Abstract
Human skin contains two distinct components: brown to black, insoluble eumelanin and light colored, alkaline‐soluble pheomelanin. Eumelanin consists of 5,6‐dihydroxyindole (DHI) and 5,6‐dihydroxyindole‐2‐carboxylic acid (DHICA) moieties, while pheomelanin consists of benzothiazine (BT) and benzothiazole (BZ) moieties. These melanin monomer units can be quantitatively analyzed through specific degradation products by high‐performance liquid chromatography (HPLC). Alkaline hydrogen peroxide oxidation (AHPO) of eumelanin gives rise to pyrrole‐2,3,5‐tricarboxylic acid (PTCA) and pyrrole‐2,3‐dicarboxylic acid (PDCA) as specific degradation products of the DHICA and DHI moieties, respectively. BZ moiety in pheomelanin can be analyzed as thiazole‐2,4,5‐tricarboxylic acid (TTCA). By reductive hydrolysis with hydroiodic acid, BT moieties in pheomelanin can be analyzed as 4‐amino‐3‐hydroxyphenylalanine (4‐AHP). As a recently improved AHPO–HPLC method enabled a better characterization of PDCA, this prompted us to address the question of DHI to DHICA ratio in human skin samples with varying degrees of constitutive pigmentation ranging from very light to dark. Results showed for the first time the ratio of 4 moieties: DHI 35%, DHICA 41%, BZ 20%, and BT 4%. The ratio is constant regardless of the degree of pigmentation. The high content of DHICA moiety may impart an antioxidant property to the epidermis melanin.
Keywords: 5,6‐dihydroxyindole; 5,6‐dihydroxyindole‐2‐carboxylic acid; eumelanin; pheomelanin; skin
Significance.
The most adapted method that allows qualitative analysis of the composition of eumelanin and pheomelanin pigments involves the measurement of chemically degraded melanin products using HPLC and has become the accepted standard for the determination of melanin composition in biological tissues. Until recently, for eumelanin, only the DHICA content could be estimated. Thanks to the improvement of the HPLC method, here, we report the quantification of DHI eumelanin content in skin of varying pigmentation.
Skin color is determined mainly by epidermal melanin pigments produced by highly specialized cells, the melanocytes. The latter produce two chemically distinct types of melanin in organelles called melanosomes: the insoluble, black to brown eumelanin, and the alkaline‐soluble, yellow to reddish pheomelanin (Alaluf et al., 2002; Barsh, 2003; Taylor, 2002). Both pigments are derived from the common precursor dopaquinone produced from tyrosine by the action of tyrosinase (Ito & Wakamatsu, 2008). The kind of pigment produced in melanosomes is determined by the availability of cysteine (Ito & Wakamatsu, 2011). Cysteine reacts rapidly and quantitatively with dopaquinone to produce 5‐S‐cysteinyldopa (5SCD) and 2‐S‐cysteinyldopa (2SCD) in a ratio of 5.3:1 (Ito & Prota, 1977). Dopaquinone then oxidizes cysteinyldopas to give benzothiazine (BT) intermediates, which gradually polymerize to form pheomelanin pigment. The benzothiazine moiety is gradually converted to benzothiazole (BZ) moiety in the late stage of pheomelanin production (Wakamatsu et al., 2009). When cysteine is depleted in melanosomes, dopaquinone spontaneously reacts to give, 5,6‐dihydroxyindole (DHI) and 5,6‐dihydroxyindole‐2‐carboxylic acid (DHICA), via dopachrome. The production of DHICA is accelerated by dopachrome tautomerase (also called tyrosinase‐related protein 2) or copper ions (Ito, Suzuki, et al., 2013). Further oxidation of these 5,6‐dihydroxyindoles produce then the eumelanin polymer.
