Abstract
Cognitive impairment has been associated with anemia and iron deficiency; however, brain electrophysiological studies correlating red blood cell (RBC) indices and iron status to cognition in adulthood are scarce. We aimed to assess neurocognitive function in young adults of the general population and its correlation with RBC indices and iron status. Neurocognitive function was investigated using scalp‐recorded event‐related potentials (ERPs) within the context of a task‐switching paradigm. ERPs and test performance were also compared across groups of “high”/“low” RBC and iron indices. Working memory was examined using the digit span test, in which mean corpuscular hemoglobin (MCH), mean corpuscular volume (MCV), and ferritin were found to be significant predictors of test performance, with higher MCH/MCV/ferritin being associated with better test scores. In the switching task, MCH, MCV, and ferritin were found to be significant predictors of task performance, with higher MCH/MCV/ferritin levels associated with a lower percentage of errors. Electrophysiological results showed that MCH and MCV were significant predictors of ERPs amplitude, with lower MCH/MCV levels associated with greater amplitude, which may reflect compensatory processes. P1, N1, P2, and P3 were greater for the low MCH/MCV groups. This is the first evidence of association between levels of MCH/MCV and brain function while engaged in an executive function task; possibly reflecting brain iron availability.
Keywords: cognitive function, event‐related potentials, iron status, red blood cell indices
Cognitive impairment has been associated with anemia and iron deficiency; however, brain electrophysiological studies correlating red blood cell (RBC) indices and iron status to cognition in adulthood are scarce. We aimed to assess neurocognitive function in young adults of the general population and its correlation with RBC indices and iron status. Neurocognitive function was investigated using scalp‐recorded event‐related potentials (ERP) within the context of a task‐switching paradigm.
INTRODUCTION
Anemia and iron deficiency (ID) are major public health problems affecting populations in developing and developed countries, with great implications for quality of life. ID is the top‐ranking cause of anemia and is more prevalent in young children and childbearing age women; but can also occur in young males. 1 Other causes of anemia include anemia due to chronic disease; anemia with normal iron status such as caused by hemodilution, typically seen among athletes; and anemia observed in carriers of inherited hemoglobinopathies, for example, β‐thalassemia carriers. 2
The prevalence of anemia, ID, and iron deficiency anemia (IDA) among young adults varies among different countries and populations. Several studies performed on young healthy Israeli adults (military recruits) reported a significant incidence of ID (10.2–31% in males and 15–61% in females), anemia (7.7–28% in males and 18–24% in females), and IDA (1.8–9% in males and 10–16.3% in females). 3 , 4 , 5 , 6 In Israel, screening and iron supplementation for the prevention of ID and IDA is only universally applied to infants 7 and recommended in pregnant women. The high prevalence of ID and IDA among young adults emphasizes the need for detection, prevention, and treatment in this age group as well. 8
ID and IDA have been reported to decrease cognitive performance and to delay mental and motor development in infancy and early childhood, principally during the period of brain growth, with possible long‐term effects. 9 , 10 , 11 , 12 , 13 , 14 While most evidence comes from very young children, it has been suggested that hemoglobin (HB) and iron status play an important role during nondevelopmental periods of life as well. Relatively few studies have focused on the effect of anemia, IDA, and ID on cognitive function in young adulthood. 15 , 16 , 17 , 18 Cognitive function has been studied mainly in young women and it was found that IDA and ID are key factors in cognitive performance and that iron supplementation and naturally iron‐rich food improves certain aspects of cognition. 17 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28
HB levels and iron status may affect brain function; however, very little is known about the possible neural mechanisms that underlie and/or mediate ID‐related and anemia‐related cognitive deficits. Scalp‐recorded event‐related potentials (ERPs) provide a noninvasive means for recording, analyzing, and characterizing the dynamic electrophysiological activity of the living brain with an excellent temporal resolution, and have great value in studying the relations between cognitive function and neural processes in various populations. ERP studies in the field of ID, anemia, and cognition are scarce; thus, further investigation in this area is required. To our knowledge, only three studies have evaluated cognitive function and brain ERPs in otherwise healthy young adults with IDA. 21 , 29 , 30 The study by Khedr et al. 21 included 28 patients with IDA and 25 controls. Compared to controls, patients demonstrated lower scores on different cognitive tests and showed significant improvement after treatment. Prolongation of ERPs’ latencies (N200 and P300) and reduction in their amplitudes (P200 and P300) were identified, with a significant increase in amplitude that occurred after treatment. The study of Kececi and Degirmenci 29 included 51 patients with IDA, who were tested before and after oral iron therapy. After therapy, central N1 amplitude and parietal P2 amplitude were increased. N2 latencies were shortened in frontal and central regions. P3 latencies were shortened in frontal, central, and parietal areas and P3 amplitude was increased in the parietal region. Both these studies used auditory stimuli in the context of a relatively simple oddball task. In the study by Wenger et al., 30 27 young women consumed iron‐biofortified beans and 28 consumed comparison beans daily for 18 weeks. They found improvement in several cognitive tasks, accompanied by ERP (P1, N1, and P2) positive changes in the treated group compared with the control group.
We recently demonstrated impairments in cognitive function and ERP alterations associated with the HB levels in adult patients with β‐thalassemia major (β‐TM; in β‐TM patients, the anemia is caused by inefficient erythropoiesis with concomitant iron overload, and they are treated with blood transfusion and iron chelation). β‐TM patients had significantly lower total performance IQ scores compared with healthy controls. Significant differences were also found in continuous performance test scores as a function of blood transfusion. Before transfusion, while they are more anemic, patients had higher rates of errors and slower response times (RTs). 31 In another study, results indicated the poorer executive function of β‐TM patients (task‐switching paradigm). HB levels were negatively correlated with error rates and RTs. Electrophysiological results indicated significant alterations in peak amplitudes of the ERP components P1, N1, and P2 in β‐TM patients relative to controls; and negative correlations were found between HB levels and P2 amplitude. 32 In yet another study, we examined attention and response inhibition function (using a stop‐signal task). Results showed impaired cognitive performance in β‐TM patients and HB was negatively correlated with RTs. Electrophysiological results indicated significant β‐TM–related alterations in neuronal activity, reflected in greater peak amplitudes of P1, N1, and P3. Negative correlations were found between levels of HB and the amplitude of all ERP components; the lower the HB, the more pronounced the ERP amplitude. 33
We hypothesize that cognitive function is affected by HB levels and iron status not only in pathological states/clinical populations but also in the general asymptomatic population. Reduced HB and iron availability are expected to negatively affect cognitive function and alter ERP patterns. The present study aimed to assess neurocognitive function in young adults and its correlation with red blood cell (RBC) indices and iron status. We investigated neural correlates of cognitive executive function using scalp‐recorded ERPs and a rather complex visual task‐switching paradigm; in which participants had to quickly and effectively switch between two different task sets. Correlations between ERPs and test performance (error rates and RTs) and various RBC indices (e.g., HB, mean corpuscular hemoglobin [MCH], mean corpuscular volume [MCV], and red cell distribution width [RDW]), and iron status (e.g., serum iron, transferrin, and ferritin) were examined. In addition, ERP amplitudes and test performance were compared across groups of high versus low RBC and iron indices. Working memory function was also examined, using Wechsler's digit span test.
METHODS
Participants
The sample included 108 first‐grade undergraduate students, native Hebrew speakers, recruited from Max Stern Yezreel‐Valley College and Tel‐Hai College (mean age: 24.8 ± 2.46 years; age range: 20–34 years). Thirty‐five were males (mean age: 26.05 ± 2.58 years; age range: 20–34 years) and 73 females (mean age: 24.18 ± 2.17 years; age range: 20–34 years). None of them had a prior history of physical, hematological, neurological, or psychiatric disorders. None of them were taking medications at the time of testing. Blood samples were taken from all participants, including complete blood count, serum iron, serum transferrin, and ferritin (Table 1). Within a week of the blood test, they arrived at the psychobiological laboratory for the experimental cognitive and ERP session. The experimenters who conducted the tests were blind to the results of the blood tests. Written informed consent was obtained from all participants and they were given course credit according to their academic requirements. The study was approved by the Emek Medical Center Helsinki committee (EMC 0017/71) and by the Yezreel Valley College and Tel‐Hai College Review Boards.
