Abstract
Purpose
To evaluate the impact of vitamin D (Vit D) supplementation on systemic biomarkers of collagen degradation, inflammation, oxidative stress, and copper metabolism in adolescent patients with keratoconus (KC).
Methods
This was a prospective observational pilot study. Twenty patients (age range, 16–19 years) presenting KC and Vit D insufficiency (<30 ng/mL) were included. Vit D supplementation was prescribed by their general practitioner as per the standard of care. Patients were followed up for 12 months. At each visit, best spectacle-corrected visual acuity (BSCVA), maximal keratometry (Kmax), and thinnest corneal thickness (TCT) were evaluated. The primary outcome of the study was the proportion of patients with Kmax progression of less than 1 D throughout the 12-month follow-up time. Blood samples were collected at different time points to evaluate Vit D levels and systemic markers of collagen degradation, inflammation, oxidative stress, and copper metabolism by ELISA or RT-PCR.
Results
Lower Vit D levels in the plasma were correlated with higher levels of systemic biomarkers of collagen degradation. Vit D supplementation increased the cell availability of copper. Moreover, stabilization of KC progression was found in 60% of patients (72% of eyes) after 12 months with Vit D supplementation. BSCVA, Kmax, and TCT rates remained stable during the observation period.
Conclusions
Our findings support that Vit D administration could affect ocular and systemic biomarkers in KC and illuminate a possible mechanism that can be used to develop new treatment alternatives.
Translational Relevance
Although KC therapy currently relies exclusively on surgical procedures, Vit D supplementation may offer a non-invasive and inexpensive alternative with minimal associated side effects.
Keywords: keratoconus, vitamin D, copper, collagen degradation, systemic disease
Introduction
Keratoconus (KC) is a progressive corneal degeneration that typically initiates and progresses in early infancy and adolescence.1–3 In a recent meta-analysis, Hashemi et al.4 calculated a global prevalence of 138/100,000, but reported that rates worldwide widely vary from values as low as 0.3/100,000 in Russia to 4.0% in a large population-based study in Iran and 5.3% in students of Arab ethnicity in Israel.
Despite extensive efforts, the etiology of KC and the factors that regulate its progression remain largely unknown.5,6 A role of specific gene mutations has been hypothesized,7 as up to 20% of first-degree KC patients’ relatives are affected.8,9 Interestingly, innate immune Toll-like receptors have been identified as early ocular changes in first-degree relatives without any abnormal corneal parameters.10,11 However, no specific gene mutation has been consistently detected in most KC patients; therefore, it is thought that, although genetic predisposition may play a role, an environmental factor is also necessary for disease manifestation.8,12 For example, eye rubbing and severe atopy can favor disease progression.8,13 In addition, hormonal imbalance has been associated with KC. Specifically, thyroid dysfunction14–16 and significant sexual hormone changes (e.g., puberty, pregnancy)17,18 can trigger KC development and/or favor its progression.
Regardless of the cause, there is unanimous consensus that reactive oxygen species are largely increased and that collagen is progressively degraded over time in the KC cornea.19 In addition, it is reasonable to hypothesize that nutritional and/or metabolic alterations could also have a role, as vitamins and metal ions significantly affect extracellular matrix and specifically collagen remodeling.20 Multiple lines of evidence support this hypothesis. First, KC prevalence is positively associated with vitamin D (Vit D) deficiency.21 Second, inefficient Vit D transport and metabolism have been demonstrated in the tear fluid of KC patients,22 suggesting a key role for this vitamin in KC pathogenesis. Additionally, copper (Cu) deposition in the cornea (i.e., Fleischer ring)23–25 and lower serum levels of Cu compared to age-matched healthy controls21,26 are frequently reported in KC patients, suggesting that a Cu imbalance may be involved in KC development.27
Although KC patients consistently show an increased incidence of certain systemic diseases and alteration of specific inflammatory mediators in the blood,28,29 no medication is available to arrest or slow down the progression of KC. Therefore, KC treatment relies exclusively on surgical procedures (corneal crosslinking, INTAC insertion, and corneal transplantation).30–33
In this paper, we aimed to evaluate the impact of Vit D supplementation on systemic biomarkers of collagen degradation, inflammation, oxidative stress, and Cu metabolism in a cohort of adolescent patients with KC and Vit D deficiency who were followed up for 12 months.
Materials and Methods
Study Design
Patient Enrollment
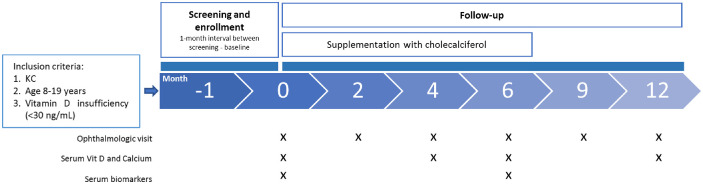
This prospective pilot study was conducted at the Cornea and Ocular Surface Unit of the San Raffaele Scientific Institute, Milan, Italy. The study was carried out in accordance with the tenets of the Declaration of Helsinki, and it was approved by the Institutional Review Board/Ethics Committee (Comitato Etico Istituto Scientifico Ospedale San Raffaele; protocol name, Kera-D; date of approval, November 10, 2021). Written informed consent was obtained from the patients at enrollment. A schematic representation of the study design is shown in Figure 1.
Figure 1.
Schematic representation of the study design. Twenty patients diagnosed with KC and presenting Vit D insufficiency (<30 ng/mL) were enrolled. They were followed up for 12 months. Full ophthalmology visits were scheduled at months 0, 2, 4, 6, 9, and 12. In addition, peripheral blood samples were collected to further analyze Vit D levels (at 0, 4, 6, and 12 months) and other biomarkers (at 0 and 6 months).
We recruited 20 patients (age range, 8–19 years) presenting KC and Vit D insufficiency (serum levels < 30 ng/mL).34 Exclusion criteria included bilateral prior surgical procedures of the cornea (including crosslinking); diagnosis of end-stage KC, defined as corneal thickness less than 300 µm and/or extensive apical leucoma and/or corneal hydrops; or diagnosis of active keratitis/conjunctivitis. The diagnosis of KC was confirmed by corneal tomography/topography (MS-39 AS-OCT; CSO, Firenze, Italy). At the screening visit, medical history was collected, including the presence of allergies and rubbing habits. All patients were instructed not to rub regardless of having the habit or not. A full ophthalmology exam was performed, including measuring (1) best spectacle-corrected visual acuity (BSCVA), (2) maximal keratometry (Kmax), and (3) epithelial and stromal minimal thickness and thinnest corneal thickness (TCT) by corneal tomography and topography. Patients were treated as per standard of care in case of Vit D insufficiency with cholecalciferol supplementation (50,000 IU once a week for the first 3 months). Maintenance treatment was then continued with 50,000 IU once a month up to month 6.
Follow-Up
After the screening visit (M0), patients were followed up for 12 months. Follow-up visits were scheduled at 2, 4, 6, 9, and 12 months from M0. A full ophthalmological exam was performed at each visit, which included measures of BSCVA and corneal tomography and topography. Kmax, TCT, and epithelial and stromal minimal thickness were evaluated. At each visit, patients were reminded not to rub. The examination was always performed at the same time of the day (14–15 hours). When crosslinking (CXL) was performed during the study, that eye was not further considered for the analysis.
Primary Outcome
The primary outcome measure was defined as the proportion of patients with Kmax progression of less than 1 diopter (D) throughout the 12-month follow-up time.
Secondary Outcomes
Secondary outcome measures were change of BSCVA, Kmax, and TCT, as well as Vit D and calcium serum levels, during the 12-month follow-up time.
