Abstract
Objective
This study was undertaken to investigate whether adjunct alteplase improves brain reperfusion following successful thrombectomy.
Methods
This single‐center, randomized, double‐blind, placebo‐controlled study included 36 patients (mean [standard deviation] = 70.8 [13.5] years old, 18 [50%] women) with large vessel occlusion undergoing thrombectomy resulting in near‐normal (expanded Thrombolysis in Cerebral Infarction [eTICI] b50/67/2c, n = 23, 64%) or normal angiographic reperfusion (eTICI 3, n = 13, 36%). Seventeen patients were randomized to intra‐arterial alteplase (0.225mg/kg), and 19 received placebo. At 48 hours, patients had brain perfusion/diffusion‐weighted magnetic resonance imaging (MRI) and MRI‐spectroscopy. The primary outcome was the difference in the proportion of patients with areas of hypoperfusion on MRI. Secondary outcomes were the infarct expansion ratio (final to initial infarction volume), and the N‐acetylaspartate (NAA) peak relative to total creatine as a marker of neuronal integrity.
Results
The prevalence of hypoperfusion was 24% with intra‐arterial alteplase, and 58% with placebo (adjusted odds ratio = 0.20, 95% confidence interval [CI] = 0.04–0.91, p = 0.03). Among 14 patients with final eTICI 3 scores, hypoperfusion was found in 1 of 7 (14%) in the alteplase group and 3 of 7 (43%) in the placebo group. Abnormal brain perfusion was associated with worse functional outcome at day 90. Alteplase significantly reduced the infarct expansion ratio compared with placebo (median [interquartile range (IQR)] = 0.7 [0.5–1.2] vs 3.2 [1.8–5.7], p = 0.01) and resulted in higher NAA peaks (median [IQR] = 1.13 [0.91–1.36] vs 1.00 [0.74–1.22], p < 0.0001).
Interpretation
There is a high prevalence of areas of hypoperfusion following thrombectomy despite successful reperfusion on angiography. Adjunct alteplase enhances brain reperfusion, which results in reduced expansion of the infarction and improved neuronal integrity. ANN NEUROL 2022;92:860–870
The no‐reflow phenomenon was first described in animal models of brain ischemia to define the persistence of microvascular hypoperfusion despite complete reopening of a proximal occlusion. 1 , 2 , 3 , 4 , 5 , 6 In these studies, platelets, leukocytes, erythrocytes, and microthrombi clogged the capillary lumina in relation to mechanisms such as calcium sensitization and vasoconstriction of parenchymal arterioles, 7 distal microembolism, 8 or overproduction of nitric oxide and superoxide in pericytes, 9 and arteriolar smooth muscle cells, 10 which leads to the formation of peroxynitrite 11 and the vasoconstriction of the microvascular bed. 9
The existence of no‐reflow is debated in human stroke, 12 although it has been occasionally described using variable definitions. 13 , 14 , 15 The absence of capillary blush on angiograms has been observed by selective injections into the distal site of occlusive thrombi prior to thrombectomy. 16 Areas of hypoperfusion have been best described at the end of thrombectomy using computed tomography (CT) perfusion, 17 or digital subtraction angiography perfusion, 18 with a prevalence of abnormal perfusion in up to 40% of the patients regardless of having a normal angiography at the end of thrombectomy. 17
Successful reperfusion is defined on digital subtraction angiography using different metrics as the antegrade restoration of capillary blush of at least 50% of the downstream territory. 19 , 20 A better outcome is associated with a higher reperfusion score, 21 , 22 but not infrequently full angiographic reperfusion may be associated with poor outcomes, and it is considered futile. In observational studies, multiple factors were associated with futile recanalization, such as old age, brain atrophy, leukoaraiosis, higher National Institutes of Health Stroke Scale (NIHSS) score, female sex, absence of collaterals, large infarct core, hypertension, hyperglycemia, delayed treatment, and number of thrombectomy passes. 23 , 24 , 25
We argue that no‐reflow may be a major contributor to suboptimal clinical efficacy of thrombectomy despite successful reperfusion on angiography. Strong evidence in support of this proposition was obtained recently in a double‐blind, placebo‐controlled, randomized study of patients with large vessel occlusion (LVO) acute ischemic stroke treated with mechanical thrombectomy and allocated to receive adjunct intra‐arterial alteplase or placebo if the procedure had been successful (Chemical Optimization of Cerebral Embolectomy (CHOICE) trial, NCT03876119). 26 The rationale to defer the administration of intra‐arterial alteplase to the end of thrombectomy was to facilitate the lysis of distal microthrombi, which might still be formed in the microcirculation and not be detected on digital subtraction angiography. In that study, although both treatment groups had similar angiographic results post‐thrombectomy, the primary outcome (proportion of patients with modified Rankin Scale [mRS] = 0–1 at day 90) was 59% in the alteplase group and 40% in the placebo group, and this difference was statistically significant. There was no significant difference in the infarct expansion ratio between the two treatment groups in the study, although a majority of the patients only had a noncontrast brain CT (NCCT) scan at baseline and at follow‐up to delineate the course of the infarction. Foreseeing the limitations of NCCT, at one study site it was planned also to perform perfusion‐weighted imaging (PWI), diffusion‐weighted imaging (DWI), and magnetic resonance (MR) spectroscopy at 48 hours after therapy whenever possible. 26 Herein, we report the results of this preplanned advanced brain imaging study.
Patients and Methods
Patients were admitted at the Hospital Clinic of Barcelona and enrolled in the CHOICE trial, but unlike the remainder of the patients included in this trial, subjects were asked to give an accompanying informed consent to also participate in a nested advanced brain imaging study. The study was approved by the ethics committee of the institution but not registered together with the main trial. The analysis plan was prespecified prior to the unblinding of the CHOICE trial results. Certified investigators performed clinical assessments at baseline, 24 and 48 hours, 5 to 7 days, and 90 days after randomization, including the mRS score for assessing global disability, and the NIHSS score for assessing neurologic impairment, as previously reported. 26 Cerebral angiographies were assessed at the central core laboratory of the clinical trial, and the CT and magnetic resonance imaging (MRI) studies were assessed by neuroradiologists blinded to treatment allocation.
