Abstract
Neurodegeneration and synaptic loss in Alzheimer's disease (AD) lead to impairment in memory functions. Neuroinflammation causes activation of microglia and astrocytes cells that locally and systemically produces inflammatory cytokines which can serve as early diagnostic markers or therapeutic targets in AD. Pro-inflammatory cytokines (Interleukins (IL-1β, IL-6 and IL-10) and tumor necrosis factor (TNF α)) levels were estimated in serum, cerebral tissue, hepatic tissue, and renal tissue in treatment groups of scopolamine-induced amnesia mice model using ELISA protocol. The results showed that cerebral tissue of AD mice exhibited elevated levels of IL1β, IL6, IL10 and TNFα which indicate contribution of pro-inflammatory cytokines in the progression of AD. A significant reduction in the concentration of IL1β, IL-10 and TNF-α were noticed in serum, cerebral tissue and hepatic tissue of animal group treated with marketed memantine tablet (Admenta), pure memantine drug (MEMp), memantine-poly (lactic-co-glycolic acid) self-assembled nanoscaffolds (MEM-PLGA) SANs, Polyethylene Glycol coated memantine-poly (lactic-co-glycolic acid) self-assembled nanoscaffolds [(PEG-MEM-PLGA) SANs] and Polyethylene Glycol coated memantine-poly [(lactic-co-glycolic acid)] self-assembled nanoscaffolds grafted with Bone Marrow Derived Stem Cell ((PEG-MEM-PLGA) SANs-BMSc), whereas a high level of IL-6 was observed in hepatic tissue, cerebral tissue and renal tissues of normal and AD induced mice which showed the emerging potential of IL-6 cytokines that can trigger either neurons survival after injury or causing neurodegeneration and cell apoptosis. The Neuroregenerative potential of stem cells helps in the proliferation of neuronal cell and thus improves cognition in AD animal model.
Keywords: Alzheimer's disease, Neuroinflammation, Neurodegeneration, Cytokines, Interleukins
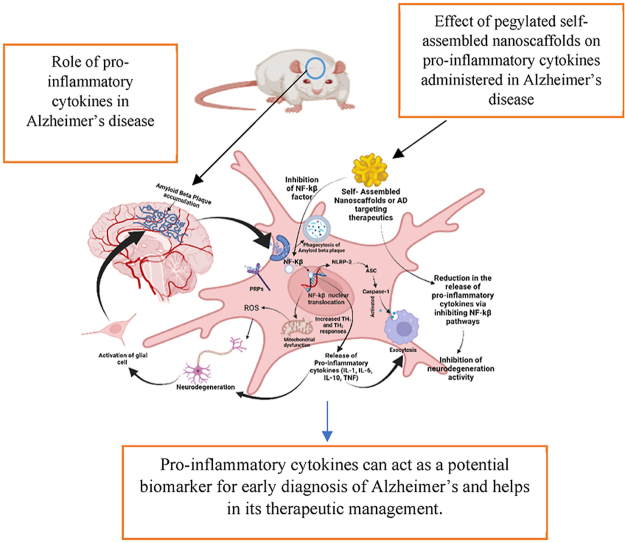
Graphical abstract
Pro-inflammatory cytokines can act as a potential biomarker for early diagnosis of Alzheimer's and helps in its therapeutic management. Effect of pegylated self-assembled nanoscaffolds on pro-inflammatory cytokines administered in Alzheimer's disease, Role of pro-inflammatory cytokines in Alzheimer's disease.
Highlights
-
•
Expression of pro-inflammatory cytokines in serum, degenerated neurons of brain, hepatic and renal tissue in Alzheimer's disease.
-
•
Locally and systemically production of inflammatory cytokines can serve as early diagnostic markers or therapeutic targets in AD.
-
•
Potential pegylated self-assembled nanoscaffolds grafted with BMSc in neuroregeneration and faster recovery from Alzheimer's disease.
-
•
Pleotropic effect of IL-6 in neurodegeneration and neuroregeneration in pegylated self-assembled nanoscaffolds treated group of Alzheimer's disease induced mice.
1. Introduction
The pro-inflammatory cytokines play a crucial role in the pathogenesis of Alzheimer's disease (AD) (Rubio-Perez and Morillas-Ruiz, 2012). These cytokines act as a biomarker for evaluating normal biological processes, pathologic processes, and biological responses after following a therapeutic intervention. Alzheimer's disease (AD), a progressive neurodegenerative disorder, is characterized by chronic inflammation in microglia, astrocytes, and other peripheral blood cells due to the accumulation of amyloid plaques and neurofibrillary tangles in the brain (Kinney et al., 2018). It has been reported that Inflammatory mediators such as cytokines have a significant role in AD linked –immunology and pathogenesis., Amyloid-beta (Aβ) and amyloid precursor protein (APP) induce cytokine and chemokine release from microglia, astrocytes, and neurons that induce the release of various cytokines such as IL-1, IL-6, IL-10 and TNF α as illustrated in Table 1 (Park et al., 2020a; Domingues et al., 2017) These proinflammatory cytokines are released early after the onset of AD, but it is unknown whether inflammation predisposes to neurological deterioration. In this study the implication of interleukin and tumor necrosis factor in early neurological worsening in the brain were studied. Beside the role of cytokines in brain but its expression and effect were also assessed in blood, renal and hepatic tissue to understand the neurodegenerative phenomena in AD. There are several biomarkers such as lymphocyte proliferation biomarkers, cell surface markers, morphological and histopathological markers which provide clinical and pathological insights to facilitate diagnosis and management of AD. However, integrating these biomarkers into clinical practice effectively and precisely is challenging and requires in-depth studies to further validate reproducibility, interpretation of data, and cost efficiency. Several literature has suggested the role and effect of proinflammatory cytokines in pathogenesis of AD. IL-1β is overexpressed in the microglia of the brain in AD due to Aβ plaque formation, tau phosphorylation, and neurofibrillary tangle formation (Park et al., 2020b; Harry and Kraft, 2008). Similarly, IL-6 is a pleiotropic cytokine having beneficial and destructive effects on inflammation, immune response, and haematopoiesis that contribute to activated B cells differentiation into antibody (Ab)-producing cells and hepatocyte-stimulating factor (HSF) in hepatocytes and nerve regeneration (Gabay, 2006). IL-6 can act as both neurodegenerative and neuroprotective based on condition of neurons. Initial stimulation of IL-6 contributes in destructive effect and later excessive neurodegeneration leads to neuroprotective action of IL-6. Research has shown that IL-6 levels increase mostly due to plaque formation in the early stages of AD (mild cases) (Wang et al., 2015a). In addition, peripheral interleukin IL-10 is associated with brain atrophy and can be used as potential biomarker for evaluation of extent of disease progression (Park et al., 2020b; Magalhães et al., 2017). Whereas, TNF-α is strongly correlated with cognitive impairment, cerebral apoptosis and neurodegeneration potentiated in the anatomical regions of the brain such as cortex, striatum, hippocampus in Mild to severe AD (Probert, 2015; Furlan et al., 2004). The pathogenesis of AD contributed by several factor in different anatomical regions. Therefore, one biomarker analysis is not adequate to define the whole pathophysiology of AD, so a combinational approach using multiple markers can help predict progression of AD pathogenesis.
Table 1.
Effect of pro-inflammatory cytokines on neurons and Aβ plaque.
| Pro-inflammatory cytokines | Origin in CNS | Effects on neurons | Effect on Aβ | References |
|---|---|---|---|---|
| IL-1β | Microglia, Astrocytes | Neurodegeneration and synaptic loss | Aβ synthesis increases | Xie et al. (2015) |
| IL-6 | Microglia, Astrocytes, endothelial cells and hepatocytes cells | Rescue of damaged neurons and prevent synaptic loss | Decrease Aβ deposition | Kummer et al. (2021) |
| IL-10 | Microglia | Pro-apoptotic | Increases Aβ synthesis | Porro et al. (2020) |
| TNF-α | Astrocytes, Microglial neurons | Pro-apoptotic, prevents apoptosis and synaptic excitotoxicity | Increases Aβ synthesis but decrease Aβ clearance | Torres-Acosta et al. (2020) |
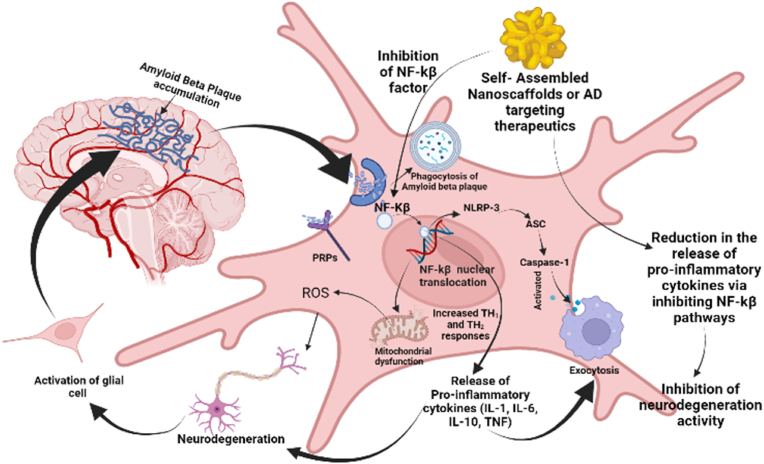
The advancements in nanomedicine intend to revolutionize the therapeutic approaches for the management of AD. Recent studies have reported the efficacy of PEGylated nanoparticles in improving memory with significant reduction in Aβ plaque deposition (Brambilla et al., 2012). Meng et al., has designed intranasal huperzine loaded lactoferrin-conjugated N-trimethylated chitosan surface modified PLGA nanoparticles with sustained-release properties for targeted delivery of drug in AD (Meng et al., 2018). The immunogenicity of these nanocarriers is primarily determined by the ingredients incorporated in the nanocarriers, their physiochemical properties and route of administration. Therefore, surface functionalization of PLGA nanoparticles has been of particular importance for transferring of drug across BBB with the purpose of increasing bioavailability of drugs in the brain, reducing immunogenicity and achieving site-specific targeting (Amo et al., 2021). The proinflammatory cytokines were released at the neurodegenerative site in brain which stimulates damage-associated molecular patterns (DAMPs) and/or pathogen-associated molecular patterns (PAMPs) are generally found in the neurodegenerative brain of Alzheimer's patient associated with pattern recognition receptors (PRRs), (Toll-like receptors (TLRs), RIG-I-like receptors (RLRs), NOD-like receptors (NLRs), and C-type lectin receptors (CLRs)) (Roh and Sohn, 2018; Kigerl et al., 2014). These receptors interact with apoptosis associated protein and lead to secretion of various cytokines at inflammatory site. It has also been proposed that activation of the nuclear factor (NF-κB) activity allows translocation of NF-κB into the nucleus and upregulation of the expression of the proinflammatory genes as shown in Fig. 1. The significant reduction in pro-inflammatory cytokines mediated by administration of therapeutic agents is generally due to inhibition of the NF-κB pathway reducing the release of these cytokines (Fig. 1) (Liu et al., 2017; Wang et al., 2015b). Furthermore, validation is required to establish the interrelationship of contributing factors in AD pathogenesis. In this study role of proinflammatory cytokines in AD pathogenesis were explored and neuroprotective behaviour of Pegylated self-assembled nanoscaffolds were estimated. The study will further help researcher to understand the therapeutic purpose of these cytokines and its contributing effect in the treatment of AD.
