Abstract
Background
Fluid treatment in sepsis is a challenge and clinical equipoise exists regarding intravenous (IV) volumes. We aimed to determine whether a 24‐h protocol restricting IV fluid was feasible in adult patients with sepsis without shock presenting to the emergency department (ED).
Methods
The REFACED Sepsis trial is an investigator‐initiated, multicenter, randomized, open‐label, feasibility trial, assigning sepsis patients without shock to 24 h of restrictive, crystal IV fluid administration or standard care. In the IV fluid restriction group fluid boluses were only permitted if predefined criteria for hypoperfusion occurred. Standard care was at the discretion of the treating team. The primary outcome was total IV crystalloid fluid volumes at 24 h after randomization. Secondary outcomes included total fluid volumes, feasibility measures, and patient‐centered outcomes.
Results
We included 123 patients (restrictive 61 patients and standard care 62 patients) in the primary analysis. A total of 32% (95% confidence interval [CI] 28%–37%) of eligible patients meeting all inclusion criteria and no exclusion criteria were included. At 24 h, the mean (±SD) IV crystalloid fluid volumes were 562 (±1076) ml versus 1370 (±1438) ml in the restrictive versus standard care group (mean difference –801 ml, 95% CI −1257 to −345 ml, p = 0.001). Protocol violations occurred in 21 (34%) patients in the fluid‐restrictive group. There were no differences between groups in adverse events, use of mechanical ventilation or vasopressors, acute kidney failure, length of stay, or mortality.
Conclusions
A protocol restricting IV crystalloid fluids in ED patients with sepsis reduced 24‐h fluid volumes compared to standard care. A future trial powered toward patient‐centered outcomes appears feasible.
INTRODUCTION
Sepsis is a global health burden, estimated to cause 11 million yearly deaths. Even in survivors, sepsis can cause permanent organ dysfunction and impaired health‐related quality of life. 1 , 2 Infections are common in emergency department (ED) patients accounting for up to one in four admissions, among these up to one in four with sepsis. 1 , 3 , 4
The treatment of sepsis includes intravenous (IV) antibiotics and fluids, source control, and supportive care. 5 The effect of IV fluids in sepsis is debated; strict fluid restriction may lead to impaired circulation and perfusion whereas liberal administration may lead to fluid overload resulting in edema and capillary leakage, and both too little and too much has been associated with organ dysfunction. 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 Although sepsis without hypotension is more common than sepsis associated hypotension and septic shock, 3 the Surviving Sepsis Campaign (SSC) only gives recommendations for fluid treatment of the latter conditions with a recommendation to give 30 ml/kg within the first 3 h. 5 However, the evidence supporting this recommendation is of low quality, the use of fluid varies, and better evidence has been requested. 4 , 9 , 18 , 19 , 20 , 21 , 22 , 23
Recent observational studies and interventional trials investigating fluid volumes in adult patients with primarily septic shock in the intensive care unit (ICU) have shown either no difference or indicated benefit with fluid restriction. 7 , 24 IV fluids given without clear indication may be harmful. 25 Two ED‐based trials from Africa in patients with sepsis and sepsis‐associated hypotension found a higher mortality rate among patients in the intervention group where patients received early, aggressive fluid therapy. 26 , 27 Whether these results are generalizable to ED sepsis patients without shock is unknown. Although trials are currently exploring fluid strategies in patients with hypotension and septic shock, 28 , 29 , 30 there appear to be no trials on fluid administration in patients with early sepsis without shock or hypotension.
The aim of the Restrictive Fluid Administration vs. Standard of Care in Emergency Department Sepsis Patients (REFACED Sepsis) feasibility trial was to test if a restrictive IV fluid protocol in ED patients with sepsis without shock is feasible and could decrease the volume of IV fluids administered compared to standard care.
METHODS
Trial registration and protocol
The REFACED Sepsis trial was registered at the EU Clinical Trials Register (EudraCT number 2021–000224‐35 [May 3, 2021]) and at ClinicalTrials.gov (identifier NCT05076435 [October 10, 2021]). The trial protocol was approved by the Committee on Health Research Ethics—Central Denmark Region (identifier 1‐10‐72‐163‐21 [June 28, 2021]). The protocol has previously been published 31 and is provided as a supplement to this paper. The trial was funded but the funding agencies had no role in the design, conduct or interpretation of the study, nor the decision to submit the manuscript for publication.
Trial design and setting
The REFACED Sepsis trial was an investigator‐initiated, multicenter, randomized, parallel‐group, open‐label, feasibility trial, assigning patients with sepsis without shock to a 24‐h restrictive fluid administration protocol or standard care. Participants were recruited in the EDs at Aarhus University Hospital, the Regional Hospital Randers, and the Regional Hospital Viborg. The three EDs serve a mixed rural–urban population of 0.9 million people and provide 24‐h emergency care to all adult acute patients except those transferred directly to catheterization laboratories, cardiology wards and stroke units, and women in labor. ED patients are either referred by a general practitioner or brought in by ambulance after an emergency call. In the three EDs, patient contacts vary between 15,000 and 63,000 per year. Emergency health care in Denmark is publicly funded.
Selection of participants
We included patients fulfilling all of the following inclusion criteria: (1) unplanned ED admission; (2) age ≥ 18 years; (3) sepsis defined as (a) infection suspected by the treating clinician, (b) blood cultures drawn, (c) IV antibiotics administered or planned, and (d) an infection‐related increase in the SOFA score ≥ 2 32 ; and (4) expected hospital stay > 24 h as deemed by the treating clinician. We excluded patients who fulfilled any of the following: (1) received ≥ 500 ml of IV fluids, (2) vasopressors or invasive ventilation started prior to screening, (3) known or suspected severe bleeding judged by the treating clinician, (4) known or suspected pregnancy, (5) prior enrollment in the trial, or (6) patients who the clinician expected not to survive the next 24 h. SOFA score was calculated automatically on the randomization website when entering laboratory values during the randomization process. A patient could be randomized as soon as the infection‐related SOFA score was 2, without awaiting all laboratory values to be available for a total SOFA score at enrollment. All laboratory blood tests for a total SOFA score calculation—except an arterial blood gas analysis—were performed prior to enrollment, results were available within a maximum of 2 h, and a total SOFA score was calculated based on these post hoc. If an arterial blood gas analysis was not performed the respiratory component of the SOFA score was assumed normal, that is, giving 0 points. Known organ dysfunction was accounted for, as described in SEPSIS‐3. 32 If there was any uncertainty about the impact of known organ dysfunction, this could be discussed with the primary investigator around the clock per telephone. Regarding exclusion criteria (1) and (2), we assumed that patients who had not received ≥500 ml of IV fluids and who had not received vasopressors at the time of inclusion were not in septic shock.
According to Danish law, patients with acute illness can only be included in a trial if all patients can provide written, informed consent or if none of the patients can provide written, informed consent, a combination is not possible. Since most sepsis patients are not able to provide informed consent, we only included patients who were unable to provide written, informed consent. As such, patients who were fully awake, oriented, and/or in no apparent distress were excluded.
Before enrollment, consent for inclusion was obtained from an independent physician followed by consent from a next of kin and/or the patient as soon as possible when they regained the capacity to provide consent. More details are presented in the protocol (Appendix S1).
