ABSTRACT
Bone stress injuries are common in athletes, resulting in time lost from training and competition. Diets that are low in energy availability have been associated with increased circulating bone resorption and reduced bone formation markers, particularly in response to prolonged exercise. However, studies have not separated the effects of low energy availability per se from the associated reduction in carbohydrate availability. The current study aimed to compare the effects of these two restricted states directly. In a parallel group design, 28 elite racewalkers completed two 6‐day phases. In the Baseline phase, all athletes adhered to a high carbohydrate/high energy availability diet (CON). During the Adaptation phase, athletes were allocated to one of three dietary groups: CON, low carbohydrate/high fat with high energy availability (LCHF), or low energy availability (LEA). At the end of each phase, a 25‐km racewalk was completed, with venous blood taken fasted, pre‐exercise, and 0, 1, 3 hours postexercise to measure carboxyterminal telopeptide (CTX), procollagen‐1 N‐terminal peptide (P1NP), and osteocalcin (carboxylated, gla‐OC; undercarboxylated, glu‐OC). Following Adaptation, LCHF showed decreased fasted P1NP (~26%; p < 0.0001, d = 3.6), gla‐OC (~22%; p = 0.01, d = 1.8), and glu‐OC (~41%; p = 0.004, d = 2.1), which were all significantly different from CON (p < 0.01), whereas LEA demonstrated significant, but smaller, reductions in fasted P1NP (~14%; p = 0.02, d = 1.7) and glu‐OC (~24%; p = 0.049, d = 1.4). Both LCHF (p = 0.008, d = 1.9) and LEA (p = 0.01, d = 1.7) had significantly higher CTX pre‐exercise to 3 hours post‐exercise but only LCHF showed lower P1NP concentrations (p < 0.0001, d = 3.2). All markers remained unchanged from Baseline in CON. Short‐term carbohydrate restriction appears to result in reduced bone formation markers at rest and during exercise with further exercise‐related increases in a marker of bone resorption. Bone formation markers during exercise seem to be maintained with LEA although resorption increased. In contrast, nutritional support with adequate energy and carbohydrate appears to reduce unfavorable bone turnover responses to exercise in elite endurance athletes. © 2022 The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).
Keywords: BONE MODELING AND REMODELING, BIOCHEMICAL MARKERS OF BONE TURNOVER, BONE‐MUSCLE INTERACTIONS, EXERCISE, NUTRITION
Introduction
Minimizing time lost due to injury is an important consideration in elite athletes( 1 ) who need to be regularly available for training and competition. Bone stress injuries (reactions and fractures) account for ~20% of annual injuries in competitive track and field athletes.( 2 ) Data from the National Collegiate Athletic Association indicates an injury rate of 4.6–7.2 stress fractures per 100,000 athlete exposures in male track athletes and 11.6–22.3 per 100,000 in females—these rates being among the highest, alongside cross‐country and women's gymnastics.( 3 ) Although participation in weight‐bearing sport has been associated with increased bone density at loaded sites,( 4 ) this benefit may be attenuated in low reproductive hormone or nutritional status.( 5 ) Although multiple factors are often at play, inadequate nutritional support is a key consideration for bone health.( 6 ) Previous studies have identified a role for both overall energy availability( 7 , 8 ) and carbohydrate availability( 9 , 10 , 11 ) in the bone turnover response to exercise. However, because energy restriction involves a relative reduction in carbohydrate intake, it has been difficult to ascertain whether the effect on bone is due to inadequate energy or lack of carbohydrate.
Assessment of the short‐term impact of nutritional or pharmacological interventions relies on the measurement of bone turnover markers (BTMs), as opposed to the longer‐term impact on bone mineral density (BMD).( 12 ) The procollagen‐1 N‐terminal peptide (P1NP) released during collagen synthesis and the C‐terminal telopeptide (CTX) during matrix dissolution represent markers of bone formation and resorption, respectively,( 13 ) and are the most commonly used clinical markers.( 12 ) Whereas carboxylated osteocalcin (gla‐OC) is involved in matrix mineralization,( 14 ) there is increasing recognition of an endocrine role for its undercarboxylated form (glu‐OC) in enhancing glucose and fatty acid uptake and catabolism during exercise.( 15 ) The current utility of BTMs lies in short‐term monitoring of the response to antiresorptive and anabolic therapies in the management of osteoporosis, allowing for more frequent clinical decision‐making than sole reliance on annual BMD measurements.( 12 ) Significant associations between BTM changes and future fracture risk have been observed, independent of BMD.( 16 ) Collectively, these BTMs reflect the bone remodeling, a measure of bone quality which, along with BMD, significantly contributes to bone strength.( 12 ) However, it must be noted that these markers are unable to indicate the status of the processes occurring at a specific site. Additionally, the utility of BTMs in both non‐osteoporotic individuals and with respect to nonpharmacological interventions is underexplored.
Energy availability is defined as the amount of energy remaining for physiological system function after accounting for the energy expended in purposeful exercise, expressed relative to fat‐free mass (FFM).( 17 ) Low energy availability may result from intentional energy restriction and/or an unintentional failure to meet high energy expenditure demands.( 18 ) Meeting energy requirements with adequate energy and nutrient intake should be a priority for the majority of the season, but athletes may periodically engage in short periods of low energy availability to achieve body composition goals, make a specific weight class, improve economy, or increase power/weight ratios.( 19 ) Although there is no absolute threshold at which various body systems are simultaneously affected, previous short‐term (5 day) controlled experiments in healthy females have shown suppression of bone formation at, and below, an energy availability of 30 kcal.kg−1 FFM.day−1, with increased bone resorption at 10 kcal.kg−1 FFM.day−1.( 7 ) Studies in males are limited, but it has been suggested that men can sustain lower energy availability without significant physiological disruption.( 20 ) Indeed, Papageorgiou and colleagues( 21 ) demonstrated that following a 5‐day intervention, women exhibited increased bone resorption and reduced bone formation at an energy availability of 15 kcal.kg−1 FFM.day−1, yet no significant effect was found in men. With this in mind, our study focused exclusively on males, expanding on the limited literature base in this population with respect to the impact of energy manipulation on bone metabolism.
In recent years, there has been an increased interest in using low carbohydrate/high fat diets to improve endurance exercise capacity through a shift toward primary use of fatty acids and ketones as a fuel source.( 22 ) However, this has been shown to reduce capacity for sustained high‐intensity race performance in competitive athletes due to an impairment of exercise economy from differences in the stoichiometry of energy production from fat versus carbohydrate oxidation.( 23 ) Further, 3.5 weeks of a ketogenic (<50 g.day−1 of carbohydrate) diet in elite racewalkers was observed to increase bone resorption (CTX) and decrease bone formation (P1NP) markers at both rest and across exercise.( 11 ) Although there are no published long‐term studies on athletes, evidence from both animal models( 24 ) and children with intractable epilepsy being treated with a ketogenic diet( 25 ) suggests that there may be a detrimental effect of the ketogenic diet on bone health, potentially due to carbohydrate restriction. A second interest in this diet is its ability to separate the effects of low carbohydrate availability from energy availability, allowing for further inquiry into the influences of macronutrients on health and performance, independent of overall energy intake. To our knowledge, only one previous study, involving a 1‐day intervention, has compared energy and carbohydrate availability directly in relation to exercise.( 26 ) Here, the comparator low carbohydrate/high fat diet was not ketogenic in nature, making it difficult to disentangle the energy and carbohydrate effects. Our study aims to expand upon this through the manipulation of macronutrient targets.
