Abstract
An increasing number of elderly people retain their natural teeth into old age and further, the prevalence of endosseous implants for supporting oral prosthesis is ever increasing. These teeth and implants now present a considerable challenge in terms of maintenance, especially when patients become dependent on care. Periodontal and peri‐implant diseases are more prevalent in elderly than in younger age cohorts. There are distinct differences related to the inflammatory response between periodontal and peri‐implant tissues, both in young and old age. The age‐related reasons for the increase in periodontal infections may be related to poor oral hygiene because of a loss of dexterity or vision, but also to immunosenescence. This term describes the aging of the immune system and the decline of its effectiveness with age. Low‐grade infections, like chronic periodontitis, may cause low‐grade inflammation and subsequently increase the likelihood of developing chronic diseases. In return, treatment of periodontitis may improve general health, as demonstrated for diabetes. A second mechanism illustrating how poor oral health translates into systemic disease is the risk of developing aspiration pneumonia. The treatment options in old age should be evaluated with regard to the issues of general health and maintenance. Systematic periodontal maintenance therapy, as performed in younger age cohorts, may be difficult to implement in elderly people experiencing institutional or hospital confinement because of logistics, barriers related to patients and caregivers, or cost. The scale of periodontal disease in old age represents a public health issue.
Keywords: edentulous mouth, endosseous dental implantation, partially edentulous jaw, quality of life
1. INTRODUCTION
Recent epidemiologic studies clearly demonstrate that more and more elderly people are retaining their natural teeth well into old age. 1 , 2 Although this evolution has been the aim of dentistry for many decades and has to be credited as a major achievement in dental public health, these natural teeth now present as a newer challenge in terms of maintenance. 3 When assistance is necessary for performing activities of daily living tasks, oral hygiene is often neglected for a number of reasons. Individuals with cognitive impairment, and those who are frail and multi‐morbid, are particularly affected. 4 , 5 The result is a high incidence of caries and/or infection of the periodontal tissues, which cause pain, and when untreated lead to tooth loss, which ultimately affects the quality of life. 6 , 7 Tooth loss‐impaired chewing capacities, ill‐fitting dentures, infections, and pain have a negative influence on dietary intake, potentially leading to protein calorie malnutrition, specifically among older patients. 8 , 9 In addition, oral infections like periodontal disease have been the focus of research for more than two decades and strong associations—causal or coincidental—have been evinced for cardiovascular disease, diabetes, rheumatoid arthritis and, more recently, cognitive impairment. 10 , 11 , 12 , 13 Of particular importance for elderly people who are dependent on care is the incidence of aspiration pneumonia, especially when swallowing disorders are present. 14 , 15 , 16 , 17 Professional oral hygiene, performed by dental professionals, may help to significantly reduce the incidence of aspiration pneumonia and the related death rates. 18 , 19 , 20 , 21 Such intervention seems to be cost‐effective, when weighing the cost of professional oral hygiene vs those of medical consultations, days of hospitalization, antibiotics, and imaging. 22 Hence, oral health in institutionalized elderly people has to be seen in a context much larger than solely focusing on the oral cavity, and oral health cannot be separated from general health and quality of life.
2. PERIODONTAL AND PERI‐IMPLANT DISEASES IN ELDERLY PEOPLE
With time, biofilm forms on any given hard surface in the oral cavity and, consequently, natural teeth are at risk of caries and periodontal disease when it is left alone to mature. The periodontium of natural teeth present inherent defense mechanisms, such as the gingival epithelium and the gingival crevicular fluid, which is a serum exudate that comports the complementary components, antibodies, neutrophils, and plasma cells necessary to prevent periodontal destruction by the specific pathogens related to periodontitis 23 (Figure 1). Complete abstinence of oral hygiene for 21 days results in clinical signs and symptoms of gingivitis, which is completely reversible in 3 weeks after resuming meticulous oral hygiene measures 24 ; the same effect was demonstrated in people aged older than 70 years. 3 , 25 Because implants are artificially inserted directly into the bone, they present an ankylotic anchorage without a surrounding tissue corresponding to the natural periodontium. Hence the natural defense mechanisms are different. 26 The reaction of the peri‐implant tissues in response to the absence of oral hygiene measures has been evaluated in split‐mouth design studies that compared a natural tooth with a control implant during the induction of an experimental gingivitis. Salvi et al 27 reported on a cohort of 58.7‐year‐old patients who showed increased signs of inflammation around the implant, and an incomplete remission 3 weeks after the uptake of oral hygiene measures. Meyer et al 3 , 28 repeated the experiments in an elderly cohort with an average age of 77.0 years and confirmed increased clinical signs of inflammation around the control implant when compared with the natural tooth (Table 1). Nevertheless, the prevalence of implant‐supported restorations in geriatric populations is steadily increasing and, according to the available evidence, implant survival rates in elderly people are not inferior to those in younger cohorts. 29 , 30 , 31 , 32 Barriers to implant placement in old age frequently consist of the patient’s unwillingness to proceed, typically because of a refusal of the surgical intervention or a lack of perceived need. 33 , 34 , 35 New, mechanically stronger alloys, and improvements in the implant surface, have enabled the development of minimally invasive treatment concepts, which are more accessible by elderly patients with reduced general health and functional dependency. 36 , 37 Hence, elderly patients can also benefit from advances in modern dentistry, and from stable and functionally optimal dentures until late in life. In an institutionalized context, oral hygiene measures in elderly people are often performed by the nursing personnel, who often have little or no relevant training or experience. 38 Occupational therapy may enhance the patient’s autonomy for the required gestures, especially in residents with mild dementia. 39 The presence of natural teeth and dental implants complicate the oral hygiene interventions and therefore require more time, adequate tools and skills. As a consequence of tooth retention and implant placement, health policymakers must ensure that there are skilled personnel for dependent elderly people to address the challenges arising from these recent and very positive developments. 40 , 41
FIGURE 1.

Principal pathways of immunosenescence (redrawn after Ebersole et al 23 )
TABLE 1.
