Abstract
Background and Aims
Autoimmune hepatitis (AIH) is a chronic inflammatory liver disease associated with an increased prevalence of extrahepatic autoimmune diseases and an increased mortality compared with the general population. The contribution of extrahepatic autoimmune diseases to the increased mortality has not been clarified. Our aim was to determine the effect of extrahepatic autoimmune diseases on mortality in AIH patients.
Methods
This nationwide register‐based cohort study included all Danish patients diagnosed with AIH between 1995 and 2019. We examined the presence of extrahepatic autoimmune diseases and compared the mortality between AIH patients with and without extrahepatic autoimmune diseases. We adjusted our analysis for age, sex, calendar year of AIH diagnosis, cirrhosis, cancer, chronic obstructive pulmonary disease and ischaemic heart disease.
Results
We included 2479 AIH patients of whom 19.8% had one extrahepatic autoimmune disease and 3.3% had multiple. The adjusted 10‐year cumulative mortality was 27.2% (95% confidence interval [CI]: 25.2–29.4) for patients with extrahepatic autoimmune diseases and 21.6% (95% CI: 19.9–23.6) for patients without. The adjusted mortality hazard ratio was 1.30 (95% CI: 1.12–1.52) for AIH patients with versus without extrahepatic autoimmune diseases; it was 1.25 (95% CI: 1.06–1.48) for patients with one extrahepatic autoimmune disease and 1.54 (95% CI: 1.15–2.05) for those with more than one.
Conclusions
Extrahepatic autoimmune diseases increased the mortality in patients with AIH. Patients with multiple extrahepatic autoimmune diseases had a higher mortality than patients with just one extrahepatic autoimmune disease.
Keywords: autoimmune diseases, autoimmune hepatitis, epidemiology, mortality, prognosis, registries
Abbreviations
- AIH
autoimmune hepatitis
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- EAD
extrahepatic autoimmune disease
- HR
hazard ratio
- ICD‐10
International Classification of Disease revision 10
- IPW
inverse probability weighting
- IQR
interquartile range
Key points.
Autoimmune hepatitis (AIH) is a chronic autoimmune liver disease.
Patients with AIH have a higher risk of death and a higher risk of having autoimmune diseases outside the liver compared with the general population.
This study followed all Danish patients with AIH and found that those with autoimmune diseases outside the liver, particularly those with multiple such diseases, had a higher risk of death than other patients with AIH. These findings contribute to our understanding of AIH.
1. INTRODUCTION
Autoimmune hepatitis (AIH) is a chronic inflammatory liver disease associated with an increased prevalence of extrahepatic autoimmune diseases (EADs) 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 and with an increased mortality compared with the general population. 12 , 13 , 14 However, it is unclear whether EADs contribute to the observed increase in mortality for patients with AIH. Only one study assessed the mortality of patients with AIH comparing those with and without EADs. 5 Wong et al. included a cohort of 562 patients from two tertiary centres. The study found no statistically significant difference in mortality in AIH patients with or without EADs. Moreover, Wong et al. did not evaluate the effect of multiple EADs on mortality, the authors did not present confounder‐adjusted analyses of the association between EADs and mortality, and it is unclear whether EADs diagnosed after AIH were included in the survival analysis. Therefore, the question about the effect of EADs on mortality remains unsettled. The answer to that question is important for our understanding of the clinical course of AIH and will enable clinicians to respond to patients' concerns about EADs' effect on mortality.
Given this background, we examined the association between EADs and all‐cause mortality in a nationwide Danish cohort of patients with AIH. Our hypothesis was that the mortality is higher in AIH patients with EADs compared with those without and that the presence of multiple EADs will increase the mortality further.