Image Acquisition and Analysis
On admission, patients had an NCCT scan, CT angiogram, and CT perfusion (CTP). Digital subtraction angiography runs were performed at the end of thrombectomy and 10 minutes after completion of the experimental treatment and evaluated by 2 senior neuroradiologists at a central core imaging laboratory to determine the expanded Treatment in Cerebral Ischemia (eTICI) score. 27 At 24 hours, NCCT was repeated for evidence of extravascular blood in the brain. At 48 (standard deviation [SD] = 24) hours, all patients underwent an MRI study using a 3‐T Prisma Siemens MR unit with a 32‐channel phase‐array coil including 3‐dimensional (3D) T1‐weighted (T1w) imaging, DWI, dynamic susceptibility‐weighted contrast‐enhanced PWI, 3D time of flight angiography, susceptibility‐weighted imaging, fluid‐attenuated inversion recovery, and multivoxel MR spectroscopic imaging (MRSI). The MRSI matrix (11.4 × 11.4 × 15mm) was individually placed to preferentially cover the ischemic hemisphere, as previously reported (Fig 1). 28
FIGURE 1.
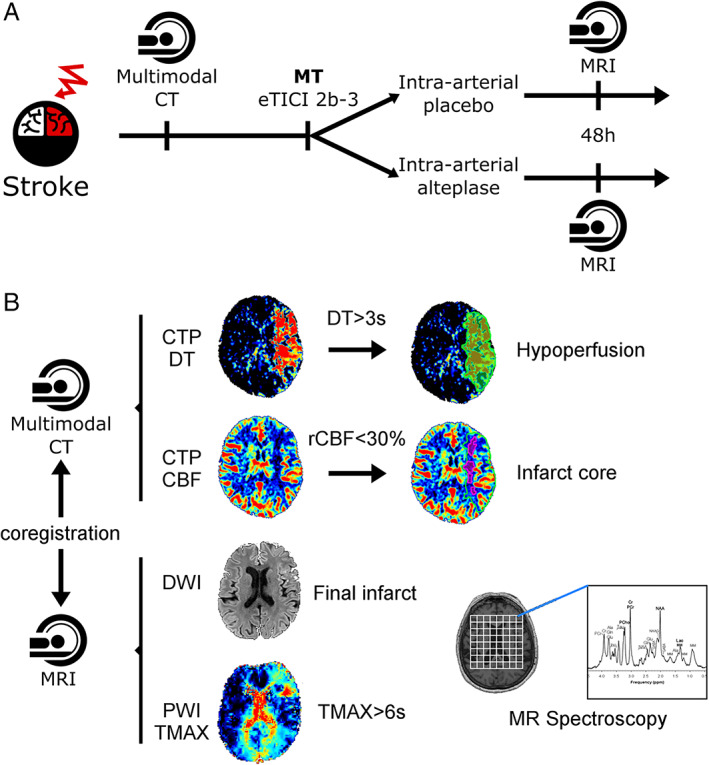
(A) Flowchart of the study. (B) Image analysis diagram. CBF = cerebral blood flow; CT = computed tomography; CTP = CT perfusion; DT = delay time; DWI = diffusion‐weighted imaging; eTICI = expanded Thrombolysis in Cerebral Infarction; MR = magnetic resonance; MRI = MR imaging; MT = mechanical thrombectomy; PWI = perfusion‐weighted imaging; rCBF = regional CBF; TMAX = time to maximum.
CTP maps were processed with MIStar (Apollo Medical Imaging Technology, Melbourne, Australia). A threshold of 3 seconds on delay time maps was used to define the hypoperfusion on admission, and infarct core was defined within the hypoperfused area with a relative cerebral blood flow threshold of 30%. 28 Final DWI lesion was segmented using Amira software (Visage Imaging, Berlin, Germany). 28 PWI maps were processed with Olea Sphere 3.0–SP6 (Olea Medical, Cambridge, MA). 29 The PWI deficits were quantified on time to maximum (TMAX) within visually segmented areas of delayed perfusion to reduce artifacts. Baseline CTP maps (including hypoperfusion and infarct core masks), T1w imaging, DWI (including final lesion mask), and MRSI were coregistered to the PWI maps for further analysis. The metabolite ratios of N‐acetylaspartate (NAA) relative to total creatine (creatine and phosphocreatine) were extracted voxel by voxel using LCModel. 30 Rejection criteria for the spectral fitting included in the software's built‐in poor baseline estimates (due to contaminating artifacts or very poor water suppression) as well as a percent standard deviation threshold of 40% to reject poorly fitted peaks. Additionally, spectra with a signal‐to‐noise ratio < 5 were removed to help eliminate poor spectra from the analysis.
