Abstract
According to the new classification, periodontitis is defined as a chronic multifactorial inflammatory disease associated with dysbiotic biofilms and characterized by progressive destruction of the tooth‐supporting apparatus. This definition, based on the current scientific evidence, clearly indicates and emphasizes, beside the microbial component dental biofilm, the importance of the inflammatory reaction in the progressive destruction of periodontal tissues. The idea to modulate this inflammatory reaction in order to decrease or even cease the progressive destruction was, therefore, a logical consequence. Attempts to achieve this goal involve various kinds of anti‐inflammatory drugs or medications. However, there is also an increasing effort in using food supplements or so‐called natural food ingredients to modulate patients’ immune responses and maybe even improve the healing of periodontal tissues. The aim of this chapter of Periodontology 2000 is to review the evidence of various food supplements and ingredients regarding their possible effects on periodontal inflammation and wound healing. This review may help researchers and clinicians to evaluate the current evidence and to stimulate further research in this area.
Keywords: food supplements, periodontitis, periodontal healing, inflammation
1. INTRODUCTION
The use of food supplements and so‐called superfoods to increase fitness and regeneration or just to improve health and well‐being is very popular these days, particularly in people living the fitness lifestyle. Some of the effects attributed to these supplements and superfoods involve tissues and processes that may also play a role in periodontal healing and regeneration. Although the number of publications investigating the effect of these products on human health and their possible use in medicine is increasing, only little is known so far regarding their effects on periodontal tissues and their possible use in periodontal treatment or medicine. The aim of this review is to provide an overview of the current evidence of some popular food supplements and superfoods that might be of interest in periodontology.
2. FISH OIL/OMEGA‐3 FATTY ACIDS
Fish oil, and particularly the enclosed omega‐3 polyunsaturated fatty acids, is assumed to be beneficial for human fitness and well‐being. Their wholesome effects are claimed to promote or participate in heart and vascular health, brain or neurological development and function, mental health and function, vision, immune system balance, body weight control, joint function, and bone and muscle mass and strength. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 Therefore, fish‐oil supplements or other supplements rich in omega‐3 polyunsaturated fatty acids are today one of the most common and widely used dietary supplements in the health and fitness sector.
There are two classes of essential fatty acids, omega‐3 and omega‐6. Omega‐3 and omega‐6 fatty acids are polyunsaturated fatty acids containing more than one cis double bond that is located between the third and the fourth carbon atoms from the methyl end in omega‐3 fatty acids and between the sixth and seventh carbon atoms in omega‐6 fatty acids. Alpha‐linolenic acid (18:3n−3) is the parent fatty acid of the omega‐3 series and linoleic acid (18:2n−6) is the parent fatty acid of the omega‐6 series. Both, alpha‐linolenic acid and linoleic acid compete for the same elongase and desaturase enzymes in the synthesis of long‐chain polyunsaturated fatty acids. 25 , 26 Humans are able to synthetize long‐chain (20 carbon atoms or more) omega‐3 polyunsaturated fatty acids, such as eicosapentaenoic acid (20:5n−3), docosapentaenoic acid (22:5n−3), and docosahexaenoic acid (22:6n−3), from alpha‐linolenic acid and long‐chain omega‐6 polyunsaturated fatty acids, such as dihomo‐γ‐linolenic acid (20:3n−6) and arachidonic acid (20:4n−6), from linoleic acid by desaturation (addition of a double bond) and elongation (addition of two carbon atoms). 27 , 28 The main natural dietary sources for alpha‐linolenic acid are plant‐based sources such as green leafy vegetables, flaxseed, chia seeds, canola, soybeans, walnuts, and pecans, but also chicken and beef. The main sources for linoleic acid are vegetable oils, safflower oil, sunflower oil, borage oil, and corn oil (Figure 1). 3 , 5 , 23 , 27 Fatty acids are absorbed in the small intestine after hydrolyzation by pancreatic enzymes and under the presence of bile salts. Under normal conditions, fat absorption throughout the small intestine is 85%‐95% efficient. 29
FIGURE 1.
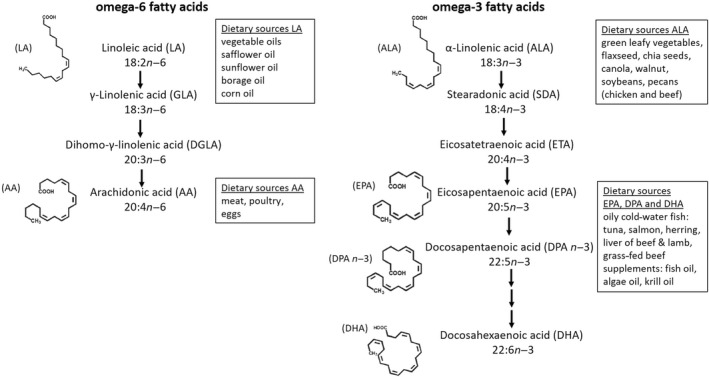
Long‐chain omega‐6 and omega‐3 polyunsaturated fatty acids deriving from their parent compound linoleic acid or alpha‐linolenic acid, including molecular structures and dietary sources 27
Fish oils contains more than 40 fatty acids. However, the claimed health benefits associated with fish oil have been attributed primarily to the most prevalent omega‐3 polyunsaturated fatty acids in fish oil, namely eicosapentaenoic acid and docosahexaenoic acid. The third most prevalent omega‐3 polyunsaturated fatty acid in fish oil, docosapentaenoic acid, has recently gained more attention by scientists. 3 , 30 Interestingly, in human breast milk, the docosapentaenoic acid content is higher than the eicosapentaenoic acid content and is comparable to the docosahexaenoic acid content, indicating an important role of docosapentaenoic acid in human development. 31 All three omega‐3 polyunsaturated fatty acids, eicosapentaenoic acid, docosapentaenoic acid, and docosahexaenoic acid, share structural similarities which may explain some of the overlapping functions. 3
Although the long‐chain omega‐3 polyunsaturated fatty acids eicosapentaenoic acid, docosapentaenoic acid, and docosahexaenoic acid can be synthetized from alpha‐linolenic acid, in reality the conversion efficiency seems to be low to absent. Studies also report that gender differences, existing diet, genetic variability in enzymes involved in the fatty acid metabolism, and lifestyle factors may have an impact on the alpha‐linolenic acid conversion rate. In healthy young men, not more than about 8% of the dietary alpha‐linolenic acid is converted to eicosapentaenoic acid and docosapentaenoic acid, and only 0%‐4% is converted to docosahexaenoic acid. In healthy young women, up to 21% of the ingested alpha‐linolenic acid is converted to eicosapentaenoic acid, 9% to docosahexaenoic acid, and 6% to docosapentaenoic acid. 32 , 33 Interestingly, docosahexaenoic acid can also be retroconverted to eicosapentaenoic acid following docosahexaenoic acid supplementation, although at a low rate. 34
Polymorphisms of the two key enzymes in fatty acid metabolism (fatty acid desaturases 1 and 2) seem to be responsible for up to 30% of the variability in blood levels of omega‐3 and omega‐6 fatty acids among individuals. 35 , 36 The already low conversion rate of alpha‐linolenic acid in long‐chain omega‐3 polyunsaturated fatty acids may be further reduced by about 40% when diets are high in omega‐6 fatty acids, as for example in a typical Western diet, since omega‐6 fatty acids compete with omega‐3 fatty acids for enzymes in biosynthetic pathways in the human body. 37 , 38 Furthermore, lifestyle factors, like alcohol consumption, have also been shown to reduce docosapentaenoic acid levels in the plasma and the liver. 39 Therefore, it is suggested that long‐chain omega‐3 polyunsaturated fatty acids may be considered as conditionally essential nutrients and an adequate supply of eicosapentaenoic acid, docosapentaenoic acid, and docosahexaenoic acid should be achieved by direct consumption from food sources. 27 , 32 , 33 Good food sources for eicosapentaenoic acid, docosapentaenoic acid, and docosahexaenoic acid are sea food, especially oily cold‐water fish (tuna, salmon, or herring), but also liver of beef and lamb, grass‐fed beef, or just supplements like fish oil, algae oil, or krill oil capsules. Unfortunately, the typical Western diet is usually rather rich in omega‐6 polyunsaturated fatty acid sources. The omega‐6 to omega‐3 polyunsaturated fatty acid ratio in a typical Western diet is about 20‐30:1, compared with a diet rich in fish or seafood with an omega‐6 to omega‐3 ratio of about 1‐2:1. 40 , 41 Therefore, supplement capsules could be a good source of long‐chain omega‐3 polyunsaturated fatty acids for people who cannot or are not keen to eat a sufficient amount of fish or seafood. 27 , 42 , 43 , 44 , 45 However, the content of eicosapentaenoic acid, docosahexaenoic acid, and docosapentaenoic acid between the numerous fish oil, krill oil, or flaxseed oil supplements may vary significantly. Therefore, it might be prudent to check the content of the different omega‐3 polyunsaturated fatty acids on the label of the particular supplement. Owing to the increased absorption rate, it is also advised to take omega‐3 polyunsaturated fatty acid supplements together with a meal. Dividing the daily dose into two or three smaller doses throughout the day may help to reduce fishy aftertaste or gastrointestinal side effects. 46 , 47 Current intake recommendations regarding omega‐3 polyunsaturated fatty acids, and particularly eicosapentaenoic acid and docosahexaenoic acid, vary between different organizations and for different indications. The European Food Safety Authority recommends an adequate intake of 250 mg/day for eicosapentaenoic acid plus docosahexaenoic acid. The World Health Organization recommends an acceptable macronutrient distribution range for eicosapentaenoic acid plus docosahexaenoic acid of 250 mg/day to 2 g/day (the upper limit applying to the secondary prevention of coronary heart disease). The International Society for the Study of Fatty Acids and Lipids recommends for healthy adults a minimum of 500 mg/day of eicosapentaenoic acid plus docosahexaenoic acid for cardiovascular health. The American Heart Association recommendation for people without documented coronary heart disease is to eat fish at least twice weekly, providing approximately 500 mg of eicosapentaenoic acid plus docosahexaenoic acid. People with documented coronary heart disease are advised to consume approximately 1 g/day of eicosapentaenoic acid plus docosahexaenoic acid, preferably from oily fish or to consider supplements in consultation with a physician. People who need to lower serum triglycerides may take 2–4 g/day of eicosapentaenoic acid plus docosahexaenoic acid supplements under a physician’s care. The Linus Pauling Institute advises that generally healthy adults should eat fish twice weekly and to consume foods rich in alpha‐linolenic acid, such as walnuts or flaxseeds. People who do not regularly eat fish should consider taking a 2 g fish oil supplement several times a week. 27
Omega‐3 and omega‐6 polyunsaturated fatty acids are important structural components of the phospholipids of all cell membranes. The composition and molecular structure of cellular membranes can be changed by diet or by the fatty acid composition of the diet. An increased intake of omega‐3 polyunsaturated fatty acids via diet will increase the omega‐3 polyunsaturated fatty acid concentration in complex lipids within the bloodstream and in phospholipid membranes of cells and tissues. 48 In humans on a typical Western diet low in omega‐3 polyunsaturated fatty acids, blood cells involved in inflammatory responses contain only about 0.5%‐1% eicosapentaenoic acid, 2%‐4% docosahexaenoic acid, but 10%‐20% arachidonic acid. An increased omega‐3 fatty acid intake has been shown to increase the omega‐3 polyunsaturated fatty acid content of the cell membranes at the expense of omega‐6 polyunsaturated fatty acids, and especially arachidonic acid. 49 , 50 , 51 , 52 , 53 Investigations with an eicosapentaenoic acid/docosahexaenoic acid supplement showed that the fraction of omega‐3 polyunsaturated fatty acids increased in red and in white blood cells following the consumption of the supplement. 54 However, whereas there was a linear relationship to the dietary dose of the intake or supplement in red blood cells, this could not be seen in white blood cells. This indicates differences in the degree of omega‐3 polyunsaturated fatty acid increase between different cell types. 10 , 54 , 55
When incorporated into phospholipids, omega‐3 and omega‐6 polyunsaturated fatty acids affect key membrane properties, such as membrane fluidity, flexibility, permeability, cell signaling, gene expression, activity of membrane‐bound enzymes, receptor activity, signaling or activation of transcription factors, membrane cation‐transport system, and the pattern of lipid mediator or eicosanoid production. 56 , 57 , 58 , 59 , 60 These lipid‐derived mediators, eicosanoids, are derived from long‐chain 20‐carbon polyunsaturated fatty acid precursors and are considered as highly potent chemical messengers and key mediators and regulators in immune and inflammatory responses (Figure 2). After stimulation by hormones, cytokines, or other factors, long‐chain polyunsaturated fatty acids are released from cell membranes and become substrates for eicosanoid production. The synthesis of eicosanoids is catalyzed by three enzyme families: cyclooxygenases, lipoxygenases, and cytochrome p450 monooxygenases. Cyclooxygenases produce prostaglandins, prostacyclins, and thromboxanes, known as prostanoids. Lipoxygenases produce leukotrienes and hydroxyl fatty acids, and cytochrome p450 monooxygenases produce hydroxyeicosatetraenoic acids and epoxides. 27 , 55 , 61 , 62 , 63 , 64
FIGURE 2.
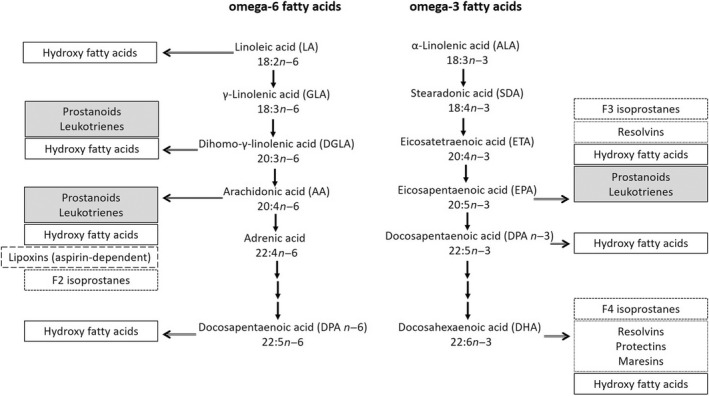
Bioactive lipid mediators derived from omega‐6 and omega‐3 fatty acids 27 , 55 , 61 , 62
However, the physiologic responses to eicosanoids deriving from long‐chain omega‐6 polyunsaturated fatty acids are quite different from those to long‐chain omega‐3 polyunsaturated fatty acid–derived eicosanoids. Eicosanoids generated from long‐chain omega‐6 polyunsaturated fatty acids, like arachidonic acid, are considered mainly proinflammatory. By contrast, eicosanoids generated from long‐chain omega‐3 polyunsaturated fatty acids are less potent inducers of inflammation, blood vessel constriction, and coagulation and are, therefore, considered as anti‐inflammatory. Arachidonic acid, for example, serves as precursor for 2‐series prostaglandins like prostaglandin E2 and 4‐series leukotrienes like leukotriene B4 via the cyclooxygenase and lipoxygenase pathways, respectively. Both eicosanoids show a high inflammatory potential, which increases production of interleukin‐6 and enhances vascular permeability and vasodilatation. Leukotriene B4 recruits neutrophils to areas of tissue damage, increases the production of interleukin‐1 and tumor necrosis factor alpha, and induces the release of reactive oxygen species from leukocytes. 5 , 65 , 66 , 67 However, it would be an oversimplification to describe all arachidonic acid–derived eicosanoids as proinflammatory. Although arachidonic acid–derived prostaglandins induce inflammation they also inhibit proinflammatory leukotrienes and cytokines and induce anti‐inflammatory lipoxins. This pathway modulates the intensity and duration of the inflammatory response via negative feedback. 55 , 68 , 69 The long‐chain omega‐3 polyunsaturated fatty acid eicosapentaenoic acid serves as a precursor for 3‐series prostaglandins such as prostaglandin E3 and 5‐series leukotrienes like leukotriene B5 (eicosanoids with a low proinflammatory potential). Their expression results in decreased vascular permeability and vasodilatation as well as reduced immune cell recruitment. 5 , 63 , 64 , 70 , 71 , 72
Dietary supplements rich in omega‐3 polyunsaturated fatty acids have been shown to reduce the concentration of 2‐series prostaglandins and increase the synthesis of 3‐series prostaglandins, which are suggested to be less inflammatory (Figure 3). Bagga et al 75 compared the effects of prostaglandin E2 and prostaglandin E3 on cell proliferation and the expression and transcriptional regulation of the cyclooxygenase‐2 in NIH 3T3 fibroblasts, as well as the production of interleukin‐6 in RAW 264.7 macrophages. Their study revealed that prostaglandin 3, unlike prostaglandin E2, is not mitogenic to NIH 3T3 fibroblasts; and although both induce cyclooxygenase‐2 messenger ribonucleic acid (mRNA) via a similar signaling mechanism, prostaglandin E3 is significantly less efficient in inducing cyclooxygenase‐2 gene expression. Furthermore, prostaglandin E3 induced interleukin‐6 synthesis in RAW 264.7 macrophages significantly less than prostaglandin E2 did. They also showed that increasing the omega‐3 content of membrane phospholipid results in a decrease in mitogen‐induced prostaglandin E2 synthesis. These results indicate that replacement of omega‐6 polyunsaturated fatty acids with omega‐3 polyunsaturated fatty acids in cell membranes can result in a decreased cellular response to mitogenic and inflammatory stimuli. 75 Furthermore, it has been assumed that, owing to the position of their unsaturations, omega‐3 polyunsaturated fatty acids are less susceptible to oxidative damage than omega‐6 polyunsaturated fatty acids are and that long‐chain polyunsaturated fatty acids of the omega‐3 series might also indirectly act as antioxidants in vascular endothelial cells, thus decreasing inflammation. 73 , 76
FIGURE 3.
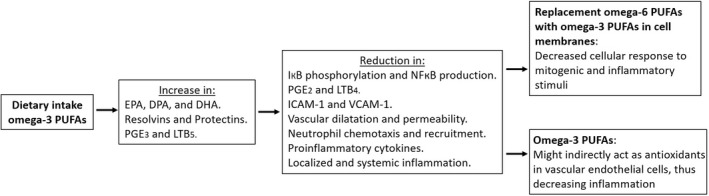
Effect of dietary intake of omega‐3 polyunsaturated fatty acids (PUFAs) on inflammatory mediators. DHA, docosahexaenoic acid; DPA, docosapentaenoic acid; EPA, eicosapentaenoic acid; ICAM‐1, intercellular adhesion molecule 1; IκB, inhibitor of kappa B; LTB4, leukotriene B4; LTB5, leukotriene B5; NFκB, nuclear factor kappa‐light‐chain‐enhancer of activated B cells; PGE2, prostaglandin E2; PGE3, prostaglandin E3; VCAM‐1, vascular cell adhesion molecule 1 12 , 73 , 74
Additionally, more recent studies revealed that long‐chain omega‐3 polyunsaturated fatty acids also serve as substrates for enzymatic conversion to a novel series of bioactive lipid mediators with anti‐inflammatory, inflammation‐resolving, and protective capabilities. 77 , 78 , 79 , 80 It is now widely recognized that resolution of inflammatory responses is an active and not, as previously considered, a passive process. Increasing evidence shows that the resolution phase is a biosynthetically active process that is governed by a superfamily of specialized pro‐resolving mediators. These potent bioactive molecules are biosynthesized from essential polyunsaturated fatty acid precursors (eg, eicosapentaenoic acid, docosahexaenoic acid, and docosapentaenoic acid) and were named resolvins, protectins, their aspirin‐triggered isomers, and more recently maresins and cysteinyl‐conjugated specialized pro‐resolving mediators. 81
Both eicosapentaenoic acid and docosahexaenoic acid can be metabolized via pathways involving cyclooxygenase and lipoxygenase and converted to E‐series resolvins, given their eicosapentaenoic acid precursor, and D‐series resolvins, biosynthesized from the precursor docosahexaenoic acid. 78 , 79 , 80 , 82 , 83 , 84 , 85 , 86 Resolvin E1 was the first discovered specialized pro‐resolving mediator derived from eicosapentaenoic acid, identified during the resolution phase of acute inflammation. The E‐series resolvins display potent anti‐inflammatory and immunoregulatory properties, control acute and chronic inflammation, neurologic disorders, and cancer, as well as stimulate tissue repair. The D‐series resolvins have been shown, for example, to stimulate macrophage phagocytosis of microbes, prevent central and peripheral inflammation and neuronal dysfunction, promote keratinocyte repair, enhance tissue regeneration and healing, reduce thrombus burden, and seem to be involved in pain regulation. Protectin D1/neuroprotectin D1 is biosynthesized from docosahexaenoic acid and has demonstrated neuroprotective actions in the brain, retina, and central nervous system. The aspirin‐triggered epimer has been shown to control polymorphonuclear neutrophils, enhance macrophage functions, and attenuate experimental stroke 18 , 79 , 80 , 81
Both resolvins E1 and D1 and protectin D1 have demonstrated activity as regulators, inhibiting the migration of neutrophils from capillaries and also limiting neutrophil infiltration into inflamed tissue, thus supporting the resolution of inflammatory processes. Furthermore, they seem to inhibit the production of tumor necrosis factor alpha and interleukin‐1β, and there are reports about an additive effect of protectin D1 when acting in concert with resolvin E1. 31 , 85 , 86 , 87 , 88 , 89
There are also reports about in vivo and in vitro synthesis of docosapentaenoic acid–derived specialized pro‐resolving mediators with potent anti‐inflammatory and tissue‐protective properties. 18 , 90 , 91
Resolvins and protectins are also generated in their respective epimeric forms when aspirin (acetylsalicylic acid) is given in mammalian systems. In the presence of aspirin, eicosapentaenoic acid and docosahexaenoic acid undergo transcellular metabolism in human cells to release various anti‐inflammatory, pro‐resolution, lipid mediators. These novel epimers are described as aspirin‐triggered resolvins and protectins. Both have demonstrated an inhibitory effect on polymorphonuclear granulocytes and the capability to prevent inflammation. Furthermore, macrophages are induced to have improved phagocytosis of bacteria and apoptotic neutrophils. Aspirin modifies the activity and specificity of cyclooxygenase‐2 and seems to be critical to the enhanced activity of the stereoisomers (18(R)‑ vs 18(S)‐resolvins). 92 , 93 , 94 , 95 , 96 , 97
In contrast to the previous understanding that resolution of inflammation was a passive process, it has become clear in recent years that it actually constitutes an active process that involves the switching on of specific pro‐resolution circuits. It is suggested that the combined use of aspirin and long‐chain omega‐3 polyunsaturated fatty acids may have the potential to turn on these specific pro‐resolution circuits and to modify the host response to chronic inflammation.
The number of studies investigating possible health benefits due to the intake of fish oil or omega‐3 polyunsaturated fatty acid supplements has increased significantly during the last decade. Possible beneficial effects of an adjunct use of fish oil or omega‐3 polyunsaturated fatty acids have been investigated for visual and neurologic development, 98 gestation and pregnancy, 99 , 100 , 101 , 102 , 103 cardiovascular disease or coronary heart disease and atherosclerosis, 104 , 105 , 106 , 107 , 108 , 109 Alzheimer disease or dementia, 102 , 110 , 111 diabetes, 112 , 113 , 114 , 115 rheumatoid arthritis, 116 , 117 Crohn disease or ulcerative colitis, 118 , 119 , 120 , 121 asthma, 122 , 123 , 124 , 125 immunoglobulin A nephropathy, 126 neuropsychiatric disorders like depression, bipolar disorder, or schizophrenia, 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 and also cancer. 137 , 138 , 139
Based on the improved understanding of function and beneficial effects of fish oils or omega‐3 polyunsaturated fatty acid supplements in the numerous systemic diseases mentioned earlier herein, attention has been drawn to the investigation of possible beneficial effects on oral tissues or diseases. The anti‐inflammatory effects, in particular, indicate their possible use as an adjunct in prevention and treatment of periodontal disease. Periodontitis is a dysbiotic inflammatory disease and the result of a host immuno‐inflammatory response to periodontopathic bacteria. The destruction of periodontal tissues is characterized by an inflammatory neutrophil‐mediated tissue injury followed by chronic infiltration of monocytes and the establishment of an acquired immune lesion. Although initiated by periodontopathic bacteria, investigations of the pathogenetic mechanisms associated with periodontal diseases have shown that the largest amount of periodontal tissue damage is not caused by bacteria directly but by the host response to infection. Important mediators in periodontal tissue destruction are prostaglandins and leukotrienes, produced from the metabolism of arachidonic acid. Attention has been focused on the role of prostaglandin E2, which, in addition to its proinflammatory action by stimulating various proinflammatory cytokines, also participates in the destruction of alveolar bone and periodontal connective tissue by activating osteoclasts and increasing the synthesis of matrix metalloproteinase‐1. 82 , 140 , 141 , 142 , 143 , 144 , 145 Therefore, it is not surprising that the number of studies investigating the effect of omega‐3 polyunsaturated fatty acids on prevention and treatment of periodontal diseases has increased significantly over the last decade.
