ABSTRACT
As biodiversity decreases worldwide, the development of effective techniques to track changes in ecological communities becomes an urgent challenge. Together with other emerging methods in ecology, acoustic indices are increasingly being used as novel tools for rapid biodiversity assessment. These indices are based on mathematical formulae that summarise the acoustic features of audio samples, with the aim of extracting meaningful ecological information from soundscapes. However, the application of this automated method has revealed conflicting results across the literature, with conceptual and empirical controversies regarding its primary assumption: a correlation between acoustic and biological diversity. After more than a decade of research, we still lack a statistically informed synthesis of the power of acoustic indices that elucidates whether they effectively function as proxies for biological diversity. Here, we reviewed studies testing the relationship between diversity metrics (species abundance, species richness, species diversity, abundance of sounds, and diversity of sounds) and the 11 most commonly used acoustic indices. From 34 studies, we extracted 364 effect sizes that quantified the magnitude of the direct link between acoustic and biological estimates and conducted a meta‐analysis. Overall, acoustic indices had a moderate positive relationship with the diversity metrics (r = 0.33, CI [0.23, 0.43]), and showed an inconsistent performance, with highly variable effect sizes both within and among studies. Over time, studies have been increasingly disregarding the validation of the acoustic estimates and those examining this link have been progressively reporting smaller effect sizes. Some of the studied indices [acoustic entropy index (H), normalised difference soundscape index (NDSI), and acoustic complexity index (ACI)] performed better in retrieving biological information, with abundance of sounds (number of sounds from identified or unidentified species) being the best estimated diversity facet of local communities. We found no effect of the type of monitored environment (terrestrial versus aquatic) and the procedure for extracting biological information (acoustic versus non‐acoustic) on the performance of acoustic indices, suggesting certain potential to generalise their application across research contexts. We also identified common statistical issues and knowledge gaps that remain to be addressed in future research, such as a high rate of pseudoreplication and multiple unexplored combinations of metrics, taxa, and regions. Our findings confirm the limitations of acoustic indices to efficiently quantify alpha biodiversity and highlight that caution is necessary when using them as surrogates of diversity metrics, especially if employed as single predictors. Although these tools are able partially to capture changes in diversity metrics, endorsing to some extent the rationale behind acoustic indices and suggesting them as promising bases for future developments, they are far from being direct proxies for biodiversity. To guide more efficient use and future research, we review their principal theoretical and practical shortcomings, as well as prospects and challenges of acoustic indices in biodiversity assessment. Altogether, we provide the first comprehensive and statistically based overview on the relation between acoustic indices and biodiversity and pave the way for a more standardised and informed application for biodiversity monitoring.
Keywords: species diversity, systematic review, ecoacoustics, soundscape, ecology, monitoring, ecological indicators, biodiversity assessment
I. INTRODUCTION
Global change is strongly altering Earth's ecosystems and leading to dramatic impacts on ecological communities (Newbold et al., 2016). Understanding biodiversity changes and predicting future scenarios is thus urgent for developing appropriate conservation programs (Pereira et al., 2013). Recent technological advances have been pushing biodiversity monitoring to the next level, with new tools for continuous spatial and temporal assessment of ecosystems at the global scale (Pettorelli et al., 2016). Techniques for habitat monitoring based on passive sensors, such as satellite and airborne remote sensing, are now crucial to estimate tthe ‘ecosystem component’ of the essential biodiversity variables framework (Pereira et al., 2013). However, some biological components are underrepresented (e.g. genetic composition, species population dynamics, community composition), since most field‐based observations are made in the short term, and a lack of standardised protocols in data sampling impairs cross‐scale (temporal and spatial) approaches (Martin, Blossey & Ellis, 2012; Proença et al., 2017; Sugai, 2020). In this sense, passive acoustic monitoring has emerged as a prospective technique to monitor biodiversity based on animal sounds (Laiolo, 2010; Blumstein et al., 2011). Recent developments in acoustic sensors are enabling simultaneous and non‐invasive monitoring at multiple sites during prolonged periods of time (Gibb et al., 2019). Consequently, passive acoustic monitoring has become a new trend in ecological data collection over recent decades for a variety of taxa worldwide (Sugai et al., 2019).
Acoustic monitoring enables animal behaviour, species diversity, phenology, species turnover, and population dynamics to be assessed using high‐temporal‐resolution sampling in the long term [e.g. over years (Llusia et al., 2013; Tucker et al., 2014; Sugai & Llusia, 2019)]. However, a fundamental challenge of this monitoring technique lies in detecting target signals within the large audio data sets generated (Priyadarshani, Marsland & Castro, 2018b ). Manual analyses are often unfeasible, while fully automatic methods for species recognition are still mostly unavailable given the overall poor audio quality of passive recordings and the need for large annotated databases (Digby et al., 2013; Ulloa et al., 2018). Alternatively, a recent proposal suggested reframing the analytical scale and focusing on the entire collection of sounds emanating from a given population, community or landscape, without using species identification (Sueur et al., 2008; Pijanowski et al., 2011b ; Farina & James, 2016). With the growing expansion of passive acoustic monitoring and the demand for efficient analyses, this shift of analytical scale has fostered a new interdisciplinary field that investigates the soundscape as a whole and its relation with ecological processes, formalised as ecoacoustics (Servick, 2014; Sueur & Farina, 2015). As such, ecoacoustics sets a challenge to develop effective methods for extracting meaningful biological and ecological information from soundscapes (Gasc et al., 2013a ; Pieretti et al., 2015; Bradfer‐Lawrence et al., 2019).
In recent years, multiple acoustic diversity indices have been proposed as a way to summarise the acoustic information contained in soundscapes into a global measure that attempts to describe animal communities effectively. Inspired by species diversity indices, acoustic indices are aimed at characterising biodiversity in space and time through the incidence, abundance, and features of sounds (Sueur et al., 2014; Eldridge et al., 2016). Since current acoustic data sets often approach ‘big‐data’ scales, the simplistic solution of using acoustic indices to represent species diversity is appealing. As a result, acoustic indices have exploded in the literature, with up to 69 developed since 2007 (Buxton et al., 2018b ). Over more than a decade of development, the perspective on the use and interpretation of acoustic indices has progressively evolved, but these novel metrics overall still assume a direct link between acoustic diversity and biodiversity. However, this fundamental assumption has conceptual and empirical shortcomings across the literature and most indices have showed contradictory relationships with species diversity. In real‐world environmental recordings, acoustic complexity is determined by multiple factors other than diversity (e.g. vocal repertoire, noise, etc.), and hence these sources of bias can critically influence the estimates of acoustic indices. Such drawbacks hinder the application of standards and guidelines for data collection, analysis, and interpretation of acoustic indices (Bradfer‐Lawrence et al., 2019), and preclude scalability across studies. While summaries of the principles and applications of acoustic indices are already available (Sueur et al., 2014; Buxton et al., 2018b ; Sugai et al., 2019), we still lack a statistically informed synthesis of the current evidence for the power of acoustic indices to estimate biodiversity.
After 15 years of research since the early proposals of acoustic indices, here we provide a comprehensive review on the use of this method as a proxy for biodiversity. For this purpose, we examine the empirical relationship between the most common acoustic indices and species diversity metrics under a meta‐analytical framework. We further identify research gaps, shortcomings, and practical considerations for the appropriate use of acoustic indices and suggest a future research agenda for ecoacoustics research.
(1). The acoustic component of ecological communities
Sound production and reception is a communication modality widespread across tetrapods (Chen & Wiens, 2020) and some arthropods (Schmidt & Balakrishnan, 2015). Among a variety of functions, acoustic signalling underpins mate choice, playing a key role in sexual selection (Gerhardt, 1994) and speciation (Tobias et al., 2014a ), since it acts as a prezygotic barrier for species recognition (Höbel & Gerhardt, 2003). Consequently, acoustic signals are usually species specific, presenting low intraspecific and high interspecific variation, and are suitable to determine taxonomic inconsistencies across species (Koehler et al., 2017). Thus, the biological component of a soundscape recorded at a given place and time is expected to reflect the local animal diversity, generating an ‘acoustic signature’ (Farina, Eldridge & Li, 2021). Additionally, social and ecological interactions within and between species can be mediated by acoustic signals, influencing individual fitness and hence determining both temporal and spatial distribution patterns (Cornec et al., 2015; Magrath et al., 2015). In this sense, acoustic monitoring relies on the detection of animal sounds that reveal not only species identity but also social, behavioural, and ecological aspects of communities (Laiolo, 2010).
Sounds can be described based on three interlinked acoustic dimensions: time, frequency, and energy. Acoustic indices are mathematical functions that rely on these three dimensions to summarise the global complexity or heterogeneity of a sound recording (Sueur et al., 2014). Differences in sounds promoted by variations in species abundance, diversity, and community composition hypothetically cause changes in acoustic indices, and therefore endorse the idea of the acoustic signature of a soundscape as a proxy for biodiversity. As animal sounds are unique in terms of acoustic features, the acoustic complexity contained in soundscapes is presumably associated, to some extent, with the composition and diversity of acoustically active species in the animal community being recorded. For instance, an audio recording with a large number of signalling species would have energy distributed across a large set of frequencies or a high rate of energy changes across frequency and time. Similarly, a recording with frequent and recurrent signals through time or with intense sound energy would reflect high levels of species activity. These relations are used as premises to advocate that the distribution of energy of all sounds in an environmental recording will be associated with taxa‐specific information of ecological communities.
The conversion of time, frequency, and energy components of an audio sample into acoustic indices alludes to the use of incidence and abundance of species to calculate indices of species diversity. As for diversity indices, acoustic indices can be divided into alpha (resembling local diversity) and beta indices (resembling inter‐site diversity; Sueur et al., 2014), with differences in conceptualisation and calculation. According to the number of indices employed, they can also be classified as first‐order (using a single index) or second‐order indices (combining more than one index; Towsey et al., 2014). Moreover, acoustic indices can be waveform indices (computed from oscillograms) or spectral indices (computed from frequency spectra and spectrograms). Over a rapid and growing evolution of their applications, these metrics have been used to represent distinct facets of animal communities, including species abundance (number of signalling individuals from identified species), species richness (number of signalling species), species diversity (heterogeneity metrics combining species richness and evenness), abundance of sounds (number of specific types of sounds or signals), diversity of sounds (number of distinct sound types), and functional diversity, phylogenetic diversity, or dissimilarity in community composition (Gasc et al., 2013a ; Sueur et al., 2014).
(2). Acoustic diversity indices: evolving applications
In the early years, ecoacoustics research was focused on developing pioneer acoustic indices aimed at estimating species richness and abundance from environmental recordings (Table 1). Bioacoustic index (BIO; Boelman et al., 2007), acoustic entropy (H; Sueur et al., 2008), and acoustic complexity index (ACI; Pieretti, Farina & Morri, 2011) were the earliest alpha indices proposed and showed promising results based on both simulations and field surveys. Promptly available in a graphical user interface software (wavesurfer), ACI became a widely used acoustic index (Farina et al., 2011; Farina, Pieretti & Morganti, 2013). Since these seminal studies, the use of acoustic indices drew much attention and encouraged new approaches in bioacoustics and ecological research. Other indices were subsequently proposed [e.g. acoustic evenness index (AEI), acoustic diversity index (ADI), normalised difference soundscape index (NDSI), acoustic richness index (AR), amplitude index (M), or number of frequency peaks (NP); Table 2] and tested in relation to ACI and H, which contributed to their consolidation as reference indices (Sueur, 2018). Nonetheless, limitations on the capacity of acoustic indices as proxies for biodiversity began to be revealed, promoting new pathways for their use (Table 1), such as mathematical optimisation with a combination of several indices (Towsey et al., 2014) or a search for links with other dimensions of biodiversity (e.g. functional diversity, phylogenetic diversity; Gasc et al., 2013a ). The application of acoustic indices beyond the representation of species richness and abundance led studies to focus mainly on higher taxonomic levels (e.g. birds, anurans, insects) and to describe their temporal activity patterns (Phillips, Towsey & Roe, 2018) and their variation among habitats (Scarpelli, Ribeiro & Teixeira, 2021).
Table 1.
Main applications of acoustic indices on the assessment of biodiversity and ecosystems
| Study parameter | Application | Representation | Example |
|---|---|---|---|
| Species richness | Surrogate of the number of signalling species from passive acoustic recordings, used to determine the diversity of local communities |
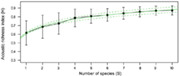
|
Sueur et al. (2008) |
| Abundance of sounds | Surrogate of the number of specific types of sounds or signals produced by a given species or animal chorus (identified or unidentified taxa), used to determine the intensity of acoustic activity |
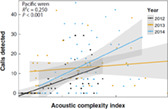
|
Pieretti et al. (2011); Buxton et al. (2016) |
| Species composition | Estimation of the similarity of soundscapes among communities or periods over time, used to identify changes in species composition or habitat structure |

|
Sueur et al. (2008); Depraetere et al. (2012) |
| Overall biological diversity | Surrogate of biological aspects of animal communities other than species richness (e.g. phylogenetic or functional diversity), used to represent a global overview of biological diversity |

|
Gasc et al. (2013b ) |
| Acoustic activity patterns | Description of temporal and spatial patterns of acoustic activity of species or communities, used to compare species' calling phenology |

|
Farina et al. (2013) |
| Soundscape composition | Determination of the relative contribution of sound sources (e.g. anthrophony and biophony) to soundscapes, used to describe their structure and dynamics |
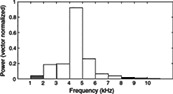
|
Kasten et al. (2012); Gage & Axel (2014) |
| Soundscape visualisation | Visual representation of long time series of audio data, used to identify acoustic events and describe their structure and dynamics |
|
Phillips et al. (2018); Towsey et al. (2018) |
| Environmental features | Surrogate of environmental features for acoustic characterisation of habitats, ecosystems, and landscapes, used to represent their structure and dynamics |

|
Elise et al. (2019); Scarpelli et al. (2021) |
| Species abundance | Surrogate of the number of signalling individuals in an animal group or chorus (identified or unidentified taxa), used as indicators of population size |

|
Papin et al. (2019) |
| Soundscape identification | Surrogate of soundscape features for automated identification of soundscape changes based on machine learning techniques, as indicator of their structure and dynamics |

|
Sethi et al. (2020) |
Table 2.
Acoustic indices selected for literature review and meta‐analysis
| Acronym | Name | Principle | Reference |
|---|---|---|---|
| BIO | Bioacoustic index | Amplitude spectrum area | Boelman et al. (2007) |
| H | Acoustic entropy index | Product of Ht and Hf | Sueur et al. (2008) |
| Ht | Temporal entropy index | Shannon entropy of the amplitude envelope | Sueur et al. (2008) |
| Hf | Spectral entropy index | Shannon entropy of the frequency spectrum | Sueur et al. (2008) |
| ACI | Acoustic complexity index | Summation of weighted amplitude differences within frequency bins | Pieretti et al. (2011) |
| ADI | Acoustic diversity index | Shannon entropy of a selection of frequency bins | Villanueva‐Rivera et al. (2011) |
| AEI | Acoustic evenness index | Gini index of a selection of frequency bins | Villanueva‐Rivera et al. (2011) |
| AR | Acoustic richness index | Weighted product of the ranks of M and Ht indices | Depraetere et al. (2012) |
| M | Amplitude index | Median of the amplitude envelope | Depraetere et al. (2012) |
| NDSI | Normalised difference soundscape index | Ratio of the spectral density of two frequency bands (anthrophony and biophony) | Kasten et al. (2012) |
| NP | Number of frequency peaks | Number of the principal peaks in a frequency spectrum | Gasc et al. (2013b ) |
Regardless of the new research pathways, the primary interest in acoustic indices as proxies for biodiversity has persisted in an increasingly growing scientific community. While the first reviews quantified 20 acoustic indices (Sueur et al., 2014), more than 60 indices were recently listed (Buxton et al., 2018b ), and multiple study cases examining the performance of acoustic indices in describing biological diversity have been published to date. The first international meeting on ecology and acoustics (in Paris in 2014) represented an important benchmark, with the definition of ecoacoustics as a new research area and the launch of the International Society of Ecoacoustics (Sueur & Farina, 2015), which promotes new research on acoustic indices and soundscapes. Since then, several books and reviews have been published (Farina, 2014; Farina & Gage, 2017; Sueur, 2018) as attempts to gain visibility and to formalise the theoretical and instrumental basis of ecoacoustics (Servick, 2014). The most recent proposals suggest the use of acoustic indices as features for classification algorithms that enable better characterisation of soundscapes and more efficient species recognition (Sethi et al., 2020).
(3). The elusive link between species and acoustic diversity
Despite the rapid uptake of acoustic indices in ecological research, evidence of a straightforward relationship between species diversity and acoustic diversity remains elusive, challenging the primary aspiration of acoustic indices of being cost‐efficient and accurate tools to quantify biological diversity. Because some of the theoretical and empirical assumptions that have supported the development of acoustic indices are controversial (see Sections IV.2 and IV.3), these metrics may present shortcomings when oriented to detect species‐related acoustic variations in environmental audio recordings (Sueur et al., 2014; Towsey et al., 2014). Several hypotheses, such as acoustic niche partitioning (Hödl, 1977), have been evoked as the theoretical framework to endorse the foundation of acoustic indices (Pijanowski et al., 2011b ; Sueur & Farina, 2015), although the support for these hypotheses is still mixed (Sugai et al., 2021a ). Moreover, soundscapes in real‐world environments are influenced by multiple factors besides species diversity, such as variation in relative species abundance, vocal repertoire size, source–sensor distance, and natural and anthropogenic noise, which lead to an unequal contribution of animal species and other sound sources to the soundscapes. Therefore, when analysing the acoustic diversity of communities, the performance and interpretation of acoustic indices depend on their ability to capture properly such variation in biological diversity.
Since the development of the first acoustic indices, the capacity of these indices as proxies for biodiversity has been investigated extensively in multiple taxa‐ and ecosystem‐oriented research, although providing ambiguous results so far. To overcome the lack of general synthesis and to supply guidance for future applications, here we aim at (i) reviewing the literature testing the link between acoustic indices and biodiversity, and (ii) providing a meta‐analysis (Fig. 1). Thereby, we determine: (a) whether acoustic indices are good direct indicators of biodiversity; (b) which acoustic indices perform better; (c) the capacity of acoustic indices to estimate distinct facets of biodiversity; and (d) whether acoustic indices are affected by different environments or sources of biological information. Finally, we identify both conceptual and practical shortcomings and pitfalls and provide general recommendations for the efficient use of these novel tools. Our findings allow the consolidation of a new research program on acoustic diversity indices that advances their application in ecology.