Randomization
Eligible patients fulfilling all inclusion criteria and no exclusion criteria were randomly assigned in a 1:1 ratio to one of the two intervention groups. The randomization was stratified by site. Randomization was performed using a centralized Web‐based system according to a computer‐generated allocation sequence list with varying block sizes (4, 6, or 8), stratified by site. The allocation sequence list and block sizes were only known by the data manager at Trial Partner, Aarhus University, who was not otherwise involved in the trial.
Intervention
Patients were assigned to either restrictive IV fluid administration or standard care for 24 h. The assigned treatment protocol was followed in the ED as well as wards or ICUs if the patient was transferred within the 24‐h period. The intervention protocol targeted IV crystalloid fluid administration. An overview of the trial, including the restrictive fluid algorithm, is provided in Figure 1.
FIGURE 1.
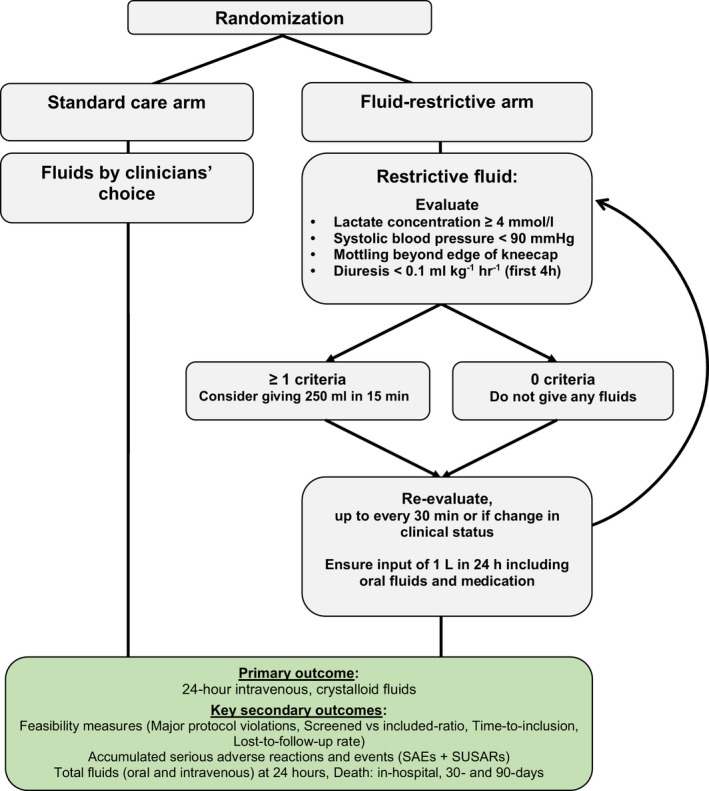
Treatment algorithms: summary of trial interventions.
In the restrictive fluid group, IV crystalloid fluids should not be given unless one of the below mentioned hypoperfusion criteria were met.
• Lactate concentration ≥ 4 mmoL/L (arterial or venous);
• Hypotension (systolic blood pressure < 90 mm Hg);
• Mottling beyond edge of kneecap (i.e., Mottling score > 2) 33 ;
• Severe oliguria, that is, diuresis < 0.1 ml/kg/h, during the first 4 h of admission.
If one or more of these criteria were met, a fluid bolus of 250 ml of isotonic crystalloid (isotonic saline or Ringer's acetate/lactate) could be administered per protocol. It was not a requirement that a fluid bolus was administered.
The treating physician could at any time violate the protocol by giving additional fluid if judged necessary. The physician had to state the reason for violating the protocol.
IV fluids could be given as carrier for medications, but the volume should be reduced if possible. In case of documented overt fluid loss (e.g., vomiting, large aspirates, diarrhea, drain losses, or ascites drainage) IV fluid could be given to correct for the loss. In case the oral/enteral route for water or electrolyte solutions was contraindicated or failed as judged by the clinical team, IV fluids could be given to correct significant electrolyte deficiencies or to ensure a total fluid input of 1 L per 24 h (counting all fluids including medications and nutrition). If a patient underwent surgery during the 24‐h inclusion period, they temporarily paused the protocol, but clinicians were encouraged to continue restrictive fluid therapy, and all intraoperative fluids were registered.
In the standard care group, fluids were administered by clinicians' choice. Aside from the fluid administration, all management of the patient care was at the discretion of the treating team. In both groups, patients were allowed to drink as much as desired or allowed by the treating team. It was not possible to blind the intervention for neither the treating team, patients, nor relatives.
Measurements
In both trial groups, oral and IV fluids were registered on a paper case report form for 24 h (Figures S1 and S2) from randomization by the treating team with help from research assistants. Data were obtained from the electronic medical record by the research team on baseline characteristics, vital signs, blood tests, use of vasopressors, mechanical ventilation and dialysis, in‐hospital course, and death and entered in an electronic case report form in REDCap.
Outcomes
The primary outcome was the total volume of IV crystalloid fluids administered during the first 24 h after randomization. The secondary outcomes were feasibility measures (number of patients randomized vs. screened positive, i.e., with all inclusion criteria fulfilled and no exclusion criteria fulfilled); time from admission to inclusion; number of patients with major protocol violations; number of patients with incomplete data on the primary outcome (e.g., due to discharge or death within 24 h); serious adverse reactions and events within 7 days; total fluid volume (oral, IV, and combined) at 24 h; mechanical ventilation within 7 days; vasopressor use within 7 days; development or worsening of acute kidney failure according to the KDIGO3 criteria 34 within 7 days of randomization; hospital length of stay; and in‐hospital, 30‐day, and 90‐day mortality.
Sample size
The sample size calculation was based on data from an observational study conducted in the Central Denmark Region in which sepsis patients meeting inclusion criteria for the current trial received a mean (±SD) of 2670 (±1695) ml IV fluids in 24 h from admission. 4 We therefore estimated that the mean (±SD) total amount of crystalloid IV fluid in the standard care group would be 2650 (±1700) ml. We considered a mean (±SD) difference of 1 L to be of clinical relevance and therefore estimated 1650 (±1700) ml in the restrictive fluid group. Based on these estimates, an alpha of 5%, a power of 90%, and a two‐sample t‐test, a sample size of 124 patients was required.
Data analysis
All analyses were conducted in a modified intention‐to‐treat population defined as all randomized patients for whom consent to use data was obtained. Baseline characteristics were compared using descriptive statistics. We used linear regression to estimate the mean difference in IV crystalloid fluid volume between the allocated groups with adjustment for the stratification variable site. As the data were not normally distributed, we performed an additional post hoc analysis using median regression to estimate the difference in medians. 35 Other continuous variables were analyzed similarly. For binary outcomes, we used logistic regression adjusted for site with differences between groups presented as odds ratios (ORs). We used summary statistics for the feasibility measures. We performed all analyses using Stata version 17 (StataCorp LP) and considered p‐values of <0.05 as statistically significant.
RESULTS
Characteristics of trial participants
From November 3, 2021, to December 18, 2021, we screened 2412 unique patients with suspected infection. Of these, 383 unique patients met all inclusion criteria and no exclusion criteria, and 124 patients were randomized (Figure 2, Table S1); 62 patients were assigned to the fluid restriction group and 62 were allocated to the standard care group. One patient in the restrictive group withdrew consent for the use of data; we thus analyzed data from 123 patients (99%). One patient was inadvertently included twice. Both admissions were included in the analyses.
