Summary
Basic volatiles like ammonia are found in insect environments, and at high concentrations cause an atypical action potential burst, followed by inhibition in multiple classes of olfactory receptor neurons (ORNs) in Drosophila melanogaster. During the period of inhibition, ORNs are unable to fire action potentials to their ligands but continue to display receptor potentials. An increase in calcium is also observed in antennal cells of Drosophila and Aedes aegypti. In the gustatory system, ammonia inhibits sugar and salt responses in a dose-dependent manner. Other amines show similar effects in both gustatory and olfactory neurons, correlated with basicity. The concentrations that inhibit neurons reduce proboscis extension to sucrose in Drosophila. In Aedes, a brief exposure to volatile ammonia abolishes attraction to human skin odor for several minutes. These findings reveal an effect that prevents detection of attractive ligands in the olfactory and gustatory systems and has potential in insect control.
Subject areas: Biological sciences, Neuroscience, Sensory neuroscience
Graphical abstract
Highlights
-
•
Amines cause bursts of activity followed by silencing of olfactory neurons
-
•
Attraction of mosquitoes to skin odor is reduced for minutes after exposure
-
•
Amines silence sugar and salt detection in the gustatory neurons
-
•
Adding amines to sugar reduces proboscis response in Drosophila
Biological sciences; Neuroscience; Sensory neuroscience
Introduction
Ammonia and amines occur commonly throughout different ecosystems at a wide range of concentrations. Low concentrations of ammonia present in human sweat act as attractive cues for disease vectors like Anopheles gambiae1,2 and Aedes aegypti,3 which can sense ammonia through grooved peg sensilla present on their antennae. Ammonia emitted by the flowering plant Rafflesia can also act as an attractive cue for certain insects facilitating pollination.4 Outside insects, ammonia has been shown to act as an important cue in prey-finding and homing behavior of sea birds such as petrels, albatrosses and shearwaters.5 It is also sensed by olfactory systems of other vertebrates like fish6 and mammals, including humans7 and mice.8
Amines are derivatives of ammonia, in which one or more hydrogens have been replaced with a hydrocarbon group. Ammonia is present in insect excreta (frass), and honey bee frass can reach high levels in soil as well.9 Amines are present in mouse urine and can act as social cues or pheromones and are detected by the olfactory epithelium.8 In other organisms, such as Caenorhabditis elegans,10 ammonium acetate can be detected by both olfactory and gustatory systems. Hence ammonia and its derivatives play a chemosensory role in a vast range of invertebrate and vertebrate animals.
A conserved class of receptors known as trace amine associated receptors (TAAR) have been identified in mice to be responsible for sensing ammonia and amines.8 Attraction toward ammonia appears to be fairly conserved in Dipteran insects too, since it is known that mosquitoes, Drosophila melanogaster, as well as a related species, Drosophila simulans, display attraction to it in olfactory behavior assays. One study identified Ir92a, an ionotropic receptor, to be necessary for olfactory detection of ammonia and certain amines at attractive concentrations.11 The same study mapped ammonia-elicited activity to VM1 glomeruli in the antennal lobes, and to VM1-PNs projecting to the lateral horns. In Anopheles coluzzii mosquitoes, mutations in the co-receptor AcIr76b lead to a complex phenotype, showing increases in responses to amines in grooved peg sensilla but a decrease in coeloconic sensilla.12 Volatile amines are also known to inhibit the CO2 receptor, which belongs to the Gustatory receptor family, in D. melanogaster and Aedes aegypti.13 In addition to members of the Ionotropic receptor family and the CO2 receptor, a study also reported the role of an ammonia transporter gene Amt in olfactory responses to ammonia.14 Amt is expressed in the auxiliary cells surrounding Ir92a-expressing ac1 neurons. Loss of Amt function greatly decreases ac1 firing frequency upon stimulation with ammonia. Interestingly the A. coluzzii mutant in the orthologous AcAmt did not show a significant difference in ammonia responses in the antenna.12 How the receptor and transporter pathways work together and contribute to ammonia-driven attractive behaviors is not fully understood as yet.
Less is known about mechanisms underlying detection of higher concentrations of ammonia, which do not always result in attractive behaviors. The higher end of the naturally-occurring concentration range (40mmol/kg) can be found in biological excrement,15 and rotting biomass in fruit and other plant parts that produce ammonia and other amines.4,16,17 Ammonia is also present in the urine and feces of various animals, including birds, present directly or as a metabolite of urea, leading to high concentrations.18,19 Ammonia is also excreted by fly larvae and can build up to considerable levels in over-crowded cultures.18,19 Finally, ammonia concentrations are extremely high in several human products and animal excreta that are found worldwide in massive quantities in environments that insects traverse regularly, such as agricultural fields treated with fertilizers (∼2–20%), household cleaners (∼5–10%) and manure (∼4–7%) (https://agcrops.osu.edu/sites/agcrops/files/imce/fertility/bulletin_604.pdf). Although the majority of past studies were directed at investigating olfactory detection of ammonia, a recent study reports physiological as well as behavioral responses to ammonium salts in the Drosophila gustatory system20. Ammonium salts evoke strong neuronal responses from Gr66a neurons in S-type sensilla of the labellum, which results in behavioral aversion in feeding experiments.
Here we test how high concentrations of ammonia alter the activity and response properties of chemosensory neurons and impact olfactory and gustatory function in the model insect D. melanogaster. We find that in response to ammonia there is a strong phasic activation followed by an atypical inhibitory response in several different classes of olfactory neurons. In both olfactory and taste neurons the tonic inhibition can completely abolish the subsequent detection of other olfactory and gustatory cues. We attribute the burst-inhibition response to a high pH effect and identify several amines that also cause strong neuronal inhibition. The observed mechanisms appear to be conserved in mosquitoes and shed light on how at higher concentrations this attractive cue becomes less so. Using these principles, we identify a compound that can mimic the effects of ammonia on the chemosensory system of both flies and mosquitoes.
Results
High concentrations of ammonia show phasic burst and tonic inhibition of olfactory neurons
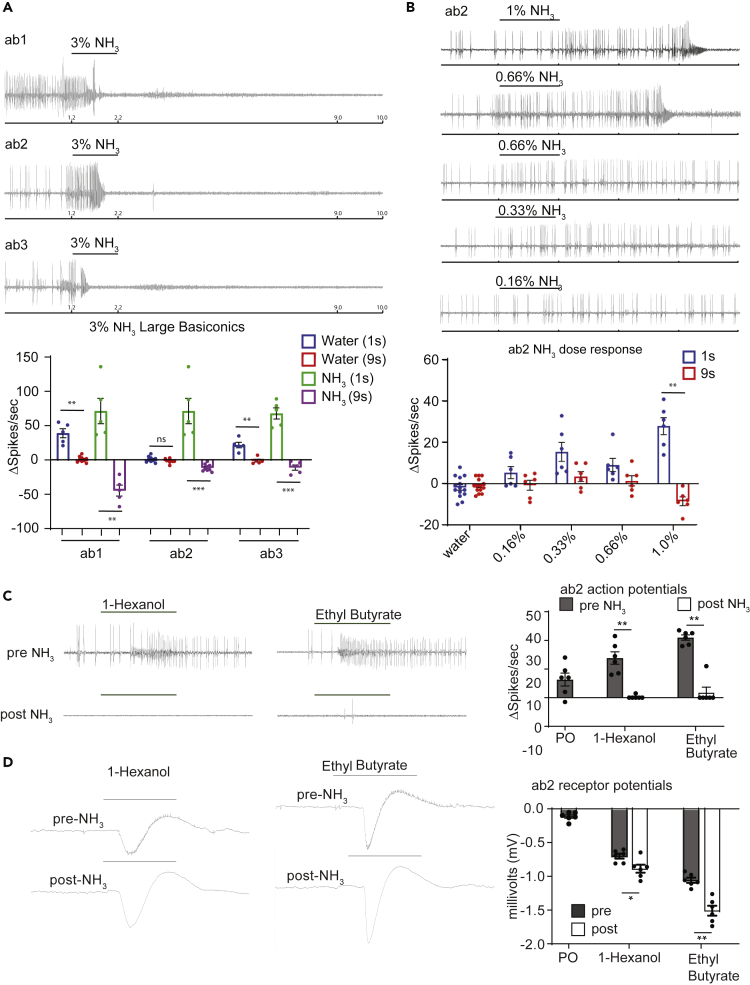
To investigate the response of olfactory neurons to high concentrations of ammonia in insects we used D. melanogaster as a model and performed single-sensillum recordings (SSRs) on the well-studied large basiconic sensilla, which are most tractable for high quality action potential traces. We began by testing headspace above 3% ammonia, which would be in the range of concentrations encountered by insects around animal excreta, fertilized fields and around cleaners used in homes. We found that in every case, a stimulus of 3% ammonia caused a brief strong phasic increase in spikes before a tonic silencing of the neuron (Figure 1A). This effect was consistent across all three large basiconic sensillar classes tested: ab1, ab2 and ab3. To determine the concentration threshold at which this unusual effect occurs, we tested ammonia on ab2 neurons across a range of concentrations. At 0.16 and 0.33%, ammonia did not cause the burst-silencing effect. At 0.66%, ammonia resulted in burst-silencing one-third of the time, and at 1.0% it always did so, indicating that the effect is concentration-dependent (Figure 1B). The length of the silencing period varied between neurons, lasting from 45 s to over 3 min. These results show that the baseline activity of olfactory neurons is suppressed after exposure to high concentrations of ammonia.
