Abstract
Azathioprine (AZA) is commonly used for many autoimmune disorders; however, the limitation of its clinical use is due to potential toxicities, including severe leukopenia. Recent studies have identified genetic NUDT15 variants strongly associated with AZA‐induced leukopenia in Asian patients. This study aimed to investigate the strength of above genetic association and evaluate the usefulness of prospective screening of the NUDT15 variants to prevent AZA‐induced leukopenia in Chinese patients. AZA‐induced leukopenia in patients with autoimmune disorders were enrolled from multiple medical centers in Taiwan/China between 2012 and 2017 to determine the strength of genetic association of NUDT15 or TPMT variants by whole exome sequencing (WES). Furthermore, a prospective study was conducted between 2018 and 2021 to investigate the incidence of AZA‐induced leukopenia with and without genetic screening. The WES result showed the genetic variants of NUDT15 R139C (rs116855232) (P = 3.7 × 10−25, odds ratio (OR) = 21.7, 95% confidence interval (95% CI) = 12.1–38.8) and NUDT15 rs746071566 (P = 4.2 × 10−9, OR = 7.1, 95% CI = 3.7–13.7), but not TPMT, were associated with AZA‐induced leukopenia and NUDT15 R139C variant shows the highest sensitivity with 92.5%. Furthermore, the targeted screening of 1,013 participants for NUDT15 R139C enabled those identified as carriers to use alternative immunosuppressants. This strategy resulted in a significant decrease in the incidence of AZA‐induced leukopenia compared with historical incidence (incidence rate = from 7.6% decreased to 0.4%; P = 9.3 × 10−20). In conclusion, the NUDT15 R139C variant was strongly associated with AZA‐induced leukopenia in Chinese patients. The genetic screening of NUDT15 R139C followed by use of alternative immunosuppressants in identified carriers effectively decreased the incidence of AZA leukopenia for patients with autoimmune disorders.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Azathioprine (AZA) may cause leukopenia, which hinders its clinical use. Genetic TPMT variants (e.g., TPMT*2, *3A, and *3C) are associated with AZA‐induced leukopenia in Western populations. However, the prevalence of these variants is exceptionally low (< 1%) in Asian populations. A strong association between NUDT15 variants and AZA‐induced leukopenia has been reported in Asian patients.
WHAT QUESTION DID THIS STUDY ADDRESS?
Still, the strength of genetic NUDT15 and TPMT associations and which genetic variant would be appropriate for prospective screening before AZA treatment in the Chinese population remains unclear.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
The genetic NUDT15 R139C variant showed the strongest association and highest sensitivity for patients with AZA‐induced leukopenia in the Chinese population. Prospective genetic testing of single NUDT15 R139C variant before AZA use can significantly decrease the incidence of AZA‐induced leukopenia.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
The pre‐emptive pharmacogenomic NUDT15 R139C screening prior to prescribing AZA for patients with autoimmune disorders effectively decreases the incidence of AZA leukopenia and provides evidence for the implementation of personalized medicine.
Azathioprine (AZA) is frequently used as an immunosuppressive agent in organ transplantation and autoimmune disorders, like rheumatoid arthritis and atopic dermatitis (AD). However, its use has been limited due to widely reported adverse effects, including severe leukopenia and liver injury. 1 , 2 Leukopenia during AZA therapy may be a sign of dose response. 3 Recent evidence in different populations showed that AZA toxicity was strongly associated with genetic polymorphisms in AZA metabolizing enzymes and drug transporters. 4 , 5 Nudix Hydrolase 15 (NUDT15) and thiopurine methyltransferase (TPMT), which are involved in purine metabolism, have been put in the spotlight because genetic variants in these two proteins may be involved in AZA toxicity. 5 , 6 , 7 , 8 , 9 , 10 In European descendants, TPMT gene variants (namely, TPMT*2, *3A, and *3C) are associated with AZA‐induced leukopenia. 9 , 10 , 11 , 12 However, in Asian populations, the prevalence of these variants is exceptionally low (< 1%). Conversely, NUDT15 has several known polymorphisms, and the missense variant rs116855232 (R139C), which is at higher prevalence in Asians but lower in European and African descendants, has shown to be strongly associated with thiopurine‐related myelosuppression and leukopenia in Asian populations. 6 , 10 , 11 , 13 , 14 , 15 , 16 , 17 In light of these findings, individuals with either TPMT or NUDT15 polymorphisms are reported to have a higher risk of developing AZA toxicity. 10 , 14 The R139C variant of NUDT15 has been shown to impair the stability of the NUDT15 protein, increasing the incorporation of 6‐TGTP and 6‐TdGTP into RNA and DNA, respectively, and resulting in myelosuppression and leukopenia. 15
To date, it has been reported that the genetic factors (such as the above‐mentioned NUDT15 and TPMT polymorphisms) are associated with the predisposition to AZA leukopenia, but it remains unclear which genetic variant of NUDT15 plays a crucial role in this process or if genetic screening would help reduce the occurrence of AZA leukopenia in Chinese patients. Therefore, the first aim of this study was to determine the strength of the genetic association of NUDT15 and TPMT variants for AZA‐induced leukopenia in Chinese patients. Furthermore, we sought to evaluate whether prospective genetic screening before AZA treatment could reduce the incidence of drug‐induced adverse effects.
METHODS
Study design
To determine the strength of genetic association for AZA‐induced leukopenia, we enrolled 40 patients with AZA‐induced leukopenia with autoimmune disorders during 2012 to 2017 from the multiple medical centers in Taiwan and China, including Chang Gung Memorial Hospital (CGMH) Health System (Linkou, Taipei, and Keelung), National Taiwan University Hospital, Taichung Veterans General Hospital, and Taipei Veterans General Hospital; and CGMH Health, Xiamen, in China. Each case was evaluated by a least two physicians. For the general population control group, we collected the DNA samples from 507 individuals with no history of AZA treatment, adverse drug reactions, or liver‐related disorders, as described previously. 18 We further enrolled 86 AZA‐tolerant patients who had received AZA for over 6 months without evidence of adverse effects, including skin rash, hair loss, or abnormal biochemical/blood parameters.
