Abstract
The habituation–dishabituation (H–D) paradigm is an established measure of sensory perception in animals. However, it has rarely been applied to canine olfaction. It proposes that animals will lose interest in, or habituate to, a stimulus after successive exposures but will regain interest in, or dishabituate to, a novel stimulus if they can perceive it. This study assessed an H–D test's practicability to determine dogs' olfactory detection thresholds (ODTs) for a neutral odorant. A random selection of mixed‐breed pet dogs (n = 26) participated in two H–D tests in a repeated‐measures crossover design. They were first habituated to a carrier odor and then presented with either ascending concentrations of n‐amyl acetate in the known ODT range (experimental condition) or repeated carrier odor presentations (control condition). No single odor concentration elicited dishabituation in the majority of the dogs. However, individual dogs dishabituated at differing experimental concentrations significantly more often than in the control condition (p = .012). These findings provide some tentative support for using this method in studying canine olfaction. However, further assessment and refinement are needed before it can be a viable alternative to traditional ODT measurement.
Keywords: odor detection, habituation, dishabituation, thresholds, dogs
There has been considerable interest in canine olfaction potential and realized applications in service roles such as in the detection of drugs, explosives and biosecurity hazards (e.g., Arner et al., 1986; Gadbois & Reeve, 2014; Helton, 2009; Kokocińska‐Kusiak et al., 2021; Moser et al., 2019; 2020; Pickel et al., 2004; Price et al., 2020). Dogs are known to have remarkably sensitive olfactory abilities, the limits of which have not yet been fully explored (Thorne, 1995; Wackermannová et al., 2016). Olfactory sensitivity can be measured by determining the perceptible lowest concentration of odorants, known as the olfactory detection threshold (ODT), through behavioral and neurological testing (e.g., Hirano et al., 2000; Marshall & Moulton, 1981; Moulton et al., 1960).
Traditional discrimination training‐based tests involve training an animal to respond to an odor, with either aversive (e.g., Krestel et al., 1984) or rewarding (e.g., Walker et al., 2006) consequences for incorrect or correct choices. They might also involve systematic decreases in the concentration of the odor until the animal no longer responds above chance performance (Angle et al., 2014; Hilliard, 2003; Johnston et al., 1994; Marshall & Moulton, 1981; Moulton et al., 1960; Polgár et al., 2016; Waggoner et al., 1997). However, this process is time‐intensive, can be confounded by several variables, and is restrictive in the dogs that can participate (due to trainability, motivation, or availability, for example). Furthermore, training improves odorant sensitivity due to perceptual learning, which likely influences the resulting detection thresholds (Engen, 1960; Hall et al., 2016; Helton, 2009; Moulton et al., 1960; Spence, 2019).
There has been considerable variation in how studies have sought to determine the ODT of dogs. Studies have differed in their experimental preparations, such as using experimental chambers to present samples (e.g., Krestel et al., 1984) or by using multiple‐choice line‐ups for the dogs to select from (e.g., Concha et al., 2019), and in their criteria for threshold detection such as designating chance performance as 50% sensitivity (e.g., Johnston et al., 1994), a lower sensitivity based on inconsistently‐determined probability (e.g., Krestel et al., 1984; Marshall & Moulton, 1981; Walker et al., 2006), or by a failure to meet a nominated raw number of correct responses (e.g., Hall et al., 2016).
The ODT of dogs is generally determined by operant conditioning and discrimination testing. Studies using operant methods to determine olfactory sensitivity have yielded varying results; for example, estimates of ODTs of dogs for n‐amyl acetate (nAA) range from 32.6 parts‐per‐billion (ppb; Krestel et al., 1984) to 1.9 parts‐per‐trillion (ppt; Walker et al., 2006). Note this organic compound has a scent similar to bananas which makes it a suitable compound for testing with dogs where there may be some applied implications in biosecurity detection. However, this has some important limitations, such as the need for time‐consuming training and the selection of trainable participant dogs.
An alternative approach, a habituation–dishabituation (H–D) test, may offer a more rapid and straightforward way of determining dogs' ODT for different odorants than other methods. This paradigm has been used across different sensory stimuli, including visual, auditory, tactile, and chemical, and with various species, including humans, other mammals, birds, fish, and insects (e.g., Farrow et al., 2020; Hostachy et al., 2019; Lejeune et al., 2021; Messina et al., 2020; Rørvang et al., 2017). It is based on the notion that animals tend to ignore familiar, irrelevant stimuli and show more interest in novel stimuli (Groves & Thompson, 1970; Pavlov, 1927; Rankin et al., 2009). The paradigm involves exposing an animal to a particular stimulus repeatedly until the animal habituates to it—specifically, when they stop responding to the stimulus presentation; following this, a novel stimulus is presented, and it is expected that if the animal can perceive a difference they would dishabituate to the stimulus and respond to its presence (Wilson, 2009; Zou et al., 2015). This approach has been used to investigate olfactory discrimination in mammals (e.g., Cleland et al., 2002; Gregg & Thiessen, 1981; Mandairon et al., 2006; Price et al., 2020; Wesson et al., 2010; Yang & Crawley, 2009; Zou et al., 2015). Furthermore, the use of this approach to measure ODT has been demonstrated in rodents (Mandairon et al., 2009; Perez‐Villalba et al., 2015; Qiu et al., 2014), and more recently with pigs (Aviles‐Rosa et al., 2020), but has not been tested in dogs. Although an H–D test appears to be a promising approach for this purpose, its outcomes are impacted by experience, expectation, or arousal (Siddle & Lip, 1997; Steiner & Barry, 2014). It therefore requires further study and scrutiny. Some studies have utilized H–D type preparations to investigate the impact of steroids on canine olfaction and if those manipulations altered the ODT of different substances, although these were not described as traditional H–D tests (e.g., Ezeh et al., 1992; Myers, 1991).
