Abstract
Aim
To present the 20‐year clinical outcomes of tissue‐level implants in partially edentulous patients previously treated for periodontitis and in periodontally healthy patients (PHP).
Material and Methods
The original population consisted of 149 partially edentulous patients consecutively enrolled in a private specialist practice and divided into three groups: PHP, moderately periodontally compromised patients (mPCP) and severely PCP (sPCP). After successful completion of periodontal/implant therapy, patients were enrolled in an individualized supportive periodontal care (SPC) programme.
Results
Eighty‐four patients rehabilitated with 172 implants reached the 20‐year examination. During the observation time, 12 implants were removed (i.e., 11 due to biological complications and 1 due to implant fracture), leading to an overall implant survival rate of 93% (i.e., 94.9% for PHP, 91.8% for mPCP and 93.1% for sPCP [p = .29]). At 20 years, PCP compliant with SPC did not present with significantly higher odds of implant loss compared with PHP compliant with SPC (p > .05). Conversely, PCP not compliant with SPC experienced implant loss with odds ratio of 14.59 (1.30–164.29, p = .03).
Conclusions
Tissue‐level implants, placed after comprehensive periodontal therapy and SPC, yield favourable long‐term results. However, patients with a history of periodontitis and non‐compliant with SPC are at higher risk of biological complications and implant loss.
Keywords: dental implants, peri‐implantitis, periodontitis, supportive periodontal therapy, tooth loss
Clinical Relevance.
Scientific rationale for study: There are only limited long‐term data about implants in periodontally compromised patients. This study presents the 20‐year clinical results of implants placed in patients with a previous history of periodontitis compared with healthy patients.
Principal findings: Supportive periodontal care (SPC), particularly in patients with a history of periodontitis, was fundamental to achieve high long‐term (i.e., 20 years) implant and teeth survival.
Practical implications: Patients in need of rehabilitation with dental implants should be properly informed before treatment that the odds for implant loss drastically increase in case of previous history of periodontitis and lack of compliance with SPC.
1. INTRODUCTION
Over the past three decades, the use of dental implants has radically changed the way to partially and totally rehabilitate edentulous patients, thus allowing clinicians to perform complex oral rehabilitations (Buser et al., 2017; Duong et al., 2022). In particular, if considering the wide body of evidence on the assessment of implant survival and success rates and peri‐implant marginal bone‐level changes, the scientific interest has recently focused on long‐term results, that is, ≥10 years (Howe et al., 2019). Indeed, since dental implants are placed with the aim of restoring missing teeth and last “forever”, it is nowadays widely accepted that studies with a limited follow‐up and reduced sample size provide limited clinical information. On the other hand, data reporting on implants placed many years apart (i.e., with at least 15‐year follow‐up) might not be representative of the contemporary situation and, therefore, preclude from external validity (Astrand et al., 2008; Mengel et al., 2019). More specifically, due to the rapid development of implant surface characteristics and prosthetic materials and technologies, the results published with a follow‐up of 20 years seem to be relevant more for historical reasons rather than for their clinical utility (Chappuis et al., 2013; Donati et al., 2018). However, even though sandblasted and acid‐etched (SLA) implant surfaces have been used for the last 25 years, they are still present in the dental market. Therefore, it seems meaningful to monitor SLA implants in order to provide long‐term clinical results, particularly in patients with a history of periodontitis displaying a 2× higher risk for implant loss compared with non‐periodontitis patients (Carra et al., 2021).
Hence, the aim of this study was to assess the 20‐year clinical outcomes of SLA implants placed in a cohort of periodontally healthy patients (PHP) compared with a group of periodontally compromised patients (PCP) of both moderate and severe extent.
2. MATERIALS AND METHODS
The 20‐year study protocol was approved by the Institutional Ethics Committee (Nr.168/2021). The investigation was conducted according to the revised principles of the Helsinki Declaration (2013). All participants signed a written informed consent before entering the study. The trial was registered at http://ClinicalTrials.gov (NCT04983758) and reported according to the STROBE guidelines.
2.1. Study population
The original population consisted of 149 patients rehabilitated with 297 sandblasted large grit and acid‐etched surface (SLA) dental implants (Straumann Group AG, Basel, Switzerland).
Details of the treatment protocol have been described in a previous publication reporting on the 10‐year outcomes (Roccuzzo et al., 2014). In brief, 123 of the 149 screened patients (mean age 50 years old; 18% smokers), attending the senior investigator's specialist periodontal practice in Torino, Italy, between December 1998 and September 2001, seeking for dental implant therapy were included in the study. Of the original population, only individuals participated in all follow‐up visits (n = 84; i.e., 10 and 20 years) were included in the present analysis.
The exclusion criteria were as follows:
complete edentulism;
presence of an implant‐supported overdenture;
any acute/chronic auto‐immune mucosal diseases (i.e., pemphigus, lichen);
alcohol and drug abuse;
pregnant or lactating females;
uncontrolled metabolic disorders;
aggressive periodontitis according to Armitage (1999);
inability or unwillingness to give informed consent.
2.2. Pre‐treatment clinical examination
Socio‐demographic characteristics, smoking status and medical history were collected during the initial visit and treatment planning. Moreover, subjects were clinically and radiographically monitored at baseline (i.e., prior to active periodontal therapy—APT). Full‐mouth plaque score (FMPS), full‐mouth bleeding score (FMBS) and pocket depth (PD) were measured at four sites per tooth for all teeth using a graduated periodontal probe (XP23/UNC 15, Hu‐Friedy, Chicago, IL), and rounded off to the nearest millimetre.
At baseline, three groups of patients were identified based on their periodontal conditions.
Patients without signs of periodontitis were classified as PHP. Patients with an initial diagnosis of periodontitis (PCP) received a score (S) on the basis of the number and depth of periodontal pockets according to the following formula:
These patients were therefore divided into two groups:
Moderate PCP (mPCP): PCP with S ≤ 25.
Severe PCP (sPCP): PCP with S > 25.
2.3. Periodontal therapy, implant therapy and prosthetic phase
After enrolment, all patients received appropriate initial therapy, consisting, depending on the cases, of motivation, oral hygiene instructions and subgingival mechanical instrumentation under local anaesthesia. Hopeless teeth were recorded and extracted. Periodontal surgery was performed as needed after re‐evaluation, including guided tissue regeneration whenever indicated. Individual treatment was thoroughly discussed with the patients and established according to their personal chief complaint. No implant surgery was performed before optimal motivation and compliance from each single patient was achieved (i.e., FMPS ≤15% and FMBS ≤15%).
After completion of APT, tissue‐level SLA implants were placed, under local anaesthesia, by the same experienced operator (M.R.), according to the manufacturer's instructions and using a standardized surgical procedure (Buser et al., 2000). No bone augmentation procedures were performed either prior to or concomitant with implant surgery. After 6–12 weeks of non‐submerged healing, abutment connection was carried out and all patients were provided with cemented implant‐supported fixed restorations (i.e., single‐unit crown [SUC] or fixed dental prosthesis [FDP]). Prosthesis delivery encompassed the collection of clinical and radiographical data.
