Abstract
Cholesterol levels have been repeatedly linked to Alzheimer's Disease (AD), suggesting that high levels could be detrimental, but this effect is likely attributed to Low‐Density Lipoprotein (LDL) cholesterol. On the other hand, High‐Density Lipoproteins (HDL) cholesterol levels have been associated with reduced brain amyloidosis and improved cognitive function. However, recent findings have suggested that HDL‐functionality, which depends upon the HDL‐cargo proteins associated with HDL, rather than HDL levels, appears to be the key factor, suggesting a quality over quantity status. In this report, we have assessed the HDL‐cargo (Cholesterol, ApoA‐I, ApoA‐II, ApoC‐I, ApoC‐III, ApoD, ApoE, ApoH, ApoJ, CRP, and SAA) in stable healthy control (HC), healthy controls who will convert to MCI/AD (HC‐Conv) and AD patients (AD). Compared to HC we observed an increased cholesterol/ApoA‐I ratio in AD and HC‐Conv, as well as an increased ApoD/ApoA‐I ratio and a decreased ApoA‐II/ApoA‐I ratio in AD. Higher cholesterol/ApoA‐I ratio was also associated with lower cortical grey matter volume and higher ventricular volume, while higher ApoA‐II/ApoA‐I and ApoJ/ApoA‐I ratios were associated with greater cortical grey matter volume (and for ApoA‐II also with greater hippocampal volume) and smaller ventricular volume. Additionally, in a clinical status‐independent manner, the ApoE/ApoA‐I ratio was significantly lower in APOE ε4 carriers and lowest in APOE ε4 homozygous. Together, these data indicate that in AD patients the composition of HDL is altered, which may affect HDL functionality, and such changes are associated with altered regional brain volumetric data.
Keywords: HDL, cholesterol, Alzheimer's disease, HDL‐cargo, ApoE, amyloid‐β
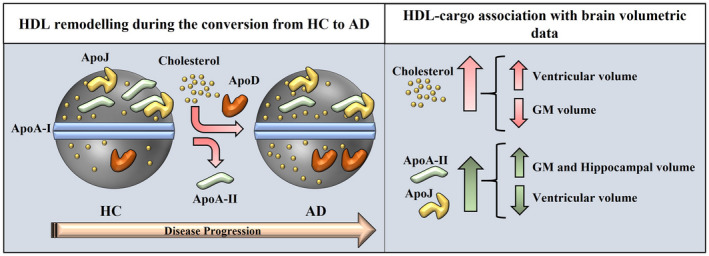
Remodeling of high‐density lipoprotein (HDL) during disease onset and progression, in which cholesterol and Apolipoprotein D levels are increased while Apolipoprotein A‐II levels are decreased on HDL (left). Additionally, increased cholesterol levels on HDL are associated with increased ventricular volume and decreased grey matter volume, while increased levels of Apolipoprotein A‐II and Apolipoprotein J are associated with increased hippocampal and grey matter volume and reduced ventricular volume (right).
![]()
Abbreviations
- AD
Alzheimer's Disease
- Aβ
amyloid‐β
- BBB
blood‐brain barrier
- CAA
cerebral amyloid angiopathy
- CRP
C‐reactive protein
- CSF
cerebrospinal fluid
- GM
grey matter
- HC
healthy control
- HC‐Conv
healthy control converter
- HDL
high‐density lipoprotein
- HL
hippocampus left
- HR
hippocampus right
- LD
lipid droplets
- LDL
low‐density lipoprotein
- LTP
long‐term potentiation
- MMSE
mini‐mental state examination
- SAA
serum amyloid A
- SUV
standardized uptake values
- SUVR
standardized uptake value ratio
- VLDL
very low‐density lipoproteins
- WM
white matter
1. INTRODUCTION
Alzheimer's disease (AD) is a neurodegenerative disease which is characterized by the extracellular deposition of amyloid β (Aβ) in the brain to form amyloid plaques and by the intracellular accumulation of hyperphosphorylated tau filaments (Masters et al., 2015). These combined events eventually are responsible for neuroinflammation, neuronal death, and reduction of brain volume, ultimately leading to the onset of disease symptoms. Several reports have indicated that diet, high cholesterol, high low‐density lipoproteins (LDL) cholesterol, and low high‐density lipoproteins (HDL) cholesterol levels are possible AD risk factors. Low cholesterol levels have been linked to Aβ precursor protein (AβPP) processing through the non‐amyloidogenic pathway (Buxbaum et al., 2001; Fassbender et al., 2001; Kojro et al., 2001; Simons et al., 1998), while high levels of intracellular cholesterol have been linked to an increase in Aβ deposition in the brain (Burns et al., 2003; Refolo et al., 2000; Wahrle et al., 2002). These results were also supported by studies in rodents indicating that high‐fat diets affect brain amyloid levels and brain mass (Levin‐Allerhand et al., 2002; Pedrini et al., 2009; Refolo et al., 2000). Many studies have therefore suggested a protective role of HDL for AD, indicating that higher HDL cholesterol levels have been associated with better cognitive outcomes, higher MMSE, a reduced risk for AD (but not to MCI), and increased brain grey matter and hippocampal volume (Atzmon et al., 2002; Bates et al., 2017; Reitz et al., 2008; Reitz et al., 2010; Ward et al., 2010; Wolf et al., 2004). Furthermore, HDL cholesterol levels have been shown to inversely correlate with brain Aβ deposits (Reed et al., 2014). Conversely, low HDL cholesterol levels were associated with white matter changes, a higher probability of memory deficits, or a greater risk of dementia (Crisby et al., 2010; Singh‐Manoux et al., 2008; Wolf et al., 2004). However, in spite of several studies indicating a protective role for HDL, other reports failed to confirm such an association (den Heijer et al., 2005; van Velsen et al., 2013). Additionally, discrepancies were also reported when assessing the overall levels of HDL cholesterol, with some studies indicating altered levels of HDL cholesterol between controls and AD, while other studies did not (den Heijer et al., 2005; Isbir et al., 2001; Reitz et al., 2004; Xiao et al., 2012; Zhang et al., 2004). These findings may indicate that HDL particles provide actions that go far beyond the classical role of excess cholesterol elimination by the liver. Many of these effects, such as anti‐inflammatory and anti‐oxidant, are mediated through HDL‐protein cargo, which comprises many apolipoproteins (and several other proteins) that are associated with and form the HDL particles, extensively described in several studies (Ronsein & Vaisar, 2019; Shah et al., 2013; Yassine et al., 2014). For example, ApoA‐I, the major constituent of plasma HDL, has shown protective effects in AD by binding to Aβ and its precursor protein (AβPP), reducing Aβ aggregation and Aβ‐induced toxicity (Koldamova et al., 2001; Paula‐Lima et al., 2009). In animal studies, overexpression of ApoA‐I reduced cognitive deficits and cerebral amyloid angiopathy (CAA), despite unaltered Aβ deposition, likely through reduced neuroinflammation (Lewis et al., 2010). Conversely, depletion of ApoA‐I increased CAA and Aβ aggregation with consequent worsening of cognitive function without altering APP processing and Aβ deposition (Lefterov et al., 2010). Altogether, it appears that ApoA‐I modulates Aβ toxicity through mechanisms other than altering APP processing and Aβ deposition. Whilst high levels of ApoA‐I have been associated with a reduced risk of dementia (Saczynski et al., 2007), reduced levels of ApoA‐I have been described in AD and correlate with the severity of the disease (Kawano et al., 1995; Liu et al., 2006; Merched et al., 2000). On the other hand, ApoE is the major constituent of CSF HDL and the ε4 isoform is considered the major risk factor for sporadic AD (Corder et al., 1993; Strittmatter et al., 1993). ApoE ε4 isoform has been associated with increased Aβ production, accumulation and oligomerization (Bales et al., 2009; Hashimoto et al., 2012; Koffie et al., 2012; Ye et al., 2005; Youmans et al., 2012) and with reduced Aβ clearance (Castellano et al., 2011; Cook et al., 2003; Deane et al., 2008; Du et al., 2009). With regard to Aβ degradation mediated by binding to ApoE, ApoE ε3 binds to Aβ more efficiently than ApoE ε4 and astrocytes from ApoE ε4 transgenic mice clear amyloid plaques less efficiently than astrocytes from ApoE ε3 transgenic mice (Simonovitch et al., 2016; Tokuda et al., 2000). Additionally, in an AD mouse models, CAA is induced more prominently by ApoE ε4 compared to ApoE ε3 (Fryer et al., 2005) and Aβ stimulation affected Long Term Potentiation (LTP) more prominently in ApoE ε4 transgenic mice rather than in ApoE ε2 or ApoE ε3 transgenic mice (Trommer et al., 2005). ApoE ε4 has also been strongly associated with increased atrophy and reduced hippocampal volume (Agosta et al., 2009; Hostage et al., 2013; Manning et al., 2014; Moffat et al., 2000; Tang et al., 2015). Finally, since low levels of ApoE and ApoE ε4 levels were found significantly reduced in AD patients (Beffert et al., 1999; Gupta et al., 2011), it is fitting that lower plasma ApoE levels are linked with the smaller hippocampus (Teng et al., 2015), while higher plasma ApoE levels are linked with reduced brain amyloidosis (Koch et al., 2018). ApoA‐II is capable to form complexes with ApoE ε2 and ApoE ε3 but not with ApoE ε4 and these complexes (ApoE ε2/ApoA‐II and ApoE ε3/ApoA‐II) increased cell viability by binding to Aβ and reducing its internalization. Such protective features were absent in ApoE ε4‐treated cells because of the absence of ApoEε4/ApoA‐II complexes (Yamauchi et al., 2000). An AD transgenic mouse model lacking ApoA‐IV displayed increased cerebral Aβ and learning deficits, likely through mechanisms that are associated with Aβ clearance (Cui et al., 2011), while the absence of ApoD was associated with an increased number of amyloid plaque in the hippocampus and, at the opposite, overexpression of ApoD was associated with a reduced number of them (Li et al., 2015). ApoJ forms complexes with Aβ (ApoJ:Aβ) for their interaction with low‐density lipoprotein receptor‐related protein 2 (Megalin), which mediates the transport and the clearance of Aβ from and to the brain (Bell et al., 2007; Hammad et al., 1997; Zlokovic et al., 1994; Zlokovic et al., 1996). Levels of ApoJ are increased in plasma and brain regions of AD patients, specifically in areas that are loaded with amyloid plaques (Bertrand et al., 1995; Gupta et al., 2016; Gupta et al., 2017; Howlett et al., 2013; Lidstrom et al., 1998; May et al., 1990; Miners et al., 2017). However, it is important to point out that the composition of plasma HDL is different from CSF HDL as not all apolipoproteins associated with HDL are produced by brain‐resident cells. In the CSF ApoE and ApoJ are the main apolipoproteins, while ApoA‐I, the main constituent of plasma HDL is not produced in the central nervous system. However, damage to the blood‐brain barrier (BBB), common in AD, allows ApoC‐III, not usually produced in the brain, to be detected in the CSF (Picard et al., 2022), while ApoA‐I can be transported across the BBB at the choroid plexus (Stukas et al., 2014). In general, lipid dysregulation in the brain affects AD like many other neurodegenerative diseases. In this regard, lipid droplets (LD) which are cytoplasmatic organelles containing cholesteryl esters which provide energy for cell metabolism and membrane synthesis (Walther & Farese Jr., 2012) were first described by Alois Alzheimer (Alzheimer et al., 1995). In AD, lipid droplet accumulation is an event that takes place before amyloid accumulation, emphasizing the importance of lipid metabolism in the disease (Hamilton et al., 2015). The importance of LD in AD and many other neurodegenerative diseases was recently assessed (Farmer et al., 2020; Ralhan et al., 2021).
Modulation of HDL cargo has been observed and reported in many diseases, such as Coronary Heart Disease, Acute Coronary Syndrome, End Stage Renal Disease and Liver Disease (Alwaili et al., 2012; Trieb et al., 2016; Vaisar et al., 2007; Weichhart et al., 2012; Yan et al., 2014). Aging has also been reported to be a factor in modulating HDL protein cargo (Holzer et al., 2013). In light of these tight connections between HDL‐protein cargo and AD and the fact that modulation of HDL protein cargo is a common factor in many other diseases, our goal in this study is to determine if HDL‐protein cargo is altered in AD and if specific changes can be directly associated with the extent of brain amyloidosis. If so, alteration of HDL‐protein cargo could serve as a direct measurement of amyloid deposits in the brain, providing a diagnostic tool that could indicate healthy controls at risk for AD.