Clinical parameters during 3 wk of experimental gingivitis/mucositis as well as 3 wk follow‐up with uptake of meticulous oral hygiene (median and interquartile range); dichotomized GI: 0 or 1 vs 2 or 3 (GI > 1)). (Table according to Meyer et al 3 )
| Plaque accumulation | Oral hygiene | |||||
|---|---|---|---|---|---|---|
| Day 0 | Day 7 | Day 14 | Day 21 | Day 28 | Day 42 | |
| Teeth | ||||||
| Mean PI | 0.00 [0.00; 0.08] | 1.88 [1.65; 2.08]* | 2.42 [2.13; 2.58]* | 2.67 [2.31; 2.77]* | 0.08 [0.00; 0.17]* | 0.13 [0.00; 0.19]* |
| GI > 1 | 0.00 [0.00; 0.00] | 0.17 [0.17; 0.33] | 0.42 [0.25; 0.60]* | 0.58 [0.42; 0.60]* | 0.00 [0.00; 0.02] | 0.00 [0.00; 0.02] |
| Mean PD [mm] | 2.38 [2.23; 2.50] | – | – | 2.58 [2.31; 2.67]* | – | 2.25 [2.08; 2.44] |
| Mean BOP | 0.00 [0.00; 0.02] | – | – | 0.58 [0.48; 0.67]* | – | 0.04 [0.00; 0.08] |
| Mean REC [mm] | 1.13 [0.65; 1.71] | – | – | 1.21 [0.56; 1.52] | – | 1.25 [0.48; 1.90] |
| Implants | ||||||
| Mean PI | 0.00 [0.00; 0.00] | 1.33 [1.25; 1.52]* | 1.83 [1.42; 2.21]* | 2.00 [1.56; 2.44]* | 0.00 [0.00; 0.08] | 0.00 [0.00; 0.08] |
| GI > 1 | 0.00 [0.00; 0.00] | 0.25 [0.17; 0.42] | 0.46 [0.31; 0.60]* | 0.63 [0.50; 0.75]* | 0.00 [0.00; 0.19]* | 0.00 [0.00; 0.00] |
| Mean PD [mm] | 2.83 [2.73; 3.08] | – | – | 3.13 [2.88; 3.33]* | – | 2.75 [2.56; 3.00] |
| Mean BOP | 0.00 [0.00; 0.10] | – | – | 0.71 [0.58; 0.85]* | – | 0.00 [0.00; 0.17] |
| Mean REC [mm] | 0.29 [0.00; 0.81] | – | – | 0.33 [0.00; 0.85] | – | 0.21 [0.00; 0.77] |
Note: Values in bold: significant difference between implants and teeth (P < .05).
Abbreviations: BOP, bleeding on probing; GI, Gingival index; PD, Probing depth; PI, Plaque index; REC, Recession.
Significant difference when compared with value at Day 0 (P < .05).
In a review, Hoben et al 42 identified barriers and facilitators with respect to good oral hygiene in a nursing home confinement. The facilitators are related to family members, care providers, organization of care services, and social interactions. Residents resisting care, care providers' lack of knowledge, education or training in providing oral care, lack of time or staff, and also a general dislike for oral care, were the identified barriers. There is still a lack of robust evidence regarding how to address these new challenges in oral care provision, especially at the large scale we are facing nowadays. 42 Furthermore, there is no consensus on a standard of care and the corresponding oral health policy. 43 Given the need for frequent maintenance in terms of oral hygiene measures in implant‐supported dentures, a tight recall system should be established at the time of implant insertion, and a follow‐up assured, even when the patient is relocated to an institution. This follow‐up should not only be limited to the prevention of peri‐implantitis, but must include denture management and desophistication of the reconstruction, along with functional decline. 44
3. AGE‐RELATED REASONS FOR PERIODONTAL INFECTIONS
The question arises why old age is associated with a higher prevalence of poor oral hygiene, and consequently higher caries and periodontal disease rates than in younger adults. 2 , 45 , 46 , 47 , 48 , 49 The main contributing factors are related to physiological aging. As vision and oral tactile perception deteriorate with increasing age, elderly people are less aware of the presence of plaque and food debris in their mouths (Figure 2). 30 , 50 , 51 , 52 At the same time, an impaired sense of smell often renders them unaware of oral halitosis. 52 Manual skills decline along with reduced hand strength and muscular coordination, especially when osteoarthritis is present. 53 Hence performing the necessary gestures for oral hygiene becomes a challenge. 39 Oral hygiene tools can be adapted to the needs of elderly people experiencing reduced manual dexterity, hand strength, and/or vision, but the prevalence of these tools and awareness of their existence remains low. 54
FIGURE 2.
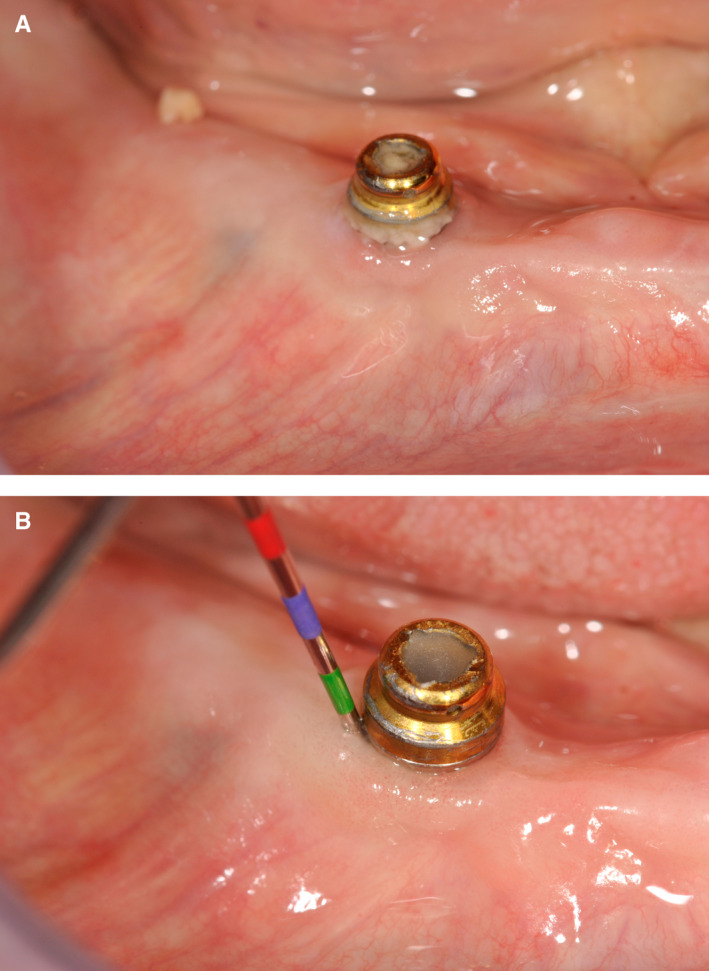
A, Typical example of biofilm accumulation around an implant in a patient aged 97 y. B, After spraying away the biofilm and simple mechanical cleaning with a toothbrush, the peri‐implant mucosa proved healthy and no pathological pockets could be measured.
