Abstract
Globally, per‐ and polyfluoroalkyl substances are common artificial ingredients in industrial and consumer products. Recently, they have been shown to be an emerging human health risk. Perfluorononanoic acid (PFNA)/perfluorononanoate and perfluorobutane sulfonic acid (PFBS)/perfluorobutane sulfonate cause reproductive toxicity and hepatotoxicity, disrupt thyroid functions, and damage embryonic development in zebrafish. However, the cardiotoxic effects of PFNA and PFBS have not been fully established. We found that PFNA and PFBS exposures repress hatchability while increasing malformation and mortality in zebrafish embryos. Hematoxylin and eosin staining as well as assessment of the transgenic zebrafish line Tg(myl7:nDsRed) revealed that exposure of embryos to PFNA increases the occurrence of severe cardiac malformations relative to exposure to PFBS. Moreover, we evaluated the differential expressions of cardiac development‐associated genes in response to PFNA and PFBS, which validated the potential cardiotoxic effects, consistent with cardiac dysfunctions. Overall, our findings reveal novel cardiotoxic effects of PFNA and PFBS in zebrafish, implying that they may exert some cardiotoxic effect in humans. To the best of our knowledge, ours is the first study to show that PFNA exerts more severe cardiotoxic effects in zebrafish when compared with PFBS. Based on these findings, studies should evaluate the mechanisms of their cardiotoxic effects. Environ Toxicol Chem 2022;41:2527–2536. © 2022 The Authors. Environmental Toxicology and Chemistry published by Wiley Periodicals LLC on behalf of SETAC.
Keywords: Environmental toxicology, Perfluoroalkyl substances, Developmental toxicity
INTRODUCTION
Rapid industrial development in recent decades has increased human convenience. However, it has also increased global pollution, causing reversible and irreversible changes to the environment, affecting humans, animals, and the ecosystem (Manzetti et al., 2014). Per‐ and polyfluoroalkyl substances (PFAS) are common artificial additives in industrial and consumer products, with broad applications since the 1940s. Due to their environmental impact and human health risks, PFAS are emerging pollutants that have attracted the attention of researchers and stakeholders (Menger et al., 2020). These organofluorine chemical compounds have high‐energy carbon–fluorine (C–F) covalent bonds, which endows them with stable physicochemical, antihydrolysis, antiphoto degradation, and antimicrobial degradation properties (Ulhaq et al., 2013). Per‐ and polyfluoroalkyl substances have extensive industrial applications, for instance they are used in the production of adhesives, water‐ and stain‐repellent surfaces, nonstick coatings, and lubricants (DeWitt et al., 2012). Paradoxically, it is their stable characteristic that facilitates their persistence in the environment and accumulation in various organisms, ultimately threatening the environmental and human health (Dasgupta et al., 2020). The toxic effects of each PFAS are associated with its carbon chain length and functional groups (Menger et al., 2020). Due to their toxicity persistent organic pollutants, and the use of some long‐chain PFAS have been prohibited by the Stockholm Convention. Among the PFAS, the short‐chain (C < 8) perfluorobutane sulfonic acid (PFBS) and the long‐chain (C ≥ 8) homologues (perfluorononanoic acid [PFNA]) are frequently detected in the environment as emerging PFAS with potential risks to human beings (Chen et al., 2018).
The sources of PFNA and short‐chain homologue PFBS are ubiquitous in the environment, which is attributed to their widespread use (Cao et al., 2019; Chen et al., 2016). During the 2005 season, fluxes of PFNA were estimated in the area north of 65°N to the Arctic, and they ranged from 73 to 860 kg/year (Young et al., 2007). The sources of PFNA as a traditional PFAS compound maybe associated with increasing production of the chemical, its stability, and its high bioaccumulation potential over time (Prevedouros et al., 2006; Young et al., 2007). The high concentrations of PFBS may be attributed to increasing usage of substitute traditional PFAS, such as N‐methyl perfluorobutane sulfonamide ethanol and related compounds, in recent years (D'eon et al., 2006; Podder et al., 2021). Khan et al. (2022) reported that the average daily flux of PFNA along the Indus River was 2.72 kg while that of PFBS was 1.71 kg. The mean value of PFNA concentration in surface water in rural areas of eastern China was 1.66 ng/L while that of PFBS was 7.22 ng/L (Chen et al., 2016). The sources of PFNA and short‐chain PFBS in groundwater include surface water, soil, atmospheric deposition, and precipitation of snow, ice, and rainfall. The concentration of PFBS was higher in groundwater surrounding a fluorochemical industrial park in China, with a mean value of 11 016.2 ng/L, whereas the concentrations of PFNA in groundwater from the Alluvial–Pluvial Plain of the Hutuo River and rural areas in eastern China were 0.24 and 4.7 ng/L, respectively (Bao et al., 2019; Sharma et al., 2016; Xu et al., 2021). Notably, PFNA and PFBS were also detected in the Arctic. The concentrations of PFNA in Arctic snow and ice ranged between 38 and 110 pg/L. Meanwhile, in the surface layer of ice core of the Arctic, the maximum concentration of PFBS reached 1500 pg/L (Cai et al., 2012).
Among aquatic organisms, relatively high concentrations of PFAS have been reported in fish and sea foods, and the major source of PFBS and PFNA in humans is fish consumption. Perfluorononanoic acid was detected in crustaceans (58 pg/g) and lean fish (77 pg/g) from the Netherlands (Noorlander et al., 2011). The concentrations of PFNA in eel ranged between 10 and 1510 pg/g while PFBS in eel was detected at concentrations slightly above 12 pg/g from the Italy (Chiesa et al., 2018). Clarke et al. (2010) evaluated the concentrations of PFNA and PFBS in a broad range of fish species from the United Kingdom. Perfluorononanoic acid was found to be broadly distributed among the species and at high concentrations, ranging between <1000 and 3000 pg/g, whereas PFBS was detected in a majority of samples at concentrations slightly above 1000 pg/g (Clarke et al., 2010). Fernandes et al. (2017) assessed 50 fish samples from the United Kingdom and found relatively high PFAS concentrations in sardines, mackerel, sprats, herring, mullet, and sea bass. The highest PFNA levels were detected in sprat, ranging between 50 and 690 pg/g; the highest concentrations of PFBS were detected in herring and ranged between 10 and 600 pg/g (Fernandes et al., 2017).
