Abstract
Haemophilus ducreyi expresses several putative virulence factors in vitro. Isogenic mutant-to-parent comparisons have been performed in a human model of experimental infection to examine whether specific gene products are involved in pathogenesis. Several mutants (momp, ftpA, losB, lst, cdtC, and hhdB) were as virulent as the parent in the human model, suggesting that their gene products did not play a major role in pustule formation. However, we could not exclude the possibility that the gene of interest was not expressed during the initial stages of infection. Biopsies of pustules obtained from volunteers infected with H. ducreyi were subjected to reverse transcription-PCR. Transcripts corresponding to momp, ftpA, losB, lst, cdtB, and hhdA were expressed in vivo. In addition, transcripts for other putative virulence determinants such as ompA2, tdhA, lspA1, and lspA2 were detected in the biopsies. These results indicate that although several candidate virulence determinants are expressed during experimental infection, they do not have a major role in the initial stages of pathogenesis.
Haemophilus ducreyi is a gram-negative bacterium that is the etiologic agent of chancroid, a genital ulcer disease that facilitates acquisition of human immunodeficiency virus type 1 (17, 35). During sexual intercourse, breaks in the epithelium may provide a portal of entry for H. ducreyi. After an incubation period of 1 to 7 days, a small erythematous papule develops. Pustules form 2 to 3 days later and eventually progress into a soft painful ulcer (25).
A human model of H. ducreyi infection was developed by our laboratory to study H. ducreyi pathogenesis (4, 5, 27, 32). Volunteers are inoculated with bacteria at multiple sites on the skin of the upper arm via puncture wounds made with an allergy testing device. In the human model, 1 to 100 CFU are sufficient to initiate infection. Papules develop in 24 h, and pustules usually form 2 to 5 days later, consistent with the natural course of disease. The histopathology of pustules resembles that of naturally occurring ulcers. For subject safety, infection is limited to the pustular stage of disease, and subjects are typically infected for 7 to 14 days.
Several putative virulence factors of H. ducreyi have been identified including pili, outer membrane proteins, toxins, and lipooligosaccharide (LOS) (8–10, 14, 15, 20, 22, 23, 26, 29, 33, 36). To study their role in pathogenesis, isogenic mutants were constructed and compared to the parent in the human model. To date, we have performed 10 mutant-to-parent comparison trials. Isogenic mutants of pal, hgbA, and dsrA were attenuated in their ability to form pustules compared to the parent (3, 7, 18). Seven mutants, including those with disruptions in hhdB, cdtC, hhdB and cdtC, momp, ftpA, lst, and losB, caused pustule formation rates that were similar to those caused by the parent (2, 28, 34, 38, 39). These results were surprising in that some of these candidate virulence factors (hhdB, cdtC, momp, losB) have roles in adherence, cell death, or serum resistance in vitro (10, 11, 19–21, 37). Although the results of human challenge trials suggested that these gene products were not required for pustule formation, we could not exclude the possibility that the gene of interest was simply not transcribed in the initial stages of infection. If a gene was transcribed and its isogenic mutant was still virulent, then it would be very unlikely that the gene product played a major role in virulence.
Previous studies indicated that sera from patients with chancroid contained elevated levels of antibodies to HgbA, TdhA, D15, Hlp, hemolysin, cytolethal distending toxin (CDT), and LOS of H. ducreyi when compared to control sera, suggesting that these factors were expressed during natural infection (1, 12, 16, 22, 24). Confocal microscopy performed on lesions from experimentally infected subjects indicated that ftpA, pal, hlp, momp, and/or ompA2 was expressed in vivo (6). However, detection of gene expression by serology and confocal microscopy is limited by the availability of appropriate antigens or antibodies.
In this study, we examined whether transcripts of candidate virulence genes of H. ducreyi were present in lesions of experimentally infected human subjects. We also estimated the number of CFU in an entire lesion and the number of CFU that allowed detection of bacterial transcripts in human tissue.
MATERIALS AND METHODS
Bacteria and culture conditions.
H. ducreyi 35000HP is a human-passaged variant of 35000 described previously (5, 31). H. ducreyi 35000HP was grown on chocolate agar plates supplemented with 1% IsoVitaleX and incubated at 33°C with 5% CO2 or in brain heart infusion broth containing 50 μg of hemin per ml, 1% IsoVitaleX, and 5% heat-inactivated fetal calf serum and incubated with aeration at 33°C.
