Abstract
Aims
Olmesartan, an angiotensin receptor blocker (ARB) used for hypertension management, is known to cause a sprue‐like enteropathy in a subset of patients. Rare cases of gastritis occurring with ARB use have also been reported, but the histological features of ARB‐induced gastritis and the response to drug cessation have not been examined in a dedicated case‐series.
Methods and results
Cases of suspected ARB‐induced gastritis were identified from the pathology archives of four institutions. Haematoxylin and eosin (H&E) slides from gastric biopsies were reviewed. Fifteen patients (14 female, one male) were identified. The most common presenting symptoms were diarrhoea (10) and weight loss (six). Gastric biopsies commonly showed a full‐thickness active chronic gastritis with surface epithelial injury involving the antrum and body. Glandular atrophy, intra‐epithelial lymphocytosis and/or subepithelial collagen thickening were also present in some cases. Duodenal involvement, including villous atrophy, intra‐epithelial lymphocytosis and/or collagenous sprue, was identified in 11 of 13 cases with concurrent duodenal biopsies. Following drug cessation, symptomatic improvement occurred in all 11 cases for which follow‐up data were available. Histological resolution occurred in five of eight cases with follow‐up gastric biopsies, with improvement seen in the remaining three biopsies.
Conclusion
ARB‐induced gastritis typically presents as active chronic gastritis, frequently with associated surface epithelial injury. Glandular atrophy, intra‐epithelial lymphocytosis and/or subepithelial collagen thickening may also be present. These gastric changes can be seen without associated duodenal injury in rare cases, and they should alert the pathologist to the possibility of ARB‐induced injury. Drug cessation results in marked symptomatic and histological improvement.
Keywords: ARB, enteropathy, gastritis, olmesartan
The histologic features of angiotensin receptor blocker (ARB)‐induced gastritis.

Introduction
Angiotensin receptor blockers (ARBs) are a drug class widely used in hypertension management. Their mechanism of action involves inhibition of angiotensin II at the angiotensin type 1 receptor, resulting in decreased vasoconstriction and aldosterone production and thus lower blood pressure. 1
A decade ago, a study published by Rubio‐Tapio et al. 2 first documented the association of the ARB olmesartan with gastrointestinal (GI) tract injury in the form of a reversible sprue‐like enteropathy. As is now widely known, patients typically present with diarrhoea, weight loss and abdominal pain. Duodenal biopsies usually show villous atrophy and frequently demonstrate increased intra‐epithelial lymphocytes (IELs), closely mimicking the histological appearance of coeliac disease. Increased subepithelial collagen (collagenous sprue) is also common in this cohort. Identifying the underlying aetiology in these cases is crucial, as withdrawal of the drug leads to both symptomatic improvement and histological resolution of the duodenal injury. 1 , 2 , 3
Histological changes have also been described in the stomachs of patients on ARBs, but the spectrum of morphological features has not been closely examined. 2 , 4 , 5 , 6 , 7 Our multi‐institutional study aims to describe the histological features of ARB‐induced gastric injury and examine the effect of drug cessation on these changes. Awareness of these findings will allow pathologists to suggest ARB therapy as a potential aetiology of gastritis in these patients.
Materials and methods
The Institutional Review Board of the University of Chicago approved this study (IRB20‐0539, 4/10/2020). The pathology archives of four institutions were searched for patients on ARB therapy who underwent upper GI endoscopy and had gastric biopsies showing histological abnormalities. Cases with Helicobacter pylori infection and/or suspected autoimmune gastritis were excluded from the study. Clinicopathological data were collected from patients' electronic medical records, including medication history, time‐line of ARB use, endoscopic findings, results of laboratory studies and treatment and follow‐up information. Slides were retrieved from the pathology archives and reviewed by subspecialist GI pathologists at each institution. Histological features assessed included presence or absence of neutrophilic activity, components of lamina propria inflammatory infiltrate, intra‐epithelial lymphocytosis (defined as > 25/100 epithelial cells), subepithelial collagen deposition (defined as collagen thickness > 10 μm), glandular atrophy (defined as loss of antral or oxyntic glands with or without associated intestinal metaplasia) and surface epithelial injury (defined as epithelial attenuation/mucin depletion, detachment, erosion and/or ulceration). Slides and/or digital images from most cases were also reviewed by a central reviewer to ensure uniformity in histological assessment.