There is an association between PFNA and markers of liver injury (Costello et al., 2022). As a traditional PFAS compound, PFNA causes reproductive toxicity and hepatotoxicity, disrupts thyroid functions, and impairs embryonic development in zebrafish (Liu et al., 2011; Zhang et al., 2012, 2016; Zheng et al., 2011). Perfluorobutane sulfonic acid, as the shorter homologue of PFNA, dysregulates pancreatic organogenesis, lipid metabolism, and embryonic development, and can exert neurologic as well as visual toxicities in zebrafish (Chen et al., 2020; Hu et al., 2020; Liu et al., 2020; Sant et al., 2019; Tang et al., 2020). Most studies on toxicities of PFNA and PFBS have focused on hepatotoxicity, thus there is a need to elucidate the cardiotoxicological effects of these two chemicals.
We investigated the cardiotoxic effects of PFNA and PFBS in zebrafish (Daniorerio) embryos, and compared the toxic effects of C8 PFAS PFNA and short chain C4 PFAS PFBS to establish the potential effects of PFAS on fish cardiac development. Our results will form the basis for assessing their underlying molecular mechanisms, in addition to improving the conservation of aquatic organisms and the protection of human and environmental health in the future.
MATERIALS AND METHODS
Chemicals
Perfluorononanoic acid (CF3(CF2)7COOH; CAS: #375‐95‐1, purity (GC AREA %: ≥96.5%)) and PFBS (CF3(CF2)3SO3K; CAS: #29420‐49‐3, TITRATION (T) NAOH 0.1 M: 97.5%–102.5%) used in the present study were purchased from Sigma‐Aldrich. Solid PFNA and solid PFBS were dissolved in water to produce the respective 10 mM stock solutions.
Zebrafish maintenance and embryo collection
Zebrafish strain AB was maintained according to standard protocols provided by the Chinese Zebrafish Resource Center. Adult zebrafish were maintained in a stable circulating filtration system and fed live brine shrimp twice a day. The water temperature was maintained at 27.5–28.5 °C and there was a 14:10‐h light: dark photoperiod. Male and female zebrafish were mated the day before spawning, and zebrafish eggs were hatched in a constant temperature incubator. Chemical treatments were performed at the indicated times (Supporting Information, Figure S1), with the treated embryos being placed in a dedicated homeothermic system for culture and normally fed seedling food after hatching. The Tg (myl7: nDsRed) transgenic zebrafish was generated by Fishman's laboratory as previously reported (Mably et al., 2003).
Chemical treatments
Zebrafish embryos were exposed to PFNA or PFBS starting at 3 h post‐fertilization (hpf) and ending at 120 hpf. All exposures were performed in sterile 10‐cm polystyrene Petri dishes with chemicals dissolved in 25 ml of egg water (embryo medium: 5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, and 0.33 mM MgSO4). The final working concentrations of each chemical treatment were 0, 0.2, 2, 20, and 200 µM per 60–70 embryos. The original nominal concentrations of PFNA and PFBS were diluted to obtain the final working concentration.
The treated embryos were cleaned three times with egg water at the end of the exposure period and placed in seedling culture bottles. The water was changed daily, and the larvae were fed a special diet twice a day. We also used this period to record the hatching, malformation, and mortality rates. The embryos were collected at appropriate intervals for subsequent experiments and analyses.
RNA sequencing analysis
We collected samples from the 20 µM PFNA, 20 µM PFBS, and control groups and subjected them to RNA‐sequencing (RNA‐seq) analysis. Total RNA was used as input material for the RNA sample preparations for the library. After the library is qualified, the different libraries are pooling according to the effective concentration and the target amount of data off the machine, then sequenced by the Illumina NovaSeq. 6000 (Novogene). Differential expression analysis of two groups was performed using the DESeq. 2R package (1.20.0). The DESeq. 2 package provided statistical routines for determining differential expression in digital gene expression data using a model based on the negative binomial distribution. The resulting p values were adjusted using the Benjamini and Hochberg approach for controlling the false discovery rate. p adj < 0.05 and |log2(foldchange)| > 1 were set as the threshold for significantly differential expression.
RNA isolation and quantitative real‐time polymerase chain reaction
Quantitative real‐time polymerase chain reaction (PCR) was used to quantify the transcriptional expression of target genes in samples according to the results of differentially expressed genes (DEGs). Each sample contained 25–30 zebrafish embryos and the total RNA was extracted from samples after lysing using the TRIzol reagent (Reagent). The total RNA was used to synthesize cDNA using a reverse transcription kit (Reagent) with 1 µg RNA of each sample. Quantitative real‐time PCR was performed using DNA Master SYBR Green I (Yeasen) on a QuantStudio 3 Real‐time PCR instrument (ABI). β‐Actin was used as the internal control. The functions of the selected genes were mainly related to heart development, and the primer sequences for the selected genes were as follows: Amhc: F (forward primer): AAGCCACTACCGCCTCTCTA, R (reverse primer): TTTGAGGCAAGGTCGTCCAA; Nppa: F: ATCCTGGGACAGAGACCGAG, R: CTATGCGATCCAGCCTTCCC; Nkx2.5: F: TCGGGATGGTAAACCGTGTC, R: TGCTCGACGGATAGTTGCAT; End1: F: ATCTTTGGGTCCAGGCACAG, R: TATGACACCGTTCGCTCTGG; Tgfb2: F: ACGCGCTTTGCAGGTATAGA, R: GCTCTTATGCTGCGACTCCA; β‐Actin: F: CTCTTCCAGCCTTCCTTCCT, R:CTTCTGCATACGGTCAGCAA.
Histological analysis
We anesthetized the zebrafish embryos and fixed them in 4% perfluoroalkoxy alkane overnight at 4 °C before embedding them in paraffin. The embedded embryos were sectioned, then 7‐µm‐thick heart sections were stained with hematoxylin and eosin (H&E) and observed under an Olympus BX53 microscope (Olympus).
Fluorescent observation
To visualize cardiomyocyte disorders after exposure in different groups, we used transgenic zebrafish expressing nuclear DsRed under the control of the cmlc2 (myl7) promoter (cmlc2: nDsRed), to label activated epicardial cells, and observations were made using a fluorescent microscope (IX83; Olympus).
Statistical analysis
All experiments were repeated three times with consistent results, and a representative set of data is shown with standard errors. Statistical significance between groups was calculated by two‐tailed Student's t test unless otherwise specified. Prism 6 was used to calculate the values. A p value <0.05 was statistically significant.
