Abstract
Objective
Intermittent rescue therapy may be used for seizure clusters, which are clinical emergencies that may persist ≥24 h and increase risk of status epilepticus, emergency room visits, and reduced quality of life for patients with epilepsy. Beyond effectiveness for aborting seizure clusters, no data exist on how intermittent rescue therapy may impact the long‐term natural course of seizure clusters. This novel analysis explores SEIzure interVAL (SEIVAL; time between seizure clusters) in patients from a long‐term safety study of diazepam nasal spray (Valtoco) to assess SEIVAL changes with intermittent rescue therapy across time.
Methods
Patients were aged 6–65 years. Age‐ and weight‐based doses of diazepam nasal spray were administered during a 12‐month treatment period with an optional follow‐up period. SEIVAL was evaluated in patients receiving two or more doses of diazepam nasal spray using 90‐day periods.
Results
Of 163 treated patients, 151 had one or more SEIVALs. One hundred twenty had SEIVALs in Period 1 and one or more other periods. An increase in SEIVAL was noted from Period 1 compared with all subsequent periods (p ≤ .001). A consistent cohort (n = 76) had one or more SEIVALs in each of Periods 1–4 (360 days); mean SEIVALs increased significantly (p < .01) from 12.2 days (Period 1) to 25.7 days (Period 4). Similar SEIVAL patterns occurred when repeat doses within a seizure cluster were eliminated and irrespective of age group, treatment duration, and change to concomitant medications. In adults, Quality of Life in Epilepsy scores were maintained with increased SEIVALs.
Significance
Across 12 months, increases in SEIVAL were demonstrated in patients using diazepam nasal spray for seizure cluster treatment in a phase 3 safety study. Increased time between seizure clusters may reflect a previously unrecognized beneficial effect of intermittent rescue therapy. These results generate a range of biological and behavioral hypotheses and warrant exploration of the impact of intermittent rescue therapy.
Keywords: acute repetitive seizures, benzodiazepine, intranasal, rescue
Key Points.
Metrics for the effectiveness of intermittent rescue therapies for seizure clusters are not well established in the literature
Seizure frequency and interval are commonly measured for daily antiseizure drugs; their meaning for intermittent rescue therapy is unclear
This post hoc analysis included patient data from a phase 3, long‐term, open‐label, repeat‐dose safety study of diazepam nasal spray
SEIVAL was used to test for any impact of intermittent rescue therapy on seizure clusters over time
We generated several biological and behavioral hypotheses to explain the significantly increased SEIVALs over time
1. INTRODUCTION
Intermittent rescue therapy aims to treat seizure clusters, and appropriate metrics are needed to assess the effectiveness of therapy. Here, we focus not on the efficacy in the acute setting, but on metrics that could capture long‐term effects of intermittent rescue therapy. The pattern over time in the number of days between two treated seizure clusters (SEIzure interVAL [SEIVAL]) is a proposed novel metric for effectiveness of intermittent rescue therapy. By analyzing changes in the recurrence rate of treated seizure clusters, SEIVAL can be used to explore the impact of intermittent rescue therapies on the natural course of seizure clusters and informs the generation of hypotheses about responsible biological and behavioral mechanisms.
Studies of the natural history of seizure clusters suggest that clusters can continue for 24 h or longer. 1 , 2 Reduction in seizure frequency and time between seizures are commonly reported as efficacy endpoints for evaluation of daily antiseizure drugs (ASDs) 3 ; however, the utility of these endpoints in regard to intermittent rescue therapy for seizure clusters has not been explored. Recurrence of seizures in a cluster (e.g., ≤24 h from the start of the cluster) has been examined, 4 and mean use of second doses of rescue medication have been calculated as a proxy measure for effectiveness. 5 , 6 However, these types of analyses may miss multiday cluster patterns or potential longer‐term effects from biological changes that occur. However, multiday changes may represent disease modification, a goal of epilepsy treatment beyond symptom suppression, as seen with, for example, inhibitors of the mechanistic target of rapamycin for seizures associated with tuberous sclerosis complex. 7
Patients with intractable epilepsy are at high risk for seizure clusters, 8 , 9 which are neurological emergencies. Seizure clusters warrant rescue treatment due to the risk for status epilepticus 10 and the disruption caused in the lives of patients and care partners, including increased emotional and financial burden and decreased quality of life. 11
Benzodiazepines are the cornerstone of intermittent rescue therapy for seizure clusters, 9 with intranasal therapies now approved for use by patients and care partners in the community. 12 , 13 Diazepam nasal spray is approved by the US Food and Drug Administration (FDA) for the acute treatment of intermittent, stereotypic episodes of frequent seizure activity (i.e., seizure clusters, acute repetitive seizures) that are distinct from a patient's usual seizure pattern in patients with epilepsy aged ≥6 years. 13 Per the FDA, administration of diazepam nasal spray by the intranasal route during a seizure event provides significantly improved ease of use compared to rectal diazepam and is thus clinically superior. 14
This analysis explores SEIVALs in patients with epilepsy and seizure clusters from a long‐term, open‐label safety study of diazepam nasal spray. The objective of the analysis was to assess whether timing between seizure clusters changes with administration of intermittent rescue therapy over time.
2. MATERIALS AND METHODS
2.1. Study design
The results presented here are from a phase 3, open‐label, repeat‐dose safety study of diazepam nasal spray (NCT02721069) conducted from April 2016 to July 2020. 6 This study evaluated long‐term safety of repeated doses of diazepam nasal spray in a broad population of patients with epilepsy and frequent seizure clusters. A 21‐day screening phase was followed by a baseline assessment period and a 12‐month treatment phase, with study visits at Day 30 and every 60 days afterward. After Day 365, patients could elect to remain on treatment. Full study methodology along with overall and subgroup safety and effectiveness results have been published. 6 The current analysis was performed post hoc.