Because adjustment of both energy and macronutrient intake is common practice in athletes and may be used as a short‐term strategy to achieve body composition or weight goals prior to competition,( 19 ) determining the health and performance effects of these diet practices is of interest. Accordingly, our aim of this study was to determine whether overall energy availability or carbohydrate availability exerted greater effects on bone metabolic responses to exercise.
Subjects and Methods
Participants
Twenty‐eight elite male racewalkers, eligible for participation in either national or international competition, were recruited for this study via convenience sampling. Based on previous work( 11 ) investigating BTM responses to either a high carbohydrate or ketogenic diet, it was estimated that including 5–10 participants per group (osteocalcin: d = 2.00; CTX: d = 1.52; P1NP: d = 1.27) was appropriate to detect statistical significance with an alpha of 0.05 and power of 0.8 (GPower version 3.1.9.6; https://g-power.apponic.com/). Screening of hormonal and metabolic health was conducted prior to the start of the study—no exclusions were required on the basis of these results. No athletes had a known medical condition or were taking medication or supplements during the study, and recent fractures within the past 3 months were ruled out. All athletes completed the study. Athlete characteristics are presented in Table 1. Athletes took part in one of two separate training camps held in January 2019 in Canberra, Australia (n = 20) and January 2021 in Melbourne, Australia (n = 8). Written informed consent was obtained following explanation of the risks and requirements of the study. The study conformed to the standards required by the Declaration of Helsinki. Ethics approval was obtained from the ethics committees of the Australian Institute of Sport (2019; ref: 20181203) and the Australian Catholic University (2021; ref: 2020‐238HC).
Table 1.
Baseline Characteristics of Athletes Allocated to Each Diet Group
| Characteristic | Unit | CON (n = 10) | LCHF (n = 8) | LEA (n = 10) |
|---|---|---|---|---|
| Age | years | 27 (21–33) | 28 (25–29) | 30 (28–33) |
| Body mass | kg | 66.6 ± 6.2 | 66.2 ± 7.7 | 67.8 ± 5.4 |
| Fat free mass | kg | 58.7 ± 5.5 | 58.6 ± 7.9 | 58.3 ± 4.9 |
| VO2max | mL.kg−1.min−1 | 63.2 ± 3.6 | 67.5 ± 5.7 | 62.1 ± 6.1 |
| BMD spine (L1–L4) | g.cm−2 | 1.19 ± 0.07 | 1.11 ± 0.10 | 1.24 ± 0.13 a |
| Z‐score | 0.02 ± 0.49 | −0.58 ± 0.65 | 0.36 ± 0.97 a | |
| BMD total hip | g.cm−2 | 1.17 ± 0.09 | 1.09 ± 0.14 | 1.10 ± 0.13 |
| Z‐score | 0.73 ± 0.66 | 0.18 ± 0.94 | 0.26 ± 1.05 |
Data are presented as mean ± standard deviation (except for age which is presented as median (interquartile range).
CON = high energy/high carbohydrate; LCHF = low carbohydrate/high fat; LEA = low energy availability.
Indicates significantly higher than LCHF (p < 0.05).
Study protocol
This study was a parallel group design. Each training camp comprised of two, 6‐day phases (Fig. 1). During phase 1 (Baseline), all athletes adhered to a high carbohydrate (~65% of total energy intake), high energy availability (>40 kcal.kg−1 FFM.day−1) control (CON) diet. For phase 2 (Adaptation), athletes were assigned to one of three diets: high carbohydrate/high energy availability (CON, n = 10), low carbohydrate/high fat/high energy availability (LCHF, n = 8), or low energy availability (LEA, n = 10). Due to the inability to blind participants to the diet, allocations to dietary interventions were based on athlete preference while matching for individual characteristics (age, 20 km personal best time, training status). During both phases, a structured training plan was followed to ensure similar training volume and intensity among groups.( 27 ) On the final day of each phase, a 25‐km racewalking protocol was performed, where venous blood samples were taken to measure BTMs.
Fig. 1.
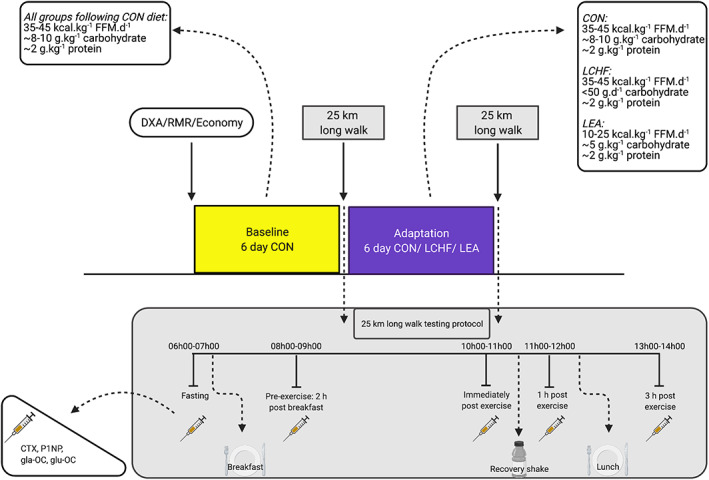
Overview of experimental protocol undertaken by elite racewalking participants, including the dietary interventions and the exercise test with timing of blood sampling. (Created with biorender.com). CON = high energy/high carbohydrate; CTX = carboxy‐terminal telopeptide; DXA = dual energy X‐ray absorptiometry; FFM = fat‐free mass; gla‐OC = carboxylated osteocalcin; glu‐OC = undercarboxylated osteocalcin; LCHF = low carbohydrate/high fat; LEA = low energy availability; P1NP = procollagen‐1 N‐terminal peptide; RMR = resting metabolic rate.
Baseline BMD
Hip and lumbar spine (L1–L4) BMD were measured at baseline via dual‐energy X‐ray absorptiometry (DXA) with Lunar iDXA machines (enCORE version 16; GE Healthcare, Chicago, IL, USA). Measurements were conducted by experienced practitioners certified in clinical bone densitometry. As body composition measurements were performed simultaneously, best practice protocols were followed.( 28 )
Dietary intervention
A brief description of dietary control is provided here; full details are specified elsewhere (manuscript in preparation). All meals were formulated and compliance with intake was monitored by a team of accredited sports dietitians, chefs, and nutritionists. Meals were devised using FoodWorks Professional Edition 9 (Xyris Software, Brisbane, Australia). The CON diet targeted an energy availability of ~40 kcal.kg−1 FFM.day−1, comprised of 65% carbohydrate, 15% protein, and 20% fat. The LCHF group was set the same energy availability target, but with low carbohydrate (<50 g.day−1), moderate protein (2.2 g.kg−1.day−1), and high fat (remainder [~80%] of target energy) intakes. The LEA group had a target energy availability of ~15 kcal.kg FFM.day−1, with a similar macronutrient composition as CON (60% of energy from carbohydrate, 25% of energy from protein, 15% of energy from fat). Calcium in the pre‐exercise meal, the other dietary characteristic known to acutely affect markers of bone turnover (Lundy et al., in review), was minimized (<50 mg) in each of standardized meals consumed prior to the long walk test protocol.
