Abstract
Osteocytes respond to mechanical forces controlling osteoblast and osteoclast function. Mechanical stimulation decreases osteocyte apoptosis and promotes bone formation. Primary cilia have been described as potential mechanosensors in bone cells. Certain osteogenic responses induced by fluid flow (FF) in vitro are decreased by primary cilia inhibition in MLO‐Y4 osteocytes. The parathyroid hormone (PTH) receptor type 1 (PTH1R) modulates osteoblast, osteoclast, and osteocyte effects upon activation by PTH or PTH‐related protein (PTHrP) in osteoblastic cells. Moreover, some actions of PTH1R seem to be triggered directly by mechanical stimulation. We hypothesize that PTH1R forms a signaling complex in the primary cilium that is essential for mechanotransduction in osteocytes and affects osteocyte‐osteoclast communication. MLO‐Y4 osteocytes were stimulated by FF or PTHrP (1−37). PTH1R and primary cilia signaling were abrogated using PTH1R or primary cilia specific siRNAs or inhibitors, respectively. Conditioned media obtained from mechanically‐ or PTHrP‐stimulated MLO‐Y4 cells inhibited the migration of preosteoclastic cells and osteoclast differentiation. Redistribution of PTH1R along the entire cilium was observed in mechanically stimulated MLO‐Y4 osteocytic cells. Preincubation of MLO‐Y4 cells with the Gli‐1 antagonist, the adenylate cyclase inhibitor (SQ22536), or with the phospholipase C inhibitor (U73122), affected the migration of osteoclast precursors and osteoclastogenesis. Proteomic analysis and neutralizing experiments showed that FF and PTH1R activation control osteoclast function through the modulation of C‐X‐C Motif Chemokine Ligand 5 (CXCL5) and interleukin‐6 (IL‐6) secretion in osteocytes. These novel findings indicate that both primary cilium and PTH1R are necessary in osteocytes for proper communication with osteoclasts and show that mechanical stimulation inhibits osteoclast recruitment and differentiation through CXCL5, while PTH1R activation regulate these processes via IL‐6.
Keywords: mechanotransduction, osteoclasts, osteocytes, primary cilia, PTH1R
1. INTRODUCTION
Mechanical loading is a key regulator of bone formation and maintenance. Osteocytes, the most abundant cells in bone, respond to mechanical stimulation altering the release of molecules that regulate osteoclast and osteoblast function (Delgado‐Calle, Sato et al., 2017; Wijenayaka et al., 2011; You et al., 2008). In the absence of mechanical stimulation, osteocytes produce signals which induce the recruitment and differentiation of osteoclast precursors stimulating bone resorption (Plotkin et al., 2015).
PTH1R, a family B G‐protein‐coupled receptor (GPCR) that can potentially trigger several intracellular signaling pathways in bone, plays a pivotal role in bone formation and remodeling (Powell WF et al., 2011; Rhee et al., 2011). Recently, we and others have described that mechanical stimulation can directly activate the PTH1R without ligand stimulation, indicating the potential role of the parathyroid hormone (PTH) receptor as a mechanosensor in osteocytes and osteoblasts (Maycas et al., 2015a; Zhang & Frangos, 2009). In this regard, our group has demonstrated that PTH1R is an important component of the mechanical signal transduction machinery in MLO‐Y4 osteocytic cells. In these cells, ligand‐independent PTH1R activation occurred immediately after mechanical stimulation by fluid flow (FF) (Maycas et al., 2015a). Moreover, FF induced an increase of PTH1R presence at the plasma membrane of MLO‐Y4 cells, and, in vivo, mechanical loading, in combination with short treatment with PTHrP‐derived peptides, prevented bone fragility induced by diabetes (Maycas et al., 2015, 2017).
Primary cilia are nonmotile microtubule‐based organelles that extend from the apical surface of nearly every cell in the human body, such as kidney epithelial cells or bone cells (J. Malicki & Johnson, 2017). Primary cilia behave as a cellular “antenna” that receive diverse extracellular signals including mechanical stimuli and chemical signals and translate them into intracellular signaling responses (Ding et al., 2020; J. J. Malicki & Johnson, 2017; P. Wang et al., 2019). The spatiotemporal localization of specific receptors and associated signaling components along the cilio‐centrosome axis, including proteins involved in the regulation of bone remodeling such as hedgehog and several receptors coupled to G proteins (GPCRs) class A, B, and F, make possible its sensing abilities (Schou et al., 2015). It has been shown that primary cilia of osteocyte‐like MLO‐Y4 cells and osteoblast‐like MC3T3‐E1 cells extend from the cell surface and deflect during FF. Nevertheless, the cellular responses of osteoblasts and osteocytes to FF was significantly inhibited after the abrogation of primary cilium formation and function (Hoey et al., 2011; Malone et al., 2007). Primary cilia in osteocytes are responsible for detecting and mediating electromagnetic stimulation signals and thus regulate osteoclastic activity and function (P. Wang et al., 2019). The recent description of the role of PTH1R as a mechanosensor at the cell membrane of osteocytes and osteoblasts (Maycas et al., 2015a; Tu et al., 2015), suggests that this GPCR receptor could be especially abundant in an organelle focused to the detection of mechanical stimuli such as the primary cilium (Martín‐Guerrero et al., 2020). In fact, PTH1R induces prosurvival actions via primary cilia‐ and Gli‐1‐dependent mechanism in both osteocytes and osteoblasts (Martín‐Guerrero et al., 2020). However, whether PTH1R can be mobilized to the primary cilia during mechanical stimulation and how this translocation affects osteocyte‐osteoclast communication is not known.
In the present study, we show that mechanical stimulation of osteocytes prevents osteoclast activation after PTH1R translocation to primary cilium through the inhibition of C‐X‐C Motif Chemokine Ligand 5 (CXCL5) secretion. Moreover, lack of PTH1R expression induces osteoclast migration and formation by an interleukin‐6 (IL‐6)‐dependent pathway. These findings suggest a mechanism, based on PTH1R mobilization to the primary cilia, that could explain the therapeutic efficiency of combined PTH or PTHrP treatment and mechanical stimulation to preserve bone mass and quality.
2. MATERIALS AND METHODS
2.1. Cell culture
Mouse osteocytic MLO‐Y4 cells (generously donated by Dr. Lynda Bonewald) were grown in α‐MEM supplemented with 2.5% fetal calf serum (FCS) and 2.5% fetal bovine serum (FBS). Murine monocyte cell line (RAW 264.7) was purchased from the American Type Culture Collection and cultured in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% FBS. All cells were cultured with penicillin (100 units/ml) and streptomycin (100 μg/ml) in a 5% CO2 humidified incubator at 37°C. MLO‐Y4 cells were plated at 5 × 105 cells/cm2 on collagen‐coated glass slides (FlexCell); the next day, fresh medium with 1% FBS was added for 24 h. Then, cells were submitted or not (static control, SC) to mechanical stress by laminar FF with a shear stress of 10 dynes/cm2 for 10 min in a Flexcell® Streamer® shear stress device. The media flowed over the cells was consistently serum‐depleted α‐MEM in every experimental condition. Or they were stimulated by PTHrP (1‐37) (Bachem) for 10 min. Cells were cultured with fresh medium (α‐MEM without serum) for an additional 18 h after being stimulates with PTHrP (1‐37), FF or SC to collect the cell conditioned medium (CM) in the different experimental groups. RNA was isolated after 6 h of the stimulation with PTHrP (1‐37), FF, or SC.
2.2. Inhibition of primary cilia, PTH1R, Gl1, cyclic AMP, and phospholipase C
Primary cilia and PTH1R were inhibited using two different approaches. Cells were treated with 1 mM aqueous chloral hydrate or with 100 nM PTHrP (7‐34) for 1 h, respectively. The other approach was transfected with a mixture of three small interfering RNAs (siRNAs; each at 20 nM) against different coding target sequences of mouseIFT88 (186729; 186730; 186731; Thermo Fisher Scientific) and against different coding target sequences of mousePTH1R (151326; 68886; 68980; Thermo Fisher Scientific) for 24 h, respectively. Transfection of siRNAs was done using lipofectamine RNAiMax (Life Technologies) overnight at 37°C, according to the manufacturer's instructions. A scrambled sequence (control siRNA‐A; Santa Cruz Biotechnology) was used as a negative control for evaluating RNA interference off‐targeted effects and to verify the accuracy of gene‐specific siRNA‐dependent changes in the different parameters evaluated. Gli transcription factor was inhibited using 10 μM Gli‐1‐Antagonist 61 (GANT61; Santa Cruz Biotechnology) for 1 h when appropriate. cAMP and phospholipase C were inhibited with 100 μM of the adenylate cyclase inhibitor SQ22536 or with 1 μM of the phospholipase C inhibitor U73122, respectively.
2.3. Cell transfection
MLO‐Y4 cells were transfected with 1 μg of a human green fluorescent protein (GFP)‐PTH1R construct (generously donated by Dr. Peter Friedman) using lipofectamine 3000 (Life Technologies) for 4 h at 37°C, according to the manufacturer's guidelines.
2.4. Inmunofluorescence
Cells were fixed with 4% p‐formaldehyde (for α‐acetylated tubulin) and permeabilized using 0.5% Triton in phosphate‐buffered saline (PBS), pH 7.4. Nonspecific binding was blocked with 5% goat serum in bovine serum albumin, followed by overnight incubation at 4°C with mouse α‐acetylated tubulin antibody (Sigma Aldrich) at dilution 1/1000. Cells were rinsed three times with PBS before incubation for 1 h with Alexa fluor 546‐conjugated anti‐mouse immunoglobulin G (IgG) secondary antibodies (Invitrogen) at dilution 1/1000. Samples were mounted in FluorSafe Reagent (Calbiochem) and examined using a Leica DMI 8 confocal microscope. Colocalization of PTH1R with primary cilia was counted from cells transfected with a GFP‐PTH1R plasmid in each condition. It was also taken into account if this colocalization was in the whole cilium or only occurred in the base of the primary cilium.
