Abstract
Introduction
People who inject drugs are at risk of hospitalisation with injection‐related infections (IRI). We audited the clinical features, microbiology and management of IRI at a tertiary service in Melbourne to describe the burden and identify quality improvement opportunities.
Methods
We performed retrospective review of IRI admissions from January 2017 to April 2019. We extracted admissions where ICD‐10 codes or triage text suggested injecting drug use, and the diagnosis suggested IRI. We reviewed these for eligibility and extracted data using a standardised form. We performed mixed‐effects logistic regression to determine predictors of unplanned discharge.
Results
From 574 extracted candidate admissions, 226 were eligible, representing 178 patients. Median age was 41 years (interquartile range 36–47), 66% (117/178) male and 49% (111/226) had unstable housing. Over 50% (96/178) had a psychiatric diagnosis and 35% (62/178) were on opioid agonist therapy (OAT) on admission. Skin and soft tissue infection was the most common IRI (119/205, 58%), followed by bacteraemia (36/205, 18%) and endocarditis (26/205, 13%). Management included addictions review (143/226, 63%), blood‐borne virus screening (115/226, 51%), surgery (77/226, 34%) and OAT commencement (68/226, 30%). Aggression events (54/226, 15%) and unplanned discharge (69/226, 30%) complicated some admissions. Opioid use without OAT was associated with almost 3‐fold increased odds of unplanned discharge compared to no opioid use (odds ratio 2.90, 95% confidence interval 1.23, 6.85, p = 0.015).
Discussion and Conclusion
Comorbidities associated with IRI may be amenable to opportunistic intervention during hospitalisation. Further research is needed to develop optimal models of care for this vulnerable patient group.
Keywords: infections, injecting drug use, injection‐related infections, substance‐related disorders
1. INTRODUCTION
People who inject drugs (PWID) are at increased risk of acute infectious diseases. These range from local injection‐related infections (IRI) such as cellulitis, abscesses and thrombophlebitis, to life‐threatening IRI complications such as bacteraemia, endocarditis and osteomyelitis [1, 2]. Estimates of lifetime incidence of acute IRI in PWID range from 6.2% to 68.6% for local skin and soft tissue infection (SSTI), and from 2.0% to 9.8% for bacteraemia [3].
IRI result in high health service utilisation, with PWID up to 50 times more likely to be admitted to hospital for treatment of a bacterial infection compared to the general population [2, 4]. The admissions can be complex and prolonged, with additional management issues of substance dependence, pain management and mental health [2, 5, 6]. Substance use is often not recognised as a discrete medical issue, and opportunities for targeted interventions, such as education about safe injecting practices, commencement of opioid agonist therapy (OAT), and screening for blood‐borne viruses, are missed [6, 7, 8, 9, 10, 11].
Unplanned discharge (including discharge against medical advice [DAMA] and absconding) is common in this population, with rates of up to 30% reported [2, 5, 12]. Unplanned discharge may result in negative health consequences, including high readmission rates, increased mortality and lack of appropriate follow‐up [13]. Recent or inpatient injection drug use, weekends and days on which social welfare payments are issued are risk factors [5, 12, 14]. Qualitative studies have identified numerous reasons for unplanned discharge from a patient perspective, including perceptions of stigmatising treatment, such as being searched or monitored for drug use, inadequate withdrawal and pain management, boredom during prolonged admissions and isolation from social supports [15].
There is increasing international and local evidence that PWID represents a growing proportion of hospitalised patients with community‐acquired infections [10, 16]. However, the burden and clinical‐spectrum of IRI among PWID have not been well described in the Australian setting, and is limited by reliance on self‐reporting methods in community settings [17, 18, 19], and focus on infective endocarditis in hospitalised patients [16, 20, 21]. In this report, we aimed to provide a comprehensive description of IRI requiring hospital‐based care in the Australian context. We reviewed the management of substance use disorder, as well as behavioural issues and discharge planning. We also sought to identify risk factors, identifiable at admission, that may predict unplanned discharge. With this report, we aim to provide data to inform optimal clinical management to improve health outcomes in PWID.
2. METHODS
2.1. Study design and setting
We performed a retrospective audit of admissions with an IRI at the Alfred Hospital (Melbourne, Australia), from January 2017 to April 2019. Eligibility criteria included admission for management of an IRI [22] and current injecting drug use. We defined IRI as: SSTI (e.g., abscess, cellulitis or thrombophlebitis); bloodstream infection; endocarditis; vertebral osteomyelitis and/or epidural abscess; osteomyelitis or septic arthritis; chest infection; deep abscess (e.g., liver, spleen, brain); central nervous system infections, including endophthalmitis; and other infection thought likely to be related to injecting drug use. We defined ‘current use’ as self‐reported injecting drug use within the preceding 6 months, documented in the medical record. For patients who would have been unable to report their history (e.g., due to reduced conscious state), a documented collateral history was accepted. Results of drug testing, where performed, were not reviewed. Ambulatory care episodes were excluded, as were emergency consultations without subsequent hospital admission. For patients with multiple admissions during the study period, each admission was included. While all information was collected at the level of individual hospital admission episodes, we identified and linked multiple admitted episodes relating to the same patient (patient‐level data) and the same infection (infection‐level data). For the purposes of data analysis, the first episode for each patient or infection was included.
