Summary
Background
Eczema is a chronic inflammatory skin disease. Domestic water with high mineral content (hard water) is a risk factor for eczema in children, but this association has not been assessed in adults.
Objectives
To examine the association between domestic hard water supply and eczema prevalence and incidence in adults aged 40–69 years and the contextual effect in eczema outcomes by postcode in adults in the UK.
Methods
We used data from the UK Biobank study collected in 2006–10 (baseline) and 2013–14 (follow‐up). Eczema prevalence at baseline (2006–10) and at follow‐up (2013–14) and incidence (new onset between baseline and follow‐up) were determined from the touchscreen questionnaires and nurse‐led interviews. Domestic hard water information was obtained in 2005 and 2013 from the local water supply companies in England, Wales and Scotland as CaCO3 concentrations. We fitted multilevel logistic regression models with random intercepts for postcode areas to examine the effect of domestic hard water on eczema outcomes, and we measured components of variance.
Results
In total, 306 531 participants with a mean age of 57 years nested across 7642 postcodes were included in the baseline analysis, and 31 036 participants nested across 3695 postcodes were included in the follow‐up analysis. We observed an increase in the odds of eczema at baseline [odds ratio (OR) 1·02, 95% confidence interval (CI) 1·01–1·04] per 50 mg L−1 of CaCO3 increase. Furthermore, exposure to domestic hard water (> 200 mg L−1 of CaCO3) was associated with increased odds of prevalent eczema at baseline (OR 1·12, 95% CI 1·04–1·22). Moreover, there was a significant linear trend (P < 0·001) in which increasing levels of hard water increased eczema prevalence risk. No association was observed with incident eczema or eczema at follow‐up. The intraclass correlation coefficient for postcode was 1·6% (95% CI 0·7–3·4), which remained unexplained by area‐level socioeconomic measures.
Conclusions
Increasing levels of domestic hard water, as measured by CaCO3 concentrations, were associated with an increased prevalence of eczema in adults but not increased incidence. Ongoing efforts to reduce hard water exposure may have a beneficial effect in reducing the burden of eczema in adults. Further research is needed to explore area‐level factors that may lead to eczema.
What is already known about this topic?
Hard water is formed when minerals are dissolved in water from filtration through sedimentary rocks.
Several studies have reported a higher prevalence of eczema in areas with hard water.
However, all studies on this topic have assessed this in infants and school‐aged children, while this association has not been explored in adults.
What does this study add?
Our findings suggest that exposure to higher concentrations of domestic hard water is associated with an increase in eczema prevalence in adults aged 40–69 years.
Ongoing efforts to reduce hard water exposure may have a beneficial effect in reducing eczema prevalence in adults.
Several studies have reported a higher prevalence of eczema in areas with hard water, high mineral content water, in infants and school‐aged children. However, this association has not been explored in adults before. Our findings suggest that higher domestic hard water concentration exposure was associated with an increase in odds of eczema in middle‐aged adults. Ongoing efforts to reduce hard water exposure may have a beneficial effect in reducing eczema prevalence in adults.

Plain language summary available online
Eczema (atopic dermatitis) is a chronic inflammatory skin disease characterized by defective skin barrier function. 1 Eczema has a heterogeneous presentation that varies by its severity, age of onset, response to treatment, and tendency to develop further atopic comorbidities. 2 Globally, it affects around 5–10% of adults and up to 20% of children. 3 Eczema is determined by an interplay of genetics, immunity and environmental factors. 2 , 4 , 5
One of the proposed environmental risk factors for eczema is domestic hard water, 6 which is water with a high mineral content. Hard water is formed when minerals are dissolved in water from filtration through sedimentary rocks. 7 The key minerals that constitute hard water are calcium in the form of calcite (CaCO3) and dolomite [CaMg(CO3)2], which are mostly associated with calcareous rocks and limestone. 8 According to the World Health Organization (WHO), domestic water is hard when the concentration of CaCO3 is > 200 mg L−1, which leads to the need for use of excess soap to achieve lather, and the formation of soap film (calcium stearate) on skin and clothes. 8 , 9
A number of studies in children have observed a higher prevalence of eczema in areas with hard water. 10 , 11 , 12 A study of UK primary‐school children aged 6–7 years suggested that those living with domestic hard water had an increased prevalence of eczema. 10 Likewise, a study conducted in Spain showed that the lifetime prevalence of eczema in schoolchildren aged 6–7 years was higher in those who lived in areas with hard water. 11 A case–control study of 80 young adults with a mean age of 26 years showed that skin sites washed with hard water significantly increased sodium lauryl sulfate (SLS) deposits, which in turn increased transepidermal water loss and caused skin irritation. 12
All of the studies in this area have assessed the impact of hard water on eczema in infants and school‐age children, while this association has not been explored in adults. 13 Consequently, a better understanding of the association between domestic hard water and adult eczema may provide evidence to generate targeted interventions to decrease eczema flares and severity. We therefore examined the association between domestic hard water supply and eczema outcomes (prevalence and incidence) in adults aged 40–69 years and the contextual associations by postcode.
