Abstract
Historically, there has been broad consensus that osseointegration represents a homeostasis between a titanium dental implant and the surrounding bone, and that the crestal bone loss characteristic of peri‐implantitis is a plaque‐induced inflammatory process. However, this notion has been challenged over the past decade by proponents of a theory that considers osseointegration an inflammatory process characterized by a foreign body reaction and peri‐implant bone loss as an exacerbation of this inflammatory response. A key difference in these two schools of thought is the perception of the relative importance of dental plaque in the pathogenesis of crestal bone loss around implants, with obvious implications for treatment. This review investigates the evidence for a persistent foreign body reaction at osseointegrated dental implants and its possible role in crestal bone loss characteristic of peri‐implantitis. Further, the role of implant‐related material release within the surrounding tissue, particularly titanium particles and corrosion by‐products, in the establishment and progression in peri‐implantitis is explored. While it is acknowledged that these issues require further investigation, the available evidence suggests that osseointegration is a state of homeostasis between the titanium implant and surrounding tissues, with little evidence that a persistent foreign body reaction is responsible for peri‐implant bone loss after osseointegration is established. Further, there is a lack of evidence for a unidirectional causative role of corrosion by‐products and titanium particles as possible non–plaque related factors in the etiology of peri‐implantitis.
Keywords: foreign body reaction, macrophages, osseointegration, peri‐implantitis, titanium particles
1. INTRODUCTION
The 2017 World Workshop on the Classification of Periodontal and Peri‐Implant Diseases and Conditions identified the plaque biofilm as the key etiological factor for the development of peri‐implant mucositis and peri‐implantitis. 1 The cause‐and‐effect relationship between the accumulation of bacterial biofilms around titanium dental implants and the development of inflammation (peri‐implant mucositis) is well established in humans. 2 , 3 , 4 , 5 Given that peri‐implantitis is an irreversible disease, it is impossible from an ethical perspective to obtain direct evidence for a causative relationship between plaque and peri‐implantitis. However, observational studies show that patients exhibiting poor plaque control and not attending regular maintenance therapy are at a higher risk of developing peri‐implantitis. 6 Furthermore, the treatment of peri‐implantitis with anti‐infective strategies has been shown to be successful in decreasing soft tissue inflammation and suppressing disease progression. 7 Therefore, the available evidence appears to strongly support the current paradigm that plaque is the primary etiological agent for peri‐implant mucositis and that in susceptible patients it will progress to peri‐implantitis, with inflammation being the key biological mechanism in the pathogenesis of both diseases. It is also recognized, however, that risk factors and indicators associated with the establishment and progression of peri‐implantitis are not fully understood. Indeed, it has been acknowledged that non–plaque‐related factors, such as peri‐implant keratinized mucosa, occlusal overload, titanium particles, bone compression necrosis, overheating, micromotion, and biocorrosion, may play a role in the etiology of peri‐implantitis, but their influence is unknown. 1
In the past decade, the potential role of non–plaque‐related factors in the pathogenesis of progressive peri‐implant bone loss has been brought to prominence in several reviews. 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 In particular, the role of the “foreign body reaction” towards the implanted material has been proposed as an important contributor to the pathogenesis of peri‐implant bone loss. 12 Therefore, this review will explore the evidence for a role of “foreign body reaction” in peri‐implantitis by examining the influence that implant‐related factors may have in initiating or exacerbating the progressive crestal bone loss that is characteristic of peri‐implantitis. The focus will be on the implant componentry that resides within soft and hard tissues (implant fixture, abutment) but will not address iatrogenic factors that have been shown to cause peri‐implant tissue complications, such as retained cement, incorrect implant positioning, inappropriate surgical technique (overheating, excessive compression), incomplete abutment or restoration seating, and occlusal trauma. In particular, three issues will be addressed:
Does osseointegration represent a return to “homeostasis” or a “chronic inflammatory” state akin to an unresolved “foreign body reaction”?
Does a foreign body reaction to an osseointegrated implant have a role in crestal bone loss characteristic of peri‐implantitis?
Following a period of function, can materials released from the implant componentry that resides within soft and hard tissues (implant fixture, abutment) initiate or exacerbate peri‐implantitis?
2. FOREIGN BODY REACTION AND OSSEOINTEGRATION
Contemporary understanding of the host response to biomaterial implantation acknowledges that there is no such thing as a totally inert biomaterial and that all implants will elicit a host response. 16 However, it is also clear that certain materials result in a “foreign body reaction” characterized by chronic inflammatory response and fibrous encapsulation, whereas others are seamlessly incorporated into the host tissue (“restitutio ad integrum”), thus achieving homeostasis. These are likely to represent two extremes of a continuum of potential responses, with contemporary biomaterial science and tissue engineering focused on strategies to attenuate the inflammatory response and encourage biomaterial integration. 17 , 18 An important question is where titanium implant osseointegration fits within this spectrum of potential outcomes; that is, whether it represents a return to “homeostasis” or whether it is a “foreign body response” characterized by a chronic inflammatory state.
2.1. The foreign body response—What is it?
The term “foreign body response” has been frequently used in the biomaterial science literature, most commonly in the context of an inappropriate response to the insertion of a material. Although there is no universally accepted definition for a foreign body response, a commonly accepted description is a “reaction composed of macrophages and foreign body giant cells (that) is the end‐stage response of the inflammatory and wound healing responses following implantation of a medical device, prosthesis, or biomaterial.” 8 Essentially, the outcome of foreign body response historically has been regarded as an adverse end‐stage outcome, with either complete rejection of the biomaterial in the short term or initial fibrous encapsulation and failure of the biomaterial in the long term. The adverse foreign body response–associated biomaterial failure is often characterized histologically by the presence of foreign body/multinucleated giant cells surrounding the biomaterial.
Detailed discussion of the various theories about the pathophysiology of foreign body response is beyond the scope of this review, and there are several published reviews on this topic. 19 , 20 , 21 , 22 In summary, an adverse foreign body response to an inserted biomaterial is understood to comprise five stages: (a) protein adsorption, (b) acute inflammation, (c) chronic inflammation, (d) foreign body giant cell formation, and (e) fibrosis/fibrous capsule formation. 16 , 19 An important consideration is that the early phases (protein adsorption and acute inflammation) will occur irrespective of any biomaterial that is surgically inserted into a host tissue. What happens thereafter is dependent on various factors, including the nature of the surgical wound, the characteristics of the biomaterial, and how the recipient tissue responds to it. Therefore, the early inflammatory wound‐healing response to the insertion of a biomaterial is critical for the downstream events that lead to implant integration or encapsulation (foreign body response).
In the context of dental implantology, the traditional understanding of osseointegration is that, following the insertion of the implant, the initial inflammatory reaction will in most cases resolve, which returns the surrounding soft and hard tissues to a state of homeostasis. This is characterized by the formation of new alveolar bone in direct contact with the implant surface. A continuation of the foreign body response, whereby tissue does not return to homeostasis, results in the rejection of the implant, which is an infrequent finding associated with early implant loss. The latter is typified by a persistent inflammatory state that leads to soft‐tissue encapsulation of the implant and the presence of foreign body/multinucleated giant cells, which is characteristic of the traditionally accepted “foreign body response” concept. Therefore, an understanding of the key inflammatory mechanisms involved in the wound healing process is crucial for understanding the nature of the implant‐host relationship following osseointegration.
2.2. Inflammation and osseointegration
Inflammation is a fundamental step during wound healing. Since implant placement involves surgical trauma of the recipient site, the initial wound healing leading to osseointegration is intimately involved with the process of peri‐implant osseous healing, which is like events during bone fracture repair (Figure 1). 23 This process is relatively complex and can be broadly characterized into four overlapping phases: hemostasis, inflammation, proliferation/matrix formation, and remodeling. 24 An inflammatory reaction is elicited rapidly following the surgical insertion of an implant, after hemostasis is achieved and protein is adsorbed onto the implant surface. A prolonged inflammatory response can potentially trigger a “foreign body response” that leads to a lack of integration of dental implants, whereas a timely resolution of the inflammation leads to repair via the formation of bone at the implant interface. Therefore, the nature of the initial inflammatory response is considered critical for the downstream events leading to osseointegration.