Clinical Parameters
BSCVA was recorded in Snellen equivalents and converted to the logMAR scale. “Counting finger” and “hand motion” BSCVA measurements were converted to 2.0 and 3.0 logMAR values, respectively.35,36 The BSCVA rate (logMAR/month) was calculated as (logMAR Mn – logMAR M0)/n, where n is the number of follow-up months and M0 is the screening visit. Therefore, an increased logMAR rate indicates decreased BSCVA. Kmax (D) was also obtained using the MS-39 AS-OCT corneal tomographer/topographer. Kmax rate was defined as (Kmax Mn – Kmax M0)/n, where n is the number of follow-up months. An increase in Kmax value will lead to an increase in Kmax rate. TCT was also obtained using the MS-39 AS-OCT corneal tomographer/topographer. The TCT rate was calculated as (TCT M0 – TCT Mn)/n, where n is the number of follow-up months. Therefore, an increased TCT rate indicates a reduction in TCT. In addition, epithelial and stromal thickness were measured.
Finally, blood samples were collected at M4, M6, and M12 to monitor serum 25-OH Vit D and calcium levels through standardized methods: electrochemiluminescence immunoassay37 and the o-cresolphthalein complexone method,38 respectively, using a Cobas C 800 autoanalyzer (Roche, Basel, Switzerland). At M0 and M6, peripheral blood mononuclear cells (PBMCs) and plasma samples were isolated using a density gradient medium (Lymphoprep; Stemcell Technologies, Vancouver, Canada). PBMCs and plasma samples were immediately stored at –80°C until analysis.
Systemic Biomarker Assessment
Enzyme-Linked Immunosorbent Assay
Plasma samples collected at M0 and M6 were centrifuged at 1000g for 15 minutes at 4°C. Supernatants were separated, diluted following the manufacturer's instructions, and immediately assessed. Highly sensitive enzyme-linked immunosorbent assay (ELISA) kits were employed following the manufacturer's instructions for the determination of matrix metalloproteinase-1 (MMP-1) (EH0232; FineTest, Wuhan, China), MMP-9 (EH0238; FineTest), type I collagen degradation product (ICTP) (EH1093; FineTest), tissue metalloproteinase inhibitor-1 (TIMP-1) (EH0294; FineTest), interleukin-1β (IL-1β) (EH0185; FineTest), ceruloplasmin (EH0662; FineTest), and vitamin D binding protein (VDBP) (ab108853, Abcam, Cambridge, UK).
Real-Time Polymerase Chain Reaction
PBMCs samples collected at M0 and M6 were used. Total RNA extraction, DNAse treatment, retrotranscription, and real-time polymerase chain reaction (RT-PCR) were performed as described elsewhere.39 The following transcripts were evaluated by the TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA): copper transporter 1 (CTR1, Hs00977266_g1), copper chaperone for superoxide dismutase (CCS, Hs00192851_m1), lysyl oxidase (LOX, Hs00942480_m1), superoxide dismutase 1 (SOD1, Hs00533490_m1), cubilin (CUBN, Hs00153607_m1), megalin (LRP2, Hs00189742_m1), amnionless (AMN, Hs00229558_m1), vitamin D receptor (VDR, Hs01045843_m1), cytochrome P450 2R1 (CYP2R1, Hs01379776_m1), cytochrome P450 27A1 (CYP27A1, Hs01017992_g1), and cytochrome P450 24A1 (CYP24A1, Hs00167999_m1), as well as glyceraldehyde 3-phosphate dehydrogenase (GAPDH, Mm99999915_g1) as a housekeeping gene. Results are shown as a relative expression (ΔΔCT method). The fold change in the expression of the different mRNA was then calculated for each patient as ΔΔCT M6/ΔΔCT M0.
Statistics
Sample Size
Published data suggest that KC progression of at least 1 D at the apex occurs in more than 80% of cases.40 We considered a reduction of KC progression to 50% after 12 months to be clinically significant in the Vit D–supplemented group as opposed to 80% in the untreated population. Hence, considering an alpha error of 0.05 and beta error of 0.2, with 0.8 power, 16 patients were necessary. Considering a potential loss at follow up of 20%, we enrolled 20 patients.
Analysis
For the primary endpoint, we considered each patient separately. For all the other analyses, we considered each eye from the same patient separately. Data were expressed as mean ± standard deviation (SD). The statistical significance of the differences between two groups for continuous variables was assessed using the Mann–Whitney unpaired test or Wilcoxon matched-pairs signed-rank test. Significant changes within groups were tested by Friedman's test, followed by Dunn's post hoc test. For categorical variables, Fisher's exact test was employed. The one-sample t-test was used to compare the fold change between M0 and M6 for the systemic biomarkers in the presence of a normal distribution according to the Kolmogorov–Smirnov test; otherwise, the one-sample Wilcoxon signed-rank test was employed. Spearman's correlation coefficient was used to evaluate the linear relationship between continuous systemic parameters. A two-sided P ≤ 0.05 was considered statistically significant. Bonferroni correction was applied when appropriate within each variable. Prism 5.0 (GraphPad Software, San Diego, CA) was used for the statistical calculations.
Results
Clinical Outcomes
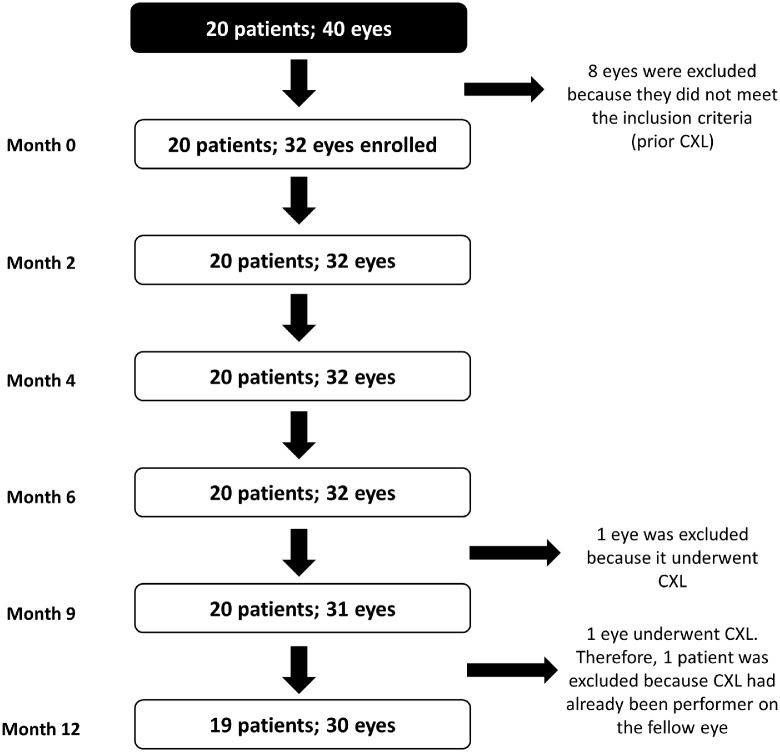
We recruited 20 patients (mean age, 16.8 ± 1.9 years; range, 12–19 years) diagnosed with KC in at least one eye who also presented Vit D insufficiency (<30 ng/mL). Eight eyes were excluded from further analysis, as the patients had undergone corneal crosslinking (Fig. 2). Three eyes were diagnosed as forme fruste KC. Most of the patients were males (85%). In addition, 35% of patients reported allergies. The patients’ demographics are summarized in Table 1.
Figure 2.
Study flow chart. Twenty patients (40 eyes) were enrolled. From these, 32 eyes were used for the analysis because CXL has already been performed in eight eyes. Patients were followed up for 12 months. Two eyes (from two different patients) underwent CXL during the study and were excluded from the final analysis.
Table 1.
Patient Demographics
| KC–Vit D Patients | |||
|---|---|---|---|
| Parameter | M0 | M12 | P |
| Patients, n | 20 | 19 | — |
| Gender, n | |||
| Male | 17 | 16 | — |
| Female | 3 | 3 | — |
| Age (y), mean (range) | 16.8 (12–19) | 17.9 (13–22) | — |
| Kmax (D), mean ± SD | 52.9 ± 7.4 | 53.1 ± 7.5 | 0.02 a |
| TCT (µM), mean ± SD | 484 ± 35 | 484 ± 39 | 0.81a |
| Uncorrected visual acuity (logMAR), mean ± SD | 0.23 ± 0.22 | 0.18 ± 0.18 | 0.06a |
| BSCVA (logMAR), mean ± SD | 0.13 ± 0.19 | 0.09 ± 0.20 | 0.03 a |
| Allergies, n (%) | 7 (35) | 7 (37) | 1.00b |
Statistically significant values (P ≤ 0.05) are shown in bold.