2.4. Supportive periodontal/peri‐implant care
Patients were enrolled in an individualized supportive periodontal care (SPC) programme, including a continuous evaluation of the occurrence and the risk of disease progression.
Patients were recalled at various intervals for oral hygiene instructions, biofilm removal and treatment of re‐infected sites were performed whenever needed. SPC was performed by the same experienced dental hygienist. If patients expressed unwillingness to attend follow‐up examinations, they were classified as “dropout”. The diagnosis and treatment of peri‐implant diseases (i.e., peri‐implant mucositis and peri‐implantitis) were performed according to the concept of cumulative interceptive supportive therapy (CIST; Lang et al., 1997; Mombelli & Lang, 1998). The number of sites treated according to CIST modalities C and D (antibiotics and/or surgery) during the 20 years was also recorded.
2.5. Clinical examinations
At the 10‐year (T1) and 20‐year (T2) follow‐up examination, implant survival rate (i.e., presence of the implant in the oral cavity) was calculated. Moreover, the same examiner with more than 15 years of experience as dental hygienist, blinded to the initial classification of the patients, did all the recordings, for each treated implant probing depth (PD) measured at four sites (mesial, buccal, distal and lingual) using a graduated periodontal probe (XP23/UNC 15; Hu‐Friedy). Measurements were rounded off to the nearest millimetre.
At the same implant sites, the presence of dental plaque (Pl), bleeding on probing (BOP) and suppuration was recorded dichotomously.
At follow‐up examinations, the following parameters were collected at patient level:
FMPS measured at four sites per tooth and implant and expressed as a percentage of examined sites;
FMBS measured at four sites per tooth and implant and expressed as a percentage of examined sites;
number of teeth lost during SPC;
adherence to SPC (yes or no): full compliance with an SPC program proposed by the principal investigator taking into account patient's needs and risk profile;
deepest PD during the SPC;
deepest PD at 10 and 20‐year follow‐ups;
number of patients requiring either C or D CIST modalities during SPC.
2.6. Statistical analysis
All analyses were performed using a statistical software (STATA BE, version 17.1, StataCorp LP, College Station, TX) and setting the level of significance at 5%. Continuous variables were presented as mean ± standard deviation (SD), categorical variables were presented as number of observations (proportion, %). Data distribution was checked through visual inspection and using the Shapiro–Wilk test. The Student's t‐test for independent samples was used for between‐group comparisons (Mann–Whitney U test for non‐normal data), while the paired t‐test (Wilcoxon's signed‐rank test for non‐normal data) was used for intra‐group comparisons. Whenever there were three groups or timepoints, one‐way ANOVA with post hoc Bonferroni correction was used for between‐group comparisons (Kruskal–Wallis test with post hoc Dunn test for non‐normal data), while ANOVA test for repeated measures (Friedman test for non‐normal data) was used for comparisons across timepoints. Categorical variables were compared using the Chi‐square test for independent samples (Cochran's Q test for paired data). Due to a significant difference between the three groups analysed, variables were adjusted for patient's age. The survival rate was calculated overall, by group and in relation to adherence to SPC between baseline and 20 years, and between 10 and 20 years. The cumulative incidence of biological complications (i.e., presence of at least one site with BOP, at least one site with PD ≥6 mm and of sites with radiographic bone loss ≥3 mm) was also calculated as percentage at implant level at the 10‐ and 20‐year follow‐ups. Crude and adjusted odds ratios (OR; 95% confidence interval [95% CI]) for implant loss were calculated using multilevel logistic regression models adjusted for clustering at patient level and considering age, number of teeth missing at baseline, baseline FMPS and FMBS as co‐variables (patient level). Smoking, compliance with SPC and periodontal status, as well as the combination of the latter two variables, were individually considered as independent variables. All computed p‐values were two‐tailed.
3. RESULTS
3.1. Patient population
Details of the original population are shown in Table 1. Of the 123 patients examined at the 10‐year follow‐up, 39 patients were lost at the 20‐year follow‐up: 16 died; 13 were not able to attend the final examination due to severe health problems or because they moved; and 10 refused the follow‐up visit. The number of patients and implants lost to follow‐up was comparable across groups (Table S1). Therefore, the population analysed at the 20‐year follow‐up included 84 subjects and 172 implants (Table 1).
TABLE 1.
Number of patients and implants through the 20‐year study period; number of implants examined and removed
| Patients | Implants | Patients lost to follow‐up | |
|---|---|---|---|
| Baseline | 149 | 297 | ‐ |
| 10 years | 123 | 246 | 26 |
| 20 years | 84 | 172 | 39 |
| List of reasons for dropout between 10 and 20 years | |||
| Death | 16 | ||
| Severe health problems | 6 | ||
| Moved | 7 | ||
| Refused to accept a visit | 10 | ||
| Total | 39 | ||
The final 20‐year analysis was performed on 22 PHP, 29 mPCP and 33 sPCP subjects, corresponding to 39, 59 and 71 implants, respectively. PHP had a statistically significant lower mean age (63.36 ± 12.11 years) compared with both mPCP (70.6 ± 9.7) and sPCP (71.03 ± 7.76; p < .001). The proportion of smokers was equally distributed across the groups (Table 2).
TABLE 2.
Characteristics of patients who reached the 20‐year examination
| Patients | Age (years) | Smokers | Teeth extracted (0–10 years) | Teeth extracted (10–20 years) | Implants removed (0–10 years) | Implants removed (10–20 years) | Patients treated with CIST C/D (0–10 years) | Patients treated with CIST C/D (10–20 years) | |
|---|---|---|---|---|---|---|---|---|---|
| PHP | 22 (26.2%) | 63.4 ± 12.1 | 4 (18.2%) | 0.6 ± 0.9 | 0.3 ± 0.6 | 0 | 2 (5.1%) | 5 (22.7%) | 7 (33.3%) |
| mPCP | 29 (34.5%) | 70.6 ± 9.7 | 4 (13.8%) | 1.3 ± 1.3 | 1.1 ± 1.2 | 2 (3.3%) | 3 (5.1%) | 13 (43.3%) | 14 (48.3%) |
| sPCP | 33 (39.3%) | 71.0 ± 7.8 | 6 (18.2%) | 1.9 ± 2.1 | 1.3 ± 1.3 | 1 (1.4%) | 4 (5.6%) | 19 (57.6%) | 19 (61.3%) |
| Statistical difference between | |||||||||
| All groups | p = .01 | p = .87 | p = .01 | p < .01 | p = .61 | p = .94 | p = .01 | p = .04 | |
| PHP vs. mPCP | p < .01 | p = .03 | p < .01 | p = .04 | p = .04 | ||||
| PHP vs. sPCP | p < .01 | p < .01 | p < .01 | p = .04 | p = .04 | ||||
| mPCP vs. sPCP | p = .46 | p = .15 | p = .18 | p = .07 | p = .06 | ||||
Note: Mean number of teeth extracted and implants removed during the first 10‐year of SPC and between 10 and 20 years of SPC. Continuous variables are expressed as mean ± standard deviation, categorical variables are expressed as number of observations (percentage). One‐way ANOVA test with post hoc Bonferroni correction (Kruskal–Wallis with post hoc Dunn test for non‐normal data) was used for inter‐group comparisons. The level of significance was set at 5%. Italic values show p < .05.