To evaluate the effectiveness of NUDT15 R139C genetic testing, we conducted a prospective study to enroll 1,056 patients with an indication for AZA treatment between 2018 and 2021. Participants of the prospective study were also recruited from multiple medical centers in Taiwan and China.
All patients gave written informed consent to participate in the study. The study was conducted according to good clinical practice guidelines and the Declaration of Helsinki and was approved by the ethics committee of the CGMH health system and other different multiple medical centers in Taiwan and China (IRB No.104‐0291B, 201701824A3C501, 201801423B0, 202001515B0, and 202000852B0).
DNA libraries quantification and whole‐exome sequencing
Genomic DNA was extracted from patients’ peripheral blood mononuclear cells by using the DNeasy Blood & Tissue Kit (QIAGEN, Hilden, Germany) and loaded on a 1% agarose gel for quality control. The capture exonic regions protocol is based on the Agilent V6 SureSelect Human All Exon platform, which targets ∼ 50 Mb of the human exonic regions. Paired‐end 150 bp whole‐exome sequencing (WES) with an insert size of 500–600 bp was performed on the Illumina NovaSeq 6000 platform with NovaSeq 6000 S4 Reagent Kit (300 cycles). DNA libraries were quantified by a Bioanalyzer. The average depth was at least > 60 for all samples.
Identification of genetic NUDT15 association with AZA‐induced leukopenia in the Chinese population
Enrolled patients with AZA‐induced leukopenia (≥ grade 2) were screened by WES, and the 507 general population controls were screened using whole genome sequencing (WGS) as established previously. 18 After performing variant calling, variants removement with a P value of Hardy–Weinberg Equilibrium < 10−6 and combination of these WES/WGS datasets, we identified a total of 1,745,379 variants (including single nucleotide variants and insertion/deletion variants) for WES data of patients with AZA‐induced leukopenia.
In this study, we performed a knowledge‐based analysis for genetic association. We mainly focused on the exonic NUDT15 and TPMT variants, which have been reported to be associated with AZA‐induced leukopenia. 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 The statistical threshold of genetic association in this study was performed by Bonferroni correction, in which significant P values were less than 2.86 × 10−8 (0.05/1,745,379). In addition, the sex and age adjustments were also performed during analysis.
Furthermore, we used the KING‐robust kinship estimator 19 , 20 on PLINK 2.0 software to evaluate relatedness in 40 patients with AZA‐induced leukopenia and 507 general population controls. A kinship value of > 0.177 indicates first‐degree relationships between the two samples. No relatedness was identified in our enrolled case and control groups.
We also compared the allelic and genotypic frequencies of 40 AZA‐induced toxicity with 86 AZA‐tolerant patients. The genotype results of NUDT15 R139C for these AZA‐tolerant patients were determined by quantitative polymerase chain reaction.
Data source and incidence analysis of AZA leukopenia in the retrospective cohort study
To assess the historical incidence rate of AZA‐related leukopenia, we gathered data between 2012 and 2017 from the multiple medical centers in Taiwan, which included 6,364 patients who have taken AZA, with or without AZA‐related leukopenia. The detailed design is shown in Figure S1 . The exclusion criteria included (1) patients with hematological malignancies (e.g., leukemia), (2) patients treated with chemotherapy and G‐CSF before taking AZA, (3) patients who were treated with cyclosporine (CsA), methotrexate (MTX), or mycophenolate mofetil (MMF) concomitantly with AZA treatment, (4) patients with sepsis or systemic lupus erythematosus (SLE) prior to AZA treatment, and (5) patients receiving AZA treatment for < 2 weeks. The diagnosis codes for the above diseases are from the International Classification of Diseases, Ninth and Tenth Revision, Clinical Modification (ICD‐9‐CM and ICD‐10‐CM; Figure S1 ), which were collected using the Anatomical Therapeutic Chemical classification system codes of the World Health Organization.
Because there is no ICD‐9‐CM and ICD‐10‐CM code for the definition of AZA‐related leukopenia, we further analyzed laboratory data to identify leukopenia, which was considered AZA‐induced if it occurred within 6 months of starting treatment. Leukopenia was graded according to white blood cell (WBC) counts as follows: grade 1, 3,000–4,000 cells/μL; grade 2, 2,000–3,000 cells/μL; grade 3, 1,000–2,000 cells/μL; and grade 4, < 1,000 cells/μL. We estimated the annual incidence of AZA‐related leukopenia in multiple medical centers in Taiwan as the annual number of toxicity cases caused by AZA divided by the annual number of new AZA users, defined as individuals who had not received the drug in the past 3 years according to the database.
The genetic screening prior AZA use in the prospective cohort study
To evaluate the effectiveness of genetic testing, between 2018 and 2021, we prospectively enrolled 1,056 patients with an indication for AZA treatment for autoimmune disorders who had not taken AZA previously. The exclusion criteria of the prospective cohort study included (1) prior treatment with AZA; (2) treatment with chemotherapy and G‐CSF prior to AZA treatment; and (3) concomitant treatment with AZA and CsA, MTX or MMF.