2. METHODS
2.1. Data sources
This study was based exclusively on data from the Danish national healthcare registries, and therefore it did not need permission from an ethics committee according to Danish law. The Danish National Patient Registry provides information on outpatient visits, inpatient admissions, discharges and discharge diagnoses. Every encounter with the healthcare system is recorded and includes a primary diagnosis that defines the main reason for the contact, and coexisting conditions are specified as secondary diagnoses. All diagnoses have been coded according to the International Classification of Disease, revision 10 (ICD‐10), since 1994. 15 , 16 We acquired data on histopathological evaluations from The Danish National Pathology Registry based on the SNOMED classification. 17 The Danish Civil Registration System provides dates of birth, death, and migration for all Danish citizens, and personal identification numbers allow individual‐level linkage of data across the registries. 18
2.2. Study population
We identified all Danish patients diagnosed with AIH (ICD‐10: K75.4 or K73.2) between 1 January 1995 and 31 December 2019. Primary and secondary diagnosis codes for AIH were included. The time of diagnosis with AIH was defined as the earliest date of an inpatient admission or outpatient visit resulting in a diagnosis code of AIH. We defined an ‘index date’ as the date six months after the time of AIH diagnosis. We excluded patients from the study population if they had been diagnosed with toxic liver disease, alcoholic liver disease, or viral hepatitis between 1 January 1994 and the index date (Table S1). These exclusion criteria were added to enhance the validity of the AIH diagnoses.
2.3. Definition of EADs
We defined 26 EADs based on primary and secondary ICD‐10 codes given between 1 January 1994 and 31 December 2019 (Table S1). All included patients were evaluated for the presence of diagnosed EADs and on this basis categorized as having or not having EADs concurrent with AIH. Additionally, we subdivided all patients as having none, one, or multiple EADs. This subdivision was added to assess the effect of multiple concurrent EADs on mortality.
2.4. Statistical analyses
The cumulative all‐cause mortality was calculated using the Kaplan–Meier estimator, and this analysis included EADs diagnosed between 1 January 1994 and the index date. We followed the patients from the index date to death, and surviving patients were censored at the study end on 30 April 2021 or at emigration. We considered the possibility that patients with or without EADs on the index date differed according to age, sex, calendar year of AIH diagnosis, and the presence of cirrhosis, cancer, chronic obstructive pulmonary disease (COPD) and ischaemic heart diseases, so we adjusted the Kaplan–Meier estimator for these characteristics using inverse probability weighting (IPW). 19 Cirrhosis was included as an indicator for the severity of AIH. Cancer, COPD and ischaemic heart disease were included because they represent three common causes of death in Denmark, 20 and they might be more prevalent in patients with EADs. We identified cirrhosis, cancer, COPD, and ischaemic heart disease based on ICD‐10 diagnosis codes received between 1 January 1994 and the index date (Table S1). χ2‐test and Wilcoxon rank‐sum test were used for a comparison of these characteristics in patients with and without EADs (Table 1).
TABLE 1.
Characteristics of autoimmune hepatitis (AIH) study population with or without extrahepatic autoimmune diseases (EADs) on the index date
| With EADs | Without EADs | Total | p value | |
|---|---|---|---|---|
| Number of AIH patients | 573 | 1906 | 2479 | N/A |
| Sex; n (%) | .390 | |||
| Female | 416 (72.6) | 1418 (74.4) | 1834 (74.0) | |
| Male | 157 (27.4) | 488 (25.6) | 645 (26.0) | |
| Age at AIH diagnosis; n (%) | ||||
| <25 | 99 (17.3) | 253 (13.3) | 352(14.2) | .016 |
| 25–49 | 178 (31.1) | 546 (28.7) | 724 (29.2) | .264 |
| 50–74 | 254 (44.3) | 916 (48.1) | 1170 (47.2) | .117 |
| 75< | 42 (7.3) | 191 (10.0) | 233 (9.4) | .053 |
| Median age (interquartile range) | 51 (32–63) | 54 (38–66) | 53 (36–66) | <.001* |
| Calendar year of AIH diagnosis; n (%) | ||||
| 1995–1999 | 62 (10.8) | 315 (16.5) | 377 (15.2) | .001 |
| 2000–2004 | 56 (9.8) | 299 (15.7) | 355 (14.3) | <.001 |
| 2005–2009 | 113 (19.7) | 353 (18.5) | 466 (18.8) | .519 |
| 2010–2014 | 166 (29.0) | 504 (26.4) | 670 (27.0) | .232 |
| 2015–2020 | 176 (30.7) | 435 (22.8) | 611 (24.7) | <.001 |
| Severity of AIH; n (%) | .217 | |||
| Cirrhosis | 70 (12.2) | 198 (10.4) | 268 (10.8) | |
| No cirrhosis | 503 (87.8) | 1708 (89.6) | 2211 (89.2) | |
| Co‐morbidities; n (%) | ||||
| Cancer | 50 (8.7) | 150 (7.9) | 200 (8.1) | .509 |
| Ischaemic heart disease | 49 (8.6) | 115 (6.0) | 164 (6.6) | .033 |
| Chronic obstructive pulmonary disease | 26 (4.5) | 58 (3.0) | 84 (3.4) | .083 |
Note: p values are estimated using χ2 test and *Wilcoxon rank‐sum test. Values in bold are significant (p < .05).
