Abstract
Aims
This pre‐specified analysis of the DELIVER trial examined whether clinical benefits of dapagliflozin in heart failure (HF) with left ventricular ejection fraction (LVEF) >40% varied by baseline New York Heart Association (NYHA) class and examined the treatment effects on NYHA class over time.
Methods and results
Treatment effects of dapagliflozin by baseline NYHA class II (n = 4713) versus III/IV (n = 1549) were examined on the primary endpoint (cardiovascular death or worsening HF event) and key secondary endpoints. Effects of dapagliflozin on change in NYHA class at 4, 16, and 32 weeks were also evaluated. Higher baseline NYHA class was associated with older age, female sex, greater comorbidity burden, lower LVEF, and higher natriuretic peptide levels. Participants with baseline NYHA class III/IV, as compared with II, were independently more likely to experience the primary endpoint (adjusted hazard ratio [HR] 1.16 [95% confidence interval, 1.02–1.33]) and all‐cause death (adjusted HR 1.22 [1.06–1.40]). Dapagliflozin consistently reduced the risk of the primary endpoint compared with placebo, irrespective of baseline NYHA class (HR 0.81 [0.70–0.94] for NYHA class II vs. HR 0.80 [0.65–0.98] for NYHA class III/IV; p interaction = 0.921). Participants with NYHA class III/IV had greater improvement in Kansas City Cardiomyopathy Questionnaire total symptom scores between baseline and 32 weeks (+4.8 [2.5–7.1]) versus NYHA class II (+1.8 [0.7–2.9]; p interaction = 0.011). Dapagliflozin was associated with higher odds of any improvement in NYHA class (odds ratio [OR] 1.32 [1.16–1.51]), as well as improvement to NYHA class I (OR 1.43 [1.17–1.75]), versus placebo at 32 weeks, with benefits seen as early as 4 weeks.
Conclusions
Among symptomatic patients with HF and LVEF >40%, treatment with dapagliflozin provided clinical benefit irrespective of baseline NYHA class and was associated with early and sustained improvements in NYHA class over time.
Keywords: Dapagliflozin, Heart failure with preserved ejection fraction, Functional status, New York Heart Association classification
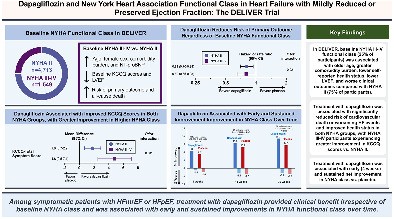
Summary of clinical profiles and treatment effects of dapagliflozin by baseline New York Heart Association (NYHA) class, as well as the impact of dapagliflozin on changes in NYHA class over time in this pre‐specified analysis of the DELIVER trial. CI, confidence interval; DELIVER, Dapagliflozin Evaluation to Improve the Lives of Patients with Preserved Ejection Fraction; HF, heart failure; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; KCCQ, Kansas City Cardiomyopathy Questionnaire; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; OR, odds ratio.
Introduction
Heart failure (HF) with mildly reduced (HFmrEF) or preserved ejection fraction (HFpEF) together constitute the majority of HF in the contemporary era and are associated with substantial death, disability, and healthcare costs. 1 , 2 , 3 Identification of therapeutic pathways to ameliorate symptom burden and functional impairment remains a central priority in the clinical care of this at‐risk population, for whom evidence‐based therapeutic options have been historically lacking. 4
Although with important limitations, 5 , 6 , 7 , 8 the New York Heart Association (NYHA) functional classification is independently predictive of mortality irrespective of left ventricular ejection fraction (LVEF), 9 , 10 , 11 is recommended in current international HF clinical practice guidelines to guide treatment eligibility, 12 , 13 and therein remains an important clinical metric widely used in clinical practice to characterize symptom burden and functional capacity. Further, NYHA class represents a key inclusion criterion for enrolment in virtually all contemporary HF clinical trials.