Abbreviations: CIST, cumulative interceptive therapy; mPCP, moderate periodontally compromised patients; PHP, periodontally healthy patients; sPCP, severe periodontally compromised patients.
The mean number of teeth lost during the SPC between 10 and 20 years was 0.27 ± 0.55 for PHP, 1.07 ± 1.23 for mPCP and 1.33 ± 1.29 for sPCP, respectively, with a statistically significant difference among the three groups (p < .001; Table 2).
At baseline (i.e., prior to APT), statistically significant differences were found among the three groups regarding both FMPS and FMBS (Table S2). Both parameters increased from PHP (27.36 ± 9.15 and 22.45 ± 9.72) to mPCP (37.47 ± 10.01 and 36.57 ± 13.52) up to sPCP (50.76 ± 23.94 and 48.97 ± 20.71). At the 20‐year examination, both FMPS and FMBS decreased in all groups and the between‐group analysis failed to show statistically significant differences (Table S2).
3.2. Clinical parameters at the 10‐ and 20‐year follow‐up
At the 20‐year examination, plaque around the tested implants was found as follows: 16.89 ± 29.59% for PHP, 19.02 ± 29.68% for mPCP and 25.75 ± 30.45% for sPCP, while BOP was found to be 25 ± 22.05%, 29.91 ± 28.35% and 30.2 ± 27.01%, respectively. BOP at implant site was comparable across groups and timepoints (p > .05), while plaque was statistically significantly lower in the PHP group compared with both PCP groups at 10 years (p = .04). At the 20‐year examination, no implants showed suppuration in the PHP and sPCP groups, while two implants presented suppuration in the mPCP group (Table 3).
TABLE 3.
Clinical parameters around the implants that reached the 20‐year examination
| Mean difference (95% CI) | ||||
|---|---|---|---|---|
| 10 years | 20 years | Intra‐group p‐value | 20–10 years | |
| Deepest PD (mm) | ||||
| Overall | 4.6 ± 1.3 | 4.3 ± 1.4 | .03 | −0.3 ± 1.3 |
| PHP | 4.4 ± 1.2A | 4 ± 1.3A | .11 | −0.4 ± 1.4 |
| mPCP | 4.6 ± 1.4B,C | 4.3 ± 1.4B,C | .35 | −0.2 ± 1.3 |
| sPCP | 4.7 ± 1.4C | 4.4 ± 1.4C | .28 | −0.3 ± 1.3 |
| Intergroup p‐value | <.01 | <.01 | .78 | |
| BOP at implant site (%) | ||||
| Overall | 34.3 ± 29.4 | 28.9 ± 26.4 | .02 | −4.7 ± 29.8 |
| PHP | 33.3 ± 27 | 25 ± 22.1 | .18 | −8.3 ± 29.2 |
| mPCP | 34.7 ± 31.2 | 29.9 ± 28.4 | .44 | −4.8 ± 27.9 |
| sPCP | 34.5 ± 29.4 | 30.2 ± 27.0 | .48 | −4.3 ± 32.3 |
| Intergroup p‐value | .68 | .73 | .83 | |
| Pl at the implant site (%) | ||||
| Overall | 29.1 ± 28.9 | 24.8 ± 28.3 | .16 | ‐ |
| PHP | 20.5 ± 21.4A | 16.9 ± 19.6 | .49 | −3.6 ± 31.2 |
| mPCP | 36.4 ± 34.5B,C | 29.0 ± 29.7 | .30 | −7.4 ± 26.7 |
| sPCP | 27.8 ± 26.2C | 25.8 ± 30.5 | .36 | −2.1 ± 31.8 |
| Intergroup p‐value | .04 | .41 | .83 | |
| Pus at the implant site (%) | ||||
| Overall | 12 (7.1%) | 2 (1.3%) | .11 | ‐ |
| PHP | 0 (0.0%) | 0 (0.0%) | ‐ | ‐ |
| mPCP | 6 (10.2%) | 2 (3.6%) | .14 | ‐ |
| sPCP | 6 (8.5%) | 0 (0.0%) | <.01 | ‐ |
| Intergroup p‐value | .11 | .17 | ‐ | |
Note: Continuous variables are expressed as mean ± standard deviation, categorical variables are expressed as number of observations (percentage). The level of significance was set at 5%. For each column, values sharing the same superscript upper‐case letter are not different at the 5% level. One‐way ANOVA test with post hoc Bonferroni correction (Kruskal–Wallis with post hoc Dunn test for non‐normal data) was used for inter‐group comparisons. Intra‐group comparisons between the 10‐ and 20‐year follow‐up were performed using the paired Student's t‐test (Wilcoxon's signed‐rank test for non‐normal data) for continuous variables. Categorical variables were compared using the Chi‐square test for independent samples (Cochran's Q for paired data). Italic values show p < .05.
Abbreviations: 95% CI, 95% confidence interval; BOP, bleeding on probing; mPCP, moderate periodontally compromised patients; PD, probing depth; PHP, periodontally healthy patients; PI, plaque; sPCP, severe periodontally compromised patients.
3.3. CIST C/D and interventions during the SPC
At 20 years, the number of patients treated with CIST C/D was statistically significantly lower in the PHP (33.3%) compared with the mPCP (48.3%) and sPCP (61.3%) groups, respectively (p = .04; Table 2). In the PHP group, around 19.1% of subjects received adjunctive antibiotic therapy and 4.8% received surgery, while in the mPCP group, 31.0% of subjects received antibiotic therapy and 17.2% underwent surgery. Finally, in the sPCP group, 8 subjects (25.81%) received adjunctive antibiotic therapy and 10 subjects underwent surgery (32.26%). The cumulative incidence of BOP at implant level was around 70% in all study groups and at both the 10‐ and 20‐year follow‐ups, without statistically significant inter‐group differences (p > .05). The occurrence of sites with PD ≥6 mm was statistically significantly higher in the sPCP (33.78%) compared with the PHP group (12.82%) at 10 years. Moreover, both at 10 and 20 years, the occurrence of sites with radiographic bone loss ≥3 mm was statistically significantly higher in the mPCP (18.33%, 33.33%) and sPCP (10.81%, 35.15%) groups compared with the PHP group (0.00%, 17.94%; p < .05; Table S3).