Some patients might be diagnosed with EADs during the follow‐up, and this may affect their mortality. We estimated the cumulative incidence of being diagnosed with a new EAD after the index date for patients without EADs at the index date or with one EAD at the index date. The analysis was conducted using ‘cumulative incidence estimations in the presence of competing risks’, 21 with death without a new diagnosis of EADs after the index date as the competing event. We adjusted the analysis for age at the time of AIH diagnosis, sex, calendar year of AIH diagnosis, cirrhosis, cancer, COPD and ischaemic heart disease.
We also conducted a time‐dependent Cox proportional hazards model with the status of EADs, that is none, one or multiple EADs, as a time‐dependent variable. This analysis included all EADs diagnosed between 1 January 1994 and 31 December 2019, and surviving patients were censored on 31 December 2019. The time‐dependent Cox proportional hazards model included age at the time of AIH diagnosis, sex, calendar year of AIH diagnosis, and cirrhosis, cancer, COPD and ischaemic heart disease as time‐dependent variables in the adjustments.
2.5. Sensitivity analysis
We conducted two sensitivity analyses to examine the effects of the choices made regarding study design. Firstly, we restricted our analyses to patients with AIH verified by a liver biopsy. Patients with a code for a liver biopsy (SNOMED code: T56xxx) less than eight months before the index date were defined as having a biopsy‐verified AIH diagnosis. Secondly, we restricted our analyses to patients who had received a primary diagnosis code for AIH.
3. RESULTS
We identified 2619 Danish patients (73.9% women) diagnosed with AIH from 1 January 1995 to 31 December 2019 of whom 2479 were alive on the index date and available for follow‐up. We found that 23.1% (n = 573) of them had EADs on the index date; of whom 85.7% (n = 491) had one EAD; and 14.3% (n = 82) had multiple EADs. Patients with EADs were younger than patients without EADs, yet, they had a higher prevalence of cancer, COPD, and ischaemic heart disease (Table 1). The median age of patients with EADs was 51 years (interquartile range [IQR]: 32–63), and of patients without EADs, it was 54 years (IQR: 38–66). Inflammatory bowel disease was the most common EAD, followed by insulin‐dependent diabetes mellitus and rheumatoid arthritis (Table 2).
TABLE 2.