Fig. 1.
Pathways of neurodegeneration in microglial cell, and release of pro-inflammatory cytokines in Alzheimer's disease.
Therefore, explorations of proinflammatory cytokines in acute and chronic neurodegeneration where indirectly implicated in disease pathogenesis. However, recent observations in animal models can act as important evidence which challenges the earlier assumptions about cytokines in neuroinflammatory processes of AD. Potentially adaptive functions of proinflammatory cytokines further contributes in mechanistic studies, and might help to alleviate the pathologic burden of disease.
2. Materials and methods
IL-1β, IL-10, IL-6 and TNF α GENLISA™ Mouse Kit were purchased from KRISHGEN Biosystems which consists of Lyophilized reconstitutable standard of (IL-1β, IL-10, IL-6 and TNF α), Biotin Conjugated Detection Antibody (BIO-CONJ), Streptavidin Horseradish Peroxidase (STRP-HRP), TMB (3,3′,5,5′-Tetramethylbenzidine) substrate Microtiter coated Plate (12 × 8), wash buffer and assay diluent.
2.1. Preparation of nanoscaffolds
Non-solvent-temperature induced phase separation (N-TIPS) method with modification was used to formulate Polyethylene glycol-memantine-poly (lactic-co-glycolic acid) self-assembled nanoscaffolds ((PEG-MEM-PLGA) SANs) (Tsujimoto et al., 2016; Cao et al., 2020). 19.18% w/v weight of 1:1 ratio mixture of PLGA (PLGA 50:50/PLGA75:25) in dioxane (solvent) were continuously stirred at 50–55 °C. 10 mg Memantine HCl (drug) and 4.98% w/v pluronics F127 were added to water and stirred continuously at 500 rpm. Drug solution was added to the PLGA solution at 500 rpm until separation of phases occurs and results in the formation of memantine-poly (lactic-co-glycolic acid) self-assembled nanoscaffolds (MEM-PLGA) SANs. (MEM-PLGA) SANs were incubated in 100 mg/ml aqueous solution of PEG 35,000, magnetically agitated for 30 min at room temperature and then lyophilized by pre-freezing at − 40 °C for 24 h followed by vacuum drying at room temperature (25 ± 5 °C) to obtain Polyethylene Glycol coated memantine-poly (lactic-co-glycolic acid) self-assembled nanoscaffolds (PEG-MEM-PLGA) SANs (Reboredo et al., 2021).
HighQC™ Human Bone Marrow Derived Stem Cell (BMSc) obtained from ACCEGEN BIOTECHNOLOGY were grown in Minimum Essential Media (MEM). 5 × 104 cells/well were placed in well plates and incubated for 24 h with tetracycline positive (TC +) and tetracycline negative (TC-) in CO2 incubator. Trypsinzed BMSc dispersed in 20% Foetal Bovine serum (FBS) were incubated with (PEG-MEM-PLGA) SANs at 37 °C % and 5% CO2 up to confluence, after which media was aspirated and the cells were fixed using 4% paraformaldehyde for 10 min to obtain Polyethylene Glycol coated memantine-poly (lactic-co-glycolic acid) self-assembled nanoscaffolds grafted with Bone Marrow Derived Stem Cell [(PEG-MEM-PLGA) SANs-BMSc]. All the experiment were performed in UV sterilized biosafety cabinet (Mitra et al., 2018; Rafal et al., 2018).
2.2. Characterization study
(PEG-MEM-PLGA) SANs were further characterized for scanning electron microscopy (SEM), Entrapment efficiency and Biodegradability study. The surface morphology of prepared (PEG-MEM-PLGA) SANs was assessed using scanning electron microscopy (SEM) (FEI QuantaTM 200, USA) possessing a secondary electron detector at an accelerated voltage of 10 kV in the size scale of 1 μm. The entrapment efficiency was determined by ultra-centrifuged at 15,000 rpm for 15 min at 4 °C in a cooling centrifuge (REMI). After centrifugation, the supernatant containing the free drug was separated and estimated using a UV–visible spectrophotometric method at 218 nm (Alshehri and Imam, 2021). The biodegradability study was evaluated by placing 10 mg (PEG-MEM-PLGA) SANs in 4 mg lysozyme/ml solution in PBS (pH = 7.4) incubated at 37 °C. The weight loss was measured as the ratio of the weight after enzymatic hydrolysis to the PLGA nanoscaffold (Nagata et al., 2022). The dialysis bag diffusion method was used for the in vitro estimation of release pattern of memantine in phosphate buffer (pH 6.8) (Zhang et al., 2007). Here, pH 6.8 has been used due to resemblance of pH at neurodegenerated site in brain, and this pH also triggering the self-assembly of nano molecules within the brain to provide a medium for growth and regeneration of neurons as extracellular matrixs (Ponto et al., 2014). Dialysis bag (cellulose membrane mol. wt. cutoff 12–14000 Da, HIMEDIA, India) was hydrated overnight in dissolution media. PEG coated memantine loaded PLGA nanoscaffold of weight 100 mg was placed in the firmly tied dialysis bag and immersed in a release chamber containing 500 ml of dissolution media under continuous stirring at 75 rpm at 37 ± 0.5 °C. 5 ml samples were withdrawn at fixed time intervals (0, 0.5, 1, 1.5, 2, 4, 6, 8, 12, 24 h, 48hr, and 72 h) with the replacement of equivalent volume of fresh media (to maintain sink conditions). The drug content was analyzed after filtration through 0.2 μm syringe filter using UV–Vis spectroscopy at 218 nm, and drug release pattern was predicted by estimating percent cumulative release in the media.
2.3. Animal model and treatment groups
The research protocol was approved by the Animal Ethical Committee of the Institute of Medicine of the Banaras Hindu University (Varanasi, India) (CPCSEA/2019/iAEC). Swiss albino mice weighing 40 ± 5 g were housed in conditioned animal cages for 12 h with alternating light and dark cycles at 20 ± 2 °C and 50–60% relative humidity. Free access to pelleted food and distilled water ad libitum were provided to the animals. Animals were sacrificed after 60 min of administration of doses. Subsequently, brain from each animal was isolated, weighed and homogenized in cold 10 mM Tris–HCl buffer, pH 7.4, containing 160 mM sucrose. The homogenates were centrifuged at 15,000 rpm for 10 min at 4 °C, and the resulting clear supernatants were used as enzyme sources that were divided into aliquots and stored at −20 °C.
Memory impairment was induced in mice by scopolamine HBr at a dose of 3 mg/kg (administered Intraperitoneally (IP)) once a week up to 21 days (Biradar and Attar, 2020). The animals were divided into seven groups (6 animals/group). T1 group were administered with a dose of 10 mg/kg memantine loaded PLGA self-assembled nanoscaffolds (MEM-PLGA) SANs intrathecally, T2 group received 10 mg/kg PEGylated memantine loaded PLGA self-assembled nanoscaffolds (PEG-MEM-PLGA) SANs intrathecally, T3 group were treated with 10 mg/kg PEGylated memantine loaded PLGA self-assembled nanoscaffolds grafted with bone marrow stem cells [(PEG-MEM-PLGA) SANs-BMSc] intrathecally, group T4 was treated with 10 mg memantine HCl tablets (Admenta) by dispersing in water via intra-oral gavaging, group T5 was administered with 10 mg/kg memantine (pure drug) (MEMp) intrathecally, and T6 control group and T7 diseased control group were administered normal saline solution (0.1 ml/10 g).
