Abstract
Background and Purpose
Recently pentoxifylline, a non‐selective phosphodiesterase inhibitor and adenosine receptor antagonist, has attracted much interest for the treatment of the increased vascular resistance and endothelial dysfunction in pre‐eclampsia. We therefore investigated the placental transfer, vascular effects and anti‐inflammatory actions of pentoxifylline in healthy and pre‐eclamptic human placentas.
Experimental Approach
The placental transfer and metabolism of pentoxifylline were studied using ex vivo placenta perfusion experiments. In wire myography experiments with chorionic plate arteries, pentoxifyllines vasodilator properties were investigated, focusing on the cGMP and cAMP pathways and adenosine receptors. Its effects on inflammatory factors were also studied in placental explants.
Key Results
Pentoxifylline transferred from the maternal to foetal circulation, reaching identical concentrations. The placenta metabolized pentoxifylline into its active metabolite lisofylline (M1), which was released into both circulations. In healthy placentas, pentoxifylline potentiated cAMP‐ and cGMP‐induced vasodilation, as well as causing vasodilation by adenosine A1 antagonism and via NO synthase and PKG. Pentoxifylline also reduced inflammatory factors secretion. In pre‐eclamptic placentas, we observed that its vasodilator capacity was preserved, however not via NO‐PKG but likely through adenosine signalling. Pentoxifylline neither potentiated vasodilation through cAMP and cGMP, nor suppressed the release of inflammatory factors from these placentas.
Conclusion and Implications
Pentoxifylline is transferred across and metabolized by the placenta. Its beneficial effects on the NO pathway and inflammation are not retained in pre‐eclampsia, limiting its application in this disease, although it could be useful for other placenta‐related disorders. Future studies might focus on selective A1 receptor antagonists as a new treatment for pre‐eclampsia.
Keywords: inflammation, nitric oxide, pentoxifylline, phosphodiesterase, placenta, pre‐eclampsia, vasodilation
Abbreviations
- GLM‐RM
general linear model – repeated measures
- M1
(±)‐lisofylline
- M4
1‐(3′‐carboxypropyl)‐3,7‐dimethylxanthine
- M5
1‐(4′‐carboxybutyl)‐3,7‐dimethylxanthine
What is already known
The non‐selective PDE inhibitor and adenosine receptor antagonist pentoxifylline improves foetal flow distribution in human.
What does this study add
Pentoxifylline crosses the placenta, while its NO‐mediated vasodilator and anti‐inflammatory effects are absent in pre‐eclampsia.
What is the clinical significance
The placental effects of pentoxifylline may be useful for pregnancy disorders other than pre‐eclampsia.
1. INTRODUCTION
Pre‐eclampsia is a serious placenta‐related pregnancy disorder, affecting approximately 5–8% of all pregnancies, for which there is currently no effective treatment (Steegers et al., 2010). Pre‐eclampsia is characterized by hypertension with an onset after 20 weeks of gestation, accompanied by proteinuria and potentially other evidence of maternal organ damage (e.g. elevated liver enzymes and pulmonary or cerebral oedema) and/or foetal growth restriction (American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy, 2013). Pre‐eclampsia has not only been associated with an increased risk of maternal and foetal complications during pregnancy but can also lead to health problems later in life for both mother and child (Bellamy et al., 2007; Goldenberg et al., 2008; Steegers et al., 2010). Early‐onset pre‐eclampsia occurs when the symptoms present before the 34th week of gestation (van der Merwe et al., 2010). The pathophysiological mechanism involved in pre‐eclampsia is believed to involve impaired placentation in early pregnancy, leading to an increased vascular resistance, generalized endothelial dysfunction and endovascular inflammation. Yet, pre‐eclampsia can only be cured by termination of pregnancy to deliver the placenta and, with it, an often‐preterm infant.
The methylxanthine‐derivative pentoxifylline has potential as a therapeutic option for the treatment of pre‐eclampsia (Azimi et al., 2015). Pentoxifylline has already been registered for intermittent claudication as it induces vasodilation and is known to have anti‐inflammatory properties, scavenge oxygen radicals, improve endothelial function, increase erythrocyte flexibility and inhibit platelet aggregation (Bhat & Madyastha, 2001; Salhiyyah et al., 2015). In a clinical study, pentoxifylline was given to pregnant women with imminent preterm labour to improve disturbances in the foetal–placental blood perfusion that frequently cause obstetricians to induce preterm delivery. In this study, Lauterbach et al. (2012) showed that pentoxifylline increased the cerebro‐placental pulsatility ratio—a measure for perinatal outcome (Gramellini et al., 1992)—by decreasing placental resistance in utero (Lauterbach et al., 2012). It also improved neonatal clinical outcome in the first 4 weeks of life (Lauterbach et al., 2012). In an experimental model for pre‐eclampsia in pregnant ewes, pentoxifylline significantly alleviated and delayed the onset of symptoms (Tálosi et al., 2001). Furthermore, pentoxifylline showed promising results as an anti‐inflammatory agent in preterm born infants with sepsis or necrotizing enterocolitis (Lauterbach et al., 1999; Salman et al., 2019; Shabaan et al., 2015).
The mechanisms behind the effects of pentoxifylline are not completely understood. As a non‐selective phosphodiesterase (PDE) inhibitor, pentoxifylline increases the intracellular concentrations of cAMP generated by adenylyl cyclase (Speer et al., 2017). Pentoxifylline can additionally inhibit adenosine signalling through interaction with adenosine receptors. These receptors consist of four subtypes:‐ A1 , A2A , A2B and A3 , which are coupled to adenylyl cyclase‐inhibitory Gi‐proteins (A1/A3 receptor) or adenylyl cyclase‐stimulatory Gs‐proteins (A2A/A2B receptor) (Fredholm et al., 2001), and as such also regulate cAMP. A2 receptor stimulation relaxes human coronary arteries in an endothelium‐independent manner by upregulating cAMP, while A1 receptor stimulation exerts the opposite effects (Sato et al., 2005). Pentoxifylline has a higher selectivity for the A1 versus the A2 receptor (Schwabe et al., 1985) and would thus be expected to induce vasodilation by blocking the effects of endogenous adenosine signalling via its A1 receptors. Simultaneously, the protective effects of pentoxifylline in pulmonary inflammation were A2A receptor‐dependent (Konrad et al., 2013). The in vivo effects of pentoxifylline have also been attributed to its metabolites, as biotransformation yields the formation of the metabolites M1–M7. While pentoxifylline rapidly disappears from the circulation, M1 (also known as lisofylline) and M5 (1‐(4′‐carboxybutyl)‐3,7‐dimethylxanthine) remain present over a longer period and hence have a potentially more significant effect in vivo (Beermann et al., 1985; Smith et al., 1986).
The anti‐inflammatory properties of pentoxifylline are of particular interest in pre‐eclamptic patients, whom display increased plasma levels of pro‐inflammatory cytokines, including IL‐6, IFN‐γ and TNF‐α (Jonsson et al., 2006; Szarka et al., 2010), and suppressed production of IL‐5 and the anti‐inflammatory cytokine IL−10 (Azizieh et al., 2005; Szarka et al., 2010). This inflammatory response may originate from the pre‐eclamptic placenta (Munno et al., 1999) and could subsequently contribute to placental dysfunction, endothelial damage and ischaemic‐reperfusion injury (Hunt et al., 1989; Nawroth & Stern, 1986; Raghupathy & Raghupathy, 2013; Stark, 1993). Speer et al. (2017) observed that pentoxifylline reduced LPS‐induced inflammation in an ex vivo placental explant model, often used to study pre‐eclampsia. However, we recently showed a decreased expression of many immune‐related genes, as well as lower numbers of regulatory and anti‐inflammatory M2‐like macrophages and mast cells in placentas from women with early‐onset pre‐eclampsia (Broekhuizen et al., 2021), while the inflammatory M1‐like macrophages were unaltered. This challenges the view of increased inflammation in pre‐eclamptic placentas.
In summary, pentoxifylline may improve placental function in pre‐eclampsia through a multitude of mechanisms. Yet, before considering treatment of pregnant women, it is important to study its placental passage and metabolism, and to discern the vascular and anti‐inflammatory effects of pentoxifylline in the human placenta. Obviously, it is also essential to investigate whether any protective effects in healthy placentas, if occurring, are retained in pre‐eclampsia. For instance, the PDE5A inhibitor sildenafil was unable to improve placental function in pre‐eclampsia, despite promising results in healthy placentas (Hitzerd et al., 2019).
The aim of this study was to evaluate the placental transfer of pentoxifylline to estimate foetal exposure and its metabolism by the placenta using ex vivo dual‐sided cotyledon perfusion of human placentas (named ex vivo placenta perfusion in short). Additionally, we investigated the vascular and anti‐inflammatory effects of pentoxifylline in the placentas of women with uncomplicated pregnancies and women with pre‐eclampsia.
2. METHODS
2.1. Patient tissue collection
Placentas of women with uncomplicated singleton pregnancies who underwent a planned caesarean section or who suffered from early‐onset pre‐eclampsia (diagnosis <34th week of gestation) were collected immediately after delivery at the Erasmus MC University Medical Center, Rotterdam, the Netherlands, from April 2019 until June 2022. The study was exempted from approval by the local institutional Medical Ethics Committee according to the Dutch medical Research with Human Subjects Law (MEC‐2016‐418 and MEC‐2017‐418) and all patients gave written consent for the use of their placenta and data prior to the experiments.
2.2. Ex vivo dual‐sided cotyledon perfusion
To study the placental transfer of pentoxifylline, ex vivo dual‐sided cotyledon perfusion was performed as described by Hitzerd et al. (2019). In short, after collection of the placenta, the amnion was removed and the foetal chorionic plate artery and corresponding vein of an intact cotyledon were cannulated and perfused with Krebs–Henseleit buffer (in mmol·L−1: NaCl 118, KCl 4.7, CaCl2 2.5, MgSO4 1.2, KH2PO4 1.2, NaHCO3 25 and glucose 8.3; pH 7.4) supplemented with heparin 2500 IU·L−1 and oxygenated with 95% O2–5% CO2. The cotyledon was placed into the perfusion chamber and the foetal flow was gradually increased up to 6 ml·min−1. The maternal circulation was re‐established by inserting four blunt cannulas into the intervillous space with a flow rate of 12 ml·min−1. After approximately 30 min perfusion, to wash out any remaining blood, the circulations were closed and replaced by fresh Krebs–Henseleit medium with the addition of bovine serum albumin (29 g·L−1 maternal and 34 g·L−1 foetal). At t = 0, pentoxifylline (10 mg·L−1) was added to the maternal circulation and samples were taken from both circulations at eight set time points over a period of 3 h and immediately stored at −80°C. To prove good overlap between the maternal and foetal circulations, antipyrine (100 mg·L−1) was also added to the maternal buffer and a foetal/maternal ratio >0.75 was considered sufficient. To control for capillary leakage, 40‐kDa fluorescein isothiocyanate (FITC)‐dextran (Sigma‐Aldrich, 36 mg·L−1) was added to the foetal circulation and its maternal/foetal ratio should have not exceeded 0.03. The concentrations of antipyrine and fluorescein isothiocyanate‐dextran were determined as described previously (Hitzerd et al., 2019).