Ethics
Zebrafish were maintained in a recirculating water system according to standard protocol. All experiments with zebrafish were approved by the ethics Committee of Tongji Medical College, Huazhong University of Science and Technology under protocol number 2022‐2793.
RESULTS
Effects of PFNA and PFBS exposures on hatchability, malformation, and mortality rates of zebrafish embryos
Fertilized embryos (3 hpf) were exposed to different concentrations of PFNA or PFBS (0, 0.2, 2, 20, and 200 µM). The specific exposure and data collection times are shown in Supporting Information, Figure SI. The hatching rate of zebrafish embryos was counted from 3 to 5 days post‐fertilization (dpf; Figure 1A), which revealed that low PFNA doses (0.2 μM) had no significant effects on hatching rate (Figure 1A1). However, 2–200 μM PFNA had significant effects on hatching rate. When embryos were exposed to low (0.2 μM) PFBS doses, there were no significant effects on hatching rate (Figure 1A2). Conversely, 2–200 μM treatments had significant effects on hatching rates. Comparisons of effects of PFNA and PFBS revealed that the hatching rate under PFBS treatment was significantly higher than under PFNA treatment, regardless of concentrations (Figure 1A3–4).
Figure 1.
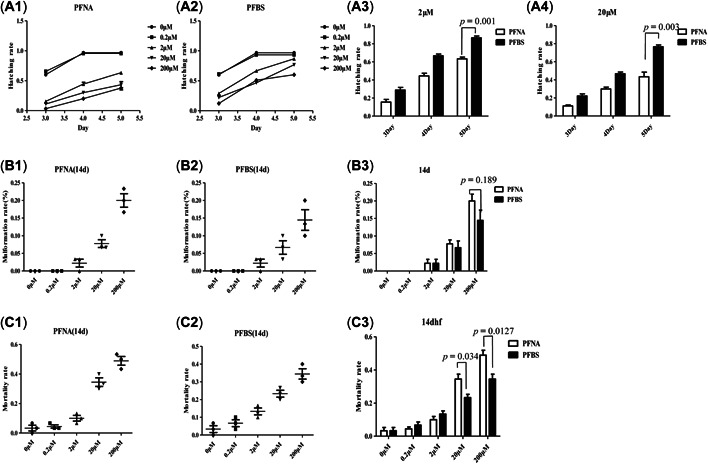
Effects of perfluorononanoic acid (PFNA) and perfluorobutane sulfonic acid (PFBS) exposure on zebrafish embryos. (A) Line chart describing the hatchability of PFNA (A1)‐ and PFBS (A2)‐treated embryos between 3 and 5 days postfertilization (dpf), and a bar graph comparing the values at 2 µM (A3) and 20 µM (A4). (B) The malformation rates of the PFNA (B1)‐ and PFBS (B2)‐exposed zebrafish embryos were evaluated over 14 days at each concentration, and the differences in rates are illustrated in the bar graph for PFNA and PFBS (B3). (C) The mortality rates of the zebrafish embryos over the 14‐day evaluation (PFNA (C1) and PFBS (C2)), illustrated in a bar graph (C3).
Malformation (including pericardial edema, swim‐bladder deficiency, yolk retention, curved body shape, curved tail, small eyes and short body length) rates of zebrafish embryos exposed to PFNA and PFBS were also evaluated and compared (Figure 1B). Significant differences were observed at 200 μM, with comparable findings at other concentrations. Specifically, at 200 μM, PNFA induced a significantly higher malformation rate than PFBS (Figure 1B3). In addition, comparisons of mortality rates at 14 dpf revealed significant differences between the chemicals (Figure 1C) at 20 and 200 μM, with the mortality rate in the PNFA group being significantly higher than that of the PFBS group at both concentrations (Figure 1C3).
Differential expressions of cardiac development and apoptosis‐related genes in PFNA‐ and PFBS‐exposed embryos
We further evaluated the transcriptional responses of developing embryos following treatment with PFNA and PFBS using samples collected at 120 hpf. Then, RNA‐seq and semi‐quantitative RT‐PCR were performed to evaluate sample transcriptomes. There were 2471 DEGs in PFBS‐ and 1178 DEGs in PFNA‐exposed embryos, compared to their respective controls. A total of 220 genes were common to PFBS‐ and PFNA‐exposed embryos (Figure 2A1), with 1257 genes being up‐regulated in PFBS‐exposed embryos and 469 genes being up‐regulated in PFNA‐exposed embryos. There were 89 overlapping DEGs between the groups (Figure 2A2). A total of 1214 genes were down‐regulated in PFBS‐exposed embryos while 709 genes were down‐regulated in PFNA‐exposed embryos, with 116 of the DEGS overlapping between the groups (Figure 2A3).
Figure 2.
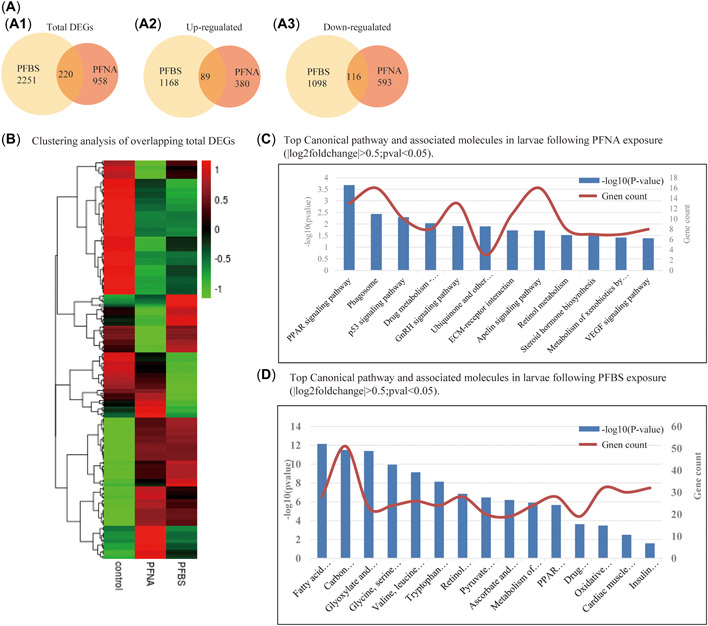
Comparison of the transcriptome data of perfluorononanoic acid (PFNA)‐ and perfluorobutane sulfonic acid (PFBS)‐exposed embryos at 120 h postfertilization (hpf). (A) Venn diagrams representing the overlapping differentially expressed genes (DEGs) in both groups of embryos (A1), the overlapping up‐regulated DEGs (A2), and the overlapping down‐regulated DEGs (A3) in the embryos. (B) Clustering analysis of the total overlapping DEGs from both groups. (C) Top Kyoto Encyclopedia of Genes and Genome (KEGG) pathways and the associated molecules identified from the PFNA‐treated larvae (|log2fold‐change| > 0.5, p < 0.05). (D) Top KEGG pathways and the associated molecules in the PFNA‐ and PFBS‐treated larvae clustered separately (|log2fold‐change| > 0.5, p < 0.05).