Figure 1.
Olfactory neuron response to high concentrations of ammonia
(A) Representative traces (top) and mean responses (bottom) for a 1-s period of stimulation from each sensillum in response to ammonia. The 1-s stimulation period is indicated by the black bar above each trace. Counting for the responses marked “1.2s–2.2s” was begun at the start of the increase in spike frequency (∼1.2 s mark on the recording). Counting for the responses marked “9s–10s” was measured during the last second (from 9s to 10s) on each recording. All recordings were obtained from 3–5 day old wildtype (CS) flies. n = 5–13 sensilla from 6 flies. Error bars indicate SEM Dots indicate responses from individual sensillum. For each sensilla type repeated measures 1-way ANOVA with Geisser-Greenhouse correction was followed by Tukey’s test for multiple comparisons. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001.
(B) Representative traces (top) and mean responses (bottom) of the ab2 sensillar neurons to a 1-s stimulus of indicated concentration of ammonia. The 1-s stimulation period is indicated by the black bar above each trace. Counting for the responses marked “1.2s–2.2s” was begun at the start of the increase in spike frequency (∼1.2 s mark on the recording). Counting for the responses marked “9s–10s” was measured during the last second (from 9s to 10s) on each recording. All recordings were obtained from 3–5 day old wildtype (CS) flies. n = 6–14 sensilla from 6 flies. Error bars indicate SEM Dots indicate individual data points. Repeated measures mixed-model test with Geisser-Greenhouse correction followed by Tukey’s test for multiple comparisons. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001.
(C) Representative traces (left) and mean responses (right) for the ab2 sensilla to headspace of 1% solutions of known stimulating odorants (10−2). The 1-s stimulation period is indicated by the black bar above each trace. “Pre-NH3” denotes that the recording precedes exposure, while “Post-NH3” denotes that the recording follows a 1-s exposure to 3% ammonia. All recordings were obtained from 3–5 day old wildtype (CS) flies. n = 5–6 sensilla from 6 flies. Error bars indicate SEM Dots indicate individual data points. Repeated measures 1-way ANOVA with Geisser-Greenhouse correction followed by Tukey’s test for multiple comparisons. ∗p < 0.05; ∗∗p < 0.01.
(D) Representative traces (left) and mean receptor potential responses (right) to 1% solutions of indicated odorants (10−2) in the ab2 sensillum both pre and post exposure to 3% ammonia. All recordings were obtained from 3–5 day old wildtype (CS) flies. n = 6 sensilla from 6 flies. Error bars indicate SEM Dots indicate individual data points. Repeated measures 1-way ANOVA with Geisser-Greenhouse correction followed by Tukey’s test for multiple comparisons. ∗p < 0.05; ∗∗p < 0.01.
Exposure to ammonia completely blocks ability to detect cognate odorants
To test whether odorants detected by ORNs could still elicit neural activity during the post-ammonia silencing period, we tested responses of neurons in the ab2 sensillum to odorant stimuli from headspace of 1% solutions of known activators (1-hexanol and ethyl butyrate) both before and 20–60 s after exposure to 3% ammonia. As observed earlier, the ORNs were silenced following an initial burst upon ammonia exposure, and subsequent stimulation with the two odorant ligands failed to produce spiking activity (Figure 1C). In a complementary electrophysiological assay, we recorded the receptor potential of the ab2 sensillum to odorant stimuli both before and after exposure to 3% ammonia. Interestingly, we found that the odor-induced sensillar receptor potentials were slightly enhanced after exposure to ammonia, although the action potentials were completely abolished (Figures 1C and 1D). This suggests that olfactory receptors are possibly still functional and may allow odor-mediated receptor potentials. However, the mechanisms underlying this phenomenon are not understood.
Ammonia causes widespread increase in calcium in antennal neurons
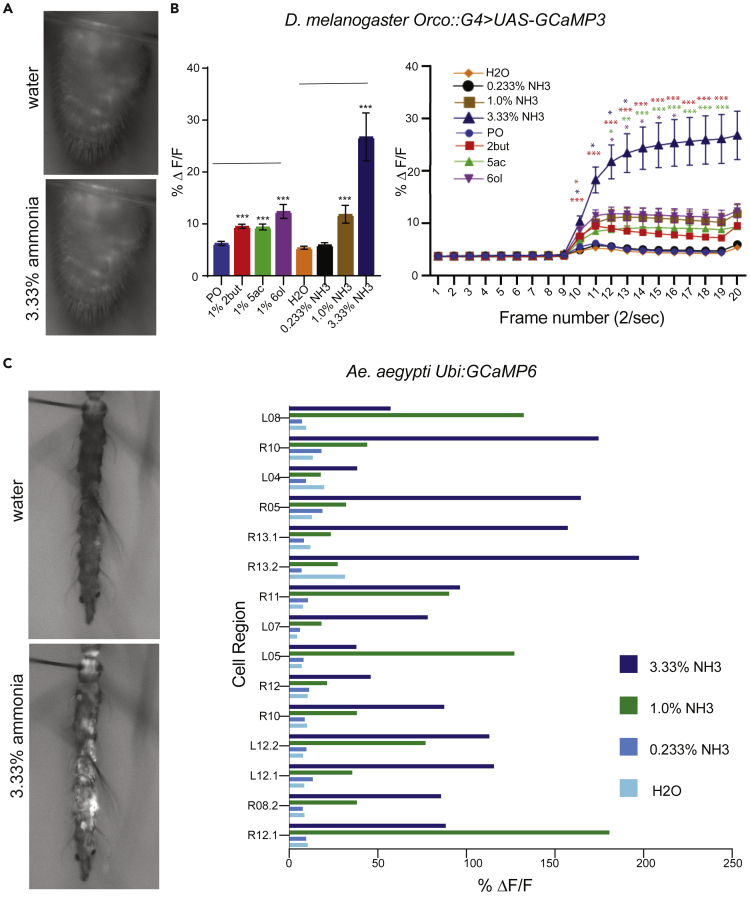
In order to measure calcium flux in response to ammonia in ORNs of the Drosophila antenna, we expressed GCaMP6m under the control of an Orco-Gal4 driver that is broadly expressed in all Or-expressing neurons, which account for ∼70% of the ORNs in the antenna. By monitoring GCaMP6m fluorescence in several cells, we were able to survey the activity of a number of ORNs simultaneously. We found that ammonia led to an increase of calcium activity in a dose-dependent manner in the antenna (Figures 2A and 2B). While 3.33 and 1% concentrations produced significant increases in signal, the 0.233% concentration did not, which is aligned with the initial strong burst of action potentials, as well as the receptor potentials observed in single-sensillar electrophysiology recordings.
Figure 2.
Increased Calcium in responses to high concentrations of ammonia
(A) Representative fluorescence micrograph of antenna exposed to indicated stimuli in OrcoGal4;UAS-GCaMP6m Drosophila melanogaster.