Results
Clinical information
Fifteen patients (14 female, one male) were identified from four institutions (Table 1). One patient received telmisartan and the rest received olmesartan. Patients ranged in age from 48 to 87 years, with a mean of 70 years. The most common presenting symptoms were diarrhoea (10), weight loss (six) and abdominal pain (six). Nausea and/or vomiting (three) and gastroesophageal reflux (two) also occurred. One patient had no documented symptoms. The duration of ARB therapy was documented in nine of 15 patients and ranged from 6 months to > 6 years, with a median of > 24 months.
Table 1.
Clinicopathological data
| Case no. | Age/sex | Symptoms | Endoscopic findings | Histological findings: stomach | Histological findings: duodenum | Follow‐up data |
|---|---|---|---|---|---|---|
| 1 | 66/F | GERD, weight loss | Antral erythema, nodular mucosa at duodenal bulb | Active chronic gastritis with glandular atrophy and surface epithelial injury | Normal | Follow‐up gastric biopsy normal |
| 2 | 65/F | Abdominal pain, weight loss | Nodular mucosa at fundus, erythematous polyps of body | Active chronic gastritis with patchy subepithelial collagen thickening and surface epithelial injury, hyperplastic polyps | Active duodenitis, patchy subepithelial collagen thickening | Symptomatic improvement. Follow‐up gastric biopsy with antral erosion and intestinal metaplasia |
| 3 | 82/F | Abdominal pain, diarrhoea | Unknown | Active chronic gastritis with glandular atrophy and surface epithelial injury | Active duodenitis with villous blunting and focally increased eosinophils | No follow‐up |
| 4 | 58/F | Diarrhoea, weight loss | Unknown | Lymphocytic gastritis | Marked villous blunting and increased IELs | No follow‐up |
| 5 | 48/M | Diarrhoea | Congestion and erythema of body and antrum | Active chronic gastritis with surface epithelial injury and focally prominent IELs | Villous blunting and a patchy mild increase in IELs | No follow‐up |
| 6 | 67/F | None documented | Gastric polyps | Active chronic gastritis with glandular atrophy, intestinal metaplasia, surface epithelial injury and foveolar hyperplasia | NA | No follow‐up |
| 7 | 74/F | Abdominal pain, diarrhoea, GERD, nausea, weight loss | Diffuse severe erythema, nodularity, friability and granularity of antrum | Active chronic gastritis with glandular atrophy, surface epithelial injury and focally prominent IELs | Active duodenitis | Symptomatic improvement Follow‐up gastric biopsy normal |
| 8 | 65/F | Abdominal pain, nausea, vomiting, weight loss | Patchy moderately erythematous and friable mucosa | Active chronic gastritis with glandular atrophy, intestinal metaplasia, and surface epithelial injury | NA | Symptomatic improvement Follow‐up gastric biopsy with decreased active chronic gastritis |
| 9 | 65/F | Diarrhoea | None in stomach | Active chronic gastritis with focally prominent IELs | Subtotal villous atrophy and increased IELs | Follow‐up gastric biopsy normal |
| 10 | 72/F | Abdominal pain, diarrhoea | Gastritis with erosion | Active chronic gastritis with surface epithelial injury and focally prominent IELs | Active duodenitis with subtotal villous atrophy and increased IELs | Symptomatic improvement |
| 11 | 82/F T. | Diarrhoea | Atrophic‐appearing | Active chronic gastritis with glandular atrophy, intestinal metaplasia, surface epithelial injury, subepithelial collagen thickening and focally prominent IELs | Collagenous sprue | No follow‐up |
| 12 | 79/F | Diarrhoea | Mild gastritis | Active chronic gastritis with focally prominent IELs | Subtotal villous atrophy and increased IELs | Symptomatic improvement Follow‐up gastric biopsy normal |
| 13 | 87/F | Weight loss | Diffuse abnormal nodularity and atrophic areas | Active chronic gastritis with glandular atrophy, intestinal metaplasia, surface epithelial injury, and focally prominent IELs | Normal | Symptomatic improvement Follow‐up gastric biopsy with persistent mild chronic gastritis |
| 14 | 65/F | Diarrhoea | Scalloped mucosa | Active chronic gastritis with surface epithelial injury and subepithelial collagen thickening | Collagenous sprue with total villous atrophy | Symptomatic improvement. Follow‐up biopsy normal |
| 15 | 74/F | Abdominal pain, diarrhoea, nausea, vomiting | Atrophy with ulceration | Active chronic gastritis with focally prominent IELs | Total villous atrophy | Symptomatic improvement |
IELs, intra‐epithelial lymphocytes; GERD, gastroesophageal reflux disease; NA, not applicable.