2.2. Data sources
We used administrative and clinical data sources to screen for potentially eligible admitted episodes. Potential injecting drug use was identified using the International Classification of Diseases, Tenth Revision, Australian Modification (ICD‐10‐AM) codes and text mining. We developed a list of ICD‐10‐AM codes potentially relating to injecting drug use and IRI from clinical knowledge and existing literature to identify these conditions in administration data [6, 7, 8] (Appendix S1, Supporting Information). In addition, we used a text‐mining approach to search emergency department free‐text triage notes for the following terms related to injecting drug use: ‘PWID’, ‘IDU’ (injecting drug use), ‘intravenous drug’, ‘IVDU’ (intravenous drug use), ‘IVU’ (intravenous use), ‘heroin’, ‘opioid replacement therapy’, ‘methadone’, ‘buprenorphine’ and ‘suboxone’. Subsequently, we extracted administrative data including unique medical record numbers, demographics, admission and separation dates and mortality status. The data used in this analysis is from AlfredHealth Data Warehouse managed by Data and Analytical Services.
2.3. Data collection
All admission episodes identified were manually reviewed to confirm eligibility, according to the criteria and definitions outlined above. For eligible admissions, we collected detailed data on patient demographics, IRI conditions, microbiology, patient management and opportunistic preventative medicine. Detailed chart review and data extraction was performed by medical officers (Freya J. Langham and Mei Jie Tang) using REDCap electronic data capture tools hosted at Alfred Health [23].
2.4. Definitions
We defined unplanned discharge as admissions ending with DAMA, or patients leaving the hospital without notice (absconding). Based on the Australian Bureau of Statistics definition, unstable housing includes living on the street as well as short‐term or emergency accommodation (such as living temporarily with friends and relatives) [24].
We considered patients to have an isolated skin or soft tissue infection if there was no documented complication, bloodstream infection or deeper focus of infection. All other patients were considered to have a complicated infection.
Admitting team refers to the medical unit with primary responsibility for the patient during their hospital stay. A consult refers to input from another specialist team or service (e.g., surgical unit, addiction medicine service) during the admission. If primary responsibility is transitioned between units during an admission, this is referred to as a takeover of care.
2.5. Ethics
We obtained approval from the Alfred Health Human Research Ethics Committee [Project 390/19], with a waiver for individual patient consent.
2.6. Statistical analysis
We performed univariate and multivariate mixed‐effects logistic regression to determine predictors of unplanned discharge. Rather than identifying causal relationships, our aim was to identify characteristics present at the time of admission that could be used to focus future interventions to avoid unplanned discharge. Based on clinical experience, we considered the following baseline characteristics as potential predictors: sex (male, female), age (18–34, 35–49, ≥50 years), opioid use (none, with OAT, without OAT) and the following binary variables: homelessness, methamphetamine use and mental health diagnosis other than substance abuse. Analysis was performed at the admission level, with patients included in the model as random effects to account for patients with repeat the admission records. For patients with unknown housing status, we performed multiple imputations using the Multiple Imputation by Chained Equations method, using all patient characteristics as covariates to generate models for five complete datasets and pooled results into one point estimate. All other covariates had no missing data. We estimated the effect of predictors and checked for completeness of data, multicollinearity and goodness of fit using a stepwise procedure with likelihood ratio tests. Adequacy of each model was assessed by Akaike's Information Criteria. Odds ratios (OR) and adjusted odds ratios (aOR) were presented with 95% confidence intervals (CI). All the statistical analyses were performed by using R Version 4.05 [26].
3. RESULTS
The ICD code and text‐mining algorithm identified 574 admissions for manual review. Among these, 226 (39%) were eligible for inclusion, involving 178 unique patients, with 205 unique infections.
3.1. Patient characteristics
The median age was 41 years (interquartile range [IQR], 36–47) and 66% (117/178) were male (Table 1). Smoking and comorbid psychiatric diagnoses other than substance abuse were highly prevalent. Eight (5%) patients were known to have HIV. Heroin was the most commonly reported injected drug used (68%, 121/178) followed by methamphetamines (56%, 100/178). At the time of admission, 36% (81/226) were already on OAT and 111 (49%) of patients were identified to have unstable housing. Fifty‐nine (33%) patients had been previously admitted to the Alfred Hospital for an IRI. Of these, 39 (66%) were due to SSTI, 12 (20%) due to endocarditis, 7 (12%) due to bone or joint infection.
TABLE 1.