Before initiating AZA therapy, all enrolled participants were screened for the NUDT15 R139C genotype. We reported the genotyping results to the participating physicians within 1–7 days. Participants with the positive NUDT15 R139C result were recommended to take alternative medicine. Those with the negative result (and who also were counseled about the risk of AZA‐induced toxicity) were started on AZA treatment. If early symptoms of AZA‐related toxicity developed, a participant was asked to return to the clinic for a hematological or biochemical test. Participants were followed up for at least 6 months to monitor the symptoms of early leukopenia or liver injury throughout the study’s duration.
Additional information regarding methods used for WES variant calling/functional annotation, genetic NUDT15 R139C, and TPMT testing are provided in the Methods section in the Supplementary Information.
Statistical analysis
SPSS was used for statistical analysis (version 20; SPSS and SAS, version 9.2; SAS Institute, IBM Corp., Armonk, NY). Descriptive statistics included the incidence analysis and basic characteristics of participants. The P values were calculated with the use of the logistic‐regression model. For the genetic association study, we conducted the statistical analysis for association by comparing the allele frequencies between cases and controls in the additive model of inheritance. A sex/age adjustment was also performed. Pc values were calculated by Bonferroni correction for the multiple comparisons (0.05/1,745,379). P < 0.05 was considered statistically significant.
RESULTS
Genetic variant NUDT15 R139C shows the strongest association with AZA‐induced leukopenia and has a high frequency in Asian patients
Forty patients with AZA‐induced leukopenia (≥ grade 2; WBC ≤ 3,000 cells/μL) were first enrolled to identify, through WES, which genetic variants of NUDT15 or TPMT were strongly associated with AZA‐induced leukopenia in the Chinese population. We also enrolled the 507 individuals with no history of adverse drug reactions as the general population control group, and 86 AZA‐tolerant controls who received AZA for more than 6 months without adverse effects, to perform case–control association analysis. Clinical characteristics of the enrolled patient and control groups are shown in Table 1 . The age, gender, percentage of AZA dose ≥ 50 mg/day, indications, and underlying diseases of the enrolled patients with AZA‐induced leukopenia compared with AZA tolerant controls showed no statistical difference (Table 1 ). The periods of AZA therapy were 68.2 ± 55.2 days and 314.9 ± 239.7 days for the enrolled patients and tolerant controls, respectively (Table 1 ). To evaluate the early symptoms of these patients with AZA‐induced leukopenia, we found that fever, gastrointestinal side effects, malaise, weakness, nausea, and chills, among others, were observed and listed in Table S1 .
Table 1.
Clinical characteristics of azathioprine‐induced leukopenia (≥ grade 2; WBC ≤ 3,000 cells/μL) patients, general population, and azathioprine tolerant control during 2012–2017
| AZA‐induced leukopenia (n = 40) | AZA tolerant control (n = 86) | General population controls (n = 507) | P value | |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||
| Age, years, mean ± SD | 47.4 ± 18.0 | 41.2 ± 19.2 | 56.0 ± 19.2 | 0.113a |
| Male, n (%) | 17 (42.5) | 41 (47.7) | 253 (49.9) | 0.530c |
| Initial AZA dose ≥2 mg/kg/day, n (%) | 11 (27.5) | 21 (24.4) | – | 0.712c |
| Periods, day | 68.2 ± 55.2 | 314.9 ± 239.7 | – | < 0.001c |
| Indication, n (%) | ||||
| Eczema/AD | 33 (82.5) | 73 (84.9) | – | 0.993c |
| Urticaria | 3 (7.5) | 9 (10.5) | – | 0.993c |
| Bullous pemphigoid | 3 (7.5) | 2 (5.0) | – | 0.993c |
| Others | 1 (2.5) | 2 (5.0) | – | 0.993c |
| Comorbidity, n (%) | ||||
| CKD | 7 (17.5) | 8 (9.3) | – | 0.310c |
| CVD | 11 (27.5) | 18 (20.9) | – | 0.648c |
| DM | 4 (10.0) | 7 (8.1) | – | 0.810c |
| Liver disorderb | 6 (15.0) | 9 (10.4) | – | 0.876c |
We excluded AZA users of SLE, sepsis, and patients with leukemia to avoid confounding factors of leukopenia.
AD, atopic dermatitis; AZA, azathioprine; CKD, chronic kidney disease; CVD, cardiovascular disease; DM, diabetes mellitus; SLE, systemic lupus erythematosus; WBC, white blood cell.
This P value was calculated by using two‐tailed Student’s t tests.
Liver disorders include all kinds of hepatitis, necrosis of liver, hepatic failure, liver replaced by transplant (see Methods section), or patient’s GPT/GOT value was twofold higher than the normal value range (36 U/L) before AZA intake.
P values were calculated with AZA‐induced leukopenia comparing to AZA tolerant control by using logistic regression model.
The result of the WES study showed three variants in NUDT15 and one in TPMT were found in the patient and control groups. After sex and age adjustment, we identified the single‐nucleotide polymorphism (SNP) of NUDT15 R139C (rs116855232; P = 3.7 × 10−25, Pc = 6.5 × 10−19; odds ratio (OR) = 21.7, 95% confidence interval (CI) = 12.1–38.8) and rs746071566 (P = 4.2 × 10−9, Pc = 0.007; OR = 7.1, 95% CI = 3.7–13.7) as being associated with AZA‐induced leukopenia (Table 2 ). Furthermore, the polymorphisms rs186364861 in NUDT15 and rs1142345 in TPMT, were absent in our patients with AZA‐induced leukopenia (Table 2 ). Based on GnomAD and 1000Genomes database, the NUDT15 R139C (rs116855232) polymorphism was at higher minor allele frequencies (~ 0.101–0.111) in the general population of Asians, but lower in European and African descendants (~ 0.001–0.036), whereas the TPMT polymorphism was much less prevalent among Asians overall, and in the studied population in particular (Table S2 ).
Table 2.