Human subjects and biopsies.
Tissue was collected from eight adults (two female and six male; mean age ± standard deviation [SD], 33.6 ± 10.4 years) who participated in several mutant-to-parent comparison trials (7, 18, 39) or who were specifically infected for this study. Informed consent was obtained from the subjects for participation and for human immunodeficiency virus serology, in accordance with the human experimentation guidelines of the U.S. Department of Health and Human Services and the Institutional Review Board of Indiana University-Purdue University at Indianapolis. Enrollment procedures and exclusion criteria are described elsewhere in detail (5, 32). Six volunteers were inoculated on the upper arm with estimated delivered doses (EDD) of 60 to 100 CFU of H. ducreyi 35000, 35000HP, or 35000.304, an isogenic cdtC and hhdB double mutant. Subjects were observed until they achieved clinical endpoint, defined as resolution of disease at all sites, 14 days of infection, or the development of a painful pustule. At clinical endpoint, a total of eight pustules were biopsied with a punch forceps.
Biopsies of uninfected skin were also obtained from two volunteers. Each biopsy of uninfected skin was divided longitudinally. Either 106 or 107 CFU of 35000HP grown to mid-logarithmic phase was added to one portion of each uninfected biopsy to serve as a positive control, while the remainder of the specimen served as a negative control.
After biopsy, all specimens were immediately placed in either 1 ml of freezing media (3% [wt/vol] tryptic soy broth–10% glycerol–10% heat-inactivated fetal calf serum) or proteose peptone saline (77 mM NaCl–1% [wt/vol] proteose peptone) and weighed. Tissue was homogenized for 2 min on ice and a portion (220 μl) of the homogenate was quantitatively cultured on chocolate agar with and without vancomycin (3 μg/ml) as described previously (31, 32).
RNA isolation and reverse transcription (RT).
The remaining homogenate was recovered and usually 600 to 700 μl was centrifuged at 16,000 × g for 5 min at 4°C. Pellets were suspended in 1 ml of Ultraspec RNA (Biotecx Laboratories, Inc., Houston, Tex.), and RNA was isolated according to the manufacturer's instructions. RNA was suspended in diethyl pyrocarbonate-treated H2O and incubated with 15 U of DNase I (GenHunter Corp, Nashville, Tenn.) at 37°C for 45 min. DNase I was heat inactivated by incubating the reaction mixture at 75°C for 6 min. For in vitro experiments, RNA was isolated as described above from approximately 108 CFU of 35000HP grown to mid-logarithmic phase.
The Advantage RT-for-PCR kit (Clontech Laboratories, Palo Alto, Calif.) was used to make cDNA according to the manufacturer's instructions from either approximately 600 ng of RNA from biopsies or RNA that represented approximately 107 CFU of 35000HP. All cDNA reaction mixtures were paired with an equal amount of RNA that was not subjected to reverse transcriptase to control for DNA contamination.
PCR.
Amplification of most target cDNAs was performed with MgCl2 (5 mM) and the appropriate primers (30 pM) in 100-μl reaction mixtures using the PCR Core Kit Plus (Roche Molecular Biochemicals, Indianapolis, Ind.) as per the manufacturer's instructions. ompA2 cDNA was amplified in the presence of MgCl2 (2.5 mM). The synthetic primers used to amplify target cDNA were based on published sequences (9, 22, 27, 30, 36) (Table 1). Robert Munson, Jr. (Ohio State University, Columbus), kindly provided the primers Cdt3 and Cdt4 (Table 1). Reaction mixtures were denatured at 95°C for 5 min, then cycled 45 times through 95°C for 1 min, 60°C for 25 s, and 72°C for 2 min, with a final extension at 72°C for 7 min and then held at 4°C using a thermal cycler (Perkin-Elmer PCR System 2400). The annealing temperature for most primers was 60°C except for ompA2 and lspA1 primers, which annealed at 63°C, and ftpA and β-actin primers, which annealed at 50 and 55°C, respectively. Genomic DNA from 35000HP and H2O were used as templates for positive and negative controls, respectively. Ten microliters of cDNA or RNA was used as template for PCRs. Amplicons were analyzed by electrophoresis of 10 μl of each PCR on 1.2% agarose gels stained with ethidium bromide.
TABLE 1.