The goal of the present study was to test the limits of olfactory sensitivity by determining the lowest concentration perceptible—the ODT of dogs for nAA in a liquid phase using an H–D test. This odorant was chosen due to its previous use in tests of olfactory sensitivity with dogs (Concha et al., 2019; Hirano et al., 2000; Krestel et al., 1984; Walker et al., 2006), with which the findings of the present study could be compared and validated. If an H–D test can be used to estimate ODTs, we expected most dogs to dishabituate and respond in the presence of liquid dilutions of between 50 ppb and one parts‐per‐million (ppm) which we considered to be a conservative floor in the present experiment.
Method
Animals
Privately‐owned pet dogs (N = 35) were recruited from 18 different owners in Armidale, Australia. The inclusion criteria for participation were that dogs were aged between 1 and 8 years with no apparent illness. They needed to be comfortable to be left with unfamiliar people and vaccinated against parvovirus, distemper, hepatitis (C3) and causes of kennel cough (CC). Of the recruited dogs, several were removed from the experiment due to the following reasons: consistently not approaching the vial due to apparent avoidance, disinterest, or fatigue (n = 4); appearing too distressed when confined (n = 2); experimenter error that compromised the test results (n = 2; in once instance the vial was dropped and in the other the camera did not record the session); or inability to undertake both tests due to rehoming (n = 1). Removing participants due to “fussiness” or not wanting to participate has been found not to systematically impact H–D test results in infants (Slaughter & Suddendorf, 2007).
All other dogs (n = 26; 22 female, 4 male) aged from 1–8 years (M = 3.53, SD = 2.23) successfully participated in both tests and were represented by 14 breeds and breed mixes (Beagle [n = 6]; Kelpie X [n = 4]; Labrador Retriever [n = 2], Miniature Dachshund [n = 2], Great Dane [n = 2], Old English Sheepdog [n = 2]; German Shepherd X [n = 1]; Jack Russell Terrier [n = 1]; Border Collie [n = 1]; Border Collie X [n = 1]; Siberian Husky [n = 1]; Siberian Husky X [n = 1]; Lhasa Apso X [n = 1]; and Silky Terrier X [n = 1]); these were categorized into “scent hound”, “herding”, and “other” breed categories. Dogs were randomly allocated to treatments such that half of the dogs (n = 13) took part in the control condition before experiencing the experimental condition, whereas the other half (n = 13) experienced the experimental condition first, before experiencing the control condition.
This research was approved by the University of New England's Animal Ethics Committee (approval number: AEC20‐014). Privately owned pet dogs participated in the study with their owners' fully informed written consent.
Materials and Apparatus
Experimental odors were plain mineral oil (MO; Sigma Aldrich; M8410) and liquid dilutions of nAA (pentyl acetate; CAS #628‐63‐7; Sigma Aldrich; W504009) in MO. MO was chosen because it is a preferred solvent for olfactory testing (Cometto‐Muñiz et al., 2003; Philpott et al., 2004) and for consistency with the most recent canine experiment on olfactory sensitivity of nAA (Concha et al., 2019).
A serial dilution procedure was used to create dilutions of one part nAA to 105, 106, 2 x 107, 4 x 107, and 1.5 x 109 parts MO (Fig. 1). The two lowest concentrations, 1:1.5 x 109 and 1:4 x 107, were chosen based on the estimated ODT range of amyl acetate in previous research with trained dogs (Concha et al., 2019). The highest concentration was expected to be perceptible for all dogs.
Figure 1.
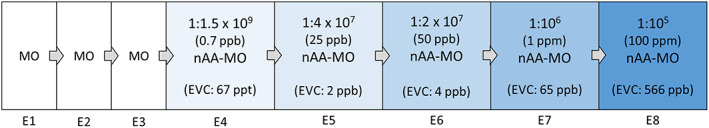
A Flowchart Depicting the Odors Presented in Each of the Eight Trials in the Experimental Condition
Note. The first three (E1‐E3) were habituation trials, and the following five (E4‐E8) were odor trials during which the experimental odor of amyl acetate was introduced in increasing concentrations in the liquid phase. Increasing color intensity reflects increasing odor intensity. nAA: n‐amyl acetate; MO: mineral oil; EVC: estimated vapor concentration.
Stock solutions of the liquid dilutions were prepared on the same day and stored in new 200 mL polypropylene jars with screw‐top lids (Sarstedt, Inc). For presentation to the dogs, 1 mL of each odor sample was pipetted into new 5 mL polypropylene vials (Sarstedt, Inc). The estimated vapor‐phase concentrations of nAA were calculated using vapor pressure data provided by Cometto‐Muñiz et al. (2003). However, these are tentative estimations, reflecting the vial's equilibrated headspace; the headspace above the vial would be expected to have lower vapor concentrations.
The research was undertaken at the Laureldale Dog Research and Training Facility (University of New England, Armidale, Australia). Experiments were conducted indoors on sealed concrete flooring. The temperature in the testing pen was 20 ± 4° C. Each trial was carried out in a 1.45 x 2.45 m pen with an exhaust fan and plastic boards to block extraneous views or odors from the sides (see Fig. 2). A clamp was attached to the front of the pen's door to hold the odor vial, usually at the height of 310 mm, but reduced to 160 mm for extra‐small dogs (e.g., Miniature Dachshunds) and increased to 470 mm for extra‐large dogs (e.g., Great Danes). A video camera (GoPro) was attached by another clamp on the side of the pen and controlled remotely.