Genotype frequencies of NUDT15 and TPMT variants in available patients with AZA‐induced leukopenia and general population controls in retrospective cohort study
| Gene | Chr. (location) | Fun. | WES/WGS in Chinese population | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Minor allele | MAF | OR (95% CI) | P value | Pc value | HWE | ||||
| Cases, N = 40 | Ctrls, N = 507 | ||||||||
| SNP | |||||||||
| NUDT15 | |||||||||
| rs116855232 | 13 (48045719) | NSV | T | 0.714 | 0.111 | 21.7 (12.1–38.8) | 3.7 × 10 −25 | 6.5 × 10 −19 | 0.303 |
| rs186364861 | 13 (48037798) | NSV | A | 0 | 0.022 | 0.0 (0.0–2.4) | 0.998 | – | 0.311 |
| rs746071566 a | 13 (48037783‐48037801) | Indel | GGAGTCGGAGTCG | 0.288 | 0.069 | 7.1 (3.7–13.7) | 4.2 × 10−9 | 0.007 | 0.020 |
|
TPMT rs1142345 |
6 (18130687) | NSV | C | 0 | 0.015 | 0.40 (0.0–6.8) | 0.999 | – | 0.965 |
P values were calculated with the use of logistic‐regression model adjusted by sex and age. Pc values were calculated by Bonferroni correction for the multiple comparisons (0.05/1,745,379). Bold values represent the significant P values for comprasion.
95% CI, 95% confidence interval; AZA, azathioprine; Chr., chromosome; Ctrls, population controls; Fun., functional variant type; HWE, Hardy–Weinberg equilibrium P values for 507 controls from the Chinese general population of Taiwan; Indel, insertion and deletion; MAF, minor allele frequency; NUDT15, nudix hydrolase 15; NSV, nonsynonymous; OR, odds ratio; SNP, single‐nucleotide polymorphism; TPMT, Thiopurine methyltransferase or thiopurine S‐methyltransferase; WES, whole exome sequencing; WGS, whole genome sequencing.
The rs554405994 was merged into rs869320766 which was further merged into rs746071566 on October 12, 2018 (based on the build 155 of NCBI dbSNP website; https://www.ncbi.nlm.nih.gov/snp/rs746071566?horizontal_tab=true). The rs746071566 belongs to inframe deletion variant.
Considering the association between AZA‐induced leukopenia and both SNPs, NUDT15 rs116855232 and rs746071566 were statistically significant, we next evaluated the linkage disequilibrium (LD) for these two NUDT15 SNPs in patients with AZA‐induced leukopenia and a general population control group. Our results showed no strong LD between NUDT15 rs116855232 and NUDT15 rs746071566 (r 2 = 0.102 and r 2 = 0.302 for AZA‐induced leukopenia cases and general population controls, respectively; Table S3 ). Therefore, in AZA‐induced leukopenia cases, NUDT15 rs116855232 does not form an extended haplotype with NUDT15 rs746071566 (r 2 = 0.102).
We further compared genotypic frequencies of NUDT15 R139C from patients with AZA‐induced leukopenia and AZA‐tolerant controls. Allelic frequency of NUDT15 R139C was both significantly higher in those patients who exhibited toxicity than in general population controls and AZA‐tolerant controls (P = 3.2 × 10−12; OR = 25.8, 95% CI = 10.4–64.5; Table 3 ).
Table 3.
Genotype frequencies of NUDT15 rs116855232 and P values in AZA‐induced leukopenia cases, population, and tolerant controls in Chinese population
| Group | Genotype frequency | Allele frequency | OR (95% CI) | P value | ||
|---|---|---|---|---|---|---|
| N/total N (%) | ||||||
| Homozygous (T/T) | Heterozygous (T/C) | Noncarrier (C/C) | (T) | |||
| AZA‐induced leukopeniaa | 20/40 (50.0%) | 17/40 (42.5%) | 3/40 (7.5%) | 0.714 | ||
| Population controls | 4/507 (0.7%) | 105/507 (20.7%) | 398/507 (78.5%) | 0.111 | 21.7 (12.1–38.8) | 3.7 × 10 −25 |
| Tolerant controls | 0/86 (0%) | 21/86 (24.4%) | 65/86 (75.6%) | 0.122 | 25.8 (10.4–64.5) | 3.2 × 10 −12 |
Tolerant control patients who had received AZA for several times and the cumulative duration was for more than 6 months without evidence of adverse reactions. We conducted the statistical analysis for the association by comparing the allele frequencies between cases and controls in the additive model of inheritance, and P values were calculated by a logistic regression model adjusted for sex and age. Bold values represent the significant P values for comprasion.
95% CI, 95% confidence interval; AZA, azathioprine; NUDT15, Nudix Hydrolase 15; OR, odds ratio.
AZA‐induced leukopenia indicates that ≥ grade 2 leukopenia (white blood cell (WBC) ≤ 3,000 cells/μL) was occurred after AZA intake.
The highest sensitivity of NUDT15 variants for patients with AZA‐induced leukopenia was R139C (rs116855232) with 92.5% (Table 2 ). We further found that patients with AZA‐induced leukopenia with NUDT15 R139C (rs116855232) positive were all co‐carried NUDT15 (rs746071566; Table S3 ), and a combination of another NUDT15 and TPMT variants could not improve the genetic sensitivity for AZA‐induced leukopenia. Therefore, we only chose the polymorphism of NUDT15 R139C (rs116855232) for the pre‐emptive genetic testing study.