Heat cluster analysis was conducted to assess all overlapping DEGs (Figure 2B) and the results used to identify the genes of interest. One of the aims of the present study was to evaluate cardiac and embryonic apoptosis‐related genes, therefore we assessed the overlapping DEGs via Gene Ontology and Kyoto Encyclopedia of Genes and Genome (KEGG) pathway analyses to identify the significantly enriched Gene Ontology terms and KEGG pathways associated with each treatment. For all evaluations, we applied a corrected p < 0.05 as the cutoff for significance. The KEGG pathway analysis revealed that PFNA DEGs were mainly enriched in peroxisome proliferator‐activated receptor (PPAR), phagosome, p53, GnRH, VEGF, and metabolism of cytochrome P450 pathways (Figure 2C). Similarly, KEGG pathway analysis in PFBS‐exposed embryos revealed that DEGs were enriched in fatty acid degradation, PPAR, insulin, and carbon (retinol, pyruvate) metabolism, and cardiac muscle contraction (Figure 2D).
Real time‐PCR was used to assess the expressions of cardiotoxic genes in zebrafish larva. At 120 hpf, 20 µM PFNA, PFBS, and control group samples were collected and analyzed. To determine whether PFNA and PFBS exposures caused cardiac malformations, we analyzed five development‐associated genes (Figure 3). The myosin, heavy chain 6 (cardiac muscle alpha, amhc) gene plays a role in atrial assembly and cardiac functions. The natriuretic peptide A (nppa) gene plays a role in cardiac muscle cell proliferation. Compared to the control group, expressions of amhc (Figure 3A) and nppa (Figure 3B) were significantly up‐regulated after PFNA exposures, but differences in expressions between PFBS‐treated and control groups were insignificant. Up‐regulated amhc expressions may be associated with recovery of cardiac dysfunctions, especially atrial dysfunction. Compared to the control group, the expressions of the cardiac transcription factor NK2 homeobox 5 (nkx2.5) gene were significantly up‐regulated after PFNA exposures, but were not significantly different from those of the PFBS exposure group (Figure 3C). Moreover, endothelin 1 (end1) expressions were significantly increased after PFNA exposures, compared to the control group, suggesting its regulatory role in vasoconstriction (Figure 3D). Finally, exposures to PFNA significantly up‐regulated the expressions of transforming growth factor beta 2 (tgfb2), implying that PFNA affects cardiomyocyte proliferation (Figure 3E). In conclusion, gene expression assessment revealed that PFNA is more toxic to the heart of zebrafish than PFBS.
Figure 3.
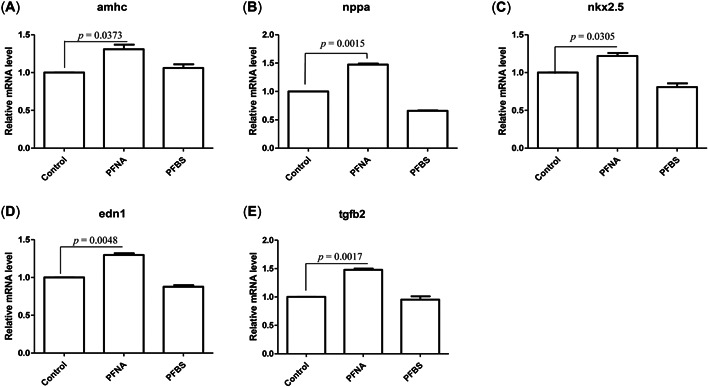
Detection of cardiac gene expression in zebrafish embryos exposed to perfluorononanoic acid (PFNA) and perfluorobutane sulfonic acid (PFBS). (A–E) Zebrafish embryos were exposed to PFNA and PFBS, and the expression changes in cardiac genes in zebrafish embryos were detected at 120 hpf. Amhc= cardiac muscle alpha; Nppa = natriuretic peptide A; Nkx2.5 = cardiac transcription factor NK2 homeobox 5; End1 = endothelin 1; Tgfb2 = transforming growth factor beta 2. Three replicates were used for all experiments, and the data were normalized to the expression of the housekeeping gene β‐actin. t‐test, p < 0.05 indicates a significant difference. Values are represented as mean ± SEM of three replicates.
Abnormalities in cardiac development for PFNA‐ and PFBS‐exposed embryos
Developmental abnormalities of the larva were evaluated using H&E‐based assays at 5 dpf. Perfluorononanoic acid was associated with more severe cardiac malformation in zebrafish larvae, relative to PFBS. In the PFNA group, compared to the wild‐type group, there were no clear boundaries of atrium and ventricle in the center of the visual field, the heart valve was thickened, and the arrangement of valve tissue was loose and movable (Figure 4A,B). In addition, heart tissues of PFBS group were filled with many red blood cells, and the structural boundaries of the atrium, ventricle, and heart valve exhibited defects, making it difficult to clearly distinguish the structures of tissues when compared to those of the control (Figure 4C).
Figure 4.
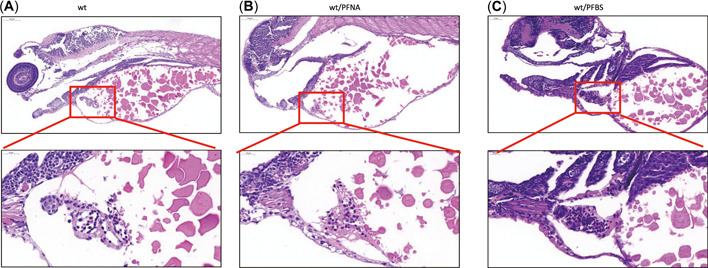
Hematoxylin and eosin (H&E) evaluation of zebrafish larval heart sections at 120 h post‐fertilization (hpf) following perfluorononanoic acid (PFNA) or perfluorobutane sulfonic acid (PFBS) exposure. (A) H&E staining of wild‐type tissues at 120 hpf, (B) H&E staining of PFNA‐treated zebrafish larvae at 120 hpf, and (C) H&E staining of PFBS zebrafish larvae at 120 hpf. wt = wild type.