2.3.1. Estimation of pro-inflammatory cytokines
The pro-inflammatory cytokines such as IL-1 β, IL-6, IL-10 and TNF-α were determined in serum, cerebral tissue, hepatic tissue and renal tissue of scopolamine induced amnesia model of mice (AD mice) following the instructions provided in the ELISA Kit manual. This was also used for the determination of effect of (MEM-PLGA) SANs, (PEG-MEM-PLGA) SANs, [(PEG-MEM-PLGA) SANs-BMSc], Memantine pure drug (MEMp) and Memantine HCL tablet (Admenta) on secretion, distribution and cellular uptake of pro-inflammatory cytokines in serum, cerebral, hepatic and renal tissues in AD mice.
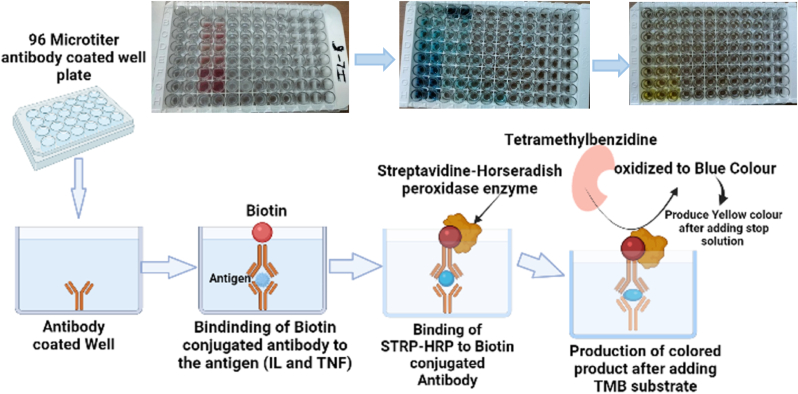
The lyophilized recombinant mouse IL-1 β, IL-6, IL-10 and TNFα was suspended in 600 μl (only for IL-1 β) and 20 μl of water and allowed to rest for 15 min after gentle agitation to prepare 1000 pg/ml, 1 μg/ml, 0.5 μg/ml and 0.5 μg/ml of stock solution respectively. Then serial dilutions of stock solution were done to obtain standard concentrations: IL-1 β – 500 pg/ml, 250 pg/ml, 125 pg/ml, 62.5 pg/ml, 31.3 pg/ml and 15.63 pg/ml from 1000 pg/ml stock solution; IL-6 -2000 pg/ml, 1000 pg/ml, 500 pg/ml, 250 pg/ml, 125 pg/ml, 62.5 pg/ml and 31.3 pg/ml; IL-10-1000 pg/ml, 500 pg/ml, 250 pg/ml, 125 pg/ml, 62.5 pg/ml, 31.3 pg/ml and 15.6 pg/ml; TNFα-1000 pg/ml, 450 pg/ml, 225 pg/ml, 112.5 pg/ml, 56.25 pg/ml, 28.13 pg/ml, 14.06 pg/ml and 3.5 pg/ml 100 μl of each standard dilution and test samples (serum, cerebral tissue, hepatic tissue and renal tissue homogenates of mice from treatment, control and diseased control group) were added to the microtitier 96 well plate, sealed and incubated for 2 h at room temperature at 18–25 °C to facilitate the immobilization of IL-1β, IL-6, IL-10 and TNF-α. The immobilization is achieved due to the binding of IL-1β or IL-6 or IL-10 or TNF-α present in sample and standard with antibody (specific to IL-1β or IL-6 or IL-10 or TNF-α) adsorbed on the surface of 96 well plates. The well plates were washed and blotted four times with wash buffer to remove non-specifically bound protein and antibody. Then, each well was filled with Biotin-conjugated detection antibody 100 μl (BIO-CONJ) and incubated for 1 h between temperature 18–25 °C. After 1 h incubation, biotin-conjugated antibody gets bound to immobilized IL-1β or IL-6 or IL-10 or TNF-α present in the wells. Further, 96 well plate was washed 4 times, streptavidin-HRP (100 μl) enzyme was added and incubated for 15–60 min at 18–25 °C. Streptavidin-HRP enzymes get covalently linked with (IL1β or IL-6 or IL-10 or TNF-α) -conjugated BIO-CONJ antibody complex, to form streptavidin HRP- (IL1β or IL-6 or IL-10 or TNF-α)-BIO-CONJ enzyme-antibody complex. Thereafter, 96 well plate was washed again and TMB (3,3′,5,5′-Tetramethylbenzidine) substrate (100 μl) was added and incubated in dark for 15–30 min at 18–25 °C. The incubation resulted in binding of TMB substrate to the streptavidin HRP-IL1β-BIO-CONJ enzyme-antibody complex with production of blue colour indicating the presence of IL-1 β or IL-6 or IL-10 or TNF-α in serum and tissue homogenates, which becomes yellow after addition of 100 μl stop solution. Finally, the and absorbance were recorded in Synergy H1 microplate reader at 450 nm for the quantitative estimation of IL-1β (Fig. 2).
Fig. 2.
Overview of Enzyme linked immunosorbent assay (ELISA) and quantitative estimation of Biomarkers including pro-inflammatory cytokines.
3. Result and discussion
3.1. Characterization study
The scanning electron microscopic studies showed that nanoscaffolds exhibited spherical structures in nano-size range with appropriate size of 100–200 nm (Fig. 3 (a)). The observed nanoscaffolds are self-assembling in nature to arranged in definite structures which can support the growth and development of neurons by mimicking an extracellular matrix. The entrapment study indicates that approximately 92.21% ± 0.28 of memantine was entrapped within (PEG-MEM-PLGA) SANs. The nanscaffolds were formulated using mixture of 75:25/50:50 PLGA which is a biodegradable polymer and forms porus structures that helps in significant entrapment of drug within the nanoscaffolds. The enzymatic degradation on (PEG-MEM-PLGA) SANs incubated four weeks in phosphate buffer saline and water containing lysozyme (Lz/PBS). After four weeks, the weight loss of SANs from 0.512 g to 0.1127 g exhibited a 77.98% degradation rate after four weeks of incubation in LZ/PBS.
Fig. 3.
(a) Scanning electron microscopy (SEM) of (PEG-MEM-PLGA) SANs at scale 1 μm (b) In-vitro drug release kinetic of MEMp, (MEM-PLGA) SANs, (PEG-MEM-PLGA) SANs and Admenta (Marketed tablet).
The in vitro release of memantine from PLGA nanoscaffolds and PEG coated PLGA nanoscaffolds were determined in phosphate buffer pH 6.8 and compared with memantine HCL (Admenta 10 mg) tablet and pure memantine drug suspension as illustrated in Fig. 3 (b). Memantine release from polymic PLGA matrix following diffusion mechanism and showed sustained release pattern with maximum release of 94.14% ± 0.20 in 72 h and PEG coated PLGA nanoscaffolds showed maximum release of 91.52% ± 0.15 in 72 h which indicates release of memantine in controlled manner for prolonged period (Fig. 3 (b)). Initially small amount of drug was release due to swelling of polymer in media but after the formation of polymer crosslinks memantine has shown an continues release in controlled manner at a definite interval that could be able to provide sustained therapeutic activity at the neurodegeneration site. Nanoscaffolds were also compared with memantine pure drug suspension and marketed memantine HCL tablet which showed maximum release of 92.02% ± 0.22 and 90.44% ± 0.34 in 24 h respectively, indicates drug release pattern from tablet and suspension exhibiting burst release pattern.
Nanoscaffold prepared by self-assembly of molecules made up of PLGA polymer which provides reservoirs for combined encapsulation of bioactive molecules. The supramolecular organization of the nanoscaffolds and their internal structure and composition, is crucial for the drug delivery outcome. PLGA is extensively used for biomedical applications due to its well-known biodegradable and mechanical properties but it has poor hydrophilicity and lacks the cell surface recognition motifs. Functionalization of PLGA incorporated nanoscaffolds have attempted to modify the surface of PLGA by blending with other hydrophilic designed polymer to provide a novel hybrid scaffold for neural tissue engineering. Manasa et al., have designed a self-assembled nanocarrier using the combination of PLGA and self-assembled biotinylated peptides (RAD16-1-BMHP1) to understand its role in the axonal regeneration especially for the peripheral nerve tissue engineering and in vitro cell compatibility in ratSchwann cells (RSC 96) for evaluating the cell proliferation, adhesion and regeneration (Nune et al., 2016). Similarily, in this study dual targeting effect of PLGA nanoscaffolds constituted with memantine for providing neuronal recovery by inhibiting glutamate excitotoxity and bone marrow stem cells potentiate the neuronal outgrowth to stimulate synaptic connection simultaneously. Surface functionalization with PEG and Pluronics F-127 enable an efficient penetration of nanocarriers brain after intrathecal injection. Pluronics F-127 is a non-ionic surfactant which helps in permeation across vascular endothelial cells and enhances the cellular uptake and absorption. Moreover, the biomimetic nature of nanoscaffold as extracellular matrix investigated for their efficiency in the induction of neuroregeneration processes towards the repair of neuronal damage. To that aim, various technical challenges for fabrication of nanodelivery systems for macromolecular drugs need to be urgently addressed. Biologically sourced materials including acellularized tissue and extracellular matrix-derived macromolecules such as collagen, chitosan, and hyaluronic acid have been advantageous and used extensively. However, biologically sourced materials carry a risk of immunogenicity and thus biodegradable and biocompatible polymers such as PLGA and PEG present an alternative with significant benefits, including reproducibility and versatile tailoring through simple modifications of nanocarriers using surfactant as Pluronics F-127. Nanosscaffolds are intended to physically support the surrounding tissue during regeneration. Earlier the scaffold shape and size were defined preimplantation, leading to surgical invasiveness and long recovery periods. The recent development of minimally invasive and in situ surgical approaches has fostered the development of intrathecal administration of nanoscaffolds which have found utility in neural repair, as they support localized treatment and minimize postsurgical complications, demonstrating versatility for translation to the clinic for neural regeneration in AD condition. In condition of nerve injury or neural degeneration due to accumulation amyloid β plaque, the distal segment of the neurons undergoes an initial degeneration that inhibits growth in the initial stage, administration of nanocarrier with bone marrow stem cells and memantine stimulates the neurogenic signalling pathways of microglial and Schwann cells and exhibiting functional nerve regeneration.
