purification and in vitro-permeabilized cell system.
BL21(DE3) carrying pET27b-tirHH was diluted into Luria broth and grown at 37°C with shaking to an optical density (A600) of ∼0.65 before inducing TirHH expression with IPTG (isopropyl-β-d-thiogalactopyranoside) (1 mM final concentration). The bacterial pellet (obtained by centrifugation at 3,000 × g for 15min at 4°C) was resuspended in lysis buffer (50 mM Tris, 1 mM EDTA, and 100 mM NaCl [pH 8], containing protease inhibitor cocktail [Sigma] and lysozyme [final concentration, 270 μg/ml]) on ice for 20 min prior to the addition of deoxycholic acid (final concentration, 1.3 mg/ml), DNase (final concentration, 11 μg/ml), and MgCl2 (final concentration, 10 mM). The lysed cells were centrifuged (12,000 × g for 10 min at 4°C), and the soluble fraction was removed. The pellet was resuspended in membrane buffer (0.5% Triton X-100, 50 mM Tris, 10 mM EDTA, 100 mM NaCl [pH 8], protease inhibitor cocktail) and recentrifuged (12,000 × g for 10 min at 4°C). The pellet containing the insoluble TirHH protein was resuspended in urea buffer (8 M urea, 10 mM imidazole, 10 mM Na2HPO4, 10 mM NaH2PO4, 500 mM NaCl [pH7.4], protease inhibitor cocktail), and the sample was passed over a nickel column (Pharmacia) per the manufacturer's protocol. TirHH bound to the column was eluted with a solution containing 255 mM imidazole, 10 mM Na2HPO4, 10 mM NaH2PO4, 500 mM NaCl [pH 7.4], and protease inhibitor cocktail.
A 100-mm-diameter plate of confluent HeLa cells was washed in kinase buffer (50 mM Tris, 10 mM MgCl2) and permeabilized by the addition of 400 μl of kinase buffer containing 0.2% saponin. Cells were scraped and placed in an Eppendorf tube. TirHH (12.5 ng) was added to 10 μl of permeabilized HeLa extract in a final volume of 50 μl containing 80 μM dATP (GIBCO-BRL), 1 mM NaF, 0.4 mM Na3VO4, and 1 mM phenylmethylsulfonyl fluoride (PMSF) for 30 min at 37°C. Samples were resuspended in Laemmlli (30) sample buffer and boiled for 5 min.
Induction of Yersinia Yop secretion.
Shaking Yersinia cultures were grown at 26°C in brain heart infusion (BHI) medium supplemented with 5 mM EGDA and 20 mM MgCl2, as previously described (14). After being diluted 1:100 into fresh medium the cells were grown for 2 h at 26°C with shaking, and half the culture was transferred to 37°C for 2 h. Supernatant and cellular samples were isolated, and the supernatant proteins were precipitated by the addition of trichloroacetic acid (TCA) (10%, vol/vol; BDH) for 60 min on ice as previously described (22). Samples were resuspended in Laemmlli (30) sample buffer and boiled for 5 min. Following resolution on polyacrylamide gels (30), protein bands were visualized by Coomassie R-250 blue staining (0.25% in 40% methanol–10% acetic acid solution; ICN Biochemicals)
Cellular fractionation and protein extraction.
Standing 37°C Luria broth EPEC and shaking 26°C BHI Yersinia cultures were usually used at a multiplicity of infection (MOI) of 100:1 to infect host cells. Preactivated EPEC was generated by diluting EPEC overnight cultures in tissue culture medium for 3 h prior to adding to host cells. Postinfection, monolayers were washed two to three times in cold phosphate-buffered saline (PBS) and permeabilized by the addition of 0.2% saponin (Calbiochem) in PBS containing 0.4 mM NaVO4, 1 mM NaF, and 0.1 mM phenylmethylsulfonyl fluoride. After 5 min of incubation on ice, samples were centrifuged (12,000 × g for 5 min at 4°C) and the soluble cytoplasmic protein fraction removed as described before (24). The insoluble pellet was washed with PBS and resuspended in Triton X-100 lysis buffer (same as saponin buffer with the addition of Triton to a 1% final concentration) as described before (24). Centrifugation (12,000 × g for 2 min at 4°C) separated the soluble membrane from insoluble fraction. For experiments involving trypsin-EDTA, infected cells were washed trice with PBS and incubated with trypsin-EDTA (0.5 and 0.2% final concentrations, respectively; Boehringer Mannheim) until cells lifted. Cells were transferred to Eppendorf tubes, resuspended in PBS containing 0.9 mM CaCl2 and 1 mM MgCl2, centrifuged (12,000 × g for 1 min at 4°C), and washed twice more. Samples were then fractionated into membrane and insoluble fractions as described above. In some experiments cytoplasmic and membrane fractions were heated to 90°C for 5 min and placed on ice for 3 min prior to centrifuging (2,500 × g for 2 min at 4°C) to pellet the heat-precipitated proteins. Samples were resuspended in Laemmlli (30) sample buffer and boiled for 5 min. For alkaline phosphatase treatment, samples were isolated in the absence of NaF or NaVO4 and incubated at 37°C for 4 h with 2 U of alkaline phosphatase (Sigma).
Western immunoblot analysis.
Protein samples were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (30), and the proteins were transferred to nitrocellulose for Western analysis as described elsewhere (36). Blots were blocked in 5% Marvel dried skimm milk and probed with anti-Tir (23), -HSV, or -T7 (Novagen) antibodies. Protein bands bound by these antibodies was detected by alkaline phosphatase-conjugated secondary antibodies (Jackson Labs) as described previously (36).
Immunofluorescence microscopy.
HeLa cells were seeded on glass coverslips and after infection the monolayers were washed with PBS and fixed in 2.5% paraformaldehyde. Cells were permeabilized with 0.1% Triton in PBS and stained for filamentous actin (using phalloidin-Texas red; Molecular Probes). Images were detected with a Zeiss Axioskop phase-contrast–epifluorescence microscope and captured using a Hamamatsu C4742-95 charge-coupled device camera and Improvision software.
Data imaging.
Raw data for the figures were scanned using an Umax (Astra 1220S) scanner and imported into Adobe Photoshop 5.02 where they were labeled prior to being printed on a Hewlett-Packard LaserJet 6P or Hewlett-Packard 2000C color printer.
RESULTS
Expression of EPEC TirHSV in host cells only leads to its partial modification.