Previous trials evaluating the role of sodium–glucose cotransporter 2 inhibitors (SGLT2i) in HF with reduced ejection fraction (HFrEF) have suggested relative treatment benefits may be attenuated among patients with higher baseline NYHA class. 14 , 15 , 16 Patients with HFpEF and HFmrEF with advanced NYHA class often exhibit a higher comorbidity burden that may be challenging to modify with HF‐specific therapies, 10 , 11 but it remains uncertain whether SGLT2i treatment effects exhibit similar heterogeneity by NYHA functional class at higher ejection fraction.
The DELIVER (Dapagliflozin Evaluation to Improve the Lives of Patients with Preserved Ejection Fraction Heart Failure) trial demonstrated an 18% improvement in cardiovascular death or worsening HF among patients with HF and LVEF >40%. 17 In this pre‐specified analysis, we aim to (i) evaluate the treatment response to dapagliflozin by baseline NYHA class, and (ii) determine the impact of dapagliflozin on changes in NYHA class over time.
Methods
Study design and patient population
The trial design, baseline characteristics, and primary results of DELIVER have been previously reported. 18 , 19 In brief, DELIVER was an international, multicentre, randomized, double‐blind, parallel‐group, event‐driven trial comparing the efficacy and safety of dapagliflozin with placebo in patients with HF and mildly reduced, preserved, or improved LVEF. DELIVER enrolled patients aged ≥40 years with HF and an LVEF >40% (documented by echocardiography or cardiac magnetic resonance imaging within the 12 months preceding enrolment), NYHA functional class II–IV symptoms, elevated concentrations of N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP), and evidence of structural heart disease (increased left atrial size or left ventricular hypertrophy). Both ambulatory and hospitalized patients were eligible for enrolment. Key exclusion criteria included recent (within 4 weeks pre‐enrolment) receipt or intolerance of an SGLT2i, type 1 diabetes, estimated glomerular filtration rate <25 ml/min/1.73 m2, uncontrolled hypertension, body mass index >50 kg/m2, the presence of an alternative diagnosis accounting for the patient's symptoms (e.g. anaemia), uncorrected primary valvular disease, and known infiltrative cardiomyopathy, myocarditis, or hypertrophic cardiomyopathy. The trial is registered with ClinicalTrials.gov (NCT03619213) and conforms with the principles outlined in the Declaration of Helsinki. The study protocol was approved by the institutional review board or ethics committee at each participating site and all participants provided written informed consent.
Assessment of New York Heart Association functional class
NYHA class was a pre‐specified subgroup in DELIVER, and treatment effects of dapagliflozin versus placebo on NYHA class over time was an exploratory endpoint pre‐specified in the study protocol. Baseline NYHA class was established by blinded clinicians at baseline, as well as at 4, 16, and 32 weeks following randomization.
Clinical outcomes in DELIVER
The primary endpoint of DELIVER was the composite of cardiovascular death or worsening HF event (inclusive of hospitalization for HF or an urgent HF visit requiring intravenous HF therapy). Secondary endpoints were cardiovascular death and total (first and recurrent) HF events, patient symptom burden as assessed by the Kansas City Cardiomyopathy Questionnaire total symptom score (KCCQ‐TSS) at 32 weeks, cardiovascular death, and all‐cause death. All clinical events were adjudicated by a blinded Clinical Endpoints Committee at Brigham & Women's Hospital (Boston, MA, USA) and University of Glasgow (Glasgow, UK).