Endoscopic appearance
Endoscopic findings were available for 13 of the 15 cases and included erythema (four), mucosal nodularity (four), friability (two), erosion/ulceration (two), polyps (two) and atrophy (one). Endoscopic changes were seen in the antrum and body in three cases, in the antrum alone in two cases and in the body alone in one case. In five cases, the location of the changes within the stomach was not specified. Biopsies were obtained from the antrum in 14 cases, while the body was sampled in nine cases (including eight cases in which the antrum was sampled).
Microscopic features
Histologically, a lymphoplasmacytic lamina propria infiltrate was seen in all 15 cases, with eosinophils readily identified as a component of the infiltrate in 10 cases. In nine cases the infiltrate involved the entire thickness of the mucosa, while in two cases the infiltrate was superficial and in two cases it involved the ‘midzone’ aspect of the mucosa. In the two remaining cases, a specific pattern of zonal involvement could not be determined. Additional findings included active inflammation (13 cases), surface epithelial injury (12 cases), intra‐epithelial lymphocytosis (nine cases), glandular atrophy (seven cases) and patchy subepithelial collagen deposition (three cases). The antrum was involved by histological changes in all 14 cases in which the antrum was sampled, while the body was involved in seven of nine cases in which the body was sampled.
Most cases (13 of 15, 87%) exhibited active chronic gastritis, with associated surface epithelial injury evident in 11 (85%) of these 13 cases (Figures 1 and 2). The antrum was involved in all 13 of these cases, and the body was involved in seven of the eight cases in which it was sampled (all seven of which also showed antral involvement). Associated glandular atrophy was seen in seven (54%) of the cases with active chronic gastritis (Figure 2), with intestinal metaplasia present in four (31%) of the active chronic gastritis cases. Intra‐epithelial lymphocytosis was seen in seven (54%) of the cases showing active chronic gastritis, three (23%) of which also demonstrated subepithelial collagen thickening (Figure 3).
Figure 1.

Four cases showing active chronic gastritis in the setting of angiotensin receptor blocker ARB. therapy. The surface epithelium is also attenuated and mucin‐depleted, with foci of epithelial detachment from the underlying basement membrane.
Figure 2.

A, Biopsy of the gastric body showing active chronic gastritis accompanied by glandular atrophy. B, A gastrin immunostain revealed no gastrin cells, supporting the diagnosis of atrophy involving gastric body mucosa. C, Follow‐up biopsies revealed histological resolution.
Figure 3.
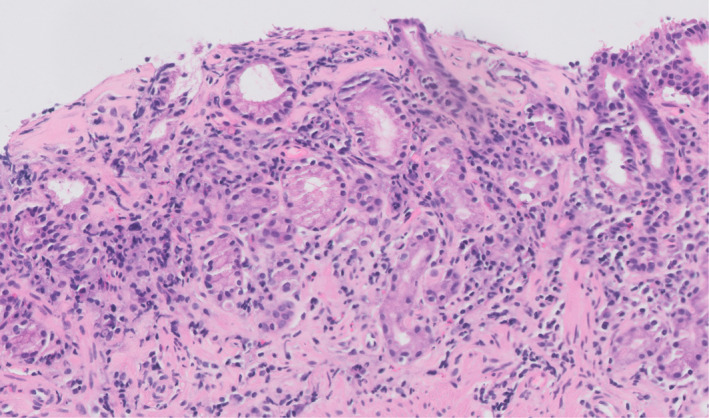
Antral biopsy showing subepithelial collagen thickening and detachment of the overlying surface epithelium.
The two cases that did not exhibit active chronic gastritis both showed intra‐epithelial lymphocytosis and a lymphoplasmacytic lamina propria infiltrate, consistent with lymphocytic gastritis (Figure 4). In one case only the antrum was sampled and involved, while in the other case only the body was sampled and involved. Surface epithelial injury was evident in the case involving the antrum but not in the case involving the body. The gastric biopsies from the one patient taking telmisartan did not exhibit any distinctive features.