Patient and methods
Design
The UK Biobank study is a population‐based prospective cohort with the aim of improving the prevention, diagnosis and treatment of a varied range of serious chronic diseases. 14 The UK Biobank represents the general population in the UK. It aimed to be as inclusive as reasonably possible, with all people aged 40–69 years who were registered with the National Health Service and living up to about 25 miles from one of the 22 study assessment centres in Scotland, England and Wales invited to participate. 14 The initial assessment visits were performed between 2006 and 2010; more details are provided elsewhere. 14 Detailed data on 502 650 people were obtained, including the participants’ demographic, socioeconomic and health‐related information. 14 Medical and medication history was collected using a touchscreen questionnaire. A nurse‐led interview was further performed, validating the medical history obtained from the touchscreen questionnaire by direct questioning. During the first repeat of the entire baseline assessment in 2012, data on around 20 000 participants were collected. 15 Furthermore, similar data were collected during the imaging visits in 2014 (Figure 1). 16
Figure 1.
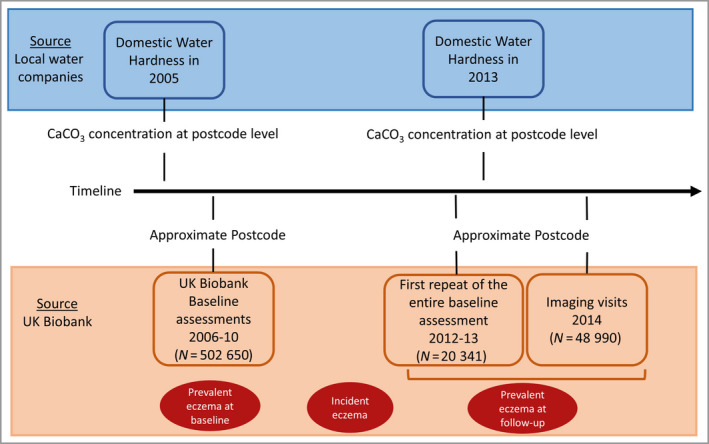
Diagram of the study data points. [Colour figure can be viewed at wileyonlinelibrary.com]
The UK Biobank has approval from the Northwest Multi‐centre Research Ethics Committee. It has also sought approval in England and Wales from the Patient Information Advisory Group to access information that would allow it to invite potential participants. The University of Melbourne Ethics Committees approved the data linkage and analysis components of this project (reference 2020‐20674‐13212‐3).
Postcode definition
The home location of each participant is based on the Ordnance Survey of Great Britain references, rounded to the nearest kilometre. We used the Ordnance Survey Code‐Point®, a database of the UK’s 1·6 million postcodes available as part of the Ordnance Survey Open Data release in 2010. A set of British National Grid coordinates is attached to each postcode in the dataset, a postcode being an abbreviated form of address made up of combinations of between five and seven alphanumeric characters. A postcode may cover between one and 100 addresses, the average number per postcode being 15 addresses. We assigned a postcode to each participant based on their approximate location and use the assigned postcodes as a link to the hard water locations.
Domestic hard water
Data on domestic water supply were obtained from local water supply companies in England, Wales and Scotland. The range of hard water concentrations in the UK is wide and covers areas of soft, hard and very hard water. Data included domestic water annual CaCO3 concentrations in mg L−1 at the area level measured 1 year before baseline (i.e. 2005) and at follow‐up (i.e. 2013) (Figure 1).
The concentration of CaCO3 is directly related to the concentrations of calcium and magnesium in the water. 17 In this study domestic hard water has been classified in three ways: (i) as a continuous variable in increments of 50 mg L−1, (ii) as a dichotomous variable (the WHO criteria 8 consider a CaCO3 concentration > 200 mg L−1 as hard water and < 200 mg L−1 as soft water), and (iii) as a categorical variable, using the United States Geological Survey classification 18 of CaCO3 concentration into four groups, namely soft water (0–60 mg L−1), moderately hard water (60–120 mg L−1), hard water (120–180 mg L−1) and very hard water (> 180 mg L−1).
Outcome definitions
Prevalent eczema
Prevalent eczema was determined using the participants’ answers to the touchscreen questionnaire and the nurse‐led interview. We used the Monthly Index of Medical Specialities online from Australia and the UK and professional advice to make a list of medication names that are indicated for treating eczema or atopic dermatitis (Table S1; see Supporting Information). Then we used this list to create a variable that represents current eczema medication at baseline and at follow‐up (repeat assessment visit and imaging visit). Participants were classified as having prevalent eczema at baseline or at follow‐up if they self‐reported both eczema and eczema medication usage at each of the assessment times.
Incident eczema
Incident eczema was defined as new eczema arising after baseline (i.e. after 2010). Participants were classified as having incident eczema if they did not report ever having had eczema at baseline and reported ever having had eczema at follow‐up. Also, participants were considered as incident cases if they reported the year of doctor diagnosis after baseline on the follow‐up (i.e. after 2010). Those with prevalent eczema at baseline were removed from analyses involving incidence.