3.2. Expression of pro-inflammatory cytokines in serum, cerebral, hepatic and renal tissues
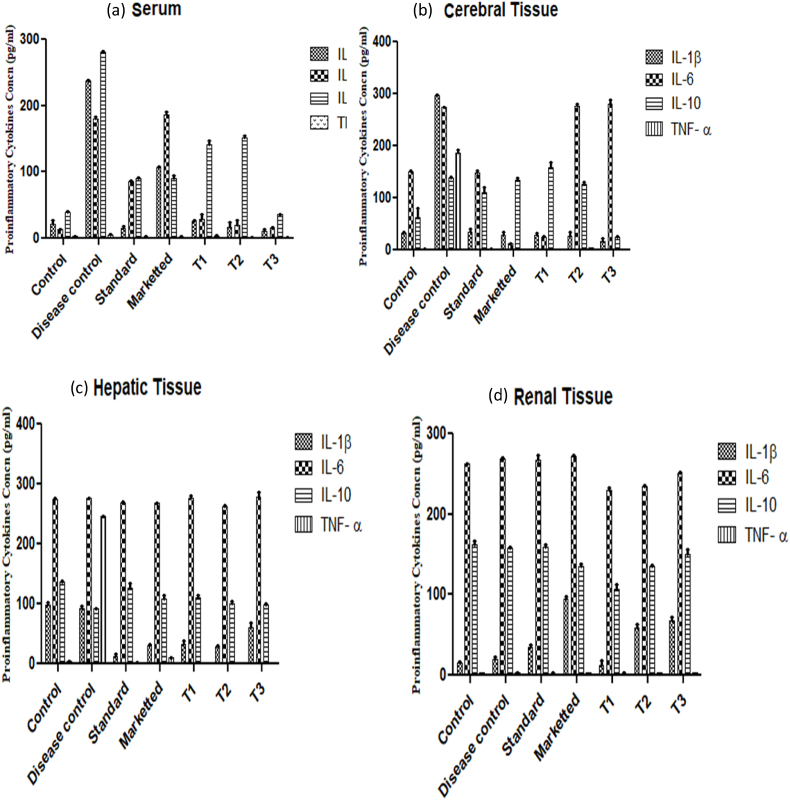
Despite modest changes in Aβ pathology, pro-inflammatory and microglial responses were found to be in the brain via following treatment of AD mice. The elevated expression of serum proinflammatory cytokines (IL-1β, IL-6, IL-10 and TNF-α) is primarily due to stimulation of blood circulating monocytes at the site of neuroinflammation and in systemic circulation (Fig. 4 (a)). Similarly, the concentration of IL-1β (296.83 ± 0.898 pg/ml) were maximally visible in cerebral tissue of AD mice followed by IL-6 concentration (274.28 ± 0.5 pg/ml) and TNF-α (185.57 ± 6.4 pg/ml) minimal concentration of IL- 10 (139.24 ± 1.91 pg/ml) which indicates higher expression of IL-1β, IL-6 and TNF-α in brain. The stimulation of IL-1β, IL-6, IL-10 and TNF-α also reported in cerebral tissue due to neurodegeneration and plaque-associated microglia formation leads to cerebral ischemia influenced by inflammatory processes (Fig. 4 (b)). Meanwhile, an extremely high level of TNF-α (245.885 ± 0.85 pg/ml) was noticed in liver tissue homogenates of scopolamine induced AD mice model compared to normal control group (3.375 ± 0.085 pg/ml) (Fig. 4 (c)). Although no any direct evidence of prominent role of TNF-α in AD were found but the enhanced TNF-α might be due to stimulation of NF-κB signalling cascades. However, it is important to note that there is no significant change in IL-1β, IL-6, IL-10 and TNF-α level were obtained in renal tissue of diseased as compared to normal healthy mice which indicates that these proinflammatory cytokines response of renal tissue did not affected by AD (Fig. 4 (d)). Several studies reported the elevation of Increased IL-1β and IL-6 levels was in the serum and brains of AD patients which are generally due to formation of diffused plaques in the early stage of plaque deposition of clinical patients with AD (Crispe, 2016; Tsartsalis et al., 2010). This study has revealed the potential of nanoscaffolds and nanoscaffolds grafted with stem cells on the alteration of proinflammatory cytokines in different organ of body that can be used for diagnosis and treatment of AD. To evaluate the effect of nanoscaffolds AD induced mice were treated with (PEG-MEM-PLGA) SANs, (MEM-PLGA) SANs, MEMp and marketed (Admenta). (PEG-MEM-PLGA)-BMsc SANs has shown prominent effect on the release of proinflammatory cytokines by showing 20-fold reduction in serum IL-1β whereas, (PEG-MEM-PLGA) SANs, (MEM-PLGA) SANs, MEMp and marketed (Admenta) has shown 13-fold, 9-fold, 15-fold and 2-fold reduction IL-1β level in serum. Similarly, (PEG-MEM-PLGA) SANs and (PEG-MEM-PLGA)-BMsc SANs has also shown 9-fold and 12-fold reduction in IL-6 level of serum respectively, whereas no reduction in expression of IL-6 level were obtained in cerebral, hepatic and renal tissue of AD induced mice. This effect might be due to protective mechanism exerted by spontaneous release of IL-6 level in brain microglial tissue and renal tissue. Meanwhile, high level of IL-6 (274.6 ± 1.22 pg/ml) was found in hepatic tissue of healthy mice due to the IL-6 is mostly expressed in hepatic cells and it contributes in activation of hepatocyte-stimulating factor (HSF) in hepatocytes. Recent studies revealed that HSF differentially regulates IL-6 production in hepatocytes and HSF express a variety of innate immune receptors and when interacts with pathogen or damage-associated molecular patterns, can produce cell-autonomous innate immune responses that may result in host defense or immunopathology (Crispe, 2016). However, it was observed that IL-6 and IL-10 were highly produced due to modulation in NF-kB and STAT3 signalling which indicates initiation of neurodegeneration, and prolonged IL-6 and IL-10 secretion can actively suppress T-cell responses by inhibiting the lipopolysaccharide- (LPS-) induced production of several inflammatory mediators in microglia. This protective response further prevents damage of neurons and helps to restore neuronal function. In summary, we can understand that the decreased level of IL-1β, IL-10 and TNF-α and elevated level of IL-6 were noticed after treatment with (PEG-MEM-PLGA) SANs and (PEG-MEM-PLGA)-BMSc SANs in scopolamine induced AD mice (Fig. 4b). The release of IL-6 during treatment of AD induced mice with MEMp, (MEM-PLGA) SANs, (PEG-MEM-PLGA) SANs, (PEG-MEM-PLGA)-BMSc SANs, contributes in downregulation of a number of pro-inflammatory cytokines, including tumor necrosis factor-α and IL-10. Therefore, modulation in IL-6 could provide potential therapeutic management mechanism for AD in clinical practice (Tsartsalis et al., 2010; Tobinick et al., 2006). In addition, some studies also shown that IL-6 contributes in stem cell regeneration that is responsible for neuronal stem cell expansion (induced pluripotent stem cells (iPSCs)) within a short time window) and regulation of neuronal outgrowth by following JAK-STAT signalling pathways (Sulistio et al., 2018). Moreover, an increased level of TNF-α in AD mice which might be responsible for the imbalance of excitation and inhibition phenomena occurring during neuronal signal conduction and can be held responsible for loss of memory functions. However, (PEG-MEM-PLGA) SANs and (PEG-MEM-PLGA)-BMsc SANs has shown almost 3-fold and 4-fold reduction in level of TNF-α in serum, cerebral, hepatic and renal tissue in treatment group of AD induced mice which indicates the recovery of neurons and memory impairment. Meanwhile, pleiotropic potential of IL-10 produces an inflammatory state that promotes chronic neurodegeneration and prolonged IL-10 production can also contribute to restore the neuronal damage associated with AD. Through the present study, it can be seen that (PEG-MEM-PLGA) SANs and (PEG-MEM-PLGA)-BMsc SANs has potentially shown 7-fold and 2-fold reduction in the secretion of IL-10 that led to neurodegeneration effect, and not involved in neuroregeneration. Overall, (PEG-MEM-PLGA)-BMsc SANs has shown greater reduction of level of pro-inflammatory cytokines as compared to (PEG-MEM-PLGA) SANs, (MEM-PLGA) SANs, MEMp and marketed (Admenta) in serum, cerebral, hepatic and renal tissue.
Fig. 4.
Expression of pro-inflammatory cytokines IL-1β, IL-6, IL-10 and TNF-α in (a) serum, (b) cerebral tissue, (c) hepatic tissue and (d) renal tissues of treatment groups of scopolamine induced AD model.