Figure 4.

Antral biopsy showing a lymphoplasmacytic lamina propria infiltrate and intra‐epithelial lymphocytosis, diagnostic of lymphocytic gastritis.
Exclusion of alternate aetiologies of gastritis
H. pylori gastritis was excluded by immunohistochemistry in 13 cases and was not histologically identified in any case. Clinical evaluation for autoimmune gastritis was not performed in most cases, with only one case known to have a negative autoimmune gastritis work‐up. However, additional histological features of autoimmune gastritis, such as enterochromaffin cell‐like (ECL) hyperplasia, were not seen. Data on polypharmacy were available in 11 of 15 cases. While polypharmacy was common (nine of 11 cases), none of the concurrently administered medications were known to cause gastritis or mimic ARB‐induced enteropathy.
Concurrent duodenal biopsies
Concurrent duodenal biopsies were obtained in 13 cases. The most common histological findings were subtotal villous atrophy and intra‐epithelial lymphocytosis (five cases) and collagenous sprue (three cases, one with total villous atrophy). Additionally, there was one case of total villous atrophy without intra‐epithelial lymphocytosis, one case of active duodenitis with villous blunting and one case of active duodenitis without villous blunting. Two cases had normal concurrent duodenal biopsies.
Follow‐up data
Follow‐up data were available for 11 cases. Olmesartan was stopped after the initial gastric biopsy in nine cases. Drug cessation took place after < 1 month in eight cases and in 2 months in one case. Additionally, in one case, olmesartan was stopped 3 years after biopsies were taken, and in another case olmesartan was stopped after an unknown duration. Symptomatic improvement of varying degrees was reported in all 11 cases. Follow‐up biopsies were obtained in eight cases, with a range of 2–92 months after the initial biopsies. There was complete histological normalisation in five of the cases. Olmesartan cessation occurred < 1 month after the initial biopsies in these five cases; the time to follow‐up biopsies ranged from 1 to 6 years. Two additional cases showed persistent mild chronic gastritis and one case showed antral erosion and intestinal metaplasia. Olmesartan was stopped < 1 month after the initial biopsies in two of these cases and 3 years after the initial biopsies in the other case. Time to follow‐up biopsies ranged from 2 to 92 months in these cases. Notably, the case that showed persistent antral erosion and intestinal metaplasia had also demonstrated subepithelial collagen thickening in the original biopsies, but this abnormality was no longer present in the follow‐up biopsies. Additionally, in the cases with persistent mild chronic gastritis on follow‐up biopsies, the degree of chronic inflammation was decreased when compared to that seen in the initial biopsies.
In two cases, the initial gastric biopsies were abnormal, but the concurrent duodenal biopsy was normal or no duodenal biopsy was obtained. In these cases, the ARB was stopped solely because of the patient's symptoms and the presence of gastritis; both cases showed symptomatic and histological improvement on follow‐up.
Duodenal biopsies following ARB cessation were obtained in six cases, with histological resolution seen in five of these cases. The remaining duodenal biopsy following ARB cessation, which showed a patchy mild increase in intra‐epithelial lymphocytes, was from a patient with no initial duodenal biopsy available for comparison.
Discussion
Olmesartan‐induced enteropathy has been described in numerous studies, 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 with cases often demonstrating duodenal villous atrophy and intra‐epithelial lymphocytosis, mimicking coeliac disease. Collagenous sprue is also well‐documented in this setting. 2 , 3 , 4 , 11 The mechanism of ARB‐induced enteropathy remains unknown, but the several‐year duration of drug exposure prior to enteropathy in many cases suggests a form of drug‐induced, cell‐mediated damage as opposed to a type I hypersensitivity reaction. 9 Inhibition of transforming growth factor (TGF)‐β by ARBs, leading to altered immune homeostasis in the gut, and increased activation of type II angiotensin receptors, resulting in enterocyte apoptosis, have been proposed as possible mechanisms. 2 , 9 Interestingly, previous studies have shown that switching from olmesartan to losartan 8 led to clinical and histological resolution of enteropathy, suggesting a drug‐specific effect as opposed to a drug class effect. However, ARB‐induced enteropathy secondary to irbesartan and telmisartan has also been reported, 1 , 10 , 13 and the one patient taking telmisartan in our study showed clinicopathological findings similar to those seen in the patients taking olmesartan. Therefore, several ARBs appear capable of producing GI tract injury.