FIGURE 1.
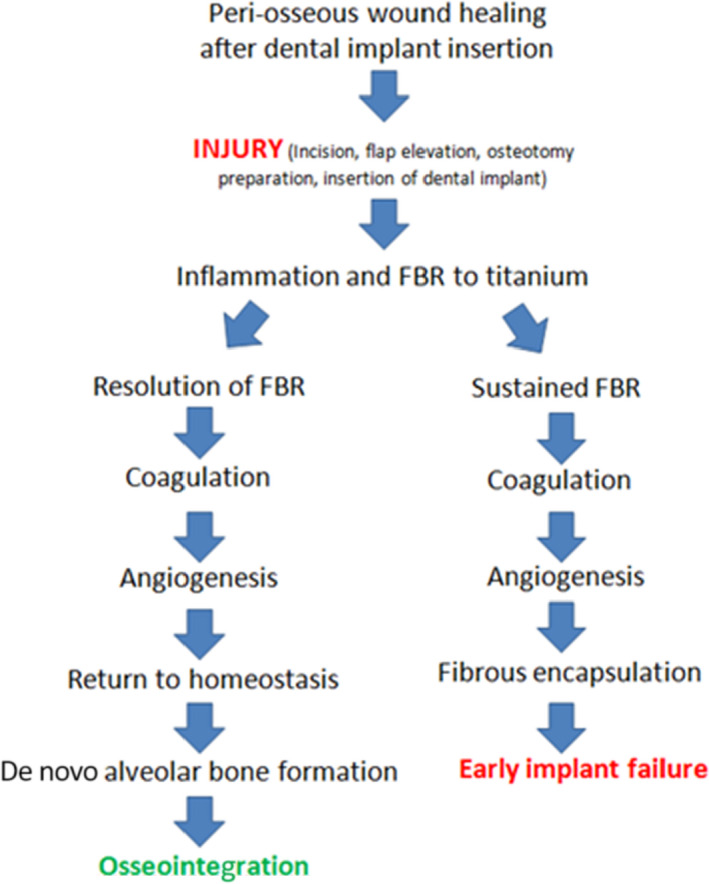
Current understanding of the possible sequelae of dental implant placement into alveolar bone. During osseointegration, the initial inflammatory response is followed by timely resolution and pro‐reparative processes leading to osseointegration and bone formation at the implant interface. The inability to resolve the initial inflammatory response leads to a chronic inflammatory state and fibrous encapsulation of the implant, which is characteristic of a foreign body response that leads to early implant failure. FBR: foreign body response
Macrophages have long been considered the key regulators of the early inflammatory response during wound healing following the insertion of a biomaterial. Macrophages are also important in the context of foreign body response, as they are the precursors to the multinucleated giant cells that are characteristic of this condition. Crucially, depending on the phenotype of the macrophages at the wound site, their secretory profile, and hence their function throughout the course of the inflammatory phase, will differ, therefore influencing downstream events in wound healing. Briefly, macrophages can be broadly divided into two subsets. 25 M1 macrophages are predominantly found in the early stages of the inflammatory phase and express high levels of proinflammatory cytokines and reactive nitrogen and oxygen intermediates. By contrast, M2 macrophages are mainly found at the conclusion at the inflammatory phase, secreting low levels of proinflammatory cytokines and high levels of anti‐inflammatory cytokines that promote wound healing and regeneration. 26 It is now recognized that M1 and M2 phenotypes represent two extremes of the macrophage phenotype that have been characterized in vitro, whereas the in vivo situation is far more complex, with a spectrum of phenotypes present that have not been fully characterized. 27 , 28 Furthermore, it is also clear that macrophages are not the only cells that are important in regulating the wound healing process, with neutrophils, lymphocytes, and other cells of the immune system playing a role. 29 Nevertheless, in the context of this review, it is important to appreciate the transition from an M1‑ to an M2‐type response as important in establishing osseointegration, whereas a persistent M1‐type response can lead to impaired healing leading to a “foreign body response.”
2.3. Osseointegration—Return to “homeostasis” or a “chronic inflammation” state?
Although “osseointegration” has been traditionally considered to represent a physiological state whereby the titanium implant is integrated into the host, it has more recently been suggested in a series of reviews that it should instead be viewed as a “foreign body response” representing a “chronic inflammatory” state. 8 , 11 , 30 However, considerable support remains for the conventional notion that osseointegration is a return to “homeostasis” rather than a “foreign body reaction/chronic inflammatory state.” 31 To fully appreciate this controversy, the available evidence regarding the healing response leading to osseointegration needs to be considered.
The conventional understanding of osseointegration as a return to homeostasis is based on the original observations showing a direct bone‐implant contact between titanium implants and the recipient alveolar bone. 32 , 33 Thereafter, the temporal events leading to inflammation have been extensively studied and show that the early inflammatory response leads to a resolution of an early inflammatory state to ultimately result in direct apposition of bone on the implant surface. 34 Complementary histological and molecular assessments of the temporal wound‐healing events leading to osseointegration in humans show that the initial inflammatory response is subsequently attenuated and replaced with anabolic biological processes, such as osteogenesis and angiogenesis, which eventually return the tissue to homeostasis and functionality. 35 , 36 Indeed, an acceleration of the transition between a proinflammatory M1 to a reparative M2 inflammatory response is considered the major mechanism for enhanced osseointegration associated with the latest generation of titanium implant surfaces. 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44
More recently, the concept that dental implant osseointegration represents a return to “homeostasis” has been challenged by a theory that an osseointegrated implant constitutes a foreign body response characterized by a mild “chronic inflammatory state.” 11 This is considered to represent a “foreign body equilibrium,” whereby the implant is “encapsulated” by bone. 11 The primary evidence provided in these narrative reviews to support this theory is the presence of multinucleated giant cells, demonstrated adjacent to an osseointegrated implant in one slide, and proposed to be “foreign body giant cells.” Interestingly, the presence of multinucleated giant cells has also been more conclusively demonstrated at the surface of osseointegrated dental implants by another group, albeit with a different interpretation of their presence. 45 In that study, multinucleated giant cells were shown to be associated with the bone‐implant interface of osseointegrated titanium and zirconia dental implants, without the persistence of any chronic inflammation and no impaired/compromised bone formation.
The question of whether osseointegration can be considered as a return to homeostasis or as a chronic inflammatory state needs to be considered in the context of our understanding that any biomaterial that is surgically inserted into a host will elicit a response, and hence cannot be considered absolutely “inert.” This then brings up the issue of whether the presence of a titanium implant changes the course of healing within a recipient osteotomy. This has been addressed in several studies, and it has been demonstrated that there are molecular mechanisms that are altered upon the insertion of an implant; but these are generally considered to be pro‐osteogenic processes important for bone formation and maintenance, rather than anything that can be associated with a foreign body response or a chronic inflammatory state. 46 , 47 Indeed, recent studies by the same group that proposed the “foreign body equilibrium theory” have explored the effect of the implant presence within the healing osteotomy, showing increased bone formation at the implant‐bone interface (compared with pristine osteotomy healing) and an enhancement of the M2 macrophage response in implant recipient sites compared with empty osteotomies. 48 These findings are consistent with the notion that titanium is a highly biocompatible and osteoconductive biomaterial, and hence it is not surprising that there is enhanced bone formation at the interface of the implant. The exceptional biocompatibility of titanium was further demonstrated by the same group in a study comparing the healing outcomes of titanium compared with copper and polyether ether ketone implants. 49 The titanium implants were shown to achieve a transition to an M2 reparative healing phenotype at a faster rate than the other implants. These findings are consistent with the notion that osseointegration of a titanium implant results in a return to “homeostasis,” providing no evidence of a persistent chronic inflammatory state.