(B) Mean response to indicated stimulus at (left) 1.5 s after stimulus (frame 12) for the indicated stimuli; and (right) averaged temporal kinetics of the calcium response detected from Drosophila antennae to the indicated odorants. All recordings were obtained from 3–5 day old flies. n = 18 antenna from 11 flies. Error bars indicate SEM T-test. ∗∗p < 0.01, ∗∗∗p < 0.01, ∗∗∗∗p < 0.001.
(C) Representative fluorescence micrograph of antenna exposed to indicated stimuli (left) and mean percentage change in fluorescence intensity of several individual cells (right) from a large number of PUbi-GCaMP female Aedes aegypti antennae to indicated stimulus (0.5 s). Each bar represents response of a single cell. Cells were measured from multiple mosquitoes and all cell are shown in Figure S1.
We then tested whether the broad ammonia-induced increase in calcium levels is conserved by imaging in the yellow fever mosquito Aedes aegypti. Using a recently developed pUbi-GCaMP6 Ae. aegypti line, we determined that a number of cells expressing GCaMP6 can be identified in the antenna of female mosquitoes and responses of individual cells can be measured20,21 (Figure 2C). Of the cell-like regions showing GCAMP6 expression, most showed increases in fluorescence in response to ammonia. By plotting the fluorescence increases of all cells that showed an increase in Ca2+ signal in response to at least one stimulus, we found that a large fraction of fluorescent cell-like regions responded to ammonia at concentrations of 3.33 and 1% (Figures 2C and S1). In general, there is a dose dependency in the response, however, we do find a few examples of regions which show higher changes in fluorescence at the 1% concentration (Figures S1 and 2C). Almost every region evaluated shows an increase in responses. This widespread activation is similar to that which we observed in Drosophila.
Ammonia inhibits sweet and salt taste neurons in the labellum
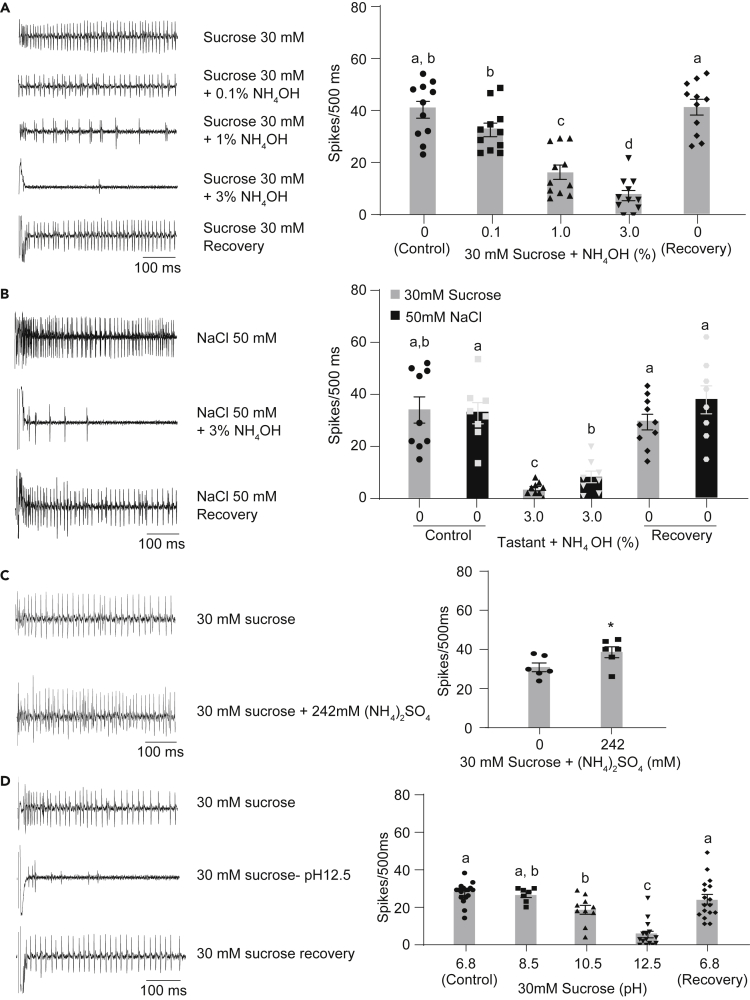
Ammonia has been shown to activate aversive bitter neurons in S-type hairs of the Drosophila labellum.22 In order to investigate if ammonia can inhibit responses of appetitive gustatory neurons, we used a tip-recording electrophysiology method to test mixtures of NH4OH with sucrose, a strong activator of sweet-sensing neurons in L-type sensilla. We found that NH4OH inhibited sucrose response in a concentration-dependent manner (Figure 3A). Exposure to ammonia did not damage the sweet taste neuron, since stimulation with 30 mM sucrose at the end of the experiment evoked a normal elevation in firing frequency.
Figure 3.
Gustatory system response to ammonia
(A) Representative traces (left) and mean responses along with individual data points recorded from labellar sensilla (L-type) for the first 500msecs when stimulated with the mentioned tastants. 5-7days old wild type (CS) flies were used for the recordings. n = 11 sensilla from 3 flies. Paired 1-way ANOVA with Tukey’s test for pairwise comparison. Error bars indicate SEM. Lower case alphabets indicate statistically different groups.
(B) Representative traces (left) and mean responses along with individual data points for the first 500 ms recorded from L-type labellar sensilla upon stimulation with the mentioned tastants. 5-7days old wild type (CS) flies were used for the recordings. n = 8–10 sensilla from 3 flies. Mixed effect analysis followed by Tukey’s test for pairwise comparison. Error bars indicate SEM Lower case alphabets indicate statistically different groups.
(C) Representative traces (left) and mean responses along with individual data points for the first 500msecs recorded when labellar L-type sensilla are stimulated with the mentioned tastants. All recordings were obtained from 5–7days old wild type (CS) flies. n = 6 sensilla from 2 flies. Paired t-test. Error bars indicate SEM ∗p < 0.05.
(D) Representative traces (left) and mean responses for the first 500msecs obtained from L-type labellar sensilla upon stimulation with the mentioned tastants. Recordings are from wild type (CS) female flies, 5–7days of age. n = 7–17 sensilla from 2–5 flies. Mixed effect analysis followed by Tukey’s test for pairwise comparison. Error bars indicate SEM Lower case alphabets indicate statistically different groups.
Sweet taste neurons house receptors that belong to the Gr (gustatory receptor) family.23,24 By contrast, appetitive salt (NaCl) taste is detected by Ir76b, a member of another receptor family called the ionotropic receptor (Ir) family.25 In order to test if ammonia can also inhibit salt detection, we mixed 50 mM NaCl with 3% NH4OH and obtained extracellular tip-recordings from L hairs. We found that exposure to ammonia inhibited Ir76b-mediated salt response as well (Figure 3B). That inhibition was not caused by irreversible damage to the neuron, was evident from the normal spiking in response to subsequent stimulation with NaCl.
Neuronal inhibition by ammonia is due to high pH
A recent study reported that NH4+ ions are responsible for the activation of bitter taste neurons by ammonium salts.22 In order to test if the presence of NH4+ ions in NH4OH is required for neuronal inhibition, we tested sucrose mixtures with 242 mM (NH4)2SO4, a concentration that matched the amount of NH4+ ions in 1% NH4OH upon complete dissociation. Neuronal firing to sucrose was not inhibited with (NH4)2SO4, on the contrary there was a slight increase in firing frequency when (NH4)2SO4 was added, indicating that it was caused by a feature other than NH4+ ions in ammonia (Figure 3C).
NH4OH is highly basic in nature. When measured, 0.1%, 1% and 3% NH4OH solutions had an approximate pH of 8.5, 10.5 and 12.5 respectively. Previous studies of other neurons have reported pH-induced changes in neuronal sensitivity.26,27,28,29 In order to test if high pH caused neuronal inhibition, we tested a range of pH by adding varying amounts of NaOH to a 30 mM sucrose solution. Although a mixed effect analysis did not exhibit a general effect, interestingly with pairwise comparisons we observed that an increase in the pH resulted in stronger inhibition of sucrose response in sweet neurons (Figure 3D). At pH 12.5 and 10.5 we observed inhibition of sucrose-evoked sweet neuron firing to levels comparable to those caused by 3 and 1% NH4OH respectively. These experiments support the view that ammonia causes neuronal inhibition due to its high pH. In every test, we observed robust neuronal responses to 30 mM sucrose from the same sensilla at the end of the experiment, confirming that any observed reductions in neuronal response were not due to irreversible damage.