Alam et al 146 showed in an early study in rats that animals fed with a diet rich in omega‐3 polyunsaturated fatty acids exhibit reduced levels of arachidonic acid, prostaglandin 2, and leukotriene B4 in gingival tissue. Campan et al 48 investigated the effect of omega‐3 polyunsaturated fatty acids in the treatment of human experimental gingivitis. Test patients received a fish oil supplement (30% omega‐3 polyunsaturated fatty acids, 18% eicosapentaenoic acid, and 12% docosahexaenoic acid) and control patients received a placebo containing olive oil (1% omega‐3 polyunsaturated fatty acids). The levels of arachidonic acid, prostaglandin E2, and leukotriene B4 were decreased in the experimental fish oil group and increased in the olive oil control group, but with no significant difference. Clinically, there was a significant reduction of the gingival index in the test group but no significant difference between the groups. Eberhard et al 147 investigated the effect of a topical application of omega‐3 or omega‐6 polyunsaturated fatty acids in a human experimental gingivitis model. The subjects were randomly assigned to two groups using a mouthrinse enriched in omega‐3 polyunsaturated fatty acids or an omega‐6 soya oil solution. However, bleeding on probing, gingival crevicular fluid, and leukotriene B4 levels were significantly increased in all groups with no differences between the control and experimental side.
A study by Bendyk et al 82 investigated the effect of omega‐3 polyunsaturated fatty acids on experimental periodontitis in mice. Adult mice were fed experimental diets containing either 10% tuna oil or Sunola, a cold‐pressed sunflower oil, for 57 days. After 14 days, the mice were inoculated orally with Porphyromonas gingivalis alone or a mixture of P. gingivalis and Fusobacterium nucleatum suspended in carboxymethyl cellulose, with carboxymethyl cellulose alone or remained untreated. At the end of the observation period the mice were killed, soft‐tissue biopsies of the oral cavity used for measurement of the polyunsaturated fatty acid concentrations, and the maxilla removed, stained, and digitally imaged to assess the bone loss around the upper molars. After 57 days, there were marked differences in the polyunsaturated fatty acid contents of the intra‐oral soft tissues of mice fed with the tuna oil–enriched diet compared with those fed with Sunola oil. The oral tissues of the tuna oil fed mice showed 10‐fold increased eicosapentaenoic acid levels and twofold increased docosahexaenoic acid levels, whereas the levels of omega‐6 polyunsaturated fatty acids were halved. Furthermore, tuna oil–fed mice inoculated with P. gingivalis alone or the combination of P. gingivalis and F. nucleatum showed 72% and 54% less alveolar bone loss, respectively, compared with the treatment control group. This indicates that (a) the omega‐3 polyunsaturated fatty acid concentrations in the oral soft tissues can be modified or influenced by diet and (b) dietary supplementation with omega‐3 polyunsaturated fatty acids, and particularly docosahexaenoic acid–rich tuna oil, significantly reduces alveolar bone loss in a murine periodontitis model. Similar observations were reported in a study by Kesavalu et al, 94 where rats were fed with fish oil or corn oil and infected with P. gingivalis. The corn oil diet contained 60% omega‐6 linoleic acid and the fish oil diet 24.6% omega‐3 polyunsaturated fatty acids (eicosapentaenoic acid and docosahexaenoic acid). Rats fed with the fish oil exhibited elevated serum levels of eicosapentaenoic acid and docosahexaenoic acid, indicating the impact of a diet rich in omega‐3 polyunsaturated fatty acids on the serum fatty acid profile of rats. Furthermore, the rats treated with the fish oil diet showed significantly less alveolar bone resorption. In contrast to the studies mentioned earlier herein, a study by Vardar‐Senguel et al 146 could not find any evidence that omega‐3 polyunsaturated fatty acid administration was effective in preventing lipopolysaccharide‐induced alveolar bone loss in rats. Experimental periodontitis in this study was induced by repeated injections of Escherichia coli lipopolysaccharide. Two different groups with daily omega‐3 polyunsaturated fatty acid supplementation (40 mg/kg, 60% eicosapentaenoic acid and 40% docosahexaenoic acid), orally gavaged, were used: One group received the omega‐3 polyunsaturated fatty acid supplementation subsequent to disease induction for 14 days, and the other group received the omega‐3 polyunsaturated fatty acid supplementation already 14 days prior to the commencement of lipopolysaccharide injections and was then continued for another 14 days. Both omega‐3 polyunsaturated fatty acid groups showed no reduction in lipopolysaccharide‐induced alveolar bone loss compared with the lipopolysaccharide control group, significantly higher interleukin‐1β and osteocalcin levels, and no effect on serum C‐reactive protein level. The authors state that the lack of a therapeutic effect of omega‐3 polyunsaturated fatty acid supplementation in their study is difficult to explain. The observed lack might be partially explained by the short periods and lower dosage of omega‐3 polyunsaturated fatty acid supplementation compared with the previously mentioned studies.
A randomized, double‐blind, placebo‐controlled study on human patients with moderate and severe chronic periodontitis investigated the effect of omega‐3 polyunsaturated fatty acid supplementation as an adjunct to scaling and root planing. 148 The test group received 300 mg of omega‐3 polyunsaturated fatty acids (180 mg eicosapentaenoic acid and 120 mg docosahexaenoic acid) orally as one capsule once daily for 12 weeks. The control group received a placebo capsule containing 300 mg of liquid paraffin orally once daily for 12 weeks. At the end of the 12 week period there was as significant reduction in gingival index, sulcus bleeding index, probing pocket depth and clinical attachment level in the test group compared to the control group. However, no statistically significant differences in serum C‐reactive protein levels were found.
A recent randomized clinical trial of Stando et al 149 evaluated the effect of dietary supplementation with omega‐3 polyunsaturated fatty acids in 30, otherwise healthy, patients with stage III and IV periodontitis. In the control group (n = 14), patients were treated with scaling and root planing only. In the test group (n = 16), patients were, in addition to scaling and root planing, supplemented with a daily dose of 2.6 g of eicosapentaenoic acid and 1.8 g of docosahexaenoic acid for 3 months. Periodontal examination 3 months following initial therapy showed a statistically significant reduction of bleeding on probing and improvement of clinical attachment level in the test group compared with the control group. Furthermore, there was a statistically significant higher percentage of closed pockets (probing pocket depth 4 mm or less without bleeding on probing) in the test group. The levels of proinflammatory cytokines/chemokines interleukin‐8 and interleukin‐17 were markedly lower in saliva samples collected from the test group compared with those from the control group at 3 months, whereas the level of anti‐inflammatory interleukin‐10 was significantly higher in the saliva samples of the omega‐3 polyunsaturated fatty acid–supplemented test group. 149
The beneficial effects of a supplementation with omega‐3 polyunsaturated fatty acids in nonsurgical treatment of periodontitis is also supported by two recently published meta‐analyses. Kruse et al 150 concluded in their systematic review and meta‐analysis that omega‐3 polyunsaturated fatty acids seem to have a positive effect on periodontal wound healing or the periodontal parameters clinical attachment level and probing pocket depth. Therefore, patients receiving periodontal treatment might benefit from nutritional counselling.
Similar to that, the meta‐analysis of Heo et al 151 suggests that supplemental or dietary intake of omega‐3 polyunsaturated fatty acids for the treatment of periodontitis may have a positive impact on the disease.
El‐Sharkawy et al 152 investigated the effect of an adjunctive treatment of chronic periodontitis patients with a combination of omega‐3 polyunsaturated fatty acids and low‐dose aspirin. The control group was treated with scaling and root planing and a placebo; the test group received scaling and root planing and dietary supplementation with 3 g fish oil (900 mg eicosapentaenoic acid/docosahexaenoic acid) and 81 mg aspirin daily. At baseline and at 3 and 6 months postbaseline, saliva samples were collected and clinical measurements recorded. Clinical measurements included plaque index, modified gingival index as well as bleeding on probing, probing pocket depth, and clinical attachment level. The unstimulated saliva samples were obtained in the morning after an overnight fast and analyzed for receptor activator of nuclear factor‐κB ligand (RANKL) and matrix metalloproteinase‐8. RANKL promotes osteoclast formation, and matrix metalloproteinase‐8 is a key player in degradation of extracellular collagen matrix and derives mainly from polymorphonuclear neutrophils during acute stages of periodontitis. 153 , 154 , 155 , 156 There were no statistically significant differences between test and control groups at different time intervals regarding plaque index and gingival index. However, there was a significantly greater reduction in probing pocket depth and gain in clinical attachment level in the test group compared with the control group at 3 months and at 6 months postbaseline. Further data analyses revealed that at 6 months the percentage of pockets with probing pocket depth <4 mm was 54.7% in the control group vs 79.5% in the test group, suggesting that 25% fewer sites required further intervention in the omega‐3 plus aspirin group. The biochemical saliva analyses showed similar outcomes. There was a statistically significant reduction in RANKL concentrations at 3 and 6 months in the omega‐3 plus aspirin group. The matrix metalloproteinase‐8 levels at 3 months were lower in the test group but not statistically significant. However, the matrix metalloproteinase‐8 level at 6 months was statistically significantly lower in the omega‐3 plus aspirin group compared with the control group.
The significant clinical and biochemical improvements in the test group are imputed to the anti‐inflammatory impact of the omega‐3 polyunsaturated fatty acids, which is further enhanced by the combination with aspirin. Resolution of inflammation is mediated by the metabolism of arachidonic acid by lipoxygenase transformation circuits leading to the production of lipoxins, endogenous anti‐inflammatory and proresolution lipid mediators. These endogenous resolution pathways are enhanced by the action of aspirin. It acetylates cyclooxygenase‐2, transforming the enzyme into an active 15(R)‐lipoxygenase, the product of which, 15(R)‐hydroxyeicosatetraenoic acid, is a substrate for conversion to a 15(R)‑ or 15‐epilipoxin, which exhibits greater activity than the native lipoxin due to its extended half‐life. Eicosapentaenoic acid and docosahexaenoic acid are metabolized into resolvins of the E and D series by the same enzyme system. These are also enhanced by aspirin transformation circuits. 5 , 152 , 154 , 157 , 158 The circulating levels of resolvins have been shown to increase after increased intake of omega‐3 polyunsaturated fatty acids. 80 , 92 , 159
The reduction of the two biomarkers, RANKL and matrix metalloproteinase‐8, investigated in this study by El‐Sharkawy et al 152 correlates with the clinical observations. The impact of omega‐3 polyunsaturated fatty acids on these biomarkers is assumed to be also mediated via resolvins. The suggested mechanisms of this impact include the reduction of upstream proinflammatory cytokines directing neutrophils to apoptosis and nonphlogistic recruitment of monocytes. Studies have indicated the inhibition of interleukin‐1β and tumor necrosis factor alpha and the reduction of the infiltration of neutrophils into inflamed tissues by resolvins. 61 , 78 , 85 , 86 , 88 , 89
Elwakeel et al 160 investigated the combination of omega‐3 polyunsaturated fatty acids and low‐dose aspirin as adjunct to nonsurgical periodontal therapy in chronic periodontitis patients with type 2 diabetes. The test group, following scaling and root planing, received dietary supplementation with omega‐3 polyunsaturated fatty acids (1 g, three times daily) plus aspirin (75 mg, once daily) for 6 months. The control group, following scaling and root planing, received placebo pills for 6 months. At baseline and at 3 and 6 months after treatment, clinical measurements (plaque index, gingival index, probing pocket depth, and clinical attachment level) were recorded and gingival crevicluar fluid samples were collected and later analyzed for interleukin‐1β and monocyte chemoattractant protein‐3. Elevated levels of interleukin‐1β are associated with numerous inflammatory disorders, including periodontitis. A significant reduction of the interleukin‐1β level indicates resolution of inflammation. 161 Monocyte chemoattractant protein‐3 is a chemotactic cytokine that is highly expressed in chronic inflammatory disorders. It has been detected in high levels in the gingival crevicular fluid of patients with chronic periodontitis and particularly in progressive periodontal lesions. Monocyte chemoattractant protein‐3 expression has been shown to be induced by proinflammatory stimuli like interleukin‐1β or tumor necrosis factor alpha, and it was mainly expressed in inflammatory leukocytes and vascular endothelium, indicating a potential role of monocyte chemoattractant protein‐3 in the recruitment of leukocytes to diseased gingival tissues. 160 , 162
Furthermore, Elwakeel et al 160 investigated the impact of the treatment in each group on the glycemic control by measurement of the glycated hemoglobin A1c in fasting venous blood samples. Statistical analyses revealed a significant reduction in probing pocket depth and gain in clinical attachment level at 3 and 6 months in the omega‐3 plus aspirin test group compared with control. The hemoglobin A1c levels showed a reduction in both groups with no significant difference. However, the test group showed a significant reduction in levels of interleukin‐1β and monocyte chemoattractant protein‐3 at 3 and 6 months compared with the placebo control.
The results of Elwakeel et al 160 corroborate the findings of El‐Sharkawy et al 152 . They also addressed the positive findings of the potent anti‐inflammatory and immune‐modulating effects of resolvins and docosatrienes. These were produced as result of the supplementation with omega‐3 polyunsaturated fatty acids plus aspirin resulting in inhibition of superoxide production, chemotaxis and migration of polymorphonuclear neutrophils, and reduction of the production of proinflammatory enzymes and cytokines. 77 , 145 , 163 , 164
Elkhouli 165 investigated the effect of the combination of omega‐3 polyunsaturated fatty acids plus aspirin on regeneration of single grade II furcation defects. Patients with at least a single grade II furcation were randomly allocated into two groups. Patients in the test group were treated with decalcified freeze‐dried bone allograft and received the combination of omega‐3 polyunsaturated fatty acids (1 g, three times daily) plus low‐dose aspirin (75 mg, once daily) for 6 months. Each omega‐3 capsule provided 300 mg docosahexaenoic acid and 150 mg eicosapentaenoic acid. Patients in the control group received the same regenerative therapy and placebo pills. At baseline and at 3 and 6 months, clinical parameters were recorded and gingival crevicluar fluid was collected and assessed for the biochemical markers interleukin‐1β and interleukin‐10. Opposed to interleukin‐1β, interleukin‐10 is an anti‐inflammatory cytokine with immunoregulatory functions, including suppression of interleukin‐1 receptor antagonist. At the end of the observation period, there was a statistically significant greater reduction in probing pocket depth and gain in clinical attachment level in the test group compared with the control group. Whereas there was also a significantly greater reduction of the mean interleukin‐1β concentrations in the test group, no significant differences between the groups were observed in mean interleukin‐10 concentrations.
In their recent randomized clinical trial, Castro dos Santos et al 166 investigated the clinical and immunological effects of orally administered omega‐3 polyunsaturated fatty acids in combination with low‐dose aspirin as adjunct to scaling and root planing for the treatment of periodontitis in patients with type 2 diabetes. The three groups investigated (n = 25 in each group) were (a) scaling and root planing plus placebo (control), (b) 3 g fish oil per day plus 100 mg aspirin per day for 2 months after scaling and root planing (test 1), and (c) 3 g fish oil per day us 100 mg aspirin per day for 2 months before scaling and root planing (test 2). Conventional periodontal parameters and gingival crevicular fluid were collected till 6 months after scaling and root planing, and gingival crevicular fluid was analyzed for cytokine levels. Ten patients (40%) in test 1 and nine patients (36%) in test 2 achieved the determined clinical endpoint for treatment (up to four sites with probing pocket depth of at least 5 mm). This was only achieved in four patients (16%) in the control group. The test 1 group also showed clinical attachment level gain in moderate and deep pockets. The levels of interferon‐gamma and interleukin‐8 decreased over time for both test groups, whereas the interleukin‐6 and hemoglobin A1c levels were lower only in the test 1 group. The authors of this study concluded that the adjunctive use of the omega‐3 polyunsaturated fatty acids and low‐dose aspirin combination, administered after periodontal debridement, provides clinical and immunological benefits to the treatment of periodontitis in patients with type 2 diabetes. 166
Vardar‐Sengul et al 167 investigated the combination of omega‐3 polyunsaturated fatty acids (40 mg/kg; 60% eicosapentaenoic acid and 40% docosahexaenoic acid) and a selective cyclooxygenase‐2 inhibitor (Celecoxib) in an experimental periodontitis model in rats. Their results indicated that the cyclooxygenase‐2 inhibitor, prophylactic omega‐3 polyunsaturated fatty acids, and the combination of both can inhibit pathologically excessive gingival tissue matrix metalloproteinase‐8 expression. The administration of therapeutic omega‐3 polyunsaturated fatty acids alone resulted in a significant increase in tissue inhibitor of matrix metalloproteinase‐1 expression in gingiva. The study showed that an adjunctive medication of omega‐3 polyunsaturated fatty acids in periodontal treatment might be beneficial because of its inhibitory effect of matrix metalloproteinase‐8 and increasing effect on tissue inhibitor of matrix metalloproteinase‐1. The prophylactic administration of omega‐3 polyunsaturated fatty acids for 2 weeks seems to provide a maximum increase in eicosapentaenoic acid and docosahexaenoic acid levels in the cell membrane, eventually maintaining membrane stability and fluidity in the physiologic state and making the cell membrane more resistant to bacterial and viral attacks. However, these finding need to be verified in clinical human studies.
In addition to the reported anti‐inflammatory or immune modulatory effects, omega‐3 polyunsaturated fatty acids such as alpha‐linolenic acid or its long‐chain derivatives eicosapentaenoic acid and docosahexaenoic acid also seem to exhibit indirect and direct effects on bone metabolism. Studies in humans report that long‐chain omega‐3 polyunsaturated fatty acids can increase bone formation, can affect peak bone mass in adolescents, and can reduce bone loss. 23 , 168 The cellular mechanisms induced by omega‐3 polyunsaturated fatty acids in bone metabolism are complex and, although not fully unveiled and understood yet, involve modulation of fatty acid metabolites such as prostaglandins, resolvins and protectins, cytokines, growth factors, and some other molecular signaling pathways.
Omega‐6 fatty acids and particularly arachidonic acid are the primary source of omega‐6 eicosanoids, produced from oxygenation of arachidonic acid by cyclooxygenase, lipoxygenase, and epoxygenase enzymes to produce prostaglandins, leukotrienes, lipoxins, and cytochrome p450 monooxygenase compounds. The long‐chain omega‐3 polyunsaturated fatty acids eicosapentaenoic acid and docosahexaenoic acid are able to replace omega‐6 fatty acids, and particularly arachidonic acid, in the membranes of neutrophils, monocytes, platelets, erythrocytes, and liver cells. This results in a change of the omega‐6/omega‐3 ratio in their membranes and subsequently in a change of their cell function, which can decrease interleukin‐1, interleukin‐6, and tumor necrosis factor alpha production, inflammatory cytokines stimulating osteoclastic bone resorption.
This modulatory effect of omega‐3 polyunsaturated fatty acids on cytokines may play an important role in bone metabolism, during bone growth or bone healing, and in the pathogenesis of diseases with a disturbed bone metabolism, such as osteoporosis. 23
Furthermore, omega‐3 polyunsaturated fatty acids can also regulate bone metabolism by decreasing the release of prostaglandin E2 and RANKL (the most important osteoclast differentiation factor) and by increasing the release of insulin‐like growth factor 1 and increasing calcium absorption and accretion in bone. 23 , 168 This way, omega‐3 polyunsaturated fatty acids may have an impact on bone metabolism by inhibiting bone resorption and preventing bone loss due to suppression or down regulation of these inflammatory cytokines and factors. 23 , 168 , 169 However, studies also suggest a stimulatory effect of omega‐3 polyunsaturated fatty acids on osteoblastic activity. Animal studies have shown that animals fed with long‐chain omega‐3 polyunsaturated fatty acids tend to show an increased rate of bone formation. Furthermore, ovariectomized rats supplemented with eicosapentaenoic acid experienced a reduced bone mineral loss. 22 , 23 Additionally, long‐chain polyunsaturated fatty acids may also be involved in bone remodeling and, in the bone marrow, in the differentiation of mesenchymal stem cells into adipocytes or osteoblasts. Derivatives of the omega‐3 and ‑6 polyunsaturated fatty acids will, depending on the existing omega‐6/omega‐3 ratio, induce the differentiation of mesenchymal stem cell precursors into adipocytes or osteoblasts. 68 , 69 , 170 More recent studies have indicated that the previously recommended omega‐6/omega‐3 ratio associated with health of 1:1 is actually much higher and between 15:1 and 16.7:1. 171 It is suggested, that polyunsaturated fatty acids also act on bone formation because metabolic products of omega‐6 and omega‐3 polyunsaturated fatty acids act directly on precursor cells of osteoblasts and adipocytes. The anti‐inflammatory effect of omega‐3 polyunsaturated fatty acids can lower the osteoclastic activity and thus reduce bone resorption. A diet rich in omega‐6 polyunsaturated fatty acids, which raises the omega‐6 to omega‐3 ratio, seems to increase the adiposity of the bone marrow by enhancing the adipogenic differentiation of mesenchymal stem cells, inhibiting their differentiation into osteoblasts. 23 , 68 , 171 A diet with a healthy proportion of omega‐6 to omega‐3 seems to avoid pathologies in bone health associated with aging, because omega‐3 polyunsaturated fatty acids do not exert as strong an adipogenesis induction capacity as that of omega‐6 polyunsaturated fatty acids, thus allowing osteoblastogenesis. This effect on mesenchymal stem cells favoring or promoting osteoblastogenesis, together with the inhibitory effect on osteoclastogenesis, may indicate a beneficial effect of omega‐3 polyunsaturated fatty acid supplementation regarding maintenance of bone mineral mass. 23 , 171 Therefore, the effect of omega‐3 polyunsaturated fatty acids on bone metabolism might be a combination of reducing bone resorption and increasing bone formation.
Longo and Ward 172 compared the bone sparing or bone protective effect of flaxseed oil (a source of alpha‐linolenic acid) and menhaden oil (a source of eicosapentaenoic acid and docosahexaenoic acid) in ovariectomized rats. Interestingly, their results suggest that alpha‐linolenic acid from flaxseed oil, but not eicosapentaenoic acid and docosahexaenoic acid from menhaden oil, may protect against ovariectomy induced bone loss. Other studies on growing or ovariectomized rats have indicated beneficial effects to the long bones of the skeleton and/or the lumbar spine with supplementation of either purified docosahexaenoic acid or fish oils with a high docosahexaenoic acid content and detrimental effects with purified eicosapentaenoic acid supplementation or fish oils with a high eicosapentaenoic acid content, although the mechanisms behind these different effects are unclear. 172
Altogether, results of multiple studies suggest that bone is responsive to supplementation with omega‐3 polyunsaturated fatty acids during periods of rapid growth or following hormone‐induced bone loss. However, different types of omega‐3 polyunsaturated fatty acids may have a different impact on bone metabolism. Docosahexaenoic acid, for example, seems to be more effective in inhibiting osteoclast differentiation and decreasing osteoclast activation and bone resorption than eicosapentaenoic acid by alleviating RANKL‐induced proinflammatory cytokine production and intracellular signaling activation. 173
Besides the effect discussed on bone metabolism, supplementation of omega‐3 polyunsaturated fatty acids ranging from 3 to 6 g/day also showed a moderate but consistent beneficial effect in joint disease. Similar to bone, synthesis of the proinflammatory mediators interleukin‐1, interleukin‐6, and tumor necrosis factor alpha was suppressed in cartilage tissue after supplementation with fish oil containing eicosapentaenoic acid and docosahexaenoic acid. 19 , 22
Another reason for the various beneficial effects of long‐chain omega‐3 polyunsaturated fatty acids might be their effect on fat metabolism. Long‐chain omega‐3 polyunsaturated fatty acids seem to be able to influence lipid metabolism in a way that they promote lipolysis, enhance hepatic fatty acid oxidation, and inhibit fatty acid synthesis and very low density lipoprotein secretion. Docosahexaenoic acid in particular seems to act as a key controller of hepatic lipid synthesis and is involved in the suppression of lipogenesis. 174 Human studies have indicated that an intake of 0.3‐3.0 g of long‐chain omega‐3 polyunsaturated fatty acids per day can reduce body weight and body fat in overweight and obese people. The underlying mechanism for the improvement of body composition by long‐chain omega‐3 polyunsaturated fatty acids is supposed to be altered gene expression favoring increased fat oxidation in adipose and skeletal muscle tissue and reduced fat deposition in adipose tissue, as well as by indirectly assisting with body fat reduction by increasing metabolic rate. An increase in vasodilator function and muscle blood flow during exercise as a result of long‐chain omega‐3 polyunsaturated fatty acid supplementation may also promote nutrient disposal by skeletal muscles and in that way reduce the availability of these nutrients for lipogenesis and storage in adipose tissues.