Wilcoxon signed-rank test.
Fisher's exact test.
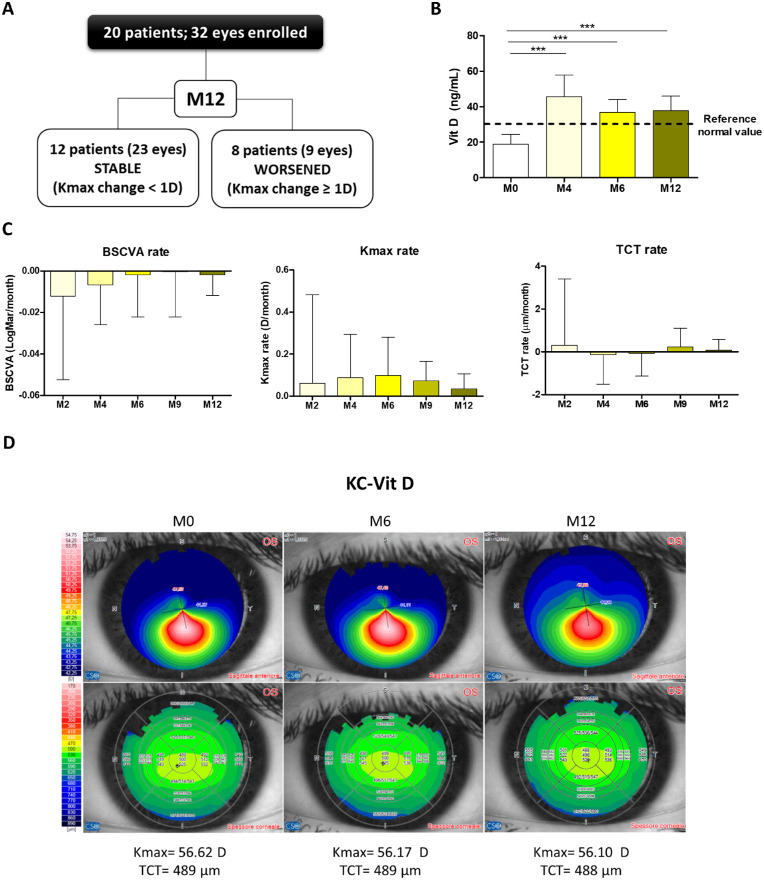
As shown in Figure 3A, 12 patients (60%) and 23 eyes (72%) remained stable (Kmax change < 1 D) by the end of the study (M12). Serum Vit D levels reached at least 30 ng/mL (reference value for sufficiency)34 during the supplementation phase (M0–M6) and remained above or equal to 30 ng/mL during the following 6 months (Fig. 3B). Calcium levels remained within the normal range (2.10–2.60 mmol/L) throughout the study (M4, 2.460 ± 0.0752 mmol/L; M6, 2.466 ± 0.0809 mmol/L; M12, 2.450 ± 0.0592 mmol/L).
Figure 3.
Vit D supplementation stabilizes KC progression. (A) Primary endpoint. Twelve patients and 23 eyes remained stable (Kmax change < 1 D) by the end of the study (month 12). (B) Vit D levels were measured at months 0, 4, 6, and 12. Vit D supplementation started following the standard of care after the screening visit (M0). (C) Patients (n = 20) were followed up for 12 months by clinical evaluation of BSCVA, Kmax, and TCT. Results are expressed in terms of rate (how much the parameter changed in the corresponding time). (D) Representative corneal tomography/topography at M0, M6, and M12 of a KC–Vit D patient. Graphs represent the mean ± SD. Statistical analysis was performed using Mann–Whitney or Friedman tests, followed by Dunn's post hoc, non-parametric tests. Bonferroni correction was applied when appropriate. *P < 0.05, ***P < 0.001.
BSCVA, Kmax, and TCT rates did not vary significantly throughout the study (Fig. 3C, Supplementary Table S1). Moreover, epithelial and stromal thickness remained stable (Table 2). The average Kmax slightly increased after 12 months (M0 = 52.9 ± 7.4 D vs. M12 = 53.1 ± 7.5 D; P = 0.02); the average change was 0.2 D/y. Interestingly, average BSCVA (logMAR) slightly improved after Vit D supplementation (M0 = 0.13 ± 0.19 vs. M12 = 0.09 ± 0.20 logMAR; P = 0.03) (Supplementary Table S1). Representative corneal topography images before and after supplementation with Vit D are shown in Figure 3D.
Table 2.
Comparison of Epithelial and Stromal Thickness Between KC Patients at M0 and M12
| KC-Vit D | |||
|---|---|---|---|
| Variables | M0 (N = 20) | M12 (N = 19) | P |
| Epithelium | |||
| 3 mm (µm) | 49 ± 7 (47, 52) | 50 ± 7 (47, 52) | 0.20 |
| 6 mm (µm) | |||
| Superior | 52 ± 5 (51, 54) | 55 ± 5 (52, 57) | 0.07 |
| Inferior | 54 ± 5 (52, 56) | 54 ± 6 (53, 56) | 0.60 |
| Nasal | 55 ± 6 (53, 58) | 57 ± 7 (54, 59) | 0.06 |
| Temporal | 52 ± 4 (51, 54) | 53 ± 4 (52, 55) | 0.10 |
| 8 mm (µm) | |||
| Superior | 49 ± 5 (48, 51) | 48 ± 4 (46, 50) | 0.02* |
| Inferior | 51± 5 (49, 53) | 51± 5 (49, 52) | 0.16 |
| Nasal | 57 ± 5 (55, 59) | 54 ± 5 (52, 56) | <0.001* |
| Temporal | 54 ± 5 (53, 56) | 53 ± 5 (51, 55) | 0.12 |
| Stroma | |||
| 3 mm (µm) | 453 ± 29 (442, 463) | 451 ± 31 (439, 463) | 0.01* |
| 6 mm (µm) | |||
| Superior | 523 ± 30 (512, 534) | 526 ± 25 (516, 535) | 0.13 |
| Inferior | 499 ± 21 (491, 507) | 501 ± 26 (492, 511) | 0.23 |
| Nasal | 508 ± 25 (499, 518) | 509 ± 25 (500, 519) | 0.58 |
| Temporal | 494 ± 28 (484, 505) | 479 ± 26 (470, 489) | <0.001* |
| 8 mm (µm) | |||
| Superior | 606 ± 43 (589, 623) | 606 ± 34 (590, 621) | 0.68 |
| Inferior | 573 ± 31 (561, 584) | 575 ± 32 (563, 587) | 0.82 |
| Nasal | 572 ± 30 (561, 583) | 579 ± 62 (556, 602) | 0.21 |
| Temporal | 530 ± 27 (520, 540) | 532 ± 25 (522, 541) | 0.18 |
Statistical analysis by Wilcoxon signed-rank test. Statistically significant values (P ≤ 0.05) are shown in bold.