The total amount of melanin can be estimated by spectrophotometry, after dissolving melanin with Soluene‐350 plus water and measuring absorbance at 500 nm (A500; Ozeki et al., 1996). The relative ratio of eumelanin to pheomelanin can be estimated by the ratio of absorbances A650/A500 (Ozeki et al., 1996). Furthermore, electron paramagnetic resonance spectroscopy allows the detection and characterization of different melanin pigments (Sarna & Swartz, 1978; Sealy, Hyde, Felix, Menon, & Prota, 1982; Sealy, Hyde, Felix, Menon, Prota, Swartz, et al., 1982; Swartz et al., 1979). The total amount of melanin and the type of eumelanin and pheomelanin in tissue samples can also be analyzed through specific degradation products by high‐performance liquid chromatography (HPLC; Figure 1). Alkaline hydrogen peroxide oxidation (AHPO) of eumelanin gives rise to pyrrole‐2,3,5‐tricarboxylic acid (PTCA) and pyrrole‐2,3‐dicarboxylic acid (PDCA) as specific degradation products of the DHICA and DHI moieties, respectively (Ito & Fujita, 1985; Ito et al., 2011). AHPO of BZ moiety in pheomelanin can be analyzed as thiazole‐2,4,5‐tricarboxylic acid (TTCA) and thiazole‐4,5‐dicarboxylic acid (TDCA; Ito et al., 2011). BT moieties in pheomelanin can be analyzed as 4‐amino‐3‐hydroxyphenylalanine (4‐AHP) and 3‐amino‐4‐hydroxyphenylalanine (3‐AHP) after reductive hydrolysis with hydroiodic acid (HI; Ito & Fujita, 1985; Wakamatsu et al., 2002). The TTCA/4‐AHP ratio is used as a marker for the conversion of BT into the BZ moiety in pheomelanin (Ito et al., 2011; Wakamatsu et al., 2012). Furthermore, AHPO of eumelanin was shown to produce, in addition to PTCA and PDCA, pyrrole‐2,3,4,5‐tetracarboxylic acid (PTeCA) that derives from cross‐linking of the C2 and C3 positions of the DHI moiety in eumelanin (Ito, Wakamatsu, et al., 2013) and is marker of photoaging of eumelanin.
FIGURE 1.
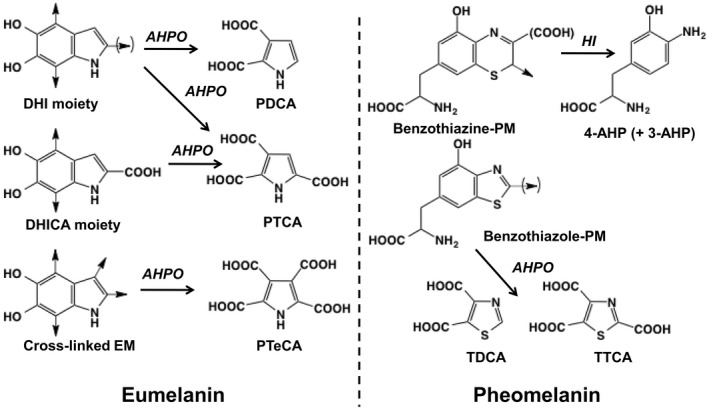
Alkaline hydrogen peroxide oxidation (AHPO) and hydroiodic acid (HI) hydrolysis of eumelanin and pheomelanin. AHPO of the 5,6‐dihydroxyindole (DHI) moiety gives pyrrole‐2,3‐dicarboxylic acid (PDCA) and pyrrole‐2,3,5‐tricarboxylic acid (PTCA), while 5,6‐dihydroxyindole‐2‐carboxylic acid (DHICA) moiety gives PTCA. The benzothiazole (BZ) moiety of pheomelanin gives thiazole‐2,4,5‐tricarboxylic acid (TTCA) and thiazole‐4,5‐dicarboxylic acid (TDCA). Upon HI hydrolysis, the benzothiazine (BT) moiety gives 4‐amino‐3‐hydroxyphenylalanine (4‐AHP) and 3‐amino‐4‐hydroxyphenylalanine (3‐AHP). Taken from Ito et al. (2020).
Using both methods, spectrophotometry after solubilization in Soluene‐350 and HPLC after chemical degradation, we analyzed for the first time the “chemical” melanin phenotype, that is, the total melanin amount and eu/pheomelanin content in a large number of skin samples, representative of a broad range of constitutive pigmentation intensities, rigorously classified according to their Individual Typology Angle (ITA°; Del Bino et al., 2015). Results showed a good correlation between constitutive pigmentation measured by ITA° and total melanin content assessed by both methods. They also showed a good correlation between ITA° and PTCA (DHICA eumelanin), TTCA (BZ moiety of pheomelanin) but not 4‐AHP (BT moiety of pheomelanin). Interestingly, HPLC analysis revealed for the first time that the human epidermis comprises approximately 74% eumelanin and 26% pheomelanin, regardless of the degree of pigmentation, the pheomelanin being mostly of the BZ type.
