Abstract
Aim
To evaluate the effect of curcumin treatment on hepatic fat content in obese individuals.
Materials and Methods
In a double‐blind, parallel‐group trial, 37 obese, non‐diabetic individuals were randomized to placebo or curcumin treatment for 6 weeks. Curcumin was dosed as lecithin‐formulated tablet; 200 mg twice daily. The primary endpoint was hepatic fat content as assessed by magnetic resonance spectroscopy (MRS). Other endpoints included anthropometric measurements, hepatic biomarkers including FibroScan measurements, metabolic variables, inflammation markers, appetite measures and ad libitum food intake.
Results
Baseline characteristics (mean ± SD) were age 46 ± 14 years, hepatic fat content 12.2% ± 8.8% points, body mass index 38.8 ± 6.1 kg/m2 and waist circumference 125.8 ± 12.3 cm. After 6 weeks of treatment with curcumin, hepatic fat content was changed by −0.86% points (95% CI −3.65; 1.94) compared with 0.71% points (95% CI − 2.08; 3.51) with placebo, thus resulting in a non‐significant estimated treatment difference of −1.57% points (95% CI −5.36; 2.22, P = .412). Compared with placebo, curcumin treatment caused small reductions in fasting plasma glucose (estimated treatment difference [ETD] − 0.24 mmol/L [95% CI −0.45; −0.03]), triglycerides (ETD [percentage change] −20.22% [95% CI −33.21; −6.03]) and gamma glutamyltransferase (ETD [percentage change] −15.70% [95% CI −23.32; −7.32]), but except for gamma glutamyltransferase, none of these differences remained statistically significant after adjusting for multiple testing. Treatment was well tolerated.
Conclusions
Compared with placebo, curcumin treatment for 6 weeks had no significant effect on MRS‐assessed hepatic fat content in obese individuals with primarily mild steatosis. Curcumin was well tolerated.
Keywords: clinical trial, fatty liver disease, insulin resistance, phenol, randomized trial
1. INTRODUCTION
The growing prevalence of obesity is strongly associated with hepatic steatosis and, thus, an increase in the prevalence of non‐alcoholic fatty liver disease (NAFLD). In the Western world, NAFLD affects 25%‐30% of the general population. 1 Ultimately, NAFLD can result in non‐alcoholic steatohepatitis, cirrhosis and hepatocellular carcinoma. 2 Despite the severe consequences of NAFLD, effective management is currently limited to lifestyle‐induced weight loss. Few drugs have shown moderately positive effects on NAFLD, 3 , 4 but the side effects and questionable durability of these drugs call for more research.
Several medical plant‐derived supplements have been explored for their potential beneficial effects in hepatic steatosis. Systematic reviews of the literature suggest that curcumin, a phenolic compound extracted from the turmeric root (Curcuma longa), reduces hepatic fat content (HFC) in NAFLD. 5 , 6 However, no studies have assessed HFC using magnetic resonance spectroscopy (MRS), which is regarded as the gold standard for non‐invasive measurement of HFC, 7 , 8 , 9 and only one study has assessed liver stiffness with FibroScan, 10 a marker of fibrosis. Also, meta‐analyses have shown that curcumin can improve several liver‐related variables in individuals with NAFLD, including circulating levels of alanine transaminase, aspartate transaminase, total and low‐density lipoprotein cholesterol, triglyceride as well as fasting plasma glucose, HbA1c, hyperinsulinaemia, insulin resistance (according to homeostatic model assessment [HOMA‐IR]), body weight, body mass index (BMI) and waist circumference. 11 , 12 , 13 , 14 , 15 Furthermore, curcumin seems to have anti‐inflammatory properties. 16 , 17 , 18 , 19
As obesity‐associated hepatic steatosis is strongly correlated with reduced glucose tolerance and the development of type 2 diabetes, it is worth mentioning that no studies have examined the effect of long‐term curcumin treatment on oral glucose tolerance or insulin, glucagon and glucagon‐like peptide‐1 (GLP‐1) responses during an oral glucose tolerance test (OGTT) in obese individuals.
Here, we used a randomized placebo‐controlled parallel group design to investigate the effect of 6 weeks of curcumin treatment on MRS‐assessed HFC in obese individuals. The majority of the individuals had mild steatosis. Also, the effects of curcumin on anthropometric measurements, hepatic biomarkers (including FibroScan measurements), metabolic variables, inflammation markers, appetite measures and ad libitum food intake were investigated.
2. MATERIALS AND METHODS
2.1. Trial design, approval and ethics
The study was a single‐centre, randomized, double‐blind, placebo‐controlled, parallel‐group trial investigating the effect of 6 weeks of curcumin treatment on HFC in individuals with obesity and was conducted from April 2019 to December 2020 at the Center for Clinical Metabolic Research, Gentofte Hospital, University of Copenhagen, Hellerup, Denmark, in accordance with the Declaration of Helsinki (2013 edition) and Good Clinical Practice guidelines. The study was approved by the Scientific–Ethical Committee of the Capital Region of Denmark (ID no.: H‐18045227) and the Danish Data Protection Agency (J. no.: VD‐2018‐385/ I‐Suite no.: 6636) and was registered at www.clinicaltrials.gov (registration no.: NCT03864783). Written informed consent was obtained from all participants before screening.
2.2. Eligibility criteria
Inclusion criteria were age above 20 years, BMI above 27 kg/m2 and the participants should have a steatosis diagnosis within the last 3 years, without significant weight loss in the subsequent 3 years. If the participant had no diagnosis of steatosis, the participant should have a BMI above 30 kg/m2 and meet two of the following criteria: steatosis on FibroScan (controlled attenuation parameter [CAP] value above 325 dB), waist circumference above 94 cm, HbA1c above 48 mmol/mol or fatty liver index score above 60%. 20 , 21 Key exclusion criteria were use of glucose‐lowering, lipid‐lowering or steatogenic drugs, known viral, inherited or alcoholic liver disease, history of alcohol abuse, adhering to a weight management programme and any condition or clinical or biochemical signs considered incompatible with participation by the investigator.
2.3. Treatment and randomization
Included participants were randomized to curcumin or placebo (1:1). Curcumin was administered as tablets containing Meriva (Indena S.p.A. Milan, Italy) 1000 mg twice daily, corresponding to 400 mg of curcuminoids. All tablets were produced and sponsored by the manufacturers who otherwise had no role in the study design, data collection, data analysis, data interpretation or publication of this manuscript. Placebo and Meriva tablets contained the same ingredients except for curcuminoids. For simplicity, Meriva tablets are referred to as curcumin in the following. Compliance was considered acceptable if more than 75% of dosages were ingested. Participants reported missed dosages, concomitant medication and adverse events during the trial. The randomization was performed by a person not otherwise involved in the study by producing a sequence of random numbers using www.random.org referring to the number on a bag containing trial product. The participants, radiologist, laboratory technicians and investigators were blinded to treatment allocation until the primary analysis was finalized.
2.4. Procedures
After inclusion, participants attended baseline MRS (including measurement of volume of visceral adipose tissue [VAT] and volume of subcutaneous adipose tissue [SAT]), experimental day #1 and filled out a 3‐day diary of food consumption and physical activity. After this, they were randomized to 6 weeks of intervention. After intervention, the assessments were repeated (Figure 1). A detailed description of the study timeline and all procedures are found in Appendix S1. MRS was performed at the Department of Radiology, Herlev Hospital, University of Copenhagen, Herlev, Denmark, using a 3.0‐T Ingenia magnetic resonance imaging (MRI) system (Philips Medical Systems, Best, The Netherlands) and the MRI protocol included planning scans and single‐voxel spectroscopy for measuring HFC. Scans were evaluated by the same radiologist and HFC was calculated as previously described. 22 At each experimental day, participants met in the research facility after an overnight 10‐hour fast. First, the participants were asked to void the urinary bladder and a urine sample was collected. Bioelectrical impedance analysis, waist circumference, hip circumference and body weight were assessed. Next, with participants positioned comfortably on a hospital bed, after at least 10 minutes of rest, blood pressure and heart rate were measured and transient elastography (FibroScan) was performed, and a cannula was inserted in a cubital vein for blood sampling. The forearm was wrapped in a heating pad for arterialization of venous blood. At time point 0 minutes, 75 g of glucose dissolved in 300 ml of tap water was ingested over 5 minutes. Blood samples were collected at time points of −15, 0, 30, 60, 90, 120, 150, 180, 210 and 240 minutes. Appetite sensations were assessed by visual analogue scale (VAS) at time points 0, 30, 60, 90, 120, 150, 180, 210 and 240 minutes and 10 minutes after termination of a subsequent ad libitum meal (see below). Between 180 and 240 minutes, resting energy expenditure was assessed by indirect calorimetry. After blood sampling at time point 240 minutes, participants voided their bladder and urine was sampled. Lastly, participants were served an ad libitum meal consisting of minced meat, pasta, corn, carrots and green pepper (1.47 kcal/g: 36% of energy as fat, 15% as protein, 48% as carbohydrates and 1% as dietary fibre) (Figure 1). A detailed description of all methods including biochemical analyses can be found in Appendix S1.