3.3. Quantification of pro-inflammatory cytokines and impact of memantine
The quantification of pro-inflammatory cytokines was mentioned as mean ± SD (n = 3) values of cytokines in mice and all values were presented in Table 2. The quantification of IL-1β showed low levels in serum (20.87 ± 6.03 pg/ml), cerebral tissue (31.42 ± 2.6 pg/ml), and renal tissue (14.56 ± 1.8 pg/ml), and comparatively higher concentration of IL-1β was observed in hepatic tissue (98.5 ± 4.1 pg/ml) of normal healthy mice (control group). Whereas IL-1β level founds drastically increased in serum (237.29 ± 0.805 pg/ml) and cerebral tissue (296.83 ± 0.898 pg/ml), and nonsignificant changes were noticed in serum IL-1β level hepatic (93.33 ± 2.6 pg/ml) and renal tissue (20.41 ± 2.2 pg/ml) after induction of AD as shown in Table 2. Significant reduction in the concentration of IL-1β was noticed in serum from 237.29 ± 0.805 pg/ml to 15.71 ± 3.14 pg/ml, cerebral tissue 296.83 ± 0.898 pg/ml to 35.24 ± 8.87 pg/ml, and hepatic tissue from 93.33 ± 2.6 pg/ml to 13.07 ± 3.8 pg/ml in standard groups treated with formulations containing memantine (MEMp). Similarly, low IL-6 level were obtained in serum (13.35 ± 0.26 pg/ml), but high IL-6 level were obtained in cerebral tissue (149.74 ± 1.66 pg/ml), hepatic tissue (274.6 ± 1.22 pg/ml) and renal tissue (262.1 ± 1.01 pg/ml) of normal healthy mice (control group). A significant increase in the concentration of IL-6 in serum from 13.35 ± 0.26 pg/ml to 180.74 ± 2.1 pg/ml was noticed after the induction of AD, whereas nonsignificant change in IL-6 concentration was observed in cerebral tissue (274.28 ± 0.5 pg/ml) hepatic tissue (275.45 ± 1.28 pg/ml) and renal tissue (268.83 ± 1.67 pg/ml) of AD induced mice illustrated in Table 2. Memantine has not showed prominent effect on reduction of IL-6 concentration in standard animal group. In addition, an increased concentration of IL-10 was recorded in serum and cerebral tissue from 39.26 ± 1.44 pg/ml to 280.66 ± 0.98 pg/ml and 61.55 ± 18.32 pg/ml to 139.24 ± 1.91 pg/ml respectively, after induction of AD in mice. Also, TNF-α showed prominent increased levels in cerebral tissue from 1.455 ± 0.68 pg/ml to 185.57 ± 6.4 pg/ml and hepatic tissue from 3.375 ± 0.085 pg/ml to 245.885 ± 0.85 pg/ml, and slight increase in TNF-α level in serum from 2.88 ± 0.255 pg/ml to 4.98 ± 1.02 pg/ml, and nonsignificant change were noticed in renal tissue of scopolamine induced AD mice compared to healthy mice (Table 2). IL-1β, IL-6, IL-10 and TNF-α were prominently expressed in brain which indicates regulatory effects of cytokines in neuroinflammation and neurodegeneration. Memantine treated mice has also shown significant reduction in TNF-α concentration in cerebral tissue whereas less influence of memantine were noticed on IL-6 and IL-10. Reduction in pro-inflammatory cytokines facilitates regulation of neuroinflammation and thus prevent neurons from degeneration.
Table 2.
Estimated pro-inflammatory cytokines concentration in serum, cerebral, hepatic and renal tissue.
| Pro-inflammatory cytokines concentration in serum (pg/ml) | ||||
|---|---|---|---|---|
| IL-1β | IL-6 | IL-10 | TNF-α | |
| Control | 20.87 ± 6.03 | 13.35 ± 0.26 | 39.26 ± 1.44 | 2.88 ± 0.255 |
| Disease Control | 237.29 ± 0.805 | 180.74 ± 2.1 | 280.66 ± 0.98 | 4.98 ± 1.02 |
| Standard | 15.71 ± 3.14 | 85.14 ± 1.67 | 89.62 ± 1.43 | 2.215 ± 0.305 |
| Marketed | 106.91 ± 1.35 | 186.97 ± 3.5 | 90.485 ± 4.56 | 1.85 ± 1.285 |
| T1 | 25.15 ± 1.57 | 28.37 ± 6.03 | 140.86 ± 7.09 | 3.72 ± 0.505 |
| T2 | 17.48 ± 6.9 | 20.03 ± 6.68 | 152.33 ± 2.56 | 1.385 ± 0.241 |
| T3 | 10.98 ± 2.71 | 15.02 ± 1.67 | 36.32 ± 0.33 | 1.035 ± 1.425 |
| Pro-inflammatory cytokines concentration in cerebral Tissue (pg/ml) | ||||
| Control | 31.42 ± 2.6 | 149.74 ± 1.66 | 61.55 ± 18.32 | 1.455 ± 0.68 |
| Disease Control | 296.83 ± 0.898 | 274.28 ± 0.5 | 139.24 ± 1.91 | 185.57 ± 6.4 |
| Standard | 35.24 ± 8.87 | 148.07 ± 4.86 | 110.8 ± 9.97 | 2.475 ± 0.465 |
| Marketed | 27.76 ± 4.7 | 11.68 ± 1.6 | 134.62 ± 3.9 | 1.200 ± 0.15 |
| T1 | 28.04 ± 4.2 | 23.36 ± 2.9 | 158.68 ± 9.97 | 1.2 ± 0.06 |
| T2 | 26.34 ± 6.1 | 277.12 ± 3.34 | 127.27 ± 3.76 | 3.815 ± 0.125 |
| T3 | 17.24 ± 5.7 | 280.96 ± 7.73 | 23.39 ± 3.55 | 2.91 ± 0.01 |
| Pro-inflammatory cytokines concentration in hepatic Tissue (pg/ml) | ||||
| Control | 98.5 ± 4.1 | 274.6 ± 1.22 | 136.62 ± 1.27 | 3.375 ± 0.085 |
| Disease Control | 93.33 ± 2.6 | 275.45 ± 1.28 | 91.62 ± 1.45 | 245.885 ± 0.85 |
| Standard | 13.07 ± 3.8 | 268.77 ± 1.02 | 125.65 ± 9.35 | 3.08 ± 0.2 |
| Marketed | 29.65 ± 2.9 | 267.94 ± 0.82 | 109.21 ± 5.09 | 10.845 ± 0.335 |
| T1 | 32.390 ± 5.7 | 277.95 ± 9.18 | 110.44 ± 3.38 | 3.3 ± 0.015 |
| T2 | 28.29 ± 2.4 | 262.1 ± 1.67 | 99.925 ± 4.57 | 2.98 ± 0.01 |
| T3 | 61.280 ± 6.1 | 277.95 ± 9.180 | 98.97 ± 1.62 | 3.21 ± 0.032 |
| Pro-inflammatory cytokines concentration in renal Tissue (pg/ml) | ||||
| Control | 14.56 ± 1.8 | 262.1 ± 1.01 | 161.8 ± 4.68 | 3.81 ± 0.05 |
| Disease Control | 20.41 ± 2.2 | 268.83 ± 1.67 | 158.06 ± 0.84 | 3.48 ± 0.17 |
| Standard | 34.58 ± 3.4 | 267.165 ± 6.615 | 159.64 ± 3.05 | 2.34 ± 0.85 |
| Marketed | 94.15 ± 4.1 | 271.26 ± 2.01 | 135.24 ± 2.59 | 3.405 ± 0.025 |
| T1 | 11.63 ± 6.4 | 230.27 ± 2.51 | 107.21 ± 5.62 | 2.005 ± 0.605 |
| T2 | 58.74 ± 5.2 | 234.38 ± 1.14 | 135 ± 2.11 | 3.335 ± 0.075 |
| T3 | 67.77 ± 4.8 | 250.41 ± 1.26 | 150.32 ± 5.73 | 2.9 ± 0.045 |
∗All the data are report as mean ± SD (n = 3).
3.4. Involvement of cytokines in AD
The conducted experimental trials suggested that interactions of pro-inflammatory cytokines and amyloid plaque in upregulation of TNF-α, IL-1β, IL-6, IL-10 by activated microglial cells lead to tau-hyperphosphorylation and neuronal death. The cerebral vasculature of Alzheimer's disease exhibits a destructive pathology in which Aβ deposition, neuronal inflammation and release of inflammatory mediators occurs. In the present study, low level of pro-inflammatory cytokines IL-1β, IL-10 and TNF-α were detected in serum, cerebral tissue, hepatic tissue in healthy mice, and these levels were further found enhanced in mice on the induction of AD using scopolamine HBr at a dose 3 mg/kg which indicates the contributory role of cytokines in pathophysiology of AD. Various studies have suggested that low levels of IL-1β are expressed in healthy central nervous system and its expression increases in CNS due to stimulation of microglial cells in neurodegenerative disorders. The contributing role of IL-1β in pathogenesis of AD were evident by the increased levels of IL-1β in serum (237.29 ± 0.805 pg/ml) and cerebral (296.83 ± 0.898 pg/ml) tissue. However, the pleiotropic effect of IL-6 was observed initially without treatment in case of neurodegeneration and then after treatment via IL-6 mediated neurodegenerative effect. Moreover, IL-6 significantly increases in the brain in order to ensure nervous tissue survival and mitigate inflammatory responses triggering several signalling pleiotropic pathways and promoting the resolution of inflammatory cascades that are important for the brain integrity and as a therapeutic or potential biomarker during neuroregenerative situation as well (Dhapola et al., 2021). Meanwhile, pleiotropic potential of IL-10 facilitates an inflammatory state that promotes chronic neurodegeneration and prolonged IL-10 production can actively suppress T helper cell responses and mediated host immune response which were not observed in this study. Moreover, a prominent level of TNF-α were obtained in cerebral tissue, hepatic tissue, serum but decreased concentration of TNF-α were observed in renal tissue of scopolamine induced AD mice compared to healthy mice. Microglial activation and upregulation of TNF-α expression is a common feature of several neurodegenerative disorders. Furthermore, TNF-α can potentiate glutamate-mediated cytotoxicity by inhibiting glutamate transport across astrocytes by triggering the surface expression of Ca +2 permeable-AMPA receptors and NMDA receptors, while decreasing inhibitory GABAA receptors on neurons. Thus, the net effect of TNF-α might be responsible for the imbalance of excitation and inhibition phenomena occurring during neuronal signal conduction that can be responsible for memory deficit. Thereby, modulation in TNF-α signalling may represent a valuable target for the therapeutic intervention in AD (Decourt et al., 2017; Ren et al., 2021).