The primary imaging endpoint was the proportion of patients with abnormal PWI. PWI abnormalities were defined as areas with delayed perfusion (TMAX > 6 seconds) on PWI, either outside or within the area of DWI lesion. 31 Only areas with delayed perfusion lying within DWI+ lesions were defined as no‐reflow. Perfusion abnormalities were quantified, and to exclude/discard/omit small areas with increased TMAX due to noise, only patients with a minimum cluster size of >10 contiguous areas were categorized as patients with abnormal PWI. The secondary imaging endpoint was the infarct expansion ratio (IER), defined as the ratio of the final infarct to initial ischemic tissue volumes. The tertiary imaging endpoint was the NAA peak on MRSI as a marker of neuronal integrity. 32
Statistical Analysis
Between‐groups differences were analyzed using Fisher exact or Mann–Whitney U test as appropriate. Nonparametric correlation coefficients assessed the associations between hypoperfusion at 48 hours on PWI‐MRI with (1) baseline CTP maps (volume of hypoperfusion, volume of infarct core, and penumbra mismatch), (2) longitudinal clinical data (NIHSS score, mRS score), and (3) infarct evolution on MRI at 48 hours (infarct expansion ratio, infarct volume; the final infarct characteristics were also assessed in linear regression models, adjusted for age, baseline infarct core, mismatch volume, and time from stroke onset to angiographic reperfusion 33 ), and (4) NAA peaks on MRSI at 48 hours. Sensitivity analyses were done in patients with a final eTICI score of 3 and in patients categorized according to the use of intravenous alteplase before thrombectomy. The treatment effect of alteplase versus placebo was analyzed using logistic regression and with hypoperfusion on post‐thrombectomy PWI as the dependent variable in a model adjusted for the stratification variable of the clinical trial (preceding use of intravenous thrombolysis). For voxel‐based MRSI comparisons, the characteristics of each patient (perfusion pattern, 3‐month mRS, treatment group) were adjudicated to every voxel. The analyses were performed using SPSS version 25.0 and R 4.0.4 (www.R-project.org), and the level of significance was established at the 0.05 level (2‐sided).
Results
Patient Characteristics
Of the 36 adults enrolled in the study, 17 patients were allocated to intra‐arterial alteplase and 19 patients were allocated to placebo. The flow diagram with reasons for exclusion in this imaging study is shown in Figure 2. A group of 21 patients enrolled in the trial was excluded in the nested study because of MRI intolerance or contraindications (n = 11), MRI unavailability (n = 7), or early discharge to another center (n = 2). The main traits of the study population were age, mean (SD) = 71 (13.5) years, 18 (50%) women, 18 (50%) men, and median baseline NIHSS score = 13, interquartile range (IQR) = 9–16. At randomization, the eTICI scores were 2b50 (n = 2), 2b67 (n = 13), 2c (n = 8), and 3 (n = 13), with a mean (SD) time from stroke onset to randomization of 384 (207) minutes and mean (SD) time from CTP to MRI of 47 (24) hours, without significant differences between the two treatment groups (Table 1). The nested population was comparable to the whole clinical trial population (data not shown).
FIGURE 2.
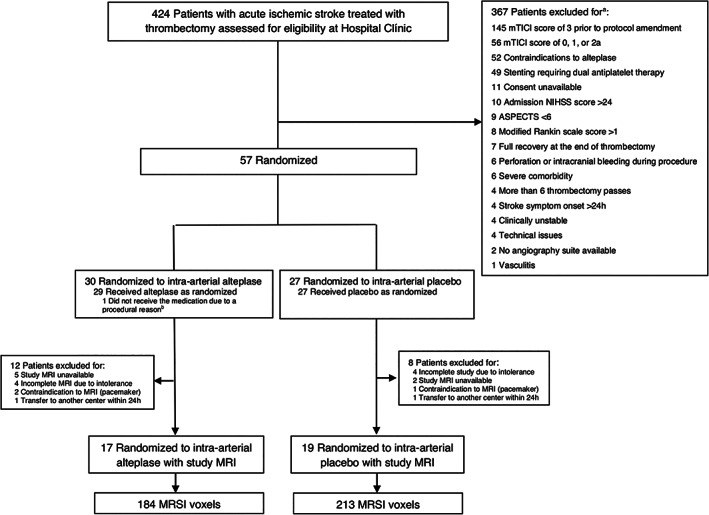
Flow diagram of the CHOICE imaging study. ASPECTS = Alberta Stroke Program Early CT Score; MRI = magnetic resonance imaging; MRSI = magnetic resonance spectroscopic imaging; mTICI = modified Thrombolysis in Cerebral Infarction; NIHSS = National Institutes of Health Stroke Scale.
TABLE 1.
Characteristics of the Patients according to Treatment and Perfusion Pattern at Follow‐up
| Characteristic | Intra‐Arterial Alteplase, n = 17 | Intra‐Arterial Placebo, n = 19 | p |
|---|---|---|---|
| Demographics | |||
| Age, yr, median (IQR) | 71 (62–85) | 75 (67–76) | 0.37 |
| Women, n (%) | 10 (59%) | 8 (42%) | 0.32 |
| Atrial fibrillation, n (%) | 0 | 3 (16%) | 0.09 |
| Diabetes mellitus, n (%) | 3 (18%) | 4 (21%) | 0.80 |
| Hypertension, n (%) | 12 (71%) | 14 (74%) | 0.84 |
| Hospital admission | |||
| SBP, mmHg, median (IQR) | 135 (120–152) | 135 (120–152) | 0.21 |
| DBP, mmHg, median (IQR) | 72 (69–81) | 73 (72–94) | 0.73 |
| Glucose, mg/dl, median (IQR) | 126 (118–137) | 113 (99–145) | 0.40 |
| NIHSS, median (IQR) | 11 (8–18) | 14 (9–18) | 0.68 |
| IV alteplase before EVT, n (%) | 7 (41%) | 7 (37%) | 0.79 |
| Time to randomization, min, median (IQR) | 297 (201–352) | 393 (301–413) | 0.22 |
| Time from CTP to MRI, h, median (IQR) | 44 (24–55) | 47 (29–67) | 0.62 |
| CTP admission | |||
| Hypoperfusion, ml, median (IQR) | 52.1 (39.4–76.9) | 54.7 (21.5–84.6) | 0.88 |
| Core median, ml (IQR) | 12.3 (9.7–17.2) | 6.2 (2.1–10.3) | 0.14 |
| Mismatch, % (95% CI) | 82 (72–87) | 90 (83–96) | 0.07 |
| eTICI score post‐thrombectomy [before randomization] | |||
| eTICI score | 0.56 | ||
| eTICI 2b50 | 1 (6%) | 1 (5%) | |
| eTICI 2b67 | 7 (41%) | 6 (32%) | |
| eTICI 2c | 2 (12%) | 6 (32%) | |
| eTICI 3 | 7 (41%) | 6 (32%) |
CI = confidence interval; CTP = computed tomography perfusion; DBP = diastolic blood pressure; eTICI: expanded Thrombolysis in Cerebral Infarction scale; EVT = endovascular treatment; IQR = interquartile range; IV = intravenous; MRI = magnetic resonance imaging; NIHSS = National Institutes of Health Stroke Scale; SBP = systolic blood pressure.