FIGURE 1.
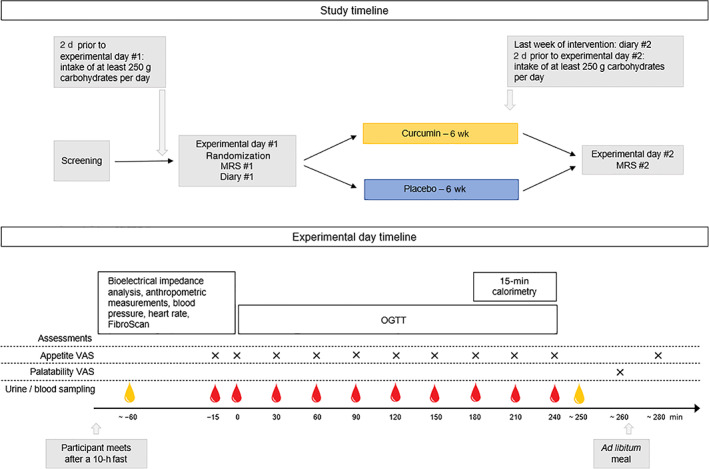
Timeline of the study and timeline of experimental days. Red drop indicates blood sampling, yellow drop indicates urine sampling. MRS, magnetic resonance spectroscopy; OGTT, oral glucose tolerance test; VAS, visual analogue scale
2.5. Calculations and statistical analysis
Calculations of fatty liver index score, FIB4 score, HOMA2‐IR, urine albumin/creatinine ratio, composite appetite score and area under the curve (AUC) for variables measured during OGTT are described in Appendix S1. Based on a previous study by Belfort et al., 23 we expected that the SD of the change in hepatitis fat content would be 3.1% points. Thus, 18 participants in each arm were needed to detect a minimum clinically relevant reduction in HFC by 15% with 80% power. For all continuous variables, a constrained linear mixed model with inherent baseline adjustment 24 was used to estimate the effect of curcumin compared with placebo. An unstructured covariance pattern was assumed to account for repeated measurements on each study participant. Skewed data were log‐transformed prior to analysis. P values for secondary and exploratory end points in turn were adjusted for multiple testing using the method of Benjamini–Hochberg,25 which controls the false discovery rate. An adjusted P value of less than .05 was considered statistically significant. In Appendix S1, the endpoints are listed grouped as secondary and exploratory endpoints. R version 4.0.3 was used for statistical analyses. All graphs were made with GraphPad Prism v. 9.0.0. Figure 1 was created in Microsoft PowerPoint.
3. RESULTS
3.1. Participant characteristics and safety
We screened 86 persons after informed consent was given. Forty‐seven were ineligible for participation. The investigator excluded two for practical reasons related to the severe acute respiratory syndrome coronavirus 2 pandemic causing national lockdown, and their results were excluded from all analyses. One participant could not cooperate with the second MRS at end‐of‐treatment (EOT) because of claustrophobia. Thirty‐seven participants completed experimental day #2, and 36 participants completed the second MRS. All participants were Caucasian. No participants had an HbA1c above 48 mmol/mol at inclusion or a BMI below 30 kg/m2. Three participants had a glucose value of 11.1 mmol/L or higher at time point 120 minutes during the first OGTT and 11 participants reached 11.1 mmol/L or higher at some point during the first OGTT. At the first OGTT, 22 individuals had a fasting glucose of 5.5 mmol/L or above and 17 individuals had plasma glucose of 7.8 mmol/L or higher at time point 120 minutes. Ten individuals had HFC below 5.5% and 17 individuals had mild steatosis in MRS #1 (the maximum HFC was 27.1%); however, all results were included in statistical analysis, regardless of the result being below or above 5.5% at baseline. Baseline characteristics for all participants are shown in Table 1. Compliance was satisfying (above 95% ingested dosages). Seven adverse events were reported in the placebo group versus nine in the curcumin group. No participants left the study because of side effects. A participant in the placebo group had a short visit to the emergency department with dyspnoea, presumably anxiety‐related and not related to study product or procedures. One participant in the curcumin group experienced a decrease in leukocyte and erythrocyte counts at experimental day #2, but showed no symptoms related to this, and values were normalized at a follow‐up visit. All other reported adverse events were mild and did not interfere with the daily life of the participants. We found no statistically significant effect of curcumin on safety measures (creatinine, urine albumin, urine creatinine, urine albumin/creatinine ratio, heart rate, systolic or diastolic blood pressure) (Table S1).
TABLE 1.
Participant baseline characteristics
| Placebo (mean ± SD) | Curcumin (mean ± SD) | |
|---|---|---|
| Men/women (‐ / ‐) | 14 / 5 | 13 / 5 |
| Age (y) | 47.7 ± 12.1 | 44.8 ± 15.8 |
| BMI (kg/m2) | 39.5 ± 6.8 | 38.0 ± 5.5 |
| Waist circumference (cm) | 127.0 ± 13.5 | 124.5 ± 11.5 |
| HFC (%) † | 14.7 ± 8.8 | 9.5 ± 8.4 |
| Liver stiffness (kPa) † | 6.0 ± 2.1 | 6.7 ± 3.5 |
| HbA1c (mmol/Mol) | 35.8 ± 4.0 | 34.2 ± 3.4 |
| Body fat percentage (%) † | 42.0 ± 7.8 | 42.3 ± 6.7 |
Abbreviations: BMI, body mass index; HFC, hepatic fat content.
HFC measured by magnetic resonance spectroscopy; liver stiffness measured by FibroScan; body fat percentage measured by bioelectrical impedance analysis.
3.2. Hepatic fat content
We found no effect of curcumin on changes in HFC from baseline to EOT (mean difference −0.86% points [95% CI −3.63; 1.94]; P = .543) or when comparing with placebo (ETD −1.57% points [95% CI −5.36; 2.22]; P = .412) (Figure 2).
FIGURE 2.

Hepatic fat content (HFC) measured by magnetic resonance spectroscopy (MRS) (mean ± SD) before and after intervention (baseline and end‐of‐treatment [EOT]) in 36 obese individuals randomized (1:1) to 6 weeks of placebo or curcumin treatment. Individual changes are shown in grey. Dagger (†) indicates P value from comparison of the estimated mean differences from baseline to EOT in the two groups, that is, estimated treatment difference. Double daggers (‡) indicate P value of the difference from baseline to EOT within each of the two groups
3.3. Anthropometric measurements
Intervention with curcumin was not associated with significant changes in BMI, waist circumference, waist–hip ratio, VAT or SAT (Table 2), or measurements from bioelectrical impedance analysis, when compared with placebo (Table S2).
TABLE 2.