3.5. Activity of memantine and nanoscaffold on inflammation in AD
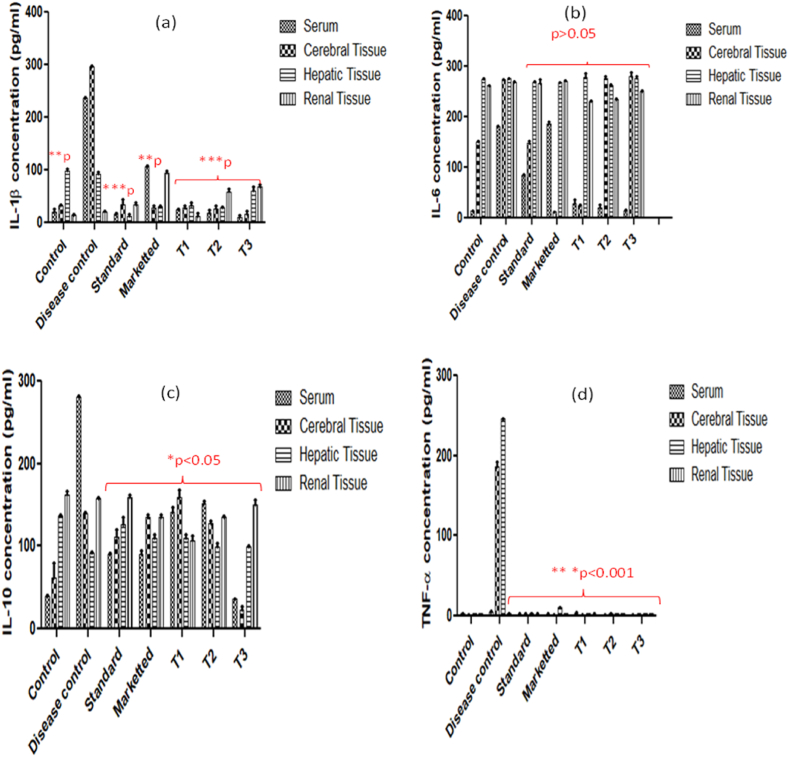
The combined drug therapy with stem cell within a nanoscaffold matrix potentiate the effect of drug and shown marked recovery in brain health. This nanoscaffolds have been designed for, site-specific delivery via intrathecal route of administration which eliminates the risk of hepatic and renal toxicity along with shortening of dosage regimen and improves patient compliance simultaneously. The therapy with memantine-loaded nanoscaffold via the intrathecal route helps to reduce the complication involved in AD by improving its BBB penetration, improved drug release, retention of drug within brain, and improving cerebral microvasculature, neuronal regrowth, and maintaining neuron signalling. In spite of contributing role of proinflammatory cytokines therapeutic activity of memantine and memantine loaded nanoscaffolds is an important aspect for maintaining mental health. Memantine is an N-methyl-D-aspartate (NMDA) receptor antagonists which provides protection against neuroinflammation and neuronal death accompanied by suppression of proliferation and activation of microglial cells and regulation of inflammatory cytokines in AD. In the present study, memantine-loaded PEG-coated self-assembled nanostructures (PEG-MEM-PLGA) SANs have been developed for penetration and delivery of drug across the BBB for prolonged action in Alzheimer's disease. Potential activity of memantine and memantine incorporated nanoscaffolds showed significant effect on reduction of TNF-α, IL-1β, IL-6, IL-10 and thus contributes in reduction of neuroinflammation. Additionally, Fig. 5 (a) significant (p∗∗∗ < 0.001) effect of MEMp, (MEM-PLGA) SANs, (PEG-MEM-PLGA) SANs and (PEG-MEM-PLGA)-BMSc on reduction in the levels of insoluble amyloid-β, hyperphosphorylated tau was observed via decrease in IL-1β concentration which leads to inhibition of NF-κB activity to restore cognition. Thus, diseased (dementia) group showed an increase of this cytokine as compared to the control group (p < 0.01), and this increase was reversed by treatment with memantine and its memantine loaded nanoscaffolds (p < 0.001). (MEM-PLGA) SANs has shown reduction in enhanced IL-1β level of AD mice from 237.29 ± 0.805 pg/ml to 25.15 ± 1.57 pg/ml in serum, 296.83 ± 0.898 pg/ml to 28.04 ± 4.2 pg/ml in cerebral tissue, 93.33 ± 2.6 pg/ml to 32.39 ± 5.7 in hepatic tissue, and 20.41 ± 2.2 pg/ml to 11.63 ± 6.4 pg/ml in renal tissue. However, (PEG-MEM-PLGA) SANs and (PEG-MEM-PLGA)-BMSc SANs treated animal group has shown prominent reduction in IL-1β level from 25.15 ± 1.57 pg/ml to 17.48 ± 6.9 pg/ml in serum, 28.04 ± 4.2 pg/ml to 26.34 ± 6.1 pg/ml in cerebral tissue, 93.33 ± 2.6 pg/ml to 28.29 ± 2.4 pg/ml in hepatic tissue as compared to (MEM-PLGA) SANs. (PEG-MEM-PLGA)-BMSc has shown the potential in reduction of neuroinflammatory response via regulating proinflammatory cytokines levels mostly in serum and cerebral tissue. (PEG-MEM-PLGA)-BMSc SANs reduces IL-1β in serum from 237.29 ± 0.805 pg/ml to 10.98 ± 2.71 pg/ml and cerebral tissue from 296.83 ± 0.898 pg/ml to17.24 ± 5.7 pg/ml (PEG-MEM-PLGA)-BMSc SANs showed 20-fold, 17-fold and 1.5-fold most reduction in IL-1β level in serum, cerebral and hepatic tissue respectively which indicates the maximized therapeutic effect in brain and blood with less distribution in other organs because BMSc facilitates neuronal recovery by regeneration confined at brain that helps in treatment of AD most effectively by incorporating in nanosacffolds loaded with memantine.
Fig. 5.
Effect of Memp, Marketed, (MEM-PLGA) SANs, (PEG-MEM-PLGA) SANs and (PEG-MEM-PLGA)-BMSc SANs on pro-inflammatory cytokines (a) IL-1β (b) IL-6 (c) IL-10 and (d) TNF-α observed in serum, cerebral, hepatic and renal tissues of treatment groups of scopolamine induced AD model vs. control and disease control (untreated cells) group and Data are represented as Mean ± SEM, (n = 3).
Fig. 5 (b) represents nonsignificant effect of IL-6 in cerebral, hepatic and renal tissue in AD mice treated with MEMp, (MEM-PLGA) SANs, (PEG-MEM-PLGA) SANs, (PEG-MEM-PLGA)-BMSc SANs. Significant reduction in IL-6 serum level from 180.74 ± 2.1 pg/ml to 85.14 ± 1.67 pg/ml, 28.37 ± 6.03 pg/ml, 20.03 ± 6.68 pg/ml and 15.02 ± 1.67 pg/ml was obtained after administration of MEMp, (MEM-PLGA) SANs, (PEG-MEM-PLGA) SANs, (PEG-MEM-PLGA)-BMSc SANs respectively. The reduction of serum IL-6 concentration is mostly due to regulation of pro-inflammatory cytokines after administration of MEMp, (MEM-PLGA) SANs, (PEG-MEM-PLGA) SANs, (PEG-MEM-PLGA)-BMSc SANs. (PEG-MEM-PLGA)-BMSc SANs has shown 10-fold, 5-fold and 3-fold greater reduction in serum IL-6 level as compared with MEMp, (MEM-PLGA) SANs, (PEG-MEM-PLGA) SANs. Furthermore, it was obtained that the level of IL-6 in cerebral tissue of mice treated with (PEG-MEM-PLGA) SANs (277.12 ± 3.34 pg/ml) and (PEG-MEM-PLGA)-BMSc SANs (280.96 ± 7.73 pg/ml) was found elevated even after following the treatment protocol which indicates the contributory role in AD and higher than that of standard group treated with MEMp ((148.07 ± 4.86 pg/ml) indicating the neuroregenerative capacity of nanoscaffolds containing bone marrow stem cells. The elevated IL-6 level indicates the neurodegenerative condition in AD mice initially without treatment and later neuroprotective behaviour of IL-6 were noticed after following treatment protocol with (PEG-MEM-PLGA) SANs and (PEG-MEM-PLGA)-BMSc SANs. The exerted neuroprotective behaviour is mainly due to prolonged neurodegeneration augmented the hippocampal levels of phosphorylated signal transducer, activator of transcription-3 (p-STAT3) and anti-apoptotic protein while diminished nuclear factor-kappaB (NF-κB) and caspase-3 (casp-3) expression. These outcomes promotes the protective effect of neurodegeneration, memory deficits and AD-like pathological condition probably via modulating NF-kB and STAT3 signalling mediated by modulating N-methyl-D-aspartate (NMDA) receptors (Alawdi et al., 2017; Yick et al., 2020).
