Abstract
The HIV‐1 often evades a robust antiretroviral‐mediated immune response, leading to persistent infection within anatomically privileged sites including the CNS. Continuous low‐level infection occurs in the presence of effective antiretroviral therapy (ART) in CD4+ T cells and mononuclear phagocytes (MP; monocytes, macrophages, microglia, and dendritic cells). Within the CNS, productive viral infection is found exclusively in microglia and meningeal, perivascular, and choroidal macrophages. MPs serve as the principal viral CNS reservoir. Animal models have been developed to recapitulate natural human HIV‐1 infection. These include nonhuman primates, humanized mice, EcoHIV, and transgenic rodent models. These models have been used to study disease pathobiology, antiretroviral and immune modulatory agents, viral reservoirs, and eradication strategies. However, each of these models are limited to specific component(s) of human disease. Indeed, HIV‐1 species specificity must drive therapeutic and cure studies. These have been studied in several model systems reflective of latent infections, specifically in MP (myeloid, monocyte, macrophages, microglia, and histiocyte cell) populations. Therefore, additional small animal models that allow productive viral replication to enable viral carriage into the brain and the virus‐susceptible MPs are needed. To this end, this review serves to outline animal models currently available to study myeloid brain reservoirs and highlight areas that are lacking and require future research to more effectively study disease‐specific events that could be useful for viral eradication studies both in and outside the CNS.
Keywords: animal models, brain, CNS, HIV‐1, humanized mice, myeloid reservoir
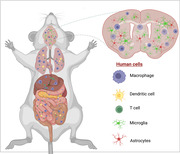
Graphical Abstract
Review on recent rodent models that contain both human myeloid and T cells for future therapeutics and viral elimination investigations in CNS.
Abbreviations
- ART
antiretroviral therapy
- BBB
blood–brain barrier
- BM
bone marrow
- cART
combinatorial antiretroviral therapy
- CSF
cerebrospinal fluid
- HAND
HIV‐associated neurocognitive disorder
- IPSC
induced pluripotent stem cells
- P2Ry12
purinergic receptor P2Y
- QVOA
quantitative viral outgrowth assay
- SAMHD1
Sterile alpha motif and histidine/aspartic acid domain‐containing protein 1
- SIV
simian immunodeficiency virus
- vDNA
viral DNA
- vRNA
viral RNA
1. INTRODUCTION
Following the initiation of combination antiretroviral therapy (cART), HIV‐infected people now lead a life largely free of comorbid conditions. 1 , 2 ART suppresses viral infection in lymphoid, genitourinary, gut, and CNS compartments. Virus commonly remains dormant in CD4+ T and myeloid cells as genome‐integrated, replication‐competent proviral DNA. The dormant virus persists for decades in HIV‐1‐infected regulatory and effector memory CD4+ T cells that have an average half‐life of 44 months 3 independent of ART‐suppressive effects on viral growth. 4 , 5 , 6 The integrated provirus can be reactivated if ART is interrupted, leading to full‐blown HIV infection. Notably, ART fails to eliminate infected cells or excise integrated proviral DNA. What follows is HIV‐1 latency. The latent viral reservoir is the principal barrier to an HIV‐1 cure. Within the CNS, a major therapeutic challenge is the presence of the blood–brain barrier (BBB), which controls entry of immune clearance mechanisms and antiretroviral drugs (ARVs). 7 For the CNS, in particular, virus resides in myeloid cells (perivascular brain macrophages and microglia) continuously throughout the course of disease. This CNS reservoir may account for the case wherein 1 patient's viral load remained undetectable for an extended time period in blood, but over time viral rebound occurred. The virus was detected within the cerebrospinal fluid (CSF), highlighting the critical importance of the CNS reservoir for HIV‐1 elimination. 8 Moreover, HIV‐1 reservoirs including the CNS are established rapidly after initial exposure. In simian immunodeficiency virus (SIV) macaque models, lymphoid tissue and the brain reservoirs have been shown to be established as early as 3 days after infection. 9 , 10 HIV‐1 was detected in the CSF of humans as early as 8 days after infection. This is obvious evidence of viral brain invasion. 11 , 12
HIV‐1 is species specific and infects human CD4 and CCR5 or CXCR4 expressing cells. The latter 2 are the viral coreceptors, which are expressed on the cell surface. 13 Although viral infection targets CD4+ T cells, infection also occurs in myeloid cells that can disseminate virus across tissue sites. 14 , 15 , 16 , 17 However, the most studied latent HIV‐1 reservoir is in resting CD4+ T‐cells. 3 , 18 Within the CNS, microglia and perivascular macrophages are the principal viral target cells that express low levels of CD4. 19 Multiple HIV‐1 variants exist through the body, and those who utilize the CCR5 coreceptor are stated as macrophage‐tropic. 20 Strain evolution and viral compartmentalization support the notion that HIV‐1 can replicate in the CNS independently of the rest of the body. 21 Viruses isolated from brain are predominantly macrophage‐tropic (M‐tropic) as compared with those obtained from the lymph nodes of the same patient. 22 The former demonstrates myeloid (perivascular macrophages and microglial) infection with low levels of CD4 and CCR5 expression. 23 , 24 Sequencing of M‐tropic viral strains show strong HIV‐1gp120 affinity to CD4. 25 HIV‐1 enters the CNS and quickly establishes a myeloid reservoir. 26 , 27
2. CNS CELL TYPES AS HIV RESERVOIRS
Myeloid HIV‐1 infection is associated with the clinical manifestations of HIV‐associated neurocognitive disorders (HAND). 26 , 28 Microglia are primary CNS MPs, and together with perivascular, meningeal, and choroid plexus brain macrophages are permissive to viral infection and serve as the primary viral reservoir. The descriptions of HIV‐1‐MP interactions have been focused on primary culture and postmortem brain studies. Such investigations may not accurately reflect human viral progression. 26 , 29 Hence, there is a need for relevant animal models to study viral neuropathogenesis.
Most myeloid cells originate in the bone marrow (BM) and yolk sac. These cells are replenished in the brain through circulating monocytes that enter the brain then differentiate into macrophages. 30 Virus–MP interactions do not lead to cytopathicity but produce abundant virus without inducing cell death. 31 Infected microglia–macrophages can live upto 20 years in the human brain and therefore contribute to a long‐lived reservoir. 32 In order to develop therapeutics and viral cure strategies, disease‐relevant animal models that can reflect human CNS infection are required and is addressed in this review.
3. DEFINING THE HIV CNS RESERVOIR
A majority of HIV‐1‐infected persons, during the course of disease, show signs and symptoms of cognitive impairment. This is seen despite effective ART and associated maximal viral suppression. 33 , 34 Minor or asymptomatic cognitive impairment are a multifactorial complication that is not simply reflective of viral infection but due to secondary factors such as neuroinflammation, nutritional deficiencies, social and environmental factors, drug toxicity including ARVs themselves, metabolic dysregulation, mitochondrial dysfunction, and substance use disorders. 35 Even with the current ART, which readily achieves sustained viral suppression, low‐level productive CNS infection contributes to disease, viral resistance, 26 , 35 and cognitive impairments. 36 , 37 , 38 Low‐level viral infection or proteins released from HIV‐infected cells can cause changes in cell and tissue homeostasis during ART. A charter of over 1500 individuals living with HIV showed that over 50% show signs and symptoms of neurocognitive impairment during ART. 39 In another group of 200 patients with undetectable viral loads for over 3 years, 27% had evidence of reduced mental fitness. 40 Whether these signs and symptoms can be reduced by improved ART remains to be established. The findings support the notion that low‐level viral infection or viral latency can by themselves contribute to cognitive impairments recorded during sustained viral infection.