Figure 2.
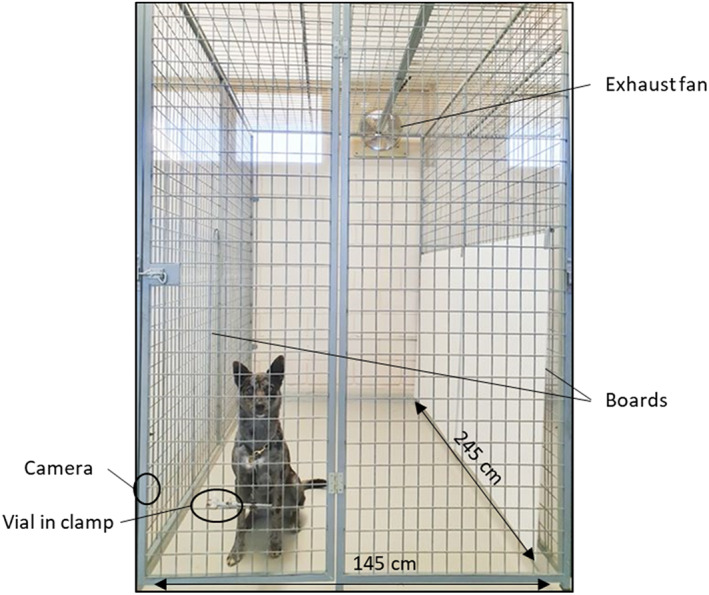
The Front View of the Testing Pen
A 1.85 x 2.70 m “resting” pen was also set up with two rubber trampoline beds and an ad libitum supply of water during intertrial intervals.
Procedure
Dogs were brought inside and were leash‐walked around the indoor facility, during which they were permitted to explore and habituate to the surroundings. They were then put in the trial pen to habituate to the odors and surroundings for approximately 1 min and then placed in the resting pen before the first trial. For the duration of the test, the participating dog was the only dog indoors.
At the beginning of each trial, dogs were led into the trial pen, the leash unclipped, and the door closed. Video recording began, and the experimenter, wearing gloves, fastened the vial containing the odor into the clamp. The experimenter called the dog's name and tapped the clamp to attract their attention. The experimenter then stood back 2 m and timed 30 s, after which the experimenter removed the vial from the clamp, praised the dog, and disposed of the vial in a separate room, separated by a closed door. Between each trial, there was a 3‐min intertrial interval (ITI) that was used in an attempt to attenuate the risk of over‐habituation in which the animal would cease to investigate the stimulus altogether, as has been suggested by others (Perez‐Villalba et al., 2015). Approximately half of the interval was spent walking in the indoor kennel area on a leash, and the other half was spent in the resting pen. This arrangement was the most accommodating for pet dogs uncomfortable with long periods of confinement. Eight trials were repeated in the same manner, comprising a single test. Each session took approximately 24 minutes in total.
The experimenter then wiped the pen's clamps and front bars with 100% isopropyl alcohol. The exhaust fan was run for at least 10 minutes to dissipate any residual odor before the next dog commenced the test. The testing pen was cleaned with hot water and detergent at the end of each day.
All dogs participated in both control and experimental conditions of an H–D test in a repeated‐measures crossover design, with a minimum of 7 days between the two tests. In the experimental condition, dogs were presented with MO for three trials, followed by five consecutive trials with increasing concentrations of nAA in MO (Fig. 1). Dogs were presented with MO only for all eight trials in the control condition.
Video recordings were reviewed to determine the amount of time the dogs spent investigating the odor vials by an observer blinded to the treatments. Investigation period was defined as the time spent actively sniffing, licking, or chewing the vial (e.g., Fig. 3). Specifically, an investigation (dishabituation) was coded if the dog's snout was oriented towards the vial combined with active head bobbing, audible sniffing, or physical contact with the vial. If the dog did not show any apparent active investigation behavior towards the vial but brought their nose within approximately 30 cm of it (and therefore could feasibly have sampled it), this was recorded as 0.01 s. If the dog did not approach the vial to this distance, indicating no interest, this was recorded as 0 s (no response).
Figure 3.

A Participant Dog Investigating the Odor Vial During a Trial
Results
To determine intrarater reliability, the primary investigator double‐coded a random selection of 20% of the recordings, blinded to the trial condition. This yielded high intrarater reliability, as determined by a nonparametric concordance coefficient, ICC = 0.93; 95% CI = 0.91, 1; p < .00001, calculated in R (R Core Team, 2019) using the nopaco package (v1.0.6; Kuiper et al., 2019; Rothery, 1979). Additionally, a reliability coder who was blind to the experimental design coded investigation period in a random selection of 20% of the recordings, yielding high interrater reliability, ICC = 0.90; 95% CI = 0.87, 1; p < .00001. Therefore, the data from the primary investigator was considered reliable and was used for analyses conducted using JMP®, Version 14.2.0 (SAS Institute, Cary, NC, 2019).
Altogether, 416 trials were included in analyses. Dogs did not approach the vial in 9.6% of these trials. Dogs showed only minimal, nonactive investigation (0.01 s) in 19.2% of trials. Across all trials, dogs' investigation periods averaged 1.25 s (SD = 1.91, Mdn = 0.60, IQR = 1.65). Investigation periods were not normally distributed (Shapiro–Wilk test: W = 0.640; p < .0001) and were heteroscedastic across the trials (Levene test: F(15, 400) = 4.908; p < .0001). As such, nonparametric tests were used.