2.5. Proteomic analysis
CM obtained from osteocytes +/‐ mechanical stimulation +/‐ PTH1R inhibition was analyzed by tandem mass tag (TMT) proteomics. Proteins secreted by osteocytes under these different conditions were relatively quantified and compared by the protein chemistry facility of CBMSO (Centro de Biología Molecular Severo Ochoa). In‐Gel Digestion (Stacking gel) was performed as previously described Moreno M. Moreno et al. (2014). TMT labeling and high pH fractionation were carried out as following. The peptide mixture from desalted proteins tryptic digest (50 µg) was labeled using chemicals from the TMT reagent sixplex Isobaric Mass Tagging Kit (reagents 126, 127, 128, and 129) (Thermo Fisher Scientific) essentially as described by manufacturer's. Peptides were dissolved in 50 μl of 100 mM triethylammonium bicarbonate, adjusted to pH 8. For labeling, each TMT reagent was dissolved in 41 μl of acetonitrile and added to the respective peptide mixture and then incubated at room temperature for 1 h. Labeling was stopped by the addition of 8 μl 5% hidroxilamine. Whole supernatants were dried down and the samples mixed to obtain the “4plex‐labeled mixture.” The mixture was analyzed by RP‐LC‐MS/MS to check the efficiency of the labeling. The sample was then fractionated using the high‐pH, reversed‐phase peptide fractionation kit (Pierce) as described with minor modifications. The sample reswollen in 0.1% TFA and then, loaded onto an equilibrated, high‐pH, reversed‐phase fractionation spin column. A step gradient of increasing acetonitrile concentrations in a volatile high pH solution was then applied to the columns to elute bound peptides into nine different fractions (5%−80% acetonitrile) collected by centrifugation. The fractions obtained from high‐pH, reversed‐phase 4plex‐labeled mixture were dried and stored until analysis by mass spectrometry for quantification. Quantitative analysis was performed by reverse phase‐liquid chromatography RP‐LC‐MS/MS as previously described Alonso Alonso et al. (2015). Peptide identification from raw data (a single search was performed with all nine raws from the fractionation) was carried out using PEAKS Studio X search engine (Bioinformatics Solutions Inc.). Database search was performed against uniprot‐Mus musculus.fasta (decoy‐fusion database).
2.6. Enzyme‐linked immunosorbent assay (ELISA)
IL‐6 and CXCL5 were evaluated in MLO‐Y4 cell‐CM using specific mouse ELISA Kits from Thermo Fisher Scientific, according to the corresponding manufacturer's instructions. Briefly, CM were collected and centrifuged at 1000 rpm for 10 min to pellet cellular debris. The optical absorbance of each well was measured in an ELISA reader (Varioskan Lux; Thermo Fisher Scientific) at 450 nm. The sensitivity of the assays was 6.5 pg/ml for IL‐6 and 35 pg/ml for CXCL5, and their inter assay variation coefficient was <12%.
2.7. Isolation of peripheral blood mononuclear cells (PBMCs) from buffy coats
PBMCs for generating human osteoclasts in vitro were isolated from buffy coats. Buffy coat was diluted with an equal volume of RPMI, carefully layer 30 ml of the diluted buffy coat on to 15 ml Ficoll and centrifuge at 800g for 40 min, with brake set to zero. Mononuclear cell layer from each tube was collected and transferred to two new 50 ml tubes. PBS was added to a volume of 45 ml per tube to wash the cells and centrifuge at 250g for 10 min. Cells were grown in α‐MEM supplemented with 10% FCS with penicillin (100 units/ml) and streptomycin (100 μg/ml) in a 5% CO2 humidified incubator at 37°C.
2.8. Transwell migration assay
Migration assay was performed using Costar transwell cell culture chamber inserts (Corning Costar Corporation) with an 8 µm pore size. Briefly, 5 × 104 RAW 264.7 cells or human osteoclasts from buffy coat were placed in the upper chamber in 200 µl of DMEM supplemented with 10% FBS or α‐MEM supplemented with 10% FCS, respectively. Standard medium with 1% FBS or FCS and containing 20% of each MLO‐Y4 cell‐conditioned medium for 18 h were placed in the lower compartment. Furthermore, in others experiments, 2 µg/ml of neutralizing antibody anti‐mCXCL5 (Thermo Fisher Scientific) and 1 µg/ml of anti‐mIL‐6 (R&D Systems) were used. Then, the medium and the upper cell layer were removed with the aid of a cotton swab, and cells on the underside of the transwell were fixed with PFA 4%, stained with crystal violet (Sigma Aldrich) and counted in five randomly selected fields at X200 magnification using Leica DM 5500B microscope.
2.9. Generation of osteoclasts for assessment of osteoclastogenesis
Cells from buffy coat were grown in α‐MEM supplemented with 10% FCS containing 20 ng/ml of macrophage colony‐stimulating factor (M‐CSF), feeding with fresh media until they reach a suitable confluency. Briefly, 2 × 104 human osteoclasts from buffy coat were seeded into wells of a 96‐well plate, supplemented with α‐MEM containing 20 ng/ml M‐CSF plus 20 ng/ml of receptor activator of nuclear factor kB ligand (RANKL). As a control, cells were incubated without RANKL were included. The following day, cells were fed with fresh α‐MEM containing M‐CSF and RANKL as appropriate, plus the accurate treatments; 20% of each MLO‐Y4 cell‐conditioned medium. Moreover, in some experiments, neutralizing antibody anti‐mCXCL5 (Thermo Fisher Scientific) and anti‐mIL‐6 (R&D Systems) were used. After 3 days, the medium was removed, cells were rinsed off with PBS to eliminate nonadherent cells. Cells were fixed with 4% p‐formaldehyde, permeabilized with 100% methanol for 20 min and stained with hematoxylin for 5 min. The differentiation of human monocytic cells into osteoclasts were determined by morphology, observing the formation of giant cells with at least three or more nuclei.
2.10. Statistical analysis
Data are expressed as means ± standard deviation. Statistical analysis was performed using GraphPad Prism (GraphPad software). Differences among conditions were evaluated by nonparametric variance analysis (Kruskal−Wallis) followed by Mann−Whitney test. The p < 0.05 was considered significant.
3. RESULTS
3.1. Secretome of mechanically‐stimulated osteocytes inhibits the migration of preosteoclastic cells and osteoclast differentiation by a mechanism dependent on the primary cilium and PTH1R
To assess the role of primary cilia and PTH1R on the effect of mechanically‐stimulated migration, we inhibited the formation of primary cilia in osteocytic cells before FF or PTHrP stimulation using two strategies; silencing IFT88, a protein required for ciliogenesis and primary cilia functional competence (Takei et al., 2018) or preincubation with chloral hydrate, a primary cilia inhibitor (Deren et al., 2016). PTH1R was also inhibited silencing the PTH1R gene, or by using the PTH/PTHrP receptor antagonist PTHrP (7‐34). The efficiency of IFT88/PTH1R silencing was confirmed by RNA assessment in MLO‐Y4 osteocytes (Figure 1a).
Figure 1.
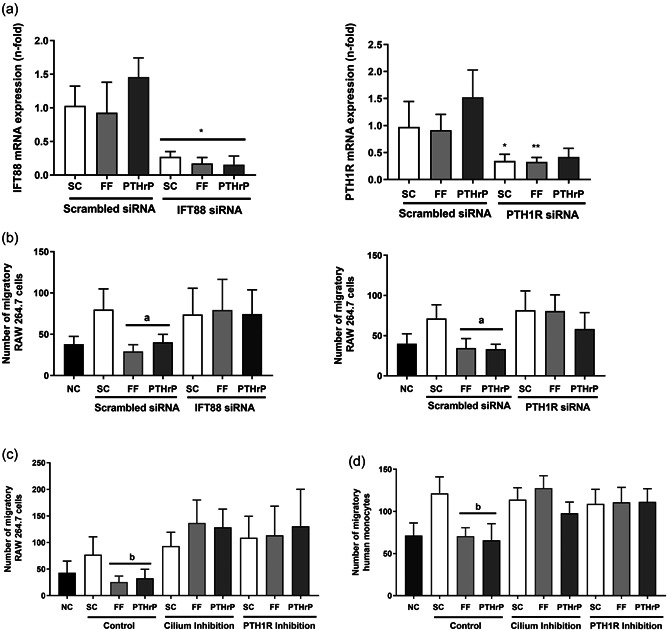
CM from mechanically‐stimulated MLO‐Y4 cells inhibit the migration of monocytes by a mechanism dependent on the primary cilium and PTH1R. MLO‐Y4 osteocytic cells were transfected with three IFT88, three PTH1R siRNAs, or with a scrambled siRNA for 24 h followed by serum‐deprivation for 24 h. The efficiency of IFT88 and PTH1R silencing was tested by real time PCR (a). Alternatively, osteocyte cells were serum‐deprived for 24 h and treated with 1 mM aqueous chloral hydrate or with 100 nM PTHrP (7‐34) for 1 h. Cells were subsequently stimulated with shear stress (10 dynes/cm2) or with 100 nM PTHrP (1‐37) for 10 min. CM was collected after 18 h (b−d). To evaluate the number of migratory cells, RAW 264.7 (b and c) or human monocytic cells from buffy coat (d) were cultivated in transwell cell culture chamber inserts with an 8 µm pore size. In the lower compartment 20% of each MLO‐Y4 cell‐conditioned medium was added. After 6 h, cells were fixed, stained with crystal violet, and counted with an inverted optical microscope. The number of monocytic cells evaluated with ImageJ software are represented. Results are the mean ± SD of triplicates. *p < 0.05 versus corresponding scrambled siRNA; **p < 0.01 versus corresponding scrambled siRNA; a p < 0.01 versus SC or corresponding IFT88 siRNA/PTH1R siRNA; b p < 0.01 versus SC or corresponding cilium or PTH1R inhibition. CM, conditioned media; FF; fluid flow; IFT88, intraflagellar transport 88 protein; mRNA, messenger RNA; NC, negative control; PTHrP, PTH‐related protein; PTH1R, PTH 1 receptor; SC, static control; SD, standard deviation; siRNA, small interfering RNA.
