Abstract
Background
This study aimed to investigate the efficacy of surgery in the treatment of small cell carcinoma of the esophagus (SCCE) and explore potential prognostic factors.
Methods
We screened patients with SCCE who underwent esophagectomy from 2010 to 2018 at three institutes. Differences in survival were analyzed using the Kaplan–Meier method and log–rank test. The prognostic factors were identified using univariate and multivariate analyses.
Results
A total of 69 patients were included. Multivariate analysis showed that TNM stage (hazard ratio [HR]: 4.10, 95% confidence interval [CI]: 1.57–10.75, p = 0.004) and adjuvant therapy (HR: 0.28, 95% CI: 0.16–0.51, p < 0.001) were independent prognostic factors. Stage I, stage IIA, and stage IIB disease were merged into the surgery response disease (SRD), whereas stage III disease into the surgery nonresponse disease (SNRD). The SRD group had significantly improved survival compared to the SNRD group (HR: 0.33, 95% CI: 0.19–0.58, p < 0.001). In addition, adjuvant therapy increased survival benefit in the SNRD group (p < 0.001) but not in the SRD group (p = 0.061).
Conclusions
Surgery alone appears to be adequate for disease control in the SRD group, whereas multimodality therapy was associated with improved survival in the SNRD group.
Keywords: adjuvant therapy, esophagectomy, prognostic factor, small cell carcinoma of the esophagus, stage
Abbreviations
- CI
confidence interval
- HR
hazard ratio
- IQR
interquartile range
- SCCE
small cell carcinoma of the esophagus
- SNRD
surgery nonresponse disease
- SRD
surgery response disease
- VALSG
the Veterans' Administration Lung Study Group
1. INTRODUCTION
Small cell carcinoma of the esophagus (SCCE) is a rare but highly aggressive neuroendocrine malignancy. 1 , 2 McKeown et al. 3 first described it in 1952, and subsequent case reports and small series confirmed its existence. 4 , 5 , 6 SCCE accounts for approximately 1.26% of all esophageal neoplasms in Chinese patients and approximately 1.6% in Western patients. 7 , 8 Similar to small cell lung cancer, 9 patients with SCCE have a poor prognosis with a 5‐year survival rate of 6.7%–12.2%. 10 , 11 To date, although various treatment options are available for SCCE, the optimal treatment has not been defined due to its rarity.
For patients with limited‐stage SCCE, surgical resection appears to be the only curative treatment option. However, the efficacy of surgical treatment remains controversial and needs to be fully explained. Meng et al. 12 have found that chemoradiotherapy had better overall survival (OS) than surgery followed by chemotherapy in limited‐stage SCCE. In contrast, several studies have showed that surgical resection was beneficial. 13 , 14 Verma et al. 15 have indicated that adding surgery or radiotherapy to chemotherapy led to improved long‐term survival outcomes. In addition, Chen et al. 16 have reported that surgical resection alone was adequate for patients with early‐stage SCCE. Thus, the role of surgery in treating resectable SCCE requires further investigation.
In this study, we aimed to investigate the therapeutic effects of surgery in the treatment of SCCE according to long‐term survival outcomes and identify the prognostic factors for patients with SCCE who underwent curative esophagectomy.
2. METHOD
2.1. Study population
This study identified consecutive patients with SCCE who underwent curative esophagectomy at West China Hospital of Sichuan University, Affiliated Hospital of North Sichuan Medical College, and Changsha Central Hospital between December 2010 and December 2018. Patient demographics, surgical procedures, pathology, adjuvant therapy and survival outcomes were collected. Inclusion criteria included (1) small cell carcinoma of esophagus; (2) limited‐stage; (3) surgical resectable; and (4) transthoracic esophagectomy. Exclusion criteria included (1) other histological types; (2) extensive‐stage; and (3) coexistence of other malignancies. Ethics approval for this study was granted by the Ethics Committee of West China Hospital of Sichuan University (No. 2021663), Affiliated Hospital of North Sichuan Medical College (No. 2021ER203‐1), and Changsha Central Hospital (No. 20213426). Patient informed consent was waived.