Baseline‐adjusted effects of curcumin and placebo on anthropometric measurements, hepatic variables, metabolic variables and inflammation markers
| Baseline outcome (95% CI) † | Δ placebo (95% CI) ‡ | Δ curcumin (95% CI) ‡ | ETD curcumin versus placebo (95% CI) ‡ | P (unadjusted/adjusted) | |
|---|---|---|---|---|---|
| Anthropometric measurements | |||||
|
BMI (kg/m2) |
38.31 (36.45; 40.28) |
0.61 (2.22; 10.00)% |
0.30 (−0.09; 0.70)% |
−0.31 (−0.84; 0.23)% |
0.262/ 0.550 |
|
Waist circumference (cm) |
125.8 (121.7; 129.9) |
−0.1 (−1.5; 1.3) |
−1.4 (−2.8; 0.0) |
−1.3 (−3.3; 0.6) |
0.184/ 0.483 |
| Waist−hip ratio |
1.1 (1.0; 1.1) |
−0.0 (−0.0; 0.0) |
−0.0 (−0.0; −0.0) |
−0.0 (−0.0; 0.0) |
0.409 / 0.872 |
|
SAT (cm3) |
499.2 (436.0; 562.4) |
−2.0 (−11.1; 7.15) |
0.5 (−8.9; 9.9) |
2.5 (−10.5; 15.4) |
0.702 / 0.920 |
|
VAT (cm3) |
261.7 (224.9; 298.6) |
−1.7 (−10.9; 7.60) |
−4.8 (−14.1; 4.5) |
−3.1 (−15.6; 9.3) |
0.616 / 0.872 |
| Hepatic biomarkers | |||||
|
CAP (dB) |
335.7 (321.2; 350.2) |
4.5 (−13.8; 22.7) |
8.0 (−11.0; 26.9) |
3.5 (−21.9; 28.9) |
0.784 / 0.920 |
|
Liver stiffness (kPa) |
5.81 (5.07; 6.66) |
4.84 (−10.14; 22.32)% |
−11.28 (−24.49; 4.24)% |
−15.37 (−31.78; 4.98)% |
0.127 / 0.438 |
| FIB4 score |
0.7 (0.6; 0.8) |
−1.7 (−13.4; 11.5)% |
9.0 (−3.6; 23.1)% |
10.9 (−6.9; 32.0)% |
0.243 / 0.550 |
|
Alanine transaminase § (U/L) |
43.11 (36.56; 49.66) |
−2.67 (−8.20; 2.85) |
−0.07 (−5.74; 5.61) |
2.61 (−5.16; 10.38) |
0.505 / 0.663 |
|
Aspartate transaminase § (U/L) |
27.11 (24.22; 29.99) |
−0.26 (−3.19; 2.67) |
0.22 (−2.79; 3.22) |
0.46 (−3.56; 4.51) |
0.815 / 0.856 |
|
Lactate dehydrogenase § (U/L) |
184 (175; 193) |
13 (3; 22) |
3 (−6;13) |
−9 (−21; 3) |
0.146 / 0.438 |
|
Alkalic phosphatase § (U/L) |
83.8 (72.9; 94.7) |
−1.0 (−4.0; 2.1) |
−2.4 (−5.6; 0.7) |
−1.5 (−5.5; 2.5) |
0.463 / 0.648 |
|
GGT § (U/L) |
28.33 (23.84; 33.66) |
13.31 (6.02; 21.10)% |
−4.48 (−10.78; 2.27)% |
−15.70 (−23.32; −7.32)% |
<0.001 / 0.013 |
|
Bilirubin § (μmol/L) |
9.87 (8.19; 11.56) |
−0.29 (−1.48; 0.89) |
0.40 (−0.78; 1.59) |
0.69 (−0.85; 2.23) |
0.371 / 0.872 |
|
Ferritin § (μg/L) |
128.0 (104.3; 151.7) |
−11.5 (−23.9; 0.9) |
−14.5 (−27.2; −1.8) |
−3.0 (20.8; 14.8) |
0.739 / 0.920 |
|
Albumin § (g/L) |
39.29 (38.54; 40.04) |
−0.02 (−0.84; 0.80) |
−0.30 (−1.17; 0.57) |
−0.28 (−1.40; 0.83) |
0.613 / 0.872 |
|
Platelets § (number of platelets × 109/L) |
258.8 (239.4; 278.2) |
7.3 (−8.3; 22.8) |
−8.6 (−23.6; 6.5) |
−15.8 (−37.3; 5.8) |
0.148 / 0.840 |
|
Estimated urea production (mmol/min) |
0.30 (0.27;0.33) |
0.02 (−0.02; 0.05) |
0.01 (−0.03; 0.05) |
−0.01 (−0.05;0.04) |
0.822 / 0.939 |
|
FGF21 § (pg/ml) |
154 (117; 203) |
3 (−20; 32) % |
−13 (−32; 13) % |
−15 (−40; 20) % |
0.352 / 0.872 |
| Metabolic variables | |||||
| HOMA2‐IR § | 2.54 (2.10; 2.97) | 0.24 (−0.09; 0.56) | −0.15 (−0.48; 0.19) | −0.38 (−0.84; 0.07) | 0.098/ 0.438 |
|
HbA1c § (mmol/mol) |
35.03 (33.78; 36.26) |
0.20 (−0.52; 0.92) |
−0.66 (−1.38; 0.07) |
−0.86 (−1.82; 0.11) |
0.080 / 0.438 |
| Glucose | |||||
|
Fasted state (mmol/L) |
5.55 (5.38; 5.72) |
0.11 (−0.04; 0.26) |
−0.13 (−0.28; 0.02) |
−0.24 (−0.45; −0.03) |
0.027 / 0.284 |
|
AUC (0‐240 min) (mmol/L × h) |
29.2 (28.0; 30.5) |
0.1 (‐0.9; 1.1) |
‐0.2 (‐1.2; 0.9) |
‐0.3 (‐1.7; 1.2) |
0.700 / 0.817 |
| Insulin | |||||
|
Fasted state (pmol/L) |
136.9 (111.5; 162.2) |
11.9 (−7.0; 30.8) |
−8.6 (−28; 10.7) |
−20.5 (−46.3; 5.2) |
0.116/ 0.438 |
|
AUC (0‐240 min) (pmol/L × h) |
2312 (1808; 2816) |
125 (‐150; 399) |
−42 (‐323; 240) |
‐166 (‐537; 205) |
0.375/ 0.648 |
| C‐peptide | |||||
|
Fasted state (pmol/L) |
781.5 (693.8; 869.1) |
20.8 (−34.2; 75.8) |
−8.5 (−64.8; 47.9) |
−29.2 (106.1; 47.6) |
0.450/ 0.648 |
|
AUC (0‐240 min) (pmol/L × h) |
8312 (7502; 9120) |
‐326 (‐774; 123) |
‐368 (‐828; 92) |
‐42 (‐669; 585) |
0.894/ 0.894 |
| Glucagon | |||||
|
Fasted state (mmol/L) |
10.4 (8.2; 12.2) |
22.4 (−7.2; 61.5)% |
1.5 (−23.6; 34.8)% |
−17.1 (−42.9; 20.4)% |
0.320 / 0.611 |
|
AUC (0‐240 min) (mmol/L × h) |
28.1 (22.3; 33.9) |
‐0.5 (‐6.3; 5.3) |
‐1.8 (‐7.7; 4.2) |
‐1.3 (‐9.6; 7.0) |
0.759 / 0.839 |
| GLP‐1 | |||||
|
Fasted state (mmol/L) |
7.3 (5.7; 8.8) |
0.4 (−1.7; 2.5) |
−0.7 (−2.9; 1.4) |
−1.1 (−4.0; 1.8) |
0.440 / 0.648 |
|
AUC (0‐240 min) (mmol/L × h) |
36.5 (30.2; 42.9) |
−5.1 (−11.3; 1.2) |
−3.0 (−9.4; 3.3) |
2.2 (−6.2; 10.6) |
0.604 / 0.746 |
|
High‐density lipoprotein § (mmol/L) |
0.99 (0.93; 1.06) |
3.03 (−1.23; 7.48)% |
−0.59 (−2.26; 9.91)% |
−3.52 (−9.02; 2.31)% |
0.228 / 0.840 |
|
Total cholesterol § (mmol/L) |
4.57 (4.35; 4.79) |
0.04 (−0.13; 0.21) |
−0.09 (−0.27; 0.08) |
−0.13 (−0.37; 0.11) |
0.284 / 0.840 |
|
Triglyceride § (mmol/L) |
1.46 (1.30; 1.63) |
10.82 (−1.90; 25.19)% |
−12.14 (−22.48; −0.04)% |
−20.22 (−33.21; −6.03)% |
0.008 / 0.275 |
|
Resting energy expenditure (estimated kcal per day) |
2108 (1959; 2257) |
23 (−65; 112) |
5 (−87; 96) |
−19 (−145; 108) |
0.769 / 0.917 |
| Inflammation markers | |||||
|
TNF‐alpha § (pg/ml) |
1.20 (1.10; 1.30) |
−3.78 (−9.02;1.75)% |
−1.24 (−6.75; 4.59)% |
2.6 (−4.98; 10.86)% |
0.502 / 0.872 |
|
IL‐6 § (pg/ml) |
1.31 (1.01; 1.07) |
−0.19 (−11.32; 12.34)% |
8.47 (−3.62; 22.09)% |
8.67 (−7.31; 27.43)% |
0.300 / 0.840 |
|
IL‐8 § (pg/ml) |
4.52 (4.05; 5.03) |
−3.20 (−12.70; 7.34)% |
−7.53 (−16.84; 2.81)% |
−4.48 (−17.29; 10.32)% |
0.528 / 0.872 |
|
IL‐10 § (pg/ml) |
0.30 (0.26; 0.34) |
−6.41 (−15.61; 3.80)% |
−1.72 (−11.37; 8.97)% |
5.00 (−7.37; 19.03)% |
0.440 / 0.872 |
|
IFN‐gamma § (pg/ml) |
6.01 (5.11;7.06) |
−5.06 (−18.61; 10.74)% |
−2.94 (−17.10; 13.65)% |
2.24 (−17.09; 26.07)% |
0.834 / 0.948 |
|
Hs‐CRP § (mg/l) |
2.27 (1.69; 3.04) |
8.47 (−13.36; 35.80)% |
8.84 (−13.10; 36.33)% |
0.34 (−25.57; 35.27)% |
0.982 / 0.989 |
|
YKL‐40 § (ug/L) |
51.60 (41.79; 61.40) |
−0.20 (−8.45; 8.04) |
−5.79 (−14.2; 2.66) |
−5.58 (−16.76; 5.60) |
0.232 / 0.840 |
|
Adiponectin § (pg × 105/ml) |
166.90 (147.65; 186.15) |
8.00 (−4.04; 20.03) |
−1.50 (−14.24; 11.29) |
‐9.50 (–26.67; 7.66) |
0.273 / 0.840 |
Note: In the last coloumn, the adjusted P value is written in italic.