Human studies of HIV‐1 latency within the CNS have proven more difficult because of the historical limitations for the use of autopsy samples and the reliance on cell‐based data sets. Alternative measures to study HIV‐1 CNS disease have relied on examinations of CSF. 41 These studies demonstrated that CSF viral RNA show divergent origins from blood and can be recorded even when virus is undetectable in blood. 42 Notably, in a study of 69 patients who were virally suppressed on ART with undetectable virus in blood, 10% had detectable virus in their CSF. 43 A replicate study of 5 individuals who died of HIV‐1 encephalitis demonstrated virus in MPs by laser microdissection and immunohistochemistry. The study also found PCR detected integrated HIV‐1. 44
Autopsy samples from patients that were virally suppressed at the time of death show HIV‐infected MPs in brain. However, most of these studies demonstrated active viral replication at the time of autopsy. 45 More recent studies from the National Neurological AIDS Bank that included 87 autopsied brain tissue samples with low or undetectable viral loads at death identified viral DNA in brain. This was identified by digital droplet PCR (ddPCR), which detected HIV DNA in 48 of the 87 brain samples. 46 A replicate study completed by the NeuroAIDS Tissue Consortium identified 16 samples from patients with undetectable plasma viral load at autopsy used DNA and RNAscope techniques to demonstrate proviral DNA in brain MPs. No viral DNA was found in astrocytes. A subset of brain tissues did demonstrate viral RNA in select brain subregions with undetectable plasma viral loads. 47 This finding supports the notion that latent HIV‐1 does exist in the brain. HIV DNA and RNA are easily detected in brains of infected persons with histories of neurocognitive impairment prior to death. Both latent (viral DNA but no RNA) and active (viral DNA and RNA) groups experienced cognitive deficits. Notably, cognitive impairments were more pronounced with active viral infection. 48 Nonetheless, the latent reservoir is thus still associated with cognitive deficits. The HIV‐1 CNS reservoir is established before signs and symptoms of HAND. In a study of 5 HIV‐1‐infected person who died of comorbid events not related to HIV‐1 infection, HIV DNA was found in brain MPs by laser dissection microscopy and PCR. 49 Most studies examine tissues from individuals infected with HIV‐1 subtype B; however, HIV‐1 subtype C is the most prevalent among people living with HIV. A study examined tissues collected from autopsy samples from Zambians infected with HIV subtype C. Three out of 4 virally suppressed subjects had detectable viral DNA and RNA in the brain as detected by ddPCR and RNA and DNAscope. Surprisingly, only 1 of 4 individuals with detectable viral loads had proviral DNA and viral RNA in brain. The HIV‐1‐infected cells in brain were found to be CD68+, indicating they were likely perivascular macrophages and microglia. 50
The BBB controls the passage of cells from the periphery to the brain. Nonetheless, activated CD14+ and CD16+ monocytes enter the brain by migrating across the BBB. Through these monocyte/macrophages and CD4+ T cells, infection can spread to other cells in the brain. These infected cells display a preference to move across the BBB compared with uninfected counterparts. 12 This cell migration helps to establish the CNS viral reservoir.
It is important to examine the mechanisms that allow MPs to become long‐lived reservoirs in the CNS. T‐cells infected with HIV experience high levels of apoptosis, or cell death, which is the principal cause of AIDS with the progression of HIV infection. 51 However, there is evidence that HIV prevents apoptosis when it comes to microglia and macrophages. Lower levels of apoptotic markers were seen in HIV‐infected myeloid cells in the brain than in uninfected cells. 51 Sections of brains from HIV+ encephalitis subjects and those from uninfected subjects were examined for the apoptotic factor TUNEL in the cerebral white matter. The percentage of TUNEL+ macrophage cells did not differ between the control and the HIV+ encephalitis samples. If the cells were not resistant to apoptosis, it would be expected that the HIV+ samples would have much higher levels of TUNEL+ macrophage and microglial cells. 51 The patient samples analyzed for this study were never under ART treatment. This result implies that macrophages and microglia are resistant to apoptosis and maintain myeloid cells as reservoirs in the CNS.
In cultured microglial cells, following a period of apoptosis after HIV infection, a subset of the cells survived. Most of these surviving cells were HIV infected, indicating that infection with HIV induces a survival mechanism in a subset of microglial cells. 31 A similar result was detected in cultured macrophages, and it was seen that in the surviving macrophages the protein Bim was up‐regulated as compared to controls, leading the authors to believe this protein may have an anti‐apoptotic effect. 31
The ability of macrophages to support low levels of viral infection and become resistant to death, which is typically induced by HIV, makes them a potent viral tissue reservoir. There are several mechanisms by which these cells can become such potent viral reservoirs. The long noncoding RNA SAF plays a role in preventing the apoptosis of infected macrophages, and if SAF is down‐regulated the apoptotic factor caspase‐3/7 was induced in HIV‐infected cells. 52 M‐CSF also enhances the survival of macrophages in the CNS and makes these cells more susceptible to viral infection by HIV. 53 M‐CSF promotes viral replication, and individuals infected with HIV have higher levels of M‐CSF in their CSF. M‐CSF was primarily found in perivascular macrophages and in macrophages present in nodules, which are also the areas of the brain that harbor the most viral reservoir. The Nef gene in HIV codes for a protein that promotes myeloid cell growth. Cell lines treated with Nef had enhanced HIV replication, and strains of relatively non‐aggressive and not progressive HIV isolated from patients were shown to have defective Nef coding regions. Nef did not appear to reduce apoptosis in myeloid cell lines, rather it stimulated greater growth through a cytokine independent pathway. 54 The transcription factor NF‐Kappa‐B also plays a role in preventing apoptosis of HIV‐infected myeloid cells. When NF‐Kappa‐B was stimulated, myeloid cell lines were protected from TNF‐alpha induced apoptosis, and restriction of NF‐Kappa‐B led to higher levels of apoptosis in infected cells. Interestingly, in non‐myeloid cell lines, NF‐Kappa‐B activation had varied effects, in some cell types protecting HIV‐infected cells from apoptosis, and in others having the opposite effect and stimulating apoptosis. 55
Macrophages throughout the body do have mechanisms to control for retroviral infections, the major restriction factor being sterile alpha motif and histidine/aspartic acid domain‐containing protein 1 (SAMHD1). 56 A study characterized monocyte‐derived macrophages and macrophages taken from the brain, lungs, and abdomen and found that only 3% of these cells allowed HIV infection under normal levels of SAMHD1. When SAMHD1 was knocked out, the level of infection increased 12 times in the cell cultures. Notably, in brain‐derived microglia, there was a 50% HIV infection rate before the knockout of SAMHD1 and complete infection after the knockout. This indicates that microglia are highly susceptible to HIV‐1 infection. 57
While these human studies have been important in understanding the effects of HIV on the brain, the inability to perform ethical in vivo experiments necessitates the use of animal models. Due to lack of access to samples and other concerns as described in Table 1 , primate models have been employed to study myeloid reservoirs of HIV, which is discussed in‐depth in the next section.
TABLE 1.