Dogs significantly decreased their overall investigation period from the first to the third trials in both the experimental (first Mdn = 1.89; third Mdn = 0.63; paired Wilcoxon signed‐rank: W = ‐116; p < .0001) and control (first Mdn = 2.01; third Mdn = 0.54; paired Wilcoxon signed‐rank: W = ‐107; p < .0001) conditions (Fig. 4). This suggests that habituation occurred as expected. However, no significant increases in the investigation period were observed across the experimental or control trials, suggesting that no trial elicited consistent dishabituation across the sample.
Figure 4.
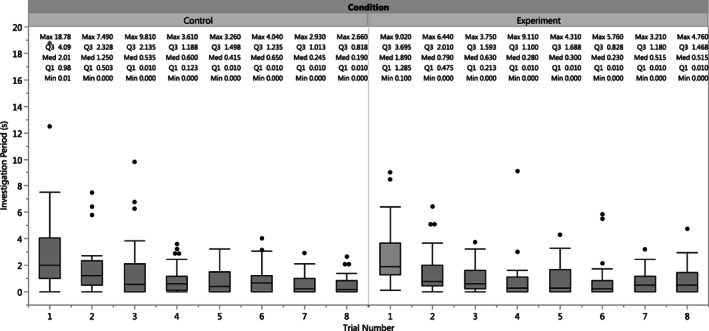
Boxplots Depicting the Medians and Ranges of Investigation Periods for Each Trial in the Control (Left) and Experimental (Right) Conditions
It was a reasonable expectation that individual dogs with differing olfactory sensitivity would demonstrate different ODTs and therefore dishabituate in other trials, possibly masking an effect at the group level. Thus, putative individual dishabituations were also considered. An investigation period increase was considered a dishabituation if it was greater than one standard deviation above the mean of the posthabituation trial of the control condition (M = 0.74, SD = 0.95) to differentiate the effect of more extended investigation periods from random variability. By this measure, an increased investigation period of ≥1.69 s was considered a dishabituation on an individual level.
When examining individual animal responses, there was a significant difference between the number of dogs that dishabituated in the experimental (11/26) compared to the control (3/26) conditions, χ2(1, n = 52) = 6.26, p = .012. As expected, dogs dishabituated only once per condition, although two dogs dishabituated in both the experimental and the control conditions (Fig. 5).
Figure 5.

The Number of Individual Dogs Showing Dishabituations in Each Trial in the Control (Left) and Experimental (Right) Conditions
Note. Different patterns represent the number of dogs from each breed category.
Other factors appeared to also influence dogs' overall investigation period. Dogs investigated for significantly longer periods in their first test condition (Mdn = 1.06) than in their second test (Mdn = 0.285), regardless of condition (2‐sample Wilcoxon test, Z = ‐6.52; p < .0001). However, the number of individual dogs that dishabituated did not differ significantly between the first (9/26) or second (5/26) test, χ2(1, n = 52) = 1.56, p = .211).
Individual dogs had significantly differing overall investigation periods, 1‐way Wilcoxon test χ2(25) = 74.4; p < .0001). Breed category appeared to influence the length of investigation periods, with beagles (Mdn = 1.72) investigating significantly longer than herding breeds (Mdn = 0.51); Wilcoxon test: Z = 5.38, p < .0001) or other breeds (Mdn = 0.52); Wilcoxon test: Z = 3.73, p < .0006), corrected using the Steel‐Dwass method (q* = 2.34, α = 0.05). There was no significant difference in investigation periods between younger dogs (less than 3 years old) (Mdn = 0.53), and older dogs (Mdn = 0.53); (1‐way Wilcoxon test: Z = 1.89; p = .058).
Discussion
This study is the first to assess an H–D method's validity to determine the detection threshold of an odorant in dogs. It was expected that an increase in investigation time, suggesting dishabituation, would be observed during the trial with the concentration corresponding to dogs' ODT range for nAA. Our results showed mixed, tentative support for using an H–D test for this purpose.
Overall, dogs habituated as expected by the third trial, consistent with previous research (e.g., Escanilla et al., 2012). However, there was no single trial in which group‐wide dishabituation was measurable. This might be explained by dogs responding in different trials due to differing individual levels of olfactory sensitivity, masking a group‐wide effect. Considerable interdog variation in olfactory detection thresholds has been observed in previous studies (e.g., Concha et al., 2019; Hilliard, 2003; Marshall & Moulton, 1981; Phelan & Barnett, 2002; Waggoner et al., 1997). On an individual level, significantly more dogs dishabituated in the experimental condition than in the control condition, although at differing concentration levels.
The absence of a group‐wide dishabituation effect in the present experiment conflicts with some reported H–D tests reported using other species, such as rodents (e.g., Escanilla et al., 2012; Mandairon et al., 2009) and pigs (Aviles‐Rosa et al., 2020). However, previous studies have tended to present stronger concentrations of odorants, which may be above the true ODT, and all have used genetically homogenous animals (Aviles‐Rosa et al., 2020; Escanilla et al., 2012; Perez‐Villalba et al., 2015); both factors which would be expected to reduce variability in responses. While a stronger odor would be more likely to engender a larger change of behavior, this may not reflect animals' actual ODT, particularly if not validated against other measures of ODTs. Conversely, Qiu et al. (2014) have successfully observed group‐wide dishabituation in a homogenous rodent sample, validated against an operant ODT measure. An olfactometer was used in a testing chamber with automatically coded investigation times (Qiu et al., 2014). This apparatus is likely to provide a more sensitive measure and may be adapted for use with dogs. However, methods that do not require specialized equipment are preferred to improve accessibility and the subsequent reproducibility of experiments.