Abbreviations: AUC, area under the curve; BMI, body mass index; CAP, controlled attenuation parameter; ETD, estimated treatment difference; FGF21, fibroblast growth factor 21; GGT, gamma glutamyltransferase; GLP‐1, glucagon‐like peptide‐1; HOMA2‐IR, homeostatic model assessment for insulin resistance; hs‐CRP, high‐sensitivity C‐reactive protein; IL, interleukin; INF‐gamma, interferon gamma; SAT, subcutaneous adipose tissue volume; TNF‐alpha, tumour necrosis factor alpha; VAT, visceral adipose tissue volume.
Baseline values are mean (95% CI) for normally distributed data and geometric mean (95% CI) for skewed data.
Delta values and ETD are differences in mean (95% CI) for normally distributed data and percentage difference in geometric mean for skewed data.
Concentrations in the fasted state.
3.4. Hepatic biomarkers
We found no effect of curcumin on FibroScan measurements of HFC (CAP value) or liver stiffness, or any biochemical hepatic variables (including urea production and FGF‐21), when comparing with placebo, except for gamma glutamyltransferase (GGT). GGT exhibited a small but statistically significant decrease compared with placebo; however, this difference was driven by a significant increase in the placebo group (Table 2).
3.5. Metabolic measures
We found no statistically significant effect of curcumin on HOMA2‐IR or HbA1c when comparing with placebo. Curcumin significantly decreased triglycerides compared with placebo, but we found no effect on HDL or total cholesterol (Table 2). LDL is not reported, as it would only be an estimate calculated from total cholesterol and HDL values. Curcumin significantly lowered fasting plasma glucose, but there was no effect on the AUC for plasma glucose, or on fasting plasma/serum concentrations or AUC for insulin, C‐peptide, glucagon or GLP‐1 when comparing with placebo (Figure 3).
FIGURE 3.
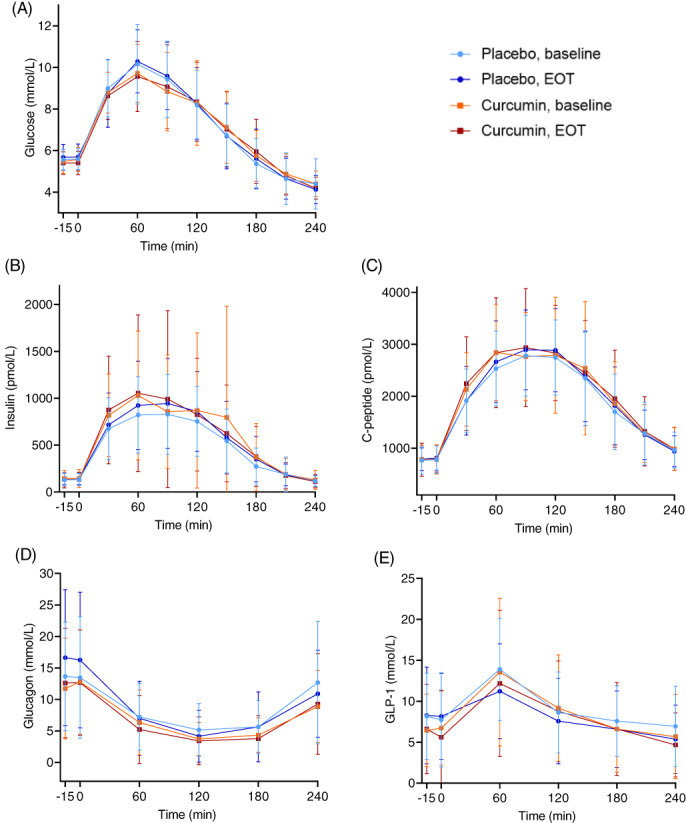
Plasma/serum concentrations of A, Glucose, B, Insulin, C, C‐peptide, D, Glucagon, and E, Glucagon‐like peptide‐1 (GLP‐1) during oral glucose tolerance test (OGTT) at baseline and at end‐of‐treatment (EOT) (mean ± SD) for all participants receiving placebo (n = 19) and curcumin (n = 18). For statistical comparisons, please refer to Table 2
3.6. Inflammation markers and adiponectin
We found no effect of curcumin on any of the measured biomarkers of inflammation (tumour necrosis factor‐alpha, interleukin [IL]‐6, IL‐8, IL‐10, interferon‐gamma, high‐sensitivity C‐reactive protein, YKL‐40) or adiponectin (Table 2). YKL‐40 is a 40 kDa glycoprotein named after its molecular weight and tyrosine (Y), lysine (K) and leucine (L). 26
3.7. Appetite measures, ad libitum meal measures and food and physical activity diaries
Curcumin appeared to decrease the sensation of prospective food intake and comfort, increase the sensation of nausea when comparing AUC with placebo AUC, and decrease the sensation of comfort 10 minutes after the ad libitum meal (Table S3). From data in the diaries, we found that participants in the curcumin group consumed a smaller amount of food and beverages compared with participants in the placebo group. However, none of these findings remained significant after adjusting for multiple testing. We found no effect of curcumin on the participants' experience of hunger, satiety, fullness or thirst, or when calculating composite appetite score. Furthermore, curcumin did not affect VAS scores on palatability sensations, meal duration or ingested kcal during the ad libitum meal. The ingested number of calories, calorie balance and ingested amount of the different constituents of the food and beverages were not affected by curcumin (Table S3).
4. DISCUSSION
Compared with placebo, curcumin did not decrease HFC in obese participants, but it resulted in small reductions in circulating levels of triglyceride, glucose and GGT during fasting, and it reduced prospective food intake and comfort and increased nausea during OGTT and resulted in decreased self‐reported amounts of ingested food and beverages. Except for the difference in GGT, which was driven by an increase in the placebo group, these differences were not statistically significant after correction for multiple comparisons.