An empirical definition of two or more seizures over a period of time outside the patient's normal seizure pattern is commonly used in the literature. 1 , 11 For the parent diazepam nasal spray study as well as for this SEIVAL analysis, an operational definition of two or more seizures treated with diazepam nasal spray in a 24‐h period was used for seizure clusters, as noted in prior published papers. 5 , 6
2.2. Patients
Enrolled patients had a clinical diagnosis of epilepsy and, in the opinion of the investigator, might need benzodiazepine treatment for seizure control at least once every other month on average (i.e., ≥6 times per year) despite a stable regimen of ASDs. Key inclusion criteria were as follows: male or female aged 6–65 years, inclusive; diagnosis of focal or generalized epilepsy with motor seizures or seizures with clear alteration of awareness; availability of a qualified care partner or medical professional who could administer study medication; no clinically significant abnormal findings in the patient's medical history or during screening; and agreement to comply with study procedures. History of status epilepticus and seasonal allergies/rhinitis was permitted; no restriction was made on concomitant use of benzodiazepines. Key exclusion criteria included major depression or a past suicide attempt or suicidal ideation and history of a clinically significant medical condition that would jeopardize the safety of the patient.
2.3. Administration and dosing
Patients and care partners were trained to administer diazepam nasal spray in age‐ and weight‐based doses (5, 10, 15, or 20 mg); administration was as needed to treat seizure clusters. Instructions were given to administer a second dose 4 to 12 h after the first dose if needed to control the seizure cluster. The dose of diazepam nasal spray could be adjusted by the study investigator as clinically warranted for effectiveness and safety. Each patient or care partner was provided with a diary for recording seizure timing and drug administration; specific seizure types were not specified in the diary during the seizure emergency. At each visit, the seizure and dosing information from the diary, including the time when the seizure occurred, when it ended, the dose, and the date and time of dosing, was recorded to the case report form.
2.4. SEIVAL analysis
The SEIVAL post hoc analysis used patient diary data from the phase 3 safety study to examine timing of recurrence of treated seizure clusters using the number of days between doses of diazepam nasal spray as a proxy for time between seizure clusters. Patients who administered two or more doses of diazepam nasal spray over the course of the study were included (i.e., two or more doses were needed to define one or more SEIVALs to examine). SEIVAL was evaluated for the safety population and for subgroups within that population.
A sensitivity analysis was performed using 90‐day periods, with doses across Period 1 and the following periods, to evaluate SEIVAL over time. Seizure clusters vary naturally across time and by patient; the 90‐day period allowed inclusion of a substantial number of patients with an assessable SEIVAL in each period. This type of analysis investigates overall patterns of seizure cluster in patients treated with diazepam nasal spray across time. A similar analysis using equal, adjacent time periods was performed with data from patients in this study to examine the potential for pharmacological tolerance to diazepam nasal spray; no statistical or clinical evidence of tolerance was found. 15
Two versions of the SEIVAL sensitivity analysis were performed. The first version included all patients with doses across Period 1 and an additional period. In the parent study, seizure clusters were defined by the 24 h following administration of the first dose. Thus, in the first version of the SEIVAL sensitivity analysis, two doses were required to define a SEIVAL, which included second doses administered during the 24 h of a single seizure cluster. In the subsequent second version of the sensitivity analysis, second doses within a seizure cluster were eliminated to ensure measurement between two seizure clusters rather than time between two doses.
In this SEIVAL analysis, four periods corresponded to 360 days, which is similar to the 365‐day treatment period of the study; thus, patients with SEIVALs across all four periods are the focus of this assessment. The analysis extended in a smaller subgroup beyond Period 4, to Period 5 (i.e., 450 days) and Period 6 (i.e., 540 days). Mean and median SEIVALs were calculated, and two‐sided paired t‐tests were used to assess statistical significance.
2.5. Consideration of potential confounder
Consistent cohorts of patients with data across consecutive periods (Periods 1–4, 1–5, and 1–6) were used to address potential confounding inherent to assessment of a variable cohort across time. This is akin to an intention‐to‐treat analysis, as it covers the possibility that patients might discontinue from the study, for example, if they did not benefit from the study medication.
Additional variables were examined to evaluate whether other potential confounders might be associated with the SEIVAL observation. These included age group (6–17, ≥18 years), study duration (<12 months vs. ≥12 months), and change in drug or dose of concomitant daily ASDs (yes vs. no) that may have influenced the findings.
Also, SEIVALs were calculated for adult patients who completed the Quality of Life in Epilepsy (QOLIE)‐31‐P tool, a tool specifically designed for use only in patients aged ≥18 years. This epilepsy‐specific instrument is used to assess health‐related quality of life based on patient responses to questions. 16 Numeric values (1–100) are assigned to the responses, with higher scores indicating better quality of life. There are seven subscales to the QOLIE‐31‐P: Seizure Worry, Overall Quality of Life, Emotional Well‐Being, Energy–Fatigue, Cognitive Functioning, Medication Effects, and Social Functioning. 16 A weighted composite of the subscales is calculated to determine the total overall score. 17
3. RESULTS
A total of 175 patients were enrolled in the phase 3 safety study of diazepam nasal spray; 163 patients received one or more doses and were included in the safety population. 6 A total of 3853 seizure clusters were treated with a total of 4390 doses of diazepam nasal spray. The mean number of doses of diazepam nasal spray per month was 2.3. The majority of patients (81.6%) had a duration of exposure to diazepam nasal spray of ≥12 months. The proportion of seizure clusters for which a second dose was administered was used as a proxy for effectiveness in the study; second doses were used in a low proportion, 12.6% (n = 485), of all seizure clusters. A total of 117 patients (71.8%) completed the study. 6
Of the 163 patients in the safety population, 12 had only one dose of diazepam nasal spray administered and were excluded from this analysis, as they had no SEIVALs to evaluate (i.e., per the definition of SEIVAL, two treated seizure clusters were required to define the interval). Therefore, 151 patients with two or more doses administered had SEIVALs and thus were included in the overall SEIVAL population (Figure 1). Aggregate data from the 151‐patient cohort show similar mean SEIVALs overall (30.7 days), which was similar in age subgroups of 6–17 years (34.3 days, n = 69) and ≥18 years (27.7 days, n = 82).