Hypoperfusion Post‐Thrombectomy
As judged by central raters, the final eTICI scores at 10 minutes of infusion of the study medication were 2b50 (n = 2), 2b67 (n = 13), 2c (n = 6), and 3 (n = 15). Overall, the final angiographic scores improved from the scores at randomization in 3 (8%) patients, and worsened in 1 (3%) patient. At 48 hours, 15 (42%) patients had an abnormal perfusion pattern, with a perfusion deficit volume of median (IQR) volume = 0.6 (0.0–3.2) ml within a DWI lesion and median (IQR) = 2.5 (1.8–9.1) ml outside the DWI lesion, which were highly correlated (rho = 0.810, p < 0.0001). Given the small size of the study cohort, we did not analyze the perfusion metrics and their corresponding clinical and radiological implications discriminating within or outside DWI lesions (Fig 3).
FIGURE 3.
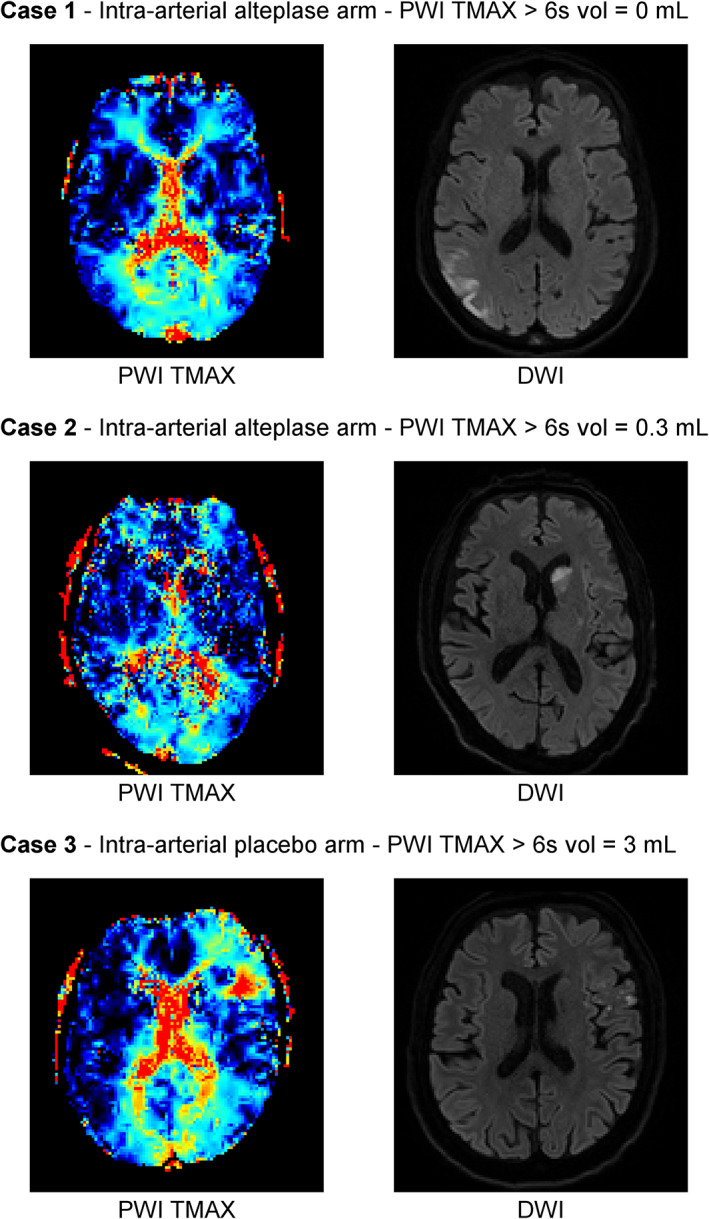
Representative cases of perfusion patterns on follow‐up magnetic resonance imaging. DWI = diffusion‐weighted imaging; PWI = perfusion‐weighted imaging; TMAX = time to maximum.
The identification of an abnormal perfusion pattern was associated with older age, higher glucose on admission, and increased infarct expansion ratio, and it was inversely correlated with the final eTICI score, although the latter difference was not statistically significant (Table 2).
TABLE 2.
Characteristics of the Patients according to the Perfusion Pattern at 48 Hours
| Characteristic | Normal Perfusion, n = 21 | Abnormal Perfusion, n = 15 | p |
|---|---|---|---|
| Demographics | |||
| Age, years, median (IQR) | 71 (57–77) | 76 (71–86) | 0.05 |
| Women, n (%) | 10 (48%) | 8 (53%) | 0.73 |
| Atrial fibrillation, n (%) | 1 (5%) | 2 (13%) | 0.35 |
| Diabetes mellitus, n (%) | 3 (14%) | 4 (27%) | 0.35 |
| Hypertension, n (%) | 14 (67%) | 12 (80%) | 0.37 |
| Hospital admission | |||
| SBP, mmHg, median (IQR) | 138 (131–157) | 145 (120–155) | 0.98 |
| DBP, mmHg, median (IQR) | 73 (71–81) | 71 (65–80) | 0.43 |
| Glucose, mg/dl, median (IQR) | 115 (98–134) | 140 (118–166) | 0.01 |
| NIHSS, median (IQR) | 9 (7–19) | 15 (11–20) | 0.55 |
| IV alteplase before EVT, n (%) | 7 (33%) | 7 (47%) | 0.41 |
| Time to randomization, min, median (IQR) | 315 (204–518) | 393 (297–450) | 0.46 |
| CTP admission | |||
| Hypoperfusion, ml, median (IQR) | 50.5 (34.1–83.8) | 55.7 (27.2–93.5) | 0.71 |
| Core median, ml (IQR) | 10.0 (7.1–14.7) | 6.2 (2.5–12.5) | 0.59 |
| Mismatch, % (95% CI) | 86 (72–96) | 88 (81–94) | 0.73 |
| Final eTICI score | |||
| eTICI score | 0.13 | ||
| eTICI 2b50 | 0 (0%) | 2 (100%) | |
| eTICI 2b67 | 6 (46%) | 7 (54%) | |
| eTICI 2c | 5 (63%) | 3 (38%) | |
| eTICI 3 | 10 (77%) | 3 (23%) | |
| Infarct course at 48 hours | |||
| Infarct expansion ratio, median (IQR) | 0.7 (0.5–2.1) | 3.2 (1.8–4.3) | 0.06 |
| Infarct volume, ml, median (IQR) | 5.7 (4.9–12.2) | 18.6 (7.7–32.1) | 0.02 |
CI = confidence interval; CTP = computed tomography perfusion; DBP = diastolic blood pressure; eTICI: expanded Thrombolysis in Cerebral Infarction scale; EVT = endovascular treatment; IQR = interquartile range; IV = intravenous; NIHSS = National Institutes of Health Stroke Scale; SBP = systolic blood pressure.