Alkaline hydrogen peroxide oxidation quantification of the five markers PTCA, PDCA, and PTeCA from eumelanin and TTCA and TDCA from pheomelanin by HPLC using the original method of 0.1 M potassium phosphate buffer (pH 2.1):methanol = 99:1 (85:15 for PTeCA) faced some problems. The markers were occasionally overlapped by interfering peaks in samples containing only trace levels of these markers, except for the major eumelanin marker PTCA. By taking advantage that all markers, PDCA, TDCA, PTCA, TTCA, and PTeCA, contain a vicinal dicarboxylic acid group, which makes them strongly acidic among carboxylic acids with low pKa, the AHPO‐HPLC method was improved by the addition of an ion pair reagent for anions, tetra‐n‐butylammonium bromide (TBA+Br−) (1 mM) (Napolitano et al., 2000; Tavazzi et al., 2005) retarded the elution of di‐, tri‐, and tetracarboxylic acids affording a better separation. The methanol concentration was increased to 17% (30% for PTeCA). This improved HPLC method was compared to the original and showed excellent correlations between both HPLC methods for PTCA and TTCA (Ito et al., 2020). With the improved AHPO method, the other markers showed an attenuation of the interfering peaks, as exemplified by the HPLC chromatograms (Figure S1).
Eumelanin consists of brown soluble in alkali DHICA moieties and black insoluble DHI moieties. Until now, only analysis of DHICA content in skin samples with varying pigmentation levels has been possible (Del Bino et al., 2015). As the improved AHPO method allows a better characterization of PDCA (Ito et al., 2020), this prompted us to quantify the DHI eumelanin content in a selection of human skin samples (n = 18) with varying degrees of constitutive pigmentation ranging from very light to dark identical to those used previously (n = 35; Del Bino et al., 2015). PTCA is the degradation product of DHICA units and DHI units linked at the 2 position. PDCA originates from only DHI units not linked at the 2 position. PTCA recovery is more than 10‐fold greater than PDCA recovery, these conditions make it impossible to calculate conversion factors for DHICA units and DHI units, solely based on the PTCA and PDCA values. For PTCA, TTCA, and 4‐AHP, the conversion factors used were as follows: eumelanin (EM): PTCA × 38 (Del Bino et al., 2015); benzothiazole‐pheomelanin (BZ‐PM): TTCA × 34 (Del Bino et al., 2015); benzothiazine‐pheomelanin (BT‐PM): 4‐AHP × 9 (Wakamatsu et al., 2002) and total melanin: A500 × 135 (Del Bino et al., 2015). DHICA mol% were estimated based on the correlation graphs for DHICA mol% versus PDCA/PTCA and PTCA/A500 (Figure 2a; Hirobe et al., 2016). As there are considerable differences between those values, we used averages of those two values. DHI mol% was thus calculated by: 100% − (DHICA % from PDCA/PTCA ratio + DHICA % from PTCA/A500 ratio)/2.
FIGURE 2.
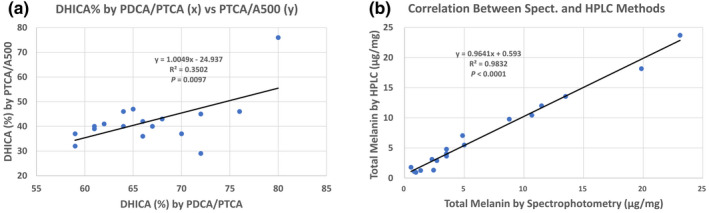
DHICA % from PDCA/PTCA ratio versus DHICA % from PTCA/A500 ratio (a). Total melanin content by spectrophotometry versus total melanin content by HPLC (b). p values were obtained with JMP 10 software (SAS Institute Inc.) to analyze bivariate fit. When the outliner (72% versus 29%) in (a) was omitted, the correlation became much better with R 2 = .5253 and p = .008.