A subset of dogs (43%) demonstrated individual dishabituation behavior at concentration thresholds within the expected range in the present study. That these dogs did this is consistent with a handful of studies that have reported using a similar procedure to determine individual dogs' ODTs, although these were not described as traditional H–D tests (e.g., Ezeh et al., 1992; Myers, 1991). In these experiments, odors were presented to dogs in order of ascending concentration while measuring behavioral investigation and electroencephalographic (EEG) responses. EEG results were slightly more sensitive than behavioral indications, but both found similar first responses to odor concentrations (Ezeh et al., 1992). The methodology in these experiments differed from the present study, with odor vials brought directly to recumbent dogs' noses and held. Our method sought to remove human influence from the odor presentation as far as possible, but these findings suggest that this might be a viable alternative methodology.
Most dogs (n = 8) that dishabituated to nAA did so within 25 ppb to 1 ppm. The observed detection range was well within the previously reported estimates of ODT, based on some findings with trained dogs (Concha et al., 2019; Krestel et al., 1984), with some adjustment to subtract perceptual learning imparted through training (Yee & Wysocki, 2001). However, it is considerably less than Walker et al. (2006) found in two dogs. EEG findings have suggested that naïve dogs (n = 6, beagles) demonstrate an ODT of 1 ppm nAA in propylene glycol (Hirano et al., 2000); however, this is a less effective solvent for odorant dispersal and could be an underestimation for MO dilutions (Philpott et al., 2004). In the present study, one dog (Labrador retriever) dishabituated at 0.7 ppb, which was more sensitive than expected for naïve dogs, but not extraordinary (e.g., Concha et al., 2019; Walker et al., 2006).
Conversely, two dogs dishabituated at 100 ppm, which was hypothesized to be higher (less sensitive) than their ODT for this odorant, while several dogs did not dishabituate at any presented concentration. Finally, we observed three instances in which dogs displayed spontaneous dishabituation in the control condition without any change in odor. Spontaneous interest recovery after a period of elapsed time has similarly been observed in neurobiological research of the H–D paradigm (Wilson, 2009). This variability and error suggest that individual dishabituation should not be taken prima facie as evidence of olfactory detection for an individual, although group patterns may be compelling.
Notably, this study's fluctuations in investigation time were subtle, meaning that differentiating true dishabituations from chance variability was difficult. Alternatively, using intrinsically motivating social odors, such as pheromones (e.g., Aviles‐Rosa et al., 2020; Laska et al., 2006) or conspecific urine (e.g., Qiu et al., 2014), are thought to elicit a more robust investigatory response upon detection. In the present study, the odorant was neutral and nonsocial and also of a very low concentration. The odorant may need to be either salient or intrinsically motivating to show large, consistent behavioral differences in an H–D test.
This study contributes some insight for the future application of olfactory H–D tests with dogs. In particular, repeated measures impacted investigation behavior, with dogs investigating significantly less in their second test despite at least a week interval between sessions. This may limit the number of tests that can be carried out per dog before they become over‐habituated and cease investigating thoroughly, which would pose issues with test–retest reliability. Secondly, although most H–D studies do not include a control condition, in this case, a control condition was valuable for comparing investigation behavior and determining the range of chance fluctuation and variability, which was considerable. It furthermore allowed for appropriate blinding. Finally, dogs were tested in a relatively large pen, with the passive presentation of the odor, where the source was out of their direct reach. Our findings were that dogs investigated the vial in most trials, with at least a close approach in 90.4% of trials and active investigation in 61.6% of trials. This was considered preferable to presenting an odor source that may elicit play or tactile engagement. However, while a smaller pen may encourage dogs to remain closer to and spend longer time investigating the odor source, it would also limit participation to dogs that do not have an aversion to being confined. Future research might elucidate whether certain individual dogs are more reliable in their responses for this testing method, perhaps due to differences in temperament or previous training.
Conclusion
Overall, while dogs did not respond consistently to a particular concentration threshold, several individual dogs appeared to dishabituate within the range of expected ODTs. Further testing and refinement of this methodology may produce more definitive results. H–D tests are appealing because they offer a more rapid and economical way of screening dogs' olfactory sensitivity without extensive training or the impact of variables related to learning (e.g., Gadbois & Reeve, 2014; Salvin et al., 2012). Although this is a promising beginning, the lack of clear validation suggests that operant‐response testing remains the best behavioral approach for determining dogs' ODT at this stage. As yet, there is not sufficient evidence that an H–D test can validly and reliably predict ODTs of dogs.
Acknowledgement
Open access publishing facilitated by University of New England, as part of the Wiley ‐ University of New England agreement via the Council of Australian University Librarians.
The authors sincerely thank the participating dogs' owners for offering their time and allowing their dogs to participate in the project. We also thank Christine Morton for her valuable comments and assistance with statistical analysis and reporting.
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Declarations of interest: None.