Early reports of olmesartan‐induced enteropathy described the presence of gastritis in some patients. In Rubio‐Tapia et al.'s 2 study of 22 olmesartan‐induced enteropathy cases, 14 patients also had gastritis. The histological features of the gastritis are not described in detail, but chronic gastritis (seven cases) with varying degrees of activity, lymphocytic gastritis (five cases) and collagenous gastritis (two cases) are reported. Follow‐up gastric biopsies were available in six of seven collagenous and lymphocytic gastritis cases. Four cases showed histological resolution, while two cases showed a residual non‐specific mild chronic gastritis. Burbure et al. 10 subsequently compiled many of the early studies on olmesartan‐induced GI tract injury and found the prevalence of lymphocytic and collagenous gastritis in patients with concurrent olmesartan‐induced enteropathy to range from 14 to 50%. Cessation of olmesartan therapy appeared to result in both symptomatic and histological improvement in these patients.
Most recently, a study by Costetti et al. 7 examined the gastric and duodenal findings in 14 patients with olmesartan‐induced enteropathy. Eleven cases with gastric biopsies were included, six of which showed active chronic gastritis with an antral predominance and four showed lymphocytic gastritis; two cases showed a combination of these two patterns. Focal subepithelial collagen thickening was seen in one case. Interestingly, mild atrophy was also noted in four cases, two of which had a background of active gastritis. Follow‐up gastric biopsies were obtained in only two of 14 cases, both of which showed histological improvement but not complete normalisation.
The present study of 15 cases, many with follow‐up gastric biopsies available for review, adds to the growing body of literature on ARB‐induced gastritis. Our study indicates that active chronic gastritis is common in these patients and is frequently accompanied by surface epithelial injury, a feature that has not been previously described. Additionally, several of our cases displayed glandular atrophy, sometimes associated with intestinal metaplasia, as was also recently reported by Costetti et al.;7 these changes may be related to the long duration of drug exposure in many of our study patients. Finally, as previously noted, intra‐epithelial lymphocytosis and subepithelial collagen thickening are evident in a subset of ARB‐induced gastric injury cases.
While active chronic gastritis is a non‐specific pattern with a broad differential, several features can point pathologists towards a diagnosis of ARB‐induced gastric injury. The lamina propria infiltrate in ARB‐induced gastritis usually involves the full thickness of the mucosa or is midzonal, which is distinct from the usual top‐heavy infiltrate seen in H. pylori gastritis. Significant antral inflammation is present in most cases, which can help to differentiate ARB‐induced gastritis from autoimmune gastritis, which would show antral sparing. Prominent surface epithelial damage can also serve as a clue to the presence of drug‐induced gastric injury and, while not always evident, intra‐epithelial lymphocytosis and/or subepithelial collagen thickening could further alert pathologists to the possibility of ARB‐induced gastric injury. While several of the histological changes seen in ARB‐induced gastritis can also be observed in non‐steroidal anti‐inflammatory drug (NSAID)‐related gastric injury, including surface epithelial damage, active inflammation and intra‐epithelial lymphocytosis, the presence of a prominent lymphoplasmacytic lamina propria infiltrate is not typical of NSAID‐related injury. Another clue to the diagnosis of ARB‐induced GI tract injury is the presence of histological changes in the duodenum. In our study, the findings in the gastric and duodenal biopsies were closely correlated in 11 of 14 cases. Three cases that showed subepithelial collagen deposition in the stomach showed similar features in the duodenum, and active inflammation and intra‐epithelial lymphocytosis also correlated well between gastric and duodenal biopsies. However, two patients with gastritis had no abnormalities in their duodenal biopsies, suggesting that isolated gastric injury due to ARB therapy can occur, and the possibility of ARB‐induced GI tract injury should not be dismissed solely based on the absence of duodenal involvement.
