Abstract
Mental health disorders, particularly depression and anxiety, affect a significant number of the global population. Several pathophysiological pathways for these disorders have been identified, including the hypothalamic‐pituitary‐adrenal axis, autonomic nervous system, and the immune system. In addition, life events, environmental factors, and lifestyle affect the onset, progression, and recurrence of mental health disorders. These may all overlap with periodontal and/or peri‐implant disease. Mental health disorders are associated with more severe periodontal disease and, in some cases, poorer healing outcomes to nonsurgical periodontal therapy. They can result in behavior modification, such as poor oral hygiene practices, tobacco smoking, and alcohol abuse, which are also risk factors for periodontal disease and, therefore, may have a contributory effect. Stress has immunomodulatory effects regulating immune cell numbers and function, as well as proinflammatory cytokine production. Stress markers such as cortisol and catecholamines may modulate periodontal bacterial growth and the expression of virulence factors. Stress and some mental health disorders are accompanied by a low‐grade chronic inflammation that may be involved in their relationship with periodontal disease and vice versa. Although the gut microbiome interacting with the central nervous system (gut‐brain axis) is thought to play a significant role in mental illness, less is understood about the role of the oral microbiome. The evidence for mental health disorders on implant outcomes is lacking, but may mainly be through behaviourial changes. Through lack of compliance withoral hygiene and maintenance visits, peri‐implant health can be affected. Increased smoking and risk of periodontal disease may also affect implant outcomes. Selective serotonin reuptake inhibitors have been linked with higher implant failure. They have an anabolic effect on bone, reducing turnover, which could account for the increased loss.
Keywords: Alzheimer disease, anxiety disorder, bipolar, depression, gut‐brain axis, mental health, microbiome, mood (affective) disorder, peri‐implant disease, periodontal disease, schizophrenia, stress, substance use disorder
1. INTRODUCTION
Periodontal diseases are characterized by microbial challenge in a susceptible individual and can result in destruction to the supporting tissues of the tooth with the potential for eventual tooth loss. 1 , 2 Peri‐implantitis appears to be a multifactorial disease that is characterized by inflammation in the peri‐implant mucosa with progressive loss of supporting bone surrounding a dental implant. 2 , 3 Epidemiologic studies have shown that periodontal and peri‐implant diseases do not affect all the population in the same way, with clear variation in phenotypic expression. 4 , 5 , 6 , 7 The presence of keystone pathogenic species with the potential to cause dysbiotic disease, coupled with variations in genes that encode the componentry of the host's immune system, sets the stage for individual differences in periodontitis risk. 8 , 9 Less is known about the heterogeneous infection that includes periodontopathic microorganisms 10 and an individual's genetic susceptibility 11 , 12 in relation to peri‐implantitis risk. It is apparent that periodontal and peri‐implant diseases have a complex pathogenesis and are multifactorial in etiology. 13 Epidemiologic and experimental evidence exists for the role of a number of risk factors in the initiation, progression, and severity of periodontal and peri‐implant diseases. For periodontal disease, these risk factors include male gender, smoking, poorly controlled diabetes mellitus and possibly obesity, osteoporosis, and low dietary calcium and vitamin D. 14 For peri‐implantitis, risk factors with strong evidence include a history of periodontitis, poor plaque control, and lack of regular maintenance. 15 In addition, implant failure may cluster in patients. 16 A substantial body of evidence indicates that psychosocial stress may play a role in periodontal diseases, with less evidence available to support the role of mental health disorders. Interestingly, there is increasing evidence to show that the gut microbiota has an important function in modulating brain functionality and, therefore, behavior. The connection has been termed the brain‐gut axis and is a significant area of study to understand mental disorders, particularly depression. 17 Little research has evaluated the relationship between the oral microflora, their interactions with the brain, and mental health disorders. Increasingly, mental health disorders and some of their pharmacotherapies have been linked to higher rates of implant failure. 18 , 19 , 20 , 21 Possible mechanisms involve inconsistent compliance with dental visits, a lack of adherence to oral hygiene regimes, and use of antidepressants. Understanding the relationship between mental health disorders, periodontal diseases, and peri‐implantitis may allow for improvements in the prevention and treatment of these diseases.
2. WHAT ARE MENTAL HEALTH DISORDERS AND WHO GETS THEM?
Mental health disorders are a group of diseases that are often chronic in nature, presenting with high comorbidity rates and poor treatment outcomes. 22 High‐prevalence mental health disorders include mood (affective) disorders. According to the International Classification of Diseases‐10, mood disorders comprise a spectrum of diseases that reflect a change of affect and mood. One end of the spectrum represents a depressed state (eg, a depressive episode), whereas the other is an elated state (eg, a manic episode). Patients can experience both; for example, in bipolar disorder. Mood disorders tend to be recurrent and often are initiated by environmental stressors. 23 Disorders such as schizophrenia, Alzheimer disease, and substance use disorders remain in other arms of the International Classification of Diseases‐10.
Depressive disorders are characterized by sadness, loss of interest and pleasure, feelings of guilt or low self‐esteem, disturbed sleep or appetite, feelings of tiredness, and poor concentration. They can be persistent and recur, leading to problems functioning at work or coping with daily life. Anxiety disorders are characterized by feelings of anxiety and fear. They can be broadly described as a group of disorders in which anxiety is induced in situations that may or may not be well defined. These situations often produce a feeling of strong avoidance, or they are endured with apprehension. Examples of physical symptoms include palpitations, shortness of breath, or feeling faint, and they are often accompanied with irrational thoughts, such as fears of dying, losing control, or going mad. Anxiety and depression are often coexistent conditions. 23
Data from 2015 indicate the prevalence of depression to be 4.4%, and more prevalent in females. This is roughly 322 million people worldwide. Prevalence appears to peak around older adulthood, 55‐74 years. Between 2005 and 2015 there was an 18.4% increase in the number of people living with depression. In the United States in 2009, US$22.8 billion were spent on the treatment of depression with the loss of productivity estimated at US$23 billion in 2011. 24 The prevalence of anxiety disorders is around 3.6% and again more common in females, equating to 264 million people worldwide. Interestingly, prevalence does not vary considerably between age groups. The largest single contributor to nonfatal health loss is depressive disorders. 25
Mental health disorders have been associated with cardiovascular disease and diabetes mellitus. This may be through lifestyle factors, such as smoking, reduced activity, poor diet, obesity, hypertension, or a lack of adherence to health programs or advice commonly seen in patients with mental health disorders. Though biological effects may be due to alterations in neurotransmitter and hormone levels such as serotonin and cortisol, which influence the immune response, systemic inflammation may also play a role through bidirectional relationships. 26
Risk factors for depression in adults include sex, age, race/ethnicity, education, marital status, geographic location, employment status, chronic illness, substance abuse, and family history of psychiatric illness. 24 Exposure to traumatic childhood events is now recognized as an antecedent to future major depressive disorder. The risk factors in older adults are slightly different and reflect a greater age. They include disability, complicated grief, chronic sleep disturbance, loneliness, and a history of depression. Different factors may influence the relapse and recurrence of depression than those that influenced the initial onset. Moreover, major depressive disorder is associated with poorer health and earlier death. This includes a higher prevalence of heart disease, diabetes, obesity, cognitive impairment, disability, and cancer. In addition, unhealthy lifestyles are common, with poorer self‐care and adverse effects of medications.
2.1. Biological mechanisms to explain mental health disorders
No established mechanism can explain all aspects of depression. 27 Major depressive disorder clusters within families, with a threefold risk for first‐degree relatives and a suggested 35% heritability. However, it is highly polygenic and involves many genes with small effects. Single‐nucleotide polymorphism analysis is inconclusive, and there has been limited success with genome‐wide association analysis. 28 This may be due to the heterogeneity of the diseases, the need for large sample sizes, the influence of environment, or patient phenotypes, which is very similar to periodontitis. Gene expression studies have shown increases in tumor necrosis factor, interleukin‐1β, cyclo‐oxygenase, inducible nitric oxide synthase, and calcium signaling genes. However, it is thought that the environment and gene‐environment interactions are likely to have large role in inflammation‐related depression. In addition, the effects of some single‐nucleotide polymorphisms may only become evident in the presence of life stressors. Stress‐dependent epigenetic deoxyribonucleic acid methylation of glucocorticoid‐response elements may lead to glucocorticoid receptor resistance. Immune genetic variants that increase risk of depression are likely to increase risk for obesity, diabetes, and cardiovascular disease and contribute to overlap between depression and other medical conditions.
There are several pathophysiological pathways that have been implicated in mental health disorders, including the hypothalamic‐pituitary‐adrenal axis, autonomic nervous system, and the immune system. Childhood exposure to risk factors for mental health disorders may persistently increase the activity of corticotropin‐releasing hormone containing neural circuits leading to reduced glucocorticoid receptor function. Hypothalamic‐pituitary‐adrenal alterations correlate with impaired cognitive activity and increased cortisol levels are a risk factor for subsequent impairment. 29 Chronic low‐grade inflammation triggers changes that contribute to mental ill health. Neuroinflammation is a term for the immune‐related process that occurs within the brain and can result from peripheral infection. It is associated with sickness behaviors such as fatigue, anhedonia, and sleep disturbance. 30 Depression has been suggested to be a chronic overextended version of sickness behavior. Any acute infection can affect mood state by the production of proinflammatory cytokines that can play role in generation of psychological phenomena. However, chronic infection is detrimental due to the effects on brain homeostasis. Although the brain lacks the adaptive arm of the immune response, peripheral immune events will affect the brain, such as immune cells entering the central nervous system or cytokine activation of brain cells. Chronic stress can cause inflammasome complex expression in the microglia of the hippocampus and other mood‐regulating areas, where prolonged activation of microglia is thought to be detrimental and may occur in depression. 31 The peripheral release of inflammatory cytokines can activate the hypothalamic‐pituitary‐adrenal axis. Nitrous oxide is an important gaseous neurotransmitter normally found at low concentrations in noninflammatory conditions, but it is increased due to induction of nitrous oxide synthase by inflammatory cytokines and can cause neuronal damage. The glucocorticoid inflammation hypothesis of depression (see later) describes how chronic stress affects glucocorticoid expression and sensitivity, which may result in less repair of damaged neural cells and an overall increase in inflammation. Reduced neuronal repair and decreased protein synthesis can contribute to changes in the brain that are seen in depression.
Recently, Miller and Raison 32 have argued for the pathogen host defense hypothesis of depression (Figure 1). They explain that an active immunity was required in early human evolution for survival, which resulted in the integration of immunological and behavioral responses to conserve energy for recovery. This immune response was “held in check” by the coevolved nonfatal immunoregulatory microflora present throughout the human body. In the clean, modern world relatively absent of the same exposure to the immunoregulatory flora, risk factors result in activation of the host defenses, leading to the release of proinflammatory cytokines and onset of sickness with depression‐like behavior. They suggest women appear to be more sensitive to the effects of inflammation on behavior, which may account for the sex differences in disease prevalence. Analyses of blood in subjects with major depressive disorders show increased levels of proinflammatory cytokines and receptors, interleukin‐6, interleukin‐8, tumor necrosis factor alpha, and toll‐like receptor 3 and 4, as well as acute‐phase reactants.
FIGURE 1.
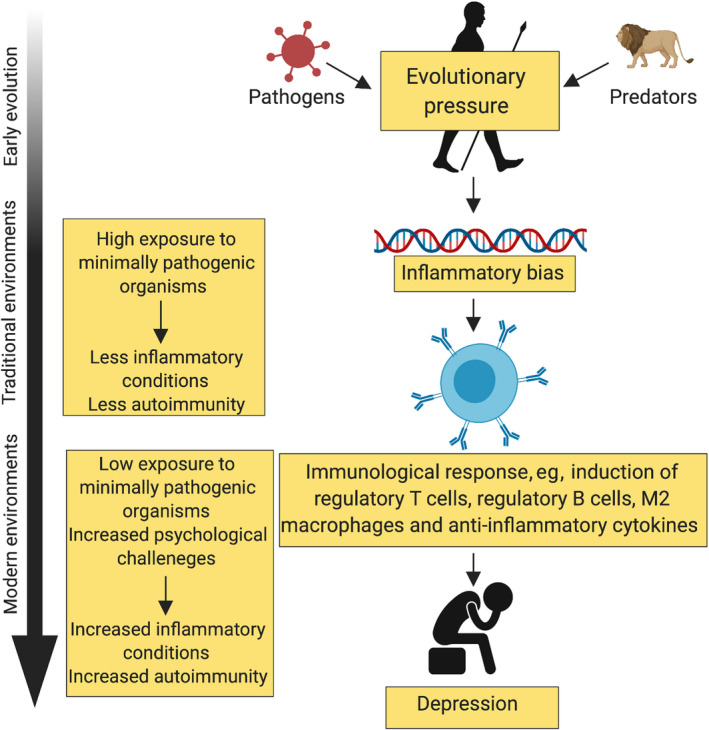
Pathogen host defense hypothesis (redrawn from Miller and Raison, 32 ). Early human evolution required an active immunity for survival that was held in check by the immunomodulatory microbiome. Modern society lacks the same exposure to an immunomodulatory microbiome, resulting in the onset of a “sickness” with depression‐like behavior
Miller and Raison 32 suggest another pathway for how inflammation affects the brain (Figure 2). Inflammasomes are cytosolic protein complexes that form in myeloid cells in response to pathogens or nonpathogenic stressors, which lead to the production of cytokines such as interleukin‐1β and interleukin‐18. There is evidence to suggest that inflammasomes may be activated by endogenous damage‐associated molecular patterns, including heat shock proteins, uric acid, and adenosine triphosphate, or microbial‐associated molecular patterns leaked from the gut. The increased levels of inflammation access the brain through humoral and neural routes. Microglial cells are also activated to an M1 proinflammatory phenotype that attracts activated myeloid cells to the brain via the cellular route, where macrophages can perpetuate central inflammatory responses. Increased levels of interleukin‐1β and tumor necrosis factor alpha can induce p38 mitogen‐activated protein kinase, which increases the expression and function of reuptake pumps and leads to decreased availability of serotonin, dopamine, and noradrenaline. Changes in the availability of these signaling molecules have important effects in guiding motivated behavior.
FIGURE 2.