Rodent studies have shown that curcumin supplementation can prevent diet‐induced steatosis. 16 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 Only a few rodent studies have examined curcumin as a treatment for established steatosis, also with positive results. 36 , 37 Randomized controlled trials have been carried out showing a decrease in ultrasound‐assessed HFC in patients diagnosed with steatosis when treating with curcumin. 38 , 39 , 40 , 41 , 42 Another study found a reduction in ultrasound‐assessed HFC in the curcumin‐treated group, but not when comparing with placebo. 43 Importantly, ultrasound is inferior to MRS 7 , 8 , 9 and considered sensitive only for HFC above 12.5%, 44 confounding the above‐mentioned results. To our knowledge, the present study is the first to investigate curcumin's effect on MRS‐assessed HFC. Curcumin is here tested in a selected cohort; thus, we cannot rule out that a cohort with steatosis but normal BMI or a cohort with diabetes or a cohort with primarily moderate or severe steatosis would exhibit different outcomes.
Previously, van Werven et al. reported MRS to have acceptable reproducibility when scanning individuals in the fasted state (the coefficient of variance was 9.5% in liver tissue of non‐obese and 4.1% in steatotic liver tissue; the repeatability coefficient was 1.3% in hepatic triglyceride content in both groups 45 ). Recently, Kovar et al. showed that feeding state and composition of the last meal before MRS may affect HFC substantially. 46 We did not measure HFC with MRS in the fasted state, nor did we standardize feeding state or meal composition before the scan, and this might explain the missing reproducibility—especially in the placebo group—in our study. On the contrary, Colgan et al. performed a study where MRS‐assessed HFC did not vary substantially with feeding state, and variation in HFC did not correlate with BMI. 47 Thus, our results reflect daily life variations, but taking the results from Kovar et al. and van Werven et al. into consideration, we cannot exclude that the lack of standardization of feeding state and meal composition prior to MRS in the present study may have contributed to the variation in MRS results and, thus, masked an effect of curcumin. Nevertheless, we performed FibroScan after 10‐12 hours of fasting and CAP values showed no effect of curcumin compared with placebo, supporting our primary finding of no effect of curcumin on MRS‐assessed HFC. In our study, post hoc analysis showed no significant correlation between changes in HFC and changes in variables known to correlate with HFC, that is, BMI, VAT, waist circumference and body fat percentage. The median percentage change for HFC was 6‐ to 15‐fold greater than the percentage change for the other variables (data not shown). Study sample size calculation was carried out before inclusion; however, the substantial individual change in HFC over time might necessitate a bigger sample size to prove if curcumin affects HFC. The sample size in the present study may, thus, be considered a limitation. Our randomization resulted in a higher baseline HFC in the placebo group. However, the difference was not significant, and we found no correlation between baseline HFC and change in HFC over time (data not shown).
We found a significant effect of curcumin on GGT, but this was driven by a significant increase of GGT in the placebo group and a non‐significant decrease in the curcumin group. Other studies have shown small reductions 43 or no effect of curcumin on GGT. 48 , 49 One study has shown a significant decrease in liver stiffness using FibroScan after curcumin treatment; however, when compared with placebo, the significance disappeared. 10 In the present study, no effects of curcumin on liver stiffness (assessed by FibroScan) or FIB4 score were observed.
Preclinical studies have shown that curcumin induces fibroblast growth factor 21 (FGF21) production via the PPAR‐alpha route. 50 , 51 As FGF21 has been shown to have anti‐steatogenic properties, 52 we included circulatory levels of FGF21 as an exploratory endpoint, but it was not affected by curcumin.
A previous study showed that 9 months of curcumin treatment significantly improved glucose tolerance (OGTT, 120 minutes) in overweight or obese prediabetic individuals 53 (an increase of ~0.7 mmol/L in the placebo group vs. a decrease of ~1.4 mmol/L in the curcumin group after 9 months of treatment). A meta‐analysis 14 and several additional studies 42 , 49 , 54 , 55 , 56 have shown curcumin‐associated reductions in fasting plasma glucose in individuals with NAFLD, impaired glucose tolerance or diabetes. By contrast, the present data do not support clinically significant glucometabolic effects of curcumin in obese individuals, as we did not observe any effect on circulating levels of glucose, insulin, C‐peptide, glucagon or GLP‐1 during OGTT, and the small effect on fasting plasma glucose was not deemed clinically relevant, and significance disappeared when we adjusted for multiple comparisons. In line with our findings, no acute effect of oral administration of 6 g of Curcuma longa on plasma glucose levels during OGTT was observed in healthy subjects. 57 Also, we observed an effect of curcumin treatment in this study on HOMA2‐IR, albeit not significant. Other studies have reported a significant decrease. 48 , 53 , 55 These studies investigated curcumin treatment of longer duration and included individuals with higher baseline HOMA‐IR. In the present study, the tendency of curcumin‐induced lowering of HbA1c, driven by a decrease in the curcumin group with no change in the placebo group, disappeared after adjustment for multiple comparisons. It is possible that a longer treatment duration would lead to a significant lowering effect of HbA1c, as seen in other studies. 14 , 40 , 53 , 55 Previous studies have shown small but significant reductions of circulating triglycerides during curcumin treatment. 40 , 55 In the present study, fasting triglyceride levels were reduced by curcumin, but the reduction was not clinically relevant, and statistical significance disappeared after adjustment for multiple comparisons.
We found a small effect of curcumin treatment on food ingestion reported in the diaries, prospective food intake, comfort and nausea levels (AUC), but significance disappeared when adjusting for multiple testing. Other studies have reported mild gastrointestinal side effects of curcumin. 40 , 42 We saw no effect of curcumin on plasma levels of GLP‐1 during OGTT, contrasting to previous preclinical findings of curcumin‐induced secretion of GLP‐1. 58 , 59 , 60 We saw no effects of curcumin on anthropometric measures, confirming negative findings from most, 42 , 43 , 49 , 61 , 62 , 63 but not all, previous human studies. 64 , 65 , 66
Obesity is associated with low‐grade inflammation, which is often considered the culprit of the development of many obesity‐related conditions and co‐morbidities. 67 , 68 Curcumin has been reported to have anti‐inflammatory properties in humans, 16 , 17 , 18 , 19 , 54 and curcumin‐induced elevation of circulating adiponectin has been suggested as a mediator. 69 In the present study, we observed no effect of curcumin on several inflammation biomarkers or adiponectin.
High amounts of Meriva and non‐formulated curcumin have been well‐tolerated in human trials. 70 , 71 The formulation of curcumin in this trial was chosen because of the bioavailability being superior to unformulated curcumin. 72 , 73 We did not measure plasma levels of curcumin in our study. Another study found a significant reduction in HFC after 8 weeks of treatment with Meriva, which was well tolerated. 39 Therefore, we chose 6 weeks of treatment with Merivain double dose. Other formulations with acceptable bioavailability could have been considered, for example, nano‐lipid‐formulations 74 or curcumin derivatives or analogues. 75 An interesting theory is that curcumin exerts its effects by improving intestinal barrier function by increasing the number and quality of tight junctions between epithelial cells, and by decreasing inflammation in the intestinal wall. Supporters of this theory therefore claim that a higher concentration of non‐formulated curcumin is the preferred treatment. 76 , 77 , 78 , 79
In conclusion, we found no effect of curcumin on MRS‐assessed HFC in our cohort (obese individuals with primarily mild steatosis), but we saw a small but statistically significant decrease in GGT. Statistical significance of the small and clinically insignificant reductions in fasting plasma glucose and triglycerides disappeared after correction for multiple comparisons. Thus, this randomized, double‐blind, placebo‐controlled, parallel‐group trial showed no clinically relevant effects of 6 weeks of curcumin treatment on HFC, glucometabolic variables, hormonal responses during OGTT and exploratory metabolic endpoints in obese individuals. Furthermore, previous reported anti‐inflammatory properties of curcumin were not evident. No safety issues related to curcumin treatment were observed.
AUTHOR CONTRIBUTIONS
PHH, JIB, TV and FKK contributed to the study design. PHH, JIB, TV, JJH, EC, MG, JSS and FKK contributed to data interpretation. PHH and KRC recruited participants and acquired data. PHH, JIB and JF performed statistical analyses. PHH and FKK wrote the manuscript and all authors revised and approved the final version of the manuscript.