Primers
| Primer | Gene | Sequence (5′ to 3′) | Amplicon size (bp) | Limit of detection (CFU)a |
|---|---|---|---|---|
| 18set5′ | pal | TTGAGAATTCAGTAGTTCATCAGGTAAAACAGATG | 400 | 101 |
| 18set3′ | TAAAGAATTCAAATTAGTACTCTAATACTGCACGG | |||
| 28set5′ | hlp | TTGAGAATTCGATAAGCCTGCAAACAACCCTGTAG | 578 | 103 |
| 28set3′ | CCAAGAATTCGGTTTATTTGTTTTCGTCAGCTTC | |||
| Pilin1 | ftpA | GACCACACAGTGCCAGGT | 220 | 101–102 |
| PilinR2 | GCGTTGCCATACGTTCAGC | |||
| Momp-for-2 | momp | CTGCACCAGATGCCAATACC | 700 | 103 |
| Momp-rev-2 | TGATAACCCTACAGAAACAGAATG | |||
| OmpA2-for-5 | ompA2 | TGGATTAGCGCGTAACGATTATAGTG | 315 | 101–102 |
| OmpA2-rev-5 | GCTCGTCCATCATCATTTGCG | |||
| TdhA-for-2 | tdhA | TGATAAAGGCAACCACTACCCAC | 550 | 102 |
| TdhA-rev-2 | CTGCGCGACAGTATTAATCCAAGG | |||
| HgbA-for-2 | hgbA | GCTCAGGTTATGCTATTCGTGGTGT | 549 | 101–102 |
| HgbA-rev-2 | CATAGCCTAGGGTAAAGCGGTTGGT | |||
| Lst-for-1 | lst | GGTTACGTGGTTTAGATGTTTATGGTGC | 423 | 101–102 |
| Lst-rev-1 | CGATATATATCAATCTTTTCTGCTTCTGTC | |||
| LosB-for-1 | losB | CGTAATAGGGATTTATTGCTTTTGAGAG | 513 | 102–103 |
| LosB-rev-1 | CAGCAATATGCACAATAGAAGTATCAGGTG | |||
| Cdt3 | cdtB | AGGCGGTTCTGATGCGGTAAG | 359 | 102 |
| Cdt4 | AGCGATCACGAACAAAACTAACAG | |||
| HhdA-for-1 | hhdA | GGCGGTCAAAATATAATGGAAAGAG | 565 | 102–103 |
| HhdA-rev-1 | TCGTGCCCGGCTTGGATACTAC | |||
| P10 | lspA1 | TTGAATTCAAGTTTCAGCAGGGACAGCAAATATC | 264 | 101–102 |
| P11 | TTTCTCGAGTCTAATTTTTCGGCGACAAGG | |||
| P12 | lspA2 | AAGTTTCAGCAAGAGCGGC | 320 | 102–103 |
| P13 | TATTGGCTGCAAGCTCTG | |||
| β-actin 5′ | β-actin | ATCTGGCACCACACCTTCTACAATGAGCTCCG | 837 | ND |
| β-actin 3′ | CGTCATACTCCTGCTTGCTGATCCACATCTGC |
Value represents the range of limits of detection for three experiments. ND, not determined.
Nucleotide sequence analysis.
Amplicons from PCRs using 35000HP as template and each primer set were sequenced on an ABI Prism 310 (Applied Biosystems) automated sequencing system employing dye terminators. Sequencing data were compared to published sequences for each gene of interest.
Limit of detection.
To determine the limit of detection for each H. ducreyi primer set, three samples of RNA prepared from 35000HP grown to mid-logarithmic phase were used to make cDNA that was subsequently serially diluted and used as template in PCR. Amplicons were analyzed as described above. The number of CFU that gave rise to each RT-PCR sample loaded on the gel was calculated. The limit of detection was defined as the lowest number of CFU whose cDNA yielded a visible RT-PCR product on an ethidium bromide-stained gel.
Analysis of amplicons prepared from biopsies.
All RT-PCR products obtained from biopsies were subjected to agarose gel electrophoresis and Southern blot analysis. DNA was transferred to a nylon membrane by a standard capillary method. Probes were prepared from amplicons that were obtained using 35000HP genomic DNA as template and the appropriate primer set. Probe labeling, hybridization, and detection were performed as described previously (34). If a band of the expected size was detected by agarose gel and Southern blotting or Southern blotting alone, the gene of interest was considered to have been transcribed in vivo. For β-actin, RT-PCR products were only analyzed by agarose gel electrophoresis and stained with ethidium bromide. The number of CFU corresponding to the RT-PCR sample loaded on the gel was calculated based on the quantitative culture for each biopsy and the portion of each biopsy that was converted to cDNA.
