Abstract
Haemophilus ducreyi produces an outer membrane protein called DsrA, which is required for serum resistance. An isogenic dsrA mutant, FX517, was constructed previously in H. ducreyi 35000. Compared to its parent, FX517 cannot survive in normal human serum. When complemented in trans with a plasmid containing dsrA, FX517 is converted to a serum-resistant phenotype (C. Elkins, K. J. Morrow, Jr., and B. Olsen, Infect. Immun. 68:1608–1619, 2000). To test whether dsrA was transcribed in vivo, we successfully amplified transcripts in five biopsies obtained from four experimentally infected human subjects. To test whether DsrA was required for virulence, six volunteers were experimentally infected with 35000 and FX517 and observed for papule and pustule formation. Each subject was inoculated with two doses (70 to 80 CFU) of live 35000 and 1 dose of heat-killed bacteria on one arm and with three doses (ranging from 35 to 800 CFU) of live FX517 on the other arm. Papules developed at similar rates at sites inoculated with the mutant or parent. However, mutant papule surface areas were significantly smaller than parent papules. The pustule formation rate was 58% (95% confidence interval [CI] of 28 to 85%) at 12 parent sites, and 0% (95% CI of 0 to 15%) at 18 mutant sites (P = 0.0004). Although biosafety regulations precluded our testing the complemented mutant in humans, these results suggest that expression of DsrA facilitates the ability of H. ducreyi to progress to the pustular stage of disease.
Haemophilus ducreyi causes chancroid, which facilitates transmission of the human immunodeficiency virus (HIV) (16, 31, 40). Usually, chancroid outbreaks occur sporadically in industrialized countries (24, 25, 39). Although now rare in the United States (12), chancroid remains a common public health problem in many developing countries (9, 10, 13, 26, 30, 37).
Recently, Elkins et al. described a 30-kDa outer membrane protein called Ducreyi serum resistance A (DsrA) (14). DsrA is expressed by all naturally occurring strains of H. ducreyi tested, except for three serum-sensitive strains that were avirulent in animal models (14). The dsrA gene was identified and sequenced (14), and the predicted amino acid sequence of DsrA was noted to be similar to the UspA2 protein of Moraxella catarrhalis and YadA of Yersinia spp. (14). Both UspA2 and YadA mediate serum resistance (1, 29). An isogenic dsrA mutant, FX517, of H. ducreyi 35000 was constructed by insertion of a chloramphenicol acetyltransferase (cat) cassette into the dsrA open reading frame and allele exchange. FX517 no longer expressed DsrA and was at least 10-fold more serum susceptible than its parent. FX517 and the three naturally occurring serum-susceptible strains that lacked DsrA were complemented in trans with a plasmid containing dsrA, and all four strains were converted to a serum-resistant phenotype.
The role of DsrA in the pathogenesis of chancroid is currently unknown. Utilizing a human challenge model, we examined whether dsrA was transcribed during experimental infection with H. ducreyi. We also compared the ability of 35000 and FX517 to cause infection in human volunteers.
MATERIALS AND METHODS
Bacteria.
H. ducreyi 35000 and FX517 were described previously (14). 35000HP is a human-passaged variant of 35000 (4, 5, 34).
Detection of dsrA transcripts in vivo.
Biopsies of pustules and normal skin were obtained from five infected and two uninfected volunteers as described in detail elsewhere (36a). RNA was isolated, and cDNA was synthesized from the biopsies, from uninfected skin, and from uninfected skin homogenized with 106 CFU of 35000HP using Ultraspec RNA (Biotecx Laboratories, Inc., Houston, Tex.) and Advantage RT-for-PCR Kit (Clontech Laboratories, Palo Alto, Calif.) as described elsewhere (36a). RNA that was not subjected to reverse transcription (RT) was included to control for DNA contamination. Amplification of target cDNA was performed with the dsrA-9 and dsrA-11 primers (14) using the PCR Core Kit Plus (Roche Molecular Biochemicals, Indianapolis, Ind.). PCR-positive and -negative control templates included genomic DNA from 35000HP and H2O, respectively. Amplicons were analyzed by electrophoresis of 10 μl of each PCR reaction on 1.2% agarose gels stained with ethidium bromide.
Human challenge protocol.