Mechanism
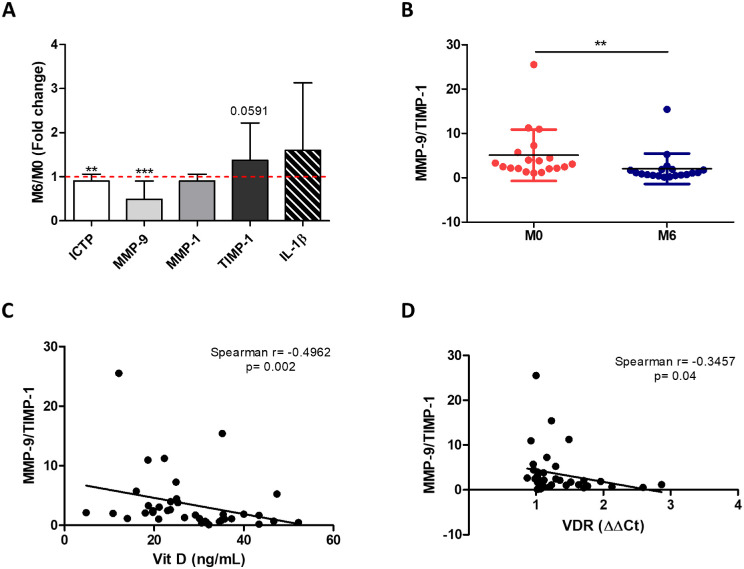
Our data show that supplementation of Vit D significantly upregulated its metabolism. This involved increased Vit D transport by means of VDBP (P = 0.04), increased Vit D activation by means of CYP27A1 levels (P = 0.02), and augmented Vit D effects by means of VDR (P = 0.03) (Table 3). Importantly, we found that Vit D supplementation inhibited systemic collagen degradation (Fig. 4A). Specifically, we observed that ICTP, a collagen degradation product, and the metalloproteinase MMP-9, a key collagenolytic enzyme, were significantly reduced in plasma after Vit D supplementation (P = 0.01 and P < 0.001, respectively). This was accompanied by a trend toward increasing levels of TIMP-1 (P = 0.0591), the main MMP-9 inhibitor. To quantify the systemic collagen degradation status, we calculated the MMP-9/TIMP-1 ratio and found that it was significantly lower after Vit D supplementation (P = 0.003) (Fig. 4B). This suggests that collagen degradation is inhibited in Vit D–supplemented patients. Indeed, the levels of MMP-9/TIMP-1 inversely correlated with Vit D levels (Spearman’s r = −0.4962; P = 0.002) (Fig. 4C) and VDR (Spearman's r = −0.3457; P = 0.04) (Fig. 4D).
Table 3.
Systemic Changes in Proteins Involved in Vit D Metabolism and Signaling Pathways After Vit D Supplementation
| M6/M0 (Fold Change) | ||||
|---|---|---|---|---|
| Mean | SD | N | P | |
| Vit D transport by VDBP | 1.299 | 0.639 | 20 | 0.04 |
| Vit D uptake | ||||
| Cubilin | 1.458 | 1.805 | 19 | 0.28 |
| Amnionless | 1.347 | 0.903 | 20 | 0.10 |
| Vit D metabolism | ||||
| CYP27A1 | 1.351 | 0.605 | 20 | 0.02 |
| CYP2R1 | 1.241 | 0.818 | 20 | 0.20 |
| CYP24A1 | 1.353 | 1.124 | 20 | 0.18 |
| VDR | 1.153 | 0.280 | 20 | 0.03 |
Statistical analysis by one-sample t-test or one-sample Wilcoxon signed-rank test.
Figure 4.
Vit D supplementation slows down KC progression by inhibiting collagen degradation systemically. (A) Plasma collagen degradation markers measured by ELISA: ICTP, MMP-9, (MMP-1, TIMP-1, and IL-1β. Results are expressed as fold change between month 0 and month 6 (M6/M0). (B) To establish the degradation status, we calculated the ratio between MMP-9 and TIMP-1 at months 0 and 6. (C, D) The MMP-9/TIMP-1 ratio inversely correlated with Vit D levels (C) and VDR expression (D) measured by RT-PCR. M0, n = 20 patients; M6, n = 20 patients. Graphs represent the mean ± SD. Statistical analysis was performed using the one-sample t-test, Wilcoxon signed-rank test, or Spearman's correlation coefficient. *P < 0.05, **P < 0.01, ***P < 0.001.
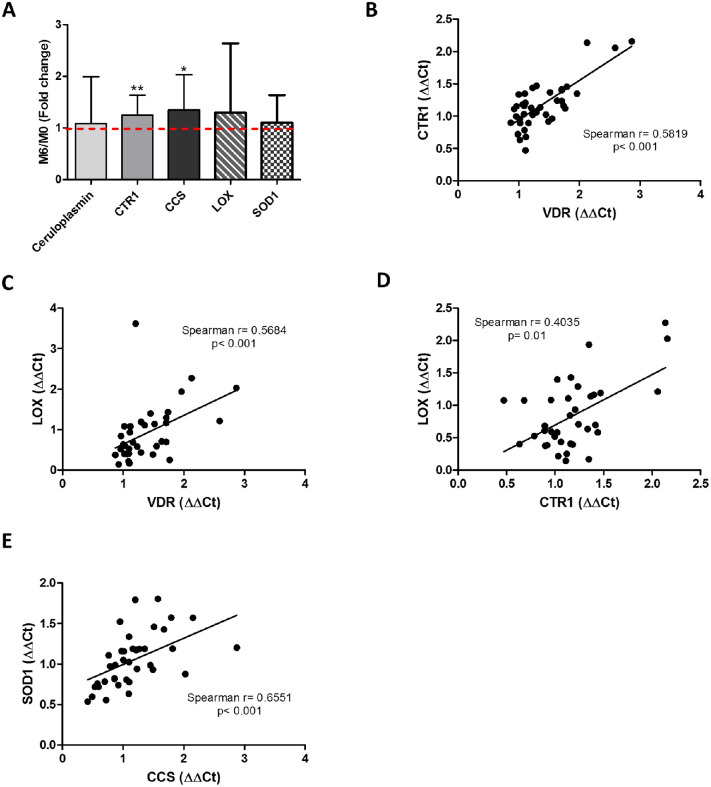
In addition, Cu metabolism was investigated, as it is a cofactor of key enzymes involved in collagen crosslinking (e.g., LOX) and with antioxidant activity (e.g., SOD). Figure 5A shows that CTR1 (P = 0.008) and intracellular CCS (P = 0.04) were systemically upregulated after Vit D supplementation. Moreover, VDR expression was directly correlated with CTR1 (Spearman’s r = 0.5819; P < 0.001) (Fig. 5B) and LOX levels (Spearman's r = 0.5684; P < 0.001) (Fig. 5C). Interestingly, higher levels of CTR1 were also associated with increased expression of LOX (Spearman's r = 0.4035; P = 0.01) (Fig. 5D); meanwhile, CCS levels positively correlated with SOD1 expression (Spearman's r = 0.6551; P < 0.001) (Fig. 5E).
Figure 5.
Vit D supplementation delays KC progression by enhancing Cu availability. (A) Systemic biomarkers related to Cu metabolism by ELISA or RT-PCR: ceruloplasmin, CTR1, CCS, LOX, and SOD1. Results are expressed as fold change between month 0 and month 6 (M6/M0). Spearman's correlation analysis found a positive correlation between (B) CTR1 and VDR expression, (C) LOX and VDR expression, (D) LOX and CTR1 expression, and (E) SOD1 and CCS levels. M0, n = 20 patients; M6, n = 20 patients. Graphs represent the mean ± SD. Statistical analysis was performed using the one-sample t-test or Spearman's correlation coefficient. *P < 0.05, **P < 0.01.