RESULTS AND DISCUSSION
Specimens.
Eight pustules were biopsied from six volunteers who were infected for 7.0 ± 1.1 days (mean ± SD). Two additional biopsies were obtained from two uninfected volunteers. Biopsies were homogenized, and a portion was cultured on selective and nonselective media. While H. ducreyi was the only bacterium recovered from pustules, no bacteria were recovered from uninfected skin.
Of the eight biopsies, four were obtained in a way that allowed us to estimate the number of CFU in an entire lesion. In other words, the biopsy encompassed the entire pustule, and the entire specimen was homogenized and quantitatively cultured. We recovered on average 2.3 × 105 CFU per biopsy (range, 1.7 × 104 to 6.0 × 105 CFU). Due to the fact that H. ducreyi aggregates, the CFU are minimal estimates of the number of bacteria present in a sample. Since the EDD and duration of infection were known, the doubling time for H. ducreyi in vivo was calculated to be 16.5 ± 3.8 h (Table 2). Since the rate of bacterial cell death in vivo is unknown, the doubling time is a minimal estimate.
TABLE 2.
Bacterial recovery
| Samplea | EDD (CFU) | Duration of infection (days) | Total CFU present in biopsy | Estimated minimal doubling time (h) |
|---|---|---|---|---|
| 142a | 60 | 7 | 1.9 × 105 | 15 |
| 142b | 60 | 7 | 1.7 × 104 | 21 |
| 165 | 93 | 6 | 6.0 × 105 | 12 |
| 164 | 93 | 7 | 7.0 × 104 | 18 |
Volunteer number. Samples a and b were collected from different sites on the same volunteer.
RT-PCR of H. ducreyi mRNA in vitro.
To verify that the target sequences were amplified, PCR products obtained from 35000HP genomic DNA and each of the H. ducreyi primer sets were sequenced. The sequence of each amplicon matched that of the expected product (data not shown).
To determine the sensitivity of each H. ducreyi primer pair, we amplified 10-fold serial dilutions of cDNA prepared from broth-grown 35000HP. Experiments were repeated with three preparations of RNA. Under the conditions studied, the limits of detection for different primer pairs varied from 101 to 103 CFU (Table 1). The results indicated that our methods had sufficient sensitivity to amplify H. ducreyi cDNA from patient samples.
Detection of H. ducreyi mRNA in control samples.
To detect potential inhibitors or false-positive signals, we performed RT-PCR on homogenates prepared from two biopsies of uninfected skin with and without H. ducreyi. No products were detected in samples from uninfected skin, but the expected PCR products were amplified from uninfected skin homogenized with H. ducreyi. To verify that cDNA was present in the PCR, β-actin was amplified from uninfected skin with and without H. ducreyi (data not shown). Although uninfected skin did not contain the same cells or genes expressed as inflamed tissue, the data suggested that the RT-PCR products were H. ducreyi specific and not eukaryotic in origin.
In preliminary experiments utilizing three biopsies, specimens were homogenized in either proteose peptone saline or freezing medium. When biopsies were homogenized in proteose peptone saline, no signal was recovered. However, homogenizing biopsies in freezing medium allowed for the detection of H. ducreyi mRNA. Preliminary experiments indicated that RNA was not stable with storage at −70°C, consistent with the instability of prokaryotic mRNA. However, cDNA stored at −70°C was stable for at least 4 months. Only biopsies that were homogenized in freezing medium and whose cDNA was made on the same day as the biopsy were included in the final analysis.
Detection of transcripts of candidate virulence genes in vivo.
Five biopsies obtained from sites inoculated with the parent strain were analyzed for the presence of H. ducreyi cDNAs. Since the products of pal, hlp, and ftpA were detected by confocal microscopy in vivo (6), these genes served as positive controls for the specimens. pal, hlp, and ftpA cDNAs were detected in all the specimens. Thus, the biopsies were also analyzed for the presence of other cDNAs.
