Abstract
Tropical pulmonary eosinophilia (TPE) is a severe asthmatic syndrome of lymphatic filariasis, in which an allergic response is induced to microfilariae (Mf) in the lungs. Previously, in a murine model for TPE, we have demonstrated that recombinant interleukin-12 (IL-12) suppresses pulmonary eosinophilia and airway hyperresponsiveness (AHR) by modulating the T helper (Th) response in the lungs from Th2- to Th1-like, with elevated gamma-interferon (IFN-γ) production and decreased IL-4 and IL-5 production. The present study examined the immunomodulatory roles of IL-4 and IFN-γ in filaria-induced AHR and pulmonary inflammation using mice genetically deficient in these cytokines. C57BL/6, IL-4 gene knockout (IL-4−/−), and IFN-γ−/− mice were first immunized with soluble Brugia malayi antigens and then inoculated intravenously with 200,000 live Mf. Compared with C57BL/6 mice, IL-4−/− mice exhibited significantly reduced AHR, whereas IFN-γ−/− mice had increased AHR. Histopathologically, each mouse strain showed increased cellular infiltration into the lung parenchyma and bronchoalveolar space compared with naïve animals. However, consistent with changes in AHR, IL-4−/− mice had less inflammation than C57BL/6 mice, whereas IFN-γ−/− mice had exacerbated pulmonary inflammation with the loss of pulmonary architecture. Systemically, IL-4−/− mice produced significantly higher IFN-γ levels compared with C57BL/6 mice, whereas IFN-γ−/− mice produced significantly higher IL-4 levels. These data indicate that IL-4 is required for the induction of filaria-induced AHR, whereas IFN-γ suppresses AHR.
The parasitic helminths that cause lymphatic filariasis, Wuchereria bancrofti and Brugia malayi, infect an estimated 130 million people around the globe (24). Much of the pathology associated with the disease is attributed to adult worms causing blockage of the lymphatics. First-stage larvae (microfilariae [Mf]), which circulate in the blood, generally do not cause pathology. However, in certain individuals, the presence of Mf in the lungs is associated with severe asthmatic symptoms and airway hyperresponsiveness (AHR) (5, 7). These patients have peripheral blood eosinophilia and increased numbers of highly activated eosinophils in bronchoalveolar lavage (BAL) fluid (30). In addition, lung biopsies from such patients show Mf surrounded by eosinophilic material (7, 17, 37). Although the mechanisms underlying these processes are not yet understood, parasites trapped in the lung microvasculature are thought to stimulate a localized inflammatory response that leads to the recruitment of eosinophils, eosinophil degranulation, and induction of severe asthmatic symptoms associated with AHR (5, 7, 30). This condition, termed tropical pulmonary eosinophilia (TPE), can be distinguished from allergic asthma by the effectiveness of anthelmintics in relieving clinical symptoms (25).
The primary clinical features of TPE can be reproduced in mice by systemic immunization with B. malayi antigens (Ags) followed by intravenous (i.v.) injection of live Mf (8). Previous studies from this laboratory demonstrated that sensitization of mice to B. malayi microfilarial antigens induces a selective T helper type 2 (Th2)-associated response (increased interleukin 4 [IL-4] and IL-5 production and decreased gamma interferon [IFN-γ] production) with eosinophilia and elevated levels of immunoglobulin E (IgE) in the serum (26–28). On subsequent i.v. inoculation, entrapment and degeneration of Mf in the lungs induces a localized inflammatory response that leads to the recruitment of eosinophils to this site. Once in the airways, eosinophils degranulate, releasing cytotoxic, cationic granule proteins, including major basic protein (MBP), which is associated with AHR (12, 36). Our recent studies have demonstrated that filaria-induced AHR is dependent on IL-5 and can be modulated by injection of recombinant IL-12 (13, 23), which suppresses pulmonary eosinophilia, deposition of MBP, and AHR by elevating IFN-γ production and decreasing IL-4 and IL-5 production. In the present study, we utilized IL-4 and IFN-γ gene knockout mice to determine more directly the regulatory roles of these cytokines in filaria-induced AHR.
MATERIALS AND METHODS
Animals.
IL-4 deficient (IL-4−/−) mice, generated from C57BL/6 and 129Sv mice, were produced as described elsewhere (19) and backcrossed to C57BL/6 mice. Breeding colonies were maintained in the animal facilities at Case Western Reserve University. C57BL/6 mice and IFN-γ−/− mice, which were backcrossed to C57BL/6 mice, were obtained from Jackson Laboratories (Bar Harbor, Maine). All animals were maintained under microisolator conditions.
Parasites and Ag.