Models for the study of CNS latent reservoirs
| Human clinical samples | Macaque models | Rodent models |
|---|---|---|
|
Advantages
|
Advantages
|
Advantages
|
|
Limitations
|
Limitations
|
Limitations
|
4. MACAQUE MODELS
HIV‐1 is highly host‐specific and can only infect human hosts; however, it is not possible to conduct in vivo mechanistic and cell‐based studies in humans due to ethical concerns, so animal models are vitally important for studying HIV latency and its mechanism of establishment. Rhesus macaque models of SIV infection are the most prevalent animal models used to study HIV, as SIV is closely related to HIV and displays similar mechanisms and disease progression. Macaques infected with SIV have high viral loads in their plasma and CSF, and often they develop SIV encephalitis. 58
Macaques have a level of SIV infection comparable to that seen in HIV‐infected patients who develop AIDS and show evidence of neurocognitive symptoms such as SIV encephalitis, and an increased rate of macrophage recruitment and accumulation in the brain tissue. 30 In contrast, animals that had lower levels of infection and no evidence of SIV encephalitis had lower levels of monocyte activation and macrophage accumulation in the brain. 59 Macaques can also be treated with ART similar or identical to those used to treat HIV infection. SIV‐infected macaques show a significant decline in the viral RNA in the CSF after ART treatment. However, there was still significant viral DNA present in the CNS. In addition, some of the infected animals still showed high levels of inflammation in the CNS. 60
In another macaque study, Avalos et al. 61 demonstrated that not only the brains of SIV‐infected macaques contain latently infected macrophages, but the virus in those macrophages was replication competent. This was accomplished using a quantitative viral outgrowth assay (QVOA) to demonstrate that over 85% of macaques that had been virally suppressed still harbored CNS macrophages with replication‐competent virus. 61 The authors posit that while SIV does not actively spread while using ART, the latent reservoir in the brain could periodically reactivate, thus leading to persistent inflammation. The same group later developed a QVOA specifically for brain MP. In macaques that were virally suppressed for over a year, all the animals possessed latently infected macrophages in the brain. These viruses were also shown to be replication‐competent and were able to infect CD4 cells, as shown using a separate assay. 62
Gama et al. 63 infected macaques with SIV and then maintained an ART regime for 500 days to maintain viral suppression. This group has previously shown that macaques infected with SIV developed SIV‐related neurocognitive issues such as encephalitis within 3 months, and that ART treatment was able to reduce viral loads to an undetectable level similar to ART suppressed HIV‐1‐infected patients. After long‐term maintenance of viral suppression, the macaques were then treated with 2 different latency‐reversing agents. After sacrifice, all the tissues from the macaques were analyzed for the presence of the virus using PCR, in situ hybridization, and phylogenetic genotyping. Despite the ongoing ART treatment, the authors observed that 1 out of the 2 animals had detectable viral load, and viral RNA in the CSF. The authors also detected the reactivated latent virus in the brain tissue. 63 Increased immune activation markers were found in the brain of the macaques that showed a rebound of SIV infection. Evidence of increased neuronal damage was observed in the same macaque brains. 46 Interestingly, the phylogenetic analysis of the viral RNA showed that the variant detected in the CSF was distinct from that detected in the plasma, which indicates that the CNS served as a genetically distinct viral reservoir. 46
Simian HIV (SHIV) is a chimeric virus that combines SIV and HIV, where the virus is primarily of SIV backbone, but contains the HIV envelope gene, which facilitates viral entry and infection. SHIV macaques have shown similar disease progression to HIV infection in humans, and infected cells were detected in the meninges of the brain. 64 The inflammation in the CNS was shown to be associated with T‐cell activation but was not strongly related to myeloid cells. 64 When archival tissues were examined from rhesus macaques infected with SHIV, 10 out of 14 had viral presence in the macrophages of the brain; however, in pigtailed macaques, 21 of 22 showed no evidence of SHIV replication in the brain. 65 In another study, when rhesus macaques were infected with SHIV, detectable SHIV DNA was found in the brains of the monkeys. 66 SHIV has been shown to invade the CNS quickly, as a study of juvenile macaques showed SHIV DNA in the cerebellum 24 h after infection. 67 Rhesus macaques were infected with SHIV and then treated with ART until all animals had an undetectable viral load. There was no detectable SHIV RNA in the CNS following necropsy; however, there were trace amounts of SHIV DNA in certain regions, primarily the spinal cord. 68 These viral DNA levels were much lower than those seen in other tissues such as the spleen or GI tract. This indicates that the brain does harbor a viral SHIV reservoir; however, the size and nature of that reservoir is unknown.
In another study, macaques were infected with SHIV as infants and then subjected to long‐term ART, and at the time of necropsy, 2 out of 6 macaques had low levels of SHIV DNA detected in their brain myeloid cells. 69 In contrast, another study of 9 SHIV‐infected rhesus macaques showed that after ART interruption none of the animals had detectable SHIV‐RNA in the CSF or in the brain tissue despite having high viral loads before the initiation of ART. 70 Colonna et al., 71 attempted to recapitulate the results from the HIV‐infected Berlin patient cured of HIV, used BM transplants on SHIV‐infected macaques; however, this did not eliminate the viral reservoir, which persisted in many tissues including the brain. These results indicate that SHIV might not be the best model for studying latency in the CNS as the results surrounding CNS infection and reservoirs are unevenly distributed. While these studies have shown that there is a viral reservoir in the brain in SHIV models, these studies are rare and do not often focus on the CNS. Macrophages have been shown to be the principal reservoir in SHIV studies in other tissue compartments such as the liver, kidney, spleen, and other tissues; however, not much analysis was performed to look at the CNS reservoir and latency. 72
Primate SIV/SHIV models are useful systems to reflect HIV infection, as SIV and HIV have similar mode of infection pattern; however, it is important to establish animal models that can look at the HIV virus directly. There are several deficits in the primate model studies (as described in Table 1), and that have shifted the focus on small animal models to study HIV reservoirs in the CNS compartment.
5. RODENT NEUROHIV MODELS
While SIV macaque models are the most well‐publicized animal models to study HIV, there are some differences between SIV and HIV that make certain CNS studies difficult to translate. 73 , 74 , 75 SIV contains Vpx, which is known to reduce a myeloid cell restriction factor, which subsequently leads to higher levels of infection in myeloid cells than would be seen in HIV studies. 76 Humans and monkeys also have different innate immune factors that control the response to HIV and SIV infection. 77 There are other limitations associated with nonhuman primate models; macaques are expensive to maintain, they are not always readily available for experiments, and most importantly, there are genetic limitations due to the dissimilarities between SIV and HIV. These limitations highlight the need for small animal models of HIV as human counterparts to study the CNS‐related complications and latent reservoirs.
As HIV‐1 is human‐specific, the only model that can recapitulate HIV disease progression in small animals is humanized mice. These models are developed on severe immunodeficiency background and require genetic and cytokine support for the successful engraftment of human cells. Multiple knockouts (scid mutation, common cytokine receptor gamma chain, recombination activating gene (Rag‐1 and −2), Flt3 ligand, CD47, H2, Ia) have been used, alone or in combination, with only varying success. 78 , 79 , 80 These models were advanced through transgenic expression of human cytokines and growth factors (M‐CSFs, IL‐3, IL‐6, GM‐CSF, and thrombopoietin, stem cell factor, HLA‐A2, ‐DR4) as well as MHC molecules. Currently, the most commonly used mouse models are either immunodeficient (NSG/NOG) mice engrafted with a functional human immune system (BM, liver, thymus) 81 or mice in which the coding region of HIV‐1gp120 is substituted with that of gp80 from ecotropic murine leukemia virus, a retrovirus that infects only rodents. 82
An ideal small animal model to study HIV‐1 pathogenesis and the cell types responsible for carrying the latent virus in the brain could accurately represent an infection in the human brain. To date, humanized mice with a human hematolymphoid system have been used to study HIV persistence in the brain and its associated neuropathology. 83 , 84 , 85 , 86 , 87 Immune deficient mice that are engrafted with a human immune system can reflect HIV‐associated brain diseases seen in humans. 84 , 87 , 88 , 89 A model known as HIV encephalitis mice was created by injecting HIV‐infected monocyte‐derived macrophages into the basal ganglia of humanized mice, and this model showed many of the hallmark signs of HIV infection in the brain, such as neuronal death, neurocognitive impairment, and the activation of microglia. 60 In another NSG‐humanized mouse model, behavioral changes were observed after HIV infection, which also displayed neuronal damage as measured using magnetic resonance spectroscopy and validated using immunofluorescence. 63 In another study, HIV infection in humanized mice led to human cells entering the brain at a higher rate, and there was detectable HIV RNA in the brain compartment. 62
In NSG‐humanized mice, HIV is not detected in the brain until 14–28 days after infection. 90 In rodent models, macrophages only have a turnover rate between 2 and 8%, which indicates that the majority of the macrophages are only replaced every few months, and some cells can persist for years, which contributes to the long‐lasting viral reservoir. 30 Humanized mice were also recently used to test a new assay to recover the replication‐competent viral reservoir from the brain. PCR can overestimate the number of viruses because inactive viruses can still be detected and sequenced, while QVOAs can underestimate the reservoir or fail to identify the virus in very small reservoirs. The brain cells of humanized mice infected with HIV and kept on ART for 3 months were adoptively transferred into uninfected humanized mice, and these mice showed viral establishment. 91 This assay shows that the cells from virally suppressed mouse brains harbor replication‐competent HIV. Under ideal conditions, up to 10% of the microglia cells in the brains of these NSG humanized mice were human, and they supported minimal HIV infection. These cells were isolated from the brains of the mice and treated with HIV latency reversing chemicals, wherein there was a sharp increase in the amount of HIV released from the cells. This demonstrates that the infected microglia in humanized mice constitute a functional reservoir. 76 An ideal model to study HIV‐1 infection in the CNS and HAND requires the repopulation of a murine brain with functional human microglial cells. Although these models could recapitulate HIV‐1 infection in the lining of meninges and perivascular areas, they lack the ability to reconstitute human microglia, a potent brain viral reservoir.