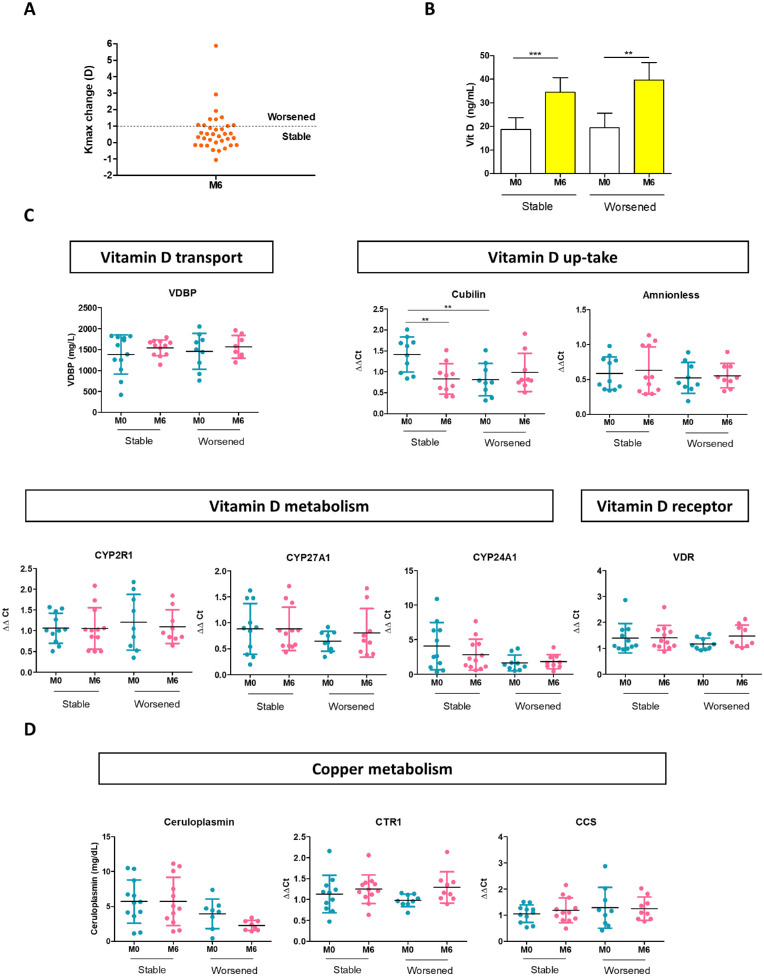
Finally, we investigated Vit D and Cu metabolism in the subgroup of patients who received supplementation but worsened (Fig. 6A). Patients who were stable and those who worsened had similar Vit D serum levels throughout the study (Fig. 6B). However, patients who worsened showed a lower expression of cubilin at M0 (P = 0.006), which remained unchanged after supplementation (Fig. 6C). In contrast, cubilin expression was reduced (P = 0.004) in stable patients at M6. Other proteins involved in Vit D metabolism showed no significant differences between both groups. Megalin expression was absent in all of the tested samples. Ceruloplasmin concentration decreased from M0 to M6 in patients who worsened (P = 0.05) (Fig. 6D) and in comparison with stable patients (P = 0.03), but these results were not significant after Bonferroni correction. Altogether, these results suggest that intracellular availability of Vit D is essential to inhibit KC progression.
Figure 6.
Evaluation of Vit D and Cu metabolism in patients who worsened even after Vit D supplementation. (A) Progression was defined as a topographic change of Kmax of more than 1 D. (B) Vit D levels in plasma were comparable between the stable and the patients who worsened. (C) Proteins related to Vit D metabolism were evaluated in the two groups of patients by ELISA or RT-PCR: VDBP, cubilin, amnionless, CYP2R1, CYP27A1, CYP24A1, and VDR. (D) Proteins related to Cu metabolism evaluated by ELISA or RT-PCR included ceruloplasmin, CTR1, and CCS. For stable patients at M0, n = 12, and at M6, n = 12. For worsened patients at M0, n = 8, and at M6, n = 8. Graphs represent the mean ± SD. Statistical analysis was performed using the Wilcoxon signed-rank test for paired samples or the Mann–Whitney test for unpaired samples. Bonferroni correction was applied when appropriate. **P < 0.01, ***P < 0.001.
Discussion
KC is the most common ectatic degeneration of the cornea12; it impairs visual acuity as it causes progressive irregular corneal astigmatism.5 Our data support that KC might be the ocular manifestation of a systemic disease in which Vit D insufficiency plays a key role. We have shown that KC remains stable over 12 months in patients supplemented with Vit D. KC has been traditionally considered an isolated ocular disease, whereas recent evidence suggests that KC is, in fact, a manifestation of a systemic disorder. Indeed, it is known that 21% to 70% of KC patients present a concomitant systemic disease, such as atopy,41,42 sleep apnea,43 mitral valve prolapse,44,45 hypothyroidism,46 or Down syndrome.47,48 Interestingly, many of these diseases are associated with Vit D insufficiency.49–53 Moreover, KC typically progresses during the adolescence growth phase and has been reported to progress in pregnancy,8,54–56 two conditions where Vit D deficiency is highly prevalent.57–59 Finally, a systemic etiology could also explain why KC has been reported to recur after corneal transplantation, although this is still matter of discussion.60–62
Quantification of KC progression can be challenging, and no unanimous consensus has been reached on which parameters should be measured.63 In our study, we considered as the primary outcome the proportion of patients with a Kmax progression of less than 1 D during the 12-month follow-up time.64–66 We found that 60% of Vit D–supplemented patients (72% of eyes) reached the primary endpoint. Interestingly, not only Kmax but also corneal thickness and BSCVA were stable at month 12. Of note, the effect on BSCVA was unexpected and is clinically relevant because it directly impacts patients’ quality of life.
We believe that our findings show promise, as the Kmax progression rate is reported to be higher than 88% in pediatric patients.2,40,67,68 In our study, only 28% of KC eyes worsened after 12 months, which means that Vit D supplementation promoted a 65% improvement. Even though the average Kmax slightly increased after 12 months, the average change (0.2 D/y) was significantly lower than the one predicted for an adolescent patient.40
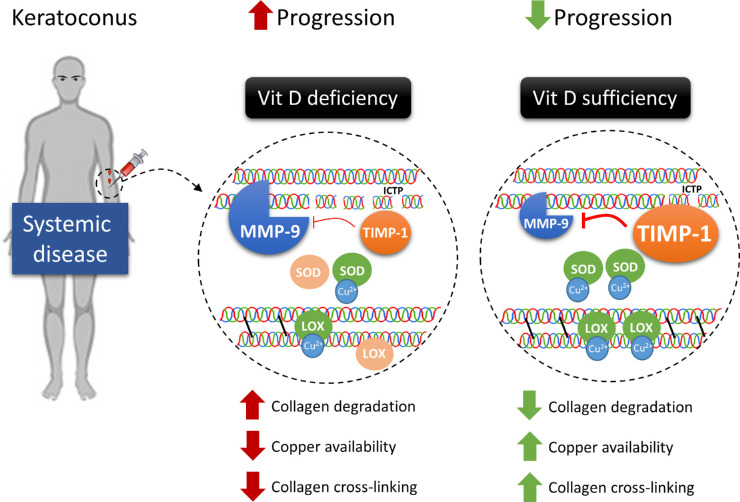
To explain the clinical stability of KC observed in supplemented patients, we identified a possible novel mechanism by which Vit D can arrest KC progression (Fig. 7). Specifically, Vit D supplementation upregulates the expression of both its own activating enzyme CYP27A1 and VDR, which mediates Vit D biological activities. Increased availability and function of Vit D result in reduced MMP-9 levels and augmented TIMP-1 expression systemically. Consequently, collagen catabolism is inhibited, as shown by the decreased MMP-9/TIMP-1 ratio69,70 and reduced serum concentration of collagen degradation products. Our data also suggest that inflammation can have a role in the process because MMPs are upregulated by many inflammatory cytokines, which are increased in the blood and the tear fluid in KC patients.71,72
Figure 7.
Vit D arrests KC progression. The increased availability of active Vit D results in the downregulation of MMP-9 levels while increasing TIMP-1 systemically. This, in turn, inhibits collagen degradation, supported by the lower levels of collagen degradation products (ICTP) observed. Additionally, Vit D halts KC progression by improving Cu availability, which would result in increased endogenous collagen CXL by LOX and antioxidant capacity by SOD.