B. malayi Mf were obtained by peritoneal lavage from male jirds (Meriones unguiculatis) which were infected with third-stage larvae (NIH contract 73262). A soluble parasite extract (Ag), for use in the enzyme-linked immunosorbent assay (ELISA) and in vitro stimulation assays, was prepared from Mf by sonication on ice until no intact worm remained, followed by centrifugation for 15 min at 10,000 × g. The supernatant was filter sterilized, and the protein concentration was determined using a Bradford assay (Bio-Rad Laboratories, Hercules, Calif.).
Immunization and i.v. injection.
Mice (4 to 6 weeks old) were immunized by three weekly subcutaneous (s.c.) injections of 100,000 killed (frozen) Mf in 0.2 ml of saline. Ten days after the final immunization, animals received a tail vein injection of 200,000 live Mf.
Isometric measurement of tracheal smooth muscle response to ACh and carbachol.
Tracheal reactivity was determined as described previously (23), using tracheae from animals that were not subjected to alveolar lavage. Briefly, tracheae were kept at 4°C in modified Krebs-Henseleit solution (118.2 mM NaCl, 25 mM Na2HCO3, 4.6 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 2.5 mM CaCl2, and 10 mM dextrose [pH 7.4]) which was continuously gassed with a mixture containing 95% O2 and 5% CO2. After removal of adventitia and fatty tissue, a 3.0-mm cylindrical section was suspended between a glass rod and a force displacement transducer (FT03; Grass Instruments, Quincy, Mass.) connected to an amplifier. Tracheal cylinders were equilibrated in an organ bath (Radnoti Glass Tech., Inc., Monrovia, Calif.) filled with 8.0 ml of Krebs-Henseleit solution which was changed every 15 min, and the temperature was maintained at 37°C by a constant-temperature circulating unit. The optimal length at which maximal isometric force developed was identified by electric-field submaximal stimulation (EFS) (5 V AC applied through platinum electrodes; 250 mA/cm2 for 10 s). Concentration response curves to acetylcholine (ACh) and carbachol were determined, and the isometric force (in grams) generated by smooth muscle was recorded.
BAL and differential cell analysis.
BAL fluid was obtained by cannulating the trachea through a small incision and performing lavage twice with 0.5 ml of phosphate-buffered saline (Sigma, St. Louis, Mo.). Total leukocyte counts in BAL fluids were determined using a hemocytometer.
For performing differential counts, cytocentrifuge preparations from BAL fluids were stained with modified Wright-Giemsa stain (Diff-Quik; Dade Diagnostics, Aguada, P.R.), and 400 cells were counted from two slides for each animal.
Detection of eosinophils in the blood.
Blood was collected from the retro-orbital plexus, erythrocytes were lysed, and total and differential leukocyte counts were determined after staining with Diff-Quik.
Histological analysis.
Lungs were fixed in 10% formalin and embedded in paraffin, and 5-μm sections were prepared for histology by standard methods. Sections were stained with hematoxylin and eosin for the assessment of overall inflammatory response.
Spleen cell preparation.
To prepare spleen cell suspensions, red cells were lysed with 0.01 M Tris (pH 7.2) containing 0.75% ammonium chloride, and splenocytes were suspended in complete medium (RPMI 1640 [BioWhittaker, Walkersville, Md.]) containing 1 mM sodium pyruvate, 2 mM l-glutamine, 20 mM HEPES, 200 U of penicillin/ml, 200 μg of streptomycin/ml, 0.5 μg of amphotericin B (Fungizone)/ml, and 10% heat-inactivated fetal calf serum (Life Technologies, Inc., New City, N.Y.]). Duplicate wells containing 5 × 105 cells were stimulated for 72 h with 2 μg of soluble anti-murine CD3 antibody (2C11; kindly provided by Thomas Forsthuber, Department of Pathology, Case Western Reserve University, Cleveland, Ohio)/ml in a final volume of 200 μl and then incubated at 37°C in 5% CO2.
Cytokine ELISA.
The concentrations of IFN-γ, IL-4, and IL-5 were measured in culture supernatants of in vitro-stimulated splenocytes using a two-site ELISA. Recombinant murine cytokines were used to generate the standard curve. Monoclonal antibodies (MAbs) R4-6A2 and XMG-1.2 were used for IFN-γ, MAbs BVD-6 and BVD-4 were used for IL-4, and MAbs TRFK-5 and TRFK-4 were used for IL-5. All reagents were obtained from PharMingen (San Diego, Calif.).
Statistical analysis.
An unpaired Student's t test was used to determine significance. A P value of <0.05 was considered statistically significant.