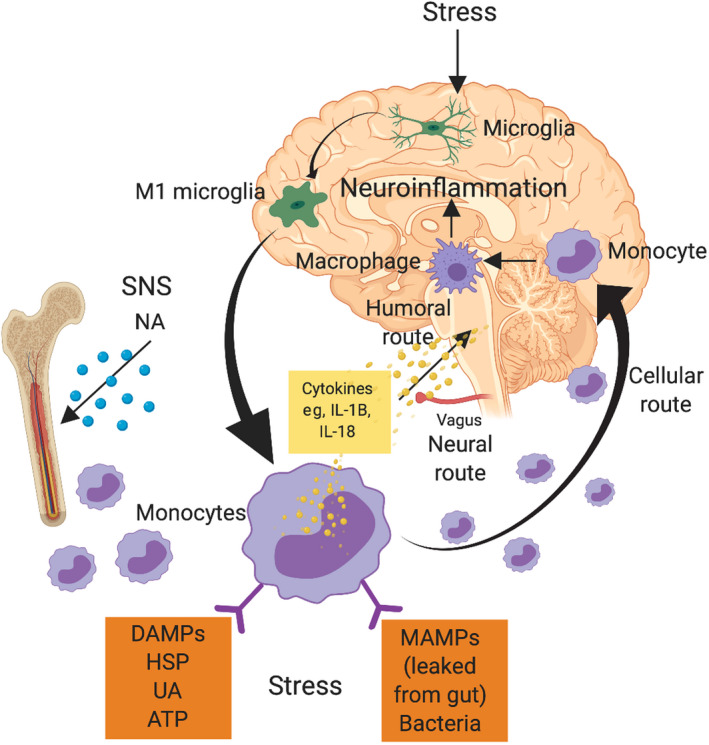
Inflammasome model of neuroinflammation (Miller and Raison 32 ). Inflammasome formation in monocytes results in the production of proinflammatory cytokines, increasing the levels of neuroinflammation through humoral, neural, and cellular routes. ATP, adenosine triphosphate; DAMPs, damage‐associated molecular patterns; HSP, heat shock protein; IL: interleukin; MAMPs, microbial‐associated molecular patterns; NA, noradrenaline; SNS, sympathetic nervous system; UA, uric acid
3. WHAT IS STRESS?
The term “stress” describes the effects of psychosocial and environmental factors on physical and/or mental well‐being. 33 These psychosocial and environmental factors are known as stressors, and they challenge the organism's normal homeostatic mechanisms, thereby eliciting a set of physiological reactions. 34 In situations where stress is acute, the stress response initiates the host's immune system for subsequent challenge. 35 By contrast, chronic stress may result in long‐term inflammatory processes that can contribute to disease either locally or systemically, such as diabetes mellitus, 36 cardiovascular disease, 37 and periodontitis. 38
3.1. Stress pathways
The duration of stress, either acute or chronic, has a significant influence on both innate and cellular immune responses and has been the focus of significant research. 39 Acute stress appears to have fast‐acting, short‐term effects resulting in the upregulation of the innate immune response, including alterations to the quantity and composition of circulating leukocytes and increases in inflammatory cytokines. 39 By contrast, chronic stress seems to have long‐term effects associated with dysregulation of innate and cellular immune responses, resulting in promoting of proinflammatory cytokine responses, decreased quantities of leukocytes, and increases quantities of regulatory/suppressor T cells. 40
The mechanisms that influence these systemic immune alterations start in the hypothalamic‐pituitary‐adrenal axis (Figure 3). At the onset of stress, the hypothalamic‐pituitary‐adrenal axis is activated, and hypothalamic secretion of corticotropin‐releasing hormone and arginine vasopressin occurs. Corticotropin‐releasing hormone, synergistically with arginine vasopressin, stimulates the pituitary gland to release adrenocorticotropic hormone. 41 Circulating adrenocorticotropic hormonecauses target organs, the adrenal glands (specifically the adrenal cortex) to increase the production and release of the glucocorticoid hormone, cortisol. Glucocorticoids are the final effectors of the hypothalamic‐pituitary‐adrenal axis and take part in regulating homeostatic mechanisms and stress. The autonomic nervous system also modulates the hypothalamic‐pituitary‐adrenal axis in stress through the release of several substances including catecholamines (eg, adrenaline and noradrenaline). 42 In turn, this can stimulate the central nervous system and peripheral nerve fibers to release substances required to counteract the stressor; for example, neuropeptides (substance P), salivary alpha‐amylase, and chromogranin A. 43
FIGURE 3.
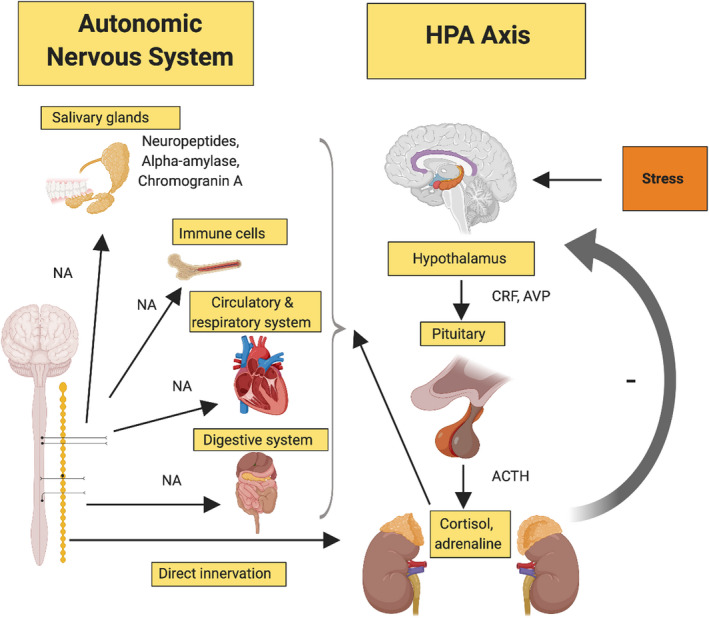
Effect of stress on the hypothalamic‐pituitary‐adrenal (HPA) axis. Activation of the hypothalamic‐pituitary‐adrenal stimulates the pituitary gland. Adrenocorticotropic hormone (ACTH) release results in increased cortisol from the adrenal glands, which then affects the autonomic nervous system. AVP, arginine vasopressin; CRF, corticotropin‐releasing factor; NA, noradrenaline
3.2. Stress and mental health
It has been suggested that psychosocial stress plays an important role in the onset, maintenance, and exacerbation of psychopathological disorders. 58 This can be illustrated by the effects of psychosocial stress on schizophrenia, where it further impairs cognitive functions but also can enhance positive or negative symptomatology, thereby increasing hallucinations or delirium and impairing social functioning to the point of complete withdrawal. 59 , 60 In subjects with dependency disorders, stress may induce maladaptive behaviors, such as alcohol consumption, tobacco smoking, drug use, and food cravings. 61 In some cases, stress can act as a direct etiological agent in disease. For example, posttraumatic stress disorder results from a well‐defined relationship that exists between a stressful life event and the onset of a clinical syndrome. Moreover, a history of childhood physical or sexual abuse has been shown to increase the risk of developing posttraumatic stress disorder following the traumatic event. 62 Major depressive episodes have been correlated with severely stressful events (eg, loss of job or divorce/separation) prior to their onset. 63 It has been demonstrated that chronic stress can impair emotionally dependent learning tasks 64 and affects the flexibility of a person's behavior 65 and their decision‐making process. 66 In addition, it may decrease social motivation and willingness for social interaction. 67 Several mental health disorders appear to be stress related resulting from a dysregulation of corticotropin‐releasing hormone. These disorders include depression, 68 anxiety, 69 and schizophrenia, 70 all resulting in hyperactivity of the hypothalamic‐pituitary‐adrenal axis. As already described, the hypothalamic‐pituitary‐adrenal axis has numerous biological functions, one of them relating to the mobilization of the immune response, thereby regulating inflammatory processes. Corticotropin‐releasing hormone dysregulation has been observed in autoimmune disorders/chronic inflammatory diseases, including rheumatoid arthritis and osteoarthritis. 71 Corticotropin‐releasing hormone dysregulation provides a biological link between stress and mental health. Considering these immunomodulatory effects that occur, it is plausible that periodontitis may also be influenced by this stress‐mental health relationship.
3.3. Stress markers and receptors in the oral cavity
The periodontal tissues have glucocorticoid receptors sensitive to the release of glucocorticoids from the hypothalamic‐pituitary‐adrenal axis. Keratinocytes have been shown to be directly responsive to adrenocorticotropic hormone and capable of producing the glucocorticoid cortisol, which may result in local immunosuppressive and anti‐inflammatory effects, 44 including inhibition of T lymphocyte cell formation 45 and suppression of macrophage 46 and natural killer cell function. 47 Blood, saliva, and gingival crevicular fluid have been used to assess the relationship between stress and periodontal diseases. Catecholamines are responsible for modulating several immune functions, including the production of proinflammatory cytokines (eg, interleukin‐1 and tumor necrosis factor alpha) 48 and suppressing lymphocyte proliferation 49 and natural killer cell function. 50 Chromogranin A is an acidic phosphorylated secretory glycoprotein that is stored and released at the same time as catecholamines from the adrenal medulla. Chromogranin A is also stored and secreted from sympathetic nerve endings 51 , 52 and the ductal cells of the human submandibular gland. 53 Chromogranin A has been shown to give rise to several bioactive peptides, such as catestatin, which may contribute to inflammation. 54 Salivary alpha‐amylase has been found to be a reliable marker reflecting sympathetic nervous system activity during stress. 55 It also displays antimicrobial activity. 56 In addition, neuropeptides such as substance P are important mediators of neurogenic inflammation, resulting in the initiation and maintenance of inflammation by stimulating proinflammatory cytokine production. 57
3.4. The role of stress in modulating the host response in periodontal diseases
A review by Monteiro da Silva et al 72 concluded that there is substantial evidence for the role of psychosocial stress as a predisposing factor in acute necrotizing ulcerative gingivitis; however, there was limited evidence for its role in periodontitis. Since this conclusion, a greater number of studies are strengthening evidence to support the notion that stress can modulate behaviors and immune system activity impacting periodontal diseases.
Correlational studies have shown positive relationships between psychosocial stress and poorer periodontal clinical parameters. Deinzer et al 73 conducted a prospective study, evaluating a cohort of medical students that had undergone a stressful period of academic examinations. Severe deterioration of gingival health was more frequently observed in stressed students when compared with their baseline levels. In addition, deterioration of gingival health was more frequently observed in stressed students than in their peer control group. A subsequent experimental study by Deinzer et al 74 evaluated medical students using a split‐mouth study design where they induced experimental gingivitis in two quadrants while maintaining high levels of oral hygiene in the remaining two quadrants. They found that students sitting examinations had significantly higher levels of interleukin‐1β in gingival crevicular fluid at both experimental gingivitis sites and sites of good oral hygiene. Interleukin‐1β is a cytokine that has been thought to play a role in the destruction of periodontal tissue during periodontal disease. 75 The authors concluded that stress may affect the health of the periodontium by suppressing the immune system and that this relationship may be more pronounced during periods of poor oral hygiene.
Proinflammatory cytokines and cortisol in serum, gingival crevicular fluid, and saliva have been investigated in relation to periodontal status. It has been shown that women on long‐term sick leave due to stress experienced greater severity of periodontitis and have increased concentrations of interleukin‐6 in gingival crevicular fluid compared with healthy controls. 76 Other studies have also observed increased quantities of proinflammatory cytokines, such as interleukin‐1β, interleukin‐6, and interleukin‐8, in the gingival crevicular fluid of periodontally diseased subjects that correlate with psychosocial stress levels. 77 Elevated levels of serum and salivary cortisol have been associated with worse clinical periodontal parameters, including bleeding on probing, clinical attachment level, and probing pocket depth. 78 , 79 Salivary cortisol and chromogranin A levels in relation to periodontal disease were evaluated by Haririan et al, 43 revealing that a positive association exists between these stress markers and the extent of periodontitis. The impact of psychological stress on the release of these stress markers was not evaluated. A more recent study by Haririan et al 80 evaluated the impact of psychological stress on the release of stress markers in saliva and serum in relation to periodontal health and disease. They found that elevated levels of stress markers (neuropeptides; eg, substance P) occurred in the saliva of patients with aggressive periodontitis and chronic periodontitis regardless of psychological stress status. A positive association between psychological stress and stress markers cannot be entirely discounted due to a low response rate (66%) to the stress evaluation questionnaire in this study. 80 Further research to elicit the relationships between psychological stress status, stress markers, and periodontal diseases is required.
In addition to psychosocial stress, there appears to be an association between an individual's ability to cope with stress and levels of periodontitis. A cross‐sectional, epidemiological study by Genco et al 81 showed a significant relationship between financial strain in relation to alveolar bone and clinical attachment loss, after adjusting for confounding factors such as age, gender, and tobacco smoking. Furthermore, individuals that had a problem‐solving coping ability for managing stress in daily life showed better periodontal clinical parameters than those that were more emotional in their focus with less‐adequate coping strategies for psychosocial strain. It was concluded that the effects of psychosocial stresses on periodontal disease can be modified by instituting adequate coping behaviors. A relationship between stress‐coping patterns and periodontitis was also found in a case‐control study by Wimmer et al, 82 where, after adjusting for smoking, age, and education, periodontitis patients with inadequate stress behavior–coping strategies were at a greater risk for severe periodontitis.
3.5. Relationship between stress and periodontal microorganisms
In recent years, our understanding of periodontal microbiology has evolved to the polymicrobial synergy and dysbiosis model. 83 This model describes the role keystone pathogens have in modulating the host's response by impairing immune surveillance and causing a change from homeostasis to dysbiosis. Considering the interaction between pathogens and the host's immune system, it is plausible that stress caused by psychosocial factors may influence the periodontal biofilm. It has been reported that the growth of Tannerella forsythia and Fusobacterium nucleatum are increased in the presence of stress hormones catecholamine, dopamine, and cortisol. 84 Also, catecholamines appear to influence periodontal bacterial growth, depending on the bacterial species. Noradrenaline can reduce the growth of Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis; however, it can also increase the growth of Eikenella corrodens, Actinomyces naeslundii, and Campylobacter gracilis. 85 Interestingly, noradrenaline has been shown to increase the expression of the gingipains, a virulence factor of P. gingivalis. 86 It can be deduced from these findings that stress‐related hormones may modulate bacterial growth and virulence factors of selected species driving a shift to dysbiosis. Interestingly, it has been hypothesized that a dysbiotic flora at the start of the alimentary system may have effects further along. 87 However, further research is required to understand these relationships and mechanisms.