After FF or PTHrP stimulation, CM from osteocytes was obtained to be used in migration and osteoclast differentiation assays. Migration of the murine monocyte RAW cell line was drastically inhibited by CM from FF‐ or PTHrP‐stimulated MLO‐Y4 cells compared to migration induced by CM from osteocytes under SC conditions (Figure 1b). In contrast, CM from IFT88‐ or PTH1R‐silenced osteocytes stimulated by either FF or PTHrP showed no inhibitory effects on monocyte migration (Figure 1b). Similarly, the CM of FF‐ or PTHrP‐stimulated osteocytes was unable to decrease murine (Figure 1c) or human (Figure 1d) monocytic cell migration when osteocytes were preincubated with the primary cilium inhibitor chloral hydrate or with the PTH1R inhibitor PTHrP (7‐34).
These data show that the secretome of osteocytes stimulated by FF or PTHrP inhibits the migration of RAW 264.7 and human monocytic cells by a mechanism dependent on the osteocytic primary cilium and PTH1R.
Following, we evaluated the putative actions of osteocytic primary cilia and PTH1R on osteoclast differentiation. We observed that CM obtained from osteocytes stimulated by FF or PTHrP, decreased the percentage of human monocytic cells that differentiate in the presence of osteoclastogenic cytokines into osteoclasts (observed as giant cells with at least three or more nuclei) compared to the percentage of differentiation induced by SC CM (Figure 2a,b). On the other hand, CM of primary cilium‐ or PTH1R‐inhibited MLO‐Y4 osteocytes induced a percentage of osteoclastogenesis that resembled that of CM of osteocytes under SC conditions. These actions were observed even if osteocytes were stimulated with FF or PTHrP (Figure 2a,b). Therefore, these results suggest that the secretome of mechanically‐ and PTHrP‐stimulated osteocytes inhibits the osteoclastogenic process of human monocytes by a mechanism dependent on PTH1R and primary cilium.
Figure 2.
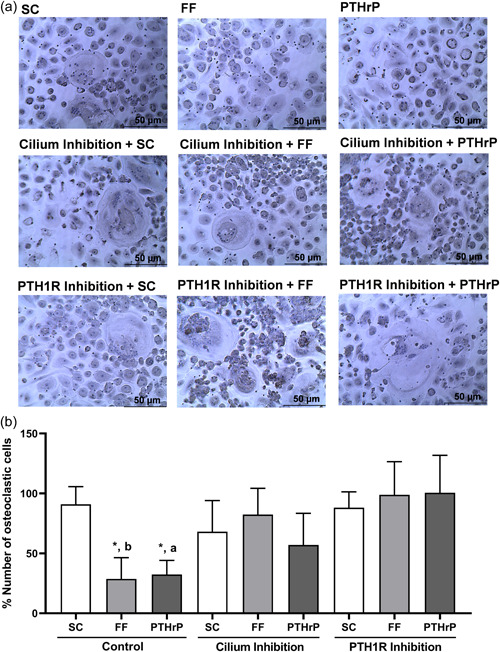
Secretome from mechanically‐stimulated MLO‐Y4 cells reduces the differentiation of human monocytes into osteoclasts by a mechanism dependent on the primary cilium and PTH1R. MLO‐Y4 cells were serum‐deprived for 24 h, treated with 1 mM aqueous chloral hydrate or with 100 nM PTHrP (7‐34) for 1 h and stimulated with shear stress (10 dynes/cm2) or with 100 nM PTHrP (1‐37) for 10 min. CM was collected after 18 h. To evaluate the differentiation of monocytic cells into osteoclasts, human monocytes were treated with 20 ng/ml M‐CSF and 20 ng/ml RANKL plus the corresponding 20% MLO‐Y4 cell‐conditioned mediums. Then, cells were fixed, permeabilized, and stained with hematoxylin. The differentiation of human monocytes into osteoclasts was determined by evaluation of the morphology, observing the formation of giant cells (≥50µm) with at least three or more nuclei. Representative images of each condition are shown (a). The percentage of cells with three or more nuclei evaluated with ImageJ software is represented (b). Results are the mean ± SD of triplicates. *p < 0.05 versus SC; **p < 0.01 versus SC; a p < 0.05 versus corresponding PTH1R inhibition; b p < 0.01 versus corresponding cilium or PTH1R inhibition. CM, conditioned media; FF; fluid flow; M‐CSF, macrophage colony‐stimulating factor; PTHrP, PTH‐related protein; PTH1R, PTH 1 receptor; RANKL, receptor activator for nuclear factor κ B ligand; RANKL and (+) α‐MEM medium; SC, static control; SD, standard deviation.
3.2. PTH1R colocalizes with primary cilium in mechanically‐stimulated osteocytes, being distributed throughout the whole cilium
We next determined the possible colocalization between the primary cilium and PTH1R in MLO‐Y4 cells and whether their colocalization could impact the functions of both mechanosensors. We found that, in static conditions, 58% of ciliated MLO‐Y4 cells showed certain colocalization of PTH1R and primary cilia. Of those, only 38% of the cells showed colocalization along the entire primary cilium whereas 20% displayed colocalization just at the base of the cilium. However, after FF stimulation, 70% of ciliated osteocytic cells manifested colocalization of PTH1R and primary cilia. Of this percentage, 56% of ciliated cells showed PTH1R localization on the whole extension of the cilium whereas 14% displayed colocalization only at the base of the cilium (Figure 3a,b). CH pretreatment abrogated cilium formation and thus, also PTH1R mobilization to it, while PTHrP (7‐34) did not affect PTH1R mobilization (Figure 3a,b). Furthermore, the length of primary cilia was increased in mechanically‐stimulated osteocytes compared to shorter length of cilia in osteocytes under SC conditions (Figure 3c). Again, this effect was inhibited by CH pretreatment but was unaffected by the PTH1R antagonist PTHrP (7‐34) (Figure 3c).
Figure 3.
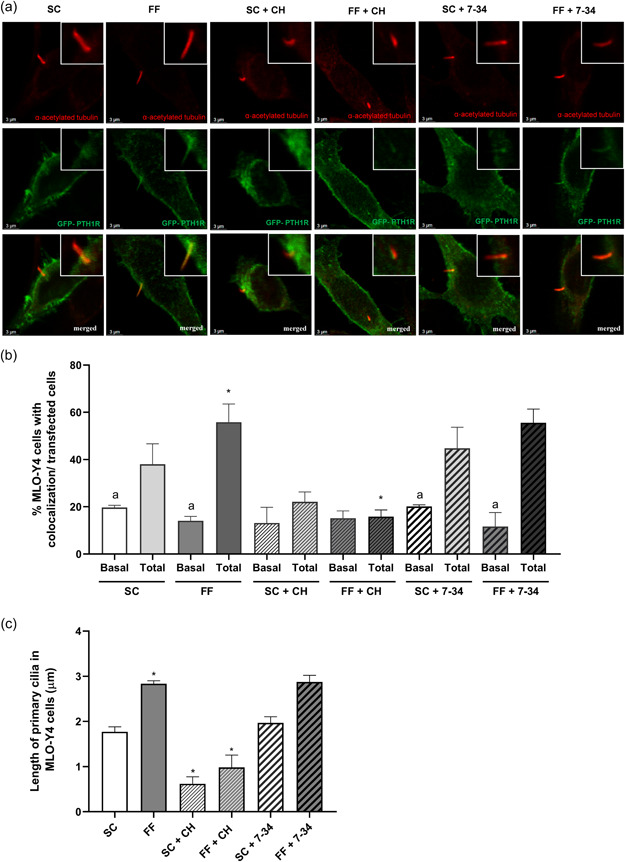
PTH1R colocalizes with primary cilium in mechanically‐stimulated MLO‐Y4 cells, being distributed throughout the whole cilium. MLO‐Y4 cells were transfected with 1 μg of a (GFP)‐PTH1R construct using lipofectamine 3000 for 4 h at 37°C. Cells were subsequently serum‐deprived for 6 h, treated with 1 mM aqueous chloral hydrate or with 100 nM PTHrP (3‐37) for 1 h and stimulated with shear stress (10 dynes/cm2) for 10 min. To evaluate the colocalization of PTH1R with primary cilia, cells were fixed, permeabilized, blocked, and incubated overnight at 4°C with mouse α‐acetylated tubulin antibody. Then, cells were incubated for 1 h with Alexa fluor 546‐conjugated anti‐mouse IgG secondary antibodies. Representative images of each condition are shown (a). The percentage of cells with PTH1R colocalization just at the base (basal) and throughout the whole (total) primary cilium was analyzed in each condition in cells transfected with a GFP‐PTH1R plasmid (b). Evaluation of the length of primary cilia in MLO‐Y4 cells using ImageJ software are represented (c). Results are the mean ± SD of triplicates. *p < 0.05 versus SC or corresponding control; a p < 0.05 versus corresponding total conditions. 7‐34, PTHrP (7‐34); CH, chloral hydrate; FF; fluid flow; (GFP)‐PTH1R, green fluorescent protein‐PTH1R; IG, immunoglobulin G; SC, static control; SD, standard deviation.
Therefore, these results show that mechanical stimulus enlarges osteocyte primary cilia and triggers significant mobilization of PTH1R along the primary cilium of these cells.