Energy availability was calculated as the difference between energy intake and exercise energy expenditure, normalized to fat free mass (measured via DXA). Exercise energy expenditure was estimated from a four‐stage incremental economy test, using collected respiratory gases inputted into the Weir equation.( 29 ) Resting metabolic rate was measured directly and subtracted from these values, which were then converted to prospective caloric estimates per kilometer. Cross‐training sessions were accounted for in metabolic equivalents. Total exercise energy expenditure was predicted by multiplying these values by the planned training sessions, and energy intake requirements calculated accordingly to achieve target energy availability. Training session completion was monitored twice daily and meals adjusted as necessary. Non‐exercise activity thermogenesis was not considered significant in this context. As a fortunate consequence of this research camp environment, on‐site researchers were able to monitor and confirm that athletes undertook very little activity outside of the prescribed training times.
Test protocol
On the last day of each dietary phase, a 25‐km racewalk was performed, combining both field and laboratory components. One athlete completed only 19 km because he was a junior athlete (18 years old). Athletes arrived in the morning following an overnight fast. An intravenous catheter was inserted and the first blood sample was drawn (6:30 a.m. ± 30 minutes). Athletes were then provided with a standardized breakfast, consisting of 2 g.kg−1 body mass of carbohydrate for all groups in the baseline phase. The same breakfast was provided to the CON group in the adaptation phase, whereas the LCHF group received an isocaloric high‐fat (~80%) option and the LEA group consumed a meal containing 1 g.kg−1 body mass carbohydrate. A pre‐exercise blood sample was taken (8:30 a.m. ± 30 minutes) 15 minutes prior to the onset of the racewalk, which commenced 2 hours after breakfast. Kilometers 1, 7, 13, 19, and 25 were performed on a treadmill at a pace equivalent to ~75% of the athlete's maximal aerobic capacity (VO2 max) and the remaining kilometers were performed at a consistent, self‐nominated pace on a flat, outdoor, road circuit. Carbohydrate gels were consumed following each treadmill bout, totaling ~60 g.h−1 for all groups during baseline and CON during adaptation. The LEA group consumed the equivalent of 30 g.h−1 during adaptation and the LCHF group ingested isocaloric (to CON) high fat snacks. Water was consumed ad libitum. Venous cannulas were flushed with ~3 mL saline after each treadmill bout. Environmental temperature and relative humidity were recorded at 30‐minute intervals with a final individualized value averaged across the exercise bout. Upon completion of the racewalk protocol, another venous blood sample was collected (10:30 a.m. ± 30 minutes). At 30 minutes post‐exercise, athletes received a standardized recovery shake (1.5 g.kg−1 body mass carbohydrate and 0.3 g.kg−1 body mass protein for all groups at baseline, or an isocaloric high‐fat low‐carbohydrate option for LCHF and 0.75 g.kg−1 body mass carbohydrate for LEA at adaptation). A further blood sample was collected at 1 hour post‐exercise (11:30 a.m. ± 30 minutes) after which lunch was provided in accordance with trial phase and dietary allocation. A final blood sample was collected at 3 hours postexercise (1:30 p.m. ± 30 minutes).
Blood analysis
Blood samples were taken at rest (fasted), pre‐exercise, and immediately, 1 hour, and 3 hours postexercise. Samples were collected into BD Vacutainer SST II tubes (East Rutherford, NJ, USA), which were left to clot at room temperature for 30 minutes prior to being centrifuged at 1500g at 4°C for 10 minutes. Serum was aliquotted into 1‐mL Eppendorf tubes and frozen at −80°C for batch analysis. Concentrations of beta‐isomerized CTX, P1NP, gla‐OC, and glu‐OC were measured from each sample. CTX and P1NP concentrations were assessed by electrochemiluminescence immunoassay (Cobas e411; Roche Diagnostics, Basel, Switzerland). Carboxylated and undercarboxylated osteocalcin measurements were performed using enzyme immunoassay (EIA) kits (Takara Bio Inc., Shiga, Japan) analyzed on a FLUROstar OPTIMA microplate reader (BMG Labtech, Ortenberg, Germany). Calculated coefficients of variation were 4.2% (CTX), 3.0% (P1NP), 10.7% (glu‐OC), and 3.9% (gla‐OC).
Statistical analysis
Statistical analysis was performed using R Studio (v1.4.1106; R Core Team, 2021) with significance set at p < 0.05. Athlete characteristics, differences in nutrient intake, and energy availability between groups and phases were analyzed with a two‐way analysis of variance (ANOVA; parametric) after verifying normality with the Shapiro‐Wilk test. Where normality was violated (age only), the Kruskal‐Wallis test (nonparametric) was used for between‐group comparisons. Bone turnover markers were analyzed in three ways: (i) using the change in fasted values from baseline to adaptation, (ii) assessing absolute concentrations across time, and (iii) by calculating area under the concentration‐time curve (AUC) for each participant (pre‐exercise to 3 hours post‐exercise), using the PKSolver add‐in (https://www.pharmpk.com/soft.html) in Microsoft Excel (v16.48; Microsoft Corp, Redmond, WA, USA), prior to further analysis in R (R Foundation for Statistical Computing, Vienna, Austria; https://www.r-project.org/). Linear mixed‐effect models were estimated for all three analyses with restricted maximum likelihood through the “lme4” package in R. As applicable, fixed effects included Diet, Phase, and Time point with random effects of subject and heat index, each nested within study, to account for inter‐individual variation and camp timing. Normality was assessed through quantile‐quantile plots—characteristic departures were not detected. Homoscedasticity was tested with the Fligner‐Killeen test. Statistical significance of fixed effects was determined using Type II Wald tests with Kenward‐Roger approximation post hoc analysis for significant effects was performed using Tukey's Honestly Significant Difference test. Where unequal variance was detected between groups, a Welch's ANOVA and post hoc Dunnett's test was applied. Cohen's d effect sizes were computed using R package “emmeans” with values of 0.2, 0.5, and 0.8 as small, medium, and large effects, respectively.
Results
Participant characteristics
Baseline characteristics are presented as mean ± standard deviation (or median and interquartile range for age) in Table 1. The athletes were well‐matched for age, body mass, fat‐free mass, and VO2max and, because all were male, sex was eliminated as a source of preanalytical variability. Here, we note the first quartile for age being lower in the CON group than the other two groups; more specifically, one athlete was 18 years old. On aggregate, statistically significantly lower spine BMD (p = 0.04) and Z‐scores (p = 0.03) were noted in the LCHF group compared to the LEA group only, yet there was no difference between groups for hip BMD or Z‐scores. Individual data indicated Z‐scores −2 < Z < −1 for two LEA athletes' hip BMD and two LCHF athletes' spine BMD; hence, the practical application to the effect on BTMs is questionable.
Dietary analysis
There was no difference in energy intake, energy availability, macronutrient, or micronutrient intake between the three diets during the baseline phase (p > 0.05; Table 2). In keeping with the study design, during the adaptation phase, energy intake and energy availability were significantly lower in the LEA group than both CON and LCHF (p < 0.001), with fat intake greatest in the LCHF group (p < 0.001). Carbohydrate intake was significantly reduced in the LCHF and LEA groups (p < 0.001) during adaptation; however, the percentage of total energy intake was largely maintained (~60%) in the LEA group.
Table 2.
