Abstract
Angiopoietin-like protein 3 (ANGPTL3) is an important regulator of lipoproteins by inhibiting both lipoprotein and endothelial lipases. It has been intensively investigated as a drug target for the treatment of dyslipidemia. In the present study, a modified small interfering RNA (siRNA) conjugated with GalNAc ANGsiR10 was characterized by in vivo and in vitro studies for its effect on ANGPTL3 silencing, the reduction of plasma triglycerides (TGs), and cholesterol levels in disease models. The results showed that ANGsiR10 displayed a significant and long-lasting efficacy in reducing blood TG and cholesterol levels in both mice and monkeys. Remarkably, the maximal reductions of plasma TG levels in the hApoC3-Tg mice, a model with high TG levels, and the spontaneous dyslipidemia model of rhesus monkey were 96.3% and 67.7%, respectively, after a single dose of ANGsiR10, with long-lasting effects up to 15 weeks. The cholesterol levels were also reduced in response to treatment, especially the non-HDL-c level, without altering the ApoA/ApoB ratio. This study showed that ANGsiR10 is effective in treating dyslipidemia and is worth further development.
Keywords: MT: Oligonucleotides: Therapies and Applications, angiopoietin-like protein 3, ANGPTL3), triglycerides, cholesterol, dyslipidemia, siRNA
Graphical abstract

A GalNAc-conjugated siRNA targeting angiopoietin-like protein 3 (ANGPTL3) was developed. It was successfully delivered to the liver and significantly inhibited the expression of ANGPTL3. TG and total cholesterol levels were remarkably reduced in both mouse and monkey models of dyslipidemia by a single-dose treatment.
Introduction
Dyslipidemia is a well-established risk factor for atherosclerosis and ischemic heart disease, including coronary heart disease and related myocardial infarction, a cluster of cardiometabolic diseases that is the leading cause of death worldwide. Although various medications can effectively reduce levels of plasma low-density lipoprotein cholesterol (LDL-c),1 the medicine for efficient reduction of triglycerides (TGs) and non-high density lipoprotein cholesterols (non-HDL-c) is still an unmet need. Recently, multiple novel targets were identified. Particularly, targeting angiopoietin-like protein 3 (ANGPTL3) was found to be promising and has been intensively studied.
ANGPTL3 is highly expressed in the liver and has been identified as an important regulator of lipoproteins by inhibiting lipoprotein lipase, which hydrolyzes TGs and phospholipids, and endothelial lipase, an extracellular lipase involved in the catabolism of HDL particles, though the precise mechanisms have yet to be elucidated.2,3 Loss-of-function mutations in ANGPTL3 resulted in decreased levels of TGs, total cholesterol, and non-esterified fatty acids (NEFAs) in mice.4 Moreover, clinical studies suggested a positive correlation between plasma ANGPLT3 levels and carotid/femoral artery intima thickness.5,6 Accordingly, genetic studies have demonstrated that heterozygous carriers of loss-of-function mutations in the ANGPTL3 gene are associated with a 34% decrease in cardiovascular events.7 In addition, ANGPTL3 may have pro-inflammatory and pro-angiogenic effects and a negative effect on cholesterol efflux, implying additional pro-atherosclerotic properties of ANGPTL3 beyond its lipid-lowering activity.8
To date, several experimental modalities have been developed for this target, including monoclonal antibodies (mAbs), small interfering RNA (siRNA), and anti-sense oligonucleotide (ASO) agents.9,10 The first drug targeting ANGPTL3, evinacumab, was approved by the US FDA for the treatment of homozygous familial hypercholesterolemia in 2021. TG reduction up to 93.2% was achieved in patients with severe hypertriglyceridemia.11 Vupanorsen, an N-acetyl galactosamine-conjugated ASO targeting hepatic ANGPTL3 mRNA, reduced TGs by 44% and very-low-density lipoprotein cholesterols (VLDL-c) by 38% in patients with hypertriglyceridaemia.12 Moreover, an siRNA targeting ANGPTL3, ARO-ANG3, was shown to reduce TGs by 47%–53% and VLDL-c by 49%–51% after 16 weeks in a phase 1/2 trial.13
RNA interference (RNAi) therapeutics provide a novel strategy to treat diseases by silencing targets that used to be “undruggable” by traditional medicines. However, there are still hurdles in the development of RNAi-based therapies, including tissue penetration, intracellular delivery and trafficking, degradation, and immune-mediated toxicities.14 Tremendous efforts have been exerted in the field, and major progress has been made. Chemical modification combined with conjugation/complexation has greatly improved the pharmacokinetic and pharmacodynamic properties of RNAi therapeutics.15 The conjugation of chemically modified siRNAs to a synthetic triantennary N-acetylgalactosamine (GalNAc) ligand represents a promising approach for safe and effective targeted delivery of RNAi therapeutics to hepatocytes in vivo,16 and three GalNAc-siRNA therapeutics have been approved in recent years.17,18 In the present study, a GalNAc-conjugated siRNA targeting ANGPTL3 was developed and evaluated for efficacy in both mouse and monkey dyslipidemia models.
Results
In vitro and in vivo screening of siRNA targeting ANGPTL3
siRNAs were designed according to human ANGPTL3 mRNA (NM_014495.3), with particular consideration to avoid highly homologous sequences like ANGPTL4 and ANGPTL8. The siRNAs used in the present study are listed in Table S1. To enhance their stability and specificity, chemical modifications with methoxyl groups, fluorine at 2′ site hydroxyl groups, or phosphorothioates at the phosphonate backbone were placed at certain sites of the sense and anti-sense strands of siRNA. To achieve liver targeting, GalNAc conjugation was placed at the 3′ end of the sense strands of siRNA (Figure S1A). The modification significantly enhanced the siRNA’s stability (Figure S1B). In addition, the tissue distribution assay showed that GalNAc-conjugated ANGsiR10 was enriched in the liver (Figure S1C).
The siRNAs were screened using the dual-luciferase assay as described before.19 The siRNAs that inhibited luciferase activity in a dose-dependent manner were identified as effective (Figure 1A). The inhibitory rate of some of siRNAs was more than 90% at the highest concentration (1 nM). The knockdown efficiency of ANGPTL3 by the siRNAs was further analyzed in Huh7 cells (Figure 1B).