Collectively, the available evidence supports the notion that all biomaterials inserted in the body will elicit an inflammatory response and that resolution of inflammation is important to return the host tissue to functionality and homeostasis. Importantly, titanium implants can facilitate the resolution of the initial immuno‐inflammatory response to favor a healing and reparative response that facilitates the formation of bone directly on its surface (Figure 1). By contrast, a nonresolving foreign body response will lead to fibrous encapsulation and implant failure, which is an infrequent finding associated with early bone loss (Figure 1). Notwithstanding the need to further elucidate the biological mechanism associated with the maintenance of osseointegration, there is currently no evidence that a chronic inflammatory state is associated with a healthy, osseointegrated dental implant.
It is important, however, to note that the apparently opposing views on implant osseointegration share many common aspects. Both acknowledge that no biomaterial is truly “inert” and that the inflammatory response plays a key role in determining the outcome of a host's response to titanium implant placement. Both recognize the classic “foreign body response” as a soft‐tissue encapsulation and a failure of osseointegration. Where they differ is on whether osseointegration represents a “mild chronic inflammatory state” or a return to “homeostasis.” This is a somewhat academic argument in the context of osseointegration, but it has more practical implications in terms of understanding, and more importantly treating, peri‐implantitis. A key consideration is whether the multinucleated giant cells present at the surface of an osseointegrated implant are involved in the etiology of peri‐implant bone loss characteristic of what has been classified as peri‐implantitis.
3. FOREIGN BODY REACTION AND PERI‐IMPLANTITIS
In the widely accepted concept of osseointegaration being a return of homeostasis, dental plaque plays a central role in initiating and progressing crestal bone loss (Figure 2), and hence forms the primary target for treatment. This approach is supported by studies that show a key role for plaque in the etiology of peri‐implant mucositis and periodontitis. For peri‐implant mucositis, a direct cause‐and‐effect relationship between biofilm and development of inflammatory responses has been demonstrated in humans. 2 , 3 , 5 It has also been demonstrated that there is a significant dose‐dependent association between plaque scores and mucositis 50 and that lack of compliance is associated with higher incidence of mucositis. 6 The key role of dental plaque in peri‐implantitis is supported by studies that show patients exhibiting poor plaque control and not attending regular maintenance therapy are at a higher risk of developing peri‐implantitis. 6 Further, anti‐infective treatment strategies are successful in decreasing soft‐tissue inflammation and in suppressing disease progression. 7
FIGURE 2.
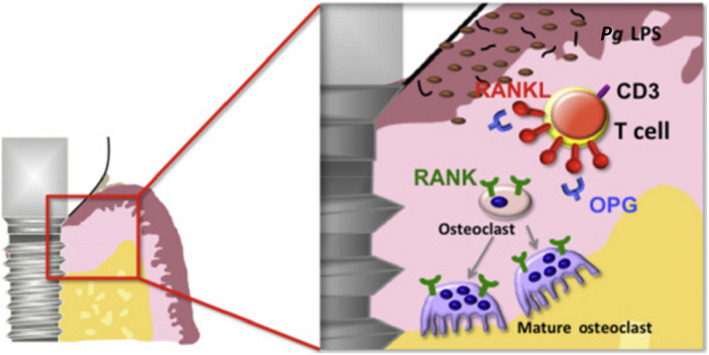
Diagrammatic representation of the plaque‐associated pathogenic mechanism for crestal bone loss. In this mechanism, an inflammatory response to biological mediators released from plaque induces an inflammatory response from the host that results in the activation of osteoclasts responsible for crestal bone loss. Pg LPS: Porphyromonas gingivalis lipopolysaccharide; OPG: osteoprotegerin; RANK: receptor activator of nuclear factor‐κB; RANKL: receptor activator of nuclear factor‐κB ligand. Diagram adapted from ref. [51]
Proponents of the “foreign body equilibrium/bone encapsulation” theory, on the other hand, consider a variety of external factors—of which dental plaque may be one factor but is not considered to be the primary one—exacerbate a latent inflammatory response, mediated by resident foreign body giant cells (Figure 3). This clearly has important implications for treatment, since the focus on managing dental plaque is far greater in the conventional compared to this alternative theory. Notably, there does not appear to be a clear strategy for the management of peri‐implant mucositis and peri‐implantitis in the context of the foreign body equilibrium concept, since the proposed etiological mechanism may be one of a variety of poorly defined and understood “external factors.” Indeed, it has been proposed that the “underlying chronic inflammation (FBE) observed around oral implants leads to the conclusion that mucositis need not automatically be coupled to disease,” and that “marginal bone loss after the implants first year in situ need not be automatically coupled to disease.” 30 Clearly, since the foreign body equilibrium concept considers inflammation and bone loss to be a physiological rather than a “pathologic” phenomenon, this downplays the role of dental plaque in peri‐implantitis. 14 , 15 This has important implications on how we view and subsequently treat inflammation and bone loss around dental implants.
FIGURE 3.
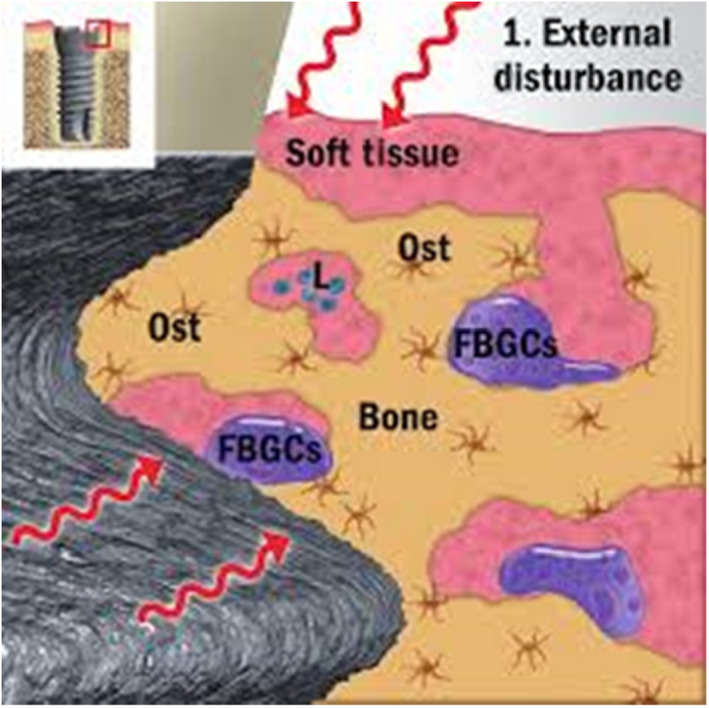
Diagrammatic representation of the “foreign body equilibrium” disturbance theory of peri‐implant bone loss. In this concept, the osseointegrated implant is considered “encapsulated in bone,” thus representing a chronic inflammatory state characterized by the presence of “foreign body giant cells” (FBGCs). In response to a variety of external stimuli, these foreign body giant cells are activated and are responsible for the peri‐implant bone loss. L: lymphocyte; Ost: osteocyte. (Diagram adapted from ref. [32])
A central premise to the foreign body equilibrium theory for peri‐implant bone loss is that the presence of “multinucleated” cells around osseointegrated dental implants—ambiguously and universally classified as “foreign body giant cells” 12 —constitutes a mild chronic inflammatory state. Subsequent disruption of this “equilibrium” leads to breakdown of osseointegration and ultimately crestal bone loss. Therefore, an understanding of the potential role of multinucleated cells associated with healthy, osseointegrated implants is necessary to appreciate their potential role in peri‐implantitis.