Clinical Correlates of the Perfusion Pattern Post‐Thrombectomy
An abnormal brain perfusion pattern at 48 hours was associated with greater neurological impairment during hospitalization (NIHSS scores), and worse functional outcomes (mRS) and poorer activities of daily living (Barthel Index) at 90 days, as compared with a normal brain perfusion pattern (Table 3), emphasizing in the study cohort the clinical relevance of the normalization of brain perfusion.
TABLE 3.
Clinical Association of the Perfusion Patterns at Follow‐up
| Normal Perfusion, n = 21 | Abnormal perfusion, n = 15 | p | |
|---|---|---|---|
| mRS score at day 90 | |||
| mRS 0–1, n (%) | 18 (86%) | 9 (60%) | 0.07 a |
| mRS 0–2—n (%) | 21 (100%) | 10 (67%) | 0.004 |
| Barthel Index at day 90 | |||
| Barthel Index > 95–100, n (%) | 20 (95%) | 7 (47%) | 0.001 |
| NIHSS course, median (IQR) | |||
| Baseline | 9 (7–19) | 15 (11–20) | 0.56 |
| 24 h | 0 | 6 (3–13) | 0.001 |
| 48 h | 0 | 3 (2–7) | 0.001 |
| Day 5–7 | 0 | 2 (1–7) | 0.005 |
| Day 90 | 0 | 0 | 0.01 |
The association between abnormal perfusion pattern at 48 hours and mRS 0–1 at day 90 was independent of the final expanded Thrombolysis in Cerebral Infarction score (odds ratio = 0.15, 95% confidence interval = 0.03–0.93, p = 0.04).
IQR = interquartile range; mRS = modified Rankin Scale; NIHSS = National Institutes of Health Stroke Scale.
Effect of the Study Drug on the Radiologic Course of the Infarct
The final angiographic score improved compared with the randomization score in 3 of 19 (16%) patients treated with placebo, and in none of 17 (0%) patients treated with alteplase. Nonetheless, adjunct intra‐arterial alteplase significantly reduced the prevalence of an abnormal brain perfusion pattern post‐thrombectomy compared with placebo (4 of 17 [24%] patients vs 11 of 19 [58%] patients, p = 0.03). This difference remained statistically significant after adjustment for the preceding use of intravenous thrombolysis [odds ratio [OR] = 0.20, 95% confidence interval [CI] = 0.04–0.91, p = 0.03), and also after further adjustment for age, stroke severity, and eTICI score (OR = 0.11, 95% CI = 0.01–0.95, p = 0.04). Correspondingly, in the adjunct alteplase group, there were fewer patients with an expanding infarct at follow‐up, and there was a statistically significant reduction in the infarct expansion ratio compared with the placebo (Table 4 and Fig 4).
TABLE 4.
Radiological Course of the Infarction according to Treatment
| Intra‐Arterial Alteplase, n = 17 | Intra‐Arterial Placebo, n = 19 | p | |
|---|---|---|---|
| Angiographic improvement, n (%) | 0 | 3 (16%) | 0.09 |
| Abnormal perfusion at 48 hours, n (%) | 4 (24%) | 11 (58%) | 0.03 |
| TMAX > 6 volume, median (IQR) | 0 | 0.76 (0.07–2.33) | 0.04 |
| Expanding infarction, n (%) | 6 (35%) | 14 (74%) | 0.02 |
| Infarct expansion ratio, median (IQR) | 0.79 (0.50–1.44) | 3.23 (1.79–5.73) | 0.02 |
“Expanding infarction” indicates infarct expansion ratio > 1.
IQR = interquartile range; TMAX = time to maximum.
FIGURE 4.
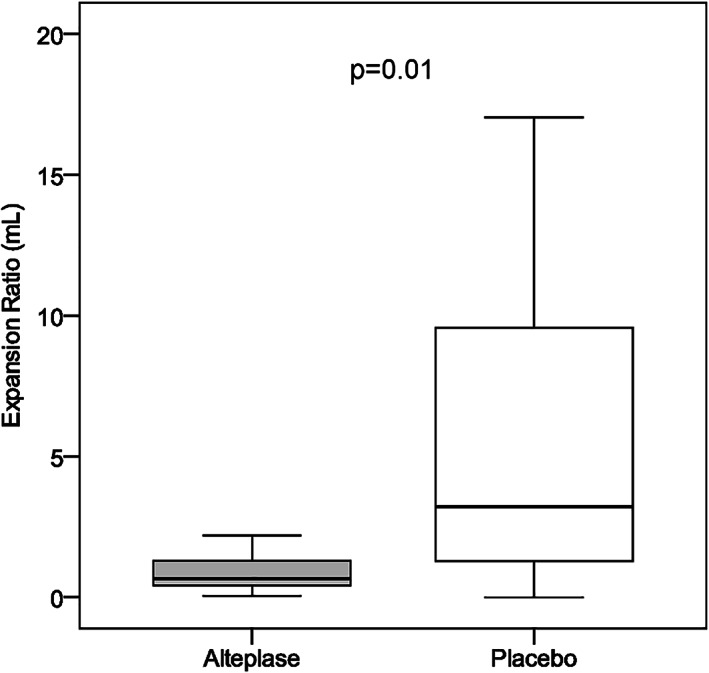
Effect of adjunct intra‐arterial alteplase on brain imaging surrogate markers. Imaging surrogate markers in patients treated with alteplase (n = 17) or placebo (n = 19) are shown in box‐whisker plot of infarct expansion ratio according to study treatment (boxes indicate 25–75% interquartile range [IQR]; central horizontal bars indicate median; outer horizontal bars indicate 10–90% IQR).