Several limitations of this study should be noted. Most of the patients (nine of 15) were on several medications in addition to an ARB, so another medication could be the source of the gastric injury in these cases. However, two patients were taking only an ARB, making this drug the most likely culprit for medication‐related gastritis in these patients. Similarly, although follow‐up data and biopsies were not available in all cases, the presence of symptomatic improvement following drug cessation in all 11 cases with clinical follow‐up data available, and the fact that histological normalisation was seen in five of the eight cases with repeat gastric biopsies following drug cessation, strongly support our theory that ARB therapy was the aetiology of gastritis in these cases. Additionally, while many of the patients' symptoms may have been the result of duodenal rather than gastric injury, the presence of concurrent normal duodenal biopsies in some cases suggests that in ARB‐induced gastric injury alone can cause gastrointestinal symptoms. Finally, the substantial variation in the number and location of the gastric biopsies hindered our ability to evaluate the extent of involvement in ARB‐induced gastritis. However, antral and body involvement were both seen, indicating that ARB‐induced gastric injury probably affects both regions, although the lack of involvement of all biopsy fragments in some cases suggests that it can be patchy.
In conclusion, this multi‐institutional series suggests that a spectrum of histological changes may occur in the stomach in association with ARB therapy. While non‐specific, active chronic gastritis and surface epithelial injury are often present in these cases, with intra‐epithelial lymphocytosis and/or subepithelial collagen thickening also sometimes seen. Such findings should alert the pathologist to the possibility of ARB‐induced gastric injury in the proper clinical context, as drug cessation may result in symptomatic and histological improvement.
Conflicts of interest
S. L. was previously involved in litigation involving olmesartan, and is currently involved in litigation involving valsartan. The other authors of this study have indicated that they have no conflicts of interest that relate to the content of this study.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Kamal A, Fain C, Park A et al. Angiotensin II receptor blockers and gastrointestinal adverse events of resembling sprue‐like enteropathy: a systematic review. Gastroenterol. Rep. 2019;7;162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rubio‐Tapia A, Herman ML, Ludvigsson JF et al. Severe spruelike enteropathy associated with olmesartan. Mayo Clin. Proc. 2012;87;732–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ianiro G, Bibbò S, Montalto M, Ricci R, Gasbarrini A, Cammarota G. Systematic review: sprue‐like enteropathy associated with olmesartan. Aliment. Pharmacol. Ther. 2014; 40; 16–23. [DOI] [PubMed] [Google Scholar]
- 4. Taylor R, Chapman C. Olmesartan‐induced collagenous gastritis, duodenitis, and colitis. Am. J. Gastroenterol. 2014; 109; S257. [Google Scholar]
- 5. Shenbagaraj L, Swift G. Olmesartan‐associated severe gastritis and enteropathy. BMJ Case Rep. 2018; 11; e226133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Choi EY, McKenna BJ. Olmesartan‐associated enteropathy: a review of clinical and histologic findings. Arch. Pathol. Lab. Med. 2015; 139; 1242–1247. [DOI] [PubMed] [Google Scholar]
- 7. Costetti M, Schiepatti A, Fraticelli S et al. Clinical and gastro‐duodenal histopathological features of enteropathy due to angiotensin II receptor blockers. Dig. Liver Dis. 2021;53;1262–1267. [DOI] [PubMed] [Google Scholar]
- 8. Adike A, Corral J, Rybnicek D, Sussman D, Shah S, Quigley E. Olmesartan‐induced enteropathy. Methodist Debakey Cardiovasc. J. 2016; 12; 230–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tran TH, Li H. Olmesartan and drug‐induced enteropathy. Pharmacol. Ther. 2014; 39; 47–50. [PMC free article] [PubMed] [Google Scholar]
- 10. Marthey L, Cadiot G, Seksik P et al. Olmesartan‐associated enteropathy: results of a national survey. Aliment. Pharmacol. Ther. 2014;40;1103–1109. [DOI] [PubMed] [Google Scholar]
- 11. Burbure N, Lebwohl B, Arguelles‐Grande C, Green PHR, Bhagat G, Lagana S. Olmesartan‐associated sprue‐like enteropathy: a systematic review with emphasis on histopathology. Hum. Pathol. 2016; 50; 127–134. [DOI] [PubMed] [Google Scholar]
- 12. Théophile H, David X‐R, M‐S G, Haramburu F. Five cases of sprue‐like enteropathy in patients treated by olmesartan. Dig. Liver Dis. 2014; 46; 465–469. [DOI] [PubMed] [Google Scholar]
- 13. Mandavdhare HS, Sharma V, Prasad KK, Kumar A, Rathi M, Rana SS. Telmisartan‐induced sprue‐like enteropathy: a case report and a review of patients using non‐olmesartan angiotensin receptor blockers. Intest. Res. 2017; 15; 419–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