Comparing admission, management and discharge data between patients with an isolated SSTI, versus a complicated infection
| Patient level characteristics | |||
|---|---|---|---|
| All patients (n = 178) | SSTI only (n = 87) | Complicated infection (n = 91) | |
| Age (median, IQR) | 41 (36–47) | 43 (36–49) | 40 (36–46) |
| Male | 118 (66%) | 60 (69%) | 58 (64%) |
| ATSI | 6 (3%) | 3 (3%) | 3 (3%) |
| Current smoker | 164 (92%) | 81 (93%) | 83 (91%) |
| Psychiatric diagnosis other than substance use disorder | 96 (54%) | 52 (60%) | 44 (48%) |
| Admission‐level characteristics | |||
|---|---|---|---|
| All admissions (n = 226) | SSTI only (n = 112) | Complicated infection (n = 114) | |
| OAT at admission | 81 (36%) | 34 (30%) | 47 (41%) |
| Previous IRI | 101 (45%) | 54 (48%) | 47 (41%) |
| Length of stay (days) a | 7 (3–19) | 4 (2–5) | 15 (9–38) |
| ICU admission | 34 (15%) | 1 (1%) | 33 (29%) |
| Housing | |||
| Stable | 111 (49%) | 42 (38%) | 69 (61%) |
| Unstable | 103 (46%) | 63 (56%) | 40 (35%) |
| Unknown | 12 (5%) | 7 (6%) | 5 (4%) |
| Infectious diseases unit involvement | |||
| Admitting team | 86 (38%) | 37 (33%) | 49 (43%) |
| TOC | 25 (11%) | 3 (3%) | 22 (19%) |
| Consult | 33 (15%) | 5 (4%) | 28 (25%) |
| None | 82 (36%) | 67 (60%) | 15 (13%) |
| Other management | |||
| Surgical review (admitting team or consult) | 151 (67%) | 66 (59%) | 85 (75%) |
| Operation performed | 77 (34%) | 37 (33%) | 40 (35%) |
| Addiction medicine review | 143 (63%) | 48 (43%) | 95 (83%) |
| Social work review | 133 (59%) | 46 (41%) | 87 (76%) |
| Hepatitis C antibody test | 115 (51%) | 33 (29%) | 82 (72%) |
| Behavioural issues (any) | 126 (56%) | 51 (23%) | 75 (33%) |
| Leaving ward | 82 (36%) | 36 (32%) | 46 (40%) |
| Substance use during admission (suspected/witnessed) | 52 (23%) | 15 (13%) | 37 (32%) |
| Aggression | 54 (24%) | 21 (19%) | 33 (15%) |
| Behavioural management | n = 126 | n = 51 | n = 75 |
| Verbal de‐escalation | 73/126 (56%) | 22/51 (43%) | 51/75 (68%) |
| Aggression event (‘Code Grey’) | 33/126 (26%) | 10/51 (20%) | 23/75 (31%) |
| Ejection from hospital | 5/126 (4%) | 1/51 (2%) | 4 (5%) |
| Discharge | |||
| Planned | 152 (67%) | 76 (68%) | 76 (67%) |
| Absconded | 37 (16%) | 20 (18%) | 17 (15%) |
| DAMA | 32 (14%) | 16 (14%) | 16 (14%) |
| Other | 3 (1%) | 0 | 3 (3%) |
| Death | 2 (1%) | 0 | 2 (2%) |
| Readmission within 30 days | 43 (19%) | 21 (19%) | 22 (19%) |
Abbreviations: ATSI, Aboriginal and Torres Strait Islander people; DAMA, discharge against medical advice; ICU, intensive care unit; IQR, interquartile range; IRI, injection‐related infection; OAT, opioid agonist therapy; SSTI, skin and soft tissue infection; TOC, takeover of care.
Median (interquartile range).
3.2. Infection data
3.2.1. Clinical syndromes
SSTI was the most common IRI reported (Figure 1). Of the reported SSTI infections, 67% had cellulitis present, while 51% had a collection or abscess. Ten (8%) patients with an SSTI were also bacteraemic.
FIGURE 1.
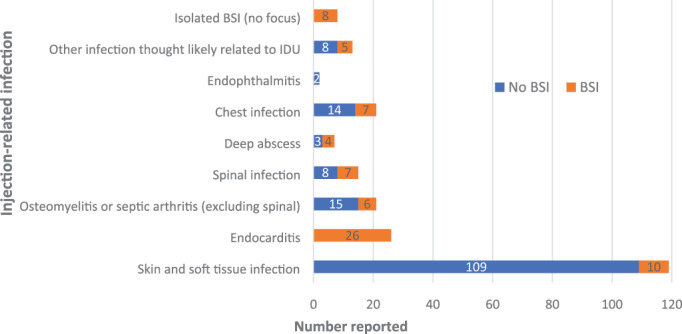
Breakdown of injection‐related infections confirmed by manual chart review, including proportion who were bacteraemic (orange shaded region). Deep abscess refers to abscess of an internal organ or deep muscle structure. Spinal infection includes vertebral osteomyelitis and septic arthritis and epidural abscess. Other infections thought likely relating to IDU included sepsis presentations without identification of a pathogen or source (n = 6), prosthetic device infections (n = 3) and purulent pericardial effusions (n = 2). BSI, bloodstream infection; IDU, injecting drug use
There were 36 episodes of bacteraemia without evidence of endocarditis. In most cases (28/36), patients were bacteraemic in association with localised anatomic site of infection, including SSTI (n = 10), spinal infection (n = 7), chest infection (n = 7), osteomyelitis/septic arthritis (n = 6), deep abscess (n = 4) and other focal site (n = 5). Eight patients had no other focal site of infection. A transthoracic echocardiogram was performed in 94% of bacteraemic patients, while a transoesophageal echocardiogram was performed in 10 patients only (30%). For the 26 episodes of endocarditis, 23 were native valve infections. The tricuspid valve was most commonly involved (n = 13), followed by mitral (n = 8) and aortic (n = 7) valves. Three patients underwent cardiothoracic surgery (12%).
There were 21 episodes of non‐vertebral osteomyelitis or septic arthritis. Median number of operations performed for these patients was one (IQR 0–1). Spinal infection occurred in 15 admissions, with overlapping clinical syndromes reported: vertebral osteomyelitis (n = 13), discitis (n = 11), epidural abscess (n = 9) and phlegmon (n = 3). Surgical intervention occurred in six cases, two patients underwent radiological aspiration and seven patients had no intervention. A deep abscess was identified in seven episodes, involving locations such as the gluteal, piriformis, psoas and iliacus muscles. Two patients underwent surgical debridement, and two had radiological aspirate; the remainder had no targeted intervention. In 21 episodes, a chest infection was identified, including nine episodes of empyema. Surgery was performed in five cases.