In older adults, omega‐3 polyunsaturated fatty acid supplementation has been shown to increase postprandial muscle protein synthesis, muscle mass, and functional capacity. 24
There is also a suggested role of long‐chain omega‐3 polyunsaturated fatty acids in appetite regulation in humans.
Furthermore, since one‐third of total circulating interleukin‐6 levels are expressed predominantly by adipocytes, reduction in fat mass could contribute to a reduction of interleukin‐6 levels, a central player in the regulation of inflammation and capable of inducing insulin resistance. 2 , 23 , 175
Altogether, there is growing evidence that supplementation with omega‐3 polyunsaturated fatty acids may have various beneficial effects in humans. Some of them affect areas that not only improve systemic health and general wellbeing but might also have a conceivable impact on periodontal health or periodontal treatment, such as immune response or immune modulation, oxidative stress, bone metabolism or resorption, joint health, and fat metabolism, as well as body weight and body composition. Nevertheless, more clinical trials are necessary to address specific questions for more detailed recommendations. The present data indicate that there are gender differences in the metabolism of long‐chain omega‐3 polyunsaturated fatty acids. Studies showed significantly higher levels of docosahexaenoic acid and lower levels of eicosapentaenoic acid circulating in serum lipids in females than in males. Females also seem to be more responsive to the metabolism of long‐chain omega‐3 polyunsaturated fatty acids and have a higher percentage of total fatty acids as docosahexaenoic acid in plasma and adipose lipids. 176 , 177 Factors like gender, age, duration and optimal dosage of supplementation, concentration and optimal eicosapentaenoic acid/docosahexaenoic acid ratio, and the best sources for omega‐3 polyunsaturated fatty acids require further consideration and need to be addressed in future clinical studies. Furthermore, caution should be used when conclusions are made regarding the effects of different omega‐3 polyunsaturated fatty acids in humans based on animal studies because of possible differences in pharmacokinetics of eicosapentaenoic acid, docosahexaenoic acid, and docosapentaenoic acid supplementation between humans and animals and because dosages used in animal studies vary significantly and are typically higher than those considered safe in humans. 177 , 178
Furthermore, possible adverse effects reported in association with different omega‐3 polyunsaturated fatty acid supplementations, although usually minor and scarce, need to be considered. Flaxseed oil supplementation, for example, is usually well tolerated, but high doses may cause loose stools or diarrhea, and there are also reports of allergic and anaphylactic reactions. Serious adverse reactions have not been reported with fish oil or eicosapentaenoic acid and docosahexaenoic acid supplements. The most common adverse effects seem to be fishy aftertaste, belching, and heartburn. Nausea and loose stools are also reported after high doses. Omega‐3 polyunsaturated fatty acids also have the potential to prolong bleeding times, which, on the other hand, possibly plays a role in its cardioprotective effects. Excessively prolonged bleeding times have been reported in Greenland Eskimos with very high eicosapentaenoic acid plus docosahexaenoic acid intakes of about 6.5 g/day. However, it is not clear if the high eicosapentaenoic acid/docosahexaenoic acid intake is alone responsible for the observed excessive bleeding time. According to the US Food and Drug Administration, long‐chain omega‐3 polyunsaturated fatty acid intakes (eicosapentaenoic acid and docosahexaenoic acid) of up to 3 g/day are unlikely to cause significant bleeding. However, caution is advised with eicosapentaenoic acid and docosahexaenoic acid supplementation in patients who are at risk of excessive bleeding or patients on anticoagulant medications. The coagulation status of those patients should be monitored regularly.
Furthermore, ex vivo studies have indicated immunosuppressive effects at doses as low as 0.9 g/day for eicosapentaenoic acid and 0.6 g/day for docosahexaenoic acid. Although these findings may not translate to impaired immune responses in vivo, it should be considered in patients with compromised immune systems. No serious adverse effects have been reported during pregnancy and lactation due to fish oil supplementation. 27 , 82 , 152 , 160 , 165
However, considering the already published indications of health benefits due to supplementation with fish oil or omega‐3 polyunsaturated fatty acids and the lack of really significant side or adverse effects, supplementation with fish oil or omega‐3 polyunsaturated fatty acids can be justified and might even exert positive effects on periodontal condition or periodontal health.
3. PROTEIN AND AMINO ACID SUPPLEMENTS
The most frequently used supplements in the fitness lifestyle are protein and amino acid supplements used to support muscle growth and muscle regeneration. Possible beneficial effects of protein and amino acid supplements have been extensively studied in orthopedic and sports medicine, and also in geriatric medicine, since proteins constitute an important structural and functional component of skeletal tissues. However, some of these so‐called muscle supplements might also have effects on periodontal tissues as well as on periodontal wound healing.
Alteration in protein turnover following tissue damage due to injury or extensive exercise is crucial to tissue repair. Increasing knowledge has indicated the need for increased protein intake during tissue repair based on its important roles supporting wound healing, maintaining tissue integrity, and promoting convalescence. An insufficient protein intake has been shown to delay wound healing and to reduce the integrity of the repaired tissue. 179 , 180 , 181
The most popular protein used in fitness and weightlifting sports is whey protein, available usually as concentrate, hydrolysate, or isolate. Whey is a milk derivative, and the whey for the protein supplement production is obtained as a by‐product when milk is coagulated during the process of cheese production. Milk contains all substrates required for infant growth and development. Thus, milk can be considered as a natural biological liquid esculent providing nutrition at a time of rapid body and particularly muscular‐skeletal growth.
The effect of dairy products or proteins on bone has been addressed in numerous studies. Most of the prospective and cross‐sectional studies support a positive relationship between protein intake and bone. Total protein intake and animal protein intake have been associated with higher bone mineral density and less bone mineral density loss over time. Conversely, a negative association between vegetable protein intake and bone mineral density was observed. The positive effects of dietary protein on bone mineral density may be due to increased levels of insulin‐like growth factor 1 and suppression of parathyroid hormone. However, the positive effect of dietary protein on bone mass seems to be most evident in those patients consuming adequate amounts of calcium (more than 1000 mg/day). High dietary protein and low calcium intake may lead to increased urinary calcium excretion and lower bone mass. 182 , 183 , 184 , 185 Studies on milk proteins have shown that fractions of whey protein possess growth‐stimulatory effects in primary cultures of osteoblasts. Further investigation of these whey protein fractions revealed that lactoferrin was a constituent in many of these fractions. 186 Lactoferrin is an 80 kDa iron‐binding protein of the transferrin family of proteins. It is present in higher concentrations in milk, particularly in colostrum, and widely distributed in body fluids, including tears and saliva. It is also present in the secretory granules of neutrophils, from which it is released during acute inflammation. In healthy people, lactoferrin serum levels are predominantly neutrophil derived and range between 2 and 7 μg/mL. However, local concentrations can increase during inflammation. 186 , 187 Studies of lactoferrin on human, rat, and mouse cell cultures of the osteoblast and osteoclast lineage and of bone marrow cultures showed that lactoferrin promotes osteoblast growth, inhibits osteoclastogenesis, and reduces osteoblast apoptosis. Interestingly, the effect of lactoferrin on proliferation and survival of osteoblasts was greater than that observed in response to established osteoblast growth factors, such as transforming growth factor beta, parathyroid hormone, insulin‐like growth factor 1, or insulin. Furthermore, lactoferrin reduced expression of RANKL in bone marrow cultures. In vivo experiments in adult mice showed a significant increase in new bone formation after administration of lactoferrin. The bone growth observed after local lactoferrin injection was significantly greater than the bone growth detected in response to factors such as insulin, C‐terminal parathyroid hormone–related peptide, or calcitonin in the same model and reached the magnitude of bone growth reported following local application of transforming growth factor beta. 186 , 188 , 189 , 190 , 191 , 192 , 193 , 194
Interestingly, besides its effects on bone metabolism, there are also reports of antimicrobial effects of lactoferrin, attributed to its action as an iron chelator, as well as of an immunomodulatory function. Lactoferrin has been shown to decrease the secretion of interleukin‐1β and tumor necrosis factor alpha and to stabilize mast cells. 195 , 196 , 197 , 198 , 199
A significant decrease in tumor necrosis factor alpha serum levels after administration of a high‐caloric protein‐rich oral supplement was also reported in a prospective randomized, double‐blind, placebo‐controlled study in patients with chronic heart failure and cachexia. 20 A more recent in vitro study using human umbilical vein endothelial cells, with or without tumor necrosis factor alpha stimulation, investigated the effect of several dairy protein compounds on inflammation. Whey protein, leucine, isoleucine, and valine normalized tumor necrosis factor alpha–induced proinflammatory gene expression in endothelial cells. This indicates that whey protein and its major amino acids, the so‐called branched‐chain amino acids, may have a protective role against inflammation in endothelial cells, supporting the preventive potential of dairy‐based functional foods for vascular health. 200
Studies in rats indicated that protein restriction or protein malnutrition reduces insulin‐like growth factor 1 levels in plasma and decreases translation, increases metabolic clearance rate, and lowers sensitivity to the anabolic effects of insulin‐like growth factor 1 in peripheral tissues. In a randomized, double‐blind, placebo‐controlled trial in elderly patients with recent hip fracture, oral protein supplementation was associated with increased serum levels of insulin‐like growth factor 1, a more favorable outcome, and a shorter stay in rehabilitation hospital. 201 , 202 , 203 This was also confirmed by a clinical trial in sarcopenic elderly patients where supplementation with whey protein, amino acids, and vitamin D increased serum insulin‐like growth factor 1 concentrations and lowered C‐reactive protein. 204 Furthermore, protein deficiency may also predispose patients to higher rates of infectious complications. 205
Protein hydrolysates have been proposed as a source of protein with beneficial characteristics. The preferred method of protein hydrolysis is enzymatic hydrolysis. As a result of the cleavage of the peptide bonds, the proteins are broken down into peptides of various sizes and free amino acids, mostly di‑ and tripeptides depending on the type of hydrolysis and the conditions under which it is performed. Compared with whole or intact proteins and free‐form amino acid mixtures, the consumption of protein hydrolysates resulted in a faster availability and uptake of amino acids. Some peptides also showed biological activity. Protein hydrolysates are also better tolerated by the gastrointestinal tract because they neutralize the acid and relieve the stomach due to the predigested nature of the hydrolysate. Altogether, these benefits have led to the incorporation of protein hydrolysates into clinical nutrition supplements for patients with digestion disorders, cancer, trauma, or burns. 180 , 206 , 207 Protein hydrolysates, and particularly casein and whey protein hydrolysates, have also been shown to promote postsurgical healing, to assist with the repair of tissue damage, and to promote a strong insulinotropic effect. This insulinotropic effect has been shown to be greater in whey protein hydrolysates than in whey protein, soy protein, or soy protein hydrolysates. It is deemed to be important since the anabolic hormone insulin reduces protein breakdown and enhances tissue uptake of branched‐chain amino acids. 208 , 209
The recommended protein intake for an average adult is suggested to be around 70 g/day. However, this can increase significantly in postsurgical patients to up to 300‐400 g/day, since surgical trauma results in an increase of whole‐body protein degradation, with the extent depending on the severity of the insult. High‐protein diets have been shown to accelerate tissue regeneration and increase tensile strength of the wound. 210 , 211 , 212 , 213 Protein hydrolysates have the potential to promote different types of tissue repair and might be useful in situations where excess protein is needed, such as tissue repair, regeneration, or wound healing. However, further well‐controlled human trials are required to confirm these findings and assess the clinical relevance in periodontal therapy.
The three proteinogenic branched‐chain amino acids leucine, isoleucine, and valine are hydrophobic essential amino acids. They account for about 20%‐25% of dietary proteins and for about 33% of essential amino acids in muscle proteins. Branched‐chain amino acids play an important role in protein synthesis, which explains the common use of branched‐chain amino acid supplements in the fitness scene. 179 , 214 In a recently published study, Lee et al 214 investigated the anti‐inflammatory and anti‐genotoxic activity of branched‐chain amino acids in lipopolysaccharide‐stimulated RAW 264.7 macrophages by measuring the production of nitric oxide, the expression of inducible nitric oxide synthase mRNA, the mRNA expression of interleukin‐6 and cyclooxygenase‐2, and by analyzing deoxyribonucleic acid (DNA) damage induced by hydrogen peroxide using the alkaline comet assay. Furthermore, they assessed the cytotoxicity of the branched‐chain amino acid concentrations used and showed that the concentrations used in their study did not affect cell viability of the RAW 264.7 macrophages. Of the branched‐chain amino acids tested, leucine showed the greatest inhibitory effect on nitric oxide production. The rate of inhibition at leucine concentration of 100 mmol/L was 81.15%. The inhibition rate of valine and isoleucine at the same concentration was less pronounced, with 29.65% and 42.95% respectively. Similar observations were made regarding suppression of inducible nitric oxide synthase mRNA expression, where 100 mmol/L leucine reduced the inducible nitric oxide synthase mRNA expression by 89.61%. Investigations of the effects of branched‐chain amino acids on the transcription levels of proinflammatory mediators interleukin‐6 and cyclooxygenase‐2 revealed that expression of interleukin‐6 mRNA was suppressed by 100 mmol/L leucine to 85.04 % and the cyclooxygenase‐2 mRNA expression was decreased by leucine, as well as by isoleucine, by more than 99%. The alkaline comet assay showed that branched‐chain amino acids have a protection effect of hydrogen peroxide–induced DNA damage with no significant difference between each of the branched‐chain amino acids. Lipopolysaccharide of gram‐negative bacteria, like most of the main periodontal pathogens, is known to induce the release of proinflammatory cytokines such as interleukin‐1β, interleukin‐6, interleukin, tumor necrosis factor‐alpha, and nitric oxide in macrophages. Overproduction of nitric oxide can be deleterious and can cause various inflammatory diseases. Inducible nitric oxide synthase can produce large amounts of nitric oxide under pathologic conditions. High serum levels of interleukin‐6 are seen in many pathologic conditions, such as inflammation and autoimmune diseases. Cyclooxygenase 2 is crucial in the conversion of arachidonic acid to prostaglandin E2. Damage to cellular DNA can jeopardize genome stability and may lead to mutations or carcinogenesis. 214 , 215 , 216 Therefore, the results of this study indicate that branched‐chain amino acids, and particularly leucine, have the potential to reduce the levels of proinflammatory cytokines and mediators in lipopolysaccharide‐stimulated macrophages. Furthermore, branched‐chain amino acids might be able to protect macrophages from DNA damage. However, although even a high dietary intake of branched‐chain amino acids should be well tolerated in people with a normal branched‐chain amino acid catabolism, some researchers indicated that people with medical conditions or limitations affecting the downstream enzymes of the branched‐chain amino acid catabolic pathway could experience negative effects upon neurologic function. Therefore, they recommended efforts to establish a safe upper limit of dietary branched‐chain amino acid intake with a branched‐chain amino acid tolerance test and clamp protocol. Furthermore, recent studies have also indicated that branched‐chain amino acids may play a role in the development of insulin resistance and might be associated with incident cardiovascular disease. 217 , 218 , 219
Glutamine is a nonessential and conditionally essential amino acid in humans and plays an important role in biosynthesis of proteins. It is the most common amino acid in the muscles, which explains its use as constituent in protein powder supplements, and also as a single supplement in the fitness scene. However, glutamine also constitutes an important fuel for some cells of the immune system and seems to exhibit some immunostimulatory effects. Immune cells increase their glutamine consumption after tissue injury and during inflammation. If the increasing glutamine requirement during these phases cannot be met by endogenous production or exogenous supplementation, the resulting glutamine deficiency can lead to a reduced ability to respond to catabolism, inflammation, and infection. 192 , 220 , 221 Glutamine has also been shown to simulate collagen synthesis through the conversion process to proline and provides 75% of the intracellular free proline in fibroblasts. A study by Murakami et al 222 has indicated that the combination of branched‐chain amino acids and glutamine is a key factor for the enhancement of skin collagen synthesis and the stimulation of the fractional synthesis rate of dermal tropo‐collagen in protein‐malnourished rats.
Amman et al 223 investigated the effect of essential amino acid supplements in adult osteoporotic rats. The essential amino acid supplements increased bone strength, prevented further decrease in bone mineral density, increased microarchitecture and cortical thickness, increased insulin‐like growth factor 1 levels, and increased bone formation and reduced bone resorption.
Unfortunately, the number of studies investigating the effect of protein and amino acid supplements on periodontal disease or therapy is very limited. Aral et al 224 investigated the effect of bodybuilding and protein supplements on periodontal tissues, comparing bodybuilders with gingivitis with nonexercising males with and without gingivitis. They assessed clinical periodontal parameters and analyzed saliva and gingival crevicular fluid samples for interleukin‐1β, apoptosis‐associated speck‐like protein containing C‐terminal caspase‐recruitment domain and caspase 1. The authors indicated that bodybuilding and supplement usage may decrease gingival inflammation by downregulating caspase 1, interleukin‐1β, and apoptosis‐associated speck‐like protein containing C‐terminal caspase‐recruitment domain. However, they conceded that owing to the lack of a bodybuilder group without supplement usage, which is presumably hard to find, it was not possible to investigate the effect of exercising alone. Another problem was that most of the participating bodybuilders also used various other supplements, such as creatinine, glutamine, and branched‐chain amino acids with unknown effects on the parameters assessed. 224
Lee et al 46 investigated the effects of a commercially available nutritional supplement drink on periodontal health or healing and tooth mobility after periodontal flap surgery. Patients with a generalized moderate chronic periodontitis were, directly after periodontal flap surgery, randomly allocated to either the intervention or the control group. The intervention group received 200 mL of a supplement drink three times a day for 8 weeks. Each supplement drink contained 13 g of protein, 24 g carbohydrates, 6 g of fat and various vitamins (A, D, E, K, B1, B2, B6, C, niacin, folate, pantothenic acid, and biotin), and minerals (sodium, potassium, calcium, phosphorus, iron, and zinc). The control group did not receive any nutritional supplementation. Clinical periodontal parameters (plaque index, gingival index and tooth mobility) were assessed at baseline and at 1, 4, and 8 weeks after surgery. After 1 week, the gingival index was significantly reduced compared with baseline in the intervention group but not in the control group. After 8 weeks, no statistically significant differences were detected anymore between the gingival index values of the interventional and control groups. As expected, tooth mobility, assessed using the Periotest M system, was significantly increased in both groups at 1 week after surgery. However, the extent of increase was less in the intervention group. After 8 weeks, tooth mobility returned to baseline levels again in both groups. The authors concluded that the use of nutritional supplementation may improve early periodontal wound healing after periodontal surgery. However, this study was subject to some limitations, amongst which were the lack of a placebo drink and the use of study subjects with a mean age of 50 years, an age at which the nutritional status is usually still good, thus limiting the effects of the supplementation.
4. GLUCOSAMINE AND CHONDROITIN SULFATE
Glucosamine is a naturally occurring amino monosaccharide that is present in the connective tissue and cartilage tissues as a component of glycosaminoglycans and is involved in maintaining strength, flexibility, and elasticity of these tissues. Therefore, glucosamine supplements are widely used in all types of sports to prevent, treat, or alleviate joint disorders, such as osteoarthritis. Numerous studies have shown the significant symptom‐modifying effect of glucosamine in osteoarthritis and its beneficial effects on joint health. 225 , 226 , 227 , 228 However, studies have also revealed that glucosamine is capable of suppressing the cytokine‐induced activation of synovial cells, such as the production of nitric oxide, prostaglandin E2, and interleukin‐8, thus possibly exerting anti‐inflammatory effects. 229 Furthermore, glucosamine may have an effect on bone and collagen metabolism. 230 , 231 There are also an increasing number of studies investigating the effects of glucosamine in combination with chondroitin sulfate in osteoarthritis therapy. The combination of glucosamine and chondroitin is among the most popular nonvitamin, nonmineral specialty supplements in the United States, often taken together as a single daily supplement for osteoarthritis. 74 Although data indicate that both agents exert an upregulation of the synthetic activity of chondrocytes, the combination of both glucosamine and chondroitin sulfate showed a greater efficacy clinically and also seems to act synergistically on articular cartridge in vitro. 232 , 233
In addition to its reported chondroprotective properties, glucosamine also seems to exhibit anti‐inflammatory properties. Glucosamine suppressed in vitro the interleukin‐1β–induced activation of synovial cells, and also synovial cell hyperplasia, cartilage destruction, and inflammatory cell infiltration in rat adjuvant arthritis. 229 , 230 , 234 Glucosamine also suppressed in vitro the tumor necrosis factor alpha–induced activation of intestinal epithelial cell HT‐29 and improved the clinical symptoms and colonic inflammation and tissue injury in dextran sulfate sodium–induced colitis in rats. 235 , 236 Furthermore, glucosamine suppressed in vitro the tumor necrosis factor alpha–induced activation of endothelial cells, the formation of atherosclerotic lesions, and the infiltration of inflammatory cells in spontaneously hyperlipidemic mice. 227 , 237 , 238
Supplementation with glucosamine has been shown to reduce inflammatory responses of joint cartilage by inhibiting the activation of nuclear factor kappa‐light‐chain‐enhancer of activated B cells, which lies upstream of inflammatory processes or mediators such as interleukin‐beta, interleukin‐8, tumor necrosis factor alpha, and C‐reactive protein. Nuclear factor kappa‐light‐chain‐enhancer of activated B cells resides in an inactive state in the cytoplasm, bound by the inhibitory subunit inhibitor of kappa B. When inhibitor of kappa B is degraded by inflammatory stimuli, nuclear factor kappa‐light‐chain‐enhancer of activated B cells freely translocates to the nucleus and potentiates the inflammatory cascade, eventually resulting in the production of both C‐reactive protein (via interleukin‐6 production) and prostaglandin E2 (via cyclooxygenases). 239 , 240 , 241 Binding of proinflammatory cytokines to their respective receptors amplifies immune response by increasing proliferation of T cells, promoting leukocyte infiltration and facilitating cell‐cell signaling. 242 , 243 , 244 , 245
Largo et al 246 investigated the effect of glucosamine sulfate administration on markers of systemic and local inflammation in rabbits with atherosclerosis aggravated by chronic arthritis. Atherosclerosis was induced by maintaining the rabbits on a hyperlipidemic diet after an endothelial lesion was produced in the femoral arteries. Arthritis was induced by repeated intra‐articular injections of ovalbumin in previously immunized rabbits. Rabbits of the test group received prophylactically a high dose of glucosamine sulfate (500 mg/kg/day) orally. After 6 weeks, the rabbits were killed, serum was extracted, peripheral blood mononuclear cells were isolated, and the femoral arteries, thoracic aorta, and synovial membranes were examined. Administration of glucosamine sulfate resulted in a reduction of the circulating levels of C‐reactive protein and interleukin‐6, lowered nuclear factor kappa‐light‐chain‐enhancer of activated B cells activation, and downregulated expression of chemokine (C‐C motif) ligand 2 (a monocyte chemoattractant protein) and cyclooxygenase‐2 genes in peripheral blood mononuclear cells. Furthermore, glucosamine sulfate administration resulted in milder wall lesions of the femoral arteries and attenuated the histologic lesions in synovial tissue. Altogether, the results of this study indicate a beneficial effect of oral glucosamine sulfate administration on systemic inflammation, mediated by nuclear factor kappa‐light‐chain‐enhancer of activated B cells. 246
Oral administration of glucosamine sulfate (10 mg/kg) in a mouse model for skin inflammation, the 12‐O‐tetradecanoyl‐13‐acetate–induced ear edema model, resulted in a decreased expression of cyclooxygenase‐2, nuclear factor kappa‐light‐chain‐enhancer of activated B cells, and transglutaminase 2. The modulation of transglutaminase 2 expression by glucosamine sulfate and the suppression of cyclooxygenase‐2 by transglutaminase 2 inhibition suggests that transglutaminase 2 might be a target for explaining the action of glucosamine sulfate and that glucosamine sulfate might be used for skin inflammation if proven in clinical trials. 247 An in vitro study in human bronchial epithelial cells by Wu et al, 248 investigating the effects of glucosamine hydrochloride on lipopolysaccharide‐mediated inflammation, showed that glucosamine hydrochloride can potently suppress lipopolysaccharide‐induced inflammatory cytokine expression (interleukin‐6 and interleukin‐8), which seems to be at least in part via attenuation of mitogen‐activated protein kinase activation. The same group also investigated in a murine model whether glucosamine is able to attenuate lung inflammation induced by cigarette smoke. 249 Cigarette smoking causes lung inflammation that is mainly regulated by redox‐sensitive pathways. Altogether, the findings of Wu and coworkers indicate a novel role of glucosamine regarding the alleviation of oxidative stress and lung inflammation induced by chronic cigarette smoke exposure in vivo, and the suppression of the cigarette smoke extract induced interleukin‐8 in vitro by inhibiting both reactive oxygen species–sensitive nicotinamide adenine dinucleotide phosphate oxidase/adenosine monophosphate–activated protein kinase/mitogen‐activated protein kinase signaling and their downstream transcriptional factors nuclear factor kappa‐light‐chain‐enhancer of activated B cells and signal transducer and activator of transcription proteins 3. Thus, this suggests the possible use of glucosamine to ameliorate lung inflammation in smokers and indicates a possible therapy option when treating chronic obstructive pulmonary disease. 248 , 249
A protective effect of glucosamine against free radical–induced damage has also been shown in erythrocytes, where glucosamine hydrochloride efficiently protected erythrocytes against free radicals. It was recommended that glucosamine hydrochloride could be used as a pharmaceutical supplement to alleviate oxidative stress. 250
The effect of glucosamine sulfate supplementation on intestinal inflammation has been investigated in a mouse model of experimental colitis. 242 Disruption of the intestinal barrier due to inflammation induces the influx of neutrophils into the mucosa followed by transepithelial migration of neutrophils, constituting a first line of defense against inflammation‐associated barrier disruption. Interleukin‐8 plays an essential role in directing the sequential process of neutrophil rolling, adhesion, and transmigration into inflamed microvasculature. Furthermore, proinflammatory mediators, such as interleukin‐1β, interleukin‐6, and tumor necrosis factor alpha increase the expression of adhesion molecules on endothelial cells and neutrophils. 242 , 251 In the Bak et al 242 study, glucosamine sulfate supplementation (0.1% w/w) resulted in a significant decrease of both circulating interleukin‐8 concentrations and colonic expression of interleukin‐1β and tumor necrosis factor alpha, which was suggested to be mediated by the observed decrease in nuclear factor kappa‐light‐chain‐enhancer of activated B cells expression. These results indicate that glucosamine sulfate protects against inflammation‐related intestinal tissue damage and related mucosal barrier disruption by suppression of the nuclear factor kappa‐light‐chain‐enhancer of activated B cells–mediated tumor necrosis factor‐alpha and interleukin‐1β production and neutrophil activation.