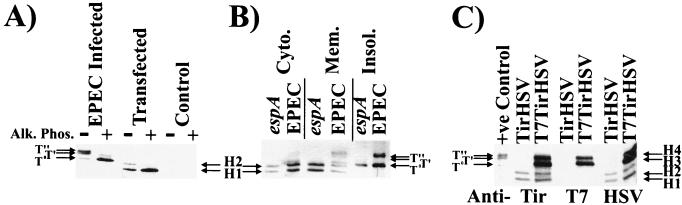
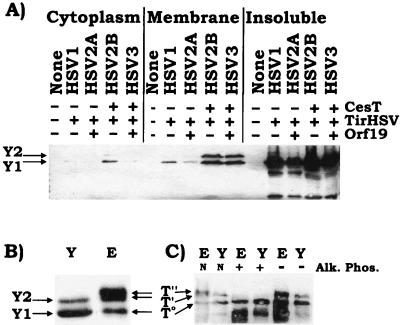
In order to determine whether the EPEC Tir molecule contains sufficient information to direct its full modification required that it be introduced into host cells in the absence of other EPEC-encoded factors. A standard approach to test this is to express the molecule within host cells from a mammalian expression plasmid. Thus, the DNA fragment encoding TirHSV was cloned into the pcDNA-3 plasmid (see Materials and Methods) and transfected into HEK293 cells. The C-terminal HSV epitope tag does not inhibit Tir delivery, modification, or actin-nucleating activity when delivered into host cells by EPEC (21). Triton X-100 (1% final concentration)-soluble fractions of transfected HEK293 cells were probed by Western analysis with anti-HSV antibodies, which revealed two HSV-related bands, H1 and H2, absent from control HEK293 cells (Fig. 1A). Both bands displayed smaller apparent molecular masses than any of the EPEC-delivered TirHSV species evident in HEK293 infected cells (Fig. 1A). Treatment of membrane fractions with alkaline phosphatase led to the disappearance of the H2, T′, and T" forms, leading to increases in the levels of the H1 and T0 forms, respectively (Fig. 1A). This implies that H1 species, like T′ and T", result from the phosphorylation of Tir. Despite the dissimilarity in apparent molecular mass between the host-expressed and EPEC-delivered TirHSV molecules, the relative difference in molecular mass between the H1 and H2 and T0 and T′ forms is similar (Fig. 1A).
FIG. 1.
Expression of Tir in host cells results in its partial (phosphate-dependent) modification (A) HEK293 cells were transfected with a mammalian expression plasmid carrying a gene encoding TirHSV or infected with EPEC Δtir expressing TirHSV. 90°C-heat-soluble Triton X-100 fractions were isolated and incubated in the presence (+) or absence (−) of alkaline phosphatase. Samples were examined by Western analysis with probing with anti-HSV antibodies. (B) HEK293 cells expressing TirHSV were infected with EPEC or the Tir translocation-defective espA mutant. Infected cells were fractionated into saponin-released cytoplasmic (Cyto.), Triton X-100-soluble membrane (Mem.), or insoluble (Insol.) fractions and analyzed as described for panel A. (C) HEK293 cells were transfected with plasmids bearing genes encoding TirHSV or T7-TirHSV, and 90°C-heat-soluble Triton X-100 fractions were isolated. Samples were examining by Western analysis probing with anti-HSV, anti-Tir, or anti-T7 antibodies. T0, T′, and T" bands indicate the position of EPEC-delivered TirHSV intermediates, while H1-H4 indicate the position of mammalian-expressed Tir-related bands.
Transfected cells were missing a TirHSV band equivalent to the EPEC-delivered T" form, indicating that this modification may require additional EPEC factors to facilitate this event, either in a direct or indirect manner. To test this, HEK293 cells expressing TirHSV were infected with EPEC or the Tir translocation-defective espA strain (23, 27) and fractionated into saponin-released cytoplasmic, Triton X-100-soluble membrane, and Triton X-100-insoluble fractions (see Materials and Methods). The latter fraction contains adherent bacteria, the host cytoskeleton and nuclei. Cytoplasmic and membrane fractions were incubated at 90°C for 5 min to enrich for Tir from host heat-precipitable proteins (23). Western analysis probing with anti-HSV antibodies revealed the mammalian expressed H1-H2 TirHSV bands in the host cytoplasmic and membrane fractions, without any evidence of any additional modification following infection with EPEC (data not shown). Productive EPEC-host cell interaction was verified by probing duplicate protein samples with anti-Tir antibodies, which detected both the EPEC delivered T0, T′, and T" Tir bands and the mammalian expressed H1-H2 TirHSV bands (Fig. 1B). As previously reported (23), the espA mutant did not deliver Tir into host cells, and thus only the Tir T0 form is apparent in the insoluble fraction, which contains the adherent Tir-expressing bacteria (Fig. 1B).
It was possible that the inability of the mammalian expressed TirHSV molecule to undergo full modifications was due to its reduced molecular mass. This is presumably due to either (i) mistranslation of tir in host cells, (ii) N-terminal cleavage of Tir, or (iii) the absence of bacterially mediated modifications (tir is predicted to encode a 56-kDa protein though it is detected as an ∼78-kDa protein within EPEC) (23). To resolve this, a mammalian vector encoding a T7-TirHSV fusion protein was introduced into HEK293 cells, and isolated membrane fractions probed with anti-T7, Tir, or HSV antibodies. The Tir antibodies revealed four T7-TirHSV-related bands (H1 to H4), of which two share the same apparent molecular mass as the TirHSV H1-H2 forms (Fig. 1C). These four bands were also detected with the HSV antibodies, with only the H3 and H4 forms detected with the T7 antibodies. The inability of the T7 antibodies to recognize the H1-H2 forms is consistent with N-terminal cleavage, or more likely the use of an alternative initiation codon by the host translational machinery. H3 and T0 have similar molecular masses, and the H4 and T′ have similar molecular masses (Fig. 1C). Slight differences in molecular mass between the EPEC-delivered T0 and T′ and host-expressed H3 and H4 forms are evident, which presumably are due to the presence of the additional T7 epitope tag on the T7-TirHSV H3-H4 bands. These results support the arguments for poor levels of expression and mistranslation of the tirHSV gene, which is greatly reduced by the addition of the T7 sequence at the 5′ end of tir. However, no additional band equivalent in size to the EPEC-delivered T" form was evident, proving that even the full-length mammalian expressed Tir molecule alone cannot be completely modified within host cells (Fig. 1C).
Incubation of Tir in host permeabilized systems also leads to only partial modification.
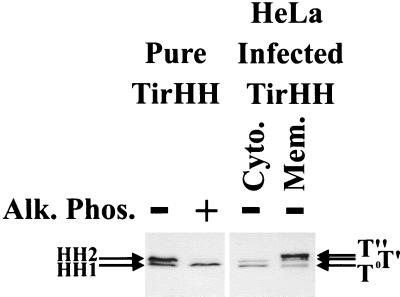
To confirm the above data, a second approach involving the incubation of purified Tir with permeabilized host cells was developed. The tir gene was cloned into the bacterial pET expression vector, generating an in-frame TirHH gene fusion for overexpression and purification via the His tag (see Materials and Methods). Purified TirHH was incubated with saponin (0.2% final concentration)-permeabilized HeLa cells before isolating Triton-soluble heat-treated samples for Western analysis for probing with anti-HSV antibodies (see Materials and Methods). This approach also identified two TirHH-related bands, HH1 and HH2, with only the HH2 form being sensitive to alkaline phosphatase treatment (Fig. 2). Comparison of these bands to the EPEC-delivered TirHH forms revealed that HH1 and T0 forms had similar molecular masses, as did the HH2 and T′ forms (Fig. 2). Thus, it appears that Tir is a substrate for a host kinase whose action leads to an increase in Tir molecular mass equivalent to the T0-to-T′ shift.
FIG. 2.
Incubation of purified Tir with permeabilized host cells results in its partial (phosphate-dependent) modification. TirHH was overexpressed and purified via its His tag (see Materials and Methods). HeLa cell monolayers were washed in kinase buffer, detached, and transferred to Eppendorf tubes. Saponin (0.2 % final concentration) was added to permeabilize the cell membrane, and purified TirHH (∼12.5 ng) was added for incubation at 37°C for 30 min. Extracts were then incubated in the presence (+) or absence (−) of alkaline phosphatase (Alk. Phos.). HeLa cells were also infected with EPEC Δtir expressing TirHH and host cells were fractionated, isolating both saponin-released cytoplasmic (Cyto.) and Triton X-100-soluble membrane (Mem.) fractions. Samples were subjected to Western analysis probing with anti-HSV antibodies. T0, T′, and T" bands indicate the position of EPEC-delivered TirHH intermediates, while HH1 and HH2 indicate the position of the purified TirHH bands.