3.2.2. Microbiology
For 205 discrete infections, 137 (66%) had at least one positive microbiological sample. The median number of positive microbiological samples per infection was two (IQR 1–2).
There were 119 positive wound swabs in the cohort. Staphylococcus aureus was isolated in 55% (65/119), of which 68% (44/65) were methicillin‐susceptible. Streptococcus pyogenes was isolated in 15% (18/119) and Group C/G streptococci in 11% (13/119).
There were 81 positive blood cultures (with discrete organisms), occurring in 64 inpatient episodes. The median duration of bacteraemia was 1 day (IQR 1–3). Overall S. aureus was the most commonly isolated organism in blood cultures, accounting for 55% (44/81) of positive blood cultures, of which 89% (39/44) were methicillin‐susceptible. Nine patients had positive blood cultures with a different organism that occurred later in their inpatient admissions; we considered these secondary infections. The microbiology differed between those considered primary bacteraemias: S. aureus (n = 44), S. pyogenes (n = 4), other streptococci (n = 3) and S. epidermidis (n = 3), and those considered a secondary infection: Candida species (n = 3), S. epidermidis (n = 2), E. faecalis (n = 1) and E. faecium (n = 1). There were also three positive cultures from indwelling‐device tips: Staphylococcus haemolyticus, Enterococcus faecalis and Enterococcus faecium. There were 54 positive samples from sterile sites (tissue, bone, aspirates), with S. aureus identified in 61% (33/54).
3.2.3. Antibiotics
A clear plan for an intended duration of antibiotics was documented in 62% (63/102) of patients with a complicated infection. Of these, 55% (35/63) were completed as planned. In 21% (13/63) the antibiotic course was not completed as planned, usually due to unplanned discharge (10/13, 77%). In 24% (15/63), antibiotic completion was unknown due to patient transfer to another institution or loss to follow‐up.
3.3. Admission data
Median length of stay for all admissions was 7 days (IQR 3–19); however, this differed when the cohort was divided into SSTI only compared to complicated infections (Table 1). Thirty‐four (15%) admissions included time spent in the intensive care unit, with median nights spent in intensive care unit of five (IQR 3–11.5). Two admissions resulted in death.
At our hospital, the most common admitting units for isolated SSTIs were infectious diseases (36%), plastic surgery (26%) and general medicine (17%). The most common admitting units for complicated infections were infectious diseases (58%), general medicine (14%) and orthopaedic surgery (11%).
At least one behavioural issue occurred in 126 admissions (56%). Patient aggression (verbal or physical) requiring security assistance (‘Code Grey’) occurred in 15% of all admissions (33/226).
The addiction medicine consultation‐liaison service (comprising nurse specialists and addiction medicine physicians) reviewed the patient in 143 (63%) of admissions. Overall, 66% (149/226) of patients did not require OAT (due to current OAT, no withdrawal symptoms or no opioid use), 30% initiated OAT (68/226) and 4% (9/226) refused OAT (Figure 2).
FIGURE 2.
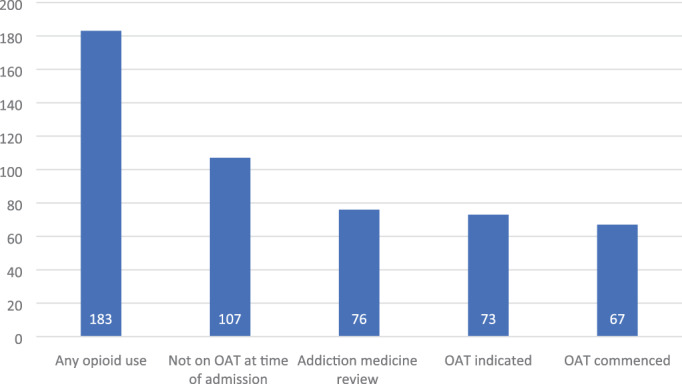
Cascade of care for patients with opioid use disorder during hospital admission for injection‐related infection. OAT, opioid agonist therapy
Screening for HIV and hepatitis B virus was performed during 101 (45%) and 105 (47%) admissions, respectively. Testing for hepatitis C virus was performed during 115 (51%) admissions; however, was more likely to occur in admissions for complicated infections (72%, 82/114) than isolated SSTI admissions (29%, 33/112). We did not take into consideration previous testing or previously known results. Twenty‐four percent (9/38) of patients with detectable hepatitis C ribonucleic acid (RNA) were commenced on direct‐acting antivirals during their hospital admission or on the day of discharge (Figure 3).
FIGURE 3.