Results were almost identical to those in Del Bino et al. (2015). This is because we used the same samples. But we obtained a better correlation between total melanin values obtained by spectrophotometry and total melanin values obtained by HPLC (Figure 2b). Eumelanin and pheomelanin contents were 76% and 24%, respectively (Figure 3 and Table S1). DHI mol% is 46% of total eumelanin on the average. DHI content was rather constant regardless of skin color, although DHI content in very light epidermis was slightly lower than the others (not statistically significant). Taken together, our results confirm the eumelanin and pheomelanin content described previously in skin with varying pigmentation levels and show for the first time the ratio of 4 moieties: DHI 35%, DHICA 41%, BZ 20%, and BT 4%. The ratio is constant regardless of the degree of pigmentation, both in eumelanin to pheomelanin ratio and DHI melanin to DHICA melanin ratio (Figure 3b). The high content of DHICA moiety may impart an antioxidant property to the epidermis melanin (Jiang et al., 2010). Also, the ratios of eumelanin to pheomelanin and BZ to BT moieties in pheomelanin are important in affecting the pro‐oxidant property of pheomelanin (Napolitano et al., 2014; Tanaka et al., 2018).
FIGURE 3.
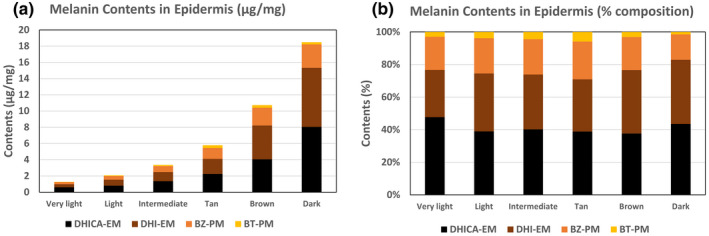
Melanin content in the different skin color groups. Contents (μg/mg) of DHI eumelanin (DHI‐EM), DHICA eumelanin (DHICA‐EM), benzothiazole‐pheomelanin (BZ‐PM) benzothiazine‐pheomelanin (BT‐PM) in the epidermis of very light to dark skin (a). Contents (% composition) in DHI‐EM, DHICA‐EM, BZ‐PM, and BT‐PM (b).
Knowledge on the different melanins content in human skin is relevant to their biological role in human skin photoprotection, knowing that the various melanins have not only distinct colors but also distinct properties. Eumelanin is known to be photoprotective, by limiting the extent of UV penetration within the epidermis, and antioxidant, due to its capabilities of scavenging reactive oxygen radicals (Corani et al., 2014; Hill et al., 1997; Kadekaro et al., 2003). In contrast, pheomelanin has photosensitizing properties, leading to the UV‐induced production of reactive oxygen species (Bustamante et al., 1993; Hill & Hill, 2000; Napolitano et al., 2014; Sarna et al., 1985). Furthermore, within eumelanin, DHICA is poorly aggregated and has superior antioxidant and free radical scavenger properties than DHI melanin which is more compact (Micillo et al., 2016; Panzella et al., 2013). DHI and DHICA have also significant differences from the standpoint of chromophore (Micillo et al., 2017). In fact, DHI melanin gives A500 value of 12.0/mg while DHICA melanin 4.0/mg (Itou et al., 2019). Being able to better quantify eumelanin through DHICA and DHI and pheomelanin through BT and BZ will in the future allow a better understanding of melanin photooxidation related to immediate and persistent pigment darkening (IPD/PPD) and melanin synthesis related to delayed tanning after exposure of human skin to solar light (Del Bino et al., 2018). Do both eumelanins (DHICA and DHI) and pheomelanin increase with tanning? What is the contribution of their oxidation in hyperpigmentation (IPD/PPD)? These are questions that still need to be thoroughly addressed.
MATERIALS AND METHODS
Eighteen human epidermal samples with varying degrees of pigmentation (3 each of very light, light, intermediate, tan, brown, and dark) were homogenized in water at a concentration of 10 mg/ml with a Ten ‐ Broeck glass homogenizer. Aliquots of 100 µl water (1 mg sample) were subjected to Soluene‐350 solubilization (Ozeki et al., 1996) to analyze absorbance at 500 nm (A500) and 650 nm (A650), the AHPO to analyze PTCA, PDCA, TTCA, TDCA, and PTeCA (Ito et al., 2011), and the HI hydrolysis to analyze 4‐AHP and 3‐AHP (Wakamatsu et al., 2002). The improved HPLC conditions for the AHPO mixtures using tetra‐n‐butylammonium bromide as an ion pair reagent were described in Ito et al. (2020).
CONFLICT OF INTEREST
SDB and JS are full time employees of L'Oreal, Research and Innovation, Aulnay, France. SI has a consulting contract with L'Oreal, Research and Innovation. KW declares no conflict of interest.