There is no doubt that multinucleated giant cells do appear around biomaterials, and several recently published reviews have explored their role in wound healing and implant rejection (foreign body response). 20 , 22 , 23 It is clear from the reviews that not all multinucleated giant cells observed histologically are similar, and it has been proposed that they may be serving different purposes (both favorable and unfavorable for integration) in various conditions. It is inappropriate,therefore, simply to brand them as “foreign body giant cells.” Indeed, it has been proposed that the multinucleated giant cells could be classified as both proinflammatory M1–multinucleated giant cells (this can be defined as the foreign body giant cells that are associated with foreign body response) and pro‐reparative M2–multinucleated giant cells, to mimic the current M1‐M2 macrophage phenotype paradigm. Indeed, within biomaterial science, it has been shown that the presence of multinucleated giant cells is frequently observed and can be considered a physiological (rather than “pathological”) response to implantation of biomaterials. 24 , 25 , 26 , 28
Aside from the mere presence of multinucleated giant cells being considered evidence of an underlying chronic inflammatory state, the other issue with the foreign body equilibrium concept of peri‐implant disease is the crestal pattern of bone loss that is characteristic of peri‐implantitis. If the resident multinucleated giant cells are indeed responsive to external stimuli and responsible for bone loss during peri‐implant disease, then their even distribution along the implant would suggest a uniform rather than a localized (crestal) pattern of bone loss that is observed in peri‐implantitis.
Notwithstanding the need for further research in this area, there is currently no evidence in the existing literature to suggest that the presence of multinucleated giant cells on a healthy, osseointegrated dental implant is a risk factor for peri‐implantitis. Given there is clear evidence that poor plaque control leads to peri‐implantitis and that the targeting of plaque leads to favorable treatment outcomes (albeit not in all cases), the weight of evidence favors the current concept that plaque is the key etiological factor in the development of peri‐implantitis, and hence an obvious primary target for therapeutic approaches for managing the disease.
4. WHAT IS THE ROLE OF THE NON–PLAQUE‐RELATED FACTORS IN THE PATHOGENESIS OF PERI‐IMPLANTITIS?
Thus far, we have established that there is no evidence to suggest that a foreign body reaction to an osseointegrated implant contributes to the crestal bone loss that is characteristic of peri‐implantitis. However, it must be acknowledged that an osseointegrated dental implant is unique compared with most other implanted biomaterials, as it is transmucosal and resides in an oral environment that favors microbial plaque formation. Further, the position of an implant‐abutment interface in the vicinity of the bone crest has the potential to influence crestal bone loss due to several anatomical, functional, iatrogenic and implant related factors. Indeed, it was acknowledged by the 2017 World Workshop on the Classification of Periodontal and Peri‐implant Diseases and Conditions that the role of several non–plaque‐related factors—including peri‐implant keratinized mucosa, occlusal overload, titanium particles, bone compression necrosis, overheating, micromotion, and biocorrosion—is not fully understood. 1 Since the focus of this review is on implant‐related factors, the possible role of titanium particles and biocorrosion will be explored in greater detail.
4.1. Titanium particles
It is indisputable that titanium particles have been found in peri‐implant tissues. Their potential role in peri‐implant inflammation and crestal bone loss has been addressed in a critical narrative review. 13 The release of titanium particles after implant placement into surrounding peri‐implant tissue, as well as in lymph nodes and various internal organs, has been documented in animal models. 52 , 53 , 54 In human studies, the presence of metal particles has been reported in macrophages located in peri‐implant hard and soft tissue. 55 , 56 Indeed, metal‐like particles have been identified both inside and outside cells in cytologic smears of tissues around both diseased and healthy implants. 57
In vitro experiments have shown the potential of titanium ions or particles to have toxic or proinflammatory effects. 58 , 59 Further, the deliberate introduction of titanium particles during osseointegration in a mouse model has been shown to induce M1 macrophage phenotype polarization and associated bone loss. 60 In vitro research has also identified factors modulating such effects, such as particle size. Nanoparticles are considered more biologically reactive and more potentially harmful than microparticles because of their greater surface‐to‐volume ratio. 61 , 62 However, these observations are not universal, as it has also been shown that microparticles (1‐3 μm) induce a greater inflammatory response by neutrophils than nanoparticles do. 63 The effect of particle size is further complicated by the fact that nanoparticles can aggregate in a microparticle size range and change their recognition by the host, hence modulating the inflammatory response. Aside from particle size, it has been shown that lower pH and higher lipopolysaccharide concentration accelerate titanium corrosion in vitro. 64
The source of titanium particles is not fully understood and remains controversial. It has been shown that titanium particles may be disseminated from local to distant sites via the bloodstream, 55 , 56 but it is also possible that they may be introduced from nonoral sources. 61 Indeed, it has been shown that titanium is present in the peri‐implant mucosa from individuals with or without titanium implants. 57 The presence of titanium particles in patients without titanium implants can be attributed to the widespread use of titanium dioxide as micro‑ or nanoparticles in foods, toothpastes, cosmetics, sunscreens, and medicine pills. 65
One obvious source of release of titanium particles is the implant‐abutment connection, and there is substantial evidence of the formation of wear debris due to mechanical stress at the implant‐abutment interface from in vitro studies. 66 , 67 , 68 Further, in a cadaver study, titanium particles in size between 0.5 and 40 μm were clearly evident in jawbone tissues associated with osseointegrated implants, although, notably, these were not affected by disease. The titanium count decreased as the distance away from the implant increased. 69 Collectively, these studies suggest that titanium particles released from the micromovement between the abutment and the implant are certainly possible under force transmission, although they are not present in all cases. 70
Samples from multiple oral sources have been assessed for the presence of implant particles, including mucosa overlying titanium cover screws during submerged healing, 71 mucosa from both peri‐implantitis lesions 72 , 73 , 74 and implants without clinical signs of pathology, 58 and gingiva from healthy teeth. 75 In a recent histopathological analysis of biopsies from both peri‐implantitis and periodontitis sites, titanium particles were identified in all peri‐implantitis specimens, but without evidence of a foreign body reaction suggestive of a direct pathological effect. 76
In summary, the available literature shows that titanium particles are commonly detected in both healthy and diseased peri‐implant mucosa, and even in gingiva of individuals without titanium implants. Thus, there is poor specificity for the association between the presence of particles and pathology. There is, however, a tendency to find more titanium particles in close proximity to the implant surface 69 and in specimens from diseased sites. 77
Released titanium particles have the potential to trigger an inflammatory response. Inflammatory cells, biofluids, and bacteria can all influence titanium particle release in a process called biocorrosion and can be further influenced by mechanical friction and wear in a phenomenon called tribocorrosion. Given the intimate and complex relationship between these factors (ie, inflammation, corrosion, particle release, and bacterial composition), the potential role of titanium particles on peri‐implant crestal bone loss needs to be considered in the context of biocorrosion and tribocorrosion.