2. MATERIAL AND METHODS
2.1. Participants
The AIBL study was conducted in accordance with the Declaration of Helsinki and was approved by the ethics committees of St. Vincent's Health and Austin Health in Melbourne and Hollywood Private Hospital and Edith Cowan University in Perth (Australia; Protocol number HPH215). All volunteers gave written and informed consent before participating in our study. A total of 213 participants were divided into stable healthy controls (HC, n = 87, HC for at least 36 months (t1 = 18 months and t2 = 36 months after HDL‐cargo analysis (t0))), healthy controls converters (HC‐Conv, n = 38, HC at the time of HDL‐cargo analysis (t0) but converted to MCI/AD within the following 36 months (either at t1 or t2)) and Alzheimer's patients (AD, n = 88, from t0 through t2) from the AIBL cohort were used in this study (Figure S1). Exclusion criteria included a history of non‐AD dementia, schizophrenia, bipolar disorder, current depression (GDS score above 5/15), Parkinson's disease, uncontrolled hypertension (systolic BP > 170 or diastolic BP > 100), cancer (other than basal cell skin carcinoma) within the last two years, symptomatic stroke, uncontrolled diabetes, or current regular alcohol use exceeding two standard drinks per day for women or four per day for men (Ellis et al., 2009). The AIBL Study clinical panel meets on a monthly basis to discuss baseline classification for each new patient and to ensure that diagnoses were made in accordance with the NINCDS‐ARDA criteria (McKhann et al., 1984; Winblad et al., 2004). Body parameters such as weight, height, blood pressure, and pulse rate were evaluated during the examinations. Blood was drawn from overnight fasting participants and collected to obtain plasma for our analysis. APOE status was determined by genotyping cells from whole blood as previously described (Gupta et al., 2011). Total cholesterol, LDL, HDL, and TG levels in plasma were also assessed.
2.2. HDL fractionation
HDL fraction was obtained by precipitating VLDL and LDL fractions using a solution identical to the one reported in the Quantolip® protocol (Reagent A: Na2HPO4x2H2O 8 g/L, NaH2PO4xH2O 11 g/L, Na2EDTA 1 g/L, Sodium Azide 0.9 g/L, Polyethylenglycol 20,000 95 g/L). Briefly, 30 μl of plasma were mixed with 60 μl of Reagent A and sat for 10 min at RT, followed by 15 min of centrifugation at 2500 g. The supernatant containing the HDL fraction was then collected, aliquoted, and stored at −80°C until analysis. HDL isolation by Polyethylenglycol precipitation has already been used in the past (Kostner et al., 1985).
2.3. HDL and HDL‐associated protein measurement in HDL fractions
HDL‐cholesterol in HDL fractions (dilution 1:75) was assessed using HDL‐Cholesterol Assay Kit (Cell Biolabs) using the manufacturer's instructions.
HDL‐cargo proteins in HDL fractions were assessed by Bioplex analysis. Serum Amyloid A (SAA) levels associated with HDL fractions (dilution 1:200) were evaluated using SAA bioplex kit (Merck Millipore) using the manufacturer's instructions. A broad panel of Apolipoprotein (ApoA‐I, ApoA‐II, ApoC‐I, ApoC‐III, ApoD, ApoE, ApoH, ApoJ, and CRP) levels associated with HDL fractions (dilution 1:10,000) were evaluated using Pro Human Apolipoprotein Panel bioplex kit (Biorad) using the manufacturer's instructions (Jóźwiak et al., 2022; Mlambo et al., 2020).
2.4. Calculations of HDL and HDL‐associated proteins
For references and calculation, in this paragraph, we will refer to cholesterol‐HDLPL as the cholesterol concentration on HDL in plasma (PL) and to cholesterol‐HDLHF as the cholesterol concentration on HDL in the HDL fraction (HF). We will also refer to Apolipoproteins/SAA/CRP as ProteinHF, for formula convenience, as all proteins were measured in the HDL fraction.
To calculate the correct amount of each Protein (in ng) or cholesterol (in μg) per μg of ApoA‐I in the HDL fraction, we used the following formulas: Protein HF (ng/ml)/ApoA‐I HF (μg/ml) and Cholesterol‐HDL HF (μg/ml)/ApoA‐I HF (μg/ml). This calculation indicates the HDL‐cargo composition with regard to ApoA‐I which is the major and more stable apolipoprotein in HDL particles.
To calculate the correct amount of each Protein (in μg) (associated to HDL) in 1 ml plasma, we used the following formula: Protein HF (μg/ml)/Cholesterol‐HDL HF (mg/ml)*Cholesterol‐HDL PL (mg/ml).
For general purposes, we will refer to HDL‐cargo to the 10 analytes assessed (Cholesterol, ApoA‐II, ApoC‐I, ApoC‐III, ApoD, ApoE, ApoH, ApoJ, CRP, SAA) compared to ApoA‐I levels.
2.5. PET scan
PET scans consisting of 30 min acquisitions were performed 40 min after injection of 370 MBq 11C‐PiB. PET images were processed using a semi‐automatic region‐of‐interested method as previously described (Villemagne et al., 2011). Standardized uptake values (SUV) for 11C‐PiB were calculated for all brain regions examined. The SUV ratio (SUVR) was calculated by dividing all regional SUV by the cerebellar cortex SUV. However, the centiloid scale was recently proposed to provide a standard quantification of Aβ‐PET images. In the centiloid scale, the Aβ burden can be expressed with values ranging from 0 (the typical Aβ burden in young controls) to 100 (the typical Aβ burden in mild AD patients) (Klunk et al., 2015). Centiloid values were generated using CapAIBL as described elsewhere (Bourgeat et al., 2018).
2.6. MRI imaging
Scanning centers either in Melbourne or Perth were used to acquire images using Siemens 3T Trio and Siemens 3T Skyra scanners (Melbourne) or Siemens 3T Verio and Siemens 1.5T Avanto scanners (Perth). The scans also included a 3D MPRAGE (Magnetization Prepared Rapid Acquisition Gradient Echo) image (voxel size 1.2 × 1 × 1 mm3, repetition time/echo time = 2300/2.98, flip angle = 9°). A 3D T2‐weighted Fluid‐attenuation inversion recovery (FLAIR) sequence, included in the image acquisition protocol, was obtained using two different sets of parameters. Gradient Recalled Echo (GRE) images used for SWI (Susceptibility‐Weighted Imaging) and QSM (Quantitative Susceptibility Mapping) were also acquired. Full details of protocols and parameters are described elsewhere (Fowler et al., 2021).
2.7. Statistical analysis
The operator was unaware of the experimental groups during the experiments and data collection. The sample size was determined using G‐Power, assuming a Cohen's D of 0.5 (medium‐size effect). For such analysis, to obtain a significance of p < 0.05, n = 64 samples were required in both HC and AD groups. Data were assessed for normality using Skewness and Kurtosis analysis and were log‐transformed when values were outside of the −2/+2 range. An interquartile range test was used to assess for outliers. Participant demographic and clinical characteristics were compared using either ANOVA or Wilcoxon Signed Ranks test for age, HDL, MMSE, and Brain Aβ deposition, and the Chi‐squared test for Gender, Site, APOE ε4 allele status, and brain Aβ deposition status. Statistical comparison of biomarker means in different groups was performed using Generalized linear models, unadjusted or adjusted for age, gender, and APOE ε4 allele status. When necessary, data were log‐transformed to better approximate normal distribution. p‐values less than 0.05 were regarded as nominally significant, with values less than 0.005 (Bonferroni adjusted value [0.05/10]) regarded as statistically significant. Partial correlation analysis was run upon adjustment for adjustment for covariates such as age, gender, and APOE ε4 allele status. Analyses were carried out using the R Statistical Environment (R Core Team, www.r‐project.org, v4.0.2) and SPSS version 27. For the Generalized linear model analyses, outliers were replaced with the median value for each marker. For the correlation analyses, outliers were not replaced.
We applied recursive feature elimination (RFE) to rank the HDL‐cargo proteins in differentiating different groups of individuals (AD, HC‐Conv, and HC) based on random forest (RF) classifier. RF is among the most robust to noise and missing data machine learning methods and RF is robust to overfitting and redundancy in predictors (Grinberg et al., 2020). RFE first fit the RF model using all the proteins as predictors, where each predictor was then ranked using its importance to the classification performance. At each iteration of the RFE process, top‐ranked predictors were retained, the model was refitted and performance was reassessed. Consequently, the proteins and factors that did not improve the classification accuracy were removed, and the top predictors were used to fit the final model. 1000 trees were used to build the RF model and root mean square error (RMSE) was used as the criterion to select the optimal model. Auto tuning was then applied to optimize the parameters of the RF. We implemented RFE with RF classification using caret package v6.0‐86 (Kuhn, 2008). This was performed by ten times five‐fold cross‐validation. This variable selection method provided insight into which variables and/or factors have the most distinguishing power in classifying Alzheimer's patients, thus those proteins and factors can serve as biomarkers for better diagnosis and prognosis. We evaluated all HDL‐cargo proteins as potential biomarkers and assessed the 5 most important with the goal to determine the best set of proteins relevant to other factors as diagnostic and prognostic biomarkers.
3. RESULTS
The basic demographics of the study participants are summarized in Table 1. In total, 87 stable healthy controls (HC), 38 HC‐Converters (HC‐Conv), and 88 Alzheimer's patients (AD) were studied (all participants were 65 years old and older). All 213 samples were evaluated for total HDL cholesterol levels and Mini‐Mental State Exam (MMSE) scores, while a smaller subset was assessed for brain amyloid deposition (assessed by PET scan) (Table 1).
TABLE 1.
Demographic characteristics, HDL levels, cognitive score, and amyloid brain deposition in HC, HC‐Conv, and AD participants
| HC | HC‐Conv | AD | p | |
|---|---|---|---|---|
| N | 87 | 38 | 88 | |
| Age | 73 ± 6 | 75 ± 7 | 77 ± 7 | 0.27 |
| Gender (M/F) | 43/44 | 19/19 | 39/49 | 0.75 |
| Site (Melbourne/Perth) | 45/42 | 19/19 | 58/30 | 0.10 |
| APOE ε4 (no/yes) | 57/30 | 19/19 | 26/62 | <0.001 |
| HDL Cholesterol (mg/dl) | 64 ± 15 | 68 ± 21 | 65 ± 17 | 1.00 |
| MMSE | 29 ± 1 | 28 ± 1 | 20 ± 4 | <0.001 |
| Brain Aβ deposition (n/y) | 60/23 | 7/4 | 1/18 | <0.001 |
Note: Values are presented as mean ± SD or as frequency. Analysis was considered significant with p < 0.05 (bold).
The levels of HDL‐cargo proteins, along with HDL cholesterol (which refers to the cholesterol associated with HDL particles), were assessed compared to the levels of ApoA‐I, which is the main apolipoprotein expressed on HDL particles. Generalized linear model (HC compared to AD) corrected for age, gender, and APOE ε4‐carrier status are shown in Table 2. Of the 10 HDL‐cargo analytes assessed among clinical groups, analyses showed statistically significant increases in cholesterol/ApoA‐I (p < 0.001 and p = 0.001, unadjusted and adjusted, respectively) and ApoD/ApoA‐I (p < 0.001 for both unadjusted and adjusted) and a significant decrease in ApoA‐II/ApoA‐I (p < 0.001 for both unadjusted and adjusted) in AD participants as compared with controls (Table 2).
TABLE 2.