RESULTS
Filaria-induced AHR is modulated by IL-4 and IFN-γ.
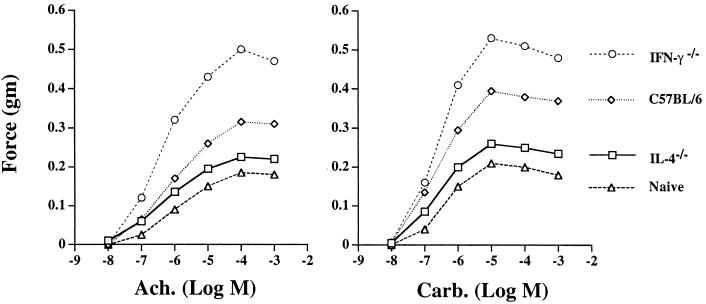
To determine the effects of IL-4 or IFN-γ deficiency on filaria-induced AHR, we measured the contractile response of tracheal smooth muscle to the cholinergic agonists ACh and carbachol, which is a standard method for measuring lung function. As shown in Fig. 1 (left panel), tracheal smooth muscle from naïve C57BL/6 mice responded to ACh in a dose-dependent manner, with the mean peak contraction (force, 0.185 g) observed at 10−4 M ACh. The responses of naïve IL-4−/− and IFN-γ−/− mice were not significantly different from those of naïve C57BL/6 mice, and i.v. injection of Mf into unsensitized mice did not induce hyperresponsiveness (data not shown). Responses of tracheae from C57BL/6 mice sensitized by s.c. immunization and challenged i.v. with live Mf were higher at each concentration of ACh and reached a significantly higher mean peak value (force) of 0.315 g. The responses of tracheae of IFN-γ−/− mice reached an even higher mean peak value (force) of 0.500 g. In contrast, the mean peak response from immunized and challenged IL-4−/− mice (force, 0.230 g) was not significantly elevated compared with that of naïve mice. Similar responses were observed when tracheae were stimulated with increasing concentrations of carbachol (Fig. 1, right panel). The contractile responses with carbachol were elevated compared with those obtained with ACh, and the peak response was observed at 10−5 M. Taken together, these findings indicate reciprocal regulatory roles for IL-4 and IFN-γ, where IL-4 is essential for the induction of AHR and IFN-γ has a critical role in the suppression of AHR.
FIG. 1.
Filaria-induced AHR in C57BL/6, IL-4−/−, and IFN-γ−/− mice. C57BL/6, IL-4−/−, and IFN-γ−/− mice were immunized three times s.c. with soluble B. malayi Ag. Ten days after the last immunization, mice were injected i.v. with 200,000 live Mf. AHR was measured as the force of tracheal smooth muscle contraction (in grams), with increased sensitivity to cholinergic agonists reflecting the severity of inflammation. Each data point represents the mean for eight mice combined from two experiments (standard deviations were <10%). P < 0.05 for C57BL/6 versus IFN-γ−/− mice, and for C57BL/6 versus IL-4−/− mice at 10−3 to 10−5 M ACh and carbachol.
IL-4 and IFN-γ regulate filaria-induced lung histopathology.
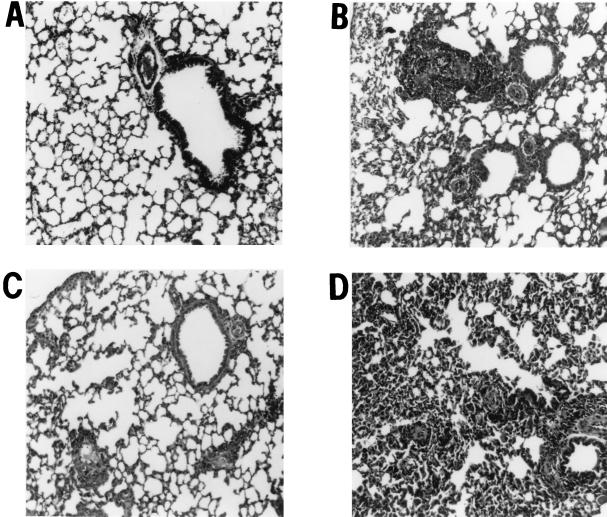
To determine if the regulatory effects of IL-4 and IFN-γ on AHR are also reflected at the level of lung histopathology, naïve C57BL/6 or immunized C57BL/6, IL-4−/−, and IFN-γ−/− mice were inoculated i.v. with live Mf and sacrificed 7 days later, and lung sections were stained with hematoxylin and eosin. As shown in Fig. 2A, lungs from naïve mice showed normal structure, with clearly defined bronchioles and alveoli. No inflammation was noted around either blood vessels or airways. In contrast, lungs from immunized and challenged C57BL/6 mice had a perivascular and peribronchial inflammatory cell infiltrate, with loss of normal lung structure (Fig. 2B). Lungs from IL-4−/− mice had less cellular infiltration, and the overall architecture of the lung was less disrupted than in C57BL/6 mice (Fig. 2C). In marked contrast to lungs from C57BL/6 and IL-4−/− mice, lungs from IFN-γ−/− mice were severely congested, with disruption of alveolar structure and a profound cellular infiltration throughout the tissue (Fig. 2D). These data show that the reciprocal modulatory effect of IL-4 and IFN-γ on AHR is reflected in histopathological changes in the lung, with IL-4 inducing and IFN-γ suppressing the development of histopathology.