Isogenic mutants of ftpA, momp, hhdB, cdtC, hhdB and cdtC, lst, and losB caused pustule formation rates that were similar to the parent in the human model of H. ducreyi infection (2, 34, 38, 39). The results suggested that the gene products were not required for pustule formation. Except for ftpA, we did not know whether these genes were transcribed in the initial stages of infection. The hemolysin and CDT are encoded by hhdBA and cdtABC gene clusters, respectively (10, 26). Thus, transcription of hhdA and cdtB were likely to represent transcription of each gene cluster. RT-PCR and Southern blotting were used to analyze five biopsies for the presence of momp, hhdA, cdtB, lst, and losB cDNAs (Fig. 1). lst, losB, and cdtB cDNAs were present in five of five biopsies, and momp and hhdA cDNAs were detected in four of five biopsies. Since the genes encoding ftpA, momp, lst, losB, cdtB, and hhdA were transcribed in vivo, these data validate the results of the human challenge trials, which indicated that these gene products made minimal contributions to virulence. The data exclude the possibility that the genes were simply not transcribed during the initial stages of infection. However, expression of these genes may be important at the ulcerative stage.
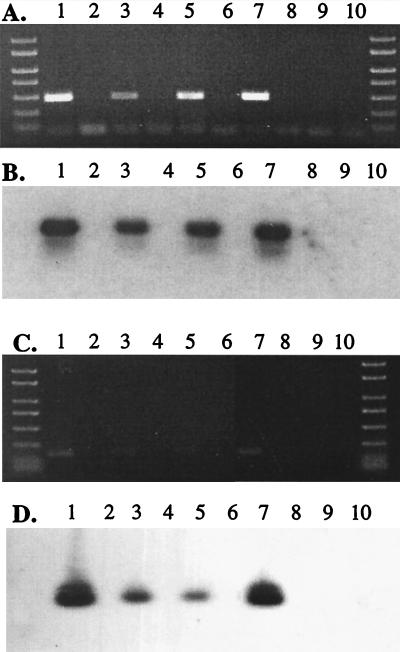
FIG. 1.
Composite agarose gels stained with ethidium bromide (A and C) and Southern blot (B and D) of PCR and RT-PCR products. PCR was performed with either losB primers (panels A and B) or ftpA primers (panels C and D). Lanes 1 and 2 contain 35000HP genomic DNA and no template, respectively. cDNA (lanes 3, 5, 7, and 9) and RNA that was not reverse transcribed (lanes 4, 6, 8, and 10) were prepared from biopsy 165 (lanes 3 and 4), biopsy 164 (lanes 5 and 6), uninfected skin homogenized with H. ducreyi (lanes 7 and 8), and uninfected skin (lanes 9 and 10).
H. ducreyi expresses two outer membrane proteins designated HgbA and TdhA, which bind hemoglobin and heme, respectively (13, 33). An isogenic hgbA mutant was attenuated in the human model of H. ducreyi infection (3). This suggested that hgbA was expressed during infection and loss of hgbA impaired the ability of the organism to survive in vivo. Thus, tdhA may not be expressed in vivo, or tdhA expression may not compensate for the loss of hgbA. hgbA cDNA was found in four of five biopsies, and tdhA cDNA was amplified from five of five biopsies. Although the isogenic hgbA mutant grew in the presence of heme in vitro (14), the data suggest that tdhA may not compensate for the loss of hgbA during infection.
ompA2, lspA1, and lspA2 are candidate virulence genes that may play a role in adherence or serum resistance (21, 23, 36). A monoclonal antibody that specifically recognizes the gene product of ompA2 is not available (23), and the gene products of lspA1 and lspA2 are secreted in vitro (36). Thus, confocal microscopy could not be used to determine if these genes were expressed in vivo. Transcripts for ompA2, lspA1, and lspA2 were detected in all five biopsies examined. Similarly, Ward et al. detected lspA1 and lspA2 cDNAs in aspirates of lesions obtained from rabbits infected with H. ducreyi (36). Since ompA2, lspA1, and lspA2 are transcribed during experimental infection, evaluation of their respective isogenic mutants in the human model will be relevant.
The actual samples amplified by RT-PCR and analyzed by agarose gel electrophoresis and Southern blotting were estimated to contain 122 ± 159 CFU (mean ± SD; range, 7 to 400 CFU). Despite an abundance of eukaryotic RNA, RT-PCR products were detected in samples estimated to represent as few as 7 CFU of H. ducreyi. Our ability to detect H. ducreyi transcripts expressed in vivo was variable in samples with <100 CFU but was more consistent when a sample contained >100 CFU (data not shown). The RNA prepared from in vitro-grown organisms lacked eukaryotic RNA, and the experiments done on the biopsies and in vitro grown bacteria were not performed simultaneously. Thus, we could not compare in vitro and in vivo levels of expression.