Fig. 1.
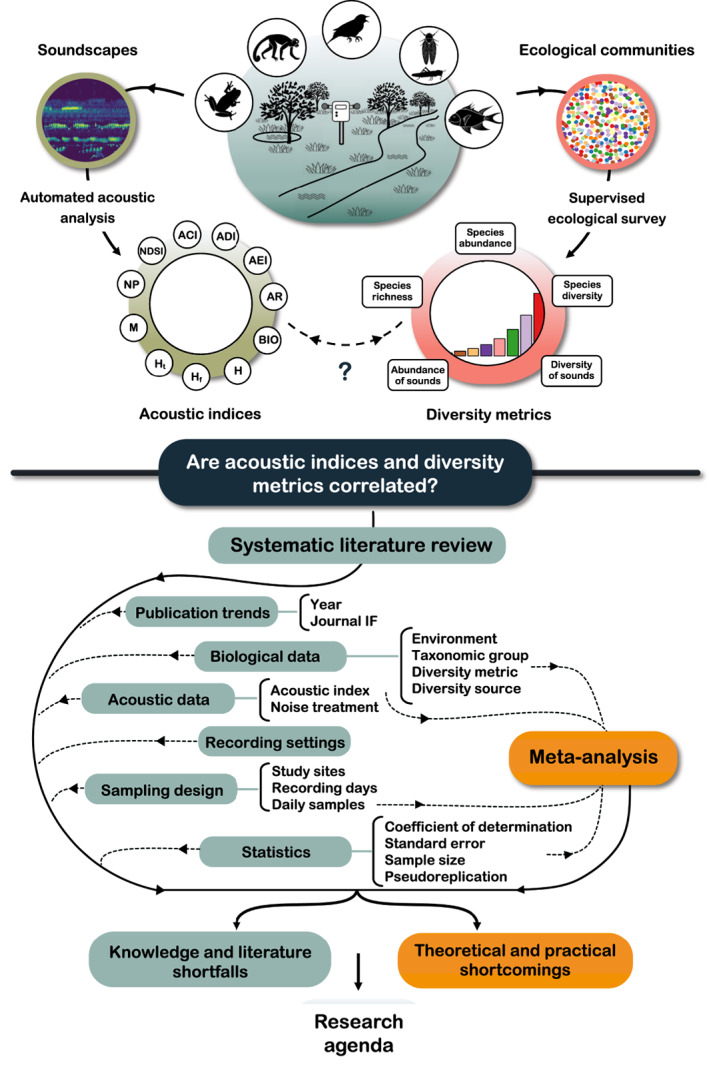
Conceptual figure outlining this review. For definitions of acoustic indices see Table 2. IF, impact factor.
II. METHODS
(1). Selection of acoustic indices and literature search strategy
We exhaustively searched the literature that tested the relationship between acoustic indices and diversity metrics, following a three‐step procedure: (i) we gathered studies by a series of systematic reviews; (ii) identified studies that reported the performance of acoustic indices; and (iii) screened studies that explicitly related acoustic indices to biodiversity (Fig. 2).
Fig. 2.
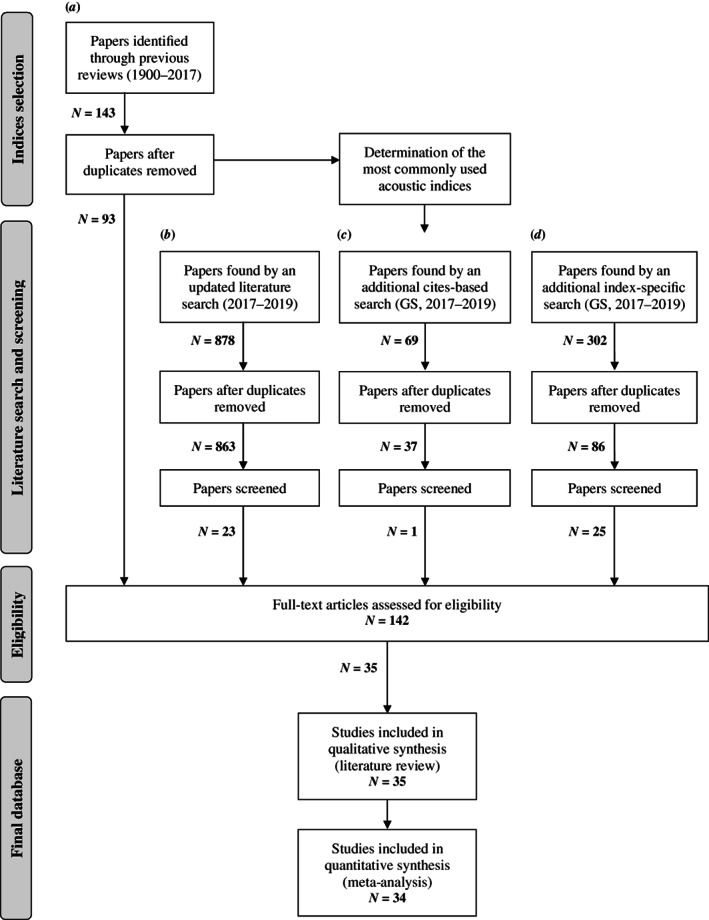
Procedures used in the literature search for articles addressing acoustic indices and diversity metrics, and the number of papers identified at each step. The literature reviews used in (a) were: Sueur et al. (2014), Buxton et al. (2018b ), and Sugai et al. (2019). The literature search in (b) was conducted in Thompson's ISI Web of Science, restricted to 2017–2019 and to nine subject areas, and was based on the key words used in Buxton et al. (2018b ). Literature searches in (c) and (d) were conducted in Google Scholar (GS), restricted to 2017–2019, and based on literature that cited the articles identified in (b) and index‐specific citations (d), respectively.
Although dozens of acoustic diversity indices have been developed, the number of publications per index is far from uniform (Buxton et al., 2018b ). To prevent including rare cases in the meta‐analysis (Koricheva, Gurevitch & Mengersen, 2013, chapter 16), our systematic review focused on the most commonly used acoustic indices. Based on three recent literature reviews on acoustic indices, biodiversity assessment, and passive acoustic monitoring (Sugai et al., 2019; Sueur et al., 2014; Buxton et al., 2018b ), we first compiled 93 articles (duplicate records removed) that represented the most comprehensive literature on acoustic indices until April 2017 (a in Fig. 2). Then, we selected those indices present in more than a single study, and excluded standard acoustic metrics (e.g. signal‐to‐noise ratio, etc.), as they are infrequently associated with complexity or diversity of acoustic communities (Sueur et al., 2014). This led to a set of 11 alpha acoustic indices (Table 2), the acoustic diversity indices most commonly used in ecoacoustics research, which are currently available in R and hence easy to implement (Sueur, 2018).
Once we identified the target indices, we updated the literature database from 2017 up to July 2019 with Thompson's ISI Web of Science (WoS), using the same combinations of key words employed by Buxton et al. (2018b ) (i.e. bioacoustic* AND ind*, ecoacoustic*, acoustic* AND biodiversity, soundscape, AND ecology). This search was restricted to nine WoS subject areas (i.e. zoology, environmental sciences ecology, behavioural sciences, biodiversity conservation, marine freshwater biology, acoustics, evolutionary biology, entomology, and remote sensing) and resulted in 878 published articles (b in Fig. 2). Additionally, we used Google Scholar to compile all literature from 2017 to July 2019 that cited (i) any of the new papers published within this period, or (ii) cited the papers originally describing the 11 selected indices (Table 2). These two complementary surveys resulted in additional 69 and 302 articles, respectively (c and d in Fig. 2). After removing all duplicates, we obtained 986 articles for screening, which, in addition to the 93 already selected in the previous step, resulted in a total of 1079 papers. The combined set of literature searches likely enabled us to identify nearly all relevant articles addressing our study question.
(2). Inclusion criteria
We screened abstract, titles, and key words of each of the 1079 identified articles and considered eligible for meta‐analysis those that met the following inclusion criteria: (i) reported data to test the efficiency of acoustic diversity indices as indicators of biodiversity; (ii) employed at least one of the target acoustic indices (Table 2); (iii) employed at least one out of five metrics to describe biodiversity, namely species abundance, species richness, species diversity (i.e. Shannon index, Simpson index, and Pielou's evenness), abundance of sounds, and diversity of sounds; and (iv) provided information of a univariate relationship between a single acoustic index and a single diversity metric. Following such inclusion criteria, we selected 142 papers as potentially eligible. To ascertain their relevance, we conducted a full‐text assessment for all these studies and finally retained 35 studies (see online Supporting Information, Tables S1 and S2; https://irene-alcocer.github.io/Acoustic-Indices/).
We excluded studies describing alternative uses of acoustic indices (e.g. Bellisario et al., 2019), reporting patterns of acoustic indices without testing their link with biodiversity (e.g. Rodriguez et al., 2014; Farina, Gage & Salutari, 2018) or examining the performance of acoustic indices in relation to other ecological parameters, such as soundscape composition (e.g. Bobryk et al., 2016) or habitat structure (e.g. Pekin et al., 2012). We also excluded studies that did not employ any of the 11 alpha indices selected (e.g. Gasc et al., 2013a ; McPherson et al., 2016), such as articles exclusively investigating dissimilarity indices (e.g. Lellouch et al., 2014) or standard acoustic metrics [e.g. sound intensity, signal‐to‐noise ratio, etc. (Torija, Ruiz & Ramos‐Ridao, 2013; Jeon & Hong, 2015; Di Iorio et al., 2018)], which are less common in the literature. Similarly, we also excluded studies combining several acoustic indices in multivariate predictive models since they are still few in number (Buxton et al., 2018b ). Models with multiple predictors were only considered if they included one single fixed factor and additional variables as random factors [e.g. linear mixed‐effects models (LMMs); Fuller et al., 2015; Buxton et al., 2016]. When graphical information was reported (e.g. scatterplots, line charts, etc.) and the study provided quantitative variables and high‐resolution images, data retrieval from both acoustic and biodiversity estimates was performed (e.g. Mammides et al., 2017; Jorge et al., 2018; Lyon et al., 2019). We included peer‐reviewed and non‐peer‐reviewed studies since the probability of publication for a given study depends on the statistical significance, magnitude, or direction of the effect (Koricheva, 2003). This bias, known as publication bias, can be mitigated by gathering the most comprehensive database possible (Lortie et al., 2007; Nakagawa et al., 2017).
(3). Data retrieval
To characterise each of the 35 identified studies, we compiled information from six main categories (i.e. publication, biological data, acoustic data, recording, sampling design, and statistics) that represented 34 features (Table S3). These features were used: (i) as descriptive variables, qualitatively to summarise ecoacoustics research; and (ii) as moderators in the meta‐analysis, to investigate their influence on the performance of acoustic indices.
Among biological data, we retrieved the type of environment (aquatic or terrestrial) and taxonomic group (invertebrates, fish, anurans, mammals, birds, or several) for which acoustic indices were calculated. We also classified the diversity metric that was related to acoustic indices into five categories: (i) species abundance (when studies examined the number of individuals of a single or several species); (ii) species richness, the simplest measure of diversity (when studies examined the number of vocal and/or non‐vocal species); (iii) species diversity, including more complex diversity indices that also consider species abundance (i.e. Shannon index, Simpson index, or species evenness); (iv) abundance of sounds (the number of sounds by identified or unidentified species); and (v) diversity of sounds (the number of sound types by identified or unidentified species). Finally, we described the method applied to obtain such biological information as diversity source, which included (i) acoustic (data extracted from audio recordings) or (ii) non‐acoustic (data extracted from literature, field surveys, etc.). Additionally, we obtained up to three parameters employed for the derivation of each acoustic index (frequency range, FFT size, and noise treatment; Table S3). The procedure used to collect sound recordings was characterised with sampling rate, audio format, recording length, and recording method, i.e. whether field recording was (i) non‐programmed (continuous recording), (ii) programmed (recordings taken at periodic time intervals by an automated device), or (iii) manual (performed by an operator).
(4). Sampling design and pseudoreplication
Acoustic indices are calculated from time series of passive audio recordings typically taken by regular sampling over hours, days, or weeks in a set of recording sites (Sugai et al., 2020). To account for differences in sampling effort among studies and to detect cases of pseudoreplication (i.e. inadequate specification of the number of independent observations when applying statistical inference), we extensively assessed the sampling design and statistics of each study. Pseudoreplication leads to a biased and incorrect inference, and it should be carefully considered (Hurlbert, 1984). Thus, we identified eight features that summarised spatial and temporal sampling and statistical tests of all the selected articles (Table S3).
Firstly, spatial sampling was defined by the number of study sites, minimal distance between sites, and number of recorders per site. To determine the number of study sites, we assumed that recorders had standard detection spaces (Llusia, Márquez & Bowker, 2011; Darras et al., 2016) and only those placed at a distance larger than 100 m from other devices were included as distinct study sites. As true spatial replicates, we considered the number of study sites, instead of the number of recorders, since nearby sensors are prone to collect similar information and can provide non‐independent observations. Secondly, temporal sampling was defined by the number of recording days per study site, the daily period when the recordings were taken, and the number of daily samples (i.e. recordings within a day per study site). Similar to spatial data, time‐series data commonly experience autocorrelation, although the degree of autocorrelation depends on the time interval between samples (Bence, 1995). As true temporal replicates, we considered the number of recording days per site, instead of the total number of recordings, since daily observations are less autocorrelated than samples taken at shorter temporal scales (e.g. hourly observations), and species' vocal activity typically follows a diel cycle (Krittika & Yadav, 2019; Gil & Llusia, 2020). Thirdly, we gathered the number of the reported replicates (sample size) from each statistical test that assessed the relationship between acoustic indices and biodiversity. When the sample size of the test was not supplied, it was calculated according to the sampling procedure described in the article (e.g. Desjonquères et al., 2015; Buxton et al., 2016; Gage et al., 2017). When the procedure was unclear, sample size was determined as the number of observations reported for the diversity metric (e.g. Bertucci et al., 2016).
After detailing the sampling design and statistics, we determined the presence of pseudoreplication in the studies by comparing the number of reported statistical replicates (sample size) and the product of the true spatial and temporal replicates (study sites × recording days). When a mismatch between these two values was identified, we classified the analysis as having inflated replication (pseudoreplication = ‘yes’; Table S1). In this case, which was rather common for simple statistical tests (i.e. Pearson, Spearman, or linear regression), our defined true replicates were used as the sample size of the study for the meta‐analysis (hereafter adjusted sample size) (Spake & Doncaster, 2017). Analyses taking into account pseudoreplication, such as tests that included random factors for daily samples and/or recorders per site [e.g. LMM (Fuller et al., 2015; Buxton et al., 2016)], as well as other statistical techniques dealing with pseudoreplication and autocorrelation (e.g. bootstrapping; Moreno‐Gómez et al., 2019), were not considered as presenting pseudoreplication.
(5). Effect size computation
We used Pearson's correlation coefficient (r) as a measure of effect size, to describe the direction and magnitude of the relationship between acoustic indices and diversity metrics. When Pearson's correlation was not reported, we extracted other statistical coefficients in the following order: Spearman's correlation, t‐values, F‐values, linear regression slope coefficients, and R 2. When only graphical information was available and authors did not provide original values, we retrieved data from figures with Web Plot Digitizer v4.2. (Rohatgi, 2019) and computed Pearson correlations, as recently suggested for meta‐analysis (Bird et al., 2019; Siviter et al., 2021). We converted all statistics to effect sizes using compute.es package in R (Re, 2013) or the formulae provided in Nakagawa & Cuthill (2007) or Koricheva et al. (2013) when the package did not offer the required functions.
We converted our effect size r to Fisher's Z in order to satisfy the normality assumption of parametric meta‐analysis (Nakagawa & Cuthill, 2007). Since Fisher's Z values are not directly interpretable by most researchers, we converted Fisher's Z back to r to ease interpretation of the meta‐analysis results. We used conventional benchmarks qualitatively to assess the absolute magnitude of the effect size [small: 0.1 > r ≤ 0.3; moderate: 0.3 > r ≤ 0.5; and large: r > 0.5 (Møller & Jennions, 2002)]. In the investigated articles, the application of statistical tests often involved pseudoreplication. Generally, the lower effect size variances of pseudoreplicated studies due to non‐independent samples inflates the relative contribution of these effect sizes in inverse‐variance weighted meta‐analysis, leading to biased meta‐estimates (Pullin & Stewart, 2006; Spake & Doncaster, 2017). However, excluding pseudoreplicated studies is undesirable, as they might contribute relevant information to the meta‐analysis (Nakagawa & Santos, 2012; Davies & Gray, 2015). To mitigate such bias while retaining all information, we used an adjusted sample size for the pseudoreplicated studies (see Section II.4). As a result of this sample size adjustment, a single study (Bolgan et al., 2018) ended up with a sample size N ≤ 3, which impeded the calculation of Fisher's Z variance using the formula 1/(N–3). For this study, we assigned an adjusted sample size of 4 to allow variance estimation. Sample size correction was also applied to studies that controlled pseudoreplication with statistical techniques, such as those using LMMs (e.g. Buxton et al., 2016) or bootstrapping (e.g. Moreno‐Gómez et al., 2019), since Fisher's Z variance is inversely proportional to sample size and does not take into account variance estimates coming from the statistical tests used in studies.