FIGURE 1.
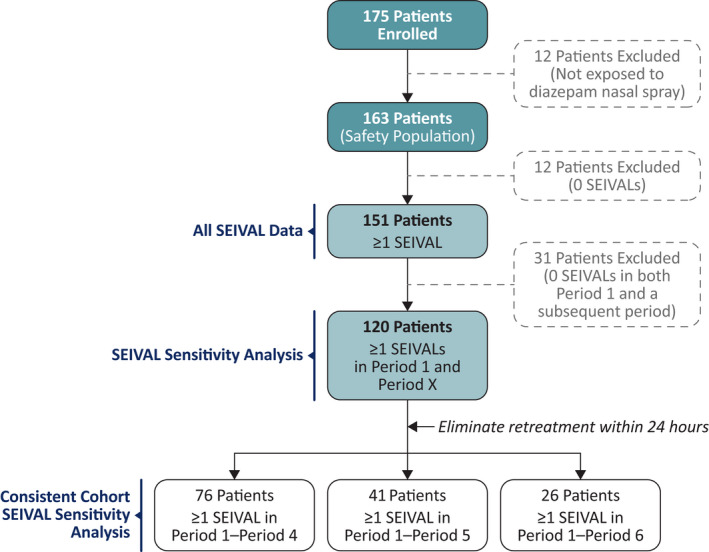
Phase 3 safety study SEIzure interVAL (SEIVAL) population disposition flowchart.
For the sensitivity analysis across time, 31 of the 151 patients with SEIVALs were excluded, as they did not have SEIVALs between two doses of diazepam nasal spray in both Period 1 and a subsequent period (i.e., both Period 1 and at least one of Period 2, 3, 4, 5, or 6; these patients may have had SEIVALs in two or more periods that did not include Period 1); thus, 120 patients had one or more SEIVALs in Period 1 and an additional subsequent period (Figure 2). Mean SEIVAL increased in Periods 2–4 compared with Period 1 (p < .001), from 14.8 days in Period 1 (0–90 days, n = 120) to 35.8 days in Period 4 (271–360 days, n = 87). Of the 120 patients, 76 had one or more SEIVALs in each of Periods 1, 2, 3, and 4 (i.e., across 360 days) and were included in the consistent cohort for Periods 1–4; this time period was similar to that of the overall study treatment period (12 months). The remaining 44 patients did not have seizure clusters in each of the consecutive periods and were not included this analysis addressing the potential for a variable cohort over time.
FIGURE 2.
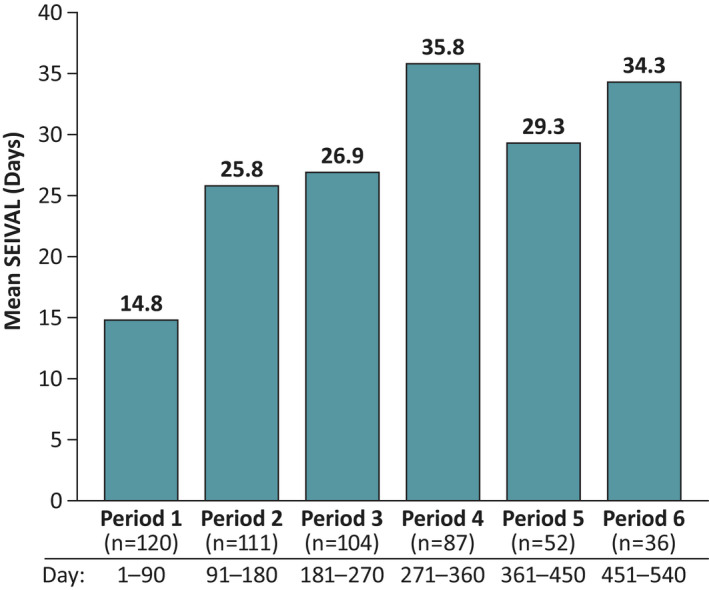
Mean SEIzure interVALs (SEIVALs) across time (sensitivity analysis; n = 120). The sensitivity analysis used consecutive 90‐day periods to show mean SEIVAL between seizure clusters across time. Included patients from the phase 3 safety study of diazepam nasal spray had one or more SEIVALs in both Period 1 (0–90 days) and an additional period to 540 days total.
Baseline demographic characteristics and safety profiles in the overall safety population (Table 1) and in the Period 1–4 SEIVAL consistent cohort were similar. In the Period 1–4 SEIVAL consistent cohort, 60.5% (n = 46) were female and 57.9% (n = 44) were adults aged ≥18 years. In these patients, treatment‐emergent adverse events (TEAEs) were reported in 93.4% (n = 71), serious TEAEs in 32.9% (n = 25), and treatment‐related TEAEs in 27.6% (n = 21); in the overall safety population, 82.2% (n = 134) reported TEAEs, 30.7% (n = 50) serious TEAEs, and 18.4% (n = 30) treatment‐related TEAEs. 6 There were no serious treatment‐related TEAEs reported in the study. Among the patients with TEAEs, 2.5% (n = 4) had a vagus nerve stimulator device implanted, and .6% (n = 1) had a brain lobectomy. Overall, one death and one discontinuation due to an adverse event were reported; neither was treatment‐related. 6
TABLE 1.