Prospective NUDT15 R139C screening
We prospectively enrolled 1,056 patients with an indication for AZA treatment of autoimmune disorders from 2018 to 2021, but without previous exposure to AZA or genetic testing. The detailed exclusion criteria of the prospective cohort study were described in the Methods. Because 43 participants were excluded due to loss of follow‐up or withdrawal by subject, a total of 1,013 participants completed the study (Figure 1 ). Two hundred forty‐eight patients (24.5%) who tested positive for NUDT15 R139C were advised against this drug and referred for an alternative immunosuppressant. Seven hundred sixty‐five patients (75.5%) who tested negative were given AZA and interviewed for 6 months to monitor symptoms throughout the study. We then analyzed the demographics (age and gender) and comorbidities for these two groups and found no significant differences between participants who tested positive or negative for NUDT15 R139C (Table S4 ).
Figure 1.
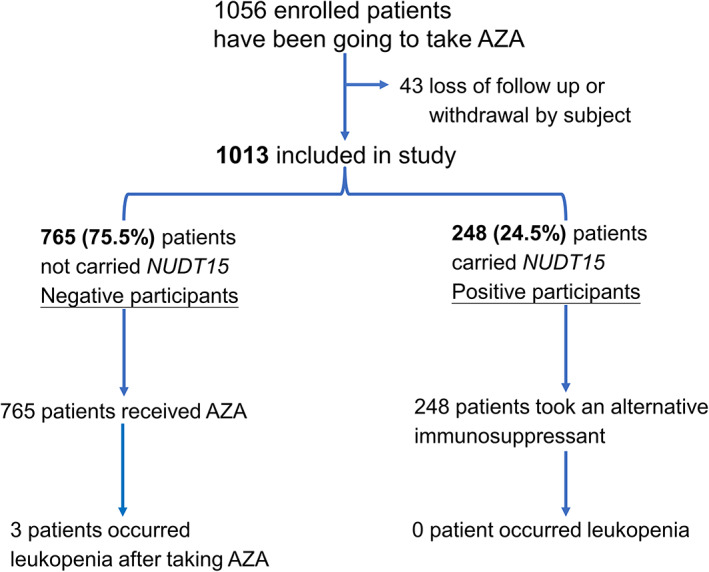
The enrollment and outcomes of prospectively genetic NUDT15 R139C testing. Azathioprine (AZA) was prescribed and provided for all participants at the time of the screening visit, but they were asked to defer taking AZA until the genetic results were available. Participants were monitored symptoms and followed for at least 6 months, with weekly or every 2‐week interviews.
Evaluation of the historical incidence of AZA‐related leukopenia
To analyze the historical incidence of AZA‐related leukopenia, we retrospectively included a total of 6,364 patients receiving AZA prescriptions in the multiple medical centers in Taiwan between 2012 and 2017. The exclusion criteria of the retrospective cohort are listed in Figure S1 . We found that the main autoimmune disorders associated with AZA treatment included eczema/AD, SLE/lupus, and urticaria, among others, as depicted in Figure S2 . After meeting the exclusion criteria to avoid confounding factors, 3,244 patients were assessed for the historical incidence of AZA‐induced leukopenia, and a total of 344 patients were identified as having AZA‐related leukopenia. The historical incidence of ≥ grade 2 and ≥ grade 4 AZA‐related leukopenia were 7.6% and 4.4%, respectively (Table 4 ).
Table 4.
Historical incidence of AZA‐induced leukopenia, compared with incidence among prospective genetic screening study participants
| Variants | Year | P value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | Total | 2018–2021 | ||
| Retrospective cohort | Prospective cohort | ||||||||
| (Historical incidence, %) | (Actual incidence, %) | ||||||||
| (95% CI) | (95% CI) | ||||||||
| AZA‐related leukopenia | |||||||||
| ≥Grade 4 | 4.4% | 4.3% | 2.6% | 5.0% | 5.7% | 4.6% | 4.4% (3.7–5.7) | 0% (0–0.5) | 5.1 ×10 −14 a |
| ≥ Grade 3 | 5.0% | 6.6% | 3.0% | 5.0% | 5.9% | 6.0% | 5.2% (4.4–6.0) | 0.3% (0–0.6) | 1.7 × 10 −13 a |
| ≥ Grade 2 | 5.7% | 8.8% | 6.3% | 8.4% | 7.8% | 10.5% | 7.6% (6.7–8.6) | 0.4% (0–0.8) | 9.3 × 10 −20 a |
Bold values represent the significant P values for comprasion.
≥ Grade 2 leukopenia indicates WBC ≤ 3,000 cells/μL after AZA intake.
≥ Grade 3 leukopenia indicates WBC ≤ 2,000 cells/μL after AZA intake.
≥ Grade 4 leukopenia indicates WBC ≤ 1,000 cells/μL after AZA intake.
AZA, azathioprine; CI, confidence interval; WBC, white blood cell.
P values were calculated by comparing the historical incidence and actual incidence among study participants.
NUDT15 R139C genetic screening significantly decreased the incidence of AZA‐induced leukopenia
After performing genetic screening for NUDT15 R139C, the actual incidence of AZA‐induced leukopenia could be decreased significantly through the use of alternative immunosuppressants in carriers of the NUDT15 R139C variant. The actual incidence of ≥ grade 2 AZA leukopenia showed the most significant decrease, from 7.6% to 0.4% (P = 9.3 × 10−20). Importantly, ≥ 4 grade AZA‐induced leukopenia, the most life‐threatening condition, was undetectable after genetic screening (in contrast with 4.4% of historical incidence; P = 5.1 × 10−14; Table 4 ).
Among the 765 patients who tested negative for NUDT15 R139C, three developed leukopenia after taking AZA (Figure 1 ). We analyzed the demographics, comorbidities, and TPMT polymorphisms in these three patients. Among these patients, two had underlying liver disorders (high GOP/GPT values), and one had cardiovascular disease, whereas none tested positive for the TPMT polymorphisms (Table S5 ). Because none of these patients carried a TPMT polymorphism, testing only NUDT15 R139C would be more relevant and cost‐effective in our population.