Previous studies have questioned if myeloid cells can serve as a source of the HIV, or if they simply ingest T cells that already harbor the virus. 92 However, humanized mice studies have disproven the ingestion hypothesis and shown that myeloid cells alone are able to harbor HIV without the presence of T cells. A team created “myeloid only mice” that did not harbor any human T cells by reconstituting mice with CD34+ stem cells to establish human B cells and myeloid cells, but no T cells. 93 The researchers found replication‐competent vDNA in the brain as well as in other body compartments. When they transferred cells from the initially infected mice to uninfected mice, the transfer conveyed HIV infection. This result confirms that myeloid cells alone can sustain HIV infection without engulfing any infected T cells.
The lack of models to repopulate microglia could be due to deficits in species‐specific cytokine support. 94 Major factors that contribute to microglial development are the CSF‐1 and IL‐34 ligands for CSF1 receptor (CSF1R) that function distinctively. 95 CSF1 contributes to the differentiation of BM‐derived progenitor cells from monocytes to tissue macrophages and dendritic cells, 96 while IL‐34 affects the development of microglia and Langerhans cells. 96 , 97 , 98 , 99 Some studies using transgenic or knock‐in mouse strains expressing human CSF‐1 failed to support spontaneous microglial development. 100 , 101 As it is difficult to obtain human microglia from tissues, induced pluripotent stem cells (iPSCs) have been examined as a productive avenue to obtain large quantities of microglia. A team developed a methodology to generate microglial cells from pluripotent stem cells. These induced microglial cells showed the identical protein profiles as microglia in vivo and also displayed a similar receptor expression profile. 102 Importantly, these induced microglial cells were distinct from other myeloid cell lineages and grouped most closely with adult and fetal microglia when subjected to functional and transcriptomic analyses. These induced microglia also displayed cytokine release in response to stress similarly to microglia in vivo. When these cells were transplanted into mice, they displayed properties consistent with normal microglial development, such as developing branches/processes, phagocytizing amyloid plaques, and responding differently in different areas of the brain. These results indicate that induced microglia developed from pluripotent stem cells could serve as an effective model for CNS disease within small rodents. Though this model may be suitable to study CNS‐related complications, it lacks the peripheral human immune system, which is vital to the study of HIV disease progression and pathogenesis.
To address this, Mathews et al. 103 overexpressed human IL‐34 (hIL‐34) in immunodeficient NOG mice under the CMV promoter that successfully supported the development of brain microglia‐like human macrophages derived from human CD34+ hematopoietic stem cells (HSCs). These microglial‐like cells expressed canonical microglia cell markers like Purinergic Receptor P2Y (P2Ry12), Triggering Receptor Expressed on Myeloid Cells 2, CX3CR1, cluster differentiation 11b, ionized calcium‐binding adaptor molecule 1, and CSF‐1R. A robust viral replication within the human microglia was achieved within 3 weeks of peripheral infection. HIV‐1 infection effectively induced human‐cell‐specific molecular changes such as immune activation, antiviral defense, and neuroinflammation. 103 This model readily characterized previously reported pathologic events of an infected human host. 104 , 105 , 106 These outcomes suggest that this approach serves as a promising new model of HIV‐1 persistence in the brain and better represents HAND progression. The ability and potential to use iPSC and stem cell therapies in humanized mice is also important and could be vital for future CNS‐related studies and its therapeutic targeting.
To look at the effect of HIV on brain cells, many researchers utilize microglial or astrocytic or neuronal cell lines; however, these isolated cell lines cannot capture the true nature of virus interactions in the CNS. To overcome this problem, recently organoids have been employed for CNS studies, as organoids were thought to better represent the functional features of a brain as compared to the cell lines. 107 Organoids are generated from stem cells that either spontaneously differentiate or are guided to differentiate by using specific signaling molecules. 107 , 108 , 109 Microglia originated from organoids are susceptible to HIV‐infection, but they need much longer exposure time as compared to cocultured cells. 107 Only microglial cells originating from cerebral organoids have been shown to be productively infected with HIV by using CCR5 coreceptor. 110 Neurons, microglia, and astrocytes isolated from human organoids were found to be infected with HIV and to secrete neuroinflammatory markers such as TNFα and IL1β 111 and thus can be used for studies targeting CNS‐related inflammation and therapeutics. More future research work is needed to look at latency using brain organoids.
6. CONCLUSIONS AND FUTURE DIRECTIONS
As of today, there are 3 widely used immunodeficient strains for HIV research. They are NOD.Cg‐PrkdcscidIl2rgtm1Wjl (NSG), NODShi.Cg‐PrkdcscidIl2rgtm1Sug (NOG), and C;129S4‐ Rag2tm1FlvIl2rgtm1Flv (commonly referred to as BALB/c‐Rag2null IL2rgnull mice or BRG) mice. 112 , 113 , 114 NSG and BRG mice lack the gamma chain (γc), whereas NOG mice have a truncated cytoplasmic domain of γc, but lacks the signaling domain. Using these backgrounds, 4 mice models are deployed to study either short‐term or long‐term HIV infection‐related peripheral and CNS complications. The human peripheral blood leukocyte (PBL) severe immune deficiency model (Hu‐PBL‐SCID) allows transient studies of human T cell function because of the development of xenogeneic graft‐versus‐host disease (GVHD). 115 The BM/liver/thymus model have all lineages of human hematopoietic cells and support a robust mucosal immune system; however, they have 2 major drawbacks: the development of GVHD‐like reactions during long‐term studies, 112 , 116 , 117 and difficulties and ethical regulations in obtaining fetal cells of human origin to generate this model. The third model is generated through the injection of human CD34 + HSCs derived from BM, umbilical cord blood, fetal liver, or G‐CSF‐mobilized peripheral blood. This model possesses BM‐generated T cells, B cells, APCs, and myeloid cells; however, these cells are found at low levels. The human T cells mature in mouse thymus and are H2 type, not HLA restricted. 118 The fourth model NSG is generated by intrahepatic injection of human CD34+ HSCs derived from human cord blood. 119 This model possesses a complete human immune system and supports long‐term HIV and latency‐related studies 120 ; however, it has 2 limitations in that the human T cells mature in murine thymus and have functionally underdeveloped lymphatic tissues. 121
While most of the current rodent models can be used to study HIV‐1 biology (viral entry, replication, and spread) after natural infection, they do not permit research on other key elements of HIV infection and AIDS, such as sustained levels of viral growth, specific antiretroviral immune responses, virus‐induced immunopathology (CD4+ T‐cell depletion), mucosal inflammation, and cellular viral tropism and latency establishment in CNS. Therefore, next‐generation mouse models that more effectively recapitulate human physiology and immunology are needed to test vaccines and immunotherapeutic approaches to prevent disease and virus transmission, and molecular approaches to achieve a cure by eliminating latent virus from host cells. Ideally, these new models would co‐express humanized genes for the primary HIV‐1 receptor (CD4), the common coreceptor in initial HIV‐1 infection (CCR5), and a glycine substitution for aspartic acid at position 106 of the mouse ortholog (C1qbp) of the human gene (C1QBP [synonyms: P32, HABP1]) that promotes viral genomic transcripts and structural proteins, resulting in assembly and release of infectious virions (unpublished data). There are several benefits of creating a triple polygenic humanized mouse (hCD4/hCCR5/hC1qbp[D106G]), which can enable testing of clinically relevant combination ARV regimens for efficacy of viral suppression while evaluating viral reservoirs, and assessing viral rebound after therapeutic cessation; thus, enabling studies of HIV‐1 pathogenesis and viral persistence in relationship to end‐organ disease including the CNS.