FIGURE 2.
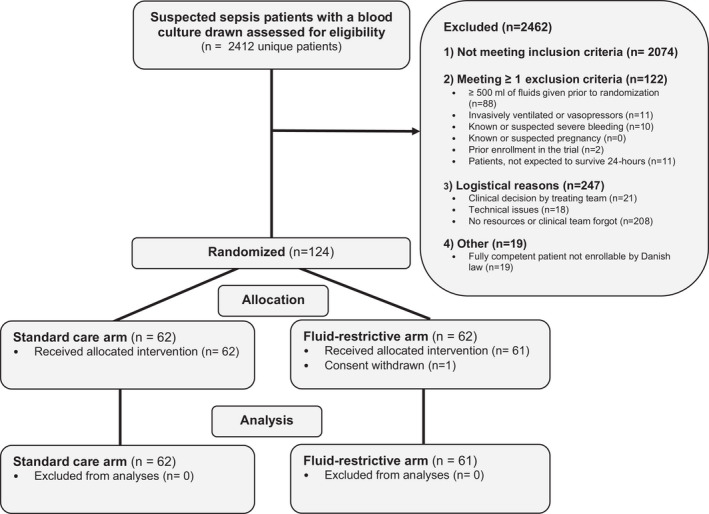
Screening, randomization, and follow‐up in the REFACED Sepsis feasibility trial. REFACED Sepsis, Restrictive Fluid Administration vs. Standard of Care in Emergency Department Sepsis Patients.
Patient characteristics are presented in Table 1 and Table S1. Overall, patients had a median (IQR) age of 76 (67–84) years and 58% were male. Most patients had not received IV fluids before randomization (Table 1).
TABLE 1.
Baseline characteristics according to group allocation
| Variable | Restrictive fluids (n = 61) | Standard care (n = 62) |
|---|---|---|
| Age (years) | 75 (67–85) | 76 (68–83) |
| Male sex | 37 (61) | 34 (55) |
| Weight (kg) | 75 (64–92) | 77 (69–90) |
| Prior history of comorbidities | ||
| Kidney failure a | 5 (8) | 9 (15) |
| Diabetes b | 11 (18) | 9 (15) |
| Heart failure c | 9 (15) | 13 (21) |
| DNI/DNAR d | 29 (48) | 18 (29) |
| Vital signs at randomization | ||
| Systolic blood pressure (mm Hg) | 130 (107–144) | 137 (126–147) |
| Diastolic blood pressure (mm Hg) | 72 (62–79) | 71 (62–83) |
| Mean arterial pressure (mm Hg) | 88 (81–101) | 94 (85–103) |
| Respiratory rate (breaths/min) | 24 (20–28) | 23 (20–28) |
| Oxygen saturation (%) | 94 (91–96) | 96 (93–97) |
| Heart rate (beats/min) | 97 (80–115) | 96 (88–110) |
| Temperature (°C) | 38.1 (37.5–38.8) | 38.6 (37.9–39.3) |
| GCS score | 15 (15–15) | 15 (15–15) |
| Blood tests before randomization | ||
| Creatinine (μmol/L) | 93 (65–136) | 91 (65–132) |
| Platelet count (×109/L) | 247 (179–299) | 230 (157–323) |
| Bilirubin (μmol/L) | 11 (7–21) | 12 (8–18) |
| Leukocytes (×109/L) | 14.2 (10.7–17.4) | 13.5 (9.6:17.9) |
| C‐reactive protein (mg/L) | 117 (47–194) | 125 (55–235) |
| Lactate (mmol/L) | 1.2 (1.0–1.8) | 1.4 (1.0–2.1) |
| Total SOFA score at randomization e | 3 (2–3) | 3 (2–3) |
| Suspected infectious source f | ||
| Respiratory [n with COVID‐19] | 45 (74) [4] | 43 (69) [3] |
| Urinary | 9 (15) | 12 (19) |
| Skin/soft tissue | 3 (5) | 1 (2) |
| Abdominal | 3 (5) | 5 (8) |
| Other/unknown | 3 (5) | 4 (6) |
| Time to IV antibiotics from admission (h) | 2.8 (1.6–3.9) | 2.9 (1.6–3.9) |
| IV fluids given prior to randomization (ml) | 0 [0–200] | 0 [0–100] |
Note: All data are presented as median (IQR) or n (%) unless otherwise stated.Abbreviations: DNI/DNAR, do not intubate or do not attempt resuscitation orders; GCS, Glasgow Coma Scale.
Renal failure defined according to KDIGO criteria (see supplemental material).
Diabetes requiring chronic oral or injection treatment.
Heart failure with history of ejection fraction ≤ 40%.
DNI and/or DNAR documented prior to or within 6 h of admission.
For SOFA subscores, see Table S1.
Some patients had more than one infectious source, why the total sum is >100%.
Feasibility measures
Feasibility measures are shown in Table 2. Overall, 32% (95% CI 28% to 37%) of patients meeting all inclusion criteria and no exclusion criteria were included (Regional Hospital Viborg 43%, Aarhus University Hospital 41%, and Regional Hospital Randers 17% [Table S2]). Randomized patients and nonrandomized patients were similar in characteristics at admission, but nonrandomized patients more often presented with abdominal complaints whereas randomized patients more of the had respiratory complaints (Table S3). The median (IQR) time from ED admission to randomization was 140 (90–194) min. One patient died and five patients were discharged within 24 h.
TABLE 2.
Feasibility measures and secondary effect parameters stratified by group allocation
| Restrictive fluids (n = 61) | Standard care (n = 62) | Overall (n = 123) | |
|---|---|---|---|
| Screened eligible/included ratio (%) | — | — |
124/383 = 32% (95% CI 28%–37%) a |
| Time from ED admission to inclusion (min) | |||
| Mean (±SD) | 149 (±76) | 161 (±106) | 155 (±92) |
| Median (IQR) | 140 (90–197) | 139 (92–179) | 140 (90–194) |
| Patients with incomplete data on primary outcome | 2 (3) | 4 (7) | 6 (5) |
| Reasons for lost to follow‐up within 24 h |
1 discharge 1 death |
4 discharges |
5 discharges 1 death |
| Patients with protocol violations | 21 (34) b | — | — |
| Patients who received no crystalloid fluid within 24 h of enrollment | 38 (62) | 15 (24) | 53 (43) |
| Accumulated adverse reactions and events within 7 days |
17 3 deaths, 1 myocardial infarction, 4 hypervolemia, 9 acute kidney injury |
18 1 death, 1 heart failure, 2 myocardial infarctions, 4 hypervolemia, 10 acute kidney injury |
35 |
Note: All data are presented as n (%) unless otherwise stated.
For site‐specific screening/included ratio and explanations, see Table S2.
IV fluids given if none of the following was true: (a) one or more hypoperfusion criteria fulfilled; (b) to correct documented fluid loss; (c) to correct significant electrolyte deficiencies; (d) fluid administered as carrier for medication (e.g., antibiotics); (e) ensure a total fluid input of 1 L per 24 h (for the specific reasons, see Table S6).