Basic amines show a burst followed by inhibition in olfactory neurons
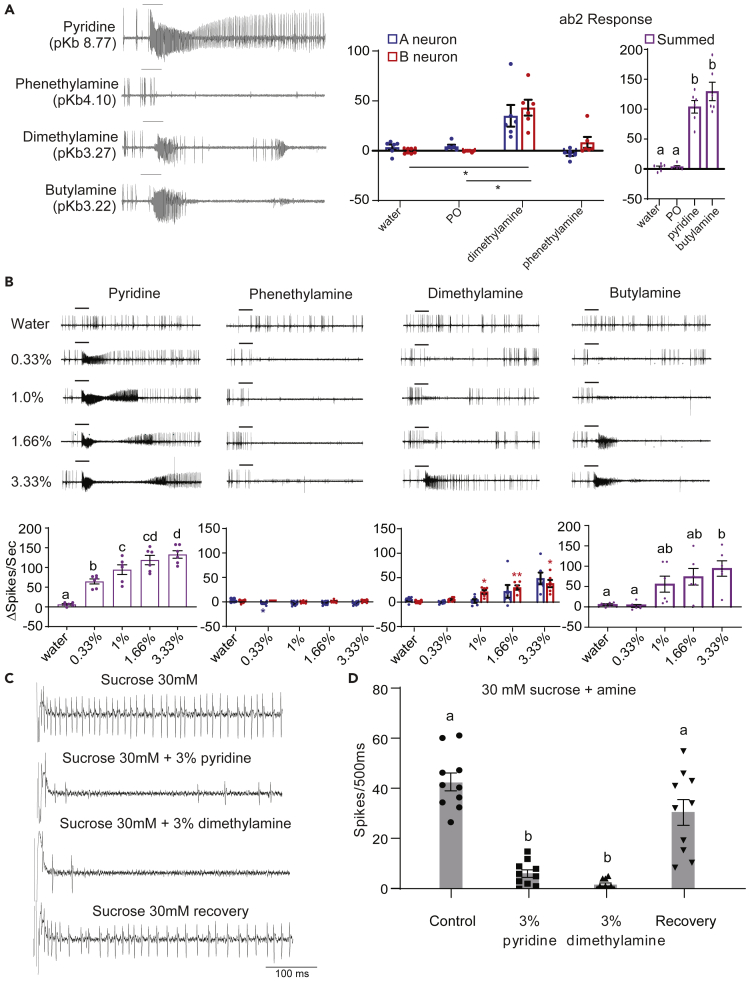
Amines are inorganic derivatives of ammonia and some occur naturally in the ecological niche of the fly. Certain amines such as dimethylamine occur naturally, whereas others enter the ecosystem through industrial effluents. Most amines are basic in pH and several are also toxic to living organisms. The amines occur both naturally and as products of industrial processes. Dimethylamine is one of the most common amines occurring ubiquitously in natural samples and piperidine and its derivatives are present in water samples.30 Piperidine occurs naturally in pepper plants and there are several derivatives that occur as natural alkaloids.31 Pyridine occurs naturally in roots and leaves of plants and is produced as a by-product in food and dairy industries.32 These compounds have a range of pKb values denoting their basicity, which will allow some degree of correlation to electrophysiological effects: pyridine (pKb 8.77) < phenethylamine (pKb 4.1) < dimethylamine (pKb 3.27) < butylamine (pKb 3.22).
To test whether amines evoke similar responses in ORNs as ammonia, we exposed ORNs to amines while recording their activity. We found that two of the most basic amines, butylamine and dimethylamine, produced the burst-silencing effect observed previously with ammonia (Figure 4A). Comparison of effects across concentrations showed that 1.66% of butylamine mimicked the burst-silencing effect seen with ammonia, whereas at concentrations lower than 0.33% it briefly inhibited the ab2A neuron while activating the ab2B neuron (Figures 4B and S2). Dimethylamine at 3.33% produced the burst-silencing effect; at lower concentrations it activated the ab2B neuron and inhibited the ab2A neuron. Two of the tested compounds that were less basic did not cause burst activity. Instead, phenethylamine silenced the ab2A neuron but did not affect the ab2B neuron, whereas the least basic volatile, pyridine, did not even cause inhibition and instead showed a strong increase in spike frequency (Figure 4A). The activation of ab2 neurons in response to pyridine exhibited dose-dependence like that of a typical activating ligand and caused strong excitation at concentrations of 1.66% and higher (Figure 4B). Phenethylamine strongly inhibited the ab2A neuron at all concentrations tested while leaving the ab2B neuron unaffected.
Figure 4.
Response to amines in the olfactory and gustatory system
(A) Representative traces (left) and mean responses from A and B neurons (middle) or summation of responses obtained from A and B neurons for a 1-s period of stimulation from the indicated compounds. For water, PO, dimethylamine and phenethylamine (middle) blue bars indicate responses from A neurons while red bars indicate responses from the B neurons. In the right figure all bars represent summation of responses obtained from both A and B types for water, PO, pyridine and butylamine. For the rightmost figure, the values shown for water and PO are actual sums of responses from A and B neurons in the middle figure. Each count was begun at the start of the increase in spike frequency. All compounds tested at 3.33% concentration in water, except phenethylamine which was dissolved in paraffin oil. All recordings were obtained from 3–5 days old wildtype (CS) flies. n = 4–6 sensilla from 6 flies. Error bars indicate SEM Dots indicate individual data points. Repeated measures 1-way ANOVA with Geisser-Greenhouse correction (between responses from same neuron from the middle figure) followed by Tukey’s test for multiple comparisons. ∗∗p < 0.01. In the rightmost figure, lower case alphabets indicate statistically different groups.
(B) Representative traces (top) and mean responses (bottom) for a 1-s period of stimulation from each concentration of the four basic compounds. Each count was begun at the start of the increase in spike frequency. For dimethylamine and phenethylamine, blue and red bars indicate responses obtained from (A and B) neurons respectively. For pyridine and butylamine, bars indicate summed responses from both neuron types. All compounds were dissolved in water, except phenethylamine which was dissolved in paraffin oil. All recordings were obtained from 3–5 days old wildtype (CS) flies. n = 6 sensilla from 6 flies. Error bars indicate SEM Dots indicate individual data points. Repeated measures 1-way ANOVA with Geisser-Greenhouse (between responses from same neuron type for dimethylamine and phenethylamine) correction followed by Tukey’s test for multiple comparisons. For phenethylamine and dimethyamine, blue and red asterisks indicate statistically significant difference from the response obtained with PO from the A neurons and the B neurons respectively. ∗p < 0.05; ∗∗p < 0.01. For pyridine and butylamine, small case alphabets indicate statistically different groups.
(C) Representative traces for the first 500 ms recorded when labellar L-type sensilla are stimulated with the mentioned tastants. All recordings were obtained from 5–7 days old wild type (CS) flies.
(D) Mean responses along with individual data points for the first 500msecs recorded when labellar L-type sensilla are stimulated with the mentioned tastants. All recordings were obtained from 5–7 days old wild type (CS) flies. n = 10 sensilla from 3 flies. Error bars indicate SEM Paired 1-way ANOVA with Tukey’s test for pairwise comparison. Lower case alphabets indicate statistically different groups.
Basic amines inhibit gustatory neurons
In order to test whether basic amines are also capable of causing the neuroinhibitory effect, we selected three compounds for mixture experiments with sucrose: 4-methylpiperidine, pyridine and dimethylamine. Tip recordings from labellar L-type sensilla of Drosophila were performed as before, with 30 mM sucrose. The two amines, pyridine and dimethylamine also had a neuroinhibitory effect (Figures 4C and 4D). The 3% solutions of pyridine and sucrose each had a pH of ∼8 (∼12.5 for dimethylamine) but resulted in a neuroinhibitory effect stronger than that caused by pH 8, invoking the involvement of additional factors in inhibition. As before we measured the response to 30 mM sucrose alone, before and after the sucrose-amine mixtures, to rule out the possibility that any observed neuronal inhibition is due to non-specific neuronal damage.