Comparison of HDL‐cholesterol and HDL‐cargo (ApoA‐II, ApoC‐I, ApoC‐III, ApoD, ApoE, ApoH, ApoJ, CRP, and SAA) expressed as a ratio to ApoA‐I in stable HC vs AD
| HC (n) | AD (n) | p | p a | |
|---|---|---|---|---|
| μgCholesterol/μgApoA‐I | 1.34 ± 0.33 (87) | 1.54 ± 0.38 (88) | 0.000183 | 0.00139 |
| ngApoA‐II/μgApoA‐I | 293 ± 87 (77) | 246 ± 53 (88) | 6.11e‐05 | 0.000403 |
| ngApoC‐I/μgApoA‐I | 298 ± 73 (75) | 273 ± 66 (85) | 0.0213 | 0.274 |
| ngApoC‐III/μgApoA‐I | 58.4 ± 20.5 (87) | 56.9 ± 19.8 (88) | 0.617 | 0.531 |
| ngApoD/μgApoA‐I | 44.3 ± 8.1 (87) | 49.8 ± 9.2 (88) | 4.36e‐05 | 0.000383 |
| ngApoE/μgApoA‐I | 13.7 ± 5.3 (87) | 10.4 ± 4.5 (88) | 2.25e‐05 | 0.0243 |
| ngApoH/μgApoA‐I | 524 ± 155 (87) | 492 ± 141 (88) | 0.149 | 0.126 |
| ngApoJ/μgApoA‐I | 46.4 ± 10.4 (87) | 45.1 ± 9.7 (88) | 0.382 | 0.751 |
| ngCRP/μgApoA‐I | 5.39 ± 3.36 (87) | 4.63 ± 2.37 (88) | 0.0867 | 0.117 |
| ngSAA/μgApoA‐I | 12.9 ± 14.8 (87) | 18.9 ± 20.1 (88) | 0.0284 | 0.107 |
Note: Values are presented as mean ± SD. Generalized Linear Model analyses were performed unadjusted (p) and adjusted for age, gender, and ApoE ε4‐carrier status (p a). Data are presented as raw values, but statistical analysis was performed on log‐transformed data to better approximate normal distribution. Analysis was considered significant with p < 0.05 (bold).
Data were adjusted for age, gender, and ApoE ε4‐carrier status.
We then assessed if such changes in HDL cholesterol and HDL‐cargo (compared to ApoA‐I) occurred before the conversion from HC to AD or if such changes were mainly associated with the ongoing disease. Generalized linear model corrected for age, gender, and APOE ε4‐carrier status for HC compared to HC‐Conv are shown in Table 3. Unlike the HC vs AD comparisons, only the levels of cholesterol/ApoA‐I were nominally significantly (increased in HC‐Conv as compared with HC; p = 0.019 and p = 0.031, unadjusted and adjusted, respectively). All other analytes in the HDL‐cargo did not statistically differ between HC and HC‐Conv, suggesting that some HDL‐cargo remodeling (such as ApoA‐II/ApoA‐I and ApoD/ApoA‐I) takes place once after the clinical onset of the disease (Table 3).
TABLE 3.
Comparison of HDL‐cholesterol and HDL‐cargo (ApoA‐II, ApoC‐I, ApoC‐III, ApoD, ApoE, ApoH, ApoJ, CRP, and SAA) expressed as a ratio to ApoA‐I in stable HC vs HC‐Converters
| HC (n) | HC‐Conv (n) | p | p a | |
|---|---|---|---|---|
| μgCholesterol/μgApoA‐I | 1.34 ± 0.33 (87) | 1.51 ± 0.38 (38) | 0.0188 | 0.0307 |
| ngApoA‐II/μgApoA‐I | 293 ± 87 (77) | 272 ± 69 (36) | 0.156 | 0.195 |
| ngApoC‐I/μgApoA‐I | 298 ± 73 (75) | 291 ± 72 (35) | 0.611 | 0.928 |
| ngApoC‐III/μgApoA‐I | 58.4 ± 20.5 (87) | 56.9 ± 17.7 (38) | 0.687 | 0.709 |
| ngApoD/μgApoA‐I | 44.3 ± 8.1 (87) | 43.8 ± 6.1 (38) | 0.711 | 0.613 |
| ngApoE/μgApoA‐I | 13.7 ± 5.3 (87) | 12.6 ± 4.1 (38) | 0.238 | 0.733 |
| ngApoH/μgApoA‐I | 524 ± 155 (87) | 516 ± 118 (38) | 0.759 | 0.742 |
| ngApoJ/μgApoA‐I | 46.4 ± 10.4 (87) | 44.1 ± 8.8 (38) | 0.211 | 0.43 |
| ngCRP/μgApoA‐I | 5.39 ± 3.36 (87) | 4.76 ± 1.18 (38) | 0.128 | 0.334 |
| ngSAA/μgApoA‐I | 12.9 ± 14.8 (87) | 9.4 ± 9.4 (38) | 0.113 | 0.152 |
Note: Values are presented as mean ± SD. Generalized Linear Model analyses were performed unadjusted (p) and adjusted for age, gender, and ApoE ε4‐carrier status (p a). Data are presented as raw values, but statistical analysis was performed on log‐transformed data to better approximate normal distribution. Analysis was considered significant with p < 0.05 (bold).
Data were adjusted for age, gender, and ApoE ε4‐carrier status.
As the cholesterol/ApoA‐I was the only analyte that differed between HC and HC‐Conv, we investigated if such change took place in the proximity of the clinical onset or years before. As the HC‐Conv group includes individuals who converted to MCI/AD at 18 and 36 months, we assessed if the increased ratio of cholesterol/ApoA‐I in HC‐Conv was affected by a similar magnitude in both HC‐Conv groups (i.e. @18 & 36 months, Figure 1). Interestingly, individuals whose conversion to MCI/AD will take place within 18 months and were, therefore, closer to conversion (HC‐Conv 18m) had a cholesterol/ApoA‐I ratio significantly higher than in HC (p < 0.001) and similar to the AD group (Figure 1). Conversely, individuals whose conversion to MCI/AD will take place in 36 months and therefore were farther from conversion (HC‐Conv 36m) had levels similar to HC (Figure 1), suggesting that cholesterol overload on HDL takes place shortly before conversion to AD (within 18 months before conversion).
FIGURE 1.
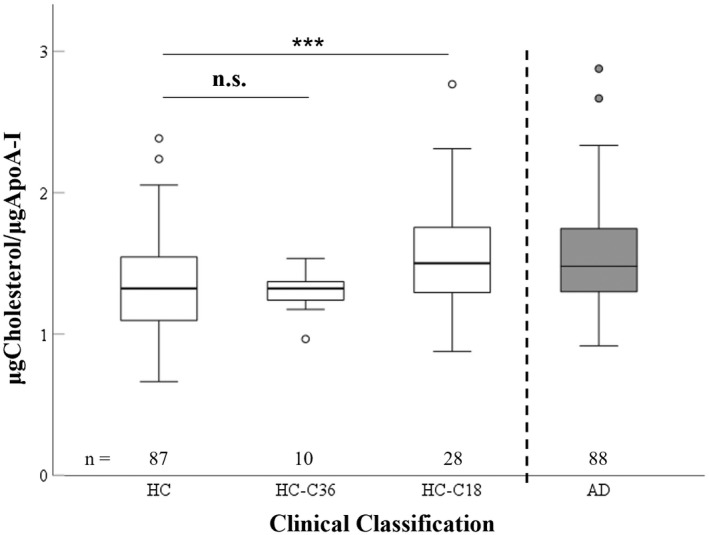
Graphical representation of cholesterol levels on HDL (to ApoA‐I levels) in healthy controls (HC), healthy controls who will convert to MCI/AD in 36 months (HC‐C36), healthy controls who will convert to MCI/AD in 18 months (HC‐C18), and AD participants (AD) (ANOVA df = 2, F = 5.633, 0 < 0.01). p‐values from pairwise comparisons unadjusted for confounders. Statistical analysis was performed using log‐transformed data to better approximate normal distribution. *p < 0.05; ***p < 0.001.
Additionally, we have analyzed if the levels of HDL‐cargo proteins were altered when assessed as levels of HDL‐associated protein per ml of plasma (Table S1). However, as many of the HDL‐protein cargo are not exclusively expressed on HDL (with the sole exception of ApoA‐I), these values do not represent the total amount of circulating protein in plasma, but only the fraction that it is directly associated with HDL particles.
To determine the effect of APOE genotype on HDL‐cargo, we then assessed if any HDL‐cargo protein was influenced by APOE genotype and clinical classification, or by APOE genotype alone. ApoE/ApoA‐I ratio was significantly affected by APOE genotype (p < 0.001) alone. However, there was no significant interaction between clinical classification and APOE genotype, indicating that differences in ApoE levels were solely affected by APOE genotype, while ApoE levels within each APOE genotype were not affected by the clinical classification (Figure 2a). When assessed by APOE genotype alone, ApoE/ApoA‐I ratio showed the highest levels in APOE ε2/3 (p < 0.001 vs ε3/3, ε3/4, and ε4/4), while APOE ε4/4 showed the lowest (p < 0.001 vs ε3/3, ε2/4 and ε3/4) (Figure 2b). Of the remaining 9 HDL‐cargo analytes, no HDL‐cargo protein (compared to ApoA‐I) was affected either by APOE genotype alone or by the interaction between clinical classification and APOE genotype, indicating that their levels were independent of APOE genotype or the clinical classification within each APOE genotype (data not shown).
FIGURE 2.
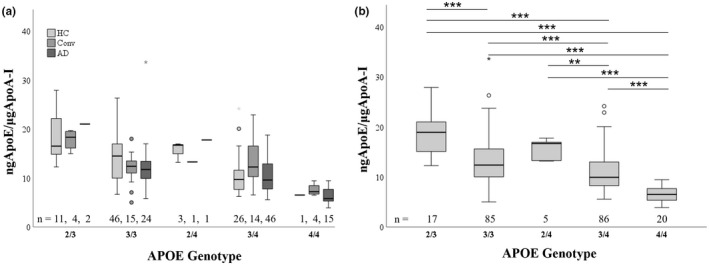
Graphic representation of ApoE levels (to ApoA‐I levels) in different APOE genotypes (ε2/3. ε3/3, ε2/4, ε3/4, and ε4/4) in different clinical groups (healthy controls (HC), healthy controls converters (HC‐Conv), and AD patients (AD)) (Figure 2a) (ANOVA df = 8, F = 1.131, p = 0.344). Graphical representation of ApoE levels (to ApoA‐I levels) in different APOE genotypes (ε2/3. ε3/3, ε2/4, ε3/4, and ε4/4). p‐values from pairwise comparisons unadjusted for confounders. Statistical analysis was performed using log‐transformed data to better approximate normal distribution. p < 0.05; **p < 0.01, ***p < 0.01 (Figure 2b) (ANOVA df = 4, F = 29.236, p < 0.001).
To determine if any of the HDL‐cargo analytes correlated with brain amyloid levels in individuals with ongoing brain amyloidosis (HC, HC‐Conv, and AD), correlation analyses were carried out (Table 4). In unadjusted correlations, only the levels of cholesterol/ApoA‐I significantly correlated with the levels of brain amyloid (p = 0.036). However, upon correction for age, gender, and APOE ε4‐carrier status, the correlation analysis only approached nominal significance (p = 0.061). None of the other HDL‐cargo analytes were significantly correlated with brain amyloid levels, regardless of whether the analysis was carried out unadjusted or adjusted for age, gender, and APOE ε4‐carrier status (Table 4).
TABLE 4.
Correlation and partial correlation of HDL‐cholesterol and HDL‐cargo (ApoA‐II, ApoC‐I, ApoC‐III, ApoD, ApoE, ApoH, ApoJ, CRP, and SAA) expressed as a ratio to ApoA‐I with brain amyloid deposition and brain amyloid deposition longitudinal changes in individuals with ongoing brain amyloidosis (PiB+)
| Centiloid t0 (0m) | ||||
|---|---|---|---|---|
| r | p | r a | p a | |
| μgCholesterol/μgApoA‐I | 0.314 | 0.036 | 0.292 | 0.061 |
| ngApoA‐II/μgApoA‐I | −0.041 | 0.790 | −0.030 | 0.850 |
| ngApoC‐I/μgApoA‐I | 0.228 | 0.142 | 0.163 | 0.314 |
| ngApoC‐III/μgApoA‐I | 0.065 | 0.685 | 0.020 | 0.900 |
| ngApoD/μgApoA‐I | −0.041 | 0.791 | −0.021 | 0.893 |
| ngApoE/μgApoA‐I | 0.009 | 0.952 | −0.077 | 0.629 |
| ngApoH/μgApoA‐I | −0.086 | 0.575 | −0.060 | 0.707 |
| ngApoJ/μgApoA‐I | −0.074 | 0.629 | −0.089 | 0.574 |
| ngCRP/μgApoA‐I | −0.186 | 0.222 | −0.189 | 0.229 |
| ngSAA/μgApoA‐I | 0.026 | 0.867 | −0.028 | 0.858 |
Note: Pearson's correlation unadjusted and adjusted for age, gender ApoE ε4‐carrier status was performed between HDL‐cargo and brain amyloid data. Analysis was considered significant with p < 0.05 (bold).