Pentoxifylline concentrations were quantified by means of a chromatography‐mass spectrometry method in a volume of 50 μl. The method was validated with pentoxifylline‐d 6 as internal standard according to the EMA validation protocol (Guideline Bioanalytical method validation www.ema.europa.eu). The analysis was performed on a Water TSQ micro system in an ISO certified laboratory. The analysis was characterized by a lower limit of quantification of 0.2 μg·L−1, an upper limit of quantification of 50 μg·L−1, a linearity of 0.2–50 μg·L−1 and a R 2 > 0.99.
2.3. Wire‐myography experiments with porcine coronary arteries
Twelve porcine hearts were collected from the slaughterhouse to perform an initial screening of pathways that may be involved in the vasodilator effects of pentoxifylline. Coronary arteries were dissected from the porcine hearts and stored overnight in Krebs–Henseleit buffer aerated with 95% O2–5% CO2 at 4°C. Vessel segments of 4‐mm length were suspended on stainless steel hooks in 15 ml‐organ baths, filled with Krebs–Henseleit buffer at 37 °C and aerated with 95% O2–5% CO2. After a period of equilibration and stretched to a stable force of about 15 mN, vessel segments were exposed to 30 mmol·L−1 KCl twice. Subsequently, maximum contractile responses were determined using 100 mmol·L−1 KCl. After washout of the KCl, segments were pre‐incubated for 30 min in the absence or presence of an inhibitor and subsequently pre‐constricted using thromboxane A2 (TP) receptor agonist U46619 (1 μmol·L−1). Segments that were pre‐incubated with SQ22536 (100 μmol·L−1) or Nω‐nitro‐l‐arginine methyl ester hydrochloride (L‐NAME, 100 μmol·L−1) were used to construct concentration–response curves to pentoxifylline (1 nmol·L−1–300 μmol·L−1. While segments that were pre‐incubated with pentoxifylline (100 μmol·L−1) were exposed to sodium nitroprusside (SNP, 1 nmol·L−1–100 μmol·L−1) or forskolin (1 nmol·L−1–30 μmol·L−1). Changes in tissue contractile force were recorded with a Harvard isometric transducer (South Natick, MA, USA).
2.4. Wire‐myography experiments with human chorionic plate arteries
Second‐order branches of chorionic plate arteries were dissected from placental tissue, cleaned from surrounding tissue and stored in Krebs–Henseleit buffer aerated with 95% O2–5% CO2 at 4°C overnight. The following day, the vessels were cut into 2 mm segments (in length) and mounted in 6‐ml organ baths (Danish Myograph Technology, Aarhus, Denmark), filled with Krebs–Henseleit buffer and aerated with 95% O2–5% CO2. After warming the organ baths to 37 °C, the tension was normalized to 90% of the estimated diameter at 38 mmHg effective transmural pressure (5.1 kPa) to mimic the physiological circumstances of placental vessels. The average diameter of the arteries after normalization was 2.74 ± 0.08 mm (total number of artery segments = 497, number of patients = 67). When the segments reached a stable baseline pressure, the maximum contractile responses to 100 mmol·L−1 KCl were determined. After washout of KCl, segments were pre‐incubated in the absence or presence of one of the following inhibitors for at least 30 min: adenylyl cyclase inhibitor SQ22536 (100 μmol·L−1), nitric oxide synthase (NOS)‐inhibitor L‐NAME, 100 μmol·L−1, protein kinase G (PKG)‐inhibitor Rp‐8‐Br‐PET‐cGMPS (Rp‐8‐BrcGMPS; 3 μmol·L−1), protein kinase A (PKA)‐inhibitor Rp‐cAMPS (10 μmol·L−1), A1 receptor antagonist 8‐cyclopentyl−1,3‐dipropylxanthine (DPCPX; 10 μmol·L−1), A2A receptor antagonist ZM 241385 (3 μmol·L−1) and A2B receptor antagonist MRS1706 (10 μmol·L−1), either alone or in combination. Antagonists were chosen using the British Journal of Pharmacology Guide to Receptors and Channels and previous studies (Alexander et al., 2011; Gabriëls et al., 2000; Hasan et al., 2000; Xi et al., 2009). All vessel segments were pre‐constricted with the thromboxane A2 agonist U46619 to ~80% of the maximum KCl constriction (10–30 nmol·L−1 U46619), to construct relaxant concentration–response curves for pentoxifylline (1 nmol·L−1–300 μmol·L−1) and M1 (1 nmol·L−1–100 μmol·L−1). Concentration–response curves for the nitric oxide (NO) donor SNP (1 nmol·L−1–100 μmol·L−1) and the adenylyl cyclase activator forskolin (1 nmol·L−1–30 μmol·L−1) were constructed in the presence or absence of pentoxifylline (10 or 100 μmol·L−1). Other concentration–response curves that were investigated without incubators included pentoxifylline metabolites M4 (1‐(3′‐carboxypropyl)‐3,7‐dimethylxanthine) and M5 (1 nmol·L−1–100 μmol·L−1) and soluble guanylyl cyclase activator BAY 60‐2770 (0.1 nmol·L−1–10 μmol·L−1). Endothelial denudation was performed by carefully rubbing a few hairs from cotton buds along the inside of the arterial segment.
2.5. Placental explant experiments
Immediately after collection of the placenta, three full thickness slices of about 0.5 cm wide were dissected from areas without visible infarction, calcification, haematoma, or damage and stored on ice. The placental tissue was thoroughly rinsed with cold PBS. After removal of the chorionic and basal plates, central villous tissue was cut into 2 × 2 mm explants. Three explants from the three different slices were combined into a 12‐well plate with 2 ml DMEM/F12 medium (Lonza) supplemented with 10% FBS, 1.95 g·L−1 NaHCO3 and 100 mg·L−1 Primocin (InvivoGen). The placental explants were left to equilibrate for 3 h at 37°C 8% O2–5% CO2. Thereafter, the villous explants were transferred to new wells with or without 100 mg·L−1 (=359 μmol·L−1) pentoxifylline. After 24 h, the culture medium was collected and stored at −80°C until further analysis. The placental explants were dried in a SpeedVac vacuum concentrator at 45°C (SPD2010, Thermo Fisher Scientific) to determine the dry weight. Experiments were performed in technical duplicates to ensure the reliability of single values.
The secretion of 24 different cytokines, chemokines, growth factors and immune regulatory molecules into the explant culture medium was measured using premixed multiplex Luminex magnetic bead assays (R&D Systems, Abingdon, United Kingdom). This analysis included C‐C motif chemokine ligand 2 (CCL2/MCP−1), C‐X3‐C motif chemokine ligand 1 (CX3CL1/fractalkine), C‐X‐C motif chemokine ligand 8 (CXCL8/IL‐8), CXCL10/IP−10, endoglin, granulocyte colony‐stimulating factor (G‐CSF), granulocyte‐macrophage colony‐stimulating factor (GM‐CSF), macrophage colony‐stimulating factor (M‐CSF), IFN‐γ, IL‐6, IL−1β, IL−1ra, IL‐2, IL‐4, IL‐5, IL−10, IL−12‐p70, IL−16, IL−18, placental growth factor (PIGF), TNF‐α, vascular endothelial growth factor (VEGF), VEGFC and VEGFR−1. Samples were measured at two‐fold dilution according to the manufacturer's standard protocol. Measurements were conducted on a Luminex MAGPIX machine and data were analysed using Bio‐Plex Manager MP software. The medium concentrations of the measured factors were normalized to the dry weight of the placental explants in each well.
2.6. Materials
Pentoxifylline (Trental®) was acquired from the hospital pharmacy of Erasmus MC, Rotterdam, the Netherlands. M1 and MRS1706 were acquired from Cayman Chemical Company (Michigan, USA), M4 and M5 from Chemodex (Nottinghamshire, UK) and BAY 60‐2770 from Bayer (Leverkusen, Germany). All other compounds were from Sigma‐Aldrich (Schnelldorf, Germany). Details of the compounds and used concentrations can be found in Table S1.
2.7. Data and statistical analysis
The declared group sizes (n) are the number of independent values, and the statistical analysis was done using these independent values. Analyses were conducted and images were produced in Graphpad Prism 8 or R 4.1 using the ggplot2 package (R Core Team, 2021; Wickham, 2016). The number of three successful ex vivo dual‐sided cotyledon perfusion experiments was based on previous experience and as well literature from comparable experiments (Hitzerd et al., 2019).
For the wire myography experiments, concentration–response curves were statistically analysed with a repeated measures ANOVA in SPSS Statistics 25; general linear model–repeated measures (GLM‐RM), sphericity was assumed. Vascular relaxation was expressed as a percentage of the contractile response to U46619. Log10‐transformed values at which the half‐maximal response (pEC50) occurred were individually estimated for pentoxifylline using sigmoid curve fitting software in GraphPad Prism 8 (n = 26 healthy, n = 18 for pre‐eclampsia). The effects of the antagonists on baseline tension were analysed with one‐sample Student's t tests versus 0, while their effects on contractions to 10 nmol·L−1 U46619 were evaluated using paired two‐samples Student's t tests; both were corrected for multiple testing using the Benjamini–Hochberg procedure (R 4.1). The effects of antagonists on concentration–response curves were statistically analysed in a two‐way repeated measures ANOVA for healthy and pre‐eclamptic experiments separately (SPSS Statistics 25; GLM‐RM, sphericity assumed). Only if F in ANOVA achieved P < 0.05, post hoc analyses were performed in a two‐way repeated measures ANOVA for each individual antagonist against the condition without antagonist (control). The differences between the pregnancy conditions (e.g. healthy and pre‐eclampsia) were statistically tested using a one‐way repeated measures ANOVA with the pregnancy condition as between‐subjects factor (SPSS Statistics 25; GLM‐RM, sphericity assumed). A power analysis using the standard deviation from previous work (Hitzerd et al., 2019), an α level of 0.05 and a statistical power of 80%, revealed a minimum sample size of six per group for the wire myography experiments.
For the placenta explant experiments, the sample size of seven placental explant experiments per group was based on previous literature (Speer et al., 2017). The effect of pentoxifylline on the release of cytokines from placental explants was compared with its own paired control experiment (100%) with a one‐sample Student's t test with Benjamini–Hochberg correction. The baseline secretions from pre‐eclamptic explants were statistically tested versus healthy explants using a Kruskal–Wallis test.
The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis (Curtis et al., 2018). Data were presented as mean ± SE or as median (range) unless described otherwise and a P value < 0.05 was considered statistically significant.
2.8. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in the IUPHAR/BPS Guide to PHARMACOLOGY http://www.guidetopharmacology.org and are permanently archived in the Concise Guide to PHARMACOLOGY 2021/22 (Alexander, Christopoulos, et al., 2021; Alexander, Fabbro, Kelly, Mathie, Peters, Veale, Armstrong, Faccenda, Harding, Pawson, Southan, Davies, Beuve, et al., 2021; Alexander, Fabbro, Kelly, Mathie, Peters, Veale, Armstrong, Faccenda, Harding, Pawson, Southan, Davies, Boison, et al., 2021).