Figure 1.
Screening and validation of siRNAs targeting ANGPTL3
(A and B) The siRNAs were screened in vitro by luciferase assay in HEK293 cells (A) and inhibition assay in Huh7 cells (B). (C–E) The effect of ANGPTL3 silencing was further evaluated in C57BL/6 mice (C), ob/ob mice (D), and hApoC3-Tg mice (E) by measuring mRNA levels of ANGPTL3 in the liver by real-time PCR. (F) The serum ANGPTL3 level was measured by ELISA in hApoC3-Tg mice. Data are represented as mean ± SEM. NC, negative control; PC1/PC, positive control. Unpaired t test was performed comparing the ANGsiR10 group with the negative control. ∗p < 0.05; ∗∗p < 0.01.
The most potent siRNAs, ANGsiR10 (sequence shown in Figure S1A) and ANGsiR11, with a sequence homologous to the target gene of not only human but also monkeys and mice, were then evaluated in vivo. Both siRNAs showed effectiveness in silencing ANGPTL3 in C57BL/6 mice in the liver (Figure 1C). Then, we used ANGsiR10 to further evaluate the efficacy of silencing ANGPTL3 in the liver of an obese mouse model, ob/ob mice. ANGsiR10 showed dose-dependent efficiency in silencing ANGPTL3 in ob/ob mice (Figure 1D). Furthermore, a dose-dependent reduction of ANGPTL3 was seen in both the liver (Figure 1E) and serum (Figure 1F) in a high TG model of hApoC3-Tg mice after the treatment of ANGsiR10. However, the alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels of the hApoC3-Tg mice were not significantly changed by the treatment (Figures S2A and S2B).
TG and cholesterol levels were remarkably decreased by ANGsiR10 in hApoC3-Tg mice
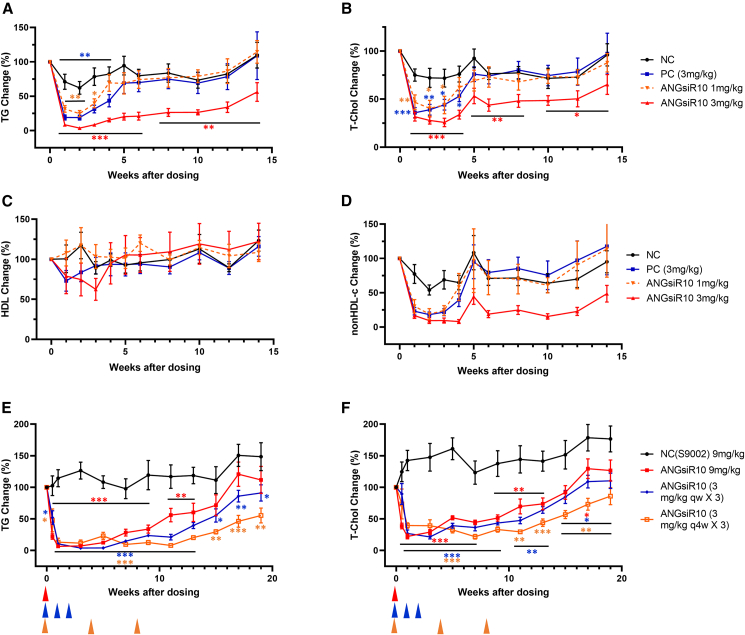
To further assess efficacy, hApoC3-Tg mice were treated with a single injection of ANGsiR10 (1 or 3 mg/kg subcutaneously [s.c.]). Serum TG and total cholesterol levels were monitored for 14 weeks. TG levels were significantly decreased in both treatment groups (Figure 2A). The highest inhibition rate was 96.3%. Total cholesterol levels were also significantly decreased up to 75.3% in both groups with dose dependence (Figure 2B). However, the HDL-c levels were not changed (Figure 2C), but the non-HDL-c levels were significantly decreased (Figure 2D). Both low and high doses of ANGsiR10 showed efficacy in lowering lipids, while the high-dose group showed a more potent and sustainable efficacy with the inhibition of TG and cholesterol sustained up to 14 weeks.
Figure 2.
ANGsiR10 significantly reduced the plasma TG and cholesterol levels in hApoC3-Tg mice
hApoC3-Tg mice was treated with a single subcutaneous injection of ANGsiR10 (1 or 3 mg/kg). (A–D) TG (A), total cholesterol (B), and HDL-c (C) levels were monitored up to 14 weeks, while non-HDL-c (D) was calculated as the level of the total cholesterol minus that of the HDL-c. Multiple-dose regimens were performed using hApoC3-Tg mice. ANGsiR10 was administered in three regimens, e.g., 9 mg/kg × 1, 3 mg/kg qw × 3, and 3 mg/kg q4w × 3. (E and F) Both TG (E) and total cholesterol (F) levels were monitored up to 19 weeks. NC, negative control; PC, positive control. Data are represented as mean ± SEM of the percentage of baseline levels. Paired t test was performed compared with the baseline level. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
To further explore the regimen, multiple-dose regimens were carried out using the hApoC3-Tg mice. ANGsiR10 was administered in three scenarios, e.g., 3 mg/kg once weekly (qw) × 3, 3 mg/kg every four weeks (q4w) × 3, and 9 mg/kg × 1. TG and total cholesterol levels were significantly decreased in all treatment groups (Figures 2E and 2F). The maximum inhibition rate was similar in the three groups. The 3 mg/kg q4w group was superior to the other groups, where the inhibition was still significant for TGs (inhibited by 45% at week 19) and total cholesterol (inhibited by 27% at week 17), when the TG and total cholesterol levels returned to the baseline of the single-dose group. The effect of the 3 mg/kg qw group was somewhere in between.
Plasma lipids were persistently decreased by single-dose treatment of ANGsiR10 in a dyslipidemic monkey model with spontaneous metabolic syndrome
To further evaluate its efficacy, ANGsiR10 was tested in a dyslipidemic monkey model with spontaneous metabolic syndrome, which is characterized by elevated blood TG levels, obesity, and other metabolic changes. Before treatment, a single-dose pharmacokinetic (PK) study was performed in one male and one female healthy monkey (Figure S3).