Healthy adult male and female volunteers over 18 years of age were recruited for the study. Informed consent was obtained from the subjects for participation and for HIV serology, in accordance with the human experimentation guidelines of the U.S. Department of Health and Human Services and the Institutional Review Board of Indiana University–Purdue University Indianapolis. The experimental challenge protocol, preparation and inoculation of the bacteria, calculation of the estimated delivered dose (EDD), and clinical observations were done exactly as described previously (3–5, 34, 35). When a papule was present, the area of erythema was calculated by measuring the greatest dimension vertically and horizontally in millimeters and then multiplying the two measurements. Subjects were observed until they reached a clinical endpoint, defined as either 14 days after inoculation, development of a painful pustule, or resolution of infection at all sites. When a clinical endpoint was achieved, the code was broken and up to two sites with active disease (one inoculated with the parent and one inoculated with the mutant), if present, were biopsied with a punch forceps. The subjects were then treated with two doses of oral ciprofloxacin as described previously.
Biopsies.
Specimens were cut into portions. One portion was fixed in formalin, and immunohistological studies were done as previously described (28, 34, 35). One portion was homogenized in 1 ml of freezing medium (3% [wt/vol] tryptic soy broth, 10% glycerol, 10% heat-inactivated fetal calf serum) for 2 min on ice and cultured semi-quantitatively as described previously (34, 35).
Phenotypes of recovered bacteria.
Individual colonies from the inocula, surface cultures, and biopsies were picked, suspended in freezing medium, and frozen in 96-well plates. The colonies were scored for susceptibility to chloramphenicol on chloramphenicol-containing chocolate agar plates. At least 30 individual colonies per specimen, if available, were analyzed. If 30 colonies had the correct phenotype, then we were confident (95% probability) that, at most, only 11% of the colonies could have the incorrect phenotype in a specimen.
RESULTS
In vivo expression of dsrA.
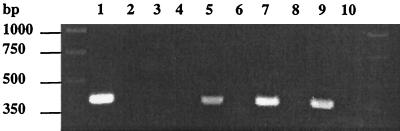
Transcription of dsrA was examined by RT-PCR of biopsies of five pustules obtained from four volunteers at sites infected with 35000 or 35000HP. RT-PCR was also performed on cDNA prepared from uninfected skin with or without added 35000HP. The expected 385-bp RT-PCR product was obtained from uninfected skin supplemented with bacteria and from the five biopsies of pustules but not from the uninfected control (Fig. 1 and data not shown). The sequence of the 385-bp amplicon generated with genomic DNA from 35000HP was identical to the published sequence of dsrA. Thus, dsrA was transcribed during experimental infection of human volunteers.
FIG. 1.
Composite agarose gel stained with ethidium bromide from PCR and RT-PCR products obtained using dsrA primers. Lanes 1 and 2 contain 35000HP genomic DNA and no template, respectively. cDNA (lanes 3, 5, 7, and 9) and RNA that was not reverse transcribed (lanes 4, 6, 8, and 10) were prepared from uninfected skin (lanes 3 and 4), uninfected skin homogenized with H. ducreyi (lanes 5 and 6), a biopsy from volunteer 160 (lanes 7 and 8), and a biopsy from volunteer 142 (lanes 9 and 10).
Human inoculation experiments.
Seven healthy adults (four females, three males; age range of 21 to 46 years; mean age ± the standard deviation, 33.9 ± 8.3 years) volunteered for the study. Three subjects (158, 159, and 160) were challenged in the first iteration, and three subjects (155, 165, and 166) were challenged in the second iteration (Table 1). Subject 161 withdrew prior to inoculation.
TABLE 1.
Response to inoculation of H. ducreyia
| Subject | Observation period (days) | Isolate | No. of initial papules | Final outcome
|
||
|---|---|---|---|---|---|---|
| No. of papules | No. of pustules | No. resolved | ||||
| 158 | 14 | |||||
| 35000 | 2 | 1 | 1 | |||
| FX517 | 2 | 2 | ||||
| 159 | 14 | |||||
| 35000 | 1 | 1 | ||||
| FX517 | 3 | 3 | ||||
| 160 | 6 | |||||
| 35000 | 2 | 2 | ||||
| FX517 | 2 | 2 | ||||
| 155 | 7 | |||||
| 35000 | 2 | 2 | ||||
| FX517 | 1 | 1 | ||||
| 165 | 9 | |||||
| 35000 | 2 | 2 | ||||
| FX517 | 1 | 1 | ||||
| 166 | 12 | |||||
| 35000 | 2 | 2 | ||||
| FX517 | 3 | 3 | ||||
Each volunteer was inoculated at two sites with parent strain 35000 and at three sites with mutant strain FX517. Volunteers 158, 159, and 160 were inoculated in the first iteration, and volunteers 155, 165, and 166 were inoculated in the second iteration.