3.3. Hegdehog, protein kinase A (PKA), and protein kinase C (PKC) pathways mediate osteocyte regulation of monocyte migration and osteoclastogenesis
PTH1R actions on bone cells have associated with different signaling pathways including overexpression of Gli transcription factor (Martín‐Guerrero et al., 2020), and PKA and PKC activation (Ardura et al., 2019). To investigate the signaling pathways involved in the regulation of migration and differentiation of osteoclasts by MLO‐Y4 cells CM, we evaluated how the inhibition of PKA, PKC, or hedgehog signaling pathways in osteocytes affects the migration of the murine monocyte line RAW 264.7 cells (Figure 4a) and human monocytic cells (Figure 4b). Pretreatment of osteocytes with Gant61, SQ22536, and U‐73122 (inhibitors of Gli, PKA, and PKC, respectively) decreased the FF‐ and PTHrP‐dependent inhibitory effects of osteocytic CM on murine and human monocyte migration (Figure 4a,b). Next, we studied the effect of the different previously described CMs on osteoclastogenesis. Similarly, CM obtained from FF‐ or PTHrP‐stimulated osteocytes pretreated with the inhibitors Gant61, SQ22536, and U‐73122 completely reversed the repression of osteoclast differentiation caused by noninhibited FF‐ or PTHrP‐stimulated osteocytes (Figure 4c).
Figure 4.
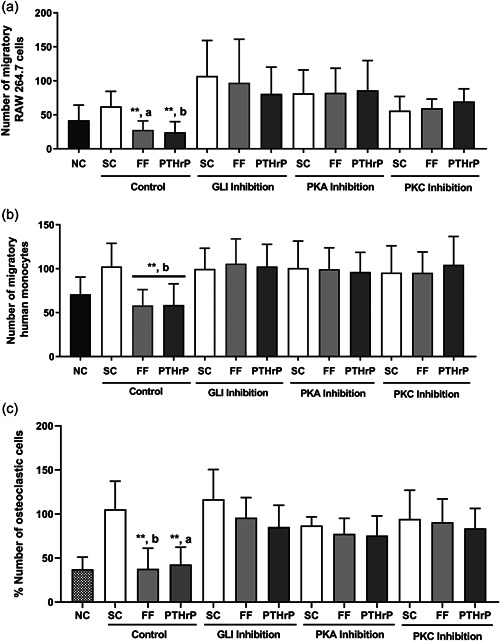
Osteocytic hegdehog, PKA, and PKC signaling pathways are involved in MLO Y4 CM‐dependent regulation of monocyte migration and osteoclastogenesis. MLO‐Y4 cells were serum‐deprived for 24 h and treated with 10 μM GANT61, 100 μM of the adenylate cyclase inhibitor SQ22536, or with 1 μM of the phospholipase C inhibitor U73122 for 1 h. Cells were subsequently stimulated with shear stress (10 dynes/cm2) or with 100 nM PTHrP (1‐37) for 10 min. CM was collected after 18 h. To evaluate the number of migratory cells, RAW 264.7 (a) or human monocytic cells from buffy coat (b) were cultured in transwell cell culture chamber inserts with an 8 µm pore size, and in the lower compartment with 20% of each MLO‐Y4 cell‐conditioned medium. After 6 h, cells were fixed, stained with crystal violet, and counted with an inverted optical microscope. The number of monocytic cells evaluated with ImageJ software are represented (a and b). To evaluate the differentiation of monocytes into osteoclasts, human monocytes were treated with 20 ng/ml M‐CSF and 20 ng/ml RANKL, plus the corresponding 20% MLO‐Y4 cell‐conditioned mediums. Cells were fixed, permeabilized, and stained with hematoxylin. The percentage of cells with three or more nuclei evaluated with ImageJ software are represented (c). Results are the mean ± SD of triplicates. **p < 0.01 versus SC; a p < 0.05 versus corresponding GLI, PKA, or PKC inhibition; b p < 0.01 versus corresponding GLI, PKA, or PKC inhibition. CM, conditioned media; FF; fluid flow; GANT61, Gli‐1‐Antagonist 61; M‐CSF, macrophage colony‐stimulating factor; NC, negative control (−) RANKL and (+) α‐MEM medium; PKA, protein kinase A; PKC, protein kinase; PTHrP, PTH‐related protein; RANKL, receptor activator for nuclear factor κ B ligand; SC, static control; SD, standard deviation.
Therefore, these data show that stimulation of osteocytes by FF and PTHrP inhibits the migration of monocytic cells and their differentiation toward osteoclasts by an effect dependent on Gli, PKA, and PKC.
3.4. Mechanical stimulation inhibits the recruitment and differentiation of osteoclasts through CXCL5 meanwhile PTH1R activation regulates osteoclastogenesis through IL‐6
We next studied the secretome of MLO‐Y4 osteocytes to analyze the role of PTH1R on the secretion of FF‐stimulated or SC osteocytes and unravel proteins secreted by osteocytes that could modulate monocyte migration and osteoclast differentiation. Secretion of 1.323 proteins was revealed in the CM of osteocytes by proteomics analysis. The secretome of FF‐stimulated osteocytes exhibited 76 proteins that increased and 39 proteins that decreased their levels of secretion compared to osteocytes subjected to SC conditions (Figure 5). Venn diagrams show secretome proteomic changes between FF‐stimulated osteocytes, PTH1R silencing + FF and PTH1R silencing + SC conditions. Proteins displaying increased secretion (fold change >1.5) are shown in Figure 5a. A total of 24 proteins are oversecreted in all the studied groups. Moreover, 31 proteins are commonly secreted by FF‐stimulated osteocytes and siPTH1R‐silenced osteocytes in SC conditions; 1 protein (Prelamin‐A/C) by FF‐stimulated osteocytes either with or without PTH1R silencing; and 5 proteins by PTH1R‐silenced osteocytes under FF or SC conditions which are related with the transforming growth factor‐β signaling pathway, plasminogen activating cascade, cholecystokinin receptor signaling pathway, and blood coagulation. Proteins showing decreased secretion (fold change >1.5) are shown in Figure 5b. Only 1 protein, ADP/ATP translocase 2, decreased in all groups. A total of 35 different proteins decreased their secretion in FF‐stimulated osteocytes with versus without PTH1R silencing and 11 proteins in PTH1R‐silenced osteocytes under SC versus FF‐stimulated. Protein secretion variations among the different conditions are also shown in a heat map where the color and intensity represent changes of protein secretion (Figure 5c). Table 1 shows representative proteins obtained from the proteomic analysis, which are related to chemotactic factors and with bone remodeling process. Some of the proteins overexpressed by osteocytes of this study, were related to inflammatory processes or to recruitment of osteoclast precursors, such as CXCL5 and IL‐6. In this regard, the proteomics data showed that FF inhibits the secretion of CXCL5, but not IL‐6. In contrast, the silencing of PTH1R induced IL‐6 secretion without affecting CXCL5 secretion. To validate these results, we determined IL‐6 and CXCL5 levels in MLO‐Y4 CM after SC and FF conditions. We confirmed that FF did not affect IL‐6 secretion but decreased CXCL5 levels (Figure 5d).
Figure 5.
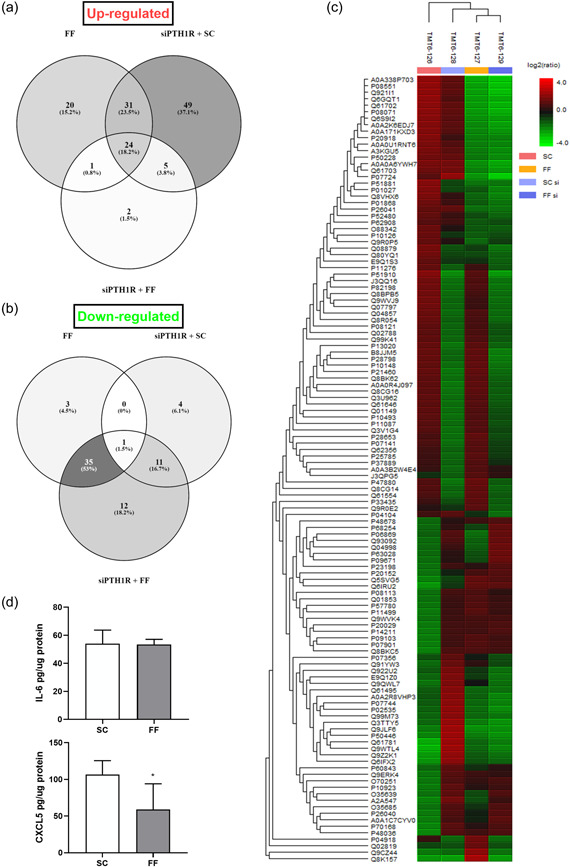
Proteomic analysis of mechanically‐stimulated MLO‐Y4 cells, +/‐ PTH1R silencing. MLO‐Y4 osteocytic cells were transfected with three PTH1R siRNAs or with a scrambled siRNA for 24 h. Cells were subsequently serum‐deprived for 24 h and stimulated with shear stress (10 dynes/cm2) for 10 min. CM were collected after 18 h, lyophilized, and analyzed by tandem mass tag mass spectrometry. Venn diagrams show the profile of secreted proteins that increase (a) or decrease (b) comparing different experimental conditions. (c) A heatmap showing protein secretion differences after proteomic analysis of osteocytes is pictured. The color and intensity of the boxes is used to represent changes (not absolute values) of protein secretion. In the picture above, red represents increased secretion and green represents decreased secretion. Black represents unchanged secretion. (d) IL‐6 and CXCL5 in the MLO‐Y4‐CM were analyzed by ELISA as described in Section 2. *p < 0.05 versus SC. CM, conditioned media; FF; fluid flow; IL‐6, interleukin‐6; PTH1R, PTH 1 receptor; SC, static control; siPTH1R, PTH1R silencing.
Table 1.