Confounder variables
A directed acyclic graph (Figure S1; see Supporting Information) was developed to assess hypothesized causal relationships and to determine the minimum set of confounders to include in the regression model to approximate a causal model. The confounder set included (i) individual‐level variables: age, ethnicity, sex and income; and (ii) area‐level variables: Index of Multiple Deprivation (IMD) and Townsend index at the postcode level. Townsend deprivation index was calculated immediately prior to the participant joining the UK Biobank based on the preceding national census output areas. 19 The overall IMD can be used to rank areas according to the deprivation experienced by their residents and to allow comparison of deprivation levels between areas. 20 To allow consistent score analysis across countries we have used UK‐adjusted IMD scores as described elsewhere. 21
Statistical analysis
The data were hierarchically structured, with individuals (level 1) living within postcode areas (level 2) in the UK. A current recommendation when analysing multilevel data is to account for the effect of clustering of participants within higher‐level units (i.e. postcodes) when fitting regression models to such data, as analysing variance gives added value. 22 , 23 We fitted multilevel logistic regressions to examine the association between domestic hard water and prevalent and incident eczema, as the assumption of independence required for standard regression modelling is likely to be violated by the correlation of outcomes between participants living in the same postcodes. 22 , 24
Firstly, we created an empty model that comprised random intercepts for postcodes to examine the general contextual effects, the extent to which the outcome varied between postcodes (model 1). If the environment in which a participant lives contributes to the likelihood of an individual developing eczema, then it is reasonable to suppose that there may be similar effects upon participants living in the same areas and experiencing similar area‐level environmental exposures. Model 2 was then created, which included the exposure (i.e. domestic hard water), individual‐level covariates (age, ethnicity, sex, income) and postcode‐specific random effects (from model 1). Finally, model 3 incorporated adjustment by the individual‐level and postcode‐level covariates (IMD and Townsend index) in addition to postcode‐specific random effects. We reported the estimated odds ratios (ORs) for multilevel logistic regression models. Furthermore, standard logistic regressions including individual‐level covariates were performed as a sensitivity analysis.
To assess the general contextual effects (i.e. the variation of the outcome by postcode), we included measures of the intraclass correlation coefficient (ICC). The ICC quantifies the proportion of observed variation in the outcome that is attributable to the effect of clustering. We also calculated the median odds ratio, presented in Appendix S1 (see Supporting Information). To assess the potential public health impact of domestic hard water in cases of eczema we calculated the population‐attributable fraction. The potential presence of nonlinearity of these associations was assessed using Stata’s ‘fracpoly’ command. Hard water and participant data linkage was performed using the QGIS geographic information system (version 3.16.16; QGIS Association; www.qgis.org). All other statistical analyses were carried out using Stata (release 16; Stata Corporation, College Station, TX, USA).
Results
In total 306 531 participants nested across 7642 postcodes were included in the baseline analysis (Figure S2; see Supporting Information). At baseline, 55·6% of the participants were female. The 56–65‐year age range was the most common. The vast majority of participants were of white ethnicity, and from England (Table 1). From the first repeat of the baseline in 2012 and the imaging visits, 31 036 participants were included in the follow‐up analysis. Those included in the follow‐up were more likely to have higher levels of income, to be white, and to live in England than those who were not followed up. Moreover, participants lost to follow‐up were more likely to live in hard water areas (Table S2; see Supporting Information).
Table 1.
Baseline characteristics of the included study sample
| Characteristic | Baseline (N = 306 531) | Follow‐up (N = 31 036) |
|---|---|---|
| Individual‐level characteristics | ||
| Sex | N = 306 531 | N = 31 036 |
| Female | 55·6% (170 462) | 51·1% (15 873) |
| Age (years) | N = 306 531 | N = 31 036 |
| < 45 | 9·1% (27 809) | 8·1% (220) |
| 46–55 | 24·9% (76 357) | 26·4% (5938) |
| 56–65 | 47·4% (145 146) | 52·2% (12 770) |
| > 65 | 18·7% (57 219) | 13·3% (12 108) |
| Ethnicity | N = 305 097 | N = 30 942 |
| White | 94·7% (288 970) | 97·5% (30 154) |
| Mixed ethnic groups | 0·6% (1695) | 0·4% (118) |
| Asian or Asian British | 2·3% (6879) | 1·1% (354) |
| Black, African, Caribbean or black British | 1·6% (4908) | 0·5% (170) |
| Other ethnic group | 0·9% (2645) | 0·5% (146) |
| Income (GBP) | N = 256 055 | N = 28 038 |
| < 18 000 | 27·6% (70 580) | 16·9% (4741) |
| 18 000–30 999 | 27·0% (69 197) | 30·5% (8561) |
| 31 000–51 999 | 24·3% (62 311) | 28·9% (8099) |
| 52 000–100 000 | 16·9% (43 198) | 18·9% (5306) |
| > 100 000 | 4·2% (10 769) | 4·8% (1331) |
| Home location | N = 298 909 | N = 30 398 |
| England | 88·3% (263 899) | 98·2% (29 859) |
| Wales | 4·4% (13 294) | 0.3% (96) |
| Scotland | 7·3% (21 716) | 1·5% (443) |
| Eczema outcomes | N = 306 531 | N = 31 036 |
| Prevalence | 1·11% (3392) | 1·03% (319) |
| Incidence | 1·21% (382) | |
| Area‐level characteristics | ||
| Postcodes | 7642 | 3695 |
| Average participants per postcode | 40 | 8 |
| England IMD, mean (SD; range)a | 18·3 (14·4; 0·61–82) | 16·5 (13·0; 1·12–81·6) |
| Townsend index, mean (SD; range)a | −1·2 (3·1; −6·3 to 10·9) | −2·0 (2·7; −6·3 to 9·7) |
| Hard water variables | N = 303 781 | N = 31 036 |
| CaCO3 level (mg L−1) | 140 (114; 5–460) | 85·1 (85·1; 1·5–477) |
| Hard water (> 200 mg L−1) | 36·3% (110 118) | 12·4% (3835) |
| Hard water category (mg L−1) | ||
| 0–60 | 37·2% (113 029) | 57·2% (17 750) |
| 60–120 | 20·2% (61 436) | 21·0% (6523) |
| 120–180 | 5·8% (17 589) | 6·7% (2074) |
| > 180 | 36·8% (111 727) | 15·1% (4689) |
The data are presented as % (n) unless stated otherwise. aA greater Index of Multiple Deprivation (IMD) or Townsend index implies a greater degree of deprivation.