3.4. Implant survival rate
The overall survival rate over 20 years was 93% (Table 4). In PHP, two implants were lost in patients non‐compliant with SPC, resulting in a survival rate of 94.9%. Five implants were lost in the mPCP and sPCP groups, respectively, yielding a survival rate of 91.8% in the former and 93.1% in the latter group. No statistically significant differences were found across groups (p > .05).
TABLE 4.
0–20 and 10–20‐year survival rate, for each group and in relation to adherence to SPC
| Implants placed | Implants lost | Survival rate (%) | p‐value | |
|---|---|---|---|---|
| Overall 0–20 years | 172 | 12 | 93.0 | |
| Group | ||||
| PHP | 39 | 2 | 94.9 | |
| Adherent to SPC | 31 | 0 | 100.0 | .06 |
| Non‐adherent to SPC | 8 | 2 | 75.0 | |
| mPCP | 61 | 5 | 91.8 | |
| Adherent to SPC | 34 | 1 | 97.1 | .64 |
| Non‐adherent to SPC | 27 | 4 | 85.2 | |
| sPCP | 72 | 5 | 93.1 | |
| Adherent to SPC | 52 | 3 | 94.2 | .49 |
| Non‐adherent to SPC | 20 | 2 | 90.0 | |
| Statistical difference between | ||||
| All groups | p = .29 | |||
| Overall 10–20 years | 169 | 9 | 94.7 | |
| Group | ||||
| PHP | 39 | 2 | 94.9 | |
| Adherent to SPC | 31 | 0 | 100.0 | .06 |
| Non‐adherent to SPC | 8 | 2 | 75.0 | |
| mPCP | 59 | 3 | 94.9 | |
| Adherent to SPC | 34 | 1 | 97.1 | .65 |
| Non‐adherent to SPC | 25 | 2 | 92.0 | |
| sPCP | 71 | 4 | 94.4 | |
| Adherent to SPC | 51 | 2 | 96.1 | .59 |
| Non‐adherent to SPC | 20 | 2 | 90.0 | |
| Statistical difference between | ||||
| All groups | p = .57 | |||
Abbreviations: 95% CI, 95% confidence interval; mPCP, moderate periodontally compromised patients; PHP, periodontally healthy patients; SPC, supportive periodontal care; sPCP, severe periodontally compromised patients.
3.5. Supportive periodontal care
In the mPCP and sPCP groups, FMPS and FMBS were statistically significantly lower in compliant vs non‐compliant subjects (p < .05; Table S2). Moreover, the number of implants with at least one site with PD ≥6 mm at 20 years was statistically significantly higher in non‐compliant compared with compliant subjects in the PHP (80% vs. 23.53%, p = .04 respectively) and sPCP groups (100% vs. 41.66%, p = .04 respectively), even though not statistically significantly higher in the mPCP group (p = .08; Table 5). After 20 years, a trend towards a higher survival rate in compliant versus non‐compliant subjects was observed, even though it did not reach statistical significance (p > .05; Table 4).
TABLE 5.
Clinical parameters at the 20‐year follow‐up in relation to adhesion to supportive periodontal therapy (SPC) in the three groups
| Adhesion to SPC | Number of patients | PI (%) 20 years | BoP (%) 20 years | Deepest PD (mm) 20 years | Teeth lost during SPC (10–20 years) | No. patients treated with CIST C/D (10 years) | No. patients treated with CIST C/D (20 years) | Implants with at least a site with deepest PD ≥6 mm at 10 years | Implants with at least a site with deepest PD ≥6 mm at 20 years | |
|---|---|---|---|---|---|---|---|---|---|---|
| PHP | No | 5 (22.7%) | 33.3 ± 25.8 | 45.8 ± 29.3 | 4.2 ± 2.6 | 0.8 ± 0.4 | 1 (20%) | 2 (40%) | 2 (40%) | 4 (80%) |
| Yes | 17 (77.3%) | 13.8 ± 17.0 | 21.0 ± 18.4 | 3.9 ± 0.9 | 0.8 ± 0.3 | 4 (23.5%) | 5 (29.4%) | 3 (17.7%) | 4 (23.5%) | |
| p‐value | .02 | .01 | .74 | .75 | .69 | .41 | .27 | .04 | ||
| mPCP | No | 13 (44.8%) | 41.3 ± 30.8 | 41.3 ± 27.8 | 4.7 ± 1.5 | 0.9 ± 0.3 | 4 (30.8%) | 8 (61.5%) | 11 (84.6%) | 10 (76.9%) |
| Yes | 16 (55.2%) | 20.5 ± 26 | 21.9 ± 26.3 | 4.1 ± 1.4 | 1.0 ± 0.2 | 9 (52.9%) | 6 (35.3%) | 5 (29.4%) | 6 (35.3%) | |
| p‐value | .01 | .01 | .11 | .22 | .28 | .14 | .02 | .08 | ||
| sPCP | No | 9 (27.3%) | 63.8 ± 26.0 | 56.8 ± 20.7 | 5.3 ± 1.0 | 1.1 ± 0.2 | 4 (44.4%) | 3 (33.3%) | 9 (100%) | 9 (100%) |
| Yes | 24 (72.7%) | 11.7 ± 17.0 | 20.4 ± 22.1 | 4.1 ± 1.3 | 1.0 ± 0.2 | 15 (62.5%) | 16 (66.7%) | 16 (66.7%) | 10 (41.7%) | |
| p‐value | <.01 | <.01 | <.01 | .04 | .44 | .38 | .04 | .04 |
Note: Continuous variables are expressed as mean ± standard deviation, categorical variables are expressed as number of observations (percentage). The level of significance was set at 5%. Intergroup comparisons (adherent vs. non‐adherent) were performed using the Student's t‐test for independent samples (Mann–Whitney U test for non‐normal data). Italic values show p < .05. Number of patients with implants with at least a site with deepest PD >=6 mm at 10‐year.
Abbreviations: 95% CI, 95% confidence interval; CIST, cumulative interceptive therapy; mPCP, moderate periodontally compromised patients; PHP, periodontally healthy patients; SPC, supportive periodontal care; sPCP, severe periodontally compromised patients.
3.6. Logistic regression models
Results of the crude and adjusted OR for implant loss are reported in Table 6. At 10 years, non‐compliant subjects and those in the PCP groups had approximately five times higher odds of implant loss compared with compliant subjects and those in the PHP group, respectively (OR = 5.63, 95% CI 1.31–70.42, p = .04; OR = 4.26, 95% CI 1.30–41.48, p = .03). At 20 years, the odds of implant loss were almost eight times higher in subjects non‐compliant with SPC compared with compliant subjects, irrespective of their periodontal status at baseline (OR = 7.65, 95% CI 1.48–39.38, p = .01). At 20 years, PCP compliant with SPC did not present with statistically significantly higher odds for implant loss compared with PHP compliant with SPC (OR = 2.18, 95% CI 0.14–34.59, p = .58; Table 6). Moreover, in non‐adherent subjects, the odds for implant loss were 8.55 (95% CI 0.51–142.07, p = .13) in PHP, while they almost doubled in non‐adherent PCP (OR = 14.59, 95% CI 1.30–164.29, p = .03), when compared with adherent PHP (reference category).