High concentrations of ammonia are behaviorally aversive to insects
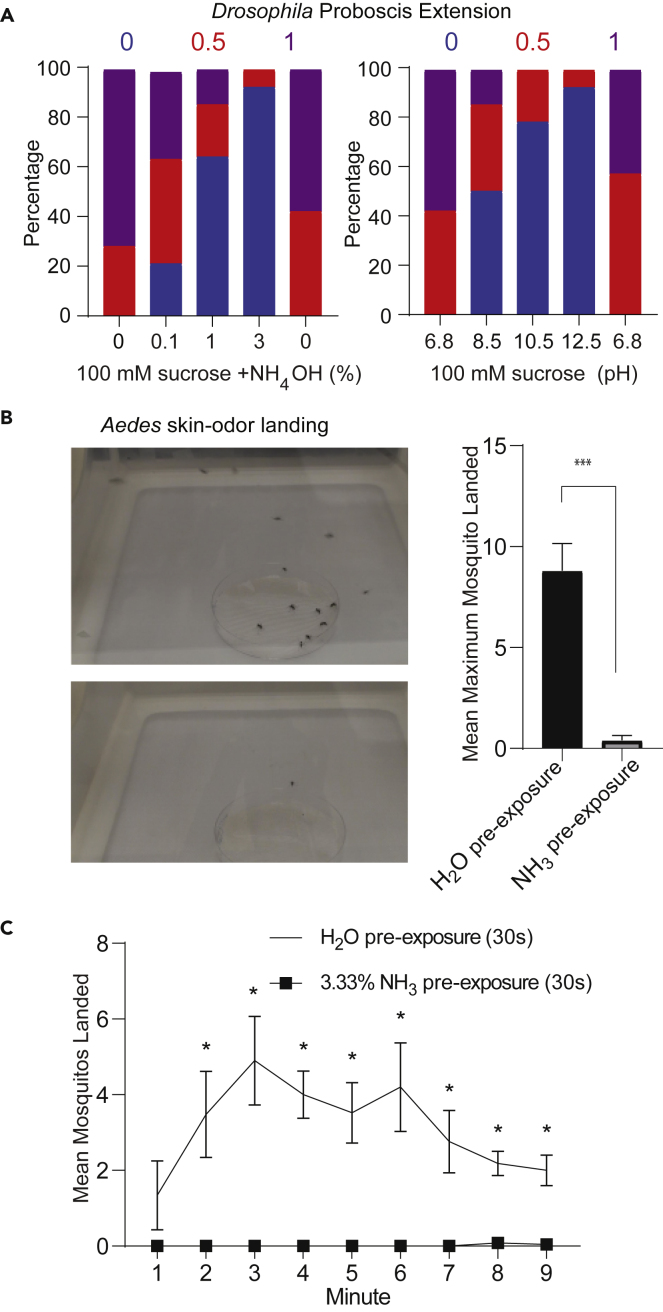
To determine if the electrophysiological effects are behaviorally relevant in gustation, we measured proboscis extension response (PER) to labellar stimulation in Drosophila. PER to 100 mM sucrose was significantly decreased by addition of increasing concentrations of NH4OH in a dose-dependent manner, as well as by increasing pH by addition of NaOH (Figures 5A and S3). Thus, neuronal inhibition of taste neurons translates to avoidance of foods contaminated with high levels of ammonia or high pH.
Figure 5.
Behavioral effects in the gustatory and olfactory system
(A) Summary of percentage of different outcomes on performing proboscis extension response assay on labellar stimulation of female CS flies to 100mM sucrose alone as well as mixed with indicated percentage of NH4OH (left panel). Summary of percentage of different outcomes on proboscis extension response assays on labellar stimulation of female CS flies to 100 mM sucrose alone (pH 6.8) as well as mixed with two different NaOH concentrations to attain the indicated pH (right panel). As indicated in numbers above each graph, percentage of flies showing full extension was indicated in purple bar and ascribed a value of +1, percentage with a half-extension or extension with immediate retraction was ascribed a red bar and a value of +0.5, and percentage with no extension in blue with a value of 0 (blue bar). n = 14 flies in both cases.
(B) Representative image (left) and mean count of maximum number of wildtype Aedes aegypti females landed on nylon mesh with human odor in a 9 min period, after a 30-s exposure to either a water control or 3.3% ammonia (right). Mated females, 10–12 days old. Maximum mosquito count frame was determined from the 90 frames during the 9-min video. n = 5 trials of 19–21 mosquitos for each condition. T test. Error bars indicate SEM ∗∗∗p < 0.001.
(C) Mean count represented over different time-points from the same experiment as above. T test. Error bars indicate SEM ∗p < 0.05.
After initial experiments with a genetic model organism, we next tested a disease vector, Aedes aegypti, in behavior assays. Since exposure to NH3 evokes such a strong prolonged silencing afterinitial activation, and responses to subsequent odorant stimuli are severely reduced, we assumed that skin odor detection may also be compromised, presumably even when NH3 is no longer present. Skin odor-mediated attraction is a well-established and robust behavioral paradigm for Aedes mosquitoes, and we used it to test the behavioral consequences of “tonic suppression” of the olfactory neurons. Mated female Aedes mosquitoes in an appropriate physiological host-seeking state were housed in holding cups pre-treated with vapors of NH3 and tested in a cage assay that provides a controlled environment. In controls, Aedes females that were mock treated with the solvent (water) were attracted to a human odor-containing nylon mesh placed in a Petri dish (Figures 5B and 5C). We found that a 30-s pre-treatment with NH3 significantly impaired this skin odor-mediated attraction over the 10 min assay (Figures 5B and 5C). The absence of NH3 in the behavior area during the testing period suggests that behavior toward skin odor is modified due to the inability of the olfactory neurons to detect the skin odorants, rather than due to avoidance of NH3 via other ORNs.
Discussion
We find that ammonia and amines, both of which have high pH, can cause a burst of activation followed by a prolonged period that lacks action potentials in the olfactory neurons. The mechanisms underlying the observed burst and subsequent inhibition are not known. The finding that odor-induced receptor potentials appear to be unaffected suggests that ion flux through odorant receptors is not drastically altered if at all, and therefore the resting membrane potential of the neuron must likewise be within its typical range. One possibility is that voltage-gated sodium channels required for action potentials are fixed in an inactive state during the period of inhibition. This would allow ions to flux across the membrane via odorant receptors, but the resulting receptor potential would fail to generate action potentials upon reaching the normal spiking threshold.
The gustatory system appears to differ somewhat in the response to basic stimuli compared to the olfactory system in not showing the “burst” prior to inhibition. While we are unable to pinpoint the contributing factors, there are many differences to consider. Architecturally the taste sensilla have a large pore at their tips, presumably allowing for a larger volume of the test solution rapid access to the sensillar lymph. Additionally, the relative buffering capacity of the sensillar lymph, and cells surrounding it, may also be different, as are the receptor family members expressed (Gr vs Or families).
The variable effects of the tested amines also raise interesting questions about the contribution of factors other than pH for each odorant-neuron interaction. However, it is notable that pyridine, which is the least basic of the compounds, acts in a manner typical of an activating ligand, causing a robust increase in spike frequency in a dose-dependent manner. Phenethylamine, which is more basic, also does not cause burst-silencing but instead inhibits the ab2 neurons. Dimethylamine inhibits, but at highest concentration causes activation. While butylamine, which is the most basic in the series, inhibits ab2 neurons in a manner similar to phenethylamine at lower concentrations, but causes burst-silencing at higher concentrations. Thus, the burst-silencing effect first seen with relatively high concentrations of ammonia and amines may be a consequence of high pH rather than the steric structure or functional groups of the ligand per se, similar to implications of gustatory neuron inhibition by high pH, and not the molecular identity, of the selected tastants.
Taken together, we show that several aspects of the host-seeking behavior, including attraction to an odor source as well as feeding are severely disrupted by NH3 at concentrations as low as ∼3%. In comparison DEET is used between 10–70% and needs to be applied directly on the skin to offer local short-distance protection. Overall, our study offers a strategy to create new insect repellents that act on both the olfactory and taste systems by blocking the ability to detect appetitive volatile and non-volatile cues. The finding that compounds with high pH can independently serve both functions expands the scope for identifying newer compounds that could become the next generation of insect repellents. Taken together, these findings guide us toward identification of a new generation of broad-spectrum insect repellents that have enhanced activities and desirable properties and also provide a foundation for discovery of even better repellents in the future.