Data were adjusted for age, gender, and ApoE ε4‐carrier status. For all analytes n = 45 except for ngApoC‐I/μgApoA‐I where n = 43.
Subsequently, we carried out a correlation analysis (adjusted for age, gender, and APOE ε4‐carrier status) to determine if any HDL‐cargo protein levels were associated with brain volumetric parameters such as grey matter volume (GM), white matter volume (WM), ventricle volume (Vent), left and right hippocampal volume (HL and HR, respectively) in individuals with ongoing brain amyloidosis (Table 5). Cholesterol/ApoA‐I ratio positively and significantly correlated with ventricular volume (p = 0.043), while negatively correlated with grey matter volume, albeit this was not quite significant (p = 0.063). ApoA‐II/ApoA‐I ratio positively correlated with grey matter and hippocampal volume (p < 0.001, p = 0.038 and p = 0.035 for GM, HL, and HR, respectively), while negatively correlated with ventricular volume (p = 0.024). ApoJ/ApoA‐I ratio positively correlated with grey matter and left hippocampal volume (p = 0.029 and p = 0.033, respectively), while right hippocampal volume only trended toward significance (p = 0.080). None of the other HDL‐cargo analytes were significantly correlated with brain volumetric parameters (Table 5).
TABLE 5.
Partial correlation of HDL‐cholesterol and HDL‐cargo (ApoA‐II, ApoC‐I, ApoC‐III, ApoD, ApoE, ApoH, ApoJ, CRP, and SAA) expressed as a ratio to ApoA‐I with brain volumetric parameters in individuals with ongoing brain amyloidosis (PiB+)
| GM t0 | WM t0 | Vent t0 | HL t0 | HR t0 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| r a | p a | r a | p a | r a | p a | r a | p a | r a | p a | |
| μgCholesterol/μgApoA‐I | −0.327 | 0.063 | −0.105 | 0.562 | 0.355 | 0.043 | −0.201 | 0.263 | −0.263 | 0.140 |
| ngApoA‐II/μgApoA‐I | 0.543 | <0.001 | 0.183 | 0.308 | −391 | 0.024 | 0.363 | 0.038 | 0.368 | 0.035 |
| ngApoC‐I/μgApoA‐I | 0.002 | 0.993 | −0.077 | 0.680 | −0.140 | 0.452 | −0.021 | 0.912 | 0.011 | 0.953 |
| ngApoC‐III/μgApoA‐I | 0.190 | 0.290 | −0.027 | 0.883 | −0.036 | 0.844 | 0.109 | 0.548 | 0.185 | 0.304 |
| ngApoD/μgApoA‐I | 0.140 | 0.438 | 0.097 | 0.592 | −0.174 | 0.333 | 0.076 | 0.673 | 0.104 | 0.564 |
| ngApoE/μgApoA‐I | −0.067 | 0.712 | −0.184 | 0.306 | −0.005 | 0.977 | 0.229 | 0.200 | 0.093 | 0.605 |
| ngApoH/μgApoA‐I | 0.301 | 0.089 | 0.055 | 0.760 | −0.129 | 0.475 | 0.163 | 0.365 | 0.125 | 0.487 |
| ngApoJ/μgApoA‐I | 0.380 | 0.029 | 0.033 | 0.855 | −0.324 | 0.066 | 0.373 | 0.033 | 0.309 | 0.080 |
| ngCRP/μgApoA‐I | 0.291 | 0.101 | 0.105 | 0.561 | −0.256 | 0.150 | 0.284 | 0.109 | 0.231 | 0.196 |
| ngSAA/μgApoA‐I | 0.204 | 0.255 | 0.127 | 0.482 | 0.029 | 0.872 | 0.007 | 0.969 | 0.003 | 0.986 |
Note: Pearson's correlation adjusted for age, gender ApoE ε4‐carrier status was performed between HDL‐cargo and brain volumetric parameters at t0. Analysis was considered significant with p < 0.05 (bold).
Abbreviations: GM, grey matter volume; HL, left hippocampal volume; HR, right hippocampal volume; Vent, ventricular volume; WM, white matter volume.
Data were adjusted for age, gender ApoE ε4‐carrier status. For all analytes n = 36 except for ngApoC‐I/μgApoA‐I where n = 34.
On assessing whether MMSE scores were associated with any HDL‐cargo analytes in AD patients, MMSE scores were found to decrease with the disease progression. No HDL‐cargo analytes were significantly associated with MMSE score or MMSE longitudinal changes, suggesting that HDL‐cargo is not involved with altered cognitive impairment changes (Table S2).
RFE variable selection indicated that among all the HDL‐cargo protein variables ApoE/ApoA‐I and ApoD/ApoA‐I have the best distinguishing powers in classifying AD and HC, followed by ApoA‐II/ApoA‐I, CRP/ApoA‐I, and cholesterol/ApoA‐I (data not shown).
4. DISCUSSION
Over the past decades, the importance of cholesterol, LDL, and HDL in AD have been extensively studied, and in this regard, it is widely accepted that high cholesterol levels and high LDL levels are considered risk factor for the disease, albeit it appears that such increased risk is present at mid‐life and disappeared with increased age (Mielke et al., 2005). As such, a clear distinction between the roles of LDL and HDL has indicated that while LDL cholesterol levels were associated with increased brain amyloid deposition (Reed et al., 2014), high HDL cholesterol levels were instead considered protective. This latest notion came from several studies in which HDL cholesterol levels were associated with lower brain amyloid deposition, a reduced risk for AD, better cognitive functions, and higher MMSE scores (Atzmon et al., 2002; Bates et al., 2017; Reitz et al., 2010). It is, however, important to note that recent evidence suggested that HDL functionality, rather than HDL overall levels, determine HDL functions (Rosenson et al., 2016).
In this report, we, therefore, assessed the HDL‐cargo composition (Cholesterol, ApoA‐II, ApoC‐I, ApoC‐III, ApoD, ApoE, ApoH, ApoJ, CRP, and SAA, all measured as ratio to ApoA‐I) in stable healthy control, healthy controls who will convert to MCI/AD within 36 months and AD to determine if any of the HDL‐cargo analytes were associated to brain amyloid deposition, brain volumetric parameters (cortical grey matter, cortical white matter, ventricular volume, hippocampal volume), and cognitive functions scores (MMSE). We found an increased amount of cholesterol/ApoA‐I ratio (in both HC‐Conv and AD, compared to HC), increased ApoD/ApoA‐I (in AD), and decreased ApoA‐II/ApoA‐I ratios (in AD). While the cholesterol/ApoA‐I ratio varies in the general population and as a function of HDL size and HDL maturation, the increased cholesterol/ApoA‐I ratio on HDL particles in our study was unexpected, as the overall plasma levels of HDL‐Cholesterol were unchanged in all clinical groups. Since ApoA‐I is solely expressed on HDL, our data also indicated that levels of HDL‐associated ApoA‐I per ml of plasma were significantly lower in AD. Such a decrease can be considered an ApoA‐I decrease in plasma and is in accordance with other reports which have indicated lower levels of ApoA‐I in AD (Kawano et al., 1995; Liu et al., 2006; Merched et al., 2000). As ApoA‐I is also the main (and more stable in number on HDL particles) Apolipoprotein on HDL, and in consideration of its reduced levels in AD, our data suggest that in AD (and in HC‐Converters, but only in those who are within 18 months from conversion to MCI/AD), there is a reduced number of HDL particles which are overloaded with cholesterol. This would explain the lower amount of ApoA‐I in plasma, but the increased amount of cholesterol on HDL particles (increased cholesterol/ApoA‐I ratio) would counterbalance the reduced numbers of HDL particles and explain the absence of different Cholesterol‐HDL plasma levels across clinical groups. Accordingly, most of the other HDL‐cargo proteins (evaluated in the same assay with ApoA‐I), which have shown reduced levels in AD compared to HC per ml of plasma (HDL‐associated protein cargo/ml plasma), do not display a different ratio to ApoA‐I, suggesting that there is no altered composition of HDL particle, but rather a reduced number.
Overall, the only exception in the composition, along with the Cholesterol/ApoA‐I ratio which occurs before the clinical conversion from HC to MCI/AD, are ApoA‐II/ApoA‐I ratio, which is decreased in AD compared to HC and ApoD/ApoA‐I ratio, which is increased in AD compared to controls. However, since both ApoA‐II/ApoA‐I and ApoD/ApoA‐I ratios are not altered in HC‐Conv compared to stable HC, it is possible that such changes take place once the disease has started, unlike the cholesterol overload which may happen before the onset of the disease (Figure 3). It has to be noted that in this study we focused on HDL composition assessed as apolipoprotein ratio to ApoA‐I on HDL particles, which may represent a better marker for conversion to AD and/or pathological changes in AD, rather than individual plasma apolipoprotein levels, which could provide confounding results as many apolipoproteins are shared among several lipoprotein particles (LDL, VLDL).
FIGURE 3.
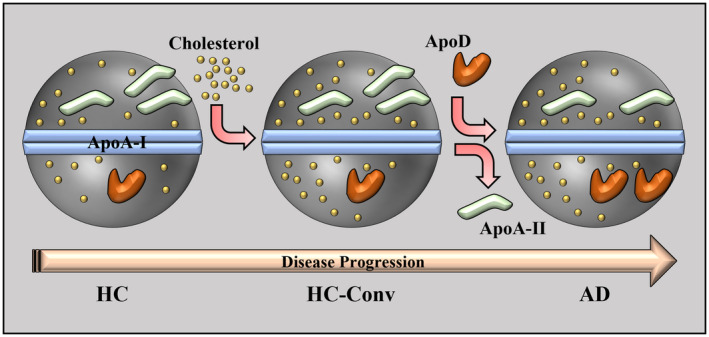
Schematic representation of HDL remodeling in which cholesterol on HDL particles increases before the conversion to AD, while the decrease in ApoA‐II and the increase in ApoD take place once the disease has started.
Interestingly, ApoE/ApoA‐I ratio was significantly lower in carriers of the allele APOE ε4 and lowest in the homozygous carrier for the allele APOE ε4. Such ApoE/ApoA‐I ratio, which defines HDL composition, was not affected by clinical classification but was solely affected by APOE genotype. As the presence of the allele APOE ε4 is widely considered the biggest risk factor for sporadic AD, such reduced ApoE presence on HDL may reduce HDL functionality, therefore providing an additional explanation for the increased risk associated with APOE genotype. This reduced binding of ApoE ε4 to HDL (with a preferential binding to chylomicrons and VLDL) is also in accordance with other studies (Poirier et al., 2014). In this regard, several studies which evaluated the effects of ApoE inducers, such as Liver‐X‐receptor (LXR) agonists, have reported protective effects in transgenic AD mice (Fitz et al., 2010; Lefterov et al., 2007; Riddell et al., 2007), supporting the notion that high levels of ApoE are beneficial in the disease. However, one limitation of this study is that it was not powered to determine the effects of ApoE genotype on disease progression.
When the influence of HDL‐cargo on regional brain volumes was assessed, we selected individuals with ongoing brain amyloidosis, in whom regional changes may be more prominent. Higher cholesterol/ApoA‐I ratio on HDL resulted to be marginally associated with lower grey matter volume. In parallel, higher cholesterol levels were also associated with greater ventricular volume. While this seems to be counterintuitive at first, as higher HDL cholesterol levels have been associated with protective features, the cholesterol increase here mentioned is per HDL particle (overall plasma HDL levels were unaffected in this study). In this report we suggest that cholesterol overload on HDL is detrimental as it affected brain volumetric parameters, suggesting that HDL quality may be more important than quantity itself. Such a hypothesis would also be in accordance with another report indicating that cholesterol overload on HDL has a negative effect on HDL anti‐atherogenic functions (Qi et al., 2015), while an increased cholesterol/ApoA‐I ratio on HDL can be a predictor of cardiovascular disease and associated mortality (Rhee et al., 2017). In accordance with these findings, a lower cholesterol/ApoA‐I ratio was linked to protective features, as higher levels of small HDL particles (known to have lower cholesterol/ApoA‐I ratio) in CSF have been associated with better cognitive performances (Martinez et al., 2022).