An escalating dose-response study was used to compare the virulence of the mutant and the parent. We initially inoculated three subjects at six sites on both arms. One arm was inoculated at three sites with EDDs of 33, 65, and 130 CFU of FX517. The other arm was inoculated at two sites with EDDs of 60 CFU of the parent strain and at a third site with 130 CFU of heat-killed FX517. Papules developed at five of six sites inoculated with the parent strain and at seven of nine sites inoculated with the mutant. All mutant papules resolved (Table 1). Pustules developed at three of six sites inoculated with the parent strain and at none of the nine sites inoculated with the mutant.
Since the mutant was impaired in its ability to cause pustules, we infected three more subjects and increased the dose of the mutant. For this group of subjects, three sites were inoculated with EDDs of 213, 427, and 855 CFU of FX517. Two sites were inoculated with 93 CFU of the parent strain, and a third site was inoculated with 855 CFU of heat-killed FX517. Six of six parent sites and five of nine mutant sites developed papules (Table 1). At the clinical endpoint, two parent papules and all five mutant papules resolved. Pustules developed at four of six parent sites and at none of nine mutant sites. Thus, FX517 was impaired in its ability to form pustules even at doses tenfold that of the parent strain.
The cumulative results for the two iterations showed that papules developed at 92% (exact binomial 95% confidence interval [CI], 61 to 99%) of sites inoculated with 35000 and at 67% (exact binomial 95% CI, 41 to 87%) of sites inoculated with FX517 (one-tailed Fisher's exact test, P = 0.125). During the trial, we noted that papules on one arm appeared smaller than papules on the other arm. We calculated the surface area of papule erythema at each site 24 and 48 h after inoculation after the code was broken. The surface areas of mutant papules were significantly smaller than the parent papules at both time points (Table 2). The surface areas of the mutant papules were statistically similar to those of the heat-killed control (Table 2). Thus, the mutant caused smaller lesions than the parent during the initial stages of infection.
TABLE 2.
Papule surface areaa
| Day | Mean papule surface area (mm2) ± SD
|
P
|
|||
|---|---|---|---|---|---|
| H | M | P | M versus H | M versus P | |
| 1 | 5.17 ± 4.45 | 5.11 ± 6.21 | 37.7 ± 37.7 | 1 | 0.0009 |
| 2 | 2.00 ± 3.49 | 2.06 ± 4.32 | 39.9 ± 35.2 | 0.998 | 0.0001 |
H, heat-killed control; M, mutant (FX517); P, parent (35000).
Overall, pustules formed at 7 of 12 (58%; exact binomial 95% CI, 28 to 85%) sites inoculated with 35000 compared to 0 of 18 (0%; exact binomial 95% CI, 0 to 15%) sites inoculated with FX517 (one-tailed Fisher's exact test, P = 0.0004). Thus, the mutant was impaired in its ability to form pustules when compared to the parent.
Bacterial recovery and cellular infiltrate from mutant and parent lesions.
From daily surface cultures, H. ducreyi was recovered intermittently from 1 of 12 parent sites, while no bacteria were recovered from the 18 mutant sites or the 6 heat-killed sites. Four subjects had parent sites that contained pustules at endpoint and were biopsied. H. ducreyi were recovered (range, 2.6 × 104 to 4.1 × 106 CFU per g of tissue) from four of four parent biopsies. We also examined the cellular infiltrate in two parent biopsies. Micropustules with polymorphonuclear leukocytes were present in the epidermis (data not shown). The dermis contained a perivascular infiltrate of mononuclear cells and some polymorphonuclear leukocytes, and the venules were lined with reactive endothelial cells. The mononuclear cells stained positively with a CD3 marker verifying their T-cell origin (data not shown). No disease was present at the mutant sites at endpoint, and no mutant sites were biopsied in accordance with our protocols.
Confirmation of the antibiotic susceptibility of the recovered bacteria.