Footnotes
Editor‐in‐Chief: Christy Alligood Associate Editor: Amanda Mahoney
References
- Angle, C. T. , Wakshlag, J. J. , Gillette, R. L. , Steury, T. , Haney, P. , Barrett, J. , & Fisher, T. (2014). The effects of exercise and diet on olfactory capability in detection dogs. Journal of Nutritional Science, 3, e44. 10.1017/jns.2014.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arner, L. D. , Johnson, G. R. , & Skovronek, H. S. (1986). Delineating toxic areas by canine olfaction. Journal of Hazardous Materials, 13(3), 375‐381. 10.1016/0304-3894(86)85009-9 [DOI] [Google Scholar]
- Aviles‐Rosa, E. O. , McGlone, J. J. , & Hall, N. J. (2020). Use of a habituation‐dishabituation paradigm to assess gilt olfaction and sensitivity to the boar pheromone. Applied Animal Behaviour Science, 231, 105086. 10.1016/j.applanim.2020.105086 [DOI] [Google Scholar]
- Cleland, T. A. , Morse, A. , Yue, E. L. , & Linster, C. (2002). Behavioral models of odor similarity. Behavioral Neuroscience, 116(2), 222‐231. 10.1037/0735-7044.116.2.222 [DOI] [PubMed] [Google Scholar]
- Cometto‐Muñiz, J., E. , Cain, W. S. , & Abraham, M. H. (2003). Quantification of chemical vapors in chemosensory research. Chemical Senses, 28(6), 467‐477. 10.1093/chemse/28.6.467 [DOI] [PubMed] [Google Scholar]
- Concha, A. R. , Guest, C. M. , Harris, R. , Pike, T. W. , Feugier, A. , Zulch, H. , & Mills, D. S. (2019). Canine olfactory thresholds to amyl acetate in a biomedical detection scenario. Frontiers in Veterinary Science, 5, 345. 10.3389/fvets.2018.00345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engen, T. (1960). Effect of practice and instruction on olfactory thresholds. Perceptual and Motor Skills, 10, 195‐198. 10.2466/pms.1960.10.3.195 [DOI] [Google Scholar]
- Escanilla, O. , Alperin, S. , Youssef, M. , Ennis, M. , & Linster, C. (2012). Noradrenergic but not cholinergic modulation of olfactory bulb during processing of near threshold concentration stimuli. Behavioral Neuroscience, 126(5), 720‐728. 10.1037/a0030006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezeh, P. I. , Myers, L. J. , Hanrahan, L. A. , Kemppainen, R. J. , & Cummins, K. A. (1992). Effects of steroids on the olfactory function of the dog. Physiology & Behavior, 51(6), 1183‐1187. 10.1016/0031-9384(92)90306-M [DOI] [PubMed] [Google Scholar]
- Farrow, L. F. , Barati, A. , & McDonald, P. G. (2020). Cooperative bird discriminates between individuals based purely on their aerial alarm calls. Behavioral Ecology, 31(2), 440–447. 10.1093/beheco/arz182 [DOI] [Google Scholar]
- Gadbois, S. , & Reeve, C. (2014). Canine olfaction: Scent, sign, and situation. In Horowitz A. (Ed.), Domestic dog cognition and behavior (pp. 3‐29). Springer‐Verlag. [Google Scholar]
- Gregg, B. , & Thiessen, D. D. (1981). A simple method of olfactory discrimination of urines for the Mongolian gerbil, Meriones unguiculatus. Physiology & Behavior, 26(6), 1133‐1136. 10.1016/0031-9384(81)90221-3 [DOI] [PubMed] [Google Scholar]
- Groves, P. M. , & Thompson, R. F. (1970). Habituation: A dual‐process theory. Psychological Review, 77(5), 419–450. https://doi.org/10/1037/h00298810 [DOI] [PubMed] [Google Scholar]
- Hall, N. J. , Smith, D. W. , & Wynne, C. D. L. (2016). Effect of odorant pre‐exposure on domestic dogs' sensitivity on an odorant detection task. Applied Animal Behaviour Science, 178, 80‐87. 10.1016/j.applanim.2016.02.003 [DOI] [Google Scholar]
- Helton, W. S. (2009). Overview of scent detection work: Issues and opportunities. In Helton W. S. (Ed.), Canine ergonomics: The science of working dogs (pp. 83‐97): CRC Press. [Google Scholar]
- Hilliard, S. (2003). The Steel Helmet Project: Canine olfactory detection of low concentrations of a surrogate chemical warfare agent. International Journal of Comparative Psychology, 16(4), 193‐208. https://escholarship.org/uc/item/5m54n59n [Google Scholar]
- Hirano, Y. , Oosawa, T. , & Tonosaki, K. (2000). Electroencephalographic olfactometry (EEGO) analysis of odour responses in dogs. Research in Veterinary Science, 69(3), 263‐265. 10.1053/rvsc.2000.0420 [DOI] [PubMed] [Google Scholar]
- Hostachy, C. , Couzi, P. , Portemer, G. , Hanafi‐Portier, M. , Murmu, M. , Deisig, N. , & Dacher, M. (2019). Exposure to conspecific and heterospecific sex‐pheromones modulates gustatory habituation in the moth Agrotis ipsilon . Frontiers in Physiology, 10, 1518. 10.3389/fphys.2019.01518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston, J. M. , Myers, L. J. , & Waggoner, L. P. , & Williams, M. (March, 1994). Determination of canine olfactory thresholds using operant laboratory methods. Paper presented at the SPIE Substance Detection Systems, Innsbruck, Austria. 10.1117/12.171244 [DOI] [Google Scholar]