Characteristics and safety profile of overall safety population, N = 163 6
| Characteristic | Value |
|---|---|
| Baseline demographics | |
| Sex, n (%) | |
| Male | 74 (45.4) |
| Female | 89 (54.6) |
| Age, years | |
| Mean (SD) | 23.1 (15.1) |
| Median (range) | 18.0 (6–65) |
| 6–17 years, n (%) | 78 (47.9) |
| ≥18 years, n (%) | 85 (52.1) |
| Race, n (%) | |
| White | 134 (82.2) |
| Black/African American | 16 (9.8) |
| Asian | 4 (2.5) |
| Native Hawaiian/Pacific Islander | 5 (3.1) |
| Other | 4 (2.5) |
| Height, cm, mean (SD) | 151.6 (24.8) a |
| Weight, kg, mean (SD) | 60.2 (33.6) b |
| During study | |
| Exposure | |
| Mean days (SD) | 528.7 (251.7) |
| Median days (range) | 458 (56–1230) |
| <6 months, n (%) | 9 (5.5) |
| 6–12 months, n (%) | 21 (12.9) |
| ≥12 months, n (%) | 133 (81.6) |
| Safety profile, n (%) | |
| Patients with TEAEs | 134 (82.2) |
| Patients with serious TEAEs | 50 (30.7) |
| Treatment‐related | 0 |
| Death | 1 (.6) |
| Discontinuation due to a TEAE | 1 (.6) |
| Treatment‐related TEAEs | 30 (18.4) |
Abbreviation: TEAE, treatment‐emergent adverse event.
n = 159.
n = 162.
3.1. SEIVAL findings for the consistent cohort
For the Period 1–4 consistent cohort, mean number of seizure clusters treated was 9.0 in Period 1, 7.9 in Period 2, 8.4 in Period 3, and 6.6 in Period 4. Mean SEIVALs increased significantly in Periods 2–4 compared with Period 1 (p < .01; Figure 3). SEIVAL increased from 12.2 days (Period 1) to 25.7 days (Period 4). The same pattern occurred with elimination of retreatments (i.e., ensuring measurements between seizure clusters by eliminating second doses of diazepam nasal spray for a single cluster within 24 h of the first dose), with increases from 13.9 days (Period 1) to 26.8 days (Period 4). Similar increases across time also were seen for the consistent cohorts for Periods 1–5 (n = 41) and Periods 1–6 (n = 26; Figures S1, S2). Both of these cohorts had significant increases from Period 1 to the final period in each analysis (p < .05) with and without elimination of second doses. Median SEIVAL results also were consistently longer in the final period (data not shown).
FIGURE 3.
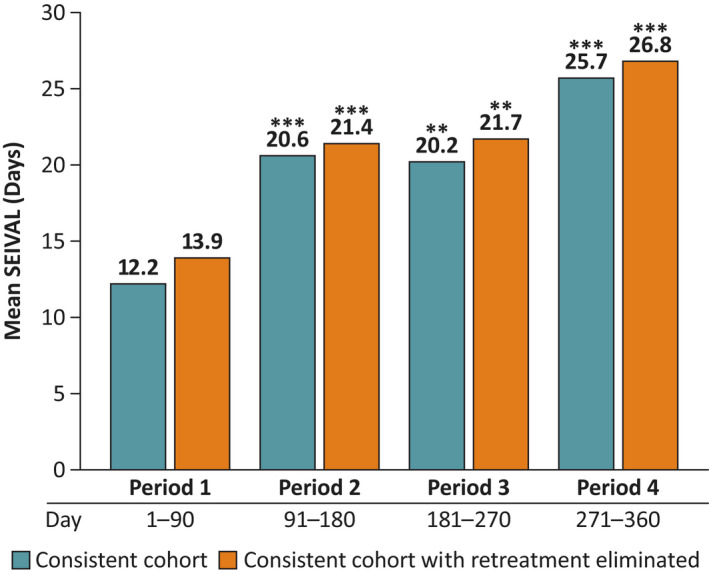
Mean SEIzure interVALs (SEIVALs) for consistent cohort for Periods 1–4 (360 days; n = 76) with and without retreatments eliminated across time. To control for survival bias across time, consistent cohorts with one or more SEIVALs between seizure clusters in each period were examined. As Periods 1–4 included 360 days, this most closely matched the 12‐month treatment period of the phase 3 safety study of diazepam nasal spray. Here, second doses administered within 24 h of the first dose were eliminated from the analysis. This ensured that time between retreatments for the same seizure cluster (defined by 24 h) were not included. Mean SEIVALs for Periods 1–4 are shown for the subgroups of patients in consistent cohort with and without retreatments eliminated. **p < .01, ***p ≤ .001 compared with Period 1.
When the Period 1–4 consistent cohort with retreatment eliminated was analyzed by age, significant mean SEIVAL increases again were observed in the 6–17‐year (n = 32) and ≥18‐year (n = 44) groups. In the pediatric group, SEIVAL increased from 13.0 days (Period 1) to 25.9 days (Period 4; p = .02); among the adults, SEIVAL increased from 14.6 days (Period 1) to 27.5 days (Period 4; p < .01).
The Period 1–4 consistent cohort also was analyzed by duration of exposure to diazepam nasal spray, and mean change in SEIVAL was similar for the small group of patients with exposure of <12 months (mean change = 28.3 days, n = 6) compared with the larger group with exposure of ≥12 months (mean change = 21.4 days, n = 81).
When a subgroup of patients with changes in concomitant daily medications (n = 56) was compared with a subgroup without such changes (n = 20), no between‐group difference was seen in mean change in SEIVAL across Periods 2–4 (Figure 4). The increase in SEIVAL from Period 1 to Period 4 for both groups was similar to the whole consistent cohort.
FIGURE 4.