Fluid results
Fluid administration during the 24‐h period in both groups is presented in Table 3, Figure 3, and Table S4 and S5. At 24 h, the mean (±SD) IV crystalloid volumes were 562 (±1076) ml vs. 1370 (±1438) ml in the restrictive versus standard care group and the mean (95% CI) difference was −801 ml (−1257 to −345; p = 0.001, corresponding to a relative decrease in fluid volume of 58%. The difference in medians was −1000 ml (95% CI −1392 to −607), using median regression. Thirty‐eight out of 61 (62%) patients in the restrictive group and 15 of 62 (24%) patients in the standard care group received no IV crystalloid fluids in the first 24 h (Figure 3, Table S4). The mean (±SD) of combined oral and IV fluids in the first 24 h was 2881 (±1295) ml in the restrictive group versus 3720 (±1623) ml in the standard care group with a mean difference of −840 ml (95% CI −1364 to −317, p = 0.002). Further details of fluid administration, type of fluid, and time intervals are shown in Table 2, Figure 3, Tables S3–S6, and Figures S4–S7.
TABLE 3.
Fluid volumes in the first 24 h stratified by group allocation
| Restrictive fluids (n = 61) | Standard care (n = 62) | Mean difference (95% CI) or difference in medians [95% CI] a | p‐value for mean difference | |
|---|---|---|---|---|
| Primary outcome | ||||
| 24‐h IV crystalloid fluid volumes (ml) | ||||
| Mean (±SD) | 562 (±1076) | 1370 (±1438) | −801 (−1257 to −345) | 0.001 |
| Median [IQR] | 0 [0–600] | 1000 [80–2000] | −1000 [−1392 to −607] a | |
| 24‐h IV crystalloid fluid volumes per kg bodyweight (ml/kg) | ||||
| Mean (±SD) | 9 (±16) | 17 (±19) | −9 (−15 to −2) | 0.007 |
| Median [IQR] | 0 [0–11] | 12.5 [1–26] | ||
| Secondary outcomes | ||||
| 24‐h oral and IV fluid volumes (ml) | ||||
| Mean (SD) | 2881 (1295) | 3720 (1623) | −840 (−1364 to −317) | 0.002 |
| Median [IQR] | 2820 [1900–3500] | 3498 [2800–4450] | −660 [−1116 to −204] a | |
| 24‐h oral and IV fluid volumes per kg bodyweight (ml/kg) | ||||
| Mean (±SD) | 38 (±20) | 48 (±22) | −9 (−17 to −2) | 0.18 |
| Median [IQR] | 36 [22–49] | 45 [32–56] | ||
| 24‐h other IV fluids b (ml) | ||||
| Mean (±SD) | 667 (±500) | 697 (±705) | −35 (−252 to 182) | 0.75 |
| Median [IQR] | 500 [400–800] | 416 [300–800] | ||
| 24‐h total IV fluid volume (ml) | ||||
| Mean (±SD) | 1229 (±1292) | 2067 (±1678) | −837 (−1374 to −298) | 0.003 |
| Median [IQR] | 792 [400–1400] | 1625 [1200–2650] | ||
| 24‐h total oral fluid volume (ml) | ||||
| Mean (SD) | 1651 (888) | 1653 (816) | −4 (−310; 302) | 0.98 |
| Median [IQR] | 1750 [1100–2225] | 1600 [950–2150] |
Note: This table shows fluid volumes in the restrictive fluid group and in the standard care group. Mean differences and differences in medians as well as p‐values are derived from the regression analyses. All mean and median differences are estimated with the standard care group as reference.
Adjusted for site. Median regression was only performed for the predefined primary and secondary outcomes.
Other IV fluids accounts for dissolved IV administered medication, glucose, plasma, albumin, blood, etc. (for further information, see Table S5).
FIGURE 3.
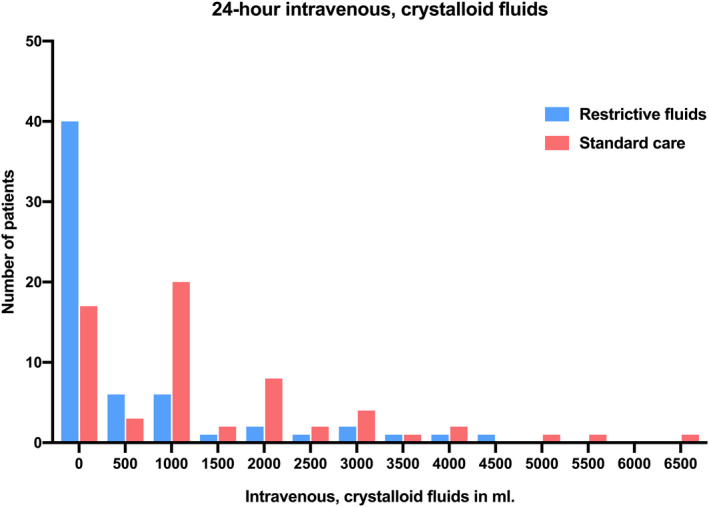
Distributions of 24‐h IV crystalloid fluids by group allocation. Histogram showing distributions of 24‐h IV, crystalloid fluids in ml by group allocation. The y‐axis represents the number of patients with the given fluid volume from each group.
In the restrictive fluid group, hypotension was the most frequently used hypoperfusion criterion for administering fluids per protocol. Twenty‐one of 61 patients had fluid administered despite no criteria fulfilled, that is, had a protocol violation. High or rising creatinine/impaired renal function was the most frequently used specific reason for giving IV fluids outside the protocol (Table 4). One patient (standard care group) underwent surgery and had the fluid resuscitation protocol temporarily suspended during surgery in accordance with the protocol and had a total 2750 ml of IV fluid and medication administered during surgery, included in total volumes but not in IV crystalloid fluid volumes.
TABLE 4.
Reasons for fluid administration and protocol violations in the restrictive fluid group
| Description of fluid indication | Number of patients with bolus/boli given for the fluid indication, N/total (%) | Number of 250 ml crystalloid boli given for the fluid indication, n |
|---|---|---|
| Hypoperfusion criteria | ||
| Lactate concentration ≥ 4 mmol/L a | 2/61 (3.3%) | 2 |
| Hypotension (sBP < 90 mm Hg) | 9/61(14.8%) | 24 |
| Mottling beyond edge of kneecap b | 1/61 (1.6%) | 1 |
| Severe oliguria, i.e., diuresis < 0.1 ml/kg/h c | 2/61 (3.3%) | 2 |
| Other allowed reasons for fluid administration | ||
| Correct significant electrolyte deficiencies | 3/61 (4.9%) | 4 |
| Replace fluid loss | 0 | 0 |
| Ensure a total fluid input of 1 L per 24 h d | 0 | 0 |
| Protocol violations | ||
| Improve circulation or low blood pressure (but sBP ≥90 mm Hg) | 5/61 (8.2%) | 9 |
| High or rising creatinine or impaired kidney function | 7/61 (11.5%) | 12 |
| Dehydration indicated by treating physician | 6/61 (9.8%) | 9 |
| Other reasons or administration by mistake e | 12/61 (19.7%) | 16 |
Abbreviation: sBP, systolic blood pressure.
Lactate measurement from an arterial or venous blood gas/blood sample.