Conversely, ApoA‐II/ApoA‐I, and ApoJ/ApoA‐I ratio displayed the opposite effect on grey matter and ventricular volume, with higher levels of both proteins being associated with higher grey matter volume and smaller ventricular volume. In addition, a higher ApoA‐II/ApoA‐I ratio was also significantly associated with higher hippocampal volume (both left and right). It is, therefore, fitting that ApoA‐II and ApoJ on HDL particles displayed protective features, as both proteins have previously been linked to AD for their capacity to bind to Aβ reducing its effects (Bell et al., 2007; Hammad et al., 1997; Yamauchi et al., 2000). Ultimately, we observed a trend between higher cholesterol levels on HDL and higher levels of brain amyloid deposition, further indicating that cholesterol overload on HDL may result in non‐functional HDL particles with consequent negative impact.
Taken together, our study suggested that in AD (a) there is a reconfiguration of HDL particles with significant increased cholesterol/ApoA‐I ratio (which may take place before the onset of the disease), increased ApoD/ApoA‐I and reduced ApoA‐II/ApoA‐I ratios on HDL which may affect HDL functionality itself; (b) such reconfiguration with the consequent increase of cholesterol/ApoA‐I ratio on HDL is associated in individuals with ongoing brain amyloidosis with lower cortical grey matter volume and greater ventricular volume; while (c) other apolipoproteins on HDL, such as ApoA‐II and ApoJ, displayed protective features and higher levels of both were associated in individuals with ongoing brain amyloidosis with higher cortical grey matter volume and smaller ventricular volume; and (d) that ApoE/ApoA‐I ratio on HDL are solely a function of APOE genotype and reduced levels of ApoE on HDL in APOE ε4 carrier may further alter HDL functionality, reduce its protective features and provide another reason for the increased risk associated with APOE genotype.
Altogether, these data are supporting the notion that the functionality of HDL is related to its protein cargo and it is independent of its absolute levels, and that HDL cargo and HDL functionality may be altered in AD as they are in many other diseases. Further studies will, however, be necessary to better define the extent of HDL functionality in relationship with HDL‐cargo in the disease.
AUTHOR CONTRIBUTIONS
Steve Pedrini: Conceptualization, Methodology, Investigation, Data analysis, Visualization, Writing ‐ original draft, Writing ‐ review/editing. James D. Doecke: Data analysis, Writing ‐ review/editing. Eugene Hone: Investigation, Writing ‐ review/editing. Penghao Wang: Data analysis, Writing ‐ review/editing. Rohith Thota: Conceptualization, Writing ‐ review/editing. Ashley I. Bush: Writing ‐ review/editing. Christopher C. Rowe: Data curation, Writing ‐ review/editing. Vincent Dore: Data curation, Writing ‐ review/editing. Victor L. Villemagne: Data curation, Writing ‐ review/editing. David Ames: Data curation, Writing ‐ review/editing. Stephanie Rainey‐Smith: Data curation, Writing ‐ review/editing. Giuseppe Verdile: Conceptualization, Writing ‐ review/editing. Hamid R. Sohrabi: Data curation, Writing ‐ review/editing. Manfred R. Raida: Conceptualization, Methodology, Investigation, Writing ‐ review/editing. Kevin Taddei: Writing ‐ review/editing, Project administration. Sam Gandy: Conceptualization, Writing ‐ review/editing. Colin L. Masters: Data curation, Writing ‐ review/editing. Pratishtha Chatterjee: Conceptualization, Writing ‐ original draft, Writing ‐ review/editing. Ralph N. Martins: Conceptualization, Writing ‐ original draft, Writing ‐ review/editing, Supervision, Project administration.
CONFLICT OF INTEREST
Ashley Bush is a shareholder in Alterity Ltd, Cogstate Ltd and Mesoblast Ltd. He is a paid consultant for, and has a profit share interest in, Collaborative Medicinal Development Pty Ltd. He has received lecture fees from Biogen and Merck Sharp & Dohme P/L. Dr. Gandy serves as a consultant for Ritrova Therapeutics and as a founder of Recuerdo Pharmaceuticals (inactive). He has served as a consultant in the past for Diagenic, and he has received research support in the past from Warner‐Lambert, Pfizer, Baxter, and Avid. He currently receives research support from the NIH. Dr. Victor Villemagne is a Senior Editor for the Journal of Neurochemistry.
Supporting information
Table S1
Table S2
Figure S1
ACKNOWLEDGMENTS
We thank all the participants who took part in this study and the clinicians who referred participants. The AIBL study (www.AIBL.csiro.au) is a consortium between Austin Health, CSIRO, Edith Cowan University, the Florey Institute (The University of Melbourne), and the National Ageing Research Institute. The study has received partial financial support from the Alzheimer's Association (US), the Alzheimer's Drug Discovery Foundation, an Anonymous foundation, the Science and Industry Endowment Fund, the Dementia Collaborative Research Centres, the Victorian Government's Operational Infrastructure Support program, the Australian Alzheimer's Research Foundation, the National Health and Medical Research Council (NHMRC), and The Yulgilbar Foundation. Numerous commercial interactions have supported data collection and analyses. In‐kind support has also been provided by Sir Charles Gairdner Hospital, Cogstate Ltd, Hollywood Private Hospital, The University of Melbourne, and St Vincent's Hospital. SRRS is supported by an NHMRC Investigator Grant (GNT1197315).
All experiments were conducted in compliance with the ARRIVE guidelines.
Pedrini, S. , Doecke, J. D. , Hone, E. , Wang, P. , Thota, R. , Bush, A. I. , Rowe, C. C. , Dore, V. , Villemagne, V. L. , Ames, D. , Rainey‐Smith, S. , Verdile, G. , Sohrabi, H. R. , Raida, M. R. , Taddei, K. , Gandy, S. , Masters, C. L. , Chatterjee, P. , Martins, R. N. , & the AIBL Research Group (2022). Plasma high‐density lipoprotein cargo is altered in Alzheimer's disease and is associated with regional brain volume. Journal of Neurochemistry, 163, 53–67. 10.1111/jnc.15681
DATA AVAILABILITY STATEMENT
AIBL data have been provided to the Global Alzheimer's Association Interactive Network (GAAIN), and software installed, thereby enabling GAAIN users to interrogate metadata and receive cohort summaries, whereupon users can request further information (and biofluid samples; blood and CSF) if needed by submitting an Expression of Interest (EoI). This mechanism ensures worldwide sharing and utilization of AIBL data (and biofluid samples) and complements the existing framework whereby AIBL imaging scans and demographic data are available on the ADNI LONI (Laboratory of Neuro Imaging, University of Southern California) website for free download and use by researchers worldwide. Up to 3 August 2019, LONI/ GAAIN data applications had been received from over 120 companies, more than 2000 organizations/institutes/departments, and over 3500 individuals, spanning 81 countries. Moreover, hundreds of EoIs from academic and industry‐based individuals have been approved resulting in further data and biofluid sample sharing. Data will also be provided to the National Institute on Aging‐funded ADOPIC (Alzheimer's Dementia Onset and Progression in International Cohorts) project which will harmonize data from the AIBL, ADNI, Washington University, St. Louis, and University of Washington, Seattle, cohorts to determine factors which influence cognitive decline in AD.
REFERENCES
- Agosta, F. , Vossel, K. A. , Miller, B. L. , Migliaccio, R. , Bonasera, S. J. , Filippi, M., Boxer, A. L. , Karydas, A., Possin, K. L. , & Gorno‐Tempini, M. L. (2009). Apolipoprotein E epsilon4 is associated with disease‐specific effects on brain atrophy in Alzheimer's disease and frontotemporal dementia. Proceedings of the National Academy of Sciences of the United States of America, 106, 2018–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alwaili, K. , Bailey, D. , Awan, Z. , Bailey, S. D. , Ruel, I. , Hafiane, A. , Krimbou, L. , Laboissiere, S. , & Genest, J. (2012). The HDL proteome in acute coronary syndromes shifts to an inflammatory profile. Biochimica et Biophysica Acta, 1821, 405–415. [DOI] [PubMed] [Google Scholar]
- Alzheimer, A. , Stelzmann, R. A. , Schnitzlein, H. N. , & Murtagh, F. R. (1995). An English translation of Alzheimer's 1907 paper, "Uber eine eigenartige Erkankung der Hirnrinde". Clinical anatomy (New York, N.Y.), 8, 429–431. [DOI] [PubMed] [Google Scholar]
- Atzmon, G. , Gabriely, I. , Greiner, W. , Davidson, D. , Schechter, C. , & Barzilai, N. (2002). Plasma HDL levels highly correlate with cognitive function in exceptional longevity. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences, 57, M712–M715. [DOI] [PubMed] [Google Scholar]
- Bales, K. R. , Liu, F. , Wu, S. , Lin, S. , Koger, D. , DeLong, C. , Hansen, J. C. , Sullivan, P. M. , & Paul, S. M. (2009). Human APOE isoform‐dependent effects on brain beta‐amyloid levels in PDAPP transgenic mice. The Journal of neuroscience: The official journal of the Society for Neuroscience, 29, 6771–6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates, K. A. , Sohrabi, H. R. , Rainey‐Smith, S. R. , Weinborn, M. , Bucks, R. S. , Rodrigues, M. , Beilby, J. , Howard, M. , Taddei, K. , Martins, G. , Paton, A. , Shah, T. , Dhaliwal, S. S. , Foster, J. K., Martins, I. J. , Lautenschlager, N. T. , Mastaglia, F. L. , Gandy, S. E. , & Martins, R. N. (2017). Serum high‐density lipoprotein is associated with better cognitive function in a cross‐sectional study of aging women. The International Journal of Neuroscience, 127, 243–252. [DOI] [PubMed] [Google Scholar]
- Beffert, U. , Cohn, J. S. , Petit‐Turcotte, C. , Tremblay, M. , Aumont, N. , Ramassamy, C. , Davignon, J. , & Poirier, J. (1999). Apolipoprotein E and beta‐amyloid levels in the hippocampus and frontal cortex of Alzheimer's disease subjects are disease‐related and apolipoprotein E genotype dependent. Brain Research, 843, 87–94. [DOI] [PubMed] [Google Scholar]
- Bell, R. D. , Sagare, A. P. , Friedman, A. E. , Bedi, G. S. , Holtzman, D. M. , Deane, R. , & Zlokovic, B. V. (2007). Transport pathways for clearance of human Alzheimer's amyloid beta‐peptide and apolipoproteins E and J in the mouse central nervous system. Journal of Cerebral Blood Flow and Metabolism: Official Journal of the International Society of Cerebral Blood Flow and Metabolism, 27, 909–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand, P. , Poirier, J. , Oda, T. , Finch, C. E. , & Pasinetti, G. M. (1995). Association of apolipoprotein E genotype with brain levels of apolipoprotein E and apolipoprotein J (clusterin) in Alzheimer disease. Brain Research Molecular Brain Research, 33, 174–178. [DOI] [PubMed] [Google Scholar]
- Bourgeat, P. , Dore, V. , Fripp, J. , Ames, D. , Masters, C. L. , Salvado, O. , Villemagne, V. L. , & Rowe, C. C. (2018). Implementing the centiloid transformation for (11)C‐PiB and beta‐amyloid (18)F‐PET tracers using CapAIBL. NeuroImage, 183, 387–393. [DOI] [PubMed] [Google Scholar]
- Burns, M. P. , Noble, W. J. , Olm, V. , Gaynor, K. , Casey, E. , LaFrancois, J. , Wang, L. , & Duff, K. (2003). Co‐localization of cholesterol, apolipoprotein E and fibrillar Abeta in amyloid plaques. Brain Research Molecular Brain Research, 110, 119–125. [DOI] [PubMed] [Google Scholar]