Molecular Signatures of Hypoperfusion: Effects of Intra‐Arterial Alteplase
An abnormal perfusion pattern was associated with statistically significantly lower peaks of NAA (median [IQR] = 1.01 [0.70–1.24] vs 1.09 [0.88–1.32], p = 0.007). Lower peaks of NAA were also identified in patients with mRS = 2 to 6 as compared with patients with mRS 0 to 1 (median [IQR] = 1.00 [0.66–1.19] vs 1.08 [0.85–1.31], p = 0.023). Conversely, adjunct intra‐arterial alteplase was associated with statistically significant higher peaks of NAA (median [IQR] = 1.13 [0.91–1.36] vs 1.00 [0.74–1.22], p < 0.0001) compared with placebo (Fig 5).
FIGURE 5.
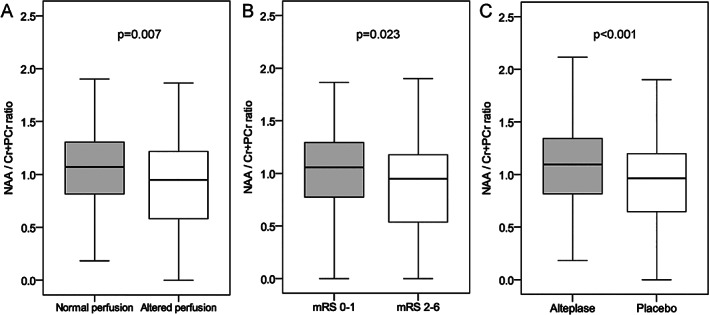
N‐Acetylaspartate (NAA) peaks according to the perfusion pattern, functional outcome, and study treatment (boxes indicate 25–75% interquartile range [IQR]; central horizontal bars indicate median; outer horizontal bars indicate 10–90% IQR). mRS = modified Rankin Scale.
Sensitivity Analyses
To minimize the confounding effects resulting from the involvement of large vessels and refine the assessment of the microvasculature, we assessed the prevalence of abnormal perfusion in a sensitivity analysis of patients (n = 14) with a complete normalization of the angiography (eTICI score = 3) at the end of treatment. In this subgroup, 4 (28%) patients had an abnormal perfusion pattern, including 1 of 7 (14%) patients in the alteplase group and 3 of 7 (43%) patients in the placebo group.
We also explored the clinical and radiological traits associated with preceding use of intravenous alteplase (Table 5). Although the small subgroups hampered the identification of significant differences, the rate of excellent outcome was numerically higher and the rate of abnormal perfusion was numerically lower in the intra‐arterial alteplase group regardless of the preceding use of intravenous alteplase.
TABLE 5.
Radiological and Clinical Course according to Treatment and Preceding Use of Intravenous Thrombolysis
| Variable | Preceding IV Alteplase/Adjunct IA Alteplase | p | |||
|---|---|---|---|---|---|
| No/No, n = 12 | No/Yes, n = 10 | Yes/No, n = 7 | Yes/Yes, n = 7 | ||
| mRS 0–1 at day 90, n (%) | 8 (67%) | 9 (90%) | 4 (57%) | 6 (86%) | 0.35 |
| Hypoperfusion at 48 hours, n (%) | 7 (58%) | 1 (10%) | 4 (57%) | 3 (43%) | 0.10 |
IA = intra‐arterial; IV = intravenous; mRS = modified Rankin Scale.
Discussion
The CHOICE trial recently showed that adjunct intra‐arterial alteplase added at the end of a successful thrombectomy significantly increased the rate of patients free of disability at 90 days (mRS = 0–1) compared with placebo. 26 Remarkably, the treatment effect occurred despite both treatment groups having similar angiographic results post‐thrombectomy, suggesting that the outcome improved from more effective thrombolytic activity within the more distal cerebral circulation, not impeded by proximal occlusions that had previously been removed successfully by thrombectomy. 26 The current nested study extends the understanding of these clinical findings by providing supportive imaging evidence of improved brain reperfusion with the use of adjunct intra‐arterial alteplase. There was a significant reduction in the prevalence of hypoperfusion at follow‐up from 58% to 24% in the alteplase group compared to the placebo group, and the effect was also significant in patients treated with intravenous alteplase before thrombectomy. In patients with successful reperfusion but incomplete normalization of the angiography (eTICI = 2b50/67/2c), it is likely that part of the thrombolytic action of adjunct alteplase took place in distal emboli clogging the macrovasculature. However, in patients with complete normalization of the angiography (eTICI = 3), there was also a reduction in the prevalence of hypoperfusion from 43% to 14% in the alteplase group compared to the placebo group, highlighting a high prevalence of no‐reflow, and the efficacy of adjunct intra‐arterial alteplase to palliate the phenomenon. Although some previous studies described a high prevalence of hypoperfusion at the completion of successful thrombectomy, 17 the current results prolonged the duration of hypoperfusion for at least 48 hours, ruling out that it represents a fleeting phenomenon.