3.6. Stress and periodontal and peri‐implant wound healing
The influence of psychosocial stress on periodontal wound healing can be divided into health‐impairing behaviors and pathophysiological effects. 88 Health‐impairing behaviors tend to increase during periods of psychosocial stress and have been shown to include tobacco smoking, 89 poor oral hygiene practices, 90 and alcohol. 91 The pathophysiologic effects of psychosocial stress and wound healing are yet to be completely elucidated; however, associations between stress and poor periodontal treatment outcomes have been made. Axtelius et al 92 investigated whether a stress system disorder played a role in the pathogenesis of therapy‐resistant periodontitis. It was observed that patients who did not respond to conventional periodontal therapy showed higher levels of psychosocial strain along with a higher prevalence of a passive‐dependent personality compared with the treatment‐responsive group. Although they are interesting observations, these findings were based on a small sample size and have not been reproduced in subsequent publications. Vettore et al 93 also evaluated the influence of psychosocial stress on the response to nonsurgical periodontal treatment in patients with chronic periodontitis. After adjusting for confounding variables, they found that 3 months following nonsurgical periodontal treatment, stressed subjects did not show a reduction in the frequency of periodontal probing depths greater than 6 mm, indicating a relationship between psychosocial stress and the response to nonsurgical periodontal therapy. The ability to cope with psychosocial stress has been shown to influence periodontal treatment outcomes. Individuals with passive coping strategies showed more advanced periodontal disease and tended to have a poor response to nonsurgical periodontal treatment, 94 whereas individuals with active coping strategies had milder periodontal disease and a more favorable response to nonsurgical periodontal treatment.
4. THE LINK BETWEEN MENTAL HEALTH AND PERIODONTAL DISEASES
The literature evaluating the relationship between anxiety and periodontal disease has shown conflicting results. Some studies have shown positive associations, 95 , 96 , 97 , 98 whereas others have not. 99 , 100 , 101 A recent case‐control study by Levin et al 97 investigated whether dental anxiety differs between individuals with and without chronic periodontitis. They found that patients with chronic periodontitis showed a significantly higher percentage of high anxiety and phobia than subjects in the control group did. In addition, patients with chronic periodontitis were significantly more likely to consider themselves as suffering from dental anxiety. This study shows an association between chronic periodontitis and anxiety; however, biological mechanism and associations were not explored. Recently, a meta‐analysis showed that periodontitis was positively correlated with anxiety, with the authors suggesting links to health risk behaviors and changes in the hypothalamic‐pituitary‐adrenal axis activity. 102
4.1. Depression and periodontitis
Evidence investigating the association between depression and periodontal diseases has received a significant amount of attention in the literature and is conflicting. A large number of studies have shown positive associations, 76 , 81 , 95 , 103 , 104 , 105 and others no association. 99 , 101 Araújo et al 106 conducted a systematic review that included 15 studies (one cohort study, six case‐control, eight cross‐sectional). A total of six studies reported a positive association between depression and periodontitis, whereas nine studies reported no association. A meta‐analysis of seven cross‐sectional studies found that there was no significant association between depression and periodontitis (odds ratio: 1.03, 95% confidence interval: 0.75‐1.41). A more recent meta‐analysis of 18 studies by Zheng et al 102 found that patients with periodontitis had higher depression‐scale scores. The authors concluded from their analysis that more high‐quality prospective studies are required to confirm the relationship as there is currently significant heterogeneity in the literature.
Limited studies have reported the influence of depression on periodontitis treatment outcomes. 107 , 108 Petit et al 108 found that patients with increased depression scores and poor coping strategies showed worsened nonsurgical periodontal therapy outcomes for the management of their advanced periodontitis. When antidepressant medications (such as selective serotonin reuptake inhibitors) have been evaluated it has been found they may have immunosuppressive effects by reducing levels of oxidative stress 109 , 110 on periodontal disease severity in animal models 111 , 112 and human studies. 113
Depression and periodontitis appear to share common risk factors, including older age, 14 , 114 low education levels, 115 , 116 ethnicity 5 , 117 and low socioeconomic status. 118 , 119 Poor lifestyle choices, such as tobacco smoking and alcohol consumption, have also been shown to be shared risk factors for both periodontitis 14 and depression. 120 Moreover, both diseases potentially share contributory genetic factors. Depression studies have reported an association with genetic polymorphisms in genes coding for brain‐derived neurotropic factor and serotonin (5‐hydroxytryptamine‐transporter‐linked promoter region). 121 Dias Corrêa et al 122 found that brain‐derived neurotropic factor genotype GG was associated with increased levels of brain‐derived neurotropic factor and tumor necrosis factor alpha in chronic periodontitis patients. Moreover, a study by Costa et al 123 suggested that 5‐hydroxytryptamine‐transporter‐linked promoter region polymorphism might be associated with aggressive periodontitis.
Proposed mechanisms by which depression contributes to periodontal disease include the dysregulation of the hypothalamic‐pituitary‐adrenal axis and changes in health behaviors. It is suggested that the dysregulation of the hypothalamic‐pituitary‐adrenal axis results in adrenal disturbances, immune system dysfunction, and excessive production of proinflammatory cytokines. 124 , 125 Now that depression is understood to be a neuroinflammatory disease it provides support for a bidirectional relationship with periodontitis. Animal studies have demonstrated that this dysregulation may influence the progression of periodontal infections. 126 , 127
Evidence suggests that periodontitis may contribute to the onset of depression. Meta‐analyses show that patients with depression are associated with chronic, low‐grade inflammatory states reflected by higher serum levels of proinflammatory cytokines, including tumor necrosis factor alpha, interleukin‐1, and interleukin‐6, 128 , 129 along with increases in acute‐phase proteins (ie, C‐reactive protein) 130 and reactive oxygen species. 131 However, the source of this inflammation is not well understood. 132 Periodontitis has also been shown to have elevated serum levels of these systemic inflammatory mediators, particularly interleukin‐6, tumor necrosis factor alpha, C‐reactive protein, and reactive oxygen species, 133 , 134 , 135 that may potentiate or exacerbate a preexisting inflammatory state, increasing susceptibility to depression. More research is required to understand the potential biological links between these two disease entities. It should be mentioned that sequelae of periodontal diseases, such as halitosis, unsightly migration of teeth, and gingival recession, may have a psychosocial impact that could lead to a depressive state in a susceptible individual. 136 At first glance, it might seem that poor mental health is the driver in the relationship between oral and mental health; however, a large‐scale (n > 60 000), longitudinal (10‐year follow‐up) study detected that there was a higher incidence of subsequent development of depression in individuals with periodontitis compared with those without periodontitis. 137
4.2. Bipolar disorder
Bipolar disorder is characterized by a variation within an affected individual's mood, thought process, and their behavior that can vary between elation (mania) and depression. These cycles often can vary in their duration and are unpredictable in nature. 138 During depressive episodes, it is not uncommon for patients to show a significant decline in oral hygiene levels, often coupled with an increase in periodontal disease. 139 , 140 , 141 Conversely, during periods of mania, heavy use of toothbrushes and interdental cleaning aids may result in mucosal or gingival lacerations. 142 Evidence shows that a chronic, low‐grade inflammation exists systemically during bipolar disorder. Proinflammatory cytokines, such as interleukin‐1, interleukin‐2, interleukin‐4, interleukin‐6, and tumor necrosis factor alpha, are elevated during periods of mania, whereas during depressive episodes only interleukin‐6 levels increase. 143 It is plausible, therefore, that inflammation could be a common factor between bipolar disorder and other inflammatory‐meditated systemic diseases, such as periodontal disease; however, more research is required.
4.3. Schizophrenia
Schizophrenia is a disorder that is distinguished by characteristic distortions of thinking and perception, along with affects that are incongruent or blunted. Normally, the individual maintains their intellectual capacities. Specific positive features include auditory hallucinations, thought broadcasting, the insertion or withdrawal of thoughts, thought disorganization, and delusional states. Specific negative features include lack of motivation, poor self‐care, and social withdrawal. 23
It has been shown that, in general, individuals with schizophrenia irregularly attend the dentist, have poorer oral health, and a greater number of missing teeth compared with the general population. 144 Eltas et al 145 evaluated the association between periodontal health and schizophrenia. They found that there was a high risk of periodontal disease in patients with schizophrenia with high scores for plaque index, bleeding on probing, probing depths, clinical attachment loss, and missing teeth. It has been shown that individuals with schizophrenia undergoing a psychotic episode have increased serum concentrations of inflammatory cytokines, including interleukin‐12, interferon‐gamma, tumor necrosis factor alpha, and C‐reactive protein. 146 Therefore, it is conceivable that the low‐grade, chronic inflammatory state of schizophrenia may contribute to immune system abnormalities that predispose patients with schizophrenia to systemic diseases. 147
4.4. Alzheimer disease
Dementia is a syndrome due to disease of the brain that is progressive in nature and results in loss of higher cortical functions, such as memory, orientation, and cognition. It can be caused by diseases such as Alzheimer disease, dementia with Lewy bodies, and vascular dementia, among others. 23 There is limited epidemiologic data available on the prevalence of dementia in Australia; therefore, estimates are commonly used. In 2016, there was an estimated 400 833 Australians living with dementia, with higher prevalence in females and those over 65 years of age. The prevalence of dementia is projected to increase by 2.75‐fold to 1 100 890 persons by 2056. 148 , 149
Evidence is emerging to support a link between Alzheimer disease and periodontitis (Figure 4). A prominent hypothesis to describe the pathogenesis of Alzheimer disease is progressive inflammation within the brain resulting in neurodegeneration. 150 It is suggested that this inflammation might be responsible for the pathologic features observed in the disease, including hyperphosphorylated tau protein, which forms neurofibrillary tangles, beta‐amyloid 1‐42 peptides found in senile plaques and components of degenerated neurons. 151 Several animal studies have supported the notion that these pathologic alterations induce glial cells to produce proinflammatory cytokines, such as tumor necrosis factor alpha, interleukin‐1β, interleukin‐6, and C‐reactive protein. 152 , 153 , 154 Through a positive feedback mechanism, the elevated proinflammatory and C‐reactive protein may stimulate glial cells further to produce additional hyperphosphorylated tau protein, beta‐amyloid 1‐42 peptide, and proinflammatory cytokines, thereby driving neurodegeneration. 155 , 156 Clinical studies supporting the link between inflammation and the pathogenesis of Alzheimer disease are limited. Holmer et al, 157 in a case‐control study, reported a correlation between periodontitis, early cognitive impairment, and Alzheimer disease, highlighting the interactions between the disease entities are biologically plausible. Other studies have shown that elevated levels of C‐reactive protein increase the likelihood of developing Alzheimer disease and cognitive decline in various populations. 158 , 159 Proinflammatory cytokines as predictors of Alzheimer disease have also been investigated, with increased levels of interleukin‐6 and interleukin‐1β suggesting an increased risk for disease. 160 , 161 Genetic evaluations indicate that the presence of interleukin‐1α‐899 and interleukin‐1β + 3953 polymorphisms have been shown to increase the risk of developing Alzheimer disease 11‐fold. 162 Further support for the role of inflammation in Alzheimer disease stems from identified effects that nonsteroidal anti‐inflammatory drugs have in delaying the onset of Alzheimer disease. 163 , 164 However, randomized control trials have not demonstrated this same positive effect. 165 , 166 It is speculated that the lack of effect in these studies could be related to the specific action of the nonsteroidal anti‐inflammatory drugs used, low dosages, and high drop‐out rates in these studies.
FIGURE 4.

Possible mechanism how periodontal disease may affect Alzheimer disease through increased chronic inflammation and infection of the brain by oral bacteria
When evaluating the role of periodontitis in Alzheimer disease, it should be considered that periodontitis is a chronic inflammatory disease, which, over years, systemically exposes the host to proinflammatory cytokines and acute‐phase proteins. Patients with moderate to severe periodontitis have been shown to have elevated systemic levels of C‐reactive protein. 167 , 168 Studies evaluating the systemic inflammatory effects of periodontitis have shown elevated levels of prostaglandin‐E2, interleukin‐1β, interleukin‐6, and tumor necrosis factor alpha. 169 , 170 It is plausible, therefore, that these periodontitis‐produced inflammatory mediators could extend to the brain through systemic and neural pathways. The periodontal pocket provides a unique opportunity for extensive numbers of periodontal bacteria to gain access to the systemic circulation and neural tissue. This has been demonstrated in a study by Riviere et al, 171 where oral Treponema species (eg, Treponema denticola), were detected in pieces of brain frontal lobe in 14 out of 16 human autopsy specimens with Alzheimer disease but only detected in four out of 18 without. The suggested mechanism by which bacterial invasion of the brain may occur is via the trigeminal nerve, where Treponema species were detected from three Alzheimer and two control specimens in the trigeminal ganglion. A recent study offered evidence that P. gingivalis and gingipains may play an important role in the pathogenesis of Alzheimer disease. In a mouse model, they showed that in vivo oral administration of gingipain inhibitors blocks gingipain‐induced neurodegeneration, reduces quantities of P. gingivalis in the brain, and decreases host beta‐amyloid response to P. gingivalis infection within the brain. These results are suggestive that gingipain inhibitors may be useful in the treatment of neurodegeneration associated with Alzheimer disease. 172 A recent systematic review evaluating 23 studies found that infection with oral pathogens correlated with developing neuropathological degeneration seen in Alzheimer disease and the detection of bacteria in the brain. In addition, when a dysbiosis is associated with oral bacteria there is evidence of a microbiological susceptibility to developing Alzheimer disease. 173
4.5. Substance use disorders
Substance use disorders include a variety of disorders that differ in their severity and clinical manifestations, but are all attributable to the use of one or more psychoactive substances (eg, alcohol, nicotine, cannabis). 23 These psychoactive substances elicit pathological changes to the brain, causing the addict to be less responsive to interpersonal and social relationships. These changes also render the addict with the inability to regulate the drive to seek drug reward. 174 The biological basis of addiction is that the psychoactive substance will cause the release of dopamine into the nucleus accumbens, the reward center of the brain, thereby reinforcing behaviors to obtain more of the substance. 175 Physiologically, repeated exposure to the same stimulus results in the development of tolerance, where progressively less dopamine is released, eventually to the point of undetectable levels unless there is a change in stimulus. 176 By contrast, continued use of psychoactive substances pharmacologically stimulates the release of dopamine and, in general, does not diminish with ongoing exposure. 177 It is this process that drives continued neuroplastic change promoting drug‐seeking behavior for biological rewards. Environmental stress has been shown to influence the development substance use disorders in susceptible individuals. 178 Studies indicate that the interactions between stress and dependency on psychoactive substances are modulated by drug‑ and stress‐induced corticotropin‐releasing factor release, responsible for the regulation of dopamine levels. 179 Furthermore, stress has the ability to enhance the addictive nature of psychoactive substances and contribute to relapse of substance dependency by increasing the strength at excitatory synapses on midbrain dopamine neurons. 180
There is clear evidence to support tobacco smoking as a risk factor for periodontitis. 181 There are numerous mechanisms by which tobacco smoking has effects on the periodontium, including microbiological shifts to periodontopathic species, 182 reduced gingival blood flow, 183 impaired polymorphonuclear cell phagocytosis, 184 increased proinflammatory cytokine production, 185 and impaired periodontal wound healing. 186 The use of marijuana has also been linked to periodontitis. Thomson et al 187 showed in a prospective cohort of young adults that, after controlling for confounding factors, regular cannabis smoking was strongly associated with periodontal disease, showing a dose‐response effect. A significant number of studies have explored the influence of alcohol on periodontitis. However, fewer studies exist evaluating the relationship with alcoholism. Khocht et al 91 investigated the effects of alcoholism and cocaine abuse on the periodontium. The results showed that persistent alcohol abuse increased loss of clinical attachment through recession of gingival margins in alcohol‐dependent subjects. No significant associations were seen between cocaine misuse and periodontal disease. 91 A systematic review including 11 cross‐sectional and five longitudinal observational studies concluded there is sound evidence to suggest alcohol consumption is a risk indicator for periodontitis. However, the available evidence is too sparse to draw a link between alcohol dependence and periodontitis. 188
5. MENTAL HEALTH AND THE MICROBIOME
Currently, no one factor can be implicated in mental health disorders. Instead, it is thought to be an interaction of many factors, as mentioned earlier herein. Increasingly, research is evaluating the influence of the microbiome on mental and neurological health, with the majority of interest being on the gut microbiome. 189 , 190 , 191 , 192 It is hypothesized that the gut microbiome can communicate and influence the brain through the gut‐brain axis. A logical extension of this is the oral cavity‐brain axis, which has been explored recently, particularly in the last 5 years.