Supporting information
Figure S1
Table S1
Del Bino, S. , Ito, S. , Sok, J. , & Wakamatsu, K. (2022). 5,6‐Dihydroxyindole eumelanin content in human skin with varying degrees of constitutive pigmentation. Pigment Cell & Melanoma Research, 35, 622–626. 10.1111/pcmr.13062
[Correction added on 5 September 2022, after first online publication: The copyright line was changed.]
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- Alaluf, S. , Atkins, D. , Barrett, K. , Blount, M. , Carter, N. , & Heath, A. (2002). The impact of epidermal melanin on objective measurements of human skin colour. Pigment Cell Research, 15(2), 119–126. 10.1034/j.1600-0749.2002.1o072.x [DOI] [PubMed] [Google Scholar]
- Barsh, G. S. (2003). What controls variation in human skin color? PLoS Biology, 1(1), E27. 10.1371/journal.pbio.0000027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante, J. , Bredeston, L. , Malanga, G. , & Mordoh, J. (1993). Role of melanin as a scavenger of active oxygen species. Pigment Cell Research, 6(5), 348–353. 10.1111/j.1600-0749.1993.tb00612.x [DOI] [PubMed] [Google Scholar]
- Corani, A. , Huijser, A. , Gustavsson, T. , Markovitsi, D. , Malmqvist, P. A. , Pezzella, A. , d'Ischia, M. , & Sundstrom, V. (2014). Superior photoprotective motifs and mechanisms in eumelanins uncovered. Journal of the American Chemical Society, 136(33), 11626–11635. 10.1021/ja501499q [DOI] [PubMed] [Google Scholar]
- Del Bino, S. , Duval, C. , & Bernerd, F. (2018). Clinical and biological characterization of skin pigmentation diversity and its consequences on UV impact. International Journal of Molecular Sciences, 19(9), 2668. 10.3390/ijms19092668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bino, S. , Ito, S. , Sok, J. , Nakanishi, Y. , Bastien, P. , Wakamatsu, K. , & Bernerd, F. (2015). Chemical analysis of constitutive pigmentation of human epidermis reveals constant eumelanin to pheomelanin ratio. Pigment Cell & Melanoma Research, 28(6), 707–717. 10.1111/pcmr.12410 [DOI] [PubMed] [Google Scholar]
- Hill, H. Z. , & Hill, G. J. (2000). UVA, pheomelanin and the carcinogenesis of melanoma. Pigment Cell Research, 13(Suppl 8), 140–144. 10.1034/j.1600-0749.13.s8.25.x [DOI] [PubMed] [Google Scholar]
- Hill, H. Z. , Li, W. , Xin, P. , & Mitchell, D. L. (1997). Melanin: A two edged sword? Pigment Cell Research, 10(3), 158–161. 10.1111/j.1600-0749.1997.tb00478.x [DOI] [PubMed] [Google Scholar]
- Hirobe, T. , Ito, S. , & Wakamatsu, K. (2016). The slaty (slt/Dct[slt] ) allele decreases the content of eumelanin, but not pheomelanin in the mouse hair. Pigment Cell & Melanoma Research, 29(1), 110–112. 10.1111/pcmr.12427 [DOI] [PubMed] [Google Scholar]
- Ito, S. , Del Bino, S. , Hirobe, T. , & Wakamatsu, K. (2020). Improved HPLC conditions to determine eumelanin and pheomelanin contents in biological samples using an ion pair reagent. International Journal of Molecular Sciences, 21(14), 5134. 10.3390/ijms21145134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, S. , & Fujita, K. (1985). Microanalysis of eumelanin and pheomelanin in hair and melanomas by chemical degradation and liquid chromatography. Analytical Biochemistry, 144(2), 527–536. [DOI] [PubMed] [Google Scholar]
- Ito, S. , Nakanishi, Y. , Valenzuela, R. K. , Brilliant, M. H. , Kolbe, L. , & Wakamatsu, K. (2011). Usefulness of alkaline hydrogen peroxide oxidation to analyze eumelanin and pheomelanin in various tissue samples: Application to chemical analysis of human hair melanins. Pigment Cell & Melanoma Research, 24(4), 605–613. 10.1111/j.1755-148X.2011.00864.x [DOI] [PubMed] [Google Scholar]