Age also affects the immune system, a phenomenon often referred to as immunosenescence. A systematic review by Preshaw et al 55 evinced three key observations that suggest the human immune system declines in effectiveness with age. Firstly, the incidence of infectious diseases increases in people aged older than 65 years compared with younger individuals, and there is increased autoimmunity and degenerative diseases that are associated with a constitutive low grade inflammation. Secondly, the effectiveness of vaccination (eg, to the influenza virus and severe acute respiratory syndrome coronavirus 2) is reduced in older age groups. 56 Thirdly, there is a delay in wound repair and healing with old age, processes linked with the function of immune cells such as macrophages. In addition to immunosenescence, other factors, such as quantitative and qualitative malnutrition, anatomic alterations (eg, prostate hypertrophy), and neurologic deficits (eg, swallowing disorders), as well as comorbidities and medications, strongly contribute to the increased risk of infection in elderly people. Malnutrition in elderly people is a major factor for decline in health, and a particularly relevant factor for the immune deficiency observed in elderly patients. The factors contributing to malnutrition in elderly people are complex (Table 2).
TABLE 2.
Factors contributing to malnutrition in elderly people
| Malnutrition in elderly people: causes |
|---|
| Increased protein requirement |
|
| Increased micronutrient requirement |
|
| Decreased nutrient intake |
|
It is still under discussion if the higher prevalence of periodontal disease in the older age strata of the population is attributable to a diminished immune defense, or simply to the accumulation of sequelae over the lifetime. 57 Nevertheless, bleeding on probing as a clinical key sign for periodontal inflammation is reduced in older patient populations enrolled in periodontal supportive therapy compared with younger ones. 58 The human immune system consists of various defense mechanisms against infections and there is increasing evidence that the immune response changes with increasing age. This age‐related dysregulation of the immune responses was termed “immunosenescence” and refers to the innate immune system. 59 The details and mechanisms on cellular and molecular processes are not completely understood yet, but there seems to be significant alterations in neutrophils and macrophages as well as inflammatory phagocytes. 60
Periodontitis constitutes an inflammation of a mostly chronic nature that leads to destruction of tissue caused by a specific biofilm and the related inflammatory response. Thus, changes in the way the immune system forms a response to such inflammation are relevant to the individual pathogenesis and progress of the disease in the elderly individual. For example, Hazeldine et al 61 reported a significant age‐related decrease in neutrophil function, and suggested an increased susceptibility to periodontal infection in elderly individuals. Furthermore, Bodineau et al 62 evinced that the ratio of CD4+ T‐lymphocyte was decreased when comparing older with younger patients with periodontitis.
A further factor contributing to poor oral hygiene may be related to social withdrawal when elderly people are hospitalized or are living in institutions, as visits from family and friends become scarce. 63 Within a context where wearing a denture is often no longer considered a “prosthetic privacy” issue, the social pressure for a well‐tended appearance is lower compared with professional adults and those who lead an active social life. When functional impairment and multi‐morbidity are dominating daily life, a shift in priority may occur, and oral hygiene and oral health may no longer be in focus. A lower perception of treatment need is well documented for elderly nursing home residents, where the objective treatment need, as defined by dental “normative” standards, is in stark contrast to the patient’s subjective perception of a treatment need. 64 This general satisfaction and subjective well‐being is documented well beyond oral health. 65 , 66 , 67 The term “overadaptation” is used for a potentially traumatizing or harmful oral situation in the absence of any subjective awareness or treatment demand of the patient. The role of the dentist in this context is to provide professional information and raise awareness, rather than “talking patients into a dental treatment”.
It also has to be taken into consideration that elderly patients often present a more complex and compromised situation for osseointegration and healthy peri‐implant tissues than younger patients because of their compromised general health. Peri‐implant mucositis is frequently reported in age cohorts older than 80 years. 32 However, in a large epidemiologic study that compared a younger with an older age cohort, time in function, and not age per se, explained bone loss around dental implants. 68 It also has to be borne in mind that many elderly patients receiving implant treatment in old age might have lost their teeth later in life and hence are unlikely to present the same risk factors as young adults who lost their teeth as a result of an aggressive periodontitis. Bryant 69 showed lower bone loss in elderly patients, and justified it with the latter argument. Furthermore, it has to be borne in mind that the bone metabolism, and hence the turnover of bone resorption and apposition, slows down with age. Other factors, like periodontal health, the number of implants placed, and treatment by a specialist, as well as micro‐ and macro‐design of the implant, play predominant roles in the onset and progression of peri‐implant disease. 70 A recent systematic review by Schimmel et al 29 could not evince increased bone loss around implants or higher implant failure rates in older patients compared with younger cohorts. In a prospective cohort study, Enkling et al 71 even demonstrated that peri‐implant bone loss was significantly lower in the 65 years or older age cohort compared with the younger cohort.