In summary, we amplified specific H. ducreyi transcripts in human lesions and estimated a minimal doubling time for H. ducreyi in vivo. Although all the candidate virulence genes examined in this study were transcribed in vivo, we cannot conclusively state that the mRNAs were translated. Nevertheless, these results strengthen the findings of several human challenge trials that suggested that several of these putative virulence determinants, while expressed in vivo, did not play a major role in pustule formation. Since we detected H. ducreyi transcripts from very few organisms, our methods should be applicable to other human skin infections such as those caused by Treponema pallidum, group A streptococi, Staphylococcus aureus, Mycobacterium leprae, and Leishmania. Future studies will be directed towards examining whether specific H. ducreyi genes are differentially expressed during human infection.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants AI27863, AI31494, and MO1RR00750. Clinical specimens were derived from trials supported by the Sexually Transmitted Diseases Clinical Trials Unit through contract no. N01-AI75329 from the NIAID.
We thank Stacy L. Nelson, Christine Ward, Margaret Bauer, Tricia Humphreys, and Byron Batteiger for advice and assistance with the manuscript and Diane Stothard for advice and providing sequence data.
REFERENCES
- 1.Alfa M J, Olson N, Degagne P, Plummer F, Namaara W, Maclean I, Ronald A R. Humoral immune response of humans to lipooligosaccharide and outer membrane proteins of Haemophilus ducreyi. J Infect Dis. 1993;167:1206–1210. doi: 10.1093/infdis/167.5.1206. [DOI] [PubMed] [Google Scholar]
- 2.Al-Tawfiq J A, Bauer M E, Fortney K R, Katz B P, Hood A F, Ketterer M, Apicella M A, Spinola S M. A pilus-deficient mutant of Haemophilus ducreyi is virulent in the human model of experimental infection. J Infect Dis. 2000;181:1176–1179. doi: 10.1086/315310. [DOI] [PubMed] [Google Scholar]
- 3.Al-Tawfiq J A, Fortney K R, Katz B P, Elkins C, Spinola S M. An isogenic hemoglobin receptor-deficient mutant of Haemophilus ducreyi is attenuated in the human model of experimental infection. J Infect Dis. 2000;181:1049–1054. doi: 10.1086/315309. [DOI] [PubMed] [Google Scholar]
- 4.Al-Tawfiq J A, Harezlak J, Katz B P, Spinola S M. Cumulative experience with Haemophilus ducreyi in the human model of experimental infection. Sex Transm Dis. 2000;27:111–114. doi: 10.1097/00007435-200002000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Al-Tawfiq J A, Thornton A C, Katz B P, Fortney K R, Todd K D, Hood A F, Spinola S M. Standardization of the experimental model of Haemophilus ducreyi infection in human subjects. J Infect Dis. 1998;178:1684–1687. doi: 10.1086/314483. [DOI] [PubMed] [Google Scholar]
- 6.Bauer M E, Spinola S M. Localization of Haemophilus ducreyi at the pustular stage of disease in the human model of infection. Infect Immun. 2000;68:2309–2314. doi: 10.1128/iai.68.4.2309-2314.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bong C T H, Throm R E, Fortney K R, Katz B P, Hood A F, Elkins C, Spinola S M. DsrA-deficient mutant of Haemophilus ducreyi is impaired in its ability to infect human volunteers. Infect Immun. 2001;69:1488–1491. doi: 10.1128/IAI.69.3.1488-1491.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bozue J A, Tullius M V, Wang J, Gibson B W, Munson R S., Jr Haemophilus ducreyi produces a novel sialyltransferase: identification of the sialyltransferase gene and construction of mutants deficient in the production of the sialic acid-containing glycoform of the lipooligosaccharide. J Biol Chem. 1999;274:4106–4114. doi: 10.1074/jbc.274.7.4106. [DOI] [PubMed] [Google Scholar]