- Ito, S. , & Prota, G. (1977). A facile one‐step synthesis of cysteinydopas using mushroom tyrosinase. Experientia, 33, 1118–1119. 10.1007/BF01946005 [DOI] [PubMed] [Google Scholar]
- Ito, S. , Suzuki, N. , Takebayashi, S. , Commo, S. , & Wakamatsu, K. (2013). Neutral pH and copper ions promote eumelanogenesis after the dopachrome stage. Pigment Cell & Melanoma Research, 26(6), 817–825. 10.1111/pcmr.12137 [DOI] [PubMed] [Google Scholar]
- Ito, S. , & Wakamatsu, K. (2008). Chemistry of mixed melanogenesis‐‐pivotal roles of dopaquinone. Photochemistry and Photobiology, 84(3), 582–592. 10.1111/j.1751-1097.2007.00238.x [DOI] [PubMed] [Google Scholar]
- Ito, S. , & Wakamatsu, K. (2011). Diversity of human hair pigmentation as studied by chemical analysis of eumelanin and pheomelanin. Journal of the European Academy of Dermatology and Venereology, 25(12), 1369–1380. 10.1111/j.1468-3083.2011.04278.x [DOI] [PubMed] [Google Scholar]
- Ito, S. , Wakamatsu, K. , Glass, K. , & Simon, J. D. (2013). High‐performance liquid chromatography estimation of cross‐linking of dihydroxyindole moiety in eumelanin. Analytical Biochemistry, 434(2), 221–225. 10.1016/j.ab.2012.12.005 [DOI] [PubMed] [Google Scholar]
- Itou, T. , Ito, S. , & Wakamatsu, K. (2019). Effects of aging on hair color, melanosome morphology, and melanin composition in Japanese females. International Journal of Molecular Sciences, 20(15), 3739. 10.3390/ijms20153739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, S. , Liu, X. M. , Dai, X. , Zhou, Q. , Lei, T. C. , Beermann, F. , Wakamatsu, K. , & Xu, S. Z. (2010). Regulation of DHICA‐mediated antioxidation by dopachrome tautomerase: Implication for skin photoprotection against UVA radiation. Free Radical Biology & Medicine, 48(9), 1144–1151. 10.1016/j.freeradbiomed.2010.01.033 [DOI] [PubMed] [Google Scholar]
- Kadekaro, A. L. , Kavanagh, R. J. , Wakamatsu, K. , Ito, S. , Pipitone, M. A. , & Abdel‐Malek, Z. A. (2003). Cutaneous photobiology. The melanocyte vs. the sun: Who will win the final round? Pigment Cell Research, 16(5), 434–447. 10.1034/j.1600-0749.2003.00088.x [DOI] [PubMed] [Google Scholar]
- Micillo, R. , Panzella, L. , Iacomino, M. , Prampolini, G. , Cacelli, I. , Ferretti, A. , Crescenzi, O. , Koike, K. , Napolitano, A. , & d'Ischia, M. (2017). Eumelanin broadband absorption develops from aggregation‐modulated chromophore interactions under structural and redox control. Scientific Reports, 7, 41532. 10.1038/srep41532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micillo, R. , Panzella, L. , Koike, K. , Monfrecola, G. , Napolitano, A. , & d'Ischia, M. (2016). “Fifty Shades” of black and red or how carboxyl groups fine tune eumelanin and pheomelanin properties. International Journal of Molecular Sciences, 17(5), 746. 10.3390/ijms17050746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napolitano, A. , Panzella, L. , Monfrecola, G. , & d'Ischia, M. (2014). Pheomelanin‐induced oxidative stress: Bright and dark chemistry bridging red hair phenotype and melanoma. Pigment Cell & Melanoma Research, 27(5), 721–733. 10.1111/pcmr.12262 [DOI] [PubMed] [Google Scholar]
- Napolitano, A. , Vincensi, M. R. , Di Donato, P. , Monfrecola, G. , & Prota, G. (2000). Microanalysis of melanins in mammalian hair by alkaline hydrogen peroxide degradation: Identification of a new structural marker of pheomelanins. Journal of Investigative Dermatology, 114(6), 1141–1147. 10.1046/j.1523-1747.2000.00977.x [DOI] [PubMed] [Google Scholar]
- Ozeki, H. , Ito, S. , Wakamatsu, K. , & Thody, A. J. (1996). Spectrophotometric characterization of eumelanin and pheomelanin in hair. Pigment Cell Research, 9(5), 265–270. 10.1111/j.1600-0749.1996.tb00116.x [DOI] [PubMed] [Google Scholar]