Mottling score > 2 as described by Ait‐Oufella et al. 33
Criteria only possible to use within first 4 h after randomization.
Total fluid input included oral fluids and fluids given with medication.
Other reasons included administering more fluid than allowed by study protocol, sparse urine output > 4 h from randomization, administration of fluid by mistake outside of protocol.
Secondary outcomes
There were no significant differences between groups in use of mechanical ventilation or vasopressors or new‐onset acute kidney failure at 7 days, nor in‐hospital length of stay or in‐hospital, 30‐day, or 90‐day mortality (Table 5).
TABLE 5.
Secondary outcomes stratified by group allocation.
| Restrictive fluids (n = 61) | Standard Care (n = 62) | Effect estimate a | p‐value for effect estimate | |
|---|---|---|---|---|
| Variable | Mean difference (95% CI) and median difference [95% CI] b | |||
| In‐hospital length of stay (days) | ||||
| Mean (SD) | 7.5 (4.9) | 6.2 (5.9) | 1.2 (−0.8; 3.1) | 0.24 c |
| Median [IQR] | 5.9 [4.0; 10.0] | 4.9 [3.0; 7.3] | 0.8 [−0.7; 2.4] |
| Odds ratio (95% CI) | ||||
|---|---|---|---|---|
| Mechanical ventilation within 7 days | 2 (3.3%) | 2 (3.2%) | 1.01 (0.14–7.43) | 0.99 |
| Vasopressors within 7 days | 2 (3.3%) | 4 (6.5%) | 0.49 (0.09–2.79) | 0.42 |
| New onset or worsening acute kidney failure within 7 days d | 9 (14.8%) | 10 (16.1%) | 0.90 (0.34–2.39) | 0.83 |
| Mortality, in‐hospital | 7 (11.5%) | 6 (9.7%) | 1.19 (0.37–3.83) | 0.80 |
| Mortality, 30 days | 9 (14.8%) | 10 (16.1%) | 0.82 (0.34–2.39) | 0.83 |
| Mortality, 90 days e | 12 (19.7%) | 15 (25.0%) | 0.73 (0.31–1.74) | 0.48 |
Note: All data are reported as numbers (%) if not otherwise stated.
Abbreviations: CI, confidence interval; IQR, interquartile range; SD, standard deviation.
All analyses of effect are adjusted for site.
Median regression adjusted for site.
p value for mean difference.
Any development or worsening of acute kidney injury, defined as the KDIGO34 creatinine score >0 compared to at randomization.
Two patients withdrew consent to obtain 90‐day mortality status, both from the standard care group.
Adverse events
There were 17 (28%) and 18 (29%) patients experiencing any of the predefined adverse events or reactions in the restrictive and standard care groups, respectively, with acute myocardial infarction, death, new‐onset acute kidney injury, and hypervolemia accounting for all events (Table 2 and Tables S7 and S8).
DISCUSSION
We conducted a randomized, multicenter trial to examine the feasibility of restricting 24‐h IV, crystalloid fluid volumes in sepsis patients without shock in three EDs. The restrictive protocol significantly reduced 24‐h IV fluid volumes and total fluid volumes.
Despite a low randomized‐to‐screened ratio, we included 124 patients within 6 weeks at three sites. Although randomization required drawing of blood cultures and results from laboratory values prior to randomization, patients were randomized within a median of 140 min of arrival to the ED and most patients did not receive IV fluids prior to randomization. Based on these feasibility measures, we consider a larger trial feasible.
Fluid volumes in the current trial were lower than those in our previous cohort study. 4 In our sample size estimation, we assumed that the total mean (±SD) amount of IV fluid in the standard care group would be 2650 (±1700) ml as it was for similar patients in our descriptive study. 4 , 36 However, the standard care group only received 2067 (±1655) ml total IV fluids in the present trial. This may represent the Hawthorne effect and/or a change in current practice toward more restrictive fluid administration in general. However, there is limited evidence on patient‐centered outcomes to support this change of practice yet. 28 , 30 , 37 , 38 On the other hand, patients in the REFACED Sepsis trial received approximately 300 ml more oral fluids than in our previous descriptive study (1650 ml compared to 1319 ml), and in general patients had a large proportion of the total 24‐h fluids through the enteral route.
The trial protocol was, in line with recent trials, 29 , 38 , 39 able to substantially reduce IV fluid volumes. Although the mean difference (801 ml) was slightly lower than the estimate used in our sample size calculation (1000 ml), the median difference was 1000 ml, and the relative reduction was large (58%). Given the relative low volume of fluid in the control group, we consider a separation of 801 ml satisfactory and the protocol successful. In ED patients with sepsis‐associated hypotension, the REFRESH trial reduced 24‐h fluids from 4250 to 3543 ml in the restrictive group, with a relative reduction of 30%. Although the absolute reduction in fluid volume administered were similar between this and the current trial, we almost doubled the relative fluid volume reduction in REFACED Sepsis (58%). The 58% reduction is more in line with the RIFTS pilot trial, where ICU patients with sepsis or septic shock received 665 ± 1119 ml in the restrictive group and 1251 ± 1588 ml in the usual care group with a mean difference of 586 (62–1109) ml in the first 24 h postrandomization and a relative reduction of 47% 38 and the ICU‐based CLASSIC septic shock feasibility trial, although the intervention in CLASSIC lasted for up to 5 days. 39 However, in all the above‐mentioned trials, patients received large fluid volumes prior to randomization in opposition to this current trial resulting in total fluids exceeding our totals but all three trials were also conducted in more severely ill patients. The REFACED Sepsis, REFRESH, and CLASSIC trials use patient specific hypoperfusion criteria for administering fluid in contrary to a “one‐size‐fits‐all” strategy for example with a fixed fluid volume for all patients. 29 , 39
The REFACED Sepsis study and its use of hypoperfusion criteria was inspired by the CLASSIC trials. 28 , 39 The four hypoperfusion criteria were chosen to represent central (systolic blood pressure), general (lactate), peripheral (mottling), and renal (oliguria) circulation and perfusion status. The cutoff value of lactate was chosen based on the former SSC guideline (2016), 40 and their 1‐h bundle 41 and data indicating that the marked increase in mortality occur at lactate values > 4 mmoL/L. 42 , 43 The mottling trigger was based on mottling score of ≥2 as described by Ait‐Oufella et al. 33 and validated in a prehospital setting. 44 Recently, some trials have used capillary refill time as a marker of peripheral perfusion, 45 , 46 which could have been used instead of mottling but it was chosen to align with the CLASSIC criteria. Severe oliguria was defined as urine output ≤ 0.1 ml/kg/h and the criterion was only to be used within the first 4 h of admission. In the REFACED Sepsis trial, a total of 29 boli of 250 ml crystalloid were given per protocol in the 61 patients in the restrictive fluid group (Table 4). The protocol was violated (i.e., giving fluids although no hypoperfusion criteria were fulfilled) in 35% in the fluid restrictive group and 24% did not receive IV, crystalloid fluids in 24 h in the standard care group, in comparison to 45% and 30%, respectively, in the CLASSIC feasibility trial. 39 Overall, the fact, that 38/61 (62%) patients in the restrictive group did not receive crystalloid fluids unless as carrier for medication, to correct electrolytes or to replace fluid loss, shows that clinicians are able to restrict fluids in a large proportion of sepsis patients.