It is clear from the above discussion that animal models with macrophages, microglia, and astrocytes of human origin in the brain are needed to study CNS latency. Because macaques cannot be infected with HIV, macaque models are less suitable for this long‐term goal. It is likely that future studies will further explore the role of myeloid cells in HIV‐induced neuropathology and behavioral deficits in HIV‐infected and cART suppressed mice as a method to better understand the HIV‐1 pathogenesis in humans and develop strategies for therapy. The IL‐34 microglia mouse can be used to test new generations of drug delivery systems with improved CNS bioavailability. This model can also help to develop better HIV cure strategies targeting specific brain cell types. However, the humanized microglia mouse model has some caveats to be considered. Although the model attained engraftment of immune cells and microglia, it lacks another potential restricted viral reservoir – astrocytes and features shown in Table 1.
Astrocytes, the other major glial cells in the brain, maintain brain homeostasis among many other functions; however, their role as a CNS reservoir is controversial. It was initially thought that astrocytes could not harbor any replication‐competent HIV virus in the brain as they do not express CD4 receptors. 122 More recent evidence suggests that astrocytes become infected through a CD4 independent mechanism, though at much lower levels than microglial cells. 123 Except a few studies (in vitro, autopsy samples and SIV studies) 44 , 124 , 125 , 126 , 127 limited work has been published examining the astrocyte's role as a latent reservoir in animal models. The support for the role of astrocytes during HIV infection came from Lutgen et al., 128 who demonstrated that astrocytes could initiate the spread of HIV‐infection through T cells and spread the disease from CNS to periphery. However, in this model, the authors first infected fetal astrocytes in vitro using VSV‐pseudo‐typed HIV and then transplanted them into brain of humanized mice, so it is not a suitable model to study latency. Previously, our group generated a humanized mouse model reconstituted with human astrocytes and human leukocytes by transplanting human neuro‐progenitor cells in the brain and HSC in the liver simultaneously in immunodeficient neonates. These mice exhibited human astrocyte‐specific antiviral responses toward systemic HIV infection and neuronal damage. 86 Overall, the role of astrocytes as latent reservoir needs more attention through suitable animal model systems of human origin.
To broadly study the CNS latency in humanized mice, the presence of both astrocytes and microglia are needed in the mouse brain. Repopulating the murine brain with functional human macrophages, astrocytes, and microglia cells can open new avenues to study HIV‐induced inflammation, neuropathogenesis, latent viral reservoirs, and myeloid cells carrying latent virus, and subsequent targeting to eliminate them in a single system.
AUTHORSHIP
P. K. D. conceived the idea. E. M. W. and P. K. D. cowrote the paper, organized the presentations, prepared the table, arranged the references, and edited the manuscript. C. Z., S. M., B. K., K. C. K. L., H. G., S. G., and L. P. cowrote and edited the manuscript. B. K. prepared the figure. All authors have read and approved the final version of the paper.
DISCLOSURE
Dr. Howard Gendelman is a cofounder of Exavir Therapeutics, Inc., a biotechnology start‐up company devoted exclusively for developing long‐acting antiretroviral medicines and HIV‐1 cure strategies. All other authors have no competing interest.
ACKNOWLEDGMENTS
This work was supported by the National Institute of Health (NIH) grant R01MH121402. The funders have no role in the preparation of the article.
Waight E, Zhang C, Mathews S, et al. Animal models for studies of HIV‐1 brain reservoirs. J Leukoc Biol. 2022;112:1285–1295. 10.1002/JLB.5VMR0322-161R
Summary Statement: Pathobiology, biomarkers, and therapeutic studies of HIV‐1 are limited by the lack of ideal human reflective rodent model system. Prior works have focused on employing transgenic, HIV‐1 system viruses or repopulation of murine immune organs with functional human leukocytes in such studies. Though the current available models allowed investigations of viral reservoirs that included lymphoid tissues and the CNS, most of them lacked cells of human origin to support precise measures of viral latency. With a focus on humanization of peripheral, lymphoid, and CNS tissue compartments, we discuss recent rodent models that contain both human myeloid and T cells for future therapeutics and viral elimination investigations.
REFERENCES
- 1. Volberding PA, Deeks SG. Antiretroviral therapy and management of HIV infection. Lancet. 2010;376:49‐62. [DOI] [PubMed] [Google Scholar]
- 2. Cahn P, Madero JS, Arribas JR, et al. Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral‐naive adults with HIV‐1 infection (GEMINI‐1 and GEMINI‐2): week 48 results from two multicentre, double‐blind, randomised, non‐inferiority, phase 3 trials. Lancet. 2019;393:143‐155. [DOI] [PubMed] [Google Scholar]
- 3. Siliciano JD, Kajdas J, Finzi D, et al. Long‐term follow‐up studies confirm the stability of the latent reservoir for HIV‐1 in resting CD4+ T cells. Nat Med. 2003;9:727‐8. [DOI] [PubMed] [Google Scholar]
- 4. Chun TW, Stuyver L, Mizell SB, et al. Presence of an inducible HIV‐1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci USA. 1997;94:13193‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lorenzo‐Redondo R, Fryer HR, Bedford T, et al. Persistent HIV‐1 replication maintains the tissue reservoir during therapy. Nature. 2016;530:51‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Deeks SG, Lewin SR, Ross AL, et al. Nature medicine. 2016;22:839‐50. International AIDS Society global scientific strategy: towards an HIV cure 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Osborne O, Peyravian N, Nair M, Daunert S, Toborek M. The paradox of HIV blood‐brain barrier penetrance and antiretroviral drug delivery deficiencies. Trends Neurosci. 2020;43:695‐708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Henrich TJ, Hanhauser E, Marty FM, et al. Antiretroviral‐free HIV‐1 remission and viral rebound after allogeneic stem cell transplantation: report of 2 cases. Ann Intern Med. 2014;161:319‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Whitney JB, Hill AL, Sanisetty S, et al. Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature. 2014;512:74‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Witwer KW, Gama L, Li M, et al. Coordinated regulation of SIV replication and immune responses in the CNS. PLoS One. 2009;4:e8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Valcour V, Chalermchai T, Sailasuta N, et al. Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis. 2012;206:275‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Veenstra M, Leon‐Rivera R, Li M, Gama L, Clements JE, Berman JW. Mechanisms of CNS viral seeding by HIV(+) CD14(+) CD16(+) monocytes: establishment and reseeding of viral reservoirs contributing to HIV‐associated neurocognitive disorders. mBio. 2017;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gorry PR, Ancuta P. Coreceptors and HIV‐1 pathogenesis. Curr HIV/AIDS Rep. 2011;8:45‐53. [DOI] [PubMed] [Google Scholar]
- 14. Borrajo A, Ranazzi A, Pollicita M, et al. Different patterns of HIV‐1 replication in MACROPHAGES is led by co‐receptor usage. Medicina (Kaunas). 2019;55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Borrajo A, Ranazzi A, Pollicita M, et al. Effects of amprenavir on HIV‐1 maturation, production and infectivity following drug withdrawal in chronically‐infected monocytes/macrophages. Viruses. 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garcia M, Buzon MJ, Benito JM, Rallon N. Peering into the HIV reservoir. Rev Med Virol. 2018;28:e1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sung JM, Margolis DM. HIV persistence on antiretroviral therapy and barriers to a cure. Adv Exp Med Biol. 2018;1075:165‐185. [DOI] [PubMed] [Google Scholar]
- 18. Finzi D, Blankson J, Siliciano JD, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV‐1, even in patients on effective combination therapy. Nature medicine. 1999;5:512‐7. [DOI] [PubMed] [Google Scholar]