Statistical analysis
Normally distributed data are reported as mean (± standard deviation), non‐normally distributed data as median (interquartile range), and categorical variables as frequencies and percentages. Study participants were categorized into two groups: NYHA class II and III/IV. Differences in baseline characteristics of trial participants by NYHA class at enrolment were summarized for each group. The association between baseline NYHA class and clinical outcomes were adjusted for covariates determined a priori including age, sex, geographic region, recent HF hospitalization within 30 days, time from HF diagnosis at baseline, body mass index, history of atrial fibrillation or flutter, history of stroke, type 2 diabetes, chronic obstructive pulmonary disease, coronary artery disease, smoking status (current or former), LVEF, systolic blood pressure, estimated glomerular filtration rate, and log‐transformed NT‐proBNP. Hazard ratios (HR) and 95% confidence intervals (CI) were calculated for time‐to‐first endpoints using Cox proportional hazard models, while rate ratios and 95% CI were calculated for recurrent event endpoints based on the Lin–Wei–Yang–Ying model. 20 Heterogeneity in treatment response to dapagliflozin on each of the primary and secondary endpoints was assessed by interaction testing. Treatment effects of dapagliflozin on NYHA functional class at each follow‐up time point was evaluated. All available data at baseline, 4, 16, and 32 weeks were included, with no imputation for missing data. Change in NYHA functional class at 4, 16, and 32 weeks following randomization was classified ordinally as ‘improved’, ‘no change’, or ‘worsened’. Only patients alive at each follow‐up visit could be assessed for NYHA class; the proportion of participants with missing data for NYHA class at each follow‐up timepoint is detailed in online supplementary Table S1 . The effect of dapagliflozin was estimated using an ordinal logistic regression model and reported as an overall odds ratio (OR) for the likelihood of attaining better versus worse NYHA class. In a separate model, the treatment effect on the odds of achieving NYHA class I symptoms at 32 weeks was assessed. A responder analysis was performed to evaluate the proportion of participants with a deterioration or improvement in KCCQ score at 32 weeks following randomization, using a ≥5 point threshold for deterioration and ≥5 (at least ‘small’), ≥10 (at least ‘moderate’), and ≥15 point (‘large’) thresholds for improvement. P‐values of <0.05 were considered statistically significant. Statistical analyses were performed using STATA version 17.0 (StataCorp, College Station, TX, USA).
Results
Overall, 6263 patients with HFmrEF or HFpEF were randomized across 350 sites and 20 countries in the DELIVER trial. A total of 4713 (75.3%) patients were classified as NYHA class II, while 1531 (24.4%) and 18 (0.3%) were NYHA class III and IV, respectively. One patient had NYHA class I symptoms at randomization and was excluded from this analysis.
Patient characteristics
Clinical profiles and medications of participants categorized by baseline NYHA functional class II versus III/IV are displayed in Table 1 . Participants with higher NYHA class were more likely to be older, female, and have a history of hypertension, type 2 diabetes, chronic obstructive pulmonary disease, sleep apnoea, any atherosclerotic cardiovascular disease, and prior HF hospitalization as compared to lower NYHA class. Higher baseline NYHA class was additionally associated with higher body mass index, longer HF duration, lower LVEF, atrial fibrillation/flutter at the time of enrolment, higher NT‐proBNP concentrations, and lower baseline estimated glomerular filtration rate. Participants with baseline NYHA class III/IV were more likely to be treated with loop diuretics, beta‐blockers, and mineralocorticoid receptor antagonists. Between‐group use of an angiotensin receptor–neprilysin inhibitor was similar at baseline. Clinical profiles and medication use patterns were well‐balanced between treatment arms, irrespective of baseline NYHA functional class group.
Table 1.