TABLE 6.
Odds ratios (ORs) for implant loss at 10 and 20 years in relation to SPC adherence, smoking status and group
| Variable | ORs for implant loss | |||||||
|---|---|---|---|---|---|---|---|---|
| Crude ORs | 95% CI | p‐value* | Adjusted a ORs | 95% CI | p‐value* | |||
| Lower | Upper | Lower | Upper | |||||
| 10‐year follow‐up | ||||||||
| Adherence to SPC | ||||||||
| Yes | REF. | .02 | REF. | .04 | ||||
| No | 4.46 | 1.08 | 50.31 | 5.63 | 1.31 | 70.42 | ||
| Smoking | ||||||||
| No | REF. | .23 | REF. | .71 | ||||
| Yes | 1.13 | 0.92 | 1.38 | 1.05 | 0.81 | 1.37 | ||
| Group | ||||||||
| PHP | REF. | .32 | REF. | .03 | ||||
| PCP | 2.72 | 0.37 | 19.72 | 4.26 | 1.30 | 41.48 | ||
| 20‐year follow‐up | ||||||||
| Adherence to SPC | ||||||||
| Yes | REF. | .03 | REF. | .01 | ||||
| No | 4.81 | 1.15 | 20.03 | 7.65 | 1.48 | 39.38 | ||
| Smoking | ||||||||
| No | REF. | .79 | REF. | .80 | ||||
| Yes | 1.03 | 0.85 | 1.24 | 3.03 | 0.82 | 5.30 | ||
| Group | ||||||||
| PHP | REF. | .95 | REF. | .53 | ||||
| PCP | 1.05 | 0.21 | 5.29 | 4.49 | 0.20 | 7.25 | ||
| Combination of SPC adherence and periodontal status | ||||||||
| Adherent, PHP | REF. | REF. | ||||||
| Adherent, PCP | 2.61 | 0.23 | 29.7 | .44 | 2.18 | 0.14 | 34.59 | .58 |
| Non‐adherent, PHP | 7.11 | 0.61 | 82.96 | .12 | 8.55 | 0.51 | 142.07 | .13 |
| Non‐adherent, PCP | 8.83 | 0.94 | 82.49 | .06 | 14.59 | 1.30 | 164.29 | .03 |
Note : p‐values written in italic refer to significant crude estimates. p‐values written in bold italic refer to significant adjusted estimates. Level of significance set at α = .05.
Abbreviations: 95% CI, 95% confidence interval; mPCP, moderate periodontally compromised patients; PHP, periodontally healthy patients; SPC, supportive periodontal care; sPCP, severe periodontally compromised patients.
Multilevel logistic regression model with implant loss at either 10 or 20 years follow‐up. Adjustments were made for age, teeth missing at baseline, baseline FMPS and FMBS.
4. DISCUSSION
Since the publication of the data from the first 10‐year analysis (Roccuzzo et al., 2014), long‐term results of implant therapy in patients with a history of periodontitis have received significant attention. Several studies, many of them with a retrospective or cross‐sectional design, have been published on this topic over the recent years (Degidi et al., 2016; Smith et al., 2017; Graetz et al., 2018).
Recently, a systematic review (Carra et al., 2021) investigated the effectiveness of implant‐supported fixed partial denture (IS‐FPD) in patients with history of periodontitis (HP) versus patients with no history of periodontitis (NHP). Seventeen articles (7 prospective and 10 retrospective) were selected, including the one reporting on the 10‐year data of the population of the present study (Roccuzzo et al., 2014). Pooled data analyses showed that overall implant survival was significantly higher in the NHP than in the HP group. This difference was noted when follow‐up period exceeded 5 years. The risk of peri‐implantitis was higher in HP than NHP patients, whereas the mean marginal bone‐level change over time was not different between the groups. The authors concluded that in partially edentulous patients receiving IS‐FPDs, an HP is associated with lower survival rate and higher risk of peri‐implantitis during a 5–10‐year period after implant loading (Carra et al., 2021).
The present investigation is, to the best of our knowledge, the first and only prospective study reporting on the 20‐year results of dental implant treatment performed on a relatively large number of patients, recruited from a specialist periodontal private clinic. The few other available studies, with data up to 20 years, reported on dental implants not commercially available anymore, hence with a limited external validity (Chappuis et al., 2013; Donati et al., 2018; Jacobs et al., 2021). On the other hand, the present dataset included SLA tissue‐level implants, which are nowadays widely used, providing unique evidence.
During the last three decades, the number of dental implants placed every year has increased dramatically (Misch, 2020) mainly due to the misleading concept among clinicians that the prognosis of complex periodontal therapy may not compare favourably with the high levels of success of treatment with implants (Rasperini et al., 2014; Lang, 2019). Consequently, more and more teeth are extracted on the assumption that implants perform better than periodontally compromised teeth and that their longevity is not affected by the individual's susceptibility to periodontitis (Lundgren et al., 2008).
The results of this investigation indicate that during 20 years of SPC, the mean number of teeth lost per patient, regardless of the reason for extraction, was 0.6 ± 1.0 for PHP, 1.3 ± 1.3 for moderate PCP and 1.9 ± 1.9 for severe PCP, with a statistically significant difference between PHP and either mPCP and sPCP (p < .001). These results are comparable with those reported in a 30‐year follow‐up study by Axelsson et al. (2004), indicating that in both of these unique cohorts of well‐maintained patients the mean number of tooth loss per patient was low.
Overall, these results confirm that PCP patients rehabilitated with dental implants but not compliant with an individualized SPC programme tend to develop more biological complications and should not be treated on the assumption that implants perform better than natural teeth.
These conclusions are similar to those reported by Pjetursson and co‐workers (Pjetursson et al., 2012) on 70 patients with a follow‐up ranging between 3 and 23 years (mean 7.9 years). The authors reported that the prevalence of peri‐implantitis was lower in the group enrolled in a well‐organized SPC programme at the University. Conversely, the current study presents excellent results in terms of overall compliance for patients enrolled in an individually tailored SPC programme in a private specialist setting (Figure 1a–f).
FIGURE 1.