The study found a significant reduction in the infarct expansion ratio and the proportion of patients with expanding infarcts (from 74% to 35%), and significantly higher peaks of NAA in the adjunct alteplase group compared with the placebo group, consistent with a more benign evolution of the infarction and increased neuronal integrity in the former group. 32
This is the first randomized study that provides supportive evidence of the need to improve the cerebral blood flow in the microvasculature to maximize the benefits of thrombectomy. The study also spotlights the current limitations of digital subtraction angiography to predict accurately the risk of impending hypoperfusion in the microcirculation. Previous studies that defined the no‐reflow as a severe focal hypoperfusion causing tissue infarction found it to be a rare occurrence, 12 although higher incidences of approximately 25% were also reported. 34 In this study, we found areas of abnormal perfusion both within and outside DWI lesions that were highly correlated. Overall, older age and higher glucose at stroke onset were found to be associated with a higher prevalence of hypoperfusion following thrombectomy, providing some mechanistic insights to explain the worse outcome that has been described associated with the use of thrombectomy in the elderly, 35 or in patients with higher glucose at the time of treatment. 36
The study has strong points, including the randomization of the therapy, the double‐blind design, the use of placebo, the setting of a central imaging core laboratory, and the implementation of a robust multimodal imaging protocol. Conversely, the study also has several limitations, the primary one being the relatively small sample size of the study, which prevented a more robust analysis of subgroups. The study population was admitted to a single center, and it represented 8% of the population undergoing thrombectomy at this site, raising some concern about generalizability of the findings. However, descriptive analyses (not shown) confirmed the similarities between the subgroup analyzed in this study and the whole trial population. Finally, the IER estimation was based on different imaging modalities, and this could have influenced the precise estimation of the course of the infarction, although this limitation certainly affected both treatment groups equally.
In conclusion, this nested analysis of a randomized study points to the effectiveness of rescue intra‐arterial alteplase compared with placebo to improve brain perfusion, limit the expansion of the brain infarction, and increase brain cellularity in patients with acute ischemic stroke undergoing successful thrombectomy. The relatively small size of the study calls for replication of these results in additional studies, although its rigorous design gives credit to a primary message that emphasizes the need to target the microvascular bed to maximize the overall benefits of thrombectomy in patients with LVO acute ischemic stroke.
Author Contributions
C.L., A.Ro., A.Re., A.M.P., X.U., and A.C. contributed to the conception and design of the study; C.L., A.Ro., L.O., M.H.‐P., A.Re., J.P., L.S.R., X.U., and A.C. contributed to the acquisition and analysis of data; C.L., X.U., and A.C. contributed to drafting the text or preparing the figures.
Potential Conflicts of Interest
A.C. reports stock from FreeOx Biotech and has received speaker honoraria from Boehringer Ingelheim. All other authors declare that they have no conflicts of interest regarding this work.
Acknowledgments
This work was supported by Fundació La Marató de TV3 (140/C/2017), and by a grant from the Spanish Ministry of Health cofinanced by the European Regional Development Fund (Instituto de Salud Carlos III, Red Temática de Investigación Cooperativa Invictus+, RD16/0019/0014), grant PI18/00444 to Á.C., and grant PI20/00901 to A.Ro. The study medication and the placebo were provided by Boehringer Ingelheim, which had no role in the design, analysis, or interpretation of the results in this study. This work was partially developed at the Centre de Recerca Biomèdica Cellex, Centre Esther Koplowitz, IDIBAPS, Barcelona, CERCA Programme/Generalitat de Catalunya. We thank the Magnetic Resonance Imaging IDIBAPS Core Facility team and 3 T Equipment (project IBPS15‐EE‐3688 cofunded by MCIU and by ERDF). The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data Availability Statement
The datasets analyzed in the current study are available from the corresponding author on reasonable request.
References
- 1. Ames A 3rd, Wright RL, Kowada M, et al. Cerebral ischemia. II. The no‐reflow phenomenon. Am. J Pathol 1968;52:437–453. [PMC free article] [PubMed] [Google Scholar]
- 2. Desilles JP, Loyau S, Syvannarath V, et al. Alteplase reduces dowstream microvascular thrombosis and improves the benefit of large artery recanalization in stroke. Stroke 2015;46:3241–3248. [DOI] [PubMed] [Google Scholar]
- 3. Hallenbeck JM, Dutka AJ, Tanishima T, et al. Polymorphonuclear leukocyte accumulation in brain regions with low blood flow during the early postischemic period. Stroke 1986;17:246–253. [DOI] [PubMed] [Google Scholar]
- 4. Garcia JH, Liu KF, Yoshida Y, et al. Brain microvessels: factors altering their patency after the occlusion of a middle cerebral artery (Wistar rat). Am J Pathol 1994;145:728–740. [PMC free article] [PubMed] [Google Scholar]
- 5. del Zoppo GJ, Schmid‐Schönbein GW, Mori E, et al. Polymorphonuclear leukocyres occlude capillaries follwing middle cerebral artery occlusion and reperfusion in baboons. Stroke 1991;22:1276–1283. [DOI] [PubMed] [Google Scholar]
- 6. Mori E, del Zoppo GJ, Chambers JD, et al. Inhibition of polymorphonuclear leukocyte adherence suppresses no‐reflow after focal cerebral ischemia in baboons. Stroke 1992;23:712–718. [DOI] [PubMed] [Google Scholar]
- 7. Cipolla MJ, Chan SL, Sweet J, et al. Postischemic reperfusion causes smooth muscle calcium sensitization and vasoconstriction of parenchymal arterioles. Stroke 2014;45:2425–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hall CN, Reynell C, Gesslein B, et al. Capillary pericytes regulates cerebral blood flow in health and disease. Nature 2014;508:55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yemisci M, Gursoy‐Ozdemir Y, Vural A, et al. Pericyte contraction induced by oxidative‐nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med 2009;15:1031–1037. [DOI] [PubMed] [Google Scholar]