Baseline characteristics by New York Heart Association functional class
| Characteristic | NYHA class II (n = 4713) | NYHA class III or IV (n = 1549) | p‐value |
|---|---|---|---|
| Age, years | 71.5 ± 9.6 | 72.1 ± 9.4 | 0.029 |
| Age groups, years | 0.06 | ||
| ≤65 | 1151 (24.4) | 353 (22.8) | |
| 65–75 | 1835 (38.9) | 577 (37.2) | |
| >75 | 1727 (36.6) | 619 (40.0) | |
| Men | 2714 (57.6) | 802 (51.8) | <0.001 |
| Race | <0.001 | ||
| White | 3201 (67.9) | 1237 (79.9) | |
| Asian | 1065 (22.6) | 209 (13.5) | |
| Black or African American | 120 (2.5) | 39 (2.5) | |
| American Indian or Alaska Native | 145 (3.1) | 44 (2.8) | |
| Other | 182 (3.9) | 20 (1.3) | |
| Geographic region | <0.001 | ||
| Europe and Saudi Arabia | 2079 (44.1) | 926 (59.8) | |
| Asia | 1030 (21.9) | 196 (12.7) | |
| Latin America | 978 (20.8) | 203 (13.1) | |
| North America | 626 (13.3) | 224 (14.5) | |
| History of AFF | 2573 (54.6) | 978 (63.1) | <0.001 |
| History of stroke | 415 (8.8) | 182 (11.7) | <0.001 |
| History of dyslipidaemia | 3069 (65.1) | 921 (59.5) | <0.001 |
| History of type 2 diabetes mellitus | 2070 (43.9) | 736 (47.5) | 0.014 |
| History of chronic obstructive pulmonary disease | 452 (9.6) | 240 (15.5) | <0.001 |
| History of non‐coronary revascularization | 115 (2.4) | 25 (1.6) | 0.06 |
| History of sleep apnoea | 334 (7.1) | 151 (9.7) | <0.001 |
| Prior myocardial infarction | 1243 (26.4) | 396 (25.6) | 0.53 |
| History of hypertension | 4135 (87.7) | 1417 (91.5) | <0.001 |
| Prior HF hospitalization | 1709 (36.3) | 830 (53.6) | <0.001 |
| Coronary artery disease | 2327 (49.4) | 837 (54.0) | 0.001 |
| Atherosclerotic cardiovascular disease | 2613 (55.4) | 939 (60.6) | <0.001 |
| Smoking status | <0.001 | ||
| Current | 363 (7.7) | 121 (7.8) | |
| Former | 1776 (37.7) | 485 (31.3) | |
| Never | 2574 (54.6) | 943 (60.9) | |
| Baseline body mass index, kg/m2 | 29.4 ± 5.9 | 31.1 ± 6.6 | <0.001 |
| Body mass index groups, kg/m2 | <0.001 | ||
| <18.5 (underweight) | 46 (1.0) | 8 (0.5) | |
| 18.5–24.9 (normal weight) | 1064 (22.6) | 279 (18.0) | |
| 25.0–29.9 (overweight) | 1633 (34.7) | 439 (28.4) | |
| 30.0–34.9 (class I obesity) | 1160 (24.6) | 414 (26.7) | |
| 35.0–39.9 (class II obesity) | 560 (11.9) | 238 (15.4) | |
| ≥40 (class III obesity) | 245 (5.2) | 170 (11.0) | |
| Time from diagnosis of HF to baseline | <0.001 | ||
| 0–3 months | 472 (10.0) | 96 (6.2) | |
| >3–6 months | 441 (9.4) | 151 (9.7) | |
| >6–12 months | 657 (14.0) | 185 (11.9) | |
| >1–2 years | 750 (15.9) | 245 (15.8) | |
| >2–5 years | 1151 (24.4) | 417 (26.9) | |
| >5 years | 1237 (26.3) | 455 (29.4) | |
| KCCQ‐TSS at baseline | 73.8 ± 20.6 | 58.4 ± 22.7 | <0.001 |
| Baseline LVEF, % | 54.5 ± 8.8 | 53.0 ± 8.5 | <0.001 |
| Pooled LVEF groups | <0.001 | ||
| ≤40% | 4 (0.1) | 0 (0.0) | |
| 41–49% | 1500 (31.8) | 612 (39.5) | |
| 50–59% | 1704 (36.2) | 552 (35.6) | |
| ≥60% | 1505 (31.9) | 385 (24.9) | |
| Improved LVEF (prior LVEF ≤40%) | 918 (19.5) | 233 (15.0) | <0.001 |
| Baseline NT‐proBNP, ng/L | 943 [583–1591] | 1263 [764–2426] | <0.001 |
| NT‐proBNP in AFF, pg/ml | 1322 [938–2055] | 1642 [1042–2806] | <0.001 |
| NT‐proBNP when no AFF, pg/ml | 673 [455–1173] | 907 [532–1910] | <0.001 |
| Baseline ECG AFF | 1882 (39.9) | 761 (49.1) | <0.001 |
| Baseline systolic blood pressure, mmHg | 128.3 ± 15.5 | 127.9 ± 15.0 | 0.34 |
| Baseline diastolic blood pressure, mmHg | 73.7 ± 10.4 | 74.7 ± 10.3 | <0.001 |
| Baseline HbA1c, % | 6.5 ± 1.4 | 6.7 ± 1.5 | <0.001 |
| Baseline pulse, bpm | 71.1 ± 11.8 | 72.6 ± 11.6 | <0.001 |
| Baseline creatinine, μmol/L | 101.5 ± 31.0 | 105.6 ± 31.2 | <0.001 |
| Baseline eGFR, ml/min/1.73 m2 | 62.0 ± 19.2 | 58.1 ± 18.7 | <0.001 |
| eGFR ≥60 ml/min/1.73 m2 | 2505 (53.2) | 686 (44.3) | <0.001 |
| Loop diuretics | 3482 (73.9) | 1328 (85.7) | <0.001 |
| ACEi | 1744 (37.0) | 551 (35.6) | 0.30 |
| ARB | 1746 (37.1) | 526 (34.0) | 0.027 |
| ARNI | 217 (4.6) | 84 (5.4) | 0.19 |
| Beta‐blocker | 3858 (81.9) | 1318 (85.1) | 0.004 |
| MRA | 1958 (41.6) | 709 (45.8) | 0.004 |
| Pacemaker | 477 (10.1) | 185 (11.9) | 0.043 |
| ICD | 82 (1.7) | 31 (2.0) | 0.50 |
Values are mean ± standard deviation, n (%), or median [interquartile range].