Extrahepatic autoimmune diseases diagnosed in Danish patients with autoimmune hepatitis between 1 January 1994 and 31 December 2019
| Extrahepatic autoimmune diseases | Number of patients; n (%) |
|---|---|
| Autoimmune skin disease | |
| Pemphigus and pemphigoid | 5 (0.5) |
| Psoriasis and psoriatic arthritis | 46 (4.3) |
| Vitiligo | 2 (0.2) |
| Autoimmune haematological disease | |
| Autoimmune haemolytic anaemia | 21 (2.0) |
| Idiopathic thrombocytopenic purpura | 13 (1.2) |
| Pernicious anaemia | 9 (0.9) |
| Autoimmune gastrointestinal disease | |
| Celiac disease and dermatitis herpetiformis | 37 (3.5) |
| Inflammatory bowel disease (IBD) | 233 (22.0) |
| Autoimmune thyroid disease | |
| Grave's disease | 56 (5.3) |
| Hashimoto's thyroiditis | 33 (3.1) |
| Autoimmune connective tissue disorder | |
| Ankylosing spondylitis (mb. Bechterew) | 11 (1.0) |
| Granulomatous polyangiitis | 5 (0.5) |
| Juvenile arthritis | 6 (0.6) |
| Polyarteritis nodosa | 2 (0.2) |
| Polymyalgia rheumatica and temporal arteritis | 45 (4.2) |
| Rheumatoid arthritis | 128 (12.1) |
| Sarcoidosis | 30 (2.8) |
| Sjogren's syndrome | 84 (7.9) |
| Systemic lupus erythematosus and local lupus erythematosus | 66 (6.2) |
| Systemic sclerosis (scleroderma) | 15 (1.4) |
| Endocrinological disease | |
| Addison's disease | 16 (1.5) |
| Insulin‐dependent diabetes mellitus | 162 (15.3) |
| Autoimmune neurological disease | |
| Guillain‐Barré syndrome | 5 (0.5) |
| Multiple sclerosis | 15 (1.4) |
| Autoimmune muscle disease | |
| Myasthenia gravis | 5 (0.5) |
| Polymyositis/dermatomyositis | 11 (1.0) |
| Total | 1061 (100) |
We had 4788 years of follow‐up on patients with EADs at the index date and 18 910 years for those without. A total of 642 patients died during the follow‐up; 506 patients did not have EADs at the index date, and 136 did have EADs at the index date. The unadjusted all‐cause mortality 10 years after the index date was 22.0% (95% confidence interval [CI]: 20.0–24.2) in patients without EADs and 25.2% (95% CI: 21.1–29.9) in patients with EADs. The confounder‐adjusted estimates of all‐cause mortality 10 years after the index date were 21.6% (95% CI: 19.9–23.6) in patients without EADs and 27.2% (95% CI: 25.2–29.4) in patients with EADs (Table 3). The adjusted 10‐year all‐cause mortality for patients with one EAD was 26.1% (95% CI: 24.0–28.4) and 35.0% (95% CI: 28.9–41.9) for patients with multiple EADs (Figure 1).
TABLE 3.
Cumulative all‐cause mortality after 1, 5 and 10 years in patients with autoimmune hepatitis (AIH) with or without extrahepatic autoimmune diseases (EADs)
| AIH patients without EADs | AIH patients with EADs | |||
|---|---|---|---|---|
| Crude (95% CI) | Crude (95% CI) | |||
| Total | Total | One | Multiple | |
| 1 year | 2.7 (2.0–3.5) | 2.8 (1.7–4.5) | 2.7 (1.6–4.5) | 3.7 (1.2–10.9) |
| 5 years | 12.1 (10.6–13.7) | 12.4 (9.9–15.6) | 12.0 (9.3–15.4) | 15.0 (8.8–24.9) |
| 10 years | 22.0 (20.0–24.2) | 25.2 (21.1–29.9) | 23.6 (19.3–28.6) | 35.4 (23.4–51.1) |
| Adjusted (95% CI) a | Adjusted (95% CI) a | |||
|---|---|---|---|---|
| Total | Total | One | Multiple | |
| 1 year | 2.7 (2.1–3.4) | 3.1 (2.5–3.9) | 3.0 (2.4–3.8) | 3.5 (2.0–6.1) |
| 5 years | 11.9 (10.6–13.3) | 13.7 (12.3–15.1) | 13.3 (11.8–14.8) | 15.8 (12.3–20.2) |
| 10 years | 21.6 (19.9–23.6) | 27.2 (25.2–29.4) | 26.1 (24.0–28.4) | 35.0 (28.9–41.9) |
Abbreviation: CI, confidence interval.
Adjusted for age at AIH diagnosis, calendar year of AIH diagnosis, sex, cirrhosis, cancer, chronic obstructive pulmonary disease and ischaemic heart disease using inverse probability weighting.
FIGURE 1.