- Buxbaum, J. D. , Geoghagen, N. S. , & Friedhoff, L. T. (2001). Cholesterol depletion with physiological concentrations of a statin decreases the formation of the Alzheimer amyloid Abeta peptide. Journal of Alzheimer's Disease, 3, 221–229. [DOI] [PubMed] [Google Scholar]
- Castellano, J. M. , Kim, J. , Stewart, F. R. , Jiang, H. , DeMattos, R. B. , Patterson, B. W. , Fagan, A. M. , Morris, J. C. , Mawuenyega, K. G. , Cruchaga, C. , Goate, A. M. , Bales, K. R. , Paul, S. M. , Bateman, R. J. , & Holtzman, D. M. (2011). Human apoE isoforms differentially regulate brain amyloid‐beta peptide clearance. Science Translational Medicine, 3, 89ra57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook, D. G. , Leverenz, J. B. , McMillan, P. J. , Kulstad, J. J. , Ericksen, S. , Roth, R. A. , Schellenberg, G. D. , Jin, L. W. , Kovacina, K. S. , & Craft, S. (2003). Reduced hippocampal insulin‐degrading enzyme in late‐onset Alzheimer's disease is associated with the apolipoprotein E‐epsilon4 allele. The American Journal of Pathology, 162, 313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder, E. H. , Saunders, A. M. , Strittmatter, W. J. , Schmechel, D. E. , Gaskell, P. C. , Small, G. W. , Roses, A. D. , Haines, J. L. , & Pericak‐Vance, M. A. (1993). Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science (New York, N.Y.), 261, 921–923. [DOI] [PubMed] [Google Scholar]
- Crisby, M. , Bronge, L. , & Wahlund, L. O. (2010). Low levels of high density lipoprotein increase the severity of cerebral white matter changes: implications for prevention and treatment of cerebrovascular diseases. Current Alzheimer Research, 7, 534–539. [DOI] [PubMed] [Google Scholar]
- Cui, Y. , Huang, M. , He, Y. , Zhang, S. , & Luo, Y. (2011). Genetic ablation of apolipoprotein A‐IV accelerates Alzheimer's disease pathogenesis in a mouse model. The American Journal of Pathology, 178, 1298–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane, R. , Sagare, A. , Hamm, K. , Parisi, M. , Lane, S. , Finn, M. B. , Holtzman, D. M. , & Zlokovic, B. V. (2008). apoE isoform‐specific disruption of amyloid beta peptide clearance from mouse brain. The Journal of Clinical Investigation, 118, 4002–4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Heijer, T. , Hofman, A. , Koudstaal, P. J. , & Breteler, M. M. (2005). Serum lipids and hippocampal volume: the link to Alzheimer's disease? Annals of Neurology, 57, 779–780 author reply 7780. [DOI] [PubMed] [Google Scholar]
- Du, J. , Chang, J. , Guo, S. , Zhang, Q. , & Wang, Z. (2009). ApoE 4 reduces the expression of Abeta degrading enzyme IDE by activating the NMDA receptor in hippocampal neurons. Neuroscience Letters, 464, 140–145. [DOI] [PubMed] [Google Scholar]
- Ellis, K. A. , Bush, A. I. , Darby, D. , De Fazio, D. , Foster, J. , Hudson, P. , Lautenschlager, N. T. , Lenzo, N. , Martins, R. N. , Maruff, P. , Masters, C. , Milner, A. , Pike, K. , Rowe, C. , Savage, G. , Szoeke, C. , Taddei, K. , Villemagne, V. , Woodward, M. , & Ames, D. (2009). The Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging: methodology and baseline characteristics of 1112 individuals recruited for a longitudinal study of Alzheimer's disease. International Psychogeriatrics, 21, 672–687. [DOI] [PubMed] [Google Scholar]
- Farmer, B. C. , Walsh, A. E. , Kluemper, J. C. , & Johnson, L. A. (2020). Lipid Droplets in Neurodegenerative Disorders. Frontiers in Neuroscience, 14, 742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender, K. , Simons, M. , Bergmann, C. , Stroick, M. , Lutjohann, D. , Keller, P. , Runz, H. , Kuhl, S. , Bertsch, T. , von Bergmann, K. , Hennerici, M. , Beyreuther, K. , & Hartmann, T. (2001). Simvastatin strongly reduces levels of Alzheimer's disease beta ‐amyloid peptides Abeta 42 and Abeta 40 in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America, 98, 5856–5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz, N. F. , Cronican, A. , Pham, T. , Fogg, A. , Fauq, A. H. , Chapman, R. , Lefterov, I. , & Koldamova, R. (2010). Liver X receptor agonist treatment ameliorates amyloid pathology and memory deficits caused by high‐fat diet in APP23 mice. The Journal of Neuroscience: The Official Journal of the SOCIETY for NEUROSCIENCE, 30, 6862–6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler, C. , Rainey‐Smith, S. R. , Bird, S. , Bomke, J. , Bourgeat, P. , Brown, B. M. , Burnham, S. C. , Bush, A. I. , Chadunow, C. , Collins, S. , Doecke, J. , Doré, V. , Ellis, K. A. , Evered, L. , Fazlollahi, A. , Fripp, J. , Gardener, S. L. , Gibson, S. , Grenfell, R. , … Ames, D. (2021). Fifteen Years of the Australian Imaging, Biomarkers and Lifestyle (AIBL) Study: Progress and Observations from 2359 Older Adults Spanning the Spectrum from Cognitive Normality to Alzheimer's Disease. Journal of Alzheimer's Disease Reports, 5, 443–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer, J. D. , Simmons, K. , Parsadanian, M. , Bales, K. R. , Paul, S. M. , Sullivan, P. M. , & Holtzman, D. M. (2005). Human apolipoprotein E4 alters the amyloid‐beta 40:42 ratio and promotes the formation of cerebral amyloid angiopathy in an amyloid precursor protein transgenic model. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 25, 2803–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinberg, N. F. , Orhobor, O. I. , & King, R. D. (2020). An evaluation of machine‐learning for predicting phenotype: studies in yeast, rice, and wheat. Machine Learning, 109, 251–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, V. B. , Doecke, J. D. , Hone, E. , Pedrini, S. , Laws, S. M. , Thambisetty, M. , Bush, A. I. , Rowe, C. C. , Villemagne, V. L. , Ames, D. , Masters, C. L. , Macaulay, S. L. , Rembach, A. , Rainey‐Smith, S. R. , Martins, R. N. (2016). Plasma apolipoprotein J as a potential biomarker for Alzheimer's disease: Australian Imaging, Biomarkers and Lifestyle study of aging. Alzheimer's & dementia (Amsterdam, Netherlands) 3, 18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, V. B. , Hone, E. , Pedrini, S. , Doecke, J. , O'Bryant, S. , James, I. , Bush, A. I. , Rowe, C. C. , Villemagne, V. L. , Ames, D. , Masters, C. L. , & Martins, R. N. (2017). Altered levels of blood proteins in Alzheimer's disease longitudinal study: Results from Australian Imaging Biomarkers Lifestyle Study of Ageing cohort. Alzheimer's & dementia (Amsterdam, Netherlands), 8, 60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, V. B. , Laws, S. M. , Villemagne, V. L. , Ames, D. , Bush, A. I. , Ellis, K. A. , Lui, J. K. , Masters, C. , Rowe, C. C. , Szoeke, C. , Taddei, K. , & Martins, R. N. (2011). Plasma apolipoprotein E and Alzheimer disease risk: The AIBL study of aging. Neurology, 76, 1091–1098. [DOI] [PubMed] [Google Scholar]
- Hamilton, L. K. , Dufresne, M. , Joppé, S. E. , Petryszyn, S. , Aumont, A. , Calon, F. , Barnabé‐Heider, F. , Furtos, A. , Parent, M. , Chaurand, P. , & Fernandes, K. J. (2015). Aberrant Lipid Metabolism in the Forebrain Niche Suppresses Adult Neural Stem Cell Proliferation in an Animal Model of Alzheimer's Disease. Cell Stem Cell, 17, 397–411. [DOI] [PubMed] [Google Scholar]
- Hammad, S. M. , Ranganathan, S. , Loukinova, E. , Twal, W. O. , & Argraves, W. S. (1997). Interaction of apolipoprotein J‐amyloid beta‐peptide complex with low density lipoprotein receptor‐related protein‐2/megalin. A mechanism to prevent pathological accumulation of amyloid beta‐peptide. The Journal of Biological Chemistry, 272, 18644–18649. [DOI] [PubMed] [Google Scholar]
- Hashimoto, T. , Serrano‐Pozo, A. , Hori, Y. , Adams, K. W. , Takeda, S. , Banerji, A. O. , Mitani, A. , Joyner, D. , Thyssen, D. H. , Bacskai, B. J. , Frosch, M. P. , Spires‐Jones, T. L. , Finn, M. B. , Holtzman, D. M. , & Hyman, B. T. (2012). Apolipoprotein E, especially apolipoprotein E4, increases the oligomerization of amyloid beta peptide. The Journal of neuroscience: The official journal of the Society for Neuroscience, 32, 15181–15192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer, M. , Trieb, M. , Konya, V. , Wadsack, C. , Heinemann, A. , & Marsche, G. (2013). Aging affects high‐density lipoprotein composition and function. Biochimica et Biophysica Acta, 1831, 1442–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostage, C. A. , Roy Choudhury, K. , Doraiswamy, P. M. , & Petrella, J. R. (2013). Dissecting the gene dose‐effects of the APOE epsilon4 and epsilon2 alleles on hippocampal volumes in aging and Alzheimer's disease. PLoS One, 8, e54483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett, D. R. , Hortobagyi, T. , & Francis, P. T. (2013). Clusterin associates specifically with Abeta40 in Alzheimer's disease brain tissue. Brain Pathology (Zurich, Switzerland), 23, 623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isbir, T. , Agachan, B. , Yilmaz, H. , Aydin, M. , Kara, I. , Eker, E. , & Eker, D. (2001). Apolipoprotein‐E gene polymorphism and lipid profiles in Alzheimer's disease. American Journal of Alzheimer's Disease and Other Dementias, 16, 77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jóźwiak, J. J. , Kasperczyk, S. , Tomasik, T. , Osadnik, T. , Windak, A. , Studziński, K. , Mastej, M. , Catapano, A. , Ray, K. K. , Mikhailidis, D. P. , Toth, P. P. , Howard, G. , Lip, G. Y. H. , Tomaszewski, M. , Charchar, F. J. , Sattar, N. , Williams, B. , MacDonald, T. M. , Krzemień, P. , … Banach, M. (2022). Design and rationale of a nationwide screening analysis from the LIPIDOGRAM2015 and LIPIDOGEN2015 studies. Archives of Medical Science. AMS, 18, 604–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano, M. , Kawakami, M. , Otsuka, M. , Yashima, H. , Yaginuma, T. , & Ueki, A. (1995). Marked decrease of plasma apolipoprotein AI and AII in Japanese patients with late‐onset non‐familial Alzheimer's disease. Clinica Chimica Acta, 239, 209–211. [DOI] [PubMed] [Google Scholar]
- Klunk, W. E. , Koeppe, R. A. , Price, J. C. , Benzinger, T. L. , Devous, M. D., Sr. , Jagust, W. J. , Johnson, K. A. , Mathis, C. A. , Minhas, D. , Pontecorvo, M. J. , Rowe, C. C. , Skovronsky, D. M. , & Mintun, M. A. (2015). The Centiloid Project: standardizing quantitative amyloid plaque estimation by PET. Alzheimers Dement, 11(1‐15), e11–e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch, M. , DeKosky, S. T. , Fitzpatrick, A. L. , Furtado, J. D. , Lopez, O. L. , Kuller, L. H. , Mackey, R. H. , Hughes, T. M. , Mukamal, K. J. , & Jensen, M. K. (2018). Apolipoproteins and Alzheimer's pathophysiology. Alzheimer's & dementia (Amsterdam, Netherlands), 10, 545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffie, R. M. , Hashimoto, T. , Tai, H. C. , Kay, K. R. , Serrano‐Pozo, A. , Joyner, D. , Hou, S. , Kopeikina, K. J. , Frosch, M. P. , Lee, V. M. , Holtzman, D. M. , Hyman, B. T. , & Spires‐Jones, T. L. (2012). Apolipoprotein E4 effects in Alzheimer's disease are mediated by synaptotoxic oligomeric amyloid‐beta. Brain: A Journal of Neurology, 135, 2155–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojro, E. , Gimpl, G. , Lammich, S. , Marz, W. , & Fahrenholz, F. (2001). Low cholesterol stimulates the nonamyloidogenic pathway by its effect on the alpha ‐secretase ADAM 10. Proceedings of the National Academy of Sciences of the United States of America, 98, 5815–5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koldamova, R. P. , Lefterov, I. M. , Lefterova, M. I. , & Lazo, J. S. (2001). Apolipoprotein A‐I directly interacts with amyloid precursor protein and inhibits A beta aggregation and toxicity. Biochemistry, 40, 3553–3560. [DOI] [PubMed] [Google Scholar]