An increase in domestic hard water was associated with an increase in the odds of prevalent eczema at baseline: OR 1·02, 95% confidence interval (CI) 1·01–1·04 per 50 mg L−1 of CaCO3 increase (Table 2, model 3). Overall, the estimates for the association between hard water and prevalent eczema at baseline were similar with and without adjustment for area‐level measures of socioeconomic status (Table 2, models 2 and 3). Participants exposed to domestic water with a CaCO3 concentration > 200 mg L−1 had an increase in the odds of prevalent eczema at baseline (OR 1·12, 95% CI 1·04–1·22) compared with participants exposed to water with < 200 mg L−1 concentration. Furthermore, participants exposed to domestic water with a concentration of 180 mg L−1 of CaCO3 had increased risk of prevalent eczema at baseline (OR 1·13, 95% CI 1·03–1·24) compared with participants exposed to concentrations < 60 mg L−1. There was a significant linear trend (P < 0·001) in which increasing levels of hard water exposure were associated with increased prevalent eczema risk at baseline. The total number of cases of eczema in the UK Biobank participants that could be attributable to domestic hard water was approximately 451 per 10 000 persons.
Table 2.
Multilevel logistic regression models for prevalent eczema at baseline (3392 of 306 531 records)
| Model 1a | Model 2b | Model 3c | |
|---|---|---|---|
| Prevalent eczema at baseline | Coefficient (95% CI) | Coefficient (95% CI) | Coefficient (95% CI) |
| Hard water (per 50 mg L−1 increase) | |||
| OR | – | 1·03 (1·01–1·05) | 1·02 (1·01–1·04) |
| General contextual effects | |||
| Postcode variance | 0·052 (0·023–0·11) | 0·054 (0·026–0·11) | 0·042 (0·017–0·10) |
| ICC | 1·6% (0·7–3·4) | 1·6% (0·7–3·4) | 1·3% (0·4–2·9) |
| Hard water (> 200 mg L−1) | |||
| OR | 1·16 (1·07–1·25) | 1·12 (1·04–1·22) | |
| General contextual effects | |||
| Postcode variance | 0·053 (0·025–0·11) | 0·043 (0·018–0·10) | |
| ICC | 1·6% (0·8–3·3) | 1·3% (0·5–2·9) | |
| Hard water groups | |||
| OR (ref. 0–60 mg L−1) | – | – | |
| 60–120 mg L−1 | 1·01 (0·91–1·12) | 1·00 (0·91–1·11) | |
| 120–180 mg L−1 | 1·08 (0·93–1·26) | 1·05 (0·90–1·23) | |
| > 180 mg L−1 | 1·17 (1·07–1·28) | 1·13 (1·03–1·24) | |
| General contextual effects | |||
| Postcode variance | 0·053 (0·020–0·10) | 0·042 (0·018–0·10) | |
| ICC | 1·6% (0·8–3·3) | 1·3% (0·4–2·8) | |
Hard water values are given as the concentration of CaCO3. CI, confidence interval; ICC, intraclass correlation coefficient; OR, cluster‐specific odds ratio of the association between domestic hard water and eczema outcomes. aNull model with no covariate adjustment. bAdjusted for sex, age, ethnicity and income. cAdjusted for sex, age, ethnicity, income, Index of Multiple Deprivation and Townsend index.
The estimates for the association between hard water and incident eczema were relatively imprecise (Table 3, models 2 and 3). Likewise, the estimates for the association between hard water and prevalent eczema at follow‐up had wide CIs in models 2 and 3 (Table 4).
Table 3.
Multilevel logistic regression models for incident eczema (511 of 31 036 records)
| Model 1a | Model 2b | Model 3c | |
|---|---|---|---|
| Incident eczema | Coefficient (95% CI) | Coefficient (95% CI) | Coefficient (95% CI) |
| Hard water (per 50 mg L−1 increase) | |||
| OR | – | 0·99 (0·93–1·05) | 0·99 (0·93–1·05) |
| General contextual effects | |||
| Postcode variance | 0·10 (0·027–0·36) | 0·10 (0·030–0·36) | 0·099 (0·027–0·37) |
| ICC | 3·0% (0·8–9·9) | 3·1% (0·9–9·9) | 2·9% (0·8–10·0) |
| Hard water (> 200 mg L−1) | |||
| OR | 0·86 (0·64–1·17) | 0·87 (0·64–1·17) | |
| General contextual effects | |||
| Postcode variance | 0·11 (0·033–0·35) | 0·10 (0·031–0·35) | |
| ICC | 3·2% (1·0–9·8) | 3·0% (0·9–9·7) | |
| Hard water groups | |||
| OR (ref. 0–60 mg L−1) | – | – | |
| 60–120 mg L−1 | 1·14 (0·93–1·39) | 1·15 (0·94–1·41) | |
| 120–180 mg L−1 | 1·04 (0·72–1·51) | 1·06 (0·73–1·53) | |
| > 180 mg L−1 | 0·92 (0·68–1·23) | 0·93 (0·69–1·25) | |
| General contextual effects | |||
| Postcode variance | 0·10 (0·030–0·35) | 0·098 (0·028–0·34) | |
| ICC | 3·0% (0·9–9·5) | 2·9% (0·9–9·6) |
Hard water values are given as the concentration of CaCO3. CI, confidence interval; ICC, intraclass correlation coefficient; OR, cluster‐specific odds ratio of the association between domestic hard water and eczema outcomes. aNull model with no covariate adjustment. bAdjusted for sex, age, ethnicity and income. cAdjusted for sex, age, ethnicity, income, Index of Multiple Deprivation and Townsend index.