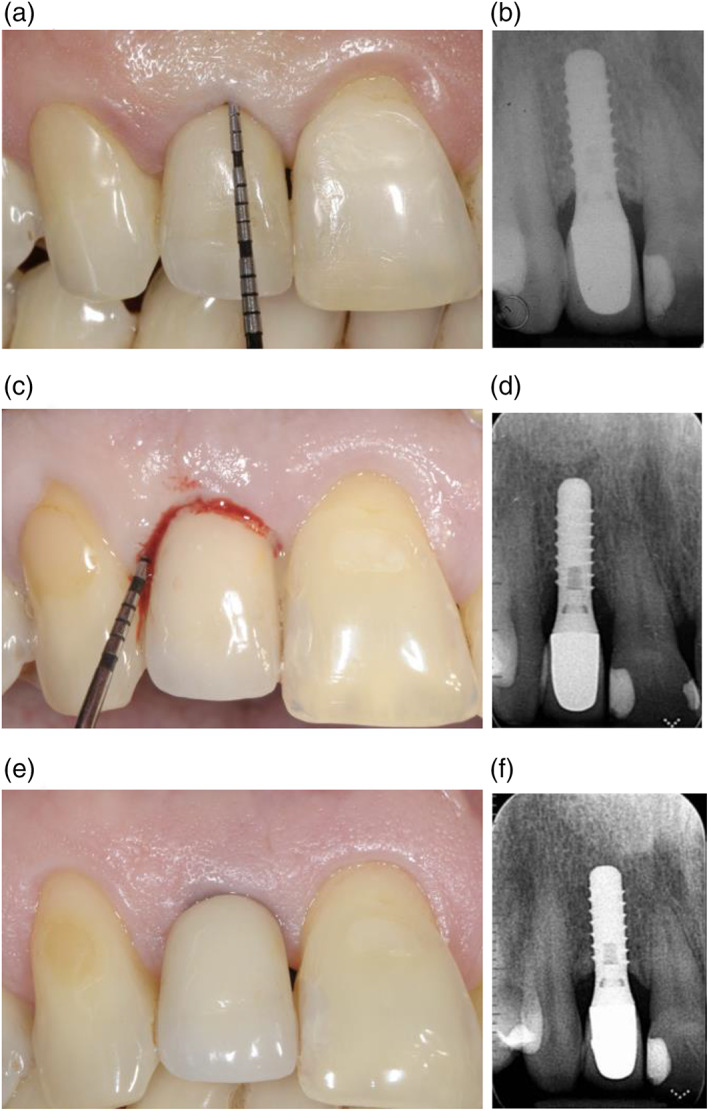
Clinical and radiographic images of an implant placed in April 2001 in an “adherent patient” (a,b); in May 2017, the site exhibited clinical signs of inflammation, bleeding on probing, increased probing depth and radiographic bone loss (c,d); clinical and radiographic images at the 20‐year follow‐up, following surgical treatment of the biological complication (e‐f)
Mir‐Mari et al. (2012) estimated the prevalence of peri‐implantitis in private practice patients, enrolled in a periodontal maintenance programme, between 12% and 22%, similar to those published in University environment samples. Nevertheless, the importance of SPC must be stressed, regardless of the fact that it is delivered in a public or private setting. In the present study, 26 of 149 (17.4%) patients were lost to follow‐up and only 16 of these (10.7%) refused the visit for various personal reasons. These values should be considered positively based on the long follow‐up period and they are somehow similar to those reported by Cardaropoli and Gaveglio (2012). Furthermore, it must be pointed out that regardless of the number of visits per year, not every patient accepted the proposed additional treatment. Therefore, patients who attended the SPC appointment, but did not accept the proposed additional treatment, were classified as a “not‐adherent”. This concept has been previously elaborated by Monje and co‐workers who, even though recommended a minimum recall interval of 5–6 months to prevent the onset of peri‐implant diseases, pointed out how SPC frequency must be tailored to the patient's risk profile (Monje et al., 2016).
The overall quality of SPC in the present investigation can be confirmed by the significant continuous reduction of the FMPS and FMBS values both at the 10‐year and 20‐year follow‐ups. These changes are more pronounced in patients compliant with SPC compared with the ones not compliant with SPC. Ideally, patients undergoing a successful SPC should have similar low plaque scores regardless of the history for periodontitis. In these groups of patients, the 20‐year FMPS, before the session of professional maintenance, were around the 25% threshold, that is, 19.4 ± 10.1% (PHP) versus 26 ± 14.5% (mPCP) versus 23.3 ± 17.3% (sPCP) with no difference among the groups.
During the entire 20‐year follow‐up period, only 12 (7%) implants had to be removed: 11 due to peri‐implantitis, while one implant with a 3.3 diameter supporting an SUC with a cantilever extension in the premolar area was lost due to fracture. This rare event has been also reported by Schmid et al. (2020) on implants with identical macro‐ and micro‐design characteristics.
Consequently, the calculated overall survival rate (i.e., 93%) is similar to those reported in recent publications with such long‐term follow‐ups (Donati et al., 2018; Jacobs et al., 2021) even though it has to be underlined that such survival rates were obtained from selected cohorts with smaller sample sizes (i.e., 32 and 10 patients, respectively).
Finally, adjunctive antibiotic delivery and/or surgical therapy were performed in 22.7% of cases in PHP, in 43.3% of cases in mPCP and in 57.6% of cases in sPCP. In other words, in order to have an elevated long‐term survival rate, it is mandatory to monitor patients frequently, especially those who lost teeth due to periodontal disease, and to organize and promptly provide cumulative non‐surgical and surgical treatments, whenever needed. Therefore, implant therapy cannot be simply proposed as “definitive”, but should be considered only as an important step in the comprehensive long‐term treatment plan of patients.
The present study retains several limitations. First, the number of dropouts (31.7%) might have impacted on the final analysis, even though it has to be underlined that this value is much lower than that reported in another 20‐year publication (i.e., 51%; Donati et al., 2018). Indeed, the reduction in the original sample due to dropouts may have lowered the power of detecting significant differences across groups. However, it should be emphasized that, even though statistically significant differences were highlighted for some variables, some estimates retain very large CIs, possibly due to the suboptimal power achieved at the 20‐year examination. Nonetheless, it should be underlined that 16 of 123 patients dropped out of the study because of death, most likely related to the elevated mean age of the present cohort. Moreover, the dropout analysis demonstrated comparable loss to follow‐ups across subgroups, hence reducing the possible risk of attrition bias. Secondly, with respect to the smoking status, it should be pointed out that patients' self‐reported data on their habit remain questionable. In addition, smoking status was assessed only at implant placement, and hence it cannot be excluded that during the observation period patients' habits might have changed, thus affecting periodontal/peri‐implant conditions (Scott et al., 2001). Thirdly, PCPs were arbitrarily divided into two groups (i.e., moderate and severe) on the basis of the number and depth of periodontal pockets at baseline examination. It is also worth mentioning that the actual classification of periodontitis was proposed and adopted in the context of the 2017 World Workshop on the Classification of Periodontal and Peri‐Implant Diseases and Conditions (Tonetti et al., 2018), more than two decades after initiation of the present study. For the same reason, the CIST protocol, even though not common in use, was chosen to record and treat biological complications because at the beginning of the study (i.e., 1998) this was the only accepted available protocol. Finally, since all implants were placed by the same experienced periodontist in a specialist private practice, the generalizability of the obtained data to a population‐based setting is difficult and does preclude from external validity (Walton & Layton, 2018).