Interestingly, the most frequently used specific reason for giving IV fluids outside the protocol in the fluid‐restrictive group was high or rising creatinine/impaired renal function. This may be due to a general perception that a low degree of prerenal kidney failure should be treated with fluids. However, the evidence for this to our knowledge is limited, and descriptive studies show improvement with less fluids. 47 , 48 , 49 , 50
The strengths of our trial include the multicenter inclusion, recruitment in both university and regional hospitals, and a short inclusion period. The fast inclusion and completion of the trial underlines the importance of the trial; sepsis patients account for a large proportion of ED patients. We believe, this patient population (i.e., older, high do not intubate/do not attempt resuscitation [DNI/DNAR] rate, unable to consent) represents a very important patient group in the ED, which have traditionally not been included in clinical trials. We consider the inclusion of this patient population a strength as it increases the generalizability of the results.
There are some important considerations for a possible future large‐scale trial. It could be of interest to include patients slightly sicker but still without septic shock at arrival, that is, including more patients with low blood pressure. Since these patients often rapidly have fluids administered prehospital or in hospital and thereby fulfill the exclusion criteria of receiving >500 ml before they could possibly have been included, it would require even closer contact to the prehospital services and first‐line in‐hospital treating team to limit IV fluid administration prior to and at arrival and thereby increase the chance of randomizing the patient. This may be appropriate since the evidence is still very sparse both prehospital and in hospital. Including the sickest sepsis patients, but still without shock, would probably increase the chance of finding a difference in outcomes between treatment arms. Also, it would increase inclusion rates and the generalizability to include more patients with abdominal infections. To ensure an even greater separation between the groups, even stricter criteria for fluid administration in the restrictive fluid group should be ensured, maybe focusing even more on changing from IV to oral fluid administration in this group. Also, the intervention period could be extended.
We found a high prevalence of DNI/DNAR orders within the sepsis population in the REFACED Sepsis trial, and the population was in general older with a median age of 76 years and a high mortality (30‐day mortality of 15%) in comparison to other sepsis studies, but similar to our descriptive study leading up to this study. 4 , 29 , 38 , 51 If an effect of fluid restriction on patient‐centered outcomes, for example, mortality or days alive at home, will be found in a future large‐scale trial, there is a potential of improving outcomes for a large patient group with significant mortality and high burden on health care systems.
LIMITATIONS
The trial was designed to show differences in IV fluid volumes and the sample size was therefore inadequate to assess clinical outcomes such as mortality. We did not prespecify any benchmarks for feasibility. It was not possible to blind the allocated intervention for neither patients, the treating team, nor investigators, which may have affected the results and potentially caused fluid in the standard care group to be quite restrictive in comparison to our previous cohort study. 4 Since patients were excluded if more than 500 ml of IV fluids had been administered prior to randomization, we may have missed patients presenting with more severe illness prehospitally or at ED admission. Also, patients who fulfilled all inclusion criteria later in their ED course may have been missed. Both above‐mentioned limitations could affect the generalizability or the results.
The proportion of elderly patients, and patients with DNI/DNAR orders was high in the REFACED Sepsis trial, resulting in a high mortality rate compared to other sepsis studies, although it was similar to the ones found in a cohort study conducted at two of the sites in REFACED Sepsis. 4 Also, the fact that 19 patients were not included since they were actually able to provide consent and there for not includable due to Danish regulations may have caused inclusion of sicker patients. The trial was conducted during autumn and winter season, during the COVID‐19‐pandemic, which likely resulted in inclusion of more patients with respiratory symptoms. At two sites, patients with a high risk of surgery within the 24 h were not enrolled (described in supplemental material), resulting in few patients with abdominal symptoms in comparison to other trials in sepsis and fluids affecting the generalizability. 29 , 38 , 39 These local conditions, as well as challenges related to COVID‐19, contributed to a low included‐to‐screened positive ratio. To keep the in‐hospital patient flow, multiple departments and clinical personnel were involved in this trial at the three hospitals causing organizational challenges and obstacles, and thus presence of investigators, research nurses, and assistants was necessary. No differences in adverse events were seen in the two groups; however, the study was not powered to show certain differences in these.
CONCLUSIONS
The results indicate that it is feasible to protocolize and restrict 24‐h intravenous fluid volumes in sepsis patients without shock in EDs. The mean difference (801 ml) was slightly lower than the estimate used in our sample size calculation (1000 ml), but the median difference was 1000 ml, and the relative reduction was large. A large‐scale trial to investigate the effect of restrictive fluids on patient‐centered outcomes appears feasible; however, modifications to the protocol may increase the separation in intravenous fluid volumes between the two intervention groups.
AUTHOR CONTRIBUTIONS
Marie K. Jessen, Lars W. Andersen, Anders Perner, Jens Aage K. Petersen, and Hans Kirkegaard conceived the study and designed the trial. Marie K. Jessen and Hans Kirkegaard obtained research funding. Lars W. Andersen, Anders Perner, Jens Aage K. Petersen, and Hans Kirkegaard supervised the conduct of the trial. Marie K. Jessen, Marie‐Louise H. Thomsen, Peter Kristensen, Wazhma Hayeri, Tina G. Messerschmidt, Christoffer G. Sølling, and Ranva E. Hassel undertook recruitment of patients. Peter Kristensen, Wazhma Hayeri, Christoffer G. Sølling, and Ranva E. Hassel were site investigators. Marie‐Louise H. Thomsen and Marie K. Jessen collected data. Marie K. Jessen managed the data, including quality control. Lars W. Andersen provided statistical advice. Marie K. Jessen drafted the manuscript, and all authors contributed substantially to its revision. Marie K. Jessen takes responsibility for the paper as a whole.
CONFLICT OF INTEREST
The Department of Intensive Care, Rigshospitalet, where AP is affiliated, receives funding for research from the Novo Nordisk Foundation, Pfizer, Fresenius Kabi, AK Pharma, and Sygeforsikringen “danmark” outside the submitted work. AP is also the sponsor of the CLASSIC trial. The authors declare no potential conflict of interest.
TRIAL REGISTRATION
EudraCT number 2021‐000224‐35 (2021‐05‐03), ClinicalTrials.gov number NCT05076435 (date 2021‐10‐13), Committee on Health Research Ethics—Central Denmark Region number 1‐10‐72‐163‐21 (date 2021‐06‐28).
Supporting information
Appendix S1
Appendix S2
Appendix S3
ACKNOWLEDGMENTS
We are very grateful to the clinical staff of doctors and nurses at the participating departments for their important contribution and to patients and relatives for their consent to participate.