4.2. Biocorrosion and tribocorrosion
Titanium and its alloys belong to a large group of oxide‐passivated metals that also include metals such as stainless steel, nickel, and cobalt and aluminum–based alloys. The inertness of titanium is largely attributed to the excellent corrosion resistance of the titanium oxide layer. Damage to the oxide film can “repair” spontaneously and rapidly if the environment contains oxygen or moisture. These features of titanium render it highly resistant to corrosion. In theory, however, certain acids can still corrode titanium, and the speed of corrosion differs depending on the type of acid. For example, titanium exhibits good corrosion resistance in strongly oxidizing nitric acid, whereas an opposite corrosion behavior is experienced in reducing hydrofluoric acid. 78 There are several types of corrosion, of which galvanic, fretting, pitting/crevice, and environmentally induced cracking are the ones mostly associated with titanium. 79
Theoretically, corrosion can lead to the breakdown of the titanium oxide layer, and in situations where the titanium oxide layer cannot be repassivated, such as an anaerobic environment, this may consequently lead to exposure of the bare metal to active corrosion and result in the release of titanium particles. The corrosion of titanium in a simulated body fluid environment has been demonstrated in vitro, and the elution of titanium ions has been shown to be influenced by immersion time, pH of the solution, acid type, mechanical stimulus, and contact with dissimilar metal. 80 It has been demonstrated that an experimental mixed biofilm initiates a decrease in pH and, therefore, leads to the corrosion of titanium in vitro. 81 Further, an in vitro study using mouse‐derived macrophages has reported that the release of active oxygen species from macrophages can induce ion release from titanium in the absence of wear and fretting. 82 These types of corrosion have been collectively named “biocorrosion” or microbiologically influenced corrosion 83 and can lead to undesirable metal ions and corrosion products. Indeed, there is substantial literature that convincingly shows that acidic environments induced by both bacterial biofilms and resultant inflammatory processes trigger surface oxidation and the release of titanium particles. 13 Additionally, there are also in vitro studies showing that certain therapeutic substances commonly used in dentistry—such as bleaching agents of fluoride and hydrogen peroxide–containing mouth rinses—can also reduce the corrosion resistance of titanium alloys or have direct biocorrosive effects. 84 , 85 , 86
The concept of biocorrosion can be further expanded by considering the influence of mechanical friction/wear, which together contribute to a degradation process called “tribocorrosion.” In tribocorrosion, a tribo system has three interrelated components: tribology (friction, wear, and lubrication), corrosion (materials and environmental factors), and biochemistry (the interactions between cells and protein). 87 In terms of possible tribocorrosion in the oral cavity, one can view this as the “irreversible transformation of metal (dental implants and its abutments) caused by simultaneous action of chemical, mechanical (wear), and electrochemical (corrosion) interactions on surface subjected to relative contact movement.” 88 It is clear that the unique dental implant environment (ie, nonshedding transmucosal structure residing in a microbial‐rich fluid environment with a submucosa connection exposed to mechanical forces) makes tribocorrosion a potential contributing mechanism to peri‐implant crestal bone loss.
Tribocorrosion is a frequently explored issue in orthopedics, as the implanted alloplastic implants (ie, total joint replacement) devices are consistently exposed to friction and wear in the presence of potentially corrosive body fluids. 89 Indeed, wear debris and ions released from medical implants and prostheses have been shown to elicit an inflammatory response. 90 , 91 In orthopedics, the inflammatory response eventually leads to a process called “aseptic loosening,” which is a common reason for revision surgery. This has been documented in cases where wear debris derived from an articulate region with various particle sizes ranging between nanometer and millimeter in uncemented metal prostheses can stimulate an immune response and elicit an inflammatory reaction. 92 The tribocorrosion behavior of titanium alloys has been documented in simulated biological environments, with none of the alloys tested found to be immune to tribocorrosion in the in vitro setting. 88 One must keep in mind, however, there are several significant differences between the orthopedic and dental setting, in that dental implants are placed within a transmucosal/open environment, not a closed environment, as in the case of total knee/joint replacements. Further, the forces involved and the materials used in orthopedics are different to those in dentistry, and hence these clinical scenarios cannot be directly compared. Interestingly, in dental implantology, aseptic loosening has been associated with zirconia implants but not with titanium implants. 93
Assuming corrosion by‐products (metal particles) are either cleared from the transmucosal environment via the peri‐implant crevicular fluid or have accumulated in the surrounding peri‐implant tissue, the effect of corrosion by‐products on the biofilm is unclear. In vitro studies exploring the effect of titanium granules on oral bacterial species have reported conflicting results, with some studies showing limited antimicrobial effects, 94 , 95 whereas more recent reports have demonstrated significant antibiofilm activity of titanium nanoparticles, either alone or in combination with other nanoparticles. 96 , 97 Indeed, titanium nanoparticles have been proposed as a commercially viable anti‐plaque and anti‐biofilm strategy. 98 Nevertheless, currently, the impact of corrosion by‐products and titanium particles on bacterial growth is unclear, and so further research is required to understand their possible effect on the formation and composition of biofilms in the peri‐implant sulcus.
In summary, wear, corrosion, titanium particles, inflammation, and microorganisms take part in a complex host response to foreign bodies. There is some biological plausibility for a link between corrosion, the presence of titanium particles, and biological complications. However, there are currently insufficient data to support a unidirectional role of titanium corrosion and metal particles in the pathogenesis of peri‐implantitis.
5. CONCLUSIONS
The influence of a foreign body response to the implant material in the etiology of crestal bone loss is both a complex and underexplored area of research in dental implantology that requires further investigation. The available literature supports the following conclusions in relation to the potential role of a foreign body response to the dental implant materials as a risk factor for crestal bone loss:
Although titanium, like all implanted biomaterials, elicits an inflammatory response upon implantation and cannot be considered absolutely “inert,” the available evidence supports the notion that the osseointegration of titanium dental implants represents a return to tissue homeostasis rather than a chronic inflammatory state that is characteristic of a “foreign body response.”
There is no evidence for a prominent role of a “foreign body response” (also characterized as “foreign body equilibrium” or a “mild chronic inflammatory state”) to an osseointegrated implant in the pathogenesis of peri‐implantitis. The available evidence supports our understanding that the dental plaque biofilm is the key modifiable etiological factor in peri‐implantitis and, hence, is the logical frontline target of both preventative and therapeutic interventions.
There is a lack of evidence for a unidirectional causative role of corrosion by‐products and titanium particles as possible non–plaque‐related factors in the etiology of peri‐implant disease, although this area remains underexplored and requires further investigation.
ACKNOWLEDGMENTS
Open access publishing facilitated by The University of Queensland, as part of the Wiley ‐ The University of Queensland agreement via the Council of Australian University Librarians.