Most of the studies (>80%) provided more than one estimate of the goodness of fit between acoustic indices and diversity metrics. Overall, studies tested several indices, both acoustic and biological, as well as the same indices for multiple taxonomic groups or recording data sets. Thus, we retrieved a total of 481 effect sizes from the 35 studies. To reduce the non‐independence of intra‐study observations while collecting effect sizes (Koricheva et al., 2013, chapter 16), we applied the following procedures (Table S3, independence). (i) Independent statistical tests, based on recordings collected in distinct geographical locations (Roca & Proulx, 2016; Eldridge et al., 2018; Moreno‐Gómez et al., 2019), temporal periods (Mammides et al., 2017) or by distinct recording methods (Jorge et al., 2018), were considered as independent units of analysis. (ii) Statistical tests based on the same recordings were only included in the meta‐analysis when researchers used a distinct frequency or temporal resolution in each analysis, which influences the calculation of the acoustic indices (Sueur, 2018). (iii) When different pre‐processing treatments [e.g. noise filtering, normalisation (Parks, Miksis‐Olds & Denes, 2014; Desjonquères et al., 2015; Harris, Shears & Radford, 2016; Bolgan et al., 2018)], or different metrics from the same data set (e.g. mean, range, etc.; Eldridge et al., 2018) were applied to the same recordings within a study, we computed a composite effect size following formulae provided in Borenstein et al. (2009, p. 230). After computing composite effect sizes between non‐independent effect sizes and removing a study (Papin et al., 2019; Table S1) due to difficulty in determining the study design, we retained for further analysis a total of 364 effect sizes extracted from 34 studies (Tables S4 and S5).
(6). Meta‐analysis
We clustered effect sizes within their corresponding studies and conducted multilevel meta‐analysis using Fisher's Z as our response variable. The multilevel structure effectively partitions the correlation structures within studies and allows the inclusion of multiple effect sizes per study (Nakagawa & Santos, 2012; Song et al., 2020). Following this scheme, we first tested whether acoustic indices were in general good proxies for biodiversity by computing an intercept‐only model. The resulting effect size estimate not only gives a clue of whether acoustic indices are performing well in estimating biodiversity across the literature, but also allows us to check if there is substantial heterogeneity in our effect sizes that could be explained by moderators. We quantified heterogeneity with the I 2 statistic, which estimates the proportion of unknown variation in effect sizes not attributed to sampling error variance (Higgins & Thompson, 2002). Due to the multilevel structure of our models, we used a modified version of the I 2 statistic to account for both within‐ and between‐cluster heterogeneity (Nakagawa & Santos, 2012). Here, within‐cluster heterogeneity corresponded to the unaccounted for variation found on effect sizes within studies, and between‐cluster heterogeneity corresponded to the unaccounted for variation between studies.
Once heterogeneity was identified, we conducted subgroup and meta‐regression analysis by extending the previous model with the inclusion of moderators as fixed factors. We focused on four moderators that could alter the accuracy of biodiversity estimation (see Table 3 for the rationale for selection of moderators), namely the factors acoustic index (N = 11 levels), diversity metric (N = 5), environment (N = 2), and diversity source (N = 2; Table 3). To retain statistical power, we refrained from including additional moderators or increasing the number of moderator levels (Borenstein et al., 2009). Additionally, to mitigate the effect of stochasticity, any moderator level with less than five studies was excluded in both subgroup and meta‐regression analysis, leading to the removal of several acoustic indices (Hf, Ht, M, and NP), and a biodiversity parameter (diversity of sounds) from model fitting procedures. Consequently, we included in our analysis seven acoustic indices (ACI, ADI, AEI, AR, BIO, H, and NDSI), four diversity metrics (species abundance, species richness, species diversity, and abundance of sounds), two types of environments (terrestrial and aquatic), and two diversity sources (acoustic and non‐acoustic). We used subgroup analysis with acoustic index as a moderator to assess which acoustic indices correlate best with biodiversity. In this analysis, we removed the model intercept specifically to test if the correlation between each acoustic index and biodiversity was significantly different from zero. Then, we used meta‐regression to check the effect of multiple moderators (namely, acoustic indices, diversity metric, environment, and diversity source) on the outcome of acoustic indices estimating biodiversity. We set as intercept the following combination of moderator levels: ACI index, species richness, terrestrial environment, and non‐acoustic diversity source. Unfortunately, due to the low study sample size between most factor level combinations, we were constrained to use an additive effects model.
Table 3.
List of moderators used to explain the variation in the relationship between acoustic indices and diversity metrics. See Table 2 for definitions of acoustic indices
| Moderator | Levels | Hypothesis | Rationale | Reference |
|---|---|---|---|---|
| Acoustic indices | ACI, AEI, ADI, AR, BIO, H, Ht, Hf, M, NP, NDSI | The performance of each acoustic index is uneven in the evaluation of biodiversity. | As each index is based on a specific mathematical formula that summarises distinct features of the soundscape, some indices might be better at estimating diversity metrics of communities or populations. | Sueur et al. (2014); Buxton et al. (2018a ) |
| Diversity metrics | Species abundance, species richness, species diversity, abundance of sounds, and diversity of sounds | Acoustic indices are particularly sensitive to changes in given biological metrics. | The biological component of soundscapes is a complex combination of sounds emitted by multiple individuals and species, and hence both diversity and abundance of vocalising animals (and their sounds) will have a large influence on the structure and dynamics of soundscapes. In consequence, acoustic indices might be able to better estimate some diversity metrics in comparison with others. | Pieretti et al. (2011); Gasc et al. (2015) |
| Environment | Aquatic or terrestrial | The environment where sounds are collected affects how well acoustic indices retrieve the biological information. | Due to the large differences in sound propagation, environmental noise, species composition, population density, animal behaviour and other aspects of aquatic and terrestrial ecosystems, acoustic indices might perform differently depending on the type of environment studied. | Gottesman et al. (2020); Roca & Opzeeland (2020) |
| Diversity source | Acoustic or non‐acoustic | The method used to extract the diversity metrics influences the fidelity of the indices to represent biodiversity. | Metrics extracted from audio recordings provide fine‐scale data that might allow a better link with acoustic estimates since both use the same source of information. Metrics extracted from field surveys, literature, or other sources often provide a more general view of the structure of the community or population and they might also be faithfully represented by the acoustic indices. | Darras et al. (2018); Melo et al. (2021) |
Collinearity among our moderators was inspected with variance inflation factor (VIF; Zuur, Ieno & Elphick, 2010) and found not to be an issue in our model (Table S6; VIF <1.7 for all moderators – threshold value = 3; Zuur et al., 2010). For all meta‐analytic models, we relied on restricted maximum likelihood estimation provided by the metafor package in R (Viechtbauer, 2010), and considered a result as significant if the effect size and corresponding 95% confidence interval (CI) did not include zero. Furthermore, we checked if our choice of moderators explained some of the variation in our effect sizes by computing an omnibus Wald‐type test on the null hypothesis that the estimates for the moderator levels are jointly equal to zero (Viechtbauer et al., 2015). This test excludes moderator levels set to be the intercept and the resulting statistic has a χ 2 distribution with degrees of freedom equal to the number of moderator levels tested. Moreover, to assess the statistical importance of the differences found in our analysis, within each moderator, we selected the moderator levels with the highest effect size estimates and contrasted them with effect size estimates from the other levels. For this, we used a Wald‐type test with one degree of freedom on the null hypothesis that the difference between the two levels is equal to zero. Statistical significance for Wald‐type tests was assumed for p < 0.05.
(7). Publication bias
One common issue in meta‐analysis is publication bias, where studies with low sample size or null effects are less likely to get published (Borenstein et al., 2009). We assessed publication bias both visually with funnel plots (Koricheva et al., 2013, chapter 14) and statistically with Egger's regression (Egger et al., 1997). A funnel plot shows the relationship between effect sizes and standard errors. In a symmetric funnel plot (i.e. hypothetically free of publication bias), the dispersion of effect sizes should get narrower as standard error decreases. Due to the multilevel structure of our data set, we used meta‐analytic residuals instead of effect sizes to meet independence assumptions (Nakagawa & Santos, 2012). Publication bias should be an issue if residuals lie outside the expected symmetry of the funnel shape, and if some of the funnel sections do not contain any residuals. To test statistically for funnel plot symmetry, we used Egger's regression with no intercept. A non‐significant inverse‐variance weighted regression of the residuals over the standard error indicates that the deviation of the residuals from the funnel plot shape is not greater than what would be expected by chance in a symmetric funnel plot (Viechtbauer, 2010).
Additionally, we checked for trends in the magnitude of effect size through time by visually inspecting a meta‐regression line fitted over publication year and effect size. Similarly, we looked for publication bias due to a potential relationship between journal impact factor and effect size (Møller & Jennions, 2002).
(8). Sensitivity analysis
To determine the influence of pseudoreplication on our meta‐analysis, we contrasted the effect size estimates for pseudoreplicated and non‐pseudoreplicated studies. For this, we conducted a meta‐analysis with pseudoreplication as the single explanatory variable and evaluated if pseudoreplicated studies provided different estimates in comparison to non‐pseudoreplicated studies. Finally, we visually inspected the presence of outlier studies using Cook's distance clustered by studies. The Cook's distance for a given study refers to an average of how far effect size estimates will move if the study in question is dropped from model fitting (Viechtbauer & Cheung, 2010). We considered a study an outlier if its Cook's distance was higher than the average of all computed Cook's distances. Outlier studies were examined to discriminate possible reasons for their influence, and meta‐regression analysis ran without the outliers to evaluate the robustness of our results (Murtaugh, 2002).
III. RESULTS
(1). Literature on the relationship between acoustic indices and biodiversity
Our systematic literature review identified 35 articles that assessed the relationship between the most common acoustic indices and biological diversity metrics. These articles were published in 18 journals (16 peer‐reviewed and two non‐peer‐reviewed) and two PhD theses. We identified two distinct periods in research: (i) from 2007 to 2013, the initial development of acoustic indices and first performance tests, and (ii) from 2014 to 2019, a rapid expansion of their application in ecology and conservation (Fig. 3). Since the first acoustic index was published in 2007 (Boelman et al., 2007), a large number of indices have been developed (Buxton et al., 2018b ), although an ad hoc assessment of their estimates has often been neglected. From a total of 142 studies that employed acoustic indices (Fig. 3), only 24.6% assessed their relationship with diversity metrics. This trend has been particularly pronounced in recent years, with a progressive decrease in the use of validation methods when employing acoustic indices (Fig. 3).
Fig. 3.
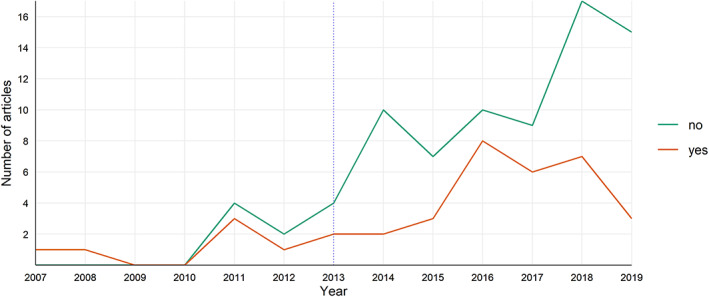
Trends (2007–2019) in publication and data validation, from a total of 142 articles. Articles that correlated the acoustic indices with real biological data are represented by an orange line and studies that did not correlate acoustic indices with such data are shown with a green line.
(2). Publication trends and shortfalls
Among the variety of indices, ACI, followed by H and ADI, have been the most popular (74, 49, and 34% of the articles, respectively; Fig. 4A), while species richness and abundance of sounds were the diversity metrics most commonly associated with acoustic indices (60 and 37% of the articles; Fig. 4C). Most studies tested more than one acoustic index (53%; Mammides et al., 2017; Retamosa Izaguirre & Ramírez‐Alán, 2018; Eldridge et al., 2018) and estimated diversity metrics from acoustic sources (68%). Over the environments and organisms investigated, research has mainly focused on terrestrial habitats (71%), birds (54%; Fig. 4B), and was conducted in the USA, followed by Australia and France (Fig. 5A). Despite the high diversity of investigated subjects, methodological approaches were overall consistent among studies, with most employing automated recordings (60%), .wav audio format (94%), recording length equal to (40%) or smaller than 1 min (40%), 44.1 kHz sampling rate (37%), and more than one site (80%). These studies recorded for an average of 44 days (range 1–282 days).
Fig. 4.
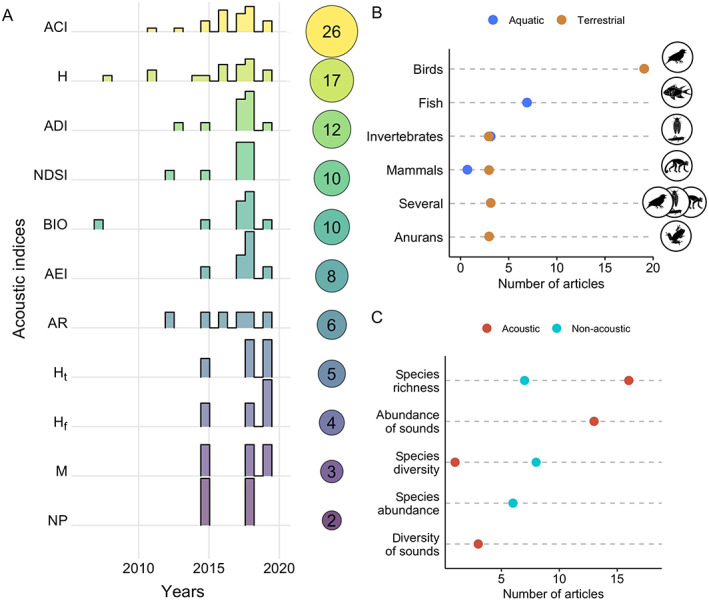
Summary of the data extracted from 35 articles identified in the systematic literature search. (A) Number of published articles per year using different acoustic indices; the sizes of the bubbles on the right represent the number of papers and the distribution of columns on each row is the frequency distribution of published articles over time, relative to the total per index (i.e. the number inside the bubble). (B) Number of articles per studied taxon in the aquatic (blue) or terrestrial (brown) environment. (C) Number of articles per diversity metric and source of data extraction (acoustic or non‐acoustic). For definitions of acoustic indices see Table 2.
Fig. 5.
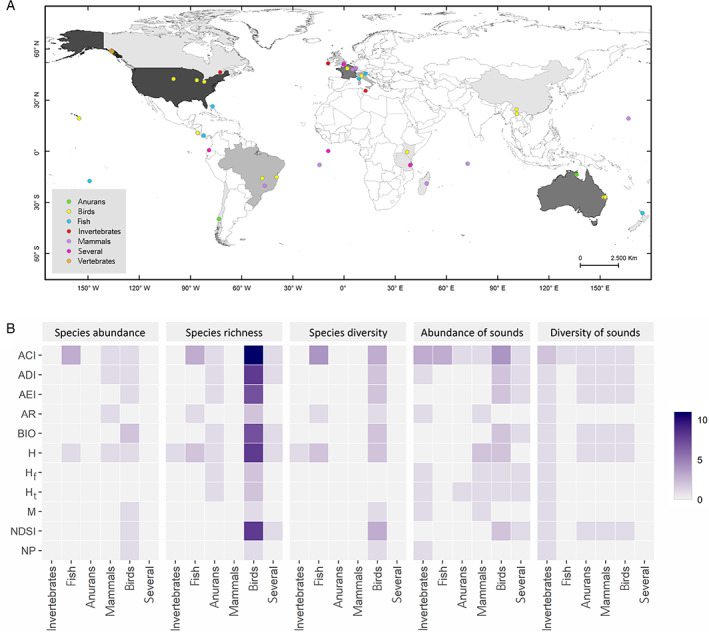
(A) The geographic distribution of the study sites corresponding to the 35 studies used in the systematic literature review. The colouring of countries exhibits a white to black gradient relative to the number of studies contributed by each country. The coloured dots discriminate between the different taxa studied. (B) Distribution of the number of articles by diversity metric, taxon and acoustic index studied (see Table 2 for definitions), from the 35 studies included in the literature review.
Although a large set of combinations of acoustic indices and diversity metrics have been tested, most relationships remained unexplored (Fig. 5B). For example, we found no study examining the relation between acoustic indices and richness and diversity of mammals, abundance and diversity of anurans, or abundance of invertebrates. Bird species richness was the sole diversity metric assessed with all the most common acoustic indices, being mainly associated with ACI (31%), followed by H, NDSI, and ADI (23%).
A large set of studies exhibited statistical deficiencies in testing the relationship between acoustic indices and biodiversity estimates, particularly pseudoreplication (40%; Fig. S1). The use of temporally non‐independent replicates was the most common type of pseudoreplication (43%), followed by spatial–temporal (36%) and spatial pseudoreplication (21%). The incidence of pseudoreplication varied significantly across indices, ranging from 20% of the tests using AR to up to 100% in the case of M and NP.
(3). Meta‐analysis
We analysed 364 effect sizes and found that acoustic indices showed an overall moderate positive correlation with biodiversity (r = 0.33, CI [0.23, 0.43]) (Fig. 6A; Table S7). The variation around the overall estimate was high (i.e. within‐study heterogeneity [I 2 study] = 17.6%; between‐study heterogeneity [I 2 entries] = 67.5%; and total heterogeneity [I 2] = 85.1%; Fig. S2), which justified the use of moderators to explain the unaccounted for variation. Subgroup analysis with acoustic indices as a single moderator showed a significant and positive correlation between diversity metrics and the ACI, ADI, H, and NDSI indices (Fig. S3; Table S8). Although the H index was the best performing acoustic index (r = 0.50, CI [0.36, 0.62]), overlapping confidence intervals between H and NDSI, ACI, ADI, and BIO indices advise against the determination of a single best acoustic index. Meta‐regression analysis provided support for the ACI, H, and NDSI indices as the best estimators of biodiversity (Fig. 6B; Table 4).
Fig. 6.