Delivery of EPEC Tir into host cells via the Y. pseudotuberculosis type III secretion apparatus.
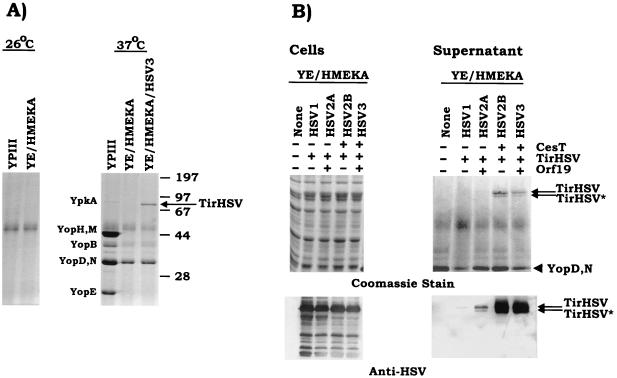
As the mechanism of Tir delivery into host cells may be important for modification, a delivery system was developed mimicking that of EPEC by exploiting the conserved nature of the type III secretion mechanisms of gram-negative pathogens. Preliminary experiments demonstrated that Y. pseudotuberculosis could secrete Tir (data not shown). Yersinia employs its type III secretion system to deliver effector molecules into host cells, where they interfere with host cellular processes (5, 18, 32). To reduce the possibility that these effector molecules would inhibit Tir delivery or modification within host cells, a Y. pseudotuberculosis YPIII strain, pIB29MEKA (referred to as YE/HMEKA hereafter in this work), was used as it is deleted for the major Yersinia effector molecules—YopH, YopM, YopE, YopK, and YpkA (14). YE/HMEKA was transformed with the plasmid pSK-HSV3 (orf19 tirHSV cesT; see Fig. 4) and examined for its ability to secrete TirHSV. The orf19 gene has recently been shown to encode a type III secreted protein that is targeted to host mitochondria (26), while cesT encodes a Tir chaperone molecule (1, 11). YE/HMEKA, with or without pSK-HSV3, and parental Yersinia (YPIII) strains were grown under conditions that induce Yersinia type III secretion. Bacterial supernatant samples were isolated and resolved by SDS-PAGE, and secreted proteins were visualized by Coomassie staining. Figure 3A confirms lack of secretion when grown at 26°C, with a typical Yop protein secretion profile (14) for the YPIII parental strain following growth at 37°C. In contrast the YE/HMEKA strain was defective for the secretion of several Yop proteins that migrate at positions corresponding to YpkA, YopE, YopH, and YopM (Fig. 3A). The presence of plasmid pSK-HSV3 resulted in the secretion of an additional band (Fig. 3A) confirmed to be TirHSV by Western blot analysis (data not shown).
FIG. 3.
Yersinia can secrete Tir independent of Orf19 and CesT. (A) Y. pseudotuberculosis (YPIII) and the isogenic YPIII/pIB29MEKA mutant strain (YE/HMEKA, with the deletion of yopH, yopM, yopE, yopK, and ypkA) with or without pSK-HSV3 (orf19 tirHSV cesT) were grown in modified BHI medium at 26°C (left panel) or subsequently shifted to 37°C (right panel), which induces type III secretion. Supernatant samples were concentrated, resolved by SDS-PAGE (12% acrylamide), and visualized by Coomassie staining. The position of the secreted TirHSV band is indicated with an arrow, while those of the molecular mass standards (in kilodaltons) are shown at the right. (B) YE/HMEKA strains carrying various plasmids (see below and Fig. 4) were grown in modified BHI medium and induced for type III secretion as described above. Cell and supernatant samples were isolated, resolved by SDS-PAGE (8% polyacrylamide), and visualized by Coomassie staining (top panels) or Western analysis (bottom panels) probing with anti-HSV antibodies. Two secreted forms of TirHSV (TirHSV and TirHSV∗) are indicated by arrows, with the position of the YopD-YopN doublet highlighted with an arrowhead. + and − indicate the presence or absence of the indicated gene product, respectively.
cesT, but not orf19, is required to obtain Coomassie stain-detectable levels of Tir secreted from Yersinia.
As pSK-HSV3 encodes three EPEC factors, Orf19, TirHSV, and CesT, the requirement of these in Yersinia-mediated Tir secretion was assessed. A series of plasmids were constructed (Fig. 4; also see Materials and Methods) which encode TirHSV alone (pSK-HSV1), Orf19 and TirHSV (pSK-HSV2A), TirHSV and CesT (pSK-HSV2B), and all three proteins (pSK-HSV3). These plasmids were transformed into YE/HMEKA, and the strains were assessed for their ability to secrete TirHSV as before. Figure 3B (top panels) reveals Coomassie stain-detectable levels of secreted TirHSV from Yersinia strains that encode both TirHSV and CesT. In Fig. 3B (top panel) two TirHSV-secreted bands (TirHSV and TirHSV∗) are detected in contrast to one in Fig. 3A, which is due to the different resolving abilities of the SDS-PAGE gels (12% polyacrylamide in Fig. 3A and 8% polyacrylamide in Fig. 3B). EPEC also secretes two Tir species that share identical N-terminal sequences (23). Western analysis of the supernatant samples revealed that both forms carried the C-terminal tag, while low levels of secreted TirHSV were evident in supernatant samples derived from Yersinia strains not expressing CesT (Fig. 3B, bottom panel). Although it appears that TirHSV secretion levels are reduced from YE/HMEKA expressing TirHSV alone, compared to the strain expressing TirHSV and Orf19, this is probably due to loading differences as reflected in the correspondingly reduced YopD-YopN secreted levels (Fig. 3B, top panels).
FIG. 8.
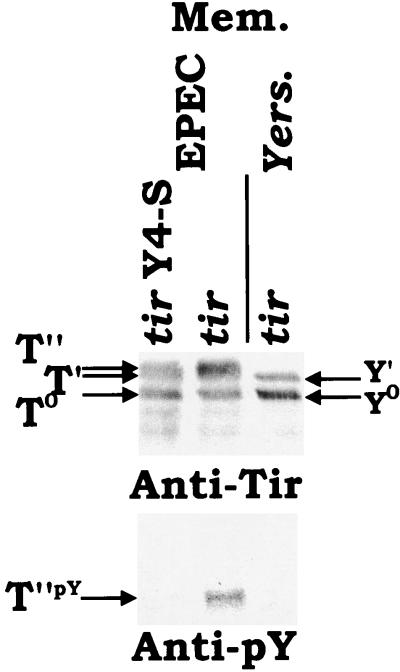
The Yersinia-delivered Y0 and Y′ bands are not tyrosine phosphorylated. HeLa cells were infected with Yersinia or EPEC Δtir strains expressing Tir. tir Y4-S indicates the presence of a specific mutation, which converts the tyrosine residue targeted for phosphorylation to a serine (21). 90°C-heat-resistant Triton X-100 fractions were isolated and probed by Western analysis with either anti-Tir or antiphosphotyrosine (anti-pY) specific antibodies. T0, T′, and T" and Y0 and Y′ indicate the position of EPEC- and Yersinia-delivered Tir bands, respectively.