Embryonic abnormalities in PFNA‐ and PFBS‐exposed transgenic zebrafish embryos
We assessed the developmental abnormalities in cardiac tissues of PFNA‐ and PFBS‐exposed larva using transgenic larva (myl7: nDsRed) at 5 dpf. Heart tissues from the PFNA group exhibited obvious abnormalities, compared to the control, confirming our H&E results. The boundaries of the atrium and ventricle in the PFNA group were blurred in both bright‐field and fluorescence images, the heart valve was thickened, and tissue structures seemed to be movable (Figure 5A,B). The heart tissues of the PFBS group exhibited considerable cardiac dilatation, and the structural margins of the atrium, ventricle, and heart valve were blurred and irregular when compared to those of the control, validating the findings of our H&E assays (Figure 5C).
Figure 5.
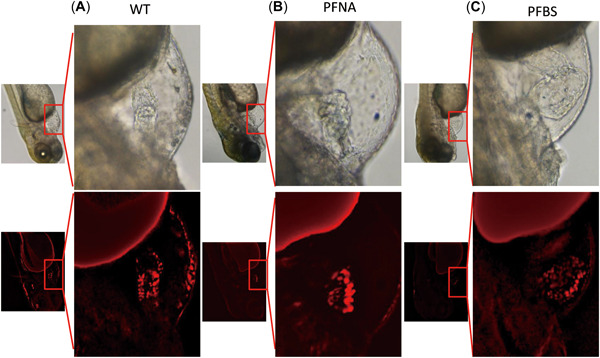
Perfluorononanoic acid (PFNA) and perfluorobutane sulfonic acid (PFBS) exposure induces cardiac abnormalities during the development of zebrafish larvae. (A) Bright‐field and fluorescence observation of various sections of transgenic fish (myl7:dsRed) at 120 h post‐fertilization (hpf). (B) Bright‐field and fluorescence observation of myl7; dsRed transgenic zebrafish at 120 hpf following PFNA (B) and PFBS (C) exposure. WT = wild type.
DISCUSSION
Per‐ and polyfluoroalkyl substances are a group of artificial chemicals that are extensively used in industrial and consumer products. These chemicals are global environmental contaminants. Selective bio‐enrichment of individual PFAS leads to partition of PFAS in the environment. Enrichment of PFAS in organisms suggests that their toxicity should be explored in addition to any potential negative effects on human and environmental health. Zebrafish is an important laboratory model for toxicological research (Gore et al., 2018; Horzmann & Freeman, 2018). It has numerous advantages and bridges the gap between in vitro models and higher mammalian biomedical models. An increasing number of studies on organ and tissue regeneration and drug toxicity risk are selecting the zebrafish as a model for toxicological studies to exploit it as an in vivo screen and reduce the number of compounds used to achieve rapid and cost‐saving outcomes.
In addition to having adverse toxic effects on skeletal and reproductive systems, PFNA and PFBS cause tumors, liver damage, and kidney damage and disrupt thyroid functions (Liu et al., 2011; Zhang et al., 2012, 2016; Zheng et al., 2011). However, few studies have reported on the cardiotoxic effects of PFNA and PFBS, especially with respect to cardiovascular development. Zebrafish is an established correlative and predictive model for assessing biomedical toxicity, particularly with regards to cardiovascular development, considering that the heart is the first organ to form and function, as early as 24 hpf (Zhou et al., 2016). Vertebrate heart development and formation is a complex and elaborate process that involves cardiac primordium emergence, cardiac tube formation, loop formation, atrial separation from the ventricle, and vascular connection (Beisaw et al., 2020; Jopling et al., 2010; Meng et al., 2020; Stoyek et al., 2015). The zebrafish heart is similar to the early human heart, a tube with an atrium, ventricle, and valves. Zebrafish heart valves originate from progenitors located between the atrium and the ventricle, which generate the valve leaflets through coordinated endocardial tissue movements. In zebrafish, the natural aortic heart valve plays an important role in preventing blood from flowing backwards, ensuring unidirectional blood circulation (Banjo et al., 2013).
Oxidative stress biomarkers have been extensively used to assess toxicologic exposures in aquatic organisms. Reactive oxygen species (ROS) accumulation diminishes the antioxidant capacity, induces DNA damage that leads to abnormal gene expression and in turn triggers cell proliferation, differentiation, and apoptosis. In the present study, compared to levels in the control group, up‐regulated levels of ROS‐related genes and enriched KEGG pathways in the PFNA‐exposed group were mainly associated with the PPAR and p53 signaling pathways. Ren et al. (2020) reported that elevated ROS levels alter the expressions of genes essential for differentiation of cardiac neural crest cells, leading to heart defects during embryonic development. Our findings indicate that PFNA and PFBS induce ROS accumulation and alter the transcriptional levels of genes related to cardiac development. Therefore, we postulated that PFNA and PFBS caused heart defects in zebrafish larva by elevating oxidative stress levels.
Exposures to toxic agents may also induce heart defects via the disruption of normal gene expression profiles in cardiomyocytes (Man et al., 2018; Yelon et al., 1999). Normal heart development is associated with normal growth of the atrium and ventricle, whereas disruption of key growth and differentiation factors often leads to various forms of congenital heart disease (Staudt & Stainier, 2012). The transcription factor (NKX2.5) is one of the earliest cardiac markers and is a key transcription factor for development of a functioning myocardium. The GATA4, GATA5, and GATA6 transcription factors comprise an evolutionarily conserved subfamily of transcription factors that are expressed within the precardiac mesoderm from the early stages and continue to be expressed in the adult heart (Staudt & Stainier, 2012). The transcriptional regulator of the T‐box gene family required in the heart for embryonic survival, Tbx5b is widely expressed during zebrafish embryo development (Albalat et al., 2010). Myosin heavy chain 6 (Myh6) is associated with atrial contraction (Singleman & Holtzman, 2012). Abnormal expressions of this gene disrupt early medial‐lateral myocardial patterning during cardiac development.