Furthermore, high plaque scores in old age may be related to the morphology of an elderly person’s dentition. A younger person’s dental morphology promotes self‐cleaning during mastication, as the interdental spaces are filled with gingival papillae and the teeth are shaped in a way that facilitates the gliding of foodstuffs into the oral vestibule. The masticatory act is performed with a muscle activity that cleans the oral cavity along with food comminution. The tongue rubs against the rugae of the palate, and again, this has a cleaning effect. With increasing age, less muscle force is used during chewing, especially when dental prostheses limit the chewing performance and suggest a diet that is easy to chew, often rich in refined carbohydrates and sugar. 48 Hence self‐cleaning is less efficient because of increasing age, and this effect is further enhanced by the presence of an unfavorable morphology of “patchwork” dental restorations and gingival recession following the accumulation of loss of periodontal tissue over the lifetime. 72 A recent tool developed in Japan tried to quantify and categorize the decline of oral function. The term “oral hypofunction” has been introduced by the Japanese Society for Gerodontology. 73 Its novelty includes an assessment of seven oral functions beyond just the presence of anatomic structures like teeth and dentures. Oral signs or symptoms comprise oral uncleanness, oral dryness, decline in occlusal force, decline in the motor function of tongue and lips, decline in tongue pressure, decline in chewing function, and decline in swallowing function, with oral hypofunction being diagnosed if the criteria for three or more signs or symptoms are fulfilled. This screening tool aims to identify patients at risk of oral dysfunction at a stage when oral dysfunction may still be prevented by a dental intervention.
Chronic diseases and, in particular, their treatment, may present further risk factors for poor oral hygiene and hence oral infection and disease. 29 Radiotherapy renders the oral mucosa sensitive and oral hygiene gestures must be performed in a gentle and not harmful manner. 74 Patients may choose not wearing their removable appliances, and treatment concepts should assure oral comfort and avoid injury without the denture. Recently, antiresorptive therapies were associated with osteonecrosis, a rare but severe complication, which may mutilate oral heath. 75 Other treatments affecting oral hygiene are related to the intake of medications, especially syrups containing sugar. 76 Antihypertensives, antidepressants or monoamine oxidase inhibitors, are known to cause a salivary gland hypofunction, but may also influence implant survival through further indirect pathways (ie, bone metabolism). 29 These medications, as well as the corresponding lack of saliva, foster adhesion of biofilm, render the oral mucosa sensitive, and finally diminish the repair capacity of initial carious lesions. But also the chronic disease itself (eg, diabetes) is associated with a lack of saliva in the oral cavity, and its related negative side effects. 77
4. EFFECT OF LOW‐GRADE INFECTIONS AND POOR ORAL HEALTH ON GENERAL HEALTH
There is increasing evidence that chronic low‐grade inflammation increases the likelihood of developing chronic diseases. Indeed, increased levels of the inflammation markers (eg, C‐reactive protein, interleukin‐6) in the young and middle‐aged predict the risk of later developing a number of highly relevant pathologies, including cardiovascular disease, 78 diabetes, 79 and dementia. 80 It has even been hypothesized that periodontal disease may be a risk factor for complications of coronavirus disease 2019. 76 Low‐grade inflammation is typically caused by low‐grade chronic infections; among these, chronic infections of the oral cavity figure prominently, in particular periodontitis. And indeed, there is increased epidemiologic evidence demonstrating a link between periodontal disease and several systemic diseases. 81 Importantly, there is now emerging evidence that treatment of gum disease is efficient in improving general health. 82 For example, intensive periodontal therapy reduced HbA1c in patients with type 2 diabetes and moderate‐to‐severe periodontitis after 12 months compared with a control therapy. 83
A second mechanism illustrating how poor oral health translates into systemic disease is the risk of developing bacterial pneumonia, which is typically caused by bacteria descending the respiratory tract and consequently infecting the pulmonary parenchyma. 84 While in younger, relatively healthy patients, the transmission of pathogenic bacteria, such as streptococcus pneumonia or mycoplasma pneumonia, is the key mechanism for the development of pneumonia, in a debilitated and frail patient, “trivial” bacteria from the oral cavity can cause aspiration pneumonia. 14 Interestingly, there is not only an association between poor oral hygiene and respiratory infection, but also a decrease in pneumonia incidence upon improved oral care. 85
5. TREATMENT OPTIONS IN OLD AGE
Several interventions have been discussed to prevent or decrease infections in elderly people (Table 3). Vaccinations in elderly people are important, but with increasing age immunosenescence decreases their efficiency. 56 For example, it is recommended that antipneumococcal vaccines are given relatively early in the course of aging. Avoiding malnutrition can contribute substantially to decrease the risk of infection in elderly people. Wherever possible, the exposure of elderly people to pathogens should be reduced. And finally, chronic infectious conditions should be treated, but these need to be distinguished from colonization.
TABLE 3.
Interventions to decrease infections in elderly people
| Interventions to decrease infections in elderly people |
|---|
| Vaccination, but |
|
| Avoid malnutrition |
|
| Decrease exposure to pathogens |
|
| Treat chronic infections? |
|
Abbreviation: COVID‐19, coronavirus disease 2019.