TABLE 1.
Red blood count and iron status characteristics of samples
| Measure |
Males (n = 35) Mean ± SD (Sample range; reference values) |
Females (n = 73) Mean ± SD (Sample range; reference values) |
|---|---|---|
| RBC (M/μl) |
4.99 ± 0.36 (4.28–5.69; 4.5–5.5) |
4.45 ± 0.27 (3.85–5; 4–5) |
| HB (g/dl) |
14.75 ± 1.04 (13.2–17; 14–17) |
12.86 ± 0.73 (10.2–14.4; 12–15) |
| HCT (%) |
43.75 ± 2.99 (38.4–50.8; 40–54) |
38.49 ± 2.21 (32.50–42.80; 37–47) |
| MCV (fl) |
87.74 ± 3.26 (81.9–95.8; 80–94) |
86.59 ± 4.10 (77.30–95.90; 80–95) |
| MCH (pg) |
29.57 ± 0.92 (27.8–31.2; 27–31) |
28.95 ± 1.45 (25–33.10; 27–31) |
| RDW (%) |
12.83 ± 0.47 (12.3–14.3; 11.5–14.5) |
12.86 ± 0.62 (11.9–15.1; 11.5–14.5) |
| Serum iron (μg/dl) |
108.52 ± 40.91 (43.1–210.2; 60–160) |
86.58 ± 36.58 (25–172; 40–145) |
| Serum transferrin (mg/dl) |
253.91 ± 26.76 (210–316; 200–360) |
300.57 ± 53.27 (211–446; 200–360) |
| Transferrin saturation (%) |
30.67 ± 11.63 (11.4–62.4; 20–50) |
22.07 ± 11.05 (4.9–54.3; 15–50) |
| Ferritin (ng/ml) |
101.25 ± 58.46 (23.9–201.3; 22–322) |
30.80 ± 20.13 (6.6–101.5; 10–291) |
Note: Of the 108 participants, 15 females met criteria for the diagnosis of iron deficiency (ID; ferritin < 15, Hb > 12) and three for iron deficiency anemia (IDA; ferritin < 15, Hb ≤ 12). None of the male participants met the criteria for ID or IDA (based on the 2020 WHO guideline on the use of ferritin concentrations to assess iron status in individuals and populations. https://www.who.int/publications/i/item/9789240000124).
Note: Reference values were taken from the hematology laboratory of Emek Medical Center, Israel.
Note: Based on participants’ ethnic origin and blood test results, none of them seemed to be a carrier of thalassemia.
Measures
Digit span test
A subtest taken from the Wechsler Adult Intelligence Scale – Third Edition (WAISIII) 34 was designed to evaluate working memory. A sequence of numbers is read out to the participant. The participant is then told to repeat the numbers that were read to them. This process continues until the participant can no longer remember either the full sequence of numbers or the correct order (maximum score = 16). In the reverse trial of the digit span test, the experimenter reads a series of numbers to the participant, and the participant is asked to repeat the sequence in the reverse order. This sequence is also continued until the participant makes an error (maximum score = 14). The scorer must add the total number of correct sequences, backward and forward (maximum score = 30).
Switching task
Digits between 1 and 9 (except for 5) were used as stimuli. The task consisted of 200 trials; in half of the trials, the digit appeared in black, and in the other half, it appeared in blue. In each trial, a single black or blue digit was presented at the center of a computer screen for 500 ms followed by a blank screen for an inter‐trial interval of 2000 ms. Participants had to switch between performing two tasks according to the color of the digit. In black‐digit trials, they had to indicate whether the digit is smaller/greater than 5 by pressing the left (smaller) or right (greater) button of a computer mouse. In blue‐digit trials, they had to indicate whether the digit is odd or even by pressing the left (odd) or right (even) button. Participants were instructed to respond as quickly as possible without compromising accuracy. The sequence of trials was unpredictable and controlled for an equal number of switch and non‐switch (repeat) trials. In non‐switch trials, the color of the concurrent stimulus was the same as in the previous trial, and in the switch trials, the color of the concurrent stimulus changed from the previous trial. RTs and error rates were recorded for the switch and non‐switch trials. The experiment was preceded by a short practice with each pure smaller/greater (eight trials) and odd/even (eight trials) task.
Electroencephalogram/ERP recording and data acquisition
During the ERP session, participants were comfortably seated in a dimly lit room at a distance of 80 cm from a 19'' computer screen. They were instructed to focus their gaze on the stimuli presented at the center of the screen and to refrain, as much as possible, from blinking or making eye movements during the session. The electroencephalogram (EEG) was recorded continuously using a 128‐channel HydroCel Geodesic Sensor Net, Net Amps 400 amplifier, and Net Station, Version 5.2 software (Electrical Geodesics Inc., Eugene, OR, USA) at 1000 Hz with 0.1 Hz high‐pass filtering. All channels were referenced to Cz during the acquisition. After the acquisition, during offline processing, the continuous EEG was referenced to an average reference, filtered with a 0.1–30 Hz bandpass filter and segmented by condition into 900 ms stimulus‐locked epochs, ranging from 100 ms prestimulus to 800 ms poststimulus. Epochs contaminated with vertical eye movement (eye blinks; ±140 μV) and horizontal eye movement (±55 μV) artifacts, as identified by a computerized algorithm and verified by visual inspection, were eliminated. In addition, a recording segment was marked as bad if it contained more than 10 bad channels (bad channel: ±200 μV for the entire segment). Individual bad channels were replaced on a segment‐by‐segment basis with spherical spline interpolation. Averaged ERP data were baseline‐corrected. All stimulus presentations and behavioral responses collections were controlled by a PC running E‐prime professional 2.0 software (Psychology SoftwareTools Inc., Sharpsburg, PA, USA).
Target‐evoked ERP components
Based on the literature regarding switching tasks–related ERPs, 32 , 35 , 36 , 37 and following a visual inspection of the grand averaged ERPs, we quantified the mean amplitudes of four ERP components within specified latency windows: P1 (70−120 ms poststimulus), N1 (120–180 ms poststimulus), P2 (190–250 ms poststimulus), and P3 (260−320 ms poststimulus). Mean amplitudes of P1, N1, P2, and P3 were quantified for 16 channels at the posterior‐parietal scalp location (average of channels 65, 66, 67, 69, 70, 71, 72, 74, 75, 76, 77, 82, 83, 84, 89, and 90), and 12 channels at the frontal scalp location (average of channels 3, 4, 5, 10, 11, 12, 16, 18, 19, 23, and 24). These electrodes were selected based on the scalp topography of the ERP components chosen for analysis as well as previous relevant studies. 32 , 36 , 38 For the electrode array, see Figure 1.
FIGURE 1.
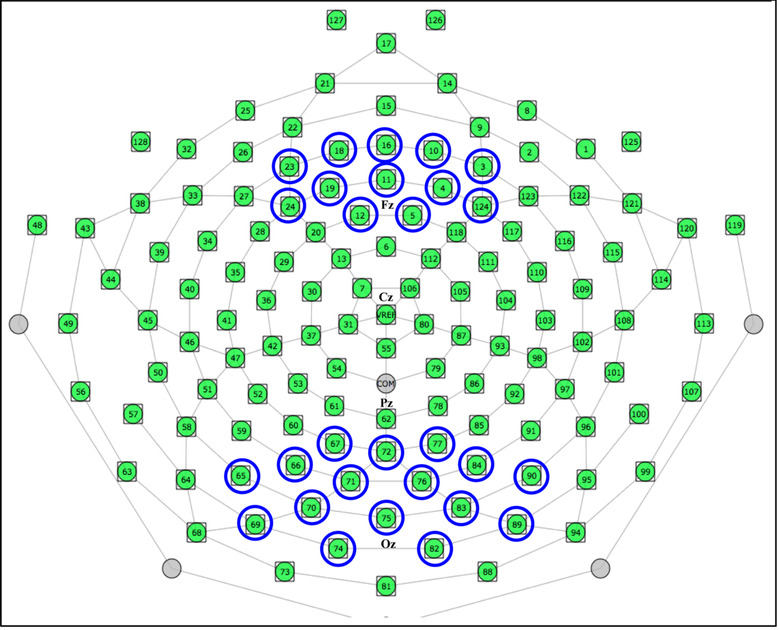
Layout of the electrode array and electrodes chosen for analysis.