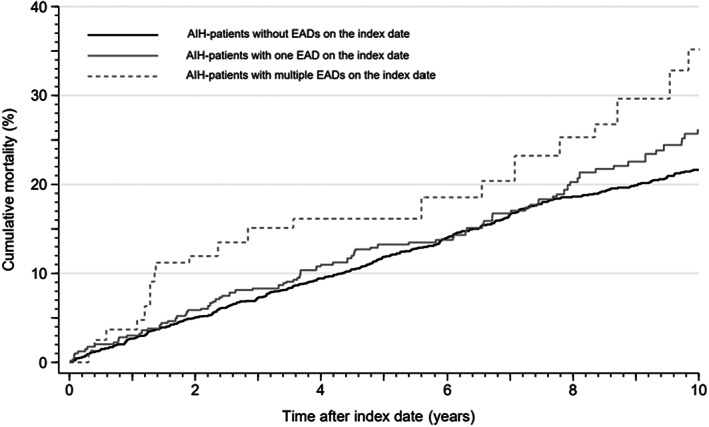
The adjusted Kaplan–Meier curve of the cumulative all‐cause mortality in autoimmune hepatitis (AIH) patients with none, one or multiple extrahepatic autoimmune diseases (EADs) on the index date. The Kaplan–Meier curve was adjusted for age at the time of AIH diagnosis, sex, calendar year at the time of AIH diagnosis, cirrhosis, cancer, chronic obstructive pulmonary disease and ischaemic heart disease using inverse probability weighting.
The cumulative incidence of being diagnosed with a new EAD within 5 years after the index date was 7.3% (95% CI: 6.2–8.6) for AIH patients without EADs on the index date (n = 1906) and 4.8% (95% CI: 3.1–7.1) for AIH patients with one EAD on the index date (n = 491). The 10‐year risks were 11.6% (95% CI: 10.1–13.2) and 8.7% (95% CI: 6.0–11.9), respectively (Figure 2).
FIGURE 2.
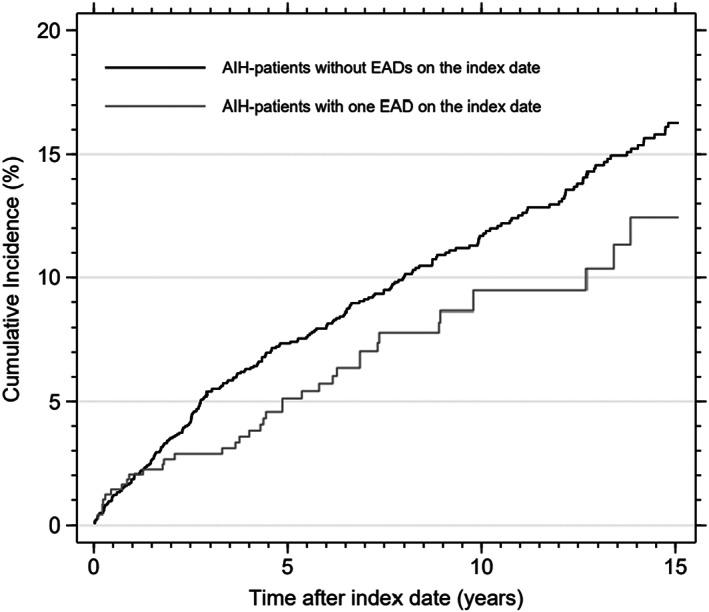
The cumulative incidence of being diagnosed with a new extrahepatic autoimmune disease (EAD) after the index date in patients with autoimmune hepatitis (AIH) stratified on not having EADs on the index date or having one EAD on the index date.
The time‐dependent mortality hazard ratios (HRs) included all 2619 Danish patients with AIH of whom 853 patients (32.6%) were diagnosed with one or multiple EADs between 1 January 1994 and 31 December 2019; 689 patients were diagnosed with only one EAD, and 164 patients were diagnosed with multiple EADs. The adjusted time‐dependent mortality HR was 1.30 (95% CI: 1.12–1.52) for having versus not having EADs concurrent with AIH, 1.25 (95% CI: 1.06–1.48) for having only one EAD versus not having EADs and 1.54 (95% CI: 1.15–2.05) for having multiple EADs versus not having EADs (Table 4).