3. RESULTS
3.1. Pentoxifylline transfers to the foetal circulation
The transplacental transfer of pentoxifylline was studied in eight ex vivo placenta perfusion experiments, of which three experiments passed all quality control measurements displaying an adequate overlap (foetal/maternal antipyrine ratio: 0.99 ± 0.01) and absence of capillary leakage between the maternal and foetal circulations (maternal/foetal 40‐kDa fluorescein isothiocyanate‐dextran ratio: 0.0053 ± 0.0008). The success rate of 38% was similar to previous research (Mathiesen et al., 2010). The clinical characteristics of the mothers and their offspring, as well as the placental characteristics, are shown in Table 1. The addition of 10 mg·L−1 pentoxifylline to the maternal circulation resulted in rapid transfer of pentoxifylline from the maternal to the foetal circulation, reaching equal concentrations in both circulations after approximately 90 min (Figure 1a,b). The total pentoxifylline concentration in both circulations decreased to 5.8 ± 0.7 μg·L−1 after 3 h of perfusion. However, the decrease in total pentoxifylline concentration was accompanied by a subsequent increase in the concentration of its metabolite M1 to 1.4 ± 0.5 μg·L−1 after 3 h of perfusion (Figure 1c). M4 and M5 were undetectable in the circulations at any time point. These data show that pentoxifylline can rapidly transfer to the foetal circulation through the placenta, which metabolizes pentoxifylline into M1.
TABLE 1.
Clinical data of the three individual perfusion experiments
| 1 | 2 | 3 | |
|---|---|---|---|
| Maternal age (years) | 23 | 43 | 36 |
| Parity (n) | 2 | 2 | 2 |
| Ethnicity | Caucasian | Caucasian | Hispanic |
| Body mass index (kg·m−2) | 29.4 | 23.0 | 30.3 |
| Highest diastolic (mmHg) | 80 | 70 | 80 |
| Highest systolic blood pressure (mmHg) | 136 | 110 | 134 |
| Highest mean arterial pressure (mmHg) | 90 | 83 | 98 |
| Mode of delivery | CS | CS | CS |
| Gestational age (weeks+days) | 38+5 | 39+3 | 39+3 |
| Foetal sex | Male | Female | Male |
| Foetal birth weight (g) | 2910 | 3370 | 3550 |
| Foetal birth centile | 12 | 51 | 54 |
| Placental weight (g) | 490 | 525 | 545 |
Abbreviation: CS, caesarean section.
FIGURE 1.
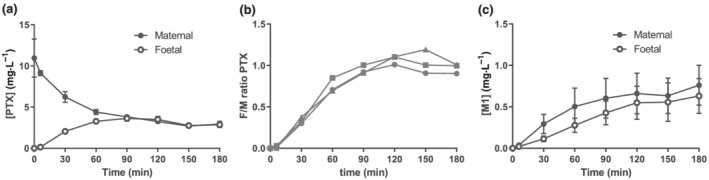
Pentoxifylline transfers completely from the maternal to the foetal circulation. Results from three ex vivo placenta perfusion experiments with 10 mg·L−1 pentoxifylline (PTX) in the maternal circulation at the start of the experiment (t = 0). (a) Absolute concentrations of pentoxifylline detected in the maternal and foetal circulations. (b) The foetal to maternal (F/M) pentoxifylline ratio. (c) Absolute concentrations of pentoxifylline metabolite M1 detected in the maternal and foetal circulations. Data are depicted as mean ± SE % relaxation of U46619 pre‐constriction.
3.2. Pentoxifylline induces vasodilation in placental arteries through A1 receptors, NO and PKG
Wire myography experiments were conducted to study the vasodilator properties of pentoxifylline. Table 2 provides the clinical characteristics of this study population. Pentoxifylline exerted vasodilation in healthy chorionic plate arteries with an Emax of 76 ± 3% of U46619 pre‐constriction and a pEC50 of −4.3 ± 0.1. Pre‐incubation of the vessel segments with the NOS inhibitor L‐NAME alone or in combination with the adenylyl cyclase inhibitor SQ22536 caused a rightward shift of the concentration–response curves in both human chorionic plate arteries (Figure 2a) and porcine coronary arteries (Figure S1A). We additionally showed that the effect of L‐NAME was endothelium‐dependent, as endothelial denudation attenuated vasodilation to pentoxifylline and L‐NAME had no effect in denuded arteries (Figure S2). SQ22536 had no effect by itself, showing that pentoxifylline‐induced vasodilation involved NO but not cAMP. Since pentoxifylline potentially also modulates adenosine signalling, we subsequently investigated its interaction with adenosine receptors. Pre‐incubation of vessel segments with the A1 receptor antagonist DPCPX attenuated pentoxifylline‐induced vasodilation, while it was not altered by inhibitors of the A2A receptor, ZM 241385 or the A2B receptor, MRS1706 (Figure 2b). To investigate whether pentoxifylline‐induced vasodilation involved specific protein kinases, experiments were also performed in the presence of the PKG inhibitor Rp‐8‐Br‐PET‐cGMPS and the PKA inhibitor Rp‐cAMPS. Pre‐incubation with Rp‐8‐Br‐PET‐cGMPS alone or in combination with Rp‐cAMPS inhibited pentoxifylline‐induced vasodilation, while Rp‐cAMPS alone had no effect (Figure 2c).
TABLE 2.
Clinical data of wire myography and placental explant experiments
| Wire myography | Explants | |||
|---|---|---|---|---|
| Healthy (n= 47) | Pre‐eclampsia (n = 20) | Healthy (n = 7) | Pre‐eclampsia (n = 7) | |
| Maternal age (years) | 33 (30–35) | 31 (29–34) | 33 (27–38) | 31 (29–40) |
| Parity | * | |||
| 0 | 14 (29.8%) | 3 (15.0%) | 2 (28.6%) | 5 (71.4%) |
| 1 | 10 (21.3%) | 12 (60.0%) | 5 (71.4%) | 1 (14.3%) |
| >1 | 23 (48.9%) | 5 (25.0%) | 0 (0.0%) | 1 (14.3%) |
| Ethnicity | ||||
| Caucasian | 31 (67.4%) | 15 (83.3%) | 4 (57.1%) | 5 (71.4%) |
| Other | 15 (32.6%) | 3 (16.7%) | 3 (42.9%) | 2 (28.6%) |
| Missing (n) | 1 | 2 | 0 | 0 |
| Body mass index (kg·m−2) | 23.2 (21.1–28.8) | 26.8 (24.8–31.2) | 23.6 (19.4–30.1) | 30.1 (24.8–35) * |
| Missing (n) | 1 | 4 | 0 | 0 |
| Highest diastolic blood pressure (mmHg) | 77 (71–80) | 108 (100–111) * | 83 (75–90) | 109 (98–135) * |
| Highest systolic blood pressure (mmHg) | 125 (120–136) | 160 (155–173) * | 129 (121–138) | 186 (140–220) * |
| Highest mean arterial pressure (mmHg) | 93 (88–99) | 124 (120–136) * | 98 (90–106) | 136 (113–163) * |
| Urinary protein/creatinine ratio (mmol·mg−1) | NM | 164 (39–482) | NM | 68 (29–813) |
| Missing (n) | 1 | 0 | ||
| Mode of delivery | ||||
| Primary caesarean section | 47 (100.0%) | 19 (95.0%) | 6 (85.7%) | 5 (71.4%) |
| Secondary caesarean section | 0 (0.0%) | 1 (5.0%) | 0 (0.0%) | 0 (0.0%) |
| Vaginally | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (14.3%) |
| Gestational age (weeks+days) | 39+0 (39+0–39+1) | 30+2 (28+1–31+4) * | 39+0 (38+4–39+2) | 31+0 (28+3–34+0) * |
| Sex | ||||
| Female | 19 (40.4%) | 12 (60.0%) | 4 (57.1%) | 3 (42.9%) |
| Male | 28 (59.6%) | 8 (40.0%) | 3 (42.9%) | 4 (57.1%) |
| Foetal birth weight (g) | 3470 (3335–3720) | 1128 (1011–1441) * | 3320 (2590–3820) | 1125 (485–3175) * |
| Foetal birth centile | * | |||
| <3rd | 0 (0.0%) | 10 (50.0%) | 0 (0.0%) | 3 (42.9%) |
| 3rd–10th | 3 (6.4%) | 6 (30.0%) | 2 (28.6%) | 1 (14.3%) |
| ≥10th | 44 (93.6%) | 4 (20.0%) | 5 (71.4%) | 3 (42.9%) |
| Placental weight (g) | 654 (561–734) | 276 (247–354) * | 510 (444–565) | 268 (152–478) * |
| Missing (n) | 9 | 1 | 1 | 1 |
Note: Numerical variables are shown as median and statistically tested by Kruskal–Wallis test. Categorical variables are shown as number of cases (%) and statistically tested by chi‐square test.
Abbreviation: NM, not measured.
P < 0.05 versus healthy.
FIGURE 2.
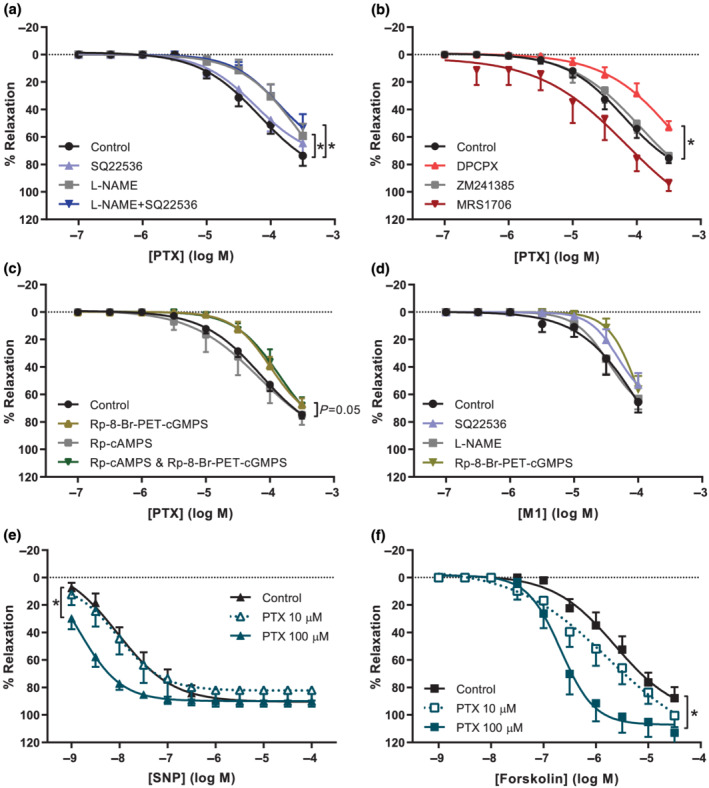
Pentoxifylline induces vasodilation and potentiates the NO and adenylyl cyclase pathways in healthy human chorionic plate arteries. Concentration–response curves (CRC) from wire myography experiments depicted as percentage relaxation of pre‐constriction by U46619. Experiments with pentoxifylline (PTX) were performed in the absence or presence of (a) NOS inhibitor L‐NAME, adenylyl cyclase inhibitor SQ22536, or both (n = 8–10), (b) A1 receptor antagonist DPCPX, A2A receptor antagonist ZM241385, A2B receptor antagonist MRS1706 (n = 6–7), or (c) PKG inhibitor Rp‐8‐Br‐PET‐cGMPS, PKA inhibitor Rp‐cAMPS, or both (n= 6–13). (d) CRC of M1 (lisofylline) without antagonists (n = 8) or with SQ22536 (n = 8), L‐NAME (n = 8), or Rp‐8‐Br‐PET‐cGMPS (n = 4). Experiments were also performed with (e) SNP in the absence (n = 9) or presence of 10 μmol·L−1 (n = 7) or 100 μmol·L−1 PTX (n= 6), and (f) forskolin in the absence (n = 8) or presence of 10 μmol·L−1 (n = 7) or 100 μmol·L−1 PTX (n = 7). Curves with antagonist were compared with curves without antagonist (control) using GLM‐RM and depicted as mean ± SE % relaxation of U46619 pre‐constriction. *P < 0.05 versus control.