Protein secretion differences after proteomic analysis of mechanically‐stimulated MLO‐Y4 cells, +/‐ PTH1R silencing are pictured
| Gene | Protein | Significance (%) | Ratio SC | Ratio FF | Ratio siPTH1R SC | Ratio siPTH1R FF | |
|---|---|---|---|---|---|---|---|
| Chemokines factors | CCL2 | C‐C motif chemokine 2 | 19.57 | 1 | 0.99 | 0.51 | 0.48 |
| CXCL3 | C‐X‐C motif chemokine 3 | 8.39 | 1 | 1.17 | 1.44 | 1.27 | |
| CXCL5 | C‐X‐C motif chemokine 5 | 32.82 | 1 | 0.41 | 0.9 | 0.33 | |
| CCL7 | C‐C motif chemokine 7 | 8.32 | 1 | 1.15 | 0.79 | 0.63 | |
| C3 | Complement C3 | 41.02 | 1 | 0.46 | 0.51 | 0.32 | |
| IL‐6 | Interleukin‐6 | 6.86 | 1 | 1.11 | 1.64 | 1.94 | |
| Pro‐plasmin factors | A2M | Alpha‐2‐macroglobulin‐P | 90.18 | 1 | 0.2 | 0.58 | 0.16 |
| PLG | Plasminogen | 14.9 | 1 | 0.32 | 0.6 | 0.31 | |
| PLAU | Urokinase‐type plasminogen activator | 19.33 | 1 | 0.89 | 1.59 | 1.79 | |
| Serpinc1 | Antithrombin‐III | 53.91 | 1 | 0.29 | 0.95 | 0.21 | |
| Pro‐anabolic factors | AHSG | Alpha‐2‐HS‐glycoprotein | 63.08 | 1 | 0.16 | 0.62 | 0.1 |
| DKK3 | Dickkopf‐related protein 3 | 11.45 | 1 | 1.52 | 0.66 | 0.84 | |
| Calcium‐binding proteins | ANXA1 | Annexin A1 | 12.51 | 1 | 1.49 | 1.66 | 1.45 |
| ANXA2 | Annexin A2 | 13.02 | 1 | 1.19 | 1.56 | 1.03 | |
| ANXA3 | Annexin A3 | 21.3 | 1 | 1.33 | 1.99 | 1.84 | |
| ANXA5 | Annexin A5 | 27.28 | 1 | 2.09 | 2.29 | 2.23 | |
| CALM1 | Calmodulin‐1 | 10.14 | 1 | 1.38 | 1.71 | 1.47 | |
| CALM2 | Calmodulin‐2 | 10.14 | 1 | 1.38 | 1.71 | 1.47 | |
| CALM3 | Calmodulin‐3 | 10.14 | 1 | 1.38 | 1.71 | 1.47 | |
| NUCB1 | Nucleobindin‐1 | 16.91 | 1 | 1.58 | 0.95 | 0.95 | |
| Transmembrane signal receptors | INSRR | Insulin receptor‐related protein | 42.17 | 1 | 2.36 | 8.06 | 1.77 |
| THBD | Thrombomodulin | 4.6 | 1 | 1.42 | 1.33 | 1.67 |
Note: The table shows the changes for each protein and the ratio for each condition relativized versus static control. Increased and decreased secretion values are shown in bold.
Abbreviations: CM, conditioned media; FF; fluid flow; PTH1R, PTH 1 receptor; SC, static control; siPTH1R, PTH1R silencing.
Based on these observations, we analyzed whether IL‐6 and CXCL5 were involved in the regulation of osteoclast migration and differentiation induced by PTH1R‐ and primary cilia‐dependent mechanisms in MLO‐Y4 cells using specific neutralizing antibodies (Figure 6). As previously shown, CM from osteocytes under SC caused an increase of monocyte migration and osteoclast differentiation; an effect that was inhibited by either pretreatment of CM of osteocytes with an anti‐CXCL5 or an anti‐IL‐6 antibody. In addition, FF decreased monocyte migration and osteoclast differentiation with or without CXCL5 or IL‐6 neutralization (Figure 6). However, CXCL5 neutralization caused no effect on monocyte migration or osteoclast differentiation when primary cilium or PTH1R from osteocytes were inhibited by CH or PTHrP (7‐34), respectively (Figure 6a−c). Conversely, anti‐IL‐6 not only reversed migration (Figure 6a,b) and osteoclastogenesis (Figure 6c) in SC conditions, but also in the presence of CH or PTHrP (7‐34) (Figure 6a−c). In this regard, osteoclastogenesis processes induced by the inhibition of Gli, PKA, or PKC pathways in FF condition were reversed by IL‐6 neutralization but not by the preincubation with CXCL5 antibody (Figure 6d).
Figure 6.
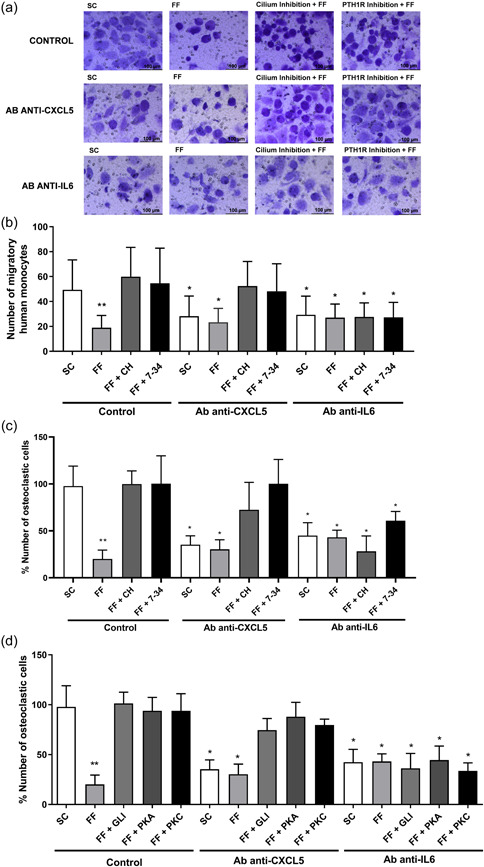
Mechanical stimulation inhibits the recruitment and differentiation of osteoclasts through a mechanism dependent on CXCL5 and IL‐6. MLO‐Y4 cells were serum‐deprived for 24 h and treated with 1 mM aqueous chloral hydrate, 100 nM PTHrP (7‐34), 10μM of the Gli inhibitor GANT61, 100 μM of the adenylate cyclase inhibitor SQ22536 or with 1 μM of the phospholipase C inhibitor U73122, for 1 h. Cells were subsequently stimulated with shear stress (10 dynes/cm2) for 10 min. CM were collected after 18 h and 2 µg/ml of neutralizing antibodies anti‐mCXCL5 or 1 µg/ml of anti‐mIL‐6 were added. To evaluate the number of migratory cells, human monocytes from buffy coat (a and b) were cultured in transwell cell culture chamber inserts with an 8 µm pore size. In the lower compartment 20% of each MLO‐Y4 cell‐conditioned medium was added plus the corresponding neutralizing antibody. After 6 h, cells were fixed, stained with crystal violet, and counted with an inverted optical microscope. Representative images of each condition are shown (a). The number of monocytic cells evaluated with ImageJ software are represented (b). To evaluate the differentiation of monocytes into osteoclasts, human monocytes were treated with 20 ng/ml M‐CSF and 20 ng/ml RANKL, plus the corresponding treatments: 20% of each MLO‐Y4 cell‐conditioned medium and the neutralizing antibody. Cells were fixed, permeabilized, and stained with hematoxylin (c and d). The percentage of cells with three or more nuclei evaluated with ImageJ software is represented (c and d). Results are the mean ± SD of triplicates. *p < 0.05 versus SC; **p < 0.01 versus SC. 7‐34, PTHrP (7‐34); Ab, antibody; CH, chloral hydrate; CM, conditioned media; CXCL5, C‐X‐C motif chemokine 5; FF; fluid flow; GANT61, Gli‐1‐Antagonist 61; IL‐6, interleukin‐6; M‐CSF, macrophage colony‐stimulating factor; PKA, protein kinase A; PKC, protein kinase C; RANKL, receptor activator for nuclear factor κ B ligand; SC, static control; SD, standard deviation.
These findings indicate that the presence of both functional primary cilium and PTH1R in osteocytes are necessary for correct communication with osteoclasts and suggest that mechanical stimulation inhibits the recruitment and differentiation of osteoclasts through CXCL5, while PTH1R activation regulate osteoclasts through IL‐6 (Figure 7).
Figure 7.
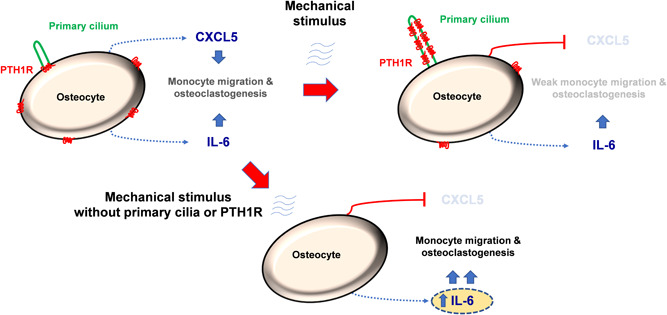
Proposed mechanism for regulation of osteoclast recruitment and differentiation by primary cilium and PTH1R in osteocytes. The presence of both functional primary cilium and PTH1R in osteocytes are necessary for correct communication with osteoclasts. Mechanical stimulation inhibits the recruitment and differentiation of osteoclasts through CXCL5, while PTH1R activation regulate osteoclasts through IL‐6. CXCL5, C‐X‐C Motif Chemokine Ligand 5; IL‐6, interleukin‐6; PTH1R, parathyroid hormone receptor type 1.
4. DISCUSSION
Osteocyte‐osteoclast communication is a key process for the physiological maintenance of bone homeostasis. In the present study, we show that PTH1R and primary cilia are part of the molecular machinery responsible for transducing mechanical stimulation in order to decrease osteoclastic cell migration and differentiation, via regulation of CXCL5 and IL‐6 secretion.