5.1. The gut‐brain axis
The gut‐brain axis is the “complex bidirectional interactions and processes utilised by the gut microbiome and brain to communicate”, 193 integrating gut function with the cognitive and emotional centers of the brain. Increased permeability of the intestine, otherwise known as a “leaky gut,” is thought to occur through immune activation, entero‐endocrine signaling, and enteric reflex, allowing microbial metabolites into the bloodstream. 193 , 194
The gut microbiome is a mixture of bacteria, viruses, fungi, and bacteriophages, 195 comprised of over 1000 microbial species. The microflora in the gut and oral cavity have coevolved with their host, and it makes sense that there is communication between using existing cellular and molecular pathways. 196 A symbiotic relationship exists between gut microbiota and the host critical for essential physiological functions within the human body. The gut microbiota has an important role in the promotion of nutrient absorption, host immune system modulation, digestion, and epithelial barrier function. 197 , 198 The brain exerts its influence on intestinal microflora and physiology through the autonomic nervous system, the hypothalamic‐pituitary‐adrenal axis, and the release of signaling molecules such as cytokines. 199 It is this complex bidirectional communication pathway that is known as the gut‐brain axis.
5.2. The oral microbiome and mental health disorders
Direct causal mechanisms have been proposed to explain the connection between oral microbiota, the brain, and how this relationship may influence the development of mental health disorders. The four direct causal mechanisms proposed are microbial and metabolite escape, neuroinflammation, central nervous system signaling, and response to neurohormones. 200 A common theme of these mechanisms is inflammation through a host reaction. In addition, exposure to physical or psychosocial stressors at a young age or continually may result in systemic and neuro‐inflammation. 201
Microbial and metabolite escape
Microbial species are commensal and usually have a resident niche where they are not pathogenic. However, as Bowland and Weyrich 200 suggest, owing to dysfunction, if a microbial species is able to disseminate via a bacteremia, then it or its products have the potential to cause disease. One of the possible mechanisms whereby the microbiota could contribute to neuroinflammation is via circulating endotoxins—that is, lipopolysaccharides, which are part of the outer membrane of Gram‐negative bacteria—or other microbe‑ or pathogen‐associated molecular patterns. In the case of lipopolysaccharide, toxicity is associated with the lipid component and immunogenicity is associated with the polysaccharide components, eliciting a variety of inflammatory responses. 202 Endotoxins can enter the circulation more readily through compromised internal barriers, such as the oral and intestinal mucosa, thereby allowing toxins to spread systemically, resulting in an inflammatory cascade in the central nervous system.
Some of the gut microbiome may affect the production and levels of monoamine neurotransmitters, such as serotonin, dopamine, and gamma‐aminobutyric acid. In some cases, bacterial strains may be able to produce these transmitters. Mazzoli et al 203 showed that Lactobacillus and Bifidobacterium secreted gamma‐aminobutyric acid, and Escherichia, Bacillus and Saccharomyces produce norepinephrine. In that study, Candida, Streptococcus, Escherichia, and Enterococcus produced serotonin, Bacillus and Serratia could produce dopamine, and Lactobacillus secreted acetylcholine. The essential amino acid tryptophan is necessary for the synthesis of serotonin. Metabolism of tryptophan by certain gut bacteria reduces the amount available to the host. 204 Regulation of these neurotransmitters and their production by the gut microbiome has important implications in the development and management of mental health disorders.
Neuroinflammation
Through the bloodstream, bacteria and their products stimulate a mild, but chronic inflammation. This systemic inflammation may induce changes in neurovascular functions, increase blood‐brain barrier permeability, reduce of nutrition, and increase neurotoxic compounds. 205 Release of reactive nitrogen and oxygen species and proinflammatory cytokines interleukin‐1β and interleukin‐6 in the presence of inflammation activate microglia and astrocytes, contributing to neural toxicity and damaging the blood‐brain barrier. 206 Additional neurotoxins may be released to the activation of the enzyme indoleamine‐2,3‐dioxygenase (which increases the metabolism of tryptophan). 207 Miller and Raison 32 also suggested that inhibition of the synthesis of the monoamine neurotransmitters serotonin, dopamine, and norepinephrine may negatively affect brain function.
Central nervous system signaling
Neurological processes may be negatively influenced by bacteria, and so affect behavior. The production of metabolites and other compounds from bacteria may have neuroactive and immunomodulatory actions. 208 These include short‐chain fatty acids, bile acids, choline and phenolic metabolites, indole derivatives, vitamins, polyamines, and lipids. 209 Caspani et al 210 suggested three mechanisms of action: (a) direct stimulation of central nervous system receptors; (b) peripheral stimulation of neural, endocrine, and immune mediators; and (c) epigenetic regulation of histone acetylation and deoxyribonucleic acid methylation. Bowland and Weyrich, 200 in their review, presented another potential mechanism of afferent signaling via the vagus nerve, which relays signals from the gut to the central nervous system and may be able to sense gut microbial metabolites.
Response to neurohormones
Hormones have been suggested to be the primary method of the interaction. 202 Catecholamines may modulate gene expression in bacteria. This has reported by Mudd et al, 211 who showed the presence of fecal Ruminococcus was negatively associated with serum cortisol and a biomarker for neurological health, N‐acetylaspartate.
Oral‐brain axis
As already discussed, there is a link between periodontal disease and mental health disorders. The oral microbiome has been implicated in a number of systemic diseases, but recently linked to mental health disorders. As outlined earlier herein, the gut microbiota has a close interaction with the central nervous system mediated through bidirectional neural, immune, and neuroendocrine pathways. 212 , 213 However, there has been little research on investigating the relationship with the oral microflora and its effect on the central nervous system. Low‐grade chronic inflammation is now recognized as an important risk factor for mental health disorders, and research has clearly shown that periodontitis and/or the bacteria involved stimulate such a response 214 (Figure 5). Though animal studies have not been a focus of this chapter, they have provided insight into the possible mechanisms between periodontitis and mental health disorders. Altogether, they show dysregulation of the hypothalamic‐pituitary‐adrenal axis with a hyperinflammatory response and behavioral changes. In addition, they may also provide evidence of direct microbial involvement. For example, Watanabe et al 215 reported that Streptococcus mutans positive for the collagen‐binding adhesin gene Cnm, normally associated with dental caries, was able to disrupt the blood‐brain barrier and induce cerebral hemorrhaging following bacteremia. Release of oral bacteria or their metabolites into the bloodstream stimulates the neuroinflammatory response with the expression of proinflammatory cytokines, resulting in a chronic inflammation. 216 This systemic inflammation may induce changes in neurovascular functions, increase blood‐brain barrier permeability, reduce nutritional uptake, and increase neurotoxic compounds. 205
FIGURE 5.
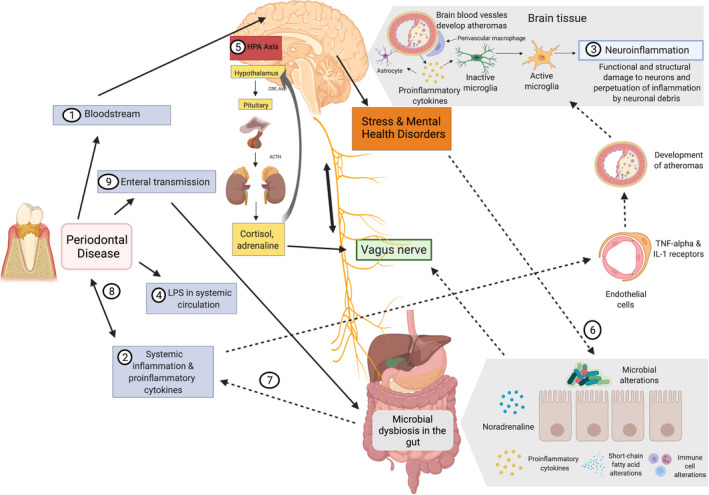
Oral‐brain axis (redrawn from Martínez et al, 201 ). (1) Bacteremia of the periodontal pathogens can reach the brain directly through the bloodstream and damaged blood‐brain barrier. (2) Activation of endothelial cells by proinflammatory cytokines can indirectly affect the central nervous system. (3) Expression of tumor necrosis factor alpha (TNF‐α) and interleukin‐1 (IL‐1) endothelial receptors activates microglia that results in inflammation. (4) Increased permeability of the periodontal vasculature results in “leaking” of lipopolysaccharide (LPS). (5) Lipopolysaccharide can activate the hypothalamic‐pituitary‐adrenal axis, thereby increasing stress hormones and/or neurotransmitters. (6) This then affects gut physiology, habitat, microbiome composition, and bacterial gene expression. (7) Changes in the gut microbiome can result in further systemic inflammation reinforcing the effect on the central nervous system. (8) Further, it may affect periodontal disease by increasing the inflammatory burden. (9) Transmission of the oral bacteria to the gut by saliva may also affect the gut microbiome composition and function. ACTH, adrenocorticotropic hormone; AVP, arginine vasopressin; CRF, corticotropin‐releasing factor
It was originally thought that the blood‐brain barrier was impermeable, but this has been shown to be incorrect. Lipopolysaccharide can adversely affect the blood‐brain barrier and lead to increased permeability. 217 It is then feasible that periodontal bacteria in the bloodstream can penetrate the central nervous system. Increased levels of lipopolysaccharide expression of toll‐like receptor 4 increases neuroinflammation further. 216 Interestingly, and similar to the vagus nerve in the gut, the facial and trigeminal nerves have been suggested as routes of egress. 218 Another form of communication is through brain‐resident microglia via leptomeninges. 219 This occurs through the activation of endothelial cells that express tumor necrosis factor beta and interleukin‐1 signal to perivascular macrophages adjacent to cerebral endothelial cells. There is then communication with microglia, microglial activation, and subsequent neuroinflammation. 220 Periodontal bacterial extracellular vesicles, such as exosomes, are potent stimulators of the immune system, increasing the inflammatory burden. 221
In comparing oral microbial studies, it is also important to consider how the samples were collected. Many studies have used saliva, whereas, although periodontal pathogens are present in saliva, subgingival plaque is more appropriate. Dubar et al 222 sampled two sites above 5 mm probing depth and a healthy control in patients with periodontitis before and after treatment. They assessed salivary cortisol levels and self‐reported stress and anxiety. Cortisol was correlated with probing depth, but not with stress or anxiety. T. forsythia was found in higher levels in the pockets of all highly stress subjects, whereas A. actinomycetemcomitans was only detected in nonanxious patients. 222 This is further supported by Simpson et al 223 in a study of adolescents with depression and anxiety symptoms, who found major depressive disorder and anxiety were associated with changes in the abundance of certain taxa, including Spirochaetaceae, Actinomyces, Firmicutes, Treponema, Fusobacterium, and Leptotrichia spp, suggesting that composition can also be associated with mental illness symptoms in this population. 223
Similar to the gut‐brain axis, the host is able to modulate the oral flora, as mentioned earlier. Chronic stress dampens the diurnal secretion of salivary glucocorticoid and catecholamine. Therefore, the abundance and/or function of oral microbes may be affected. Roberts et al 224 noted host modulation of oral bacterial gene expression through cortisol. The level of stress may also affect the variability and levels of the microbiome. 225
The interrelationship between the gut and oral microbiome
Given the oral cavity is the start of the alimentary system, it is not unreasonable to consider the transmission of bacteria from the oral cavity to the intestine. About 1.5 L of saliva is swallowed every day. 226 However, the acidity of the stomach, barrier functions along the gastrointestinal tract, and the commensal gut microbiome prevent colonization. 227 Dysregulation or disruption of the normal gut physiology or flora may allow the oral bacteria to colonize. The subsequent disruption and/or dysbiosis of the gut flora could affect central nervous system functioning. In addition, a dysbiotic oral microflora increases overall inflammation, with a knock‐on effect of altering the gut flora making the composition more proinflammatory.
With all research we need to recognize its limitations. Sampling the intestinal flora is complex, and stool samples are unlikely to be completely representative. 228 Confounding variables relating behaviors associated with mental health disorders may influence study outcomes, as may variations in diagnostic criteria for cases of periodontitis.
6. MENTAL HEALTH, STRESS, AND IMPLANT OUTCOMES
There are limited studies examining mental health disorders and implant survival and success. 229 This may in part be because mental disorders are more common in low or middle socioeconomic groups who may not have access or the resources to afford an implant. 230 Patients with severe mental health disorders are unlikely to attend for implant treatment.