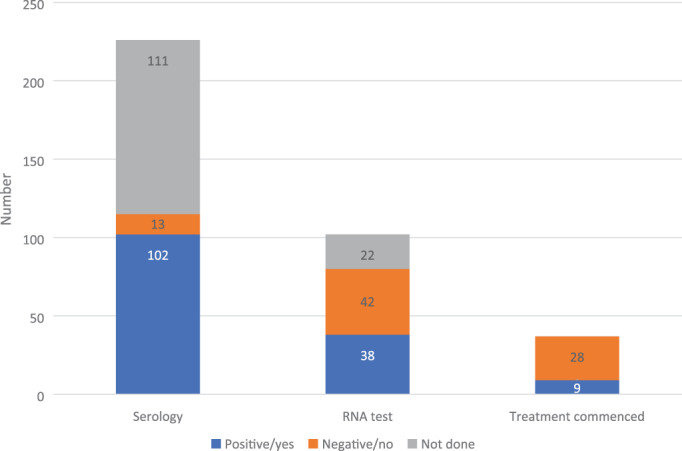
Screening for and management of hepatitis C. RNA, ribonucleic acid
3.4. Unplanned discharge
Discharge was unplanned in 31% (69/226) of admissions (Table 1). The proportion of unplanned discharges (due to absconding or DAMA), and readmission within 30 days, were approximately the same between the SSTI‐only group and those with complicated infections. Two patients had planned discharges to Hospital in the Home services.
Univariate analyses identified unstable housing and opioid use without OAT as predictors of unplanned discharge. In the multivariate model, opioid use without OAT was associated with an almost three‐fold increase in the odds of unplanned discharge when compared to no opioid use (OR 2.90, 95% CI 1.23, 6.85, p = 0.015) (Table 2).
TABLE 2.
Predictors of unplanned discharge
| Outcome | N | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p‐value | OR | 95% CI | p‐value | ||
| Sex | |||||||
| Male | 138 | Reference | |||||
| Female | 88 | 1.53 | 0.82, 2.85 | 0.181 | |||
| Age, years | |||||||
| 18–34 | 41 | Reference | |||||
| 35–49 | 140 | 0.66 | 0.27, 1.59 | 0.356 | |||
| ≥50 | 45 | 0.58 | 0.20, 1.68 | 0.316 | |||
| Housing | |||||||
| Stable | 156 | Reference | Reference | ||||
| Unstable | 70 | 1.93 | 1.05, 3.52 | 0.033 | 1.84 | 0.96, 3.54 | 0.065 |
| Opioid use | |||||||
| None | 59 | Reference | Reference | ||||
| With OAT | 68 | 0.72 | 0.27, 1.94 | 0.521 | 0.85 | 0.33, 2.17 | 0.727 |
| Without OAT | 99 | 3.03 | 1.16, 7.93 | 0.024 | 2.90 | 1.23,6.85 | 0.015 |
| Methamphetamine use | |||||||
| No | 94 | Reference | |||||
| Yes | 132 | 1.12 | 0.58, 2.15 | 0.732 | |||
| Mental health diagnosis other than substance abuse | |||||||
| No | 100 | Reference | |||||
| Yes | 126 | 1.39 | 0.72, 2.70 | 0.323 | |||
Note: Results from univariable and multivariable mixed effects logistic regression.
Abbreviations: CI, confidence interval; OAT, opioid agonist therapy; OR, odds ratio.
4. DISCUSSION
This data is among the first to demonstrate the burden, complexity and outcomes of IRI events at an Australian hospital. Our study highlights the breadth of infections related to injecting drug use, and subsequent challenges associated with hospitalisation and management. We have identified a number of areas for improving care for PWID.
In keeping with previous literature, SSTI was the most common reported IRI [2, 3]. In our cohort, patients with an isolated SSTI had shorter length‐of‐stay, but were less likely to be reviewed by the infectious diseases unit and less likely to receive opportunistic care (e.g., addiction support, OAT commencement, blood‐borne virus screening) compared to those with complicated IRI. Admission with an SSTI may represent an opportunity for intervention, to potentially prevent subsequent admission with a severe or complicated IRI [4].
Patients with complicated IRI have longer hospital stays. These admissions represent a significant burden of health‐care utilisation, but are an opportunity for ongoing harm reduction. However, prolonged admissions and the need for intravenous antibiotics also have potential negative consequences, including line‐associated or hospital‐acquired infections. A previous cohort of 420 episodes of infective endocarditis among PWID in Ontario, Canada reported 82 episodes of new bloodstream infection (with a different microorganism) occurring while patients were still receiving parenteral antimicrobial therapy [25]. New bloodstream infections were more common in patients receiving inpatient treatment [25]. Only two patients in our cohort were discharged with Hospital in the Home despite growing evidence that PWID can be safely managed in the outpatient setting [20, 26]. Expanding the use of outpatient parenteral antibiotic therapy may represent an opportunity to reduce hospitalisation burden, while improving engagement in care and overall patient outcomes.
Unplanned discharge occurred in 30% of all admissions, which is on the upper end of reported rates in international literature of 14–30% [2, 5, 12, 14]. This results in incomplete treatment courses and inadequate discharge planning, and is associated with worse outcomes [13]. Opioid use without OAT use (at time of admission) was found to be associated with higher likelihood of unplanned discharge in our cohort. This may be a causal association (i.e., high risk of withdrawal on admission to hospital) or may highlight a cohort of patients with longstanding barriers to health‐care engagement. Regardless, opioid use without OAT could potentially be a useful indicator for the need of active strategies to maintain engagement and avoid unplanned discharge.