2.2. Surgery
An esophagoscope, contrast‐enhanced computed tomography of the neck, chest, and abdomen, endoscopic ultrasonography, bone scan, and magnetic resonance imaging were used for perioperative tumor staging. The standard surgical approach was minimally invasive esophagectomy or open thoracotomy. However, the surgical approach was not associated with complete resection. The extent of lymphadenectomy included two‐field lymph node dissections and was conducted in most patients. Esophagogastrostomy was performed using circular staplers or hand‐sewn double layer sutures.
2.3. Pathology
Two staging systems were used to determine the pathologic stages: the Veterans' Administration Lung Study Group (VALSG) staging system for small cell lung cancer and the 8th edition of the American Joint Committee on Cancer TNM staging system.
2.4. Follow‐up
OS was defined as the time from surgery to death. Patients alive or lost to follow‐up were censored at the date of last follow‐up. In the first 2 years, patients were observed every 3 months and then every 6 months thereafter. Information on follow‐up was available 5 years after surgery or at the time of death.
2.5. Statistical analysis
Nonnormally distributed continuous variables are presented as the median with interquartile range, and categorical variables are presented as frequencies and percentages. Survival curves were calculated using the Kaplan–Meier method, and the log‐rank test was used to compare the differences between survival curves. For multiple comparisons, the p value was adjusted using the Benjamini and Hochberg method by the “fdrtool” package in R. Univariate and multivariate analyses were performed with the Cox proportional hazards regression model. Survival analyses were analyzed using the ‘survival’ package (version 3.2–10) in R. Survival curves were plotted using the “survminer” package (version 0.4.9) in R. Statistical analyses were performed using SPSS Statistics (version 24, IBM) and the R programming language (version 3.6.3). Statistical significance was set at a two‐sided p value less than 0.05.
3. RESULTS
3.1. Patient characteristics
A total of 69 patients with limited‐disease SCCE underwent curative esophagectomy in our center between December 2010 and December 2018. The baseline characteristics of the patients are summarized in Table 1. All patients were defined as having limited disease according to the VALSG staging system. By the TNM staging system, 17 patients (24.6%) had stage I disease, 7 patients (10.1%) had stage IIA disease, 10 patients (14.5%) had stage IIB disease, and 35 patients (50.7%) had stage III disease. When considering pathological components, 40 patients had pure small cell carcinoma, and 29 patients presented mixed squamous cell or adenocarcinoma.
Table 1.
Patient characteristics
| Characteristic (n = 69) | Total |
|---|---|
| Age (years), median (IQR) | 62 (53–66) |
| Gender, n (%) | |
| Male | 53 (76.8%) |
| Female | 16 (23.2%) |
| Length (cm), median (IQR) | 4 (3–5) |
| Location, n (%) | |
| Upper | 5 (7.2%) |
| Middle | 43 (62.3%) |
| Lower | 21 (30.4%) |
| Surgical approach, n (%) | |
| Sweet | 21 (30.4%) |
| Ivor Lewis | 15 (21.7%) |
| McKeown | 33 (47.8%) |
| pT, n (%) | |
| T1 | 28 (40.6%) |
| T2 | 23 (33.3%) |
| T3 | 18 (26.1%) |
| pN, n (%) | |
| N0 | 26 (37.7%) |
| N1 | 20 (29.0%) |
| N2 | 14 (20.3%) |
| N3 | 9 (13.0%) |
| Pathological component, n (%) | |
| Pure small cell | 40 (58.0%) |
| Adenocarcinoma | 1 (1.4%) |
| Squamous cell | 28 (40.6%) |
| Lymphovascular invasion, n (%) | |
| Yes | 6 (8.7%) |
| No | 63 (91.3%) |
| No. of resected nodes, median (IQR) | 15 (11–21) |
| No. of involved nodes, median (IQR) | 1 (0–3) |
| Adjuvant therapy, n (%) | |
| No | 36 (52.2%) |
| Chemotherapy | 12 (17.4%) |
| Chemoradiotherapy | 21 (30.4%) |
Abbreviation: IQR, interquartile range.
3.2. Surgical outcomes
All the patients were assessed to have resectable disease preoperatively, and all patients had an R0 resection in postoperative pathology evaluation. Postoperative major complications occurred in 4 patients (5.8%): 1 patient had pneumonia, and 3 patients experienced anastomotic leakage. No patient died within 30 days postoperatively. Patients with lymph node involvement in the resected specimen were recommended to receive adjuvant therapy. In this study, approximately half of the patients received adjuvant chemotherapy/chemoradiotherapy. Because of poor performance status, several patients did not receive further therapy.