To confirm that the inocula were correct and that no cross-contamination of sites had occurred during infection, individual colonies from each of the broth cultures used to prepare the inocula, from surface cultures, and from biopsy specimens were analyzed for chloramphenicol susceptibility (Cms). For the two parent and two mutant broth cultures used to prepare the inocula, all 88 parent colonies and 88 mutant colonies tested were phenotypically correct (mutant, Cmr; parent, Cms). All 40 colonies obtained from surface cultures of parent sites were phenotypically correct (Cms). A total of 192 parent colonies obtained from biopsies were phenotypically correct (Cms). Thus, all colonies tested from the inocula, surface cultures, and biopsies had the expected antibiotic susceptibility.
DISCUSSION
In this study, we amplified dsrA cDNA from RNA of pustules of experimentally infected subjects, suggesting that DsrA was expressed in vivo. We also tested the ability of an isogenic DsrA-deficient mutant (FX517) to infect human volunteers. In our volunteer group, the mutant formed papules at a rate similar to that of the parent strain, but mutant papules were significantly smaller and did not progress to the pustular stage of disease. In previous studies, isogenic hgbA receptor and pal mutants were impaired in their ability to form pustules in humans (3, 17). This is the third demonstration that a putative virulence factor of H. ducreyi facilitates pustule formation in humans.
Our institutional biosafety committee does not permit the testing of mutants complemented with plasmids in human subjects. We cannot exclude the possibility that the decreased virulence of FX517 was due to a secondary mutation or a polar effect of the cat cassette. However, complementation of FX517 in trans restores expression of DsrA and serum resistance to the mutant (14). Thus, it is highly likely that the decreased virulence of FX517 was due to the mutation of dsrA.
The bactericidal activity of human serum is an important component of innate host defenses, and many bacterial pathogens are serum resistant. H. ducreyi 35000 is resistant to normal human serum at concentrations of up to 50% (18). Although initial investigations by Odumeru and colleagues suggested that truncations in the lipooligosaccharide of H. ducreyi mediated serum sensitivity (27), recent studies showed that the loss of serum resistance was due to the loss of DsrA and not to truncations in lipooligosaccharide (14, 18, 36). In natural infection, H. ducreyi is thought to gain access to the skin via wounds that occur during intercourse (25). Puncture wounds are required to initiate experimental infection (35). FX517 may have been less efficient at establishing and maintaining infection in our volunteers because it was killed by serum that leaks into the skin during wound healing and inflammation (20).
DsrA is a member of a growing family of proteins that share homology at their C termini and confer serum resistance and/or the ability to adhere to human cells on their respective organisms (1, 2, 14, 21, 23, 29, 32, 33). This family includes the YadA protein of Yersinia spp., the UspA2 protein of M. catarrhalis and the Eib protein of certain Escherichia coli strains. Each protein appears to have a unique mechanism of diverting complement-mediated antibody killing. For example, YadA binds complement factor H (29), UspA2 binds the complement regulatory protein vitronectin (23), and the Eib proteins bind immunoglobulins via the Fc portion of the molecule (32, 33). The mechanism by which DsrA mediates serum resistance is not known and is currently under investigation.
Attachment of bacteria to host cells is a critical step in the establishment of infection. H. ducreyi attaches to human keratinocytes and fibroblasts in vitro (6, 11, 19, 22, 38). Elkins and colleagues tested the ability of FX517 to attach to a keratinocyte cell line and found that FX517 adhered significantly less efficiently than the parent strain (L. E. Cole, C. Elkins, T. Kawula, Abstr. 100th Gen. Meet. Am. Soc. Microbiol., abstr. B-122, p. 69, 2000). The complemented mutant regained its ability to attach. However, confocal microscopy data failed to show that H. ducreyi binds to keratinocytes throughout the course of experimental infection of human subjects (8; unpublished observations). Thus, the significance of the in vitro observations and their relevance to human disease is not clear.
Throughout the course of experimental infection, H. ducreyi colocalizes with collagen in the upper dermis (unpublished observations). H. ducreyi binds to collagen in vitro (7). YadA mediates binding to collagen through repeated NSVAIG-S motifs in the amino-terminal half of the protein (15). DsrA lacks sequences homologous to the N-terminal half of YadA, and it is unlikely that DsrA mediates collagen binding through a similar motif.