Dietary Intake for Each Diet Group During Both Baseline and Adaptation Phases
| Baseline | Adaptation | ||||||
|---|---|---|---|---|---|---|---|
| Parameter | Unit | CON (n = 10) | LCHF (n = 8) | LEA (n = 10) | CON (n = 10) | LCHF (n = 8) | LEA (n = 10) |
| Energy intake | kcal.d−1 | 3824 ± 623 | 3843 ± 462 | 3727 ± 335 | 3970 ± 537 | 3730 ± 411 | 2335 ± 238 a , b , c |
| kcal.kg−1 | 58 ± 6 | 59 ± 4 | 55 ± 2 | 61 ± 5 | 57 ± 3 | 35 ± 3 a , b , c | |
| Energy availability | kcal.kg−1 FFM.day−1 | 40 ± 3 | 41 ± 1 | 41 ± 4 | 41 ± 4 | 41 ± 2 | 15 ± 2 a , b , c |
| Carbohydrate | g.day−1 | 613 ± 102 | 616 ± 76 | 599 ± 53 | 639 ± 87 | 36 ± 6 a , b | 338 ± 33 a , b , c |
| g.kg−1.day−1 | 9.4 ± 1.0 | 9.5 ± 0.6 | 8.9 ± 0.4 | 9.8 ± 0.8 | 0.5 ± 0.1 a , b | 5.0 ± 0.4 a , b , c | |
| Protein | g.day−1 | 144 ± 22 | 144 ± 17 | 141 ± 14 | 148 ± 21 | 145 ± 16 | 141 ± 14 |
| g.kg−1.day−1 | 2.2 ± 0.2 | 2.2 ± 0.1 | 2.1 ± 0.1 | 2.3 ± 0.2 | 2.2 ± 0.0 | 2.1 ± 0.1 | |
| Fat | g.day−1 | 83 ± 15 | 84 ± 11 | 79 ± 9 | 84 ± 13 | 330 ± 39 a , b | 40 ± 7 a , b , c |
| g.kg−1.day−1 | 1.3 ± 0.2 | 1.3 ± 0.1 | 1.2 ± 0.1 | 1.3 ± 0.2 | 5.1 ± 0.4 a , b | 0.6 ± 0.1 a , b , c | |
Data are presented as mean ± standard deviation.
CON = high energy/high carbohydrate; LCHF = low carbohydrate/high fat; LEA = low energy availability.
Indicates significant difference to baseline (p < 0.05).
Indicates significant difference to CON (p < 0.05).
Indicates significant difference to LCHF (p < 0.05).
Percent change in fasting concentrations
From Baseline to Adaptation (Fig. 2), all groups showed an increase (p < 0.001) in fasting CTX concentrations with no significant difference between groups (~8%–16%; p = 0.60). The LCHF group showed decreased fasting P1NP (−26% ± 4%; p < 0.0001, d = 3.6), gla‐OC (−22% ± 7%; p = 0.01, d = 1.8), and glu‐OC (−41% ± 8%; p = 0.004, d = 2.1), with these percent changes all significantly different to CON (p < 0.01). The LEA group also demonstrated significant, although smaller, reductions in P1NP (−14% ± 3%; p = 0.02, d = 1.7) and glu‐OC (−24% ± 7%; p = 0.049, d = 1.4) with a nonsignificant reduction in gla‐OC (−7% ± 6%; p = 0.81, d = 0.56); P1NP and gla‐OC percent changes were significantly lower than CON (p < 0.001 and p = 0.03, respectively). In contrast, the CON group showed an increase in P1NP (7% ± 3%; p = 0.76, d = 0.65) and gla‐OC (16% ± 6%; p = 0.25, d = 1.1) and an unchanged glu‐OC (3% ± 8%; p = 1.00, d = 0.12). No differences between LCHF and LEA were found for these percent changes (p > 0.05).
Fig. 2.
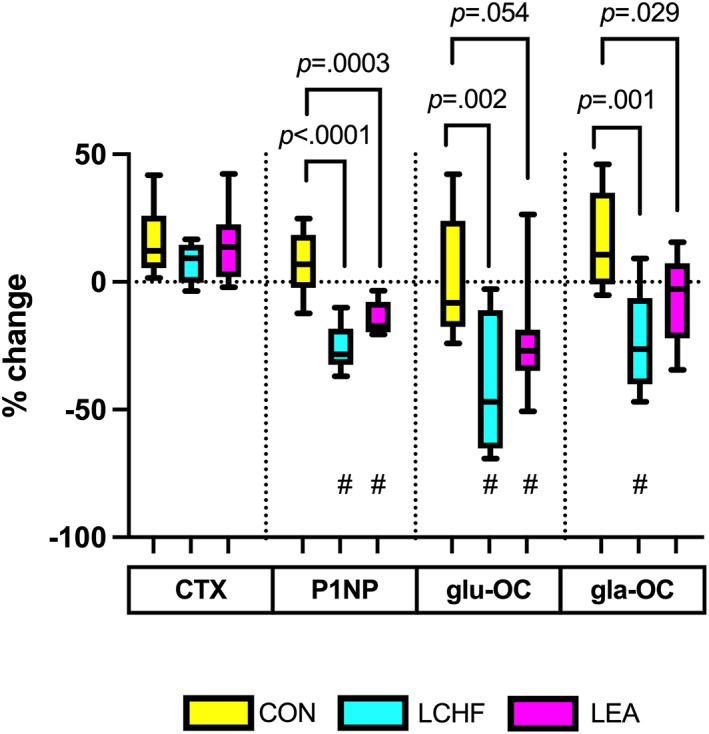
Percent change in fasted concentrations of bone turnover markers from baseline to adaptation. Raw data presented in box plot as median, upper and lower quartiles, and minimum and maximum. Values of p on figure represent comparisons to CON (between group); # represents significant change within group from baseline to adaptation with p values in text. CON = high energy/high carbohydrate; CTX = carboxy‐terminal telopeptide; gla‐OC = carboxylated osteocalcin; glu‐OC = undercarboxylated osteocalcin; LCHF = low carbohydrate/high fat; LEA = low energy availability; P1NP = procollagen‐1 N‐terminal peptide.
Changes across time and exercise
Peak CTX concentrations occurred in the fasted state (p < 0.05) for all groups, decreasing by ~45% prior to the onset of exercise, signifying a clear effect from consuming a meal (Fig. 3A,B ). At 1 hour postexercise, CTX concentrations were greater than pre‐exercise for all groups (~20%; p < 0.001), demonstrating a possible acute response to exercise. Both LCHF (p = 0.0001, d = 1.4) and LEA (p = 0.02, d = 0.91) demonstrated elevated CTX overall following Adaptation, with CON remaining similar to Baseline (p = 0.67, d = 0.42).
Fig. 3.