- 10. Hill RA, Tong L, Yuan P, et al. Regional blood flow in the normal and ischemic brain is controlled by arteriolar smooth muscle cell contractility snf not by capillary pericytes. Neuron 2015;87:95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 2007;87:315–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ter Schiphorst A, Charron S, Hassen WB, et al. Tissue no‐reflow despite full recanalization following thrombectomy for anterior circulation stroke with proximal occlusion: a clinical study. J Cereb Blood Flow Metab 2021;41:253–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baird AE, Donnan GA, Austin MC, et al. Reperfusion after thrombolytic therapy in ischemic stroke measured by single‐photon emission computed tomography. Stroke 1994;25:79–85. [DOI] [PubMed] [Google Scholar]
- 14. Albers GW, Thijs VN, Wechsler L, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol 2006;60:508–517. [DOI] [PubMed] [Google Scholar]
- 15. De Silva DA, Fink JN, Christensen S, et al. Assessing reperfusion and recanalization as markers of clinical outcomes after intravenous thrombolysis in the echoplanar imaging thrombolytic evaluation trial (EPITHET). Stroke 2009;40:2872–2874. [DOI] [PubMed] [Google Scholar]
- 16. Arsava EM, Arat A, Topcuoglu MA, et al. Angiographic microcirculatory obstructions distal to occlusion signify poor outcome after endovascular treatment for acute ischemic stroke. Transl Stroke Res 2018;9:44–50. [DOI] [PubMed] [Google Scholar]
- 17. Rubiera M, Garcia‐Tornel A, Olivé‐Gadea M, et al. Computed tomography perfusion after thrombectomy: an immediate surrogate marker of outcome after recanalization in acute stroke. Stroke 2020;51:1736–1742. [DOI] [PubMed] [Google Scholar]
- 18. Kosior JC, Buck B, Wannamaker R, et al. Exploring reperfusion following endovascular thrombectomy. Stroke 2019;50:2389–2395. [DOI] [PubMed] [Google Scholar]
- 19. Higashida RT, Furlan AJ, Roberts H, et al. Trial design and reporting standards for intra‐arterial cerebral thrombolysis for acute ischemic stroke. Stroke 2003;34:e109–e137. [DOI] [PubMed] [Google Scholar]
- 20. Zaidat OO, Yoo AJ, Khatri P, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke 2013;44:2650–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Saver JL, Goyal M, van der Lugt A, et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta‐analysis. JAMA 2016;316:1279–1288. [DOI] [PubMed] [Google Scholar]
- 22. Chamorro Á, Blasco J, López A, et al. Complete reperfusion is required for maximal benefits of mechanical thrombectomy in stroke patients. Sci Rep 2017;7:11636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kitano T, Todo K, Yoshimura S, et al. Futile complete recanalization: patients characteristics and its time course. Sci Rep 2020;10:4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rabinstein AA, Albers GW, Brinjikji W, Koch S. Factors that may contribute to poor outcome despite good reperfusion after acute endovascular stroke therapy. Int J Stroke 2019;14:23–31. [DOI] [PubMed] [Google Scholar]
- 25. Deng G, Xiao J, Yu H, et al. Predictors of futile recanalization after endovascular treatment in acute ischemic stroke: a meta‐analysis. J Neurointerv Surg 2021; neurintsurg‐2021‐017963. [DOI] [PubMed] [Google Scholar]
- 26. Renú A, Millán M, San Román L, et al. Effect of intra‐arterial alteplase vs placebo following successful thrombectomy on functional outcomes in patients with large vessel occlusion acute ischemic stroke: the CHOICE randomized clinical trial. JAMA 2022;327:826–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liebeskind DS, Bracard S, Guillemin F, et al. eTICI reperfusion: defining success in endovascular stroke therapy. J Neurointerv Surg 2019;11:433–438. [DOI] [PubMed] [Google Scholar]
- 28. Laredo C, Renú A, Tudela R, et al. The accuracy of ischemic core perfusion thresholds varies according to time to recanalization in stroke patients treated with mechanical thrombectomy: a comprehensive whole‐brain computed tomography perfusion study. J Cereb Blood Flow Metab 2020;40:966–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nicoli F, Scalzo F, Saver JL, et al. The combination of baseline magnetic resonance perfusion‐weighted imaging‐derived tissue volume with severely prolonged arterial‐tissue delay and diffusion‐weighted imaging lesion volume is predictive of MCA‐M1 recanalization in patients treated with endovascular thrombectomy. Neuroradiology 2014;56:117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed 2001;14:260–264. [DOI] [PubMed] [Google Scholar]
- 31. Inoue M, Mlynash M, Straka M, et al. Clinical outcomes strongly associated with the degree of reperfusion achieved in target mismatch patients: pooled data from the diffusion and perfusion imaging evaluation for understanding stroke evolution studies. Stroke 2013;44:1885–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li Y, Wang T, Zhang T, et al. Fast high‐resolution metabolic imaging of acute stroke with 3D magnetic resonance spectroscopy. Brain 2020;143:3225–3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ng FC, Churilov L, Yassi N, et al. Prevalence and significance of impaired microvascular tissue reperfusion despite macrovascular angiographic reperfusion (no‐reflow). Neurology 2022;98:e790–e801. [DOI] [PubMed] [Google Scholar]
- 34. Dalkara T, Arsava EM. Can restoring incomplete microcirculatory reperfusion improve stroke outcome after thrombolysis? J Cereb Blood Flow Metab 2012;32:2091–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alawieh A, Starke RM, Chatterjee AR, et al. Outcomes of endovascular thrombectomy in the elderly: a ‘real‐world’ multicenter study. J Neurointerv Surg 2019;11:545–553. [DOI] [PubMed] [Google Scholar]
- 36. Chamorro Á, Brown S, Amaro S, et al. Glucose modifies the effect of endovascular thrombectomy in patients with acute stroke. Stroke 2019;50:690–696. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed in the current study are available from the corresponding author on reasonable request.