Twelve dyslipidemic monkeys were divided into two groups, e.g., the ANGsiR10 group (n = 8), which was treated with a single dose of ANGsiR10 (9 mg/kg, s.c.), and the control group, treated with a single dose of scramble siRNA (9 mg/kg, s.c.) (n = 4). Plasma lipids and lipoproteins were monitored for up to 15 weeks. Liver biopsy was performed before dosing and at 4 and 15 weeks after dosing.
TG levels were decreased after 1 week of treatment and reached maximal reduction of 67.7% compared with control at 4 weeks after treatment (Figures 3A and S4A). At the end of the study (week 15), the inhibitory effect on TG levels was still maintained with a 21.9% decrease compared with baseline levels. The total cholesterol and non-HDL-c levels were also significantly decreased with a bottom at 4 weeks after the dosing (Figures 3B, 3C, S4B, and S4C), while the HDL-c and LDL-c levels were not changed (Figures 3D, 3E, S4D, and S4E). The changes in total cholesterol correlated well with non-HDL-c (Figure S4F), indicating that the decrease in total cholesterol was mainly from non-HDL-c. Lipoprotein A (LPA) levels were transiently decreased 1–2 weeks after the treatment of ANGsiR10 but recovered at week 3 (Figure S4G). ApoA levels were significantly decreased (Figures 3F and S4H), while ApoB levels showed a downward trend but were only significant at week 3 (Figures 3G and S4I). Hence, the ApoA/ApoB ratio was not significantly changed in response to treatment (Figures 3H and S4J). ApoC3 levels were moderately decreased from week 9 and went back to basal levels at week 15 (Figure S4K). No obvious change in liver fat deposition was found after treatment as shown by oil red O staining (Figure S5).
Figure 3.
Percentage change in plasma lipids in dyslipidemic monkeys after the treatment of ANGsiR10
Twelve monkeys were divided into two groups and administered ANGsiR10 (9 mg/kg) or scramble siRNA as a negative control. (A–G) Blood samples were collected before and after treatment (weekly up to 5 weeks and then biweekly) and analyzed for TG (A), total cholesterol (B), non-HDL-c (C), HDL-c (D), LDL-c (E), ApoA (F), and ApoB (G). (H) The ApoA/ApoB ratio was calculated. Data are represented as mean ± SEM of the percentage of baseline levels. For each parameter, paired t test was performed comparing the value after treatment with the average baseline level. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
The silencing efficiency of ANGsiR10 was assessed by measuring the expression levels of ANGPTL3 in liver (mRNA) as well as in serum (protein) using real-time PCR and ELISA, respectively. In accordance with the lipid-lowering effect, mRNA levels of ANGPTL3 in the liver were decreased by 88.8% 4 weeks after dosing and remained depressed at 39.1% at the end of the study (Figure 4A). The serum levels of ANGPTL3 were also decreased by 85.1% at week 4 and 40.3% at the end of the study (Figure 4B). Histopathology of the liver biopsy samples did not show signs of inflammation and/or damage after treatment (Figure 4C). The levels of ALT and AST were significantly decreased after treatment in both the control and treatment groups (Figures 4D and 4E), but the potential mechanisms and clinical relevance remain to be elucidated.
Figure 4.
The expression of ANGPTL3 was inhibited in the monkeys treated with ANGsiR10
Before and after treatment with ANGsiR10 or scramble siRNA as a negative control, liver biopsy and blood sampling were performed and analyzed for the expression of ANGPTL3 by real-time PCR for mRNA and ELISA for protein. (A and B) The relative mRNA levels in the liver (A) and the protein levels in the serum (B) were significantly decreased in the ANGsiR10-treated group. (C) No sign of inflammation and/or tissue damage was observed in H&E-stained sections of livers in either the control or treatment group. (D and E) The ALT (D) and AST (E) levels in plasma were measured. Data are represented as mean ± SEM. Paired t test was performed compared with the baseline level. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
In addition, body weight and waist size were significantly decreased at the end of the study in the ANGsiR10 group (Figures 5A and 5B). Blood pressure was not affected by treatment (Figures 5C and 5D). Fasting plasma glucose and insulin levels were not changed compared with baseline (Figures 5E and 5F). There were no significant changes in white blood cell counts or high-sensitivity C-reactive protein (hsCRP) during the study (Figures 5G and 5H).
Figure 5.
The effect of ANGsiR10 on physiological parameters was investigated in monkeys
The body weight (BW) (A); waist (B); systolic/diastolic blood pressure (SBP/DBP) (C and D); fasting plasma glucose (FPG) (E); insulin (F); white blood cell (WBC) count (G); and high-sensitivity C-reactive protein (hsCRP) (H) were measured at baseline and 4 and 15 weeks after the dosing. Scramble siRNA was used as a negative control. Data are represented as mean ± SEM. Paired t test was performed compared with the baseline level. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
Discussion
In the present study, we developed a chemically modified siRNA ANGsiR10 conjugated to GalNAc that efficiently reduced the expression of ANGPTL3 both in vivo and in vitro. Its efficacy was assessed using both mouse models and a dyslipidemic monkey model of spontaneous metabolic syndrome. In particular, beneficial effects of lowering plasma lipids and body weight were observed in dyslipidemic monkeys.
The reduction of plasma TG levels was the most robust phenotype in response to treatment with ANGsiR10, which is consistent with effects of antibody treatment, as well as ASO targeting of ANGPTL3.20,21,22 Inhibition of TGs by ANGsiR10 was significant up to 15 weeks, while the effects from antibody lasted only 5 weeks in the respective monkey studies,20 indicating the advantage of siRNA therapeutics. Elevated plasma TG levels are strongly associated with increasing risks of atherosclerosis, myocardial infarction, coronary heart disease, and ischemic stroke.23,24,25,26 Our results showed that ANGsiR10 has a profound effect in lowering plasma TGs and might provide more benefits in reducing the risk of cardiovascular diseases.
Mounting evidence has established the link between cholesterol levels and atherosclerosis.27 Increased LDL-c and decreased HDL-c levels are well-established biomarkers for increased risk of atherosclerosis.28,29 The ratio of ApoA, the major apolipoprotein of HDL, and ApoB, the major apolipoprotein of atherogenic lipoproteins such as VLDLs, intermediate-density lipoproteins (IDLs), and LDLs, is also a widely used biomarker for the development and progression of atherosclerosis and cardiovascular diseases.30,31,32 We measured these proteins in the animal models to assess the effect of ANGsiR10 in lowering lipid levels and risk reduction of cardiovascular disease.