Finally, we found that Cu metabolism is altered in KC patients. Indeed, previous evidence shows that Cu levels are reduced in these subjects.21,26 Cu is an essential cofactor for the enzyme controlling endogenous CXL (LOX)73 and the free radical scavenger SOD.74–77 The activity of these enzymes is profoundly reduced in KC patients,78,79 although the reason is unclear. We report, for the first time, to the best of our knowledge, that the serum levels of the Cu carrier ceruloplasmin are largely below reference levels in KC adolescent patients. In this scenario, we found that Vit D supplementation enhances copper availability by increasing the expression of the principal Cu membrane transporter CTR1 and the chaperone CCS. Moreover, we found that VDR and LOX expression are positively correlated, suggesting that Vit D directly promotes endogenous CXL.
In conclusion, Vit D may also control KC progression by improving Cu metabolism, which would result in increased endogenous CXL and antioxidant capacity (Fig. 7). Because there was a subgroup of patients who worsened after Vit D supplementation, we investigated whether in this group Vit D metabolism was altered. Interestingly, these patients showed lower baseline levels of cubilin—one of the membrane receptors mediating Vit D endocytosis—which remained unchanged throughout the study. On the other hand, stable patients could modulate cubilin expression based on Vit D availability. Because Vit D levels were normal and similar in both patients who were stable and those who worsened, differential cubilin expression points to reduced cellular uptake of Vit D in patients who worsened.
The reason why cubilin expression is impaired in patients who worsened requires further elucidation. It is well known, however, that the absence of cubilin or inhibition of its function markedly reduces cellular uptake of Vit D. Indeed, specific mutations of the cubilin gene differentially impact its function.80 Therefore, it is possible that patients who worsened harbor genetic mutations that impair cubilin function.
Corneal CXL is currently the only available treatment to arrest KC progression. This is a surgical procedure associated with significant postoperative pain,81 rare but potentially sight-threatening complications,82 and a substantial cost ($US2000–$US4000/eye).83 Of note, CXL cannot reduce MMP9 and TIMP expression70,84,85 or stabilize corneal thickness.64,86–88 Instead, Vit D supplementation significantly reduced the MMP-9/TIMP-1 ratio and collagen catabolism. Furthermore, Vit D supplementation is a medical treatment with minimal, if any, associated side effects.89 Among them, severe hypercalcemia is the most relevant that is related to excessive long-term Vit D intake. In any case, calcium levels can be easily determined by a blood draw and monitored during the supplementation phase. We acknowledge that our pilot study enrolled a small number and a specific subset of KC patients (adolescents) where disease progression is most likely, and then only those with Vit D insufficiency. Therefore, the role of Vit D in KC patients with normal cholecalciferol levels remains to be studied. Finally, this study included a follow-up period of 1 year, which may be short considering the chronicity of the disease; therefore, the patients’ KC status after longer periods remains to be evaluated.
Although a randomized clinical trial is needed to definitively prove the role of Vit D in KC progression, our observational pilot study suggests for the first time that Vit D supplementation has profound implications on the systemic metabolism of collagen and copper in KC patients, stabilizing the disease in nearly two-thirds of the eyes.
Supplementary Material
Acknowledgments
Supported by a grant from the Assocheratocono Foundation (N OBLFERRARI to GF).
Disclosure: R.M. Lasagni Vitar, None; P. Fonteyne, None; K.A. Knutsson, None; F. Bertuzzi, None; L. Galli, None; P. Rama, None; G. Ferrari, None
References
- 1. Anitha V, Vanathi M, Raghavan A, Rajaraman R, Ravindran M, Tandon R.. Pediatric keratoconus - current perspectives and clinical challenges. Indian J Ophthalmol. 2021; 69(2): 214–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mukhtar S, Ambati BK.. Pediatric keratoconus: a review of the literature. Int Ophthalmol. 2018; 38(5): 2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chan E, Chong EW, Lingham G, et al.. Prevalence of keratoconus based on Scheimpflug imaging: the Raine Study. Ophthalmology. 2021; 128(4): 515–521. [DOI] [PubMed] [Google Scholar]
- 4. Hashemi H, Heydarian S, Hooshmand E, et al.. The prevalence and risk factors for keratoconus: a systematic review and meta-analysis. Cornea. 2020; 39(2): 263–270. [DOI] [PubMed] [Google Scholar]
- 5. Davidson AE, Hayes S, Hardcastle AJ, Tuft SJ.. The pathogenesis of keratoconus. Eye (Lond). 2014; 28(2): 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ferrari G, Rama P.. The keratoconus enigma: a review with emphasis on pathogenesis. Ocul Surf. 2020; 18(3): 363–373. [DOI] [PubMed] [Google Scholar]
- 7. Loukovitis E, Sfakianakis K, Syrmakesi P, et al.. Genetic aspects of keratoconus: a literature review exploring potential genetic contributions and possible genetic relationships with comorbidities. Ophthalmol Ther. 2018; 7(2): 263–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Santodomingo-Rubido J, Carracedo G, Suzaki A, Villa-Collar C, Vincent SJ, Wolffsohn JS.. Keratoconus: an updated review. Cont Lens Anterior Eye. 2022; 45(3): 101559. [DOI] [PubMed] [Google Scholar]
- 9. Rabinowitz YS, Galvis V, Tello A, Rueda D, García JD.. Genetics vs. chronic corneal mechanical trauma in the etiology of keratoconus. Exp Eye Res. 2021; 202: 108328. [DOI] [PubMed] [Google Scholar]
- 10. Regueiro U, López-López M, Hervella P, Sobrino T, Lema I.. Corneal and conjunctival alteration of innate immune expression in first-degree relatives of keratoconus patients. Graefes Arch Clin Exp Ophthalmol. 2021; 259(2): 459–467. [DOI] [PubMed] [Google Scholar]
- 11. Malfeito M, Regueiro U, Pérez-Mato M, Campos F, Sobrino T, Lema I.. Innate immunity biomarkers for early detection of keratoconus. Ocul Immunol Inflamm. 2019; 27(6): 942–948. [DOI] [PubMed] [Google Scholar]
- 12. Gordon-Shaag A, Millodot M, Shneor E, Liu Y.. The genetic and environmental factors for keratoconus. Biomed Res Int. 2015; 2015: 795738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Balasubramanian SA, Pye DC, Willcox MDP.. Effects of eye rubbing on the levels of protease, protease activity and cytokines in tears: relevance in keratoconus. Clin Exp Optom. 2013; 96(2): 214–218. [DOI] [PubMed] [Google Scholar]
- 14. Thanos S, Oellers P, Meyer Zu Hörste M, et al.. Role of thyroxine in the development of keratoconus. Cornea. 2016; 35(10): 1338–1346. [DOI] [PubMed] [Google Scholar]
- 15. El-Massry A, Doheim MF, Iqbal M, et al.. Association between keratoconus and thyroid gland dysfunction: a cross-sectional case-control study. J Refract Surg. 2020; 36(4): 253–257. [DOI] [PubMed] [Google Scholar]
- 16. Stachon T, Stachon A, Hartmann U, Seitz B, Langenbucher A, Szentmáry N. Urea, uric acid, prolactin and fT4 concentrations in aqueous humor of keratoconus patients. Curr Eye Res. 2017; 42(6): 842–846. [DOI] [PubMed] [Google Scholar]
- 17. Bilgihan K, Hondur A, Sul S, Ozturk S.. Pregnancy-induced progression of keratoconus. Cornea. 2011; 30(9): 991–994. [DOI] [PubMed] [Google Scholar]