While these basic compounds are not appropriate for topical human use due to their safety and smell characteristics, these proof-of-principle experiments demonstrate that we will be able to identify repellents that are not topically applied to skin. The volatile repellents could expand possibilities for use in different formulations such as treatments on surfaces like windowsills, eves of huts, house entryways, backyards, candles, storage areas, entryways of animal shelters, and next to crops in fields.
Limitations of the study
While we show that basic volatiles like amines cause olfactory neurons in Drosophila to burst with activity and then become quiescent for a short period, our study does not uncover the mechanism underlying this broad effect. The phenomenon corelates with basicity and occurs mainly in OR-expressing neurons, while the Gr-expressing CO2 neuron is instead inhibited as seen previously.13 We show that the olfactory neurons are unable to respond to their endogenous odorant ligands during the amine-induced quiescent phase, however we do not know why. We then demonstrate that A. aegypti mosquitoes exposed to basic ammonia volatiles lose their ability to navigate and land on human skin odor, for minutes afterward. While we hypothesize that this behavioral deficit is due to the loss of ability to detect skin odor due to the quiescence of the olfactory neurons, we have not evaluated it directly. In the gustatory system we observe that sugar and salt responses are inhibited with increasing basicity of the tastant solution. A recent study has shown a requirement for three members of the Ir family (Ir51a, Ir25a and Ir76b) in activation of bitter neurons to nitrogenous waste compounds.33 However, a determination of whether the observed inhibition is dependent on these or any other chemosensory receptors, or whether it is mediated by some other aspects of cellular function, awaits further molecular genetic investigation.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Sucrose | Sigma-Aldrich | Cat #S7903 |
| Ammonium hydroxide | Sigma-Aldrich | Cat # 44273 |
| Sodium hydroxide | Sigma-Aldrich | Cat #S5881 |
| Pyridine | Sigma-Aldrich | Cat #P3776 |
| Dimethyl amine | Sigma-Aldrich | Cat # 426458 |
| Phenethylamine | Sigma-Aldrich | Cat # 241008 |
| Ethyl butyrate | Sigma-Aldrich | Cat #W242705 |
| Hexanol | Sigma-Aldrich | Cat #H13303 |
| Paraffin Oil | Sigma-Aldrich | Cat # 18512 |
| Tricholine citrate | Sigma-Aldrich | Cat #T0252 |
| Dextrose | Sigma-Aldrich | Cat #G8270 |
| Propionic Acid | Sigma-Aldrich | Cat # 402907 |
| Sodium chloride | Macron Fine chemicals | Cat # 7647-14-5 |
| Yellow cornmeal | Quaker | Cat # 57020 |
| Inactive dry yeast | Lynside | Cat # 75570 |
| Tegosept | Apex chemical and reagent | Cat # 20-258 |
| Tetramin® Fish food tablets | Tetra Gmbh. Melle, Germany | Cat # 16110 |
| Defibrinated bovine blood | HemoStat Laboratories | Cat #J62036 |
| Experimental models: Organisms/strains | ||
| Drosophila melanogaster: CS | Bloomington Drosophila Stock Center | Cat # BL64349 |
| Drosophila melanogaster: Orco-GAL4;w1118;P{w[+mC]=Orco-GAL4.K}97.1 | Bloomington Drosophila Stock Center | Cat # BL23292 |
| Drosophila melanogaster: UAS-GCaMP;w1118;P{y[+t7.7]w[+mC]=20XUAS-IVS-GCaMP6m}attP40 | Bloomington Drosophila Stock Center | Cat # BL42748 |
| Aedes aegypti: pUb-GCaMP6s | Akbari Laboratory, University of California, San Diego | Bui et al. (2019) |
| Aedes aegypti: Orlando strain | Leslie Vosshall Laboratory, Rockefeller University | N/A |
| Software and algorithms | ||
| Prism | software | https://www.graphpad.com/scientific-software/prism/ |
| Fiji | software | https://imagej.net/Fiji |
| Autospike | Syntech | http://www.ockenfels-syntech.com/products/signal-acquisition-systems-2/ |
| Other | ||
| Glass capillaries | World Precision Instruments | Cat # 1B100F-4 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Anandasankar Ray (anand.ray@ucr.edu).
Materials availability
The study did not generate new, unique reagents.
Experimental model and subject details
D. melanogaster
Flies were reared at 22°C–25°C in standard cornmeal-dextrose media. Wild type flies used in the experiments were Canton-S (BL 64349) obtained from Bloomington stock center. Orco-GAL4 (BL 23292) and UAS-GCaMP6m (BL 42748) flies were also obtained from Bloomington stock center. Following is the composition of the media per 100 food vials: dextrose-100 gms, inactive dry yeast-50 gms cornmeal-70 gms, Drosophila agar-6 gms, propionic acid-6 mL, tegosept-12 mL, water-1025 mL.
Aedes aegypti
All mosquito strains were maintained in an insectary at ∼27°C, 70–80% humidity on a 14:10 h (Light: Dark) photoperiod. Lights on at 0700hrs. Adults were held in the insectary in 30 cm3 Bugdorm cages and given access to 10% sucrose solution. Females were offered blood (defibrinated bovine blood, HemoStat laboratories) using artificial blood feeding system and allowed to lay eggs. Upon hatching, larvae were thinned into 6qt size (5.7L) plastic containers filled with about 900 mL deionized water and fed on Tetramin® fish food tablets until pupation. Ae. aegypti wildtype (Orlando strain), was obtained from Dr. Leslie Vosshall’s laboratory at Rockefeller University. pUb-GCaMP6s mosquitoes were obtained from Dr. Omar Akbari’s laboratory at University of California, San Diego.
Method details
Chemicals
Sucrose (S7903), ammonium hydroxide (44273), sodium hydroxide (S5881), pyridine (P3776), dimethyl amine (426458) Sucrose (84097), phenethylamine (241008), ethyl butyrate (W242705) and hexanol (H13303) were all obtained from Sigma. For PER assays, water was used as the solvent. Odorants were dissolved in paraffin oil (18512, Sigma) or water as mentioned for single sensillum recordings. Tastants were dissolved in 30 mM TCC (Sigma, T0252) for extracellular tip recordings.
For making fly food yellow cornmeal (57020, Quaker), dextrose (G8270, Sigma), inactive dry yeast (75570, Lynside), propionic acid (402907, Sigma) and tegosept (20-258, Apex chemical and reagent) were used. Tetramin® fish food tablets (16110) used for feeding mosquito larvae were obtained from Tetra Gmbh. Melle, Germany. Adult mosquitoes were blood-fed with defibrinated bovine blood.
Quantification and statistical analysis
Electrophysiology
Olfaction
Adult male flies were tested at 3–7 days post-eclosion. Individual flies were inserted into a 1.0 mL pipette tip and secured with molding clay. A glass coverslip was placed underneath the head and a glass pipette was used as a rod to secure an antenna in place. A reference electrode (1B100F-4, World Precision Instruments) filled with sensillar lymph ringer solution as in Kaissling and Thorson 1980, was inserted into the eye. All compounds were dissolved in water (unless otherwise mentioned) to the designated dilutions. A 3 × 35 mm filter paper strip was placed in each 5¾” glass Pasteur pipette to form odor cartridges. Ten μL of each compound was inserted into each cartridge, except in Figure 2 where 50 μL of the activating compounds were added. Cartridges were allowed to sit and volatilize for 15 min before use. Each cartridge was used no more than twice before being discarded. A glass tube was used to maintain a humidified airstream of 10 mL/s on the preparation, provided by a gas tank routed through a water-filled beaker. The odor cartridge was inserted into the side of a glass tube through an opening into the carrier humidified airstream.