The vasodilator capacities of the pentoxifylline metabolites M1, M4 and M5 were studied in a next set of experiments. M1 induced vasodilation to a similar extent as pentoxifylline, while M4 and M5 did not induce vasodilation in healthy chorionic plate arteries (Figure S3). Pre‐incubation of chorionic plate arteries with the PKG inhibitor Rp‐8‐Br‐PET‐cGMPS tended to cause a rightward shift of the M1 concentration‐response curve, although this was not statistically significant. L‐NAME and SQ22536 had no statistically significant effects on M1‐induced vasodilation (Figure 2d).
3.3. Pentoxifylline potentiates vasodilation to SNP and forskolin
Pre‐incubation of healthy chorionic plate arteries with 100 μmol·L−1 pentoxifylline resulted in a significant leftward shift of the concentration–response curves of the NO donor SNP and the adenylyl cyclase activator forskolin (Figure 2e,f). These effects were only evident at a concentration of 100 μmol·L−1 pentoxifylline, but not at 10 μmol·L−1. The same tendencies were observed in porcine coronary arteries (Figure S1B,C). These data suggest that in addition to a direct vasodilator effect of pentoxifylline, its PDE inhibitor capacity improved vasodilation through the NO and adenylyl cyclase pathways.
3.4. Pentoxifylline‐induced vasodilation is preserved in pre‐eclamptic arteries
Although pentoxifylline dilated pre‐eclamptic chorionic plate arteries to the same extent (Emax 80 ± 4%, pEC50 ‐4.3 ± 0.1) as arteries from healthy placentas, contrastingly, neither L‐NAME nor Rp‐8‐Br‐PET‐cGMPS blocked this response in pre‐eclamptic arteries (Figures 3a,b and 4a). Like in arteries from healthy placentas, SQ22536 did not alter the effect of pentoxifylline in pre‐eclamptic arteries (Figure 3a). The effects of DPCPX and endothelial denudation were studied in four pre‐eclamptic placentas due to limited availability of such tissue and the preliminary data are shown in Figure S4. In two placentas, endothelial denudation was successful and attenuated vasodilation by pentoxifylline. In three out of four experiments, DPCPX inhibited pentoxifylline‐induced vasodilation as well. Furthermore, pentoxifylline no longer potentiated the vasodilation induced by SNP or forskolin (Figure 3c,d). Responses to SNP, forskolin and the soluble guanylyl cyclase activator BAY 60‐2770 were however unaltered in pre‐eclamptic versus healthy arteries (Figure 4b–d), implying that the guanylyl and adenylyl cyclase pathways are still fully functional.
FIGURE 3.
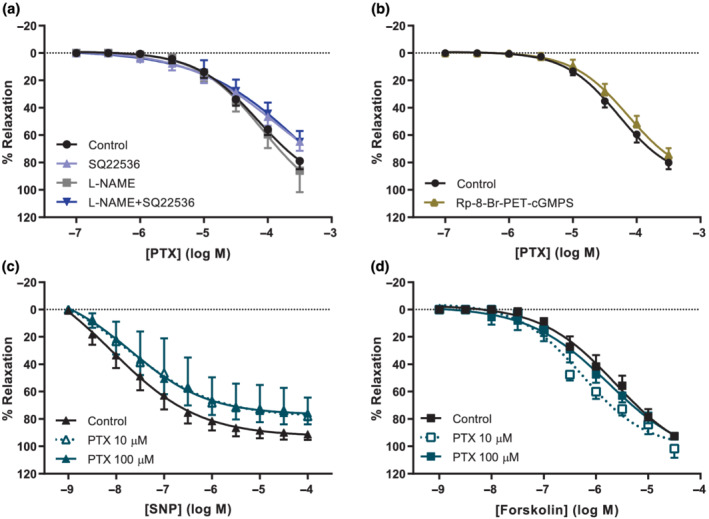
In preeclamptic chorionic plate arteries, pentoxifylline induces vasodilation but does not potentiate vasodilation by NO or adenylyl cyclase. Concentration–response curves (CRC) from wire myography experiments depicted as percentage relaxation of pre‐constriction by U46619. (a) CRC of pentoxifylline (PTX), in the absence (n = 11) or presence of L‐NAME (n = 10), SQ22536 (n = 7), or both (n = 9), or (b) in the absence (n = 6) or presence of Rp‐8‐Br‐PET‐cGMPS (n = 6). (c) CRC with SNP in the absence (n = 10) or presence of 10 μmol·L−1 (n = 4) or 100 μmol·L−1 PTX (n = 6). (d) CRC with forskolin in the absence (n = 11) or presence 10 μmol·L−1 (n = 5) or 100 μmol·L−1 PTX (n = 6). Curves with antagonist were compared with curves without antagonist (control) using GLM‐RM and depicted as mean ± SE % relaxation of U46619 pre‐constriction.
FIGURE 4.
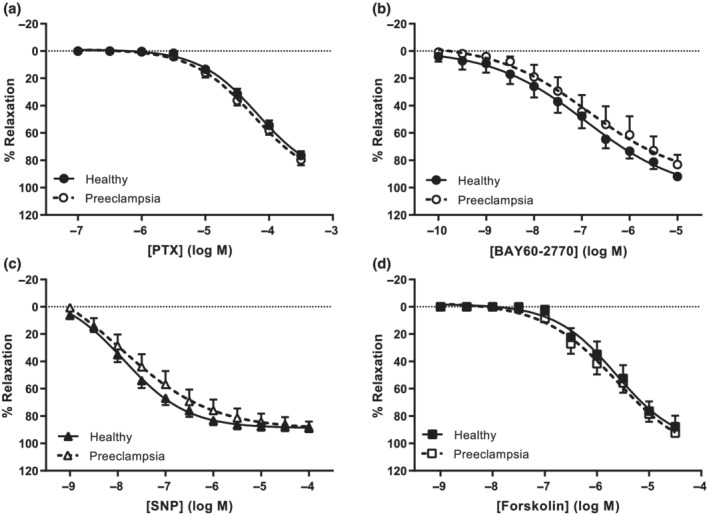
Comparisons of vasodilation responses between healthy and pre‐eclamptic chorionic plate arteries. Concentration‐response curves (CRC) from wire myography experiments depicted as percentage relaxation of pre‐constriction by U46619. (a) CRC of pentoxifylline (PTX, healthy n = 26, pre‐eclampsia n = 18). (b) CRC of soluble guanylyl cyclase activator BAY 60‐2770 (healthy n = 8, pre‐eclampsia n = 6). (c) CRC of NO donor SNP (healthy n= 19, pre‐eclampsia n= 13). (d) CRC of adenylyl cyclase activator forskolin (healthy n = 8, pre‐eclampsia n = 11). Pre‐eclampsia curves were compared with healthy curves using GLM‐RM and depicted as mean ± SE % relaxation of U46619 pre‐constriction.
3.5. L‐NAME affects baseline tension in healthy arteries only
Incubation with L‐NAME, alone or in combination with SQ22536, elevated the baseline tension in healthy, but not pre‐eclamptic arteries (Figure S5A). None of the other antagonists affected baseline tension. Constrictions to 10 nmol·L−1 U46619 were similar under all conditions (Figure S5B).
3.6. Pentoxifylline reduces cytokine secretion from healthy but not pre‐eclamptic placentas
Since pre‐eclamptic placentas are often described to be inflammatory, we compared the baseline secretions between the control and pre‐eclamptic explants. VEGF, VEGFC and IL‐5 were below the detection limit in most of the experiments and therefore exempted from further analysis. Endoglin and VEGFR−1 concentrations were higher in the medium of pre‐eclamptic compared with healthy placentas, while the other measured factors were unaltered (Table 3). Pentoxifylline reduced the secretion of CX3CL1/fractalkine, IFN‐γ, IL−1β, IL−12p70, IL−1Rα, CXCL8/IL‐8, CCL2/MCP−1 and TNF‐α from healthy placental explants (Figure 5a). In pre‐eclamptic placental explants in contrast, pentoxifylline only reduced the secretion of CCL2/MCP−1 (Figure 5b).
TABLE 3.
Baseline secretion of cytokines and (anti)angiogenic factors from pre‐eclamptic and healthy placental explants
| Healthy (n = 5–7) a | Pre‐eclampsia (n = 7) | |
|---|---|---|
| Endoglin | 261.4 (176.7, 337.1) | 630.8 (359.2, 1268.7) * |
| VEGFR−1 | 972.6 (959.1, 1819.7) | 3494.3 (2039.0, 4854.0) * |
| CCL2/MCP−1 | 205.9 (203.7, 271.8) | 97.0 (88.6, 201.3) |
| CXCL10/IP−10 | 4.2 (4.1, 10.1) | 9.3 (7.6, 20.0) |
| M‐CSF | 289.4 (163.5, 291.1) | 161.1 (132.7, 196.5) |
| PIGF | 2.3 (1.5, 3.0) | 4.0 (2.6, 5.8) |
| G‐CSF | 27.3 (21.8, 43.0) | 39.3 (25.3, 65.4) |
| IL‐6 | 396.4 (267.4, 495.3) | 276.7 (243.5, 456.4) |
| IL−1‐beta | 2.9 (1.9, 5.6) | 3.8 (3.6, 5.7) |
| TNF‐alpha | 2.6 (1.4, 5.5) | 3.6 (3.3, 5.0) |
| CX3CL1/fractalkine | 1090.7 (418.8, 2797.6) | 1479.6 (1155.9, 2077.3) |
| GM‐CSF | 5.1 (3.0, 11.9) | 7.2 (5.8, 9.9) |
| IFN‐gamma | 21.5 (6.6, 36.7) | 26.8 (21.0, 39.2) |
| IL‐4 | 64.8 (29.9, 104.3) | 74.8 (59.6, 106.6) |
| IL−12‐p70 | 77.7 (25.7, 134.6) | 96.0 (80.8, 136.2) |
| IL−16 | 64.9 (53.2, 91.7) | 75.6 (66.8, 94.7) |
| IL−18 | 19.1 (9.5, 33.3) | 21.7 (19.5, 32.2) |
| IL−1ra | 370.9 (257.3, 552.3) | 408.4 (328.0, 556.7) |
| IL‐2 | 30.9 (28.4, 51.2) | 38.4 (30.6, 55.7) |
| CXCL8/IL‐8 | 1953.3 (1791.4, 3182.4) | 2311.5 (1803.5, 3636.3) |
| IL−10 | 3.6 (1.7, 7.9) | 5.4 (4.0, 6.8) |
Note: The concentrations are given in pg·ml−1·mg−1 dry tissue weight as median (Q1, Q3). VEGF, VEGFC and IL‐5 were undetectable.
n = 7 in healthy for endoglin and GSF, and n = 5 for all other variables due to a technical error.
P < 0.05 versus healthy using a Kruskal–Wallis test.
FIGURE 5.