The high positive and negative predictive values of genetic NUDT15 R139C screening
We further analyzed the positive predictive value (PPV) and negative predictive value (NPV) of NUDT15 R139C to prevent AZA leukopenia, and found the PPV and NPV were 26.14% (95% CI = 25.61–26.67%) and 99.22% (95% CI = 99.15–99.28%), respectively (Table 5 ). Theoretically, one patient could be prevented from experiencing AZA leukopenia for at least 15 (95% CI = 13–17) individuals screened (Table 5 ).
Table 5.
Calculations used for number needed to screen to prevent one case of AZA‐induced leukopenia and the positive/negative predictive values with polymorphism of NUDT15 R139C (rs116855232)
| NUDT15 R139C | Positive | Negative | Total | Notes |
|---|---|---|---|---|
| AZA‐induced leukopenia | 7,030 | 570 | 7,600 |
Incidence = 7.60% Sensitivity = 92.5% |
| Control | 19,866 | 72,534 | 92,400 | Specificity = 78.5% |
| Total | 26,896 | 73,104 | 100,000 | |
|
PPV = 26.14% (25.61–26.67%) |
NPV = 99.22% (99.15–99.28%) |
NNS to prevent one case = 15 (13–17) |
A theoretical population of 100,000 has been assumed, and the number of individuals with the ADR calculated based on previous reported prevalence, and the number of population controls calculated by subtracting this figure from 100,000. The sensitivity and specificity of the genetic NUDT15 R139C (rs116855232) test for the c AZA‐induced leukopenia have been taken from Table 3 and the numbers in each of the four cells (a, b, c, and d) calculated. The PPV, NPV, and NNS were then calculated from the numbers in each cell (a, b, c, and d).
ADR, adverse drug reactions; AZA, azathioprine; NNS, number needed to screen; NPV, negative predictive value; PPV, positive predictive value.
DISCUSSION
The genes, TPMT and NUDT15, are known to be involved in the metabolism of thiopurines. 21 Variants within these genes are associated with the functional impairment of AZA metabolizing enzymes or drug transporters, leading to AZA‐indued hepatotoxicity and leukopenia. 4 , 11 , 13 , 22 , 23 AZA may also induce acute pancreatitis in patients with inflammatory bowel diseases 24 ; however, we did not find any of our enrolled patients who developed pancreatitis during AZA therapy. This may be explained by mainly enrolling patients with autoimmune diseases in our study.
Although the association between TPMT polymorphisms and AZA‐induced leukopenia is well‐established, these variants are less common among Asian patients than among White patients, 25 , 26 and recent studies showed that NUDT15 polymorphisms were associated with thiopurine‐induced toxicity in Asians, including Chinese, Japanese Korean, and Indian populations. 5 , 15 , 16 , 27 , 28 , 29 , 30 Preventative screening approaches can be very effective in preventing treatment failure and adverse reactions with severe complications. 31 , 32 Our previous studies have shown important clinical implementations of pharmacogenomic testing to prevent severe adverse drug reactions. 22 , 33 , 34 , 35 , 36 , 37 Therefore, this study aimed to investigate the strength of genetic NUDT15 and TPMT associations with AZA‐induced leukopenia in Chinese patients and which genetic variant would be appropriate for prospective screening before AZA treatment.
A high incidence of AZA‐induced leukopenia among NUDT15 R139C carriers was observed in our Chinese population (the carrier prevalence of NUDT15 R139C is 24.4%). However, real‐world and large‐scale studies of genetic NUDT15 R139C screening prior to AZA therapy in Asian patients with autoimmune disorders are still lacking. In this study, we conducted a prospective screening of NUDT15 R139C for 1,013 participants with autoimmune disorders before initiating AZA therapy. Prospective genetic screening and subsequent treatment adjustment for NUDT15 R139C carriers resulted in a significant and substantial decrease in the incidence of AZA‐induced leukopenia; therefore, targeted genetic screening of NUDT15 R139C can be a valuable strategy to avoid AZA‐induced leukopenia in the Chinese population. A strong association between NUDT15 R139C and thiopurine‐induced leukopenia has also been observed in other Asian populations, suggesting that NUDT15 R139C screening may benefit other Asian populations, including Japanese, Korean, and Indian populations by identifying carriers of the NUDT15 variant who then can receive alternative immunosuppressants. However, a small number of patients who screened negative for NUDT15 R139C still had leukopenia induced by AZA, suggesting that additional variables could contribute to between‐patient variability in AZA toxicity. Our results showed that the few patients who screened negative for NUDT15 R139C, but nonetheless experienced AZA leukopenia, did not carry TPMT variants, suggesting that genetic testing for TMPT polymorphisms is not needed for Chinese patients. Moreover, in this study, patients who tested positive for NUDT15 R139C were advised against AZA and referred for an alternative immunosuppressant. We did not reduce the AZA dose for NUDT15 R139C carriers. Future research is therefore necessary to determine with certainty the exact point at which a reduced AZA dose in decreasing the incidence of AZA‐induced leukopenia for patients who carried NUDT15 R139C.
We evaluated the historical incidence of AZA‐related leukopenia based on the hospital database from multiple medical centers in Taiwan. However, there are still several limitations, including (1) all results relied exclusively on electronic medical records and laboratory data, and misclassification of diseases were defined based on registry‐based data set; and (2) we could not ascertain whether the patients had continued exposure to AZA beyond the index prescription or in another hospital.