FIG. 2.
Lung histopathology in C57BL/6, IL-4−/−, and IFN-γ−/− mice. C57BL/6, IL-4−/−, and IFN-γ−/− mice were immunized and injected i.v. with 200,000 live Mf as described above. One week later, animals were sacrificed, lungs were fixed in formalin, and 5-μm sections were stained with hematoxylin and eosin. Shown are representative lung sections from similar sites in the left lobe for naïve mice (A), immunized, Mf-challenged C57BL/6 mice (B), IL-4−/− mice (C), and IFN-γ−/− mice (D). Peribronchial and perivascular cell infiltrates can be detected in panels B and D. In panel D, normal lung architecture is also lost. Sections are representative of five mice per group from two repeat experiments.
IL-4 and IFN-γ have reciprocal regulatory effects on pulmonary eosinophilia.
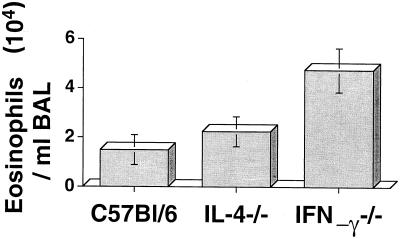
As previous studies showed an essential role for IL-5 and eosinophils in the development of filaria-induced AHR (13), we next determined if the observed regulatory effects of IL-4 and IFN-γ on AHR and histopathology are associated with pulmonary eosinophilia. BAL cells recovered by alveolar lavage were examined by differential staining. As shown in Fig. 3, total eosinophil numbers were similar in IL-4−/− mice and C57BL/6 mice. However, BAL eosinophils from IFN-γ−/− mice were >2.0-fold elevated compared with those from C57BL/6 mice (P < 0.05). BAL eosinophil numbers reflected those in the lung parenchyma (data not shown). There was no significant difference in total mononuclear cell numbers between these groups, and neutrophils comprised <2% of total cells in all the groups. Together, these data indicate that the histopathological changes are associated with the differential effects of IL-4 and IFN-γ on eosinophil recruitment to the lungs.
FIG. 3.
Eosinophils in BAL fluid of C57BL/6, IL-4−/−, and IFN-γ−/− mice. C57BL/6, IL-4−/−, and IFN-γ−/− mice were immunized and injected i.v. with 200,000 live Mf as described above. One week later, mice were sacrificed, lungs were lavaged, and the total number of eosinophils per milliliter of lavage fluid was determined as described in Materials and Methods. Data are means ± standard deviations for five mice per group. Significantly more eosinophils were recovered from IFN-γ−/− mice than from C57BL/6 or IL-4−/− mice (P < 0.05). This experiment was repeated twice with similar results.
To determine if the effects of IFN-γ and IL-4 on pulmonary eosinophilia are due to altered eosinophil production and release into the peripheral blood, we determined peripheral blood eosinophilia in immunized and challenged C57BL/6, IL-4−/−, and IFN-γ−/− mice. The numbers of peripheral eosinophils per microliter were not significantly different among these mouse strains (114 ± 79.3 for C57BL/6 mice, 151 ± 118 for IL-4−/− mice, and 131 ± 62.6 for IFN-γ−/− mice), indicating that IL-4 or IFN-γ deficiency does not significantly affect eosinophil production.
Cytokine production in IFN-γ- and IL-4-deficient mice.
Since results from this and from our previous study (23) indicate that IFN-γ down-modulates filaria-induced AHR, we determined if the decreased AHR and pulmonary inflammation in IL-4-deficient mice is associated with elevated IFN-γ production. Conversely, since IL-4 appears to induce filaria-induced AHR, we determined if the increased airway disease in IFN-γ-deficient mice is associated with elevated IL-4 production. We therefore examined cytokine production by splenocytes from immunized, Mf-challenged C57BL/6, IL-4−/−, and IFN-γ−/− mice.