PTH1R is expressed in osteoblast lineage cells in bone and is needed for normal bone formation and remodeling (Delgado‐Calle, Xioalin et al., 2017). PTH1R signaling exerts direct effects on osteocytes to promote bone formation via suppression of sclerostin and through the induction of RANKL to increase osteoclastic resorption (Estell & Rosen, 2021). The two natural agonists of PTH1R are PTH, secreted by parathyroid glands, and PTHrP, which is secreted by a wide variety of tissues where it acts locally (Ardura et al., 2019). PTH1R activation can induce bone anabolism, after an intermittent administration of PTH or PTHrP, or catabolism, after sustained PTH elevation (Ardura et al., 2019; Maycas et al., 2017; Rhee et al., 2011; Tu et al., 2015). In the last few years, several studies have shown that PTH1R might be activated directly by mechanical stimulation. Zhang & Frangos, (2009) demonstrated that shear stress leads to ligand‐independent conformational changes of the PTH1R in osteoblastic MC3T3‐E1 cells. Interestingly, PTH and mechanical stimulation exert different conformational changes of the receptor, suggesting that although both stimuli may stimulate PTH1R, the mechanisms underlying activation of the receptor and its intracellular responses may also be different (Zhang & Frangos, 2009). In this regard, a previous study from our group showed that mechanical stimulation by hypotonic shock or FF activates PTH1R in a ligand independent manner (Maycas et al., 2015b). In that study, we described that mechanical stretching rapidly (<1 min) stimulated Cai 2+ transients in PTH1R‐overexpressing HEK‐293 cells and in MLO‐Y4 cells. Moreover, calcium signaling was unaffected after PTHrP gene silencing but was inhibited by knocking down PTH1R (Maycas et al., 2015b). On the other hand, it has also been described that mechanical loading such as physical exercise and PTH/PTHrP peptides can synergically potentiate each other actions (Gardinier et al., 2016, 2019; Maycas et al., 2017; Robinson et al., 2021; Sugiyama et al., 2008; Y. Wang et al., 2007). In fact, physical activity as running or swimming induces transient PTH secretion (Gardinier et al., 2016), and mechanical stimulation increases PTHrP production in osteocytes, which might induce PTH1R activation (Chen et al., 2005; Maycas et al., 2015a). In a similar way, the anabolic action of PTH or PTHrP is enhanced by mechanical loading (Maycas et al., 2017; Sugiyama et al., 2008).
Primary cilia act as mechanosensors, receiving mechanical signals and transducing them through their specific machinery. The primary cilium organelle is a nonmotile unique protrusion of the cell membrane that is formed during the G0 phase of the cell cycle and functions as an antenna. There are a great number of receptors and ion channels that bind to the membrane of the primary cilium. Moreover, primary cilium has been shown to mediate mechanical loading‐induced bone formation (Martín‐Guerrero et al., 2020; Rais et al., 2015) by coordinating bone‐forming osteoblast proliferation and continuous replenishment of osteoblasts from mesenchymal stem cell populations in response to paracrine factors secreted by mechanosensitive osteocytes (Hoey et al., 2011; Leucht et al., 2013). This organelle has also been involved in mechanically‐stimulated recruitment of osteoblasts and posterior differentiation into embedded, fully mature osteocytes (Hoey et al., 2011; Moore et al., 2019).
We have recently shown that PTH1R localizes into primary cilia, both in control or PTHrP‐stimulated conditions in osteocyte and osteoblast cells (Martín‐Guerrero et al., 2020). Moreover, a recent study demonstrated that mechanical stress promotes transport of PTH1R to primary cilia, increasing PTH signaling in nucleus pulposus cells from the intervertebral disc (Zheng et al, 2018). However, to our knowledge, PTH1R redistribution along the whole primary cilium after mechanical stimulation by FF in bone cells has not been described before. The present data suggest that mechanical stimulation may enhance PTHrP effectiveness by potentiating a more accessible distribution of its receptor, PTH1R, to external mechanical stimuli such as PTH1R natural agonists. These could explain, at least in part, the therapeutic efficiency of combined PTH or PTHrP treatment and mechanical stimulation to preserve bone mass and quality.
Mechanical loading plays an essential role in the maintenance of bone quality and quantity, and osteocytes are critical for sensing mechanical stimulation (Bellido, 2014; Choy et al., 2020). Osteocytes respond to mechanical stimulation by releasing proteins that regulate osteoclast and osteoblast activity, including Sclerostin, RANKL, osteoprotegerin (OPG), and M‐CSF. These paracrine factors can regulate osteoclast differentiation from monocyte precursors (Delgado‐Calle, Sato et al., 2017; Wijenayaka et al., 2011; You et al., 2008). In this regard, RANKL and M‐CSF promote osteoclast differentiation and activity (Takayanagi, 2007). Osteocyte‐like MLO‐Y4 cells promote osteoclast formation by increasing surface bound RANKL and secreting M‐CSF (Simfia et al., 2020; Xiong et al., 2011; Zhao et al., 2002). WNT/β‐catenin signaling pathway regulates production of RANKL, M‐CSF, and OPG by osteocytes. Differentiated osteoblasts also regulate osteoclast formation by activating β‐catenin/Wnt pathway, that inhibits the secretion of the RANKL decoy molecule, OPG (Baron & Kneissel, 2013). Other osteocyte‐derived factors that contribute to osteoclast differentiation and function include IL‐6, tumor necrosis factor alpha (perhaps through osteocyte‐derived apoptotic bodies), and high mobility group box 1 (Robling & Bonewald, 2020). Many inflammatory cytokines regulate osteoclastogenesis. For example, IL‐4 and IL‐10 inhibit osteoclast formation whereas stromal cell‐derived factor‐1 and monocyte chemoattractant protein‐1 stimulate it (H. R. Kim et al., 2014; M. S. Kim et al., 2006; Takayanagi, 2009). While the field of osteocyte control of osteoclastogenesis has made great strides in recent years, several RANKL/OPG independent mechanisms are currently under investigation (O'brien et al., 2016). In the present study, we have found that mechanical stimulation by FF inhibits preosteoclast migration and differentiation by a mechanism dependent of PTH1R and primary cilia.
Proteomic analysis and neutralizing experiments point to CXCL5 as a cytokine regulated by osteocytes that, when is secreted, controls osteoclast migration and differentiation. CXCL5 is a chemokine involved in leukocyte recruitment (Nouailles et al., 2014; Yoshida et al., 2014). This chemokine binds to CXC receptor 1 and CXC receptor 2, which are both expressed in osteoclast precursors (Grassi et al., 2003; Li et al., 2011). Moreover, CXCL5 has been describe to increase RANKL expression in Paget's disease in bone (Sundaram et al., 2013). Other study shows that microgravity promotes osteoclast formation through the induction of syncytin‐A expression in RAW264.7 preosteoclast cells without RANKL stimulation. The effects of osteotropic factors such as CXCL5 to regulate the expression of syncytin‐A in preosteoclast cells subjected to microgravity compared to ground based cultures has been confirmed (Ethiraj et al., 2018). However, in the present study when PTH1R gene expression was silenced CXCL5 was not affected, although FF effects on osteoclast migration and differentiation were completely reversed. This observation suggests that secretion of CXCL5 is highly dependent on mechanical stimulation but does not change whether PTH1R is activated or not.
We also observed that IL‐6 secretion was increased when PTH1R was silenced, both in static and FF conditions. Previous studies found that IL‐6 enhances osteocyte‐mediated osteoclastogenesis by promoting JAK2 and RANKL activity (Wu et al., 2017). Kazuhiro's study demonstrated that a combination of TNF and IL‐6 can induce bone‐resorptive activity in osteoclast‐like cells (Yokota et al., 2014). Indeed, IL‐6 plays a significant role as a biochemical regulator during osteoclastogenesis and bone resorption and regeneration (Cheung et al., 2012). Similar to this study, osteoclast formation was enhanced after the secretion of both IL‐6 and soluble IL‐6 receptor (Palmqvist et al., 2002). Accordingly, we show herein that IL‐6 neutralizing antibody decreased cell migration and differentiation when PTH1R was inhibited. However, CXCL5 neutralizing antibody had no effect on this regard.
We have observed that PTH1R silencing is associated with increased monocyte migration and osteoclastogenesis and also with IL‐6 over‐secretion by osteocytes even when cells were stimulated by FF. Given that IL‐6 neutralization under these conditions decreases both migration and osteoclasts differentiation, these data suggest that secretion of high levels of IL‐6 can overcome FF‐dependent under‐secretion of CXCL5 and maintain monocyte migration and osteoclastogenesis. In a similar way, we showed that primary cilia inhibition is also associated with increased osteoclast function, even in mechanical stimulated conditions, and IL‐6 neutralization reversed this effect. Collectively, these data suggest that abrogation of primary cilia might induce an IL‐6 over‐secretion that overcome CXCL5 under‐secretion, as occur when PTH1R is silenced. However, it is also possible that primary cilia under FF condition could modulate other alternative cytokines involved in osteoclast communication. Still, IL‐6 neutralization was enough to avoid migration and differentiation of osteoclast precursors.
Our findings support that functional primary cilium and PTH1R are required in osteocytes to regulate the secretome of these cells and their communication with osteoclasts. Thus, mechanically stimulated‐osteocytes inhibit osteoclast recruitment and differentiation by decreasing CXCL5 secretion, while activation of osteocytic PTH1R regulate osteoclasts via modulation of Il‐6 secretion.
ACKNOWLEDGMENT
Funding was available from Fundación Española de Investigación Ósea y Metabolismo Mineral (FEIOMM) and grant PID2019‐109659RB‐I00 from the Spanish Ministerio de Ciencia e Innovación. Eduardo Martin‐Guerrero and Sara Heredero‐Jimenez were recipients of PhD fellowships from San Pablo CEU University. We thank CBMSO PROTEIN CHEMISTRY FACILITY that belongs to ProteoRed, PRB2‐ISCIII, supported by grant PT13/0001 for the proteomic analysis.
Tirado‐Cabrera, I. , Martin‐Guerrero, E. , Heredero‐Jimenez, S. , Ardura, J. A. , & Gortázar, A. R. (2022). PTH1R translocation to primary cilia in mechanically‐stimulated ostecytes prevents osteoclast formation via regulation of CXCL5 and IL‐6 secretion. Journal of Cellular Physiology, 237, 3927–3943. 10.1002/jcp.30849
Juan A. Ardura and Arancha R. Gortazar have same senior status.