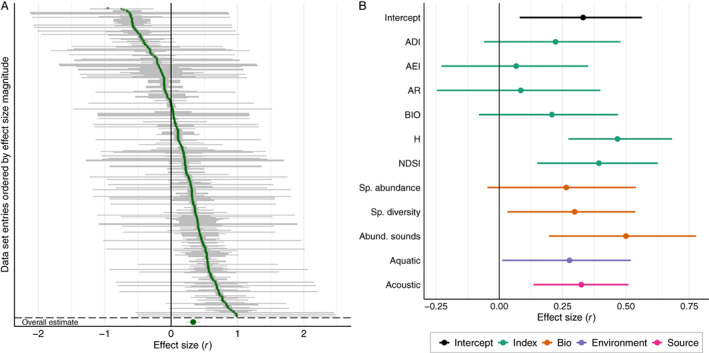
Meta‐analysis results. (A) Pearson correlation effect sizes (r) in ascending order of magnitude from all data set entries. Effect sizes larger than 0 (vertical line) represent a positive correlation between acoustic indices and diversity. Effect sizes below 0 indicate a negative correlation between acoustic indices and diversity. Above the dashed horizontal line, the green circles are effect sizes means, with corresponding 95% confidence intervals (grey horizontal lines). Below the dashed line, the green circle is the overall effect size, with a corresponding 95% confidence interval, obtained from the intercept‐only meta‐analysis. (B) Mean estimates (circles) and corresponding 95% confidence intervals (horizontal lines) represented as Pearson correlation (r) effect sizes. Each estimate (except the intercept) corresponds to the additive effect of each coefficient as obtained with the predict_rma function from metafor R package. Estimated effect sizes whose 95% confidence intervals do not overlap zero (black vertical line) indicate a positive correlation between acoustic indices and diversity if they are to the right of zero, or a negative correlation if they are to the left of zero. Moderators are acoustic indices (Index), diversity metrics (Bio), environment (Environment) and acoustic source (Source). See Table 2 for definitions of acoustic indices.
Table 4.
Meta‐regression coefficients for the model intercepts (ACI index, species richness, terrestrial environment, and non‐acoustic source) and the levels of each moderator, based on Pearson (r) correlation (‘Estimate’). The standard error of the estimates (SE) and the lower and upper bounds of the confidence intervals (CI.lb and CI.ub) are shown. See Table 2 for definitions of acoustic indices
| Moderator | Coefficient | Estimate | SE | CI.lb | CI.ub |
|---|---|---|---|---|---|
| Intercept | 0.344 | 0.141 | 0.081 | 0.563 | |
| Acoustic index | ADI | −0.129 | 0.117 | −0.344 | 0.1 |
| AEI | −0.284 | 0.123 | −0.488 | −0.05 | |
| AR | −0.267 | 0.147 | −0.511 | 0.017 | |
| BIO | −0.144 | 0.12 | −0.363 | 0.091 | |
| H | 0.195 | 0.109 | −0.016 | 0.39 | |
| NDSI | 0.084 | 0.125 | −0.162 | 0.319 | |
| Diversity metric | Species abundance | −0.081 | 0.158 | −0.374 | 0.226 |
| Species diversity | −0.042 | 0.095 | −0.224 | 0.143 | |
| Abundance of sounds | 0.254 | 0.146 | −0.028 | 0.499 | |
| Environment | Aquatic | −0.066 | 0.147 | −0.342 | 0.221 |
| Diversity source | Acoustic | −0.009 | 0.145 | −0.288 | 0.271 |
Additionally, acoustic indices had a higher correlation with abundance of sounds (r = 0.25, CI [−0.03, 0.50]) than with any other diversity metric, although such difference was weak as indicated by the wide confidence intervals of abundance of sounds and the non‐significant Wald‐type tests comparing abundance of sounds with all other diversity metrics (see Tables S9–S12 for results of Wald‐type tests). The meta‐regression results showed that the moderators diversity metrics, environment, and diversity source had low additional explanatory power compared to the results of the subgroup analysis (Table 4). Indeed, the omnibus test revealed that even though our choice of moderators explained some variation in effect sizes (Wald [χ 2] = 27.43, p = 0.004, df = 11), only the acoustic indices moderator was found to be statistically important in that regard (Wald [χ 2] = 22.35, p = 0.001, df = 6).
Regarding pseudoreplication, we found that this statistical issue did not influence effect size estimates (contrast between non‐pseudoreplicated and pseudoreplicated estimates = −0.08, CI [−0.31, 0.16]; Table S13), suggesting that sampling design and statistical analysis were not crucial in explaining the variation among effect sizes in our meta‐analysis. Additionally, publication bias seems a minor issue in our data set, as indicated by inspection of the funnel plot (Fig. 7) and a non‐significant relationship between residuals and effect size precision (Egger's regression on the null hypothesis of plot symmetry: β = 0.15, df = 294, p = 0.51). However, we observed a tendency for effect size values to decrease over time from 2007 to 2019 (Fig. 8A), with a less pronounced decrease since 2015 (Fig. 8B). No linear trend was found for the relation between effect size and journal impact factor (Fig. S4).
Fig. 7.
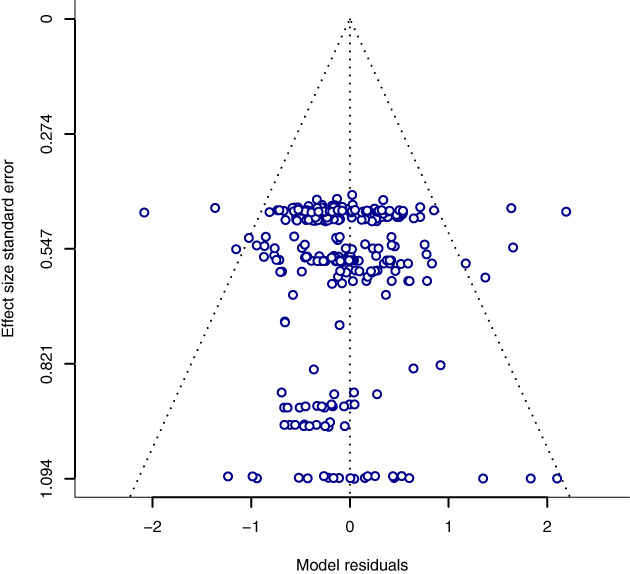
Funnel plot (dashed triangle) showing the relationship between model residuals from the meta‐regression model and effect size standard error. Absence of publication bias is represented by a scattered and symmetric distribution of values (circles) within the funnel. We tested funnel plot symmetry with Egger's regression and failed to reject the null hypothesis of funnel symmetry (p = 0.51).
Fig. 8.
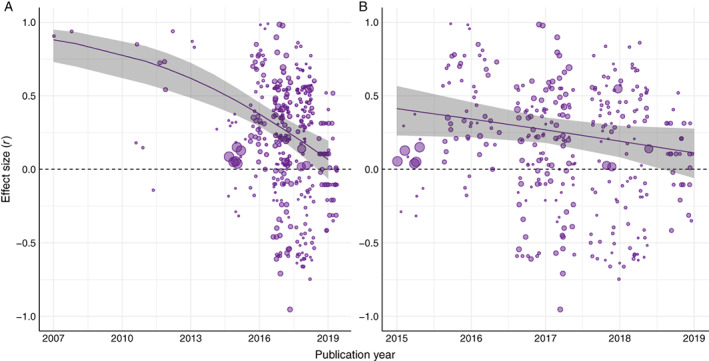
Relationship between reported mean effect sizes and publication year. Circle size indicates the relative sample size for each effect size. The fitted line is a meta‐regression over the year of publication with the corresponding 95% confidence interval region shaded grey. The dashed horizontal line represents an effect size of 0. Effect size mean values are positioned along the publication year axis with minor random noise to reduce overlapping. (A) Relationship between effect size and publication year for the entire data set (2007–2019), corresponding to all literature identified up to 2019 assessing the performance of acoustic indices as proxies for biodiversity. Model statistics in Pearson correlation (r), intercept 1.00 [1.00, 1.00], slope –0.11 [−0.15, −0.06], estimate [CI]. The computed model is a linear model using Fisher's Z as effect size. The transformation from Fisher's Z unbounded values to Pearson correlation values bounded between −1 and 1 creates the curved pattern for larger effect sizes. (B) Relationship for the subset of effect sizes published between 2015 and 2019 (inclusive) during which there was a prominent rise in publications (see Fig. 3). Model statistics in Pearson correlation (r), intercept 1.00 [−0.29, 1.00], slope –0.08 [−0.16, 0.00], estimate [CI].
Finally, we identified the presence of two outliers, i.e. studies with a Cook's distance value greater than the mean of all computed Cook's distance values (Fig. S5). Both of these studies investigated birds. The study with the largest Cook's distance (Mammides et al., 2017) had a total of 84 entries in our data set, comprising 28% of our effect sizes. The effect sizes for this study were over‐dispersed and mostly skewed towards the negative side of the meta‐analytic residuals (Fig. S6). The other study (Gage et al., 2017) had extremely high effect size values at both ends of the effect size scale (−1 and 1), specifically an extremely strong negative correlation between ADI and species richness (r = −0.95, N adjusted = 60), and almost perfect correlations between H and abundance of sounds (r = 0.99, N adjusted = 60) and between AEI and species richness (r = 0.99, N adjusted = 60). Meta‐regression analysis with the exclusion of these studies resulted in reinforcement of the magnitude of the correlation between acoustic indices and biodiversity, with all predicted effect sizes becoming significantly positive (Table S14; Fig. S7), perhaps because the study with the largest Cook's distance (i.e. Mammides et al., 2017) had meta‐analytic residuals on the negative side of the original meta‐regression model. Moreover, there was some reduction in effect size heterogeneity (total I 2 = 83%). The latter changes are possibly linked to the joint effect of the removal of the extreme values of one of the outlier studies (Gage et al., 2017) and the removal of the over‐dispersed effect sizes in the other (Mammides et al., 2017). These changes had a minor effect on the relative positions between model estimates when compared to our original model (Fig. S7), and thus we retain our conclusions from the model including the full data set.
IV. DISCUSSION
Animal sounds comprise a major source of variation in natural soundscapes, making the use of acoustic indices potentially suitable to describe and track changes in ecological communities. Although acoustic indices are increasingly employed in biodiversity assessment, empirical evidence of their efficiency as proxies for biological diversity is mixed, hampering the generalisation of these indicators in ecological research. Over a decade since their release, we found 35 studies that investigated the relationship between acoustic indices and biodiversity in a wide range of subjects. The majority of the studies were related to bird richness in the northern hemisphere and multiple combinations of acoustic indices, diversity metrics, animal groups, and regions remain to be examined. Surprisingly, we identified pseudoreplication as a frequent shortcoming in the literature and future research should consider statistical analysis more rigorously. Moreover, we found that effect sizes tended to decrease over time and that a large and growing number of studies used indices without testing their link with biodiversity, which may impair the interpretation of estimates and lead to spurious findings. Overall, our meta‐analytical framework showed that acoustic indices have a moderate positive correlation with biodiversity, although effect sizes were highly variable both within and among studies. Specifically, acoustic indices detected changes in abundance of sounds better than changes in other diversity metrics, with H, NDSI, and ACI having the best performance. Other factors (type of environment and information source) had no influence on effect sizes, suggesting that additional parameters might be crucial to explain variation in the indices estimates. Altogether, our findings highlight that caution should be exercised when using acoustic indices as ecological indicators (see Sections IV.1–4), since they are far from being direct proxies for biodiversity. However, these novel tools are able partially to capture changes in diversity metrics and show a certain capacity to generalise their application across habitats and taxa, making them promising bases for future developments. We provide below an overview of the prospects and challenges of acoustic indices in biodiversity assessment, and outline recommendations for their use and future research (see Sections IV.4 and IV.5).
(1). Do acoustic indices reflect local biodiversity? A meta‐analytical overview
The moderate relationship between acoustic indices and metrics characterising local biodiversity corroborates, to a certain degree, the original rationale behind acoustic diversity indices, aimed at summarising the acoustic features of communities and soundscapes in order to represent or estimate biological diversity. Acoustic indices partially inform about species diversity patterns and hence might be used as ecological indicators, although our analysis revealed a lack of evidence for a strong correlation. In complex systems such as animal communities and ecosystems, the relationship between measurable variables is rarely high, as shown by a review of meta‐analyses in ecology (r = 0.18, CI [0.15, 0.21]; Møller & Jennions, 2002). However, it is also true that our results preclude us from considering acoustic indices as direct proxies for biodiversity, especially when used as single predictors in simple linear models, such as those investigated in this study and which are the most common in the literature so far. The high variability in the performance of the acoustic indices, both within and among studies, and even when applying the same index, advises against their generalisation as surrogates of diversity metrics without validation of their predictions. Thus, caution should be used when employing these indices and interpreting their outputs (e.g. combining multiple indices, validating estimates, etc.). Moreover, further research is needed to enhance the application of these novel metrics in biodiversity appraisal (see Section IV.5).
We found H, NDSI, and ACI to be better indicators of biodiversity than the other common acoustic indices examined in the meta‐analysis (i.e. ADI, AEI, AR, and BIO). Our results highlight the most suitable acoustic indices to be selected in future research investigating local biodiversity through single linear models. However, given the ability of each index to capture distinct aspects of the acoustic complexity of sound samples, the combination of several indices is particularly advised for characterising biodiversity (Towsey et al., 2014; Buxton et al., 2018b ). It remains to be addressed in the future whether H, NDSI, and ACI perform better when using a combination of indices.
Overall, the performance of acoustic indices was unrelated to biological moderators. Specifically, we found no influence of the type of environment where studies were conducted, namely terrestrial (N = 24 studies) versus aquatic (N = 10), nor of the information source used to estimate the diversity metrics, namely acoustic (N = 26) versus non‐acoustic (N = 11). This consistent performance of acoustic indices across environments and methods used to extract biological data suggests that indices‐based estimates might be generalisable and encourages their use as a complementary approach to traditional survey methods (Greenhalgh et al., 2020; Melo et al., 2021). However, our findings showed that acoustic indices correlate better with abundance of sounds than with other metrics, indicating a better capacity to detect changes in the number of recorded signals than in species abundance, richness, or diversity. This difference, although apparently weak, suggests that predicting species‐based metrics with acoustic diversity and heterogeneity will be challenging. These are complex and variable features that are hard to capture with single acoustic indices computed from passive recordings. By contrast, a high sensitivity to variation in sound energy enables acoustic indices to estimate the abundance of specific types of sounds from identified or unidentified sources more efficiently.
The lack of explanatory power of biological moderators raises two main considerations. First, other moderators not explored here, such as recording settings, parameters of index calculation, etc., might also modulate the magnitude and/or direction of the correlation between acoustic indices and diversity metrics (Metcalf et al., 2020a ). Second, our additive meta‐regression model with two (out of four) binary moderators might have failed to capture interactions between moderator levels or underlying variation at finer scales (e.g. using taxa as moderator levels, instead of environment type). The design of our meta‐analysis was constrained by the low number of moderator level combinations. Future research will be able to investigate these points as more studies and effect sizes reporting the link between acoustic indices and diversity metrics become available.
Surprisingly, we found that studies progressively reported smaller effect sizes over time. This implies that the broader application of acoustic indices over recent years has revealed limitations on their capacity to quantify local biodiversity efficiently. In the early years of their application (2007–2013), the studies that described and tested new indices often provided overly optimistic assessments of their performance, which likely led to a false sense of optimality. When indices started to be applied to a variety of taxa, environments, and acoustic conditions, researchers reported a more balanced performance for these indices. Indeed, the most recent studies have provided results closer to a distribution around the overall mean effect size (r = 0.3). This is probably related to the growth in papers trying to provide guidelines and sheds light on the use and interpretation of acoustic indices during the later period in ecoacoustics research (2014–2019). An alternative hypothesis is that the expansion of ecoacoustics, with more numerous and diverse practitioners, might have led to a decline in the appropriate application of acoustic indices, leading to an apparently less‐efficient performance of these tools over time. However, there is little evidence for this hypothesis; a majority of pioneering researchers in ecoacoustics continue to contribute publications and no study has linked past experience in ecoacoustics with the effect sizes reported by researchers. Thus, together with the rapid spread of acoustic indices, additional efforts will be required to clarify the significance, interpretation, and efficient usage of these metrics in biodiversity appraisal (Gasc et al., 2015; Buxton et al., 2018b ; Bradfer‐Lawrence et al., 2019; Metcalf et al., 2020a ) (see Section IV.5). Our review enabled the identification of shortcomings when applying acoustic indices that should be addressed in future ecoacoustics research. These shortcomings derive from two main sources: (a) the theoretical framework, and (b) practical implementation.
(2). Shortcomings in the theoretical framework
The ecological literature has long been dominated by the classical perspective of deterministic forces driving the assembly of ecological communities (MacArthur & Levins, 1967; Diamond, 1975). Specifically, ecological niche partitioning and environmental selection (e.g. environmental filtering and species sorting) are mechanisms predicting that (i) competition promotes segregation of ecological niches and (ii) the environment filters out species lacking specific attributes that enable their persistence in such environments. Similarly, the two main hypotheses underlying the theoretical background of ecoacoustics posit similar expectations for the acoustic output (or acoustic space) of coexisting species (Sueur & Farina, 2015): the acoustic niche hypothesis (ANH; Hödl, 1977) and the acoustic adaptation hypothesis (AAH; Morton, 1975). Both hypotheses can be nested within a general framework where sensory systems (including sound emission, propagation, reception, and signal design) are under selective pressure to maximise information transfer [sensory‐drive hypothesis (Endler, 1992; Ryan & Cummings, 2013)].
The ANH predicts the acoustic output of communities to be partitioned in time and frequency given the potential impact of signal interference and recognition errors among co‐occurring species (Hödl, 1977; Duellman & Pyles, 1983; Brumm, 2013; de Araújo et al., 2020). Consequently, the higher the number of vocally active species in a given community, the higher the expected diversity of acoustic signals in the acoustic space. Hence, the acoustic space would be segregated and show low overlap between species acoustic signals. These ideas are rooted in the process of character displacement and specifically, reproductive character displacement, where closely related species with similar phenotypes are subject to higher competition and hybridisation potential (Pfennig & Pfennig, 2009; Hoskin & Higgie, 2010). Evidence for character displacement in acoustic signals comes mainly from studies of pairs of sympatric/syntopic and allopatric/allotopic lineages (Loftus‐Hills & Littlejohn, 1992; Lemmon, 2009; Kirschel, Blumstein & Smith, 2009). However, it is likely that most divergence in species traits may have accumulated while in allopatry, before secondary contact. Trait divergence is often a result of larger evolutionary ages of interacting lineages (Tobias et al., 2014a ; Laiolo et al., 2017), especially when in allopatry (Drury et al., 2018), and thus a pattern of acoustic partitioning in ecological communities may be absent or driven by evolutionary forces unrelated to sensory systems. Accordingly, disentangling the roles of sexual, ecological, and sensory‐driven selection on acoustic divergence requires testing multiple hypotheses in the same system (e.g. Sugai et al., 2021a ), ideally while considering genetic differences (Wilkins, Seddon & Safran, 2013). Therefore, the degree of acoustic partitioning and signal diversity is not necessarily related to the number of co‐occurring species and relying on the ANH to identify a direct relationship between acoustic and species diversity could be misleading.