Potential strategies to reduce the incidence of AZA‐induced leukopenia may include: (1) NUDT15 R139C genetic screening before prescribing AZA to individuals among populations with a high prevalence of the risk allele; and (2) the use of alternative drug(s) for NUDT15 R139C‐positive patients. Additional research would be necessary to calculate the cost‐effectiveness of genetic screening for NUDT15 R139C. Furthermore, in countries where the allele frequency is low (about 1%) of NUDT15, restricting the screening of this allele, or including additional polymorphisms, such as TPMT, in higher‐risk patients could also be a strategy to prevent or reduce the occurrence of AZA toxicity. Further studies to estimate the prevalence are suggested.
In conclusion, this study revealed that the genetic variant NUDT15 R139C (rs116855232) had the strongest association with AZA‐induced leukopenia in the Chinese population and a high incidence of AZA leukopenia is observed in Taiwan. Prospective screening of the NUDT15 R139C variant, coupled with alternative drug treatment for carriers, is highly effective and can significantly decrease AZA leukopenia incidence in Chinese patients. One case of AZA induced‐leukopenia can be prevented for every 15 patients screened, highlighting the potential benefits of personalized medicine and genetic testing in preventing adverse drug reactions in the clinical setting. Therefore, physicians are recommended to perform genetic screening for NUDT15 R139C before prescribing AZA therapy for Chinese patients to prevent AZA‐induced leukopenia.
FUNDING
This work was supported by grants from the Ministry of Science and Technology, Taiwan (MOST 109‐2326‐B‐182A‐001‐, 108‐2314‐B‐182A‐104 ‐MY3, and 108‐2320‐B‐182A‐024 ‐MY2), and Chang Gung Memorial Hospital (CMRPG3K2181, CORPG3J0321‐3, and CORPG1J0011‐3). We also thank Sanofi Taiwan Co. Ltd. for financial support of incidence analysis.
CONFLICT OF INTEREST
All authors declared no competing interests for this work.
AUTHOR CONTRIBUTIONS
C.W.W. and M.H.C. wrote the manuscript. C.W.W., M.H.C., Chi‐J.C., Chee‐J.C., and Y.J.L. analyzed the data. C.W.W., R.C.Y.H., and W.H.C. designed the research. M.H.C., T.F.T., K.H.Y., H.W.K., H.C.C., C.B.C., C.W.L., W.T.C., Y.C.C., Y.T.C., Y.J.J.W., Y.H.H., H.Y.H., C.Y.N., P.W.H., R.C.Y.H., and W.H.C. performed the research.
ETHICS APPROVAL
This study was approved by the institutional review board (IRB) the ethics committee of the CGMH health system and other different multiple medical centers in Taiwan and China (IRB No. 104‐0291B, 201701824A3C501, 201801423B0, 202001515B0, and 202000852B0). Informed consent was obtained from each participant.
Supporting information
Supporting Information 1
Supporting Information 2
ACKNOWLEDGMENTS
The authors thank the statistical assistance and wish to acknowledge the support of the Maintenance Project of the Biostatistical Consultation Center (Grant CLRPG2C0021‐4 and CLRPG2G0081‐3) at Chang Gung Memorial Hospital for study design and monitor, data analysis, and interpretation.
References
- 1. Bjornsson, E.S. et al. Azathioprine and 6‐mercaptopurine‐induced liver injury: clinical features and outcomes. J. Clin. Gastroenterol. 51, 63–69 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Siramolpiwat, S. & Sakonlaya, D. Clinical and histologic features of azathioprine‐induced hepatotoxicity. Scand. J. Gastroenterol. 52, 876–880 (2017). [DOI] [PubMed] [Google Scholar]
- 3. Luber, R.P. , Honap, S. , Cunningham, G. & Irving, P.M. Can we predict the toxicity and response to thiopurines in inflammatory bowel diseases? Front. Med. (Lausanne) 6, 279 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yang, J. et al. NUDT15 and TPMT genetic polymorphisms are related to azathioprine intolerance in Chinese patients with rheumatic diseases. Genet. Test Mol. Biomarkers 23, 751–757 (2019). [DOI] [PubMed] [Google Scholar]
- 5. Moriyama, T. et al. NUDT15 polymorphisms alter thiopurine metabolism and hematopoietic toxicity. Nat. Genet. 48, 367–373 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Matsuoka, K. NUDT15 gene variants and thiopurine‐induced leukopenia in patients with inflammatory bowel disease. Intest Res. 18, 275–281 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heckmann, J.M. , Lambson, E.M. , Little, F. & Owen, E.P. Thiopurine methyltransferase (TPMT) heterozygosity and enzyme activity as predictive tests for the development of azathioprine‐related adverse events. J. Neurol. Sci. 231, 71–80 (2005). [DOI] [PubMed] [Google Scholar]
- 8. Ben Salem, C. , Ben Salah, L. , Belajouza, C. & Bouraoui, K. Azathioprine‐induced severe cholestatic hepatitis in patient carrying TPMT*3C polymorphism. Pharm. World Sci. 32, 701–703 (2010). [DOI] [PubMed] [Google Scholar]
- 9. Budhiraja, P. & Popovtzer, M. Azathioprine‐related myelosuppression in a patient homozygous for TPMT*3A. Nat. Rev. Nephrol. 7, 478–484 (2011). [DOI] [PubMed] [Google Scholar]
- 10. Relling, M.V. et al. Clinical pharmacogenetics implementation consortium guideline for thiopurine dosing based on TPMT and NUDT15 genotypes: 2018 update. Clin. Pharmacol. Ther. 105, 1095–1105 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Walker, G.J. et al. Association of genetic variants in NUDT15 with thiopurine‐induced myelosuppression in patients with inflammatory bowel disease. JAMA 321, 773–785 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Avallone, E.V. , Pica, R. , Cassieri, C. , Zippi, M. , Paoluzi, P. & Vernia, P. Azathioprine treatment in inflammatory bowel disease patients: type and time of onset of side effects. Eur. Rev. Med. Pharmacol. Sci. 18, 165–170 (2014). [PubMed] [Google Scholar]