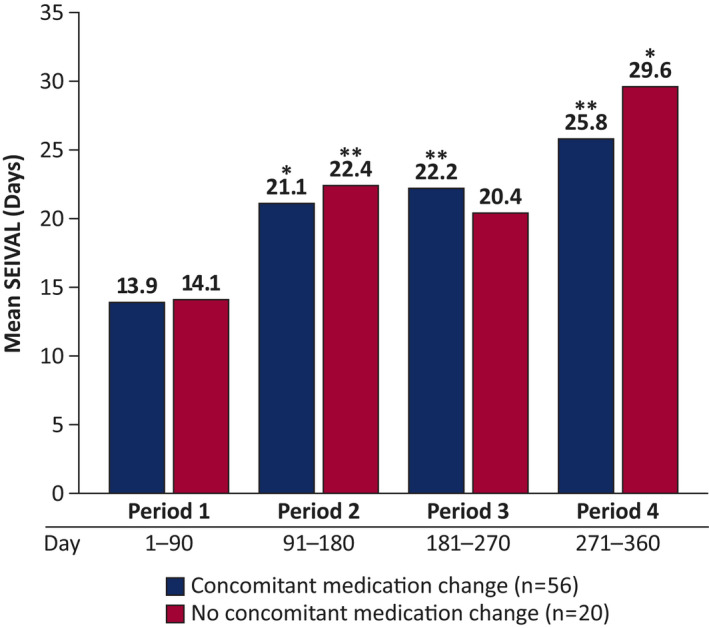
Mean SEIzure interVALs (SEIVALs) in subgroups with and without concomitant medication changes from Periods 1–4 (n = 76). Changes to concomitant daily antiseizure drugs (e.g., change of drug or dose of drug) were examined across time. This was done to determine whether such changes had an impact on SEIVAL between seizure clusters treated with diazepam nasal spray. Mean SEIVALs for Periods 1–4 are shown for the subgroups of patients with and without changes to concomitant medications. *p < .05, **p < .01 compared with Period 1.
A separate analysis of QOLIE‐31‐P responses (n = 74) from adults in the study demonstrated that quality‐of‐life scores were maintained across the 12‐month study period, generally with small numeric increase (directional improvement) in mean overall scores. 18 Within the SEIVAL Periods 1–4 consistent cohort, the subgroup of adult patients completing the QOLIE‐31‐P at Day 0 and Day 365 (n = 35) had similar change in SEIVAL between Period 1 and Period 4 (12.2 days) to the total consistent cohort (12.9 days, n = 76; Figure 5). Similar differences in overall composite QOLIE‐31‐P scores from Day 0 to Day 365 were seen for all patients with QOLIE‐31‐P data and those in the consistent cohort with QOLIE‐31‐P data (−.1 and 2.0, respectively). Two subscales of the QOLIE‐31‐P, Seizure Worry and Social Functioning, were hypothesized by the investigators to be particularly relevant to patients with seizure clusters. Similar differences in scores from Day 0 to Day 365 were seen for (1) all patients with QOLIE‐31‐P data and (2) those in the consistent cohort with QOLIE‐31‐P data, for the Seizure Worry subscale (8.7 and 6.4 points, respectively) and Social Functioning subscale (8.1 and 7.7 days, respectively) of the QOLIE‐31‐P (Figure S3). Minimally important change has been defined as 7.4 for the Seizure Worry subscale and 4.0 for the Social Functioning subscale. 19 Thus, clinically meaningful improvement was shown for all patients with QOLIE‐31‐P data on both subscales and for the SEIVAL consistent cohort group on the Social Functioning subscale.
FIGURE 5.
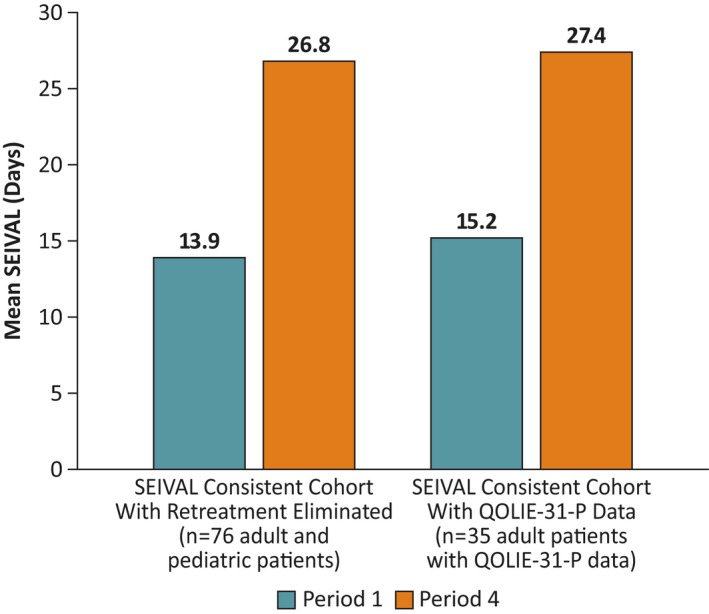
Mean SEIzure interVALs (SEIVALs) comparing Periods 1–4 overall consistent cohort and subgroup with Quality of Life in Epilepsy (QOLIE)‐31‐P data. The QOLIE‐31‐P epilepsy‐specific instrument was used to assess adult patient quality of life in the phase 3 safety study of diazepam nasal spray. Shown are mean SEIVALs at Period 1 and Period 4 for the consistent cohort with retreatments eliminated and for the subgroup of adult patients from the consistent cohort who provided QOLIE‐31‐P data.
4. DISCUSSION
This novel analysis examined SEIVAL data from epilepsy patients with seizure clusters treated intermittently with diazepam nasal spray in a phase 3 safety study. The analysis found a consistent and statistically significant pattern of increased SEIVAL over time, across all the analyzed populations. A doubling of mean SEIVALs was demonstrated between Period 1 (12.2 days) and Period 4 (25.7 days) for the 76‐patient consistent cohort treated with diazepam nasal spray across 360 days. Decreased use of this benzodiazepine formulation supports adoption to diazepam nasal spray. Potential for reduced use of this benzodiazepine over time also may mitigate concerns about the risk of dependence and misuse that are associated with this class of drugs for patients who are using these medications. 20 An interim analysis from the phase 3 safety study demonstrated a lack of observed pharmacological tolerance with use of diazepam nasal spray over time. 15 Additionally, increased SEIVAL suggests potential for fewer seizure emergencies, fewer injuries, less stress, and better quality of life.