3.3. Survival
We used the reverse Kaplan–Meier method to calculate the median follow‐up time. The median follow‐up time was 124.9 months (95% confidence interval [CI]: 113.9–135.8). The Kaplan–Meier survival curve in the entire cohort was displayed in Figure 1. Our results showed that the median OS was 19.6 months (95% CI: 14.2–30.0) for patients with SCCE undergoing curative resection, and the 1‐, 3‐, and 5‐year OS rates were 44.9%, 25.3%, and 11.8%, respectively.
Figure 1.
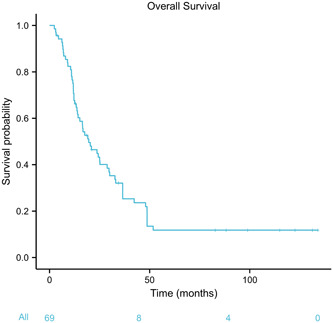
Kaplan–Meier curve of overall survival for the entire cohort
3.4. Cox proportional hazards model
The results of the Cox proportional hazards model were presented in Table 2. In univariate analysis, TNM staging (p < 0.001), adjuvant therapy (p < 0.001), lymph node involvement (p = 0.006) and tumor length (p = 0.058) were potential prognostic factors. Statistically significant variables (p < 0.10) in univariate analysis were entered into the multivariate analysis. However, multivariate analysis showed that TNM staging (p = 0.004) and adjuvant therapy (p < 0.001) were independent prognostic factors for OS. Notably, stage III disease had significantly worse OS than stage I‐II disease (hazard ratio [HR]: 4.10, 95% CI: 1.57–10.75, p = 0.004) in SCCE. In addition, patients receiving adjuvant therapy were associated with prolonged OS benefit compared to patients not receiving adjuvant therapy (HR: 0.28, 95% CI: 0.16–0.51, p < 0.001).
Table 2.
Cox proportional hazards model for survival in small cell carcinoma of the esophagus
| Characteristics | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Age | 1.00 (0.97–1.04) | 0.810 | ||
| Gender | ||||
| Female | Reference | |||
| Male | 0.99 (0.53–1.85) | 0.975 | ||
| Location | ||||
| Upper/middle | Reference | |||
| Lower | 1.17 (0.65–2.12) | 0.606 | ||
| Length | 1.15 (0.99–1.34) | 0.058* | 0.97 (0.82–1.15) | 0.731 |
| Surgical approach | ||||
| Ivor Lewis/McKeown | Reference | |||
| Sweet | 0.75 (0.43–1.34) | 0.331 | ||
| Pathologic component | ||||
| Pure small cell | Reference | |||
| Not pure small cell | 0.84 (0.49–1.43) | 0.525 | ||
| Lymphovascular invasion | 1.89 (0.83–4.29) | 0.127 | ||
| Lymph node involvement | 2.18 (1.25–3.80) | 0.006* | 1.12 (0.44–2.81) | 0.813 |
| TNM stage | ||||
| I–II | Reference | |||
| III | 2.99 (1.73–5.17) | <0.001* | 4.10 (1.57–10.74) | 0.004** |
| Adjuvant therapy | 0.40 (0.24–0.69) | <0.001* | 0.28 (0.16–0.51) | <0.001** |
Abbreviations: CI, confidence interval; HR, hazard ratio.
*p < 0.10.
**p < 0.05.
3.5. Classification according to surgery response
By the TNM staging system, no significant survival differences were found among stages I, IIA, and IIB disease; however, stage III disease had obviously worse OS than any other stage (stage I vs. stage IIA: adjusted p = 1.000; stage I vs. stage IIB: adjusted p = 1.000; stage I vs. stage III: adjusted p = 0.001; stage IIA vs. stage IIB: adjusted p = 1.000; stage IIA vs. stage III: adjusted p = 0.036; stage IIB vs. stage III: adjusted p = 0.020) (Figure 2A). As a result, stages I, IIA, and IIB disease were merged into a new group called surgery response disease (SRD), whereas stage III disease was named surgery nonresponse disease (SNRD). Kaplan–Meier survival analysis showed that the SRD group had better median OS than the SNRD group (HR: 0.33, 95% CI: 0.19–0.58, p < 0.001) (Figure 2B).
