Monitoring and treatment of congestion are key pillars of modern heart failure (HF) management. Intravascular volume derangement in HF is a well-recognized problem; however, routine assessment of congestion poses challenges in clinical practice as the signs and symptoms are neither sensitive nor specific (1). In fact, no commonly used surrogate of volume status (i.e., physical signs, biomarkers, thoracic impedance, or calculated intravascular volume estimates) consistently correlates with absolute circulating blood volume (BV). CardioMEMS (Abbott), an implantable pulmonary arterial pressure (PAP) monitor, provides real-time hemodynamic information (2). Clinical trials and post-market studies have shown value of pulmonary arterial diastolic pressure (PAD)-guided management to adjust HF medications and prevent hospitalizations. Despite pressure-guided HF management, an excess in HF events including hospitalization and mortality remains. The common belief that almost all patients with HF are intravascularly volume overloaded has been challenged, and the resulting, near-universal treatment with diuretics may lead to the mistreatment of patients who are euvolemic or even hypovolemic. Basing volume-adjusting therapies on proxy measures of pressure might not accurately account for the complex nature of congestion. We sought to test pressure-volume phenotypes in patients with ambulatory HF managed with CardioMEMS.
Data collection occurred across two centers (Baptist, Memphis and Duke University, Durham) with IRB-approval, in consecutive patients undergoing initial implantation of CardioMEMS or upon subsequent outpatient follow-up. Blood volume analysis (BVA) (BVA-100™, Daxor Corporation) is clinically approved and utilizes the gold-standard indicator dilution technique with an Iodine131-tagged albumin tracer to provide quantitative measurement of total BV (TBV), plasma volume (PV) and red blood cell volume (RBCV) (3). Radiotracer injection is followed by at least 3 timed blood samples. The BVA-report provides absolute values (ml) and deviation from ideal TBV, PV, and RBCV (expressed as absolute deviation and excess or deficit in mL and %-deviation)(4). A TBV deviation ≥±8% indicates either an excess or deficit of volume. We employed a previously described simulation-based method for estimating stressed blood volume (eSBV) based on widely used models of the cardiovascular system (5). eSBV was simulated using heart rate, cardiac output, central venous pressure, pulmonary capillary wedge pressure, systolic and diastolic systemic arterial and PAP and left ventricular ejection fraction. To account for differences in patient sizes, eSBV values are presented as ml/70 kg body weight.
A total of 20 patients were included in the analysis. Average age was 61±13 years,13 were men with an average body mass index of 30±5kg/m2. Of them, 35% were Caucasian, 60% were Black and 5% were Native-American, 70% had a LVEF≤40% and one patient had a left ventricular assist device. The majority of patients (75%) had New York Heart Association (NYHA) Class III HF symptoms, while 25% were NYHA Class IV and 70% were classified as Class C HF, while 30% were classified as Class D. In the preceding 6 months the average HF hospitalization rate was 1.3 events/patient and 0.75 events/patient in the 6 months thereafter. Average hematocrit was 39±6% and NT-proBNP was 3,712±5,533pg/mL. The average PAD (± standard deviation) at the time of BVA was 20.8±7.7mmHg. The average TBV was 5,464±1,461mL with absolute deviation of 112±1033mL (relative +0.5±17.5%) from ideal, the average PV was 3530±391mL with absolute deviation of 275±797mL (relative +7±19.8%) from ideal, and the average RBCV was 1,934±644mL with absolute deviation of −135±369mL (relative −9.2±19.3%) from ideal. There was lack of correlation between PAD and BV metrics (PAD vs TBV, R2=0.002; PAD vs PV, R2=0.001; PAD vs RBCV, R2=0.025) (Figure). The average absolute eSBV was 3302±1602mL with an average SBV/70kg was 2086±486mL. PAD and eSBV/70kg had moderate correlation of R2=0.237 and a stronger correlation between measured TBV and eSBV was R2=0.339.