Alternatively, as predicted by the AAH, the acoustic output of communities in similar habitats can converge in time and frequency given evolutionary pressures for the optimisation of signal transmission (Morton, 1975). Experimental tests have shown that signal attenuation and degradation during propagation through the environment can affect signal detection and the encoded information aimed at a receiver (Forrest, 1994; Slabbekoorn, Ellers & Smith, 2002; Ringler et al., 2017). In agreement with these observations, habitat‐dependent selection has been found to influence signal design over evolutionary timescales (Goutte et al., 2016; Derryberry et al., 2018; Pearse et al., 2018). However, empirical evidence on AAH as a driver of the acoustic output of ecological communities is also mixed (Ey & Fischer, 2009; Tobias et al., 2010), and meta‐analytical support reveals that the overall effect size is weak (Boncoraglio & Saino, 2007).
Moreover, selection on ecological traits that are correlated with acoustic features (e.g. body size) can also shape the extent of variation in the acoustic output of ecological communities (Derryberry et al., 2018), and thus a convergent acoustic pattern of communities in similar habitats may be unrelated to the role of AAH. For instance, agonistic character displacement can promote convergence in traits associated with competitor recognition given mutual benefits in reducing interspecific aggression and competition for ecological resources (Cody, 1973; Grether et al., 2009; Losin et al., 2016). Under this perspective, species‐diverse communities shaped by interspecific interactions would be composed of species with similar acoustic signals and/or signalling strategies, leading to low variability in the acoustic output of communities (communication networks hypothesis), as suggested for tropical bird communities (Tobias et al., 2014b ). As a consequence, an acoustic output with low acoustic variability should not be interpreted as a low species diversity soundscape, since the same pattern can be driven by other forces, such as habitat‐dependent selection and species interactions. Hence, acoustic assessments would benefit from including covariates characterising habitats and species relatedness (Erdtmann & Lima, 2013; Wilkins et al., 2013). In conclusion, we identify significant shortcomings in the theoretical framework behind the rationale of acoustic indices, which may partly explain their moderate link with diversity metrics and why estimates often deviate from expectations. These shortfalls include weak support for ANH and AAH and the disregard of multiple deterministic and stochastic processes influencing the acoustic output of ecological communities.
(3). Shortcomings in practical implementation
From a practical point of view, one challenge is to find accurate solutions that enable the assessment of biological diversity by automated and synthetic analysis of sound samples. First, the mathematical computation of each acoustic index involves reducing the entire acoustic dimensions (time, frequency, and energy) into a single value. Thus, it seems reasonable to question to what extent an analytical strategy based on such extreme information reduction can properly describe biodiversity in a wide range of complex soundscapes, communities, and ecosystems (Buxton et al., 2018b ). Second, multiple factors strongly affect the acoustic diversity of environmental sound samples in real‐world scenarios, representing confounding factors that often mislead the estimates of acoustic indices (Depraetere et al., 2012; Gasc et al., 2015).
Sound produced by non‐target animal groups, weather conditions, or abiotic elements (e.g. rain, wind, streams), as well as noise generated by human activities (e.g. traffic noise), might cause sound distortion and mask signals of interest, all of which can greatly interfere with acoustic metrics of biodiversity (Towsey et al., 2014; Fairbrass et al., 2019). This issue is particularly severe in the presence of intermittent rather than continuous noise (Pieretti et al., 2011; Depraetere et al., 2012). Thus, it is advisable to reduce noise in recordings, either by placing acoustic sensors far from noise sources (Machado, Aguiar & Jones, 2017; Mammides et al., 2017) or by applying noise filtering (Harris et al., 2016; Eldridge et al., 2018) or automated noise detection to the collected audio files (Metcalf et al., 2020b ), before indices calculation. Some indices can also be employed in specific frequency bands, enabling avoidance of narrow‐band noise. However, when noise covers a wide frequency range and masks the target signals, removing recordings from the sample might likely be the only solution (Depraetere et al., 2012).
In addition to noise, there are other deterministic and stochastic sources of variability that are critical confounding factors when implementing acoustic indices, such as the number of signals emitted by each species or the sound diversity within and among species. The contribution of each taxon to a given soundscape is largely determined by its relative abundance. As shown by our meta‐analysis, acoustic indices may be particularly sensitive to the number of sounds of a given species registered in the environmental recordings (e.g. conspecific choruses). Thus, differences in species relative abundance are expected to influence estimates regardless of species richness. The same pattern can be driven by variation in behavioural motivation of the signallers (leading to interspecific differences in the number of recorded signals) and signaller–receiver distance (leading to interspecific differences in the amplitude of recorded signals). These sources of variability alter the features of the soundscapes to be analysed (Priyadarshani, Castro & Marsland, 2018a ; Priyadarshani et al., 2018b ) and consequently influence the acoustic estimates. As passive sensors typically record sounds over a wide variety of conditions and distances, standardising or accounting for these parameters (relative abundance, call rate, or sound amplitude) is unfeasible, which may cause noticeably biased values when using acoustic indices to estimate species diversity.
Moreover, the number of sound types emitted by each species (within‐species sound diversity) is highly variable, particularly for certain groups such as mammals and birds. While the vocal repertoire of some species is restricted to few simple stereotyped signals, other species may produce dozens or hundreds of distinct sounds (leading to an unequal contribution of species to the acoustic space). Thus, variation in the vocal repertoire size exhibited by signalling species may critically influence the outputs and is an additional issue to be considered when using and interpreting acoustic indices. As a result, a low‐diversity community may give rise to audio recordings characterised by high acoustic diversity, and vice versa (Mammides et al., 2021). Other confounding sources to take into account when relating acoustic indices to biological diversity are the ecological and evolutionary drivers of interspecific differences in acoustic features (among‐species sound diversity; see Section IV.2). For instance, phylogenetic inertia of animal signals implies higher acoustic similarities in closely related species, and hence a species‐diverse community may exhibit lower acoustic diversity when comprising closely related species. Overall, community assembly is influenced by non‐exclusive deterministic (e.g. competition and environmental filtering) and stochastic forces (e.g. dispersal and random population fluctuations) that shape the organisation of the acoustic space (Chek, Bogart & Lougheed, 2003; Roca & Proulx, 2016; Sugai et al., 2021a , b ).
As acoustic indices are mathematical formulae that summarise the entire complexity of an audio sample into a single value, the interpretation and limitations of their estimates should be closely linked to these formulae and to the soundscapes recorded. Despite increasing efforts to provide guidelines for their use (Gasc et al., 2015; Buxton et al., 2018b ; Sueur, 2018; Bradfer‐Lawrence et al., 2019; Metcalf et al., 2020a ), the high diversity of acoustic indices in terms of conceptualisation and calculation, with intrinsic variations in the derivation of each index, still limits a full understanding of the performance of these indicators. For example, acoustic indices can be calculated as scalar quantities or as vectors corresponding to frequency bins, based on ranks, evenness, or area under the curve, and computed as averages, ratios, or normalised values (Towsey, 2017; Sueur, 2018). Thus, enhancing our understanding of how a particular sound event or parameter contributes to the output values of a given index is a key, but largely unknown, aspect of these novel tools. The choice of specific settings (e.g. time and frequency bins) for the computation of some acoustic indices has been shown recently to play a key role in their fidelity to the representation of ecological communities (Metcalf et al., 2020a ). When selecting proper time and spectral scales, indices are likely to correlate better with diversity metrics related to a target biological group. Similarly, the use of multiple indices and measures of statistical dispersion, such as standard errors (rather than only mean values), can provide a more complete understanding of soundscape patterns over time and space (Bradfer‐Lawrence et al., 2020).
(4). Ecoacoustic literature
Together with the recent and growing expansion of this research area, our literature review identified a series of trends and shortfalls in the studies testing the relationship between acoustic indices and biodiversity. The development of these novel tools has been closely linked with the emergence of passive acoustic monitoring in ecological research, which enables cost‐efficient remote sampling of environmental sounds at multiple sites and over long periods (Sugai et al., 2019). We found that studies typically collect sounds using short time windows (1 min or less), although the use of continuous recording has recently been advocated (Bradfer‐Lawrence et al., 2019), now supported by advances in technology (recording units and data storage). In contrast with the highly diverse recording schedules applied in terrestrial passive acoustic monitoring (Sugai et al., 2020), more consistent sampling designs seem to be used in ecoacoustics research. As expected, differences in sampling rate between studies were often related to differences in focal animal groups. This parameter has a direct influence on the spectral range and resolution of the recordings and hence it should be carefully considered as it may potentially impact the acoustic estimates. Noise treatment is commonly taken into account as a required pre‐step (Depraetere et al., 2012; Gasc et al., 2015) and a variety of methods have been employed so far, due to a lack of a consensus on the most efficient solution. Furthermore, studies generally collected biodiversity information from the same audio recordings used for indices calculation, and less frequently from field surveys (e.g. point counts; Bibby et al., 1992) or from the literature. In line with the earliest papers (Sueur et al., 2008; Pieretti et al., 2011; Depraetere et al., 2012), most studies used a set of acoustic indices and tested their correlation with diversity metrics, particularly species richness and abundance of sounds. Thus, the published literature evaluating biodiversity with acoustic indices fails to cover a broad collection of diversity metrics in a balanced way.
We found a large proportion of studies addressing bird species richness. This is likely rooted in the traditional use of birds as study species in ecological research, where they are highly overrepresented in comparison with other taxa (Titley, Snaddon & Turner, 2017). Well‐documented avian bioacoustics as well as the widespread use of autonomous recording units for bird surveys (Shonfield & Bayne, 2017; Sugai et al., 2019) might have contributed to this uneven representation of studies applying acoustic indices. As birds are highly vocal animals, a better understanding of bird species richness using acoustic methods could be an efficient way to assess the contemporary status of bird communities to support conservation programs. Particularly noticeable is a lack of studies on bats, which is the group most commonly monitored by passive acoustics (Sugai et al., 2019), reinforcing the idea that ecoacoustics has focused on birds as an especially suitable model system. Primary studies were also highly biased towards terrestrial environments, coherent with the fact that all these indices were first tested in terrestrial communities. The first study correlating acoustic indices with biodiversity in marine ecosystems was published in 2013, in which the abundance of sounds in benthic habitats was evaluated using ACI (McWilliam & Hawkins, 2013). It was not until 2015 that acoustic indices were first employed in freshwater environments (Desjonquères et al., 2015). In addition, research has mostly focused on temperate regions, as identified in previous reviews (Buxton et al., 2018b ; Sugai et al., 2019), with typically less saturated and complex acoustic environments than tropical regions (Pijanowski et al., 2011a ). New research on less‐studied taxa and environments will be crucial for a comprehensive understanding of acoustic communities and their link with acoustic indices. Some recent studies are providing the first steps to fill this knowledge gap (Greenhalgh et al., 2020; Retamosa Izaguirre et al., 2021).
A common statistical issue in the reviewed studies was pseudoreplication. Pseudoreplication has been well documented in biological sciences (Colegrave & Ruxton, 2018) and occurs when samples are considered statistically independent when they are not (Hurlbert, 1984). We found that 40% of the 35 studies relied on pseudoreplicated data without taking into account the absence of data independence, and hence violating this general assumption of statistical tests. The percentage of pseudoreplicated studies was similar to those recently identified in other scientific areas (e.g. Lazic, 2010; Waller et al., 2013). Ecoacoustics research is prone to pseudoreplication as samples are often time series of recordings taken at regular intervals over long periods and from multiple sites. Failure to account for the temporal and spatial autocorrelation of such observations artificially inflates sample size, leading to overestimated effect sizes, an increase in type‐I error rates, and thus a higher probability of drawing incorrect conclusions. These and other conceptual and analytical issues regarding the use of entropy index in ecoacoustics have been discussed previously (Sandoval, Barrantes & Wilson, 2019).
For studies using passive recordings, we strongly encourage the adoption of basic procedures to avoid pseudoreplication. First, proper identification of replicates (true independent samples) and pseudoreplicates (non‐independent samples) must be achieved by critically assessing the survey design. Observations at the same site or within the same time period (e.g. hour or day) should be considered non‐independent samples as they are likely correlated (see Section II.4). Also, if samples are nested, collected in subsets, or hierarchically organised, pseudoreplicates are common since all potentially eligible samples (sample universe or population) had unequal chances of being selected. Second, a proper application of statistical analyses must take into account the structure of the data set. As pseudoreplication is a statistical issue, rather than a design error, non‐independent data does not entail a pseudoreplicated study (Colegrave & Ruxton, 2018). Several strategies can be applied to prevent pseudoreplication: (i) to summarise data into new variables that are less autocorrelated and represent true replicates (e.g. mean per site), although this strategy involves information loss; or (ii) to apply statistical analyses that enable the use of the whole data set (including pseudoreplicates) by properly incorporating the true structure of randomness present in the data. These analyses include: repeated‐measures analysis of variance (rANOVA), which allows the addition of within‐subject factors; mixed models (e.g. LMM), which incorporate random and fixed factors, as an improved version of rANOVA (see Fuller et al., 2015); or bootstrapping techniques which enable the control of autocorrelation by iterative processes of resampling and testing (see Llusia et al., 2013; Moreno‐Gómez et al., 2019). Other strategies ranging from simple design‐based resolutions to more complex statistical tests have also been described (Forstmeier, Wagenmakers & Parker, 2017).
In our meta‐analysis, after adjusting sample sizes, we found that pseudoreplicated studies gave effect size estimates comparable to those of non‐pseudoreplicated studies. We included them because they may contain relevant ecological information which is needed for a broad overview of the correlation between acoustic indices and biodiversity. In ecological research, such studies can be collectively informative when used in meta‐analysis (Spake & Doncaster, 2017), while large amounts of valuable data would be lost if all pseudoreplicated studies were removed from analysis (Davies & Gray, 2015).
(5). Research agenda
The novelty of emerging technologies for audio recording has promoted rapid collection of large amounts of data at the cost of lacking standards and guidelines for spatial and temporal sampling (Sugai et al., 2020) and acoustic analysis (Metcalf et al., 2020a ). As a result, recommendations aimed at reducing the sources of variation and improving efficacy when using acoustic indices as proxies for biodiversity have been proposed (Gasc et al., 2015; Buxton et al., 2018b ; Bradfer‐Lawrence et al., 2019; Sugai et al., 2020; Metcalf et al., 2020a ). Our findings support these practical guidelines as important foundations for future research. In addition, we call attention to the general shortcomings and knowledge gaps highlighted herein (see Sections (2), (3), (4)). We argue that addressing these key issues will vastly improve our understanding of the relationship between acoustic indices and biodiversity.
We also look forward to new avenues of research, beyond the applications explored herein. The combined use of multiple acoustic indices to characterise biodiversity has shown promising results (Towsey et al., 2014; Gómez, Isaza & Daza, 2018; Buxton et al., 2018b ; Dema et al., 2020; Bradfer‐Lawrence et al., 2020). This approach encompasses the transformation of several indices into new metrics (e.g. Towsey et al., 2014), the application of multivariate analysis based on classification algorithms (e.g. Buxton et al., 2018b ; Bradfer‐Lawrence et al., 2020), or the fitting of multiple regression models (e.g. Retamosa Izaguirre et al., 2021). Despite being proposed early on, few studies have applied these methods so far, likely because computation and data analysis are more complex and time‐consuming than the use of indices as single predictors (e.g. correlation, simple linear regression, etc.). The accessibility of the method may thus be important for wider use of this alternative approach. Another key point is to understand how acoustic indices can better represent distinct components of animal sounds in environmental recordings and which combinations of complementary indices could lead to more accurate estimates of biodiversity. For example, understanding how the resolution of time, frequency, and energy affect the relations between indices and diversity metrics would increase our understanding about the redundancy and uniqueness of the indices to be combined. Moreover, combinations of indices might mitigate the observed limitations in the performance of acoustic indices, partly derived from the extreme information reduction (in both dimensionality and data) related to index calculation.
Beta acoustic indices have also been proposed to assess biodiversity by examining the similarity or dissimilarity of acoustic diversity (Gasc et al., 2013b ; Depraetere et al., 2012; Sueur et al., 2014). For example, these indices were recently used to analyse differences in soundscapes between an oil plantation and surrounding forests (Hayashi et al., 2020). Probably due to the same challenges as the approach based on a combination of indices, they have attracted less attention than alpha indices so far. We encourage scientists to continue employing beta indices, not only to enhance our knowledge on their efficiency for biodiversity assessment, but also to study other biological aspects such as functional and phylogenetic beta‐diversity (Gasc et al., 2013a ).
We found large research gaps due to the bias in studies addressing the relationship of acoustic indices and diversity metrics, with a paucity of analysis on distinct combinations of indices, animal groups, and ecosystems, which should be overcome in future research. Currently, the lack of data validation in the literature, with a large and growing number of articles assuming a direct link between acoustic indices and alpha biodiversity indices, is a major shortcoming that needs to be addressed in ecoacoustic studies, particularly considering the moderate association identified in our meta‐analysis. Furthermore, we suggest that future research should adopt experimental approaches based on simulations to disentangle the roles of distinct factors (e.g. types of organism, environment, recording setting, and analytical procedure) in the estimates provided by acoustic indices (Gasc et al., 2015). Thereby, acoustic conditions can be fully controlled, assisting in testing the performance of acoustic indices in their relationship with species abundance, richness, and diversity. Overall, it is necessary to improve our understanding of the capacity of acoustic indices to retrieve biological information. Enhancing collaborative science to create large open databases will be a beneficial strategy to increase temporal and spatial replicates and allow evaluation of the performance of different indices at the community level (Eldridge et al., 2018; Bradfer‐Lawrence et al., 2020).