Total cholesterol plasma levels were also significantly reduced by ANGsiR10 treatment in our animal models. The non-HDL-c levels were also obviously reduced upon ANGsiR10 treatment, while the reduction of LDL-c levels was not significant, which is consistent with studies of anti-ANGPTL3 mAbs in monkeys.20 This finding was consistent with the LDL-c-lowering effect of ARO-ANG3, reported only in patients with familial hypercholesterolemia with elevated LDL-c.33 Whether the knockdown of ANGPTL3 is effective in patients with moderately increased LDL-c levels awaits further verification.
The levels of ApoA and ApoB, but not the ApoA/ApoB ratio, were significantly decreased after treatment, and there was a transient reduction in the HDL-c levels at the beginning of treatment, with a return to normal levels after a couple of weeks in our study. These results are consistent with a human genetic study.34,35 These results, together with previous studies, suggest that the dosage of the siRNA and the treatment regimen for silencing ANGPTL3 might be critical for the protective effects of HDL-c and, therefore, worthy of further careful characterization. In addition, the mouse study showed that reduction of ANGPTL3 expression is protective not only against hyperlipidemia but also atherosclerosis, perhaps due to the enhanced catabolism and clearance of TG-rich lipoproteins.36 Nevertheless, the anti-atherogenic effect of anti-ANGPTL3 treatment awaits further investigation in populations at high risk for atherosclerosis. Vupanorsen, an ASO of ANGPTL3 mRNA, reduced ApoC3 by 58%.12 However, ApoC3 was only transiently decreased in the monkey study.
The majority of the siRNA is rapidly taken up by liver.37 ANGPTL3 is exclusively expressed in the liver, making it an ideal target for RNAi therapeutics since delivery to liver is one of the most mature techniques in the field.38 The delivery techniques of RNAi therapeutics have continuously advanced to overcome its innate deficiencies, such as instability, poor PK profiles, poor cellular internalization, etc., which will facilitate future applications in clinics.39,40,41,42 Our PK study in monkeys showed a typical PK profile for GalNAc-conjugated siRNA that peaked around 2–4 h after dosing and was eliminated from the circulation after 48 h. However, the effects on ANGPTL3 silencing and the reduction of plasma lipids were long lasting in both mice and monkeys. More importantly, the lowering effect of a single-dose treatment on blood lipids in mice achieved a comparable efficacy with multiple dosing, which is in agreement with the properties of other GalNAc-siRNA drugs and supportive of less-frequent administration for clinical applications.
Besides therapies targeting only ANGPTL3, targeting the ANGPTL3/8 complex has also attracted attention. Very recently, an antibody blocking the binding of the ANGPTL3/8 complex to lipoprotein lipase (LPL) was developed and significantly reduced serum TG levels in a hypertriglyceric mice model.43 ANGPTL3 inhibition of LPL requires complex formation with ANGPTL8, which is not required for its inhibition of endothelial lipase (EL).44 The effect of the anti-ANGPTL3/8 complex antibody on HDL-c and LDL-c may be different from the treatment of ANGPTL3 silencing since the effects seem to be EL dependent.45,46
In summary, a comprehensive preclinical evaluation of an siRNA therapeutic, ANGsiR10, was performed. The results showed that ANGsiR10 effectively inhibited the expression of ANGPTL3 and reduced the plasma levels of TG and cholesterol in both mice and monkey. Further investigation is needed to explore the potential clinical application.
Materials and methods
Animals
All mouse procedures were conducted in compliance with protocols approved by the Ribo Life Science Institutional Animal Care and Use Committee. All mice were maintained in a temperature-controlled barrier facility under specific-pathogen-free conditions with a 12 h light/dark cycle and were given free access to water and food.
The study with dyslipidemic monkeys was approved by the Ethics Committee of Peking University and carried out in the animal facility of Peking University accredited by the Association for Accreditation of Laboratory Animal Care (AAALAC). Monkeys were individually housed with a 12 h light/dark cycle. The environmental temperature was between 18°C and 24°C, and the humidity was between 40% and 70%. The monkeys had free access to water and were fed ad libitum with standard pellet monkey chow (Beijing HFK Bio-Technology, Beijing, China).
All experiments involving animals conformed to the regulations for experimental animals of the People’s Republic of China.
Characterization of siRNA targeting ANGPTL3
All siRNAs used in this study were designed and synthesized by Suzhou Ribo Life Science (Kunshan, China) (Table S1). siRNAs targeting human ANGPTL3 were designed and screened using the dual-luciferase (Firefly-Renilla) assay. HEK293A cells were plated into 24-well plates at ∼1 × 105 cells/well 24 h before transfection. A psi-CHECK vector (100 ng/well), which carries both the Firefly luciferase gene and Renilla luciferase gene, as well as the target site of the siRNA to be tested, was transfected into HEK293A cells using Lipofectamine 2000 (Invitrogen, Waltham, MA, USA) at ∼70% confluence, together with the siRNA at the appropriate concentration. The siRNA target sequence was inserted into the multiple cloning site downstream of the coding region of Renilla luciferase (Renilla). Firefly luciferase (Firefly) was the internal reference. The activity of both luciferases was determined 24 h after transfection using a Synergy HT fluorometer (BioTek Instruments, Winooski, VT, USA). Cells were lysed with passive lysis buffer (Promega, Madison, WI, USA). Cell lysate (10 μL) was transferred into a 96-well plate, and the substrate reagents were added. Firefly and Renilla luciferase activities were evaluated using the dual-luciferase reporter assay system (Promega) according to the manufacturer’s instructions, and Renilla activity was normalized by the Firefly activity. A non-specific siRNA, not targeting any gene in transcripts of human, mouse, or monkey, was used as the negative control in all screening assays. The positive control (PC1) in the luciferase assay is an siRNA targeting the psi-CHECK vector to monitor whether the screening system worked. All experiments were repeated at least three times.