Almost two‐thirds of our cohort were reviewed by the addiction medicine consultant liaison service. While still suboptimal, this is a much higher proportion than has been recorded in previous studies, although comparison is limited by heterogeneity in reporting, and varying terminology of what constitutes an addiction review [6, 10, 11, 27, 28]. This may reflect improved access to addiction medicine services in public hospitals in Australia, while the majority of the literature originates in North America. While not explored in this review, barriers to referral to addiction medicine service at our institution may include lack of acknowledgement of substance use disorder by treating teams in the absence of active withdrawal, lack of awareness of service availability and scope (i.e., beyond opioid agonist therapy prescription), and short inpatient admissions that may end before referral and review occurs.
Initiation of OAT, where appropriate, is one harm reduction strategy that may be implemented during a hospital admission. Inpatient OAT improves infection‐related outcomes [27, 28, 29] and reduces post‐hospital substance use [30]; however, impact on DAMA rates is mixed [12, 29, 31]. Hospitalisation with IRI is an opportunity to engage individuals with addiction care at a time when they are open to doing so [7]. However, OAT is only relevant to opioid‐dependent PWID, and thus only applies to a subset of our cohort. Addiction care should include interventions targeted at non‐opioid‐based addictions, including safe injecting education and counselling, however, this is less well established.
Addressing underlying social and economic determinants of health, such as homelessness, is also key to improving overall health outcomes. People experiencing homelessness have high rates of health care usage [32], and there is a clear association between unstable housing and bacterial infection risk [1], including injection site infections [33]. This may be due to increased likelihood of public injecting, which is associated with poorer hygiene and injection techniques [34]. Almost 50% of our cohort experienced unstable housing. Whether providing housing to PWID directly reduces infection is difficult to demonstrate, but provision of housing is an effective intervention for reducing substance use and injecting frequency, and reducing medical services utilisation [35, 36]. In our cohort, unstable housing was associated with unplanned discharge. This is consistent with other studies, and highlights the vulnerability of this cohort, who may have competing demands and unique needs to support an inpatient stay [37]. Further opportunistic care may be directed at comorbid psychiatric conditions and cigarette smoking, which were both highly prevalent in this cohort.
Finally, testing for and treating blood‐borne viruses is an important intervention that should be performed during all admissions for IRI [8]. In Australia, an estimated 1.3% of PWID have HIV (range 1.0–1.6%), 3.8% (2.4–5.2%) have active hepatitis B (sAg positive) and 53.5% (50.2–56.9%) are hepatitis C virus (HCV) antibody positive [38]. In our cohort, 89% of those tested were found to be HCV antibody positive, which may reflect a testing or selection bias, as this is markedly higher than population surveys in Australia which have found less than 50% HCV antibody positivity in PWID since 2017 [39]. Similarly, the proportion with detectable HCV RNA (active infection) was higher (48%) in our cohort than in Australian population surveys, and reflect levels closer to those seen prior to the introduction of direct‐acting anti‐viral therapy in March 2016 [39]. Reasons for this discrepancy may relate to factors that also increase risk of IRI, such as injecting frequency, and sharing needles, or our cohort may reflect patients who are less engaged in health care in general. In Australia, hepatitis C treatment can only be prescribed to outpatients, so eligible patients cannot start treatment during their hospital admission and must be provided with a script on discharge, with variable uptake as a result. In our cohort, only 24% of eligible patients were commenced on direct‐acting antivirals. Reasons for this may include unplanned discharge prior to prescription, return of RNA results after discharge, and lack of awareness of treatment availability by treating teams. Treatment during a hospital admission with IRI, which potentially requires several weeks of supervised intravenous antibiotics and medication administration, would allow more people access to hepatitis C cure.
There are limitations inherent in a retrospective, single‐centre audit, however, we have been able to outline 226 inpatient admissions in significant detail. We attempted to overcome the limitations of ICD‐10‐AM codes by also text‐mining key words in emergency triage documentation. Our cohort is likely an underestimate of the true number of admissions for IRI, due to underlying deficiencies in documentation of issues such as injecting drug use in the medical record [9]. Similarly, poor documentation of factors related to patients' social demographics such as housing status, and behavioural issues and their management was evident. Finally, data reflect the demographics and hospital experience of an inner‐city referral hospital in Melbourne, Australia and may not reflect the experiences of other health centres.
Our study has important implications for optimising management of PWID during hospital admissions. We identified that numerous medical and surgical teams are involved in the care of this population, and management strategies need to be multidisciplinary. Education related to the management of addiction, and associated behavioural issues, should be offered to all staff engaged in care. Improvement in early recognition of substance use disorder as a medical issue may improve interventions and outcomes, even during brief hospitalisations [40].
5. CONCLUSION
We have described a large cohort of admissions due to IRI in Australia, demonstrating the heterogeneity of admissions for infections related to injecting drug use. Our data also provide a comprehensive overview of the comorbidities associated with injecting drug use, which are co‐risk factors for infection, and also influence treatment outcomes. This highlights the complexities of management of infections in this population, and the need for comprehensive multidisciplinary management that addresses issues beyond the IRI. There are many barriers to health care among PWID. To date, the majority of interventions for PWID admitted to hospital have focused on the provision of OAT, however, this only addresses a subset of the PWID population and has mixed outcomes. Further data describing this cohort, and successful programs to optimise hospital care are required, in order to develop more comprehensive models of care.
AUTHOR CONTRIBUTIONS
FL, SC, JD, OV and AS conceived and/or designed the work. BJ designed the text mining and extracted the data. FL and MT performed the manual eligibility assessment and data extraction. FL and SC analysed the data, supervised by AS, with input from all authors. FL drafted the manuscript, and all other authors revised it critically for important intellectual content. All authors approved the final version of the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Appendix S1 International Classification of Diseases, Tenth Revision, Australian Modification (ICD‐10‐AM) codes used to identify hospital admission episodes for people who inject drugs with an injecting related infection.