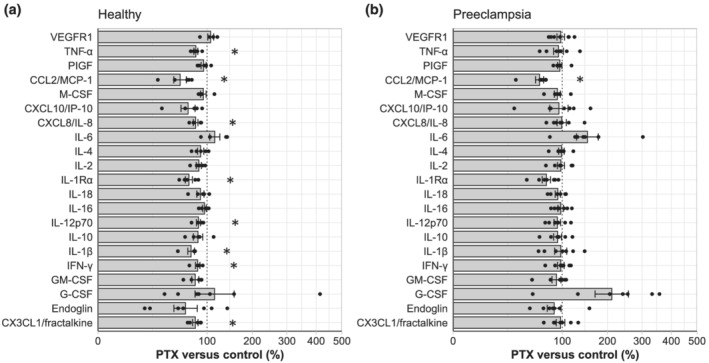
The effects of pentoxifylline on the release of proteins from placental explants. Placental explants from healthy (a, n = 5–7) and pre‐eclamptic (b, n = 7) women were incubated with or without 100 mg·L−1 (=359 μmol·L−1) pentoxifylline (PTX). The release of cytokines from placental explants with pentoxifylline was normalized to the release of cytokines from its own paired control experiment and displayed as percentage change versus control (mean ± SE). *P < 0.05 versus 100% in one‐sample Student's t test with Benjamini–Hochberg correction.
4. DISCUSSION
In a previous study, Lauterbach et al. (2012) showed that pentoxifylline can improve the foetal flow distribution in utero and neonatal outcome in the first 4 weeks of life in women with imminent preterm labour. In the present study, we investigated whether pentoxifylline could be a therapeutic strategy for pre‐eclampsia, by studying its effects in healthy and pre‐eclamptic placentas. Our data display that pentoxifylline rapidly transfers from the maternal to the foetal circulation, where it can reduce placental resistance through interaction with the A1 receptor, the NO‐cGMP‐PKG pathway and the cAMP pathway. This provides an explanation for the improved cerebro‐placental ratio in the human foetus following maternal treatment in vivo (Lauterbach et al., 2012). Additionally, pentoxifylline reduced the release of inflammatory cytokines from the placenta. In pre‐eclampsia, the direct vasodilator capacity of pentoxifylline was unaltered, although its ability to improve vasodilation through the NO‐cGMP‐PKG and cAMP pathways and the anti‐inflammatory effects of pentoxifylline were not retained.
4.1. Vascular effects of pentoxifylline
To the best of our knowledge, we are the first to show that pentoxifylline can affect vascular function in human placental arteries. Figure 6 provides a schematic illustration of the proposed mechanisms of action of pentoxifylline in chorionic plate arteries. Pentoxifylline enhanced the vasodilator effects of both SNP and forskolin in healthy chorionic plate arteries, confirming that it is capable of non‐selective inhibiting PDE (Kabbesh et al., 2012; Kaputlu & Sadan, 1994; Sheridan et al., 1997), thereby potentiating both the cGMP‐ and cAMP‐mediated responses. Enhancement of the cAMP‐mediated response seems specific for human placental tissue, as this was not observed in our experiments with porcine tissue nor in rat and equine studies (Berkenboom et al., 1991; Kabbesh et al., 2012; Kaputlu & Sadan, 1994). Remarkably, the effects of pentoxifylline as an unselective PDE inhibitor were lost in pre‐eclamptic arteries. This is reminiscent of what we observed with the selective PDE inhibitors sildenafil and vinpocetine in pre‐eclampsia (Hitzerd et al., 2019). Together with the inability of L‐NAME to inhibit the pentoxifylline‐induced relaxation, this suggests a reduced importance of the NO pathway in pre‐eclampsia. The NO pathway involves the activation of soluble guanylyl cyclase by NO, resulting in the formation of cGMP, which subsequently acts via PKG. Gao et al. (2016) showed a reduced expression and activity of soluble guanylyl cyclase and a consequently lower cGMP content in pre‐eclamptic placenta vessels. In the current study however, the responses to both the NO donor SNP and the soluble guanylyl cyclase activator BAY 60‐2770 were unaltered in pre‐eclampsia. This contrasts with previous studies (Kossenjans et al., 2000; Ong et al., 2002). A unifying concept might be that the NO‐mediated response is only modestly attenuated. Despite not matching the statistical significance that was reached in the two previous studies, the present study did display a tendency for a reduced function of the NO pathway. A further possibility is that the endogenous NO generation is diminished in pre‐eclampsia, affecting the capacity of pentoxifylline to up‐regulate such NO generation. Our observation that L‐NAME elevated the baseline tension in healthy, but not pre‐eclamptic arteries supports this concept. Irrespective of the outcome, the present data clearly show that PDE inhibition does not improve vasodilation in early‐onset pre‐eclampsia. This argues against placental PDE upregulation as a causal factor in pre‐eclampsia, in agreement with our previous work in which PDE1A and PDE5A were not found to be differentially expressed in pre‐eclamptic placentas (Hitzerd et al., 2019). It rather points to a reduced significance of placental PDE in this condition and thus inhibition of PDE does not seem a logical choice to treat placental dysfunction in pre‐eclampsia, as shown previously with sildenafil (Hitzerd et al., 2019). However, PDE inhibition might still be beneficial to target the increased serum PDE activity in pre‐eclampsia (Pinheiro da Costa et al., 2006).
FIGURE 6.
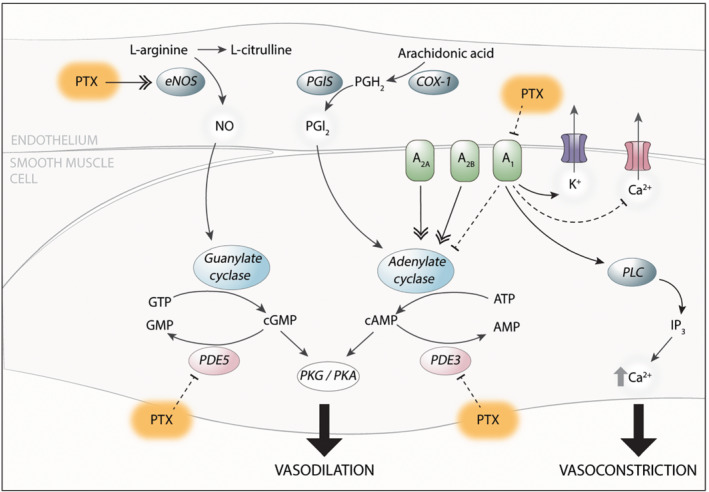
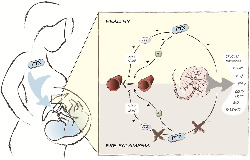
Schematic illustration of the potential effects of pentoxifylline (PTX) in placental blood vessels. Pentoxifylline can improve vasodilation of healthy chorionic plate arteries by enhancement of NOS‐mediated NO synthesis and/or inhibition of PDEs. It can also inhibit vasoconstriction through antagonism of the adenosine type 1 receptor (A1). A2A/A2B, adenosine type 2A/2B receptor; ATP, adenosine triphosphate; (c)AMP, (cyclic) adenosine monophosphate; (c)GMP, (cyclic) guanosine monophosphate; GTP, guanosine triphosphate; IP3, inositol triphosphate; PGH2, prostaglandin H2; PGI2, prostacyclin; PKG/PKA, protein kinase G/A; PLC, phospholipase C
Animal work supports a range of mechanisms that might underlie the vasodilator function of pentoxifylline (Berkenboom et al., 1991; Crowell et al., 1990; Hansen, 1994; Hoeffner et al., 1989; Kabbesh et al., 2012; Kaputlu & Sadan, 1994; Marukawa et al., 1994). Our observation that pentoxifylline enhanced endogenous NO‐cGMP‐PKG signalling, at least in healthy placentas, mimics similar findings in rat mesenteric arteries (Hansen, 1994), rat aorta (Marukawa et al., 1994) and equine digital veins (Kabbesh et al., 2012). Like in equine digital veins, vasodilation by pentoxifylline was not affected by inhibition of the cAMP pathway and thus did not depend on potentiation of endogenous cAMP (Kabbesh et al., 2012), although pentoxifylline did potentiate exogenous activation of the cAMP pathway with forskolin, probably due to its PDE‐inhibitory capacity.
We additionally confirmed that the metabolite M1, which was converted from pentoxifylline in our ex vivo placenta perfusion experiments, evokes vasodilation (Ruddock & Hirst, 2005) via a mechanism that involves PKG but not cAMP. The metabolites M4 and M5 did not have any vasodilator properties in placental vessels and were not formed by the placenta ex vivo.
Given the absence of pentoxifylline‐induced NO‐cGMP‐PKG signalling in pre‐eclamptic arteries, while its net relaxant effects remained unaltered, alternative pathways must have contributed to the observed relaxation in pre‐eclampsia. Here, interaction of pentoxifylline with adenosine receptors might come into play. Indeed, pentoxifylline was shown to antagonize the A1 receptor and thereby block coronary vascular constriction (Sato et al., 2005). A2 receptor antagonism would be expected to exert the opposite effect (Farmer et al., 1988; Sato et al., 2005), but the affinity of pentoxifylline for A1 receptors is higher than that for A2 receptors (Schwabe et al., 1985). Importantly, the concentrations required to block adenosine receptors are around one order of magnitude higher than those needed to block cAMP‐degrading PDE (Ki: 180 ± 2 versus 37.7 ± 3.5 μmol·L−1) (Miyamoto et al., 1993). Our data with A1, A2A and A2B receptor antagonists support an effect via A1 receptors at pentoxifylline concentrations that are 10–20 times higher than those reported in patients (Beermann et al., 1985; Lauterbach et al., 2012; Smith et al., 1986). Although the inhibitory function of the A1 receptor has been suggested to involve adenylyl cyclase (Sato et al., 2005), the lack of effect of the adenylyl cyclase inhibitor SQ22536 towards pentoxifylline‐induced vasodilation argues against this concept. Therefore, in chorionic plate arteries, A1 receptor effects might be accomplished through other mechanisms including (1) elevation of inositol 1,4,5‐trisphosphate and intracellular calcium levels through activation of phospholipase C‐β, and (2) interaction with pertussis toxin‐sensitive G proteins and KATP channels, as well as Q‐, P‐,and N‐type Ca2+ channels (Borea et al., 2018) (Figure 6). A further alternative is that pentoxifylline does not antagonize the constrictor effects of endogenous adenosine via A1 receptors, but instead directly induces vasodilation via these receptors. Future studies should evaluate these possibilities, as well as their potential alterations in pre‐eclampsia. Preliminary data support that the A1 receptor‐dependent effect of pentoxifylline can be observed in pre‐eclamptic arteries (Figure S4).