Although the cause of the observed increase in SEIVAL across time is unknown, explanatory biological factors can be hypothesized. One possibility is that intermittent treatment with diazepam nasal spray may alter the underlying biology of clusters, although no such mechanism has yet been identified for formulations that include diazepam. Treatments may demonstrate both symptomatic and disease‐modifying effects in neurologic conditions such as multiple sclerosis. 21 , 22 In addition, symptomatic treatments may be found to also have preventive properties. For example, the oral calcitonin gene‐related peptide receptor antagonist rimegepant that was initially approved by the FDA for acute treatment of migraine in adults also was later shown to be effective as a preventive treatment 23 and has been theorized to potentially alter evolution of progressive anatomical transformation (e.g., structural change) in migraine. 24
Due to the high cost and cognitive, behavioral, and social burdens of epilepsy, disease‐modifying therapies are needed. 25 The goals of such therapies include slowing or preventing progression in the severity of epilepsy and modifying the manifestations of epilepsy (e.g., preventing increase in seizure frequency or reducing or eliminating seizures over time). 25 Such beneficial outcomes may result from modification of the ongoing epileptogenic process. 26 Currently, medical therapies for epilepsy are predominantly considered symptomatic, as their goal is to control seizures and not to specifically address the disease process. 25 However, ASDs may have disease‐modifying properties that have not been evaluated as part of the regulatory approval process. 25
Use of second doses of diazepam nasal spray was low across 24 h in the phase 3 study, which potentially suggests alteration of the natural history of individual seizure clusters with treatment. 5 Although these findings may be suggestive of a disease‐modifying effect of diazepam nasal spray—which would be an ideal effect of any therapy—this hypothesis, along with the other hypotheses discussed here, needs to be examined and verified in subsequent studies. Also, it is unclear at this point whether the change in SEIVAL is a demonstration of fewer seizure clusters or less severe seizure clusters that caregivers chose not to treat.
Another hypothesis suggests that behavioral change and personal beliefs may affect adherence to epilepsy medication over time, in particular within the setting of a clinical trial. Medicine‐taking behavior varies with individuals, and nonadherence may be intentional or unintentional; in some cases, nonadherence may arise from lack of motivation to take medications as prescribed. 27 In a UK study, patients with epilepsy were mailed questionnaires assessing their perceptions (N = 398 respondents); results showed that adherence to ASDs may be linked to patient beliefs about the necessity of taking medication and concerns about negative effects of the medication. 27 A Turkish study showed that patients with epilepsy (N = 174) had low medication adherence; they had high belief scores about the potential harms of medications and low belief scores about their personal treatment needs. 28 On the other hand, behavioral factors associated with efficient epilepsy medication self‐management/adherence were examined in a study using computer‐based assessments (N = 317). 29 This study found that interactions among such psychosocial variables as self‐efficacy, social support, and patient satisfaction directly influence management of medication. 29
Potential behavioral reasons for the observed SEIVAL increase in this study can be hypothesized. The patient/care partner may have initially treated a wider range of possible seizure cluster events but over time learned to better identify seizure clusters and used intermittent rescue therapy only in situations similar to the ones for which there was previously a benefit. Another possibility is that there was a patient/care partner change in perceived need for treatment, although many patients had received rescue prior to enrollment, so such a learning effect would be expected to be limited. Also, there may have been enthusiasm for the new therapy that waned over time.
Finally, in epilepsy studies, improvement in seizure frequency may be due to regression to the mean. For patients with chronic, uncontrolled epilepsy, seizures may be unpredictable, with fluctuations across time. 30 Patients experiencing higher than average frequency are more likely to seek medical attention or meet eligibility for a trial. Following enrollment, irrespective of treatment efficacy, seizure frequency is expected to have a natural tendency to regress toward the average for the patient. This may explain why the response to active treatment may be greater in uncontrolled studies than in randomized controlled trials. 30 In simulations based on seizure diaries, regression to the mean effects appeared to taper off after 3–6 months. 31 It has been suggested that a delayed start for a clinical trial may attenuate the effects of regression to the mean by allowing patients to return first to their mean state. 31 However, the durations examined in this analysis were longer—up to and beyond 1 year—and the patterns shown were consistent across time out to Period 6 (540 days).
Looking at treated seizure frequency over the long term, similar patterns have been associated with ASDs. In an open‐label, multicenter extension study of brivaracetam as adjunctive therapy for adult patients with epilepsy (N = 729), consistent median percent reductions in the frequency of focal seizures were demonstrated that were maintained over time to 96 months. 32 In that study, QOLIE‐31‐P scores in the patients with focal seizures generally remained stable or improved to the last value in Year 2 compared with baseline in the previous trial. 32 In a retrospective study looking at a 2‐year period for adult patients with epilepsy who initiated ASDs at a single site (N = 394 charts), the number of seizures over time decreased in a linear pattern, which the authors hypothesized might reflect the treatment effects of ASDs. 33
Our analysis explored several potential explanations for the increased SEIVAL/reduced dosing of diazepam nasal spray. Similar SEIVAL changes were seen regardless of changes to concomitant ASDs, suggesting that a change in those medications or their dosing was not the reason for better seizure cluster control. Additionally, the use of a consistent cohort allowed for controlling for the potential for retention bias. Here, the same findings were demonstrated when patients dropped out of the study. Furthermore, the high completion rate of the study and the stability of QOLIE‐31‐P scores over time argue that patients perceived a treatment benefit with diazepam nasal spray, suggesting that lack of tolerability is unlikely to have led to reduced use. As the study drug was provided without charge in this clinical trial, cost burden of diazepam nasal spray would not have been a factor.
This post hoc analysis has limitations. In the overall study, there was no precise definition of a seizure cluster, as there is no agreed‐upon definition in the literature, 34 and the administration instructions for diazepam nasal spray were tailored per protocol to the individual patient by the associated study investigator. Although this analysis did not examine untreated clusters, the study protocol directed use of treatment for all seizure clusters. Additionally, this analysis did not examine timing intervals for overall seizure frequency (i.e., including individual seizures), only specifically for seizure clusters. Also, the natural history of SEIVAL over time is not known, and there was no comparison group in the analysis. Per protocol, the study did not collect data on the patients' severity of epilepsy or types of seizures in treated seizure clusters or changes in counseling regarding medication adherence. In addition, the analysis used 90‐day epochs and did not examine other timing, and the numbers of patients for the consistent cohorts out to Periods 5 and 6 were small (41 and 26 patients, respectively). Also, as data on underlying seizure disorders and causes for seizure disorders were not systematically collected, the subgroups of patients with and without changes to concomitant medications could not be compared based on these criteria. To address these limitations, future studies in selected animal models and/or in humans should be designed to further explore these novel findings.