Jessen MK, Andersen LW, Thomsen M‐L, et al. Restrictive fluids versus standard care in adults with sepsis in the emergency department (REFACED): A multicenter, randomized feasibility trial. Acad Emerg Med. 2022;29:1172‐1184. doi: 10.1111/acem.14546
Funding informationFunding for the trial was provided by Carl and Ellen Hertz foundation (DKK 15,000), Frimodt‐Heineke Foundation (DKK 25,000), Ruth & Holger Hesses Memorial Fund (DKK 60,000), Health Research Foundation of Central Denmark Region (DKK 215,000), “Akutpuljen” Central Denmark Region (DKK 582,000), and Aarhus University (salary for primary investigator, DKK 1.5 mio). The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Supervising Editor: Dr. Michael Puskarich
REFERENCES
- 1. Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990‐2017: analysis for the global burden of disease study. Lancet. 2020;395(10219):200‐211. doi: 10.1016/s0140-6736(19)32989-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Winters BD, Eberlein M, Leung J, Needham DM, Pronovost PJ, Sevransky JE. Long‐term mortality and quality of life in sepsis: a systematic review. Crit Care Med. 2010;38(5):1276‐1283. doi: 10.1097/CCM.0b013e3181d8cc1d [DOI] [PubMed] [Google Scholar]
- 3. Henriksen DP, Laursen CB, Jensen TG, Hallas J, Pedersen C, Lassen AT. Incidence rate of community‐acquired sepsis among hospitalized acute medical patients‐a population‐based survey. Crit Care Med. 2015;43(1):13‐21. doi: 10.1097/ccm.0000000000000611 [DOI] [PubMed] [Google Scholar]
- 4. Jessen MK, Andersen LW, Thomsen MH, et al. Twenty‐four‐h fluid administration in emergency department patients with suspected infection: a multicenter, prospective, observational study. Acta Anaesthesiol Scand. 2021;65(8):1122‐1142. doi: 10.1111/aas.13848 [DOI] [PubMed] [Google Scholar]
- 5. Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021;49(11):e1063‐e1143. doi: 10.1097/ccm.0000000000005337 [DOI] [PubMed] [Google Scholar]
- 6. Ueyama H, Kiyonaka S. Predicting the need for fluid therapy‐does fluid responsiveness work? J Intensive Care. 2017;5:34. doi: 10.1186/s40560-017-0210-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Malbrain ML, Marik PE, Witters I, et al. Fluid overload, de‐resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. Anaesthesiol Intensive Ther. 2014;46(5):361‐380. doi: 10.5603/ait.2014.0060 [DOI] [PubMed] [Google Scholar]
- 8. Seymour CW, Gesten F, Prescott HC, et al. Time to treatment and mortality during mandated emergency care for Sepsis. N Engl J Med. 2017;376(23):2235‐2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marik PE, Linde‐Zwirble WT, Bittner EA, Sahatjian J, Hansell D. Fluid administration in severe sepsis and septic shock, patterns and outcomes: an analysis of a large national database. Intensive Care Med. 2017;43(5):625‐632. doi: 10.1007/s00134-016-4675-y [DOI] [PubMed] [Google Scholar]
- 10. Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39(2):259‐265. doi: 10.1097/CCM.0b013e3181feeb15 [DOI] [PubMed] [Google Scholar]
- 11. Kelm DJ, Perrin JT, Cartin‐Ceba R, Gajic O, Schenck L, Kennedy CC. Fluid overload in patients with severe sepsis and septic shock treated with early goal‐directed therapy is associated with increased acute need for fluid‐related medical interventions and hospital death. Shock. 2015;43(1):68‐73. doi: 10.1097/shk.0000000000000268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu X, Hu Z, Yuan H, Chen L, Li Y, Zhao C. Fluid resuscitation and markers of glycocalyx degradation in severe sepsis. Open Med (Wars). 2017;12:409‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Malbrain M, Van Regenmortel N, Saugel B, et al. Principles of fluid management and stewardship in septic shock: it is time to consider the four D's and the four phases of fluid therapy. Ann Intensive Care. 2018;8(1):66. doi: 10.1186/s13613-018-0402-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sethi M, Owyang CG, Meyers C, Parekh R, Shah KH, Manini AF. Choice of resuscitative fluids and mortality in emergency department patients with sepsis. Am J Emerg Med. 2018;36(4):625‐629. doi: 10.1016/j.ajem.2017.09.042 [DOI] [PubMed] [Google Scholar]
- 15. Shaw AD, Raghunathan K, Peyerl FW, Munson SH, Paluszkiewicz SM, Schermer CR. Association between intravenous chloride load during resuscitation and in‐hospital mortality among patients with SIRS. Intensive Care Med. 2014;40(12):1897‐1905. doi: 10.1007/s00134-014-3505-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Byrne L, Obonyo NG, Diab SD, et al. Unintended consequences: fluid resuscitation worsens shock in an ovine model of endotoxemia. Am J Respir Crit Care Med. 2018;198(8):1043‐1054. doi: 10.1164/rccm.201801-0064OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Messmer AS, Zingg C, Müller M, Gerber JL, Schefold JC, Pfortmueller CA. Fluid overload and mortality in adult critical care patients‐a systematic review and meta‐analysis of observational studies. Crit Care Med. 2020;48(12):1862‐1870. doi: 10.1097/ccm.0000000000004617 [DOI] [PubMed] [Google Scholar]
- 18. Hjortrup PB, Haase N, Wetterslev J, Perner A. Associations of hospital and patient characteristics with fluid resuscitation volumes in patients with severe sepsis: post hoc analyses of data from a multicentre randomised clinical trial. PLoS One. 2016;11(5):e0155767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Angus DC, Barnato AE, Bell D, et al. A systematic review and meta‐analysis of early goal‐directed therapy for septic shock: the ARISE, ProCESS and ProMISe investigators. Intensive Care Med. 2015;41(9):1549‐1560. doi: 10.1007/s00134-015-3822-1 [DOI] [PubMed] [Google Scholar]
- 20. Keijzers G, Macdonald SP, Udy AA, et al. The Australasian Resuscitation in SEPSIS Evaluation: Fluids or Vasopressors in Emergency Department Sepsis (ARISE FLUIDS), a multi‐Centre observational study describing current practice in Australia and New Zealand. Emerg Med Australas. 2020;32(4):586‐598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alhazzani W, Moller MH, Arabi YM, et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID‐19). Crit Care Med. 2020;48:e440‐e469. doi: 10.1097/ccm.0000000000004363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harris T, Coats TJ, Elwan MH. Fluid therapy in the emergency department: an expert practice review. Emerg Med J. 2018;35(8):511‐515. doi: 10.1136/emermed-2017-207245 [DOI] [PubMed] [Google Scholar]
- 23. Perner A, Gordon AC, Angus DC, et al. The intensive care medicine research agenda on septic shock. Intensive Care Med. 2017;43(9):1294‐1305. doi: 10.1007/s00134-017-4821-1 [DOI] [PubMed] [Google Scholar]
- 24. Meyhoff TS, Møller MH, Hjortrup PB, Cronhjort M, Perner A, Wetterslev J. Lower vs higher fluid volumes during initial management of sepsis: a systematic review with meta‐analysis and trial sequential analysis. Chest. 2020;157(6):1478‐1496. doi: 10.1016/j.chest.2019.11.050 [DOI] [PubMed] [Google Scholar]