4.2. Anti‐inflammatory effects of pentoxifylline
The anti‐inflammatory effects of pentoxifylline in healthy placental explants were absent in pre‐eclamptic placentas but might have resulted from its conversion into M1 (van Furth et al., 1997; Wójcik‐Pszczoła et al., 2016; Yang et al., 2005). In a mouse model of LPS‐induced pulmonary inflammation, the anti‐inflammatory effects of pentoxifylline were suggested to be A2A receptor‐dependent (Konrad et al., 2013). Yet, the vasodilator effects of pentoxifylline in the present study involved A1 receptor antagonism and PDE inhibition. Only the latter effect was absent in pre‐eclampsia. A more likely explanation of our observations is therefore that the anti‐inflammatory effects involve PDE inhibition. Importantly, our data differ from those of Speer et al. (2017), who mimicked pre‐eclamptic conditions by exposing healthy placental explants to LPS. This illustrates that LPS‐induced inflammation does not fully resemble pre‐eclampsia. The pre‐eclamptic placental explants in the present study did maintain their in vivo phenotype, evidenced by increased endoglin and VEGFR−1 secretions, as expected based on elevated circulating levels of endoglin and soluble VEGFR−1 in women with pre‐eclampsia (Levine et al., 2006; Romero et al., 2008). The secretion of other measured cytokines and angiogenic factors was not different between the two conditions. It should be noted that this was based on the unadjusted P values since our study was not powered to detect differences between the baseline secretion of proteins from pre‐eclamptic and healthy explants. Nevertheless, our results indicate that the placenta may not be the source of the elevated circulating cytokine and chemokine concentrations (including CCL2/MCP−1) in women with pre‐eclampsia (Ma et al., 2019; Szarka et al., 2010). This conclusion agrees with our recent data that depict a suppressed rather than pro‐inflammatory placental immune system in early‐onset pre‐eclampsia (Broekhuizen et al., 2021). It additionally implies that the pro‐inflammatory state in pre‐eclampsia might originate in tissue other than the placenta, for example it may reflect generalized endothelial dysfunction. Whether pentoxifylline would act outside the placenta cannot be concluded based on our results.
Nevertheless, in the pre‐eclamptic placenta, the potentiating effects of pentoxifylline on the cGMP and cAMP pathway, as well as the anti‐inflammatory effects, have disappeared. At the same time, its direct relaxant effects remained fully intact and since these involved A1 receptors, future studies should focus on adenosine receptor responsiveness in pre‐eclampsia, in particular because elevated maternal and foetal adenosine levels have been reported in pre‐eclampsia and related to placental insufficiency (Espinoza et al., 2011; Takeuchi et al., 2001; Yoneyama et al., 1996; Yoneyama et al., 2002).
In conclusion, we have shown that pentoxifylline exerts vasodilator and anti‐inflammatory effects in the human placenta, involving the NO and cAMP pathways, as well as the A1 receptor. Not all these effects are preserved in pre‐eclampsia, questioning its application in this disease. Yet, an obvious advantage of pentoxifylline as a novel treatment is that it is already available for clinical use allowing quick implementation. To the best of our knowledge, no teratogenic effects have been observed in either animal or clinical studies. It is also already safely used in preterm infants, suggesting less risks of treatment during the third trimester of pregnancy. Pentoxifylline could nevertheless provide an opportunity to treat other placenta‐related diseases such as foetal growth restriction or chorioamnionitis. Here it should be noted that pentoxifylline is metabolized by the placenta and does reach the foetus, so that a thorough pharmacokinetic study in pregnant women is required to define safe dosing regimens. Regarding pre‐eclampsia, our observation that pentoxifylline induces vasodilation via blockade of the adenosine A1 receptor, combined with evidence for elevated adenosine levels in this disorder (Espinoza et al., 2011; Takeuchi et al., 2001; Yoneyama et al., 1996; Yoneyama et al., 2002), raises the possibility that selective adenosine A1 receptor antagonists might serve as new therapeutic tools.
CONFLICT OF INTEREST
We have no conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
The study was designed by MB, AD, SS and EH. Data collection was performed by MB, RV, MS and EH, while data analysis was performed by MB and EH. All authors contributed to interpreting the data and writing the article and approved the submitted version.
PERMISSION TO REPRODUCE MATERIAL FROM OTHER SOURCES
Not applicable.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for Design and Analysis and as recommended by funding agencies, publishers and other organizations engaged with supporting research.
Supporting information
Table S1. Summary table of the compounds used for wire myography experiments.
Figure S1. Results of the wire myography experiments using porcine coronary arteries. Concentration‐response curves (CRC) from wire myography experiments displayed as % relaxation of U46619 pre‐contraction. A) CRC of pentoxifylline (PTX) in control segments (circles) or after incubation with SQ22536 (triangles) or L‐NAME (squares, n = 9). B) CRC of sodium nitroprusside (SNP) in control segments (black triangles) or after incubation with 100 μmol l−1 pentoxifylline (cyan triangles). Pentoxifylline itself had no significant effect but the interaction between the SNP concentration and % relaxation was significantly different (n = 5). C) CRC of forskolin in control segments (black squares) or after incubation with 100 μmol l−1 (cyan squares, n = 5‐6). Curves with antagonist were compared to curves without antagonist (control) using GLM‐RM and data are depicted as mean ± SE % relaxation of U46619 pre‐constriction. * P < 0.05.
Figure S2. Exploratory data: Pentoxifylline‐induced vasodilation is endothelium dependent in healthy placenta arteries. Concentration‐response curves of pentoxifylline constructed using wire myography experiments in intact segments as well as in segments of which the endothelium was removed (Denuded) and depicted as percentage relaxation of pre‐constriction by U46619. Denudation attenuated vasodilation to pentoxifylline (black open squares, n = 4), similar to L‐NAME (grey filled circles, n = 3), when compared to intact artery segments (black filled squares, n = 4). L‐NAME did not further inhibit vasodilation in denuded arteries (grey open squares, n =4). Data are depicted as mean ± SE % relaxation of U46619 pre‐constriction.
Figure S3. The effects of pentoxifylline metabolites in healthy chorionic plate arteries. Concentration‐response curves of pentoxifylline and its metabolites M1 (lisofylline), M4 and M5 from wire myography experiments with human chorionic plate arteries (n = 6−12). Data are depicted as mean ± SE % relaxation of U46619 pre‐constriction. Comparisons of curves with antagonist to curves without antagonist (control) in GLM‐RM did not result in statistically significant results.
Figure S4. The effects of DPCPX and endothelial denudation on pentoxifylline‐induced vasodilation in four individual experiments with chorionic plate arteries from pregnancies with pre‐eclampsia (PE). Data are depicted as % relaxation of U46619 pre‐constriction and each data point represents the average value from two duplicate experiments.
Figure S5. The baseline effects of antagonists on healthy and pre‐eclamptic chorionic plate arteries in wire myography experiments. A) Incubation with L‐NAME, alone or in combination with SQ22536 elevated the baseline tension when compared to the vessels without antagonist (control) in healthy placental arteries only. B) None of the antagonist altered the contractile response to 10 nmol l−1 U46619. * P < 0.05 in one sample Student's t tests with Benjamini‐Hogbergh correction versus 0.
ACKNOWLEDGEMENTS
We would like to thank all women who donated their placenta.
Broekhuizen, M. , de Vries, R. , Smits, M. A. W. , Dik, W. A. , Schoenmakers, S. , Koch, B. C. P. , Merkus, D. , Reiss, I. K. M. , Danser, A. H. J. , Simons, S. H. P. , & Hitzerd, E. (2022). Pentoxifylline as a therapeutic option for pre‐eclampsia: a study on its placental effects. British Journal of Pharmacology, 179(22), 5074–5088. 10.1111/bph.15931
Part of this research was funded by the Netherlands Organisation for Health Research and Development ZonMW (grant number 848082002).
Funding information ZonMW, Grant/Award Number: 848082002
DATA AVAILABILITY STATEMENT
All supporting data are available within the article and in the Supporting Information.
REFERENCES
- Alexander, S. P. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Southan, C. , Davies, J. A. , Abbracchio, M. P. , Alexander, W. , Al‐hosaini, K. , Bäck, M. , Barnes, N. M. , Bathgate, R. , … Ye, R. D. (2021). THE CONCISE GUIDE TO PHARMACOLOGY 2021/22: G protein‐coupled receptors. British Journal of Pharmacology, 178(S1), S27–S156. 10.1111/bph.15538 [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Southan, C. , Davies, J. A. , Beuve, A. , Brouckaert, P. , Bryant, C. , Burnett, J. C. , Farndale, R. W. , Friebe, A. , Garthwaite, J. , … Waldman, S. A. (2021). THE CONCISE GUIDE TO PHARMACOLOGY 2021/22: Catalytic receptors. British Journal of Pharmacology, 178(S1), S264–S312. 10.1111/bph.15541 [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Southan, C. , Davies, J. A. , Boison, D. , Burns, K. E. , Dessauer, C. , Gertsch, J. , Helsby, N. A. , Izzo, A. A. , Koesling, D. , … Wong, S. S. (2021). THE CONCISE GUIDE TO PHARMACOLOGY 2021/22: Enzymes. British Journal of Pharmacology, 178(S1), S313–S411. 10.1111/bph.15542 [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Mathie, A. , & Peters, J. A. (2011). Guide to receptors and channels (GRAC), 5th edition. British Journal of Pharmacology, 164(s1), S1–S2. 10.1111/j.1476-5381.2011.01649_1.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy . (2013). Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' task force on hypertension in pregnancy. Obstetrics and Gynecology, 122(5), 1122–1131. 10.1097/01.AOG.0000437382.03963.88 [DOI] [PubMed] [Google Scholar]
- Azimi, A. , Ziaee, S. M. , Farhadi, P. , & Sagheb, M. M. (2015). Hypothesis: Pentoxifylline explores new horizons in treatment of preeclampsia. Medical Hypotheses, 85(4), 468–474. 10.1016/j.mehy.2015.06.031 [DOI] [PubMed] [Google Scholar]
- Azizieh, F. , Raghupathy, R. , & Makhseed, M. (2005). Maternal cytokine production patterns in women with pre‐eclampsia. American Journal of Reproductive Immunology, 54(1), 30–37. 10.1111/j.1600-0897.2005.00278.x [DOI] [PubMed] [Google Scholar]