Figure:
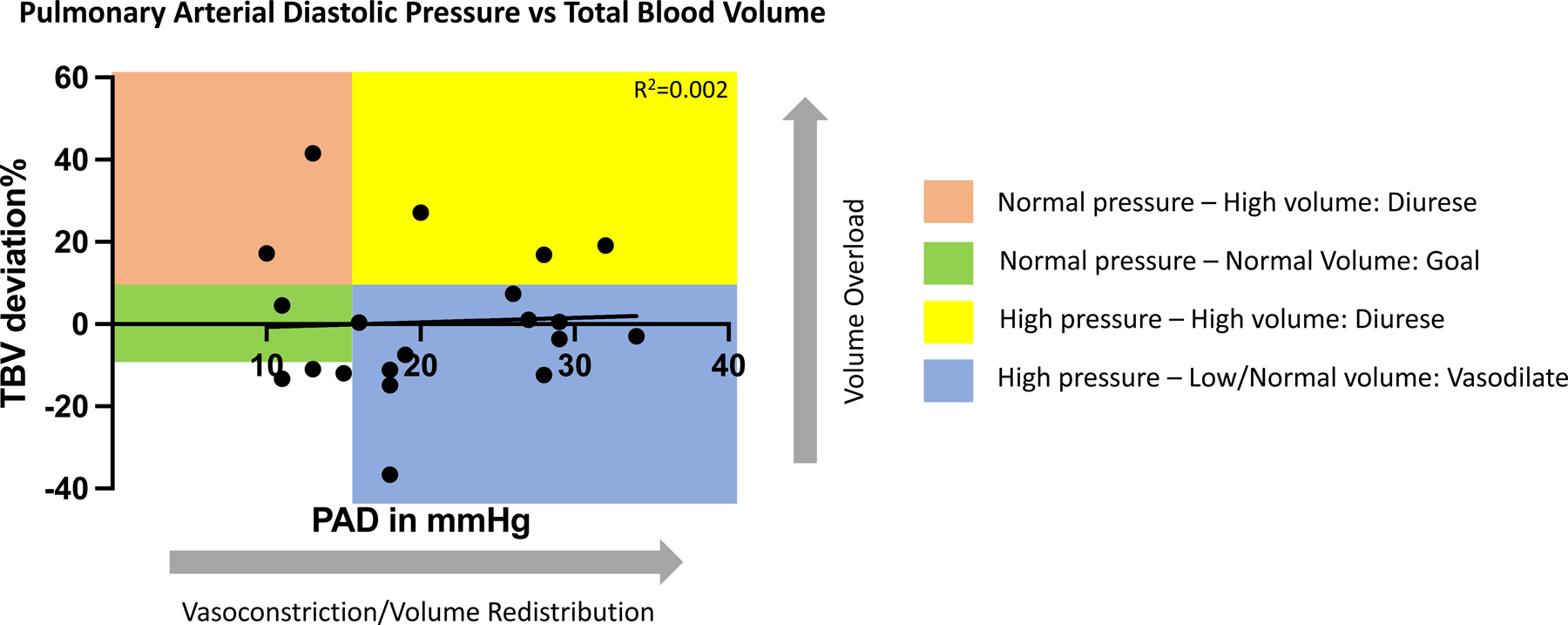
The correlation between PAD and TBV. Color boxes demonstrate the proposed pressure-volume phenotypes (and proposed action) using PAD of 15mmHg and the TBV ±8% deviation from ideal as cutoffs.
Patients with >+8% of ideal TBV (N=5) had in total only 1 hospitalization in follow-up (rate of 0.2 events/patient), and patients with a TBV 8% or less (N=15) had a higher rate of 0.93 events/patient.