As part of the Vitamins and Lifestyle biomarker study, Kantor et al 240 investigated the association between the use of glucosamine and chondroitin supplements and various inflammatory markers, such as plasma high‐sensitivity C‐reactive protein, interleukin‐1β, interleukin‐6, tumor necrosis factor alpha, and urinary prostaglandin E metabolite (PGE‐M). Altogether, 217 men and women aged 50‐75 years were included in the study. Participants were interviewed regarding frequency of use of glucosamine and chondroitin supplements, and blood and urine samples were collected for analysis of the inflammatory markers. Glucosamine and chondroitin usage were classified regarding average number of pills per week of use: nonusers, low users (fewer than 14 pills per week), and high users (at least 14 pills per week). High‐users of chondroitin showed a 36% lower high‐sensitivity C‐reactive protein and 27% lower prostaglandin E metabolite than nonusers did. High users of glucosamine showed a 28% lower high‐sensitivity C‐reactive protein and 24% lower prostaglandin E metabolite. Weak and nonstatistically significant reductions were seen in this study regarding interleukin‐1β, interleukin‐6, interleukin‐8, and tumor necrosis factor alpha. The reduced inflammation of both glucosamine and chondroitin was suggested to be mediated via inhibition of nuclear factor kappa‐light‐chain‐enhancer of activated B cells. 243 , 245 The observed reduction of high‐sensitivity C‐reactive protein following supplementation with glucosamine and chondroitin has also been confirmed by the National Health and Nutrition Examination Survey including nearly 10 000 adults. 252
However, glucosamine also seems to exhibit interesting effects on bone and collagen metabolism (Figure 4).
FIGURE 4.
Schematic representation of the assumed effects of glucosamine (GlcN) and N‐acetyl‐d‐glucosamine (GlcNAc) on the osteoblastic and osteoclastic cell differentiation according to Nagaoka et al. 227 Glucosamine and N‐acetyl‐d‐glucosamine increase mineralization of mature osteoblasts and expression of middle‑ and late‐stage markers—osteopontin (OPN) and osteocalcin (OCN)—during osteoblastic differentiation and reduce expression of receptor activator of nuclear factor kappa Β ligand (RANKL), a differentiation and activation factor for osteoclasts, thus possibly increasing bone matrix deposition and decreasing bone resorption to promote bone formation
A study of Nagaoka et al investigated the effect of glucosamine and N‐acetyl‐d‐glucosamine on mineralization of differentiated mouse osteoblastic MC3T3‐E1 cells. After incubation for 21 days, both 0.1 and 1 mmol/L glucosamine as well as 1 mmol/L N‐acetyl‐d‐glucosamine significantly increased the mineralization compared to control with glucosamine being more potent at the same concentration. Furthermore, effects of glucosamine and N‐acetyl‐d‐glucosamine on osteoblastic differentiation of MC3T3‐E1 cells was measured, using type I collagen and alkaline phosphatase as markers for early stage, osteopontin as a marker for middle stage and osteocalcin as a marker for late stage of osteoplastic differentiation. 227 , 253 , 254 Whereas the expression of type I collagen and alkaline phosphatase was not significantly changed compared with control, the expression of osteopontin apparently was and the expression of osteocalcin significantly increased after incubation with both glucosamine and N‐acetyl‐d‐glucosamine. RANKL is expressed and secreted by mature osteoblasts and is a key factor in osteoclastogenesis. Evaluation of the expression of RANKL showed that both glucosamine and N‐acetyl‐d‐glucosamine (1 mmol/L) significantly suppressed the RANKL expression after incubation for 21 days. 227 , 255 These results indicate that glucosamine increases bone mineral density, induces osteoblastic differentiation, especially at middle and late stages, and also suppresses osteoclastic cell differentiation, thereby increasing bone matrix deposition, decreasing bone resorption, and promoting bone formation. 227 , 256
Furthermore, glucosamine and chondroitin sulfate also seem to have an effect on collagen synthesis and degradation. Lippiello 231 showed in an in vitro study that a commercially available glucosamine and chondroitin sulfate combination effectively stimulated the neosynthesis of collagen in cell cultures of cartilage, tendon, and ligament tissue.
An in vitro study in osteosarcoma cells showed that glucosamine sulfate has a pronounced suppressive effect on matrix metalloproteinases, particularly on matrix metalloproteinase‐3 and to a lesser extent on matrix metalloproteinase‐9. No reduction was found in matrix metalloproteinase‐2 expression, where even a modest but statistically insignificant increase was seen. These observations suggest that glucosamine sulfate is not a broad matrix metalloproteinase inhibitor but may be useful for more specific matrix metalloproteinase suppression. 257 The most distinct effect of glucosamine sulfate was observed at a concentration of 10 μg/mL, which is within the range of human serum concentrations that can be achieved by oral glucosamine intake. 258
Although these in vitro data cannot be extrapolated to in vivo circumstances, they may justify further exploration of glucosamine and chondroitin sulfate in situations where collagen degeneration is occurring or an accelerated collagen repair process after trauma or surgery would be beneficial.
Besides all the beneficial effects mentioned herein thus far, data from animal studies have raised concerns that glucosamine might adversely affect glucose metabolism and might cause insulin resistance. 259 Some recent studies suggested that glucosamine may also affect glucose transport and insulin resistance in humans, especially in patients with impaired glucose tolerance. 260 However, these studies had considerable heterogeneity in terms of dose, route, and duration of glucosamine administration. Furthermore, risk factors for diabetes development are elevated triglycerides, blood pressure, body mass index, and family history of diabetes. The most prevalent risk factors for osteoarthritis are overweight or obesity, higher age, and female sex. This means that some people are at increased risk for both diseases. 261 To address these problems, in a recent placebo‐controlled study, Gommans et al 261 investigated the effect of a prolonged glucosamine sulfate usage on hemoglobin A1c levels and new‐onset diabetes mellitus in 407 overweight and obese middle‐aged women. The findings of this study indicate that there is no effect of glucosamine sulfate on mean hemoglobin A1c level or on obtaining a high hemoglobin A1c level or new‐onset diabetes mellitus over 6.5 years, especially in participants with a normal hemoglobin A1c level at baseline. A possible effect of glucosamine sulfate could not be ruled out in the subgroup with a high hemoglobin A1c level at baseline, although these results were not statistically significant. These findings indicate that, for now, glucosamine sulfate is safe to use, certainly in people with a normal hemoglobin A1c level at baseline. 261
Interestingly, a study in rats showed that glucosamine can increase relative bioavailability of paracetamol. This increased paracetamol bioavailability seems to be caused mostly by metabolic enzyme inhibition but not through paracetamol absorption or protein binding. The data of this study also suggest that glucosamine may reduce paracetamol liver toxicity. 262 However, further studies to investigate this particular effect of glucosamine are required.
The results of an in vitro study using inferior nerve preparation in a rat mandible suggest that d‐glucosamine hydrochloride has a pain relief effect on patients with dental pain. 263
4.1. Natural herbal products and seeds “functional food or super food”
In recent years, the increasing number of people suffering from cardiovascular diseases, obesity, diabetes mellitus, neurologic diseases, dementia, cancer, and other related diseases has shifted the focus from disease treatment to healthy lifestyle changes. Epidemiologic studies have shown that physical inactivity and unhealthy diet containing high amounts of refined carbohydrates combined with saturated fatty acids but lacking fiber, minerals, and antioxidant micronutrients may be an important risk factor in the development of pathologic conditions.
Two of the greatest shifts in human evolution from pre‐Neolithic to Neolithic and from Neolithic to Industrial Revolution societies resulted in the largest changes in food production. Results from samples of calculus collected from 34 early European skeletons suggested that the transition from hunter‐gatherer to farming and later to processed food technologies shifted the oral microbial community to a disease‐associated configuration. Whereas pre‐Neolithic oral bacterial ecosystems were more diverse and dominated by the nonpathogenic family of Ruminococcaceae, modern oral ecosystems are less diverse with an abundance of periodontopathogens, such as P. gingivalis, Tannarella, and Treponema, and cariogenic species, such as Streptococcus mutans. 264
In 2009, Baumgartner et al 265 illustrated that diet may have a significant impact on periodontal inflammatory status. The Swiss study on 10 adults who were placed in a “Stone Age” environment and on diet rich in fibers, fish oils, and micronutrients showed significant reduction in bleeding on probing and pocket depth compared with baseline, even in the absence of oral hygiene. 265 , 266
An evidence‐based review, based on 31 human studies that explored the relationship between food supplements and periodontitis, showed substantial evidence of beneficial outcomes for treatment of periodontal diseases from nutritional intervention. It also suggested guidelines for micronutrient supplement intake (mainly vitamins C and D) that may improve results in the treatment of periodontitis, especially in cases of refractory disease. 267 Furthermore, it has been shown that the use of nutritional agents as adjuvants to nonsurgical periodontal therapy significantly reduced the periodontal disease severity, improved treatment prognosis in the short term (2‐6 months), and reduced susceptibility toward periodontal disease. 268
In the past few years, emerging evidence from the studies have increased the awareness of the industry and consumers related to the possible nutritional and health attributes of certain natural herbal products. They are now manufactured and widely distributed as food supplements all over the world, labeled as supporting health and preventing disease. Some of these herbal natural supplements (Table 1) contain ingredients with known health benefits, as opposed to supplements in which the composition and effect may not have be fully defined. 298
TABLE 1.
Natural herbal products: bioactive compounds and potential health benefits
| Natural herbal products and seeds | Significant health‐contributing and bioactive compounds | Evidence‐based potential health benefits |
|---|---|---|
|
Chia seeds (Salvia hispanica L.) |
Vitamins: thiamine, riboflavin, niacin, folic and ascorbic acids Minerals: calcium, phosphorus, potassium, and magnesium Antioxidants: polyphenols, chlorogenic acid, caffeic acid, quercetin, kaempferol |
Improvement of lipid profile; reduction of risk of diabetes and cardiovascular diseases 271 , 272 , 273 |
|
Quinoa (Cehnopodium quinoa Wild.) |
Vitamins: pyridoxine (vitamin B6), folic acid, ascorbic acid, and vitamin E Minerals: calcium, iron, and magnesium Fatty acids: linoleic, linolenic Phytochemicals: saponins, phytosterols, and phytoecdysteroids 274 |
Improvement of lipid profile; prevention of cardiovascular diseases; prevention of malnutrition in early childhood; safe dietary option for individuals with celiac disease 275 , 276 , 277 , 278 , 279 , 280 |
|
Spirulina (Arthrospira platensis) |
Vitamins: B‐complex, β‐carotenes, and vitamin K Minerals: iron, magnesia, zinc, copper, selenium, and chromium Antioxidants: carotenoids and C‐phycocyanin 281 |
Protection and symptom relief in patients with allergic rhinitis; improvement of clinical parameters in treatment of periodontitis; regression of oral leucoplakia; reduction of inflammation in rheumatoid arthritis; prevention of cardiovascular diseases and diabetes 282 , 283 , 284 , 285 , 286 , 287 |
|
Tumeric (Curcuma longa) |
Curcumin (curcumin I), demethoxycurcumin (curcumin II) and bisdemethoxycurcumin (curcumin III) 288 | Therapy of inflammatory conditions, osteoarthritis, neurological conditions, and cancers; prevention and therapy of gingivitis and periodontitis 289 , 290 , 291 , 292 , 293 |
|
Açai‐berry (Euterpe oleracea Martius) |
Phytochemicals: anthocyanins, flavonoids Fatty acids: mono‑ and polyunsaturated 294 |
Protection of cells from reactive oxidative species; reduction of pain and improvement of range of motion in patients with osteoarthritis; neuroprotective and cognitive effects 294 , 295 , 296 , 297 |
Chia seeds. Salvia hispanica L., locally known as chia (Nahuatl word chian, which means “oily”), is a plant that originates from the American continent and is well known after its seeds. Chia seed contains 15%‐25% of proteins, 30%‐33% of fats, 26%‐41% of carbohydrates, 18%‐30% of dietary fibers. Apart from that, it is a source of many vitamins (thiamin, riboflavin, niacin, and folic and ascorbic acids) and minerals (calcium, phosphorus, potassium, and magnesium), as well as compounds with antioxidant properties. 269 , 270
Owing to its high content and adequate ratio of polyunsaturated fatty acids, such as omega−3 alpha‐linolenic acid and omega−6 alpha‐linoleic acid, chia seed–supplemented food may play an important role in improvement of lipid profile and reduction of risk of diabetes and cardiovascular disease. A randomized single‐blind controlled trial on 20 men and women with type 2 diabetes who were supplemented with 37 g of chia seeds per day as addition to their daily conventional therapy, for 12 weeks showed significant reduction in systolic blood pressure and C‐reactive protein concentration in plasma. 271
To date, only a few clinical trials related to the therapeutic property of chia used as food supplement have been carried out. Trials conducted on healthy adults showed that, 120 min after consumption of chia seeds, postprandial glycemia significantly reduced. 272
Furthermore, some studies showed that a chia‐supplemented diet over a period of 3 months reduced body weight and total cholesterol but increased low‐density lipoprotein cholesterol in the group consuming chia flour. 273
Chia seeds extract has been tested in a recent in vitro study and demonstrated excellent antimicrobial efficacy against three periodontal pathogens: P. gingivalis, Aggregatibacter actinomycetemcomitans, and F. nucleatum. Its inhibitory potential was similar to 0.2% chlorhexidine, which was used as positive control. 299 As there are no clinical trials related to the preventive or therapeutic properties of chia seeds on the diseases that affect oral mucosa and periodontium, we can only suggest that the systemic anti‐inflammatory potential of chia seeds shown in some studies may play a role in the prevention and treatment of periodontal diseases. It can also be stipulated that their antioxidative potential may have an effect on oxidative stress that orchestrates proinflammatory cascades that underpin tissue destruction in periodontitis and other inflammatory conditions associated with periodontitis, such as type 2 diabetes, cardiovascular disease, and obesity and related metabolic dysregulation. The seeds’ mineral content may also improve the quality of bone and prevent osteoporosis and its effect on the periodontal status of the patients. Although the presence of active ingredients in chia seeds contributes to health benefits, the studies on the efficacy and safety of chia seeds are still limited and have shown inconclusive results.
Quinoa. Cehnopodium Quinoa Willd. is a grain‐like pseudo‐cereal that belongs to the Chenopodiacea family, which also includes spinach and beet. It originates in South America and has been domesticated in the Andean region. Quinoa seeds, leaves, and sprouts are used as human and animal food owing to their nutritional values. Quinoa has been described as “one of the grains of the 21st century,” and its production, preservation, and consumption were promoted by the Food and Agriculture Organization of the United Nations in 2013.
Quinoa is superior to many grains, such as rice, rye, barley, and oat, in relation to protein and lipid content. It contains 13.1%‐16.7% of high‐quality proteins with well‐balanced essential amino acid content that satisfies the amino acid requirements for adults suggested by the Food and Agriculture Organization of the United Nations/World Health Organization/United Nations University. It contains a significant amount of essential amino acids, such as lysine, methionine, and threonine, that is higher than in essential cereals, such as wheat and maize. 300
The total fiber content of quinoa is close to that of wheat, whereas the main carbohydrate component, starch, constitutes 52%‐69% of the plant. Other sugars, such as maltose, d‐galactose, d‐ribose, fructose, and glucose, contribute by only 3%. Quinoa’s composition of lipids is such that it is considered as an alternative oil seed with a lipid profile like soybean. It contains both saturated and unsaturated fatty acids. Linoleic and alpha‐linolenic acids are the major unsaturated essential fatty acids and constitute 88% of the total fatty acid amount of quinoa seed. Palmitic fatty acid, the basic saturated fatty acid in quinoa, contributes 10% to its entire lipid content. The content of micronutrients, such as vitamins and minerals, is also of great importance, as the seed is rich in pyridoxine (vitamin B6), folic acid, ascorbic acid (vitamin C), and vitamin E. Mineral content, such as calcium, iron, and magnesium, is considerably higher than in other commonly used grains, such as wheat and corn. 274
Quinoa also contains saponins, phytosterols, and phytoecdysteroids, which are biologically active compounds known as phytochemicals. These components are known to exhibit a wide range of health benefits, such as antifungal, antiviral, antibacterial, and cancer‐suppressing effects. They also exhibit hypoglycemic, antithrombotic, diuretic, anti‐inflammatory, anabolic, antidiabetic, anti‐osteoporotic, and anti‐obesity properties. 275
The evidence of some of these benefits is demonstrated in limited numbers of animal and human studies.
Several animal studies have demonstrated that consumption of quinoa seeds may improve lipid profile and glucose level in Wistar rats fed a fructose‐enriched diet. 301 Substitution of a high‐fat food with quinoa for 3 weeks resulted in modulation of adipokine expression, thus significantly preventing diet‐induced obesity in mice. 276 Furthermore, it has been shown that diets containing quinoa extract enriched in 20‐hydroxyecdysone (a steroid hormone) may influence oxidative status and increase antioxidant capacity of rats with induced oxidative stress, by increasing the activity of antioxidant enzymes and reducing levels of malondialdehyde in plasma. 277
Several human studies have confirmed positive health benefits of a diet supplemented with quinoa seeds. In a study conducted in Ecuador, it was observed that feeding 50‑ to 65‐month‐old boys with quinoa‐supplemented food twice a day for 15 days significantly augmented the insulin‐like growth factor levels in plasma and provided proteins and other nutritional microelements sufficient to prevent malnutrition in early childhood. 278
Subsequent human short‐time studies that involved different groups of participants, such as healthy students and overweight postmenopausal women, showed that daily consumption of quinoa over a short period of time has the potential to prevent cardiovascular disease and modulate some metabolic parameters, such as total cholesterol, low‐density lipoprotein, and triglyceride level. 279 , 302 Moreover, individuals with celiac disease taking a quinoa‐supplemented diet over 6 months showed that it may be a safe alternative gluten‐free dietary option. 280
The antibacterial activity of quinoa against oral bacteria has rarely been reported. A recent in vitro study showed that alkali‐transformed saponins derived from quinoa husk were efficient against three halitosis‐related bacteria: P. gingivalis, Clostridium perfringens, and F. nucleatum. The saponins altered membrane potential and morphology, as well as interfered with its permeability, causing leakage of nucleic acids and proteins. The results of the study indicated that saponins derived from quinoa husk may have an important role in a new drug delivery system against oral halitosis caused by oral microorganisms. 303
Spirulina. Arthrospira platensis is a microscopic single‐cell alga that inhabits fresh and marine waters. It derives its name from its spiral shape as seen under the microscope, and it smells and tastes like seaweed. It contains two pigments, blue (phycocyanin) and green (chlorophyll), that give the algae its color. Owing to its nutritional value, spirulina has been claimed to be “best food for the future.” Spirulina contains up to 70% of proteins; it is also rich source of vitamins (B‐complex, β‐carotenes, and vitamin K) and minerals (iron, magnesia, zinc, copper, selenium, and chromium). It can be easily cultivated, harvested, and processed into a variety of final products, such as powder, tablets, flakes, and other edible profiles. 281
Owing to the high content of carotenoids and the protein‐bound pigment C‐phycocyanin this blue‐green algae has been shown to have antioxidant and immunomodulatory properties in in vitro and in vivo studies. These substances may act as scavengers of reactive oxygen species mainly generated by host defense cells during an inflammatory reaction and increased oxidative stress. 281 Oxidative stress was first described by Sies in 1985, and some years later it was revealed that it underpins the pathogenesis of numerous of inflammatory diseases, such as periodontitis, diabetes, cardiovascular disease, and obesity/metabolic dysregulation. 267 , 304
The antioxidative potential of spirulina has been demonstrated in several human studies conducted on geriatric patients and on healthy individuals after exercise. Food supplemented by spirulina for 16 weeks showed significantly increased levels of antioxidant status in plasma of geriatric patients. 282 It has also reduced production of creatine kinase and delayed exhaustion time during physical exercise. 283
There is evidence that spirulina has an impact on the immune system by stimulating phagocytosis, modulating production of antibodies and cytokines. The photosynthetic pigment C‐phycocyanin may modulate inflammatory response in a dose‐dependent manner through its inhibitory effects on cyclooxygenase‐2 activity and suppression of production of proinflammatory substances such as prostaglandin E2 and tumor necrosis factor alpha. 284 Spirulina’s protective and symptomatic relief role through inhibition of histamine release from mast cells in patients with allergic rhinitis has been confirmed in animal and human studies. 283 , 285 It has also been noticed that spirulina may reduce inflammation in patients with rheumatoid arthritis through stimulation of secretion of interleukin‐2. 281
Several animal studies have shown that spirulina extract taken orally may have chemopreventive activity and reduce the incidence of some types of tumors. In Malaysia, 33% of medical practitioners who also practice alternative medicine use spirulina to augment anticancer therapy, especially for pediatric patients. Although there is no evidence‐based proof to use spirulina for preventive or remedial properties in patients with pre‐malignancies and malignancies, one of the first human studies showed that consumption of 1 g/day of Spirulina fusiformis for 1 year could have contributed to complete regression of oral leukoplakia in 45% of pan tobacco chewers as opposed to only 7% in the placebo group. 286 The findings in the serum of the study participants did not show any increase of β‐carotene or retinol, which may imply that other constituents of spirulina may have been responsible for the anticancer effect. 283 , 286 Although their results seem to be promising, the trial was unblinded and nonrandomized and, as such, cannot be taken as a reliable piece of evidence.