- Beermann, B. , Ings, R. , Månsby, J. , Chamberlain, J. , & McDonald, A. (1985). Kinetics of intravenous and oral pentoxifylline in healthy subjects. Clinical Pharmacology and Therapeutics, 37(1), 25–28. 10.1038/clpt.1985.6 [DOI] [PubMed] [Google Scholar]
- Bellamy, L. , Casas, J. P. , Hingorani, A. D. , & Williams, D. J. (2007). Pre‐eclampsia and risk of cardiovascular disease and cancer in later life: Systematic review and meta‐analysis. BMJ, 335(7627), 974. 10.1136/bmj.39335.385301.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkenboom, G. , Fang, Z.‐Y. , Unger, P. , Goldman, M. , & Fontaine, J. (1991). Endothelium‐dependent effects of pentoxifylline in rat aorta. European Journal of Pharmacology, 193(1), 81–86. 10.1016/0014-2999(91)90203-3 [DOI] [PubMed] [Google Scholar]
- Bhat, V. B. , & Madyastha, K. M. (2001). Antioxidant and radical scavenging properties of 8‐oxo derivatives of xanthine drugs pentoxifylline and lisofylline. Biochemical and Biophysical Research Communications, 288(5), 1212–1217. 10.1006/bbrc.2001.5922 [DOI] [PubMed] [Google Scholar]
- Borea, P. A. , Gessi, S. , Merighi, S. , Vincenzi, F. , & Varani, K. (2018). Pharmacology of adenosine receptors: The state of the art. Physiological Reviews, 98(3), 1591–1625. 10.1152/physrev.00049.2017 [DOI] [PubMed] [Google Scholar]
- Broekhuizen, M. , Hitzerd, E. , van den Bosch, T. P. P. , Dumas, J. , Verdijk, R. M. , van Rijn, B. B. , Danser, A. H. J. , van Eijck, C. H. J. , Reiss, I. K. M. , & Mustafa, D. A. M. (2021). The placental innate immune system is altered in early‐onset preeclampsia, but not in late‐onset preeclampsia. Frontiers in Immunology, 12(5386), 780043. 10.3389/fimmu.2021.780043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell, R. E. , Chick, T. W. , & Reed, W. P. (1990). Pentoxifylline relaxes isolated pulmonary arteries after preconstriction with norepinephrine. Respiration, 57(1), 45–50. 10.1159/000195818 [DOI] [PubMed] [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , Giembycz, M. A. , Hoyer, D. , Insel, P. A. , Izzo, A. A. , Ji, Y. , MacEwan, D. J. , Sobey, C. G. , Stanford, S. C. , Teixeira, M. M. , Wonnacott, S. , & Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175(7), 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza, J. , Espinoza, A. F. , & Power, G. G. (2011). High fetal plasma adenosine concentration: A role for the fetus in preeclampsia? American Journal of Obstetrics and Gynecology, 205(5), 485.e24–485.e27. 10.1016/j.ajog.2011.06.034 [DOI] [PubMed] [Google Scholar]
- Farmer, S. G. , Canning, B. J. , & Wilkins, D. E. (1988). Adenosine receptor‐mediated contraction and relaxation of guinea‐pig isolated tracheal smooth muscle: Effects of adenosine antagonists. British Journal of Pharmacology, 95(2), 371–378. 10.1111/j.1476-5381.1988.tb11655.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm, B. B. , Ijzerman, A. P. , Jacobson, K. A. , Klotz, K.‐N. , & Linden, J. (2001). International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacological Reviews, 53(4), 527. 10.1097/00005344-200002000-00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriëls, G. , Endlich, K. , Rahn, K. H. , Schlatter, E. , & Steinhausen, M. (2000). In vivo effects of diadenosine polyphosphates on rat renal microcirculation. Kidney International, 57(6), 2476–2484. 10.1046/j.1523-1755.2000.00106.x [DOI] [PubMed] [Google Scholar]
- Gao, Q. , Tang, J. , Li, N. , Zhou, X. , Zhu, X. , Li, W. , Liu, B. , Feng, X. , Tao, J. , Han, B. , Zhang, H. , Sun, M. , & Xu, Z. (2016). New conception for the development of hypertension in preeclampsia. Oncotarget, 7(48), 78387–78395. 10.18632/oncotarget.13410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg, R. L. , Culhane, J. F. , Iams, J. D. , & Romero, R. (2008). Epidemiology and causes of preterm birth. Lancet, 371(9606), 75–84. 10.1016/S0140-6736(08)60074-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramellini, D. , Folli, M. C. , Raboni, S. , Vadora, E. , & Merialdi, A. (1992). Cerebral‐umbilical Doppler ratio as a predictor of adverse perinatal outcome. Obstetrics and Gynecology, 79(3), 416–420. 10.1097/00006250-199203000-00018 [DOI] [PubMed] [Google Scholar]
- Hansen, P. R. (1994). In vitro studies on responses to pentoxifylline and aminophylline of rat mesenteric resistance vessels. European Journal of Pharmacology, 261(1), 105–110. 10.1016/0014-2999(94)90307-7 [DOI] [PubMed] [Google Scholar]
- Hasan, A. Z. M. A. , Abebe, W. , & Mustafa, S. J. (2000). Antagonism of coronary artery relaxation by adenosine A2A‐receptor antagonist ZM241385. Journal of Cardiovascular Pharmacology, 35(2), 322–325. 10.1097/00005344-200002000-00022 [DOI] [PubMed] [Google Scholar]
- Hitzerd, E. , Broekhuizen, M. , Mirabito Colafella, K. M. , Glisic, M. , de Vries, R. , Koch, B. C. P. , de Raaf, M. A. , Merkus, D. , Schoenmakers, S. , Reiss, I. K. M. , Danser, A. H. J. , & Simons, S. H. P. (2019). Placental effects and transfer of sildenafil in healthy and preeclamptic conditions. eBioMedicine, 45, 447–455. 10.1016/j.ebiom.2019.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffner, U. , Aarhus, L. L. , Katusic, Z. S. , & Vanhoutte, P. M. (1989). Pharmacology of pentoxifylline in isolated canine arteries and veins. Journal of Cardiovascular Pharmacology, 14(6), 899–907. 10.1097/00005344-198912000-00017 [DOI] [PubMed] [Google Scholar]
- Hunt, J. S. , Soares, M. J. , Lei, M. G. , Smith, R. N. , Wheaton, D. , Atherton, R. A. , & Morrison, D. C. (1989). Products of lipopolysaccharide‐activated macrophages (tumor necrosis factor‐alpha, transforming growth factor‐beta) but not lipopolysaccharide modify DNA synthesis by rat trophoblast cells exhibiting the 80‐kDa lipopolysaccharide‐binding protein. Journal of Immunology, 143(5), 1606–1613. https://www.jimmunol.org/content/143/5/1606.long [PubMed] [Google Scholar]
- Jonsson, Y. , Rubèr, M. , Matthiesen, L. , Berg, G. , Nieminen, K. , Sharma, S. , Ernerudh, J. , & Ekerfelt, C. (2006). Cytokine mapping of sera from women with preeclampsia and normal pregnancies. Journal of Reproductive Immunology, 70(1‐2), 83–91. 10.1016/j.jri.2005.10.007 [DOI] [PubMed] [Google Scholar]
- Kabbesh, N. , Gogny, M. , Chatagnon, G. , Noireaud, J. , Thorin, C. , Desfontis, J. C. , & Mallem, M. Y. (2012). Vasodilatory effect of pentoxifylline in isolated equine digital veins. The Veterinary Journal, 192(3), 368–373. 10.1016/j.tvjl.2011.09.005 [DOI] [PubMed] [Google Scholar]
- Kaputlu, I. , & Sadan, G. (1994). Pentoxifylline‐induced vasodilatation is not endothelium‐dependent in rabbit aorta. Journal of Basic and Clinical Physiology and Pharmacology, 5(3–4), 295–304. 10.1515/jbcpp.1994.5.3-4.295 [DOI] [PubMed] [Google Scholar]
- Konrad, F. M. , Neudeck, G. , Vollmer, I. , Ngamsri, K. C. , Thiel, M. , & Reutershan, J. (2013). Protective effects of pentoxifylline in pulmonary inflammation are adenosine receptor A2A dependent. The FASEB Journal, 27(9), 3524–3535. 10.1096/fj.13-228122 [DOI] [PubMed] [Google Scholar]
- Kossenjans, W. , Eis, A. , Sahay, R. , Brockman, D. , & Myatt, L. (2000). Role of peroxynitrite in altered fetal‐placental vascular reactivity in diabetes or preeclampsia. American Journal of Physiology. Heart and Circulatory Physiology, 278(4), H1311–H1319. 10.1152/ajpheart.2000.278.4.H1311 [DOI] [PubMed] [Google Scholar]
- Lauterbach, R. , Pawlik, D. , Kowalczyk, D. , Ksycínski, W. , Helwich, E. , & Zembala, M. (1999). Effect of the immunomodulating agent, pentoxifylline, in the treatment of sepsis in prematurely delivered infants: A placebo‐controlled, double‐blind trial. Critical Care Medicine, 27(4), 807–814. 10.1097/00003246-199904000-00042 [DOI] [PubMed] [Google Scholar]
- Lauterbach, R. , Rytlewski, K. , Pawlik, D. , Hurkała, J. , Wójtowicz, A. , Bręborowicz, G. , & Szymankiewicz, M. (2012). Effect of Pentoxifylline, administered in preterm labour, on the foetal–placental circulation and neonatal outcome: A randomized, prospective pilot study. Basic & Clinical Pharmacology & Toxicology, 110(4), 342–346. 10.1111/j.1742-7843.2011.00809.x [DOI] [PubMed] [Google Scholar]
- Levine, R. J. , Lam, C. , Qian, C. , Yu, K. F. , Maynard, S. E. , Sachs, B. P. , Sibai, B. M. , Epstein, F. H. , Romero, R. , Thadhani, R. , Karumanchi, S. A. , & CPEP Study Group . (2006). Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. The New England Journal of Medicine, 355(10), 992–1005. 10.1056/NEJMoa055352 [DOI] [PubMed] [Google Scholar]
- Ma, Y. , Ye, Y. , Zhang, J. , Ruan, C. C. , & Gao, P. J. (2019). Immune imbalance is associated with the development of preeclampsia. Medicine (Baltimore), 98(14), e15080. 10.1097/MD.0000000000015080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marukawa, S. , Hatake, K. , Wakabayashi, I. , & Hishida, S. (1994). Vasorelaxant effects of oxpentifylline and theophylline on rat isolated aorta. Journal of Pharmacy and Pharmacology, 46(5), 342–345. 10.1111/j.2042-7158.1994.tb03809.x [DOI] [PubMed] [Google Scholar]
- Mathiesen, L. , Mose, T. , Mørck, T. J. , Nielsen, J. K. S. , Nielsen, L. K. , Maroun, L. L. , Dziegiel, M. H. , Larsen, L. G. , & Knudsen, L. E. (2010). Quality assessment of a placental perfusion protocol. Reproductive Toxicology, 30(1), 138–146. 10.1016/j.reprotox.2010.01.006 [DOI] [PubMed] [Google Scholar]
- Miyamoto, K. , Yamamoto, Y. , Kurita, M. , Sakai, R. , Konno, K. , Sanae, F. , Ohshima, T. , Takagi, K. , & Hasegawa, T. (1993). Bronchodilator activity of xanthine derivatives substituted with functional groups at the 1‐ or 7‐position. Journal of Medicinal Chemistry, 36(10), 1380–1386. 10.1021/jm00062a010 [DOI] [PubMed] [Google Scholar]
- Munno, I. , Chiechi, L. M. , Lacedra, G. , Putignano, G. , Patimo, C. , Lobascio, A. , & Loizzi, P. (1999). Spontaneous and induced release of prostaglandins, interleukin (IL)−1beta, IL‐6, and tumor necrosis factor‐alpha by placental tissue from normal and preeclamptic pregnancies. American Journal of Reproductive Immunology, 42(6), 369–374. 10.1111/j.1600-0897.1999.tb00114.x [DOI] [PubMed] [Google Scholar]
- Nawroth, P. P. , & Stern, D. M. (1986). Modulation of endothelial cell hemostatic properties by tumor necrosis factor. The Journal of Experimental Medicine, 163(3), 740–745. 10.1084/jem.163.3.740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong, S. S. , Crocker, I. P. , Warren, A. Y. , & Baker, P. N. (2002). Functional characteristics of chorionic plate placental arteries from normal pregnant women and women with pre‐eclampsia. Hypertension in Pregnancy, 21(3), 175–183. 10.1081/prg-120015844 [DOI] [PubMed] [Google Scholar]