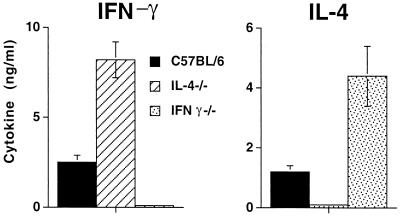
As shown in Fig. 4, IFN-γ production was significantly higher in IL-4−/− mice than in C57BL/6 mice, whereas IFN-γ−/− mice produced significantly more IL-4 than C57BL/6 mice. All three strains produced IL-5 in response to antigen stimulation, with no significant differences among them. Together these data strengthen the hypothesis that IFN-γ and IL-4 have reciprocal roles in modulating filaria-induced AHR.
FIG. 4.
Cytokine responses in splenocytes from C57BL/6, IL-4−/−, and IFN-γ−/− mice. Splenocytes were isolated from C57BL/6, IL-4−/−, and IFN-γ−/− mice after immunization and injection with B. malayi Mf as described above and were stimulated in vitro with 2 μg of anti-CD3/ml. Culture supernatants were recovered 72 h later, and IFN-γ, IL-4, and IL-5 levels were measured by ELISA. Results are means ± standard deviations for five mice per group, and data are representative of three experiments. P < 0.05 for differences in IFN-γ production between C57BL/6 and IL-4−/− mice and for differences in IL-4 production between C57BL/6 and IFN-γ−/− mice.
DISCUSSION
In the murine model for TPE, we and others have previously shown that (i) prior immunization is necessary for the development of parasite-specific cytokine responses, eosinophilia, and induction of AHR (8, 23), (ii) IL-5 and eosinophils are required for filaria-induced AHR, as IL-5 gene knockout mice do not develop pulmonary eosinophilia and do not exhibit AHR (13), and (iii) AHR and immunopathologic responses associated with TPE can be suppressed by administration of recombinant IL-12 at the time of initial sensitization, which induces a 25-fold increase in IFN-γ production (23).
In the present study, we used gene knockout mice to demonstrate more directly the suppressing effect of Th1 cytokines on filaria-induced AHR, as IFN-γ−/− mice had significantly increased AHR, lung histopathology, and recruitment of eosinophils to the lungs. Further, we also demonstrated that IL-4−/− mice had significantly elevated IFN-γ production, with diminished AHR, lung histopathology, and recruitment of eosinophils to the lungs. Development of pulmonary inflammation induced by filarial parasites appears to result from an early inductive phase, in which parasite Ags stimulate a predominantly Th2-associated response (elevated IL-4 and IL-5 production and decreased IFN-γ production), and a secondary effector stage, in which Mf trapped in lung capillaries stimulate a local inflammatory response. With regard to the inductive phase, earlier studies from this laboratory showed that inoculation of mice with B. malayi Mf or immunization with soluble Ags selectively induced a Th2-like response associated with elevated serum IgE and eosinophil production (26–28, 34). The present study showed that IL-4 and IFN-γ modulation of pulmonary inflammation did not occur at the inductive phase of parasite-specific IL-5 and eosinophil production, as these responses were not significantly altered in knockout mice. This is consistent with previous reports that IL-5 and eosinophils are produced in the absence of IL-4 in response to B. malayi Mf, Onchocerca volvulus L3, O. volvulus adult worm Ags, and Onchocerca lienalis Mf (15, 16, 20, 29, 34, 35). Similarly, in the mouse models of aeroallergen-induced eosinophilic inflammation, lung histopathology, and AHR, IL-4 deficiency has been found to affect the recruitment of eosinophils into the lungs; however, IL-5 production and peripheral eosinophilia remained unaffected (3, 4, 10, 14).
Given the absence of a modulatory effect on IL-5 and eosinophil production, results from the present study indicate that the effects of IL-4 and IFN-γ on filaria-induced AHR occur at the effector cell recruitment stage. The mechanism has yet to be determined; however, IL-4 up-regulates the expression of vascular cell adhesion molecule-1 (VCAM-1), which is involved in the transmigration of eosinophils across endothelial cells (1, 2). In various animal models for allergen-induced pulmonary inflammation and AHR, blockade of VCAM-1 and/or very late activation antigen-4 (VLA-4), which is expressed on eosinophils, has been found to inhibit recruitment of eosinophils into the lungs and AHR (6, 9, 32). In addition, expression of chemotactic cytokines may augment cell recruitment, as many of these chemokines, including eotaxin, which is specific for eosinophils (11, 22, 31), may be up-regulated. It has been shown recently that IL-4-stimulated human vascular endothelial cells selectively expressed a novel CC chemokine, eotaxin-3, which exhibits potent activity toward eosinophils (33). Future studies will decipher the role of IL-4 in the expression of vascular adhesion molecules and chemokines in the mouse model for TPE.