- 18. Kennedy RH, Bourne WM, Dyer JA.. A 48-year clinical and epidemiologic study of keratoconus. Am J Ophthalmol. 1986; 101(3): 267–273. [DOI] [PubMed] [Google Scholar]
- 19. Kenney MC, Brown DJ.. The cascade hypothesis of keratoconus. Cont Lens Anterior Eye. 2003; 26(3): 139–146. [DOI] [PubMed] [Google Scholar]
- 20. Lasagni Vitar RM, Bonelli F, Rama P, Ferrari G. Nutritional and metabolic imbalance in keratoconus. Nutrients. 2022; 14(4): 913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zarei-Ghanavati S, Yahaghi B, Hassanzadeh S, Mobarhan MG, Hakimi HR, Eghbali P.. Serum 25-hydroxyvitamin D, selenium, zinc and copper in patients with keratoconus. J Curr Ophthalmol. 2020; 32(1): 26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. López-López M, Regueiro U, Bravo SB, et al.. Tear proteomics in keratoconus: a quantitative SWATH-MS analysis. Invest Ophthalmol Vis Sci. 2021; 62(10): 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Avetisov SE, Mamikonian VR, Novikov IA.. The role of tear acidity and Cu-cofactor of lysyl oxidase activity in the pathogenesis of keratoconus. Vestn Oftalmol. 2011; 127(2): 3–8. [PubMed] [Google Scholar]
- 24. Avetisov SE, Mamikonyan VR, Novikov IA, Pateyuk LS, Osipyan GA, Kiryushchenkova NP.. Abnormal distribution of trace elements in keratoconic corneas. Vestn Oftalmol. 2015; 131(6): 34. [DOI] [PubMed] [Google Scholar]
- 25. Hu P, Lin L, Wu Z, Jin X, Ni H. Kayser-Fleischer ring with keratoconus: a coincidence? A case report. BMC Ophthalmol. 2020; 20(1): 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bamdad S, Owji N, Bolkheir A.. Association between advanced keratoconus and serum levels of zinc, calcium, magnesium, iron, copper, and selenium. Cornea. 2018; 37(10): 1306–1310. [DOI] [PubMed] [Google Scholar]
- 27. Dudakova L, Liskova P, Jirsova K.. Is copper imbalance an environmental factor influencing keratoconus development? Med Hypotheses. 2015; 84(5): 518–524. [DOI] [PubMed] [Google Scholar]
- 28. Galvis V, Sherwin T, Tello A, Merayo J, Barrera R, Acera A.. Keratoconus: an inflammatory disorder? Eye (Lond). 2015; 29(7): 843–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ionescu C, Corbu CG, Tanase C, et al.. Inflammatory biomarkers profile as microenvironmental expression in keratoconus. Dis Markers. 2016; 2016: 1243819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Galvis V, Tello A, Laiton AN, Salcedo SLL.. Indications and techniques of corneal transplantation in a referral center in Colombia, South America (2012–2016). Int Ophthalmol. 2019; 39(8): 1723–1733. [DOI] [PubMed] [Google Scholar]
- 31. Galvis V, Tello A, Carreño NI, et al.. Corneal cross-linking (with a partial deepithelization) in keratoconus with five years of follow-up. Ophthalmol Eye Dis. 2016; 8: OED.S38364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Larkin DFP, Chowdhury K, Burr JM, et al.. Effect of corneal cross-linking versus standard care on keratoconus progression in young patients: the KERALINK randomized controlled trial. Ophthalmology. 2021; 128(11): 1516–1526. [DOI] [PubMed] [Google Scholar]
- 33. Thanitcul C, Varadaraj V, Canner JK, Woreta FA, Soiberman US, Srikumaran D.. Predictors of receiving keratoplasty for keratoconus. Am J Ophthalmol. 2021; 231: 11–18. [DOI] [PubMed] [Google Scholar]
- 34. Holick MF. Medical progress: vitamin D deficiency. N Engl J Med. 2007; 357(3): 266–281. [DOI] [PubMed] [Google Scholar]
- 35. Lasagni Vitar RM, Triolo G, Fonteyne P, et al.. Epidemiology of corneal neovascularization and its impact on visual acuity and sensitivity: a 14-year retrospective study. Front Med (Lausanne). 2021; 8: 733538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Holladay JT. Proper method for calculating average visual acuity. J Refract Surg. 1997; 13(4): 388–391. [DOI] [PubMed] [Google Scholar]
- 37. Arneson WL, Arneson DL.. Current methods for routine clinical laboratory testing of vitamin D levels. Lab Med. 2013; 44(1): e38–e42. [Google Scholar]
- 38. Connerty H V., Briggs AR.. Determination of serum calcium by means of orthocresolphthalein complexone. Am J Clin Pathol. 1966; 45(3): 290–296. [DOI] [PubMed] [Google Scholar]
- 39. Lasagni Vitar RM, Barbariga M, Fonteyne P, Bignami F, Rama P, Ferrari G. Modulating ocular surface pain through neurokinin-1 receptor blockade. Invest Ophthalmol Vis Sci. 2021; 62(3): 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chatzis N, Hafezi F.. Progression of keratoconus and efficacy of corneal collagen cross-linking in children and adolescents. J Refract Surg. 2012; 28(11): 753–758. [DOI] [PubMed] [Google Scholar]
- 41. Rahi A, Davies P, Ruben M, Lobascher D, Menon J.. Keratoconus and coexisting atopic disease. Br J Ophthalmol. 1977; 61: 761–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bawazeer AM, Hodge WG, Lorimer B.. Atopy and keratoconus: a multivariate analysis. Br J Ophthalmol. 2000; 84(8): 834–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pedrotti E, Demasi CL, Fasolo A, et al.. Obstructive sleep apnea assessed by overnight polysomnography in patients with keratoconus. Cornea. 2018; 37(4): 470–473. [DOI] [PubMed] [Google Scholar]
- 44. Sharif KW, Casey TA, Coltart J.. Prevalence of mitral valve prolapse in keratoconus patients. J R Soc Med. 1992; 85(8): 446–448. [PMC free article] [PubMed] [Google Scholar]
- 45. Beardsley TL, Foulks GN.. An association of keratoconus and mitral valve prolapse. Ophthalmology. 1982; 89(1): 35–37. [DOI] [PubMed] [Google Scholar]
- 46. Lee R, Hafezi F, Bradley Randleman J. Bilateral keratoconus induced by secondary hypothyroidism after radioactive iodine therapy. J Refract Surg. 2018; 34(5): 351–353. [DOI] [PubMed] [Google Scholar]
- 47. Marsack JD, Benoit JS, Kollbaum PS, Anderson HA.. Application of topographical keratoconus detection metrics to eyes of individuals with Down syndrome. Optom Vis Sci. 2019; 96(9): 664–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Alio JL, Vega-Estrada A, Sanz P, et al.. Corneal morphologic characteristics in patients with Down syndrome. JAMA Ophthalmol. 2018; 136(9): 971–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hollams EM, Hart PH, Holt BJ, et al.. Vitamin D and atopy and asthma phenotypes in children: a longitudinal cohort study. Eur Respir J. 2011; 38(6): 1320–1327. [DOI] [PubMed] [Google Scholar]
- 50. Archontogeorgis K, Nena E, Papanas N, Steiropoulos P. The role of vitamin D in obstructive sleep apnoea syndrome. Breathe. 2018; 14(3): 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Eren E, Ellidag HY, Cekin Y, Ayoglu RU, Sekercioglu AO, Yilmaz N.. Heart valve disease: the role of calcidiol deficiency, elevated parathyroid hormone levels and oxidative stress in mitral and aortic valve insufficiency. Redox Rep. 2014; 19(1): 34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stagi S, Lapi E, Romano S, et al.. Determinants of vitamin D levels in children and adolescents with Down syndrome. Int J Endocrinol. 2015; 2015: 896758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mackawy AMH, Al-Ayed BM, Al-Rashidi BM. Vitamin D deficiency and its association with thyroid disease. Int J Health Sci (Qassim). 2013; 7(3): 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kennedy RH, Bourne WM, Dyer JA.. A 48-year clinical and epidemiologic study of keratoconus. Am J Ophthalmol. 1986; 101(3): 267–273. [DOI] [PubMed] [Google Scholar]