Beyond acoustic indices, machine learning is being used for automated signal analysis, with increasing success (Ulloa et al., 2018; Stowell et al., 2019; Wood et al., 2021). This alternative research path could enable retrieval of ecological data from passive recordings, mainly in species‐oriented research, providing detailed information on species presence and activity, and being an attractive monitoring method for poorly documented habitats. Yet, machine learning typically demands manual labelling for the development of large annotated data sets, which can be arduous and time‐consuming. Recently, a combination of acoustic features, indices, and unsupervised algorithms that do not require annotated information has been proposed to characterise soundscapes and to visualise long periods of acoustic data in a single image (Phillips et al., 2018; Sethi et al., 2020, 2022), although the complexity of these innovative approaches still precludes wide application in ecological research.
V. CONCLUSIONS
(1) Novel tools are required for efficient monitoring of rapidly declining biodiversity at the global scale. For this purpose, a large number of acoustic indices has recently been developed to support passive acoustic monitoring and offer an automated method to characterise and track changes in soundscapes. Despite being increasingly employed in biodiversity assessment, whether these indicators are able to capture local biodiversity accurately remains unclear. Our systematic literature review and meta‐analysis represent the first attempt to assess the overall performance of acoustic indices as proxies for biodiversity and hence provide new insights for future studies using this innovative approach.
(2) After 15 years of research on acoustic indices, we found 35 studies that have explored their link with biodiversity. Overall, our meta‐analysis revealed that these indices have a moderate positive correlation with diversity metrics, implying that they are able partially to inform on changes in biodiversity. However, the inconsistent performance of the acoustic indices, with highly variable effect sizes, both within and among studies, highlights that caution should be exercised (e.g. validating estimates, etc.) when using them as surrogates of diversity metrics, especially if employed as single predictors. Moreover, studies have been progressively reporting smaller effect sizes over the research trajectory, revealing limitations on their capacity to quantify local biodiversity efficiently. Although our findings endorse to some extent the rationale behind acoustic indices and support their use as promising bases for future developments, we confirmed that these tools are far from being direct proxies for biodiversity.
(3) The indices H, NDSI, and ACI had the highest predictive performance in comparison with other commonly used acoustic indices. Abundance of sounds was the metric that best correlated with acoustic indices, although large confidence intervals prevent establishing clear differences with other diversity variables. We found no effect of the type of monitored environment (terrestrial versus aquatic) and the procedure for extracting biological information (acoustic versus non‐acoustic), suggesting a certain capacity to generalise their application across research contexts. Further studies are required to test finer moderators and interactions, and to provide a better understanding of how other factors affect the performance of acoustic indices.
(4) We outlined significant theoretical and practical shortcomings of acoustic indices in biodiversity assessment. The literature provides weak support for two main hypotheses proposed as cornerstones of the ecoacoustic framework (acoustic niche hypothesis and acoustic adaptation hypothesis), as the acoustic output of ecological communities will be influenced by multiple deterministic and stochastic processes beyond acoustic partitioning and environmental filtering. Additionally, a variety of factors strongly affect the acoustic diversity of soundscapes in real‐world scenarios, driving an unequal contribution of species to the acoustic space and representing confounding factors that often mislead the acoustic estimates (e.g. noise, among‐species differences in abundance, vocal repertoire size, or behavioural motivation, etc.).
(5) A trend towards neglecting the validation of the predictive models over time was identified, with studies increasingly assuming a correlation between acoustic indices and biodiversity without testing this assumption. Assessing the accuracy of the acoustic estimates is a crucial step for study design and interpretation of results, according to our results (i.e. moderate correlation, high variability in effect sizes, etc.). Moreover, pseudoreplication was a common issue across the literature, as ecoacoustics research is prone to the collection of autocorrelated data. Researchers should consider more carefully the non‐independence of time series of audio recordings given its potential to undermine statistical inference. Finally, the performance of acoustic indices remains unexplored for multiple combinations of indices, diversity metrics, animal groups, and regions, making further research needed to overcome these knowledge shortfalls.
(6) Future research should reinforce and promote the accessibility of new approaches based on combinations of indices, expand the application of beta indices, and address the identified shortcomings and knowledge gaps. Recent developments have proposed the use of multivariate analysis and machine learning based on a suite of acoustic indices for a more effective characterisation of soundscapes and biodiversity. Altogether, we believe an increasing knowledge of the relationship between ecological communities and soundscapes will allow us to integrate acoustic indices with new techniques and provide a comprehensive understanding of the use of these promising tools in biodiversity assessment.
Supporting information
Table S1. Data set used in the study.
Table S2. Variable descriptions for Table S1.
Table S3. List of the 34 features used to characterise studies that tested the relationship between acoustic indices and diversity metrics.
Table S4. Number of effect sizes collected from each of the 34 studies included in the meta‐analysis.
Table S5. Number of effect sizes and studies per moderator levels.
Fig. S1. Pseudoreplication summary.
Table S6. VIF (Variance Inflation Factor) values obtained for each moderator level.
Table S7. Model estimates from the intercept‐only model.
Fig. S2. Visual representation of the distribution of variance over the multilevel structure of the intercept‐only model.
Table S8. Model estimates for the sub‐group analysis.
Fig. S3. Effect size mean estimates and corresponding 95% confidence intervals obtained from the sub‐group meta‐analysis with acoustic indices as the moderating factor.
Table S9. Results of Wald‐type tests for all moderators, and for each moderator separately.
Table S10. Results of Wald‐type tests for the contrasts between acoustic index H and all other acoustic indices.
Table S11. Results of Wald‐type tests for the contrasts between acoustic index NDSI and all other acoustic indices.
Table S12. Results of Wald‐type tests for the contrasts between the diversity metric ‘abundance of sounds’ and all other diversity metrics.
Table S13. Model estimates for the meta‐analysis inspecting differences between pseudoreplicated and non‐pseudoreplicated studies.
Fig. S4. Relationship between reported mean effect sizes and journal impact factor.
Fig. S5. Cook's distance values for each study and average Cook's distance over all studies.
Fig. S6. Boxplot and distribution of effect size values of the two studies identified as outliers.
Table S14. Model estimates for the meta‐regression over the data set without outliers.
Fig. S7. Contrast of model estimates obtained with meta‐regression analysis using the full data set and over the data set with outliers removed.
ACKNOWLEDGEMENTS
This study was supported by a research project funded by the Comunidad de Madrid and the European Social Fund (PEJ‐2018‐AI/AMB‐9957, to D. L.). We thank Camille Desjonquères for her valuable comments on the study design, Alison Cooper for her exhaustive and insightful revision of the manuscript, and anonymous reviewers for their significant contribution. I. A. and L. S. M. S. acknowledge research grants provided by the Comunidad de Madrid (PEJ‐2018‐AI/AMB‐9957, to D. L.) and the Ministerio de Economía, Industria y Competitividad of Spain (PEJ‐2018‐004603‐A, to D. L.), respectively, together with the support of the European Social Fund. H. L. was supported by the FPI program of the Ministerio de Ciencia e Innovación of Spain (grant CGL2017‐86926‐P). D. L. also acknowledges a post‐doctoral grant provided by the Comunidad de Madrid (2020‐T1/AMB‐20636, Atracción de Talento Investigador, Spain) and a research project funded by the Ministerio de Economía, Industria y Competitividad (CGL2017‐88764‐R, MINECO/AEI/FEDER, Spain).
REFERENCES
References identified with an asterisk (*) were used in the meta‐analysis.
- Bellisario, K. M. , Broadhead, T. , Savage, D. , Zhao, Z. , Omrani, H. , Zhang, S. , Springer, J. & Pijanowski, B. C. (2019). Contributions of MIR to soundscape ecology. Part 3: tagging and classifying audio features using a multi‐labeling k‐nearest neighbor approach. Ecological Informatics 51, 103–111. [Google Scholar]
- Bence, J. R. (1995). Analysis of short time series: correcting for autocorrelation. Ecology 76, 628–639. [Google Scholar]
- * Bertucci, F. , Parmentier, E. , Lecellier, G. , Hawkins, A. D. & Lecchini, D. (2016). Acoustic indices provide information on the status of coral reefs: an example from Moorea Island in the South Pacific. Scientific Reports 6, 33326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibby, C. J. , Burgess, N. D. , Hillis, D. , Hill, D. & Mustoe, S. (1992). Bird Census Techniques. Academic Press, London. [Google Scholar]
- Bird, G. , Kaczvinsky, C. , Wilson, A. E. & Hardy, N. B. (2019). When do herbivorous insects compete? A phylogenetic meta‐analysis. Ecology Letters 22(5), 875–883. [DOI] [PubMed] [Google Scholar]
- Blumstein, D. T. , Mennill, D. J. , Clemins, P. , Girod, L. , Yao, K. , Patricelli, G. , Deppe, J. L. , Krakauer, A. H. , Clark, C. , Cortopassi, K. A. , Hanser, S. F. , McCowan, B. , Ali, A. M. & Kirschel, A. N. G. (2011). Acoustic monitoring in terrestrial environments using microphone arrays: applications, technological considerations and prospectus. Journal of Applied Ecology 48, 758–767. [Google Scholar]
- Bobryk, C. W. , Rega‐Brodsky, C. C. , Bardhan, S. , Farina, A. , He, H. S. & Jose, S. (2016). A rapid soundscape analysis to quantify conservation benefits of temperate agroforestry systems using low‐cost technology. Agroforestry Systems 90, 997–1008. [Google Scholar]
- * Boelman, N. T. , Asner, G. P. , Hart, P. J. & Martin, R. E. (2007). Multi‐trophic invasion resistance in Hawaii: bioacoustics, field surveys, and airborne remote sensing. Ecological Applications 17, 2137–2144. [DOI] [PubMed] [Google Scholar]
- * Bolgan, M. , Amorim, M. C. P. , Fonseca, P. J. , Di Iorio, L. & Parmentier, E. (2018). Acoustic complexity of vocal fish communities: a field and controlled validation. Scientific Reports 8, 10559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boncoraglio, G. & Saino, N. (2007). Habitat structure and the evolution of bird song: a meta‐analysis of the evidence for the acoustic adaptation hypothesis. Functional Ecology 21, 134–142. [Google Scholar]
- Borenstein, M. , Hedges, L. V. , Higgins, J. P. & Rothstein, H. R. (2009). Introduction to Meta‐Analysis. John Wiley & Sons, Ltd, Chichester. [Google Scholar]
- Bradfer‐Lawrence, T. , Bunnefeld, N. , Gardner, N. , Willis, S. G. & Dent, D. H. (2020). Rapid assessment of avian species richness and abundance using acoustic indices. Ecological Indicators 115, 106400. [Google Scholar]
- Bradfer‐Lawrence, T. , Gardner, N. , Bunnefeld, L. , Bunnefeld, N. , Willis, S. G. & Dent, D. H. (2019). Guidelines for the use of acoustic indices in environmental research. Methods in Ecology and Evolution 10, 1796–1807. [Google Scholar]
- Brumm, H. (2013). Animal Communication and Noise. Springer, Berlin, Heidelberg. [Google Scholar]
- * Buscaino, G. , Ceraulo, M. , Pieretti, N. , Corrias, V. , Farina, A. , Filiciotto, F. , Maccarrone, V. , Grammauta, R. , Caruso, F. , Giuseppe, A. & Mazzola, S. (2016). Temporal patterns in the soundscape of the shallow waters of a Mediterranean marine protected area. Scientific Reports 6, 34230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton, R. T. , Agnihotri, S. , Robin, V. V. , Goel, A. & Balakrishnan, R. (2018. a). Acoustic indices as rapid indicators of avian diversity in different land‐use types in an Indian biodiversity hotspot. Journal of Ecoacoustics 2, 1–17. [Google Scholar]
- * Buxton, R. T. , Brown, E. , Sharman, L. , Gabriele, C. M. & McKenna, M. F. (2016). Using bioacoustics to examine shifts in songbird phenology. Ecology and Evolution 6, 4697–4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton, R. T. , McKenna, M. F. , Clapp, M. , Meyer, E. , Stabenau, E. , Angeloni, L. M. , Crooks, K. & Wittemyer, G. (2018. b). Efficacy of extracting indices from large‐scale acoustic recordings to monitor biodiversity. Conservation Biology 32, 1174–1184. [DOI] [PubMed] [Google Scholar]
- Chek, A. A. , Bogart, J. P. & Lougheed, S. C. (2003). Mating signal partitioning in multi‐species assemblages: a null model test using frogs. Ecology Letters 6, 235–247. [Google Scholar]
- Chen, Z. & Wiens, J. J. (2020). The origins of acoustic communication in vertebrates. Nature Communications 11, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cody, M. L. (1973). Character convergence. Annual Review of Ecology and Systematics 4, 189–211. [Google Scholar]
- Colegrave, N. & Ruxton, G. D. (2018). Using biological insight and pragmatism when thinking about pseudoreplication. Trends in Ecology & Evolution 33, 28–35. [DOI] [PubMed] [Google Scholar]
- Cornec, C. , Hingrat, Y. , Robert, A. & Rybak, F. (2015). The meaning of boom calls in a lekking bird: identity or quality information? Animal Behaviour 109, 249–264. [Google Scholar]
- Darras, K. , Batáry, P. , Furnas, B. , Celis‐Murillo, A. , Van Wilgenburg, S. L. , Mulyani, Y. A. & Tscharntke, T. (2018). Comparing the sampling performance of sound recorders versus point counts in bird surveys: a meta‐analysis. Journal of Applied Ecology 55, 2575–2586. [Google Scholar]
- Darras, K. , Pütz, P. , Fahrurrozi, R. K. & Tscharntke, T. (2016). Measuring sound detection spaces for acoustic animal sampling and monitoring. Biological Conservation 201, 29–37. [Google Scholar]
- Davies, G. M. & Gray, A. (2015). Don't let spurious accusations of pseudoreplication limit our ability to learn from natural experiments (and other messy kinds of ecological monitoring). Ecology and Evolution 5, 5295–5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araújo, C. B. , Furtado, S. , Vieira, G. H. C. & Simões, C. R. (2020). O nicho acústico: integrando a física, ecologia e teoria da comunicação. Oecologia Australis 24, 769. [Google Scholar]
- Dema, T. , Towsey, M. , Sherub, S. , Sonam, J. , Kinley, K. , Truskinger, A. , Brereton, M. & Roe, P. (2020). Acoustic detection and acoustic habitat characterisation of the critically endangered white‐bellied heron (Ardea insignis) in Bhutan. Freshwater Biology 65, 153–164. [Google Scholar]
- * Depraetere, M. , Pavoine, S. , Jiguet, F. , Gasc, A. , Duvail, S. & Sueur, J. (2012). Monitoring animal diversity using acoustic indices: implementation in a temperate woodland. Ecological Indicators 13, 46–54. [Google Scholar]
- Derryberry, E. P. , Seddon, N. , Derryberry, G. E. , Claramunt, S. , Seeholzer, G. F. , Brumfield, R. T. & Tobias, J. A. (2018). Ecological drivers of song evolution in birds: disentangling the effects of habitat and morphology. Ecology and Evolution 8, 1890–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Desjonquères, C. , Rybak, F. , Depraetere, M. , Gasc, A. , Viol, I. L. , Pavoine, S. & Sueur, J. (2015). First description of underwater acoustic diversity in three temperate ponds. PeerJ 3, e1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Iorio, L. , Raick, X. , Parmentier, E. , Boissery, P. , Valentini‐Poirier, C.‐A. & Gervaise, C. (2018). ‘Posidonia meadows calling’: a ubiquitous fish sound with monitoring potential. Remote Sensing in Ecology and Conservation 4, 248–263. [Google Scholar]
- Diamond, J. M. (1975). Assembly of species communities. In Ecology and Evolution of Communities (Volume 60, eds Diamond J. M. and Cody M. L.), Cambridge: The Belknap Press of Harvard University Press. pp. 342–444. [Google Scholar]
- Digby, A. , Towsey, M. , Bell, B. D. & Teal, P. D. (2013). A practical comparison of manual and autonomous methods for acoustic monitoring. Methods in Ecology and Evolution 4, 675–683. [Google Scholar]
- Drury, J. P. , Tobias, J. A. , Burns, K. J. , Mason, N. A. , Shultz, A. J. & Morlon, H. (2018). Contrasting impacts of competition on ecological and social trait evolution in songbirds. PLoS Biology 16, e2003563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duellman, W. E. & Pyles, R. A. (1983). Acoustic resource partitioning in anuran communities. Copeia 3, 639–649. [Google Scholar]