CONFLICT OF INTEREST
All authors declare that there are no conflicts of interests associated with this manuscript.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14804.
Supporting information
Appendix S1:Supporting Information
Table S1. Baseline‐adjusted effects of curcumin and placebo on blood pressure, heart rate, creatinine and urine concentrations of creatinine and albumin measured in the fasted state. †Baseline values are mean (95% CI) for normally distributed data and geometric mean (95% CI) for skewed data. ‡ Delta (∆) values and ETD are differences in mean (95% CI) for normally distributed data and percentage difference in geometric mean for skewed data. ETD, estimated treatment difference.
Table S2. Baseline‐adjusted effects of curcumin and placebo on body composition data from bioelectrical impedance analysis † Baseline values are mean (95% CI) for normally distributed data and geometric mean (95% CI) for skewed data. ‡ Delta (∆) values and ETD are differences in mean (95% CI) for normally distributed data and percentage difference in geometric mean for skewed data.
Table S3. Baseline‐adjusted effects of curcumin and placebo on data from appetite questionnaires during oral glucose tolerance test and after the ad libitum, data from the ad libitum meal and data from food and physical activity diary. †Baseline values are mean (95% CI) for normally distributed data and geometric mean (95% CI) for skewed data. ‡ Delta (∆) values and ETD are differences in mean (95% CI) for normally distributed data and percentage difference in geometric mean for skewed data. AUC, area under the curve.
ACKNOWLEDGEMENTS
We thank all participants and colleagues in the Center for Clinical Metabolic Research and Steno Diabetes Center Copenhagen, who contributed with help and expertise. Also, a special thanks to Giorgia Centorame, who helped during experimental days and to Julia Sidenius Johansen (Herlev Hospital, University of Copenhagen), who contributed with analysis of YKL‐40. We also thank Indena S.p.A. (Milan, Italy) for sponsoring the study product. The study was sponsored by Augustinus Fonden and A. P. Møller Fonden.
Hellmann PH, Bagger JI, Carlander KR, et al. The effect of curcumin on hepatic fat content in individuals with obesity. Diabetes Obes Metab. 2022;24(11):2192‐2202. doi: 10.1111/dom.14804
Funding information A. P. Møller Fonden; Augustinus Fonden
DATA AVAILABILITY STATEMENT
Data is available on request by contacting corresponding author.
REFERENCES
- 1. Younossi ZM. Non‐alcoholic fatty liver disease ‐ A global public health perspective. J Hepatol. 2019;70(3):531‐544. [DOI] [PubMed] [Google Scholar]
- 2. Pais R, Maurel T. Natural history of NAFLD. J Clin Med. 2021;10(6):1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aller R, Fernández‐Rodríguez C, Lo Iacono O, et al. Consensus document. Management of non‐alcoholic fatty liver disease (NAFLD). Clinical practice guideline. Gastroenterol Hepatol. 2018;41(5):328‐349. [DOI] [PubMed] [Google Scholar]
- 4. Polyzos SA, Kang ES, Boutari C, Rhee EJ, Mantzoros CS. Current and emerging pharmacological options for the treatment of nonalcoholic steatohepatitis. Metabolism. 2020;111S:154203. [DOI] [PubMed] [Google Scholar]
- 5. Cicero AFG, Colletti A, Bellentani S. Nutraceutical approach to non‐alcoholic fatty liver disease (NAFLD): the available clinical evidence. Nutrients. 2018;10(9):1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. White CM, Lee JY. The impact of turmeric or its curcumin extract on nonalcoholic fatty liver disease: a systematic review of clinical trials. Pharm Pract. 2019;17(1):1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Besutti G, Valenti L, Ligabue G, et al. Accuracy of imaging methods for steatohepatitis diagnosis in non‐alcoholic fatty liver disease patients: A systematic review. Liver Int. 2019;39(8):1521‐1534. [DOI] [PubMed] [Google Scholar]
- 8. Wang XM, Zhang XJ, Ma L. Diagnostic performance of magnetic resonance technology in detecting steatosis or fibrosis in patients with nonalcoholic fatty liver disease: A meta‐analysis. Medicine. 2018;97(21):e10605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Makhija N, Vikram NK, Kaur G, et al. Role of magnetic resonance imaging in the monitoring of patients with nonalcoholic fatty liver disease: comparison with ultrasonography, lipid profile, and body mass index. J Clin Exp Hepatol. 2020;10(2):139‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Saadati S, Sadeghi A, Mansour A, et al. Curcumin and inflammation in non‐alcoholic fatty liver disease: a randomized, placebo controlled clinical trial. BMC Gastroenterol. 2019;19(1):133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baziar N, Parohan M. The effects of curcumin supplementation on body mass index, body weight, and waist circumference in patients with nonalcoholic fatty liver disease: A systematic review and dose‐response meta‐analysis of randomized controlled trials. Phytother Res PTR. 2020;34(3):464‐474. [DOI] [PubMed] [Google Scholar]
- 12. Goodarzi R, Sabzian K, Shishehbor F, Mansoori A. Does turmeric/curcumin supplementation improve serum alanine aminotransferase and aspartate aminotransferase levels in patients with nonalcoholic fatty liver disease? A systematic review and meta‐analysis of randomized controlled trials. Phytother Res. 2019;33(3):561‐570. [DOI] [PubMed] [Google Scholar]
- 13. Jalali M, Mahmoodi M, Mosallanezhad Z, Jalali R, Imanieh MH, Moosavian SP. The effects of curcumin supplementation on liver function, metabolic profile and body composition in patients with non‐alcoholic fatty liver disease: A systematic review and meta‐analysis of randomized controlled trials. Complement Ther Med. 2020;48:102283. [DOI] [PubMed] [Google Scholar]
- 14. de Melo ISV, Dos Santos AF, Bueno NB. Curcumin or combined curcuminoids are effective in lowering the fasting blood glucose concentrations of individuals with dysglycemia: systematic review and meta‐analysis of randomized controlled trials. Pharmacol Res. 2018;128:137‐144. [DOI] [PubMed] [Google Scholar]
- 15. Wei Z, Liu N, Tantai X, et al. The effects of curcumin on the metabolic parameters of non‐alcoholic fatty liver disease: a meta‐analysis of randomized controlled trials. Hepatol Int. 2019;13(3):302‐313. [DOI] [PubMed] [Google Scholar]
- 16. Inzaugarat ME, De Matteo E, Baz P, et al. New evidence for the therapeutic potential of curcumin to treat nonalcoholic fatty liver disease in humans. PLoS One. 2017;12(3):e0172900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Panahi Y, Hosseini MS, Khalili N, et al. Effects of curcumin on serum cytokine concentrations in subjects with metabolic syndrome: apost‐hoc analysis of a randomized controlled trial. Biomed Pharmacother Biomedecine Pharmacother. 2016;82:578‐582. [DOI] [PubMed] [Google Scholar]
- 18. Derosa G, Maffioli P, Simental‐Mendía LE, Bo S, Sahebkar A. Effect of curcumin on circulating interleukin‐6 concentrations: asystematic review and meta‐analysis of randomized controlled trials. Pharmacol Res. 2016;111:394‐404. [DOI] [PubMed] [Google Scholar]
- 19. Ghandadi M, Sahebkar A. Curcumin: an effective inhibitor of Interleukin‐6. Curr Pharm Des. 2017;23(6):921‐931. [DOI] [PubMed] [Google Scholar]
- 20. Bedogni G, Bellentani S, Miglioli L, et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Motamed N, Sohrabi M, Ajdarkosh H, et al. Fatty liver index vs waist circumference for predicting non‐alcoholic fatty liver disease. World J Gastroenterol. 2016;22(10):3023‐3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chabanova E, Bille DS, Thisted E, Holm JC, Thomsen HS. MR spectroscopy of liver in overweight children and adolescents: investigation of 1H T2 relaxation times at 3T. Eur J Radiol. 2012;81(5):811‐814. [DOI] [PubMed] [Google Scholar]