In summary, our data show that dsrA is transcribed in vivo and suggests that expression of DsrA is required for the virulence of H. ducreyi. The homology among DsrA, YadA, UspA2, and the Eib proteins suggests that DsrA may have multiple functions, including serum resistance and cell adherence. Therefore, FX517 may have been attenuated in experimental infection for multiple reasons. Future studies will focus on delineating the mechanisms by which DsrA mediates serum resistance and its possible role in adherence.
ACKNOWLEDGMENTS
This work was supported by grants AI27863, AI31494, AI40263, and AI31496 from the National Institutes of Health (NIH). The clinical trial was supported by the Sexually Transmitted Diseases Clinical Trials Unit through contract N01-AI75329 from the NIAID and by grant MO1RR00750 to the GCRC at Indiana University.
REFERENCES
- 1.Aebi C, LaFontane E R, Cope L D, Latimer J L, Lumbley S L, McCracken G H, Jr, Hansen E J. Phenotypic effect of isogenic uspA1 and uspA2 mutations on Moraxella catarrhalis 035E. Infect Immun. 1998;66:3113–3119. doi: 10.1128/iai.66.7.3113-3119.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aebi C, Maciver I, Latimer J L, Cope L D, Stevens M K, Thomas S E, McCracken G H, Jr, Hansen E J. A protective epitope of Moraxella catarrhalis is encoded by two different genes. Infect Immun. 1997;65:4367–4377. doi: 10.1128/iai.65.11.4367-4377.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Tawfiq J A, Fortney K R, Katz B P, Elkins C, Spinola S M. An isogenic hemoglobin receptor-deficient mutant of Haemophilus ducreyi is attenuated in the human model of experimental infection. J Infect Dis. 2000;181:1049–1054. doi: 10.1086/315309. [DOI] [PubMed] [Google Scholar]
- 4.Al-Tawfiq J A, Harezlak J, Katz B P, Spinola S M. Cumulative experience with Haemophilus ducreyi in the human model of experimental infection. Sex Transm Dis. 2000;27:111–114. doi: 10.1097/00007435-200002000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Al-Tawfiq J A, Thornton A C, Katz B P, Fortney K R, Todd K D, Hood A F, Spinola S M. Standardization of the experimental model of Haemophilus ducreyi infection in human subjects. J Infect Dis. 1998;178:1684–1687. doi: 10.1086/314483. [DOI] [PubMed] [Google Scholar]
- 6.Alfa M J, Degagne P, Hollyer T. Haemophilus ducreyi adheres to but does not invade cultured human foreskin cells. Infect Immun. 1993;61:1735–1742. doi: 10.1128/iai.61.5.1735-1742.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bauer M E, Spinola S M. Binding of Haemophilus ducreyi to extracellular matrix proteins. Infect Immun. 1999;67:2649–2653. doi: 10.1128/iai.67.5.2649-2652.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bauer M E, Spinola S M. Localization of Haemophilus ducreyi at the pustular stage of disease in the human model of infection. Infect Immun. 2000;68:2309–2314. doi: 10.1128/iai.68.4.2309-2314.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Behets F M-T, Andriamiadana J, Randrianasolo D, Randriamanga R, Rasamilalao D, Chen C-Y, Weiss J B, Morse S A, Dallabetta G, Cohen M S. Chancroid, primary syphilis, genital herpes, and lymphogranuloma venereum in Antananarivo, Madagascar. J Infect Dis. 1999;180:1382–1385. doi: 10.1086/315005. [DOI] [PubMed] [Google Scholar]
- 10.Behets F M-T, Brathwaite A R, Hylton-Kong T, Chen C-Y, Hoffman I, Weiss J B, Morse S A, Dallabetta G, Cohen M S, Figueroa J P. Genital ulcers: etiology, clinical diagnosis, and associated human immunodeficiency virus infection in Kingston, Jamaica. Clin Infect Dis. 1999;28:1086–1090. doi: 10.1086/514751. [DOI] [PubMed] [Google Scholar]
- 11.Brentjens R J, Spinola S M, Campagnari A A. Haemophilus ducreyi adheres to human keratinocytes. Microb Pathog. 1994;16:243–247. doi: 10.1006/mpat.1994.1025. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Summary of notifiable diseases, United States, 1998. Morb Mortal Wkly Rep. 1999;47:53. [PubMed] [Google Scholar]