Figure 2.
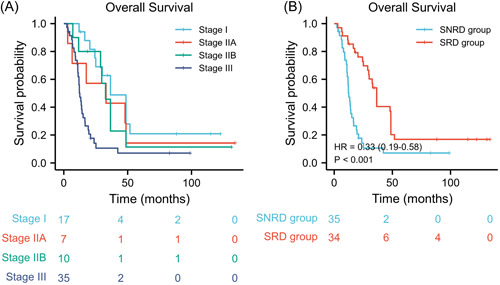
Kaplan–Meier curves of overall survival stratified by (A) TNM stage and (B) surgery response. Stages I, IIA, and IIB patients were merged into the surgery response disease (SRD) group, whereas stage III patients were merged into the surgery nonresponse disease (SNRD) group
3.6. Adjuvant therapy
We also investigated the role of adjuvant therapy in SCCE. The median OS was 30.0 months (95% CI: 11.8–24.3) for patients with adjuvant therapy compared with 12.5 months (95% CI: 11.8–24.3) in patients without adjuvant therapy. In the entire cohort, adjuvant therapy improved the survival of patients with SCCE after surgery (HR: 0.43, 95% CI: 0.25–0.74, p = 0.001) (Figure 3). In the subgroup analysis, adjuvant therapy did not significantly improve the median OS in the SRD group (HR: 0.50, 95% CI: 0.23–1.12, log–rank p = 0.061) (Figure 4A). However, adjuvant therapy significantly improved the median OS in the SNRD group (HR: 0.29, 95% CI: 0.14–0.62, log–rank p < 0.001) (Figure 4B).
Figure 3.
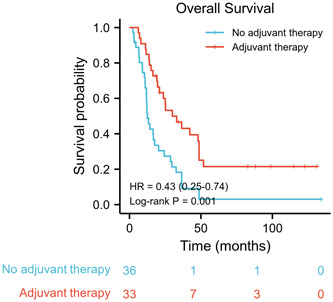
Kaplan–Meier curves of overall survival stratified by adjuvant therapy
Figure 4.
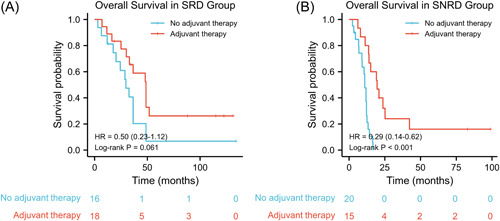
Kaplan–Meier curves of overall survival stratified by adjuvant therapy (A) in the surgery response disease (SRD) group; (B) in the surgery nonresponse disease (SNRD) group
4. DISCUSSION
In this study, our results showed that SCCE had an extremely poor prognosis, with a median OS of 19.6 months. The 1‐, 3‐, and 5‐year OS rates were 44.9%, 25.3%, and 11.8%, respectively. These results parallel the results of a previous study by Fan et al., 17 with a median OS of 17.4 months and 1‐, 3‐, and 5‐year OS rates of 66.3%, 24.1%, and 21.4%, respectively. However, their median follow‐up time of 73.0 months was shorter than our study of 124.9 months. Notably, insufficient follow‐up time might potentially overestimate the survival rate. In addition, we found that TNM stage and adjuvant therapy were independent prognostic factors for patients with SCCE undergoing curative esophagectomy. Likewise, Miao et al. 18 also found that surgery‐based multimodality treatment was an independent prognostic factor. However, they did not find that TNM stage had a significant impact on survival in multivariate analysis. The potential explanation may be that their study included both limited‐stage and extensive‐stage SCCE.
Currently, no specific staging system is established for SCCE due to its rarity. The VALSG staging system is the most commonly used in SCCE, which is classified as limited‐stage and extensive‐stage. 19 Another commonly used option is the TNM staging system for esophageal cancer by the American Joint Committee on Cancer. In this study, all the patients had limited disease according to the VALSG staging system. However, this classification was not conclusive. When analyzed by the TNM staging system, no significant survival differences were found among stages I, IIA, and IIB patients, but stage III patients had significantly worse survival than any other stage. Therefore, it is reasonable to identify the favorable population, and patients were grouped into the SRD group and SNRD group.