ACEi, angiotensin‐converting enzyme inhibitor; AFF, atrial fibrillation/flutter; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor–neprilysin inhibitor; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin; HF, heart failure; ICD, implantable cardioverter‐defibrillator; KCCQ‐TSS, Kansas City Cardiomyopathy Questionnaire total symptom score; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association.
Clinical outcomes by baseline NYHA class
As compared to participants with NYHA class II symptoms and functional status at baseline, those with baseline NYHA class III/IV were independently at higher risk of the primary outcome of time‐to‐first cardiovascular death or worsening HF event (adjusted HR 1.16, 95% CI 1.02–1.33; p = 0.029) and all‐cause death (adjusted HR 1.22, 95% CI 1.06–1.40; p = 0.006) (online supplementary Figure S1 ). Participants with baseline NYHA class III/IV experienced higher rates of HF hospitalization (adjusted HR 1.15, 95% CI 0.98–1.36; p = 0.087) and cardiovascular death (adjusted HR 1.21, 95% CI 0.99–1.49; p = 0.062) compared with those with NYHA class II, but these did not reach statistical significance after covariate adjustment.
Treatment effects of dapagliflozin by baseline NYHA class
Treatment with dapagliflozin reduced the risk of the primary composite outcome irrespective of baseline NYHA class (HR 0.81 [95% CI 0.70–0.94] for NYHA class II vs. HR 0.80 [95% CI 0.65–0.98] for NYHA class III/IV; p interaction = 0.921). There was no evidence of treatment heterogeneity by baseline NYHA class for any of the individual components of the primary endpoint, all‐cause mortality, and composite total HF events and cardiovascular death (Figure 1 ).
Figure 1.
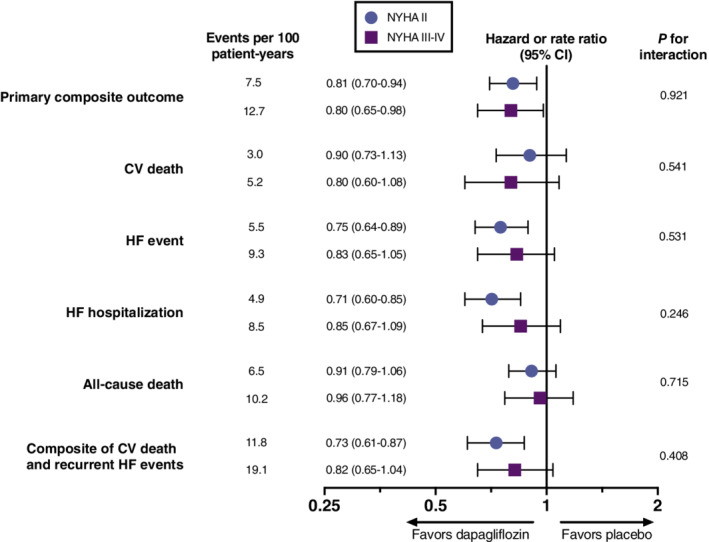
Effect of dapagliflozin versus placebo on primary and secondary endpoints by baseline New York Heart Association (NYHA) functional class. CI, confidence interval; CV, cardiovascular; HF, heart failure.