- 19. Herz J, Filiano AJ, Smith A, Yogev N, Kipnis J. Myeloid cells in the central nervous system. Immunity. 2017;46:943‐956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Joseph SB, Arrildt KT, Sturdevant CB, Swanstrom R. HIV‐1 target cells in the CNS. J Neurovirol. 2015;21:276‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schnell G, Joseph S, Spudich S, Price RW, Swanstrom R. HIV‐1 replication in the central nervous system occurs in two distinct cell types. PLoS Pathog. 2011;7:e1002286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peters PJ, Duenas‐Decamp MJ, Sullivan WM, Clapham PR. Variation of macrophage tropism among HIV‐1 R5 envelopes in brain and other tissues. J Neuroimmune Pharmacol. 2007;2:32‐41. [DOI] [PubMed] [Google Scholar]
- 23. Gorry PR, Bristol G, Zack JA, et al. Macrophage tropism of human immunodeficiency virus type 1 isolates from brain and lymphoid tissues predicts neurotropism independent of coreceptor specificity. J Virol. 2001;75:10073‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gorry PR, Taylor J, Holm GH, et al. Increased CCR5 affinity and reduced CCR5/CD4 dependence of a neurovirulent primary human immunodeficiency virus type 1 isolate. J Virol. 2002;76:6277‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gorry PR, Francella N, Lewin SR, Collman RG. HIV‐1 envelope‐receptor interactions required for macrophage infection and implications for current HIV‐1 cure strategies. J Leukoc Biol. 2014;95:71‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wallet C, De Rovere M, Van Assche J, et al. Microglial cells: the main HIV‐1 reservoir in the brain. Front Cell Infect Microbiol. 2019;9:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wong ME, Jaworowski A, Hearps AC. The HIV reservoir in monocytes and macrophages. Front Immunol. 2019;10:1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Borrajo Lopez A, Penedo MA, Rivera‐Baltanas T, et al. Microglia: the real foe in HIV‐1‐associated neurocognitive disorders? Biomedicines. 2021;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rojas‐Celis V, Valiente‐Echeverria F, Soto‐Rifo R, Toro‐Ascuy D. New challenges of HIV‐1 infection: how HIV‐1 attacks and resides in the central nervous system. Cells. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nowlin BT, Burdo TH, Midkiff CC, Salemi M, Alvarez X, Williams KC. SIV encephalitis lesions are composed of CD163(+) macrophages present in the central nervous system during early SIV infection and SIV‐positive macrophages recruited terminally with AIDS. Am J Pathol. 2015;185:1649‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Castellano P, Prevedel L, Eugenin EA. HIV‐infected macrophages and microglia that survive acute infection become viral reservoirs by a mechanism involving. Bim Sci Rep. 2017;7:12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reu P, Khosravi A, Bernard S, et al. The lifespan and turnover of microglia in the human brain. Cell Rep. 2017;20:779‐784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rumbaugh JA, Tyor W. HIV‐associated neurocognitive disorders: five new things. Neurol Clin Pract. 2015;5:224‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Eggers C, Arendt G, Hahn K, et al. HIV‐1‐associated neurocognitive disorder: epidemiology, pathogenesis, diagnosis, and treatment. J Neurol. 2017;264:1715‐1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Strain MC, Letendre S, Pillai SK, et al. Genetic composition of human immunodeficiency virus type 1 in cerebrospinal fluid and blood without treatment and during failing antiretroviral therapy. Journal of virology. 2005;79:1772‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang Y, Liu M, Lu Q, et al. Global prevalence and burden of HIV‐associated neurocognitive disorder: a meta‐analysis. Neurology. 2020;95:e2610‐e2621. [DOI] [PubMed] [Google Scholar]
- 37. Bougea A, Spantideas N, Galanis P, Gkekas G, Thomaides T. Optimal treatment of HIV‐associated neurocognitive disorders: myths and reality. A critical review. Ther Adv Infect Dis. 2019;6:2049936119838228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Underwood J, Robertson KR, Winston A. Could antiretroviral neurotoxicity play a role in the pathogenesis of cognitive impairment in treated HIV disease? AIDS. 2015;29:253‐61. [DOI] [PubMed] [Google Scholar]
- 39. Heaton RK, Franklin DR, Ellis RJ, et al. HIV‐associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Simioni S, Cavassini M, Annoni JM, et al. Cognitive dysfunction in HIV patients despite long‐standing suppression of viremia. AIDS. 2010;24:1243‐50. [DOI] [PubMed] [Google Scholar]
- 41. Gianella S, Kosakovsky Pond SL, Oliveira MF, et al. Compartmentalized HIV rebound in the central nervous system after interruption of antiretroviral therapy. Virus Evol. 2016;2:vew020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dahl V, Peterson J, Fuchs D, Gisslen M, Palmer S, Price RW. Low levels of HIV‐1 RNA detected in the cerebrospinal fluid after up to 10 years of suppressive therapy are associated with local immune activation. AIDS. 2014;28:2251‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Eden A, Fuchs D, Hagberg L, et al. HIV‐1 viral escape in cerebrospinal fluid of subjects on suppressive antiretroviral treatment. J Infect Dis. 2010;202:1819‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Churchill MJ, Gorry PR, Cowley D, et al. Use of laser capture microdissection to detect integrated HIV‐1 DNA in macrophages and astrocytes from autopsy brain tissues. J Neurovirol. 2006;12:146‐52. [DOI] [PubMed] [Google Scholar]
- 45. Fischer‐Smith T, Croul S, Sverstiuk AE, et al. CNS invasion by CD14+/CD16+ peripheral blood‐derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J Neurovirol. 2001;7:528‐41. [DOI] [PubMed] [Google Scholar]
- 46. Lamers SL, Rose R, Maidji E, et al. HIV DNA is frequently present within pathologic tissues evaluated at autopsy from combined antiretroviral therapy‐treated patients with undetectable viral loads. J Virol. 2016;90:8968‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ko A, Kang G, Hattler JB, et al. Macrophages but not astrocytes harbor HIV DNA in the brains of HIV‐1‐infected aviremic individuals on suppressive antiretroviral therapy. J Neuroimmune Pharmacol. 2019;14:110‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Desplats P, Dumaop W, Smith D, et al. Molecular and pathologic insights from latent HIV‐1 infection in the human brain. Neurology. 2013;80:1415‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thompson KA, Cherry CL, Bell JE, McLean CA. Brain cell reservoirs of latent virus in presymptomatic HIV‐infected individuals. Am J Pathol. 2011;179:1623‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tso FY, Kang G, Kwon EH, et al. Brain is a potential sanctuary for subtype C HIV‐1 irrespective of ART treatment outcome. PLoS One. 2018;13:e0201325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cosenza MA, Zhao ML, Lee SC. HIV‐1 expression protects macrophages and microglia from apoptotic death. Neuropathol Appl Neurobiol. 2004;30:478‐90. [DOI] [PubMed] [Google Scholar]
- 52. Boliar S, Gludish DW, Jambo KC, et al. Inhibition of the lncRNA SAF drives activation of apoptotic effector caspases in HIV‐1‐infected human macrophages. Proc Natl Acad Sci USA. 2019;116:7431‐7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gerngross L, Fischer T. Evidence for cFMS signaling in HIV production by brain macrophages and microglia. J Neurovirol. 2015;21:249‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Briggs SD, Scholtz B, Jacque JM, Swingler S, Stevenson M, Smithgall TE. HIV‐1 Nef promotes survival of myeloid cells by a Stat3‐dependent pathway. J Biol Chem. 2001;276:25605‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. DeLuca C, Kwon H, Pelletier N, Wainberg MA, Hiscott J. NF‐kappaB protects HIV‐1‐infected myeloid cells from apoptosis. Virology. 1998;244:27‐38. [DOI] [PubMed] [Google Scholar]