We also found that curative esophagectomy combined with adjuvant therapy significantly improved prognosis compared to surgery alone in all patients. Furthermore, adjuvant therapy had no significant impact on survival in the SRD group but was associated with survival benefit in the SNRD group. These results also parallel the findings by Zou et al., 20 in which adjuvant therapy improved survival in limited‐stage II disease but not in limited‐stage I disease. This may be due to locoregional therapy has good control in stage I/II SCCE. However, stage III SCCE appears to be a systematic disease that necessitates systemic therapy. 21 , 22
The strengths of the study include that this multicenter, long‐term follow‐up, retrospective study mainly focused on patients with SCCE undergoing surgery‐based treatment. Furthermore, our results revealed that stage I and stage II patients with SCCE were a favorable population for surgical resection, whereas multimodality treatment remains the main treatment approach for stage III patients. To our knowledge, few published studies have addressed this topic.
The potential weakness of the study included that the therapeutic effect of neoadjuvant therapy was not investigated in this study. As neoadjuvant therapy was not well established in China before 2018, 23 only two patients in this study received neoadjuvant therapy. However, most patients with nodal disease received adjuvant therapy. Cai et al. 24 reported that preoperative chemotherapy plus surgery improved SCCE patient survival compared with surgery alone. However, their study included many cases of R1/R2 resection, which possibly weakens the conclusion.
There are also some limitations in this study. First, it is a retrospective study with inherent flaws. Second, although this study had a relatively larger sample size than the previous literature by Fan et al. 17 and Miao et al. 18 The sample size of 69 patients warrants further investigation with multicenter and large sample sizes.
5. CONCLUSIONS
SCCE is a rare but highly aggressive malignancy. Patients with limited‐stage SCCE could be further grouped into the SRD group and SNRD group according to TNM staging. For the SRD group, surgery alone appears to be adequate; for the SNRD group, adjuvant therapy adds more benefit to patient survival.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
SYNOPSIS
Small cell carcinoma of the esophagus is a rare but highly aggressive malignancy. However, the role of surgery in treatment remains unclear and further explanation. Our study highlighted that surgery alone appears to be adequate for disease control in the surgery response disease group, whereas multimodality therapy was associated with improved survival in the surgery nonresponse disease group.
ACKNOWLEDGMENTS
This research was supported by National Natural Science Foundation of China (82000514) and Key Projects of Sichuan Provincial Department of Science and Technology (2021YFS0222).
Gu Y‐M, Yang Y‐S, Shi G‐D, et al. Limited‐stage small cell carcinoma of the esophagus treated with curative esophagectomy: A multicenter retrospective cohort study. J Surg Oncol. 2022;126:1396‐1402. 10.1002/jso.27073
Yi‐Min Gu and Yu‐Shang Yang contributed equally to this work.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Ku GY, Minsky BD, Rusch VW, Bains M, Kelsen DP, Ilson DH. Small‐cell carcinoma of the esophagus and gastroesophageal junction: review of the memorial Sloan‐Kettering experience. Ann Oncol. 2008;19(3):533‐537. [DOI] [PubMed] [Google Scholar]
- 2. Vos B, Rozema T, Miller RC, et al. Small cell carcinoma of the esophagus: a multicentre rare cancer network study. Dis Esophagus. 2011;24(4):258‐264. [DOI] [PubMed] [Google Scholar]
- 3. McKeown F. Oat‐cell carcinoma of the oesophagus. J Pathol Bacteriol. 1952;64(4):889‐891. [DOI] [PubMed] [Google Scholar]
- 4. Caldwell CB, Bains MS, Burt M. Unusual malignant neoplasms of the esophagus. Oat cell carcinoma, melanoma, and sarcoma. J Thorac Cardiovasc Surg. 1991;101(1):100‐107. [PubMed] [Google Scholar]
- 5. Pantvaidya GH, Pramesh CS, Deshpande MS, Jambhekar NA, Sharma S, Deshpande RK. Small cell carcinoma of the esophagus: the Tata Memorial Hospital experience. Ann Thorac Surg. 2002;74(6):1924‐1927. [DOI] [PubMed] [Google Scholar]