Our findings suggest that PFNA exposures led to more severe cardiac disorders in zebrafish larva than PFBS. We hypothesize that such defects are caused by ROS accumulation during cardiac development and changes in expression profiles of cardiac development‐related genes. Using a zebrafish model to explore PFNA and PFBS toxicities provided new evidence that could facilitate the assessment of their toxic effects in aquatic organisms and humans. Our study focused on the toxic effects of PFNA and PFBS on the heart of zebrafish larva. Further studies should explore the toxic effects of PFNA and PFBS on other organ systems.
CONCLUSIONS
To the best of our knowledge, ours is the first study to show that exposures of zebrafish embryos to PFNA results in more severe cardiac malformations than exposure to PFBS. We found that PFNA and PFBS inhibit hatchability, while increasing malformation and mortality in zebrafish embryos. Differential expressions of cardiac development‐associated genes in PFNA‐ and PFBS‐exposed embryos could be the underlying mechanisms of the cardiotoxic effects. Ours is also the first study to evaluate PFNA‐ and PFBS‐mediated cardiotoxicity in zebrafish, offering additional perspectives into the underlying mechanisms of their cardiotoxic effects, which could facilitate further research in other organ systems or animal models.
Supporting Information
The Supporting information is available on the Wiley Online Library at https://doi.org/10.1002/etc.5447.
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions Statement
Hongjian Gong: Investigation; Data curation. JuanDu: Formal analysis; Writing—original draft. Jia Xu: Supervision. Yuan Yang: Supervision. Hui Lu: Writing—review & editing. Han Xiao: Conceptualization; Methodology; Writing—review & editing; Funding acquisition.
Supporting information
This article includes online‐only Supporting Information.
Supplementary information.
Acknowledgments
The present study was supported by the Natural Science Foundation of Hubei Province (Grant Number 2020CFB741 to H. Xiao) and Top Medical Young Talents (2019) of Hubei Province (H. Xiao).
Hongjian Gong and Juan Du contributed equally to this work.
Data Availability Statement
All data that support the findings of the present study are available within the article and its Supporting Information.
REFERENCES
- Albalat, R. , Baquero, M. , & Minguillón, C. (2010). Identification and characterisation of the developmental expression pattern of tbx5b, a novel tbx5 gene in zebrafish. Gene Expression Patterns, 10(1), 24–30. 10.1016/j.gep.2009.11.003 [DOI] [PubMed] [Google Scholar]
- Bao, J. , Yu, W. J. , Liu, Y. , Wang, X. , Jin, Y. H. , & Dong, G. H. (2019). Perfluoroalkyl substances in groundwater and home‐produced vegetables and eggs around a fluorochemical industrial park in China. Ecotoxicology and Environmental Safety, 171, 199–205. 10.1016/j.ecoenv.2018.12.086 [DOI] [PubMed] [Google Scholar]
- Banjo, T. , Grajcarek, J. , Yoshino, D. , Osada, H. , Miyasaka, K. Y. , Kida, Y. S. , Ueki, Y. , Nagayama, K. , Kawakami, K. , Matsumoto, T. , Sato, M. , & Ogura, T. (2013). Haemodynamically dependent valvulogenesis of zebra fish heart is mediated by flow‐dependent expression of miR‐21. Nature Communications, 4, 1978. 10.1038/ncomms2978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisaw, A. , Kuenne, C. , Guenther, S. , Dallmann, J. , Wu, C. C. , Bentsen, M. , Looso, M. , & Stainier, D. (2020). AP‐1 contributes to chromatin accessibility to promote sarcomere disassembly and cardiomyocyte protrusion during zebrafish heart regeneration. Circulation Research, 126(126), 1760–1778. 10.1161/CIRCRESAHA.119.316167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, M. , Zhao, Z. , Yin, Z. , Ahrens, L. , Huang, P. , Cai, M. , Yang, H. , He, J. , Sturm, R. , Ebinghaus, R. , & Xie, Z. (2012). Occurrence of perfluoroalkyl compounds in surface waters from the North Pacific to the Arctic Ocean. Environmental Science & Technology, 46(2), 661–668. 10.1021/es2026278 [DOI] [PubMed] [Google Scholar]
- Cao, X. , Wang, C. , Lu, Y. , Zhang, M. , Khan, K. , Song, S. , Wang, P. , & Wang, C. (2019). Occurrence, sources and health risk of polyfluoroalkyl substances (PFASs) in soil, water and sediment from a drinking water source area. Ecotoxicology and Environmental Safety, 174, 208–217. 10.1016/j.ecoenv.2019.02.058 [DOI] [PubMed] [Google Scholar]
- Chen, L. , Lam, J. , Tang, L. , Hu, C. , Liu, M. , Lam, P. , & Zhou, B. (2020). Probiotic modulation of lipid metabolism disorders caused by perfluorobutanesulfonate pollution inzebrafish. Environmental Science & Technology, 54(12), 7494–7503. 10.1021/acs.est.0c02345 [DOI] [PubMed] [Google Scholar]
- Chen, L. , Tsui, M. , Shi, Q. , Hu, C. , Wang, Q. , Zhou, B. , Lam, P. , & Lam, J. (2018). Accumulation of perfluorobutanesulfonate (PFBS) and impairment of visual function in the eyes of marine medaka after a life‐cycle exposure. Aquatic Toxicology, 201, 1–10. 10.1016/j.aquatox.2018.05.018 [DOI] [PubMed] [Google Scholar]
- Chen, S. , Jiao, X. C. , Gai, N. , Li, X. J. , Wang, X. C. , Lu, G. H. , Piao, H. T. , Rao, Z. , & Yang, Y. L. (2016). Perfluorinated compounds in soil, surface water, and groundwater from rural areas in eastern China. Environmental Pollution, 211, 124–131. 10.1016/j.envpol.2015.12.024 [DOI] [PubMed] [Google Scholar]
- Chiesa, L. M ., & Nobile, M . (2018). Detection of perfluoroalkyl acids and sulphonates in Italian eel samples by HPLC‐HRMS Orbitrap. Chemosphere, 193, 358–364. 10.1016/j.chemosphere.2017.10.082 [DOI] [PubMed] [Google Scholar]