- 56. Laguette N, Sobhian B, Casartelli N, et al. SAMHD1 is the dendritic‐ and myeloid‐cell‐specific HIV‐1 restriction factor counteracted by Vpx. Nature. 2011;474:654‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cenker JJ, Stultz RD, McDonald D. Brain Microglial Cells Are Highly Susceptible to HIV‐1 Infection and Spread. AIDS Res Hum Retroviruses. 2017;33:1155‐1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zink MC, Suryanarayana K, Mankowski JL, et al. High viral load in the cerebrospinal fluid and brain correlates with severity of simian immunodeficiency virus encephalitis. J Virol. 1999;73:10480‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Williams K, Lackner A, Mallard J. Non‐human primate models of SIV infection and CNS neuropathology. Curr Opin Virol. 2016;19:92‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zink MC, Brice AK, Kelly KM, et al. Simian immunodeficiency virus‐infected macaques treated with highly active antiretroviral therapy have reduced central nervous system viral replication and inflammation but persistence of viral DNA. J Infect Dis. 2010;202:161‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Avalos CR, Abreu CM, Queen SE, et al. Brain macrophages in simian immunodeficiency virus‐infected, antiretroviral‐suppressed macaques: a functional latent reservoir. mBio. 2017;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Abreu C, Shirk EN, Queen SE, et al. Brain macrophages harbor latent, infectious simian immunodeficiency virus. AIDS. 2019;33:S181‐S188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gama L, Abreu CM, Shirk EN, et al. Reactivation of simian immunodeficiency virus reservoirs in the brain of virally suppressed macaques. AIDS. 2017;31:5‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hsu DC, Sunyakumthorn P, Wegner M, et al. Central nervous system inflammation and infection during early, nonaccelerated simian‐human immunodeficiency virus infection in rhesus macaques. J Virol. 2018;92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Buch SJ, Villinger F, Pinson D, et al. Innate differences between simian‐human immunodeficiency virus (SHIV)(KU‐2)‐infected rhesus and pig‐tailed macaques in development of neurological disease. Virology. 2002;295:54‐62. [DOI] [PubMed] [Google Scholar]
- 66. Bauer AM, Ziani W, Lindemuth E, et al. Novel transmitted/founder simian‐human immunodeficiency viruses for human immunodeficiency virus latency and cure research. J Virol. 2020;94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Miyake A, Enose Y, Ohkura S, et al. The quantity and diversity of infectious viruses in various tissues of SHIV‐infected monkeys at the early and AIDS stages. Arch Virol. 2004;149:943‐55. [DOI] [PubMed] [Google Scholar]
- 68. North TW, Higgins J, Deere JD, et al. Viral sanctuaries during highly active antiretroviral therapy in a nonhuman primate model for AIDS. J Virol. 2010;84:2913‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Obregon‐Perko V, Bricker KM, Mensah G, et al. Simian‐human immunodeficiency virus SHIV.C.CH505 persistence in ART‐suppressed infant macaques is characterized by elevated SHIV RNA in the gut and a high abundance of intact SHIV DNA in naive CD4(+) T Cells. J Virol. 2020;95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hsu DC, Silsorn D, Inthawong D, et al. Impact of analytical treatment interruption on the central nervous system in a simian‐HIV model. AIDS. 2019;33:S189‐S196. [DOI] [PubMed] [Google Scholar]
- 71. Colonna L, Peterson CW, Schell JB, et al. Evidence for persistence of the SHIV reservoir early after MHC haploidentical hematopoietic stem cell transplantation. Nat Commun. 2018;9:4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Igarashi T, Brown CR, Endo Y, et al. Macrophage are the principal reservoir and sustain high virus loads in rhesus macaques after the depletion of CD4+ T cells by a highly pathogenic simian immunodeficiency virus/HIV type 1 chimera (SHIV): implications for HIV‐1 infections of humans. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:658‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sharer LR, Baskin GB, Cho ES, Murphey‐Corb M, Blumberg BM, Epstein LG. Comparison of simian immunodeficiency virus and human immunodeficiency virus encephalitides in the immature host. Ann Neurol. 1988;23:S108‐12. [DOI] [PubMed] [Google Scholar]
- 74. Post K, Olson ED, Naufer MN, et al. Mechanistic differences between HIV‐1 and SIV nucleocapsid proteins and cross‐species HIV‐1 genomic RNA recognition. Retrovirology. 2016;13:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Pollom E, Dang KK, Potter EL, et al. Comparison of SIV and HIV‐1 genomic RNA structures reveals impact of sequence evolution on conserved and non‐conserved structural motifs. PLoS Pathog. 2013;9:e1003294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Llewellyn GN, Alvarez‐Carbonell D, Chateau M, Karn J, Cannon PM. HIV‐1 infection of microglial cells in a reconstituted humanized mouse model and identification of compounds that selectively reverse HIV latency. J Neurovirol. 2018;24:192‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Williams KC, Burdo TH. HIV and SIV infection: the role of cellular restriction and immune responses in viral replication and pathogenesis. APMIS. 2009;117:400‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG‐1‐deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869‐77. [DOI] [PubMed] [Google Scholar]
- 79. Iwabuchi R, Ikeno S, Kobayashi‐Ishihara M, et al. Introduction of human Flt3‐L and GM‐CSF into humanized mice enhances the reconstitution and maturation of myeloid dendritic cells and the development of Foxp3(+)CD4(+) T Cells. Front Immunol. 2018;9:1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Strowig T, Rongvaux A, Rathinam C, et al. Transgenic expression of human signal regulatory protein alpha in Rag2‐/‐gamma(c)‐/‐ mice improves engraftment of human hematopoietic cells in humanized mice. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:13218‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lavender KJ, Pang WW, Messer RJ, et al. BLT‐humanized C57BL/6 Rag2‐/‐gammac‐/‐CD47‐/‐ mice are resistant to GVHD and develop B‐ and T‐cell immunity to HIV infection. Blood. 2013;122:4013‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Potash MJ, Chao W, Bentsman G, et al. A mouse model for study of systemic HIV‐1 infection, antiviral immune responses, and neuroinvasiveness. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:3760‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Boska MD, Dash PK, Knibbe J, et al. Associations between brain microstructures, metabolites, and cognitive deficits during chronic HIV‐1 infection of humanized mice. Mol Neurodegener. 2014;9:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Gorantla S, Makarov E, Finke‐Dwyer J, et al. Links between progressive HIV‐1 infection of humanized mice and viral neuropathogenesis. Am J Pathol. 2010;177:2938‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Dash PK, Gorantla S, Gendelman HE, et al. Loss of neuronal integrity during progressive HIV‐1 infection of humanized mice. J Neurosci. 2011;31:3148‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Li W, Gorantla S, Gendelman HE, Poluektova LY. Systemic HIV‐1 infection produces a unique glial footprint in humanized mouse brains. Dis Model Mech. 2017;10:1489‐1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Gorantla S, Poluektova L, Gendelman HE. Rodent models for HIV‐associated neurocognitive disorders. Trends Neurosci. 2012;35:197‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gorantla S, Gendelman HE, Poluektova LY. Can humanized mice reflect the complex pathobiology of HIV‐associated neurocognitive disorders? J Neuroimmune Pharmacol. 2012;7:352‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Dash PK, Gorantla S, Poluektova L, et al. Humanized mice for infectious and neurodegenerative disorders. Retrovirology. 2021;18:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Su H, Cheng Y, Sravanam S, et al. Immune activations and viral tissue compartmentalization during progressive HIV‐1 infection of humanized mice. Front Immunol. 2019;10:340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Su H, Sravanam S, Sillman B, et al. Recovery of latent HIV‐1 from brain tissue by adoptive cell transfer in virally suppressed humanized mice. J Neuroimmune Pharmacol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Calantone N, Wu F, Klase Z, et al. Tissue myeloid cells in SIV‐infected primates acquire viral DNA through phagocytosis of infected T cells. Immunity. 2014;41:493‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Honeycutt JB, Wahl A, Baker C, et al. Macrophages sustain HIV replication in vivo independently of T cells. J Clin Invest. 2016;126:1353‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Scheerlinck J‐P, (2014) Cytokine species‐specificity and humanized mice. 93–108.