A puff of air was released through the odor cartridge with a Syntech device for 1 s. Spikes were counted during the 0.25 s–1.25 s post-stimulus window, owing to a slight delay caused by the increased distance to the antennae. The baseline activity from 1 s before the stimulus onset was subtracted from the total activity to obtain a net increase in spikes. The injection of the cartridge headspace (5 mL/s) into a humidified airstream (10 mL/s) reduces vapor concentrations by two-thirds, therefore the ammonium hydroxide solution on the filter paper cartridge was adjusted to a higher concentration. Thus, the concentration of ammonia fluxing over the antennae is equivalent to a 1% solution stimulus if a 3% solution is added to the filter paper. All stimulus percentages listed represent the two-thirds reduction caused by mixing.
Taste
Single sensillum extracellular recordings were obtained from L hairs of the labellum. Female CS flies aged 5–7 days were used for recordings. All the tastants were mixed in 30 mM tricholine citrate electrolyte. A reference electrode was used to pierce the dorsal thorax and maneuver the labellum into a fully extended position. For recordings with NH4OH, the solutions were always freshly prepared just before the experiment. Test solutions were exposed to the hairs in order of ascending concentration, whenever applicable. The taste solutions were back-filled into recording electrodes (1B100F-4, World Precision Instruments). The tastant-induced action potentials were amplified and digitized using IDAC-4 data acquisition software. Autospike software (Syntech) was utilized to manually count and visualize the spikes. In order to quantify neuronal responses, the number of spikes in the first 500ms after stimulus contact was counted.
Calcium imaging
From flies
Flies of genotype OrcoGAL4/CyO; UAS-GCaMP6 were used for calcium imaging after they were 2 weeks old essentially as described earlier. The flies used for calcium imaging experiments were raised at 25°C.
A fly was immobilized inside a 200 ul yellow pipette tip (tip cut back) with their antennae protruding out. One antenna was held down using a glass electrode (1B100F-4, World Precision Instruments) on a thin layer of 70% glycerol that enhanced imaging of fluorescence. Odorants in water or paraffin oil (100ul) were applied onto 2 Whatman filter paper strips (2 × 3 cm) placed inside 5 mL plastic syringes and delivered manually over the preparation. Fresh solutions were prepared each time. Imaging was performed on an Olympus BX51WI fluorescence microscope using a 20× LMPLANFL N air objective with a Hamamatsu Orca-flash 4.0 camera and Metamorph software. Fluorescent intensity changes were computed from a region of interest across the antenna and analysis was performed offline using ImageJ/FIJI software.
From mosquitoes
Female A. aegypti between 3 and 8 days age with the homozygous Ubi-GCaMP6 transgene were prepared for imaging on a glass slide. A custom Macro was used to assist in the analysis which included partitioning the mosquito antenna into grids for cell numbering by region, and background control stimulus subtraction before calculating the ΔF values. The controlled odor stimulus was delivered using a Syntech CS-55 unit from odor cartridges put together from disposable glass Pasteur pipettes containing a piece of filter paper, as is used in single-unit electrophysiology recordings. The placement of the cartridge was similar to that of the SSR recordings.
Proboscis extension response assays (flies)
The assay was conducted as explained by Shiraiwa and Carlson, 2007 with some modifications. We collected flies aged 0–2 days and aged them in vials containing 10 males and 10 females until they were 5–7 days. Prior to the experiments, the flies were starved on water-soaked Kimwipes for 24 h. The flies were placed within truncated P-20 tips so that only the head protruded outside. The opposite tip end was sealed with clay. Flies were presented with taste stimuli on paper wicks. They were allowed to drink water to the point of satiation at the beginning of the assay and also between successive stimuli. Any fly that kept on drinking for more than 1 min was discarded. They were then exposed to 100 mM sucrose, and those that did not respond were discarded likewise. Ammonia or NaOH solutions were tested from lowest to highest concentration with water applied between each test stimulus. The stimulus was applied for 2 s following which we waited for 7 s to observe the fly’s response. A full extension was ascribed a value of +1, a half-extension or extension with immediate retraction was ascribed a value of +0.5, and no extension a value of 0. Average value obtained from all flies tested was calculated as PER index. All experiments were repeated over the course of at least two days.
Pre-exposure assay (mosquitoes)
Testing cage and nylon meshes were washed in odorless soap and allowed to dry over-night. 19–21 Aedes aegypti females of age 10–12 days old, starved over-night, were aspirated into a plastic container with a nylon mesh top and allowed to acclimate in the testing area for 30 min. After the acclimation period, mosquitos were treated by placing a filter paper treated with 500 μL of water(control) or ammonia on top of the plastic container for 30 s. Immediately after treatment mosquitos were released into a 30x30 cage containing a human odor landing surface, activated with a 4 s puff of CO2 by aspirator, and video recorded for 9 min. Human odor was provided by nylon mesh worn inside of sock for 2 h; during that period 8 flights of stairs were climbed up and down to provide a baseline physical activity.
Videos were analyzed by manually counting mosquitos on human odor surface (HOS) every 6 s for the duration of the 9-min video. Mosquitos’ ability to navigate to HOS was measured across 9 time points, by taking an average mosquito count from 10 frames for every minute time point. Maximum number of mosquitos landed at one time only considered the 90 frames originally counted for every trial. The maximum count for every trial was then averaged for each condition and compared.
Statistical analyses
Prism9 (Graphpad) was used for all statistical analyses. For olfactory recordings, we used either repeated measures 1-way ANOVA with Geisser-Greenhouse correction or repeated measures two-way ANOVA with Geisser-Greenhouse correction as suitable for the experimental design and the dataset. For post hoc pairwise comparisons we performed Tukey’s test that corrected for multiple comparisons.
For gustatory recordings, since multiple stimulus were tested on the same flies, the data was paired. Hence, we used repeated measures ANOVA or mixed models test depending on whether all data points were present in every single fly tested or not. We chose to conduct 1-way or two-way ANOVA based on the number of effects for which we analyzed the results. For pairwise comparisons we used Tukey’s test that also corrected for multiple comparisons. For experiments where there were only two columns were compared (eg. Figure 3C), we used paired T-test.
Outcomes of PER experiments represented categorical data (3 possible outcomes of each trial being 0, 0.5 and 1) which is also non-parametric. Hence, we used Friedman’s test and followed it up with Dunn’s test for pairwise comparisons that also corrected for multiple comparisons.
In all figures mean values are stated unless otherwise mentioned in the figure legends. Error bars in all figures represent SEM Statistical tests used for each figure are stated in the respective figure legends. Information about the exact value of n and what n represents for each figure is also available in the respective figure legends.
In each figure alphabets in small case represent statistically different groups. Asterisks indicate statistically significant groups. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.005.
Acknowledgments
We thank NIH, NIDCD (R01DC014092) to A.R. for support of the of the study. The funders had no role in designing the study.
Author contributions
J.T.C. designed, performed and analyzed the data for the olfactory electrophysiology experiments and helped write the manuscript; A.G. designed, performed and analyzed the data for the gustatory electrophysiology and behavior experiments and helped write the manuscript; J.E. performed the Ca imaging experiments; M. L. performed the mosquito behavior experiments; A.D. supervised the gustatory experiments and helped edit the manuscript; A.R. conceptualized the study, obtained funding, supervised the experiments and wrote the final version of the manuscript.
Declaration of interests
A.R. is Founder and President of two startups, Sensorygen Inc and Remote Epigenetics Inc. and is listed as inventor on multiple patents filed by UC Riverside.
Published: January 20, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2022.105777.
Supplemental information
Data and code availability
-
•
The published article includes all the data that are generated for the study and the raw data is available in other formats upon request from the lead contact.
-
•
This study did not generate any unique code.