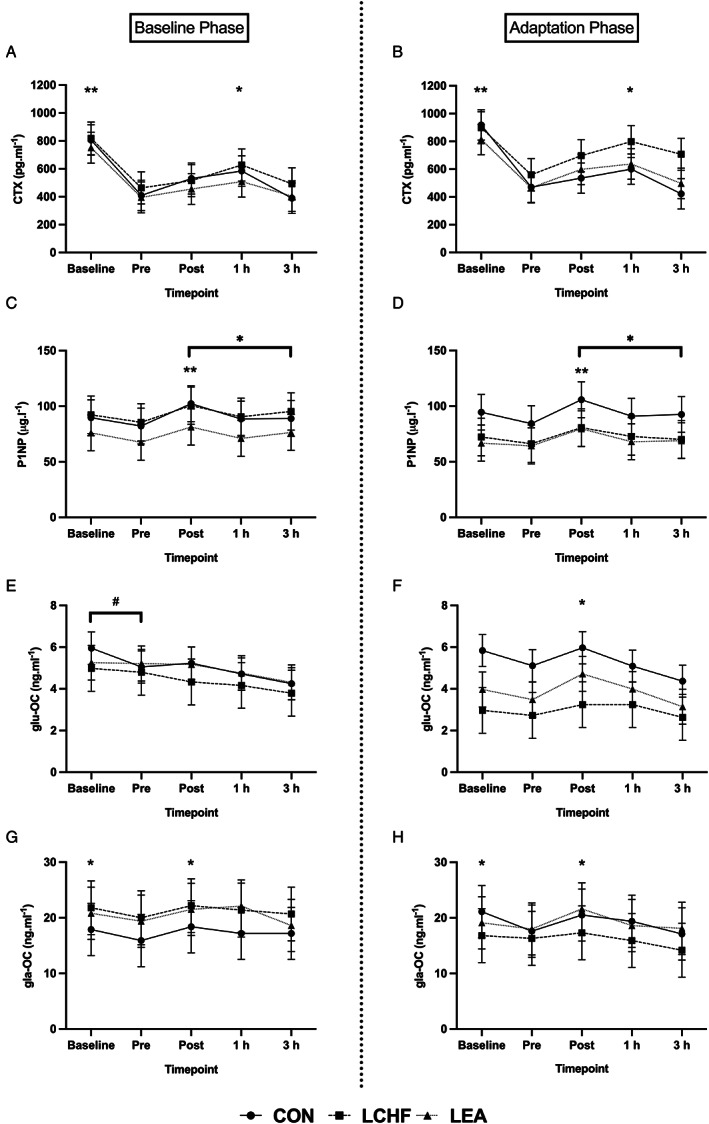
Concentration of bone turnover markers for each diet group in each phase across time. 3A and 3B: CTX concentrations across time per group in the Baseline and Adaptation phases, respectively. 3C and 3D: P1NP concentrations across time per group in the Baseline and Adaptation phases, respectively. 3E and 3F: glu‐OC concentrations across time per group in the Baseline and Adaptation phases, respectively. 3G and 3H: gla‐OC concentrations across time per group in the Baseline and Adaptation phases, respectively. *Time effect: Significantly higher than pre‐exercise in all groups (p < 0.05). **Time effect: Significantly higher than all other time points in all groups (p < 0.05). #Time by trial effect: Significantly higher than adaptation at specific time point (p < 0.05). CON = high energy/high carbohydrate; CTX = carboxy‐terminal telopeptide; gla‐OC = carboxylated osteocalcin; glu‐OC = undercarboxylated osteocalcin; LCHF = low carbohydrate/high fat; LEA = low energy availability; P1NP = procollagen‐1 N‐terminal peptide.
A meal effect, although of small magnitude, was also suggested to occur with P1NP (Fig. 3C,D ) and gla‐OC (Fig. 3G,H ) with pre‐exercise concentrations being ~8% lower than fasted for both markers (p < 0.0001, d = 1.0; and p = 0.01, d = 0.62, respectively). The subsequent influence of exercise was evidenced by an increase in both markers immediately post‐exercise compared to pre‐exercise values (p < 0.0001; P1NP: d = 2.5, gla‐OC: d = 0.86). Similarly, immediately post‐exercise glu‐OC concentrations were higher than pre‐exercise for all groups in the Adaptation phase (Fig. 3F ; p = 0.01, d = 0.97) and those at 3 hours post‐exercise in both phases (Fig. 3E,F ; p < 0.05, Baseline: d = 0.89, Adaptation: d = 1.4).
Exercise‐associated AUC
Both LCHF (p = 0.008, d = 1.9) and LEA (p = 0.01, d = 1.7) groups had significantly higher CTX AUC values following Adaptation (28.0% and 30.9% increase, respectively; Fig. 4A ) but only the LCHF group showed significantly reduced (21.1%; p < 0.0001, d = 3.2) P1NP AUC values (Fig. 4B ). Although both the LCHF (p = 0.003, d = 2.1) and LEA (p = 0.01, d = 1.7) groups had lower exercise‐associated glu‐OC AUC after Adaptation (29.2% and 19.8% reduction, respectively; Fig. 4C ), only the LCHF group also had lower (23.2%) gla‐OC (Fig. 4D ; p = 0.001, d = 2.4). Exercise‐associated AUC remained unchanged for all markers for CON (p > 0.10).
Fig. 4.
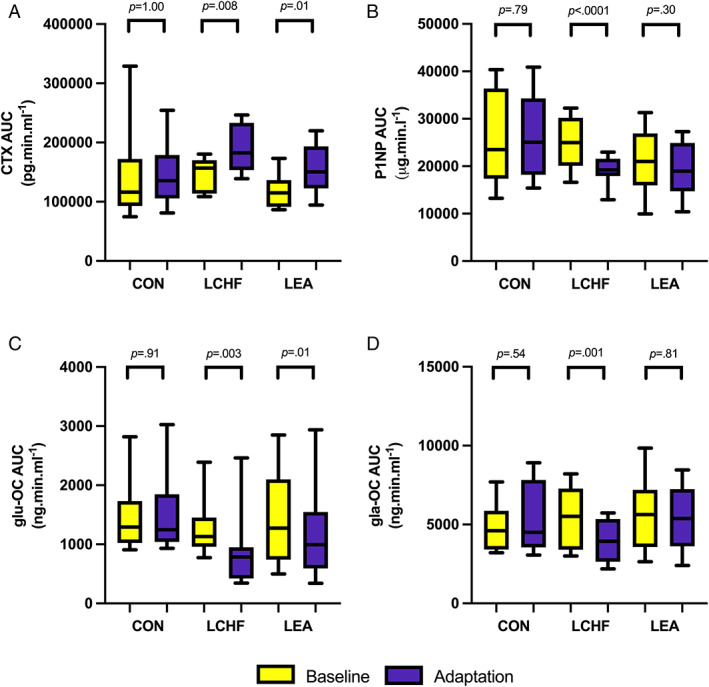
Exercise‐related area under the curve concentrations of bone turnover markers (pre‐exercise to 3 hours post‐exercise) for each diet group in each phase. (A) CTX AUC per group in the Baseline and Adaptation phases. (B) P1NP AUC per group in the Baseline and Adaptation phases. (C) glu‐OC AUC per group in the Baseline and Adaptation phases. (D) gla‐OC AUC per group in the Baseline and Adaptation phases. Raw data presented in box plot as median, upper and lower quartiles, and minimum and maximum. Values of p represent difference from baseline to adaptation. AUC = area under the curve; CON = high energy/high carbohydrate; CTX = carboxy‐terminal telopeptide; gla‐OC = carboxylated osteocalcin; glu‐OC = undercarboxylated osteocalcin; LCHF = low carbohydrate/high fat; LEA = low energy availability; P1NP = procollagen‐1 N‐terminal peptide.
Discussion
This is the first study to compare the effect of short‐term (6‐day) adherence to high energy availability/high carbohydrate (CON), high energy availability/ketogenic (LCHF), and low energy availability (LEA) diets on markers of bone resorption and formation around a prolonged bout of exercise. Our main findings indicate that, in comparison to undertaking exercise with high energy/high carbohydrate dietary support (CON), a marker of bone resorption was increased when exercise was undertaken following either the LEA or LCHF ketogenic diet. In contrast, bone formation markers during exercise were negatively affected by the LCHF ketogenic diet only. In addition, fasted concentrations of P1NP and glu‐OC declined in both LEA and LCHF, yet CTX was similar between groups. Therefore, this study suggests that carbohydrate may be key for maintaining bone formation during prolonged exercise, but that both overall energy and carbohydrate are necessary to support bone formation at rest and limit exercise‐related bone resorption.