- 6. Brenner B, Shah MA, Gonen M, Klimstra DS, Shia J, Kelsen DP. Small‐cell carcinoma of the gastrointestinal tract: a retrospective study of 64 cases. Br J Cancer. 2004;90(9):1720‐1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lu XJ, Luo JD, Ling Y, et al. Management of small cell carcinoma of esophagus in China. J Gastrointest Surg. 2013;17(7):1181‐1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huncharek M, Muscat J. Small cell carcinoma of the esophagus. The Massachusetts General Hospital experience, 1978 to 1993. Chest. 1995;107(1):179‐181. [DOI] [PubMed] [Google Scholar]
- 9. Bunn PA Jr., Minna JD, Augustyn A, et al. Small cell lung cancer: can recent advances in biology and molecular biology be translated into improved outcomes? J Thorac Oncol. 2016;11(4):453‐474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lv J, Liang J, Wang J, et al. Primary small cell carcinoma of the esophagus. J Thorac Oncol. 2008;3(12):1460‐1465. [DOI] [PubMed] [Google Scholar]
- 11. Lu J, Xue LY, Lu N, Zou SM, Liu XY, Wen P. Superficial primary small cell carcinoma of the esophagus: clinicopathological and immunohistochemical analysis of 15 cases. Dis Esophagus. 2010;23(2):153‐159. [DOI] [PubMed] [Google Scholar]
- 12. Meng MB, Zaorsky NG, Jiang C, et al. Radiotherapy and chemotherapy are associated with improved outcomes over surgery and chemotherapy in the management of limited‐stage small cell esophageal carcinoma. Radiother Oncol. 2013;106(3):317‐322. [DOI] [PubMed] [Google Scholar]
- 13. Tanaka T, Matono S, Nagano T, et al. Surgical management for small cell carcinoma of the esophagus. Dis Esophagus. 2010;23(6):502‐505. [DOI] [PubMed] [Google Scholar]
- 14. Koide N, Saito H, Suzuki A, et al. Clinicopathologic features and histochemical analyses of proliferative activity and angiogenesis in small cell carcinoma of the esophagus. J Gastroenterol. 2007;42(12):932‐938. [DOI] [PubMed] [Google Scholar]
- 15. Verma V, Sleightholm RL, Fang P, Ryckman JM, Lin C. National Cancer Database report of nonmetastatic esophageal small cell carcinoma. Cancer Med. 2018;7(12):6365‐6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen WW, Wang F, Chen S, et al. Detailed analysis of prognostic factors in primary esophageal small cell carcinoma. Ann Thorac Surg. 2014;97(6):1975‐1981. [DOI] [PubMed] [Google Scholar]
- 17. Fan N, Wang Z, Huang Y, Tan Z, Yang H, Lin P. A retrospective study of 52 patients with primary small cell carcinoma of the esophagus treated with radical surgery. Cancer Control. 2021;28:10732748211027147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miao H, Li R, Chen D, Hu J, Chen Y, Wen Z. Survival outcomes and prognostic factors of primary small cell carcinoma of the esophagus. J Thorac Dis. 2021;13(5):2790‐2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zelen M. Keynote address on biostatistics and data retrieval. Cancer Chemother Rep 3. 1973;4(2):31‐42. [PubMed] [Google Scholar]
- 20. Zou B, Li T, Zhou Q, et al. Adjuvant therapeutic modalities in primary small cell carcinoma of esophagus patients: a retrospective cohort study of multicenter clinical outcomes. Medicine (Baltimore). 2016;95(17):e3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Isolauri J, Mattila J, Kallioniemi OP. Primary undifferentiated small cell carcinoma of the esophagus: clinicopathological and flow cytometric evaluation of eight cases. J Surg Oncol. 1991;46(3):174‐177. [DOI] [PubMed] [Google Scholar]
- 22. Atsumi K, Shioyama Y, Nomoto S, et al. Chemoradiation for small cell esophageal carcinoma: report of 11 cases from multi‐institution experience. J Radiat Res. 2010;51(1):15‐20. [DOI] [PubMed] [Google Scholar]
- 23. Yang H, Liu H, Chen Y, et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): a phase III multicenter, randomized, open‐label clinical trial. J Clin Oncol. 2018;36(27):2796‐2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cai G, Wang J, Zou B, et al. Preoperative chemotherapy for limited‐stage small cell carcinoma of the esophagus. Ann Thorac Surg . 2021. 10.1016/j.athoracsur.2021.08.059 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