In addition to its unproven role as an antioxidant and immunomodulator, spirulina has been reported to improve blood lipid profile, which may be of importance in prevention of diabetes and cardiovascular diseases. 281
Recently, Spirulina (Arthrospira) maxima was tested on rats as a potential agent in treatment of periodontitis. SD rats at 8 weeks old with induced periodontitis were orally treated with S. maxima at different doses (test treatment: 100, 200, 400 mg/kg) and compared with indomethacin (positive control: 5 mg/kg) after 14 days of treatment. 305 Gingival tissue of rats treated with S. maxima showed reduced concentrations of proinflammatory cytokines, such as tumor necrosis factor alpha, interleukin‐1β, interleukin‐6, and inflammatory transcription factor nuclear factor kappa‐light‐chain‐enhancer of activated B cells. Activity of myeloperoxidase and expression of matrix metalloproteinases were also decreased in periodontal tissue of test rats. In addition, treatment with S. maxima increased concentration of anti‐inflammatory cytokine interleukin‐4 and the osteoprotegerin/RANKL expression ratio. S. maxima‐treated groups showed reduced numbers of osteoclasts and less bone loss, as well as increased production of osteoblasts and osteogenesis‐related factors. 305
Scarce evidence so far exists on the effect of spirulina on periodontal health and disease. One randomized controlled clinical study tested the benefits of local application of spirulina‐based gel as adjunct to nonsurgical treatment (scaling and root planing) of chronic periodontitis. The results showed significant improvement of clinical parameters, such as probing depth reduction and clinical attachment gain, in the experimental group when compared with the control group (scaling and root planing alone). During the course of treatment, spirulina gel did not cause any side or adverse effects. 287 As this study is one of the first to use spirulina as a local adjunct agent in the treatment of periodontitis, further studies, including more relevant clinical and biochemical parameters, are necessary to confirm the findings and explore underlying mechanisms.
Although rare, some adverse reactions, such as skin rash, headache, muscle pain, flushing of the face, sweating, and difficulty concentrating, have been documented in individuals taking 1 g of spirulina orally. At the same time, concerns have been raised regarding potential toxic substances called microcystins that may be present in spirulina and cause serious health problems. The presence of such substances and other potentially toxic elements are highly dependent on the process of cultivation of spirulina. 281
Turmeric is a dietary spice whose active ingredient, curcumin, is isolated from the rhizomes of Curcuma longa, a plant that belongs to the ginger family. Turmeric is yellow in color and is most used in Asian and Indian cuisine. It has also been used for medicinal purposes for thousands of years. There is a plethora of available material related to curcumin’s health benefits, such as published articles (over 6000), audio and video recordings. Curcumin was first discovered by Vogel and Pelletier in 1815; however, its chemical structure was first reported by Lampe and Milobedzka in 1913. 288 , 289
The powder of turmeric contains volatile and nonvolatile oils, proteins, fats, minerals, carbohydrates, and curcuminoids. Commercially available curcumin is a mix of three biologically active components, collectively called curcuminoids: 77% of curcumin (curcumin I), 17% of demethoxycurcumin (curcumin II) and 3% of bisdemethoxycurcumin (or curcumin III). 290
Curcumin has been approved by the US Food and Drug Administration to be a safe food supplement, and a daily intake of curcumin at a dose of 0.1‐3 mg/kg body weight has been considered as an acceptable dose by the Food and Agriculture Organization of the United Nations/World Health Organization Expert Committee on Food Additives, 1996. 289
The systemic bioavailability of curcumin after consumption is relatively low; therefore, various curcumin derivatives (man‐made analogues) have been designed and tested to overcome this problem. 290
Curcumin (mainly its analogues) has shown chemopreventive and chemotherapeutic properties in different cancer studies. It has been shown in vivo (paw edema model) to have analgesic and anti‐inflammatory activity through suppression of gene expression and inhibition of secretion of proinflammatory substances, such as tumor necrosis factor alpha, monocyte chemoattractant protein‐1, interleukin‐10, and interleukin‐6. Few studies have confirmed curcumin free‐radical scavenging activity and antioxidant effects, as well as its effect on regulation of blood glucose level. 290
Curcumin was first tested in a human study by Oppenheimer in 1937. Since then, many human studies reported beneficial properties of curcumin, used either as monotherapy or in combination with other agents, in the treatment of diseases such as cancer, inflammatory conditions, osteoarthritis, neurologic conditions, and diabetes. It has been delivered in the form of nanoparticles, tablets, capsules, powder, or solution in doses from 0.18 to 8 g/d. 290
In clinical trials, patients with periodontal diseases usually use curcumin as adjuvant therapy following subgingival instrumentation. Curcumin is commonly applied topically as a solution (mouthwash) or in solid form (curcumin sustained‐release tablet, gel, or chip). 306
A comprehensive review summarized the existing evidence related to the effects of turmeric in the prevention and treatment of gingivitis. Only five studies with a total of 290 participants, published between 2010 and 2016, satisfied the selection criteria and were analyzed. All trials were conducted in India, with the main objective to compare the efficacy of turmeric and chlorhexidine, formulated either as a mouthwash or gel, in the prevention and treatment of gingivitis. 291
Three studies on prevention showed that, as an adjunct to mechanical plaque control, turmeric‐based mouthwash significantly reduced plaque accumulation and gingival inflammation after the experimental period (14‐21 days). However, when compared with chlorhexidine, it was less efficient. 291 , 292
Two studies tested the therapeutical efficacy of turmeric and chlorhexidine as an adjunct to mechanical treatment of gingivitis and compared them with mechanical treatment alone. Both studies showed significant improvement of clinical parameters (plaque index and gingival index) at 14 and 21 days compared with baseline, for all treatment modalities. The turmeric and chlorhexidine groups demonstrated significantly superior results related to plaque index and gingival inflammation reduction when compared with the group that received mechanical treatment alone. There was no significant difference between the turmeric and chlorhexidine groups at any time interval. The studies also showed that the patients favored turmeric gel owing to it sweet taste, pleasant odor, and lesser staining compared with the chlorhexidine gel. 291 The main limitations of these studies were small population size, short duration of therapy, nonstandardized protocol, and use of different formulations of turmeric (mouthwash and gel) with different concentrations (0.1%‐20%). As the studies were conducted only in India, the results may not be applicable to other demographic groups of patients.
Subsequent studies that tested use of curcumin as an adjunct to subgingival instrumentation have shown that its topical application significantly improved periodontal condition. Curcumin can directly and indirectly inhibit the activation of nuclear factor kappa‐light‐chain‐enhancer of activated B cells, Janus kinase/signal transducer and activator of transcription, and mitogen‐activated protein kinase signaling pathways, thus inhibiting the inflammatory cascade reaction involved in development of a variety of inflammatory diseases, as well as periodontitis. 306
It is important to mention that curcumin can produce a large amount of active oxygen under controlled light conditions (300‐500 nm wavelength range). It has been widely used in photodynamic therapy of cancers owing to its low cost and high efficacy. The light produced by photodynamic therapy can enhance the ability of curcumin to penetrate biofilm. On the other hand, curcumin can enhance the bacteriostatic effect of photodynamic therapy. 306 De Paula Zago et al 307 have shown that curcumin can significantly inhibit the growth of oral pathogens while used as a photosensitizer. In a clinical study, Sreedhar et al 308 used curcumin gel as a photosensitizer in photodynamic therapy following subgingival instrumentation with ultrasonic scaling in 15 patients with deep periodontal pockets. Curcumin showed enhanced antimicrobial properties against P. gingivalis, A. actinomycetemcomitans, and Prevotella intermedia. Interestingly, antibacterial effects almost doubled when curcumin gel was irradiated with light of 470 nm wavelength and 620 mW/cm2 power intensity. These results were improved when the multiple applications of photodynamic therapy were performed. The curcumin binds to the cell wall of periodontal pathogens and when irradiated with light of specific wavelength produces reactive oxygen species, which can destroy the pathogens in the immediate vicinity. 308
It is still controversial whether curcumin has more advanced therapeutic effects than traditional photosensitizers, such as methylene blue or toluidine blue O. However, being cheap, safe, and less prone to stain teeth and esthetic restorations makes curcumin a new option for photodynamic therapy.
A recent animal study showed that intra‐gastric administration of curcumin might reduce bone loss in rats with ligature‐induced periodontitis via suppression of RANKL/receptor activator of nuclear factor‐κB/osteoprotegerin expression and reduction of proinflammatory cytokines, mainly tumor necrosis factor alpha and interleukin‐6. 293 This and other preliminary in vivo studies provide initial evidence that curcumin may offer periodontists a complementary approach to the conventional periodontal therapy through either systemic or local application. 293
Nanoparticulate drug delivery systems have been shown to be effective in increasing the efficacy of therapeutic agents. Integration of curcumin in these delivery systems has resulted in improved solubility, bioavailability, transmembrane permeability, prolonged plasma half‐life, long‐term stability, target‐specific delivery, and upgraded therapeutic effects. 306
In that context, curcumin was incorporated in the structure of a nanofibrous asymmetric collagen membrane along with aspirin‐loaded poly(lactic‐co‐glycolic acid) nanoparticles. The membrane showed promising effect on bone regeneration and bone healing, as well as a strong antibacterial effect,an in animal study. 309
Açai‐berry, the fruit of the Amazonian palm, Euterpe oleracea Martius, has been extensively studied not only for its nutritional properties but also its anti‐inflammatory, antioxidant, and bioactivity properties. Açai pulp fraction contains a remarkable number of phytochemicals and mono‑ and polyunsaturated fatty acids. 294
Phytochemical analyses indicate that açai extract is rich in anthocyanins and possesses a high number of polyphenols, especially flavonoids, that exhibit promising therapeutic potential. Current evidence from a human randomized placebo‐controlled cross‐over clinical trial that involved 12 healthy participants between 19 and 55 years of age shows that consumption of a juice blend, containing predominantly açai berry, had several health benefits. The study reported significant reduction in lipid peroxidation during oxidative stress and a rapid increase of serum antioxidant capacity in protecting cells from reactive oxygen species. 294 Earlier in vitro studies demonstrated that acai extract may exhibit potent anti‐inflammatory, neuroprotective, and anticarcinogenic properties. 294 Furthermore, it has been demonstrated in vitro that acai‐berry extract may reduce osteoclast formation, differentiation, and activity through modulation of secretion of osteoclastogenesis‐promoting cytokines (tumor necrosis factor alpha, interleukin‐1α, interleukin‐6) and osteoclastogenesis‐inhibiting cytokines (interleukin‐3, interleukin‐4, interleukin‐13, and interferon‐gamma). 295 These findings may be of importance for further testing and development of novel therapeutic agents with potential to reduce inflammatory bone loss that occurs as a result of periodontitis.
Tested on mice, açai extract has been shown to produce analgesic effects that have been further investigated in a clinical study. An open‐label clinical pilot study on 14 participants with chronic pain and reduced range of motion, mainly due to osteoarthritis, showed that daily consumption of 120 mL of an açai fruit and berry blend significantly reduced pain and increased range of motion after 12 weeks. The significant correlation between antioxidant status and improvements in physical well‐being of the participants was also observed in the study. 296 These findings, as well as emerging evidence that food rich in polyphenols may have neuroprotective and cognitive effects, warrants further well‐designed clinical studies with large groups. 297
4.2. Minerals
Minerals belong to the group of minor/micronutrients that are present in food in small amounts, measured by microgram quantities. Minerals can be classified as major minerals (more than 100 mg/day) and those traditionally called “trace minerals” (<100 mg/day) depending on a daily intake and need. They are an integral part of our hard tissues, such as bones and teeth. They also maintain the acid‐base homeostasis and are key constituents of electrolytes that are important for muscle contraction and nerve conduction. 298 Minerals act as catalysts in a variety of enzyme systems, either as ionic enzymatic cofactors or metalloenzymes. In this respect, they play an important role in the functional and structural integrity of the tissues, and any deficiency below the required limit may cause physiologic or structural abnormalities. Regular daily intake of food rich in minerals is usually sufficient to maintain health; however, in some cases, pharmacological supplements are used to maintain satisfactory levels or treat deficiencies. 310
Sodium is the cation and main major mineral in extracellular fluid. It plays a key role in cellular membrane potential and nerve conduction, and together with calcium, potassium, and magnesium has an important influence on cardiac output and peripheral vascular resistance, the main determinants of blood pressure level. 298 The modern diets provide sodium in markedly higher amounts than recommended and in different ratios related to other minerals that may further cause the breakdown of cardiovascular and renal homeostasis, rendering blood hypertension a common serious problem of modern society. 310 Sodium is mainly consumed as sodium chloride, “dietary salt,” but may be found in food additives, too. The value of adequate daily intake for sodium has been revised recently and for healthy adults is set at 460‐920 mg/day (20‐40 mmol/day) as per NHMRC revised guidelines from 2017. 311
Potassium is the key cation in intracellular fluid with a similar role to sodium. Potassium is known to have a protective effect on the cardiovascular system, and its anti‐atherosclerotic properties have attracted attention in the recent years. Other health benefits of potassium may be related to diabetic patients and improvement of their glucose tolerance. 310 A low potassium level in plasma may result in muscle cramps, confusion, and may also lead to life‐threatening events such as arrhythmia. 298 The adequate daily intake of potassium for healthy adults is 2800 mg/day (72 mmol/day) for woman and 3800 mg/day (100 mmol/day) for men. 311
Calcium is the main component of hydroxyapatite, a mineral that is present in our skeletal system and teeth. It is important for normal bone turnover, nerve conduction, and blood coagulation. 298 Metabolism of calcium is regulated by parathyroid hormone and calcitonin, and its active resorption through intestinal wall is highly dependent on vitamin D. In the event of reduced calcium level in serum due to low dietary intake or increased demand (growth and pregnancy), production of active vitamin D (1,25‐dihydroxycholecalciferol) increases in order to improve intestinal resorption of the calcium. If the former mechanism is insufficient to rebalance serum concentration of calcium, parathyroid hormone will mobilize calcium from the skeletal system, including the alveolar bone, and increase its reabsorption at the level of the renal distal tubule. 312 Numerous clinical studies have emphasized the importance of calcium intake in bone mineral density maintenance and tooth retention, especially in the elderly population. Vitamin D deficiency is common in the world, with an estimate that more than 1 billion people suffer from its insufficiency or deficiency. 313 The beneficial effect of supplementation with vitamin D and calcium has been well documented and recognized in the treatment of rickets, osteomalacia, and osteoporosis. In recent times, vitamin D and calcium have also been considered as candidates to modulate periodontal disease, as some studies have found that their intake may reduce alveolar bone loss, gingival inflammation, and attachment loss. Caution should be considered with patients reporting a risk of bowel cancer. 314
The Third National Health and Nutrition Examination Survey large cohort of up to 12 000 subjects suggested that low dietary intake of calcium results in more severe periodontal disease and progressive attachment loss in a dose‐dependent manner. 313 Another study that used data from the Third National Health and Nutrition Examination Survey reported an inverse association between the prevalence of periodontal disease and the intake of dairy products, a common dietary source of calcium and vitamin D. 315 A recent cross‐sectional study on 51 subjects on periodontal maintenance therapy resulted in a trend toward better clinical (gingival inflammation, probing depth, and attachment loss and furcation involvement) and radiological parameters (cemento‐enamel junction to alveolar crest distance) of periodontal disease in patients who were voluntarily taking calcium (at least 1000 mg/day) and vitamin D (at least 400 IU/day) supplements for more than 18 months (average of 10.6 years) prior to commencement of the study. 313 Based on the findings, the authors suggested that the role and dose of vitamin D and calcium in maintenance of periodontal diseases should be further investigated and their supplementation may be advocated as a component of periodontal disease management. Although some studies implied benefits of daily supplementation with vitamin D and calcium, use of these microelements in healthy patients with periodontal disease requires further evidence. Recommended daily intake of calcium for adults ranges from 1000 to 1300 mg/day. 311
Magnesium is second most prominent intracellular cation and is present in all tissues, with majority (two‐thirds) stored in bones. It plays a crucial role in ion and energy transfer, stabilization of membranes, and is necessary for many physiologic functions. 267 , 298 Imbalances in magnesium metabolism may be associated with different pathologic conditions such as cardiovascular diseases, diabetes, pre‐eclampsia, eclampsia, and sickle cell disease. 267 Low magnesium intake has been linked to periodontitis. 316 In a cross‐sectional epidemiologic study involving 4290 subjects from 20 to 80 years of age, periodontal health was determined and correlated to concentrations of serum magnesium and calcium. It was demonstrated that a higher magnesium/calcium ratio was significantly associated with reduced probing depth (P < 0.001), less attachment loss (P = 0.006), and higher number of remaining teeth (P = 0.005). In a matched study, the periodontal status of 60 subjects from the same population using magnesium drugs was compared with 120 nonusers. Subjects taking magnesium showed less attachment loss (P < 0.01) and a higher number of remaining teeth than did their counterparts. The findings of the study indicate that magnesium supplementation may improve periodontal status and improve tooth retention. 317 Recommended daily intake of magnesium for adults is 320 mg/day for women and 420 mg/day for men. 311
5. CONCLUSION
In summary, an increasing number of studies have revealed aspects and effects of these so‐called lifestyle or fitness supplements and superfoods that may have an impact on periodontal health and healing after treatment. Against the background of periodontal disease as a chronic inflammatory disease involving bone and connective tissue degradation, a deeper insight and understanding of the potential anti‐inflammatory effects of supplements and their effects on bone and connective tissue metabolism could help to develop new prevention and treatment strategies. However, some the current evidence is of a very low quality, and more validated scientific data are required before their possible use in prevention or treatment of periodontal diseases can be made.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest with respect to the authorship and/or publication of this article.
ACKNOWLEDGMENT
Open access publishing facilitated by The University of Sydney, as part of the Wiley ‐ The University of Sydney agreement via the Council of Australian University Librarians. Open access publishing facilitated by The University of Sydney, as part of the Wiley ‐ The University of Sydney agreement via the Council of Australian University Librarians.
Spahr A, Divnic‐Resnik T. Impact of health and lifestyle food supplements on periodontal tissues and health. Periodontol 2000. 2022;90:146‐175. doi: 10.1111/prd.12455
Funding information
This project was self‐funded by the authors
Contributor Information
Axel Spahr, Email: axel.spahr@sydney.edu.au.
Tihana Divnic‐Resnik, Email: tihana.divnic-resnik@sydney.edu.au.
REFERENCES
- 1. Bhattacharya A, Rahman M, Banu J, et al. Inhibition of osteoporosis in autoimmune disease prone MRL/Mpj‐Fas lpr mice by n−3 fatty acids. J Am Coll Nutr. 2005;24(3):200‐209. [DOI] [PubMed] [Google Scholar]
- 2. Buckley JD, Howe PRC. Anti‐obesity effects of long‐chain omega‐3 polyunsaturated fatty acids. Obes Rev. 2009;10(6):648‐659. [DOI] [PubMed] [Google Scholar]
- 3. Byelashov OA, Sinclair AJ, Kaur G. Dietary sources, current intakes, and nutritional role of omega‐3 docosapentaenoic acid. Lipid Technol. 2015;27(4):79‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chalon S, Vancassel S, Zimmer L, Guilloteau D, Durand G. Polyunsaturated fatty acids and cerebral function: focus on monoaminergic neurotransmission. Lipids. 2001;36(9):937‐944. [DOI] [PubMed] [Google Scholar]
- 5. Dawson DR, Branch‐Mays G, Gonzalez OA, Ebersole JL. Dietary modulation of the inflammatory cascade. Periodontol 2000. 2013;64(1):161‐197. [DOI] [PubMed] [Google Scholar]
- 6. Ditschuneit HH, Flechtner‐Mors M, Johnson TD, Adler G. Metabolic and weight‐loss effects of a long‐term dietary intervention in obese patients. Am J Clin Nutr. 1999;69(2):198‐204. [DOI] [PubMed] [Google Scholar]
- 7. Galland L. Diet and inflammation. Nutr Clin Pract. 2010;25(6):634‐640. [DOI] [PubMed] [Google Scholar]
- 8. Geusens P, Wouters C, Nijs J, Jiang Y, Dequeker J. Long‐term effect of omega‐3 fatty acid supplementation in active rheumatoid arthritis. Arthr Rheum. 1994;37(6):824‐829. [DOI] [PubMed] [Google Scholar]
- 9. Hamazaki K, Hamazaki T, Inadera H. Abnormalities in the fatty acid composition of the postmortem entorhinal cortex of patients with schizophrenia, bipolar disorder, and major depressive disorder. Psychiatry Res. 2013;210(1):346‐350. [DOI] [PubMed] [Google Scholar]
- 10. Harris WS. Omega‐3 fatty acids in cardiac biopsies from heart transplantation patients: correlation with erythrocytes and response to supplementation. Circulation. 2004;110(12):1645‐1649. [DOI] [PubMed] [Google Scholar]
- 11. Harris WS, Miller M, Tighe AP, Davidson MH, Schaefer EJ. Omega‐3 fatty acids and coronary heart disease risk: clinical and mechanistic perspectives. Atherosclerosis. 2008;197(1):12‐24. [DOI] [PubMed] [Google Scholar]
- 12. Innis SM. Dietary omega 3 fatty acids and the developing brain. Brain Res. 2008;1237:35‐43. [DOI] [PubMed] [Google Scholar]
- 13. Jeffrey BG, Weisinger HS, Neuringer M, Mitchell DC. The role of docosahexaenoic acid in retinal function. Lipids. 2001;36(9):859‐871. [DOI] [PubMed] [Google Scholar]
- 14. Lau DCW, Dhillon B, Yan H, Szmitko PE, Verma S. Adipokines: molecular links between obesity and atheroslcerosis. Am J Physiol Heart Circ Physiol. 2005;288(5):H2031‐H2041. [DOI] [PubMed] [Google Scholar]
- 15. Markhus MW, Skotheim S, Graff IE, et al. Low omega‐3 index in pregnancy is a possible biological risk factor for postpartum depression. PLoS ONE. 2013;8(7):e67617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nakatani T, Kim H‐J, Kaburagi Y, Yasuda K, Ezaki O. A low fish oil inhibits SREBP‐1 proteolytic cascade, while a high‐fish‐oil feeding decreases SREBP‐1 mRNA in mice liver. J Lipid Res. 2002;44(2):369‐379. [DOI] [PubMed] [Google Scholar]
- 17. SanGiovanni JP, Chew EY. The role of omega‐3 long‐chain polyunsaturated fatty acids in health and disease of the retina. Prog Retin Eye Res. 2005;24(1):87‐138. [DOI] [PubMed] [Google Scholar]
- 18. Serhan CN. Pro‐resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shapiro JA, Koepsell TD, Voigt LF, Dugowson CE, Kestin M, Nelson JL. Diet and rheumatoid arthritis in women. Epidemiology. 1996;7(3):256‐263. [DOI] [PubMed] [Google Scholar]
- 20. Sun D, Krishnan A, Zaman K, Lawrence R, Bhattacharya A, Fernandes G. Dietary n−3 fatty acids decrease osteoclastogenesis and loss of bone mass in ovariectomized mice. J Bone Miner Res. 2003;18(7):1206‐1216. [DOI] [PubMed] [Google Scholar]
- 21. Taha AY, Cheon Y, Ma K, Rapoport SI, Rao JS. Altered fatty acid concentrations in prefrontal cortex of schizophrenic patients. J Psychiatr Res. 2013;47(5):636‐643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Watkins BA, Li Y, Lippman HE, Seifert MF. Omega‐3 polyunsaturated fatty acids and skeletal health. Exp Biol Med. 2001;226(6):485‐497. [DOI] [PubMed] [Google Scholar]
- 23. Kajarabille N, Díaz‐Castro J, Hijano S, López‐Frías M, López‐Aliaga I, Ochoa JJ. A new insight to bone turnover: role of ω−3 polyunsaturated fatty acids. ScientificWorldJournal. 2013;2013:589641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mangano KM, Sahni S, Kerstetter JE, Kenny AM, Hannan MT. Polyunsaturated fatty acids and their relation with bone and muscle health in adults. Curr Osteoporos Rep. 2013;11(3):203‐212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jump DB, Depner CM, Tripathy S. Omega‐3 fatty acid supplementation and cardiovascular disease. J Lipid Res. 2012;53(12):2525‐2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nakamura MT, Nara TY. Structure, function, and dietary regulation of Δ6, Δ5, and Δ9 desaturases. Ann Rev Nutr. 2004;24(1):345‐376. [DOI] [PubMed] [Google Scholar]
- 27. Linus Pauling Institute MIC, Oregon State University . Essential fatty acids. 2014. https://lpi.oregonstate.edu/mic/other‐nutrients/essential‐fatty‐acids.