- 9.Brentjens R J, Ketterer M, Apicella M A, Spinola S M. Fine tangled pili expressed by Haemophilus ducreyi are a novel class of pili. J Bacteriol. 1996;178:808–816. doi: 10.1128/jb.178.3.808-816.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cope L D, Lumbley S, Latimer J L, Klesney-Tait J, Stevens M K, Johnson L S, Purven M, Munson R S, Jr, Lagergard T, Radolf J D, Hansen E J. A diffusible cytotoxin of Haemophilus ducreyi. Proc Natl Acad Sci USA. 1997;94:4056–4061. doi: 10.1073/pnas.94.8.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cortes-Bratti X, Chaves-Olarte E, Lagergard T, Thelestam M. The cytolethal distending toxin from the chancroid bacterium Haemophilus ducreyi induces cell-cycle arrest in the G2 phase. J Clin Investig. 1999;103:107–115. doi: 10.1172/JCI3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dutro S M, Wood G E, Totten P A. Prevalence of, antibody response to, and immunity induced by Haemophilus ducreyi hemolysin. Infect Immun. 1999;67:3317–3328. doi: 10.1128/iai.67.7.3317-3328.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elkins C. Identification and purification of a conserved heme-regulated hemoglobin-binding outer membrane protein from Haemophilus ducreyi. Infect Immun. 1995;63:1241–1245. doi: 10.1128/iai.63.4.1241-1245.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elkins C, Chen C-J, Thomas C E. Characterization of the hgbA locus encoding a hemoglobin receptor from Haemophilus ducreyi. Infect Immun. 1995;63:2194–2200. doi: 10.1128/iai.63.6.2194-2200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elkins C, Morrow K J, Olsen B. Serum resistance in Haemophilus ducreyi requires outer membrane protein DsrA. Infect Immun. 2000;68:1608–1619. doi: 10.1128/iai.68.3.1608-1619.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elkins C, Yi K, Olsen B, Thomas C, Thomas K, Morse S. Development of a serological test for Haemophilus ducreyi for seroprevalence studies. J Clin Microbiol. 2000;38:1520–1526. doi: 10.1128/jcm.38.4.1520-1526.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fleming D T, Wasserheit J N. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999;75:3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fortney K R, Young R S, Bauer M E, Katz B P, Hood A F, Munson R S, Jr, Spinola S M. Expression of peptidoglycan-associated lipoprotein is required for virulence in the human model of Haemophilus ducreyi infection. Infect Immun. 2000;68:6441–6448. doi: 10.1128/iai.68.11.6441-6448.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gelfanova V, Hansen E J, Spinola S M. Cytolethal distending toxin of Haemophilus ducreyi induces apoptotic death of Jurkat T cells. Infect Immun. 1999;67:6394–6402. doi: 10.1128/iai.67.12.6394-6402.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gibson B W, Campagnari A A, Melaugh W, Phillips N J, Apicella M A, Grass S, Wang J, Palmer K L, Munson R S., Jr Characterization of a transposon Tn916-generated mutant of Haemophilus ducreyi 35000 defective in lipooligosaccharide biosynthesis. J Bacteriol. 1997;179:5062–5071. doi: 10.1128/jb.179.16.5062-5071.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hiltke T J, Bauer M E, Klesney-Tait J, Hansen E J, Munson R S, Jr, Spinola S M. Effect of normal and immune sera on Haemophilus ducreyi 35000HP and its isogenic MOMP and LOS mutants. Microb Pathog. 1999;26:93–102. doi: 10.1006/mpat.1998.0250. [DOI] [PubMed] [Google Scholar]
- 22.Hiltke T J, Campagnari A A, Spinola S M. Characterization of a novel lipoprotein expressed by Haemophilus ducreyi. Infect Immun. 1996;64:5047–5052. doi: 10.1128/iai.64.12.5047-5052.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klesney-Tait J, Hiltke T J, Maciver I, Spinola S M, Radolf J D, Hansen E J. The major outer membrane protein of Haemophilus ducreyi consists of two OmpA homologs. J Bacteriol. 1997;179:1764–1773. doi: 10.1128/jb.179.5.1764-1773.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lagergard T. The role of Haemophilus ducreyi bacteria, cytotoxin, endotoxin and antibodies in animal models for study of chancroid. Microb Pathog. 1992;13:203–217. doi: 10.1016/0882-4010(92)90021-f. [DOI] [PubMed] [Google Scholar]