ACKNOWLEDGEMENTS
No specific funding was received for this project. Andrew J. Stewardson is supported by an Australian National Health and Medical Research Council Early Career Fellowship (GNT1141398). Open access publishing facilitated by Monash University, as part of the Wiley ‐ Monash University agreement via the Council of Australian University Librarians.
Langham FJ, Curtis SJ, Tang MJ, Jomon B, Doyle JS, Vujovic O, et al. Acute injection‐related infections requiring hospitalisation among people who inject drugs: Clinical features, microbiology and management. Drug Alcohol Rev. 2022;41(7):1543–1553. 10.1111/dar.13525
Funding information National Health and Medical Research Council, Grant/Award Number: GNT1141398
REFERENCES
- 1. Dahlman D, Berge J, Björkman P, Nilsson AC, Håkansson A. Both localized and systemic bacterial infections are predicted by injection drug use: a prospective follow‐up study in Swedish criminal justice clients. PLoS One. 2018;13:e0196944‐e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lewer D, Hope VD, Harris M, Kelleher M, Jewell A, Pritchard M, et al. Incidence and treatment costs of severe bacterial infections among people who inject heroin: a cohort study in South London, England. Drug Alcohol Depend. 2020;212:108057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Larney S, Peacock A, Mathers BM, Hickman M, Degenhardt L. A systematic review of injecting‐related injury and disease among people who inject drugs. Drug Alcohol Depend. 2017;171:39–49. [DOI] [PubMed] [Google Scholar]
- 4. Binswanger IA, Takahashi TA, Bradley K, Dellit TH, Benton KL, Merrill JO. Drug users seeking emergency care for soft tissue infection at high risk for subsequent hospitalization and death. J Stud Alcohol Drugs. 2008;69:924–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eaton EF, Westfall AO, McClesky B, Paddock CS, Lane PS, Cropsey KL, et al. In‐hospital illicit drug use and patient‐directed discharge: barriers to care for patients with injection‐related infections. Open Forum Infect Dis. 2020;7:ofaa074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jicha C, Saxon D, Lofwall M, Fanucchi L. Substance use disorder assessment, diagnosis, and management for patients hospitalized with severe infections due to injection drug use. J Addict Med. 2019;13:69–74. [DOI] [PubMed] [Google Scholar]
- 7. Velez CM, Nicolaidis C, Korthuis PT, Englander H. “It's been an experience, a life learning experience”: a qualitative study of hospitalized patients with substance use disorders. J Gen Intern Med. 2017;32:296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Valerio H, Alavi M, Law M, McManus H, Tillakeratne S, Bajis S, et al. Opportunities to enhance linkage to hepatitis C care among hospitalised people with recent drug dependence in New South Wales, Australia: a population‐based linkage study. Clin Infect Dis. 2021;73:2037–44. [DOI] [PubMed] [Google Scholar]
- 9. Miller AC, Polgreen PM. Many opportunities to record, diagnose, or treat injection drug‐related infections are missed: a population‐based cohort study of inpatient and emergency department settings. Clin Infect Dis. 2019;68:1166–75. [DOI] [PubMed] [Google Scholar]
- 10. Gray ME, Rogawski McQuade ET, Scheld WM, Dillingham RA. Rising rates of injection drug use associated infective endocarditis in Virginia with missed opportunities for addiction treatment referral: a retrospective cohort study. BMC Infect Dis. 2018;18:532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rosenthal ES, Karchmer AW, Theisen‐Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use‐associated infective endocarditis. Am J Med. 2016;129:481–5. [DOI] [PubMed] [Google Scholar]
- 12. Ti L, Ti L. Leaving the hospital against medical advice among people who use illicit drugs: a systematic review. Am J Public Health. 2015;105:e53–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Southern WN, Nahvi S, Arnsten JH. Increased risk of mortality and readmission among patients discharged against medical advice. Am J Med. 2012;125:594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chan AC, Palepu A, Guh DP, Sun H, Schechter MT, O'Shaughnessy MV, et al. HIV‐positive injection drug users who leave the hospital against medical advice: the mitigating role of methadone and social support. J Acquir Immune Defic Syndr. 2004;35:56–9. [DOI] [PubMed] [Google Scholar]
- 15. Pollini RA, Paquette CE, Drvar T, Marshalek P, Ang‐Rabanes M, Feinberg J, et al. A qualitative assessment of discharge against medical advice among patients hospitalized for injection‐related bacterial infections in West Virginia. Int J Drug Policy. 2021;94:103206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tung MK, Light M, Giri R, Lane S, Appelbe A, Harvey C, et al. Evolving epidemiology of injecting drug use‐associated infective endocarditis: a regional Centre experience. Drug Alcohol Rev. 2015;34:412–7. [DOI] [PubMed] [Google Scholar]
- 17. Salmon AM, Dwyer R, Jauncey M, van Beek I, Topp L, Maher L. Injecting‐related injury and disease among clients of a supervised injecting facility. Drug Alcohol Depend. 2009;101:132–6. [DOI] [PubMed] [Google Scholar]
- 18. Topp L, Iversen J, Conroy A, Salmon AM, Maher L. NSPs obotCoA. Prevalence and predictors of injecting‐related injury and disease among clients of Australia's needle and syringe programs. Aust N Z J Public Health. 2008;32:34–7. [DOI] [PubMed] [Google Scholar]