Statistical analysis
RBC and iron indices were standardized relative to the mean scores of the current sample, separately for males and females, and all statistical analyses were performed on the resulting standardized scores. To test the relations between blood count results and iron status and behavioral and ERP responses, the sample was divided into two groups of “low” and “high” for each of the blood indices according to the median value. Notice that participants were classified into the low and high groups based only on the median of the present sample taken from the general population. That is not to say that they have lower or higher levels of blood and iron indices than what is considered normal. In fact, the averages of both the low and high groups fell within the normal range.
Digit span test
A hierarchical regression analysis was conducted to analyze the effect of gender and blood indices standard scores on test performance. The first step of the regression consisted of gender, and blood indices (e.g., ferritin) were added as the second step. Univariate general linear model (GLM) analyses with gender as a covariate were used to examine differences in test performance between groups of low and high in the relevant blood indices.
Switching task
Behavioral results
A hierarchical regression analysis was conducted to analyze the effect of gender and blood indices standard scores on test performance (percent of errors) in the switch and non‐switch conditions of the task. The first step of each regression consisted of gender, and blood indices were added as the second step. To examine group differences in error rates and RTs, we conducted mixed‐model 2 × 2 ANOVAs, with Condition (switch/non‐switch) as the within‐subject factor, and Group (low/high) as the between‐subject factor.
ERP results
A hierarchical regression analysis was conducted to analyze the effect of gender and blood indices standard scores on ERPs (P1, N1, P2, and P3) mean amplitude at frontal and posterior‐parietal locations in the switch and non‐switch conditions of the task. The first step of each regression consisted of gender, and blood indices were added as the second step. To further assess the relationship between blood indices and brain activity, we used mixed‐model 2 × 2 ANOVAs to analyze mean amplitudes for the preselected ERP components at the frontal and posterior‐parietal channels. Condition (switch/non‐switch) was the within‐subject factor, and Group (low/high) was the between‐subject factor. Throughout the behavioral and electrophysiological analyses, the Bonferroni correction was used where appropriate to control for multiple comparisons. Only results that remained statistically significant after the correction are reported. SPSS‐25 was used to perform statistical analyses and Excel was used to produce figures.
RESULTS
Digit span test
Regression analyses showed that gender was a significant predictor of memory test performance, with higher test scores in males. After controlling for gender, MCH, MCV, and ferritin were found to be significant predictors of test performance, with higher MCH/MCV/ferritin being associated with better test scores (Table 2). Univariate GLM analyses with gender as covariate revealed significant differences in test performance between groups of low and high MCH (F (1,105) = 13.75, p < 0.001, η p 2 = 0.12), MCV (F (1,105) = 7.47, p = 0.007, η p 2 = 0.07), and ferritin (F (1,105) = 6.49, p = 0.012, η p 2 = 0.06), such that the low MCH/MCV/ferritin group had poorer test performance (Figure 2).
TABLE 2.
Regression analyses showing gender and MCH/MCV/ferritin, as predictors of performance (scores) on the digit span test
| Cumulative | Simultaneous | |||
|---|---|---|---|---|
| Variable | R 2 change | F change | β | p |
| Step 1 | ||||
| Gender | 0.05 | F (1,106) = 5.43 | −0.22 | 0.02 |
| Step 2 | ||||
| MCH | 0.14 | F (1,105) = 18.08 | 0.37 | 0.000 |
| Step 2 | ||||
| MCV | 0.09 | F (1,105) = 10.80 | 0.30 | 0.001 |
| Step 2 | ||||
| Ferritin | 0.03 | F (1,105) = 3.78 | 0.18 | 0.05 |
Note: Gender: 1, male; 2, female.
Note: Results of “step 1” are the same for the three regression analyses performed.
FIGURE 2.
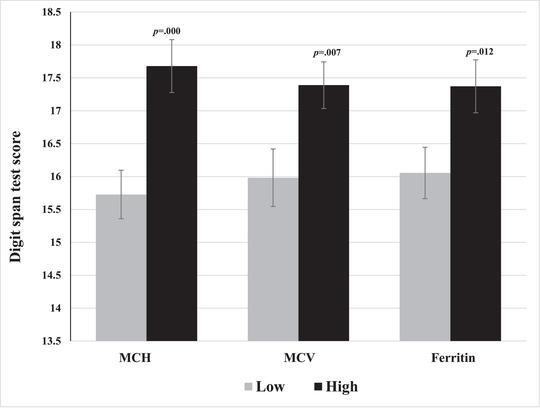
Mean digit span test scores for groups of high and low MCH, MCV, and ferritin. Error bars represent SEM.
Switching task
Behavioral results
Regression analyses showed that gender was not a predictor of performance on the switching task; absolutely no correlation was found between gender and percentage of errors. However, MCH, MCV, and ferritin were all found to be significant predictors of switching task performance, with higher MCH/MCV/ferritin levels associated with a lower percentage of errors (i.e., better test performance) (Table 3). Mixed‐model 2 × 2 ANOVAs revealed significant within‐subject effects of Condition for MCH (F (1,106) = 84.98, p < 0.001, η p 2 = 0.45), MCV (F (1,106) = 86.37, p < 0.001, η p 2 = 0.45), and ferritin (F (1,106) = 84.72, p < 0.001, η p 2 = 0.45) with higher error rates on switch trials than on non‐switch trials. More importantly, there was a significant between‐subject effect of Group (low/high) for MCH (F (1,106) = 5.07, p = 0.026, η p 2 = 0.05), MCV (F (1,106) = 6.27, p = 0.014, η p 2 = 0.06), and ferritin (F (1,106) = 8.00, p = 0.006, η p 2 = 0.07). On average, participants with lower levels of MCH, MCV, and ferritin had higher error rates (poorer performance) compared with those with high levels (Figure 3).a No interaction effects were found between Condition and Group. No between‐group main effects or interactions were found for RTs.
TABLE 3.
Regression analyses showing gender and MCH/MCV/ferritin, as predictors of performance (percent of errors) on the switching task
| Switch condition | Non‐switch condition | |||||||
|---|---|---|---|---|---|---|---|---|
| Cumulative | Simultaneous | Cumulative | Simultaneous | |||||
| Variable | R 2 change | F change | β | p | R 2 change | F change | β | p |
| Step 1 | ||||||||
| Gender | 0.00 | F (1,106) = 0.17 | 0.04 | N.S. | 0.00 | F (1,106) = 0.08 | 0.03 | N.S. |
| Step 2 | ||||||||
| MCH | 0.09 | F (1,105) = 9.77 | −0.29 | 0.002 | 0.06 | F (1,105) = 7.17 | −0.25 | 0.009 |
| Step 2 | ||||||||
| MCV | 0.04 | F (1,105) = 4.78 | −0.21 | 0.03 | 0.03 | F (1,105) = 3.15 | −0.17 | N.S. |
| Step 2 | ||||||||
| Ferritin | 0.07 | F (1,105) = 8.14 | −0.27 | 0.005 | 0.08 | F (1,105) = 8.51 | −0.27 | 0.004 |
Note: Gender: 1, male; 2, female.
Note: Results of “step 1” are the same for the three regression analyses performed.
FIGURE 3.
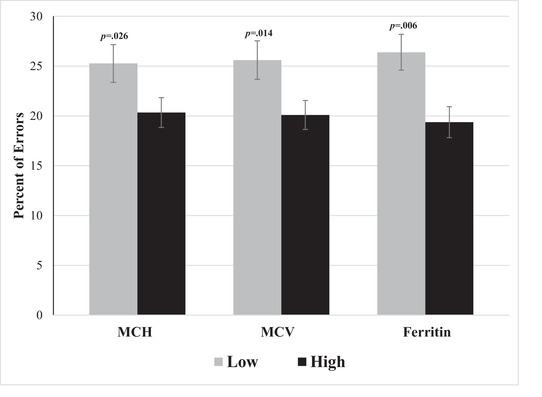
Mean error rates of the switching task for groups of high and low MCH, MCV, and ferritin. The switch and non‐switch conditions are combined. Error bars represent SEM.