Participants with NYHA functional class III/IV exhibited more severe patient‐reported symptoms at baseline as compared with NYHA functional class II, based on assessment with KCCQ‐TSS. When compared with placebo, participants in both NYHA groups treated with dapagliflozin experienced significant improvement in patient‐reported health status by week 32, with greater improvement among participants with NYHA functional class III/IV (mean difference in KCCQ‐TSS, +1.8 [95% CI 0.7–2.9] for NYHA class II vs. +4.8 [95% CI 2.5–7.1] for NYHA class III/IV; p interaction = 0.011). Similar findings were observed for KCCQ clinical summary score (CSS) and KCCQ overall summary score (OSS) (Figure 2 ). A responder analysis depicting the proportion of participants with clinically meaningful changes (≤5 point deterioration and ≥5, ≥10, and ≥15 point improvement) in KCCQ scores with dapagliflozin compared to placebo by baseline NYHA functional class at 32 weeks is presented in online supplementary Figure S2 . For each KCCQ summary score, treatment effects of dapagliflozin on clinically meaningful improvement or deterioration in health status were similar among participants with baseline NYHA functional class II versus III/IV (online supplementary Table S2 ).
Figure 2.
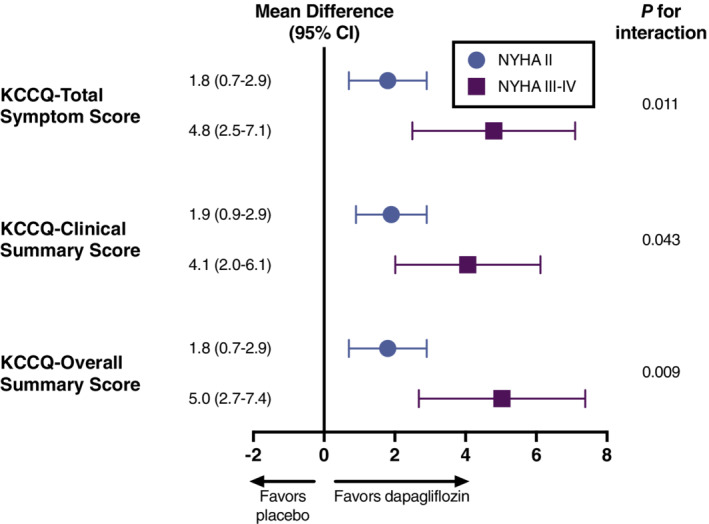
Effect of dapagliflozin versus placebo on symptom burden and other measures of health status by baseline New York Heart Association (NYHA) functional class. Values displayed as mean difference in Kansas City Cardiomyopathy Questionnaire (KCCQ) assessment score between dapagliflozin and placebo groups at week 32 compared with week 0. CI, confidence interval.
Dapagliflozin was well‐tolerated, with no effect modification by baseline NYHA functional class on the risk of any adverse events with dapagliflozin as compared with placebo (online supplementary Table S3 ).
Treatment effects of dapagliflozin on NYHA class over time
Participants randomized to treatment with dapagliflozin more often experienced any improvement in NYHA class by week 4 (11.0% vs. 8.7%), 16 (15.8% vs. 13.2%), and 32 (18.7% vs. 14.5%) as compared with placebo. Treatment with dapagliflozin was additionally associated with a lower likelihood of deterioration in NYHA class at each timepoint. Participants treated with dapagliflozin were more likely than participants treated with placebo to experience an improvement rather than worsening in NYHA functional class as early as 4 weeks (OR 1.37, 95% CI 1.17–1.60; p < 0.001) and sustained through 32 weeks (OR 1.32, 95% CI 1.16–1.51; p < 0.001) (Figure 3 ). By week 32, participants randomized to the dapagliflozin arm numerically more often experienced a one‐class improvement (18% vs. 14%) and two‐class improvement (0.7% vs. 0.5%), and less often experienced a one‐class deterioration (3.0% vs. 3.6%) and two‐class deterioration (0.1% vs. 0.2%), as compared with placebo. Additionally, participants treated with dapagliflozin compared to placebo were significantly more likely to experience improvement to NYHA functional class I at 32 weeks (OR 1.43, 95% CI 1.17–1.75; p < 0.001). The distribution of NYHA class by treatment arm at week 32 is displayed in online supplementary Table S4 .