Table 4.
Multilevel logistic regression models for prevalent eczema at follow‐up (319 of 31 036 records)
| Model 1a | Model 2b | Model 3c | |
|---|---|---|---|
| Prevalent eczema at follow‐up | Coefficient (95% CI) | Coefficient (95% CI) | Coefficient (95% CI) |
| Hard water (per 50 mg L−1 increase) | |||
| OR | – | 0·98 (0·91–1·05) | 1·00 (0·94–1·07) |
| General contextual effects | |||
| Postcode variance | 0·053 (0·005–5·25) | 0·033 (0·001–52) | 0·034 (0·001–43·2) |
| ICC | 1·6% (0·2–11·4) | 4·1% (1·4–11·6) | 1·0% (0·1–92·9) |
| Hard water (> 200 mg L−1) | |||
| OR | 0·92 (0·64–1·33) | 1·00 (0·70–1·42) | |
| General contextual effects | |||
| Postcode variance | 0·046 (0·001–9·7) | 0·043 (0·001–13·6) | |
| ICC | 1·4% (0·1–74·6) | 1·3% (0·1–80·5) | |
| Hard water groups | |||
| OR (ref. 0–60 mg L−1) | – | – | |
| 60–120 mg L−1 | 0·88 (0·66–1·18) | 0·95 (0·71–1·27) | |
| 120–180 mg L−1 | 1·34 (0·90–2·00) | 1·39 (0·93–2·07) | |
| > 180 mg L−1 | 0·90 (0·63–1·28) | 0·99 (0·70–1·39) | |
| General contextual effects | |||
| Postcode variance | 0·030 (0·001–140) | 0·029 (0·001–172) | |
| ICC | 0·9% (0·1–97·8) | 0·9% (0·1–98·1) |
Hard water values are given as the concentration of CaCO3. CI, confidence interval; ICC, intraclass correlation coefficient; OR, cluster‐specific odds ratio of the association between domestic hard water and eczema outcomes. aNull model with no covariate adjustment. bAdjusted for sex, age, ethnicity and income. cAdjusted for sex, age, ethnicity, income, Index of Multiple Deprivation and Townsend index.
The estimated general contextual effects (variance components) were considered low in all models (Tables 2 and 3). For all outcomes, the variance estimates in model 1 (null model) were higher than those estimated in model 3. This means that the clustering effect seen in model 1 was partly explained by the addition of individual‐level and area‐level covariates (IMD and Townsend index).
The estimated ORs are conditional on the random effect on postcodes being held constant. 22 We can estimate the population‐average OR based on the cluster‐specific regression OR using shrinkage factors. 25 Considering that the variance estimates for each eczema outcome were low, the corresponding shrinkage factor for prevalent eczema at baseline was 1·007, for incident eczema 1·017, and for prevalent eczema at follow‐up 1·005. As the shrinkage factors are almost one, the population‐average ORs are essentially equal to the cluster‐specific ORs (to two decimal places).
As a sensitivity test, we used a model that only included individual‐level covariates in a standard (i.e. single‐level) logistic regression without considering postcode influence and postcode‐level variables (Table S3; see Supporting Information). The association estimates between hard water and eczema outcomes were similar to those from the multilevel logistic models.
Discussion
In this large population‐based cohort of adults from the UK we estimated the association between domestic hard water and eczema in adults. We showed that increasing levels of domestic hard water, as measured by CaCO3 concentrations, were associated with an increase in prevalent eczema at baseline. The absence of an association found between domestic hard water and incident eczema may be due to a lack of statistical power. Furthermore, we observed minor variations in eczema outcomes across postcodes; postcode‐level measures of deprivation explained a minor proportion of this variation. The reduction of domestic hard water concentrations may therefore lead to a relatively small reduction in eczema prevalence in adults.
To the best of our knowledge this is the first study to evaluate the association between domestic hard water and eczema in a cohort of adults. The results from most studies assessing this association in infants and school‐age children report higher risk of eczema prevalence associated with exposure to higher concentrations of hard water. 10 , 11 , 26 , 27 , 28 , 29 One observational study in children 30 suggested that water hardness was not associated with eczema; however, the comparison groups were not very different in terms of CaCO3 levels. Likewise, a randomized controlled trial reported no good evidence that a water softener (a device that reduces tap hard water concentration) provided additional benefit for patients with eczema in comparison with usual care. However, this trial only had a duration of 12 weeks and assessed eczema severity, not prevalence. 31
A recent systematic review reported a pooled OR of 1·28 (95% CI 1·09–1·50; I 2 = 63%) based on 385 901 participants from different studies, showing increased odds of eczema prevalence in infants and children exposed to hard water compared with those exposed to soft water. 13 While the direction of association is concordant with what we have observed at baseline for eczema prevalence in adults when comparing participants living in areas of more vs. less than 200 mg L−1 of CaCO3, the strength of association in this adult cohort is somewhat lower (OR 1·12, 95% CI 1·04–1·22). It is possible that the association wanes over time as eczema becomes less prevalent in adults aged around 55 years. 32 Alternatively, individuals who have eczema that is exacerbated by hard water may employ other ways to protect their skin, including lotions or creams, different shower duration, use of rainwater for washing, or selectively moving away from areas with hard water.