- Panzella, L. , Gentile, G. , D'Errico, G. , Della Vecchia, N. F. , Errico, M. E. , Napolitano, A. , Carfagna, C. , & d'Ischia, M. (2013). Atypical structural and π‐electron features of a melanin polymer that lead to superior free‐radical‐scavenging properties. Angewandte Chemie International Edition in English, 52(48), 12684–12687. 10.1002/anie.201305747 [DOI] [PubMed] [Google Scholar]
- Sarna, T. , Menon, I. A. , & Sealy, R. C. (1985). Photosensitization of melanins: A comparative study. Photochemistry and Photobiology, 42(5), 529–532. 10.1111/j.1751-1097.1985.tb01605.x [DOI] [PubMed] [Google Scholar]
- Sarna, T. , & Swartz, H. M. (1978). Identification and characterization of melanin in tissues and body fluids. Folia Histochemica et Cytochemica (Krakow), 16(4), 275–286. [PubMed] [Google Scholar]
- Sealy, R. C. , Hyde, J. S. , Felix, C. C. , Menon, I. A. , & Prota, G. (1982). Eumelanins and pheomelanins: Characterization by electron spin resonance spectroscopy. Science, 217(4559), 545–547. 10.1126/science.6283638 [DOI] [PubMed] [Google Scholar]
- Sealy, R. C. , Hyde, J. S. , Felix, C. C. , Menon, I. A. , Prota, G. , Swartz, H. M. , Persad, S. , & Haberman, H. F. (1982). Novel free radicals in synthetic and natural pheomelanins: Distinction between dopa melanins and cysteinyldopa melanins by ESR spectroscopy. Proceedings of the National Academy of Sciences of the United States of America, 79(9), 2885–2889. 10.1073/pnas.79.9.2885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz, H. M. , Sarna, T. , & Varma, R. R. (1979). On the natural and excretion of the hepatic pigment in the Dubin‐Johnson syndrome. Gastroenterology, 76(5 Pt 1), 958–964. [PubMed] [Google Scholar]
- Tanaka, H. , Yamashita, Y. , Umezawa, K. , Hirobe, T. , Ito, S. , & Wakamatsu, K. (2018). The pro‐oxidant activity of pheomelanin is significantly enhanced by UVA irradiation: Benzothiazole moieties are more reactive than benzothiazine moieties. International Journal of Molecular Sciences, 19, 2889. 10.3390/ijms19102889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazzi, B. , Lazzarino, G. , Leone, P. , Amorini, A. M. , Bellia, F. , Janson, C. G. , Di Pietro, V. , Ceccarelli, L. , Donzelli, S. , Francis, J. S. , & Giardina, B. (2005). Simultaneous high performance liquid chromatographic separation of purines, pyrimidines, N‐acetylated amino acids, and dicarboxylic acids for the chemical diagnosis of inborn errors of metabolism. Clinical Biochemistry, 38(11), 997–1008. 10.1016/j.clinbiochem.2005.08.002 [DOI] [PubMed] [Google Scholar]
- Taylor, S. C. (2002). Skin of color: Biology, structure, function, and implications for dermatologic disease. Journal of the American Academy of Dermatology, 46(2), S41–S62. 10.1067/mjd.2002.120790 [DOI] [PubMed] [Google Scholar]
- Wakamatsu, K. , Ito, S. , & Rees, J. L. (2002). The usefulness of 4‐amino‐3‐hydroxyphenylalanine as a specific marker of pheomelanin. Pigment Cell Research, 15(3), 225–232. 10.1034/j.1600-0749.2002.02009.x [DOI] [PubMed] [Google Scholar]
- Wakamatsu, K. , Nakanishi, Y. , Miyazaki, N. , Kolbe, L. , & Ito, S. (2012). UVA‐induced oxidative degradation of melanins: Fission of indole moiety in eumelanin and conversion to benzothiazole moiety in pheomelanin. Pigment Cell & Melanoma Research, 25(4), 434–445. 10.1111/j.1755-148X.2012.01011.x [DOI] [PubMed] [Google Scholar]
- Wakamatsu, K. , Ohtara, K. , & Ito, S. (2009). Chemical analysis of late stages of pheomelanogenesis: Conversion of dihydrobenzothiazine to a benzothiazole structure. Pigment Cell & Melanoma Research, 22(4), 474–486. 10.1111/j.1755-148X.2009.00580.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Table S1
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