PAP and PAD are surrogate markers for BV status and are often used to guide volume-adjusting therapy. However, several studies have found poor correlation between intra-cardiac pressures and direct measurement of circulating BV, including in HF (1,3). In other words, pressure overload does not always equal volume overload, and congestion is a product of a distinct cardiovascular pressure-volume interplay. Notably, we found no relationship between PAD and actually measured intravascular volume yet found a moderate relationship between PAD and eSBV. This finding suggests that PAD is more so determined by SBV (volume distribution due to the central vascular compartment and venous tone) rather than blood volume itself. To date our understanding of the pressure-volume relationship (or disconnect thereof) is limited to a single timepoint and we lack longitudinal evidence of the trend between PAD and TBV. Findings of this nature emphasize the need to study the longitudinal cardiovascular pressure-volume relationships in the dynamic clinical environment of HF. These findings do indicate that pressure-based assessment of congestion in ambulatory HF patients does not accurately represent intravascular volume, nevertheless pressure changes remain indicative of HF exacerbations. Additional volume-based phenotyping may be required to guide decongestion strategies in patients with HF. Our data provides initial evidence that patients with low/normal volume (independent of PAD) are at highest risk of HF hospitalization. This finding suggests variable pressure/volume phenotypes, with a previously unappreciated variable risk profile. Further studies are needed to explore if clinical outcomes of pressure-guided HF management could be improved upon with volume-guided phenotyping.
Funding Sources:
Mario Family Award
Disclosures:
Dr Fudim was supported by the National Heart, Lung, and Blood Institute (NHLBI) (K23HL151744), the American Heart Association (20IPA35310955), Mario Family Award, Duke Chair’s Award, Translating Duke Health Award, Bayer, Bodyport and BTG Specialty Pharmaceuticals. He receives consulting fees from AxonTherapies, Bodyport, Boston Scientific, CVRx, Daxor, Edwards LifeSciences, Fire1, Inovise, NXT Biomedical, Viscardia, Zoll. Dr Jefferies receives research support from NIH, Abbott, Medtronic, Chiesi, Nuwellis, Myokardia, Novartis, Pfizer, Alynylam, Regeneron, Sanofi Genzyme, Biocardia, Bristol Myers Squibb, Verun, Ionis, Eli Lilly, Innolife, V Wave, HeartBeam, Daxor. He consults for Stealth Biotherapeutics, Abbott, Medtronic, Novartis, Pfizer, AstraZeneca, Chiesi, Sanofi Genzyme, Daxor, Bayer, Audentes, HeartBeam, Davor, Nuwellis. Dr Silver is a Medical Advisor for Daxor. Dr Rao was supported by an NIH T32 training grant. Dr. Burkhoff received institutional grants from Abiomed, Ancora and Fire 1; consulting fees from PVLoops LLC and Axon Therapeutics. All other authors report no relevant disclosures.
Abbreviations:
- BV
blood volume
- BVA
blood volume analysis
- HF
heart failure
- PAP
pulmonary arterial pressure
- PAD
pulmonary arterial diastolic pressure
- PV
plasma volume
- RBC
red blood cell volume
- TBV
total blood volume
References:
- 1.Androne AS, Hryniewicz K, Hudaihed A, Mancini D, Lamanca J, Katz SD. Relation of unrecognized hypervolemia in chronic heart failure to clinical status, hemodynamics, and patient outcomes. The American journal of cardiology 2004;93:1254–9. [DOI] [PubMed] [Google Scholar]
- 2.Lindenfeld J, Zile MR, Desai AS et al. Haemodynamic-guided management of heart failure (GUIDE-HF): a randomised controlled trial. Lancet 2021;398:991–1001. [DOI] [PubMed] [Google Scholar]
- 3.Miller WL, Sorimachi H, Grill DE, Fischer K, Borlaug BA. Contributions of cardiac dysfunction and volume status to central haemodynamics in chronic heart failure. European journal of heart failure 2021;23:1097–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feldschuh J, Enson Y. Prediction of the normal blood volume. Relation of blood volume to body habitus. Circulation 1977;56:605–12. [DOI] [PubMed] [Google Scholar]
- 5.Doshi D, Burkhoff D. Cardiovascular Simulation of Heart Failure Pathophysiology and Therapeutics. J Card Fail 2016;22:303–11. [DOI] [PubMed] [Google Scholar]