- Kokocińska‐Kusiak, A. , Woszczyło, M. , Zybala, M. , Maciocha, J. , Barłowska, K. , & Dzięcioł, M. (2021). Canine olfaction: Physiology, behavior, and possibilities for practical applications. Animals, 11(8), 2463. 10.3390/ani11082463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krestel, D. , Passe, D. , Smith, J. C. , & Jonsson, L. (1984). Behavioral determination of olfactory thresholds to amyl acetate in dogs. Neuroscience & Biobehavioral Reviews, 8(2), 169‐174. 10.1016/0149-7634(84)90037-X [DOI] [PubMed] [Google Scholar]
- Kuiper, R. , Hoogenboezem, R. , Huisman, S. , Sonneveld, P. , & van Duin, M. (2019). nopaco: a non‐parametric concordance coefficient. R package version, 1(6). https://CRAN.R-project.org/package=nopaco. [Google Scholar]
- Laska, M. , Wieser, A. , & Salazar, L. T. (2006). Sex‐specific differences in olfactory sensitivity for putative human pheromones in nonhuman primates. Journal of Comparative Psychology, 120(2), 106‐112. 10.1037/0735-7036.120.2.106 [DOI] [PubMed] [Google Scholar]
- Lejeune, F. , Delacroix, E. , Gentaz, E. , Berne‐Audéoud, F. , Marcus, L. , & Debillon, T. (2021). Influence of swaddling on tactile manual learning in preterm infants. Early Human Development, 153, 105288. 10.1016/j.earlhumdev.2020.105288 [DOI] [PubMed] [Google Scholar]
- Mandairon, N. , Stack, C. , Kiselycznyk, C. , & Linster, C. (2006). Enrichment to odors improves olfactory discrimination in adult rats. Behavioral Neuroscience, 120(1), 173‐179. 10.1037/0735-7044.120.1.173 [DOI] [PubMed] [Google Scholar]
- Mandairon, N. , Sultan, S. , Rey, N. , Kermen, F. , Moreno, M. , Busto, G. , & Didier, A. (2009). A computer‐assisted odorized hole‐board for testing olfactory perception in mice. Journal of Neuroscience Methods, 180(2), 296‐303. 10.1016/j.jneumeth.2009.04.008 [DOI] [PubMed] [Google Scholar]
- Marshall, M. , & Moulton, D. G. (1981). Olfactory sensitivity to α‐ionone in humans and dogs. Chemical Senses, 6(1), 53‐61. 10.1093/chemse/6.1.53 [DOI] [Google Scholar]
- Messina, A. , Potrich, D. , Schiona, I. , Sovrano, V. A. , Fraser, S. E. , Brennan, C. H. , & Vallortigara, G. (2020). Response to change in the number of visual stimuli in zebrafish: A behavioural and molecular study. Scientific Reports, 10(1), 5769. 10.1038/s41598-020-62608-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser, A. Y. , Bizo, L. , & Brown, W. Y. (2019). Olfactory generalization in detector dogs. Animals, 9, 702. 10.3390/ani9090702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser, A. Y. , Brown, W. Y. , Bizo, L. A. , Andrew, N. , & Taylor, M. (2020). Dogs detect live insects after training with odour‐proxy training aids: Scent extract and dead specimens. Chemical Senses, 45(3), 179‐186. 10.1093/chemse/bjaa001 [DOI] [PubMed] [Google Scholar]
- Moulton, D. G. , Ashton, E. H. , & Eayrs, J. T. (1960). Studies in olfactory acuity. Relative detectability of n‐aliphatic acids by the dog. Animal Behaviour, 8(3‐4), 117‐128. 10.1016/0003-3472(60)90019-1 [DOI] [Google Scholar]
- Myers, L. J. (1991). Use of innate behaviors to evaluate sensory function in the dog. Veterinary Clinics of North America: Small Animal Practice, 21(2), 389‐399. 10.1016/s0195-5616(91)50040-1 [DOI] [PubMed] [Google Scholar]
- Pavlov, I. P. (1927). Conditioned reflexes: An investigation of the physiological activity of the cerebral cortex. Oxford University Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez‐Villalba, A. , Palop, M. J. , Pérez‐Sánchez, F. , & Fariñas, I. (2015). Assessment of olfactory behavior in mice: Odorant detection and habituation‐dishabituation tests. Bio‐protocol, 5(13), e1518. 10.21769/BioProtoc.1518 [DOI] [Google Scholar]
- Phelan, J. M. , & Barnett, J. L. (August, 2002). Chemical sensing thresholds for mine detection dogs. Paper presented at the Detection and Remediation Technologies for Mines and Minelike Targets VII, Orlando, FL, United States. 10.1117/12.479126 [DOI] [Google Scholar]
- Philpott, C. M. , Goodenough, P. C. , Wolstenholme, C. R. , & Murty, G. E. (2004). Which solvent for olfactory testing? Clinical Otolaryngology & Allied Sciences, 29(6), 667‐671. 10.1111/j.1365-2273.2004.00890.x. [DOI] [PubMed] [Google Scholar]
- Pickel, D. , Manucy, G. P. , Walker, D. B. , Hall, S. B. , & Walker, J. C. (2004). Evidence for canine olfactory detection of melanoma. Applied Animal Behaviour Science, 89(1‐2), 107‐116. 10.1016/j.applanim.2004.04.008 [DOI] [Google Scholar]
- Polgár, Z. , Kinnunen, M. , Újváry, D. , Miklósi, Á. , & Gácsi, M. (2016). A test of canine olfactory capacity: Comparing various dog breeds and wolves in a natural detection task. PLOS one, 11(5), e0154087. 10.1371/journal.pone.0154087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, C. J. , Banks, P. B. , Brown, S. , Latham, M. C. , Latham, A. D. M. , Pech, R. P. , & Norbury, G. L. (2020). Invasive mammalian predators habituate to and generalize avian prey cues: A mechanism for conserving native prey. Ecological Applications, 30(8), e02200. 10.1002/eap.2200 [DOI] [PubMed] [Google Scholar]