There are a number of biological mechanisms by which hard water may lead to increased risk of individuals developing and having more severe and persistent eczema by damaging the skin barrier. 9 The most recognized mechanism involves the increase of use and deposition of detergents such as SLS on the skin. SLS causes skin irritation and skin‐barrier impairment, the extent of which is dependent on the hardness of the wash water. 12 SLS residues left on the skin alter protein secondary structure, solubilize stratum corneum lipids, and elevate skin‐surface pH in a dose‐dependent manner. 12 Soap also reacts with calcium in hard water to form small chalk particles that can irritate the skin. 9 In addition, calcium and magnesium are alkaline metals that form a basic solution with water, which can increase skin pH (normally mildly acidic) and compromise barrier function. 12 Increased concentrations of the metal ion (Ca2+) in hard water may also alter calcium signalling in the epidermis and contribute to impaired skin‐barrier function. 12 , 33 These various potential mechanisms can then lead to increased allergen penetration and bacterial colonization of the skin, which are risk factors for eczema development and progression. 34
We evaluated the general contextual effect (clustering effect) of individuals with eczema outcomes by postcode. Previous studies provided evidence that the socioeconomic characteristics of the neighbourhood environment were related to individual risk of eczema. 35 , 36 The variance components estimated in our study showed that there is a minor contextual effect that influences eczema outcomes. The addition of individual‐level variables and the deprivation measures did not explain a major proportion of the variance. As such, there are other contextual characteristics that may lead to this unexplained variance in eczema. These may include air pollution, area effects (i.e. neighbourhood greenness or blueness) or other neighbourhood‐level variables.
The strengths of our study include access to a large population‐based prospective cohort study of around half a million participants at baseline, extensive collection of covariates, and confirmation of responses from the touchscreen questionnaire during the nurse‐led interviews. Finally, we used multilevel analysis to formally examine the magnitude of the effect of clustering and account for it in our association estimates. An important study limitation was that we did not have access to data on the participants’ usage of water softeners. Informal data suggested that in 2016 only 3% of UK households had a water softener fitted. Another limitation was that very mild cases of eczema that did not require any type of treatment will have been missed. Finally, there was a substantial and selective reduction in participation at the follow‐up, and participants lost to follow‐up were more likely to come from hard water areas, which reduced our power to test associations with eczema incidence and eczema at follow‐up.
In conclusion, our findings suggest that exposure to higher concentrations of domestic hard water is associated with an increase in odds of eczema prevalence in adults aged 40–69 years from the UK. Ongoing efforts to reduce hard water exposure in adults may have a relatively small beneficial effect in reducing eczema prevalence in adults. Furthermore, the estimates of clustering effect by postcode in eczema outcomes, although small, remain mostly unexplained by area‐level socioeconomic measures, so further research is needed to explore which geographical factors may lead to eczema.
Author contributions
Diego Lopez: Conceptualization (equal); data curation (lead); formal analysis (lead); funding acquisition (lead); investigation (lead); methodology (equal); software (lead); validation (equal); visualization (equal); writing – original draft (lead); writing – review and editing (equal). Ankur Singh: Conceptualization (supporting); data curation (equal); formal analysis (equal); investigation (supporting); methodology (equal); supervision (supporting); validation (supporting); visualization (supporting); writing – review and editing (equal). Caroline Lodge: Data curation (equal); formal analysis (supporting); funding acquisition (supporting); investigation (supporting); supervision (supporting); validation (supporting); visualization (supporting); writing – review and editing (equal). Nilakshi Waidyatillake: Formal analysis (supporting); investigation (equal); methodology (equal); resources (equal); validation (supporting); visualization (supporting); writing – review and editing (equal). John C Su: Formal analysis (supporting); investigation (equal); methodology (equal); resources (equal); validation (supporting); visualization (supporting); writing ‐‐ review and editing (equal). Dinh Bui: Data curation (supporting); funding acquisition (supporting); investigation (supporting); methodology (supporting); resources (equal); software (supporting); validation (equal); visualization (supporting); writing – review and editing (equal). Shyamali Dharmage: Data curation (supporting); formal analysis (equal); funding acquisition (supporting); investigation (supporting); methodology (supporting); project administration (equal); supervision (supporting); validation (equal); visualization (supporting); writing – review and editing (equal). Adrian J Lowe: Conceptualization (lead); data curation (supporting); formal analysis (supporting); funding acquisition (supporting); investigation (supporting); methodology (supporting); project administration (equal); resources (equal); supervision (lead); validation (lead); visualization (lead); writing – original draft (equal); writing – review and editing (equal).
Funding sources
The Population Health Investing in Research Students’ Training from the University of Melbourne funded the UK Biobank’s access fee. D.J.L. was supported by the University of Melbourne and Becas Carlos Antonio Lopez scholarships. The funding agencies had no direct role in the conduct of the study; the collection, management, statistical analysis or interpretation of the data; or the preparation or approval of the manuscript.