REFERENCES
- Alonso, R. , Pisa, D. , Marina, A. I. , Morato, E. , Rábano, A. , Rodal, I. , & Carrasco, L. (2015). Evidence for fungal infection in cerebrospinal fluid and brain tissue from patients with amyotrophic lateral sclerosis. International Journal of Biological Sciences, 11(5), 546–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardura, J. A. , Portal‐Núñez, S. , Alonso, V. , Bravo, B. , & Gortazar, A. R. (2019). Handling parathormone receptor type 1 in skeletal diseases: Realities and expectations of abaloparatide. Trends in Endocrinology and Metabolism, 30(10), 756–766. [DOI] [PubMed] [Google Scholar]
- Baron, R. , & Kneissel, M. (2013). WNT signaling in bone homeostasis and disease: From human mutations to treatments. Nature Medicine, 19(2), 179–192. 10.1038/nm.3074 [DOI] [PubMed] [Google Scholar]
- Bellido, T. (2014). Osteocyte‐driven bone remodeling. Calcified Tissue International, 94(1), 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X. , Macica, C. M. , Ng, K. W. , & Broadus, A. E. (2005). Stretch‐induced PTH—Related protein gene expression in osteoblasts. Journal of Bone and Mineral Research, 20(8), 1454–1461. [DOI] [PubMed] [Google Scholar]
- Cheung, W. Y. , Simmons, C. A. , & You, L. (2012). Osteocyte apoptosis regulates osteoclast precursor adhesion via osteocytic IL‐6 secretion and endothelial ICAM‐1 expression. Bone, 50(1), 104–110. 10.1016/j.bone.2011.09.052 [DOI] [PubMed] [Google Scholar]
- Choy, M. H. V. , Wong, R. M. Y. , Chow, S. K. H. , Li, M. C. , Chim, Y. N. , Li, T. K. , Ho, W. T. , Cheng, J. C. Y. , & Cheung, W. H. (2020). How much do we know about the role of osteocytes in different phases of fracture healing? A systematic review. Journal of Orthopaedic Translation, 21, 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado‐Calle, J. , Sato, A. Y. , & Bellido, T. (2017). Role and mechanism of action of sclerostin in bone. Bone, 96, 29–37. 10.1016/j.bone.2016.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado‐Calle, J. , Xiaolin, J. , Pacheco‐Costa, R. , McAndrews, K. , Edwards, R. , Pellegrini1, G. , et al. (2017). Control of bone anabolism in response to mechanical loading and PTH by distinct mechanisms downstream of the PTH receptor. Journal of Bone and Mineral Research, 33(3), 522–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deren, M. E. , Yang, X. , Guan, Y. , & Chen, Q. (2016). Biological and chemical removal of primary cilia affects mechanical activation of chondrogenesis markers in chondroprogenitors and hypertrophic chondrocytes. International Journal of Molecular Sciences, 17(2), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, D. , Yang, X. , Luan, H. , Wu, X. , He, C. , Sun, L. , & Fan, Y. (2020). Pharmacological regulation of primary cilium formation affects the mechanosensitivity of osteocytes. Calcified Tissue International, 107(6), 625–635. 10.1007/s00223-020-00756-6 [DOI] [PubMed] [Google Scholar]
- Estell, E. G. , & Rosen, C. J. (2021). Emerging insights into the comparative effectiveness of anabolic therapies for osteoporosis. Nature Reviews Endocrinology, 17(1), 31–46. 10.1038/s41574-020-00426-5 [DOI] [PubMed] [Google Scholar]
- Ethiraj, P. , Link, J. R. , Sinkway, J. M. , Brown, G. D. , Parler, W. A. , & Reddy, S. V. (2018). Microgravity modulation of syncytin‐A expression enhance osteoclast formation. Journal of Cellular Biochemistry, 119(7), 5696–5703. [DOI] [PubMed] [Google Scholar]
- Gardinier, J. D. , Al‐Omaishi, S. , Morris, M. D. , & Kohn, D. H. (2016). PTH signaling mediates perilacunar remodeling during exercise. Matrix Biology, 52–54, 162–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardinier, J. D. , Daly‐Seiler, C. , Rostami, N. , Kundal, S. , & Zhang, C. (2019). Loss of the PTH/PTHrP receptor along the osteoblast lineage limits the anabolic response to exercise. PLoS One. 14(1):e0211076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi, F. , Piacentini, A. , Cristino, S. , Toneguzzi, S. , Cavallo, C. , Facchini, A. , & Lisignoli, G. (2003). Human osteoclasts express different CXC chemokines depending on cell culture substrate: Molecular and immunocytochemical evidence of high levels of CXCL10 and CXCL12. Histochemistry and Cell Biology, 120(5), 391–400. [DOI] [PubMed] [Google Scholar]
- Hoey, D. A. , Kelly, D. J. , & Jacobs, C. R. (2011). A role for the primary cilium in paracrine signaling between mechanically stimulated osteocytes and mesenchymal stem cells. Biochemical and Biophysical Research Communications, 412(1), 182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H. R. , Kim, K. W. , Kim, B. M. , Jung, H. G. , Cho, M. L. , & Lee, S. H. (2014). Reciprocal activation of cd4+ t‐cells and synovial fibroblasts by stromal cell‐derived factor 1 promotes rankl expression and osteoclastogenesis in rheumatoid arthritis. Arthritis Rheumatol, 66(3), 538–548. [DOI] [PubMed] [Google Scholar]
- Kim, M. S. , Day, C. J. , Selinger, C. I. , Magno, C. L. , Stephens, S. R. J. , & Morrison, N. A. (2006). MCP‐1‐induced human osteoclast‐like cells are tartrate‐resistant acid phosphatase, NFATc1, and calcitonin receptor‐positive but require receptor activator of NFκB ligand for bone resorption. Journal of Biological Chemistry, 281(2), 1274–1285. [DOI] [PubMed] [Google Scholar]
- Leucht, P. , Monica, S. D. , Temiyasathit, S. , Lenton, K. , Manu, A. , Longaker, M. T. , Jacobs, C. R. , Spilker, R. L. , Guo, H. , Brunski, J. B. , & Helms, J. A. (2013). Primary cilia act as mechanosensors during bone healing around an implant. Medical Engineering & Physics, 35(3), 392–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, A. , King, J. , Moro, A. , Sugi, M. D. , Dawson, D. W. , Kaplan, J. , Li, G. , Lu, X. , Strieter, R. M. , Burdick, M. , Go, V. L. , Reber, H. A. , Eibl, G. , & Hines, O. J. (2011). Overexpression of CXCL5 is associated with poor survival in patients with pancreatic cancer. American Journal of Pathology, 178(3), 1340–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malicki, J. , & Johnson, C. (2017). The cilium: Cellular antenna and central processing unit. Trends in Cellular Biology [Internet], 27(2), 126–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malicki, J. J. , & Johnson, C. A. (2017). The cilium: cellular antenna and central processing unit. Trends in Cellular Biology [Internet], 27(2), 126–140. 10.1016/j.tcb.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone, A. M. D. , Anderson, C. T. , Tummala, P. , Kwon, R. Y. , Johnston, T. R. , Stearns, T. , & Jacobs, C. R. (2007). Primary cilia mediate mechanosensing in bone cells by a calcium‐independent mechanism. Proceedings of the National Academy of Sciences of the United States of America, 104(33), 13325–13330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín‐Guerrero, E. , Tirado‐Cabrera, I. , Buendía, I. , Alonso, V. , Gortázar, A. R. , & Ardura, J. A. (2020). Primary cilia mediate parathyroid hormone receptor type 1 osteogenic actions in osteocytes and osteoblasts via Gli activation. Journal of Cellular Physiology, 235(10), 7356–7369. [DOI] [PubMed] [Google Scholar]
- Maycas, M. , Ardura, J. A. , De Castro, L. F. , Bravo, B. , Gortázar, A. R. , & Esbrit, P. (2015a). Role of the parathyroid hormone type 1 receptor (PTH1R) as a mechanosensor in osteocyte survival. Journal of Bone and Mineral Research, 30(7), 1231–1244. [DOI] [PubMed] [Google Scholar]
- Maycas, M. , Ardura, J. A. , De Castro, L. F. , Bravo, B. , Gortázar, A. R. , & Esbrit, P. (2015b). Role of the parathyroid hormone type 1 receptor (PTH1R) as a mechanosensor in osteocyte survival. Journal of Bone and Mineral Research, 30(7), 1231–1244. [DOI] [PubMed] [Google Scholar]
- Maycas, M. , Fernández de Castro, L. , Bravo, B. , García de Durango, C. , Forriol, F. , Gortázar, A. R. , & Esbrit, P. (2015). The VEGF (VEGFR2) 2 receptor and PTH (PTH1R) 1 receptor act as mediators in the anti‐apoptotic response to mechanical stimulus in MLO‐Y4 osteocyte‐like cell. Revista de Osteoporosis y Metabolismo Mineral, 7(4), 91–97. [Google Scholar]
- Maycas, M. , McAndrews, K. A. , Sato, A. Y. , Pellegrini, G. G. , Brown, D. M. , Allen, M. R. , Plotkin, L. I. , Gortazar, A. R. , Esbrit, P. , & Bellido, T. (2017). PTHrP‐derived peptides restore bone mass and strength in diabetic mice: Additive effect of mechanical loading. Journal of Bone and Mineral Research, 32(3), 486–497. [DOI] [PubMed] [Google Scholar]
- Moore, E. R. , Chen, J. C. , & Jacobs, C. R. (2019). Prx1‐expressing progenitor primary cilia mediate bone formation in response to mechanical loading in mice. Stem Cells International, 2019, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno, M. L. , Escobar, J. , Izquierdo‐Álvarez, A. , Gil, A. , Pérez, S. , Pereda, J. , Zapico, I. , Vento, M. , Sabater, L. , Marina, A. , Martínez‐Ruiz, A. , & Sastre, J. (2014). Disulfide stress: A novel type of oxidative stress in acute pancreatitis. Free Radical Biology and Medicine, 70, 265–277. [DOI] [PubMed] [Google Scholar]