In contrast to IL-4, IFN-γ impairs the development of pulmonary inflammation in response to filarial parasites. This finding is consistent with our previous observation that treatment of C57BL/6 mice with recombinant IL-12 diminishes pulmonary inflammation and is associated with increased IFN-γ production (23). The inhibitory role of IFN-γ in lung immunopathology has also been demonstrated in mice expressing the transgene for IFN-γ in the lungs, as these animals had diminished pulmonary eosinophilia and decreased AHR (21). Similarly, treatment of mice with IFN-γ cDNA was found to suppress the Th2 pathway in a murine model of atopic allergy using ovalbumin as the Ag (18). Conversely, IFN-γ−/− mice, given IL-12 treatment prior to i.v. injection of Schistosoma mansoni eggs, had greatly enlarged lung granulomas, consistent with elevated production of Th2-associated cytokines (38).
In summary, these results demonstrate clearly that inflammatory cell recruitment to the lungs and the severity of filaria-induced lung immunopathology are tightly regulated by Th-associated cytokines. Future studies will examine the mechanisms associated with these phenomena and may indicate strategies for immune intervention in this and other, related pulmonary diseases.
ACKNOWLEDGMENTS
We acknowledge the excellent technical assistance of Gina Diaconu, Fred Hazlett, and Alan Higgins. We thank David Bardenstein for assistance with histology. We also thank Peter Zimmerman for discussion and support.
This study was funded by Burroughs Wellcome New Investigator Award 0720 (E.P.) and National Institutes of Health grant HL50527 (M.A.H.).
REFERENCES
- 1.Atsuta J, Sterbinsky S A, Plitt J, Schwiebert L M, Bochner B S, Schleimer R P. Phenotyping and cytokine regulation of the BEAS-2B human bronchial epithelial cell: demonstration of inducible expression of the adhesion molecules VCAM-1 and ICAM-1. Am J Respir Cell Mol Biol. 1997;17:571–582. doi: 10.1165/ajrcmb.17.5.2685. [DOI] [PubMed] [Google Scholar]
- 2.Bochner B S, Klunk D A, Sterbinsky S A, Coffman R L, Schleimer R P. IL-13 selectively induces vascular cell adhesion molecule-1 expression in human endothelial cells. J Immunol. 1995;154:799–803. [PubMed] [Google Scholar]
- 3.Brusselle G, Kips J, Joos G, Bluethmann H, Pauwels R. Allergen-induced airway inflammation and bronchial responsiveness in wild-type and interleukin-4-deficient mice. Am J Respir Cell Mol Biol. 1995;12:254–259. doi: 10.1165/ajrcmb.12.3.7873190. [DOI] [PubMed] [Google Scholar]
- 4.Brusselle G G, Kips J C, Tavernier J H, van der Heyden J G, Cuvelier C A, Pauwels R A, Bluethmann H. Attenuation of allergic airway inflammation in IL-4 deficient mice. Clin Exp Allergy. 1994;24:73–80. doi: 10.1111/j.1365-2222.1994.tb00920.x. [DOI] [PubMed] [Google Scholar]
- 5.Chhabra S, Gaur S. Airway hyperreactivity in tropical pulmonary eosinophilia. Chest. 1988;93:1105–1106. doi: 10.1378/chest.93.5.1105. [DOI] [PubMed] [Google Scholar]
- 6.Chin J E, Hatfield C A, Winterrowd G E, Brashler J R, Vonderfecht S L, Fidler S F, Griffin R L, Kolbasa K P, Krzesicki R F, Sly L M, Staite N D, Richards I M. Airway recruitment of leukocytes in mice is dependent on α4-integrins and vascular cell adhesion molecule-1. Am J Physiol. 1997;272:L219–L229. doi: 10.1152/ajplung.1997.272.2.L219. [DOI] [PubMed] [Google Scholar]
- 7.Danaraj T J, Pacheco G, Shanmugaratnam K, Beaver P C. The etiology and pathology of eosinophilic lung (tropical eosinophilia) Am J Trop Med Hyg. 1966;15:183–189. doi: 10.4269/ajtmh.1966.15.183. [DOI] [PubMed] [Google Scholar]
- 8.Egwang T G, Kazura J W. The BALB/c mouse as a model for immunological studies of microfilariae-induced pulmonary eosinophilia. Am J Trop Med Hyg. 1990;43:61–66. doi: 10.4269/ajtmh.1990.43.61. [DOI] [PubMed] [Google Scholar]