- 13.Chen C Y, Ballard R C, Beck-Sague C M, Dangor Y, Radebe F, Schmid S, Weiss J B, Tshabalala V, Fehler G, Htun Y, Morse S A. Human immunodeficiency virus infection and genital ulcer disease in South Africa. The herpetic connection. Sex Transm Dis. 2000;27:21–29. doi: 10.1097/00007435-200001000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Elkins C, Morrow K J, Olsen B. Serum resistance in Haemophilus ducreyi requires outer membrane protein DsrA. Infect Immun. 2000;68:1608–1619. doi: 10.1128/iai.68.3.1608-1619.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El Tahir Y, Kuusela P, Skurnik M. Functional mapping of the Yersinia enterocolitica adhesin YadA. Identification of eight NSVAIG-S motifs in the amino-terminal half of the protein involved in collagen binding. Mol Microbiol. 2000;37:192–206. doi: 10.1046/j.1365-2958.2000.01992.x. [DOI] [PubMed] [Google Scholar]
- 16.Fleming D T, Wasserheit J N. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999;75:3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fortney K R, Young R S, Bauer M E, Katz B P, Hood A F, Jr, Munson R S, Spinola S M. Expression of peptidoglycan-associated lipoprotein is required for virulence in the human model of Haemophilus ducreyi infection. Infect Immun. 2000;68:6441–6448. doi: 10.1128/iai.68.11.6441-6448.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hiltke T J, Bauer M E, Klesney-Tait J, Hansen E J, Munson R S, Jr, Spinola S M. Effect of normal and immune sera on Haemophilus ducreyi 35000HP and its isogenic MOMP and LOS mutants. Microb Pathog. 1999;26:93–102. doi: 10.1006/mpat.1998.0250. [DOI] [PubMed] [Google Scholar]
- 19.Hobbs M M, Paul T R, Wyrick P B, Kawula T H. Haemophilus ducreyi infection causes basal keratinocyte cytotoxicity and elicits a unique cytokine induction pattern in an in vitro human skin model. Infect Immun. 1998;66:2914–2921. doi: 10.1128/iai.66.6.2914-2921.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirsner R S, Eaglstein W H. The wound healing process. Dermatol Clin. 1993;11:629–640. [PubMed] [Google Scholar]
- 21.LaFontaine E R, Cope L D, Aebi C, Latimer J L, McCracken G H, Jr, Hansen E J. The UspA1 protein and a second type of UspA2 protein mediate adherence of Moraxella catarrhalis to human epithelial cells in vitro. J Bacteriol. 2000;182:1364–1373. doi: 10.1128/jb.182.5.1364-1373.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lammel C J, Dekker N P, Palefsky J, Brooks G F. In vitro model of Haemophilus ducreyi adherence to and entry into eukaryotic cells of genital origin. J Infect Dis. 1993;167:642–650. doi: 10.1093/infdis/167.3.642. [DOI] [PubMed] [Google Scholar]
- 23.McMichael J C, Fiske M J, Fredenberg R A, Chakravarti D N, VanDerMeid K R, Barniak V, Caplan J, Bortell E, Baker S, Arumugham R, Chen D. Isolation and characterization of two proteins from Moraxella catarrhalis that bear a common epitope. Infect Immun. 1998;66:4374–4381. doi: 10.1128/iai.66.9.4374-4381.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mertz K J, Trees D, Levine W C, Lewis J S, Litchfield B, Pettus K S, Morse S A, St. Louis M E, Weiss J B, Schwebke J, Dickes J, Kee R, Reynolds J, Hutcheson D, Green D, Dyer I, Richwald G A, Novotny J, Weisfuse I, Goldberg M, O'Donnell J A, Knaup R. Etiology of genital ulcers and prevalence of human immunodeficiency virus coinfection in 10 U.S. cities. J Infect Dis. 1998;178:1795–1798. doi: 10.1086/314502. [DOI] [PubMed] [Google Scholar]
- 25.Morse S A. Chancroid and Haemophilus ducreyi. Clin Microbiol Rev. 1989;2:137–157. doi: 10.1128/cmr.2.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morse S A, Trees D L, Htun Y, Radebe F, Orle K A, Dangor Y, Beck-Sague C M, Schmid S, Fehler G, Weiss J B, Ballard R C. Comparison of clinical diagnosis and standard laboratory and molecular methods for the diagnosis of genital ulcer disease in Lesotho: association with human immunodeficiency virus infection. J Infect Dis. 1997;175:583–589. doi: 10.1093/infdis/175.3.583. [DOI] [PubMed] [Google Scholar]