5. CONCLUSIONS
Across 12 months and beyond, a statistically significant increase in mean SEIVAL was demonstrated in patients who used diazepam nasal spray for treatment of seizure clusters in consecutive periods in a phase 3 safety study. The pattern was consistent regardless of age group, study duration, and change to concomitant medications, and with no effect on QOLIE‐31‐P scores. This previously undetected pattern of increased time between seizure clusters needing intervention presents a fresh opportunity for hypothesis generation regarding the potential impact of intermittent rescue therapy on the natural course of seizure clusters, such as the possibility of a role in altering the underlying biology of clusters. These results also support previous findings of lack of pharmacological tolerance to diazepam nasal spray. Further investigation and corroboration of these findings in prospective clinical or preclinical studies are needed, and identification of a germane database may help to address the current lack of a control group.
AUTHOR CONTRIBUTIONS
All authors contributed to conception, design, and development of the article, and all authors revised the manuscript critically for important intellectual content. All authors read and approved the final version of this manuscript for submission to Epilepsia.
FUNDING INFORMATION
This study was funded by Neurelis, Inc.
CONFLICT OF INTEREST
S.N.M. is an employee of and has received stock options from Neurelis. M.R.S. has received compensation for speaking at continuing medical education programs from Medscape, Projects for Knowledge, International Medical Press, and UCB Pharma. He has consulted for Medtronic, Neurelis, and Johnson & Johnson. He has received research support from Eisai, Medtronic, Neurelis, SK Life Science, Takeda, Xenon, Cerevel, UCB Pharma, Janssen, Equilibre, and Engage Pharmaceuticals. He receives royalties from Oxford University Press and Cambridge University Press. V.R.R. has served as a consultant for NeuroPace, manufacturer of the RNS System. J.M.P. has served as a speaker and consultant for Neurelis, SK Life Science, and Jazz Pharmaceuticals. C.D. is a consultant to Neurelis. E.C. is an employee of and received stock and stock options from Neurelis. A.L.R. is an employee of and has received stock options from Neurelis. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
INSTITUTIONAL REVIEW BOARD STATEMENT
Prior to study initiation, the study protocol, informed consent form, and other relevant study documentation were approved by ethics committees or institutional review boards at each site.
Supporting information
FIGURE S1–S3
ACKNOWLEDGMENTS
Medical writing support was provided at the direction of the authors by Laura J. Herold, MA, of The Curry Rockefeller Group (Tarrytown, NY), which also provided additional editorial assistance including formatting and proofreading. This support was funded by Neurelis (San Diego, CA).
Misra SN, Sperling MR, Rao VR, Peters JM, Davis C & Carrazana E et al. Significant improvements in SEIzure interVAL (time between seizure clusters) across time in patients treated with diazepam nasal spray as intermittent rescue therapy for seizure clusters. Epilepsia. 2022;63:2684–2693. 10.1111/epi.17385
DATA AVAILABILITY STATEMENT
All relevant data are within the paper.
REFERENCES
- 1. Fisher RS, Bartfeld E, Cramer JA. Use of an online epilepsy diary to characterize repetitive seizures. Epilepsy Behav. 2015;47:66–71. [DOI] [PubMed] [Google Scholar]
- 2. Haut SR, Lipton RB, LeValley AJ, Hall CB, Shinnar S. Identifying seizure clusters in patients with epilepsy. Neurology. 2005;65(8):1313–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Loscher W, Klein P. The pharmacology and clinical efficacy of antiseizure medications: from bromide salts to cenobamate and beyond. CNS Drugs. 2021;35(9):935–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ferastraoaru V, Schulze‐Bonhage A, Lipton RB, Dumpelmann M, Legatt AD, Blumberg J, et al. Termination of seizure clusters is related to the duration of focal seizures. Epilepsia. 2016;57(6):889–95. [DOI] [PubMed] [Google Scholar]
- 5. Sperling MR, Wheless JW, Hogan RE, Dlugos D, Cascino GD, Liow K, et al. Use of second doses of Valtoco® (diazepam nasal spray) across 24 hours after the initial dose for out‐of‐hospital seizure clusters: results from a phase 3, open‐label, repeat‐dose safety study. Epilepsia. 2022;63(4):836–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wheless JW, Miller I, Hogan RE, Dlugos D, Biton V, Cascino GD, et al. Final results from a phase 3, long‐term, open‐label, repeat‐dose safety study of diazepam nasal spray for seizure clusters in patients with epilepsy. Epilepsia. 2021;62(10):2485–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moavero R, Muhlebner A, Luinenburg MJ, Craiu D, Aronica E, Curatolo P. Genetic pathogenesis of the epileptogenic lesions in tuberous sclerosis complex: therapeutic targeting of the mTOR pathway. Epilepsy Behav. 2022;131(Pt B):107713. [DOI] [PubMed] [Google Scholar]
- 8. Asadi‐Pooya AA, Nei M, Sharan A, Sperling MR. Seizure clusters in drug‐resistant focal epilepsy. Epilepsia. 2016;57(9):e187–90. [DOI] [PubMed] [Google Scholar]
- 9. Jafarpour S, Hirsch LJ, Gainza‐Lein M, Kellinghaus C, Detyniecki K. Seizure cluster: definition, prevalence, consequences, and management. Seizure. 2019;68:9–15. [DOI] [PubMed] [Google Scholar]
- 10. Haut SR. Seizure clusters: characteristics and treatment. Curr Opin Neurol. 2015;28(2):143–50. [DOI] [PubMed] [Google Scholar]
- 11. Penovich PE, Buelow J, Steinberg K, Sirven J, Wheless J. Burden of seizure clusters on patients with epilepsy and caregivers: survey of patient, caregiver, and clinician perspectives. Neurologist. 2017;22(6):207–14. [DOI] [PubMed] [Google Scholar]
- 12. NAYZILAM® (midazolam nasal spray). Full prescribing information. Smyrna, GA: UCB; 2021.