- 23. Belfort R, Harrison SA, Brown K, et al. A placebo‐controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297‐2307. [DOI] [PubMed] [Google Scholar]
- 24. Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. 2nd ed. Hoboken, NJ: John Wiley & Sons; 2011. [Google Scholar]
- 25. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995;57(1):289‐300. [Google Scholar]
- 26. Junker N, Johansen JS, Hansen LT, Lund EL, Kristjansen PEG. Regulation of YKL‐40 expression during genotoxic or microenvironmental stress in human glioblastoma cells. Cancer Sci. 2005;96(3):183‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ding L, Li J, Song B, et al. Curcumin rescues high fat diet‐induced obesity and insulin sensitivity in mice through regulating SREBP pathway. Toxicol Appl Pharmacol. 2016;304:99‐109. [DOI] [PubMed] [Google Scholar]
- 28. Hasan ST, Zingg JM, Kwan P, Noble T, Smith D, Meydani M. Curcumin modulation of high fat diet‐induced atherosclerosis and steatohepatosis in LDL receptor deficient mice. Atherosclerosis. 2014;232(1):40‐51. [DOI] [PubMed] [Google Scholar]
- 29. Liu Y, Cheng F, Luo Y, et al. PEGylated curcumin derivative attenuates hepatic steatosis via CREB/PPAR‐γ/CD36 pathway. Biomed Res Int. 2017;11:8234507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maithilikarpagaselvi N, Sridhar MG, Swaminathan RP, Sripradha R, Badhe B. Curcumin inhibits hyperlipidemia and hepatic fat accumulation in high‐fructose‐fed male Wistar rats. Pharm Biol. 2016;54(12):2857‐2863. [DOI] [PubMed] [Google Scholar]
- 31. Shao W, Yu Z, Chiang Y, et al. Curcumin prevents high fat diet induced insulin resistance and obesity via attenuating lipogenesis in liver and inflammatory pathway in adipocytes. PloS One. 2012;7(1):e28784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yiu WF, Kwan PL, Wong CY, et al. Attenuation of fatty liver and prevention of hypercholesterolemia by extract of Curcuma longa through regulating the expression of CYP7A1, LDL‐receptor, HO‐1, and HMG‐CoA reductase. J Food Sci. 2011;76(3):H80‐H89. [DOI] [PubMed] [Google Scholar]
- 33. Zhao L, Pan Y, Peng K, et al. Inhibition of 11β‐HSD1 by LG13 improves glucose metabolism in type 2 diabetic mice. J Mol Endocrinol. 2015;55(2):119‐131. [DOI] [PubMed] [Google Scholar]
- 34. Yang JW, Yeo HK, Yun JH, Lee JU. Theracurmin (highly bioavailable curcumin) prevents high fat diet‐induced hepatic steatosis development in mice. Toxicol Res. 2019;35(4):403‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee DE, Lee SJ, Kim SJ, Lee HS, Kwon OS. Curcumin ameliorates nonalcoholic fatty liver disease through inhibition of O‐GlcNAcylation. Nutrients. 2019;11(11):1‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pan MH, Chen JW, Kong ZL, Wu JC, Ho CT, Lai CS. Attenuation by Tetrahydrocurcumin of adiposity and hepatic steatosis in mice with high‐fat‐diet‐induced obesity. J Agric Food Chem. 2018;66(48):12685‐12695. [DOI] [PubMed] [Google Scholar]
- 37. Feng WW, Kuang SY, Tu C, et al. Natural products berberine and curcumin exhibited better ameliorative effects on rats with non‐alcohol fatty liver disease than lovastatin. Biomed Pharmacother Biomedecine Pharmacother. 2018;99:325‐333. [DOI] [PubMed] [Google Scholar]
- 38. Panahi Y, Kianpour P, Mohtashami R, Soflaei SS, Sahebkar A. Efficacy of phospholipidated curcumin in nonalcoholic fatty liver disease: a clinical study. J Asian Nat Prod Res. 2019;21(8):798‐805. [DOI] [PubMed] [Google Scholar]
- 39. Panahi Y, Kianpour P, Mohtashami R, Jafari R, Simental‐Mendía LE, Sahebkar A. Efficacy and safety of phytosomal curcumin in non‐alcoholic fatty liver disease: arandomized controlled trial. Drug Res. 2017;67(4):244‐251. [DOI] [PubMed] [Google Scholar]
- 40. Rahmani S, Asgary S, Askari G, et al. Treatment of non‐alcoholic fatty liver disease with curcumin: arandomized placebo‐controlled trial. Phytother Res PTR. 2016;30(9):1540‐1548. [DOI] [PubMed] [Google Scholar]
- 41. Selmanovic S, Beganlic A, Salihefendic N, Ljuca F, Softic A, Smajic E. Therapeutic effects of curcumin on ultrasonic morphological characteristics of liver in patients with metabolic syndrome. Acta Inform Medica. 2017;25(3):169‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jazayeri‐Tehrani SA, Rezayat SM, Mansouri S, et al. Nano‐curcumin improves glucose indices, lipids, inflammation, and Nesfatin in overweight and obese patients with non‐alcoholic fatty liver disease (NAFLD): a double‐blind randomized placebo‐controlled clinical trial. Nutr Metab. 2019;16:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jarhahzadeh M, Alavinejad P, Farsi F, Husain D, Rezazadeh A. The effect of turmeric on lipid profile, malondialdehyde, liver echogenicity and enzymes among patients with nonalcoholic fatty liver disease: a randomized double blind clinical trial. Diabetol Metab Syndr. 2021;13:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bril F, Ortiz‐Lopez C, Lomonaco R, et al. Clinical value of liver ultrasound for the diagnosis of nonalcoholic fatty liver disease in overweight and obese patients. Liver Int. 2015;35(9):2139‐2146. [DOI] [PubMed] [Google Scholar]
- 45. van Werven JR, Hoogduin JM, Nederveen AJ, et al. Reproducibility of 3.0 Tesla magnetic resonance spectroscopy for measuring hepatic fat content. J Magn Reson Imaging. 2009;30(2):444‐448. [DOI] [PubMed] [Google Scholar]
- 46. Kovar J, Dusilova T, Sedivy P, et al. Acute responses of hepatic fat content to consuming fat, glucose and fructose alone and in combination in non‐obese non‐diabetic individuals with non‐alcoholic fatty liver disease. J Physiol Pharmacol. 2021;72(1):45‐53. [DOI] [PubMed] [Google Scholar]
- 47. Colgan TJ, Van Pay AJ, Sharma SD, Mao L, Reeder SB. Diurnal variation of proton density fat fraction in the liver using quantitative chemical shift encoded magnetic resonance imaging. J Magn Reson Imaging. 2020;51(2):407‐414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cicero AFG, Sahebkar A, Fogacci F, Bove M, Giovannini M, Borghi C. Effects of phytosomal curcumin on anthropometric parameters, insulin resistance, cortisolemia and non‐alcoholic fatty liver disease indices: a double‐blind, placebo‐controlled clinical trial. Eur J Nutr. 2020;59(2):477‐483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Funamoto M, Shimizu K, Sunagawa Y, et al. Effects of highly absorbable curcumin in patients with impaired glucose tolerance and non‐insulin‐dependent diabetes mellitus. J Diabetes Res. 2019;7:8208237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tian L, Zeng K, Shao W, et al. Short‐term curcumin gavage sensitizes insulin signaling in dexamethasone‐treated C57BL/6 mice. J Nutr. 2015;145(10):2300‐2307. [DOI] [PubMed] [Google Scholar]
- 51. Zeng K, Tian L, Patel R, et al. Diet polyphenol curcumin stimulates hepatic Fgf21 production and restores its sensitivity in high‐fat‐diet–fed male mice. Endocrinology. 2017;158(2):277‐292. [DOI] [PubMed] [Google Scholar]