Western analysis of the corresponding bacterial extracts revealed identical TirHSV banding patterns in all but the Yersinia plasmidless strains (Fig. 3B). The prominent band corresponds to the full-length TirHSV band, with the minor bands presumably representing minor levels of N-terminal (detected via the C-terminal tag) cleaved TirHSV breakdown products.
Yersinia can deliver Tir into host cell in a CesT-independent manner.
To assess whether Yersinia can deliver TirHSV into host cells and the possible role of orf19 and cesT, HeLa cells were infected with YE/HMEKA strains carrying the various tir plasmid constructs (see Fig. 4 and Materials and Methods). Following infection the host cells were incubated with trypsin-EDTA (to degrade extracellular TirHSV) and fractionated into saponin-released cytoplasmic, Triton X-100-soluble membrane and Triton X-100-insoluble fractions, as before. Western analysis of the insoluble fraction (which contains adherent bacteria) revealed the bacterial TirHSV and minor N-terminally cleaved degradation products in all samples except those obtained from the cells infected with the plasmidless Yersinia strain (Fig. 5A). TirHSV bands were also detected in the cytoplasmic and membrane fractions of all but the plasmidless Yersinia-infected cells. These bands have resisted trypsin digestion of intact cells, as found for the EPEC-delivered Tir species (21), demonstrating that Yersinia can also deliver Tir into host cells. TirHSV delivery was independent of CesT or Orf19 coexpression, though CesT was required for maximal efficiency of TirHSV delivery (Fig. 5A). A single TirHSV band, equivalent in size to the bacterial unmodified (Y1) form, was apparent in all but the control plasmidless Yersinia-infected host cytoplasmic and membrane fractions. A second, slower-migrating form (Y2) was just visible in the membrane fraction of host cells infected with Yersinia pSK-HSV2A, but readily detectable when infecting Yersinia coexpressed CesT and TirHSV (Fig. 5A).
FIG. 5.
Yersinia-directed delivery of TirHSV into host cells is independent of Orf19 and CesT, with the latter acting as an efficiency factor. (A) YPIII/pIB29MEKA (YE/HMEKA) strains, with or without the tir region plasmids, were use to infect HeLa cells. pSK-HSV1 carries tirHSV alone, pSK-HSV2A carries orf19 and tirHSV, and pSK-HSV2B carries tirHSV and cesT, while pSK-HSVs carries all three genes (Fig. 4). Host cells were treated with trypsin-EDTA to degrade extracellular secreted proteins and then fractionated into saponin-released cytoplasmic, Triton-soluble membrane, and insoluble fractions. Samples were resolved by SDS-PAGE (6% polyacrylamide) for Western analysis and probed with anti-HSV antibodies. (B) 90°C-heat-resistant membrane fractions from Yersinia pSK-HSV3 (Y)- and EPEC Δtir/pSK-HSV3 (E)-infected cells were isolated for Western analysis probing with anti-HSV antibodies. (C) membrane fractions, isolated as in panel B were left untreated (N) or incubated in the presence (+) or absence (−) of alkaline phosphate (Alk. Phos.) prior to Western analysis probing with anti-HSV antibodies. The positions of the Yersinia (Y1 and Y2)- and EPEC (T0, T′, and T")-delivered TirHSV species are indicted with arrows.
Comparison of the apparent molecular masses of the Yersinia-delivered TirHSV forms to those of EPEC revealed that the Y1 and T0 displayed similar apparent molecular masses as did the Y2 and T′ forms (Fig. 5B). Like the T′ and T" forms, the Y2 form was susceptible to alkaline phosphatase treatment (Fig. 5C). Given the similarity of the Y1 and T0 and Y2 and T′ forms, Y1 and Y2 were renamed Y0 and Y′, respectively. This third approach confirms that the Tir molecule alone only contains sufficient information to direct the T0-to-T′, but not T" equivalent, modification.
Kinetics of TirHSV delivery and Y°-to-Y′ modification.
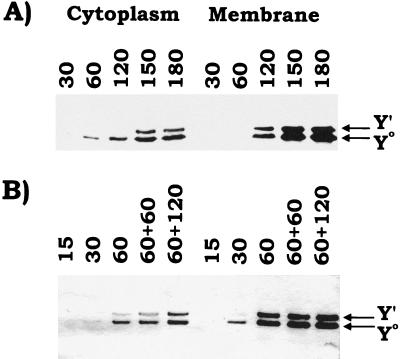
The Yersinia delivery system was also used to study the kinetics of TirHSV delivery and Y0-to-Y′ modification. To do this HeLa cells were infected with Yersinia (YE/HMEKA) carrying pSK-HSV3 (orfU tirHSV cesT) at two different MOIs—approximately 10:1 and 100:1—for various time periods prior to isolating host fractions. Western analysis of fractions isolated from host cells infected at the lower MOI (10:1) revealed the Y0 form in the cytoplasmic and membrane fractions by 60 and 120 min postinfection, respectively (Fig. 6A). In contrast, the Y′ form is first apparent in the membrane fraction (120 min postinfection) and then in the cytoplasm (150 min postinfection). Increased infection times did not appear to increase the level of TirHSV delivered or the ratio of Y0 and Y′ species in either the cytoplasmic or the membrane fractions (Fig. 6A).
FIG. 6.
Kinetics of Yersinia-directed Tir delivery and Y0 to Y′ modification. HeLa cells were infected at two MOIs—∼10:1 (bacteria/host cells) (A) and 100:1 (B)—for the time periods (minutes) indicated. Host cells were then fractionated by isolating both saponin-released cytoplasmic (Cyto.) and Triton X-100-soluble membrane (Mem) fractions. Samples were resolved by SDS-PAGE (6% polyacrylamide) for Western analysis probing with anti-HSV antibodies. (B) HeLa cells infected with Yersinia for 60 min at the higher MOI were treated with gentamicin for the indicated time (minutes), to kill the Yersinia, before fractionation. The positions of the Yersinia-delivered TirHSV Y0 and Y′ forms are indicated with arrows.
Infection at the higher MOI (∼100:1) led to a more rapid delivery of TirHSV into host cells, with the Y0 species detectable in the membrane fraction by 30 min postinfection and with lower levels apparent in the corresponding cytoplasmic fraction (Fig. 6B). By 60 min postinfection a similar cellular distribution and ratio of Y0 to Y′ species was apparent as seen in the 150 min postinfection samples of host cells infected with Yersinia at the lower m.o.i. (compare Fig. 6B and A). This pattern remained unchanged following a further 2-h incubation period of the infected host cells in the presence of gentamicin sulfate (final concentration, 100 μg/ml) which kills the majority of the Yersinia (data not shown). These results suggest that the modification process is saturable, as is the capacity of the membrane to retain the partially modified Tir species.
The Yersinia-delivered Tir Y′ species is not modified to the Y" form following coinfection with EPEC.