- Qiu, Q. , Scott, A. , Scheerer, H. , Sapkota, N. , Lee, D. K. , Ma, L. , & Yu, C. R. (2014). Automated analyses of innate olfactory behaviors in rodents. PLOS one, 9(4), e93468. 10.1371/journal.pone.0093468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing. https://www.r-project.org/
- Rankin, C. H. , Abrams, T. , Barry, R. J. , Bhatnagar, S. , Clayton, D. F. , Colombo, J. , Coppola, G. , Geyer, M. A. , Glanzman, D. L. , Marsland, S. , McSweeney, F. , Wilson, D. A. , Wu, C.‐F. , & Thompson, R. F. (2009). Habituation revisited: An updated and revised description of the behavioral characteristics of habituation. Neurobiology of Learning and Memory, 92(2), 135–138. 10.1016/j.nlm.2008.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rørvang, M. V. , Jensen, M. B. , & Nielsen, B. L. (2017). Development of test for determining olfactory investigation of complex odours in cattle. Applied Animal Behaviour Science, 196, 84–90. 10.1016/j.applanim.2017.07.008 [DOI] [Google Scholar]
- Rothery, P. (1979). A nonparametric measure of intraclass correlation. Biometrika, 66(3), 629–639. 10.2307/2335185 [DOI] [Google Scholar]
- Salvin, H. E. , McGrath, C. , McGreevy, P. D. , & Valenzuela, M. J. (2012). Development of a novel paradigm for the measurement of olfactory discrimination in dogs (Canis familiaris): A pilot study. Journal of Veterinary Behavior, 7(1), 3‐10. 10.1016/j.jveb.2011.04.005 [DOI] [Google Scholar]
- SAS Institute, Cary, NC. (2019). JMP®, Version 14.2.0. [computer software]. https://www.jmp.com/en_us/home.html
- Siddle, D. A. T. , & Lipp, O. V. (1997). Orienting, habituation, and information processing: The effects of omission, the role of expectancy, and the problem of dishabituation. In Lang P. J. (Ed.), Attention and orienting: Sensory and motivational processes, (pp. 23‐40). Psychology Press. [Google Scholar]
- Slaughter, V. , & Suddendorf, T. (2007). Participant loss due to "fussiness" in infant visual paradigms: A review of the last 20 years. Infant Behavior& Development, 30(3), 505‐514. 10.1016/j.infbeh.2006.12.006 [DOI] [PubMed] [Google Scholar]
- Spence, C. (2019). Perceptual learning in the chemical senses: A review. Food Research International, 123, 746‐761. 10.1016/j.foodres.2019.06.005 [DOI] [PubMed] [Google Scholar]
- Steiner, G. Z. , & Barry, R. J. (2014). The mechanism of dishabituation. Frontiers in Integrative Neuroscience, 8, 14. 10.3389/fnint.2014.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne, C. (1995). Feeding behaviour of domestic dogs and the role of experience. In Serpell J. (Ed.), The domestic dog: Its evolution, behaviour and interactions with people (pp. 103‐113). Cambridge University Press. [Google Scholar]
- Wackermannová, M. , Pinc, L. , & Jebavý, L. (2016). Olfactory sensitivity in mammalian species. Physiological Research, 65(1), 369‐390. 10.33549/physiolres.932955 [DOI] [PubMed] [Google Scholar]
- Waggoner, L. P. , Johnston, J. M. , Williams, M. , Jackson, J. , Jones, M. H. , Boussom, T. , & Petrousky, J. A. (February, 1997). Canine olfactory sensitivity to cocaine hydrochloride and methyl benzoate. Paper presented at the SPIE, Chemistry‐ and Biology‐Based Technologies for Contraband Detection, Boston, MA, United States. 10.1117/12.266775 [DOI] [Google Scholar]
- Walker, D. B. , Walker, J. C. , Cavnar, P. J. , Taylor, J. L. , Pickel, D. H. , Hall, S. B. , & Suarez, J. C. (2006). Naturalistic quantification of canine olfactory sensitivity. Applied Animal Behaviour Science, 97(2‐4), 241‐254. 10.1016/j.applanim.2005.07.009 [DOI] [Google Scholar]
- Wesson, D. W. , Levy, E. , Nixon, R. A. , & Wilson, D. A. (2010). Olfactory dysfunction correlates with amyloid‐β burden in an Alzheimer's disease mouse model. Journal of Neuroscience, 30(2), 505‐514. 10.1523/JNEUROSCI.4622-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, D. A. (2009). Olfaction as a model system for the neurobiology of mammalian short‐term habituation. Neurobiology of Learning and Memory, 92(2), 199‐205. 10.1016/j.nlm.2008.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, M. , & Crawley, J. N. (2009). Simple behavioral assessment of mouse olfaction. Current Protocols in Neuroscience, 8(24). 10.1002/0471142301.ns0824s48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee, K. K. , & Wysocki, C. J. (2001). Odorant exposure increases olfactory sensitivity: Olfactory epithelium is implicated. Physiology & Behavior, 72(5), 705‐711. 10.1016/s0031-9384(01)00428-0 [DOI] [PubMed] [Google Scholar]
- Zou, J. , Wang, W. , Pan, Y. W. , Lu, S. , & Xia, Z. (2015). Methods to measure olfactory behavior in mice. Current Protocols in Toxicology, 63, 11.18.1‐11.18.21. 10.1002/0471140856.tx1118s63 [DOI] [PMC free article] [PubMed] [Google Scholar]