The success rates of periodontal treatment in old age have only been studied rarely. The presence of removable prostheses and poorly shaped or ill‐fitting fixed restorations are the “natural enemy” of periodontal health, and make the success of a periodontal treatment especially difficult. 86 Concerning peri‐implant treatment, the situation is even more difficult as, to the best of our knowledge, the treatment outcomes of peri‐implant disease in elderly patients have not been studied yet. 32 As previously mentioned, the clinical signs of inflammation are stronger around implants compared with natural teeth when immunosenescence is present. 3 , 27 , 28 However, the critical point of the long‐term success of periodontal therapy in elderly compared with younger patients is the maintenance. As for the survival of teeth in removable partial denture wearers, Tada et al 86 demonstrated a significant effect of regular periodontal maintenance on tooth survival. A similar effect might apply to dental implants, but this has not yet been proven in a high‐level evidence clinical trial. Adhesion to a systematic recall system may be difficult because of access to care, mobility problems, financial limitations, and motivation, whereas maintaining a high level of oral hygiene is a challenge in itself, for all of the aforementioned reasons. Furthermore, in an institutionalized context, the knowledge and skills of the caregivers are a limiting factor, and protected time for oral hygiene of the nursing personnel remains a crucial issue in care for the elderly. 41 Professional oral hygiene regularly performed by dental personnel (ie, dental hygienists or dentists) rather than medical care staff would not only be helpful to avoid the biofilm from maturing, but could also prevent death from aspiration pneumonia. 20 However, the numbers of dental personnel who are skilled and interested in treating this special care population are low, and more staff need to be trained in vocational training as well as via structured undergraduate and postgraduate education programs. Considering the extent of human resources needed for preventive oral healthcare, physicians and primary healthcare providers might play a role in screening for oral disease and delivering preventive measures. 87 The presence of natural teeth and implants in contemporary old and very old cohorts has become a public health issue, and several calls for action have been published by experts in the field. 57 , 88 , 89
6. SUMMARY AND CONCLUSION
Periodontal and peri‐implant diseases are very frequent and they increase with age. In old age, periodontal and peri‐implant diseases should be evaluated in the context of general health, physiological aging of the immune system, and general body function. Systematic periodontal maintenance therapy, as performed in younger age cohorts, may be difficult to implement in elderly people experiencing institutional and hospital confinement because of logistics and barriers related to patients, caregivers, or cost. The scale of periodontal disease in old age represents a considerable public health issue.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
ACKNOWLEDGMENT
Open access funding provided by Universite de Geneve. Open access funding provided by Universite de Geneve.
Müller F, Srinivasan M, Krause K‐H, Schimmel M. Periodontitis and peri‐implantitis in elderly people experiencing institutional and hospital confinement. Periodontol 2000. 2022;90:138‐145. doi: 10.1111/prd.12454
REFERENCES
- 1. Jordan AR, Micheelis W. Fünfte Deutsche Mundgesundheitsstudie (DMS V). Fifth German Oral Health Survey. Deutscher Zahnärzteverlag Köln; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schneider C, Zemp E, Zitzmann NU. Oral health improvements in Switzerland over 20 years. Eur J Oral Sci. 2017;125(1):55‐62. [DOI] [PubMed] [Google Scholar]
- 3. Meyer S, Giannopoulou C, Courvoisier D, Schimmel M, Müller F, Mombelli A. Experimental mucositis and experimental gingivitis in persons aged 70 or over. Clinical and biological responses. Clin Oral Implants Res. 2017;28(8):1005‐1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chalmers JM, Carter KD, Spencer AJ. Oral diseases and conditions in community‐living older adults with and without dementia. Spec Care Dentist. 2003;23(1):7‐17. [DOI] [PubMed] [Google Scholar]
- 5. Gil‐Montoya JA, Ponce G, Sanchez Lara I, Barrios R, Llodra JC, Bravo M. Association of the oral health impact profile with malnutrition risk in Spanish elders. Arch Gerontol Geriatr. 2013;57(3):398‐402. [DOI] [PubMed] [Google Scholar]
- 6. Peltola P, Vehkalahti MM, Wuolijoki‐Saaristo K. Oral health and treatment needs of the long‐term hospitalised elderly. Gerodontology. 2004;21(2):93‐99. [DOI] [PubMed] [Google Scholar]
- 7. Stenman U, Ahlqwist M, Bjorkelund C, Hakeberg M. Oral health‐related quality of life – associations with oral health and conditions in Swedish 70‐year‐old individuals. Gerodontology. 2012;29(2):e440‐e446. [DOI] [PubMed] [Google Scholar]
- 8. Sheiham A, Steele JG, Marcenes W, et al. The relationship among dental status, nutrient intake, and nutritional status in older people. J Dent Res. 2001;80:408‐413. [DOI] [PubMed] [Google Scholar]
- 9. Schimmel M, Katsoulis J, Genton L, Müller F. Masticatory function and nutrition in old age. Swiss Dent J. 2015;125(4):449‐454. [DOI] [PubMed] [Google Scholar]
- 10. Ryden L, Buhlin K, Ekstrand E, et al. Periodontitis increases the risk of a first myocardial infarction: a report from the PAROKRANK Study. Circulation. 2016;133(6):576‐583. [DOI] [PubMed] [Google Scholar]