- 13. Yang, S.K. et al. A common missense variant in NUDT15 confers susceptibility to thiopurine‐induced leukopenia. Nat. Genet. 46, 1017–1020 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miao, Q. et al. Association of genetic variants in TPMT, ITPA, and NUDT15 with azathioprine‐induced myelosuppression in Southwest China patients with autoimmune hepatitis. Sci. Rep. 11, 7984 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kakuta, Y. et al. NUDT15 R139C causes thiopurine‐induced early severe hair loss and leukopenia in Japanese patients with IBD. Pharmacogenomics J. 16, 280–285 (2016). [DOI] [PubMed] [Google Scholar]
- 16. Tanaka, Y. et al. Susceptibility to 6‐MP toxicity conferred by a NUDT15 variant in Japanese children with acute lymphoblastic leukaemia. Br. J. Haematol. 171, 109–115 (2015). [DOI] [PubMed] [Google Scholar]
- 17. Kham, S.K. et al. Thiopurine S‐methyltransferase activity in three major Asian populations: a population‐based study in Singapore. Eur. J. Clin. Pharmacol. 64, 373–379 (2008). [DOI] [PubMed] [Google Scholar]
- 18. Wang, C.W. et al. Whole genome sequencing identifies genetic variants associated with co‐trimoxazole hypersensitivity in Asians. J. Allergy Clin. Immunol. 147, 1402–1412 (2021). [DOI] [PubMed] [Google Scholar]
- 19. Yang, J. , Lee, S.H. , Goddard, M.E. & Visscher, P.M. GCTA: a tool for genome‐wide complex trait analysis. Am. J. Hum. Genet. 88, 76–82 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Manichaikul, A. , Mychaleckyj, J.C. , Rich, S.S. , Daly, K. , Sale, M. & Chen, W.M. Robust relationship inference in genome‐wide association studies. Bioinformatics 26, 2867–2873 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pristup, J. et al. Molybdenum cofactor catabolism unravels the physiological role of the drug metabolizing enzyme thiopurine S‐methyltransferase. Clin. Pharmacol. Ther. 112, 808–816 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chung, W.H. , Wang, C.W. & Dao, R.L. Severe cutaneous adverse drug reactions. J. Dermatol. 43, 758–766 (2016). [DOI] [PubMed] [Google Scholar]
- 23. Shiohara, T. & Mizukawa, Y. Drug‐induced hypersensitivity syndrome (DiHS)/drug reaction with eosinophilia and systemic symptoms (DRESS): an update in 2019. Allergol. Int. 68, 301–308 (2019). [DOI] [PubMed] [Google Scholar]
- 24. Teich, N. et al. Azathioprine‐induced acute pancreatitis in patients with inflammatory bowel diseases – a prospective study on incidence and severity. J. Crohns Colitis 10, 61–68 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. de Boer, N.K. , Mulder, C.J. & van Bodegraven, A.A. Myelotoxicity and hepatotoxicity during azathioprine therapy. Neth. J. Med. 63, 444–446 (2005). [PubMed] [Google Scholar]
- 26. Severine, W. , Xavier, K. & Jean‐Charles, C. A rare case of azathioprine‐induced leukopenia in an European woman. Acta Clin. Belg. 77, 163–167 (2022). [DOI] [PubMed] [Google Scholar]
- 27. Kim, S.Y. et al. NUDT15 p.R139C variant is common and strongly associated with azathioprine‐induced early leukopenia and severe alopecia in Korean patients with various neurological diseases. J. Neurol. Sci. 378, 64–68 (2017). [DOI] [PubMed] [Google Scholar]
- 28. Fei, X. et al. NUDT15 R139C variation increases the risk of azathioprine‐induced toxicity in Chinese subjects: case report and literature review. Medicine (Baltimore) 97, e0301 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fei, X. et al. NUDT15 R139C variants increase the risk of azathioprine‐induced leukopenia in Chinese autoimmune patients. Front. Pharmacol. 9, 460 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Banerjee, R. et al. NUDT15 C415T variant compared with TPMT genotyping in predicting azathioprine‐induced leucopenia: prospective analysis of 1014 inflammatory bowel disease patients in India. Aliment. Pharmacol. Ther. 52, 1683–1694 (2020). [DOI] [PubMed] [Google Scholar]
- 31. Leopold, J.A. & Loscalzo, J. Emerging role of precision medicine in cardiovascular disease. Circ. Res. 122, 1302–1315 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang, C.W. , Preclaro, I.A.C. , Lin, W.H. & Chung, W.H. An updated review of genetic associations with severe adverse drug reactions: translation and implementation of pharmacogenomic testing in clinical practice. Front. Pharmacol. 13, 886377 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chung, W.H. et al. Medical genetics: a marker for Stevens‐Johnson syndrome. Nature 428, 486 (2004). [DOI] [PubMed] [Google Scholar]
- 34. Chen, P. et al. Carbamazepine‐induced toxic effects and HLA‐B*1502 screening in Taiwan. N. Engl. J. Med. 364, 1126–1133 (2011). [DOI] [PubMed] [Google Scholar]
- 35. Hung, S.I. et al. HLA‐B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc. Natl. Acad. Sci. USA 102, 4134–4139 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ko, T.M. et al. Use of HLA‐B*58:01 genotyping to prevent allopurinol induced severe cutaneous adverse reactions in Taiwan: national prospective cohort study. BMJ 351, h4848 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang, C.W. , Dao, R.L. & Chung, W.H. Immunopathogenesis and risk factors for allopurinol severe cutaneous adverse reactions. Curr. Opin. Allergy Clin. Immunol. 16, 339–345 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information 1
Supporting Information 2