Conflicts of interest
S.C.D., C.J.L. and A.J.L. declare they have received research funds from GSK's competitively awarded Investigator Sponsored Studies programme, for unrelated research. A.J.L. has also received donations of interventional product (EpiCeram) from Primus Pharmaceuticals for unrelated research. J.C.S. has been a consultant/speaker/investigator for AbbVie, Amgen, Bioderma, Bristol Myers Squibb, Ego Pharmaceuticals, Eli‐Lilly, Janssen, LEO Pharma, L'Oreal, Mayne, Novartis, Pfizer, Pierre‐Fabre, and Sanofi. The other authors declare they have no conflicts of interest.
Ethics statement
The UK Biobank has approval from the Northwest Multi‐centre Research Ethics Committee. It has also sought approval in England and Wales from the Patient Information Advisory Group to access information that would allow it to invite potential participants. The University of Melbourne Ethics Committees approved the data linkage and analyses components of this project (reference 2020‐20674‐13212‐3).
Supporting information
Appendix S1 Calculation of median odds ratio.
Figure S1 Directed acyclic graph of hypothesized causal relationships and confounders.
Figure S2 Participant flowchart.
Table S1 Medications indicated for treating eczema or atopic dermatitis.
Table S2 Baseline characteristics of patients lost to follow‐up.
Table S3 Odds ratios for logistic regression models for the adjusted association between water hardness definitions and eczema outcomes.
Acknowledgments
We would like to acknowledge the UK Biobank (application ID 70068) for providing the data for this analysis. We acknowledge the contributions of the founders, participants and researchers of the UK Biobank cohort study. We would also like to acknowledge Affinity Water, Anglian Water, Bournemouth Water, Bristol Water; Dŵr Cymru Welsh Water, Essex & Suffolk Water, Northumbrian Water Limited, Portsmouth Water Company, SES Water, South East Water, South West Region UK, Scottish Water, Thames Water, United Utilities, Wessex Water, and Yorkshire Water for providing the water hardness data. Open access publishing facilitated by The University of Melbourne, as part of the Wiley ‐ The University of Melbourne agreement via the Council of Australian University Librarians.
Plain language summary available online
Data availability
The participant data that support the findings of this study are available from the UK Biobank. Restrictions apply to the availability of these data, which were used under license for this study. Data are available at https://www.ukbiobank.ac.uk/enable‐your‐research/apply‐for‐access with the permission of the UK biobank. The hard water data that support the findings of this study are available from the local water companies upon reasonable request.
References
- 1. Weidinger S, Beck LA, Bieber T et al. Atopic dermatitis. Nat Rev Dis Primers 2018; 4:1. [DOI] [PubMed] [Google Scholar]
- 2. Fu T, Keiser E, Linos E et al. Eczema and sensitization to common allergens in the United States: a multiethnic, population‐based study. Pediatr Dermatol 2014; 31:21–6. [DOI] [PubMed] [Google Scholar]
- 3. Barbarot S, Auziere S, Gadkari A et al. Epidemiology of atopic dermatitis in adults: results from an international survey. Allergy 2018; 73:1284–93. [DOI] [PubMed] [Google Scholar]
- 4. Powers CE, McShane DB, Gilligan PH et al. Microbiome and pediatric atopic dermatitis. J Dermatol 2015; 42:1137–42. [DOI] [PubMed] [Google Scholar]
- 5. Ahn K. The role of air pollutants in atopic dermatitis. J Allergy Clin Immunol 2014; 134:993–9. [DOI] [PubMed] [Google Scholar]
- 6. Kantor R, Silverberg JI. Environmental risk factors and their role in the management of atopic dermatitis. Expert Rev Clin Immunol 2017; 13:15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Akram S, Rehman F. Hardness in drinking‐water, its sources, its effects on humans and its household treatment. J Chem Appl 2018; 4:4. [Google Scholar]
- 8. World Health Organization (WHO) . Guidelines for Drinking‐Water Quality. Geneva: WHO, 2011. [Google Scholar]
- 9. Jabbar‐Lopez ZK, Craven J, Logan K et al. Longitudinal analysis of the effect of water hardness on atopic eczema: evidence for gene–environment interaction. Br J Dermatol 2020; 183:285–93. [DOI] [PubMed] [Google Scholar]
- 10. McNally NJ, Williams HC, Phillips DR et al. Atopic eczema and domestic water hardness. Lancet 1998; 352:527–31. [DOI] [PubMed] [Google Scholar]
- 11. Arnedo‐Pena A, Bellido‐Blasco J, Puig‐Barbera J et al. [Domestic water hardness and prevalence of atopic eczema in Castellon (Spain) school children]. Salud Publica Mex 2007; 49:295–301 (in Spanish). [DOI] [PubMed] [Google Scholar]
- 12. Danby SG, Brown K, Wigley AM et al. The effect of water hardness on surfactant deposition after washing and subsequent skin irritation in atopic dermatitis patients and healthy control subjects. J Invest Dermatol 2018; 138:68–77. [DOI] [PubMed] [Google Scholar]
- 13. Jabbar‐Lopez ZK, Ung CY, Alexander H et al. The effect of water hardness on atopic eczema, skin barrier function: a systematic review, meta‐analysis. Clin Exp Allergy 2021; 51:430–51. [DOI] [PubMed] [Google Scholar]