Ivanovski S, Bartold PM, Huang Y‐S. The role of foreign body response in peri‐implantitis: What is the evidence? Periodontol 2000. 2022;90:176‐185. doi: 10.1111/prd.12456
REFERENCES
- 1. Berglundh T, Armitage G, Araujo MG, et al. Peri‐implant diseases and conditions: consensus report of Workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri‐Implant Diseases and Conditions. J Clin Periodontol. 2018;45(Suppl 20):S286‐S291. [DOI] [PubMed] [Google Scholar]
- 2. Meyer S, Giannopoulou C, Courvoisier D, Schimmel M, Müller F, Mombelli A. Experimental mucositis and experimental gingivitis in persons aged 70 or over. Clinical and biological responses. Clin Oral Implants Res. 2017;28(8):1005‐1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pontoriero R, Tonelli MP, Carnevale G, Mombelli A, Nyman SR, Lang NP. Experimentally induced peri‐implant mucositis. A clinical study in humans. Clin Oral Implants Res. 1994;5(4):254‐259. [DOI] [PubMed] [Google Scholar]
- 4. Salvi GE, Aglietta M, Eick S, Sculean A, Lang NP, Ramseier CA. Reversibility of experimental peri‐implant mucositis compared with experimental gingivitis in humans. Clin Oral Implants Res. 2012;23(2):182‐190. [DOI] [PubMed] [Google Scholar]
- 5. Zitzmann NU, Berglundh T, Marinello CP, Lindhe J. Experimental peri‐implant mucositis in man. J Clin Periodontol. 2001;28(6):517‐523. [DOI] [PubMed] [Google Scholar]
- 6. Oliveira Costa F , Takenaka‐Martinez S, Miranda Cota LO , Diniz Ferreira S , Magalhães Silva GL , Costa JE. Peri‐implant disease in subjects with and without preventive maintenance: a 5‐year follow‐up. J Clin Periodontol 2012; 39(2):173–181. [DOI] [PubMed] [Google Scholar]
- 7. Berglundh T, Wennström JL, Lindhe J. Long‐term outcome of surgical treatment of peri‐implantitis. A 2‐11–year retrospective study. Clin Oral Implants Res. 2018;29(4):404‐410. [DOI] [PubMed] [Google Scholar]
- 8. Albrektsson T, Buser D, Sennerby L. Crestal bone loss and oral implants. Clin Implant Dent Relat Res. 2012;14(6):783‐791. [DOI] [PubMed] [Google Scholar]
- 9. Albrektsson T, Buser D, Sennerby L. On crestal/marginal bone loss around dental implants. Int J Prosthodont. 2012;25(4):320‐322. [PubMed] [Google Scholar]
- 10. Albrektsson T, Canullo L, Cochran D, De Bruyn H. "Peri‐implantitis:" a complication of a foreign body or a man‐made "disease." Facts and fiction. Clin Implant Dent Relat Res. 2016;18(4):840‐849. [DOI] [PubMed] [Google Scholar]
- 11. Albrektsson T, Dahlin C, Jemt T, Sennerby L, Turri A, Wennerberg A. Is marginal bone loss around oral implants the result of a provoked foreign body reaction? Clin Implant Dent Relat Res. 2014;16(2):155‐165. [DOI] [PubMed] [Google Scholar]
- 12. Trindade R, Albrektsson T, Tengvall P, Wennerberg A. Foreign body reaction to biomaterials: on mechanisms for buildup and breakdown of osseointegration. Clin Implant Dent Relat Res. 2016;18(1):192‐203. [DOI] [PubMed] [Google Scholar]
- 13. Mombelli A, Hashim D, Cionca N. What is the impact of titanium particles and biocorrosion on implant survival and complications? A critical review. Clin Oral Implants Res. 2018;29(Suppl 18):37‐53. [DOI] [PubMed] [Google Scholar]
- 14. Albrektsson T, Becker W, Coli P, Jemt T, Mölne J, Sennerby L. Bone loss around oral and orthopedic implants: an immunologically based condition. Clin Implant Dent Relat Res. 2019;21(4):786‐795. [DOI] [PubMed] [Google Scholar]
- 15. Coli P, Jemt T. Are marginal bone level changes around dental implants due to infection? Clin Implant Dent Relat Res. 2021;23(2):170‐177. [DOI] [PubMed] [Google Scholar]
- 16. Williams DF. On the nature of biomaterials. Biomaterials. 2009;30(30):5897‐5909. [DOI] [PubMed] [Google Scholar]
- 17. Franz S, Rammelt S, Scharnweber D, Simon JC. Immune responses to implants—a review of the implications for the design of immunomodulatory biomaterials. Biomaterials. 2011;32(28):6692‐6709. [DOI] [PubMed] [Google Scholar]
- 18. Zhou G, Groth T. Host responses to biomaterials and anti‐inflammatory design—a brief review. Macromol Biosci. 2018;18(8):1800112. [DOI] [PubMed] [Google Scholar]
- 19. Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20(2):86‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gibon E, Córdova LA, Lu L, et al. The biological response to orthopedic implants for joint replacement. II: polyethylene, ceramics, PMMA, and the foreign body reaction. J Biomed Mater Res B Appl Biomater. 2017;105(6):1685‐1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Klopfleisch R, Jung F. The pathology of the foreign body reaction against biomaterials. J Biomed Mater Res A. 2017;105(3):927‐940. [DOI] [PubMed] [Google Scholar]
- 22. Major MR, Wong VW, Nelson ER, Longaker MT, Gurtner GC. The foreign body response: at the interface of surgery and bioengineering. Plast Reconstr Surg. 2015;135(5):1489‐1498. [DOI] [PubMed] [Google Scholar]
- 23. Giannoudis PV, Einhorn TA, Marsh D. Fracture healing: the diamond concept. Injury. 2007;38(Suppl 4):S3‐S6. [DOI] [PubMed] [Google Scholar]
- 24. Enoch S, Leaper DJ. Basic science of wound healing. Surgery. 2008;26(2):31‐37. [Google Scholar]
- 25. Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677‐686. [DOI] [PubMed] [Google Scholar]
- 26. Mahdavian Delavary B, van der Veer WM, van Egmond M, Niessen FB, Beelen RH. Macrophages in skin injury and repair. Immunobiology. 2011;216(7):753‐762. [DOI] [PubMed] [Google Scholar]
- 27. Liu YC, Zou XB, Chai YF, Yao YM. Macrophage polarization in inflammatory diseases. Int J Biol Sci. 2014;10(5):520‐529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Murray PJ. Macrophage polarization. Annu Rev Physiol. 2017;79:541‐566. [DOI] [PubMed] [Google Scholar]
- 29. Julier Z, Park AJ, Briquez PS, Martino MM. Promoting tissue regeneration by modulating the immune system. Acta Biomater. 2017;53:13‐28. [DOI] [PubMed] [Google Scholar]
- 30. Albrektsson T, Jemt T, Mölne J, Tengvall P, Wennerberg A. On inflammation‐immunological balance theory—a critical apprehension of disease concepts around implants: mucositis and marginal bone loss may represent normal conditions and not necessarily a state of disease. Clin Implant Dent Relat Res. 2018;21(1):183‐189. [DOI] [PubMed] [Google Scholar]
- 31. Liddell RS, Ajami E, Li Y, Bajenova E, Yang Y, Davies JE. The influence of implant design on the kinetics of osseointegration and bone anchorage homeostasis. Acta Biomater. 2021;121:514‐526. [DOI] [PubMed] [Google Scholar]
- 32. Brånemark PI, Adell R, Breine U, Hansson BO, Lindström J, Ohlsson A. Intra‐osseous anchorage of dental prostheses: I. Experimental studies. Scand J Plast Reconstr Surg. 1969;3(2):81‐100. [DOI] [PubMed] [Google Scholar]
- 33. Salvi GE, Bosshardt DD, Lang NP, et al. Temporal sequence of hard and soft tissue healing around titanium dental implants. Periodontol 2000. 2015;68(1):135‐152. [DOI] [PubMed] [Google Scholar]
- 34. Schroeder A, Pohler O, Sutter F. Tissue reaction to an implant of a titanium hollow cylinder with a titanium surface spray layer. SSO Schweiz Monatsschr Zahnheilkd. 1976;86(7):713‐727. [PubMed] [Google Scholar]