TABLE 4.
Mortality hazard ratios (HRs) for autoimmune hepatitis (AIH) patients with versus without extrahepatic autoimmune diseases (EADs)
| AIH patients with vs. without EADs | ||
|---|---|---|
| Crude HR (95% CI) | Adjusted HR (95% CI) a | |
| One or multiple EADs | 1.20 (1.03–1.40) | 1.30 (1.12–1.52) |
| One EAD | 1.14 (0.96–1.34) | 1.25 (1.06–1.48) |
| Multiple EADs | 1.54 (1.16–2.04) | 1.54 (1.15–2.05) |
Note: All diagnoses of EADs before and after the index date were included as a time‐dependent variable.
Abbreviation: Cl, confidence interval.
Adjusted for age at AIH diagnosis, calendar year of AIH diagnosis, sex, cirrhosis, cancer, chronic obstructive pulmonary disease and ischaemic heart disease.
3.1. Sensitivity analysis
A total of 1643 (62.7%) AIH patients had a liver biopsy‐verified AIH diagnosis. This restricted cohort had a slightly lower mortality than the full cohort evaluated in our primary analyses. Restricting the cohort to the 2361 (90.1%) patients with a primary AIH diagnosis did not change our estimates (Table S2).
4. DISCUSSION
This nationwide study of Danish patients with AIH showed that the mortality was slightly increased in AIH patients with EADs compared with AIH patients without EADs. Our findings further showed that patients with multiple EADs had higher mortality than those with only one EAD.
Our study was based on data from the well‐established Danish healthcare registries, and reporting to the registries is mandatory. In addition, Denmark has free healthcare that provides a good basis for all Danish citizens to be diagnosed and treated in case of disease irrespective of their socio‐economic status. 15 Considering this, it is reasonable to presume that all Danish citizens diagnosed with AIH are registered in the Danish Healthcare registries and thereby included in our study population.
The validity of data in the Danish healthcare registries is high. 15 , 22 However, a key concern is whether misdiagnosed patients without AIH were included in our study population. When we explored this concern by restricting our study population to patients with a liver biopsy‐verified AIH diagnosis, we observed a slight decrease in the 1‐, 5‐, and 10‐year mortality leading to a slight increase in the effect of EADs on mortality, yet no clinically significant change in the estimates was observed. When we restricted our cohort to primary diagnoses of AIH, we still found no particular change in our estimates. Given this, we surmise that any inclusion of patients without AIH did not materially affect our findings.
We conducted adjusted analyses including the major confounders of sex, age and calender year of AIH diagnosis, the severity of AIH (expressed as cirrhosis) and the co‐morbidities of cancer, COPD and ischaemic heart disease. However, confounding attributable to differences in lifestyle, physical activity or nutrition may remain. We found that AIH patients with EADs had a higher prevalence of cancer, COPD, and ischaemic heart disease, although they were younger. We argue that to some extent, we adjusted for the differences in everyday lifestyle caused by these co‐morbidities when adjusting for them. Yet, it is plausible that patients with EADs differ from those without EADs in their general habits given that their everyday life might directly be affected by the symptoms or treatment of their EADs. Therefore, some of the excess mortality that we attribute to EADs might in reality be attributable to a less healthy lifestyle.