Derks and co‐workers (Derks et al., 2016), when analysing the effectiveness of implant therapy in a Swedish population sample, demonstrated higher implant loss among smokers and patients with an initial diagnosis of periodontitis, in accordance with the results of the present investigation. Moreover, the multilevel analysis revealed lower OR for loss of tissue‐level implants, which are of the same type employed in the present study. According to these findings, the question of which implant surface and surgical protocol should be considered ideal for the treatment of PCP remains open.
5. CONCLUSIONS
PHP who, after adequate implant therapy, comply with a tailored SPC program experience fewer biological complications than patients with an HP, in the long‐term (i.e., 20 years).
Considering the low number of teeth lost, the approach for strategic tooth extractions and replacement with dental implants, based on the assumption that implants perform better than teeth, is not scientifically supported.
Excellent values of long‐term survival rate can be obtained even in PCP, if SPC is associated with a continuous evaluation of the risk for biological complications.
PCP, even though enrolled in an adequate long‐term SPC programme, may need additional non‐surgical and surgical therapies for the treatment of biological complications.
AUTHOR CONTRIBUTIONS
Andrea Roccuzzo collected, analysed the data and led to the writing; Crystal Marruganti performed the statistical analysis and contributed to the writing; Jean‐Claude Imber and Giovanni E. Salvi analysed the data and contributed to the writing; Guglielmo Ramieri critically revised the manuscript; Mario Roccuzzo conceived the idea, performed the surgeries and critically revised the manuscript.
FUNDING INFORMATION
Andrea Roccuzzo was the recipient of a 3‐year scholarship from the Clinical Research Foundation (CFR) for the Promotion of Oral Health, Brienz, Switzerland. Andrea Roccuzzo is the recipient of a 1‐year scholarship from the International Team for Implantology (ITI). The study was self‐funded; no external funding was available for this research.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Supporting information
Table S1 Number of dental implants and patients dropped out for each study group.
Table S2. Clinical parameters at patient level at the 20‐year examination.
Table S3. Cumulative incidence of biological complications at implant level at the 10‐ and 20‐year examination.
ACKNOWLEDGEMENTS
The authors thank Ms Giulia Fontana and Dr Dario Pittoni for their valuable help in data collection. Open access funding provided by Universitat Bern.
Roccuzzo, A. , Imber, J.‐C. , Marruganti, C. , Salvi, G. E. , Ramieri, G. , & Roccuzzo, M. (2022). Clinical outcomes of dental implants in patients with and without history of periodontitis: A 20‐year prospective study. Journal of Clinical Periodontology, 49(12), 1346–1356. 10.1111/jcpe.13716
Funding information The study was self‐funded; no external funding was available for this research.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Armitage, G. C. (1999). Development of a classification system for periodontal diseases and conditions. Annals of Periodontology, 4(1), 1–6. 10.1902/annals.1999.4.1.1 [DOI] [PubMed] [Google Scholar]
- Astrand, P. , Ahlqvist, J. , Gunne, J. , & Nilson, H. (2008). Implant treatment of patients with edentulous jaws: A 20‐year follow‐up. Clinical Implant Dentistry and Related Research, 10(4), 207–217. 10.1111/j.1708-8208.2007.00081.x [DOI] [PubMed] [Google Scholar]
- Axelsson, P. , Nystrom, B. , & Lindhe, J. (2004). The long‐term effect of a plaque control program on tooth mortality, caries and periodontal disease in adults. Results after 30 years of maintenance. Journal of Clinical Periodontology, 31(9), 749–757. 10.1111/j.1600-051X.2004.00563.x [DOI] [PubMed] [Google Scholar]
- Buser, D. , Sennerby, L. , & De Bruyn, H. (2017). Modern implant dentistry based on osseointegration: 50 years of progress, current trends and open questions. Periodontology 2000, 73(1), 7–21. 10.1111/prd.12185 [DOI] [PubMed] [Google Scholar]
- Buser, D. , von Arx, T. , ten Bruggenkate, C. , & Weingart, D. (2000). Basic surgical principles with ITI implants. Clinical Oral Implants Research, 11(Suppl 1), 59–68. 10.1034/j.1600-0501.2000.011s1059.x [DOI] [PubMed] [Google Scholar]
- Cardaropoli, D. , & Gaveglio, L. (2012). Supportive periodontal therapy and dental implants: An analysis of patients' compliance. Clinical Oral Implants Research, 23(12), 1385–1388. 10.1111/j.1600-0501.2011.02316.x [DOI] [PubMed] [Google Scholar]
- Carra, M. C. , Range, H. , Swerts, P. J. , Tuand, K. , Vandamme, K. , & Bouchard, P. (2022). Effectiveness of implant‐supported fixed partial denture in patients with history of periodontitis: A systematic review and meta‐analysis. Journal of Clinical Periodontology, 49(Suppl 24):208–223. 10.1111/jcpe.13481 [DOI] [PubMed] [Google Scholar]
- Chappuis, V. , Buser, R. , Bragger, U. , Bornstein, M. M. , Salvi, G. E. , & Buser, D. (2013). Long‐term outcomes of dental implants with a titanium plasma‐sprayed surface: A 20‐year prospective case series study in partially edentulous patients. Clinical Implant Dentistry and Related Research, 15(6), 780–790. 10.1111/cid.12056 [DOI] [PubMed] [Google Scholar]
- Degidi, M. , Nardi, D. , & Piattelli, A. (2016). 10‐year prospective cohort follow‐up of immediately restored XiVE implants. Clinical Oral Implants Research, 27(6), 694–700. 10.1111/clr.12642 [DOI] [PubMed] [Google Scholar]
- Derks, J. , Schaller, D. , Hakansson, J. , Wennstrom, J. L. , Tomasi, C. , & Berglundh, T. (2016). Effectiveness of implant therapy analyzed in a Swedish population: Prevalence of peri‐implantitis. Journal of Dental Research, 95(1), 43–49. 10.1177/0022034515608832 [DOI] [PubMed] [Google Scholar]
- Donati, M. , Ekestubbe, A. , Lindhe, J. , & Wennstrom, J. L. (2018). Marginal bone loss at implants with different surface characteristics—A 20‐year follow‐up of a randomized controlled clinical trial. Clinical Oral Implants Research, 29(5), 480–487. 10.1111/clr.13145 [DOI] [PubMed] [Google Scholar]