- 9.Fryer A D, Costello R W, Yost B L, Lobb R R, Tedder T F, Steeber D A, Bochner B S. Antibody to VLA-4, but not to l-selectin, protects neuronal M2 muscarinic receptors in antigen-challenged guinea pig airways. J Clin Investig. 1997;99:2036–2044. doi: 10.1172/JCI119372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gavett S H, O'Hearn D J, Karp C L, Patel E A, Schofield B H, Finkelman F D, Wills-Karp M. Interleukin-4 receptor blockade prevents airway responses induced by antigen challenge in mice. Am J Physiol. 1997;272:L253–L261. doi: 10.1152/ajplung.1997.272.2.L253. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalo J A, Lloyd C M, Kremer L, Finger E, Martinez A C, Siegelman M H, Cybulsky M, Gutierrez-Ramos J C. Eosinophil recruitment to the lung in a murine model of allergic inflammation: the role of T cells, chemokines and adhesion receptors. J Clin Investig. 1996;98:2332–2345. doi: 10.1172/JCI119045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gundel R, Letts L, Gleich G. Human eosinophil major basic protein induces airway constriction and airway hyperresponsiveness in primates. J Clin Investig. 1991;87:1470–1473. doi: 10.1172/JCI115155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall L R, Mehlotra R K, Higgins A W, Haxhiu M A, Pearlman E. An essential role for IL-5 and eosinophils in helminth-induced airway hyperresponsiveness. Infect Immun. 1998;66:4425–4430. doi: 10.1128/iai.66.9.4425-4430.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hogan S P, Mould A, Kikutani H, Ramsay A J, Foster P S. Aeroallergen-induced eosinophilic inflammation, lung damage, and airway hyperreactivity in mice can occur independently of IL-4 and allergen-specific immunoglobulins. J Clin Investig. 1997;99:1329–1339. doi: 10.1172/JCI119292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hogarth P, Taylor M, Bianco A. IL-5-dependent immunity to microfilariae is independent of IL-4 in a mouse model of onchocerciasis. J Immunol. 1998;160:5436–5440. [PubMed] [Google Scholar]
- 16.Johnson E, Schynder-Candrian S, Rajan T, Nelson F, Lustigman S, Abraham D. Immune responses to third stage larvae of Onchocerca volvulus in interferon-gamma and interleukin-4 knockout mice. Parasite Immunol. 1998;20:319–324. doi: 10.1046/j.1365-3024.1998.00148.x. [DOI] [PubMed] [Google Scholar]
- 17.Joshi V V, Udwadia F E, Gadgil R K. Etiology of tropical eosinophilia. A study of lung biopsies and review of published reports. Am J Trop Med Hyg. 1969;18:231–240. [PubMed] [Google Scholar]
- 18.Kang K W, Kim T S, Kim K M. Interferon-gamma- and interleukin-4-targeted gene therapy for atopic allergic disease. Immunology. 1999;97:462–465. doi: 10.1046/j.1365-2567.1999.00802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kopf M, Le Gros G, Bachmann M, Lamers M C, Bluethmann H, Kohler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993;362:245–248. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- 20.Lange A, Yutanawiboonchai W, Scott P, Abraham D. IL-4- and IL-5-dependent immunity to Onchocerca volvulus infective larvae in BALB/cBYJ mice. J Immunol. 1994;153:205–211. [PubMed] [Google Scholar]
- 21.Li X-M, Chopra R K, Chou T-Y, Schofield C H, Wills-Karp M, Huang S-K. Mucosal IFN-γ gene transfer inhibits pulmonary allergic responses in mice. J Immunol. 1996;157:3216–3219. [PubMed] [Google Scholar]
- 22.Lilly C M, Nakamura H, Kesselman H, Nagler-Anderson C, Assano K, Garcia-Zepeda E A, Rothenberg M E, Drazen J M, Luster A D. Expression of eotaxin by human lung epithelial cells: induction by cytokines and inhibition by glucocorticoids. J Clin Investig. 1997;99:1767–1773. doi: 10.1172/JCI119341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehlotra R K, Hall L R, Higgins A W, Dreshaj I A, Haxhiu M A, Kazura J W, Pearlman E. Interleukin-12 suppresses filaria-induced pulmonary eosinophilia, deposition of eosinophil major basic protein and airway hyperresponsiveness. Parasite Immunol. 1998;20:455–462. doi: 10.1046/j.1365-3024.1998.00174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michael E, Bundy D A P. Global mapping of lymphatic filariasis. Parasitol Today. 1997;13:472–476. doi: 10.1016/s0169-4758(97)01151-4. [DOI] [PubMed] [Google Scholar]