The IL-10 concentration in cerebral tissue and serum found diminished in animals subjected to administration of MEMp, (MEM-PLGA) SANs, (PEG-MEM-PLGA) SANs, (PEG-MEM-PLGA)-BMSc SANs. IL-10 concentration was significantly reduced in serum from 280.66 ± 0.98 pg/ml to 36.32 ± 0.33 pg/ml, and cerebral tissue from 139.24 ± 1.91 pg/ml to 23.39 ± 3.55 pg/ml in AD induced animal group treated with (PEG-MEM-PLGA)-BMSc SANs which showed 4-fold and 5-fold more potent than standard, marketed, (MEM-PLGA) SANs and (PEG-MEM-PLGA) SANs respectively. (MEM-PLGA) SANs, (PEG-MEM-PLGA) SANs and (PEG-MEM-PLGA)-BMSc SANs treated group also showed slight increase in hepatic tissue IL-10 level as compared to AD mice. Similarly, IL-10 level in renal tissue was found to be slightly reduced in AD induced animal groups treated with marketed formulation (Admenta) (135.24 ± 2.59 pg/ml), (MEM-PLGA) SANs (107.21 ± 5.62 pg/ml, (PEG-MEM-PLGA) SANs (135 ± 2.1 pg/ml) and (PEG-MEM-PLGA)-BMSc SANs (150.32 ± 5.73 pg/ml compared to untreated AD mice (158.06 ± 0.84 pg/ml) (Table 2) (Fig. 5 (c)). The finding indicates the contributory role of IL-10 predominately in serum and cerebral tissue whereas hepatic tissue and renal tissue were not involved in the pathogenesis of AD and regulation of proinflammatory cytokines in the therapeutic management of AD. The observed neurodegenerative effect is primarily due to non-competitive NMDA receptor antagonist activity of memantine that is responsible for attenuation of B cell receptor (BCR) and Toll-like receptor 4 (TLR4) for mediating B-cell signalling and can affect the production of IL-10 (Simma et al., 2014).
The results revealed that TNF-α were predominately expressed in cerebral and hepatic cells after induction of AD whereas TNF-α found lesser expressed in serum and renal tissue as compared to IL-1β, IL-6, IL-10. The TNF-α concentration were found significantly increased in cerebral and hepatic tissue of AD induced mice as compared to normal healthy mice which was reduced after administration of MEMp, marketed (Admenta), (MEM-PLGA) SANs, (PEG-MEM-PLGA) SANs and (PEG-MEM-PLGA)-BMSc SANs. (PEG-MEM-PLGA)-BMSc SANs showed almost 14-fold and 15-fold greater reduction in cerebral and hepatic TNF-α level as compared to MEMp, marketed (Admenta), (MEM-PLGA) SANs, (PEG-MEM-PLGA) SANs. TNF-α level in renal tissue varied non-significantly amongst the groups (Table 2) (Fig. 5 (d)). In addition, the incorporation of bone marrow stem cell therapy fastens the recovery of neurons and nanoscaffolds provides an environment for growth and regeneration of neurons. Furthermore, (PEG-MEM-PLGA)-BMSc incorporated with bone marrow stem cell stimulate regeneration of neurons facilitating improvement in cognitive function in AD.
3.6. Statistical analysis
The absorbance (Abs) obtained in the microtiter wells was subtracted by blank Abs to obtain original absorbance of cytokines and by using interpolation method, concentration of pro-inflammatory cytokines was determined. The statistical analysis was performed and the results were interpreted by the interpolation method using GraphPad prism software (version 5). Data has been presented as mean ± standard deviation. Statistical analysis has been performed using two-way ANOVA followed by Bonferroni post-hoc test for comparison among the groups and P value (p < 0.05) was regarded as significant. The analyzed data showed significant effect of therapeutics (standard, marketed and SANs) in cognitive improvement by affecting IL-1 β pro-inflammatory cytokines among standard, (PEG-MEM-PLGA) SANs and (PEG-MEM-PLGA) SANs-BMSc treated groups showing ∗∗∗p < 0.001, and marketed has also shown significant effect by showing ∗∗p < 0.01 compared to diseased group (Fig. 5). Moreover, significant effect was observed on the level of pro-inflammatory cytokines mainly IL-10 and TNF-α in serum, cerebral and hepatic tissue with values of ∗p < 0.05 and ∗∗p < 0.001, respectively as compared to diseased group. Whereas insignificant effect on IL-6 and IL-10 and TNF-α in renal tissue were observed.
4. Conclusion
Restorative therapies of neurological disorders require new strategies for repair and stimulation of neurogenesis against mental deterioration and neurodegenerative disorders. The recent observations help to understanding contribution of proinflammatory cytokines in the pathogenesis of CNS in triggering adaptive innate immune response during the course of chronic neurodegenerative disease. Despite the profound impact of AD, the conventional treatments are unable to achieve satisfactory therapeutic effects or stop the progression of the disease. Our finding of novel treatments for AD has become neuroprotective agents including N-methyl-D-aspartate (NMDA) receptor modulators, and brain stimulation in neuroinflammatory and neurodegenerative processes. These findings can also influence the balance between beneficial and detrimental outcomes and potentiation of such adaptive cytokines driven responses in chronic neurodegenerative disease for providing new avenues for therapeutic intervention. Hence, identification of biomarkers is an important step to improve the accuracy of early AD diagnosis and management of therapy. We found that after induction of AD in mice the level of cytokines spiked unexpectedly which indicates that neurodegeneration or neuroinflammation also impacts on release of cytokines. The level of cytokines might be beneficial as a tool or biomarker to detect inflammation trigged neurodegeneration. Although these markers are not specific to the disease but effects of IL-1β, IL-6, IL, and TNF-α were found insightful for the significance to act as biomarkers. IL-6 level in serum, hepatic and renal tissue and IL-1β in serum and cerebral tissue, IL-10 in serum, and TNF-α in cerebral tissue or cerebrospinal fluid was found enhanced in diseased conditions. The expression of each cytokine is different in different organs so understanding cytokines individually in organs will further help to develop a suitable identifiable marker for the detection of neurodegeneration. Furthermore, incorporation of serum, cerebral and hepatic biomarkers can provide more diagnostic/prognostic information by detecting abnormally low or high level of pro-inflammatory cytokines that can help predict pathological changes associated with AD. Until now, numerous potential biomarkers for AD in peripheral blood have been reported, but large discrepancies exist among different studies thus validation of results and method becomes a crucial aspect to develop an effective technique in diagnosis and treatment of AD. Also, additional studies are required to further understand the role of pro-inflammatory cytokines as biomarkers in neuroinflammation, neurodegeneration, and for disease-modifying therapies for AD.
Funding details
This research study has not received any research grant. Authors are greateful to the director of Indian Institute of Technology (Banaras Hindu University) for supporting this work.
CRediT authorship contribution statement
Varsha Rani: Conceptualization, Methodology, Formal analysis, Investigation, Writing – original draft. Rinki Verma: Validation, Visualization, Writing – review & editing. Krishan Kumar: Validation, Data curation. Ruchi Chawla: Visualization, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
The data that has been used is confidential.