- Clarke, D. B ., Bailey, V. A ., Routledge, A ., Lloyd, A. S ., Hird, S ., Mortimer, D. N ., & Gem, M . (2010). Dietary intake estimate for perfluorooctanesulphonic acid (PFOS) and other perfluorocompounds (PFCs) in UK retail foods following determination using standard addition LC‐MS/MS. Food Additives and Contaminants: Part A, 27(4), 530–545. 10.1080/19440040903476590 [DOI] [PubMed] [Google Scholar]
- Costello, E. , Rock, S. , Stratakis, N. , Eckel, S. P. , Walker, D. I. , Valvi, D. , Cserbik, D. , Jenkins, T. , Xanthakos, S. A. , Kohli, R. , Sisley, S. , Vasiliou, V. , La Merrill, M. A. , Rosen, H. , Conti, D. V. , McConnell, R. , & Chatzi, L. (2022). Exposure to per‐ and polyfluoroalkylsubstances and markers of liverinjury: A systematic review and meta‐analysis. Environmental Health Perspectives, 130(4), 46001. 10.1289/EHP10092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta, S. , Reddam, A. , Liu, Z. , Liu, J. , & Volz, D. C. (2020). High‐content screening inzebrafish identifies perfluorooctanesulfonamide as a potent developmental toxicant. Environmental Pollution, 256, 113550. 10.1016/j.envpol.2019.113550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt, J. C. , Peden‐Adams, M. M. , Keller, J. M. , & Germolec, D. R. (2012). Immunotoxicity of perfluorinated compounds: Recent developments. Toxicologic Pathology, 40(2), 300–311. 10.1177/0192623311428473 [DOI] [PubMed] [Google Scholar]
- D'eon, J. C. , Hurley, M. D. , Wallington, T. J. , & Mabury, S. A. (2006). Atmospheric chemistry of N‐methyl perfluorobutanesulfonamidoethanol, C4F9SO2N(CH3)CH2CH2OH: Kinetics and mechanism of reaction with OH. Environmental Science & Technology, 40(6), 1862–1868. 10.1021/es0520767 [DOI] [PubMed] [Google Scholar]
- Fernandes, A. R ., Mortimer, D ., Holmes, M ., Rose, M ., Zhihua, L ., Huang, X ., Smith, F ., Panton, S ., & Marshall, L . (2017).Occurrence and spatial distribution of chemical contaminants in edible fish species collected from UK and proximate marine waters. Environment International, 114, 219–230. 10.1016/j.envint.2018.02.047 [DOI] [PubMed] [Google Scholar]
- Gore, A. V. , Pillay, L. M. , VeneroGalanternik, M. , & Weinstein, B. M. (2018). The zebrafish: A fintastic model for hematopoietic development and disease. Wiley Interdisciplinary Reviews. Developmental Biology, 7(3), e312. 10.1002/wdev.312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horzmann, K. A. , & Freeman, J. L. (2018). Making waves: New developments in toxicology with the zebrafish. Toxicological Sciences, 163(1), 5–12. 10.1093/toxsci/kfy044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, C. , Tang, L. , Liu, M. , Lam, P. K. , Lam, J. C. , & Chen, L. (2020). Probiotic modulation of perfluorobutanesulfonate toxicity in zebrafish: Disturbances in retinoid metabolism and visual physiology. Chemosphere, 258, 127409. 10.1016/j.chemosphere.2020.127409 [DOI] [PubMed] [Google Scholar]
- Jopling, C. , Sleep, E. , Raya, M. , Martí, M. , Raya, A. , & Izpisúa Belmonte, J. C. (2010). Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature, 464(7288), 606–609. 10.1038/nature08899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, K. , Younas, M. , Zhou, Y. , Sharif, H. , Li, X. , Yaseen, M. , Ibrahim, S. M. , Baninla, Y. , Cao, X. , & Lu, Y. (2022). First report of perfluoroalkyl acids (PFAAs) in the Indus Drainage System: Occurrence, source and environmental risk. Environmental Research, 211, 113113. 10.1016/j.envres.2022.113113 [DOI] [PubMed] [Google Scholar]
- Liu, M. , Song, S. , Hu, C. , Tang, L. , Lam, J. C. , Lam, P. K. , & Chen, L. (2020). Dietary administration of probiotic Lactobacillus rhamnosus modulates the neurological toxicities of perfluorobutanesulfonate in zebrafish. Environmental Pollution, 265, 114832. 10.1016/j.envpol.2020.114832 [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Wang, J. , Fang, X. , Zhang, H. , & Dai, J. (2011). The thyroid‐disrupting effects of long‐term perfluorononanoate exposure on zebrafish (Daniorerio). Ecotoxicology, 20(1), 47–55. 10.1007/s10646-010-0555-3 [DOI] [PubMed] [Google Scholar]
- Mably, J. D. , Mohideen, M. A. , Burns, C. G. , Chen, J. N. , & Fishman, M. C. (2003). Heart of glass regulates the concentric growth of the heart in zebrafish. Current Biology, 13(24), 2138–2147. 10.1016/j.cub.2003.11.055 [DOI] [PubMed] [Google Scholar]
- Man, J. , Barnett, P. , & Christoffels, V. M. (2018). Structure and function of the Nppa‐Nppb cluster locus during heart development and disease. Cellular and Molecular Life Sciences, 75(8), 1435–1444. 10.1007/s00018-017-2737-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzetti, S. , van der Spoel, E. R. , & van der Spoel, D. (2014). Chemical properties, environmental fate, and degradation of seven classes of pollutants. Chemical Research in Toxicology, 27(5), 713–737. 10.1021/tx500014w [DOI] [PubMed] [Google Scholar]
- Meng, Y. , Zhong, K. , Xiao, J. , Huang, Y. , Wei, Y. , Tang, L. , Chen, S. , Wu, J. , Ma, J. , Cao, Z. , Liao, X. , & Lu, H. (2020). Exposure to pyrimethanil induces developmental toxicity and cardiotoxicity in zebrafish. Chemosphere, 255, 126889. 10.1016/j.chemosphere.2020.126889 [DOI] [PubMed] [Google Scholar]
- Menger, F. , Pohl, J. , Ahrens, L. , Carlsson, G. , & Örn, S. (2020). Behavioural effects and bioconcentration of per‐ and polyfluoroalkyl substances (PFASs) in zebrafish (Daniorerio) embryos. Chemosphere, 245, 125573. 10.1016/j.chemosphere.2019.125573 [DOI] [PubMed] [Google Scholar]