Fasted P1NP and both forms of osteocalcin (glu‐OC, gla‐OC) declined to a greater degree in the LCHF group than in LEA, without significant differences in CTX in either group compared to the CON diet. Although data on healthy young males need to be expanded, there is evidence of an intraindividual weekly biological variation of ~9% for CTX, ~7% for P1NP,( 30 ) and ~10% for osteocalcin.( 31 ) Comparing these values with the magnitude of changes seen in our study and, noting the additional factors of tight dietary and activity control as well as well‐matched groups, it is plausible that a true effect was observed. Our results align with the findings of a previous study of low energy availability in well‐trained young male distance runners, in which energy balanced (~40 kcal.kg−1 FFM.day−1) and restricted (50% of balanced condition) diets were compared over a 5‐day period. Here the authors reported an ~15% reduction in fasting P1NP concentrations in the energy‐restricted group without significant change in urine N‐terminal telopeptide (NTX) or serum OC.( 32 ) Murphy and colleagues( 8 ) demonstrated a reduction in P1NP (15%–25%, compared to 14% in the current study) and up to 6% increase in CTX following 5‐day adherence to low energy availability (~15 kcal.kg−1 FFM.day−1) in recreationally active males performing concurrent cycling. In contrast, Papageorgiou and colleagues( 21 ) showed no difference in P1NP nor CTX in the recreationally active young male cohort of their study, which compared low energy availability (~15 kcal.kg−1 FFM.day−1) and control (~45 kcal.kg−1 FFM.day−1) diets over 5 days of running. Further studies are required to confirm these discrepant results of low energy availability in males on fasted bone resorption markers and related magnitude, but it appears that bone formation markers may be more consistently affected and to a similar magnitude over a short‐term period.
The novel aspect of this study was the inclusion of a high‐energy LCHF group against which to compare the relative effects on bone. Though a longer intervention period than the current study, our group has previously reported( 11 ) that 3.5 weeks of an LCHF diet in elite racewalkers resulted in a ~22% increase in fasted CTX concentrations, an ~14% decline in P1NP, and an ~25% decline in total OC. In contrast, in the current study, our LCHF group exhibited smaller increases in fasted CTX (~8%) but greater reductions in P1NP (~26%) and OC (gla‐OC ~22%, glu‐OC ~41%). Because the participants were similar demographically, it could be speculated that these differences are attributable to the different lengths of the diet intervention. Nevertheless, the current study may suggest that the short‐term low‐carbohydrate availability suppresses bone formation markers at rest to a greater extent than both high‐energy availability/high‐carbohydrate and low‐energy availability diets, while exerting a smaller effect on bone resorption markers. The translation of circulating biomarkers to structural bone changes requires further research, because currently there is no evidence to indicate site‐specific remodeling or for the prediction of fracture risk in non‐osteoporotic populations.
It is further noted that both LCHF and LEA groups demonstrated lower fasted glu‐OC following adaptation than the CON group. It is increasingly recognized that glu‐OC may play an important role in energy metabolism, where lower glu‐OC has been associated with impaired glucose metabolism, increased adiposity, and lower testosterone.( 33 ) Although it should be noted that these studies, from animal models and cross‐sectional studies of humans, require further support from well‐controlled intervention studies, restriction of carbohydrate and energy intake while expending substantial energy via high‐intensity exercise may lead to unfavorable metabolic adaptations.
Our study not only explores the effect of short‐term energy and macronutrient manipulation on fasted concentrations of BTMs but also the response to feeding. Pre‐exercise concentrations were lower than fasted concentrations for CTX, P1NP, and gla‐OC, although the magnitude was substantially larger for CTX (~45% versus ~8%). The effect of feeding was explicitly examined by Scott and colleagues,( 34 ) where comparisons were made between undertaking exercise fasted or 2 hours following breakfast (2.3 MJ, 60% carbohydrate, 32% fat, 8% protein). Here, CTX was lower in the fed condition from 1 hour post‐meal until 1 hour after a 60‐minute run. In contrast, P1NP was unaffected by time or food intake prior to exercise, with OC declining prior to exercise regardless of food consumption. Similarly, Bjarnason and colleagues( 35 ) demonstrated a 50% reduction in CTX over 2 hours following an oral glucose tolerance test as opposed to fasting, yet no response in osteocalcin was observed. In a similar population of racewalkers to those in the current study, we previously showed that CTX decreased 2 hours following breakfast as well, but this was not seen for P1NP or osteocalcin.( 11 ) Although the meal effect is clear for CTX, it is less so for the other markers of bone turnover. These findings underline the importance of standardized procedures when comparing BTMs to each other and across time, especially when monitoring the effects of a nutritional intervention or pharmacological therapy.
Regardless of diet or phase, exercise seemed to have an effect on markers of bone turnover, with both P1NP and CTX increasing over exercise, then declining either to, or below, fasted levels by 3 hours. However, we note that, without a resting control, the acute effect of exercise on these markers is inconclusive. This response over time needs to be viewed against the background of circadian variation in these markers, which, although heavily influenced by feeding,( 35 ) typically follows a peak in the early morning hours (~2:00 a.m. to 5:00 a.m.) and a nadir in the afternoon (~12:00 p.m. to 4:00 p.m.).( 36 ) In our study, the exercise bout occurred between 8:00 a.m. and 11:00 a.m. with blood sampling and feeding on either side as described. Thus, although circadian variability could have influenced our results, some insight can be gleaned from the between‐group comparisons. In the LCHF group there was an increase in CTX and a decrease in P1NP from pre‐exercise to 3 hours post‐exercise. In contrast, the higher carbohydrate intake in the other groups resulted in maintenance of bone formation, marginally offsetting the increased bone resorption from short‐term reduced energy availability. The acute effects of carbohydrate consumption around exercise have been shown previously. Sale and colleagues( 10 ) compared an 8% carbohydrate solution to placebo consumed by physically active men before, during, and immediately after a 120‐minute treadmill run at 70% VO2max, where the carbohydrate group demonstrated lower CTX and P1NP concentrations up to 2 hours following exercise. The similar findings in the current study now extend this evidence of acute carbohydrate provision on bone turnover to periods of short‐term (~1 week) carbohydrate manipulation. It must be noted that, although the majority of circulating CTX and P1NP is derived from bone, the possibility of derivation from other type 1 collagen sources, such as tendons and cartilage, remains.( 37 ) Nevertheless, taken together with the lower bone‐specific gla‐OC in the LCHF group, a role for carbohydrate in supporting bone formation is suggested.
Of further interest is the crosstalk between bone and other systems, and the influence of exercise and nutrition thereon. In the same study by Sale and colleagues,( 10 ) carbohydrate provision resulted in lower interleukin‐6 (IL‐6) concentrations up to 2 hours after exercise, with a strong correlation between post‐exercise IL‐6 and CTX.( 10 ) IL‐6 is released from the muscle during exercise and serves to increase glucose availability, particularly in situations of low glycogen.( 38 ) This myokine has also been shown to be involved in a feedforward loop with osteocalcin, which itself may increase uptake and catabolism of fatty acids and glucose in the muscle,( 15 ) creating a crosstalk between muscle and bone. Higher IL‐6 concentrations were evident following a short‐term LCHF diet compared to LEA or CON, with these data reported elsewhere( 27 ); however, no significant correlations between post‐exercise IL‐6 and BTMs were apparent. In vitro studies have shown that IL‐6 increases the expression of receptor activator of nuclear factor κ‐Β ligand (RANKL) and decreases osteoprotegerin (OPG) expression in osteoblasts, resulting in increased bone resorption (CTX) and production of glu‐OC.( 15 ) Although we observed a decrease in glu‐OC following adaptation in both the LCHF and LEA groups, both had significant increases in CTX across exercise, with LCHF also exhibiting a decline in P1NP and gla‐OC. Therefore, in support of the higher IL‐6 concentrations,( 27 ) short‐term LCHF appears to result in greater exercise‐related bone resorption than an isoenergetic high‐carbohydrate diet or a low energy availability diet.