Electrophysiological results
Gender was not found as a predictor of ERP amplitude in any of the regression analyses performed. MCH and MCV were found to be significant predictors of ERP amplitude, with lower MCH/MCV levels associated with greater amplitude (more positive at posterior‐parietal, i.e., negative correlation, and more negative at frontal channels, i.e., positive correlation); especially in the switch condition, which is the more challenging part of the task where executive function ability is being put to the test. Notice that almost all cases where no significant correlations were found were in the non‐switch condition (which is the easier, less challenging part of the task) (Table 4).
TABLE 4.
Regression analyses showing MCH and MCV as predictors of ERPs (P1, N1, P2, and P3) mean amplitude for switch and non‐switch stimuli at frontal and posterior‐parietal scalp locations, after controlling for gender
| Switch condition | Non‐switch condition | |||||||
|---|---|---|---|---|---|---|---|---|
| Cumulative | Simultaneous | Cumulative | Simultaneous | |||||
| Variable (Step 2) | R 2 change | F change | β | p | R 2 change | F change | β | p |
| P1‐Frontal | ||||||||
| MCH | 0.08 | F (1,102) = 8.51 | 0.28 | 0.004 | 0.06 | F (1,102) = 6.45 | 0.25 | 0.013 |
| MCV | 0.04 | F (1,101) = 4.70 | 0.21 | 0.033 | 0.01 | F (1,101) = 1.15 | 0.10 | N.S. |
| P1‐Posterior‐parietal | ||||||||
| MCH | 0.04 | F (1,102) = 4.72 | −0.21 | 0.032 | 0.04 | F (1,102) = 4.16 | −0.20 | 0.044 |
| MCV | 0.04 | F (1,101) = 4.14 | −0.20 | 0.045 | 0.01 | F (1,101) = 1.13 | −0.11 | N.S. |
| N1‐Frontal | ||||||||
| MCH | 0.10 | F (1,102) = 11.18 | 0.31 | 0.001 | 0.10 | F (1,102) = 11.23 | 0.32 | 0.001 |
| MCV | 0.05 | F (1,101) = 4.94 | 0.22 | 0.028 | 0.00 | F (1,101) = 0.32 | 0.05 | N.S. |
| N1‐Posterior‐parietal | ||||||||
| MCH | 0.07 | F (1,102) = 7.15 | −0.26 | 0.009 | 0.07 | F (1,102) = 8.05 | −0.27 | 0.006 |
| MCV | 0.04 | F (1,101) = 4.08 | −0.20 | 0.046 | 0.02 | F (1,101) = 1.54 | −0.12 | N.S. |
| P2‐Frontal | ||||||||
| MCH | 0.07 | F (1,102) = 7.48 | 0.26 | 0.007 | 0.04 | F (1,102) = 4.51 | 0.21 | 0.036 |
| MCV | 0.05 | F (1,101) = 5.21 | 0.22 | 0.025 | 0.00 | F (1,101) = 0.02 | 0.01 | N.S. |
| P2‐Posterior‐parietal | ||||||||
| MCH | 0.06 | F (1,102) = 5.95 | −0.23 | 0.016 | 0.02 | F (1,102) = 1.58 | −0.12 | N.S. |
| MCV | 0.06 | F (1,101) = 6.05 | −0.24 | 0.016 | 0.04 | F (1,101) = 3.81 | −0.19 | N.S. |
| P3‐Frontal | ||||||||
| MCH | 0.07 | F (1,102) = 6.66 | 0.25 | 0.011 | 0.05 | F (1,102) = 5.15 | 0.22 | 0.025 |
| MCV | 0.04 | F (1,101) = 4.15 | 0.20 | 0.044 | 0.03 | F (1,101) = 3.47 | 0.18 | N.S. |
| P3‐Posterior‐parietal | ||||||||
| MCH | 0.04 | F (1,102) = 3.50 | −0.18 | N.S. | 0.02 | F (1,102) = 2.38 | −0.15 | N.S. |
| MCV | 0.04 | F (1,101) = 3.95 | −0.20 | 0.05 | 0.02 | F (1,101) = 2.48 | −0.16 | N.S. |
Note: Gender was not found as a significant predictor of ERPs amplitude in any of the regression analyses performed; therefore, the results of “step 1” are not shown in Table 4.
Mixed‐model 2 × 2 ANOVAs revealed significant between‐group differences in ERP mean amplitudes for MCH and MCV at frontal and posterior‐parietal locations. Results are presented in Table 5. Figure 4 depicts the grand averaged ERPs to switch and non‐switch conditions by low MCV and high MCV groups over posterior‐parietal (A) and frontal (B) channels.
TABLE 5.
Between‐group results of ANOVAs of ERPs mean amplitude within high and low MCH and MCV groups at frontal and posterior‐parietal location
| MCH | MCV | ||||||
|---|---|---|---|---|---|---|---|
| ERP component | Scalp location | F (1,102) | p | η p 2 | F (1,101) | p | η p 2 |
| P1 | Frontal | 13.95 | 0.000 | 0.12 | 12.23 | 0.001 | 0.11 |
| Posterior‐parietal | 8.19 | 0.005 | 0.07 | 12.60 | 0.001 | 0.11 | |
| N1 | Frontal | 18.57 | 0.000 | 0.16 | 13.63 | 0.000 | 0.12 |
| Posterior‐parietal | 14.15 | 0.000 | 0.12 | 13.64 | 0.000 | 0.12 | |
| P2 | Frontal | 10.40 | 0.002 | 0.09 | 8.20 | 0.005 | 0.08 |
| Posterior‐parietal | 7.37 | 0.008 | 0.07 | 10.15 | 0.002 | 0.09 | |
| P3 | Frontal | 7.61 | 0.007 | 0.07 | 7.61 | 0.007 | 0.07 |
| Posterior‐parietal | 4.98 | 0.028 | 0.05 | 7.12 | 0.009 | 0.07 | |
FIGURE 4.
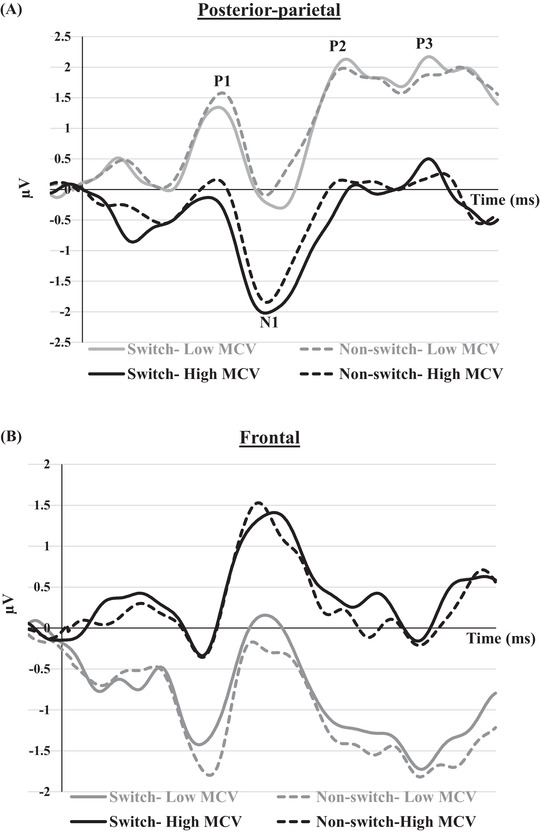
Grand averaged event‐related potentials to switch and non‐switch conditions in posterior‐parietal channels (A) and frontal channels (B) for groups of high and low MCV. The grand averaged ERPs waveform for groups of high and low MCH is highly similar to that of MCV and is, therefore, not presented in the graph.
P1, P2, and P3 mean amplitudes to switch and non‐switch conditions were greater (more positive at posterior‐parietal channels and more negative at frontal channels) for the low MCH/MCV group compared with the high MCH/MCV group. N1 amplitude was more negative at posterior‐parietal channels and more positive at frontal channels for the high MCH/MCV group compared with the low MCH/MCV group. Figures 5, 6, 7, 8 represent the mean amplitudes of P1 (Figure 5), N1 (Figure 6), P2 (Figure 7), and P3 (Figure 8) at posterior‐parietal and frontal locations within the low and high MCH and MCV groups.b The pattern of ferritin results was very similar to that of MCH and MCV but did not reach statistical significance.