- Noorlander, C. W. , van Leeuwen, S. P. , TeBiesebeek, J. D. , Mengelers, M. J. , & Zeilmaker, M. J. (2011). Levels of perfluorinated compounds in food and dietary intake of PFOS and PFOA in The Netherlands. Journal of Agricultural and Food Chemistry, 59(13), 7496–7505. 10.1021/jf104943p [DOI] [PubMed] [Google Scholar]
- Podder, A. , Sadmani, A.A. , Reinhart, D. , Chang, N. B. , & Goel, R. (2021). Per and poly‐fluoroalkyl substances (PFAS) as a contaminant of emerging concern in surface water: A transboundary review of their occurrences and toxicity effects. Journal of Hazardous Materials, 419, 126361. 10.1016/j.jhazmat.2021.126361 [DOI] [PubMed] [Google Scholar]
- Prevedouros, K. , Cousins, I. T. , Buck, R. C. , & Korzeniowski, S. H. (2006). Sources, fate and transport of perfluorocarboxylates. Environmental Science & Technology, 40(1), 32–44. 10.1021/es0512475 [DOI] [PubMed] [Google Scholar]
- Ren, F. , Huang, Y. , Tao, Y. , Ji, C. , Aniagu, S. , Jiang, Y. , & Chen, T. (2020). Resveratrol protects against PM2.5‐induced heart defects in zebrafishembryos as an antioxidant rather than as an AHR antagonist. Toxicology and Applied Pharmacology, 398, 115029. 10.1016/j.taap.2020.115029 [DOI] [PubMed] [Google Scholar]
- Sant, K. E. , Venezia, O. L. , Sinno, P. P. , & Timme‐Laragy, A. R. (2019). Perfluorobutanesulfonic acid disrupts pancreatic organogenesis and regulation of lipid metabolism in the zebrafish, Daniorerio . Toxicological Sciences, 167(1), 258–268. 10.1093/toxsci/kfy237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, B. M. , Bharat, G. K. , Tayal, S. , Larssen, T. , Becanova, J. , Karaskova, P. , Whitehead, P. G. , Futter, M. N. , Butterfield, D. , & Nizzetto, L. (2016). Perfluoroalkyl substances (PFAS) in river and ground/drinking water of the Ganges River basin: Emissions and implications for human exposure. Environmental Pollution, 208(Pt B), 704–713. 10.1016/j.envpol.2015.10.050 [DOI] [PubMed] [Google Scholar]
- Singleman, C. , & Holtzman, N. G. (2012). Analysis of postembryonic heart development and maturation in the zebrafish, Daniorerio . Developmental Dynamics, 241(12), 1993–2004. 10.1002/dvdy.23882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudt, D. , & Stainier, D. (2012). Uncovering the molecular and cellular mechanisms of heart development using the zebrafish. Annual Review of Genetics, 46, 397–418. 10.1146/annurev-genet-110711-155646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoyek, M. R. , Croll, R. P. , & Smith, F. M. (2015). Intrinsic and extrinsic innervation of the heart in zebrafish (Daniorerio). Journal of Comparative Neurology, 523(11), 1683–1700. 10.1002/cne.23764 [DOI] [PubMed] [Google Scholar]
- Tang, L. , Song, S. , Hu, C. , Liu, M. , Lam, P. K. , Zhou, B. , Lam, J. C. , & Chen, L. (2020). Parental exposure to perfluorobutanesulfonate disturbs the transfer of maternal transcripts and offspring embryonic development in zebrafish. Chemosphere, 256, 127169. 10.1016/j.chemosphere.2020.127169 [DOI] [PubMed] [Google Scholar]
- Ulhaq, M. , Orn, S. , Carlsson, G. , Morrison, D. A. , & Norrgren, L. (2013). Locomotor behavior in zebrafish (Daniorerio) larvae exposed to perfluoroalkyl acids. Aquatic Toxicology, 144–145, 332–340. 10.1016/j.aquatox.2013.10.021 [DOI] [PubMed] [Google Scholar]
- Xu, B. , Liu, S. , Zhou, J. , Zheng, C. , Weifeng, J. , Chen, B. , Zhang, T. , & Qiu, W. (2021). PFAS and their substitutes in groundwater: Occurrence, transformation and remediation. Journal of Hazardous Materials, 412, 125159. 10.1016/j.jhazmat.2021.125159 [DOI] [PubMed] [Google Scholar]
- Yelon, D. , Horne, S. A. , & Stainier, D. Y. (1999). Restricted expression of cardiac myosin genes reveals regulated aspects of heart tube assembly in zebrafish. Developmental Biology, 214(1), 23–37. 10.1006/dbio.1999.9406 [DOI] [PubMed] [Google Scholar]
- Young, C. J. , Furdui, V. I. , Franklin, J. , Koerner, R. M. , Muir, D. C. , & Mabury, S. A. (2007). Perfluorinated acids in Arctic snow: New evidence for atmospheric formation. Environmental Science & Technology, 41(10), 3455–3461. 10.1021/es0626234 [DOI] [PubMed] [Google Scholar]
- Zhang, W. , Liu, Y. , Zhang, H. , & Dai, J. (2012). Proteomic analysis of male zebrafish livers chronically exposed to perfluorononanoic acid. Environment International, 42, 20–30. 10.1016/j.envint.2011.03.002 [DOI] [PubMed] [Google Scholar]
- Zhang, W. , Sheng, N. , Wang, M. , Zhang, H. , & Dai, J. (2016). Zebrafish reproductive toxicity induced by chronic perfluorononanoateexposure. Aquatic Toxicology, 175, 269–276. 10.1016/j.aquatox.2016.04.005 [DOI] [PubMed] [Google Scholar]
- Zheng, X. M. , Liu, H. L. , Shi, W. , Wei, S. , Giesy, J. P. , & Yu, H. X. (2011). Effects of perfluorinated compounds on development of zebrafishembryos. Environmental Science and Pollution Research, 19(7), 2498–2505. 10.1007/s11356-012-0977-y [DOI] [PubMed] [Google Scholar]
- Zhou, T. , Dong, Q. , Shen, Y. , Wu, W. , Wu, H. , Luo, X. , Liao, X. , & Wang, G. (2016). PEG‐b‐PCL polymeric nano‐micelle inhibits vascular angiogenesis by activating p53‐dependent apoptosis in zebrafish. International Journal of Nanomedicine, 11, 6517–6531. 10.2147/IJN.S112658 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This article includes online‐only Supporting Information.
Supplementary information.
Data Availability Statement
All data that support the findings of the present study are available within the article and its Supporting Information.