To our knowledge, the only other study to compare the separate effects of energy and carbohydrate availability in relation to exercise is by Hammond and colleagues.( 26 ) Following a morning high‐intensity interval training (HIIT) run, participants consumed one of three diets in a randomized crossover order: isocaloric (60 kcal.kg−1 FFM) high carbohydrate (12 g.kg−1 body mass [BM]) or non‐ketogenic low‐carbohydrate high‐fat (3 g.kg−1 BM), or energy and carbohydrate restricted (20 kcal.kg−1 FFM, 3 g.kg−1). Three‐and‐a‐half hours later, they performed a second HIIT run and then continued the diets for a further 17 hours. Both conditions where lower carbohydrate was consumed, regardless of energy availability, showed increased CTX concentrations prior to and up to 3 hours after the afternoon HIIT, with no effect of the diet on P1NP. Here, the authors concluded that carbohydrate may have a more important (energy independent) influence on limiting bone resorption during exercise. In contrast, our study suggests that carbohydrate may be important for maintaining bone formation but both adequate energy and carbohydrate are needed to limit exercise‐related bone resorption. Differences in intervention period lengths, dietary composition (both carbohydrate and energy availability targets), participant characteristics, and study design features may account for the slight difference in results.
The strengths of this study lie in the tight dietary and activity control as well as the closely matched groups in terms of baseline characteristics. We acknowledge the small sample size as a limitation yet highlight the corresponding small pool of elite athletes in the population from which it is feasible to draw upon at any given time.( 39 ) We also note the inclusion of younger athletes in the CON group who may have had higher bone turnover. However, although increased turnover may be more likely for males younger than 20 years old, there seems to be less variability between age groups thereafter.( 40 ) With the majority aged in their mid to late 20s, as indicated by the interquartile ranges, and the aforementioned strengths of control in this study, we expect that this contribution to preanalytical variability has been somewhat moderated. Furthermore, the statistically significant difference in spine BMD between the LEA and LCHF groups could have influenced BTM concentrations. Yet with little evidence demonstrating a correlation between BTMs and BMD in young males (possibly due to different site accrual rates( 41 )) and the individuals' distribution between groups, we are hesitant about the clinical relevance. Here we emphasize the related and final limitation of our study in extrapolating circulating BTM changes to infer implications to bone structure or function over the longer term in this population with the currently available evidence.
Conclusion
Short‐term carbohydrate restriction appears to result in reduced circulating markers of bone formation at rest and during exercise with a further exercise‐related increase in a bone resorption marker. Although short‐term LEA seemed to be better tolerated with relatively unchanged bone formation markers across exercise, the marker of bone resorption was still increased. Both carbohydrate and energy restriction may further impair energy metabolism through the reduced endocrine action of OC. In contrast, the provision of a diet with adequate energy and carbohydrate to support training in elite athletes appears to prevent the unfavorable imbalance between bone resorption and formation markers and may improve energy metabolism. We acknowledge the short‐term nature of this study and the limitations of translating these findings to longer term outcomes. Nevertheless, because BTMs provide insight into bone quality,( 12 ) may predict fracture risk in some populations,( 16 ) and offer a more accessible and shorter‐term monitoring tool than DXA,( 12 ) they are a useful tool to evaluate responses to nutritional or pharmacological interventions, and deserve further attention for use in athletic populations. Athletes may frequently engage in short‐term periods of dietary manipulation throughout the season, and the potential long‐term impact of accumulating these short, periodic cycles on bone health is of interest. Although the effects on future bone strength warrants further investigation, the current study supports the notion that a short‐term low‐energy diet may be better tolerated than a ketogenic diet, provided that adequate protein and carbohydrate is sustained.
AUTHOR CONTRIBUTIONS
Nikita C. Fensham: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; resources; software; validation; visualization; writing – original draft; writing – review and editing. Ida A. Heikura: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; resources; software; validation; writing – review and editing. Alannah K.A. McKay: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; resources; software; supervision; validation; writing – review and editing. Nicolin Tee: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; resources; validation; writing – review and editing. Kathryn E. Ackerman: Funding acquisition; resources; supervision; validation; writing – review and editing. Louise M. Burke: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; resources; supervision; validation; writing – review and editing.
Conflicts of Interest
The authors and funding agents do not have any conflicts of interest.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/jbmr.4658.
Acknowledgments
The study was funded by a Program Grant from the Australian Catholic University Research Funds to LMB and funding from the Wu Tsai Human Performance Alliance and Boston Children's Hospital via KEA. Specific thanks to Brent Vallance for coordinating the training program and Dr. Jamie Whitfield for oversight of the laboratory testing. We thank our research colleagues and supporters of the Supernova research camps and extend our gratitude to the elite racewalking athletes.
Authors’ roles: The study was designed by LMB, IAH, AKA, NT, and NCF. Data were collected and analyzed by LMB, AKA, IAH, NT, and NCF, and interpreted by LMB, AKA, IAH, NT, KEA, and NCF. All authors contributed to drafting the manuscript or revising it critically for important intellectual content. All authors approved the final version of the submitted manuscript. Open access publishing facilitated by Australian Catholic University, as part of the Wiley ‐ Australian Catholic University agreement via the Council of Australian University Librarians.