- 11. Detert J, Pischon N, Burmester GR, Buttgereit F. The association between rheumatoid arthritis and periodontal disease. Arthritis Res Ther. 2010;12(5):218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Olsen I, Singhrao SK. Can oral infection be a risk factor for Alzheimer’s disease? J Oral Microbiol. 2015;7:29143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Martande SS, Pradeep AR, Singh SP, et al. Periodontal health condition in patients with Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2014;29(6):498‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. O’Donnell LE, Smith K, Williams C, et al. Dentures are a reservoir for respiratory pathogens. J Prosthodont. 2016;25(2):99‐104. [DOI] [PubMed] [Google Scholar]
- 15. van der Maarel‐Wierink CD, Vanobbergen JN, Bronkhorst EM, Schols JM, de Baat C. Meta‐analysis of dysphagia and aspiration pneumonia in frail elders. J Dent Res. 2011;90(12):1398‐1404. [DOI] [PubMed] [Google Scholar]
- 16. Baumgartner W, Schimmel M, Müller F. Oral health and dental care of elderly adults dependent on care. Swiss Dent J. 2015;125(4):417‐426. [DOI] [PubMed] [Google Scholar]
- 17. Müller F. Oral hygiene reduces the mortality from aspiration pneumonia in frail elders. J Dent Res. 2015;94(3):14S‐16S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Iinuma T, Arai Y, Abe Y, et al. Denture wearing during sleep doubles the risk of pneumonia in the very elderly. J Dent Res. 2015;94(3):28S‐36S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nishizawa T, Niikura Y, Akasaka K, et al. Pilot study for risk assessment of aspiration pneumonia based on oral bacteria levels and serum biomarkers. BMC Infect Dis. 2019;19(1):761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sjögren P, Wardh I, Zimmerman M, Almstahl A, Wikstrom M. Oral care and mortality in older adults with pneumonia in hospitals or nursing homes: systematic review and meta‐analysis. J Am Geriatr Soc. 2016;64(10):2109‐2115. [DOI] [PubMed] [Google Scholar]
- 21. Yoneyama T, Yoshida M, Matsui T, Sasaki H. Oral care and pneumonia. Oral Care Working Group. Lancet. 1999;354(9177):515. [DOI] [PubMed] [Google Scholar]
- 22. Schwendicke F, Stolpe M, Müller F. Professional oral health care for preventing nursing home‐acquired pneumonia: a cost‐effectiveness and value of information analysis. J Clin Periodontol. 2017;44(12):1236‐1244. [DOI] [PubMed] [Google Scholar]
- 23. Ebersole JL, Graves CL, Gonzalez OA, et al. Aging, inflammation, immunity and periodontal disease. Periodontol 2000. 2016;72(1):54‐75. [DOI] [PubMed] [Google Scholar]
- 24. Loe H, Theilade E, Jensen SB. Experimental gingivitis in man. J Periodontol. 1965;36:177‐187. [DOI] [PubMed] [Google Scholar]
- 25. Holm‐Pedersen P, Agerbaek N, Theilade E. Experimental gingivitis in young and elderly individuals. J Clin Periodontol. 1975;2(1):14‐24. [DOI] [PubMed] [Google Scholar]
- 26. Salvi GE, Cosgarea R, Sculean A. Prevalence and mechanisms of peri‐implant diseases. J Dent Res. 2017;96(1):31‐37. [DOI] [PubMed] [Google Scholar]
- 27. Salvi GE, Aglietta M, Eick S, Sculean A, Lang NP, Ramseier CA. Reversibility of experimental peri‐implant mucositis compared with experimental gingivitis in humans. Clin Oral Implants Res. 2012;23(2):182‐190. [DOI] [PubMed] [Google Scholar]
- 28. Meyer S, Giannopoulou C, Cancela J, Courvoisier D, Müller F, Mombelli A. Experimental mucositis gingivitis in persons aged 70 or over: microbiological findings and predictoin. Clin Oral Investig. 2019;23(10):3855‐3863. [DOI] [PubMed] [Google Scholar]
- 29. Schimmel M, Srinivasan M, McKenna G, Müller F. Effect of advanced age and/or systemic medical conditions on dental implant survival: a systematic review and meta‐analysis. Clin Oral Implants Res. 2018;29(Suppl 16):311‐330. [DOI] [PubMed] [Google Scholar]
- 30. Schimmel M, Müller F, Suter V, Buser D. Implants for elderly patients. Periodontol 2000. 2017;73(1):228‐240. [DOI] [PubMed] [Google Scholar]
- 31. Müller F, Barter S. ITI Treatment Guide 9: Implant Therapy in the Geriatric Patient. Quintessence; 2016. [Google Scholar]
- 32. Kowar J, Eriksson A, Jemt T. Fixed implant‐supported prostheses in elderly patients: a 5‐year retrospective comparison between partially and completely edentulous patients aged 80 years or older at implant surgery. Clin Implant Dent Relat Res. 2013;15(1):37‐46. [DOI] [PubMed] [Google Scholar]
- 33. Leles CR, Dias DR, Nogueira TE, McKenna G, Schimmel M, Jordao LMR. Impact of patient characteristics on edentulous subjects' preferences for prosthodontic rehabilitation with implants. Clin Oral Implants Res. 2019;30(3):285‐292. [DOI] [PubMed] [Google Scholar]
- 34. Walton JN, MacEntee MI. Choosing or refusing oral implants: a prospective study of edentulous volunteers for a clinical trial. Int J Prosthodont. 2005;18(6):483‐488. [PubMed] [Google Scholar]
- 35. Müller F, Salem K, Barbezat C, Herrmann FR, Schimmel M. Knowledge and attitude of elderly persons towards dental implants. Gerodontology. 2012;29(2):e914‐e923. [DOI] [PubMed] [Google Scholar]
- 36. Müller F, Al‐Nawas B, Storelli S, et al. Small‐diameter titanium grade IV and titanium‐zirconium implants in edentulous mandibles: five‐year results from a double‐blind, randomized controlled trial. BMC Oral Health. 2015;15(1):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Batisse C, Bonnet G, Bessadet M, et al. Stabilization of mandibular complete dentures by four mini implants: impact on masticatory function. J Dent. 2016;50:43‐50. [DOI] [PubMed] [Google Scholar]
- 38. Chebib N, Waldburger TC, Boire S, et al. Oral care knowledge, attitude and practice: caregivers' survey and observation. Gerodontology. 2021;38(1):95‐103. [DOI] [PubMed] [Google Scholar]