- 25. Nijssen EC, Rennenberg RJ, Nelemans PJ, et al. Prophylactic hydration to protect renal function from intravascular iodinated contrast material in patients at high risk of contrast‐induced nephropathy (AMACING): a prospective, randomised, phase 3, controlled, open‐label, non‐inferiority trial. Lancet. 2017;389(10076):1312‐1322. doi: 10.1016/s0140-6736(17)30057-0 [DOI] [PubMed] [Google Scholar]
- 26. Andrews B, Semler MW, Muchemwa L, et al. Effect of an early resuscitation protocol on in‐hospital mortality among adults with sepsis and hypotension: a randomized clinical trial. JAMA. 2017;318(13):1233‐1240. doi: 10.1001/jama.2017.10913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Andrews B, Muchemwa L, Kelly P, Lakhi S, Heimburger DC, Bernard GR. Simplified severe sepsis protocol: a randomized controlled trial of modified early goal‐directed therapy in Zambia. Crit Care Med. 2014;42(11):2315‐2324. doi: 10.1097/ccm.0000000000000541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meyhoff TS, Hjortrup PB, Møller MH, et al. Conservative vs liberal fluid therapy in septic shock (CLASSIC) trial‐protocol and statistical analysis plan. Acta Anaesthesiol Scand. 2019;63(9):1262‐1271. doi: 10.1111/aas.13434 [DOI] [PubMed] [Google Scholar]
- 29. Macdonald SPJ, Keijzers G, Taylor DM, et al. Restricted fluid resuscitation in suspected sepsis associated hypotension (REFRESH): a pilot randomised controlled trial. Intensive Care Med. 2018;44(12):2070‐2078. doi: 10.1007/s00134-018-5433-0 [DOI] [PubMed] [Google Scholar]
- 30. Self WH, Semler MW, Bellomo R, et al. Liberal versus restrictive intravenous fluid therapy for early septic shock: rationale for a randomized trial. Ann Emerg Med. 2018;72:457‐466. doi: 10.1016/j.annemergmed.2018.03.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jessen MK, Andersen LW, Thomsen MH, et al. Restrictive Fluid Administration vs. Standard of Care in Emergency Department Sepsis Patients (REFACED Sepsis)—protocol for a multicenter, randomized, clinical, proof‐of‐concept trial. Pilot Feasibility Stud. 2022;8(1):75. doi: 10.1186/s40814-022-01034-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis‐3). JAMA. 2016;315(8):801‐810. doi: 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ait‐Oufella H, Lemoinne S, Boelle PY, et al. Mottling score predicts survival in septic shock. Intensive Care Med. 2011;37(5):801‐807. doi: 10.1007/s00134-011-2163-y [DOI] [PubMed] [Google Scholar]
- 34. KDIGO clinical practice guideline for acute kidney injury. Kidney International Supplements. 2012;2:1. [Google Scholar]
- 35. Staffa SJ, Zurakowski D. Calculation of confidence intervals for differences in medians between groups and comparison of methods. Anesth Analg. 2020;130(2):542‐546. doi: 10.1213/ane.0000000000004535 [DOI] [PubMed] [Google Scholar]
- 36. Jessen MK, Andersen LW, Thomsen MH, et al. Restrictive Fluid Administration vs. Standard of Care in Emergency Department Sepsis Patients (REFACED Sepsis)—protocol for a multicenter, randomized, clinical, proof‐of‐concept trial. Pilot and Feasibility Studies 2022; Accepted for publication. doi: 10.1186/s40814-022-01034-y [DOI] [PMC free article] [PubMed]
- 37. Australasian Resuscitation In Sepsis Evaluation : FLUid or Vasopressors In Emergency Department Sepsis (ARISE FLUIDS). ClinicalTrials.gov NCT04569942. First posted September 3, 2020. Access date: November 21, 2021. https://clinicaltrials.gov/ct2/show/record/NCT04569942.
- 38. Corl KA, Prodromou M, Merchant RC, et al. The restrictive IV fluid trial in severe sepsis and septic shock (RIFTS): a randomized pilot study. Crit Care Med. 2019;47(7):951‐959. doi: 10.1097/ccm.0000000000003779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hjortrup PB, Haase N, Bundgaard H, et al. Restricting volumes of resuscitation fluid in adults with septic shock after initial management: the CLASSIC randomised, parallel‐group, multicentre feasibility trial. Intensive Care Med. 2016;42(11):1695‐1705. doi: 10.1007/s00134-016-4500-7 [DOI] [PubMed] [Google Scholar]
- 40. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017;45(3):486‐552. doi: 10.1097/ccm.0000000000002255 [DOI] [PubMed] [Google Scholar]
- 41. Levy MM, Evans LE, Rhodes A. The surviving sepsis campaign bundle: 2018 update. Intensive Care Med. 2018;44(6):925‐928. doi: 10.1007/s00134-018-5085-0 [DOI] [PubMed] [Google Scholar]
- 42. Wacharasint P, Nakada TA, Boyd JH, Russell JA, Walley KR. Normal‐range blood lactate concentration in septic shock is prognostic and predictive. Shock. 2012;38(1):4‐10. doi: 10.1097/SHK.0b013e318254d41a [DOI] [PubMed] [Google Scholar]
- 43. Webb AL, Kramer N, Rosario J, et al. Delta lactate (three‐hour lactate minus initial lactate) prediction of in‐hospital death in sepsis patients. Cureus. 2020;12(4):e7863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jouffroy R, Saade A, Tourtier JP, et al. Skin mottling score and capillary refill time to assess mortality of septic shock since pre‐hospital setting. Am J Emerg Med. 2019;37(4):664‐671. doi: 10.1016/j.ajem.2018.07.010 [DOI] [PubMed] [Google Scholar]
- 45. Castro R, Kattan E, Ferri G, et al. Effects of capillary refill time‐vs. lactate‐targeted fluid resuscitation on regional, microcirculatory and hypoxia‐related perfusion parameters in septic shock: a randomized controlled trial. Ann Intensive Care. 2020;10(1):150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hernández G, Ospina‐Tascón GA, Damiani LP, et al. Effect of a resuscitation strategy targeting peripheral perfusion status vs serum lactate levels on 28‐day mortality among patients with septic shock: the ANDROMEDA‐SHOCK randomized clinical trial. Jama. 2019;321(7):654‐664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Manzoor H, Bhatt H. Prerenal Kidney Failure. StatPearls Publishing LLC; 2022. [PubMed] [Google Scholar]
- 48. Montomoli J, Donati A, Ince C. Acute kidney injury and fluid resuscitation in septic patients: are we protecting the kidney? Nephron. 2019;143:1‐4. doi: 10.1159/000501748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rice DM, Ratliff PD, Judd WR, Kseibi SA, Eberwein KA. Assessing the impact of CKD on outcomes in septic shock patients receiving standard vs reduced initial fluid volume. Am J Emerg Med. 2020;38(10):2147‐2150. doi: 10.1016/j.ajem.2020.07.055 [DOI] [PubMed] [Google Scholar]
- 50. Truong TN, Dunn AS, McCardle K, et al. Adherence to fluid resuscitation guidelines and outcomes in patients with septic shock: reassessing the “one‐size‐fits‐all” approach. J Crit Care. 2019;51:94‐98. doi: 10.1016/j.jcrc.2019.02.006 [DOI] [PubMed] [Google Scholar]
- 51. Douglas IS, Alapat PM, Corl KA, et al. Fluid response evaluation in sepsis hypotension and shock: a randomized clinical trial. Chest. 2020;158(4):1431‐1445. doi: 10.1016/j.chest.2020.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Appendix S2
Appendix S3