Our study is the first to explore the effect of EADs on mortality in a nationwide cohort of AIH patients. The only other study by Wong et al. investigated the difference in mortality between AIH patients from tertiary centres with or without EADs. 5 Wong et al. did not find an effect of EADs on mortality in patients with AIH, and overall, they found lower mortality among AIH patients with and without EADs than we did. The disparities might be explained by a difference in the definition of EADs, cohort selection and adjustments for confounders. Wong et al. found a larger proportion of AIH patients with EADs compared with our findings (42% in their study vs. 32.6% in our study). A possible explanation for this difference is that they defined 58 EADs, and our study only defined 26. Wong et al. included a cohort of patients from two tertiary centres, whereas our cohort was nationwide, thus, representing a broad spectrum of AIH patients corresponding to the wide range of clinical presentations and clinical courses. AIH patients with milder manifestations of EADs compared with our study population might have been included in Wong et al. maybe because of a more thorough screening for EADs in the United Kingdom tertiary centres than in Denmark as a whole. Furthermore, Wong et al. did not adjust for confounders in the survival analysis in contrast to our calculations that adjusted for age, sex, calendar year of AIH diagnosis, cirrhosis and co‐morbidities. Finally, it is unclear whether Wong et al. evaluated the mortality with the inclusion of all EADs diagnosed both before and after AIH, whereas we evaluated the effect of both perspectives.
The effect of multiple EADs on mortality in AIH patients was not assessed by Wong et al., and we were the first to evaluate this aspect. We found a 10‐year mortality of 35% in patients with multiple EADs. The patients in our study had up to five different EADs, but most frequently only two concurrent EADs were observed, and we did not find a pattern in the combination of multiple EADs. The most common combination of two EADs was found in 7 out of 82 patients and consisted of systemic/local lupus erythematosus and rheumatoid arthritis.
This study improves the understanding of EADs' effect on mortality in AIH patients, and our findings will enable clinicians to provide answers for patients regarding their mortality risk. Our findings suggest that AIH patients with multiple EADs have increased mortality. The excess mortality might partly be explained by symptoms or treatment of EADs resulting in a less healthy lifestyle: for instance, this might be the case for rheumatoid arthritis restraining the patient's ability to be physically active, or the general fatigue associated with many inflammatory diseases also limiting physical activity, or it may be high‐dose steroid treatment for rheumatic diseases resulting in obesity. It could also be that patients with multiple autoimmune diseases are more difficult to treat due to polypharmacy and pharmacological interaction and thereby decreased patient compliance—but we do not have the data to pursue such speculations. Nevertheless, our findings are clinically relevant to the patients and their clinicians and suggest the need for thorough interdisciplinary clinical care for these patients.
In conclusion, this nationwide study found that EADs increase mortality among patients with AIH. The effect is small for single EADs, whereas multiple EADs have a clinically significant effect.
FUNDING INFORMATION
This study is funded by the Novo Nordisk Foundation and the A.P. Møller Foundation for the Advancement of Medical Science.
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interest with respect to this study.
Supporting information
Table S1
Table S2
Birn‐Rydder R, Jensen MD, Jepsen P, Grønbæk L. Extrahepatic autoimmune diseases in autoimmune hepatitis: Effect on mortality. Liver Int. 2022;42:2466‐2472. doi: 10.1111/liv.15382
Handling Editor: Alessio Aghemo
DATA AVAILABILITY STATEMENT
According to Danish law, we can only access the data through a secure connection to a remote server hosted by The Danish Health Data Authority. We cannot download the data, let alone share it. We will perform additional analyses and share our code upon reasonable request.