It can be extrapolated that the relationship between mental health disorders, stress, and peri‐implant disease are similar to what is reported with periodontitis. It seems likely that peri‐implant disease and wound healing following implant placement may be influenced by the same dysregulation of the hypothalamic‐pituitary‐adrenal axis and adrenergic nerve signaling and health risk behaviors as seen in periodontitis. It has been suggested that all wound healing phases are influenced by stress, resulting in dysregulation to cytokine and immune function, cellular function, matrix metalloproteases, and increases in hypoxia. 231 Changes in inflammatory and glucocorticoid signaling as a result of stress affect functional brain circuits, including the affective‐salience circuit, which is important in guiding motivated behavior. 32 As already discussed, health risk behaviors tend to increase with mental health disorders and stress 232 ; in particular, lack of compliance with professional and home care and smoking appear to increase and are considered risk factors for peri‐implant disease. 15
Associations between antidepressant medications and implant failure have been found. 20 In particular, associations between selective serotonin reuptake inhibitors and implant failure have been shown in numerous studies. 21 A common side effect of antidepressants is xerostomia, where increased discomfort on brushing may result in increased plaque accumulation and the development of peri‐implant mucositis. The role of serotonin in bone homeostasis provides the biologically plausible link between selective serotonin reuptake inhibitor use and implant failure. Serotonin receptors are found in nervous tissue, cells of the digestive tract, platelets, and bone cells (osteocytes, osteoclasts, and osteoblasts). When acting on bone cells, serotonin blocks serotonin transporters on bone cells, which decreases bone formation by increasing osteoclast formation and decreasing osteoblast formation, 233 resulting in decreased bone mineral density and bone mass. 234
Wu et al 18 showed that treatment of depression with selective serotonin reuptake inhibitors was linked to an increased failure rate of osseointegrated implants compared with those not taking selective serotonin reuptake inhibitors after controlling for a number of factors. They suggested that the main reason for implant failure due to selective serotonin reuptake inhibitors was likely related to problems with the mechanical loading of the implants, since it has been shown in vivo that serotonin plays a critical role in the response of bone to mechanical loading. 235
Recently, it has been reported that serotonin‐norepinephrine reuptake inhibitors and tricyclic antidepressants pose the highest risk for failure when compared with non–antidepressant users. 20 In this study it was found that serotonin‐norepinephrine reuptake inhibitors yielded a much higher risk of implant failure than selective serotonin reuptake inhibitor antidepressants did. Although the link between serotonin and bone metabolism appears clearer, it is not the same for norepinephrine. It has been demonstrated in mice that lack of norepinephrine reuptake can lead to reduced bone formation and increased bone resorption, leading to poor bone mass and suboptimal bone mechanical properties. 236 Similarly, it is suggested that tricyclic antidepressants exhibit their actions and increase the risk of implant failure through serotonin and norepinephrine pathways; however, there have been limited investigations.
Further well‐designed prospective studies are required to understand the effects on dental implant biology.
7. CONCLUSION
Current evidence suggests a positive relationship between stress, mental health disorders and periodontal disease. It appears that stress and mental health disorders, such as anxiety disorders, depression, bipolar, schizophrenia, Alzheimer disease, and substance use disorders, are associated with more severe periodontal disease and in some cases poorer healing outcomes to nonsurgical periodontal therapy. Evidence suggests that stress and mental health disorders can result in behavior modification, such as poor oral hygiene practices, tobacco smoking, and alcohol abuse, which are also risk factors for periodontal disease and which, therefore, may have a contributory effect. In addition, stress has immunomodulatory effects regulating immune cell numbers and function, as well as proinflammatory cytokine production. The biological pathways between psychosocial stress and systemic pathophysiology have received significant scientific attention; however, the roles of the hypothalamic‐pituitary‐adrenal axis and the sympathetic nervous system are insufficiently understood. Psychosocial stress and a number of mental health disorders (ie, anxiety disorders, depression, bipolar, schizophrenia, and Alzheimer disease) appear to be accompanied by a low‐grade chronic inflammation. Systemic effects of inflammation have been shown to have adverse systemic health effects, influencing diseases such as cardiovascular disease. 237 Therefore, it is plausible that the presence of low‐grade chronic inflammation may be involved in a bidirectional relationship between stress, mental health disorders and periodontal and peri‐implant disease. There is less evidence available evaluating the relationship between mental health, stress, and peri‐implant disease. Associations have been made between antidepressant use and implant failure, which seem biologically plausible, particularly for selective serotonin reuptake inhibitor and serotonin‐norepinephrine reuptake inhibitor medications. With increasing interest, over the last few years, it has become increasingly clear that there is a bidirectional relationship between the brain/central nervous system and the gut microbiome. There is evidence to suggest that alterations or dysbiosis of the gut flora and consequent inflammation or changes in hormone levels may promote the development of mental health disorders. There is less available evidence exploring the link between the oral microbiome, stress, and mental health disorders.
These findings suggest that stress management and adequate treatment of mental health disorders may be a valuable adjunct in the periodontal treatment of patients. It is pertinent to note that, on review of the literature, significant heterogeneity exists between studies in relation to study methodology and analysis of data (eg, adjusting for confounding variables), making it difficult to draw definitive conclusions on such relationships. The heterogeneity of mental health disorders in relation to their etiology, psychopathology, symptomatology, and level of disability further complicates the task of analyzing potential links with periodontal and peri‐implant diseases. To date, only associations have been made.
ACKNOWLEDGMENT
Open access publishing facilitated by The University of Melbourne, as part of the Wiley ‐ The University of Melbourne agreement via the Council of Australian University Librarians. Open access publishing facilitated by The University of Melbourne, as part of the Wiley ‐ The University of Melbourne agreement via the Council of Australian University Librarians.
Ball J, Darby I. Mental health and periodontal and peri‐implant diseases. Periodontol 2000. 2022;90:106‐124. doi: 10.1111/prd.12452
REFERENCES
- 1. American Academy of Periodontology . American Academy of Periodontology Task Force report on the update to the 1999 classification of periodontal diseases and conditions. J Periodontol. 2015;86(7):835‐838. [DOI] [PubMed] [Google Scholar]
- 2. Lang NP, Berglundh T, Working Group 4 of Seventh European Workshop on Periodontology . Periimplant diseases: where are we now?–Consensus of the Seventh European Workshop on Periodontology. J Clin Periodontol. 2011;38 (suppl 11):178‐181. [DOI] [PubMed] [Google Scholar]
- 3. Lindhe J, Meyle J, Group D of European Workshop on Periodontology . Peri‐implant diseases: consensus report of the Sixth European Workshop on Periodontology. J Clin Periodontol. 2008;35(8 suppl):282‐285. [DOI] [PubMed] [Google Scholar]
- 4. Albandar JM, Rams TE. Global epidemiology of periodontal diseases: an overview. Periodontol 2000. 2002;29(1):7‐10. [DOI] [PubMed] [Google Scholar]
- 5. Eke PI, Dye B, Wei L, Thornton‐Evans G, Genco R. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012;91(10):914‐920. [DOI] [PubMed] [Google Scholar]
- 6. Derks J, Tomasi C. Peri‐implant health and disease. A systematic review of current epidemiology. J Clin Periodontol. 2015;42(42 suppl):S158‐S171. [DOI] [PubMed] [Google Scholar]
- 7. Lee C‐T, Huang Y‐W, Zhu L, Weltman R. Prevalences of peri‐implantitis and peri‐implant mucositis: systematic review and meta‐analysis. J Dent. 2017;62:1‐12. [DOI] [PubMed] [Google Scholar]
- 8. Laine ML, Crielaard W, Loos BG. Genetic susceptibility to periodontitis. Periodontol 2000. 2012;58(1):37‐68. [DOI] [PubMed] [Google Scholar]
- 9. Hajishengallis G, Darveau RP, Curtis MA. The keystone‐pathogen hypothesis. Nat Rev Microbiol. 2012;10(10):717‐725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lafaurie GI, Sabogal MA, Castillo DM, et al. Microbiome and microbial biofilm profiles of peri‐implantitis: a systematic review. J Periodontol. 2017;88(10):1066‐1089. [DOI] [PubMed] [Google Scholar]
- 11. Dereka X, Mardas N, Chin S, Petrie A, Donos N. A systematic review on the association between genetic predisposition and dental implant biological complications. Clin Oral Implants Res. 2012;23(7):775‐788. [DOI] [PubMed] [Google Scholar]
- 12. Lafuente‐Ibáñez de Mendoza I, Setien‐Olarra A, García‐De la Fuente AM, Aguirre‐Urizar JM, Marichalar‐Mendia X. Role of proinflammatory mutations in peri‐implantitis: systematic review and meta‐analysis. Int J Implant Dent. 2022;8(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Laine M, Moustakis V, Koumakis L, Potamias G, Loos B. Modeling susceptibility to periodontitis. J Dent Res. 2013;92(1):45‐50. [DOI] [PubMed] [Google Scholar]
- 14. Genco RJ, Borgnakke WS. Risk factors for periodontal disease. Periodontol 2000. 2013;62(1):59‐94. [DOI] [PubMed] [Google Scholar]
- 15. Schwarz F, Derks J, Monje A, Wang HL. Peri‐implantitis. J Clin Periodontol. 2018;45:S246‐S266. [DOI] [PubMed] [Google Scholar]
- 16. Jemt T, Häger P. Early complete failures of fixed implant‐supported prostheses in the edentulous maxilla: a 3‐year analysis of 17 consecutive cluster failure patients. Clin Implant Dent Relat Res. 2006;8(2):77‐86. [DOI] [PubMed] [Google Scholar]
- 17. Dinan TG, Cryan JF. Gut instincts: microbiota as a key regulator of brain development, ageing and neurodegeneration. J Physiol. 2017;595(2):489‐503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu X, Al‐Abedalla K, Rastikerdar E, et al. Selective serotonin reuptake inhibitors and the risk of osseointegrated implant failure: a cohort study. J Dent Res. 2014;93(11):1054‐1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chrcanovic BR, Kisch J, Albrektsson T, Wennerberg A. Analysis of risk factors for cluster behavior of dental implant failures. Clin Implant Dent Relat Res. 2017;19(4):632‐642. [DOI] [PubMed] [Google Scholar]
- 20. Hakam AE, Vila G, Mendes Duarte P, et al. Effects of different antidepressant classes on dental implant failure: a retrospective clinical study. J Periodontol. 2021;92(2):196‐204. [DOI] [PubMed] [Google Scholar]
- 21. Chappuis V, Avila‐Ortiz G, Araújo MG, Monje A. Medication‐related dental implant failure: systematic review and meta‐analysis. Clin Oral Implants Res. 2018;29(S16):55‐68. [DOI] [PubMed] [Google Scholar]
- 22. Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575‐1586. [DOI] [PubMed] [Google Scholar]
- 23. World Health Organization . The ICD‐10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. World Health Organization; 1992. [Google Scholar]
- 24. Siu AL, Bibbins‐Domingo K, Grossman DC, et al. Screening for depression in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;315(4):380‐387. [DOI] [PubMed] [Google Scholar]
- 25. World Health Organization . Depression and other Common Mental Disorders: Global Health Estimates. World Health Organization; 2017. [Google Scholar]
- 26. Prince M, Patel V, Saxena S, et al. No health without mental health. Lancet. 2007;370(9590):859‐877. [DOI] [PubMed] [Google Scholar]
- 27. Otte C, Gold SM, Penninx BW, et al. Major depressive disorder. Nat Rev Dis Primers. 2016;2:16065. [DOI] [PubMed] [Google Scholar]
- 28. Barnes J, Mondelli V, Pariante CM. Genetic contributions of inflammation to depression. Neuropsychopharmacology. 2017;42(1):81‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wingenfeld K, Wolf OT. HPA axis alterations in mental disorders: impact on memory and its relevance for therapeutic interventions. CNS Neurosci Ther. 2011;17(6):714‐722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Leonard BE. Inflammation and depression: a causal or coincidental link to the pathophysiology? Acta Neuropsychiatr. 2018;30(1):1‐16. [DOI] [PubMed] [Google Scholar]
- 31. Iwata M, Ota KT, Duman RS. The inflammasome: pathways linking psychological stress, depression, and systemic illnesses. Brain Behav Immun. 2013;31:105‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16(1):22‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Esch T, Stefano GB, Fricchione GL, Benson H. The role of stress in neurodegenerative diseases and mental disorders. Neuroendocrinol Lett. 2002;23(3):199‐208. [PubMed] [Google Scholar]
- 34. Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5(7):374‐381. [DOI] [PubMed] [Google Scholar]
- 35. Dhabhar FS. Stress‐induced augmentation of immune function—the role of stress hormones, leukocyte trafficking, and cytokines. Brain Behav Immun. 2002;16(6):785‐798. [DOI] [PubMed] [Google Scholar]
- 36. Chida Y, Hamer M. An association of adverse psychosocial factors with diabetes mellitus: a meta‐analytic review of longitudinal cohort studies. Diabetologia. 2008;51(21):2168‐2178. Springer. [DOI] [PubMed] [Google Scholar]