- 28. Trumbo P, Schlicker S, Yates AA, Poos M. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Am Diet Assoc. 2002;102(11):1621‐1630. [DOI] [PubMed] [Google Scholar]
- 29. Lichtenstein AH, Jones PJH. Lipids: absorption and transport. In: Erdman JW Jr, Macdonald IA, Zeisel SH, eds. Present Knowledge in Nutrition. Wiley‐Blackwell; 2012:118‐131. [Google Scholar]
- 30. Kaur G, Cameron‐Smith D, Garg M, Sinclair AJ. Docosapentaenoic acid (22:5n−3): a review of its biological effects. Prog Lipid Res. 2011;50(1):28‐34. [DOI] [PubMed] [Google Scholar]
- 31. Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation‐resolution programmes. Nature. 2007;447(7146):869‐874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Burdge GC, Jones AE, Wootton SA. Eicosapentaenoic and docosapentaenoic acids are the principal products of α‐linolenic acid metabolism in young men. Br J Nutr. 2002;88(04):355. [DOI] [PubMed] [Google Scholar]
- 33. Burdge GC, Wootton SA. Conversion of α‐linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br J Nutr. 2002;88(04):411. [DOI] [PubMed] [Google Scholar]
- 34. Brossard N, Croset M, Pachiaudi C, Riou JP, Tayot JL, Lagarde M. Retroconversion and metabolism of [13C]22:6n−3 in humans and rats after intake of a single dose of [13C]22:6n−3‐triacylglycerols. Am J Clin Nutr. 1996;64(4):577‐586. [DOI] [PubMed] [Google Scholar]
- 35. Cho HP, Nakamura M, Clarke SD. Cloning, expression, and fatty acid regulation of the human Δ‐5 desaturase. J Biol Chem. 1999;274(52):37335‐37339. [DOI] [PubMed] [Google Scholar]
- 36. Davidson MH. Omega‐3 fatty acids. Curr Opin Lipidol. 2013;24(6):467‐474. [DOI] [PubMed] [Google Scholar]
- 37. Kris‐Etherton PM, Taylor DS, Yu‐Poth S, et al. Polyunsaturated fatty acids in the food chain in the United States. Am J Clin Nutr. 2000;71(1):179S‐188S. [DOI] [PubMed] [Google Scholar]
- 38. Simopoulos AP. The importance of the ratio of omega‐6/omega‐3 essential fatty acids. Biomed Pharmacother. 2002;56(8):365‐379. [DOI] [PubMed] [Google Scholar]
- 39. Pawlosky RJ, Salem N. Ethanol exposure causes a decrease in docosahexaenoic acid and an increase in docosapentaenoic acid in feline brains and retinas. Am J Clin Nutr. 1995;61(6):1284‐1289. [DOI] [PubMed] [Google Scholar]
- 40. Bang HO, Dyerberg J, Sinclair HM. The composition of the Eskimo food in north western Greenland. Am J Clin Nutr. 1980;33(12):2657‐2661. [DOI] [PubMed] [Google Scholar]
- 41. Eaton SB, Eaton SB III, Sinclair AJ, Cordain L, Mann NJ. Dietary intake of long‐chain polyunsaturated fatty acids during the paleolithic. In: Simopoulos AP, ed. The Return of ω3 Fatty Acids into the Food Supply. World Review of Nutrition and Dietetics. Vol 83. Karger; 1998:12‐23. [DOI] [PubMed] [Google Scholar]
- 42. Droulez V, Williams PG, Levy G, Stobaus T, Sinclair A. Composition of Australian red meat 2002. 2. Fatty acid profile. Food Australia. 2006;58(7):335‐341. [Google Scholar]
- 43. Elvevoll EO, Barstad H, Breimo ES, et al. Enhanced incorporation of n−3 fatty acids from fish compared with fish oils. Lipids. 2006;41(12):1109‐1114. [DOI] [PubMed] [Google Scholar]
- 44. Miller E, Kaur G, Larsen A, et al. A short‐term n−3 DPA supplementation study in humans. Eur J Nutr. 2012;52(3):895‐904. [DOI] [PubMed] [Google Scholar]
- 45. Rahmawaty S, Charlton K, Lyons‐Wall P, Meyer BJ. Dietary intake and food sources of EPA, DPA and DHA in Australian children. Lipids. 2013;48(9):869‐877. [DOI] [PubMed] [Google Scholar]
- 46. Alonso L, Marcos ML, Blanco JG, et al. Anaphylaxis caused by linseed (flaxseed) intake. J Allergy Clin Immunol. 1996;98(2):469‐470. [DOI] [PubMed] [Google Scholar]
- 47. DC N, Friman C, Konttinen YT, VE H, Nasu Y, Antila E. Alpha‐linolenic acid in the treatment of rheumatoid arthritis. A double‐blind, placebo‐controlled and randomized study: flaxseed vs. safflower seed. Rheumatol Int. 1995;14(6):231‐234. [DOI] [PubMed] [Google Scholar]
- 48. Campan P, Planchand P‐O, Duran D. Pilot study on n−3 polyunsaturated fatty acids in the treatment of human experimental gingivitis. J Clin Periodontol. 1997;24(12):907‐913. [DOI] [PubMed] [Google Scholar]
- 49. Calder PC. n−3 fatty acids, inflammation and immunity: new mechanisms to explain old actions. Proceed Nutr Soc. 2013;72(03):326‐336. [DOI] [PubMed] [Google Scholar]
- 50. Gibney M, Hunter B. The effects of short‐and long‐term supplementation with fish oil on the incorporation of n−3 polyunsaturated fatty acids into cells of the immune system in healthy volunteers. Eur J Clin Nutr. 1993;47(4):255‐259. [PubMed] [Google Scholar]
- 51. Healy DA, Wallace FA, Miles EA, Calder PC, Newsholme P. Effect of low‐to‐moderate amounts of dietary fish oil on neutrophil lipid composition and function. Lipids. 2000;35(7):763‐768. [DOI] [PubMed] [Google Scholar]
- 52. Lee TH, Hoover RL, Williams JD, et al. Effect of dietary enrichment with eicosapentaenoic and docosahexaenoic acids on in vitro neutrophil and monocyte leukotriene generation and neutrophil function. N Engl J Med. 1985;312(19):1217‐1224. [DOI] [PubMed] [Google Scholar]
- 53. Yaqoob P, Pala HS, Cortina‐Borja M, Newsholme EA, Calder PC. Encapsulated fish oil enriched in alpha‐tocopherol alters plasma phospholipid and mononuclear cell fatty acid compositions but not mononuclear cell functions. Eur J Clin Invest. 2000;30(3):260‐274. [DOI] [PubMed] [Google Scholar]
- 54. Witte TR, Salazar AJ, Ballester OF, Hardman WE. RBC and WBC fatty acid composition following consumption of an omega 3 supplement: lessons for future clinical trials. Lipids Health Dis. 2010;9(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Calder PC. Polyunsaturated fatty acids and inflammatory processes: new twists in an old tale. Biochimie. 2009;91(6):791‐795. [DOI] [PubMed] [Google Scholar]
- 56. Calder PC. n−3 polyunsaturated fatty acids and immune cell function. Adv Enzyme Regul. 1997;37:197‐237. [DOI] [PubMed] [Google Scholar]
- 57. Calder PC. Immunomodulation by omega‐3 fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2007;77(5‐6):327‐335. [DOI] [PubMed] [Google Scholar]
- 58. Calder PC. The relationship between the fatty acid composition of immune cells and their function. Prostaglandins Leukot Essent Fatty Acids. 2008;79(3‐5):101‐108. [DOI] [PubMed] [Google Scholar]
- 59. Stillwell W, Wassall SR. Docosahexaenoic acid: membrane properties of a unique fatty acid. Chem Phys Lipids. 2003;126(1):1‐27. [DOI] [PubMed] [Google Scholar]
- 60. Swanson JE, Lokesh BR, Kinsella JE. Ca2+‐Mg2+ ATPase of mouse cardiac sarcoplasmic reticulum is affected by membrane n−6 and n−3 polyunsaturated fatty acid content. J Nutr. 1989;119(3):364‐372. [DOI] [PubMed] [Google Scholar]
- 61. Bannenberg G, Serhan CN. Specialized pro‐resolving lipid mediators in the inflammatory response: an update. Biochim Biophys Acta. 2010;1801(12):1260‐1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Palombo JD, DeMichele SJ, Boyce PJ, et al. Effect of short‐term enteral feeding with eicosapentaenoic and γ‐linolenic acids on alveolar macrophage eicosanoid synthesis and bactericidal function in rats. Crit Care Med. 1999;27(9):1908‐1915. [DOI] [PubMed] [Google Scholar]
- 63. German JB, Lokesh B, Kinsella JE. The effect of dietary fish oils on eicosanoid biosynthesis in peritoneal macrophages is influenced by both dietary n−6 polyunsaturated fats and total dietary fat. Prostaglandins Leukot Essent Fatty Acids. 1988;34(1):37‐45. [DOI] [PubMed] [Google Scholar]
- 64. Kinsella JE, Lokesh B, Stone RA. Dietary n−3 polyunsaturated fatty acids and amelioration of cardiovascular disease: possible mechanisms. Am J Clin Nutr. 1990;52(1):1‐28. [DOI] [PubMed] [Google Scholar]
- 65. Calder PC. n−3 fatty acids, inflammation, and immunity—relevance to postsurgical and critically ill patients. Lipids. 2004;39(12):1147‐1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Calder PC. n−3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83(6):1505S‐1519S. [DOI] [PubMed] [Google Scholar]
- 67. Raffaelli L, Serini S, Piccioni E, et al. n−3 polyunsaturated fatty acid effect in periodontal disease: state of art and possible mechanisms involved. Int J Immunopathol Pharmacol. 2008;21(2):261‐266. [DOI] [PubMed] [Google Scholar]
- 68. Casado‐Díaz A, Santiago‐Mora R, Dorado G, Quesada‐Gómez JM. The omega‐6 arachidonic fatty acid, but not the omega‐3 fatty acids, inhibits osteoblastogenesis and induces adipogenesis of human mesenchymal stem cells: potential implication in osteoporosis. Osteoporosis Int. 2012;24(5):1647‐1661. [DOI] [PubMed] [Google Scholar]
- 69. Schmitz G, Ecker J. The opposing effects of n−3 and n−6 fatty acids. Prog Lipid Res. 2008;47(2):147‐155. [DOI] [PubMed] [Google Scholar]
- 70. Caughey GE, Mantzioris E, Gibson RA, Cleland LG, James MJ. The effect on human tumor necrosis factor alpha and interleukin 1 beta production of diets enriched in n−3 fatty acids from vegetable oil or fish oil. Am J Clin Nutr. 1996;63(1):116‐122. [DOI] [PubMed] [Google Scholar]
- 71. De Caterina R, Cybulsky MA, Clinton SK, Gimbrone MA, Libby P. Omega‐3 fatty acids and endothelial leukocyte adhesion molecules. Prostaglandins Leukot Essent Fatty Acids. 1995;52(2‐3):191‐195. [DOI] [PubMed] [Google Scholar]
- 72. Goldman DW, Pickett WC, Goetzl EJ. Human neutrophil chemotactic and degranulating activities of leukotriene B5 (LTB5) derived from eicosapentaenoic acid. Biochem Biophys Res Commun. 1983;117(1):282‐288. [DOI] [PubMed] [Google Scholar]
- 73. Sculley DV. Periodontal disease: modulation of the inflammatory cascade by dietary n−3 polyunsaturated fatty acids. J Periodontal Res. 2014;49:277‐281. [DOI] [PubMed] [Google Scholar]
- 74. Qato DM, Alexander GC, Conti RM, Johnson M, Schumm P, Lindau ST. Use of prescription and over‐the‐counter medications and dietary supplements among older adults in the United States. JAMA. 2008;300(24):2867‐2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bagga D, Wang L, Farias‐Eisner R, Glaspy JA, Reddy ST. Differential effects of prostaglandin derived from ω−6 and ω−3 polyunsaturated fatty acids on COX‐2 expression and IL‐6 secretion. Proc Natl Acad Sci USA. 2003;100(4):1751‐1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Richard D, Kefi K, Barbe U, Bausero P, Visioli F. Polyunsaturated fatty acids as antioxidants. Pharmacol Res. 2008;57(6):451‐455. [DOI] [PubMed] [Google Scholar]
- 77. Campbell EL, Louis NA, Tomassetti SE, et al. Resolvin E1 promotes mucosal surface clearance of neutrophils: a new paradigm for inflammatory resolution. FASEB J. 2007;21(12):3162‐3170. [DOI] [PubMed] [Google Scholar]
- 78. Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti‐inflammatory and pro‐resolution lipid mediators. Nat Rev Immunol. 2008;8(5):349‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Serhan CN, Gotlinger K, Hong S, Arita M. Resolvins, docosatrienes, and neuroprotectins, novel omega‐3–derived mediators, and their aspirin‐triggered endogenous epimers: an overview of their protective roles in catabasis. Prostaglandins Other Lipid Mediat. 2004;73(3‐4):155‐172. [DOI] [PubMed] [Google Scholar]
- 80. Serhan CN, Hong S, Gronert K, et al. Resolvins. J Exp Med. 2002;196(8):1025‐1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Chiang N, Serhan CN. Specialized pro‐resolving mediator network: an update on production and actions. Essays Biochem. 2020;64(3):443‐462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bendyk A, Marino V, Zilm PS, Howe P, Bartold PM. Effect of dietary omega‐3 polyunsaturated fatty acids on experimental periodontitis in the mouse. J Periodontal Res. 2009;44(2):211‐216. [DOI] [PubMed] [Google Scholar]
- 83. Hasturk H, Kantarci A, Ohira T, et al. RvE1 protects from local inflammation and osteoclast‐mediated bone destruction in periodontitis. FASEB J. 2006;20(2):401‐403. [DOI] [PubMed] [Google Scholar]
- 84. Hong S, Gronert K, Devchand PR, Moussignac R‐L, Serhan CN. Novel docosatrienes and 17S‐resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. J Biol Chem. 2003;278(17):14677‐14687. [DOI] [PubMed] [Google Scholar]
- 85. Marcheselli VL, Hong S, Lukiw WJ, et al. Novel docosanoids inhibit brain ischemia‐reperfusion‐mediated leukocyte infiltration and pro‐inflammatory gene expression. J Biol Chem. 2003;278(44):43807‐43817. [DOI] [PubMed] [Google Scholar]
- 86. Schwab J, Serhan C. Lipoxins and new lipid mediators in the resolution of inflammation. Curr Opin Pharmacol. 2006;6(4):414‐420. [DOI] [PubMed] [Google Scholar]
- 87. Bannenberg GL, Chiang N, Ariel A, et al. Molecular circuits of resolution: formation and actions of resolvins and protectins. J Immunol. 2005;174(7):4345‐4355. [DOI] [PubMed] [Google Scholar]
- 88. Duffield JS, Hong S, Vaidya VS, et al. Resolvin D series and protectin D1 mitigate acute kidney injury. J Immunol. 2006;177(9):5902‐5911. [DOI] [PubMed] [Google Scholar]
- 89. Serhan CN, Gotlinger K, Hong S, et al. Anti‐inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy‐containing docosatrienes. J Immunol. 2006;176(3):1848‐1859. [DOI] [PubMed] [Google Scholar]
- 90. Aursnes M, Tungen JE, Vik A, et al. Total synthesis of the lipid mediator PD1 n−3 DPA: configurational assignments and anti‐inflammatory and pro‐resolving actions. J Nat Prod. 2014;77(4):910‐916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Dalli J, Colas RA, Serhan CN. Novel n−3 immunoresolvents: structures and actions. Sci Rep. 2013;3(1):1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Arita M, Clish CB, Serhan CN. The contributions of aspirin and microbial oxygenase to the biosynthesis of anti‐inflammatory resolvins: novel oxygenase products from ω−3 polyunsaturated fatty acids. Biochem Biophys Res Commun. 2005;338(1):149‐157. [DOI] [PubMed] [Google Scholar]
- 93. Chen LY, Lawson DL, Mehta JL. Reduction in human neutrophil superoxide anion generation by n−3 polyunsaturated fatty acids: role of cyclooxygenase products and endothelium‐derived relaxing factor. Thrombosis Res. 1994;76(4):317‐322. [DOI] [PubMed] [Google Scholar]
- 94. Kesavalu L, Vasudevan B, Raghu B, et al. Omega‐3 fatty acid effect on alveolar bone loss in rats. J Dent Res. 2006;85(7):648‐652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Smith MR, O’Malley AJ, Keating NL. Gonadotrophin‐releasing hormone agonists, diabetes and cardiovascular disease in men with prostate cancer: which metabolic syndrome? BJU International. 2008;101(11):1335‐1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Vardar S, Buduneli E, Baylas H, Hüseyinov Berdeli A, Buduneli N, Atilla G. Individual and combined effects of selective cyclooxygenase‐2 inhibitor and omega‐3 fatty acid on endotoxin‐induced periodontitis in rats. J Periodontol. 2005;76(1):99‐106. [DOI] [PubMed] [Google Scholar]
- 97. Kesavalu L, Bakthavatchalu V, Rahman MM, et al. Omega‐3 fatty acid regulates inflammatory cytokine/mediator messenger RNA expression in Porphyromonas gingivalis–induced experimental periodontal disease. Oral Microbiol Immunol. 2007;22(4):232‐239. [DOI] [PubMed] [Google Scholar]
- 98. Guesnet P, Alessandri J‐M. Docosahexaenoic acid (DHA) and the developing central nervous system (CNS)—implications for dietary recommendations. Biochimie. 2011;93(1):7‐12. [DOI] [PubMed] [Google Scholar]
- 99. Filion KB, El Khoury F, Bielinski M, Schiller I, Dendukuri N, Brophy JM. Omega‐3 fatty acids in high‐risk cardiovascular patients: a meta‐analysis of randomized controlled trials. BMC Cardiovasc Dis. 2010;10(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Gibson RA, Kneebone GM. Fatty acid composition of human colostrum and mature breast milk. Am J Clin Nutr. 1981;34(2):252‐257. [DOI] [PubMed] [Google Scholar]
- 101. Horvath A, Koletzko B, Szajewska H. Effect of supplementation of women in high‐risk pregnancies with long‐chain polyunsaturated fatty acids on pregnancy outcomes and growth measures at birth: a meta‐analysis of randomized controlled trials. Br J Nutr. 2007;98(02):253‐259. [DOI] [PubMed] [Google Scholar]
- 102. Marchioli R. Early protection against sudden death by n−3 polyunsaturated fatty acids after myocardial infarction: time‐course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)‐Prevenzione. Circulation. 2002;105(16):1897‐1903. [DOI] [PubMed] [Google Scholar]
- 103. Szajewska H, Horvath A, Koletzko B. Effect of n−3 long‐chain polyunsaturated fatty acid supplementation of women with low‐risk pregnancies on pregnancy outcomes and growth measures at birth: a meta‐analysis of randomized controlled trials. Am J Clin Nutr. 2006;83(6):1337‐1344. [DOI] [PubMed] [Google Scholar]
- 104. Jakobsen MU, O’Reilly EJ, Heitmann BL, et al. Major types of dietary fat and risk of coronary heart disease: a pooled analysis of 11 cohort studies. Am J Clin Nutr. 2009;89(5):1425‐1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kromhout D. Omega‐3 fatty acids and coronary heart disease. The final verdict? Curr Opin Lipidol. 2012;23(6):554‐559. [DOI] [PubMed] [Google Scholar]
- 106. Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med. 2009;169(7):659‐669. [DOI] [PubMed] [Google Scholar]
- 107. Mozaffarian D, Micha R, Wallace S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta‐analysis of randomized controlled trials. PLoS Med. 2010;7(3):e1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Mozaffarian D, Wu JHY. Omega‐3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58(20):2047‐2067. [DOI] [PubMed] [Google Scholar]
- 109. Zheng J, Huang T, Yu Y, Hu X, Yang B, Li D. Fish consumption and CHD mortality: an updated meta‐analysis of seventeen cohort studies. Public Health Nutr. 2011;15(04):725‐737. [DOI] [PubMed] [Google Scholar]
- 110. Kalmijn S, Launer LJ, Ott A, Witteman JCM, Hofman A, Breteler MMB. Dietary fat intake and the risk of incident dementia in the Rotterdam study. Ann Neurol. 1997;42(5):776‐782. [DOI] [PubMed] [Google Scholar]
- 111. Morris MC, Evans DA, Bienias JL, et al. Consumption of fish and n−3 fatty acids and risk of incident Alzheimer disease. Arch Neurol. 2003;60(7):940. [DOI] [PubMed] [Google Scholar]
- 112. Farmer A, Montori V, Dinneen S, Clar C. Fish oil in people with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2001;(3):CD003205. [DOI] [PubMed] [Google Scholar]
- 113. Friedberg CE, Janssen MJFM, Heine RJ, Grobbee DE. Fish oil and glycemic control in diabetes: a meta‐analysis. Diabetes Care. 1998;21(4):494‐500. [DOI] [PubMed] [Google Scholar]
- 114. Jeppesen C, Schiller K, Schulze MB. Omega‐3 and omega‐6 fatty acids and type 2 diabetes. Curr Diab Rep. 2013;13(2):279‐288. [DOI] [PubMed] [Google Scholar]
- 115. Montori VM, Farmer A, Wollan PC, Dinneen SF. Fish oil supplementation in type 2 diabetes: a quantitative systematic review. Diabetes Care. 2000;23(9):1407‐1415. [DOI] [PubMed] [Google Scholar]
- 116. Lee Y‐H, Bae S‐C, Song G‐G. Omega‐3 polyunsaturated fatty acids and the treatment of rheumatoid arthritis: a meta‐analysis. Arch Med Res. 2012;43(5):356‐362. [DOI] [PubMed] [Google Scholar]
- 117. Miles EA, Calder PC. Influence of marine n−3 polyunsaturated fatty acids on immune function and a systematic review of their effects on clinical outcomes in rheumatoid arthritis. Br J Nutr. 2012;107(S2):S171‐S184. [DOI] [PubMed] [Google Scholar]
- 118. Cabré E, Mañosa M, Gassull MA. Omega‐3 fatty acids and inflammatory bowel diseases—a systematic review. Br J Nutr. 2012;107(S2):S240‐S252. [DOI] [PubMed] [Google Scholar]
- 119. Feagan BG, Sandborn WJ, Mittmann U, et al. Omega‐3 free fatty acids for the maintenance of remission in Crohn disease: the EPIC randomized controlled trials. JAMA. 2008;299(14):1690‐1697. [DOI] [PubMed] [Google Scholar]
- 120. Swan K, Allen PJ. Omega‐3 fatty acid for the treatment and remission of Crohn’s disease. J Complement Integr Med. 2013;10(1):221‐228. [DOI] [PubMed] [Google Scholar]
- 121. Turner D, Shah PS, Steinhart AH, Zlotkin S, Griffiths AM. Maintenance of remission in inflammatory bowel disease using omega‐3 fatty acids (fish oil): a systematic review and meta‐analyses. Inflamm Bowel Dis. 2011;17(1):336‐345. [DOI] [PubMed] [Google Scholar]
- 122. Reisman J, Schachter HM, Dales RE, et al. Treating asthma with omega‐3 fatty acids: where is the evidence? A systematic review. BMC Complement Altern Med. 2006;6(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Schachter HM, Reisman J, Tran K, et al. Health effects of omega‐3 fatty acids on asthma. Evid Rep Technol Assess (Summ). 2004;91:1‐7. [PMC free article] [PubMed] [Google Scholar]
- 124. Thien FC, De Luca S, Woods RK, Abramson MJ. Dietary marine fatty acids (fish oil) for asthma in adults and children. Cochrane Database Syst Rev. 2002;(4):CD001283. [DOI] [PubMed] [Google Scholar]
- 125. Wong KW. Clinical efficacy of n−3 fatty acid supplementation in patients with asthma. J Am Diet Assoc. 2005;105(1):98‐105. [DOI] [PubMed] [Google Scholar]
- 126. Liu L‐L, Wang L‐N. Omega‐3 fatty acids therapy for IgA nephropathy: a meta‐analysis of randomized controlled trials. Clin Nephrol. 2012;77(02):119‐125. [DOI] [PubMed] [Google Scholar]
- 127. Akter K, Gallo DA, Martin SA, et al. A review of the possible role of the essential fatty acids and fish oils in the aetiology, prevention or pharmacotherapy of schizophrenia. J Clin Pharm Ther. 2011;37(2):132‐139. [DOI] [PubMed] [Google Scholar]
- 128. Hibbeln JR. Fish consumption and major depression. Lancet. 1998;351(9110):1213. [DOI] [PubMed] [Google Scholar]