- 19. Ivan M, van Beek I, Wand H, Maher L. Surveillance of injecting‐related injury and diseases in people who inject drugs attending a targeted primary health care facility in Sydney's kings cross. Aust N Z J Public Health. 2015;39:182–7. [DOI] [PubMed] [Google Scholar]
- 20. Low ZM, Krishnaswamy S, Woolley IJ, Stuart RL, Boers A, Barton TL, et al. Burden of infective endocarditis in an Australian cohort of people who inject drugs. Intern Med J. 2020;50:1240–6. [DOI] [PubMed] [Google Scholar]
- 21. Hilbig A, Cheng A. Infective endocarditis in the intravenous drug use population at a tertiary hospital in Melbourne. Australia Heart Lung Circ. 2020;29:246–53. [DOI] [PubMed] [Google Scholar]
- 22. World Health Organization . Regional Office for South‐East a. Management of common health problems of drug users. New Delhi: WHO Regional Office for South‐East Asia; 2008. [Google Scholar]
- 23. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)‐‐a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Australian Institute of Health and Welfare. Homelessness and homelessness services. Canberra: AIHW; 2021. [Google Scholar]
- 25. Tan C, Shojaei E, Wiener J, Shah M, Koivu S, Silverman M. Risk of new bloodstream infections and mortality among people who inject drugs with infective endocarditis. JAMA Netw Open. 2020;3:e2012974‐e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Suzuki J, Johnson J, Montgomery M, Hayden M, Price C. Outpatient parenteral antimicrobial therapy among people who inject drugs: a review of the literature. Open Forum Infect Dis. 2018;5:ofy194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rodger L, Glockler‐Lauf SD, Shojaei E, Sherazi A, Hallam B, Koivu S, et al. Clinical characteristics and factors associated with mortality in first‐episode infective endocarditis among persons who inject drugs. JAMA Netw Open. 2018;1:e185220‐e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marks LR, Munigala S, Warren DK, Liang SY, Schwarz ES, Durkin MJ. Addiction medicine consultations reduce readmission rates for patients with serious infections from opioid use disorder. Clin Infect Dis. 2019;68:1935–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jo Y, Nosal R, Vittori A, Cordova L, Vandever C, Alvarez C, et al. Effect of initiation of medications for opioid use disorder on hospitalization outcomes for endocarditis and osteomyelitis in a large private hospital system in the United States, 2014‐18. Addiction. 2021;116:2127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wakeman SE, Metlay JP, Chang Y, Herman GE, Rigotti NA. Inpatient addiction consultation for hospitalized patients increases post‐discharge abstinence and reduces addiction severity. J Gen Intern Med. 2017;32:909–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Suzuki J, Robinson D, Mosquera M, Solomon DA, Montgomery MW, Price CD, et al. Impact of medications for opioid use disorder on discharge against medical advice among people who inject drugs hospitalized for infective endocarditis. Am J Addict. 2020;29:155–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fazel S, Geddes JR, Kushel M. The health of homeless people in high‐income countries: descriptive epidemiology, health consequences, and clinical and policy recommendations. Lancet. 2014;384:1529–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hope VD, Marongiu A, Parry JV, Ncube F. The extent of injection site infection in injecting drug users: findings from a national surveillance study. Epidemiol Infect. 2010;138:1510–8. [DOI] [PubMed] [Google Scholar]
- 34. Small W, Rhodes T, Wood E, Kerr T. Public injection settings in Vancouver: physical environment, social context and risk. Int J Drug Policy. 2007;18:27–36. [DOI] [PubMed] [Google Scholar]
- 35. Fitzpatrick‐Lewis D, Ganann R, Krishnaratne S, Ciliska D, Kouyoumdjian F, Hwang SW. Effectiveness of interventions to improve the health and housing status of homeless people: a rapid systematic review. BMC Public Health. 2011;11:638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fortier E, Sylvestre MP, Artenie AA, Minoyan N, Jutras‐Aswad D, Roy É, et al. Associations between housing stability and injecting frequency fluctuations: findings from a cohort of people who inject drugs in Montréal, Canada. Drug Alcohol Depend. 2020;206:107744. [DOI] [PubMed] [Google Scholar]
- 37. Wang A, Pridham KF, Nisenbaum R, Pedersen C, Brown R, Hwang SW. Factors associated with readmission among general internal medicine patients experiencing homelessness. J Gen Intern Med. 2021;36:1944–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Degenhardt L, Peacock A, Colledge S, Leung J, Grebely J, Vickerman P, et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health. 2017;5:e1192–e207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Heard S, Iversen J, Geddes L, Maher L. Australian NSP survey: prevalence of HIV, HCV and injecting and sexual behaviour among NSP attendees, 25‐year National Data Report 1995–2019. Sydney: The Kirby Institute; 2020. [Google Scholar]
- 40. Englander H, Weimer M, Solotaroff R, Nicolaidis C, Chan B, Velez C, et al. Planning and designing the improving addiction care team (IMPACT) for hospitalized adults with substance use disorder. J Hosp Med. 2017;12:339–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 International Classification of Diseases, Tenth Revision, Australian Modification (ICD‐10‐AM) codes used to identify hospital admission episodes for people who inject drugs with an injecting related infection.