- 37. Backé E‐M, Seidler A, Latza U, Rossnagel K, Schumann B. The role of psychosocial stress at work for the development of cardiovascular diseases: a systematic review. Int Arch Occup Environ Health. 2012;85(1):67‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stabholz A, Soskolne WA, Shapira L. Genetic and environmental risk factors for chronic periodontitis and aggressive periodontitis. Periodontol 2000. 2010;53(1):138‐153. [DOI] [PubMed] [Google Scholar]
- 39. Rohleder N. Stress and inflammation—the need to address the gap in the transition between acute and chronic stress effects. Psychoneuroendocrinology. 2019;105:164‐171. [DOI] [PubMed] [Google Scholar]
- 40. Dhabhar FS, McEwen BS. Enhancing versus suppressive effects of stress hormones on skin immune function. Proc Natl Acad Sci USA. 1999;96(3):1059‐1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lamberts S, Verleun T, Oosterom R, de Jong F, Hackeng W. Corticotropin‐releasing factor (ovine) and vasopressin exert a synergistic effect on adrenocorticotropin release in man. J Clin Endocrinol Metab. 1984;58(2):298‐303. [DOI] [PubMed] [Google Scholar]
- 42. Gaillet S, Lachuer J, Malaval F, Assenmacher I, Szafarczyk A. The involvement of noradrenergic ascending pathways in the stress‐induced activation of ACTH and corticosterone secretions is dependent on the nature of stressors. Exp Brain Res. 1991;87(1):173‐180. [DOI] [PubMed] [Google Scholar]
- 43. Haririan H, Bertl K, Laky M, et al. Salivary and serum chromogranin A and α‐amylase in periodontal health and disease. J Periodontol. 2012;83(10):1314‐1321. [DOI] [PubMed] [Google Scholar]
- 44. Cirillo N, Prime SS. Keratinocytes synthesize and activate cortisol. J Cell Biochem. 2011;112(6):1499‐1505. [DOI] [PubMed] [Google Scholar]
- 45. Dhabhar FS, Mcewen BS. Acute stress enhances while chronic stress suppresses cell‐mediated immunityin vivo: a potential role for leukocyte trafficking. Brain Behav Immun. 1997;11(4):286‐306. [DOI] [PubMed] [Google Scholar]
- 46. Baybutt H, Holsboer F. Inhibition of macrophage differentiation and function by cortisol. Endocrinology. 1990;127(1):476‐480. [DOI] [PubMed] [Google Scholar]
- 47. Gatti G, Cavallo R, Sartori ML, et al. Inhibition by cortisol of human natural killer (NK) cell activity. J Steroid Biochem. 1987;26(1):49‐58. [DOI] [PubMed] [Google Scholar]
- 48. Johnson J, Campisi J, Sharkey C, et al. Catecholamines mediate stress‐induced increases in peripheral and central inflammatory cytokines. Neuroscience. 2005;135(4):1295‐1307. [DOI] [PubMed] [Google Scholar]
- 49. Bergquist J, Josefsson E, Tarkowski A, Ekman R, Ewing A. Measurements of catecholamine‐mediated apoptosis of immunocompetent cells by capillary electrophoresis. Electrophoresis. 1997;18(10):1760‐1766. [DOI] [PubMed] [Google Scholar]
- 50. Schedlowski M, Falk A, Rohne A, et al. Catecholamines induce alterations of distribution and activity of human natural killer (NK) cells. J Clin Immunol. 1993;13(5):344‐351. [DOI] [PubMed] [Google Scholar]
- 51. Weiler R, Steiner H‐J, Fischer‐Colbrie R, Schmid K, Winkler H. Undegraded chromogranin A is present in serum and enters the endocytotic lysosomal pathway in kidney. Histochemistry. 1991;96(5):395‐399. [DOI] [PubMed] [Google Scholar]
- 52. Landsberg L. Chromogranin A. N Engl J Med. 1984;311(12):794‐795. [DOI] [PubMed] [Google Scholar]
- 53. Saruta J, Tsukinoki K, Sasaguri K, et al. Expression and localization of chromogranin A gene and protein in human submandibular gland. Cells Tissues Organs. 2005;180(4):237‐244. [DOI] [PubMed] [Google Scholar]
- 54. Aung G, Niyonsaba F, Ushio H, et al. Catestatin, a neuroendocrine antimicrobial peptide, induces human mast cell migration, degranulation and production of cytokines and chemokines. Immunology. 2011;132(4):527‐539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bosch JA, Brand HS, Ligtenberg TJ, Bermond B, Hoogstraten J, Nieuw Amerongen AV. Psychological stress as a determinant of protein levels and salivary‐induced aggregation of Streptococcus gordonii in human whole saliva. Psychosom Med. 1996;58(4):374‐382. [DOI] [PubMed] [Google Scholar]
- 56. Arhakis A, Karagiannis V, Kalfas S. Salivary alpha‐amylase activity and salivary flow rate in young adults. Open Dent J. 2013;7:7‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bartold P, Kylstra A, Lawson R. Substance P: an immunohistochemical and biochemical study in human gingival tissues. A role for neurogenic inflammation? J Periodontol. 1994;65(12):1113‐1121. [DOI] [PubMed] [Google Scholar]
- 58. Tost H, Champagne FA, Meyer‐Lindenberg A. Environmental influence in the brain, human welfare and mental health. Nat Neurosci. 2015;18(10):1421‐1431. [DOI] [PubMed] [Google Scholar]
- 59. Huppert JD, Smith TE. Anxiety and schizophrenia: the interaction of subtypes of anxiety and psychotic symptoms. CNS Spectr. 2005;10(9):721‐731. [DOI] [PubMed] [Google Scholar]
- 60. Corcoran C, Walker E, Huot R, et al. The stress cascade and schizophrenia: etiology and onset. Schizophr Bull. 2003;29(4):671‐692. [DOI] [PubMed] [Google Scholar]
- 61. C‐sR L, Sinha R. Inhibitory control and emotional stress regulation: neuroimaging evidence for frontal‐limbic dysfunction in psycho‐stimulant addiction. Neurosci Biobehav Rev. 2008;32(3):581‐597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Boney‐McCoy S, Finkelhor D. Prior victimization: a risk factor for child sexual abuse and for PTSD‐related symptomatology among sexually abused youth. Child Abuse Negl. 1995;19(12):1401‐1421. [DOI] [PubMed] [Google Scholar]
- 63. Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156(6):837‐841. [DOI] [PubMed] [Google Scholar]
- 64. Mineur Y, Belzung C, Crusio W. Functional implications of decreases in neurogenesis following chronic mild stress in mice. Neuroscience. 2007;150(2):251‐259. [DOI] [PubMed] [Google Scholar]
- 65. Cerqueira JJ, Mailliet F, Almeida OF, Jay TM, Sousa N. The prefrontal cortex as a key target of the maladaptive response to stress. J Neurosci. 2007;27(11):2781‐2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dias‐Ferreira E, Sousa JC, Melo I, et al. Chronic stress causes frontostriatal reorganization and affects decision‐making. Science. 2009;325(5940):621‐625. [DOI] [PubMed] [Google Scholar]
- 67. Sandi C, Haller J. Stress and the social brain: behavioural effects and neurobiological mechanisms. Nat Rev Neurosci. 2015;16(5):290‐304. [DOI] [PubMed] [Google Scholar]
- 68. Holsboer F, Gerken A, Von Bardeleben U, et al. Human corticotropin‐releasing hormone in depression—correlation with thyrotropin secretion following thyrotropin‐releasing hormone. Biol Psychiatry. 1986;21(7):601‐611. [DOI] [PubMed] [Google Scholar]
- 69. Bremner JD, Licinio J, Darnell A, et al. Elevated CSF corticotropin‐releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry. 1997;154(5):624‐629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. O'Connor L, Nemeroff CB. CSF corticotropin‐releasing factor–like immunoreactivity in depression and schizophrenia. Am J Psychiatry. 1987;144(7):873‐877. [DOI] [PubMed] [Google Scholar]
- 71. Crofford LJ, Sano H, Karalis K, et al. Corticotropin‐releasing hormone in synovial fluids and tissues of patients with rheumatoid arthritis and osteoarthritis. J Immunol. 1993;151(3):1587‐1596. [PubMed] [Google Scholar]
- 72. Monteiro da Silva A, Newman H, Oakley D. Psychosocial factors in inflammatory periodontal diseases. J Clin Periodontol. 1995;22(7):516‐526. [DOI] [PubMed] [Google Scholar]
- 73. Deinzer R, Rüttermann S, Möbes O, Herforth A. Increase in gingival inflammation under academic stress. J Clin Periodontol. 1998;25(5):431‐433. [DOI] [PubMed] [Google Scholar]
- 74. Deinzer R, Förster P, Fuck L, Herforth A, Stiller‐Winkler R, Idel H. Increase of crevicular interleukin 1b under academic stress at experimental gingivitis sites and at sites of perfect oral hygiene. J Clin Periodontol. 1999;26(1):1‐8. [DOI] [PubMed] [Google Scholar]
- 75. Wilton J, Mcpton J, Griffiths G, et al. Interleukin‐1 beta (IL‐1β) levels in gingival crevicular fluid from adults with previous evidence of destructive periodontitis. J Clin Periodontol. 1992;19(1):53‐57. [DOI] [PubMed] [Google Scholar]
- 76. Johannsen A, Rydmark I, Söder B, Åsberg M. Gingival inflammation, increased periodontal pocket depth and elevated interleukin‐6 in gingival crevicular fluid of depressed women on long‐term sick leave. J Periodontal Res. 2007;42(6):546‐552. [DOI] [PubMed] [Google Scholar]
- 77. Giannopoulou C, Kamma JJ, Mombelli A. Effect of inflammation, smoking and stress on gingival crevicular fluid cytokine level. J Clin Periodontol. 2003;30(2):145‐153. [DOI] [PubMed] [Google Scholar]
- 78. Ishisaka A, Ansai T, Soh I, et al. Association of salivary levels of cortisol and dehydroepiandrosterone with periodontitis in older Japanese adults. J Periodontol. 2007;78(9):1767‐1773. [DOI] [PubMed] [Google Scholar]
- 79. Ishisaka A, Ansai T, Soh I, et al. Association of cortisol and dehydroepiandrosterone sulphate levels in serum with periodontal status in older Japanese adults. J Clin Periodontol. 2008;35(10):853‐861. [DOI] [PubMed] [Google Scholar]
- 80. Haririan H, Andrukhov O, Böttcher M, et al. Salivary neuropeptides, stress, and periodontitis. J Periodontol. 2018;89(1):9‐18. [DOI] [PubMed] [Google Scholar]
- 81. Genco R, Ho A, Grossi S, Dunford R, Tedesco L. Relationship of stress, distress, and inadequate coping behaviors to periodontal disease. J Periodontol. 1999;70(7):711‐723. [DOI] [PubMed] [Google Scholar]
- 82. Wimmer G, Janda M, Wieselmann‐Penkner K, Jakse N, Polansky R, Pertl C. Coping with stress: its influence on periodontal disease. J Periodontol. 2012;73(11):1343‐1351. [DOI] [PubMed] [Google Scholar]
- 83. Hajishengallis G, Lamont RJ. Beyond the red complex and into more complexity: the polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol Oral Microbiol. 2012;27(6):409‐419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Jentsch H, März D, Krüger M. The effects of stress hormones on growth of selected periodontitis related bacteria. Anaerobe. 2013;24:49‐54. [DOI] [PubMed] [Google Scholar]
- 85. Roberts A, Matthews J, Socransky S, Freestone P, Williams P, Chapple I. Stress and the periodontal diseases: effects of catecholamines on the growth of periodontal bacteria in vitro. Mol Oral Microbiol. 2002;17(5):296‐303. [DOI] [PubMed] [Google Scholar]
- 86. Saito T, Inagaki S, Sakurai K, Okuda K, Ishihara K. Exposure of P. gingivalis to noradrenaline reduces bacterial growth and elevates ArgX protease activity. Arch Oral Biol. 2011;56(3):244‐250. [DOI] [PubMed] [Google Scholar]
- 87. Olsen I, Yamazaki K. Can oral bacteria affect the microbiome of the gut? J Oral Microbiol. 2019;11(1):1586422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Boyapati L, Wang HL. The role of stress in periodontal disease and wound healing. Periodontol 2000. 2007;44(1):195‐210. [DOI] [PubMed] [Google Scholar]
- 89. Monteiro da Silva A, Newman H, Oakley D, O'leary R. Psychosocial factors, dental plaque levels and smoking in periodontitis patients. J Clin Periodontol. 1998;25(6):517‐523. [DOI] [PubMed] [Google Scholar]
- 90. Kurer J, Watts T, Weinman J, Gower D. Psychological mood of regular dental attenders in relation to oral hygiene behaviour and gingival health. J Clin Periodontol. 1995;22(1):52‐55. [DOI] [PubMed] [Google Scholar]
- 91. Khocht A, Janal M, Schleifer S, Keller S. The influence of gingival margin recession on loss of clinical attachment in alcohol‐dependent patients without medical disorders. J Periodontol. 2003;74(4):485‐493. [DOI] [PubMed] [Google Scholar]
- 92. Axtelius B, Söderfeldt B, Nilsson A, Edwardsson S, Attström R. Therapy‐resistant periodontitis. Psychosocial characteristics. J Clin Periodontol. 1998;25(6):482‐491. [DOI] [PubMed] [Google Scholar]
- 93. Vettore M, Quintanilha RS, Monteiro da Silva AM, Lamarca GA, Leão AT. The influence of stress and anxiety on the response of non‐surgical periodontal treatment. J Clin Periodontol. 2005;32(12):1226‐1235. [DOI] [PubMed] [Google Scholar]
- 94. Wimmer G, Köhldorfer G, Mischak I, Lorenzoni M, Kallus KW. Coping with stress: its influence on periodontal therapy. J Periodontol. 2005;76(1):90‐98. [DOI] [PubMed] [Google Scholar]
- 95. Ng SKS, Keung Leung W. A community study on the relationship between stress, coping, affective dispositions and periodontal attachment loss. Community Dent Oral Epidemiol. 2006;34(4):252‐266. [DOI] [PubMed] [Google Scholar]
- 96. Johannsen A, Åsberg M, Söder PÖ, Söder B. Anxiety, gingival inflammation and periodontal disease in non‐smokers and smokers—an epidemiological study. J Clin Periodontol. 2005;32(5):488‐491. [DOI] [PubMed] [Google Scholar]
- 97. Levin L, Zini A, Levine J, et al. Demographic profile, oral health impact profile and dental anxiety scale in patients with chronic periodontitis: a case‐control study. Int Dent J. 2018;68:269‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Vettore M, Leao A, Monteiro Da Silva A, Quintanilha R, Lamarca G. The relationship of stress and anxiety with chronic periodontitis. J Clin Periodontol. 2003;30(5):394‐402. [DOI] [PubMed] [Google Scholar]
- 99. Castro G, Oppermann R, Haas A, Winter R, Alchieri J. Association between psychosocial factors and periodontitis: a case‐control study. J Clin Periodontol. 2006;33(2):109‐114. [DOI] [PubMed] [Google Scholar]
- 100. Delgado‐Angulo EK, Sabbah W, Suominen AL, et al. The association of depression and anxiety with dental caries and periodontal disease among Finnish adults. Community Dent Oral Epidemiol. 2015;43(6):540‐549. [DOI] [PubMed] [Google Scholar]
- 101. Solis A, Lotufo R, Pannuti C, Brunheiro E, Marques A, Lotufo‐Neto F. Association of periodontal disease to anxiety and depression symptoms, and psychosocial stress factors. J Clin Periodontol. 2004;31(8):633‐638. [DOI] [PubMed] [Google Scholar]
- 102. Zheng DX, Kang XN, Wang YX, et al. Periodontal disease and emotional disorders: A meta‐analysis. J Clin Periodontol. 2021;48(2):180‐204. [DOI] [PubMed] [Google Scholar]