Data Availability Statement
The data that support the findings of this study are openly available in “figshare” at https://doi.org/10.6084/m9.figshare.17352176.v2
References
- 1. Feddermann‐Demont N, Junge A, Edouard P, Branco P, Alonso J‐M. Injuries in 13 international athletics championships between 2007–2012. Br J Sports Med. 2014;48(7):513‐522. [DOI] [PubMed] [Google Scholar]
- 2. Bennell KL, Malcolm SA, Thomas SA, Wark JD, Brukner PD. The incidence and distribution of stress fractures in competitive track and field athletes. A twelve‐month prospective study. Am J Sports Med. 1996;24(2):211‐217. [DOI] [PubMed] [Google Scholar]
- 3. Rizzone KH, Ackerman KE, Roos KG, Dompier TP, Kerr ZY. The epidemiology of stress fractures in collegiate student‐athletes, 2004‐2005 through 2013‐2014 academic years. J Athl Train. 2017;52(10):966‐975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tenforde AS, Fredericson M. Influence of sports participation on bone health in the young athlete: a review of the literature. PM R. 2011;3(9):861‐867. [DOI] [PubMed] [Google Scholar]
- 5. Tenforde AS, Carlson JL, Sainani KL, et al. Sport and triad risk factors influence bone mineral density in collegiate athletes. Med Sci Sports Exerc. 2018;50(12):2536‐2543. [DOI] [PubMed] [Google Scholar]
- 6. Sale C, Elliott‐Sale KJ. Nutrition and athlete bone health. Sports Med. 2019;49(Suppl 2):139‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ihle R, Loucks AB. Dose‐response relationships between energy availability and bone turnover in young exercising women. J Bone Miner Res. 2004;19(8):1231‐1240. [DOI] [PubMed] [Google Scholar]
- 8. Murphy C, Bilek LDD, Koehler K. Low energy availability with and without a high‐protein diet suppresses bone formation and increases bone resorption in men: a randomized controlled pilot study. Nutrients. 2021;13(3):802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Sousa MV, Pereira RM, Fukui R, Caparbo VF, da Silva ME. Carbohydrate beverages attenuate bone resorption markers in elite runners. Metabolism. 2014;63(12):1536‐1541. [DOI] [PubMed] [Google Scholar]
- 10. Sale C, Varley I, Jones TW, et al. Effect of carbohydrate feeding on the bone metabolic response to running. J Appl Physiol. 2015;119(7):824‐830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heikura IA, Burke LM, Hawley JA, et al. A short‐term ketogenic diet impairs markers of bone health in response to exercise. Front Endocrinol. 2019;10:880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu CH, Chang YF, Chen CH, et al. Consensus statement on the use of bone turnover markers for short‐term monitoring of osteoporosis treatment in the Asia‐Pacific region. J Clin Densitom. 2021;24(1):3‐13. [DOI] [PubMed] [Google Scholar]
- 13. Song L. Calcium and bone metabolism indices. Adv Clin Chem. 2017;82:1‐46. [DOI] [PubMed] [Google Scholar]
- 14. Moser SC, Van Der Eerden BCJ. Osteocalcin—a versatile bone‐derived hormone. Front Endocrinol (Lausanne). 2019;9:794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mera P, Laue K, Ferron M, et al. Osteocalcin signaling in myofibers is necessary and sufficient for optimum adaptation to exercise. Cell Metab. 2016;23(6):1078‐1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tian A, Ma J, Feng K, et al. Reference markers of bone turnover for prediction of fracture: a meta‐analysis. J Orthop Surg Res. 2019;14(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Loucks AB, Kiens B, Wright HH. Energy availability in athletes. J Sports Sci. 2011;29(Suppl 1):S7‐S15. [DOI] [PubMed] [Google Scholar]
- 18. Loucks AB. Energy balance and body composition in sports and exercise. J Sports Sci. 2004;22(1):1‐14. [DOI] [PubMed] [Google Scholar]
- 19. Burke LM, Close GL, Lundy B, Mooses M, Morton JP, Tenforde AS. Relative energy deficiency in sport in male athletes: a commentary on its presentation among selected groups of male athletes. Int J Sport Nutr Exerc Metab. 2018;28(4):364. [DOI] [PubMed] [Google Scholar]
- 20. De Souza MJ, Koltun KJ, Williams NI. The role of energy availability in reproductive function in the female athlete triad and extension of its effects to men: an initial working model of a similar syndrome in male athletes. Sports Med. 2019;49(Suppl 2):125‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Papageorgiou M, Elliott‐Sale KJ, Parsons A, et al. Effects of reduced energy availability on bone metabolism in women and men. Bone. 2017;105:191‐199. [DOI] [PubMed] [Google Scholar]
- 22. Volek JS, Noakes T, Phinney SD. Rethinking fat as a fuel for endurance exercise. Eur J Sport Sci. 2015;15(1):13‐20. [DOI] [PubMed] [Google Scholar]
- 23. Burke LM, Sharma AP, Heikura IA, et al. Crisis of confidence averted: impairment of exercise economy and performance in elite race walkers by ketogenic low carbohydrate, high fat (LCHF) diet is reproducible. PLoS One. 2020;15(6):e0234027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bielohuby M, Matsuura M, Herbach N, et al. Short‐term exposure to low‐carbohydrate, high‐fat diets induces low bone mineral density and reduces bone formation in rats. J Bone Miner Res. 2010;25(2):275‐284. [DOI] [PubMed] [Google Scholar]
- 25. Simm PJ, Bicknell‐Royle J, Lawrie J, et al. The effect of the ketogenic diet on the developing skeleton. Epilepsy Res. 2017;136:62‐66. [DOI] [PubMed] [Google Scholar]
- 26. Hammond KM, Sale C, Fraser W, et al. Post‐exercise carbohydrate and energy availability induce independent effects on skeletal muscle cell signalling and bone turnover: implications for training adaptation. J Physiol. 2019;597(18):4779‐4796. [DOI] [PubMed] [Google Scholar]
- 27. McKay AKA, Peeling P, Pyne DB, et al. Six days of low carbohydrate, not energy availability, alters the iron and immune response to exercise in elite athletes. Med Sci Sports Exerc. 2022;54(3):377‐387. [DOI] [PubMed] [Google Scholar]
- 28. Nana A, Slater GJ, Stewart AD, Burke LM. Methodology review: using dual‐energy x‐ray absorptiometry (DXA) for the assessment of body composition in athletes and active people. Int J Sport Nutr Exerc Metab. 2015;25(2):198‐215. [DOI] [PubMed] [Google Scholar]
- 29. Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109(1‐2):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang S, Mu R, Zhang X, Yun K, Shang H, Zhao M. Biological variation in serum bone turnover markers. Ann Clin Biochem. 2020;57(2):144‐150. [DOI] [PubMed] [Google Scholar]
- 31. Panteghini M, Pagani F. Biological variation in bone‐derived biochemical markers in serum. Scand J Clin Lab Invest. 1995;55(7):609‐616. [DOI] [PubMed] [Google Scholar]
- 32. Zanker CL, Swaine IL. Responses of bone turnover markers to repeated endurance running in humans under conditions of energy balance or energy restriction. Eur J Appl Physiol. 2000;83(4‐5):434‐440. [DOI] [PubMed] [Google Scholar]
- 33. Zoch ML, Clemens TL, Riddle RC. New insights into the biology of osteocalcin. Bone. 2016;82:42‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Scott JP, Sale C, Greeves JP, Casey A, Dutton J, Fraser WD. Effect of fasting versus feeding on the bone metabolic response to running. Bone. 2012;51(6):990‐999. [DOI] [PubMed] [Google Scholar]
- 35. Bjarnason NH, Henriksen EE, Alexandersen P, Christgau S, Henriksen DB, Christiansen C. Mechanism of circadian variation in bone resorption. Bone. 2002;30(1):307‐313. [DOI] [PubMed] [Google Scholar]
- 36. Hannon R, Eastell R. Preanalytical variability of biochemical markers of bone turnover. Osteoporos Int. 2000;11(Suppl 6):S30‐S44. [DOI] [PubMed] [Google Scholar]
- 37. Vasikaran S, Eastell R, Bruyere O, et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int. 2011;22(2):391‐420. [DOI] [PubMed] [Google Scholar]
- 38. Hennigar SR, McClung JP, Pasiakos SM. Nutritional interventions and the IL‐6 response to exercise. FASEB J. 2017;31(9):3719‐3728. [DOI] [PubMed] [Google Scholar]
- 39. McKay AKA, Stellingwerff T, Smith ES, et al. Defining training and performance caliber: a participant classification framework. Int J Sports Physiol Perform. 2022;17(2):317‐331. [DOI] [PubMed] [Google Scholar]
- 40. Shao J, Zhou SS, Qu Y, Liang BB, Yu QH, Wu J. Correlation between bone turnover and metabolic markers with age and gender: a cross‐sectional study of hospital information system data. BMC Musculoskelet Disord. 2020;21(1):603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Szulc P, Garnero P, Munoz F, Marchand F, Delmas PD. Cross‐sectional evaluation of bone metabolism in men. J Bone Miner Res. 2001;16(9):1642‐1650. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available in “figshare” at https://doi.org/10.6084/m9.figshare.17352176.v2