The inhibitory rate of ANGPTL3 by the siRNAs was evaluated using both in vitro and in vivo models. The siRNAs were transfected into Huh7 cell at a final concentration of 50 nM, and the mRNA levels of ANGPTL3 were measured by real-time PCR. The PC was an unmodified siRNA with an identical sequence to ARO-ANG3. The most potent siRNAs in inhibiting ANGPTL3 expression levels were then conjugated with Tris-GalNAc at the 3′ end of the sense strand. GalNAc can specifically bind to the asialoglycoprotein receptor (ASGPR), which is highly expressed on hepatocytes, and results in effective liver-targeted delivery.47,48 GalNAc-conjugated siRNAs were further assessed in vivo.
To evaluate the stability of GalNAc-conjugated ANGsiR10, the siRNA was incubated with human serum (Xenotech, Kansas City, KS, USA) at a final concentration of 2 μM at 37°C up to 72 h and analyzed on 15% PAGE gel with the unmodified ANGsiR10 as the control. Tissue distribution of GalNAc-conjugated ANGsiR10 was also investigated in 6- to 8-week-old male C57BL/6 mice (n = 3) and one 6-year-old male rhesus monkey. A single dose of ANGsiR10 was administered s.c. (3 mg/kg), and multiple tissues were harvested 24 h later. The level of ANGsiR10 was determined by liquid chromatography-mass spectrometry (LC-MS).
siRNAs were administered subcutaneously in 6- to 8-week-old female C57BL/6 mice (Beijing Vital River Laboratory Animal Technology, Beijing, China) (n = 5 per group). The livers were collected on the eighth day after dosing and analyzed for ANGPTL3 expression levels by real-time PCR. The best siRNA ANGsiR10 was further characterized at three doses (0.3, 1, and 3 mg/kg) in the 6- to 8-week-old female ob/ob mice (Cavens Biogle [Suzhou] Model Animal Research, Suzhou, China) (n = 3–7 per group) and in high TG hApoC3 transgenic mice (The Jackson Laboratory, Bar Harbor, ME, USA) (n = 8–10 per group, male:female [M:F] = 1:1) at two doses (1 and 3 mg/kg). The inhibition of ANGPTL3 expression in liver was evaluated by real-time PCR. The serum levels of ANGPTL3 were measured in the hApoC3 transgenic mice by ELISA (R&D, cat. no. MANL30). In addition, those serum samples were sent to Dian Diagnostics to measure ALT and AST by biochemical analyzer.
Evaluation of the efficacy in hApoC3-Tg mice
hApoC3 transgenic mice (6 to 8 weeks old) were purchased from The Jackson Laboratory and bred in the Beijing Laboratory Animal Research Center. Mice were maintained on regular chow diet (Beijing Keao Xieli Feed, Beijing, China). To establish a baseline lipid profile, blood samples were collected prior to the first dosing. On study day 0, mice were sorted into treatment groups (n = 6 per group) and then administered a single s.c. injection of ANGsiR10 at two different doses, e.g., 1 and 3 mg/kg. A non-specific siRNA, not targeting any gene in transcripts of human, mouse, or monkey, was used as the negative control. ANG65695, an siRNA prepared following the protocol provided in the patent of ARO-ANG3 (patent no. US 2019/0,078,089 A1)49 was used as the PC. Blood samples were collected weekly for up to 6 weeks and then biweekly up to 14 weeks after dosing, and serum TG, total cholesterol, and HDL-c levels were analyzed by the automated chemistry analyzer (Cobas 8000 cc701, Roche, Basel, Switzerland). ANGPTL3 mRNA levels were measured by real-time PCR.
Multiple-dose regimens were also performed using the hApoC3 transgenic mice. Three treatment groups (n = 4–6 per group) were set up to evaluate three regimes, e.g., 9 mg/kg × 1, 3 mg/kg qw × 3, and 3 mg/kg q4w × 3. Blood samples were collected at 0.5 and 1 week and then biweekly up to 19 weeks after dosing, and serum TG and total cholesterol levels were analyzed by the automated chemistry analyzer (Cobas 8000 cc701, Roche). ANGPTL3 mRNA levels were measured by real-time PCR.
Spontaneous dyslipidemic monkey model
In brief, 12 male rhesus monkeys with spontaneous metabolic syndrome (dyslipidemic) having significantly elevated TG levels as described in a previous study were used in the present study.50 The animals were divided into two groups, 8 in the ANGsiR10 treatment group and 4 in the control group treated with scramble GalNAc-siRNA. Both baseline blood and liver biopsy samples were collected prior to dosing. The liver biopsy was performed using a biopsy needle (SC18/10, GMT Medical, Beijing, China) under the guidance of ultrasound (GE vivid E9) in anesthetized animals. A single dose of either ANGsiR10 or scramble siRNA (9 mg/kg) was administered s.c., and animals were followed via blood samples for up to 15 weeks to test efficacy. Biochemistry analysis was performed using the automated chemistry analyzer (Cobas C311, Roche). Insulin was analyzed by the Roche Cobas e411 analyzer. Blood cell counting was performed using IDEXX ProCyte Dx. (IDEXX Laboratories, Westbrook, ME, USA). Plasma levels of LPA and ApoC3 were analyzed using monkey ELISA kits (Bluegene, cat. nos. E09L0258 and E09A0516, respectively).
For H&E staining, the liver tissue was fixed with 4% paraformaldehyde immediately after biopsy, paraffin embedded, dewaxed, rehydrated, and then stained with H&E using standard protocols. For oil red O staining, frozen sections were stained with oil red O according to standard protocols.
The single-dose PK profile was also analyzed in one male and one female healthy monkey. Blood samples were collected at 0, 0.25, 0.5, 1, 2, 4, 6, 24, and 48 h after dosing. The serum was analyzed by mass spectrum (Thermo Fisher Scientific Q Exactive FocusTM HRAM) for both the sense and anti-sense strands of AGNsiR10.51,52
Statistical analyses
Data were analyzed by unpaired t tests (Figures 1, 2, and 3) or paired t tests (Figures 4, 5, and S4) where ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001. GraphPad Prism 9 was used for statistical analyses.
Acknowledgments
We express our sincere appreciation to Suzhou Ribo Life Science Co., Ltd., for providing all siRNAs and for technical support in siRNA delivery. The present study was supported by the National Key Research and Development Program of China (2018YFA0801405); Suzhou Ribo Life Science Co., Ltd.; National Key Research and Development Program of China (2018YFA0507603), and National Natural Science Foundation of China (81970690, 81630008, and 81770376).