- 103. Monteiro da Silva A, Oakley D, Newman H, Nohl F, Lloyd H. Psychosocial factors and adult onset rapidly progressive periodontitis. J Clin Periodontol. 1996;23(8):789‐794. [DOI] [PubMed] [Google Scholar]
- 104. Moss ME, Beck JD, Kaplan BH, et al. Exploratory case‐control analysis of psychosocial factors and adult periodontitis. J Periodontol. 1996;67(10 suppl):1060‐1069. [DOI] [PubMed] [Google Scholar]
- 105. Li Q, Xu C, Wu Y, et al. Relationship between the chronic periodontitis and the depression anxiety psychological factor. J Cent South Univ (Med Sci). 2011;36(1):88‐92. [DOI] [PubMed] [Google Scholar]
- 106. Araújo MM, Martins CC, Costa LCM, et al. Association between depression and periodontitis: a systematic review and meta‐analysis. J Clin Periodontol. 2016;43(3):216‐228. [DOI] [PubMed] [Google Scholar]
- 107. Elter JR, White BA, Gaynes BN, Bader JD. Relationship of clinical depression to periodontal treatment outcome. J Periodontol. 2002;73(4):441‐449. [DOI] [PubMed] [Google Scholar]
- 108. Petit C, Anadon‐Rosinach V, Rettig L, et al. Influence of psychological stress on non‐surgical periodontal treatment outcomes in patients with severe chronic periodontitis. J Periodontol. 2021;92(2):186‐195. [DOI] [PubMed] [Google Scholar]
- 109. Eren I, Nazıroğlu M, Demirdaş A, et al. Venlafaxine modulates depression‐induced oxidative stress in brain and medulla of rat. Neurochem Res. 2007;32(3):497‐505. [DOI] [PubMed] [Google Scholar]
- 110. Eren İ, Nazıroğlu M, Demirdaş A. Protective effects of lamotrigine, aripiprazole and escitalopram on depression‐induced oxidative stress in rat brain. Neurochem Res. 2007;32(7):1188‐1195. [DOI] [PubMed] [Google Scholar]
- 111. Branco‐de‐Almeida LS, Franco GC, Castro ML, et al. Fluoxetine inhibits inflammatory response and bone loss in a rat model of ligature‐induced periodontitis. J Periodontol. 2012;83(5):664‐671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Aguiar J, Gomes E, Fonseca‐Silva T, et al. Fluoxetine reduces periodontal disease progression in a conditioned fear stress model in rats. J Periodontal Res. 2013;48(5):632‐637. [DOI] [PubMed] [Google Scholar]
- 113. Bhatia A, Sharma RK, Tewari S, Khurana H, Narula SC. Effect of fluoxetine on periodontal status in patients with depression: a cross‐sectional observational study. J Periodontol. 2015;86(8):927‐935. [DOI] [PubMed] [Google Scholar]
- 114. Allan C, Valkanova V, Ebmeier K. Depression in older people is underdiagnosed. Practitioner. 2014;258(1771):19‐22. 2–3. [PubMed] [Google Scholar]
- 115. Hong JS, Tian J. Prevalence of anxiety and depression and their risk factors in Chinese cancer patients. Support Care Cancer. 2014;22(2):453‐459. [DOI] [PubMed] [Google Scholar]
- 116. Boillot A, El Halabi B, Batty GD, Rangé H, Czernichow S, Bouchard P. Education as a predictor of chronic periodontitis: a systematic review with meta‐analysis population‐based studies. PLoS One. 2011;6(7):e21508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Dunlop DD, Song J, Lyons JS, Manheim LM, Chang RW. Racial/ethnic differences in rates of depression among preretirement adults. Am J Public Health. 2003;93(11):1945‐1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Miech RA, Shanahan MJ. Socioeconomic status and depression over the life course. J Health Soc Behav. 2000;41(2):162‐176. [Google Scholar]
- 119. Haas AN, Gaio EJ, Oppermann RV, Rösing CK, Albandar JM, Susin C. Pattern and rate of progression of periodontal attachment loss in an urban population of South Brazil: a 5‐years population‐based prospective study. J Clin Periodontol. 2012;39(1):1‐9. [DOI] [PubMed] [Google Scholar]
- 120. Klimkiewicz A, Klimkiewicz J, Jakubczyk A, Kieres‐Salomoński I, Wojnar M. Comorbidity of alcohol dependence with other psychiatric disorders. Part I. Epidemiology of dual diagnosis. Psychiatr Pol. 2015;49(2):265‐275. [DOI] [PubMed] [Google Scholar]
- 121. Roy M, Tapadia MG, Joshi S, Koch B. Molecular and genetic basis of depression. J Genet. 2014;93(3):879‐892. [DOI] [PubMed] [Google Scholar]
- 122. Dias Corrêa J, Sirineu Pereira D, Fernandes Moreira Madeira M, et al. Brain‐derived neurotrophic factor in chronic periodontitis. Mediators Inflamm. 2014;2014:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Costa JE, Gomes CC, Cota LO, et al. Polymorphism in the promoter region of the gene for 5‐HTT in individuals with aggressive periodontitis. J Oral Sci. 2008;50(2):193‐198. [DOI] [PubMed] [Google Scholar]
- 124. Murri MB, Pariante C, Mondelli V, et al. HPA axis and aging in depression: systematic review and meta‐analysis. Psychoneuroendocrinology. 2014;41:46‐62. [DOI] [PubMed] [Google Scholar]
- 125. Zorrilla EP, Luborsky L, McKay JR, et al. The relationship of depression and stressors to immunological assays: a meta‐analytic review. Brain Behav Immun. 2001;15(3):199‐226. [DOI] [PubMed] [Google Scholar]
- 126. Breivik T, Opstad PK, Gjermo P, Thrane PS. Effects of hypothalamic‐pituitary‐adrenal axis reactivity on periodontal tissue destruction in rats. Eur J Oral Sci. 2000;108(2):115‐122. [DOI] [PubMed] [Google Scholar]
- 127. Breivik T, Thrane P, Gjermo P, Opstad P, Pabst R, Von Hörsten S. Hypothalamic‐pituitary‐adrenal axis activation by experimental periodontal disease in rats. J Periodontal Res. 2001;36(5):295‐300. [DOI] [PubMed] [Google Scholar]
- 128. Dowlati Y, Herrmann N, Swardfager W, et al. A meta‐analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446‐457. [DOI] [PubMed] [Google Scholar]
- 129. Black C, Miller BJ. Meta‐analysis of cytokines and chemokines in suicidality: distinguishing suicidal versus nonsuicidal patients. Biol Psychiatry. 2015;78(1):28‐37. [DOI] [PubMed] [Google Scholar]
- 130. Berk M, Wadee A, Kuschke R, O'Neill‐Kerr A. Acute phase proteins in major depression. J Psychosom Res. 1997;43(5):529‐534. [DOI] [PubMed] [Google Scholar]
- 131. Maes M, Christophe A, Delanghe J, Altamura C, Neels H, Meltzer HY. Lowered ω3 polyunsaturated fatty acids in serum phospholipids and cholesteryl esters of depressed patients. Psychiatry Res. 1999;85(3):275‐291. [DOI] [PubMed] [Google Scholar]
- 132. Berk M, Williams LJ, Jacka FN, et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013;11(1):200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. D'Aiuto F, Parkar M, Andreou G, et al. Periodontitis and systemic inflammation: control of the local infection is associated with a reduction in serum inflammatory markers. J Dent Res. 2004;83(2):156‐160. [DOI] [PubMed] [Google Scholar]
- 134. Bretz WA, Weyant RJ, Corby PM, et al. Systemic inflammatory markers, periodontal diseases, and periodontal infections in an elderly population. J Am Geriatr Soc. 2005;53(9):1532‐1537. [DOI] [PubMed] [Google Scholar]
- 135. Fredriksson MI, Gustafsson AK, Bergström KG, Åsman BE. Constitutionally hyperreactive neutrophils in periodontitis. J Periodontol. 2003;74(2):219‐224. [DOI] [PubMed] [Google Scholar]
- 136. Durham J, Fraser HM, McCracken GI, Stone KM, John MT, Preshaw PM. Impact of periodontitis on oral health–related quality of life. J Dent. 2013;41(4):370‐376. [DOI] [PubMed] [Google Scholar]
- 137. Hsu C‐C, Hsu Y‐C, Chen H‐J, et al. Association of periodontitis and subsequent depression: a nationwide population‐based study. Medicine (Baltimore). 2015;94(51):e2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Clark DB. Dental care for the patient with bipolar disorder. J Can Dent Assoc. 2003;69(1):20‐24. [PubMed] [Google Scholar]
- 139. Barnes GP, Allen EH, Parker WA, Lyon TC, Armentrout W, Cole JS. Dental treatment needs among hospitalized adult mental patients. Spec Care Dentist. 1988;8(4):173‐177. [DOI] [PubMed] [Google Scholar]
- 140. Velasco E, Bullon P. Periodontal status and treatment needs among Spanish hospitalized psychiatric patients. Spec Care Dentist. 1999;19(6):254‐258. [DOI] [PubMed] [Google Scholar]
- 141. Sjögren R, Nordström G. Oral health status of psychiatric patients. J Clin Nurs. 2000;9(4):632‐638. [DOI] [PubMed] [Google Scholar]
- 142. Friedlander AA, Brill NQ. The dental management of patients with bipolar disorder. Oral Surg Oral Med Oral Pathol. 1986;61(6):579‐581. [DOI] [PubMed] [Google Scholar]
- 143. Brietzke E, Stertz L, Fernandes BS, et al. Comparison of cytokine levels in depressed, manic and euthymic patients with bipolar disorder. J Affect Disord. 2009;116(3):214‐217. [DOI] [PubMed] [Google Scholar]
- 144. McCreadie R, Stevens H, Henderson J, et al. The dental health of people with schizophrenia. Acta Psychiatr Scand. 2004;110(4):306‐310. [DOI] [PubMed] [Google Scholar]
- 145. Eltas A, Kartalcı Ş, Eltas Ş, Dündar S, Uslu M. An assessment of periodontal health in patients with schizophrenia and taking antipsychotic medication. Int J Dent Hyg. 2013;11(2):78‐83. [DOI] [PubMed] [Google Scholar]
- 146. Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta‐analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70(7):663‐671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Leonard BE, Schwarz M, Myint AM. The metabolic syndrome in schizophrenia: is inflammation a contributing cause? J Psychopharmacol. 2012;26(5_suppl):33‐41. [DOI] [PubMed] [Google Scholar]
- 148. Brown L, Hansnata E, La HA. Economic Cost of Dementia in Australia: 2016‐2056. Institute for Governance and Policy Analysis; 2017. [Google Scholar]
- 149. Anstey KJ, Burns RA, Birrell CL, Steel D, Kiely KM, Luszcz MA. Estimates of probable dementia prevalence from population‐based surveys compared with dementia prevalence estimates based on meta‐analyses. BMC Neurol. 2010;10(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. McGeer PL, McGeer EG. Inflammation, autotoxicity and Alzheimer disease. Neurobiol Aging. 2001;22(6):799‐809. [DOI] [PubMed] [Google Scholar]
- 151. Akiyama H, Barger S, Barnum S, et al. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21(3):383‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Heyser CJ, Masliah E, Samimi A, Campbell IL, Gold LH. Progressive decline in avoidance learning paralleled by inflammatory neurodegeneration in transgenic mice expressing interleukin 6 in the brain. Proc Natl Acad Sci USA. 1997;94(4):1500‐1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Ledeboer A, Brevé JJ, Poole S, Tilders FJ, Van Dam AM. Interleukin‐10, interleukin‐4, and transforming growth factor‐β differentially regulate lipopolysaccharide‐induced production of pro‐inflammatory cytokines and nitric oxide in co‐cultures of rat astroglial and microglial cells. Glia. 2000;30(2):134‐142. [DOI] [PubMed] [Google Scholar]
- 154. Patel NS, Paris D, Mathura V, Quadros AN, Crawford FC, Mullan MJ. Inflammatory cytokine levels correlate with amyloid load in transgenic mouse models of Alzheimer's disease. J Neuroinflammation. 2005;2(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. El Khoury JB, Moore KJ, Means TK, et al. CD36 mediates the innate host response to β‐amyloid. J Exp Med. 2003;197(12):1657‐1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Lue LF, Rydel R, Brigham EF, et al. Inflammatory repertoire of Alzheimer's disease and nondemented elderly microglia in vitro. Glia. 2001;35(1):72‐79. [DOI] [PubMed] [Google Scholar]
- 157. Holmer J, Eriksdotter M, Schultzberg M, Pussinen PJ, Buhlin K. Association between periodontitis and risk of Alzheimer's disease, mild cognitive impairment and subjective cognitive decline: a case‐control study. J Clin Periodontol. 2018;45(11):1287‐1298. [DOI] [PubMed] [Google Scholar]
- 158. Yaffe K, Kanaya A, Lindquist K, et al. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA. 2004;292(18):2237‐2242. [DOI] [PubMed] [Google Scholar]
- 159. Engelhart MJ, Geerlings MI, Meijer J, et al. Inflammatory proteins in plasma and the risk of dementia: the Rotterdam Study. Arch Neurol. 2004;61(5):668‐672. [DOI] [PubMed] [Google Scholar]
- 160. Kalman J, Juhasz A, Laird G, et al. Serum interleukin‐6 levels correlate with the severity of dementia in Down syndrome and in Alzheimer's disease. Acta Neurol Scand. 1997;96(4):236‐240. [DOI] [PubMed] [Google Scholar]
- 161. Holmes C, El‐Okl M, Williams A, Cunningham C, Wilcockson D, Perry V. Systemic infection, interleukin 1β, and cognitive decline in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2003;74(6):788‐789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Nicoll JA, Mrak RE, Graham DI, et al. Association of interleukin‐1 gene polymorphisms with Alzheimer's disease. Ann Neurol. 2000;47(3):365‐368. [PMC free article] [PubMed] [Google Scholar]
- 163. McGeer P, McGeer E. Anti‐inflammatory drugs in the fight against Alzheimer's disease. Ann N Y Acad Sci. 1996;777(1):213‐220. [DOI] [PubMed] [Google Scholar]
- 164. Stewart WF, Kawas C, Corrada M, Metter EJ. Risk of Alzheimer's disease and duration of NSAID use. Neurology. 1997;48(3):626‐632. [DOI] [PubMed] [Google Scholar]
- 165. Aisen PS, Schafer KA, Grundman M, et al. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA. 2003;289(21):2819‐2826. [DOI] [PubMed] [Google Scholar]
- 166. Thal LJ, Ferris SH, Kirby L, et al. A randomized, double‐blind, study of rofecoxib in patients with mild cognitive impairment. Neuropsychopharmacology. 2005;30(6):1204‐1215. [DOI] [PubMed] [Google Scholar]
- 167. Craig RG, Yip JK, So MK, Boylan RJ, Socransky SS, Haffajee AD. Relationship of destructive periodontal disease to the acute‐phase response. J Periodontol. 2003;74(7):1007‐1016. [DOI] [PubMed] [Google Scholar]
- 168. Slade G, Offenbacher S, Beck J, Heiss G, Pankow J. Acute‐phase inflammatory response to periodontal disease in the US population. J Dent Res. 2000;79(1):49‐57. [DOI] [PubMed] [Google Scholar]
- 169. Shapira L, Soskolne WA, Sela M, Offenbacher S, Barak V. The secretion of PGE2, IL‐1β, IL‐6, and TNFα by adherent mononuclear cells from early onset periodontitis patients. J Periodontol. 1994;65(2):139‐146. [DOI] [PubMed] [Google Scholar]
- 170. Salvi GE, Collins JG, Yalda B, Arnold RR, Lang NP, Offenbacher S. Monocytic TNFα secretion patterns in IDDM patients with periodontal diseases. J Clin Periodontol. 1997;24(1):8‐16. [DOI] [PubMed] [Google Scholar]