- 25.Ottesen E A, Nutman T B. Tropical pulmonary eosinophilia. Annu Rev Med. 1992;43:417–424. doi: 10.1146/annurev.me.43.020192.002221. [DOI] [PubMed] [Google Scholar]
- 26.Pearlman E, Hazlett F E, Boom W H, Kazura J W. Induction of murine T-helper-cell responses to the filarial nematode Brugia malayi. Infect Immun. 1993;61:1105–1112. doi: 10.1128/iai.61.3.1105-1112.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pearlman E, Kazura J W, Hazlett F E, Boom W H. Modulation of murine cytokine responses to mycobacterial antigens by helminth-induced T helper 2 cell responses. J Immunol. 1993;151:4857–4864. [PubMed] [Google Scholar]
- 28.Pearlman E, Kroeze W K, Hazlett F E, Chen S S, Mawhorter S D, Boom W H, Kazura J W. Brugia malayi: acquired resistance to microfilariae in BALB/c mice correlates with local Th2 responses. Exp Parasitol. 1993;76:200–208. doi: 10.1006/expr.1993.1023. [DOI] [PubMed] [Google Scholar]
- 29.Pearlman E, Lass J, Bardenstein D, Diaconu E, Hazlett F J, Albright J, Higgins A, Kazura J. Onchocerca volvulus-mediated keratitis: cytokine production by IL-4-deficient mice. Exp Parasitol. 1996;84:274–281. doi: 10.1006/expr.1996.0113. [DOI] [PubMed] [Google Scholar]
- 30.Pinkston P, Vijayan V K, Nutman T B, Rom W N, O'Donnell K M, Cornelius M J, Kumaraswami V, Ferrans V J, Takemura T, Yenokida G, Thiruvengadam K V, Tripathi S P, Ottesen E A, Crystal R G. Acute tropical pulmonary eosinophilia. Characterization of the lower respiratory tract inflammation and its response to therapy. J Clin Investig. 1987;80:216–225. doi: 10.1172/JCI113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rothenberg M E, MacLean J A, Pearlman E, Leder P. Targeted disruption of the chemokine eotaxin partially reduces peripheral blood and antigen induced tissue eosinophilia. J Exp Med. 1997;185:785–790. doi: 10.1084/jem.185.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schleimer R P, Bochner B S. The role of adhesion molecules in allergic inflammation and their suitability as targets of antiallergic therapy. Clin Exp Allergy. 1998;28(Suppl. 3):15–23. [PubMed] [Google Scholar]
- 33.Shinkai A, Yoshisue H, Koike M, Shoji E, Nakagawa S, Saito A, Takeda T, Imabeppu S, Kato Y, Hanai N, Anazawa H, Kuga T, Nishi T. A novel human CC chemokine, eotaxin-3, which is expressed in IL-4-stimulated vascular endothelial cells, exhibits potent activity toward eosinophils. J Immunol. 1999;163:1602–1610. [PubMed] [Google Scholar]
- 34.Subramanian G, Kazura J W, Pearlman E, Jia X, Malhotra I, King C L. B7–2 requirement for helminth-induced granuloma formation and CD4 type 2 helper cell cytokine expression. J Immunol. 1997;158:5914–5920. [PubMed] [Google Scholar]
- 35.Taylor M J, van Es R P, Shay K, Folkard S G, Townson S, Bianco A E. Protective immunity against Onchocerca volvulus and O. lienalis infective larvae in mice. Trop Med Parasitol. 1994;45:17–23. [PubMed] [Google Scholar]
- 36.Uchida D, Ackerman S, Coyle A, Larsen G, Weller P, Freed J, Irvin C. The effect of human eosinophil granule major basic protein on airway responsiveness in the rat in vivo. A comparison with polycations. Am Rev Respir Dis. 1993;147:982–988. doi: 10.1164/ajrccm/147.4.982. [DOI] [PubMed] [Google Scholar]
- 37.Webb G K G, Job C K, Gault E W. Tropical eosinophilia. Demonstration of microfilariae in lung, liver and lymph nodes. Lancet. 1960;i:835–842. doi: 10.1016/s0140-6736(60)90730-3. [DOI] [PubMed] [Google Scholar]
- 38.Wynn T A, Jankovic D, Hieny S, Zioncheck K, Jardieu P, Cheever A W, Sher A. IL-12 exacerbates rather than suppresses T helper 2-dependent pathology in the absence of endogenous IFN-gamma. J Immunol. 1995;154:3999–4009. [PubMed] [Google Scholar]