- 39. Bellomo F, de Preux F, Chung JP, Julien N, Budtz‐Jorgensen E, Müller F. The advantages of occupational therapy in oral hygiene measures for institutionalised elderly adults. Gerodontology. 2005;22(1):24‐31. [DOI] [PubMed] [Google Scholar]
- 40. McKenna G, Allen F, Schimmel M, Müller F. Editorial: Who’s picking up the bill? Gerodontology. 2015;32(3):161‐162. [DOI] [PubMed] [Google Scholar]
- 41. Heitz‐Mayfield LJ, Aaboe M, Araujo M, et al. Group 4 ITI Consensus Report: risks and biologic complications associated with implant dentistry. Clin Oral Implants Res. 2018;29(Suppl 16):351‐358. [DOI] [PubMed] [Google Scholar]
- 42. Hoben M, Clarke A, Huynh KT, et al. Barriers and facilitators in providing oral care to nursing home residents, from the perspective of care aides: a systematic review and meta‐analysis. Int J Nurs Stud. 2017;73:34‐51. [DOI] [PubMed] [Google Scholar]
- 43. Charadram N, Maniewicz S, Maggi S, et al. Development of a European consensus from dentists, dental hygienists and physicians on a standard for oral health care in care‐dependent older people: an e‐Delphi study. Gerodontology. 2021;38(1):41‐56. [DOI] [PubMed] [Google Scholar]
- 44. Müller F, Schimmel M. Revised success criteria: a vision to meet frailty and dependency in implant patients. Int J Oral Maxillofac Implants. 2016;31(1):15. [PubMed] [Google Scholar]
- 45. Billings M, Holtfreter B, Papapanou PN, Mitnik GL, Kocher T, Dye BA. Age‐dependent distribution of periodontitis in two countries: findings from NHANES 2009 to 2014 and SHIP‐TREND 2008 to 2012. J Periodontol. 2018;89(Suppl 1):S140‐S158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Slade GD, Akinkugbe AA, Sanders AE. Projections of U.S. Edentulism prevalence following 5 decades of decline. J Dent Res. 2014;93(10):959‐965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yao CS, MacEntee MI. Inequity in oral health care for elderly Canadians: part 2. Causes and ethical considerations. J Can Dent Assoc. 2014;80:e10. [PubMed] [Google Scholar]
- 48. Sato N, Ono T, Kon H, et al. Ten‐year longitudinal study on the state of dentition and subjective masticatory ability in community‐dwelling elderly people. J Prosthodont Res. 2016;60(3):177‐184. [DOI] [PubMed] [Google Scholar]
- 49. Stock C, Jurges H, Shen J, Bozorgmehr K, Listl S. A comparison of tooth retention and replacement across 15 countries in the over‐50s. Community Dent Oral Epidemiol. 2016;44(3):223‐231. [DOI] [PubMed] [Google Scholar]
- 50. Jonas JB, Cheung CMG, Panda‐Jonas S. Updates on the epidemiology of age‐related macular degeneration. Asia Pac J Ophthalmol (Phila). 2017;6(6):493‐497. [DOI] [PubMed] [Google Scholar]
- 51. Avivi‐Arber L, Sessle BJ. Jaw sensorimotor control in healthy adults and effects of ageing. J Oral Rehabil. 2018;45(1):50‐80. [DOI] [PubMed] [Google Scholar]
- 52. Welge‐Lussen A. Olfactory disorders – history, classification and implications. Ther Umsch. 2016;73(4):219‐223. [DOI] [PubMed] [Google Scholar]
- 53. Gracia‐Ibanez V, Agost MJ, Bayarri‐Porcar V, Granell P, Vergara M, Sancho‐Bru JL. Hand kinematics in osteoarthritis patients while performing functional activities. Disabil Rehabil. 2022. (online ahead of print). [DOI] [PubMed] [Google Scholar]
- 54. Srinivasan M, Delavy J, Schimmel M, et al. Prevalence of oral hygiene tools amongst hospitalised elders: a cross‐sectional survey. Gerodontology. 2019;36(2):125‐133. [DOI] [PubMed] [Google Scholar]
- 55. Preshaw PM, Henne K, Taylor JJ, Valentine RA, Conrads G. Age‐related changes in immune function (immune senescence) in caries and periodontal diseases: a systematic review. J Clin Periodontol. 2017;44(Suppl 18):S153‐S177. [DOI] [PubMed] [Google Scholar]
- 56. Arregoces‐Castillo L, Fernandez‐Nino J, Rojas‐Botero M, et al. Effectiveness of COVID‐19 vaccines in older adults in Colombia: a retrospective, population‐based study of the ESPERANZA cohort. Lancet Healthy Longev. 2022;3(4):e242‐e252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tonetti MS, Bottenberg P, Conrads G, et al. Dental caries and periodontal diseases in the ageing population: call to action to protect and enhance oral health and well‐being as an essential component of healthy ageing – consensus report of group 4 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. J Clin Periodontol. 2017;44(Suppl 18):S135‐S144. [DOI] [PubMed] [Google Scholar]
- 58. Ramseier CA, Fischer JR, Fischer G, Schimmel M. Effect of age on bleeding on probing (BOP) as an indicator of periodontal inflammation in patients enrolled in supportive periodontal therapy. Oral Health Prev Dent. 2021;19(1):43‐50. [DOI] [PubMed] [Google Scholar]
- 59. Gomez CR, Nomellini V, Faunce DE, Kovacs EJ. Innate immunity and aging. Exp Gerontol. 2008;43(8):718‐728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hajishengallis G. Too old to fight? Aging and its toll on innate immunity. Mol Oral Microbiol. 2010;25(1):25‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hazeldine J, Harris P, Chapple IL, et al. Impaired neutrophil extracellular trap formation: a novel defect in the innate immune system of aged individuals. Aging Cell. 2014;13(4):690‐698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bodineau A, Coulomb B, Tedesco AC, Seguier S. Increase of gingival matured dendritic cells number in elderly patients with chronic periodontitis. Arch Oral Biol. 2009;54(1):12‐16. [DOI] [PubMed] [Google Scholar]
- 63. Schimmel M, Schoeni P, Zulian GB, Müller F. Utilisation of dental services in a university hospital palliative and long‐term care unit in Geneva. Gerodontology. 2008;25(2):107‐112. [DOI] [PubMed] [Google Scholar]