- 171. Riviere GR, Riviere K, Smith K. Molecular and immunological evidence of oral Treponema in the human brain and their association with Alzheimer's disease. Mol Oral Microbiol. 2002;17(2):113‐118. [DOI] [PubMed] [Google Scholar]
- 172. Dominy SS, Lynch C, Ermini F, et al. Porphyromonas gingivalis in Alzheimer's disease brains: evidence for disease causation and treatment with small‐molecule inhibitors. Sci Adv. 2019;5(1):eaau3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Parra‐Torres V, Melgar‐Rodríguez S, Muñoz‐Manríquez C, et al. Periodontal bacteria in the brain—implication for Alzheimer's disease: a systematic review. Oral Dis. 2021. doi: 10.1111/odi.14054 [DOI] [PubMed] [Google Scholar]
- 174. Reid AG, Lingford‐Hughes AR, Cancela L, Kalivas PW. Substance abuse disorders. Handb Clin Neurol. 2012;106(1):419‐431. [DOI] [PubMed] [Google Scholar]
- 175. Wise RA. Brain reward circuitry: insights from unsensed incentives. Neuron. 2002;36(2):229‐240. [DOI] [PubMed] [Google Scholar]
- 176. Schultz W. Neural coding of basic reward terms of animal learning theory, game theory, microeconomics and behavioural ecology. Curr Opin Neurobiol. 2004;14(2):139‐147. [DOI] [PubMed] [Google Scholar]
- 177. Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162(8):1403‐1413. [DOI] [PubMed] [Google Scholar]
- 178. Moghaddam B. Stress activation of glutamate neurotransmission in the prefrontal cortex: implications for dopamine‐associated psychiatric disorders. Biol Psychiatry. 2002;51(10):775‐787. [DOI] [PubMed] [Google Scholar]
- 179. Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You Z‐B. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin‐releasing factor: a role in stress‐induced relapse to drug seeking. J Neurosci. 2005;25(22):5389‐5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37(4):577‐582. [DOI] [PubMed] [Google Scholar]
- 181. Leite FRM, Nascimento GG, Scheutz F, López R. Effect of smoking on periodontitis: a systematic review and meta‐regression. Am J Prev Med. 2018;54(6):831‐841. [DOI] [PubMed] [Google Scholar]
- 182. Zambon JJ, Grossi SG, Machtei EE, Ho AW, Dunford R, Genco RJ. Cigarette smoking increases the risk for subgingival infection with periodontal pathogens. J Periodontol. 1996;67(10 Suppl):1050‐1054. [DOI] [PubMed] [Google Scholar]
- 183. Bergström J, Boström L. Tobacco smoking and periodontal hemorrhagic responsiveness. J Clin Periodontol. 2001;28(7):680‐685. [DOI] [PubMed] [Google Scholar]
- 184. Güntsch A, Erler M, Preshaw P, Sigusch B, Klinger G, Glockmann E. Effect of smoking on crevicular polymorphonuclear neutrophil function in periodontally healthy subjects. J Periodontal Res. 2006;41(3):184‐188. [DOI] [PubMed] [Google Scholar]
- 185. Petropoulos G, McKay IJ, Hughes FJ. The association between neutrophil numbers and interleukin‐1α concentrations in gingival crevicular fluid of smokers and non‐smokers with periodontal disease. J Clin Periodontol. 2004;31(5):390‐395. [DOI] [PubMed] [Google Scholar]
- 186. James JA, Sayers NM, Drucker DB, Hull PS. Effects of tobacco products on the attachment and growth of periodontal ligament fibroblasts. J Periodontol. 1999;70(5):518‐525. [DOI] [PubMed] [Google Scholar]
- 187. Thomson WM, Poulton R, Broadbent JM, et al. Cannabis smoking and periodontal disease among young adults. JAMA. 2008;299(5):525‐531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. da Silva Furtado Amaral C, Vettore MV, Leão A. The relationship of alcohol dependence and alcohol consumption with periodontitis: a systematic review. J Dent. 2009;37(9):643‐651. [DOI] [PubMed] [Google Scholar]
- 189. Liang S, Wu X, Jin F. Gut‐brain psychology: rethinking psychology from the microbiota‐gut‐brain axis. Front Integr Neurosci. 2018;12:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Stilling RM, Dinan TG, Cryan JF. Microbial genes, brain & behaviour—epigenetic regulation of the gut‐brain axis. Genes Brain Behav. 2014;13(1):69‐86. [DOI] [PubMed] [Google Scholar]
- 191. Lam YY, Ha CWY, Hoffmann JMA, et al. Effects of dietary fat profile on gut permeability and microbiota and their relationships with metabolic changes in mice. Obesity (Silver Spring, Md). 2015;23(7):1429‐1439. [DOI] [PubMed] [Google Scholar]
- 192. Gasmi Benahmed A, Gasmi A, Doşa A, et al. Association between the gut and oral microbiome with obesity. Anaerobe. 2021;70:102248. [DOI] [PubMed] [Google Scholar]
- 193. Carabotti M, Scirocco A, Maselli MA, Severi C. The gut‐brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28(2):203‐209. [PMC free article] [PubMed] [Google Scholar]
- 194. Arneth BM. Gut‐brain axis biochemical signalling from the gastrointestinal tract to the central nervous system: gut dysbiosis and altered brain function. Postgrad Med J. 2018;94(1114):446‐452. [DOI] [PubMed] [Google Scholar]
- 195. Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017;474(11):1823‐1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Lyte M. Microbial endocrinology in the microbiome‐gut‐brain axis: how bacterial production and utilization of neurochemicals influence behavior. PLoS Pathog. 2013;9(11):e1003726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Wang Y, Kasper LH. The role of microbiome in central nervous system disorders. Brain Behav Immun. 2014;38:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313‐323. Erratum. Nat Rev Immunol. 2009; 9: 600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Sherwin E, Sandhu KV, Dinan TG, Cryan JF. May the force be with you: the light and dark sides of the microbiota‐gut‐brain axis in neuropsychiatry. CNS Drugs. 2016;30(11):1019‐1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Bowland GB, Weyrich LS. The oral‐microbiome‐brain axis and neuropsychiatric disorders: an anthropological perspective. Front Psychiatry. 2022;13:810008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Martínez M, Postolache TT, García‐Bueno B, et al. The role of the oral microbiota related to periodontal diseases in anxiety, mood and trauma‑ and stress‐related disorders. Front Psychiatry. 2021;12:814177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Heumann D, Roger T. Initial responses to endotoxins and gram‐negative bacteria. Clin Chim Acta. 2002;323(1):59‐72. [DOI] [PubMed] [Google Scholar]
- 203. Mazzoli R, Pessione E. The neuro‐endocrinological role of microbial glutamate and GABA signaling. Front Microbiol. 2016;7:1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Almeida C, Oliveira R, Soares R, Barata P. Influence of gut microbiota dysbiosis on brain function: a systematic review. Porto Biomed J. 2020;5(2):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Xia M‐X, Ding X, Qi J, Gu J, Hu G, Sun X‐L. Inhaled budesonide protects against chronic asthma‐induced neuroinflammation in mouse brain. J Neuroimmunol. 2014;273(1):53‐57. [DOI] [PubMed] [Google Scholar]
- 206. Kacimi R, Giffard RG, Yenari MA. Endotoxin‐activated microglia injure brain derived endothelial cells via NF‐κB, JAK‐STAT and JNK stress kinase pathways. J Inflamm (Lond). 2011;8(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Kynurenine pathway metabolism and the microbiota‐gut‐brain axis. Neuropharmacology. 2017;112(Pt B):399‐412. [DOI] [PubMed] [Google Scholar]
- 208. Averina OV, Zorkina YA, Yunes RA, et al. Bacterial metabolites of human gut microbiota correlating with depression. Int J Mol Sci. 2020;21(23):9234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Baer RA. Mindfulness training as a clinical intervention: a conceptual and empirical review. Clin Psychol (New York). 2003;10(2):125‐143. [Google Scholar]
- 210. Caspani G, Kennedy S, Foster JA, Swann J. Gut microbial metabolites in depression: understanding the biochemical mechanisms. Microb Cell. 2019;6(10):454‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Mudd AT, Berding K, Wang M, Donovan SM, Dilger RN. Serum cortisol mediates the relationship between fecal Ruminococcus and brain N‐acetylaspartate in the young pig. Gut Microbes. 2017;8(6):589‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Yang I, Arthur RA, Zhao L, et al. The oral microbiome and inflammation in mild cognitive impairment. Exp Gerontol. 2021;147:111273. [DOI] [PubMed] [Google Scholar]
- 213. Cunha FA, Cota LOM, Cortelli SC, et al. Periodontal condition and levels of bacteria associated with periodontitis in individuals with bipolar affective disorders: a case‐control study. J Periodontal Res. 2019;54(1):63‐72. [DOI] [PubMed] [Google Scholar]
- 214. Cecoro G, Annunziata M, Iuorio MT, Nastri L, Guida L. Periodontitis, low‐grade inflammation and systemic health: a scoping review. Medicina (Kaunas). 2020;56(6):272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Watanabe I, Kuriyama N, Miyatani F, et al. Oral Cnm‐positive Streptococcus mutans expressing collagen binding activity is a risk factor for cerebral microbleeds and cognitive impairment. Sci Rep. 2016;6(1):38561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Xue L, Zou X, Yang X‐Q, Peng F, Yu D‐K, Du J‐R. Chronic periodontitis induces microbiota‐gut‐brain axis disorders and cognitive impairment in mice. Exp Neurol. 2020;326:113176. [DOI] [PubMed] [Google Scholar]
- 217. Frister A, Schmidt C, Schneble N, et al. Phosphoinositide 3‐kinase γ affects LPS‐induced disturbance of blood–brain barrier via lipid kinase‐independent control of cAMP in microglial cells. Neuromolecular Med. 2014;16(4):704‐713. [DOI] [PubMed] [Google Scholar]
- 218. Yu X‐C, Yang J‐J, Jin B‐H, et al. A strategy for bypassing the blood‐brain barrier: facial intradermal brain‐targeted delivery via the trigeminal nerve. J Control Release. 2017;258:22‐33. [DOI] [PubMed] [Google Scholar]
- 219. Liu Y, Wu Z, Zhang X, et al. Leptomeningeal cells transduce peripheral macrophages inflammatory signal to microglia in reponse to Porphyromonas gingivalis LPS. Mediators Inflamm. 2013;2013:407562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Perry VH. The influence of systemic inflammation on inflammation in the brain: implications for chronic neurodegenerative disease. Brain Behav Immun. 2004;18(5):407‐413. [DOI] [PubMed] [Google Scholar]
- 221. Tobón‐Arroyave SI, Celis‐Mejía N, Córdoba‐Hidalgo MP, Isaza‐Guzmán DM. Decreased salivary concentration of CD9 and CD81 exosome‐related tetraspanins may be associated with the periodontal clinical status. J Clin Periodontol. 2019;46(4):470‐480. [DOI] [PubMed] [Google Scholar]
- 222. Dubar M, Clerc‐Urmès I, Baumann C, Clément C, Alauzet C, Bisson C. Relations of psychosocial factors and cortisol with periodontal and bacterial parameters: a prospective clinical study in 30 patients with periodontitis before and after non‐surgical treatment. Int J Environ Res Public Health. 2020;17(20):7651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Simpson CA, Adler C, du Plessis MR, et al. Oral microbiome composition, but not diversity, is associated with adolescent anxiety and depression symptoms. Physiol Behav. 2020;226:113126. [DOI] [PubMed] [Google Scholar]
- 224. Roberts A, Matthews JB, Socransky SS, Freestone PPE, Williams PH, Chapple ILC. Stress and the periodontal diseases: growth responses of periodontal bacteria to Escherichia coli stress‐associated autoinducer and exogenous Fe. Oral Microbiol Immunol. 2005;20(3):147‐153. [DOI] [PubMed] [Google Scholar]
- 225. Poole AC, Goodrich JK, Youngblut ND, et al. Human salivary amylase gene copy number impacts oral and gut microbiomes. Cell Host Microbe. 2019;25(4):553‐564. [DOI] [PubMed] [Google Scholar]
- 226. Humphrey SP, Williamson RT. A review of saliva: normal composition, flow, and function. J Prosthet Dent. 2001;85(2):162‐169. [DOI] [PubMed] [Google Scholar]
- 227. Ding T, Schloss PD. Dynamics and associations of microbial community types across the human body. Nature (London). 2014;509(7500):357‐360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Leite GGS, Weitsman S, Parodi G, et al. Mapping the segmental microbiomes in the human small bowel in comparison with stool: a REIMAGINE study. Dig Dis Sci. 2020;65(9):2595‐2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Bera R, Tripathi R, Bhattacharjee B, Singh A, Kanojia S, Kumar V. Implant survival in patients with neuropsychiatric, neurocognitive, and neurodegenerative disorders: a meta‐analysis. Natl J Maxillofac Surg. 2021;12(2):162‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Kiely KM, Leach LS, Olesen SC, Butterworth P. How financial hardship is associated with the onset of mental health problems over time. Soc Psychiatry Psychiatr Epidemiol. 2015;50(6):909‐918. [DOI] [PubMed] [Google Scholar]
- 231. Decker AM, Kapila YL, Wang HL. The psychobiological links between chronic stress‐related diseases, periodontal/peri‐implant diseases, and wound healing. Periodontol 2000. 2021;87(1):94‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Kisely S, Sawyer E, Siskind D, Lalloo R. The oral health of people with anxiety and depressive disorders—a systematic review and meta‐analysis. J Affect Disord. 2016;200:119‐132. [DOI] [PubMed] [Google Scholar]
- 233. Tsapakis E, Gamie Z, Tran G, et al. The adverse skeletal effects of selective serotonin reuptake inhibitors. Eur Psychiatry. 2012;27(3):156‐169. [DOI] [PubMed] [Google Scholar]
- 234. Battaglino R, Fu J, Späte U, et al. Serotonin regulates osteoclast differentiation through its transporter. J Bone Miner Res. 2004;19(9):1420‐1431. [DOI] [PubMed] [Google Scholar]
- 235. Sibilia V, Pagani F, Dieci E, et al. Dietary tryptophan manipulation reveals a central role for serotonin in the anabolic response of appendicular skeleton to physical activity in rats. Endocrine. 2013;44(3):790‐802. [DOI] [PubMed] [Google Scholar]
- 236. Ma Y, Krueger JJ, Redmon SN, et al. Extracellular norepinephrine clearance by the norepinephrine transporter is required for skeletal homeostasis. J Biol Chem. 2013;288(42):30105‐30113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135‐1143. [DOI] [PubMed] [Google Scholar]