- 13. Valtoco (diazepam nasal spray). Full Prescribing Information. San Diego, CA: Neurelis; 2022.
- 14. US Food and Drug Administration . Clinical superiority findings [cited 2020 Jun 23]. Available from: https://www.fda.gov/industry/designating‐orphan‐product‐drugs‐and‐biological‐products/clinical‐superiority‐findings
- 15. Cascino GD, Tarquinio D, Wheless JW, Hogan RE, Sperling MR, Liow K, et al. Lack of observed tolerance to diazepam nasal spray (Valtoco®) after long‐term rescue therapy in patients with epilepsy: interim results from a phase 3, open‐label, repeat‐dose safety study. Epilepsy Behav. 2021;120:107983. [DOI] [PubMed] [Google Scholar]
- 16. Cramer JA, Van Hammee G, N132 Study Group . Maintenance of improvement in health‐related quality of life during long‐term treatment with levetiracetam. Epilepsy Behav. 2003;4(2):118–23. [DOI] [PubMed] [Google Scholar]
- 17. Cramer JA, Perrine K, Devinsky O, Bryant‐Comstock L, Meador K, Hermann B. Development and cross‐cultural translations of a 31‐item quality of life in epilepsy inventory. Epilepsia. 1998;39(1):81–8. [DOI] [PubMed] [Google Scholar]
- 18. Misra SN, Faught E, Davis C, Carrazana E, Rabinowicz AL. Results for the Quality of Life in Epilepsy Scale from a long‐term safety study of diazepam nasal spray for seizure clusters. Paper prresented at: American Epilepsy Society Annual Meeting; December 3–7, 2021; Chicago, Illinois.
- 19. Borghs S, de la Loge C, Cramer JA. Defining minimally important change in QOLIE‐31 scores: estimates from three placebo‐controlled lacosamide trials in patients with partial‐onset seizures. Epilepsy Behav. 2012;23(3):230–4. [DOI] [PubMed] [Google Scholar]
- 20. Mattsson M, Boland F, Kirke C, Flood M, Quinn E, Walsh M, et al. Evaluation of policies and practices to support safe and appropriate analgesic and sedative prescribing: the CDRx (controlled drug prescribing) protocol. Res Social Adm Pharm. 2022;18:3588–95. [DOI] [PubMed] [Google Scholar]
- 21. Morant AV, Jagalski V, Vestergaard HT. Labeling of disease‐modifying therapies for neurodegenerative disorders. Front Med (Lausanne). 2019;6:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Doody RS. We should not distinguish between symptomatic and disease‐modifying treatments in Alzheimer's disease drug development. Alzheimers Dement. 2008;4(1 Suppl 1):S21–5. [DOI] [PubMed] [Google Scholar]
- 23. Croop R, Lipton RB, Kudrow D, Stock DA, Kamen L, Conway CM, et al. Oral rimegepant for preventive treatment of migraine: a phase 2/3, randomised, double‐blind, placebo‐controlled trial. Lancet. 2021;397(10268):51–60. [DOI] [PubMed] [Google Scholar]
- 24. Andreou AP, Edvinsson L. Mechanisms of migraine as a chronic evolutive condition. J Headache Pain. 2019;20(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. French JA, Bebin M, Dichter MA, Engel J Jr, Hartman AL, Jozwiak S, et al. Antiepileptogenesis and disease modification: clinical and regulatory issues. Epilepsia Open. 2021;6(3):483–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Clossen BL, Reddy DS. Novel therapeutic approaches for disease‐modification of epileptogenesis for curing epilepsy. Biochim Biophys Acta Mol Basis Dis. 2017;1863(6):1519–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chapman SC, Horne R, Chater A, Hukins D, Smithson WH. Patients' perspectives on antiepileptic medication: relationships between beliefs about medicines and adherence among patients with epilepsy in UK primary care. Epilepsy Behav. 2014;31:312–20. [DOI] [PubMed] [Google Scholar]
- 28. Dayapoglu N, Turan GB, Ozer Z. Evaluation of medication adherence and medication beliefs among patients with epilepsy. Epilepsy Behav. 2021;124:108366. [DOI] [PubMed] [Google Scholar]
- 29. DiIorio C, Shafer PO, Letz R, Henry TR, Schomer DL, Yeager K, et al. Project EASE: a study to test a psychosocial model of epilepsy medication managment. Epilepsy Behav. 2004;5(6):926–36. [DOI] [PubMed] [Google Scholar]
- 30. Perucca E, Wiebe S. Not all that glitters is gold: a guide to the critical interpretation of drug trials in epilepsy. Epilepsia Open. 2016;1(1–2):9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Goldenholz DM, Goldenholz SR. Response to placebo in clinical epilepsy trials—old ideas and new insights. Epilepsy Res. 2016;122:15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ben‐Menachem E, Baulac M, Hong SB, Cleveland JM, Reichel C, Schulz AL, et al. Safety, tolerability, and efficacy of brivaracetam as adjunctive therapy in patients with focal seizures, generalized onset seizures, or Unverricht‐Lundborg disease: an open‐label, long‐term follow‐up trial. Epilepsy Res. 2021;170:106526. [DOI] [PubMed] [Google Scholar]
- 33. Raru TB, Geremew BM, Tamirat KS. Change in the frequency of seizure attacks and associated factors among adult epilepsy patients at Amanuel Mental Specialized Hospital (AMSH): a generalized linear mixed model (GLMM). Neuropsychiatr Dis Treat. 2021;17:2529–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Buchhalter J, Shafer PO, Buelow JM, French JA, Gilchrist B, Hirsch LJ, et al. Preferred practices for rescue treatment of seizure clusters: a consensus‐driven, multi‐stakeholder approach. Epilepsy Behav. 2021;117:107836. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1–S3
Data Availability Statement
All relevant data are within the paper.