- Egger, M. , Smith, G. D. , Schneider, M. & Minder, C. (1997). Bias in meta‐analysis detected by a simple, graphical test. BMJ 315, 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge, A. , Casey, M. , Moscoso, P. & Peck, M. (2016). A new method for ecoacoustics? Toward the extraction and evaluation of ecologically‐meaningful soundscape components using sparse coding methods. PeerJ 4, e2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Eldridge, A. , Guyot, P. , Moscoso, P. , Johnston, A. , Eyre‐Walker, Y. & Peck, M. (2018). Sounding out ecoacoustic metrics: avian species richness is predicted by acoustic indices in temperate but not tropical habitats. Ecological Indicators 95, 939–952. [Google Scholar]
- Elise, S. , Urbina‐Barreto, I. , Pinel, R. , Mahamadaly, V. , Bureau, S. , Penin, L. , Adjeroud, M. , Kulbicki, M. & Bruggemann, J. H. (2019). Assessing key ecosystem functions through soundscapes: a new perspective from coral reefs. Ecological Indicators 107, 105623. [Google Scholar]
- Endler, J. A. (1992). Signals, signal conditions, and the direction of evolution. The American Naturalist 139, S125–S153. [Google Scholar]
- Erdtmann, L. K. & Lima, A. P. (2013). Environmental effects on anuran call design: what we know and what we need to know. Ethology Ecology & Evolution 25, 1–11. [Google Scholar]
- Ey, E. & Fischer, J. (2009). The “acoustic adaptation hypothesis”—a review of the evidence from birds, anurans and mammals. Bioacoustics 19, 21–48. [Google Scholar]
- Fairbrass, A. J. , Firman, M. , Williams, C. , Brostow, G. J. , Titheridge, H. & Jones, K. E. (2019). CityNet—deep learning tools for urban ecoacoustic assessment. Methods in Ecology and Evolution 10, 186–197. [Google Scholar]
- * Fairbrass, A. J. , Rennert, P. , Williams, C. , Titheridge, H. & Jones, K. E. (2017). Biases of acoustic indices measuring biodiversity in urban areas. Ecological Indicators 83, 169–177. [Google Scholar]
- Farina, A. (2014). Soundscape Ecology: Principles, Patterns, Methods and Applications. Springer, New York. [Google Scholar]
- Farina, A. , Eldridge, A. & Li, P. (2021). Ecoacoustics and multispecies semiosis: naming, semantics, semiotic characteristics, and competencies. Biosemiotics 14, 141–165. [Google Scholar]
- Farina, A. & Gage, S. H. (2017). Ecoacoustics: The Ecological Role of Sounds. John Wiley & Sons, Oxford. [Google Scholar]
- Farina, A. , Gage, S. H. & Salutari, P. (2018). Testing the ecoacoustics event detection and identification (EEDI) approach on Mediterranean soundscapes. Ecological Indicators 85, 698–715. [Google Scholar]
- Farina, A. & James, P. (2016). The acoustic communities: definition, description and ecological role. Biosystems 147, 11–20. [DOI] [PubMed] [Google Scholar]
- Farina, A. , Lattanzi, E. , Malavasi, R. , Pieretti, N. & Piccioli, L. (2011). Avian soundscapes and cognitive landscapes: theory, application and ecological perspectives. Landscape Ecology 26, 1257–1267. [Google Scholar]
- Farina, A. , Pieretti, N. & Morganti, N. (2013). Acoustic patterns of an invasive species: the Red‐billed Leiothrix (Leiothrix lutea Scopoli 1786) in a Mediterranean shrubland. Bioacoustics 22, 175–194. [Google Scholar]
- * Ferreira, L. M. , Oliveira, E. G. , Lopes, L. C. , Brito, M. R. , Baumgarten, J. , Rodrigues, F. H. & Sousa‐Lima, R. S. (2018). What do insects, anurans, birds, and mammals have to say about soundscape indices in a tropical savanna. Journal of Ecoacoustics 2, PVH6YZ. [Google Scholar]
- Forrest, T. G. (1994). From sender to receiver: propagation and environmental effects on acoustic signals. American Zoologist 34, 644–654. [Google Scholar]
- Forstmeier, W. , Wagenmakers, E. & Parker, T. H. (2017). Detecting and avoiding likely false‐positive findings – a practical guide. Biological Reviews 92, 1941–1968. [DOI] [PubMed] [Google Scholar]
- * Fuller, S. , Axel, A. C. , Tucker, D. & Gage, S. H. (2015). Connecting soundscape to landscape: which acoustic index best describes landscape configuration? Ecological Indicators 58, 207–215. [Google Scholar]
- Gage, S. H. & Axel, A. C. (2014). Visualization of temporal change in soundscape power of a Michigan lake habitat over a 4‐year period. Ecological Informatics 21, 100–109. [Google Scholar]
- * Gage, S. H. , Wimmer, J. , Tarrant, T. & Grace, P. R. (2017). Acoustic patterns at the Samford Ecological Research Facility in South East Queensland, Australia: the Peri‐Urban SuperSite of the Terrestrial Ecosystem Research Network. Ecological Informatics 38, 62–75. [Google Scholar]
- Gasc, A. , Pavoine, S. , Lellouch, L. , Grandcolas, P. & Sueur, J. (2015). Acoustic indices for biodiversity assessments: analyses of bias based on simulated bird assemblages and recommendations for field surveys. Biological Conservation 191, 306–312. [Google Scholar]
- Gasc, A. , Sueur, J. , Jiguet, F. , Devictor, V. , Grandcolas, P. , Burrow, C. , Depraetere, M. & Pavoine, S. (2013. a). Assessing biodiversity with sound: do acoustic diversity indices reflect phylogenetic and functional diversities of bird communities? Ecological Indicators 25, 279–287. [Google Scholar]
- Gasc, A. , Sueur, J. , Pavoine, S. , Pellens, R. & Grandcolas, P. (2013. b). Biodiversity sampling using a global acoustic approach: contrasting sites with microendemics in New Caledonia. PLoS One 8, e65311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt, H. C. (1994). The evolution of vocalization in frogs and toads. Annual Review of Ecology and Systematics 25, 293–324. [Google Scholar]
- Gibb, R. , Browning, E. , Glover‐Kapfer, P. & Jones, K. E. (2019). Emerging opportunities and challenges for passive acoustics in ecological assessment and monitoring. Methods in Ecology and Evolution 10, 169–185. [Google Scholar]
- Gil, D. & Llusia, D. (2020). The bird dawn chorus revisited. In Coding Strategies in Vertebrate Acoustic Communication (eds Aubin T. and Mathevon N.), pp. 45–90. Springer International Publishing, Cham. [Google Scholar]
- Gómez, W. E. , Isaza, C. V. & Daza, J. M. (2018). Identifying disturbed habitats: a new method from acoustic indices. Ecological Informatics 45, 16–25. [Google Scholar]
- Gottesman, B. L. , Francomano, D. , Zhao, Z. , Bellisario, K. , Ghadiri, M. , Broadhead, T. , Gasc, A. & Pijanowski, B. C. (2020). Acoustic monitoring reveals diversity and surprising dynamics in tropical freshwater soundscapes. Freshwater Biology 65, 117–132. [Google Scholar]
- Goutte, S. , Dubois, A. , Howard, S. D. , Marquez, R. , Rowley, J. J. , Dehling, J. M. , Grandcolas, P. , Rongchuan, X. & Legendre, F. (2016). Environmental constraints and call evolution in torrent‐dwelling frogs. Evolution 70, 811–826. [DOI] [PubMed] [Google Scholar]
- Greenhalgh, J. A. , Genner, M. J. , Jones, G. & Desjonquères, C. (2020). The role of freshwater bioacoustics in ecological research. Wiley Interdisciplinary Reviews: Water 7, e1416. [Google Scholar]
- Grether, G. F. , Losin, N. , Anderson, C. N. & Okamoto, K. (2009). The role of interspecific interference competition in character displacement and the evolution of competitor recognition. Biological Reviews 84, 617–635. [DOI] [PubMed] [Google Scholar]
- * Harris, S. A. , Shears, N. T. & Radford, C. A. (2016). Ecoacoustic indices as proxies for biodiversity on temperate reefs. Methods in Ecology and Evolution 7, 713–724. [Google Scholar]
- Hayashi, K. , Erwinsyah, Lelyana, V. D. & Yamamura, K. (2020). Acoustic dissimilarities between an oil palm plantation and surrounding forests: analysis of index time series for beta‐diversity in South Sumatra, Indonesia. Ecological Indicators 112, 106086. [Google Scholar]
- Higgins, J. P. & Thompson, S. G. (2002). Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 21, 1539–1558. [DOI] [PubMed] [Google Scholar]
- Höbel, G. & Gerhardt, H. C. (2003). Reproductive character displacement in the acoustic communication system of green tree frogs (Hyla cinerea). Evolution 57, 894–904. [DOI] [PubMed] [Google Scholar]
- Hödl, W. (1977). Call differences and calling site segregation in anuran species from Central Amazonian floating meadows. Oecologia 28, 351–363. [DOI] [PubMed] [Google Scholar]
- Hoskin, C. J. & Higgie, M. (2010). Speciation via species interactions: the divergence of mating traits within species. Ecology Letters 13, 409–420. [DOI] [PubMed] [Google Scholar]
- Hurlbert, S. H. (1984). Pseudoreplication and the design of ecological field experiments. Ecological Monographs 54, 187–211. [Google Scholar]
- * Indraswari, K. , Bower, D. S. , Tucker, D. , Schwarzkopf, L. , Towsey, M. & Roe, P. (2018). Assessing the value of acoustic indices to distinguish species and quantify activity: a case study using frogs. Freshwater Biology 65, 142–152. [Google Scholar]
- Jeon, J. Y. & Hong, J. Y. (2015). Classification of urban park soundscapes through perceptions of the acoustical environments. Landscape and Urban Planning 141, 100–111. [Google Scholar]
- * Joo, W. , Gage, S. H. & Kasten, E. P. (2011). Analysis and interpretation of variability in soundscapes along an urban–rural gradient. Landscape and Urban Planning 103, 259–276. [Google Scholar]
- * Jorge, F. C. , Machado, C. G. , da Cunha Nogueira, S. S. & Nogueira‐Filho, S. L. G. (2018). The effectiveness of acoustic indices for forest monitoring in Atlantic rainforest fragments. Ecological Indicators 91, 71–76. [Google Scholar]
- Kasten, E. P. , Gage, S. H. , Fox, J. & Joo, W. (2012). The remote environmental assessment laboratory's acoustic library: an archive for studying soundscape ecology. Ecological Informatics 12, 50–67. [Google Scholar]
- Kirschel, A. N. G. , Blumstein, D. T. & Smith, T. B. (2009). Character displacement of song and morphology in African tinkerbirds. Proceedings of the National Academy of Sciences 106, 8256–8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler, J. , Jansen, M. , Rodriguez, A. , Kok, P. J. , Toledo, L. F. , Emmrich, M. , Glaw, F. , Haddad, B. , Celio, F. , Roedel, M.‐O. & Vences, M. (2017). The use of bioacoustics in anuran taxonomy: theory, terminology, methods and recommendations for best practice. Zootaxa 4251, 1–124. [DOI] [PubMed] [Google Scholar]
- Koricheva, J. (2003). Non‐significant results in ecology: a burden or a blessing in disguise? Oikos 102, 397–401. [Google Scholar]
- Koricheva, J. , Gurevitch, J. & Mengersen, K. (2013). Handbook of Meta‐Analysis in Ecology and Evolution. Princeton University Press, Princeton. [Google Scholar]
- Krittika, S. & Yadav, P. (2019). Circadian clocks: an overview on its adaptive significance. Biological Rhythm Research 51, 1109–1132. [Google Scholar]
- Laiolo, P. (2010). The emerging significance of bioacoustics in animal species conservation. Biological Conservation 143, 1635–1645. [Google Scholar]
- Laiolo, P. , Seoane, J. , Obeso, J. R. & Illera, J. C. (2017). Ecological divergence among young lineages favours sympatry, but convergence among old ones allows coexistence in syntopy. Global Ecology and Biogeography 26, 601–608. [Google Scholar]
- Lazic, S. E. (2010). The problem of pseudoreplication in neuroscientific studies: is it affecting your analysis? BMC Neuroscience 11, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lellouch, L. , Pavoine, S. , Jiguet, F. , Glotin, H. & Sueur, J. (2014). Monitoring temporal change of bird communities with dissimilarity acoustic indices. Methods in Ecology and Evolution 5, 495–505. [Google Scholar]
- Lemmon, E. M. (2009). Diversification of conspecific signals in sympatry: geographic overlap drives multidimensional reproductive character displacement in frogs. Evolution 63, 1155–1170. [DOI] [PubMed] [Google Scholar]
- Llusia, D. , Márquez, R. , Beltrán, J. F. , Benítez, M. & do Amaral, J. P. (2013). Calling behaviour under climate change: geographical and seasonal variation of calling temperatures in ectotherms. Global Change Biology 19, 2655–2674. [DOI] [PubMed] [Google Scholar]
- Llusia, D. , Márquez, R. & Bowker, R. (2011). Terrestrial sound monitoring systems, a methodology for quantitative calibration. Bioacoustics 20, 277–286. [Google Scholar]
- Loftus‐Hills, J. J. & Littlejohn, M. J. (1992). Reinforcement and reproductive character displacement in Gastrophryne carolinensis and G. olivacea (Anura: Microhylidae): a reexamination. Evolution 46, 896–906. [DOI] [PubMed] [Google Scholar]
- Lortie, C. J. , Aarssen, L. W. , Budden, A. E. , Koricheva, J. K. , Leimu, R. & Tregenza, T. (2007). Publication bias and merit in ecology. Oikos 116, 1247–1253. [Google Scholar]
- Losin, N. , Drury, J. P. , Peiman, K. S. , Storch, C. & Grether, G. F. (2016). The ecological and evolutionary stability of interspecific territoriality. Ecology Letters 19, 260–267. [DOI] [PubMed] [Google Scholar]
- * Lyon, R. P. , Eggleston, D. B. , Bohnenstiehl, D. R. , Layman, C. A. , Ricci, S. W. & Allgeier, J. E. (2019). Fish community structure, habitat complexity, and soundscape characteristics of patch reefs in a tropical, back‐reef system. Marine Ecology Progress Series 609, 33–48. [Google Scholar]
- MacArthur, R. & Levins, R. (1967). The limiting similarity, convergence, and divergence of coexisting species. The American Naturalist 101, 377–385. [Google Scholar]
- * Machado, R. B. , Aguiar, L. & Jones, G. (2017). Do acoustic indices reflect the characteristics of bird communities in the savannas of Central Brazil? Landscape and Urban Planning 162, 36–43. [Google Scholar]
- Magrath, R. D. , Haff, T. M. , Fallow, P. M. & Radford, A. N. (2015). Eavesdropping on heterospecific alarm calls: from mechanisms to consequences. Biological Reviews 90, 560–586. [DOI] [PubMed] [Google Scholar]
- * Mammides, C. , Goodale, E. , Dayananda, S. K. , Kang, L. & Chen, J. (2017). Do acoustic indices correlate with bird diversity? Insights from two biodiverse regions in Yunnan Province, south China. Ecological Indicators 82, 470–477. [Google Scholar]
- Mammides, C. , Goodale, E. , Dayananda, S. K. , Luo, K. & Chen, J. (2021). On the use of the acoustic evenness index to monitor biodiversity: a comment on “Rapid assessment of avian species richness and abundance using acoustic indices” by Bradfer‐Lawrence et al. (2020) [Ecological Indicators, 115, 106400]. Ecological Indicators 126, 107626. [Google Scholar]
- Martin, L. J. , Blossey, B. & Ellis, E. (2012). Mapping where ecologists work: biases in the global distribution of terrestrial ecological observations. Frontiers in Ecology and the Environment 10, 195–201. [Google Scholar]
- * McLaren, J. & DeGroote, L. (2012). Monitoring Techniques for Temperate Bird Diversity: Uncovering Relationships between Soundscape Analysis and Point Counts. University of Notre Dame Environmental Research Center, Land O'Lakes, WI, 2012, vol. 54540.
- McPherson, C. , Martin, B. , MacDonnell, J. & Whitt, C. (2016). Examining the value of the Acoustic Variability Index in the characterisation of Australian marine soundscapes. In Proceedings of Acoustics, pp. 9–11, Brisbane, Australia.
- * McWilliam, J. N. & Hawkins, A. D. (2013). A comparison of inshore marine soundscapes. Journal of Experimental Marine Biology and Ecology 446, 166–176. [Google Scholar]
- Melo, I. , Llusia, D. , Bastos, R. P. & Signorelli, L. (2021). Active or passive acoustic monitoring? Assessing methods to track anuran communities in tropical savanna wetlands. Ecological Indicators 132, 108305. [Google Scholar]
- Metcalf, O. C. , Barlow, J. , Devenish, C. , Marsden, S. , Berenguer, E. & Lees, A. C. (2020. a). Acoustic indices perform better when applied at ecologically meaningful time and frequency scales. Methods in Ecology and Evolution 12, 421–431. [Google Scholar]
- Metcalf, O. C. , Lees, A. C. , Barlow, J. , Marsden, S. J. & Devenish, C. (2020. b). hardRain: an R package for quick, automated rainfall detection in ecoacoustic datasets using a threshold‐based approach. Ecological Indicators 109, 105793. [Google Scholar]
- Møller, A. P. & Jennions, M. D. (2002). How much variance can be explained by ecologists and evolutionary biologists? Oecologia 132, 492–500. [DOI] [PubMed] [Google Scholar]
- * Moreno‐Gómez, F. N. , Bartheld, J. , Silva‐Escobar, A. A. , Briones, R. , Márquez, R. & Penna, M. (2019). Evaluating acoustic indices in the Valdivian rainforest, a biodiversity hotspot in South America. Ecological Indicators 103, 1–8. [Google Scholar]
- Morton, E. S. (1975). Ecological sources of selection on avian sounds. The American Naturalist 109, 17–34. [Google Scholar]
- Murtaugh, P. A. (2002). Journal quality, effect size, and publication bias in meta‐analysis. Ecology 83, 1162–1166. [Google Scholar]
- Nakagawa, S. & Cuthill, I. C. (2007). Effect size, confidence interval and statistical significance: a practical guide for biologists. Biological Reviews 82, 591–605. [DOI] [PubMed] [Google Scholar]
- Nakagawa, S. , Noble, D. W. A. , Senior, A. M. & Lagisz, M. (2017). Meta‐evaluation of meta‐analysis: ten appraisal questions for biologists. BMC Biology 15, 18–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa, S. & Santos, E. S. (2012). Methodological issues and advances in biological meta‐analysis. Evolutionary Ecology 26, 1253–1274. [Google Scholar]
- Newbold, T. , Hudson, L. N. , Arnell, A. P. , Contu, S. , De Palma, A. , Ferrier, S. , Hill, S. L. L. , Hoskins, A. J. , Lysenko, I. , Phillips, H. R. P. , Burton, V. J. , Chng, C. W. T. , Emerson, S. , Gao, D. , Pask‐Hale, G. , et al. (2016). Has land use pushed terrestrial biodiversity beyond the planetary boundary? A global assessment. Science 353, 288–291. [DOI] [PubMed] [Google Scholar]
- * Paisley‐Jones, C. (2011). Monitoring agroecosystem biodiversity using bioacoustics and remote recording units . Doctoral Thesis: The Ohio State University.