- 95. Muñoz‐Garcia J, Cochonneau D, Télétchéa S, et al. The twin cytokines interleukin‐34 and CSF‐1: masterful conductors of macrophage homeostasis. Theranostics. 2021;11:1568‐1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wei S, Nandi S, Chitu V, et al. Functional overlap but differential expression of CSF‐1 and IL‐34 in their CSF‐1 receptor‐mediated regulation of myeloid cells. J Leukoc Biol. 2010;88:495‐505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Baghdadi M, Umeyama Y, Hama N, et al. Interleukin‐34, a comprehensive review. J Leukoc Biol. 2018;104:931‐951. [DOI] [PubMed] [Google Scholar]
- 98. Chitu V, Gokhan Ş, Nandi S, Mehler MF, Stanley ER. Emerging roles for CSF‐1 receptor and its ligands in the nervous system. Trends Neurosci. 2016;39:378‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Tong L, Gong Y, Wang P, et al. Microglia loss contributes to the development of major depression induced by different types of chronic stresses. Neurochem Res. 2017;42:2698‐2711. [DOI] [PubMed] [Google Scholar]
- 100. Svoboda DS, Barrasa MI, Shu J, et al. Human iPSC‐derived microglia assume a primary microglia‐like state after transplantation into the neonatal mouse brain. Proc Natl Acad Sci U S A. 2019;116:25293‐25303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Willinger T, Rongvaux A, Takizawa H, et al. Human IL‐3/GM‐CSF knock‐in mice support human alveolar macrophage development and human immune responses in the lung. Proc Natl Acad Sci U S A. 2011;108:2390‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Abud EM, Ramirez RN, Martinez ES, et al. iPSC‐derived human microglia‐like cells to study neurological diseases. Neuron. 2017;94:278‐293. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Mathews S, Branch Woods A, Katano I, et al. Human Interleukin‐34 facilitates microglia‐like cell differentiation and persistent HIV‐1 infection in humanized mice. Mol Neurodegener. 2019;14:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Borjabad A, Brooks AI, Volsky DJ. Gene expression profiles of HIV‐1‐infected glia and brain: toward better understanding of the role of astrocytes in HIV‐1‐associated neurocognitive disorders. J Neuroimmune Pharmacol. 2010;5:44‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Polyak MJ, Vivithanaporn P, Maingat FG, et al. Differential type 1 interferon‐regulated gene expression in the brain during AIDS: interactions with viral diversity and neurovirulence. FASEB j. 2013;27:2829‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Sanna PP, Repunte‐Canonigo V, Masliah E, Lefebvre C. Gene expression patterns associated with neurological disease in human HIV infection. PLoS One. 2017;12:e0175316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Gumbs SBH, Kubler R, Gharu L, et al. Human microglial models to study HIV infection and neuropathogenesis: a literature overview and comparative analyses. J Neurovirol. 2022;28:64‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Wang H. Modeling neurological diseases with human brain organoids. Front Synaptic Neurosci. 2018;10:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Premeaux TA, Mediouni S, Leda A, et al. Next‐generation human cerebral organoids as powerful tools to advance NeuroHIV research. mBio. 2021;12:e0068021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Gumbs SBH, Berdenis van Berlekom A, Kubler R, et al. Characterization of HIV‐1 infection in microglia‐containing human cerebral organoids. Viruses. 2022;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Dos Reis RS, Sant S, Keeney H, Wagner MCE, Ayyavoo V. Modeling HIV‐1 neuropathogenesis using three‐dimensional human brain organoids (hBORGs) with HIV‐1 infected microglia. Sci Rep. 2020;10:15209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Shultz LD, Brehm MA, Garcia‐Martinez JV, Greiner DL. Humanized mice for immune system investigation: progress, promise and challenges. Nat Rev Immunol. 2012;12:786‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Zschaler J, Schlorke D, Arnhold J. Differences in innate immune response between man and mouse. Crit Rev Immunol. 2014;34:433‐54. [PubMed] [Google Scholar]
- 114. Theocharides AP, Rongvaux A, Fritsch K, Flavell RA, Manz MG. Humanized hemato‐lymphoid system mice. Haematologica. 2016;101:5‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Mosier DE, Gulizia RJ, Baird SM, Wilson DB. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 1988;335:256‐9. [DOI] [PubMed] [Google Scholar]
- 116. Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7:118‐30. [DOI] [PubMed] [Google Scholar]
- 117. Ai M, Curran MA. Immune checkpoint combinations from mouse to man. Cancer Immunol Immunother. 2015;64:885‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Watanabe Y, Takahashi T, Okajima A, et al. The analysis of the functions of human B and T cells in humanized NOD/shi‐scid/gammac(null) (NOG) mice (hu‐HSC NOG mice). Int Immunol. 2009;21:843‐58. [DOI] [PubMed] [Google Scholar]
- 119. Ishikawa F, Yasukawa M, Lyons B, et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood. 2005;106:1565‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Dash PK, Kaminski R, Bella R, et al. Sequential LASER ART and CRISPR treatments eliminate HIV‐1 in a subset of infected humanized mice. Nat Commun. 2019;10:2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Akkina R, Allam A, Balazs AB, et al. Improvements and limitations of humanized mouse models for HIV research: nIH/NIAID “Meet the Experts” 2015 workshop summary. AIDS Res Hum Retroviruses. 2016;32:109‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Gonzalez‐Scarano F, Martin‐Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69‐81. [DOI] [PubMed] [Google Scholar]
- 123. Ash MK, Al‐Harthi L, Schneider JR. HIV in the brain: identifying viral reservoirs and addressing the challenges of an HIV cure. Vaccines (Basel). 2021:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Churchill MJ, Wesselingh SL, Cowley D, et al. Extensive astrocyte infection is prominent in human immunodeficiency virus‐associated dementia. Ann Neurol. 2009;66:253‐8. [DOI] [PubMed] [Google Scholar]
- 125. Thompson KA, Churchill MJ, Gorry PR, et al. Astrocyte specific viral strains in HIV dementia. Ann Neurol. 2004;56:873‐7. [DOI] [PubMed] [Google Scholar]
- 126. Li GH, Anderson C, Jaeger L, Do T, Major EO, Nath A. Cell‐to‐cell contact facilitates HIV transmission from lymphocytes to astrocytes via CXCR4. AIDS. 2015;29:755‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Overholser ED, Coleman GD, Bennett JL, et al. Expression of simian immunodeficiency virus (SIV) nef in astrocytes during acute and terminal infection and requirement of nef for optimal replication of neurovirulent SIV in vitro. J Virol. 2003;77:6855‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Lutgen V, Narasipura SD, Barbian HJ, et al. HIV infects astrocytes in vivo and egresses from the brain to the periphery. PLoS Pathog. 2020;16:e1008381. [DOI] [PMC free article] [PubMed] [Google Scholar]