- 35. Hamlet S, Alfarsi M, George R, Ivanovski S. The effect of hydrophilic titanium surface modification on macrophage inflammatory cytokine gene expression. Clin Oral Implants Res. 2012;23(5):584‐590. [DOI] [PubMed] [Google Scholar]
- 36. Milleret V, Tugulu S, Schlottig F, Hall H. Alkali treatment of microrough titanium surfaces affects macrophage/monocyte adhesion, platelet activation and architecture of blood clot formation. Eur Cell Mater. 2011;21:430‐444. [DOI] [PubMed] [Google Scholar]
- 37. Alfarsi MA, Hamlet SM, Ivanovski S. Titanium surface hydrophilicity modulates the human macrophage inflammatory cytokine response. J Biomed Mater Res A. 2014;102(1):60‐67. [DOI] [PubMed] [Google Scholar]
- 38. Donos N, Hamlet S, Lang NP, et al. Gene expression profile of osseointegration of a hydrophilic compared with a hydrophobic microrough implant surface. Clin Oral Implants Res. 2011;22(4):365‐372. [DOI] [PubMed] [Google Scholar]
- 39. Hamlet S, Ivanovski S. Inflammatory cytokine response to titanium chemical composition and nanoscale calcium phosphate surface modification. Acta Biomater. 2011;7(5):2345‐2353. [DOI] [PubMed] [Google Scholar]
- 40. Hotchkiss KM, Ayad NB, Hyzy SL, Boyan BD, Olivares‐Navarrete R. Dental implant surface chemistry and energy alter macrophage activation in vitro . Clin Oral Implants Res. 2017;28(4):414‐423. [DOI] [PubMed] [Google Scholar]
- 41. Hotchkiss KM, Clark NM, Olivares‐Navarrete R. Macrophage response to hydrophilic biomaterials regulates MSC recruitment and T‐helper cell populations. Biomaterials. 2018;182:202‐215. [DOI] [PubMed] [Google Scholar]
- 42. Hotchkiss KM, Reddy GB, Hyzy SL, Schwartz Z, Boyan BD, Olivares‐Navarrete R. Titanium surface characteristics, including topography and wettability, alter macrophage activation. Acta Biomater. 2016;31:425‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Refai AK, Textor M, Brunette DM, Waterfield JD. Effect of titanium surface topography on macrophage activation and secretion of proinflammatory cytokines and chemokines. J Biomed Mater Res A. 2004;70(2):194‐205. [DOI] [PubMed] [Google Scholar]
- 44. Tan KS, Qian L, Rosado R, Flood PM, Cooper LF. The role of titanium surface topography on J774A.1 macrophage inflammatory cytokines and nitric oxide production. Biomaterials. 2006;27(30):5170‐5177. [DOI] [PubMed] [Google Scholar]
- 45. Chappuis V, Cavusoglu Y, Gruber R, Kuchler U, Buser D, Bosshardt DD. Osseointegration of zirconia in the presence of multinucleated giant cells. Clin Implant Dent Relat Res. 2016;18(4):686‐698. [DOI] [PubMed] [Google Scholar]
- 46. Nishimura I. Genetic networks in osseointegration. J Dent Res. 2013;92(Suppl 12):109S‐118S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ogawa T, Nishimura I. Genes differentially expressed in titanium implant healing. J Dent Res. 2006;85(6):566‐570. [DOI] [PubMed] [Google Scholar]
- 48. Trindade R, Albrektsson T, Galli S, Prgomet Z, Tengvall P, Wennerberg A. Osseointegration and foreign body reaction: titanium implants activate the immune system and suppress bone resorption during the first 4 weeks after implantation. Clin Implant Dent Relat Res. 2018;20(1):82‐91. [DOI] [PubMed] [Google Scholar]
- 49. Trindade R, Albrektsson T, Galli S, Prgomet Z, Tengvall P, Wennerberg A. Bone immune response to materials, part I: titanium, PEEK and copper in comparison to sham at 10 days in rabbit tibia. J Clin Med. 2018;7(12):526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ferreira SD, Silva GL, Cortelli JR, Costa JE, Costa FO. Prevalence and risk variables for peri‐implant disease in Brazilian subjects. J Clin Periodontol. 2006;33(12):929‐935. [DOI] [PubMed] [Google Scholar]
- 51. Shuto T, Wachi T, Shinohara Y, Nikawa H, Makihira S. Increase in receptor activator of nuclear factor κB ligand/osteoprotegerin ratio in peri‐implant gingiva exposed to Porphyromonas gingivalis lipopolysaccharide. J Dent Sci. 2016;11(1):8‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Meyer U, Bühner M, Büchter A, Kruse‐Lösler B, Stamm T, Wiesmann HP. Fast element mapping of titanium wear around implants of different surface structures. Clin Oral Implants Res. 2006;17(2):206‐211. [DOI] [PubMed] [Google Scholar]
- 53. Schliephake H, Reiss G, Urban R, Neukam FW, Guckel S. Metal release from titanium fixtures during placement in the mandible: an experimental study. Int J Oral Maxillofac Implants. 1993;8(5):502‐511. [PubMed] [Google Scholar]
- 54. Wennerberg A, Ide‐Ektessabi A, Hatkamata S, et al. Titanium release from implants prepared with different surface roughness: an in vitro and in vivo study. Clin Oral Implants Res. 2004;15(5):505‐512. [DOI] [PubMed] [Google Scholar]
- 55. Olmedo D, Guglielmotti MB, Cabrini RL. An experimental study of the dissemination of titanium and zirconium in the body. J Mater Sci Mater Med. 2002;13(8):793‐796. [DOI] [PubMed] [Google Scholar]
- 56. Urban RM, Jacobs JJ, Tomlinson MJ, Gavrilovic J, Black J, Peoc'h M. Dissemination of wear particles to the liver, spleen, and abdominal lymph nodes of patients with hip or knee replacement. J Bone Joint Surg Am. 2000;82(4):457‐476. [DOI] [PubMed] [Google Scholar]
- 57. Cionca N, Hashim D, Mombelli A. Zirconia dental implants: where are we now, and where are we heading? Periodontol 2000. 2017;73(1):241‐258. [DOI] [PubMed] [Google Scholar]
- 58. Pettersson M, Kelk P, Belibasakis GN, Bylund D, Molin Thorén M, Johansson A. Titanium ions form particles that activate and execute interleukin‐1β release from lipopolysaccharide‐primed macrophages. J Periodontal Res. 2017;52(1):21‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pioletti DP, Takei H, Kwon SY, Wood D, Sung KL. The cytotoxic effect of titanium particles phagocytosed by osteoblasts. J Biomed Mater Res. 1999;46(3):399‐407. [DOI] [PubMed] [Google Scholar]
- 60. Wang X, Li Y, Feng Y, Cheng H, Li D. Macrophage polarization in aseptic bone resorption around dental implants induced by Ti particles in a murine model. J Periodontal Res. 2019;54(4):329‐338. [DOI] [PubMed] [Google Scholar]
- 61. Guglielmotti MB, Domingo MG, Steimetz T, Ramos E, Paparella ML, Olmedo DG. Migration of titanium dioxide microparticles and nanoparticles through the body and deposition in the gingiva: an experimental study in rats. Eur J Oral Sci. 2015;123(4):242‐248. [DOI] [PubMed] [Google Scholar]
- 62. Okuda‐Shimazaki J, Takaku S, Kanehira K, Sonezaki S, Taniguchi A. Effects of titanium dioxide nanoparticle aggregate size on gene expression. Int J Mol Sci. 2010;11(6):2383‐2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kumazawa R, Watari F, Takashi N, Tanimura Y, Uo M, Totsuka Y. Effects of Ti ions and particles on neutrophil function and morphology. Biomaterials. 2002;23(17):3757‐3764. [DOI] [PubMed] [Google Scholar]
- 64. Barão VA, Mathew MT, Assunção WG, Yuan JC, Wimmer MA, Sukotjo C. The role of lipopolysaccharide on the electrochemical behavior of titanium. J Dent Res. 2011;90(5):613‐618. [DOI] [PubMed] [Google Scholar]