- Pinheiro da Costa, B. E. , Scocco, C. , Poli de Figueiredo, C. E. , & Guimarães, J. A. (2006). Maternal medicine: Increased serum phosphodiesterase activity in women with pre‐eclampsia. BJOG: An International Journal of Obstetrics & Gynaecology, 113(5), 577–579. 10.1111/j.1471-0528.2006.00916.x [DOI] [PubMed] [Google Scholar]
- R Core Team . (2021). R: A language and environment for statistical computing. In R Foundation for Statistical Computing. https://www.R-project.org/
- Raghupathy, R. , & Raghupathy, R. (2013). Cytokines as key players in the pathophysiology of preeclampsia. Medical Principles and Practice, 22(Suppl. 1), 8–19. 10.1159/000354200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero, R. , Nien, J. K. , Espinoza, J. , Todem, D. , Fu, W. , Chung, H. , Kusanovic, J. P. , Gotsch, F. , Erez, O. , Mazaki‐Tovi, S. , Gomez, R. , Edwin, S. , Chaiworapongsa, T. , Levine, R. J. , & Karumanchi, S. A. (2008). A longitudinal study of angiogenic (placental growth factor) and anti‐angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor−1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. The Journal of Maternal‐Fetal & Neonatal Medicine, 21(1), 9–23. 10.1080/14767050701830480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddock, M. W. , & Hirst, D. G. (2005). Pentoxifylline inhibits agonist‐induced vasoconstriction in vascular smooth muscle and spontaneous peristalsis in isolated ileum. Oncology Research, 15(2), 81–86. https://www.ncbi.nlm.nih.gov/pubmed/16119005, 10.3727/096504005775082011 [DOI] [PubMed] [Google Scholar]
- Salhiyyah, K. , Forster, R. , Senanayake, E. , Abdel‐Hadi, M. , Booth, A. , Michaels, J. A. , & Cochrane Vascular Group . (2015). Pentoxifylline for intermittent claudication. Cochrane Database of Systematic Reviews, 9, CD005262. 10.1002/14651858.CD005262.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salman, S. , Hibbert, J. , Page‐Sharp, M. , Manning, L. , Simmer, K. , Doherty, D. A. , Patole, S. , Batty, K. T. , & Strunk, T. (2019). Effects of maturation and size on population pharmacokinetics of pentoxifylline and its metabolites in very preterm infants with suspected late‐onset sepsis or necrotizing enterocolitis: A pilot study incorporating clinical outcomes. British Journal of Clinical Pharmacology, 85(1), 147–159. 10.1111/bcp.13775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, A. , Terata, K. , Miura, H. , Toyama, K. , Loberiza, F. R. , Hatoum, O. A. , Saito, T. , Sakuma, I. , & Gutterman, D. D. (2005). Mechanism of vasodilation to adenosine in coronary arterioles from patients with heart disease. American Journal of Physiology. Heart and Circulatory Physiology, 288(4), H1633–H1640. 10.1152/ajpheart.00575.2004 [DOI] [PubMed] [Google Scholar]
- Schwabe, U. , Ukena, D. , & Lohse, M. J. (1985). Xanthine derivatives as antagonists at A1 and A2 adenosine receptors. Naunyn‐Schmiedeberg's Archives of Pharmacology, 330(3), 212–221. 10.1007/bf00572436 [DOI] [PubMed] [Google Scholar]
- Shabaan, A. E. , Nasef, N. , Shouman, B. , Nour, I. , Mesbah, A. , & Abdel‐Hady, H. (2015). Pentoxifylline therapy for late‐onset sepsis in preterm infants: A randomized controlled trial. The Pediatric Infectious Disease Journal, 34(6), e143–e148. 10.1097/INF.0000000000000698 [DOI] [PubMed] [Google Scholar]
- Sheridan, B. C. , McIntyre, R. C. , Meldrum, D. R. , & Fullerton, D. A. (1997). Pentoxifylline treatment attenuates pulmonary vasomotor dysfunction in acute lung injury. Journal of Surgical Research, 71(2), 150–154. 10.1006/jsre.1997.5144 [DOI] [PubMed] [Google Scholar]
- Smith, R. V. , Waller, E. S. , Doluisio, J. T. , Bauza, M. T. , Puri, S. K. , Ho, I. , & Lassman, H. B. (1986). Pharmacokinetics of orally administered pentoxifylline in humans. Journal of Pharmaceutical Sciences, 75(1), 47–52. 10.1002/jps.2600750111 [DOI] [PubMed] [Google Scholar]
- Speer, E. M. , Lin, X. , Murthy, A. , Hou, W. , Islam, S. , & Hanna, N. (2017). Pentoxifylline inhibits lipopolysaccharide‐induced inflammatory mediators in human second trimester placenta explants. Placenta, 58, 60–66. 10.1016/j.placenta.2017.08.006 [DOI] [PubMed] [Google Scholar]
- Stark, J. M. (1993). Pre‐eclampsia and cytokine induced oxidative stress. British Journal of Obstetrics and Gynaecology, 100(2), 105–109. 10.1111/j.1471-0528.1993.tb15203.x [DOI] [PubMed] [Google Scholar]
- Steegers, E. A. , von Dadelszen, P. , Duvekot, J. J. , & Pijnenborg, R. (2010). Pre‐eclampsia. Lancet, 376(9741), 631–644. 10.1016/S0140-6736(10)60279-6 [DOI] [PubMed] [Google Scholar]
- Szarka, A. , Rigó, J. , Lázár, L. , Bekő, G. , & Molvarec, A. (2010). Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunology, 11(1), 59. 10.1186/1471-2172-11-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi, T. , Yoneyama, Y. , Suzuki, S. , Sawa, R. , Otsubo, Y. , & Araki, T. (2001). Regulation of platelet aggregation in vitro by plasma adenosine in preeclampsia. Gynecologic and Obstetric Investigation, 51(1), 36–39. 10.1159/000052888 [DOI] [PubMed] [Google Scholar]
- Tálosi, G. , Németh, I. , & Pintér, S. (2001). Inhibitory effects of methylxanthines on the pre‐eclamptic‐like symptoms in ewes. European Journal of Obstetrics, Gynecology, and Reproductive Biology, 99(1), 25–32. 10.1016/s0301-2115(01)00351-7 [DOI] [PubMed] [Google Scholar]
- van der Merwe, J. L. , Hall, D. R. , Wright, C. , Schubert, P. , & Grove, D. (2010). Are early and late preeclampsia distinct subclasses of the disease—What does the placenta reveal? Hypertension in Pregnancy, 29(4), 457–467. 10.3109/10641950903572282 [DOI] [PubMed] [Google Scholar]
- van Furth, A. M. , Verhard‐Seijmonsbergen, E. M. , van Furth, R. , & Langermans, J. A. (1997). Effect of lisofylline and pentoxifylline on the bacterial‐stimulated production of TNF‐alpha, IL−1 beta IL−10 by human leucocytes. Immunology, 91(2), 193–196. 10.1046/j.1365-2567.1997.00252.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham, H. (2016). ggplot2: Elegant graphics for data analysis. Springer‐Verlag. 10.1007/978-3-319-24277-4 [DOI] [Google Scholar]
- Wójcik‐Pszczoła, K. , Hińcza, K. , Wnuk, D. , Kądziołka, D. , Koczurkiewicz, P. , Sanak, M. , Madeja, Z. , Pękala, E. , & Michalik, M. (2016). Pentoxifylline and its active metabolite lisofylline attenuate transforming growth factor β1‐induced asthmatic bronchial fibroblast‐to‐myofibroblast transition. Acta Biochimica Polonica, 63(3), 437–442. 10.18388/abp.2016_1357 [DOI] [PubMed] [Google Scholar]
- Xi, J. , McIntosh, R. , Shen, X. , Lee, S. , Chanoit, G. , Criswell, H. , Zvara, D. A. , & Xu, Z. (2009). Adenosine A2A and A2B receptors work in concert to induce a strong protection against reperfusion injury in rat hearts. Journal of Molecular and Cellular Cardiology, 47(5), 684–690. 10.1016/j.yjmcc.2009.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Z. , Chen, M. , Ellett, J. D. , Carter, J. D. , Brayman, K. L. , & Nadler, J. L. (2005). Inflammatory blockade improves human pancreatic islet function and viability. American Journal of Transplantation, 5(3), 475–483. 10.1111/j.1600-6143.2005.00707.x [DOI] [PubMed] [Google Scholar]
- Yoneyama, Y. , Sawa, R. , Suzuki, S. , Shin, S. , Power, G. G. , & Araki, T. (1996). The relationship between uterine artery Doppler velocimetry and umbilical venous adenosine levels in pregnancies complicated by preeclampsia. American Journal of Obstetrics and Gynecology, 174(1), 267–271. 10.1016/S0002-9378(96)70406-4 [DOI] [PubMed] [Google Scholar]
- Yoneyama, Y. , Suzuki, S. , Sawa, R. , Yoneyama, K. , Power, G. G. , & Araki, T. (2002). Increased plasma adenosine concentrations and the severity of preeclampsia. Obstetrics & Gynecology, 100(6), 1266–1270. 10.1016/S0029-7844(02)02247-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Summary table of the compounds used for wire myography experiments.
Figure S1. Results of the wire myography experiments using porcine coronary arteries. Concentration‐response curves (CRC) from wire myography experiments displayed as % relaxation of U46619 pre‐contraction. A) CRC of pentoxifylline (PTX) in control segments (circles) or after incubation with SQ22536 (triangles) or L‐NAME (squares, n = 9). B) CRC of sodium nitroprusside (SNP) in control segments (black triangles) or after incubation with 100 μmol l−1 pentoxifylline (cyan triangles). Pentoxifylline itself had no significant effect but the interaction between the SNP concentration and % relaxation was significantly different (n = 5). C) CRC of forskolin in control segments (black squares) or after incubation with 100 μmol l−1 (cyan squares, n = 5‐6). Curves with antagonist were compared to curves without antagonist (control) using GLM‐RM and data are depicted as mean ± SE % relaxation of U46619 pre‐constriction. * P < 0.05.
Figure S2. Exploratory data: Pentoxifylline‐induced vasodilation is endothelium dependent in healthy placenta arteries. Concentration‐response curves of pentoxifylline constructed using wire myography experiments in intact segments as well as in segments of which the endothelium was removed (Denuded) and depicted as percentage relaxation of pre‐constriction by U46619. Denudation attenuated vasodilation to pentoxifylline (black open squares, n = 4), similar to L‐NAME (grey filled circles, n = 3), when compared to intact artery segments (black filled squares, n = 4). L‐NAME did not further inhibit vasodilation in denuded arteries (grey open squares, n =4). Data are depicted as mean ± SE % relaxation of U46619 pre‐constriction.
Figure S3. The effects of pentoxifylline metabolites in healthy chorionic plate arteries. Concentration‐response curves of pentoxifylline and its metabolites M1 (lisofylline), M4 and M5 from wire myography experiments with human chorionic plate arteries (n = 6−12). Data are depicted as mean ± SE % relaxation of U46619 pre‐constriction. Comparisons of curves with antagonist to curves without antagonist (control) in GLM‐RM did not result in statistically significant results.
Figure S4. The effects of DPCPX and endothelial denudation on pentoxifylline‐induced vasodilation in four individual experiments with chorionic plate arteries from pregnancies with pre‐eclampsia (PE). Data are depicted as % relaxation of U46619 pre‐constriction and each data point represents the average value from two duplicate experiments.
Figure S5. The baseline effects of antagonists on healthy and pre‐eclamptic chorionic plate arteries in wire myography experiments. A) Incubation with L‐NAME, alone or in combination with SQ22536 elevated the baseline tension when compared to the vessels without antagonist (control) in healthy placental arteries only. B) None of the antagonist altered the contractile response to 10 nmol l−1 U46619. * P < 0.05 in one sample Student's t tests with Benjamini‐Hogbergh correction versus 0.
Data Availability Statement
All supporting data are available within the article and in the Supporting Information.