References
- 1.Meijerink J., Braks M.A., Van Loon J.J. Olfactory receptors on the antennae of the malaria mosquito Anopheles gambiae are sensitive to ammonia and other sweat-borne components. J. Insect Physiol. 2001;47:455–464. doi: 10.1016/s0022-1910(00)00136-0. [DOI] [PubMed] [Google Scholar]
- 2.Semmelhack J.L., Wang J.W. Select Drosophila glomeruli mediate innate olfactory attraction and aversion. Nature. 2009;459:218–223. doi: 10.1038/nature07983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geier M., Bosch O.J., Boeckh J. Ammonia as an attractive component of host odour for the yellow fever mosquito, Aedes aegypti. Chem. Senses. 1999;24:647–653. doi: 10.1093/chemse/24.6.647. [DOI] [PubMed] [Google Scholar]
- 4.Davis C.C., Latvis M., Nickrent D.L., Wurdack K.J., Baum D.A. Floral gigantism in Rafflesiaceae. Science. 2007;315:1812. doi: 10.1126/science.1135260. [DOI] [PubMed] [Google Scholar]
- 5.O’Dwyer T.W., Nevitt G.A. Individual Odor Recognition in Procellariiform Chicks: potential role for the major histocompatibility complex. Ann. N. Y. Acad. Sci. 2009;1170:442–446. doi: 10.1111/j.1749-6632.2009.03887.x. [DOI] [PubMed] [Google Scholar]
- 6.Barimo J.F., Walsh P.J. Use of urea as a chemosensory cloaking molecule by a bony fish. J. Exp. Biol. 2006;209:4254–4261. doi: 10.1242/jeb.02533. [DOI] [PubMed] [Google Scholar]
- 7.Wallrabenstein I., Kuklan J., Weber L., Zborala S., Werner M., Altmüller J., Becker C., Schmidt A., Hatt H., Hummel T., Gisselmann G. Human trace amine-associated receptor TAAR5 can Be activated by trimethylamine. PLoS One. 2013;8 doi: 10.1371/journal.pone.0054950. e54950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liberles S.D., Buck L.B. A second class of chemosensory receptors in the olfactory epithelium. Nature. 2006;442:645–650. doi: 10.1038/nature05066. [DOI] [PubMed] [Google Scholar]
- 9.Mishra A., Afik O., Cabrera M.L., Delaplane K.S., Mowrer J.E. Inorganic nitrogen derived from foraging honey bees could have adaptive benefits for the plants they visit. PLoS One. 2013;8 doi: 10.1371/journal.pone.0070591. e70591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frøkjaer-Jensen C., Ailion M., Lockery S.R. Ammonium-acetate is sensed by gustatory and olfactory neurons in Caenorhabditis elegans. PLoS One. 2008;3 doi: 10.1371/journal.pone.0002467. e2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Min S., Ai M., Shin S.A., Suh G.S. Dedicated olfactory neurons mediating attraction behavior to ammonia and amines in Drosophila. Proc. Natl. Acad. Sci. USA. 2013;110 doi: 10.1073/pnas.1215680110. E1321–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye Z., Liu F., Sun H., Ferguson S.T., Baker A., Ochieng S.A., et al. Discrete roles of Ir76b ionotropic coreceptor impact olfaction, blood feeding, and mating in the malaria vector mosquito Anopheles coluzzii. Proc Natl Acad Sci USA. 2022;119 doi: 10.1073/pnas.2112385119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacWilliam D., Kowalewski J., Kumar A., Pontrello C., Ray A. Signaling mode of the broad-spectrum conserved CO2 receptor is one of the important determinants of odor valence in Drosophila. Neuron. 2018;97:1153–1167.e4. doi: 10.1016/j.neuron.2018.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menuz K., Larter N.K., Park J., Carlson J.R. An RNA-seq screen of the Drosophila antenna identifies a transporter necessary for ammonia detection. PLoS Genet. 2014;10 doi: 10.1371/journal.pgen.1004810. e1004810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dai X., Karring H. A determination and comparison of urease activity in feces and fresh manure from pig and cattle in relation to ammonia production and pH changes. PLoS One. 2014;9 doi: 10.1371/journal.pone.0110402. e110402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiss J., Korbász M., Sass-Kiss A. Study of amine composition of botrytized grape berries. J. Agric. Food Chem. 2006;54:8909–8918. doi: 10.1021/jf061578g. [DOI] [PubMed] [Google Scholar]
- 17.Ough C.S., Daudt C.E., Crowell E.A. Identification of new volatile amines in grapes and wines. J. Agric. Food Chem. 1981;29:938–941. doi: 10.1021/jf00107a012. [DOI] [PubMed] [Google Scholar]
- 18.Borash D.J., Pierce V.A., Gibbs A.G., Mueller L.D. Evolution of ammonia and urea tolerance in Drosophila melanogaster: resistance and cross-tolerance. J. Insect Physiol. 2000;46:763–769. doi: 10.1016/s0022-1910(99)00165-1. [DOI] [PubMed] [Google Scholar]
- 19.Ménsua J.L., Moya A. Stopped development in overcrowded cultures of Drosophila melanogaster. Heredity. 1983;(Pt 1):347–352. doi: 10.1038/hdy.1983.39. [DOI] [PubMed] [Google Scholar]
- 20.Bui M., Shyong J., Lutz E.K., Yang T., Li M., Truong K., Arvidson R., Buchman A., Riffell J.A., Akbari O.S. Live calcium imaging of Aedes aegypti neuronal tissues reveals differential importance of chemosensory systems for life-history-specific foraging strategies. BMC Neurosci. 2019;20:27. doi: 10.1186/s12868-019-0511-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lahondère C., Vinauger C., Okubo R.P., Wolff G.H., Chan J.K., Akbari O.S., Riffell J.A. The olfactory basis of orchid pollination by mosquitoes. Proc. Natl. Acad. Sci. USA. 2020;117:708–716. doi: 10.1073/pnas.1910589117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delventhal R., Menuz K., Joseph R., Park J., Sun J.S., Carlson J.R. The taste response to ammonia in Drosophila. Sci. Rep. 2017;7:43754. doi: 10.1038/srep43754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dahanukar A., Foster K., van der Goes van Naters W.M., Carlson J.R. A Gr receptor is required for response to the sugar trehalose in taste neurons of Drosophila. Nat. Neurosci. 2001;4:1182–1186. doi: 10.1038/nn765. [DOI] [PubMed] [Google Scholar]
- 24.Dahanukar A., Lei Y.-T., Kwon J.Y., Carlson J.R. Two Gr genes underlie sugar reception in Drosophila. Neuron. 2007;56:503–516. doi: 10.1016/j.neuron.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelber C., Rössler W., Kleineidam C.J. Multiple olfactory receptor neurons and their axonal projections in the antennal lobe of the honeybee Apis mellifera. J. Comp. Neurol. 2006;496:395–405. doi: 10.1002/cne.20930. [DOI] [PubMed] [Google Scholar]
- 26.Fukuda Y., Loeschcke H.H. Effect of H+ on spontaneous neuronal activity in the surface layer of the rat medulla oblongata in vitro. Pflugers Arch. 1977;371:125–134. doi: 10.1007/BF00580780. [DOI] [PubMed] [Google Scholar]
- 27.Fukuda Y., See W.R., Honda Y. Sensitivity and pattern of discharge of neurons in the chemosensitive areas of the ventral medulla oblongata of rats in vitro. Pflugers Arch. 1980;388:53–61. doi: 10.1007/BF00582628. [DOI] [PubMed] [Google Scholar]
- 28.Petroff E., Snitsarev V., Gong H., Abboud F.M. Acid sensing ion channels regulate neuronal excitability by inhibiting BK potassium channels. Biochem. Biophys. Res. Commun. 2012;426:511–515. doi: 10.1016/j.bbrc.2012.08.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruusuvuori E., Kaila K. Carbonic anhydrases and brain pH in the control of neuronal excitability. Subcell. Biochem. 2014;75:271–290. doi: 10.1007/978-94-007-7359-2_14. [DOI] [PubMed] [Google Scholar]
- 30.Neurath G.B., Dünger M., Pein F.G., Ambrosius D., Schreiber O. Primary and secondary amines in the human environment. Food Cosmet. Toxicol. 1977;15:275–282. doi: 10.1016/s0015-6264(77)80197-1. [DOI] [PubMed] [Google Scholar]
- 31.Henry T.A. The Plant Alkaloids. Philadelphia: Blakiston and Son. 1949:p562. [Google Scholar]
- 32.Buttery R.G., Seifert R.M., Guadagni D.G., Ling L.C. Characterization of volatile pyrazine and pyridine components of potato chips. J. Agric. Food Chem. 1971;19:969–971. [Google Scholar]
- 33.Dhakal S., Sang J., Aryal B., Lee Y. Ionotropic receptors mediate nitrogenous waste avoidance in Drosophila melanogaster. Commun. Biol. 2021;4:1281. doi: 10.1038/s42003-021-02799-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
The published article includes all the data that are generated for the study and the raw data is available in other formats upon request from the lead contact.
-
•
This study did not generate any unique code.