- Nouailles, G. , Dorhoi, A. , Koch, M. , Zerrahn, J. , Weiner, J. , Faé, K. C. , Arrey, F. , Kuhlmann, S. , Bandermann, S. , Loewe, D. , Mollenkopf, H. J. , Vogelzang, A. , Meyer‐Schwesinger, C. , Mittrücker, H. W. , McEwen, G. , & Kaufmann, S. H. (2014). CXCL5‐secreting pulmonary epithelial cells drive destructive neutrophilic inflammation in tuberculosis. Journal of Clinical Investigation, 124(3), 1268–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'brien, W. , Fissel, B. M. , Maeda, Y. , Yan, J. , Ge, X. , Gravallese, E. M. , Aliprantis, A. O. , & Charles, J. F. (2016). RANK‐independent osteoclast formation and bone erosion in inflammatory arthritis. Arthritis Rheumatol, 68(12), 2889–2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmqvist, P. , Persson, E. , Conaway, H. H. , Lerner, U. H. , Palmqvist, P. , Persson, E. , Conaway, H. H. , & Lerner, U. H. (2002). IL‐6, leukemia inhibitory factor, and oncostatin M stimulate bone resorption and regulate the expression of receptor activator of NF‐kappa B ligand, osteoprotegerin, and receptor activator of NF‐kappa B in mouse calavariae. Journal of Immunology, 6(23), 3353–3362. [DOI] [PubMed] [Google Scholar]
- Plotkin, L. I. , Gortazar, A. R. , Davis, H. M. , Condon, K. W. , Gabilondo, H. , Maycas, M. , Allen, M. R. , & Bellido, T. (2015). Inhibition of osteocyte apoptosis prevents the increase in osteocytic receptor activator of nuclear factor κb ligand (RANKL) but does not stop bone resorption or the loss of bone induced by unloading. Journal of Biological Chemistry, 290(31), 18934–18942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell WF, J. r , Barry, K. J. , Tulum, I. , Kobayashi, T. , Harris, S. E. , Bringhurst, F. R. , & Pajevic, P. D. (2011). Targeted ablation of the PTH/PTHrP receptor in osteocytes impairs bone structure and homeostatic calcemic responses. Journal of Endocrinology, 209(1), 21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rais, Y. , Reich, A. , Simsa‐Maziel, S. , Moshe, M. , Idelevich, A. , Kfir, T. , Miosge, N. , & Monsonego‐Ornan, E. (2015). The growth plate's response to load is partially mediated by mechano‐sensing via the chondrocytic primary cilium. Cellular and Molecular Life Science, 72(3), 597–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee, Y. , Allen, M. R. , Condon, K. , Lezcano, V. , Ronda, A. C. , Galli, C. , Olivos, N. , Passeri, G. , O'Brien, C. A. , Bivi, N. , Plotkin, L. I. , & Bellido, T. (2011). PTH receptor signaling in osteocytes governs periosteal bone formation and intracortical remodeling. Journal of Bone and Mineral Research, 26(5), 1035–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, S. , Shyu, P. , & Guo, X. (2021). Mechanical loading and parathyroid hormone effects and synergism in bone vary by site and modeling/remodeling regime. Bone, 153, 116171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robling, A. G. , & Bonewald, L. F. (2020). The osteocyte: New insights. Annual Review of Physiology, 82, 485–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schou, K. B. , Pedersen, L. B. , & Christensen, S. T. (2015). Ins and outs of GPCR signaling in primary cilia. EMBO Reports, 16(9), 1099–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simfia, I. , Schiavi, J. , & McNamara, L. M. (2020). Alterations in osteocyte mediated osteoclastogenesis during estrogen deficiency and under ROCK‐II inhibition: An in vitro study using a novel postmenopausal multicellular niche model. Exp Cell Res [Internet], 392(1), 112005. 10.1016/j.yexcr.2020.112005 [DOI] [PubMed] [Google Scholar]
- Sugiyama, T. , Saxon, L. K. , Zaman, G. , Moustafa, A. , Sunters, A. , Price, J. S. , & Lanyon, L. E. (2008). Mechanical loading enhances the anabolic effects of intermittent parathyroid hormone (1−34) on trabecular and cortical bone in mice. Bone, 43(2), 238–248. [DOI] [PubMed] [Google Scholar]
- Sundaram, K. , Rao, D. S. , Ries, W. L. , & Reddy, S. V. (2013). CXCL5 stimulation of RANK ligand expression in Paget's disease of bone. Laboratory in Investigation, 93(4), 472–479. [DOI] [PubMed] [Google Scholar]
- Takayanagi, H. (2007). Osteoclast differentiation and activation. Clinical calcium, 17(4), 484–492. [PubMed] [Google Scholar]
- Takayanagi, H. (2009). Osteoimmunology and the effects of the immune system on bone. Nature Reviews Rheumatology, 5(12), 667–676. 10.1038/nrrheum.2009.217 [DOI] [PubMed] [Google Scholar]
- Takei, R. , Katoh, Y. , & Nakayama, K. (2018). Robust interaction of IFT70 with IFT52–IFT88 in the IFT‐B complex is required for ciliogenesis. Biology Open, 7(5), bio033241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu, X. , Delgado‐Calle, J. , Condon, K. W. , Maycas, M. , Zhang, H. , Carlesso, N. , Taketo, M. M. , Burr, D. B. , Plotkin, L. I. , & Bellido, T. (2015). Osteocytes mediate the anabolic actions of canonical Wnt/β‐catenin signaling in bone. Proceedings of the National Academy of Sciences of the United States of America, 112(5), E478–E486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, P. , Tang, C. , Wu, J. , Yang, Y. , Yan, Z. , Liu, X. , Shao, X. , Zhai, M. , Gao, J. , Liang, S. , Luo, E. , & Jing, D. (2019). Pulsed electromagnetic fields regulate osteocyte apoptosis, RANKL/OPG expression, and its control of osteoclastogenesis depending on the presence of primary cilia. Journal of Cellular Physiology, 234(7), 10588–10601. [DOI] [PubMed] [Google Scholar]
- Wang, Y. , McNamara, L. M. , & Schaffler, M. B. W. S. (2007). A model for the role of integrins in flow induced mechanotransduction in osteocytes. Proceedings of the National Academy of Sciences of the United States of America, 104(40), 15941–15946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijenayaka, A. R. , Kogawa, M. , Lim, H. P. , Bonewald, L. F. , Findlay, D. M. , & Atkins, G. J. (2011). Sclerostin stimulates osteocyte support of osteoclast activity by a RANKL‐dependent pathway. PLoS One, 6(10), 25900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Q. , Zhou, X. , Huang, D. , Ji, Y. , & Kang, F. (2017). IL‐6 enhances osteocyte‐mediated osteoclastogenesis by promoting JAK2 and RANKL activity in vitro. Cellular Physiology and Biochemistry, 41(4), 1360–1369. [DOI] [PubMed] [Google Scholar]
- Xiong, J. , Onal, M. , Jilka, R. L. , Weinstein, R. S. , Manolagas, S. C. , & O'Brien, C. A. (2011). Matrix‐embedded cells control osteoclast formation. Nature Medicine, 17(10), 1235–1241. 10.1038/nm.2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota, K. , Sato, K. , Miyazaki, T. , Kitaura, H. , Kayama, H. , Miyoshi, F. , Araki, Y. , Akiyama, Y. , Takeda, K. , & Mimura, T. (2014). Combination of tumor necrosis factor α and interleukin‐6 induces mouse osteoclast‐like cells with bone resorption activity both in vitro and in vivo. Arthritis & Rheumatology, 66(1), 121–129. [DOI] [PubMed] [Google Scholar]
- Yoshida, K. , Korchynskyi, O. , Tak, P. P. , Isozaki, T. , Ruth, J. H. , Campbell, P. L. , Baeten, D. L. , Gerlag, D. M. , Amin, M. A. , & Koch, A. E. (2014). Citrullination of epithelial neutrophil‐activating peptide 78/CXCL5 results in conversion from a non‐monocyte‐recruiting chemokine to a monocyte‐recruiting chemokine. Arthritis & Rheumatology, 66(10), 2716–2727. [DOI] [PubMed] [Google Scholar]
- You, L. , Temiyasathit, S. , Lee, P. , Kim, C. H. , Tummala, P. , Yao, W. , Kingery, W. , Malone, A. M. , Kwon, R. Y. , & Jacobs, C. R. (2008). Osteocytes as mechanosensors in the inhibition of bone resorption due to mechanical loading. Bone, 42(1), 172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. L. , & Frangos, J. A. C. M. (2009). Mechanical stimulus alters conformation of type 1 parathyroid hormone receptor in bone cells. American Journal of Physiology: Cell Physiology, 296(6), C1391–C1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, S. , Kato, Y. , Zhang, Y. , Harris, S. , Ahuja, S. S. , & Bonewald, L. F. (2002). MLO‐Y4 osteocyte‐like cells support osteoclast formation and activation. Journal of Bone and Mineral Research, 17(11), 2068–2079. [DOI] [PubMed] [Google Scholar]
- Zheng, L. , Cao, Y. , Ni, S. , Qi, H. , Ling, Z. , Xu, X. , Zou, X. , Wu, T. , Deng, R. , Hu, B. , Gao, B. , Chen, H. , Li, Y. , Zhu, J. , Tintani, F. , Demehri, S. , Jain, A. , Kebaish, K. M. , Liao, S. , … Cao, X. (2018). Ciliary parathyroid hormone signaling activates transforming growth factor‐β to maintain intervertebral disc homeostasis during aging. Bone Research, 6(1), 21. [DOI] [PMC free article] [PubMed] [Google Scholar]