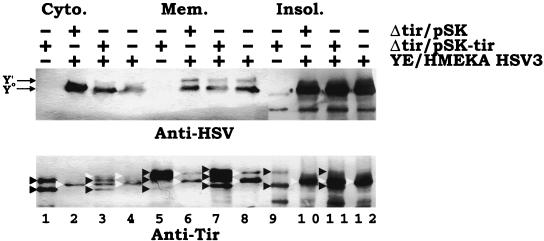
As the Yersinia delivery system mimics that of EPEC, it might deliver Tir in a manner that enables its further modification following exposure of the Yersinia-infected cells to EPEC—to enable it to provide accessory factors or stimulate host activities required for further modification. To test this, HeLa cells were preinfected with EPEC for 3 h to enable EPEC adherence, and nonadherent bacteria were removed prior to infection with Yersinia-expressing TirHSV. Probing extracts with anti-HSV antibodies, to specifically detect the Yersinia-delivered Tir species, revealed the presence of only Y0 and Y′, in the absence of the Y" form in Yersinia-EPEC dually infected cultures (Fig. 7, top panel). To confirm EPEC's productive interaction with the host cells, duplicate samples were probed with anti-Tir antibodies to detect both the EPEC Tir and Yersinia TirHSV delivered bands. Host cells infected with EPEC alone generated the reported banding pattern (21) of two (T0 and T′), three (T0, T′, and T"), and two (T0 and T") bands in the cytoplasmic, membrane, and insoluble fractions, respectively (Fig. 7, bottom panel, lanes 1, 5, and 9). As shown earlier, infection with Yersinia alone produced two bands (Y0 and Y′) in the cytoplasm and membrane fractions and one (Y0) in the insoluble fraction (Fig. 7, bottom panel, lanes 4, 8, and 12). A similar banding pattern, except for the inability to detect the Y′ form in the cytoplasm fraction, was apparent in cells infected with EPEC Δtir/pSK and Yersinia pSK-HSV3 (Fig. 7, bottom panel, lanes 2, 6, and 10). However, cells coinfected with Yersinia pSK-HSV3 and EPEC Δtir/pSK-tir contained a composite banding pattern of EPEC Tir and Yersinia TirHSV delivered bands (Fig. 7, bottom panel, lanes 3, 7, and 11). Although host cell populations can support the delivery of Tir by both EPEC and Yersinia species, EPEC infection does not stimulate further modification of the Yersinia-delivered Tir molecule, although the EPEC-delivered Tir molecule was fully modified.
FIG. 7.
Dual EPEC-Yersinia infections do not lead to the further modification of the Yersinia-delivered TirHSV molecule. HeLa cells were left uninfected or infected with EPEC Δtir carrying pSK or pSK-tir (orf19 tir cesT) for 3 h. Monolayers were washed, and the presence of adherent EPEC colonies was confirmed by phase-contrast microscopy. Cells were then infected (MOI, 100:1) with YPIII/pIB29MEKA (YE/HMEKA) carrying pSK-HSV3 (orf19 tirHSV cesT) for a further 3 h before fractionating into saponin-released cytoplasmic (Cyto.), Triton-soluble membrane (Mem.), and insoluble (Insol.) fractions. Samples were processed for Western analysis (SDS-PAGE [6% polyacrylamide]) and probed with anti-HSV (top panel) or anti-Tir (bottom panel) antibodies. + and − indicates the presence and absence of the strain during infection, respectively. In the top panel, arrows indicate the position of the Yersinia-delivered Y0 and Y′ TirHSV species. Note the absence of an additional band corresponding to the Y" form. In the bottom panel, black arrowheads indicate the position of EPEC-delivered Tir species, with white arrowheads indicating the position of Yersinia-delivered TirHSV species. EPEC-delivered Tir species are lacking the HSV epitope and thus display a smaller apparent molecular mass than the corresponding Yersinia delivered species. Lanes are numbered sequentially.
To counteract the possibility that different subsets of the host cells were infected with EPEC and Yersinia in the dual-infection experiments, the study was repeated with analysis of individual cells infected with both strains. Phase-contrast microscopy readily detected cells with both adherent characteristic EPEC microcolonies and dispersed elongated yersiniae (data not shown). Staining of dually infected cells with phalloidin trimethyl rhodamine isocyanate to detect polymerized actin (see Materials and Methods) was used to determine the presence or absence of fully modified Tir, as predelivered fully modified Tir can be sequestered by intimin-expressing strains to trigger actin nucleation (21, 24, 36). As previously reported (21) EPEC Δtir/pSK-tir mediated Tir-intimin actin nucleation in host cells, which was evident under the EPEC microcolonies but not the Yersinia pSK-HSV3 bacteria (which do not express intimin) in dually infected cells (data not shown). No such actin polymerization was evident under the intimin-expressing EPEC Δtir/pSK bacteria on cells in the absence or presence of adherent Yersinia pSK-HSV3 bacteria (data not shown).
Similar results were found when host cells were infected first with YE/HMEKA pSK-HSV3 (MOI, 100:1 [for 60 min to deliver TirHSV]) followed by EPEC Δtir/pSK or pSK-tir infections. In these experiments gentamicin was added following Yersinia infection (preventing further signalling or translocation activity), and then host cells were infected in the absence of gentamicin with the EPEC strains (data not shown). Together these results suggest that EPEC cannot induce further modification of the Yersinia-delivered Tir molecule whether it is delivered before, at the same time, or after EPEC infection.
The Yersinia TirHSV partially modified species are not tyrosine phosphorylated.
We also examined whether the Yersinia-delivered TirHSV molecule was tyrosine phosphorylated. This modification is essential for Tir actin-nucleating activity but does not contribute to the observed shifts in apparent molecular mass (21). Thus, HeLa cells were infected with Yersinia or EPEC Δtir carrying pSK-tir (orf19 tir cesT). As a control EPEC carrying pSK-tir Y4-S was also used, where Y4-S indicates the presence of a substitution in tir that converts the tyrosine 474 residue (Y), targeted for phosphorylation, to a serine (S). Analysis of 90°C heat-treated membrane fractions with anti-Tir antibodies demonstrated similar levels of the EPEC- and Yersinia-delivered T0 and T′ and Y0 and Y′ forms, respectively, with an additional T" form evident in the EPEC pSK-tir and pSK-tir Y4-S, but not Yersinia pSK-tir, infected samples (Fig. 8). Probing duplicate samples with antiphosphotyrosine-specific antibodies revealed that only the EPEC wild-type T" Tir form was tyrosine phosphorylated (Fig. 8).
DISCUSSION
We have previously reported that EPEC translocates the Tir protein into host cells, where it acts as a receptor for the bacterial outer membrane protein intimin (23). Tir-intimin interaction triggers signalling events that induce the nucleation of host cytoskeletal proteins beneath the bacteria to form characteristic pedestal-like structures (20, 23, 37), a process correlated with full virulence in in vivo infection model systems (10, 35). Tir undergoes a series of phosphorylation events within host cells, of which at least one (phosphorylation on a single tyrosine residue) is essential for its actin-nucleating function (21). The role of the other nontyrosine phosphorylation events is unknown, yet in contrast to Tir tyrosine phosphorylation, such events lead to increases in apparent molecular mass (21). These shifts are thought to reflect phosphorylation-induced conformational changes in Tir, perhaps facilitating its correct insertion into the host plasma membrane (21).
One question arising from earlier studies is whether Tir encodes sufficient information to facilitate its complete phosphorylation within host cells, or whether it requires additional EPEC factors to facilitate these events. To test this, the Tir molecule was introduced into host cells in three different approaches involving (i) expression from a mammalian vector, (ii) incubation of purified Tir with permeabilized host cells, and (iii) “injecting” Tir into intact host cells using the Yersinia type III secretion apparatus. In the last approach, a Yersinia strain with a deletion of the major Yop effector molecules was employed, as these effector molecules are cytotoxic to host cells (5, 32) and could interfere, either directly or indirectly, with Tir delivery and/or modification or function. Although this strain encodes other effector molecules, they did not have any discernible deleterious effect on host cells, as Yersinia-infected cells supported subsequent EPEC infection, leading to Tir translocation and/or modification (Fig. 7) and actin-nucleating activity (as seen by epifluorescence microscopy) (data not shown).