- 65. Weir A, Westerhoff P, Fabricius L, Hristovski K, von Goetz N. Titanium dioxide nanoparticles in food and personal care products. Environ Sci Technol. 2012;46(4):2242‐2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Blum K, Wiest W, Fella C, et al. Fatigue induced changes in conical implant‐abutment connections. Dent Mater. 2015;31(11):1415‐1426. [DOI] [PubMed] [Google Scholar]
- 67. Klotz MW, Taylor TD, Goldberg AJ. Wear at the titanium‐zirconia implant‐abutment interface: a pilot study. Int J Oral Maxillofac Implants. 2011;26(5):970‐975. [PubMed] [Google Scholar]
- 68. Stimmelmayr M, Edelhoff D, Güth JF, Erdelt K, Happe A, Beuer F. Wear at the titanium‐titanium and the titanium‐zirconia implant‐abutment interface: a comparative in vitro study. Dent Mater. 2012;28(12):1215‐1220. [DOI] [PubMed] [Google Scholar]
- 69. He X, Reichl FX, Wang Y, et al. Analysis of titanium and other metals in human jawbones with dental implants—a case series study. Dent Mater. 2016;32(8):1042‐1051. [DOI] [PubMed] [Google Scholar]
- 70. Olmedo DG, Nalli G, Verdú S, Paparella ML, Cabrini RL. Exfoliative cytology and titanium dental implants: a pilot study. J Periodontol. 2013;84(1):78‐83. [DOI] [PubMed] [Google Scholar]
- 71. Mercan S, Bӧlükbaşı N, Bӧlükbaşı MK, Yayla M, Cengiz S. Titanium element level in peri‐implant mucosa. Biotechnol Biotechnol Equip. 2013;27(4):4002‐4005. [Google Scholar]
- 72. Fretwurst T, Buzanich G, Nahles S, Woelber JP, Riesemeier H, Nelson K. Metal elements in tissue with dental peri‐implantitis: a pilot study. Clin Oral Implants Res. 2016;27(9):1178‐1186. [DOI] [PubMed] [Google Scholar]
- 73. Wilson TG Jr, Valderrama P, Burbano M, et al. Foreign bodies associated with peri‐implantitis human biopsies. J Periodontol. 2015;86(1):9‐15. [DOI] [PubMed] [Google Scholar]
- 74. Berryman Z, Bridger L, Hussaini HM, Rich AM, Atieh M, Tawse‐Smith A. Titanium particles: an emerging risk factor for peri‐implant bone loss. Saudi Dent J. 2020;32(6):283‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tawse‐Smith A, Ma S, Duncan WJ, Gray A, Reid MR, Rich AM. Implications of wear at the titanium‐zirconia implant‐abutment interface on the health of peri‐implant tissues. Int J Oral Maxillofac Implants. 2017;32(3):599‐609. [DOI] [PubMed] [Google Scholar]
- 76. Rakic M, Radunovic M, Petkovic‐Curcin A, Tatic Z, Basta‐Jovanovic G, Sanz M. Study on the immunopathological effect of titanium particles in peri‐implantitis granulation tissue: a case‐control study. Clin Oral Implants Res. 2022;33(6):656‐666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Safioti LM, Kotsakis GA, Pozhitkov AE, Chung WO, Daubert DM. Increased levels of dissolved titanium are associated with peri‐implantitis—a cross‐sectional study. J Periodontol. 2017;88(5):436‐442. [DOI] [PubMed] [Google Scholar]
- 78. Brunette DM, Tengvall P, Textor M, Thomsen P, eds Titanium in Medicine: Material Science, Surface Science, Engineering, Biological Responses and Medical Applications. Springer‐Verlag; 2012. [Google Scholar]
- 79. Gittens RA, Olivares‐Navarrete R, Tannenbaum R, Boyan BD, Schwartz Z. Electrical implications of corrosion for osseointegration of titanium implants. J Dent Res. 2011;90(12):1389‐1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Suito H, Iwawaki Y, Goto T, Tomotake Y, Ichikawa T. Oral factors affecting titanium elution and corrosion: an in vitro study using simulated body fluid. PLoS One. 2013;8(6):e66052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Souza JC, Henriques M, Oliveira R, Teughels W, Celis JP, Rocha LA. Do oral biofilms influence the wear and corrosion behavior of titanium? Biofouling. 2010;26(4):471‐478. [DOI] [PubMed] [Google Scholar]
- 82. Mu Y, Kobayashi T, Sumita M, Yamamoto A, Hanawa T. Metal ion release from titanium with active oxygen species generated by rat macrophages in vitro . J Biomed Mater Res. 2000;49(2):238‐243. [DOI] [PubMed] [Google Scholar]
- 83. Cadosch D, Chan E, Gautschi OP, Filgueira L. Metal is not inert: role of metal ions released by biocorrosion in aseptic loosening—current concepts. J Biomed Mater Res A. 2009;91(4):1252‐1262. [DOI] [PubMed] [Google Scholar]
- 84. Mabilleau G, Bourdon S, Joly‐Guillou ML, Filmon R, Baslé MF, Chappard D. Influence of fluoride, hydrogen peroxide and lactic acid on the corrosion resistance of commercially pure titanium. Acta Biomater. 2006;2(1):121‐129. [DOI] [PubMed] [Google Scholar]
- 85. Faverani LP, Barão VA, Ramalho‐Ferreira G, Ferreira MB, Garcia‐Júnior IR, Assunção WG. Effect of bleaching agents and soft drink on titanium surface topography. J Biomed Mater Res B Appl Biomater. 2014;102(1):22‐30. [DOI] [PubMed] [Google Scholar]
- 86. Souza JC, Barbosa SL, Ariza EA, et al. How do titanium and Ti6Al4V corrode in fluoridated medium as found in the oral cavity? An in vitro study. Mater Sci Eng C Mater Biol Appl. 2015;47:384‐393. [DOI] [PubMed] [Google Scholar]
- 87. Mathew MT, Kerwell S, Lundberg HJ, Sukotjo C, Mercuri LG. Tribocorrosion and oral and maxillofacial surgical devices. Br J Oral Maxillofac Surg. 2014;52(5):396‐400. [DOI] [PubMed] [Google Scholar]
- 88. Souza JC, Henriques M, Teughels W, Ponthiaux P, Celis J‐P, Roch LA. Wear and corrosion interactions on titanium in oral environment: literature review. J Bio Tribo Corros. 2015;1:13. [Google Scholar]
- 89. Mathew M, Srinivas Pai P, Pourzal R, Fischer A, Wimmer MA. Significance of tribocorrosion in biomedical applications: overview and current status. Adv Tribol. 2009;2009:250986. [Google Scholar]
- 90. Wang JJ, Sanderson BJ, Wang H. Cyto‐and genotoxicity of ultrafine TiO2 particles in cultured human lymphoblastoid cells. Mutat Res. 2007;628(2):99‐106. [DOI] [PubMed] [Google Scholar]
- 91. Goodman SB, Gibon E, Yao Z. The basic science of periprosthetic osteolysis. Instr Course Lect. 2013;62:201‐206. [PMC free article] [PubMed] [Google Scholar]
- 92. Holt G, Murnaghan C, Reilly J, Meek RM. The biology of aseptic osteolysis. Clin Orthop Relat Res. 2007;460:240‐252. [DOI] [PubMed] [Google Scholar]
- 93. Cionca N, Muller N, Mombelli A. Two‐piece zirconia implants supporting all‐ceramic crowns: a prospective clinical study. Clin Oral Implants Res. 2015;26(4):413‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Jiang W, Mashayekhi H, Xing B. Bacterial toxicity comparison between nano‑ and micro‐scaled oxide particles. Environ Pollut. 2009;157(5):1619‐1625. [DOI] [PubMed] [Google Scholar]
- 95. Leonhardt Å, Dahlén G. Effect of titanium on selected oral bacterial species in vitro . Eur J Oral Sci. 1995;103(6):382‐387. [DOI] [PubMed] [Google Scholar]
- 96. Lavaee F, Faez K, Faez K, Hadi N, Modaresi F. Antimicrobial and antibiofilm activity of silver, titanium dioxide and iron nano particles. Am J Dent. 2016;29(6):315‐320. [PubMed] [Google Scholar]
- 97. Vargas‐Reus MA, Memarzadeh K, Huang J, Ren GG, Allaker RP. Antimicrobial activity of nanoparticulate metal oxides against peri‐implantitis pathogens. Int J Antimicrob Agents. 2012;40(2):135‐139. [DOI] [PubMed] [Google Scholar]
- 98. Tabrez Khan S, Al‐Khedhairy A, Musarrat J. ZnO and TiO2 nanoparticles as novel antimicrobial agents for oral hygiene: a review. J Nanopart Res. 2015;17:276‐291. [Google Scholar]