Author contributions
J.W., X.Z., S.G., and R.-p.X. conceived the idea of the study and contributed to the writing and revisions; J.W., W.Z., and S.Z. analyzed the data. W.Z., S.Z., Y.Y., W.W., W.C., L.X., H.S., and H.Z. collected the data.
Declaration of interests
The present study was partially supported by Suzhou Ribo Life Science Co., Ltd. S.Z., H.Z., and S.G. are employees of Ribo. We have fully disclosed these interests and have developed an approved plan to manage any potential conflicts that may arise from such an arrangement.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.omtn.2022.11.023.
Contributor Information
Rui-Ping Xiao, Email: xiaor@pku.edu.cn.
Shan Gao, Email: gaos@ribolia.com.
Xiuqin Zhang, Email: zhangxq@pku.edu.cn.
Supplemental information
Data availability
All data included in this study are available upon request by contacting the corresponding authors (zhangxq@pku.edu.cn or gaos@ribolia.com).
References
- 1.Tall A.R., Thomas D.G., Gonzalez-Cabodevilla A.G., Goldberg I.J. Addressing dyslipidemic risk beyond LDL-cholesterol. J. Clin. Invest. 2022;132:e148559. doi: 10.1172/JCI148559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kersten S. Angiopoietin-like 3 in lipoprotein metabolism. Nat. Rev. Endocrinol. 2017;13:731–739. doi: 10.1038/nrendo.2017.119. [DOI] [PubMed] [Google Scholar]
- 3.Dewey F.E., Gusarova V., Dunbar R.L., O'Dushlaine C., Schurmann C., Gottesman O., McCarthy S., Van Hout C.V., Bruse S., Dansky H.M., et al. Genetic and pharmacologic inactivation of ANGPTL3 and cardiovascular disease. N. Engl. J. Med. 2017;377:211–221. doi: 10.1056/NEJMoa1612790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koishi R., Ando Y., Ono M., Shimamura M., Yasumo H., Fujiwara T., Horikoshi H., Furukawa H. Angptl3 regulates lipid metabolism in mice. Nat. Genet. 2002;30:151–157. doi: 10.1038/ng814. [DOI] [PubMed] [Google Scholar]
- 5.Hatsuda S., Shoji T., Shinohara K., Kimoto E., Mori K., Fukumoto S., Koyama H., Emoto M., Nishizawa Y. Association between plasma angiopoietin-like protein 3 and arterial wall thickness in healthy subjects. J. Vasc. Res. 2007;44:61–66. doi: 10.1159/000098153. [DOI] [PubMed] [Google Scholar]
- 6.Ruscica M., Macchi C., Fogacci F., Ferri N., Grandi E., Rizzoli E., D'Addato S., Borghi C., Cicero A.F., Brisighella Heart Study Group Angiopoietin-like 3 and subclinical peripheral arterial disease: evidence from the brisighella heart study. Eur. J. Prev. Cardiol. 2020;27:2251–2254. doi: 10.1177/2047487319884378. [DOI] [PubMed] [Google Scholar]
- 7.Stitziel N.O., Khera A.V., Wang X., Bierhals A.J., Vourakis A.C., Sperry A.E., Natarajan P., Klarin D., Emdin C.A., Zekavat S.M., et al. ANGPTL3 deficiency and protection against coronary artery disease. J. Am. Coll. Cardiol. 2017;69:2054–2063. doi: 10.1016/j.jacc.2017.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lupo M.G., Ferri N. Angiopoietin-like 3 (ANGPTL3) and atherosclerosis: lipid and non-lipid related effects. J. Cardiovasc. Dev. Dis. 2018;5:39. doi: 10.3390/jcdd5030039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jia X., Liu J., Mehta A., Ballantyne C.M., Virani S.S. Lipid-lowering biotechnological drugs: from monoclonal antibodies to antisense therapies-a clinical perspective. Cardiovasc. Drugs Ther. 2021;35:1269–1279. doi: 10.1007/s10557-020-07082-x. [DOI] [PubMed] [Google Scholar]
- 10.Watts G.F., Raal F.J., Chan D.C. Transcriptomic therapy for dyslipidemias utilizing nucleic acids targeted at ANGPTL3. Future Cardiol. 2022;18:143–153. doi: 10.2217/fca-2021-0096. [DOI] [PubMed] [Google Scholar]
- 11.Ahmad Z., Pordy R., Rader D.J., Gaudet D., Ali S., Gonzaga-Jauregui C., Ponda M.P., Shumel B., Banerjee P., Dunbar R.L. Inhibition of angiopoietin-like protein 3 with evinacumab in subjects with high and severe hypertriglyceridemia. J. Am. Coll. Cardiol. 2021;78:193–195. doi: 10.1016/j.jacc.2021.04.091. [DOI] [PubMed] [Google Scholar]
- 12.Gaudet D., Karwatowska-Prokopczuk E., Baum S.J., Hurh E., Kingsbury J., Bartlett V.J., Figueroa A.L., Piscitelli P., Singleton W., Witztum J.L., et al. Vupanorsen, an N-acetyl galactosamine-conjugated antisense drug to ANGPTL3 mRNA, lowers triglycerides and atherogenic lipoproteins in patients with diabetes, hepatic steatosis, and hypertriglyceridaemia. Eur. Heart J. 2020;41:3936–3945. doi: 10.1093/eurheartj/ehaa689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hussain A., Ballantyne C.M., Saeed A., Virani S.S. Triglycerides and ASCVD risk reduction: recent insights and future directions. Curr. Atheroscler. Rep. 2020;22:25. doi: 10.1007/s11883-020-00846-8. [DOI] [PubMed] [Google Scholar]
- 14.Pecot C.V., Calin G.A., Coleman R.L., Lopez-Berestein G., Sood A.K. RNA interference in the clinic: challenges and future directions. Nat. Rev. Cancer. 2011;11:59–67. doi: 10.1038/nrc2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Fougerolles A., Vornlocher H.P., Maraganore J., Lieberman J. Interfering with disease: a progress report on siRNA-based therapeutics. Nat. Rev. Drug Discov. 2007;6:443–453. doi: 10.1038/nrd2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuda S., Keiser K., Nair J.K., Charisse K., Manoharan R.M., Kretschmer P., Peng C.G., V Kel'in A., Kandasamy P., Willoughby J.L.S., et al. siRNA conjugates carrying sequentially assembled trivalent N-acetylgalactosamine linked through nucleosides elicit robust gene silencing in vivo in hepatocytes. ACS Chem. Biol. 2015;10:1181–1187. doi: 10.1021/cb501028c. [DOI] [PubMed] [Google Scholar]