Each of the three approaches demonstrated that Tir is a natural substrate for a host kinase whose action results in a shift in molecular mass similar to the T0-to-T′ shift observed in EPEC-infected cells. Although we have not shown that this shift represents an identical modification(s) in each system, this is likely given the similar ca. 5- to 6-kDa increase in apparent molecular mass. While the addition of phosphate groups will alter the apparent molecular mass of a protein, this modification usually goes undetected (phosphate has a molecular mass of less than 0.1 kDa), as demonstrated for the tyrosine phosphorylation of Tir (21). Detectable shifts in protein molecular mass are usually associated with changes in protein conformation (17). Thus, it is highly unlikely that phosphorylation of Tir at different sites would lead to the similar large shift in Tir molecular mass, implying that the same host kinase(s) is responsible for the modification. In support of this we have recently identified a host kinase whose phosphorylation of Tir on a single serine residue leads to the T0-to-T′ shift in molecular mass, and we are currently investigating the role of this event in Tir function (J. Warawa and B. Kenny, unpublished data). However, T0-to-T′ modification of the Yersinia-delivered Tir molecule was found to be dependent on this serine, indicating that Yersinia delivers Tir in a similar manner to EPEC.
An interesting finding from these studies was that host cells were unable to completely convert the unmodified Tir T0 population to the T′ form. Studies with the Yersinia delivery system revealed that even following gentamicin killing of the bacteria the host cells could not subsequently convert the unmodified Tir molecule to the modified form. These data imply (i) that there is limited kinase activity, (ii) that there is a phosphorylation-dephosphorylation equilibrium, or (iii) that the unmodified form, if not immediately modified, is no longer compatible for modification. Tir intermediates were only evident in in vitro infection systems when Tir was expressed from a multicopy plasmid in the infecting EPEC strain (21), suggesting that there is a saturable rate-limiting modification step that can lead to the accumulation of the intermediate forms. This would provide a reason for EPEC to regulate Tir delivery levels, perhaps using some feedback mechanism through intimin-Tir interaction. A similar regulation has been described for the delivery of EspB, required for Tir delivery, into host cells (42).
The Yersinia delivery system also suggests that the unmodified Tir form is sequestered from the cytoplasm into the membrane fraction in a saturable manner to then start to accumulate in the cytoplasmic fraction (Fig. 6). The membrane-associated unmodified form appears to be the substrate for modification to the T′ form, though this process again seems to be saturable, leading to the loss of the modified form into the cytoplasmic fraction. Although it is possible that Tir is modified in the cytoplasm and is immediately recruited to the membrane fraction, the detection of the host Tir-modifying activity within the host membrane fraction argues against this (Warawa and Kenny, unpublished data) and supports the former possibility. In either case, the data suggest that the T′ form is associated with the membrane fraction, while its later accumulation within the cytoplasmic fraction suggests there is an active saturable retention process in operation. It is presumably this membrane-associated partially modified form that is the substrate for additional phosphorylation leading to the T" and T"pY (tyrosine phosphorylated) forms.
Although all three approaches revealed that Tir can undergo T0-to-T′ modification, they also revealed its inability to undergo the T′-to-T" modification, as demonstrated by the lack of an additional ca. 2- to 3-kDa shift in Tir molecular mass evident in EPEC-infected host cells. This deficiency in further modification does not appear to be due to insufficient levels of Tir, as each system contained levels of Tir equivalent to those evident in EPEC-infected host cells resulting in full modification. In contrast, EPEC may provide additional factors to facilitate this second modification, possibly in a direct manner (for example, a cofactor or chaperone molecule) or by an indirect mechanism (for example, activation or recruitment of host activities). However, EPEC infection of host cells either prior to, at the same time as, or after Yersinia infection did not result in further modification of the Yersinia-delivered Tir molecule to the T" form (data not shown). This implies that other EPEC factors need to be coexpressed or codelivered with Tir to enable its full modification in the host cell. This could reflect a labile or nondiffusible nature of factors injected into the host cell or the requirement for such factors to associate with or modify Tir prior to its delivery into the host cell. Unsuccessful attempts have been made to identify these factors by cointroducing DNA fragments from the 3′ region of LEE (encodes all known EPEC type III secreted and translocated factors) with the tir region into Yersinia. Possible explanations for this include the participation of multiple nonadjacent gene products, the absence of a required gene(s) from the 3′ or entire LEE region, or the inability of Yersinia to deliver such factors into host cells via its type III machinery. Alternative approaches will be required to identify the putative Tir modification accessory factors.
The absence of a fully modified functional Tir molecule following its introduction into host cells in an EPEC-independent mechanism was supported by the finding that the Yersinia-delivered partially unmodified Tir molecule was not tyrosine phosphorylated, an event essential for Tir function (21). This also suggests that this Tir tyrosine phosphorylation requires either prior modification of Tir to the T" form or additional EPEC accessory factors or stimulation of host activities.
A recent publication (13) suggests that the detection of Tir in the host cell cytoplasmic fraction may be due to its release from the bacterial cells following their exposure to Triton during the fractionation procedure. It should be noted that although this is a possible form of contamination, it appears that in our hands, this process is minor and insignificant. Previous work demonstrated that Triton X-100 (1% final concentration) extraction of radiolabeled EPEC, grown under conditions used to infect host cells, did not release significant levels of bacterial proteins (25). In addition, no Tir-related bands were detected in Triton X-100-extracted soluble fractions infected with EPEC Tir translocation-defective strains (23). This is reiterated in Fig. 1B, where the Tir unmodified form is only detected in the insoluble fraction of the espA mutant infected cells, unlike EPEC (3-h) infection, where it is evident in all three fractions. Analysis of the detergent-fractionated samples provided by Gauthier et al. (13) reveals multiple Tir-related breakdown products in the insoluble fraction as reported earlier (21, 23). However, unlike Gauthier et al., we do not detect these Tir breakdown forms in the membrane fraction (21, 23) (this work and unpublished results). These authors also suggest that the detection of the contaminating Tir forms might be due to the increased sensitivity of their anti-Tir monoclonal antibody. This does not appear to be a suitable explanation as we do not detect such TirHSV breakdown products with anti-HSV monoclonal antibodies in the detergent-isolated cytoplasmic or membrane fractions, though they are detected in the insoluble fraction (reference 21 and this work). The Tir host distribution profile we consistently obtain is very similar to that obtained by their ultracentrifugation procedure. Together this argues that the detergent lysis of EPEC observed by these authors (13) might be due to the quality of the reagents used or their fractionation procedure. These authors also detect the unmodified and modified Tir forms in the ultracentrifugation-isolated cytoplasmic and membrane fractions (in the absence of Tir lysis breakdown products), supporting the saturable nature of Tir modification, which then leads to the gradual accumulation of intermediates in the cytoplasmic fraction at later time points.