FIGURE 5.
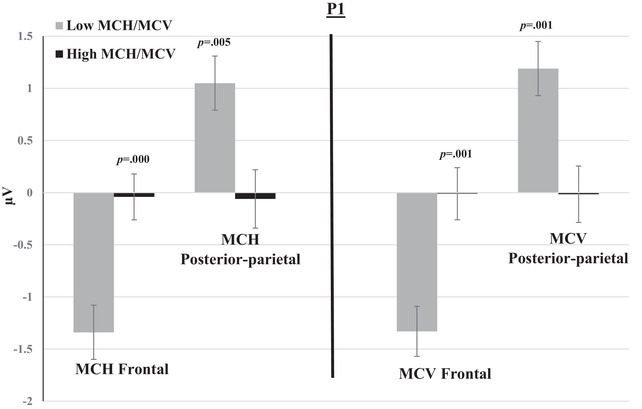
Mean amplitudes of P1 within groups of low and high MCH/MCV in the posterior‐parietal and frontal channels. The switch and non‐switch conditions are combined. Error bars represent SEM.
FIGURE 6.
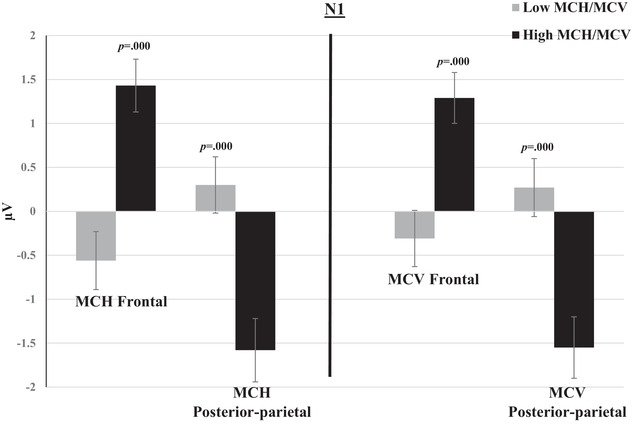
Mean amplitudes of N1 within groups of low and high MCH/MCV in the posterior‐parietal and frontal channels. The switch and non‐switch conditions are combined. Error bars represent SEM.
FIGURE 7.
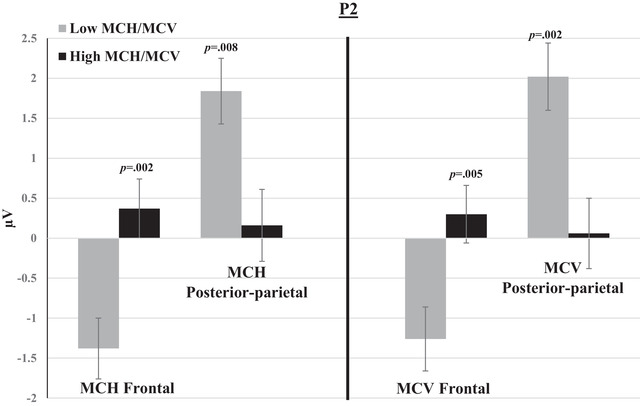
Mean amplitudes of P2 within groups of low and high MCH/MCV in posterior‐parietal and frontal channels. The switch and non‐switch conditions are combined. Error bars represent SEM.
FIGURE 8.
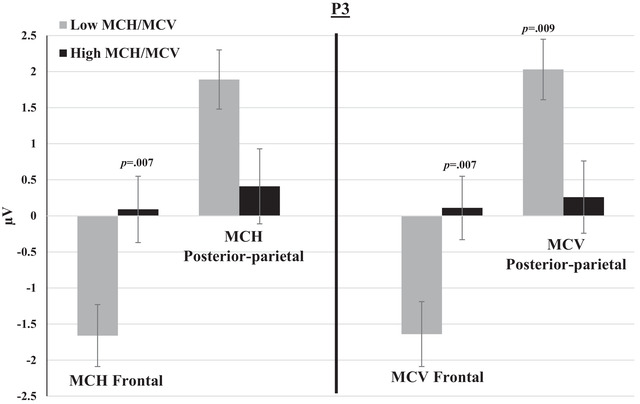
Mean amplitudes of P3 within groups of low and high MCH/MCV in posterior‐parietal and frontal channels. The switch and non‐switch conditions are combined. Error bars represent SEM.
DISCUSSION
The aim of the present study was to examine the relationship between RBC indices levels, body iron status, and neurocognitive function in young adults. Results of the digit span test, which evaluates working memory, showed that MCH, MCV, and ferritin were positively correlated with test performance. When the sample is divided into two groups of “high” and “low” according to the median of each of these indices, we find that those with higher levels of MCH, MCV, and ferritin score significantly better on the memory test.
Results of the switching task, designed to evaluate a higher executive function, showed that MCH, MCV, and ferritin levels were negatively correlated with percentage of errors made in the test; the higher the level of MCH, MCV, and ferritin, the lower the percentage of errors (i.e., the better the test performance). This trend of results recurred when groups of low and high were compared; the high MCH/MCV/ferritin groups had significantly lower error rates on the switching task compared with the low MCH/MCV/ferritin groups. These results are consistent with other studies that have reported associations between iron status and cognitive function in young adults. 18 , 21 , 23 , 39 , 40 , 41
Significant findings regarding MCH and MCV also emerged upon analysis of the electrophysiological results: lower levels of MCH and MCV were correlated with greater (more positive at posterior‐parietal and more negative at frontal channels) P1, N1, P2, and P3 amplitude. Moreover, these correlations were more pronounced and consistent for the more difficult and demanding part of the task, the switching condition, which is considered an indicator of executive function ability. Further corroboration for this pattern of results emerges when splitting the sample into high and low MCH/MCV groups; the low MCH/MCV groups had significantly more negative (i.e., −μV) ERPs responses at the frontal location and more positive (i.e., +μV) ERPs responses at the posterior‐parietal location than the high MCH/MCV groups.
Of all the blood indices examined in this study, MCH and MCV yielded the most consistent and unequivocal findings and were found to be the most sensitive to changes in cognitive and brain function. As mentioned, ferritin results, while not statistically significant, were quite similar to those of MCH and MCV. MCH is a measure of the average amount of HB in the RBCs, irrespective of cell size. MCV measures the average size of RBCs. Both measures are related to HB and mainly to iron status and may aid in determining the etiology of anemia. Lower than normal levels of MCH and MCV are commonly seen in iron‐deficient anemia; therefore, these measures had been suggested as potentially important parameters for screening and detecting IDA. 42 , 43 , 44 , 45 , 46 Very few studies directly examined the relations between MCH and MCV and cognitive function in young and middle‐aged healthy adults. For example, Winchester et al. 47 found in a very large sample of healthy adults that MCH (together with RDW) was the RBC measure most strongly associated with cognitive outcomes; lower MCH was associated with lower verbal–numeric reasoning and lower numeric memory performance and slower reaction times. Using Mendelian randomization, they also showed that MCH has a causative relationship with cognitive performance.
Brain ERP studies correlating HB and iron status to cognition in adulthood are extremely scarce, and ERP studies concerning MCH/MCV that are related mainly to ID are completely lacking in the literature. As far as we know, this is the first evidence of an association between levels of MCH/MCV and brain function while engaged in a rather complicated executive function task. The difference between the high and low MCH/MCV groups is noticeable throughout the continuous ERP waveform (from the early P1 to the later P3) both at the frontal and posterior‐parietal scalp locations.