- 25.Morse S A. Chancroid and Haemophilus ducreyi. Clin Microbiol Rev. 1989;2:137–157. doi: 10.1128/cmr.2.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palmer K L, Munson R S., Jr Cloning and characterization of the genes encoding the haemolysin of Haemophilus ducreyi. Mol Microbiol. 1995;18:821–830. doi: 10.1111/j.1365-2958.1995.18050821.x. [DOI] [PubMed] [Google Scholar]
- 27.Palmer K L, Schnizlein-Bick C T, Orazi A, John K, Chen C-Y, Hood A F, Spinola S M. The immune response to Haemophilus ducreyi resembles a delayed-type hypersensitivity reaction throughout experimental infection of human subjects. J Infect Dis. 1998;178:1688–1697. doi: 10.1086/314489. [DOI] [PubMed] [Google Scholar]
- 28.Palmer K L, Thornton A C, Fortney K R, Hood A F, Munson R S, Jr, Spinola S M. Evaluation of an isogenic hemolysin-deficient mutant in the human model of Haemophilus ducreyi infection. J Infect Dis. 1998;178:191–199. doi: 10.1086/515617. [DOI] [PubMed] [Google Scholar]
- 29.Spinola S M, Griffiths G E, Bogdan J A, Menegus M A. Characterization of an 18,000-molecular-weight outer membrane protein of Haemophilus ducreyi that contains a conserved surface-exposed epitope. Infect Immun. 1992;60:385–391. doi: 10.1128/iai.60.2.385-391.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spinola S M, Hiltke T J, Fortney K, Shanks K. The conserved 18,000-molecular-weight outer membrane protein of Haemophilus ducreyi has homology to PAL. Infect Immun. 1996;64:1950–1955. doi: 10.1128/iai.64.6.1950-1955.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spinola S M, Orazi A, Arno J N, Fortney K, Kotylo P, Chen C-Y, Campagnari A A, Hood A F. Haemophilus ducreyi elicits a cutaneous infiltrate of CD4 cells during experimental human infection. J Infect Dis. 1996;173:394–402. doi: 10.1093/infdis/173.2.394. [DOI] [PubMed] [Google Scholar]
- 32.Spinola S M, Wild L M, Apicella M A, Gaspari A A, Campagnari A A. Experimental human infection with Haemophilus ducreyi. J Infect Dis. 1994;169:1146–1150. doi: 10.1093/infdis/169.5.1146. [DOI] [PubMed] [Google Scholar]
- 33.Thomas C E, Olsen B, Elkins C. Cloning and characterization of tdhA, a locus encoding a tonB-dependent heme receptor from Haemophilus ducreyi. Infect Immun. 1998;66:4254–4262. doi: 10.1128/iai.66.9.4254-4262.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Throm R E, Al-Tawfiq J A, Fortney K R, Katz B P, Hood A F, Hansen E J, Spinola S M. Evaluation of an isogenic MOMP-deficient mutant in the human model of Haemophilus ducreyi infection. Infect Immun. 2000;68:2602–2607. doi: 10.1128/iai.68.5.2602-2607.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trees D L, Morse S A. Chancroid and Haemophilus ducreyi: an update. Clin Microbiol Rev. 1995;8:357–375. doi: 10.1128/cmr.8.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ward C, Lumbley S, Latimer J, Cope L, Hansen E. Haemophilus ducreyi secretes a filamentous hemagglutinin-like protein. J Bacteriol. 1998;180:6013–6022. doi: 10.1128/jb.180.22.6013-6022.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wood G E, Dutro S M, Totten P A. Target cell range of Haemophilus ducreyi hemolysin and its involvement in invasion of human epithelial cells. Infect Immun. 1999;67:3740–3749. doi: 10.1128/iai.67.8.3740-3749.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young R S, Fortney K, Haley J C, Hood A F, Campagnari A A, Wang J, Bozue J A, Munson R S, Jr, Spinola S M. Expression of sialylated or paragloboside-like lipooligosaccharides are not required for pustule formation by Haemophilus ducreyi in human volunteers. Infect Immun. 1999;67:6335–6340. doi: 10.1128/iai.67.12.6335-6340.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Young R S, Fortney K R, Gelfanova V, Phillips C L, Katz B P, Hood A F, Latimer J L, Munson R S, Jr, Hansen E J, Spinola S M. Expression of cytolethal distending toxin and hemolysin is not required for pustule formation by Haemophilus ducreyi in human volunteers. Infect Immun. 2001;69:1938–1942. doi: 10.1128/IAI.69.3.1938-1942.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]