- Papin, M. , Aznar, M. , Germain, E. , Guérold, F. & Pichenot, J. (2019). Using acoustic indices to estimate wolf pack size. Ecological Indicators 103, 202–211. [Google Scholar]
- * Parks, S. E. , Miksis‐Olds, J. L. & Denes, S. L. (2014). Assessing marine ecosystem acoustic diversity across ocean basins. Ecological Informatics 21, 81–88. [Google Scholar]
- Pearse, W. D. , Morales‐Castilla, I. , James, L. S. , Farrell, M. , Boivin, F. & Davies, T. J. (2018). Global macroevolution and macroecology of passerine song. Evolution 72, 944–960. [DOI] [PubMed] [Google Scholar]
- Pekin, B. K. , Jung, J. , Villanueva‐Rivera, L. J. , Pijanowski, B. C. & Ahumada, J. A. (2012). Modeling acoustic diversity using soundscape recordings and LIDAR‐derived metrics of vertical forest structure in a neotropical rainforest. Landscape Ecology 27, 1513–1522. [Google Scholar]
- Pereira, H. M. , Ferrier, S. , Walters, M. , Geller, G. N. , Jongman, R. H. G. , Scholes, R. J. , Bruford, M. W. , Brummitt, N. , Butchart, S. H. M. & Cardoso, A. C. (2013). Essential biodiversity variables. Science 339, 277–278. [DOI] [PubMed] [Google Scholar]
- Pettorelli, N. , Wegmann, M. , Skidmore, A. , Mücher, S. , Dawson, T. P. , Fernandez, M. , Lucas, R. , Schaepman, M. E. , Wang, T. , O'Connor, B. , Jongman, R. H. G. , Kempeneers, P. , Sonnenschein, R. , Leidner, A. K. , Böhm, K. S. H. , et al. (2016). Framing the concept of satellite remote sensing essential biodiversity variables: challenges and future directions. Remote Sensing in Ecology and Conservation 2, 122–131. [Google Scholar]
- Pfennig, K. & Pfennig, D. (2009). Character displacement: ecological and reproductive responses to a common evolutionary problem. The Quarterly Review of Biology 84, 253–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips, Y. F. , Towsey, M. & Roe, P. (2018). Revealing the ecological content of long‐duration audio‐recordings of the environment through clustering and visualisation. PLoS One 13, e0193345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Picciulin, M. , Colla, S. , Pranovi, F. , Malavasi, S. , Fiorin, R. & Bolgan, M. (2016). The soundscape of a mussel farm: biophony and man‐made noise levels. Proceedings of Meetings on Acoustics 27, 010016. [Google Scholar]
- Pieretti, N. , Duarte, M. H. L. , Sousa‐Lima, R. S. , Rodrigues, M. , Young, R. J. & Farina, A. (2015). Determining temporal sampling schemes for passive acoustic studies in different tropical ecosystems. Tropical Conservation Science 8, 215–234. [Google Scholar]
- * Pieretti, N. , Farina, A. & Morri, D. (2011). A new methodology to infer the singing activity of an avian community: the Acoustic Complexity Index (ACI). Ecological Indicators 11, 868–873. [Google Scholar]
- Pijanowski, B. C. , Farina, A. , Gage, S. H. , Dumyahn, S. L. & Krause, B. L. (2011. a). What is soundscape ecology? An introduction and overview of an emerging new science. Landscape Ecology 26, 1213–1232. [Google Scholar]
- Pijanowski, B. C. , Villanueva‐Rivera, L. J. , Dumyahn, S. L. , Farina, A. , Krause, B. L. , Napoletano, B. M. , Gage, S. H. & Pieretti, N. (2011. b). Soundscape ecology: the science of sound in the landscape. BioScience 61, 203–216. [Google Scholar]
- Priyadarshani, N. , Castro, I. & Marsland, S. (2018. a). The impact of environmental factors in birdsong acquisition using automated recorders. Ecology and Evolution 8, 5016–5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priyadarshani, N. , Marsland, S. & Castro, I. (2018. b). Automated birdsong recognition in complex acoustic environments: a review. Journal of Avian Biology 49, jav‐01447. [Google Scholar]
- Proença, V. , Martin, L. J. , Pereira, H. M. , Fernandez, M. , McRae, L. , Belnap, J. , Böhm, M. , Brummitt, N. , García‐Moreno, J. , Gregory, R. D. , Honrado, J. P. , Jürgens, N. , Opige, M. , Schmeller, D. S. , Tiago, P. , et al. (2017). Global biodiversity monitoring: from data sources to essential biodiversity variables. Biological Conservation 213, 256–263. [Google Scholar]
- Pullin, A. S. & Stewart, G. B. (2006). Guidelines for systematic review in conservation and environmental management. Conservation Biology 20, 1647–1656. [DOI] [PubMed] [Google Scholar]
- * Raynor, E. J. , Whalen, C. E. , Brown, M. B. & Powell, L. A. (2017). Grassland bird community and acoustic complexity appear unaffected by proximity to a wind energy facility in the Nebraska Sandhills. The Condor 119, 484–496. [Google Scholar]
- Re, A. C. D. (2013). Compute. es: Compute effect sizes. R Package.
- Retamosa Izaguirre, M. , Barrantes‐Madrigal, J. , Segura Sequeira, D. , Spínola‐Parallada, M. & Ramírez‐Alán, O. (2021). It is not just about birds: what do acoustic indices reveal about a Costa Rican tropical rainforest? Neotropical Biodiversity 7, 431–442. [Google Scholar]
- * Retamosa Izaguirre, M. & Ramírez‐Alán, O. (2018). Acoustic indices applied to biodiversity monitoring in a Costa Rica dry tropical forest. Journal of Ecoacoustics 2, 1–5. [Google Scholar]
- Ringler, M. , Szipl, G. , Hödl, W. , Khil, L. , Kofler, B. , Lonauer, M. , Provin, C. & Ringler, E. (2017). Acoustic ranging in poison frogs—it is not about signal amplitude alone. Behavioral Ecology and Sociobiology 71, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca, I. T. & Opzeeland, I. V. (2020). Using acoustic metrics to characterize underwater acoustic biodiversity in the Southern Ocean. Remote Sensing in Ecology and Conservation 6, 262–273. [Google Scholar]
- * Roca, I. T. & Proulx, R. (2016). Acoustic assessment of species richness and assembly rules in ensiferan communities from temperate ecosystems. Ecology 97, 116–123. [DOI] [PubMed] [Google Scholar]
- Rodriguez, A. , Gasc, A. , Pavoine, S. , Grandcolas, P. , Gaucher, P. & Sueur, J. (2014). Temporal and spatial variability of animal sound within a neotropical forest. Ecological Informatics 21, 133–143. [Google Scholar]
- Rohatgi, A. (2019). WebPlotDigitizer User Manual, version 4.2. Pacifica. https://automeris.io/WebPlotDigitizer [Google Scholar]
- Ryan, M. J. & Cummings, M. E. (2013). Perceptual biases and mate choice. Annual Review of Ecology, Evolution, and Systematics 44, 437–459. [Google Scholar]
- Sandoval, L. , Barrantes, G. & Wilson, D. R. (2019). Conceptual and statistical problems with the use of the Shannon‐Weiner entropy index in bioacoustic analyses. Bioacoustics 28, 297–311. [Google Scholar]
- Scarpelli, M. D. A. , Ribeiro, M. C. & Teixeira, C. P. (2021). What does Atlantic Forest soundscapes can tell us about landscape? Ecological Indicators 121, 107050. [Google Scholar]
- Schmidt, A. K. & Balakrishnan, R. (2015). Ecology of acoustic signalling and the problem of masking interference in insects. Journal of Comparative Physiology A 201, 133–142. [DOI] [PubMed] [Google Scholar]
- Servick, K. (2014). Eavesdropping on ecosystems. Science 343, 834–837. [DOI] [PubMed] [Google Scholar]
- Sethi, S. S. , Ewers, R. M. , Jones, N. S. , Sleutel, J. , Shabrani, A. , Zulkifli, N. & Picinali, L. (2022). Soundscapes predict species occurrence in tropical forests. Oikos 2022, e08525. [Google Scholar]
- Sethi, S. S. , Jones, N. S. , Fulcher, B. D. , Picinali, L. , Clink, D. J. , Klinck, H. , Orme, C. D. L. , Wrege, P. H. & Ewers, R. M. (2020). Characterizing soundscapes across diverse ecosystems using a universal acoustic feature set. Proceedings of the National Academy of Sciences 117, 17049–17055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonfield, J. & Bayne, E. M. (2017). Autonomous recording units in avian ecological research: current use and future applications. Avian Conservation and Ecology 12, 14. [Google Scholar]
- Siviter, H. , Bailes, E. J. , Martin, C. D. , Oliver, T. R. , Koricheva, J. , Leadbeater, E. & Brown, M. J. (2021). Agrochemicals interact synergistically to increase bee mortality. Nature 596(7872), 389–392. [DOI] [PubMed] [Google Scholar]
- Slabbekoorn, H. , Ellers, J. & Smith, T. B. (2002). Birdsong and sound transmission: the benefits of reverberations. The Condor 104, 564–573. [Google Scholar]
- Song, C. , Peacor, S. D. , Osenberg, C. W. & Bence, J. R. (2020). An assessment of statistical methods for nonindependent data in ecological meta‐analyses. Ecology 101, e03184. [DOI] [PubMed] [Google Scholar]
- Spake, R. & Doncaster, C. P. (2017). Use of meta‐analysis in forest biodiversity research: key challenges and considerations. Forest Ecology and Management 400, 429–437. [Google Scholar]
- * Staaterman, E. , Ogburn, M. , Altieri, A. , Brandl, S. , Whippo, R. , Seemann, J. , Goodison, M. & Duffy, J. (2017). Bioacoustic measurements complement visual biodiversity surveys: preliminary evidence from four shallow marine habitats. Marine Ecology Progress Series 575, 207–215. [Google Scholar]
- Stowell, D. , Wood, M. D. , Pamuła, H. , Stylianou, Y. & Glotin, H. (2019). Automatic acoustic detection of birds through deep learning: the first Bird Audio Detection challenge. Methods in Ecology and Evolution 10, 368–380. [Google Scholar]
- Sueur, J. (2018). Sound Analysis and Synthesis with R. Springer, Culemborg. [Google Scholar]
- Sueur, J. & Farina, A. (2015). Ecoacoustics: the ecological investigation and interpretation of environmental sound. Biosemiotics 8, 493–502. [Google Scholar]
- Sueur, J. , Farina, A. , Gasc, A. , Pieretti, N. & Pavoine, S. (2014). Acoustic indices for biodiversity assessment and landscape investigation. Acta Acustica united with Acustica 100, 772–781. [Google Scholar]
- * Sueur, J. , Pavoine, S. , Hamerlynck, O. & Duvail, S. (2008). Rapid acoustic survey for biodiversity appraisal. PLoS One 3, e4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugai, L. S. , Llusia, D. , Siqueira, T. & Silva, T. S. (2021. a). Revisiting the drivers of acoustic similarities in tropical anuran assemblages. Ecology 102, e03380. [DOI] [PubMed] [Google Scholar]
- Sugai, L. S. M. (2020). Pandemics and the need for automated systems for biodiversity monitoring. The Journal of Wildlife Management 84, 1424–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugai, L. S. M. , Desjonquères, C. , Silva, T. S. F. & Llusia, D. (2020). A roadmap for survey designs in terrestrial acoustic monitoring. Remote Sensing in Ecology and Conservation 6, 220–235. [Google Scholar]
- Sugai, L. S. M. & Llusia, D. (2019). Bioacoustic time capsules: using acoustic monitoring to document biodiversity. Ecological Indicators 99, 149–152. [Google Scholar]
- Sugai, L. S. M. , Silva, T. S. F. , Llusia, D. & Siqueira, T. (2021. b). Drivers of assemblage‐wide calling activity in tropical anurans and the role of temporal resolution. Journal of Animal Ecology 90, 673–684. [DOI] [PubMed] [Google Scholar]
- Sugai, L. S. M. , Silva, T. S. F. , Ribeiro, J. W. & Llusia, D. (2019). Terrestrial passive acoustic monitoring: review and perspectives. BioScience 69, 15–25. [Google Scholar]
- Titley, M. A. , Snaddon, J. L. & Turner, E. C. (2017). Scientific research on animal biodiversity is systematically biased towards vertebrates and temperate regions. PLoS One 12, e0189577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias, J. A. , Aben, J. , Brumfield, R. T. , Derryberry, E. P. , Halfwerk, W. , Slabbekoorn, H. & Seddon, N. (2010). Song divergence by sensory drive in Amazonian birds. Evolution 64, 2820–2839. [DOI] [PubMed] [Google Scholar]
- Tobias, J. A. , Cornwallis, C. K. , Derryberry, E. P. , Claramunt, S. , Brumfield, R. T. & Seddon, N. (2014. a). Species coexistence and the dynamics of phenotypic evolution in adaptive radiation. Nature 506, 359–363. [DOI] [PubMed] [Google Scholar]
- Tobias, J. A. , Planqué, R. , Cram, D. L. & Seddon, N. (2014. b). Species interactions and the structure of complex communication networks. Proceedings of the National Academy of Sciences 111, 1020–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torija, A. J. , Ruiz, D. P. & Ramos‐Ridao, A. F. (2013). Application of a methodology for categorizing and differentiating urban soundscapes using acoustical descriptors and semantic‐differential attributes. The Journal of the Acoustical Society of America 134, 791–802. [DOI] [PubMed] [Google Scholar]
- * Torti, V. , Valente, D. , De Gregorio, C. , Comazzi, C. , Miaretsoa, L. , Ratsimbazafy, J. , Giacoma, C. & Gamba, M. (2018). Call and be counted! Can we reliably estimate the number of callers in the indri's (Indri indri) song? PLoS One 13, e0201664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towsey (2017). The calculation of acoustic indices derived from long‐duration recordings of the natural environment. Technical report. QUT ePrints. Ecoacoustics Research Group. Queensland University of Technology, Brisbane, Australia. https://eprints.qut.edu.au/110634/
- Towsey, M. , Wimmer, J. , Williamson, I. & Roe, P. (2014). The use of acoustic indices to determine avian species richness in audio‐recordings of the environment. Ecological Informatics 21, 110–119. [Google Scholar]
- Towsey, M. , Znidersic, E. , Broken‐Brow, J. , Indraswari, K. , Watson, D. M. , Phillips, Y. , Truskinger, A. & Roe, P. (2018). Long‐duration, false‐colour spectrograms for detecting species in large audio data‐sets. Journal of Ecoacoustics 2, IUSWUI. [Google Scholar]
- Tucker, D. , Gage, S. H. , Williamson, I. & Fuller, S. (2014). Linking ecological condition and the soundscape in fragmented Australian forests. Landscape Ecology 29, 745–758. [Google Scholar]
- Ulloa, J. S. , Aubin, T. , Llusia, D. , Bouveyron, C. & Sueur, J. (2018). Estimating animal acoustic diversity in tropical environments using unsupervised multiresolution analysis. Ecological Indicators 90, 346–355. [Google Scholar]
- Viechtbauer, W. (2010). Conducting meta‐analyses in R with the metafor package. Journal of Statistical Software 36, 1–48. [Google Scholar]
- Viechtbauer, W. & Cheung, M. W.‐L. (2010). Outlier and influence diagnostics for meta‐analysis. Research Synthesis Methods 1, 112–125. [DOI] [PubMed] [Google Scholar]
- Viechtbauer, W. , López‐López, J. A. , Sánchez‐Meca, J. & Marín‐Martínez, F. (2015). A comparison of procedures to test for moderators in mixed‐effects meta‐regression models. Psychological Methods 20, 360–374. [DOI] [PubMed] [Google Scholar]
- Villanueva‐Rivera, L. J. , Pijanowski, B. C. , Doucette, J. & Pekin, B. (2011). A primer of acoustic analysis for landscape ecologists. Landscape Ecology 26, 1233–1246. [Google Scholar]
- * wa Maina, C. , Muchiri, D. & Njoroge, P. (2016). A bioacoustic record of a conservancy in the Mount Kenya Ecosystem. Biodiversity Data Journal 4, e9906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller, B. M. , Warmelink, L. , Liebal, K. , Micheletta, J. & Slocombe, K. E. (2013). Pseudoreplication: a widespread problem in primate communication research. Animal Behaviour 86, 483–488. [Google Scholar]
- Wilkins, M. R. , Seddon, N. & Safran, R. J. (2013). Evolutionary divergence in acoustic signals: causes and consequences. Trends in Ecology & Evolution 28, 156–166. [DOI] [PubMed] [Google Scholar]
- Wood, C. M. , Kah, S. , Chaon, P. , Peery, M. Z. & Klinck, H. (2021). Survey coverage, recording duration, and community composition affect observed species richness in passive acoustic surveys. Methods in Ecology and Evolution 12, 885–896. [Google Scholar]
- * Zhang, L. , Towsey, M. , Zhang, J. & Roe, P. (2015). Computer‐assisted sampling of acoustic data for more efficient determination of bird species richness. In 2015 IEEE International Conference on Data Mining Workshop (ICDMW), pp. 798–805. IEEE, Atlantic City. [Google Scholar]
- Zuur, A. F. , Ieno, E. N. & Elphick, C. S. (2010). A protocol for data exploration to avoid common statistical problems. Methods in Ecology and Evolution 1, 3–14. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Data set used in the study.
Table S2. Variable descriptions for Table S1.
Table S3. List of the 34 features used to characterise studies that tested the relationship between acoustic indices and diversity metrics.
Table S4. Number of effect sizes collected from each of the 34 studies included in the meta‐analysis.
Table S5. Number of effect sizes and studies per moderator levels.
Fig. S1. Pseudoreplication summary.
Table S6. VIF (Variance Inflation Factor) values obtained for each moderator level.
Table S7. Model estimates from the intercept‐only model.
Fig. S2. Visual representation of the distribution of variance over the multilevel structure of the intercept‐only model.
Table S8. Model estimates for the sub‐group analysis.
Fig. S3. Effect size mean estimates and corresponding 95% confidence intervals obtained from the sub‐group meta‐analysis with acoustic indices as the moderating factor.
Table S9. Results of Wald‐type tests for all moderators, and for each moderator separately.
Table S10. Results of Wald‐type tests for the contrasts between acoustic index H and all other acoustic indices.
Table S11. Results of Wald‐type tests for the contrasts between acoustic index NDSI and all other acoustic indices.
Table S12. Results of Wald‐type tests for the contrasts between the diversity metric ‘abundance of sounds’ and all other diversity metrics.
Table S13. Model estimates for the meta‐analysis inspecting differences between pseudoreplicated and non‐pseudoreplicated studies.
Fig. S4. Relationship between reported mean effect sizes and journal impact factor.
Fig. S5. Cook's distance values for each study and average Cook's distance over all studies.
Fig. S6. Boxplot and distribution of effect size values of the two studies identified as outliers.
Table S14. Model estimates for the meta‐regression over the data set without outliers.
Fig. S7. Contrast of model estimates obtained with meta‐regression analysis using the full data set and over the data set with outliers removed.