The ability of Yersinia to secrete the EPEC Tir molecule, presumably through its type III secretion apparatus, was not a surprising finding, as many type III substrates have been found to be secreted by homologous systems in other pathogens (2, 38). Indeed, Tir is also secreted by Salmonella enterica serovar Typhimurium (B. Kenny, unpublished data) and Shigella flexneri (11). What was more surprising was the ability of Yersinia to inject Tir into host cells, which was also informative on the role of the Tir chaperone, CesT. In the Yersinia system both Tir secretion and translocation processes were found to be able to function, albeit very inefficiently, in the absence of CesT. This supports the results of Elliott et al. (11), who in contrast to Abe et al. (1) reported an EPEC CesT-independent secretion mechanism, which they postulated was analogous to that reported for the Yersinia YopE protein (3). The Yersinia system also revealed that CesT is an efficiency factor for Tir secretion and translocation into host cells. However, in contrast to the EPEC system where CesT was required for Tir stability (1, 11) no such role was apparent with the Yersinia system (Fig. 3B). This indicates the absence of a protease activity in Yersinia (a potential regulatory mechanism), or the ability of a Yersinia-encoded chaperone protein to interact with and stabilize Tir. The absence of a CesT stability function within Yersinia implies that it acts at another level, for example by targeting Tir to the secretion apparatus or maintaining it in a secretion-competent conformation.
In conclusion, we have shown using three different approaches that the Tir molecule does not encode sufficient information in its sequence to enable its complete modification within host cells, leading to its intimin-dependent actin-nucleating activity. Our studies predict that EPEC encodes additional factors that have to be coexpressed or codelivered with Tir to facilitate full modification in host cells. Our use of an effector-depleted Yersinia strain as a delivery vector of heterologous type III secreted proteins should prove useful in studying functional requirements of other type III effector molecules or investigating putative dual functions of molecules which are required for the delivery process.
ACKNOWLEDGMENTS
This work was funded by the Wellcome Trust under a career development fellowship to B.K., with additional support provided by a Royal Society research grant.
Thanks go to Hans Wolf-Watz (Umea University, Umea, Sweden) for providing the Yersinia multiple deletion strain, H. Mellor (Biochemistry Department, Bristol University) for providing HEK293 cells, J. Tavare (Biochemistry Department, Bristol University) for providing pcDNA3, and Mark Jepson (Cell Imaging Facility, Bristol University) for his constructive evaluation of the manuscript.
REFERENCES
- 1.Abe A, de Grado M, Pfuetzner R A, Sánchez-SanMartín C, DeVinney R, Puente J L, Strynadka N C J, Finlay B B. Enteropathogenic Escherichia coli translocated intimin receptor, Tir, requires a specific chaperone for stable secretion. Mol Microbiol. 1999;33:1162–1175. doi: 10.1046/j.1365-2958.1999.01558.x. [DOI] [PubMed] [Google Scholar]
- 2.Anderson D M, Fouts D E, Collmer A, Schneewind O. Reciprocal secretion of proteins by the bacterial type III machines of plant and animal pathogens suggests universal recognition of mRNA targeting signals. Proc Natl Acad Sci USA. 1999;96:12839–12843. doi: 10.1073/pnas.96.22.12839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson D M, Schneewind O. A mRNA signal for the type III secretion of Yop proteins by Yersinia enterocolitica. Science. 1997;278:1140–1143. doi: 10.1126/science.278.5340.1140. [DOI] [PubMed] [Google Scholar]
- 4.Batchelor M, Prasannan S, Daniell S, Reece S, Connerton I, Bloomberg G, Dougan G, Frankel G, Matthews S. Structural basis for recognition of the translocated intimin receptor (Tir) by intimin from enteropathogenic Escherichia coli. EMBO J. 2000;19:2452–2464. doi: 10.1093/emboj/19.11.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornelis G R, Boland A, Boyd A P, Geuijen C, Iriarte M, Neyt C, Sory M P, Stainier I. The virulence plasmid of Yersinia, an antihost genome. Microbiol Mol Biol Rev. 1998;62:1315–1352. doi: 10.1128/mmbr.62.4.1315-1352.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Grado M, Abe A, Gauthier A, Steele-Mortimer O, DeVinney R, Finlay B B. Identification of the intimin-binding domain of Tir of enteropathogenic Escherichia coli. Cell Microbiol. 1999;1:7–17. doi: 10.1046/j.1462-5822.1999.00001.x. [DOI] [PubMed] [Google Scholar]
- 7.Deibel C, Kramer S, Chakraborty T, Ebel F. EspE, a novel secreted protein of attaching and effacing bacteria, is directly translocated into infected host cells, where it appears as a tyrosine-phosphorylated 90 kDa protein. Mol Microbiol. 1998;28:463–474. doi: 10.1046/j.1365-2958.1998.00798.x. [DOI] [PubMed] [Google Scholar]
- 8.Donnenberg M S, Calderwood S B, Donohue-Rolfe A, Keusch G T, Kaper J B. Construction and analysis of TnphoA mutants of enteropathogenic Escherichia coli unable to invade HEp-2 cells. Infect Immun. 1990;58:1565–1571. doi: 10.1128/iai.58.6.1565-1571.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donnenberg M S, Kaper J B, Finlay B B. Interactions between enteropathogenic Escherichia coli and host epithelial cells. Trends Microbiol. 1997;5:109–114. doi: 10.1016/S0966-842X(97)01000-7. [DOI] [PubMed] [Google Scholar]
- 10.Donnenberg M S, Tacket C O, James S P, Losonsky G, Nataro J P, Wasserman S S, Kaper J B, Levine M M. Role of the eaeA gene in experimental enteropathogenic Escherichia coli infection. J Clin Investig. 1993;92:1412–1417. doi: 10.1172/JCI116717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elliott S J, Hutcheson S W, Dubois M S, Mellies J L, Wainwright L A, Batchelor M, Frankel G, Knutton S, Kaper J B. Identification of CesT, a chaperone for the type III secretion of Tir in enteropathogenic Escherichia coli. Mol Microbiol. 1999;33:1176–1189. doi: 10.1046/j.1365-2958.1999.01559.x. [DOI] [PubMed] [Google Scholar]
- 12.Elliott S J, Wainwright L A, McDaniel T K, Jarvis K G, Deng Y K, Lai L C, McNamara B P, Donnenberg M S, Kaper J B. The complete sequence of the locus of enterocyte effacement (LEE) from enteropathogenic Escherichia coli E2348/69. Mol Microbiol. 1998;28:1–4. doi: 10.1046/j.1365-2958.1998.00783.x. [DOI] [PubMed] [Google Scholar]
- 13.Gauthier A, de Grado M, Finlay B B. Mechanical fractionation reveals structural requirements for enteropathogenic Escherichia coli Tir insertion into host membranes. Infect Immun. 2000;68:4344–4348. doi: 10.1128/iai.68.7.4344-4348.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hakansson S, Galyov E E, Rosqvist R, Wolf-Watz H. The Yersinia YpkA Ser/Thr kinase is translocated and subsequently targeted to the inner surface of the HeLa cell plasma membrane. Mol Microbiol. 1996;20:593–603. doi: 10.1046/j.1365-2958.1996.5251051.x. [DOI] [PubMed] [Google Scholar]