- 17.Hu B., Zhong L., Weng Y., Peng L., Huang Y., Zhao Y., Liang X.J. Therapeutic siRNA: state of the art. Signal Transduct. Target. Ther. 2020;5:101. doi: 10.1038/s41392-020-0207-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garrelfs S.F., Frishberg Y., Hulton S.A., Koren M.J., O'Riordan W.D., Cochat P., Deschênes G., Shasha-Lavsky H., Saland J.M., Van't Hoff W.G., et al. Lumasiran, an RNAi therapeutic for primary hyperoxaluria type 1. N. Engl. J. Med. 2021;384:1216–1226. doi: 10.1056/NEJMoa2021712. [DOI] [PubMed] [Google Scholar]
- 19.Song X., Wang X., Ma Y., Liang Z., Yang Z., Cao H. Site-specific modification using the 2'-methoxyethyl group improves the specificity and activity of siRNAs. Mol. Ther. Nucleic Acids. 2017;9:242–250. doi: 10.1016/j.omtn.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gusarova V., Alexa C.A., Wang Y., Rafique A., Kim J.H., Buckler D., Mintah I.J., Shihanian L.M., Cohen J.C., Hobbs H.H., et al. ANGPTL3 blockade with a human monoclonal antibody reduces plasma lipids in dyslipidemic mice and monkeys. J. Lipid Res. 2015;56:1308–1317. doi: 10.1194/jlr.M054890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graham M.J., Lee R.G., Brandt T.A., Tai L.J., Fu W., Peralta R., Yu R., Hurh E., Paz E., McEvoy B.W., et al. Cardiovascular and metabolic effects of ANGPTL3 antisense oligonucleotides. N. Engl. J. Med. 2017;377:222–232. doi: 10.1056/NEJMoa1701329. [DOI] [PubMed] [Google Scholar]
- 22.Lim G.B. Novel lipid-lowering therapies targeting ANGPTL3 and Lp(a) Nat. Rev. Cardiol. 2022;19:349. doi: 10.1038/s41569-022-00715-8. [DOI] [PubMed] [Google Scholar]
- 23.Nordestgaard B.G., Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384:626–635. doi: 10.1016/S0140-6736(14)61177-6. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz G.G., Abt M., Bao W., DeMicco D., Kallend D., Miller M., Mundl H., Olsson A.G. Fasting triglycerides predict recurrent ischemic events in patients with acute coronary syndrome treated with statins. J. Am. Coll. Cardiol. 2015;65:2267–2275. doi: 10.1016/j.jacc.2015.03.544. [DOI] [PubMed] [Google Scholar]
- 25.Raposeiras-Roubin S., Rosselló X., Oliva B., Fernández-Friera L., Mendiguren J.M., Andrés V., Bueno H., Sanz J., Martínez de Vega V., Abu-Assi E., et al. Triglycerides and residual atherosclerotic risk. J. Am. Coll. Cardiol. 2021;77:3031–3041. doi: 10.1016/j.jacc.2021.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marston N.A., Giugliano R.P., Im K., Silverman M.G., O'Donoghue M.L., Wiviott S.D., Ference B.A., Sabatine M.S. Association between triglyceride lowering and reduction of cardiovascular risk across multiple lipid-lowering therapeutic classes: a systematic review and meta-regression analysis of randomized controlled trials. Circulation. 2019;140:1308–1317. doi: 10.1161/CIRCULATIONAHA.119.041998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duan Y., Gong K., Xu S., Zhang F., Meng X., Han J. Regulation of cholesterol homeostasis in health and diseases: from mechanisms to targeted therapeutics. Signal Transduct. Target. Ther. 2022;7:265. doi: 10.1038/s41392-022-01125-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castelli W.P., Anderson K., Wilson P.W., Levy D. Lipids and risk of coronary heart disease. The Framingham Study. Ann. Epidemiol. 1992;2:23–28. doi: 10.1016/1047-2797(92)90033-m. [DOI] [PubMed] [Google Scholar]
- 29.Fujihara K., Suzuki H., Sato A., Kodama S., Heianza Y., Saito K., Iwasaki H., Kobayashi K., Yatoh S., Takahashi A., et al. Carotid artery plaque and LDL-to-HDL cholesterol ratio predict atherosclerotic status in coronary arteries in asymptomatic patients with type 2 diabetes mellitus. J. Atheroscler. Thromb. 2013;20:452–464. doi: 10.5551/jat.14977. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt C., Fagerberg B., Wikstrand J., Hulthe J. apoB/apoA-I ratio is related to femoral artery plaques and is predictive for future cardiovascular events in healthy men. Atherosclerosis. 2006;189:178–185. doi: 10.1016/j.atherosclerosis.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 31.Walldius G., Jungner I. The apoB/apoA-I ratio: a strong, new risk factor for cardiovascular disease and a target for lipid-lowering therapy--a review of the evidence. J. Intern. Med. 2006;259:493–519. doi: 10.1111/j.1365-2796.2006.01643.x. [DOI] [PubMed] [Google Scholar]
- 32.Kampoli A.M., Tousoulis D., Antoniades C., Siasos G., Stefanadis C. Biomarkers of premature atherosclerosis. Trends Mol. Med. 2009;15:323–332. doi: 10.1016/j.molmed.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 33.Watts G.F., Schwabe C., Scott R., Gladding P., Sullivan D., Baker J., Clifton P., Hamilton J., Given B., San Martin J., et al. Pharmacodynamic effect of ARO-ANG3, an investigational RNA interference targeting hepatic angiopoietin-like protein 3, in patients with hypercholesterolemia. Circulation. 2020;142:15751. [Google Scholar]