- Kostner, G. M. , Molinari, E. , & Pichler, P. (1985). Evaluation of a new HDL2/HDL3 quantitation method based on precipitation with polyethylene glycol. Clinica Chimica Acta, 148, 139–147. [DOI] [PubMed] [Google Scholar]
- Kuhn, M. (2008). Building Predictive Models in R Using the caret Package. Journal of Statistical Software, 28, 1–26.27774042 [Google Scholar]
- Lefterov, I. , Bookout, A. , Wang, Z. , Staufenbiel, M. , Mangelsdorf, D. , & Koldamova, R. (2007). Expression profiling in APP23 mouse brain: inhibition of Abeta amyloidosis and inflammation in response to LXR agonist treatment. Molecular Neurodegeneration, 2, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefterov, I. , Fitz, N. F. , Cronican, A. A. , Fogg, A. , Lefterov, P. , Kodali, R. , Wetzel, R. , & Koldamova, R. (2010). Apolipoprotein A‐I deficiency increases cerebral amyloid angiopathy and cognitive deficits in APP/PS1DeltaE9 mice. The Journal of Biological Chemistry, 285, 36945–36957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin‐Allerhand, J. A. , Lominska, C. E. , & Smith, J. D. (2002). Increased amyloid‐ levels in APPSWE transgenic mice treated chronically with a physiological high‐fat high‐cholesterol diet. The Journal of Nutrition, Health & Aging, 6, 315–319. [PubMed] [Google Scholar]
- Lewis, T. L. , Cao, D. , Lu, H. , Mans, R. A. , Su, Y. R. , Jungbauer, L. , Linton, M. F. , Fazio, S. , LaDu, M. J. , & Li, L. (2010). Overexpression of human apolipoprotein A‐I preserves cognitive function and attenuates neuroinflammation and cerebral amyloid angiopathy in a mouse model of Alzheimer disease. The Journal of Biological Chemistry, 285, 36958–36968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H. , Ruberu, K. , Muñoz, S. S. , Jenner, A. M. , Spiro, A. , Zhao, H. , Rassart, E. , Sanchez, D. , Ganfornina, M. D. , Karl, T. , & Garner, B. (2015). Apolipoprotein D modulates amyloid pathology in APP/PS1 Alzheimer's disease mice. Neurobiology of Aging, 36, 1820–1833. [DOI] [PubMed] [Google Scholar]
- Lidstrom, A. M. , Bogdanovic, N. , Hesse, C. , Volkman, I. , Davidsson, P. , & Blennow, K. (1998). Clusterin (apolipoprotein J) protein levels are increased in hippocampus and in frontal cortex in Alzheimer's disease. Experimental Neurology, 154, 511–521. [DOI] [PubMed] [Google Scholar]
- Liu, H. C. , Hu, C. J. , Chang, J. G. , Sung, S. M. , Lee, L. S. , Yuan, R. Y. , & Leu, S. J. (2006). Proteomic identification of lower apolipoprotein A‐I in Alzheimer's disease. Dementia and Geriatric Cognitive Disorders, 21, 155–161. [DOI] [PubMed] [Google Scholar]
- Manning, E. N. , Barnes, J. , Cash, D. M. , Bartlett, J. W. , Leung, K. K. , Ourselin, S. , & Fox, N. C. (2014). APOE epsilon4 is associated with disproportionate progressive hippocampal atrophy in AD. PLoS One, 9, e97608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez, A. E. , Weissberger, G. , Kuklenyik, Z. , He, X. , Meuret, C. , Parekh, T. , Rees, J. C. , Parks, B. A. , Gardner, M. S. , King, S. M. , Collier, T. S. , Harrington, M. G. , Sweeney, M. D. , Wang, X. , Zlokovic, B. V. , Joe, E. , Nation, D. A. , Schneider, L. S. , Chui, H. C. , … Yassine, H. N. (2022). The small HDL particle hypothesis of Alzheimer's disease. Alzheimer's & Dementia. Epub ahead of print. 10.1002/alz.12649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters, C. L. , Bateman, R. , Blennow, K. , Rowe, C. C. , Sperling, R. A. , & Cummings, J. L. (2015). Alzheimer's disease. Nature Reviews Disease Primers, 1, 15056. [DOI] [PubMed] [Google Scholar]
- May, P. C. , Lampert‐Etchells, M. , Johnson, S. A. , Poirier, J. , Masters, J. N. , & Finch, C. E. (1990). Dynamics of gene expression for a hippocampal glycoprotein elevated in Alzheimer's disease and in response to experimental lesions in rat. Neuron, 5, 831–839. [DOI] [PubMed] [Google Scholar]
- McKhann, G. , Drachman, D. , Folstein, M. , Katzman, R. , Price, D. , & Stadlan, E. M. (1984). Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology, 34, 939–944. [DOI] [PubMed] [Google Scholar]
- Merched, A. , Xia, Y. , Visvikis, S. , Serot, J. M. , & Siest, G. (2000). Decreased high‐density lipoprotein cholesterol and serum apolipoprotein AI concentrations are highly correlated with the severity of Alzheimer's disease. Neurobiology of Aging, 21, 27–30. [DOI] [PubMed] [Google Scholar]
- Mielke, M. M. , Zandi, P. P. , Sjögren, M. , Gustafson, D. , Ostling, S. , Steen, B. , & Skoog, I. (2005). High total cholesterol levels in late life associated with a reduced risk of dementia. Neurology, 64, 1689–1695. [DOI] [PubMed] [Google Scholar]
- Miners, J. S. , Clarke, P. , & Love, S. (2017). Clusterin levels are increased in Alzheimer's disease and influence the regional distribution of Abeta. Brain Pathology (Zurich, Switzerland), 27, 305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlambo, Z. P. , Varaden, D. , Moodley, J. , & Naicker, T. (2020). Are concentrations of clusterin and beta‐2‐glycoprotein I dysregulated in HIV associated preeclampsia? European Journal of Obstetrics, Gynecology, and Reproductive Biology, 251, 1–7. [DOI] [PubMed] [Google Scholar]
- Moffat, S. D. , Szekely, C. A. , Zonderman, A. B. , Kabani, N. J. , & Resnick, S. M. (2000). Longitudinal change in hippocampal volume as a function of apolipoprotein E genotype. Neurology, 55, 134–136. [DOI] [PubMed] [Google Scholar]
- Paula‐Lima, A. C. , Tricerri, M. A. , Brito‐Moreira, J. , Bomfim, T. R. , Oliveira, F. F. , Magdesian, M. H. , Grinberg, L. T. , Panizzutti, R. , & Ferreira, S. T. (2009). Human apolipoprotein A‐I binds amyloid‐beta and prevents Abeta‐induced neurotoxicity. The International Journal of Biochemistry & Cell Biology, 41, 1361–1370. [DOI] [PubMed] [Google Scholar]
- Pedrini, S. , Thomas, C. , Brautigam, H. , Schmeidler, J. , Ho, L. , Fraser, P. , Westaway, D. , Hyslop, P. S. , Martins, R. N. , Buxbaum, J. D. , Pasinetti, G. M. , Dickstein, D. L., Hof, P. R. , Ehrlich, M. E. , Gandy, S. (2009). Dietary composition modulates brain mass and solubilizable Abeta levels in a mouse model of aggressive Alzheimer's amyloid pathology. Molecular Neurodegeneration, 4, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard, C. , Nilsson, N. , Labonté, A. , Auld, D. , Rosa‐Neto, P. , Initiative, A.'s. D. N. , Ashton, N. J. , Zetterberg, H. , Blennow, K. , Breitner, J. C. B. , Villeneuve, S. , & Poirier, J. (2022). Apolipoprotein B is a novel marker for early tau pathology in Alzheimer's disease. Alzheimers Dement, 18, 875–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier, J. , Miron, J. , Picard, C. , Gormley, P. , Théroux, L. , Breitner, J. , & Dea, D. (2014). Apolipoprotein E and lipid homeostasis in the etiology and treatment of sporadic Alzheimer's disease. Neurobiology of Aging, 35(Suppl 2), S3–S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi, Y. , Fan, J. , Liu, J. , Wang, W. , Wang, M. , Sun, J. , Liu, J. , Xie, W. , Zhao, F. , Li, Y. , & Zhao, D. (2015). Cholesterol‐overloaded HDL particles are independently associated with progression of carotid atherosclerosis in a cardiovascular disease‐free population: A community‐based cohort study. Journal of the American College of Cardiology, 65, 355–363. [DOI] [PubMed] [Google Scholar]
- Ralhan, I. , Chang, C. L. , Lippincott‐Schwartz, J. , & Ioannou, M. S. (2021). Lipid droplets in the nervous system. The Journal of Cell Biology, 220, e202102136. 10.1083/jcb.202102136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed, B. , Villeneuve, S. , Mack, W. , DeCarli, C. , Chui, H. C. , & Jagust, W. (2014). Associations between serum cholesterol levels and cerebral amyloidosis. JAMA Neurology, 71, 195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refolo, L. M. , Malester, B. , LaFrancois, J. , Bryant‐Thomas, T. , Wang, R. , Tint, G. S. , Sambamurti, K. , Duff, K. , & Pappolla, M. A. (2000). Hypercholesterolemia accelerates the Alzheimer's amyloid pathology in a transgenic mouse model. Neurobiology of Disease, 7, 321–331. [DOI] [PubMed] [Google Scholar]
- Reitz, C. , Tang, M. X. , Luchsinger, J. , & Mayeux, R. (2004). Relation of plasma lipids to Alzheimer disease and vascular dementia. Archives of Neurology, 61, 705–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitz, C. , Tang, M. X. , Manly, J. , Schupf, N. , Mayeux, R. , & Luchsinger, J. A. (2008). Plasma lipid levels in the elderly are not associated with the risk of mild cognitive impairment. Dementia and Geriatric Cognitive Disorders, 25, 232–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitz, C. , Tang, M. X. , Schupf, N. , Manly, J. J. , Mayeux, R. , & Luchsinger, J. A. (2010). Association of higher levels of high‐density lipoprotein cholesterol in elderly individuals and lower risk of late‐onset Alzheimer disease. Archives of Neurology, 67, 1491–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee, E. J. , Byrne, C. D. , & Sung, K. C. (2017). The HDL cholesterol/apolipoprotein A‐I ratio: an indicator of cardiovascular disease. Current Opinion in Endocrinology, Diabetes, and Obesity, 24, 148–153. [DOI] [PubMed] [Google Scholar]
- Riddell, D. R. , Zhou, H. , Comery, T. A. , Kouranova, E. , Lo, C. F. , Warwick, H. K. , Ring, R. H. , Kirksey, Y. , Aschmies, S. , Xu, J. , Kubek, K. , Hirst, W. D. , Gonzales, C. , Chen, Y. , Murphy, E. , Leonard, S. , Vasylyev, D. , Oganesian, A. , Martone, R. L. , … Jacobsen, J. S. (2007). The LXR agonist TO901317 selectively lowers hippocampal Abeta42 and improves memory in the Tg2576 mouse model of Alzheimer's disease. Molecular and Cellular Neurosciences, 34, 621–628. [DOI] [PubMed] [Google Scholar]
- Ronsein, G. E. , & Vaisar, T. (2019). Deepening our understanding of HDL proteome. Expert Review of Proteomics, 16, 749–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenson, R. S. , Brewer, H. B., Jr. , Ansell, B. J. , Barter, P. , Chapman, M. J. , Heinecke, J. W. , Kontush, A. , Tall, A. R. , & Webb, N. R. (2016). Dysfunctional HDL and atherosclerotic cardiovascular disease. Nature Reviews Cardiology, 13, 48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saczynski, J. S. , White, L. , Peila, R. L. , Rodriguez, B. L. , & Launer, L. J. (2007). The relation between apolipoprotein A‐I and dementia: the Honolulu‐Asia aging study. American Journal of Epidemiology, 165, 985–992. [DOI] [PubMed] [Google Scholar]
- Shah, A. S. , Tan, L. , Long, J. L. , & Davidson, W. S. (2013). Proteomic diversity of high density lipoproteins: our emerging understanding of its importance in lipid transport and beyond. Journal of Lipid Research, 54, 2575–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonovitch, S. , Schmukler, E. , Bespalko, A. , Iram, T. , Frenkel, D. , Holtzman, D. M. , Masliah, E. , Michaelson, D. M. , & Pinkas‐Kramarski, R. (2016). Impaired Autophagy in APOE4 Astrocytes. Journal of Alzheimer's Disease, 51, 915–927. [DOI] [PubMed] [Google Scholar]