- 27.Odumeru J A, Wiseman G M, Ronald A R. Virulence factors of Haemophilus ducreyi. Infect Immun. 1984;43:607–611. doi: 10.1128/iai.43.2.607-611.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palmer K L, Schnizlein-Bick C T, Orazi A, John K, Chen C-Y, Hood A F, Spinola S M. The immune response to Haemophilus ducreyi resembles a delayed-type hypersensitivity reaction throughout experimental infection of human subjects. J Infect Dis. 1998;178:1688–1697. doi: 10.1086/314489. [DOI] [PubMed] [Google Scholar]
- 29.Pilz D, Vocke T, Heesemann J, Brade V. Mechanism of YadA-mediated serum resistance of Yersinia enterocolitica serotype O3. Infect Immun. 1992;60:189–195. doi: 10.1128/iai.60.1.189-195.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Risbud A, Chan-Tack K, Gadkari D, Gangakhedkar R R, Shepherd M E, Bollinger R, Mehendale S, Gaydos C, Divekar A, Rompalo A, Quinn T C. The etiology of genital ulcer disease by multiplex polymerase chain reaction and relationship to HIV infection among patients attending sexually transmitted disease clinics in Pune, India. Sex Transm Dis. 1999;26:55–62. doi: 10.1097/00007435-199901000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Royce R A, Sena A, Cates W, Cohen M S. Current concepts: sexual transmission of HIV. N Engl J Med. 1997;336:1072–1078. doi: 10.1056/NEJM199704103361507. [DOI] [PubMed] [Google Scholar]
- 32.Sandt C H, Hill C W. Four different genes responsible for nonimmune immunoglobulin-binding activities within a single strain of Escherichia coli. Infect Immun. 2000;68:2205–2214. doi: 10.1128/iai.68.4.2205-2214.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sandt C H, Wang Y-D, Wilson R, Hill C W. Escherichia coli strains with nonimmune immunoglobulin-binding activity. Infect Immun. 1997;65:4572–4579. doi: 10.1128/iai.65.11.4572-4579.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spinola S M, Orazi A, Arno J N, Fortney K, Kotylo P, Chen C-Y, Campagnari A A, Hood A F. Haemophilus ducreyi elicits a cutaneous infiltrate of CD4 cells during experimental human infection. J Infect Dis. 1996;173:394–402. doi: 10.1093/infdis/173.2.394. [DOI] [PubMed] [Google Scholar]
- 35.Spinola S M, Wild L M, Apicella M A, Gaspari A A, Campagnari A A. Experimental human infection with Haemophilus ducreyi. J Infect Dis. 1994;169:1146–1150. doi: 10.1093/infdis/169.5.1146. [DOI] [PubMed] [Google Scholar]
- 36.Sun S, Schilling B, Tarantino L, Tullius M V, Gibson B W, Munson R S. Cloning and characterization of the lipooligosaccharide galactosyltransferase II gene of Haemophilus ducreyi. J Bacteriol. 2000;182:2292–2298. doi: 10.1128/jb.182.8.2292-2298.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36a.Throm R E, Spinola S M. Transcription of candidate virulence genes of Haemophilus ducreyi during infection of human volunteers. Infect Immun. 2001;69:1483–1487. doi: 10.1128/IAI.69.3.1483-1487.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Totten P A, Kuypers J M, Chen C-Y, Alfa M J, Parsons L M, Dutro S M, Morse S A, Kiviat N B. Etiology of genital ulcer disease in Dakar, Senegal, and comparison of PCR and serologic assays for detection of Haemophilus ducreyi. J Clin Microbiol. 2000;38:268–273. doi: 10.1128/jcm.38.1.268-273.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Totten P A, Lara J C, Norn D V, Stamm W E. Haemophilus ducreyi attaches to and invades human epithelial cells in vitro. Infect Immun. 1994;62:5632–5640. doi: 10.1128/iai.62.12.5632-5640.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trees D L, Morse S A. Chancroid and Haemophilus ducreyi: an update. Clin Microbiol Rev. 1995;8:357–375. doi: 10.1128/cmr.8.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wasserheit J N. Epidemiological synergy. Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex Transm Dis. 1992;19:61–77. [PubMed] [Google Scholar]