- 129. Hoen WP, Lijmer JG, Duran M, Wanders RJA, van Beveren NJM, de Haan L. Red blood cell polyunsaturated fatty acids measured in red blood cells and schizophrenia: a meta‐analysis. Psychiatry Res. 2013;207(1‐2):1‐12. [DOI] [PubMed] [Google Scholar]
- 130. Locke CA, Stoll AL. Omega‐3 fatty acids in major depression. In: Simopoulos AP, Pavlou KN, eds. Nutrition and Fitness: Diet, Genes, Physical Activity and Health World Review of Nutrition and Dietetics. Vol 89. Karger; 2001:173‐185. [DOI] [PubMed] [Google Scholar]
- 131. Maes M, Christophe A, Delanghe J, Altamura C, Neels H, Meltzer HY. Lowered ω3 polyunsaturated fatty acids in serum phospholipids and cholesteryl esters of depressed patients. Psychiatry Res. 1999;85(3):275‐291. [DOI] [PubMed] [Google Scholar]
- 132. Noaghiul S, Hibbeln JR. Cross‐national comparisons of seafood consumption and rates of bipolar disorders. Am J Psychiatry. 2003;160(12):2222‐2227. [DOI] [PubMed] [Google Scholar]
- 133. Ortega RM, Rodríguez‐Rodríguez E, López‐Sobaler AM. Effects of omega 3 fatty acids supplementation in behavior and non‐neurodegenerative neuropsychiatric disorders. Br J Nutr. 2012;107(S2):S261‐S270. [DOI] [PubMed] [Google Scholar]
- 134. Peet M, Murphy B, Shay J, Horrobin D. Depletion of omega‐3 fatty acid levels in red blood cell membranes of depressive patients. Biol Psychiatry. 1998;43(5):315‐319. [DOI] [PubMed] [Google Scholar]
- 135. Prior PL, Galduróz JCF. (n–3) fatty acids: molecular role and clinical uses in psychiatric disorders. Adv Nutr. 2012;3(3):257‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Tiemeier H, van Tuijl HR, Hofman A, Kiliaan AJ, Breteler MMB. Plasma fatty acid composition and depression are associated in the elderly: the Rotterdam Study. Am J Clin Nutr. 2003;78(1):40‐46. [DOI] [PubMed] [Google Scholar]
- 137. Siena L, Cipollina C, Di Vincenzo S, et al. Electrophilic derivatives of omega‐3 fatty acids counteract lung cancer cell growth. Cancer Chemother Pharmacol. 2018;81(4):705‐716. [DOI] [PubMed] [Google Scholar]
- 138. Vernieri C, Nichetti F, Raimondi A, et al. Diet and supplements in cancer prevention and treatment: clinical evidences and future perspectives. Crit Rev Oncol Hematol. 2018;123:57‐73. [DOI] [PubMed] [Google Scholar]
- 139. Zanoaga O, Jurj A, Raduly L, et al. Implications of dietary ω−3 and ω−6 polyunsaturated fatty acids in breast cancer. Exp Ther Med. 2017;15(2):1167‐1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15(1):30‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Kantarci A, Van Dyke TE. Lipoxin signaling in neutrophils and their role in periodontal disease. Prostaglandins Leukot Essent Fatty Acids. 2005;73(3‐4):289‐299. [DOI] [PubMed] [Google Scholar]
- 142. Offenbacher S. Periodontal diseases: pathogenesis. Ann Periodontol. 1996;1(1):821‐878. [DOI] [PubMed] [Google Scholar]
- 143. Page RC. The role of inflammatory mediators in the pathogenesis of periodontal disease. J Periodontal Res. 1991;26(3):230‐242. [DOI] [PubMed] [Google Scholar]
- 144. Reddy MS, Geurs NC, Gunsolley JC. Periodontal host modulation with antiproteinase, anti‐inflammatory, and bone‐sparing agents. A systematic review. Ann Periodontol. 2003;8(1):12‐37. [DOI] [PubMed] [Google Scholar]
- 145. Van Dyke TE, Serhan CN. Resolution of inflammation: a new paradigm for the pathogenesis of periodontal diseases. J Dent Res. 2003;82(2):82‐90. [DOI] [PubMed] [Google Scholar]
- 146. Alam SQ, Bergens BM, Alam BS. Arachidonic acid, prostaglandin E2 and leukotriene C4 levels in gingiva and submandibular salivary glands of rats fed diets containing n−3 fatty acids. Lipids. 1991;26(11):895‐900. [DOI] [PubMed] [Google Scholar]
- 147. Eberhard J, Heilmann F, Acil Y, Albers HK, Jepsen S. Local application of n−3 or n−6 polyunsaturated fatty acids in the treatment of human experimental gingivitis. J Clin Periodontol. 2002;29(4):364‐369. [DOI] [PubMed] [Google Scholar]
- 148. Deore GD, Gurav AN, Patil R, Shete AR, NaikTari RS, Inamdar SP. Omega 3 fatty acids as a host modulator in chronic periodontitis patients: a randomised, double‐blind, palcebo‐controlled, clinical trial. J Periodontal Implant Sci. 2014;44(1):25‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Stando MPP, Namiecinska M, Lewkowicz P, et al. Omega‐3 polyunsaturated fatty acids EPA and DHA as an adjunct to non‐surgical treatment of periodontitis: a randomized clinical trial. 2020;12(9):2614‐2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Kruse AB, Kowalski CD, Leuthold S, Vach K, Ratka‐Krüger P, Woelber JP. What is the impact of the adjunctive use of omega‐3 fatty acids in the trestment of periodontitis? A systematic review and meta‐analysis. Lipids Health Dis. 2020;19(1):100‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Heo H, Bae JH, Amano A, Park T, Choi YH. Supplemental or dietary intake of omega‐3 fatty acids for the treatment of periodontitis: a meta‐analysis. J Clin Periodontol. 2022;49(4):362‐377. [DOI] [PubMed] [Google Scholar]
- 152. El‐Sharkawy H, Aboelsaad N, Eliwa M, et al. Adjunctive treatment of chronic periodontitis with daily dietary supplementation with omega‐3 fatty acids and low‐dose aspirin. J Periodontol. 2010;81(11):1635‐1643. [DOI] [PubMed] [Google Scholar]
- 153. Cochran DL. Inflammation and bone loss in periodontal disease. J Periodontol. 2008;79(8s):1569‐1576. [DOI] [PubMed] [Google Scholar]
- 154. Kawai T, Paster BJ, Komatsuzawa H, et al. Cross‐reactive adaptive immune response to oral commensal bacteria results in an induction of receptor activator of nuclear factor‐κB ligand (RANKL)‐dependent periodontal bone resorption in a mouse model. Oral Microbiol Immunol. 2007;22(3):208‐215. [DOI] [PubMed] [Google Scholar]
- 155. Passoja A, Ylipalosaari M, Tervonen T, Raunio T, Knuuttila M. Matrix metalloproteinase‐8 concentration in shallow crevices associated with the extent of periodontal disease. J Clin Periodontol. 2008;35(12):1027‐1031. [DOI] [PubMed] [Google Scholar]
- 156. Rajasekhar L, Liou L, Chan C, Tsai W, Cheng C. Matrix metalloproteinase‐8 in sera and from polymorphonuclear leucocytes in rheumatoid arthritis: in vitro characterization and correlation with disease activity. Clin Exp Rheumatol. 2004;22:597‐602. [PubMed] [Google Scholar]
- 157. Arita M, Bianchini F, Aliberti J, et al. Stereochemical assignment, antiinflammatory properties, and receptor for the omega‐3 lipid mediator resolvin E1. J Exp Med. 2005;201(5):713‐722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Chiang N, Bermudez EA, Ridker PM, Hurwitz S, Serhan CN. Aspirin triggers antiinflammatory 15‐epi‐lipoxin A4 and inhibits thromboxane in a randomized human trial. Proc Natl Acad Sci USA. 2004;101(42):15178‐15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Bannenberg G, Arita M, Serhan CN. Endogenous receptor agonists: resolving inflammation. ScientificWorldJournal. 2007;7:1440‐1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Elwakeel NM, Hazaa HH. Effect of omega 3 fatty acids plus low‐dose aspirin on both clinical and biochemical profiles of patients with chronic periodontitis and type 2 diabetes: a randomized double blind placebo‐controlled study. J Periodont Res. 2015;50(6):721‐729. [DOI] [PubMed] [Google Scholar]
- 161. Engebretson SP, Grbic JT, Singer R, Lamster IB. GCF IL‐1β profiles in periodontal disease. J Clin Periodontol. 2002;29(1):48‐53. [DOI] [PubMed] [Google Scholar]
- 162. Dezerega A, Pozo P, Hernández M, et al. Chemokine monocyte chemoattractant protein‐3 in progressive periodontal lesions in patients with chronic periodontitis. J Periodontol. 2010;81(2):267‐276. [DOI] [PubMed] [Google Scholar]
- 163. Kang JX, Weylandt KH. Modulation of inflammatory cytokines by omega‐3 fatty acids. Subcellular Biochem. 2008;49:133‐143. [DOI] [PubMed] [Google Scholar]
- 164. Vardar S, Buduneli E, Türkoǧlu O, et al. Therapeutic versus prophylactic plus therapeutic administration of omega‐3 fatty acid on endotoxin‐induced periodontitis in rats. J Periodontol. 2004;75(12):1640‐1646. [DOI] [PubMed] [Google Scholar]
- 165. Elkhouli A. The efficacy of host response modulation therapy (omega‐3 plus low‐dose aspirin) as an adjunctive treatment of chronic periodontitis (Clinical and biochemical study). J Periodont Res. 2011;46(2):261‐268. [DOI] [PubMed] [Google Scholar]
- 166. Castro Dos Santos NC, NMRB A, Araujo CF, et al. Omega‐3 PUFA and aspirin as adjuncts to periodontal debridement in patients with periodontitis and type 2 diabetes mellitus. Randomized clinical trial. J Periodontol. 2020;91(10):1318‐1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Vardar‐Sengul S, Buduneli E, Turkoglu O, et al. The effects of selective COX‐2 inhibitor/celecoxib and omega‐3 fatty acid on matrix metalloproteinases, TIMP‐1, and laminin‐5γ2‐chain immunolocalization in experimental periodontitis. J Periodontol. 2008;79(10):1934‐1941. [DOI] [PubMed] [Google Scholar]
- 168. Griel AE, Kris‐Etherton PM, Hilpert KF, Zhao G, West SG, Corwin RL. An increase in dietary n−3 fatty acids decreases a marker of bone resorption in humans. Nutr J. 2007;6(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Ozaki Y, Morozumi T, Watanabe K, et al. Inhibitory effect of omega‐3 fatty acids on alveolar bone resorption and osteoclasts differentiation. J Oral Sci. 2020;62(3):298‐302. [DOI] [PubMed] [Google Scholar]
- 170. Watkins BA, Hutchins H, Li Y, Seifert MF. The endocannabinoid signaling system: a marriage of PUFA and musculoskeletal health. J Nutr Biochem. 2010;21(12):1141‐1152. [DOI] [PubMed] [Google Scholar]
- 171. Salari P, Abdollahi M. Controversial effects of non‐steroidal anti‐inflammatory drugs on bone: a review. Inflamm Allergy Drug Targets. 2009;8(3):169‐175. [DOI] [PubMed] [Google Scholar]
- 172. Longo AB, Ward WE. Providing flaxseed oil but not menhaden oil protects against OVX induced bone loss in the mandible of Sprague‐Dawley rats. Nutrients. 2016;8(10):597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Rahman MM, Bhattacharya A, Fernandes G. Docosahexaenoic acid is more potent inhibitor of osteoclast differentiation in RAW 264.7 cells than eicosapentaenoic acid. J Cell Physiol. 2008;214(1):201‐209. [DOI] [PubMed] [Google Scholar]
- 174. Rothacker DQ, Watemberg S. Short‐term hunger intensity changes following ingestion of a meal replacement bar for weight control. Int J Food Properties. 2004;7(3):553‐559. [DOI] [PubMed] [Google Scholar]
- 175. Makki K, Froguel P, Wolowczuk I. Adipose tissue in obesity‐related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflamm. 2013;2013:139239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Oliver E, McGillicuddy FC, Harford KA, et al. Docosahexaenoic acid attenuates macrophage‐induced inflammation and improves insulin sensitivity in adipocytes‐specific differential effects between LC n−3 PUFA. J Nutr Biochem. 2012;23(9):1192‐1200. [DOI] [PubMed] [Google Scholar]
- 177. Thorsdottir I, Tomasson H, Gunnarsdottir I, et al. Randomized trial of weight‐loss‐diets for young adults varying in fish and fish oil content. Int J Obes. 2007;31(10):1560‐1566. [DOI] [PubMed] [Google Scholar]
- 178. Golub N, Geba D, Mousa S, Williams G, Block R. Greasing the wheels of managing overweight and obesity with omega‐3 fatty acids. Med Hypotheses. 2011;77(6):1114‐1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Brosnan JT, Brosnan ME. Branched‐chain amino acids: enzyme and substrate regulation. J Nutr. 2006;136(1):207S‐211S. [DOI] [PubMed] [Google Scholar]
- 180. Thomson RL, Buckley JD. Protein hydrolysates and tissue repair. Nutr Res Rev. 2011;24(02):191‐197. [DOI] [PubMed] [Google Scholar]
- 181. Tui C. The value of protein and its chemical components (amino acids) in surgical repair. Bull N Y Acad Med. 1945;21(12):631‐635. [PMC free article] [PubMed] [Google Scholar]
- 182. Promislow JHE, Goodman‐Gruen D, Slymen DK, Barrett‐Connor E. Protein consumption and bone mineral density in the elderly: the Rancho Bernardo study. Am J Epidemiol. 2002;155(7):636‐644. [DOI] [PubMed] [Google Scholar]
- 183. Vatanparast H, Bailey DA, Baxter‐Jones ADG, Whiting SJ. The effects of dietary protein on bone mineral mass in young adults may be modulated by adolescent calcium intake. J Nutr. 2007;137(12):2674‐2679. [DOI] [PubMed] [Google Scholar]
- 184. Weaver CM, Gordon CM, Janz KF, et al. The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. Osteoporosis Int. 2016;27(4):1281‐1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Weaver CM, Proulx WR, Heaney R. Choices for achieving adequate dietary calcium with a vegetarian diet. Am J Clin Nutr. 1999;70(3):543s‐548s. [DOI] [PubMed] [Google Scholar]
- 186. Cornish J, Callon KE, Naot D, et al. Lactoferrin is a potent regulator of bone cell activity and increases bone formation in vivo . Endocrinology. 2004;145(9):4366‐4374. [DOI] [PubMed] [Google Scholar]
- 187. Caccavo D, Sebastiani GD, Di Monaco C, et al. Increased levels of lactoferrin in synovial fluid but not in serum from patients with rheumatoid arthritis. Int J Clin Lab Res. 1999;29(1):30‐35. [DOI] [PubMed] [Google Scholar]
- 188. Cornish J. Parathyroid hormone‐related protein‐(107‐139) inhibits bone resorption in vivo . Endocrinology. 1997;138(3):1299‐1304. [DOI] [PubMed] [Google Scholar]
- 189. Cornish J, Callon KE, Lin CQ, et al. Comparison of the effects of calcitonin gene‐related peptide and amylin on osteoblasts. J Bone Miner Res. 1999;14(8):1302‐1309. [DOI] [PubMed] [Google Scholar]
- 190. Cornish J, Callon KE, Reid IR. Insulin increases histomorphometric indices of bone formation in vivo . Calcif Tissue Int. 1996;59(6):492‐495. [DOI] [PubMed] [Google Scholar]
- 191. Hangoc G, Falkenburg J, Broxmeyer H. Influence of T‐lymphocytes and lactoferrin on the survival‐promoting effects of IL‐1 and IL‐6 on human bone marrow granulocyte‐macrophage and erythroid progenitor cells. Exp Hematol. 1991;19(7):697‐703. [PubMed] [Google Scholar]
- 192. Lorget F, Clough J, Oliveira M, Daury M‐C, Sabokbar A, Offord E. Lactoferrin reduces in vitro osteoclast differentiation and resorbing activity. Biochem Biophys Res Commun. 2002;296(2):261‐266. [DOI] [PubMed] [Google Scholar]
- 193. Mackie EJ, Trechsel U. Stimulation of bone formation in vivo by transforming growth factor‐β: remodeling of woven bone and lack of inhibition by indomethacin. Bone. 1990;11(4):295‐300. [DOI] [PubMed] [Google Scholar]
- 194. Marcelli C, Yates AJ, Mundy GR. In vivo effects of human recombinant transforming growth factor β on bone turnover in normal mice. J Bone Miner Res. 2009;5(10):1087‐1096. [DOI] [PubMed] [Google Scholar]
- 195. Baveye S, Elass E, Mazurier J, Spik G, Legrand D. Lactoferrin: a multifunctional glycoprotein involved in the modulation of the inflammatory process. Clin Chem Lab Med. 1999;37(3):281‐286. [DOI] [PubMed] [Google Scholar]
- 196. He S, McEuen AR, Blewett SA, et al. The inhibition of mast cell activation by neutrophil lactoferrin: uptake by mast cells and interaction with tryptase, chymase and cathepsin G. Biochem Pharmacol. 2003;65(6):1007‐1015. [DOI] [PubMed] [Google Scholar]
- 197. Kimber I, Cumberbatch M, Dearman RJ, Headon DR, Bhushan M, Griffiths CE. Lactoferrin: influences on Langerhans cells, epidermal cytokines, and cutaneous inflammation. Biochem Cell Biol. 2002;80(1):103‐107. [DOI] [PubMed] [Google Scholar]
- 198. Weinberg ED. Human lactoferrin: a novel therapeutic with broad spectrum potential. J Pharm Pharmcol. 2001;53(10):1303‐1310. [DOI] [PubMed] [Google Scholar]
- 199. Zimecki M, Właszczyk A, Zagulski T, Kübler A. Lactoferrin lowers serum interleukin 6 and tumor necrosis factor alpha levels in mice subjected to surgery. Arch Immunol Ther Exp (Warsz). 1998;46(2):97‐104. [PubMed] [Google Scholar]
- 200. Da Silva MS, Bigo C, Barbier O, Rudkowska I. Whey protein hydrolysate and branched‐chain amino acids downregulate inflammation‐related genes in vascular endothelial cells. Nutr Res. 2017;38:43‐51. [DOI] [PubMed] [Google Scholar]
- 201. Schürch M‐A, Rizzoli R, Slosman D, Vadas L, Vergnaud P, Bonjour JP. Protein supplements increase serum insulin‐like growth factor–I levels and attenuate proximal femur bone loss in patients with recent hip fracture. A randomized, double‐blind, placebo‐controlled trial. Ann Int Med. 1998;128(10):801‐809. [DOI] [PubMed] [Google Scholar]
- 202. Thissen J‐P, Ketelslegers J‐M, Underwood LE. Nutritional regulation of the insulin‐like growth factors. Endocr Rev. 1994;15(1):80‐101. [DOI] [PubMed] [Google Scholar]
- 203. Thissen J‐P, Underwood LE, Maiter D, Maes M, Clemmons DR, Ketelslegers J‐M. Failure of insulin‐like growth factor–I (IGF‐I) infusion to promote growth in protein‐restricted rats despite normalization of serum IGF‐I concentrations. Endocrinology. 1991;128(2):885‐890. [DOI] [PubMed] [Google Scholar]
- 204. Rondanelli M, Klersy C, Terracol G, et al. Whey protein, amino acids, and vitamin D supplementation with physical activity increases fat‐free mass and strength, functionality, and quality of life and decreases inflammation in sarcopenic elderly. Am J Clin Nutr. 2016;103(3):830‐840. [DOI] [PubMed] [Google Scholar]
- 205. Tkatch L, Rapin CH, Rizzoli R, et al. Benefits of oral protein supplementation in elderly patients with fracture of the proximal femur. J Am Coll Nutr. 1992;11(5):519‐525. [DOI] [PubMed] [Google Scholar]
- 206. Clemente A. Enzymatic protein hydrolysates in human nutrition. Trends Food Sci Technol. 2000;11(7):254‐262. [Google Scholar]
- 207. Sinha R, Radha C, Prakash J, Kaul P. Whey protein hydrolysate: functional properties, nutritional quality and utilization in beverage formulation. Food Chem. 2007;101(4):1484‐1491. [Google Scholar]
- 208. Manninen AH. Hyperinsulinaemia, hyperaminoacidaemia and post‐exercise muscle anabolism: the search for the optimal recovery drink. Br J Sports Med. 2006;40(11):900‐905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Morifuji M, Ishizaka M, Baba S, et al. Comparison of different sources and degrees of hydrolysis of dietary protein: effect on plasma amino acids, dipeptides, and insulin responses in human subjects. J Agric Food Chem. 2010;58(15):8788‐8797. [DOI] [PubMed] [Google Scholar]
- 210. Clague MB, Keir MJ, Wright PD, Johnston IDA. The effects of nutrition and trauma on whole‐body protein metabolism in man. Clin Sci. 1983;65(2):165‐175. [DOI] [PubMed] [Google Scholar]
- 211. Harvey SC, Howes EL. Effect of high protein diet on the velocity of growth of fibroblasts in the healing wound. Ann Surg. 1930;91(5):641‐650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Levenson SM, Lund CC. Protein metabolism in surgical patients. Am J Nurs. 1948;48(7):415‐418. [PubMed] [Google Scholar]
- 213. Tashiro T, Yamamori H, Takagi K, Morishima Y, Nakajima N. Increased contribution by myofibrillar protein to whole‐body protein breakdown according to severity of surgical stress. Nutrition. 1996;12(10):685‐689. [DOI] [PubMed] [Google Scholar]
- 214. Lee JH, Park E, Jin HJ, et al. Anti‐inflammatory and anti‐genotoxic activity of branched chain amino acids (BCAA) in lipopolysaccharide (LPS) stimulated RAW 264.7 macrophages. Food Sci Biotechnol. 2017;26(5):1371‐1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Kim ND, Kim EM, Kang KW, Cho MK, Choi SY, Kim SG. Ginsenoside Rg3 inhibits phenylephrine‐induced vascular contraction through induction of nitric oxide synthase. Br J Pharmacol. 2003;140(4):661‐670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Sharma JN, Al‐Omran A, Parvathy SS. Role of nitric oxide in inflammatory diseases. Inflammopharmacology. 2007;15(6):252‐259. [DOI] [PubMed] [Google Scholar]
- 217. Bloomgarden Z. Diabetes and branched‐chain amino acids: what is the link? J Diab. 2018;10(5):350‐352. [DOI] [PubMed] [Google Scholar]
- 218. Harris RA, Joshi M, Jeoung NH, Obayashi M. Overview of the molecular and biochemical basis of branched‐chain amino acid catabolism. J Nutr. 2005;135(6):1527S‐1530S. [DOI] [PubMed] [Google Scholar]
- 219. Tobias DK, Lawler PR, Harada PH, et al. Circulating branched‐chain amino acids and incident cardiovascular disease in a prospective cohort of US women. Circ Genom Precis Med. 2018;11(4):e002157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Castell LM, Newsholme EA. Glutamine and the effects of exhaustive exercise upon the immune response. Can J Physiol Pharmacol. 1998;76(5):524‐532. [DOI] [PubMed] [Google Scholar]
- 221. Hall JC, Heel K, McCauley R. Glutamine. Br J Surg. 1996;83(3):305‐312. [DOI] [PubMed] [Google Scholar]
- 222. Murakami H, Shimbo K, Takino Y, Kobayashi H. Combination of BCAAs and glutamine enhances dermal collagen protein synthesis in protein‐malnourished rats. Amino Acids. 2012;44(3):969‐976. [DOI] [PubMed] [Google Scholar]
- 223. Ammann P, Laib A, Bonjour JP, Meyer JM, Rüegsegger P, Rizzoli R. Dietary essential amino acid supplements increase bone strength by influencing bone mass and bone microarchitecture in ovariectomized adult rats fed an isocaloric low‐protein diet. J Bone Miner Res. 2002;17(7):1264‐1272. [DOI] [PubMed] [Google Scholar]
- 224. Aral K, Berdeli E, Aral CA, Berdeli A, Atan M. Effects of bodybuilding and protein supplements in saliva, gingival crevicular fluid, and serum. J Oral Sci. 2017;59(1):121‐130. [DOI] [PubMed] [Google Scholar]