- 15.Hartland E L, Batchelor M, Delahay R M, Hale C, Matthews S, Dougan G, Knutton S, Connerton I, Frankel G. Binding of intimin from enteropathogenic Escherichia coli to Tir and to host cells. Mol Microbiol. 1999;32:151–158. doi: 10.1046/j.1365-2958.1999.01338.x. [DOI] [PubMed] [Google Scholar]
- 16.Hartland E L, Daniell S J, Delahay R M, Neves B C, Wallis T, Shaw R K, Hale C, Knutton S, Frankel G. The type III protein translocation system of enteropathogenic Escherichia coli involves EspA-EspB protein interactions. Mol Microbiol. 2000;35:1483–1492. doi: 10.1046/j.1365-2958.2000.01814.x. [DOI] [PubMed] [Google Scholar]
- 17.Hubbard S R, Mohammadi M, Schlessinger J. Autoregulatory mechanisms in protein-tyrosine kinases. J Biol Chem. 1998;273:11987–11990. doi: 10.1074/jbc.273.20.11987. [DOI] [PubMed] [Google Scholar]
- 18.Hueck C J. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev. 1998;62:379–433. doi: 10.1128/mmbr.62.2.379-433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jarvis K G, Kaper J B. Secretion of extracellular proteins by enterohemorrhagic Escherichia coli via a putative type III secretion system. Infect Immun. 1996;64:4826–4829. doi: 10.1128/iai.64.11.4826-4829.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalman D, Weiner O D, Goosney D L, Sedat J W, Finlay B B, Abo A, Bishop J M. Enteropathogenic E. coli acts through WASP and Arp2/3 complex to form actin pedestals. Nat Cell Biol. 1999;1:389–391. doi: 10.1038/14087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kenny B. Phosphorylation of tyrosine 474 of the enteropathogenic Escherichia coli (EPEC) Tir receptor molecule is essential for actin nucleating activity and is preceded by additional host modifications. Mol Microbiol. 1999;31:1229–1241. doi: 10.1046/j.1365-2958.1999.01265.x. [DOI] [PubMed] [Google Scholar]
- 22.Kenny B, Abe A, Stein M, Finlay B B. Enteropathogenic E. coli (EPEC) protein secretion is induced in response to factors similar to those of the gastrointestinal tract. Infect Immun. 1997;65:2606–2612. doi: 10.1128/iai.65.7.2606-2612.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kenny B, DeVinney R, Stein M, Reinscheid D J, Frey E A, Finlay B B. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell. 1997;91:511–520. doi: 10.1016/s0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- 24.Kenny B, Finlay B B. Intimin-dependent binding of enteropathogenic Escherichia coli to host cells triggers novel signaling events, including tyrosine phosphorylation of phospholipase C gamma. Infect Immun. 1997;65:2528–2536. doi: 10.1128/iai.65.7.2528-2536.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kenny B, Finlay B B. Protein secretion by enteropathogenic Escherichia coli is essential for transducing signals to epithelial cells. Proc Natl Acad Sci USA. 1995;92:7991–7995. doi: 10.1073/pnas.92.17.7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kenny B, Jepson M. Targeting of an enteropathogenic E. coli (EPEC) effector protein to host mitochondria. Cell Microbiol. 2000;2:579–590. doi: 10.1046/j.1462-5822.2000.00082.x. [DOI] [PubMed] [Google Scholar]
- 27.Kenny B, Lai L C, Finlay B B, Donnenberg M S. EspA, a protein secreted by enteropathogenic Escherichia coli, is required to induce signals in epithelial cells. Mol Microbiol. 1996;20:313–323. doi: 10.1111/j.1365-2958.1996.tb02619.x. [DOI] [PubMed] [Google Scholar]
- 28.Knutton S, Rosenshine I, Pallen M J, Nisan I, Neves B C, Bain C, Wolff C, Dougan G, Frankel G. A novel EspA-associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. EMBO J. 1998;17:2166–2176. doi: 10.1093/emboj/17.8.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kresse A U, Rohde M, Guzman C A. The EspD protein of enterohemorrhagic Escherichia coli is required for the formation of bacterial surface appendages and is incorporated in the cytoplasmic membranes of target cells. Infect Immun. 1999;67:4834–4842. doi: 10.1128/iai.67.9.4834-4842.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laemmli U. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 31.Lee C A. Type III secretion systems: machines to deliver bacterial proteins into eukaryotic cells? Trends Microbiol. 1997;5:148–156. doi: 10.1016/S0966-842X(97)01029-9. [DOI] [PubMed] [Google Scholar]
- 32.Lee V T, Schneewind O. Type III secretion machines and the pathogenesis of enteric infections caused by Yersinia and Salmonella spp. Immunol Rev. 1999;168:241–255. doi: 10.1111/j.1600-065x.1999.tb01296.x. [DOI] [PubMed] [Google Scholar]
- 33.Levine M M, Nataro J P, Karch H, Baldini M M, Kaper J B, Black R E, Clements M L, O'Brien A D. The diarrheal response of humans to some classic serotypes of enteropathogenic Escherichia coli is dependent on a plasmid encoding an enteroadhesiveness factor. J Infect Dis. 1985;152:550–559. doi: 10.1093/infdis/152.3.550. [DOI] [PubMed] [Google Scholar]
- 34.Luo Y, Frey E A, Pfuetzner R A, Creagh A L, Knoechel D G, Haynes C A, Finlay B B, Strynadka N C. Crystal structure of enteropathogenic Escherichia coli intimin-receptor complex. Nature. 2000;405:1073–1077. doi: 10.1038/35016618. [DOI] [PubMed] [Google Scholar]
- 35.Marchès O, Nougayrède J P, Boullier S, Mainil J, Charlier G, Raymond I, Pohl P, Boury M, De Rycke J, Milon A, Oswald E. Role of Tir and intimin in the virulence of rabbit enteropathogenic Escherichia coli serotype O103:H2. Infect Immun. 2000;68:2171–2182. doi: 10.1128/iai.68.4.2171-2182.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenshine I, Donnenberg M S, Kaper J B, Finlay B B. Signal transduction between enteropathogenic Escherichia coli (EPEC) and epithelial cells: EPEC induces tyrosine phosphorylation of host cell proteins to initiate cytoskeletal rearrangement and bacterial uptake. EMBO J. 1992;11:3551–3560. doi: 10.1002/j.1460-2075.1992.tb05438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenshine I, Ruschkowski S, Stein M, Reinscheid D J, Mills S D, Finlay B B. A pathogenic bacterium triggers epithelial signals to form a functional bacterial receptor that mediates actin pseudopod formation. EMBO J. 1996;15:2613–2624. [PMC free article] [PubMed] [Google Scholar]
- 38.Rosqvist R, Hakansson S, Forsberg A, Wolf-Watz H. Functional conservation of the secretion and translocation machinery for virulence proteins of yersiniae, salmonellae and shigellae. EMBO J. 1995;14:4187–4195. doi: 10.1002/j.1460-2075.1995.tb00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Studier F W, Rosenberg A H, Dunn J J, Dubendorff J W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- 40.Wainwright L A, Kaper J B. EspB and EspD require a specific chaperone for proper secretion from enteropathogenic Escherichia coli. Mol Microbiol. 1998;27:1247–1260. doi: 10.1046/j.1365-2958.1998.00771.x. [DOI] [PubMed] [Google Scholar]
- 41.Warawa J, Finlay B B, Kenny B. Type III secretion-dependent hemolytic activity of enteropathogenic Escherichia coli. Infect Immun. 1999;67:5538–5540. doi: 10.1128/iai.67.10.5538-5540.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolff C, Nisan I, Hanski E, Frankel G, Rosenshine I. Protein translocation into host epithelial cells by infecting enteropathogenic Escherichia coli. Mol Microbiol. 1998;28:143–155. doi: 10.1046/j.1365-2958.1998.00782.x. [DOI] [PubMed] [Google Scholar]