- 34.Romeo S., Yin W., Kozlitina J., Pennacchio L.A., Boerwinkle E., Hobbs H.H., Cohen J.C. Rare loss-of-function mutations in ANGPTL family members contribute to plasma triglyceride levels in humans. J. Clin. Invest. 2009;119:70–79. doi: 10.1172/JCI37118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Minicocci I., Santini S., Cantisani V., Stitziel N., Kathiresan S., Arroyo J.A., Martí G., Pisciotta L., Noto D., Cefalù A.B., et al. Clinical characteristics and plasma lipids in subjects with familial combined hypolipidemia: a pooled analysis. J. Lipid Res. 2013;54:3481–3490. doi: 10.1194/jlr.P039875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ando Y., Shimizugawa T., Takeshita S., Ono M., Shimamura M., Koishi R., Furukawa H. A decreased expression of angiopoietin-like 3 is protective against atherosclerosis in apoE-deficient mice. J. Lipid Res. 2003;44:1216–1223. doi: 10.1194/jlr.M300031-JLR200. [DOI] [PubMed] [Google Scholar]
- 37.McDougall R., Ramsden D., Agarwal S., Agarwal S., Aluri K., Arciprete M., Brown C., Castellanos-Rizaldos E., Charisse K., Chong S., et al. The nonclinical disposition and pharmacokinetic/pharmacodynamic properties of N-Acetylgalactosamine-Conjugated small interfering RNA are highly predictable and build confidence in translation to human. Drug Metab. Dispos. 2022;50:781–797. doi: 10.1124/dmd.121.000428. [DOI] [PubMed] [Google Scholar]
- 38.Cui H., Zhu X., Li S., Wang P., Fang J. Liver-targeted delivery of oligonucleotides with N-acetylgalactosamine conjugation. ACS Omega. 2021;6:16259–16265. doi: 10.1021/acsomega.1c01755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y. Delivery systems for RNA interference therapy: current technologies and limitations. Curr. Gene Ther. 2020;20:356–372. doi: 10.2174/1566523220666201005110726. [DOI] [PubMed] [Google Scholar]
- 40.Hu B., Li B., Li K., Liu Y., Li C., Zheng L., Zhang M., Yang T., Guo S., Dong X., et al. Thermostable ionizable lipid-like nanoparticle (iLAND) for RNAi treatment of hyperlipidemia. Sci. Adv. 2022;8:eabm1418. doi: 10.1126/sciadv.abm1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Semple S.C., Akinc A., Chen J., Sandhu A.P., Mui B.L., Cho C.K., Sah D.W.Y., Stebbing D., Crosley E.J., Yaworski E., et al. Rational design of cationic lipids for siRNA delivery. Nat. Biotechnol. 2010;28:172–176. doi: 10.1038/nbt.1602. [DOI] [PubMed] [Google Scholar]
- 42.Kamerkar S., LeBleu V.S., Sugimoto H., Yang S., Ruivo C.F., Melo S.A., Lee J.J., Kalluri R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546:498–503. doi: 10.1038/nature22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Balasubramaniam D., Schroeder O., Russell A.M., Fitchett J.R., Austin A.K., Beyer T.P., Chen Y.Q., Day J.W., Ehsani M., Heng A.R., et al. An anti-ANGPTL3/8 antibody decreases circulating triglycerides by binding to a LPL-inhibitory leucine zipper-like motif. J. Lipid Res. 2022;63:100198. doi: 10.1016/j.jlr.2022.100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang R. The potential of ANGPTL8 antagonism to simultaneously reduce triglyceride and increase HDL-cholesterol plasma levels. Front. Cardiovasc. Med. 2021;8:795370. doi: 10.3389/fcvm.2021.795370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shimamura M., Matsuda M., Yasumo H., Okazaki M., Fujimoto K., Kono K., Shimizugawa T., Ando Y., Koishi R., Kohama T., et al. Angiopoietin-like protein3 regulates plasma HDL cholesterol through suppression of endothelial lipase. Arterioscler. Thromb. Vasc. Biol. 2007;27:366–372. doi: 10.1161/01.ATV.0000252827.51626.89. [DOI] [PubMed] [Google Scholar]
- 46.Wu L., Soundarapandian M.M., Castoreno A.B., Millar J.S., Rader D.J. LDL-cholesterol reduction by ANGPTL3 inhibition in mice is dependent on endothelial lipase. Circ. Res. 2020;127:1112–1114. doi: 10.1161/CIRCRESAHA.120.317128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Springer A.D., Dowdy S.F. GalNAc-siRNA conjugates: leading the way for delivery of RNAi therapeutics. Nucleic Acid Ther. 2018;28:109–118. doi: 10.1089/nat.2018.0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thangamani L., Balasubramanian B., Easwaran M., Natarajan J., Pushparaj K., Meyyazhagan A., Piramanayagam S. GalNAc-siRNA conjugates: prospective tools on the frontier of anti-viral therapeutics. Pharmacol. Res. 2021;173:105864. doi: 10.1016/j.phrs.2021.105864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mohamed F., Botha T.C., Raal F.J. Inhibition of angiopoietin-like 3 for the management of severe hypercholesterolemia. Curr. Opin. Lipidol. 2021;32:213–218. doi: 10.1097/MOL.0000000000000755. [DOI] [PubMed] [Google Scholar]
- 50.Zhang X., Zhang R., Raab S., Zheng W., Wang J., Liu N., Zhu T., Xue L., Song Z., Mao J., et al. Rhesus macaques develop metabolic syndrome with reversible vascular dysfunction responsive to pioglitazone. Circulation. 2011;124:77–86. doi: 10.1161/CIRCULATIONAHA.110.990333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zou Y., Tiller P., Chen I.W., Beverly M., Hochman J. Metabolite identification of small interfering RNA duplex by high-resolution accurate mass spectrometry. Rapid Commun. Mass Spectrom. 2008;22:1871–1881. doi: 10.1002/rcm.3561. [DOI] [PubMed] [Google Scholar]
- 52.Husser C., Brink A., Zell M., Müller M.B., Koller E., Schadt S. Identification of GalNAc-conjugated antisense oligonucleotide metabolites using an untargeted and generic approach based on high resolution mass spectrometry. Anal. Chem. 2017;89:6821–6826. doi: 10.1021/acs.analchem.7b01244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data included in this study are available upon request by contacting the corresponding authors (zhangxq@pku.edu.cn or gaos@ribolia.com).