- Simons, M. , Keller, P. , De Strooper, B. , Beyreuther, K. , Dotti, C. G. , & Simons, K. (1998). Cholesterol depletion inhibits the generation of beta‐amyloid in hippocampal neurons. Proceedings of the National Academy of Sciences of the United States of America, 95, 6460–6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh‐Manoux, A. , Gimeno, D. , Kivimaki, M. , Brunner, E. , & Marmot, M. G. (2008). Low HDL cholesterol is a risk factor for deficit and decline in memory in midlife: the Whitehall II study. Arteriosclerosis, Thrombosis, and Vascular Biology, 28, 1556–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter, W. J. , Saunders, A. M. , Schmechel, D. , Pericak‐Vance, M. , Enghild, J. , Salvesen, G. S. , & Roses, A. D. (1993). Apolipoprotein E: high‐avidity binding to beta‐amyloid and increased frequency of type 4 allele in late‐onset familial Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America, 90, 1977–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stukas, S. , Robert, J. , Lee, M. , Kulic, I. , Carr, M. , Tourigny, K. , Fan, J. , Namjoshi, D. , Lemke, K. , DeValle, N. , Chan, J. , Wilson, T. , Wilkinson, A. , Chapanian, R. , Kizhakkedathu, J. N. , Cirrito, J. R. , Oda, M. N. , & Wellington, C. L. (2014). Intravenously injected human apolipoprotein A‐I rapidly enters the central nervous system via the choroid plexus. Journal of the American Heart Association, 3, e001156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, X. , Holland, D. , Dale, A. M. , & Miller, M. I. (2015). APOE affects the volume and shape of the amygdala and the hippocampus in mild cognitive impairment and alzheimer's disease: Age matters. Journal of Alzheimer's Disease, 47, 645–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng, E. , Chow, N. , Hwang, K. S. , Thompson, P. M. , Gylys, K. H. , Cole, G. M. , Jack, C. R., Jr. , Shaw, L. M. , Trojanowski, J. Q. , Soares, H. D. , Weiner, M. W. , & Apostolova, L. G. (2015). Low plasma ApoE levels are associated with smaller hippocampal size in the Alzheimer's disease neuroimaging initiative cohort. Dementia and Geriatric Cognitive Disorders, 39, 154–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda, T. , Calero, M. , Matsubara, E. , Vidal, R. , Kumar, A. , Permanne, B. , Zlokovic, B. , Smith, J. D. , Ladu, M. J. , Rostagno, A. , Frangione, B. , & Ghiso, J. (2000). Lipidation of apolipoprotein E influences its isoform‐specific interaction with Alzheimer's amyloid beta peptides. The Biochemical Journal, 348(Pt 2), 359–365. [PMC free article] [PubMed] [Google Scholar]
- Trieb, M. , Horvath, A. , Birner‐Gruenberger, R. , Spindelboeck, W. , Stadlbauer, V. , Taschler, U. , Curcic, S. , Stauber, R. E. , Holzer, M. , Pasterk, L. , Heinemann, A. , & Marsche, G. (2016). Liver disease alters high‐density lipoprotein composition, metabolism and function. Biochimica et Biophysica Acta, 1861, 630–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trommer, B. L. , Shah, C. , Yun, S. H. , Gamkrelidze, G. , Pasternak, E. S. , Stine, W. B. , Manelli, A. , Sullivan, P. , Pasternak, J. F. , & LaDu, M. J. (2005). ApoE isoform‐specific effects on LTP: blockade by oligomeric amyloid‐beta1‐42. Neurobiology of Disease, 18, 75–82. [DOI] [PubMed] [Google Scholar]
- Vaisar, T. , Pennathur, S. , Green, P. S. , Gharib, S. A. , Hoofnagle, A. N. , Cheung, M. C. , Byun, J. , Vuletic, S. , Kassim, S. , Singh, P. , Chea, H. , Knopp, R. H. , Brunzell, J. , Geary, R. , Chait, A. , Zhao, X. Q. , Elkon, K. , Marcovina, S. , Ridker, P. , … Heinecke, J. W. (2007). Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. The Journal of Clinical Investigation, 117, 746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Velsen, E. F. , Vernooij, M. W. , Vrooman, H. A. , van der Lugt, A. , Breteler, M. M. , Hofman, A. , Niessen, W. J. , & Ikram, M. A. (2013). Brain cortical thickness in the general elderly population: The Rotterdam Scan Study. Neuroscience Letters, 550, 189–194. [DOI] [PubMed] [Google Scholar]
- Villemagne, V. L. , Pike, K. E. , Chételat, G. , Ellis, K. A. , Mulligan, R. S. , Bourgeat, P. , Ackermann, U. , Jones, G. , Szoeke, C. , Salvado, O. , Martins, R. , O'Keefe, G. , Mathis, C. A. , Klunk, W. E. , Ames, D. , Masters, C. L. , & Rowe, C. C. (2011). Longitudinal assessment of Abeta and cognition in aging and Alzheimer disease. Annals of Neurology, 69, 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahrle, S. , Das, P. , Nyborg, A. C. , McLendon, C. , Shoji, M. , Kawarabayashi, T. , Younkin, L. H. , Younkin, S. G. , & Golde, T. E. (2002). Cholesterol‐dependent gamma‐secretase activity in buoyant cholesterol‐rich membrane microdomains. Neurobiology of Disease, 9, 11–23. [DOI] [PubMed] [Google Scholar]
- Walther, T. C. , & Farese, R. V., Jr. (2012). Lipid droplets and cellular lipid metabolism. Annual Review of Biochemistry, 81, 687–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward, M. A. , Bendlin, B. B. , McLaren, D. G. , Hess, T. M. , Gallagher, C. L. , Kastman, E. K. , Rowley, H. A. , Asthana, S. , Carlsson, C. M. , Sager, M. A. , & Johnson, S. C. (2010). Low HDL cholesterol is associated with lower gray matter volume in cognitively healthy adults. Frontiers in Aging Neuroscience, 2, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichhart, T. , Kopecky, C. , Kubicek, M. , Haidinger, M. , Döller, D. , Katholnig, K. , Suarna, C. , Eller, P. , Tölle, M. , Gerner, C. , Zlabinger, G. J. , van der Giet, M. , Hörl, W. H. , Stocker, R. , & Säemann, M. D. (2012). Serum amyloid A in uremic HDL promotes inflammation. Journal of the American Society of Nephrology: JASN, 23, 934–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winblad, B. , Palmer, K. , Kivipelto, M. , Jelic, V. , Fratiglioni, L. , Wahlund, L. O. , Nordberg, A. , Bäckman, L. , Albert, M. , Almkvist, O. , Arai, H. , Basun, H. , Blennow, K. , de Leon, M. , DeCarli, C. , Erkinjuntti, T. , Giacobini, E. , Graff, C. , Hardy, J. , … Petersen, R. C. (2004). Mild cognitive impairment‐‐beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. Journal of Internal Medicine, 256, 240–246. [DOI] [PubMed] [Google Scholar]
- Wolf, H. , Hensel, A. , Arendt, T. , Kivipelto, M. , Winblad, B. , & Gertz, H. J. (2004). Serum lipids and hippocampal volume: the link to Alzheimer's disease? Annals of Neurology, 56, 745–748. [DOI] [PubMed] [Google Scholar]
- Xiao, Z. , Wang, J. , Chen, W. , Wang, P. , Zeng, H. , & Chen, W. (2012). Association studies of several cholesterol‐related genes (ABCA1, CETP and LIPC) with serum lipids and risk of Alzheimer's disease. Lipids in Health and Disease, 11, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi, K. , Tozuka, M. , Hidaka, H. , Nakabayashi, T. , Sugano, M. , Kondo, Y. , Nakagawara, A. , & Katsuyama, T. (2000). Effect of apolipoprotein AII on the interaction of apolipoprotein E with beta‐amyloid: some apo(E‐AII) complexes inhibit the internalization of beta‐amyloid in cultures of neuroblastoma cells. Journal of Neuroscience Research, 62, 608–614. [DOI] [PubMed] [Google Scholar]
- Yan, L. R. , Wang, D. X. , Liu, H. , Zhang, X. X. , Zhao, H. , Hua, L. , Xu, P. , & Li, Y. S. (2014). A pro‐atherogenic HDL profile in coronary heart disease patients: an iTRAQ labelling‐based proteomic approach. PLoS One, 9, e98368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassine, H. N. , Jackson, A. M. , Borges, C. R. , Billheimer, D. , Koh, H. , Smith, D. , Reaven, P. , Lau, S. S. , & Borchers, C. H. (2014). The application of multiple reaction monitoring and multi‐analyte profiling to HDL proteins. Lipids in Health and Disease, 13, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, S. , Huang, Y. , Mullendorff, K. , et al. (2005). Apolipoprotein (apo) E4 enhances amyloid beta peptide production in cultured neuronal cells: apoE structure as a potential therapeutic target. Proceedings of the National Academy of Sciences of the United States of America, 102, 18700–18705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans, K. L. , Tai, L. M. , Nwabuisi‐Heath, E. , Jungbauer, L. , Kanekiyo, T. , Gan, M. , Kim, J. , Eimer, W. A. , Estus, S. , Rebeck, G. W. , Weeber, E. J. , Bu, G. , Yu, C. , & Ladu, M. J. (2012). APOE4‐specific changes in Abeta accumulation in a new transgenic mouse model of Alzheimer disease. The Journal of Biological Chemistry, 287, 41774–41786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, B. , Matsunaga, A. , Saku, K. , Nakano, S. , & Yamada, T. (2004). Associations among plasma lipoprotein subfractions as characterized by analytical capillary isotachophoresis, apolipoprotein E phenotype, Alzheimer disease, and mild cognitive impairment. Arteriosclerosis, Thrombosis, and Vascular Biology, 24, e144–e146. [DOI] [PubMed] [Google Scholar]
- Zlokovic, B. V. , Martel, C. L. , Mackic, J. B. , Matsubara, E. , Wisniewski, T. , McComb, J. G. , Frangione, B. , & Ghiso, J. (1994). Brain uptake of circulating apolipoproteins J and E complexed to Alzheimer's amyloid beta. Biochemical and Biophysical Research Communications, 205, 1431–1437. [DOI] [PubMed] [Google Scholar]
- Zlokovic, B. V. , Martel, C. L. , Matsubara, E. , McComb, J. G. , Zheng, G. , McCluskey, R. T. , Frangione, B. , & Ghiso, J. (1996). Glycoprotein 330/megalin: probable role in receptor‐mediated transport of apolipoprotein J alone and in a complex with Alzheimer disease amyloid beta at the blood‐brain and blood‐cerebrospinal fluid barriers. Proceedings of the National Academy of Sciences of the United States of America, 93, 4229–4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Figure S1
Data Availability Statement
AIBL data have been provided to the Global Alzheimer's Association Interactive Network (GAAIN), and software installed, thereby enabling GAAIN users to interrogate metadata and receive cohort summaries, whereupon users can request further information (and biofluid samples; blood and CSF) if needed by submitting an Expression of Interest (EoI). This mechanism ensures worldwide sharing and utilization of AIBL data (and biofluid samples) and complements the existing framework whereby AIBL imaging scans and demographic data are available on the ADNI LONI (Laboratory of Neuro Imaging, University of Southern California) website for free download and use by researchers worldwide. Up to 3 August 2019, LONI/ GAAIN data applications had been received from over 120 companies, more than 2000 organizations/institutes/departments, and over 3500 individuals, spanning 81 countries. Moreover, hundreds of EoIs from academic and industry‐based individuals have been approved resulting in further data and biofluid sample sharing. Data will also be provided to the National Institute on Aging‐funded ADOPIC (Alzheimer's Dementia Onset and Progression in International Cohorts) project which will harmonize data from the AIBL, ADNI, Washington University, St. Louis, and University of Washington, Seattle, cohorts to determine factors which influence cognitive decline in AD.