- 225. Henrotin Y, Chevalier X, Herrero‐Beaumont G, et al. Physiological effects of oral glucosamine on joint health: current status and consensus on future research priorities. BMC Res Notes. 2013;6(1):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. McAlindon TE, LaValley MP, Gulin JP, Felson DT. Glucosamine and chondroitin for treatment of osteoarthritis: a systematic quality assessment and meta‐analysis. JAMA. 2000;283(11):1469‐1475. [DOI] [PubMed] [Google Scholar]
- 227. Nagaoka I, Igarashi M, Sakamoto K. Biological activities of glucosamine and its related substances. In: Kim S‐K, ed. Marine Medicinal Foods: Implications and Applications—Animals and Microbes. Advances in Food and Nutrition Research. Vol 65. Academic Press; 2012:337‐352. [DOI] [PubMed] [Google Scholar]
- 228. Reginster JY, Deroisy R, Rovati LC, et al. Long‐term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo‐controlled clinical trial. Lancet. 2001;357(9252):251‐256. [DOI] [PubMed] [Google Scholar]
- 229. Hua J, Sakamoto K, Kikukawa T, Abe C, Kurosawa H, Nagaoka I. Evaluation of the suppressive actions of glucosamine on the interleukin‐1β‐mediated activation of synoviocytes. Inflamm Res. 2007;56(10):432‐438. [DOI] [PubMed] [Google Scholar]
- 230. Igarashi M, Sakamoto K, Nagaoka I. Effect of glucosamine, a therapeutic agent for osteoarthritis, on osteoblastic cell differentiation. Int J Mol Med. 2011;28(3):373‐379. [DOI] [PubMed] [Google Scholar]
- 231. Lippiello L. Collagen synthesis in tenocytes, ligament cells and chondrocytes exposed to a combination of glucosamine HCl and chondroitin sulfate. Evid Based Complement Alternat Med. 2007;4(2):219‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Clegg DO, Reda DJ, Harris CL, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354(8):795‐808. [DOI] [PubMed] [Google Scholar]
- 233. Lippiello L, Woodward J, Karpman R, Hammad TA. In vivo chondroprotection and metabolic synergy of glucosamine and chondroitin sulfate. Clin Orthop Relat Res. 2000;381:229‐240. [DOI] [PubMed] [Google Scholar]
- 234. Hua J, Suguro S, Hirano S, Sakamoto K, Nagaoka I. Preventive actions of a high dose of glucosamine on adjuvant arthritis in rats. Inflamm Res. 2005;54(3):127‐132. [DOI] [PubMed] [Google Scholar]
- 235. Yomogida S, Hua J, Sakamoto K, Nagaoka I. Glucosamine suppresses interleukin‐8 production and ICAM‐1 expression by TNF‐α‐stimulated human colonic epithelial HT‐29 cells. Int J Mol Med. 2008;22(2):205‐211. [PubMed] [Google Scholar]
- 236. Yomogida S, Kojima Y, Tsutsumi‐Ishii Y, Hua J, Sakamoto K, Nagaoka I. Glucosamine, a naturally occurring amino monosaccharide, suppresses dextran sulfate sodium–induced colitis in rats. Int J Mol Med. 2008;22(3):317‐323. [PubMed] [Google Scholar]
- 237. Ju Y, Hua J, Sakamoto K, Ogawa H, Nagaoka I. Modulation of TNF‐α–induced endothelial cell activation by glucosamine, a naturally occurring amino monosaccharide. Int J Mol Med. 2008;22(6):809‐815. [PubMed] [Google Scholar]
- 238. Yomogida S, Kojima Y, Hua J, Ju Y, Sakamoto K, Nagaoka I. Evaluation of the effect of glucosamine on atherosclerosis using atherosclerotic B6·KOR·Apoeshl mice. Chitin Chitosan Res. 2008;14:55‐61. [Google Scholar]
- 239. Black SKI, Salmos D. C‐reactive protein. J Biol Chem. 2004;279:48487‐48490. [DOI] [PubMed] [Google Scholar]
- 240. Kantor ED, Lampe JW, Navarro SL, Song X, Milne GL, White E. Associations between glucosamine and chondroitin supplement use and biomarkers of systemic inflammation. J Altern Complement Med. 2014;20(6):479‐485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Pahl HL. Activators and target genes of Rel/NF‐κB transcription factors. Oncogene. 1999;18(49):6853‐6866. [DOI] [PubMed] [Google Scholar]
- 242. Bak YK, Lampe JW, Sung MK. Effects of dietary supplementation of glucosamine sulfate on intestinal inflammation in a mouse model of experimental colitis. J Gastroenterol Hepatol. 2014;29(5):957‐963. [DOI] [PubMed] [Google Scholar]
- 243. Largo R, Alvarez‐Soria M, Dıez‐Ortego I, et al. Glucosamine inhibits IL‐1β–induced NFκB activation in human osteoarthritic chondrocytes. Osteoarthritis Cartilage. 2003;11(4):290‐298. [DOI] [PubMed] [Google Scholar]
- 244. Mosmann TR, Sad S. The expanding universe of T‐cell subsets: Th1, Th2 and more. Immunol Today. 1996;17(3):138‐146. [DOI] [PubMed] [Google Scholar]
- 245. Xu C‐X, Jin H, Chung Y‐S, et al. Chondroitin sulfate extracted from ascidian tunic inhibits phorbol ester‐induced expression of inflammatory factors VCAM‐1 and COX‐2 by blocking NF‐κB activation in mouse skin. J Agric Food Chem. 2008;56(20):9667‐9675. [DOI] [PubMed] [Google Scholar]
- 246. Largo R, Martínez‐Calatrava MJ, Sánchez‐Pernaute O, et al. Effect of a high dose of glucosamine on systemic and tissue inflammation in an experimental model of atherosclerosis aggravated by chronic arthritis. Am J Physiol Heart Circ Physiol. 2009;297(1):H268‐H276. [DOI] [PubMed] [Google Scholar]
- 247. Park MK, Cho SA, Lee HJ, et al. Suppression of transglutaminase‐2 is involved in anti‐inflammatory actions of glucosamine in 12‐O‐tetradecanoylphorbol‐13‐acetate–induced skin inflammation. Biomol Ther. 2012;20(4):380‐385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Wu Y‐L, Kou YR, Ou H‐L, et al. Glucosamine regulation of LPS‐mediated inflammation in human bronchial epithelial cells. Eur J Pharmacol. 2010;635(1‐3):219‐226. [DOI] [PubMed] [Google Scholar]
- 249. Wu Y‐L, Lin A‐H, Chen C‐H, et al. Glucosamine attenuates cigarette smoke‐induced lung inflammation by inhibiting ROS‐sensitive inflammatory signaling. Free Radic Biol Med. 2014;69:208‐218. [DOI] [PubMed] [Google Scholar]
- 250. Jamialahmadi K, Arasteh O, Riahi MM, Mehri S, Riahi‐Zanjani B, Karimi G. Protective effects of glucosamine hydrochloride against free radical‐induced erythrocytes damage. Environ Toxicol Pharmacol. 2014;38(1):212‐219. [DOI] [PubMed] [Google Scholar]
- 251. Ardite E, Panes J, Miranda M, et al. Effects of steroid treatment on activation of nuclear factor κB in patients with inflammatory bowel disease. Br J Pharmacol. 1998;124(3):431‐433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Kantor ED, Lampe JW, Vaughan TL, Peters U, Rehm CD, White E. Association between use of specialty dietary supplements and C‐reactive protein concentrations. Am J Epidemiol. 2012;176(11):1002‐1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Ibaraki K, Termine JD, Whitson SW, Young MF. Bone matrix mRNA expression in differentiating fetal bovine osteoblasts. J Bone Miner Res. 1992;7(7):743‐754. [DOI] [PubMed] [Google Scholar]
- 254. Weinreb M, Shinar D, Rodan GA. Different pattern of alkaline phosphatase, osteopontin, and osteocalcin expression in developing rat bone visualized by in situ hybridization. J Bone Miner Res. 1990;5(8):831‐842. [DOI] [PubMed] [Google Scholar]
- 255. Kobayashi Y, Udagawa N, Takahashi N. Action of RANKL and OPG for osteoclastogenesis. Crit Rev Eukaryot Gene Expr. 2009;19(1):61‐72. [DOI] [PubMed] [Google Scholar]
- 256. Shimizu T, Nakatani S, Kobata K, Wada M. Oral administration of N‐cetyl‐d‐glucosamine and d‐glucosamine chydrochlorite affects bone mineral density in C57BL/6J mice. Chitin Chitonas Res. 2011;17:74‐78. [Google Scholar]
- 257. Pohlig F, Ulrich J, Lenze U, et al. Glucosamine sulfate suppresses the expression of matrix metalloproteinase‐3 in osteosarcoma cells in vitro . BMC Complement Altern Med. 2016;16(1):313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Biggee BA, Blinn CM, McAlindon TE, Nuite M, Silbert JE. Low levels of human serum glucosamine after ingestion of glucosamine sulphate relative to capability for peripheral effectiveness. Ann Rheum Dis. 2006;65(2):222‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Hussain MA. A case for glucosamine. Eur J Endocrinol. 1998;139(5):472‐475. [DOI] [PubMed] [Google Scholar]
- 260. Dostrovsky NR, Towheed T, Hudson RW, Anastassiades TP. The effect of glucosamine on glucose metabolism in humans: a systematic review of the literature. Osteoarthritis Cartilage. 2011;19(4):375‐380. [DOI] [PubMed] [Google Scholar]
- 261. YMM G, Runhhaar J, Jacobs ML, Bierma‐ Zeinstra SMA. The effect of prolonged glucosamine usage on HbA1c levels and new‐onset diabetes mellitus in overweight and obese middle‐aged women. Am J Med. 2017;130(6):731‐737. [DOI] [PubMed] [Google Scholar]
- 262. Qinna NA, Shubbar MH, Matalka KZ, Al‐Jbour N, Ghattas MA, Badwan AA. Glucosamine enhances paracetamol bioavailability by reducing its metabolism. J Pharm Sci. 2015;104(1):257‐265. [DOI] [PubMed] [Google Scholar]
- 263. Kaida K, Yamashita H, Toda K, Hayashi Y. Suppressive effects of d‐glucosamine on the 5‐HT sensitive nociceptive units in the rat tooth pulpal nerve. Biomed Res Int. 2014;2014:187989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Adler CJ, Dobney K, Weyrich LS, et al. Sequencing ancient calcified dental plaque shows changes in oral microbiota with dietary shifts of the Neolithic and Industrial revolutions. Nat Genet. 2013;45(4):450‐455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Baumgartner SIT, Schicht O, Baumgartner S, et al. The impact of the Stone Age diet on gingival conditions in the absence of oral hygiene. J Periodontol. 2009;80:759‐768. [DOI] [PubMed] [Google Scholar]
- 266. ILC C, Milward MR, Ling‐Mountford N, et al. Adjunctive daily supplementation with encapsulated fruit, vegetable and berry juice powder concentrates and clinical periodontal outcomes: a double blind RCT. J Clin Periodontol. 2012;39:61‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Van der Velden U, Kuzmanova D, Chapple ILC. Micronutritional approaches to periodontal therapy. J Clin Periodontol. 2010;38(suppl 11):142‐158. [DOI] [PubMed] [Google Scholar]
- 268. Sousa Né YG, Voss Martins B, Lopes Castro MM, et al. Is nutritional intervention an improvement factor in the management of periodontitis? A systematic review. Clin Nutr. 2020;39(9):2639‐2646. [DOI] [PubMed] [Google Scholar]
- 269. Ali NM, Yeap SW, Ho WY, Beh BK, Tan SW, Tan SG. The promising future of chia, Salvia hispanica L. J Biomed Biotechnol. 2012;2012:171956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Ixtaina VY, Nolasco SM, Tomas MC. Physical properties of chia (Salvia hispanica L.) seeds. Industrial Crops Prod. 2008;28(3):286‐293. [Google Scholar]
- 271. Vuksan V, Whitham D, Sievenpiper JL, et al. Supplementation of conventional therapy with the novel grain salba (Salvia hispanica L.) improves major and emerging cardiovascular risk factors in type 2 diabetes: results of a randomized controlled trial. Diabetes Care. 2007;30(11):2804‐2810. [DOI] [PubMed] [Google Scholar]
- 272. Vuksan V, Jenkins AL, Dias AG, et al. Reduction of postprandial glucose excursion and prolongation of satiety: possible explanation of the long‐term effects of whole grain salba (Salvia hispanica L). Eur J Clin Nutr. 2010;64(4):436‐438. [DOI] [PubMed] [Google Scholar]
- 273. Taveras Toscano L, Taveras Toscano L, Leite Tavares R, da Olivera Silva CS, Silva AS. Chia induces clinically discrete weight loss and improves lipid profile only in altered previous values. Nutr Hosp. 2015;31(3):1176‐1182. [DOI] [PubMed] [Google Scholar]
- 274. Navruz‐Varli S, Sanlier N. Nutritional and health benefits of quinoa (Chenopodium quinoa Willd.). J Cereal Sci. 2016;69:371‐376. [Google Scholar]
- 275. Vilcacundo R, Hernández‐Ledesma B. Nutritional and biological value of quinoa (Chenopodium quinoa Willd.). Curr Opin Food Sci. 2017;14:1‐6. [Google Scholar]
- 276. Pasko P, Zagrodzki P, Barton H, Chlopicka J, Gorinstein S. Effects of quinoa seeds (Chenopodium quinoa) in diet on some biochemical parameters and essential elements in blood of high fructose‐fed rats. Plant Foods Hum Nutr. 2010;65:333‐338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Foucault A‐S, Mathé V, Lafont R, et al. Quinoa extract enriched in 20‐hydroxyecdysone protects mice from diet induced obesity and modulates adipokines expression. Obesity. 2011;20:270‐277. [DOI] [PubMed] [Google Scholar]
- 278. Ruales J, de Grijalva Y, Lopez‐Jaramillo P, Nair BM. The nutritional quality of an infant food from quinoa and its effect on the plasma level of insulin‐like growth factor–1 (IGF‐1) in undernourished children. Int J Food Sci Nutr. 2002;53(2):143‐154. [DOI] [PubMed] [Google Scholar]
- 279. De Carvalho FG, Ovídio PP, Padovan GJ, Jordão Junior AA, Marchini JS, Navarro AM. Metabolic parameters of postmenopausal women after quinoa or corn flakes intake—a prospective and double‐blind study. Int J Food Sci Nutr. 2014;65(3):380‐385. [DOI] [PubMed] [Google Scholar]
- 280. Zevallos VF, Herencia LI, Chang F, Donnelly S, Ellis HJ, Ciclitira PJ. Gastrointestinal effects of eating quinoa (Chenopodium quinoa Willd.) in celiac patients. Am J Gastroenterol. 2014;109(2):270‐278. [DOI] [PubMed] [Google Scholar]
- 281. Ravi M, De LS, Azharuddin S, SFD P. The beneficial effects of spirulina focusing on its immunomodulatory and antioxidant properties. Nutr Dietary Suppl. 2010;2010:73‐83. [Google Scholar]
- 282. Park HJ, Lee YJ, Ryu HK, Kim MH, Chung HW, Kim WY. A randomized double‐blind, placebo‐controlled study to establish the effects of spirulina in elderly Koreans. Ann Nutr Metab. 2008;52(4):322‐328. [DOI] [PubMed] [Google Scholar]
- 283. Karkos P, Leong S, Karkos C, Sivaji N, Assimakopoulos D. Spirulina in clinical practice: evidence‐based human applications. Evid Based Complement Alternat Med. 2011;2011:531053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Romay C, Armesto J, Remirez D, Gonzáles R, Ledon N, García I. Antioxidant and anti‐inflammatory properties of C‐phycocyanin from blue‐green algae. Inflamm Res. 1998;47(1):36‐41. [DOI] [PubMed] [Google Scholar]
- 285. Cingi C, Conk‐Dalay M, Cakli H, Bal C. The effects of spirulina on allergic rhinitis. Eur Arch Otorhinolaryngol. 2008;265(10):1219‐1223. [DOI] [PubMed] [Google Scholar]
- 286. Mathew B, Sankaranarayanan R, Nair PP, et al. Evaluation of chemoprevention of oral cancer with Spirulina fusiformis . Nutr Cancer. 1995;24(2):197‐202. [DOI] [PubMed] [Google Scholar]
- 287. Mahendra J, Mahendra L, Muthu J, John L, Romanos GE. Clinical effects of subgingivally delivered spirulina gel in chronic periodontitis cases: a placebo controlled clinical trial. J Clin Diag Res. 2013;7(10):2330‐2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Aggarwal BB, Bhatt ID, Ichikawa H, et al. Curcumin ‐ Biological and Medicinal Properties. In: Tumeric ‐ The genus Curcuma. Boca Raton: CRC Press;2007;45:297‐368. [Google Scholar]
- 289. Ghosh S, Banerjee S, Sil PC. The beneficial role of curcumin on inflammation, diabetes and neurodegenerative disease: a recent update. Food Chem Toxicol. 2015;83:111‐124. [DOI] [PubMed] [Google Scholar]
- 290. Prasad S, Gupta SC, Tyagi AK, Aggarwal BB. Curcumin, a component of golden spice: from bedside to bench and back. Biotechnol Adv. 2014;32(6):1053‐1064. [DOI] [PubMed] [Google Scholar]
- 291. Stoyell KA, Mappus JL, Gandhi MA. Clinical efficacy of turmeric use in gingivitis: a comprehensive review. Complement Ther Clin Pract. 2016;25:13‐17. [DOI] [PubMed] [Google Scholar]
- 292. Waghmare P, Chaudhari A, Karhadkar V, Jamkhande A. Comparative evaluation of turmeric and chlorhexidinegluconate mouthwash in prevention of plaque formation and gingivitis: a clinical and microbiological study. J Contemp Dent Pract. 2011;12(4):221‐224. [DOI] [PubMed] [Google Scholar]
- 293. Zhou T, Chen D, Li Q, Sun X, Song Y, Wang C. Curcumin inhibits inflammatory response and bone loss during experimental periodontitis in rats. Acta Odontol Scand. 2013;71(2):349‐356. [DOI] [PubMed] [Google Scholar]
- 294. Jensen GS, Wu X, Patterson KM, et al. In vitro and in vivo antioxidant and anti‐inflammatory capacities of an antioxidant‐rich fruit and berry juice blend. Results of a pilot and randomized, double‐blinded, placebo‐controlled, crossover study. J Agric Food Chem. 2008;56(18):8326‐8333. [DOI] [PubMed] [Google Scholar]
- 295. Brito C, Stavroullakis A, Ferreira A, et al. Extract of acai‐berry inhibits osteoclast differentiation and activity. Arch Oral Biol. 2016;68:29‐34. [DOI] [PubMed] [Google Scholar]
- 296. Jensen G, Ager D, Redman K, et al. Pain reduction and improvement of range of motion after consumption of an antioxidant‐rich fruit‑ and berry‐juice blend. J Med Food. 2011;14:702‐711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Poulose SM, Fisher DR, Larson J, et al. Anthocyanin‐rich açai (Euterpe oleracea Mart.) fruit pulp fractions attenuate inflammatory stress signaling in mouse brain BV‐2 microglial cells. J Agric Food Chem. 2012;60(4):1084‐1093. [DOI] [PubMed] [Google Scholar]
- 298. Robert SE. Periodontal disease and nutrition: separating the evidence from current fads. Periodontol 2000. 2009;50:78‐89. [DOI] [PubMed] [Google Scholar]
- 299. Divyapriya GK, Veeresh DJ, Yavagal PC. Evaluation of antibacterial efficacy of chia (Salvia hispanica) seeds extract against Porphyromonas gingivalis, Fusobacterium nucleatum and Aggregatibacter actinomycetemcomitans—an in vitro study. Int J Ayurveda Pharma Res. 2016;4(4):22‐26. [Google Scholar]
- 300. Joint FAO/WHO/UNU Expert Consultation on Protein and Amino Acid Requirements in Human Nutrition . Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint FAO/WHO/UNU Expert Consultation . WHO Technical Report Series, no. 935. WHO, Geneva, Switzerland.
- 301. Pasko P, Barton H, Zagrodzki P, et al. Effect of diet supplemented with quinoa seeds on oxidative status in plasma and selected tissues of high fructose‐fed rats. Plant Foods Hum Nutr. 2010;65:146‐151. [DOI] [PubMed] [Google Scholar]
- 302. Vasques Farinazzi‐Machado FM, Barbalho SM, Oshiiwa M, Goulart R, Pessan Junior O. Use of cereal bars with quinoa (Chenopodium quinoa W.) to reduce risk factors related to cardiovascular diseases. Ciênc Tecnol Aliment Campinas. 2012;32(2):239‐244. [Google Scholar]
- 303. Sun X, Yang X, Xue P, Zhang Z, Ren G. Improved antibacterial effects of alkali‐transformed saponin from quinoa husks against halitosis‐related bacteria. BMC Complement Altern Med. 2019;19(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Sies H, Cadenas E. Oxidative stress: damage to intact cells and organs. Phil Trans R Soc Lond B. 1985;311(1152):617‐631. [DOI] [PubMed] [Google Scholar]
- 305. Kang MS, Moon JH, Park SC, Jang YP, Choung SY. Spirulina maxima reduces inflammation and alveolar bone loss in Porphyromonas gingivalis‐induced periodontitis. Phytomedicine. 2021;81:153420. [DOI] [PubMed] [Google Scholar]
- 306. Li Y, Jiao J, Qi Y, et al. Curcumin: a review of experimental studies and mechanisms related to periodontitis treatment. J Periodontal Res. 2021;56(5):837‐847. [DOI] [PubMed] [Google Scholar]
- 307. de Paula Zago LH, de Annunzio SR, de Oliveira KT, et al. Antimicrobial photodynamic therapy against metronidazole‐resistant dental plaque bacteria. J Photochem Photobiol B. 2020;209:111903. [DOI] [PubMed] [Google Scholar]
- 308. Sreedhar A, Sarkar I, Rajan P, et al. Comparative evaluation of the efficacy of curcumin gel with and without photo activation as an adjunct to scaling and root planing in the treatment of chronic periodontitis: a split mouth clinical and microbiological study. J Nat Sci Biol Med. 2015;6(Suppl 1):S102‐S109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Ghavimi MA, Bani Shahabadi A, Jarolmasjed S, Memar MY, Maleki Dizaj S, Sharifi S. Nanofibrous asymmetric collagen/curcumin membrane containing aspirin‐loaded PLGA nanoparticles for guided bone regeneration. Sci Rep. 2020;10(1):18200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Karppanen H, Karppanen P, Mervaala E. Why and how to implement sodium, potassium, calcium, and magnesium changes in food items and diets? J Hum Hypertens. 2005;19(S3):S10. [DOI] [PubMed] [Google Scholar]
- 311. National Health and Medical Research Council, Australian Government Department of Health and Ageing, New Zealand Ministry of Health . Nutrient Reference Values for Australia and New Zealand, Including Recommended Dietary Intakes. Version 1.2. National Health and Medical Research Council; https://www.nhmrc.gov.au/guidelines‐publications/n35‐n36‐n37. [Google Scholar]
- 312. Christakos S, Dhawan P, Porta A, Mady LJ, Seth T. Vitamin D and intestinal calcium absorption. Mol Cell Endocrinol. 2011;347(1‐2):25‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Miley DD, Garcia MN, Hildebolt CF, et al. Cross‐sectional study of vitamin D and calcium supplementation effects on chronic periodontitis. J Periodontol. 2009;80(9):1433‐1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Crockett SD, Barry EL, Mott LA, et al. Calcium and vitamin D supplementation and increased risk of serrated polyps: results from a randomised clinical trial. Gut. 2019;68:475‐486 Gut. 2018 Mar 1. pii: gutjnl‐2017‐315242. doi: 10.1136/gutjnl-2017-315242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Al‐Zahrani MS. Increased intake of dairy products is related to lower periodontitis prevalence. J Periodontol. 2006;77(2):289‐294. [DOI] [PubMed] [Google Scholar]
- 316. Staudte H, Kranz S, Völpel A, Schütze J, Sigusch BW. Comparison of nutrient intake between patients with periodontitis and healthy subjects. Quintessence Int. 2012;43:907‐916. [PubMed] [Google Scholar]
- 317. Meisel P, Schwahn C, Luedemann J, John U, Kroemer HK, Kocher T. Magnesium deficiency is associated with periodontal disease. J Dent Res. 2005;84(10):937‐941. [DOI] [PubMed] [Google Scholar]