- Duong, H. Y., Roccuzzo, A., Stähli, A., Salvi, G. E., Lang, N. P., & Sculean, A. (2022). Oral health‐ related quality of life of patients rehabilitated with fixed and removable implant‐supported dental prostheses. Periodontol 2000, 88(1), 201–237. 10.1111/prd.12419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graetz, C. , El‐Sayed, K. F. , Geiken, A. , Plaumann, A. , Salzer, S. , Behrens, E. , Wiltfang, J. , & Dorfer, C. E. (2018). Effect of periodontitis history on implant success: A long‐term evaluation during supportive periodontal therapy in a university setting. Clinical Oral Investigations, 22(1), 235–244. 10.1007/s00784-017-2104-4 [DOI] [PubMed] [Google Scholar]
- Howe, M. S. , Keys, W. , & Richards, D. (2019). Long‐term (10‐year) dental implant survival: A systematic review and sensitivity meta‐analysis. Journal of Dentistry, 84, 9–21. 10.1016/j.jdent.2019.03.008 [DOI] [PubMed] [Google Scholar]
- Jacobs, R. , Gu, Y. , Quirynen, M. , De Mars, G. , Dekeyser, C. , van Steenberghe, D. , Vrombaut, D. , Shujaat, S. , & Naert, I. (2021). A 20‐year split‐mouth comparative study of two screw‐shaped titanium implant systems. Int J Oral Implantol, 14(4), 421–430. [PubMed] [Google Scholar]
- Lang, N. P. (2019). Oral implants: The paradigm shift in restorative dentistry. Journal of Dental Research, 98(12), 1287–1293. 10.1177/0022034519853574 [DOI] [PubMed] [Google Scholar]
- Lang, N. P. , Mombelli, A. , Tonetti, M. S. , Bragger, U. , & Hammerle, C. H. (1997). Clinical trials on therapies for peri‐implant infections. Annals of Periodontology, 2(1), 343–356. 10.1902/annals.1997.2.1.343 [DOI] [PubMed] [Google Scholar]
- Lundgren, D. , Rylander, H. , & Laurell, L. (2008). To save or to extract, that is the question. Natural teeth or dental implants in periodontitis‐susceptible patients: clinical decision‐making and treatment strategies exemplified with patient case presentations. Periodontology 2000, 47(1), 27–50. 10.1111/j.1600-0757.2007.00239.x [DOI] [PubMed] [Google Scholar]
- Mengel, R. , Wendt, J. , & Peleska, B. (2019). Prosthodontic treatment outcomes in periodontally compromised patients: A 6‐ to 20‐year long‐term cohort study. The International Journal of Prosthodontics, 32(2), 153–161. 10.11607/ijp.5917 [DOI] [PubMed] [Google Scholar]
- Mir‐Mari, J. , Mir‐Orfila, P. , Figueiredo, R. , Valmaseda‐Castellon, E. , & Gay‐Escoda, C. (2012). Prevalence of peri‐implant diseases. A cross‐sectional study based on a private practice environment. Journal of Clinical Periodontology, 39(5), 490–494. 10.1111/j.1600-051X.2012.01872.x [DOI] [PubMed] [Google Scholar]
- Misch, C. M. (2020). Editorial: The global dental implant market: Everything has a price. Int J Oral Implantol, 13(4), 311–312. [PubMed] [Google Scholar]
- Mombelli, A. , & Lang, N. P. (1998). The diagnosis and treatment of peri‐implantitis. Periodontology 2000, 2000(17), 63–76. 10.1111/j.1600-0757.1998.tb00124.x [DOI] [PubMed] [Google Scholar]
- Monje, A. , Aranda, L. , Diaz, K. T. , Alarcon, M. A. , Bagramian, R. A. , Wang, H. L. , & Catena, A. (2016). Impact of maintenance therapy for the prevention of peri‐implant diseases: A systematic review and meta‐analysis. Journal of Dental Research, 95(4), 372–379. 10.1177/0022034515622432 [DOI] [PubMed] [Google Scholar]
- Pjetursson, B. E. , Helbling, C. , Weber, H. P. , Matuliene, G. , Salvi, G. E. , Bragger, U. , Schmidlin, K. , Zwahlen, M. , & Lang, N. P. (2012). Peri‐implantitis susceptibility as it relates to periodontal therapy and supportive care. Clinical Oral Implants Research, 23(7), 888–894. 10.1111/j.1600-0501.2012.02474.x [DOI] [PubMed] [Google Scholar]
- Rasperini, G. , Siciliano, V. I. , Cafiero, C. , Salvi, G. E. , Blasi, A. , & Aglietta, M. (2014). Crestal bone changes at teeth and implants in periodontally healthy and periodontally compromised patients. A 10‐year comparative case‐series study. Journal of Periodontology, 85(6), e152–e159. 10.1902/jop.2013.130415 [DOI] [PubMed] [Google Scholar]
- Roccuzzo, M. , Bonino, L. , Dalmasso, P. , & Aglietta, M. (2014). Long‐term results of a three arms prospective cohort study on implants in periodontally compromised patients: 10‐year data around sandblasted and acid‐etched (SLA) surface. Clinical Oral Implants Research, 25(10), 1105–1112. 10.1111/clr.12227 [DOI] [PubMed] [Google Scholar]
- Schmid, E. , Morandini, M. , Roccuzzo, A. , Ramseier, C. A. , Sculean, A. , & Salvi, G. E. (2020). Clinical and radiographic outcomes of implant‐supported fixed dental prostheses with cantilever extension. A retrospective cohort study with a follow‐up of at least 10 years. Clinical Oral Implants Research, 31(12), 1243–1252. 10.1111/clr.13672 [DOI] [PubMed] [Google Scholar]
- Scott, D. A. , Palmer, R. M. , & Stapleton, J. A. (2001). Validation of smoking status in clinical research into inflammatory periodontal disease. Journal of Clinical Periodontology, 28(8), 715–722. 10.1034/j.1600-051x.2001.280801.x [DOI] [PubMed] [Google Scholar]
- Smith, M. M. , Knight, E. T. , Al‐Harthi, L. , & Leichter, J. W. (2017). Chronic periodontitis and implant dentistry. Periodontology 2000, 74(1), 63–73. 10.1111/prd.12190 [DOI] [PubMed] [Google Scholar]
- Tonetti, M. S. , Greenwell, H. , & Kornman, K. S. (2018). Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. Journal of Clinical Periodontology, 45(Suppl 20), S149–S161. 10.1111/jcpe.12945 [DOI] [PubMed] [Google Scholar]
- Walton, T. R. , & Layton, D. M. (2018). Intra‐ and inter‐examiner agreement when assessing radiographic implant bone levels: Differences related to brightness, accuracy, participant demographics and implant characteristics. Clinical Oral Implants Research, 29(7), 756–771. 10.1111/clr.13290 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Number of dental implants and patients dropped out for each study group.
Table S2. Clinical parameters at patient level at the 20‐year examination.
Table S3. Cumulative incidence of biological complications at implant level at the 10‐ and 20‐year examination.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