- 52. Lin W, Zhang T, Zhou Y, Zheng J, Lin Z. Advances in biological functions and clinical studies of FGF21. Diabetes Metab Syndr Obes Targets Ther. 2021;14:3281‐3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chuengsamarn S, Rattanamongkolgul S, Luechapudiporn R, Phisalaphong C, Jirawatnotai S. Curcumin extract for prevention of type 2 diabetes. Diabetes Care. 2012;35(11):2121‐2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Maithili Karpaga Selvi N, Sridhar MG, Swaminathan RP, Sripradha R. Efficacy of turmeric as adjuvant therapy in type 2 diabetic patients. Indian . J Clin Biochem. 2015;30(2):180‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Na LX, Li Y, Pan HZ, et al. Curcuminoids exert glucose‐lowering effect in type 2 diabetes by decreasing serum free fatty acids: a double‐blind, placebo‐controlled trial. Mol Nutr Food Res. 2013;57(9):1569‐1577. [DOI] [PubMed] [Google Scholar]
- 56. Navekar R, Rafraf M, Ghaffari A, Asghari‐Jafarabadi M, Khoshbaten M. Turmeric supplementation improves serum glucose indices and leptin levels in patients with nonalcoholic fatty liver diseases. J Am Coll Nutr. 2017;36(4):261‐267. [DOI] [PubMed] [Google Scholar]
- 57. Wickenberg J, Ingemansson SL, Hlebowicz J. Effects of Curcuma longa (turmeric) on postprandial plasma glucose and insulin in healthy subjects. Nutr J. 2010;9(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tsuda T. Possible abilities of dietary factors to prevent and treat diabetes via the stimulation of glucagon‐like peptide‐1 secretion. Mol Nutr Food Res. 2015;59(7):1264‐1273. [DOI] [PubMed] [Google Scholar]
- 59. Kato M, Nishikawa S, Ikehata A, et al. Curcumin improves glucose tolerance via stimulation of glucagon‐like peptide‐1 secretion. Mol Nutr Food Res. 2017;61(3):1600471. [DOI] [PubMed] [Google Scholar]
- 60. Alli‐Oluwafuyi AM, Luis PB, Nakashima F, et al. Curcumin induces secretion of glucagon‐like peptide‐1 through an oxidation‐dependent mechanism. Biochimie. 2019;165:250‐257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Saadati S, Hatami B, Yari Z, et al. The effects of curcumin supplementation on liver enzymes, lipid profile, glucose homeostasis, and hepatic steatosis and fibrosis in patients with non‐alcoholic fatty liver disease. Eur J Clin Nutr. 2019;73(3):441‐449. [DOI] [PubMed] [Google Scholar]
- 62. Saraf‐Bank S , Ahmadi A, Paknahad Z, Maracy M, Nourian M. Effects of curcumin on cardiovascular risk factors in obese and overweight adolescent girls: a randomized clinical trial. Sao Paulo Med J Rev Paul Med. 2019;137(5):414‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Moradi Kelardeh B, Rahmati‐Ahmadabad S, Farzanegi P, Helalizadeh M, Azarbayjani MA. Effects of non‐linear resistance training and curcumin supplementation on the liver biochemical markers levels and structure in older women with non‐alcoholic fatty liver disease. J Bodyw Mov Ther. 2020;24(3):154‐160. [DOI] [PubMed] [Google Scholar]
- 64. Mirhafez SR, Rezai A, Dehabeh M, et al. Efficacy of phytosomal curcumin among patients with non‐alcoholic fatty liver disease. Int J Vitam Nutr Res Int Z Vitam‐ Ernahrungsforschung J Int Vitaminol Nutr. 2019;10:1‐9. [DOI] [PubMed] [Google Scholar]
- 65. Panahi Y, Khalili N, Sahebi E, et al. Effects of curcuminoids plus piperine on glycemic, hepatic and inflammatory biomarkers in patients with type 2 diabetes mellitus: arandomized double‐blind placebo‐controlled trial. Drug Res. 2018;68(7):403‐409. [DOI] [PubMed] [Google Scholar]
- 66. DiPierro F, Bressan A, Ranaldi D, Rapacioli G, Giacomelli L, Bertuccioli A. Potential role of bioavailable curcumin in weight loss and omental adipose tissue decrease: preliminary data of a randomized, controlled trial in overweight people with metabolic syndrome. Preliminary study. Eur Rev Med Pharmacol Sci. 2015;19(21):4195‐4202. [PubMed] [Google Scholar]
- 67. Pirola L, Ferraz JC. Role of pro‐ and anti‐inflammatory phenomena in the physiopathology of type 2 diabetes and obesity. World J Biol Chem. 2017;8(2):120‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. 2017;127(1):1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Clark CCT, Ghaedi E, Arab A, Pourmasoumi M, Hadi A. The effect of curcumin supplementation on circulating adiponectin: a systematic review and meta‐analysis of randomized controlled trials. Diabetes Metab Syndr. 2019;13(5):2819‐2825. [DOI] [PubMed] [Google Scholar]
- 70. Lao CD, Ruffin MT, Normolle D, et al. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med. 2006;6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Chainani‐Wu N, Madden E, Lozada‐Nur F, Silverman S. High‐dose curcuminoids are efficacious in the reduction in symptoms and signs of oral lichen planus. J Am Acad Dermatol. 2012;66(5):752‐760. [DOI] [PubMed] [Google Scholar]
- 72. Marczylo TH, Verschoyle RD, Cooke DN, Morazzoni P, Steward WP, Gescher AJ. Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine. Cancer Chemother Pharmacol. 2007;60(2):171‐177. [DOI] [PubMed] [Google Scholar]
- 73. Cuomo J, Appendino G, Dern AS, et al. Comparative absorption of a standardized curcuminoid mixture and its lecithin formulation. J Nat Prod. 2011;74(4):664‐669. [DOI] [PubMed] [Google Scholar]
- 74. Ban C, Jo M, Park YH, et al. Enhancing the oral bioavailability of curcumin using solid lipid nanoparticles. Food Chem. 2020;302:125328. [DOI] [PubMed] [Google Scholar]
- 75. Noureddin SA, El‐Shishtawy RM, Al‐Footy KO. Curcumin analogues and their hybrid molecules as multifunctional drugs. Eur J Med Chem. 2019;182:111631. [DOI] [PubMed] [Google Scholar]
- 76. Feng D, Zou J, Su D, et al. Curcumin prevents high‐fat diet‐induced hepatic steatosis in ApoE−/− mice by improving intestinal barrier function and reducing endotoxin and liver TLR4/NF‐κB inflammation. Nutr Metab. 2019;16:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Burge K, Gunasekaran A, Eckert J, Chaaban H. Curcumin and intestinal inflammatory diseases: molecular mechanisms of protection. Int J Mol Sci. 2019;20(8):1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ghosh S. Curcumin as a potential therapeutic option for NAFLD and other metabolic diseases: need for establishing the underlying mechanism(s) of action. Hepatol Int. 2019;13(3):245‐247. [DOI] [PubMed] [Google Scholar]
- 79. Wang J, Ghosh SS, Ghosh S. Curcumin improves intestinal barrier function: modulation of intracellular signaling, and organization of tight junctions. Am J Physiol Cell Physiol. 2017;312(4):C438‐C445. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1:Supporting Information
Table S1. Baseline‐adjusted effects of curcumin and placebo on blood pressure, heart rate, creatinine and urine concentrations of creatinine and albumin measured in the fasted state. †Baseline values are mean (95% CI) for normally distributed data and geometric mean (95% CI) for skewed data. ‡ Delta (∆) values and ETD are differences in mean (95% CI) for normally distributed data and percentage difference in geometric mean for skewed data. ETD, estimated treatment difference.
Table S2. Baseline‐adjusted effects of curcumin and placebo on body composition data from bioelectrical impedance analysis † Baseline values are mean (95% CI) for normally distributed data and geometric mean (95% CI) for skewed data. ‡ Delta (∆) values and ETD are differences in mean (95% CI) for normally distributed data and percentage difference in geometric mean for skewed data.
Table S3. Baseline‐adjusted effects of curcumin and placebo on data from appetite questionnaires during oral glucose tolerance test and after the ad libitum, data from the ad libitum meal and data from food and physical activity diary. †Baseline values are mean (95% CI) for normally distributed data and geometric mean (95% CI) for skewed data. ‡ Delta (∆) values and ETD are differences in mean (95% CI) for normally distributed data and percentage difference in geometric mean for skewed data. AUC, area under the curve.
Data Availability Statement
Data is available on request by contacting corresponding author.