REFERENCES
- 1. Floreani A, De Martin S, Secchi MF, Cazzagon N. Extrahepatic autoimmunity in autoimmune liver disease. Eur J Intern Med. 2019;59:1‐7. [DOI] [PubMed] [Google Scholar]
- 2. Fogel R, Comerford M, Chilukuri P, Orman E, Chalasani N, Lammert C. Extrahepatic autoimmune diseases are prevalent in autoimmune hepatitis patients and their first‐degree relatives: survey study. Interact J Med Res. 2018;7(2):e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grønbæk L, Vilstrup H, Pedersen L, Jepsen P. Extrahepatic autoimmune diseases in patients with autoimmune hepatitis and their relatives: a Danish nationwide cohort study. Liver Int. 2019;39(1):205‐214. [DOI] [PubMed] [Google Scholar]
- 4. Guo L, Zhou L, Zhang N, Deng B, Wang B. Extrahepatic autoimmune diseases in patients with autoimmune liver diseases: a phenomenon neglected by gastroenterologists. Gastroenterol Res Pract. 2017;2017:2376231‐2376237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wong GW, Yeong T, Lawrence D, Yeoman AD, Verma S, Heneghan MA. Concurrent extrahepatic autoimmunity in autoimmune hepatitis: implications for diagnosis, clinical course and long‐term outcomes. Liver Int. 2017;37(3):449‐457. [DOI] [PubMed] [Google Scholar]
- 6. Efe C, Wahlin S, Ozaslan E, et al. Autoimmune hepatitis/primary biliary cirrhosis overlap syndrome and associated extrahepatic autoimmune diseases. Eur J Gastroenterol Hepatol. 2012;24(5):531‐534. [DOI] [PubMed] [Google Scholar]
- 7. Manns MP, Lohse AW, Vergani D. Autoimmune hepatitis—update 2015. J Hepatol. 2015;62(Suppl 1):S100‐S111. [DOI] [PubMed] [Google Scholar]
- 8. Lohse AW, Mieli‐Vergani G. Autoimmune hepatitis. J Hepatol. 2011;55(1):171‐182. [DOI] [PubMed] [Google Scholar]
- 9. Tanaka A. Autoimmune hepatitis: 2019 update. Gut Liver. 2020;14(4):430‐438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wong GW, Heneghan MA. Association of Extrahepatic Manifestations with autoimmune hepatitis. Dig Dis. 2015;33(Suppl 2):25‐35. [DOI] [PubMed] [Google Scholar]
- 11. Sucher E, Sucher R, Gradistanac T, Brandacher G, Schneeberger S, Berg T. Autoimmune hepatitis‐immunologically triggered liver pathogenesis‐diagnostic and therapeutic strategies. J Immunol Res. 2019;2019:9437043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ngu JH, Gearry RB, Frampton CM, Stedman CA. Mortality and the risk of malignancy in autoimmune liver diseases: a population‐based study in Canterbury, New Zealand. Hepatology. 2012;55(2):522‐529. [DOI] [PubMed] [Google Scholar]
- 13. Sharma R, Verna EC, Söderling J, Roelstraete B, Hagström H, Ludvigsson JF. Increased mortality risk in autoimmune hepatitis: a Nationwide population‐based cohort study with histopathology. Clin Gastroenterol Hepatol. 2021;19(12):2636‐2647.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grønbæk L, Vilstrup H, Jepsen P. Autoimmune hepatitis in Denmark: incidence, prevalence, prognosis, and causes of death. A nationwide registry‐based cohort study. J Hepatol. 2014;60(3):612‐617. [DOI] [PubMed] [Google Scholar]
- 15. Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39(Suppl 7):30‐33. [DOI] [PubMed] [Google Scholar]
- 17. Bjerregaard B, Larsen OB. The Danish Pathology Register. Scand J Public Health. 2011;39(Suppl 7):72‐74. [DOI] [PubMed] [Google Scholar]
- 18. Schmidt M, Pedersen L, Sørensen HT. The Danish Civil Registration system as a tool in epidemiology. Eur J Epidemiol. 2014;29(8):541‐549. [DOI] [PubMed] [Google Scholar]
- 19. Hernán MA, Robins JM. Causal Inference: What If. Chapman & Hall/CRC; 2020. [Google Scholar]
- 20. Statistics Denmark . Deaths. Accessed April 4, 2022. https://www.dst.dk/en/Statistik/emner/borgere/befolkning/doedsfald
- 21. Cleves M, Gould WW, Marchenko YV, eds. Competing risks. In: An Introduction to Survival Analysis Using Stata. 3rd ed. Stata Press; 2016:381‐408. [Google Scholar]
- 22. Andersen TF, Madsen M, Jørgensen J, Mellemkjoer L, Olsen JH. The Danish National Hospital Register. A valuable source of data for modern health sciences. Dan Med Bull. 1999;46(3):263‐268. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Data Availability Statement
According to Danish law, we can only access the data through a secure connection to a remote server hosted by The Danish Health Data Authority. We cannot download the data, let alone share it. We will perform additional analyses and share our code upon reasonable request.