References
- Alawdi S.H., El-Denshary E.S., Safar M.M., Eidi H., David M.O., Abdel-Wahhab M.A. Neuroprotective effect of nanodiamond in Alzheimer's disease rat model: a pivotal role for modulating NF-κB and STAT3 signaling. Mol. Neurobiol. 2017;54:1906–1918. doi: 10.1007/S12035-016-9762-0/FIGURES/9. [DOI] [PubMed] [Google Scholar]
- Alshehri S., Imam S.S. Formulation and evaluation of butenafine loaded PLGA-nanoparticulate laden chitosan nano gel. Drug Deliv. 2021;28:2348. doi: 10.1080/10717544.2021.1995078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amo L Del, Cano A., Ettcheto M., Souto E.B., Espina M., Camins A., et al. Surface functionalization of PLGA nanoparticles to increase transport across the BBB for Alzheimer's disease. Appl. Sci. 2021;11:4305. doi: 10.3390/APP11094305. 2021;11:4305. [DOI] [Google Scholar]
- Biradar P.R., Attar V. Pharmacological evaluation of Salvadora persica on scopolamine-induced memory disorder. 2020. [DOI]
- Brambilla D., Verpillot R., Le Droumaguet B., Nicolas J., Taverna M., Kóňa J., et al. PEGylated nanoparticles bind to and alter amyloid-beta peptide conformation: toward engineering of functional nanomedicines for Alzheimer's disease. ACS Nano. 2012;6:5897–5908. doi: 10.1021/NN300489K. [DOI] [PubMed] [Google Scholar]
- Cao Y., Han W., Pu Z., Wang X., Wang B., Liu C., et al. Fabrication of hierarchically porous superhydrophilic polycaprolactone monolith based on nonsolvent-thermally induced phase separation. RSC Adv. 2020;10:26319–26325. doi: 10.1039/D0RA04687F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispe I.N. Hepatocytes as immunological agents. J. Immunol. 2016;196:17–21. doi: 10.4049/JIMMUNOL.1501668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decourt B., Lahiri D.K., Sabbagh M.N. Targeting tumor necrosis factor alpha for Alzheimer's disease. Curr. Alzheimer Res. 2017;14:412. doi: 10.2174/1567205013666160930110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhapola R., Hota S.S., Sarma P., Bhattacharyya A., Medhi B., Reddy D.H.K. Recent advances in molecular pathways and therapeutic implications targeting neuroinflammation for Alzheimer's disease. Inflammopharmacology. 2021;29:1669–1681. doi: 10.1007/S10787-021-00889-6/TABLES/1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingues C., da Cruz e Silva O.A.B., Henriques A.G. Impact of cytokines and chemokines on Alzheimer's disease neuro-pathological hallmarks. Curr. Alzheimer Res. 2017;14:870. doi: 10.2174/1567205014666170317113606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlan R., Villa P., Senaldi G., Martino G. TNFalpha in experimental diseases of the CNS. Methods Mol. Med. 2004;98:171–190. doi: 10.1385/1-59259-771-8:171. [DOI] [PubMed] [Google Scholar]
- Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res. Ther. 2006;8:S3. doi: 10.1186/AR1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harry G.J., Kraft A.D. Neuroinflammation and Microglia: considerations and approaches for neurotoxicity assessment. Expet Opin. Drug Metabol. Toxicol. 2008;4:1265. doi: 10.1517/17425255.4.10.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kigerl K.A., de Rivero Vaccari J.P., Dietrich W.D., Popovich P.G., Keane R.W. Pattern recognition receptors and central nervous system repair. Exp. Neurol. 2014;258:5. doi: 10.1016/J.EXPNEUROL.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney J.W., Bemiller S.M., Murtishaw A.S., Leisgang A.M., Salazar A.M., Lamb B.T. Inflammation as a central mechanism in Alzheimer's disease. Alzheimer’s Dement Transl Res Clin Interv. 2018;4:575. doi: 10.1016/J.TRCI.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummer K.K., Zeidler M., Kalpachidou T., Kress M. Role of IL-6 in the regulation of neuronal development, survival and function. Cytokine. 2021;144 doi: 10.1016/J.CYTO.2021.155582. [DOI] [PubMed] [Google Scholar]
- Liu T., Zhang L., Joo D., Sun S.C. NF-κB signaling in inflammation. Signal Transduct. Targeted Ther. 2017;2 doi: 10.1038/SIGTRANS.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhães C.A., Carvalho M.D.G., De Sousa L.P., Caramelli P., Gomes K.B. Alzheimer's disease and cytokine IL-10 gene polymorphisms: is there an association? Arq Neuropsiquiatr. 2017;75:649–656. doi: 10.1590/0004-282X20170110. [DOI] [PubMed] [Google Scholar]
- Meng Q., Wang A., Hua H., Jiang Y., Wang Y., Mu H., et al. Intranasal delivery of Huperzine A to the brain using lactoferrin-conjugated N-trimethylated chitosan surface-modified PLGA nanoparticles for treatment of Alzheimer's disease. Int. J. Nanomed. 2018;13:705–718. doi: 10.2147/IJN.S151474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra S., Ghosh N., Banerjee E.R. Carboxymethyl Guar Gum nanoscaffold as matrix for cell growth in vitro. J Lung, Pulm Respir Res. 2018;ume 5 doi: 10.15406/JLPRR.2018.05.00156. [DOI] [Google Scholar]
- Nagata K., Ashikaga R., Mori W., Zako T., Shimazaki Y. Analysis of the enzymatic degradation of lysozyme fibrils using a combination method of non-denaturing gel electrophoresis and double staining with Coomassie Brilliant Blue G-250 and R-250 dyes. Anal. Sci. 2022 doi: 10.1007/S44211-022-00229-W. [DOI] [PubMed] [Google Scholar]
- Nune M., Krishnan U.M., Sethuraman S. PLGA nanofibers blended with designer self-assembling peptides for peripheral neural regeneration. Mater. Sci. Eng. C. 2016;62:329–337. doi: 10.1016/j.msec.2016.01.057. [DOI] [PubMed] [Google Scholar]
- Park J.C., Han S.H., Mook-Jung I. Peripheral inflammatory biomarkers in Alzheimer's disease: a brief review. BMB Rep. 2020;53:10. doi: 10.5483/BMBREP.2020.53.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.C., Han S.H., Mook-Jung I. Peripheral inflammatory biomarkers in Alzheimer's disease: a brief review. BMB Rep. 2020;53:10–19. doi: 10.5483/BMBREP.2020.53.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponto L., Schultz S., Jacob M., Menda Y., Wemmie J., Magnotta V. Brain pH and Alzheimer's pathology. J. Nucl. Med. 2014;55 [Google Scholar]
- Porro C., Cianciulli A., Panaro M.A. The regulatory role of IL-10 in neurodegenerative diseases. Biomolecules. 2020;10:1–15. doi: 10.3390/BIOM10071017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probert L. TNF and its receptors in the CNS: the essential, the desirable and the deleterious effects. Neuroscience. 2015;302:2–22. doi: 10.1016/J.NEUROSCIENCE.2015.06.038. [DOI] [PubMed] [Google Scholar]
- Rafal H.A., Nahi Yosef Y., Shahlla M.S., Maeda H.M., Ahmed Majeed A.-S. Direct and simple method for mesenchymal stem cells isolation, culturing and detection. Int. J. Stem Cell Res. Transplant. 2018;5 doi: 10.23937/2469-570X/1410054. [DOI] [Google Scholar]
- Reboredo C., González-Navarro C.J., Martínez-Oharriz C., Martínez-López A.L., Irache J.M. Preparation and evaluation of PEG-coated zein nanoparticles for oral drug delivery purposes. Int J Pharm. 2021;597 doi: 10.1016/J.IJPHARM.2021.120287. [DOI] [PubMed] [Google Scholar]
- Ren S., Breuillaud L., Yao W., Yin T., Norris K.A., Zehntner S.P., et al. TNF-α–mediated reduction in inhibitory neurotransmission precedes sporadic Alzheimer's disease pathology in young Trem2R47H rats. J. Biol. Chem. 2021:296. doi: 10.1074/JBC.RA120.016395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh J.S., Sohn D.H. Damage-associated molecular patterns in inflammatory diseases. Immune Netw. 2018;18 doi: 10.4110/IN.2018.18.E27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Perez J.M., Morillas-Ruiz J.M. A review: inflammatory process in Alzheimer's disease, role of cytokines. Sci. World J. 2012;2012 doi: 10.1100/2012/756357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simma N., Bose T., Kahlfuß S., Mankiewicz J., Lowinus T., Lühder F., et al. NMDA-receptor antagonists block B-cell function but foster IL-10 production in BCR/CD40-activated B cells. 2014. [DOI] [PMC free article] [PubMed]
- Sulistio Y.A., Lee H.K., Jung S.J., Heese K. Interleukin-6-Mediated induced pluripotent stem cell (iPSC)-Derived neural differentiation. Mol. Neurobiol. 2018;55:3513–3522. doi: 10.1007/S12035-017-0594-3. [DOI] [PubMed] [Google Scholar]
- Tobinick E., Gross H., Weinberger A., Cohen H. TNF-alpha modulation for treatment of Alzheimer's disease: a 6-month pilot study. Medsc. Gen. Med. 2006;8:25. [PMC free article] [PubMed] [Google Scholar]
- Torres-Acosta N., O'Keefe J.H., O'Keefe E.L., Isaacson R., Small G. Therapeutic potential of TNF- α inhibition for Alzheimer's disease prevention. J. Alzheim. Dis. 2020;78:619–626. doi: 10.3233/JAD-200711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsartsalis S., Tomos C., Karanikola T., Mironidou-Tzouveleki M. The effect of memantine on cerebral cortex tumor necrosis factor alpha exression in a rat model of acute hyperammonemia. Ann. Gen. Psychiatr. 2010;9 doi: 10.1186/1744-859X-9-S1-S181. [DOI] [Google Scholar]
- Tsujimoto T., Hosoda N., Uyama H. Fabrication of porous poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) monoliths via thermally induced phase separation. Polymer. 2016;8:66. doi: 10.3390/POLYM8030066. 2016;8:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W.Y., Tan M.S., Yu J.T., Tan L. Role of pro-inflammatory cytokines released from microglia in Alzheimer's disease. Ann. Transl. Med. 2015;3 doi: 10.3978/J.ISSN.2305-5839.2015.03.49. 7–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W.Y., Tan M.S., Yu J.T., Tan L. Role of pro-inflammatory cytokines released from microglia in Alzheimer's disease. Ann. Transl. Med. 2015;3 doi: 10.3978/J.ISSN.2305-5839.2015.03.49. 7–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L., Lai Y., Lei F., Liu S., Liu R., Wang T. Exploring the association between interleukin-1β and its interacting proteins in Alzheimer's disease. Mol. Med. Rep. 2015;11:3219–3228. doi: 10.3892/MMR.2015.3183/HTML. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yick L.W., Tang C.H., Ma O.K.F., Kwan J.S.C., Chan K.H. Memantine ameliorates motor impairments and pathologies in a mouse model of neuromyelitis optica spectrum disorders. J. Neuroinflammation. 2020;17:1–16. doi: 10.1186/S12974-020-01913-2/FIGURES/7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Chen L., Gu W., Xu Z., Gao Y., Li Y. In vitro and in vivo evaluation of donepezil-sustained release microparticles for the treatment of Alzheimer's disease. Biomaterials. 2007;28:1882–1888. doi: 10.1016/J.BIOMATERIALS.2006.12.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.