- 14. Hewitt J, Walters M, Padmanabhan S, Dawson J. Cohort profile of the UK Biobank: diagnosis and characteristics of cerebrovascular disease. BMJ Open 2016; 6:e009161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sudlow C, Gallacher J, Allen N et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLOS Med 2015; 12:e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Petersen SE, Matthews PM, Bamberg F et al. Imaging in population science: cardiovascular magnetic resonance in 100,000 participants of UK Biobank – rationale, challenges and approaches. J Cardiovasc Magn Res 2013; 15:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Clune JW, Cravotta CA III. Drinking water health standards comparison and chemical analysis of groundwater for 72 domestic wells in Bradford County, Pennsylvania, 2016. Scientific Investigations Report 2018‐5170. 10.3133/sir20185170. [DOI] [Google Scholar]
- 18. Durfor CN, Becker E. Public Water Supplies of the 100 Largest Cities in the United States, 1962. Washington, DC: US Government Printing Office, 1964. [Google Scholar]
- 19. Townsend P, Phillimore P, Beattie A. Health and Deprivation: Inequality and the North. Abingdon‐on‐Thames: Routledge, 1988. [Google Scholar]
- 20. Payne RA, Abel GA. UK indices of multiple deprivation – a way to make comparisons across constituent countries easier. Health Stat Q 2012; 53:2015–16. [Google Scholar]
- 21. Abel GA, Barclay ME, Payne RA. Adjusted indices of multiple deprivation to enable comparisons within and between constituent countries of the UK including an illustration using mortality rates. BMJ Open 2016; 6:e012750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Austin PC, Merlo J. Intermediate and advanced topics in multilevel logistic regression analysis. Stat Med 2017; 36:3257–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Merlo J, Wagner P, Ghith N, Leckie G. An original stepwise multilevel logistic regression analysis of discriminatory accuracy: the case of neighbourhoods and health. PLOS ONE 2016; 11:e0153778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Merlo J, Chaix B, Ohlsson H et al. A brief conceptual tutorial of multilevel analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Commun Health 2006; 60:290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Larsen K, Merlo J. Appropriate assessment of neighborhood effects on individual health: integrating random and fixed effects in multilevel logistic regression. Am J Epidemiol 2005; 161:81–8. [DOI] [PubMed] [Google Scholar]
- 26. Chaumont A, Voisin C, Sardella A, Bernard A. Interactions between domestic water hardness, infant swimming and atopy in the development of childhood eczema. Environ Res 2012; 116:52–7. [DOI] [PubMed] [Google Scholar]
- 27. Perkin MR, Craven J, Logan K et al. Association between domestic water hardness, chlorine, and atopic dermatitis risk in early life: a population‐based cross‐sectional study. J Allergy Clin Immunol 2016; 138:509–16. [DOI] [PubMed] [Google Scholar]
- 28. Engebretsen KA, Bager P, Wohlfahrt J et al. Prevalence of atopic dermatitis in infants by domestic water hardness and season of birth: cohort study. J Allergy Clin Immunol 2017; 139:1568–74. [DOI] [PubMed] [Google Scholar]
- 29. Miyake Y, Yokoyama T, Yura A et al. Ecological association of water hardness with prevalence of childhood atopic dermatitis in a Japanese urban area. Environ Res 2004; 94:33–7. [DOI] [PubMed] [Google Scholar]
- 30. Font‐Ribera L, Gracia‐Lavedan E, Esplugues A et al. Water hardness and eczema at 1 and 4 y of age in the INMA birth cohort. Environ Res 2015; 142:579–85. [DOI] [PubMed] [Google Scholar]
- 31. Thomas KS, Dean T, O’Leary C et al. A randomised controlled trial of ion‐exchange water softeners for the treatment of eczema in children. PLOS Med 2011; 8:e1000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rönmark EP, Ekerljung L, Lötvall J et al. Eczema among adults: prevalence, risk factors and relation to airway diseases. Results from a large‐scale population survey in Sweden. Br J Dermatol 2012; 166:1301–8. [DOI] [PubMed] [Google Scholar]
- 33. Lee SE, Lee SH. Skin barrier and calcium. Ann Dermatol 2018; 30:265–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. Tanaka A, Takai M, Yoshinari Y, Matsuda H. Ultrapure soft water reduces growth of Staphylococcus aureus adopted on skins of the barrier‐disrupted animal model. Allergy 2009; 64:abstract 1458.19416142 [Google Scholar]
- 35. Zhang J, Zhang Y, Sundell J et al. Association between home environment and allergies among children in Beijing, China. Proc Eng 2015; 121:477–84. [Google Scholar]
- 36. McKenzie C, Silverberg JI. Associations of unsafe, unsupportive, and underdeveloped neighborhoods with atopic dermatitis in US children. Ann Allergy Asthma Immunol 2019; 122:198–203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Calculation of median odds ratio.
Figure S1 Directed acyclic graph of hypothesized causal relationships and confounders.
Figure S2 Participant flowchart.
Table S1 Medications indicated for treating eczema or atopic dermatitis.
Table S2 Baseline characteristics of patients lost to follow‐up.
Table S3 Odds ratios for logistic regression models for the adjusted association between water hardness definitions and eczema outcomes.
Data Availability Statement
The participant data that support the findings of this study are available from the UK Biobank. Restrictions apply to the availability of these data, which were used under license for this study. Data are available at https://www.ukbiobank.ac.uk/enable‐your‐research/apply‐for‐access with the permission of the UK biobank. The hard water data that support the findings of this study are available from the local water companies upon reasonable request.