The P1 component is generated by extrastriate visual areas in the perceptual stage of information processing. It is the first component of the visual ERP waveform to be reliably modulated by voluntary attention and is considered an index of attention‐related processes and mobilization of attentional resources. Augmentation of P1 is thought to reflect the recruitment of additional brain resources for perceptual processing of stimuli. 48 , 49 , 50 The N1 component is considered an index of selective attention. It is considered to reflect the operation of a discriminative process. The N1 attention effect seems to be found only when subjects are required to make a discrimination, and it is absent when subjects must merely detect the presence of a stimulus. Selective attention mechanisms regulate which information has the most impact on behavior by enhancing the sensory processing of relevant information and suppressing that of irrelevant information. Selectivity prevents us from reacting reflexively to stimuli in the environment and enables behavioral flexibility. Whereas the P1 may reflect the rather exogenous facilitation of early sensory processing within the focus of attention, the N1 may reflect additional top–down modulation of this early sensory processing. 51 P2 is thought to represent inhibition of further processing of sensory input via automatic stimulus identification and discrimination/classification, or inhibition of other channels of information competing for attention and further processing. In the context of switching tasks, it has been reported that the amplitude of P2 is greater for switch trials than for repeat trials. This suggests that the effect of task switching on the P2 reflects the recruitment of processes that are generally related to switching a task set. 37 , 52 To explain this, Brown et al. 52 suggested a “change detector” process, which is thought to operate through a phasic change in neural activity associated with an alternation in the task set that leads to updating the representation of the task set. P3 is assumed to reflect endogenous or cognitive aspects of “context updating,” that is, the comparison of the attributes of incoming stimuli with an internal model and the subsequent revision of the model. In task switching, the larger P3 for switch than for repetition trials has been interpreted as reflecting updating of task sets in working memory. 53 , 54
Switching tasks involve high perceptual loads. Perceptual load elicits greater mobilization of attentional resources and calls for a higher level of concentrated resources for information processing and response planning and initiation. 51 In the present study, the lower MCH/MCV groups exhibited overall larger ERP amplitudes than the higher MCH/MCV groups. Increased brain activity was correlated with poorer switching performance, possibly reflecting compensatory processes recruited in those with lower MCH/MCV. 55 A possible interpretation of these results is that lower levels of MCH and MCV, which may reflect low brain iron, are associated with the recruitment of additional brain resources when dealing with a rather difficult cognitive challenge, such as a switching task. Our findings may indicate lower efficiency of neural activation associated with poorer cognitive performance among individuals with lower MCH/MCV. Interestingly, the present findings are generally in line with our previous study conducted on β‐thalassemia major patients. 32 β‐TM is characterized by chronic severe hemolytic anemia without ID. β‐TM is also characterized by microcytosis (low MCV). We found that HB levels were negatively correlated with error rates and RTs, and that β‐TM patients had higher error rates compared to healthy controls. Electrophysiological results indicated significant alterations in amplitudes of the ERP components in β‐TM patients relative to controls such that the continuous ERP waveform (from the early to later components) was more positive at posterior‐parietal region for the β‐TM group compared with healthy controls.
Interpretation of the present findings should be taken with caution. At this point, the underlying mechanisms of the observed cognitive and neural associations with HB and iron‐reflecting RBC indices (with an emphasis on MCH and MCV) are not clear enough. Additional studies are needed to replicate, confirm, extend, and explain these results. Importantly, this study was conducted on a general asymptomatic population of young adults. Comparisons in this study were not made between extreme groups of anemia, ID, or IDA sufferers versus healthy controls. For example, mean MCV for the low group was 83.98 fl and for the high group, it was 93.18 fl; both fall within the normal range of MCV. The same is true for MCH where the low group had a normal mean of 28.13 pg and the high group had a normal mean of 30.23 pg. Given the fact that significant and consistent cognitive and neural findings have emerged despite the relatively small overall sample variance, and the relatively small between‐groups blood indices differences, it is possible that even more salient effects would be found should more distinct/extreme groups be compared. It is also possible that comparison between more extreme groups will yield significant findings for additional indices (e.g., significant ERP results not only for MCH and MCV but also for HB, RDW, and ferritin). The measure of HB (g/dl) did not correlate with any of the cognitive or ERP variables studied here. It is suggested that in a normal, nonanemic population, MCH and MCV may provide more sensitive indicators for subclinical ID. This is in line with a recent study conducted on a large nonanemic sample, 56 which found significant differences in MCH and MCV between subclinical iron deficiency (SID) and non‐SID groups. The authors noted that MCH was the best discriminator of SID in both males and females.
Looking at the behavioral results of both the digit span test and the switching task raises the possibility that it is the iron status that contributes most to the alterations in neurocognitive function, since, aside from MCH and MCV, results were also found for ferritin, and partly for serum iron, transferrin saturation, and serum transferrin. Microcytic hypochromic anemia (i.e., below normal range of MCV and MCH) secondary to ID is the most common type of anemia and has been linked to cognitive dysfunction. 18 , 57 Currently, there is not enough knowledge on whether the total body iron sources are directly correlated with the iron content of brain neurons. The brain iron metabolism requires very small amounts of iron compared to the iron required for normal erythropoiesis. Then, the results of the present study may suggest that low brain iron, not detected by low serum ferritin, affects cognition. Subsequently, the findings of the present study conducted on a general normative population highlight the utility and sensitivity of the MCH and MCV indices in detecting subtle alterations in the efficacy and quality of neurocognitive function. The question remains whether MCH and MCV reflect brain iron availability, which ultimately affects cognitive function, 58 and the possibility of correcting early ID to maximize cognitive activity. More studies are needed to elucidate the complicated relations between RBC and iron indices and neurocognitive outcomes in adulthood.
AUTHOR CONTRIBUTIONS
Conception and design: S.R., C.L., and A.K. Acquisition of data: S.R. and C.L. Analysis of data: S.R. Interpretation of data: S.R., C.L., and A.K. Drafting of the manuscript: S.R. Revision of intellectual content of the manuscript: S.R., C.L., and A.K. Approval of final version of the manuscript: S.R., C.L., and A.K. Responsibility for the integrity of the data analysis: S.R. and C.L.
COMPETING INTERESTS
The authors declare no competing interests.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/nyas.14877.
ACKNOWLEDGMENT
This study was funded by the Israeli Ministry for Development of the Negev and the Galilee.
Raz, S. , Koren, A. , & Levin, C. (2022). Associations between red blood cell indices and iron status and neurocognitive function in young adults: Evidence from memory and executive function tests and event‐related potentials. Ann NY Acad Sci., 1517, 300–313. 10.1111/nyas.14877
Footnotes
Since no Condition × Group interactions emerged, the switch and non‐switch conditions were combined for the purpose of graphical presentation.
Since no within‐subject effects of Condition (switch/non‐switch) nor Condition × Group interactions emerged, the switch and non‐switch conditions were combined for the purpose of graphical presentation.