- 55. Stock RA, Thumé T, Bonamigo EL.. Acute corneal hydrops during pregnancy with spontaneous resolution after corneal cross-linking for keratoconus: a case report. J Med Case Rep. 2017; 11(1): 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bilgihan K, Hondur A, Sul S, Ozturk S.. Pregnancy-induced progression of keratoconus. Cornea. 2011; 30(9): 991–994. [DOI] [PubMed] [Google Scholar]
- 57. Hollis BW, Wagner CL.. Vitamin D deficiency during pregnancy: an ongoing epidemic. Am J Clin Nutr. 2006; 84: 273. [DOI] [PubMed] [Google Scholar]
- 58. Manios Y, Moschonis G, Lambrinou CP, et al.. A systematic review of vitamin D status in southern European countries. Eur J Nutr. 2018; 57(6): 2001–2036. [DOI] [PubMed] [Google Scholar]
- 59. Huh SY, Gordon CM.. Vitamin D deficiency in children and adolescents: epidemiology, impact and treatment. Rev Endocr Metab Disord. 2008; 9(2): 161–170. [DOI] [PubMed] [Google Scholar]
- 60. Brookes NH, Niederer RL, Hickey D, McGhee CNJ, Sherwin T.. Recurrence of keratoconic pathology in penetrating keratoplasty buttons originally transplanted for keratoconus. Cornea. 2009; 28(6): 688–693. [DOI] [PubMed] [Google Scholar]
- 61. Yoshida J, Murata H, Miyai T, et al.. Characteristics and risk factors of recurrent keratoconus over the long term after penetrating keratoplasty. Graefes Arch Clin Exp Ophthalmol. 2018; 256(12): 2377–2383. [DOI] [PubMed] [Google Scholar]
- 62. Yoshida J, Toyono T, Shirakawa R, Miyai T, Usui T.. Risk factors and evaluation of keratoconus progression after penetrating keratoplasty with anterior segment optical coherence tomography. Sci Rep. 2020; 10(1): 18594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gomes JAP, Tan D, Rapuano CJ, et al.. Global consensus on keratoconus and ectatic diseases. Cornea. 2015; 34(4): 359–369. [DOI] [PubMed] [Google Scholar]
- 64. Hersh PS, Greenstein SA, Fry KL.. Corneal collagen crosslinking for keratoconus and corneal ectasia: one-year results. J Cataract Refract Surg. 2011; 37(1): 149–160. [DOI] [PubMed] [Google Scholar]
- 65. Wu H, Li L, Luo S, et al.. Safety and efficacy of repeated crosslinking assisted by transepithelial double-cycle iontophoresis in keratoconus progression after primary corneal crosslinking. Eye (Lond). 2021; 35(11): 3020–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wang YM, Chan TC, Yu MCY, Jhanji V.. Comparative evaluation of progression rate in keratoconus before and after collagen crosslinking. Br J Ophthalmol. 2018; 102(8): 1109–1113. [DOI] [PubMed] [Google Scholar]
- 67. Ferdi AC, Nguyen V, Gore DM, Allan BD, Rozema JJ, Watson SL.. Keratoconus natural progression: a systematic review and meta-analysis of 11 529 eyes. Ophthalmology. 2019; 126(7): 935–945. [DOI] [PubMed] [Google Scholar]
- 68. Hamilton A, Wong S, Carley F, Chaudhry N, Biswas S. Tomographic indices as possible risk factors for progression in pediatric keratoconus. J AAPOS. 2016; 20(6): 523–526. [DOI] [PubMed] [Google Scholar]
- 69. Zhou P, Yang C, Zhang S, et al.. The imbalance of MMP-2/TIMP-2 and MMP-9/TIMP-1 contributes to collagen deposition disorder in diabetic non-injured skin. Front Endocrinol (Lausanne). 2021; 12: 1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jia HZ, Pang X, Peng XJ.. Changes of matrix metalloproteinases in the stroma after corneal cross-linking in rabbits. Int J Ophthalmol. 2021; 14(1): 26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sobrino T, Regueiro U, Malfeito M, et al.. Higher expression of Toll-like receptors 2 and 4 in blood cells of keratoconus patients. Sci Rep. 2017; 7(1): 12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wisse RPL, Kuiper JJW, Gans R, Imhof S, Radstake TRDJ, Van Der Lelij A.. Cytokine Expression in keratoconus and its corneal microenvironment: a systematic review. Ocul Surf. 2015; 13(4): 272–283. [DOI] [PubMed] [Google Scholar]
- 73. Kagan HM, Trackman PC.. Properties and function of lysyl oxidase. Am J Respir Cell Mol Biol. 1991; 5(3): 206–210. [DOI] [PubMed] [Google Scholar]
- 74. Ortak H, Söǧüt E, Taş U, Mesci C, Mendil D.. The relation between keratoconus and plasma levels of MMP-2, zinc, and SOD. Cornea. 2012; 31(9): 1048–1051. [DOI] [PubMed] [Google Scholar]
- 75. Cantemir A, Alexa AI, Ciobica A, et al.. Evaluation of antioxidant enzymes in keratoconus. Rev Chim. 2016; 67(8): 1538–1541. [Google Scholar]
- 76. Tekin S, Seven E.. Assessment of serum catalase, reduced glutathione, and superoxide dismutase activities and malondialdehyde levels in keratoconus patients. Eye (Lond). 2022; 36(10): 2062–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kiliç R, Bayraktar AC, Bayraktar S, Kurt A, Kavutçu M.. Evaluation of serum superoxide dismutase activity, malondialdehyde, and zinc and copper levels in patients with keratoconus. Cornea. 2016; 35(12): 1512–1515. [DOI] [PubMed] [Google Scholar]
- 78. Udar N, Atilano SR, Brown DJ, et al.. SOD1: a candidate gene for keratoconus. Invest Ophthalmol Vis Sci. 2006; 47(8): 3345–3351. [DOI] [PubMed] [Google Scholar]
- 79. Bykhovskaya Y, Li X, Epifantseva I, et al.. Variation in the lysyl oxidase (LOX) gene is associated with keratoconus in family-based and case-control studies. Invest Ophthalmol Vis Sci. 2012; 53(7): 4152–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Nykjaer A, Fyfe JC, Kozyraki R, et al.. Cubilin dysfunction causes abnormal metabolism of the steroid hormone 25(OH) vitamin D3. Proc Natl Acad Sci USA. 2001; 98(24): 13895–13900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ghanem VC, Ghanem RC, De Oliveira R.. Postoperative pain after corneal collagen cross-linking. Cornea. 2013; 32(1): 20–24. [DOI] [PubMed] [Google Scholar]
- 82. Dhawan S, Rao K, Natrajan S.. Complications of corneal collagen cross-linking. J Ophthalmol. 2011; 2011: 869015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Godefrooij DA, Mangen MJJ, Chan E, et al.. Cost-effectiveness analysis of corneal collagen crosslinking for progressive keratoconus. Ophthalmology. 2017; 124(10): 1485–1495. [DOI] [PubMed] [Google Scholar]
- 84. Balasubramanian SA, Mohan S, Pye DC, Willcox MDP.. Proteases, proteolysis and inflammatory molecules in the tears of people with keratoconus. Acta Ophthalmol. 2012; 90(4): e303–e309. [DOI] [PubMed] [Google Scholar]
- 85. Kolozsvári BL, Berta A, Petrovski G, et al.. Alterations of tear mediators in patients with keratoconus after corneal crosslinking associate with corneal changes. PLoS One. 2013; 8(10): e76333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kim TG, Kim KY, Bin Han J, Jin KH. The long-term clinical outcome after corneal collagen cross-linking in Korean patients with progressive keratoconus. Korean J Ophthalmol. 2016; 30(5): 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. De Bernardo M, Capasso L, Lanza M, et al.. Long-term results of corneal collagen crosslinking for progressive keratoconus. J Optom. 2015; 8(3): 180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Aixinjueluo W, Usui T, Miyai T, Toyono T, Sakisaka T, Yamagami S.. Accelerated transepithelial corneal cross-linking for progressive keratoconus: a prospective study of 12 months. Br J Ophthalmol. 2017; 101(9): 1244–1249. [DOI] [PubMed] [Google Scholar]
- 89. Marcinowska-Suchowierska E, Kupisz-Urbanska M, Lukaszkiewicz J, Pludowski P, Jones G.. Vitamin D toxicity–a clinical perspective. Front Endocrinol (Lausanne). 2018; 9: 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.