- 64. Stuck AE, Chappuis C, Flury H, Lang NP. Dental treatment needs in an elderly population referred to a geriatric hospital in Switzerland. Community Dent Oral Epidemiol. 1989;17(5):267‐272. [DOI] [PubMed] [Google Scholar]
- 65. MacEntee MI. Quality of life as an indicator of oral health in older people. J Am Dent Assoc. 2007;138(Suppl):47S‐52S. [DOI] [PubMed] [Google Scholar]
- 66. Steele JG, Sanders AE, Slade GD, et al. How do age and tooth loss affect oral health impacts and quality of life? A study comparing two national samples. Community Dent Oral Epidemiol. 2004;32(2):107‐114. [DOI] [PubMed] [Google Scholar]
- 67. Stone AA, Schwartz JE, Broderick JE, Deaton A. A snapshot of the age distribution of psychological well‐being in the United States. Proc Natl Acad Sci U S A. 2010;107(22):9985‐9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Derks J, Schaller D, Hakansson J, Wennstrom JL, Tomasi C, Berglundh T. Effectiveness of implant therapy analyzed in a Swedish population: prevalence of peri‐implantitis. J Dent Res. 2016;95(1):43‐49. [DOI] [PubMed] [Google Scholar]
- 69. Bryant SR. The effects of age, jaw site, and bone condition on oral implant outcomes. Int J Prosthodont. 1998;11(5):470‐490. [PubMed] [Google Scholar]
- 70. Derks J, Schaller D, Hakansson J, Wennstrom JL, Tomasi C, Berglundh T. Peri‐implantitis – onset and pattern of progression. J Clin Periodontol. 2016;43(4):383‐388. [DOI] [PubMed] [Google Scholar]
- 71. Enkling N, Moazzin R, Geers G, Kokoschka S, Abou‐Ayash S, Schimmel M. Clinical outcomes and bone‐level alterations around one‐piece mini dental implants retaining mandibular overdentures: 5‐year follow‐up of a prospective cohort study. Clin Oral Implants Res. 2020;31(6):549‐556. [DOI] [PubMed] [Google Scholar]
- 72. Katafuchi M, Weinstein BF, Leroux BG, Chen YW, Daubert DM. Restoration contour is a risk indicator for peri‐implantitis: a cross‐sectional radiographic analysis. J Clin Periodontol. 2018;45(2):225‐232. [DOI] [PubMed] [Google Scholar]
- 73. Minakuchi S, Tsuga K, Ikebe K, et al. Oral hypofunction in the older population: position paper of the Japanese Society of Gerodontology in 2016. Gerodontology. 2018;35(4):317‐324. [DOI] [PubMed] [Google Scholar]
- 74. Schiegnitz E, Al‐Nawas B, Kammerer PW, Grotz KA. Oral rehabilitation with dental implants in irradiated patients: a meta‐analysis on implant survival. Clin Oral Investig. 2014;18(3):687‐698. [DOI] [PubMed] [Google Scholar]
- 75. Stavropoulos A, Bertl K, Pietschmann P, Pandis N, Schiodt M, Klinge B. The effect of antiresorptive drugs on implant therapy: systematic review and meta‐analysis. Clin Oral Implants Res. 2018;29(Suppl 18):54‐92. [DOI] [PubMed] [Google Scholar]
- 76. Storbeck T, Qian F, Marek C, Caplan D, Marchini L. Dose‐dependent association between xerostomia and number of medications among older adults. Spec Care Dentist. 2022;42(3):225‐231. [DOI] [PubMed] [Google Scholar]
- 77. Baer AN, Walitt B. Sjogren syndrome and other causes of sicca in older adults. Clin Geriatr Med. 2017;33(1):87‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Koenig W, Sund M, Fröhlich F, et al. C‐reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle‐aged men. Circulation. 1999;99(2):237‐242. [DOI] [PubMed] [Google Scholar]
- 79. Freeman DJ, Norrie J, Caslake MJ, et al. C‐reactive protein is an independent predictor of risk for the development of diabetes in the West of Scotland Coronary Prevention Study. Diabetes. 2002;51(5):1596‐1600. [DOI] [PubMed] [Google Scholar]
- 80. Engelhart MJ, Geerlings MI, Meijer J, et al. Inflammatory proteins in plasma and the risk of dementia: the Rotterdam study. Arch Neurol. 2004;61(5):668‐672. [DOI] [PubMed] [Google Scholar]
- 81. Fi C, Wo W. Periodontal disease and systemic diseases: an overview on recent progresses. J Biol Regul Homeost Agents. 2021;35(Suppl 1):1‐9. [PubMed] [Google Scholar]
- 82. Jeffcoat MK, Jeffcoat RL, Gladowski PA, Bramson JB, Blum JJ. Impact of periodontal therapy on general health: evidence from insurance data for five systemic conditions. Am J Prev Med. 2014;47(2):166‐174. [DOI] [PubMed] [Google Scholar]
- 83. D’Aiuto F, Gkranias N, Bhowruth D, et al. Systemic effects of periodontitis treatment in patients with type 2 diabetes: a 12 month, single‐centre, investigator‐masked, randomised trial. Lancet Diabetes Endocrinol. 2018;6(12):954‐965. [DOI] [PubMed] [Google Scholar]
- 84. Scannapieco FA. Poor oral health in the etiology and prevention of aspiration pneumonia. Dent Clin N Am. 2021;65(2):307‐321. [DOI] [PubMed] [Google Scholar]
- 85. Pace CC, McCullough GH. The association between oral microorgansims and aspiration pneumonia in the institutionalized elderly: review and recommendations. Dysphagia. 2010;25(4):307‐322. [DOI] [PubMed] [Google Scholar]
- 86. Tada S, Allen PF, Ikebe K, Matsuda K, Maeda Y. Impact of periodontal maintenance on tooth survival in patients with removable partial dentures. J Clin Periodontol. 2015;42(1):46‐53. [DOI] [PubMed] [Google Scholar]
- 87. Kossioni AE, Hajto‐Bryk J, Janssens B, et al. Practical guidelines for physicians in promoting oral health in frail older adults. J Am Med Dir Assoc. 2018;19(12):1039‐1046. [DOI] [PubMed] [Google Scholar]
- 88. Kossioni AE, Hajto‐Bryk J, Maggi S, et al. An expert opinion from the European College of Gerodontology and the European Geriatric Medicine Society: European policy recommendations on oral health in older adults. J Am Geriatr Soc. 2018;66(3):609‐613. [DOI] [PubMed] [Google Scholar]
- 89. Petersen PE, Yamamoto T. Improving the oral health of older people: the approach of the WHO Global Oral Health Programme. Community Dent Oral Epidemiol. 2005;33:81‐92. [DOI] [PubMed] [Google Scholar]