REFERENCES
- 1. Camaschella, C. (2015). Iron‐deficiency anemia. New England Journal of Medicine, 372(19), 1832–1843. 10.1056/NEJMra1401038 [DOI] [PubMed] [Google Scholar]
- 2. Merkel, D. , Huerta, M. , Grotto, I. , Blum, D. , Rachmilewitz, E. , Fibach, E. , Epstein, Y. , & Shpilberg, O. (2009). Incidence of anemia and iron deficiency in strenuously trained adolescents: Results of a longitudinal follow‐up study. Journal of Adolescent Health, 45(3), 286–291. 10.1016/j.jadohealth.2009.02.003 [DOI] [PubMed] [Google Scholar]
- 3. Dubnov, G. , Foldes, A. J. , Mann, G. , Magazanik, A. , Siderer, M. , & Constantini, N. (2006). High prevalence of iron deficiency and anemia in female military recruits. Military Medicine, 171(9), 866–869. 10.7205/MILMED.171.9.866 [DOI] [PubMed] [Google Scholar]
- 4. Epstein, D. , Borohovitz, A. , Merdler, I. , Furman, M. , Atalli, E. , Sorkin, A. , Stainfeld, Y. , Isenberg, Y. , Mashiach, T. , Shapira, S. , Weisshof, R. , & Dann, E. J. (2018). Prevalence of iron deficiency and iron deficiency anemia in strenuously training male army recruits. Acta Haematologica, 139(3), 141–147. 10.1159/000485736 [DOI] [PubMed] [Google Scholar]
- 5. Israeli, E. , Merkel, D. , Constantini, N. , Yanovich, R. , Evans, R. K. , Shahar, D. A. N. I. T. , & Moran, D. S. (2008). Iron deficiency and the role of nutrition among female military recruits. Medicine and Science in Sports and Exercise, 40(11), S685–S690. [DOI] [PubMed] [Google Scholar]
- 6. Yanovich, R. , Merkel, D. , Israeli, E. , Evans, R. K. , Erlich, T. , & Moran, D. S. (2011). Anemia, iron deficiency, and stress fractures in female combatants during 16 months. Journal of Strength & Conditioning Research, 25(12), 3412–3421. 10.1519/JSC.0b013e318215f779 [DOI] [PubMed] [Google Scholar]
- 7. Levin, C. , Harpaz, S. , Muklashi, I. , Lumelsky, N. , Komisarchik, I. , Katzap, I. , Abu Hanna, M. , & Koren, A. (2016). Iron deficiency and iron‐deficiency anemia in toddlers ages 18 to 36 months: A prospective study. Journal of Pediatric Hematology/Oncology, 38(3), 205–209. 10.1097/MPH.0000000000000539 [DOI] [PubMed] [Google Scholar]
- 8. Camaschella, C. (2019). Iron deficiency. Blood, 133(1), 30–39. 10.1182/blood-2018-05-815944 [DOI] [PubMed] [Google Scholar]
- 9. Carter, R. C. , Jacobson, J. L. , Burden, M. J. , Armony‐Sivan, R. , Dodge, N. C. , Angelilli, M. L. , Lozoff, B. , & Jacobson, S. W. (2010). Iron deficiency anemia and cognitive function in infancy. Pediatrics, 126(2), 427–434. 10.1542/peds.2009-2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Halterman, J. S. , Kaczorowski, J. M. , Aligne, C. A. , Auinger, P. , & Szilagyi, P. G. (2001). Iron deficiency and cognitive achievement among school‐aged children and adolescents in the United States. Pediatrics, 107(6), 1381–1386. 10.1542/peds.107.6.1381 [DOI] [PubMed] [Google Scholar]
- 11. Logan, E. C. M. , Yates, J. M. , Stewart, R. M. , Fielding, K. , & Kendrick, D. (2002). Investigation and management of iron deficiency anaemia in general practice: A cluster randomised controlled trial of a simple management prompt. Postgraduate Medical Journal, 78(923), 533–537. 10.1136/pmj.78.923.533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lozoff, B. , & Georgieff, M. K. (2006). Iron deficiency and brain development. Seminars in Pediatric Neurology, 13, 158–165. 10.1016/j.spen.2006.08.004 [DOI] [PubMed] [Google Scholar]
- 13. Lozoff, B. , Armony‐Sivan, R. , Kaciroti, N. , Jing, Y. , Golub, M. , & Jacobson, S. W. (2010). Eye‐blinking rates are slower in infants with iron‐deficiency anemia than in nonanemic iron‐deficient or iron‐sufficient infants. Journal of Nutrition, 140(5), 1057–1061. 10.3945/jn.110.120964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Algarin, C. , Nelson, C. A. , Peirano, P. , Westerlund, A. , Reyes, S. , & Lozoff, B. (2013). Iron‐deficiency anemia in infancy and poorer cognitive inhibitory control at age 10 years. Developmental Medicine & Child Neurology, 55(5), 453–458. 10.1111/dmcn.12118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Agrawal, S. , Kumar, S. , Ingole, V. , Acharya, S. , Wanjari, A. , Bawankule, S. , & Raisinghani, N. (2019). Does anemia affects cognitive functions in neurologically intact adult patients: Two year cross sectional study at rural tertiary care hospital. Journal of Family Medicine and Primary Care, 8(9), 3005. 10.4103/jfmpc.jfmpc_599_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Falkingham, M. , Abdelhamid, A. , Curtis, P. , Fairweather‐Tait, S. , Dye, L. , & Hooper, L. (2010). The effects of oral iron supplementation on cognition in older children and adults: A systematic review and meta‐analysis. Nutrition Journal, 9(1), 1–16. 10.1186/1475-2891-9-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jáuregui‐Lobera, I. (2014). Iron deficiency and cognitive functions. Neuropsychiatric Disease and Treatment, 10, 2087. 10.2147/NDT.S72491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sharief, S. M. , Shaik, A. P. , Parveen, S. A. , & Hussain, S. M. (2019). Correlation of iron deficiency anemia with cognitive function in young adults. IOSR Journal of Dental and Medical Sciences, 18(5), 47–54. 10.9790/0853-1805114754 [DOI] [Google Scholar]
- 19. Blanton, C. (2014). Improvements in iron status and cognitive function in young women consuming beef or non‐beef lunches. Nutrients, 6(1), 90–110. 10.3390/nu6010090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blanton, C. A. , Green, M. W. , & Kretsch, M. J. (2013). Body iron is associated with cognitive executive planning function in college women. British Journal of Nutrition, 109(5), 906–913. 10.1017/S0007114512002620 [DOI] [PubMed] [Google Scholar]
- 21. Khedr, E. , Hamed, S. A. , Elbeih, E. , El‐Shereef, H. , Ahmad, Y. , & Ahmed, S. (2008). Iron states and cognitive abilities in young adults: Neuropsychological and neurophysiological assessment. European Archives of Psychiatry and Clinical Neuroscience, 258(8), 489–496. 10.1007/s00406-008-0822-y [DOI] [PubMed] [Google Scholar]
- 22. Leonard, A. J. , Chalmers, K. A. , Collins, C. E. , & Patterson, A. J. (2014). A study of the effects of latent iron deficiency on measures of cognition: A pilot randomised controlled trial of iron supplementation in young women. Nutrients, 6(6), 2419–2435. 10.3390/nu6062419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Murray‐Kolb, L. E. , & Beard, J. L. (2007). Iron treatment normalizes cognitive functioning in young women. American Journal of Clinical Nutrition, 85(3), 778–787. 10.1093/ajcn/85.3.778 [DOI] [PubMed] [Google Scholar]
- 24. Scott, S. P. , & Murray‐Kolb, L. E. (2016). Iron status is associated with performance on executive functioning tasks in nonanemic young women. Journal of Nutrition, 146(1), 30–37. 10.3945/jn.115.223586 [DOI] [PubMed] [Google Scholar]
- 25. Murray‐Kolb, L. E. , Wenger, M. J. , Scott, S. P. , Rhoten, S. E. , Lung'aho, M. G. , & Haas, J. D. (2017). Consumption of iron‐biofortified beans positively affects cognitive performance in 18‐ to 27‐year‐old Rwandan female college students in an 18‐week randomized controlled efficacy trial. Journal of Nutrition, 147(11), 2109–2117. 10.3945/jn.117.255356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Scott, S. P. , Murray‐Kolb, L. E. , Wenger, M. J. , Udipi, S. A. , Ghugre, P. S. , Boy, E. , & Haas, J. D. (2018). Cognitive performance in Indian school‐going adolescents is positively affected by consumption of iron‐biofortified pearl millet: A 6‐month randomized controlled efficacy trial. Journal of Nutrition, 148(9), 1462–1471. 10.1093/jn/nxy113 [DOI] [PubMed] [Google Scholar]
- 27. Soleimani, N. (2011). Relationship between anaemia, caused from the iron deficiency, and academic achievement among third grade high school female students. Procedia‐Social and Behavioral Sciences, 29, 1877–1884. 10.1016/j.sbspro.2011.11.437 [DOI] [Google Scholar]
- 28. Wenger, M. J. , Murray‐Kolb, L. E. , Nevins, J. E. , Venkatramanan, S. , Reinhart, G. A. , Wesley, A. , & Haas, J. D. (2017). Consumption of a double‐fortified salt affects perceptual, attentional, and mnemonic functioning in women in a randomized controlled trial in India. Journal of Nutrition, 147(12), 2297–2308. 10.3945/jn.117.251587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kececi, H. , & Degirmenci, Y. (2008). Quantitative EEG and cognitive evoked potentials in anemia. Neurophysiologie Clinique/Clinical Neurophysiology, 38(2), 137–143. 10.1016/j.neucli.2008.01.004 [DOI] [PubMed] [Google Scholar]
- 30. Wenger, M. J. , Rhoten, S. E. , Murray‐Kolb, L. E. , Scott, S. P. , Boy, E. , Gahutu, J. B. , & Haas, J. D. (2019). Changes in iron status are related to changes in brain activity and behavior in Rwandan female university students: Results from a randomized controlled efficacy trial involving iron‐biofortified beans. Journal of Nutrition, 149(4), 687–697. 10.1093/jn/nxy265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Raz, S. , Koren, A. , Dan, O. , & Levin, C. (2016). Cognitive functions in adults with β‐thalassemia major: Before and after blood transfusion and comparison with healthy controls. Annals of the New York Academy of Sciences, 1375(1), 19–27. 10.1111/nyas.13103 [DOI] [PubMed] [Google Scholar]
- 32. Raz, S. , Koren, A. , Dan, O. , & Levin, C. (2016). Executive function and neural activation in adults with β‐thalassemia major: An event‐related potentials study. Annals of the New York Academy of Sciences, 1386(1), 16–29. 10.1111/nyas.13279 [DOI] [PubMed] [Google Scholar]
- 33. Raz, S. , Koren, A. , & Levin, C. (2019). Attention, response inhibition and brain event‐related potential alterations in adults with beta‐thalassaemia major. British Journal of Haematology, 186(4), 580–591. 10.1111/bjh.15957 [DOI] [PubMed] [Google Scholar]
- 34. Wechsler, D. (1997). Wechsler Intelligence Scale for Adults, Third Edition. San Antonio, TX: Psychological Cooperation. [Google Scholar]
- 35. Barceló, F. , Periáñez, J. A. , & Nyhus, E. (2008). An information theoretical approach to task‐switching: Evidence from cognitive brain potentials in humans. Frontiers in Human Neuroscience, 2, 13. 10.3389/neuro.09.013.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gajewski, P. D. , Ferdinand, N. K. , Kray, J. , & Falkenstein, M. (2018). Understanding sources of adult age differences in task switching: Evidence from behavioral and ERP studies. Neuroscience & Biobehavioral Reviews, 92, 255–275. 10.1016/j.neubiorev.2018.05.029 [DOI] [PubMed] [Google Scholar]
- 37. West, R. , Langley, M. M. , & Bailey, K. (2011). Signaling a switch: Neural correlates of task switching guided by task cues and transition cues. Psychophysiology, 48(5), 612–623. 10.1111/j.1469-8986.2010.01123.x [DOI] [PubMed] [Google Scholar]
- 38. Zhang, R. , Stock, A. K. , Rzepus, A. , & Beste, C. (2017). Self‐regulatory capacities are depleted in a domain‐specific manner. Frontiers in Systems Neuroscience, 11, 70. 10.3389/fnsys.2017.00070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Beard, J. L. , & Connor, J. R. (2003). Iron status and neural functioning. Annual Review of Nutrition, 23(1), 41–58. 10.1146/annurev.nutr.23.020102.075739 [DOI] [PubMed] [Google Scholar]
- 40. Greig, A. J. , Patterson, A. J. , Collins, C. E. , & Chalmers, K. A. (2013). Iron deficiency, cognition, mental health and fatigue in women of childbearing age: A systematic review. Journal of Nutritional Science, 2, E14. 10.1017/jns.2013.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pollitt, E. (1993). Iron deficiency and cognitive function. Annual Review of Nutrition, 13(1), 521–537. 10.1146/annurev.nu.13.070193.002513 [DOI] [PubMed] [Google Scholar]
- 42. Alquaiz, J. M. , Abdulghani, H. M. , Khawaja, R. A. , & Shaffi‐Ahamed, S. (2012). Accuracy of various iron parameters in the prediction of iron deficiency anemia among healthy women of child bearing age, Saudi Arabia. Iranian Red Crescent Medical Journal, 14(7), 397–401. [PMC free article] [PubMed] [Google Scholar]
- 43. Clark, S. F. (2008). Iron deficiency anemia. Nutrition in Clinical Practice, 23(2), 128–141. 10.1177/0884533608314536 [DOI] [PubMed] [Google Scholar]
- 44. Goddard, A. F. , James, M. W. , McIntyre, A. S. , & Scott, B. B. (2011). Guidelines for the management of iron deficiency anaemia. Gut, 60(10), 1309–1316. [DOI] [PubMed] [Google Scholar]
- 45. Guyatt, G. H. , Patterson, C. , Ali, M. , Levine, M. , Turpie, I. , Meyer, R. , & Singer, J. (1990). Diagnosis of iron‐deficiency anemia in the elderly. American Journal of Medicine, 88(3), 205–209. 10.1016/0002-9343(90)90143-2 [DOI] [PubMed] [Google Scholar]
- 46. Maner, B. S. (2020). Mean corpuscular volume. StatPearls Publishing. [PubMed] [Google Scholar]
- 47. Winchester, L. M. , Powell, J. , Lovestone, S. , & Nevado‐Holgado, A. J. (2018). Red blood cell indices and anaemia as causative factors for cognitive function deficits and for Alzheimer's disease. Genome Medicine, 10(1), 1–12. 10.1186/s13073-018-0556-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hopfinger, J. B. , & Mangun, G. R. (2001). Tracking the influence of reflexive attention on sensory and cognitive processing. Cognitive, Affective, & Behavioral Neuroscience, 1(1), 56–65. 10.3758/CABN.1.1.56 [DOI] [PubMed] [Google Scholar]
- 49. Luck, S. J. , Woodman, G. F. , & Vogel, E. K. (2000). Event‐related potential studies of attention. Trends in Cognitive Sciences, 4(11), 432–440. 10.1016/S1364-6613(00)01545-X [DOI] [PubMed] [Google Scholar]
- 50. Luck, S. J. (2012). Event‐related potentials. In Long, D. L. (Ed.). APA handbook of research methods in psychology (pp. 1–18). American Psychological Association. [Google Scholar]
- 51. Vogel, E. K. , & Luck, S. J. (2000). The visual N1 component as an index of a discrimination process. Psychophysiology, 37(2), 190–203. 10.1111/1469-8986.3720190 [DOI] [PubMed] [Google Scholar]
- 52. Brown, J. W. , Reynolds, J. R. , & Braver, T. S. (2007). A computational model of fractionated conflict‐control mechanisms in task‐switching. Cognitive Psychology, 55(1), 37–85. 10.1016/j.cogpsych.2006.09.005 [DOI] [PubMed] [Google Scholar]
- 53. Barcelo, F. , Escera, C. , Corral, M. J. , & Periáñez, J. A. (2006). Task switching and novelty processing activate a common neural network for cognitive control. Journal of Cognitive Neuroscience, 18(10), 1734–1748. 10.1162/jocn.2006.18.10.1734 [DOI] [PubMed] [Google Scholar]
- 54. Jost, K. , Mayr, U. , & Rösler, F. (2008). Is task switching nothing but cue priming? Evidence from ERPs. Cognitive, Affective, & Behavioral Neuroscience, 8(1), 74–84. 10.3758/CABN.8.1.74 [DOI] [PubMed] [Google Scholar]
- 55. Jimura, K. , & Braver, T. S. (2010). Age‐related shifts in brain activity dynamics during task switching. Cerebral Cortex, 20(6), 1420–1431. 10.1093/cercor/bhp206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nah, E. H. , Cho, H. I. , Cho, S. , & Kim, S. (2020). Subclinical iron deficiency in non‐anemic individuals: A retrospective analysis of Korean health examinees. Acta Haematologica, 143(1), 26–32. 10.1159/000500630 [DOI] [PubMed] [Google Scholar]
- 57. De Franceschi, L. , Iolascon, A. , Taher, A. , & Cappellini, M. D. (2017). Clinical management of iron deficiency anemia in adults: Systemic review on advances in diagnosis and treatment. European Journal of Internal Medicine, 42, 16–23. 10.1016/j.ejim.2017.04.018 [DOI] [PubMed] [Google Scholar]
- 58. Thompson, K. J. , Shoham, S. , & Connor, J. R. (2001). Iron and neurodegenerative disorders. Brain Research Bulletin, 55(2), 155–164. 10.1016/S0361-9230(01)00510-X [DOI] [PubMed] [Google Scholar]