Figure 3.
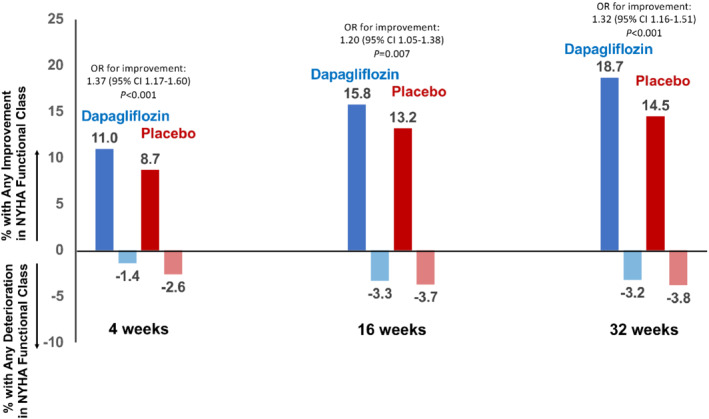
Effect of dapagliflozin versus placebo on New York Heart Association (NYHA) functional class over time. Values displayed as percentage of participants with any improvement or deterioration in NYHA functional class. Odds ratios (OR) represent OR for improvement rather than worsening NYHA functional class at each timepoint. CI, confidence interval.
Discussion
In this pre‐specified analysis of DELIVER, we show that dapagliflozin reduced the risk of cardiovascular death or worsening HF events as compared with placebo among symptomatic patients irrespective of baseline NYHA functional class. Patients with more advanced functional class (III/IV) experienced greater improvement in symptom burden and other domains of health status with dapagliflozin compared with those with NYHA functional class II. Additionally, participants treated with dapagliflozin were significantly more likely to experience any improvement, more likely to achieve NYHA functional class I, and less likely to experience any deterioration in NYHA class over time. Improvement in NYHA functional class was observed early (4 weeks) and sustained through 32 weeks. Taken together, these findings demonstrate that treatment with dapagliflozin provides significant, early, and sustained benefit on NYHA class across a wide spectrum of functional impairment (Graphical Abstract).
Prior trials evaluating the efficacy of SGLT2i in HFrEF have suggested potential attenuation of relative treatment benefits among those with higher (i.e. III/IV) baseline NYHA class. A meta‐analysis of the EMPEROR‐Reduced (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction) and DAPA‐HF (Dapagliflozin and Prevention of Adverse outcomes in Heart Failure) trials demonstrated a nominally significant treatment‐by‐subgroup interaction for NYHA functional class, with potentially less clinical benefit in those with NYHA class III/IV as compared with NYHA class II patients. 16 The observed discrepancy in the consistency of the effect of SGLT2i across NYHA function classes by HF phenotype may be attributable to differences in characteristics of the trial participants and/or pathophysiologic mechanisms in HFrEF as compared to HFpEF and HFmrEF. As compared with both EMPEROR‐Reduced and DAPA‐HF, wherein the incidence rates of HF hospitalization and cardiovascular death events were more balanced, 14 , 15 HF hospitalization accounted for a relatively higher proportion of primary events in DELIVER. This heightened burden of HF hospitalization in HFmrEF and HFpEF 21 may be similarly modified by SGLT2i across a range of baseline functional status. Further, as compared with EMPEROR‐Reduced and DAPA‐HF, DELIVER included participants with a higher proportion of cardiometabolic comorbidities, including obesity, hypertension, and atrial fibrillation/flutter, 19 which in turn are particularly strongly linked with impaired health status and functional limitation in HFpEF. 10 , 11 , 22 , 23 , 24 , 25 , 26 , 27 However, comorbid cardiac and non‐cardiac conditions especially prevalent in this population, including anaemia, chronic kidney disease, hypertension, and obesity, may be favourably modified by SGLT2i. 28 , 29 These factors underscore the importance of evaluating the therapeutic effects of SGLT2i across a range of baseline functional limitations in HFmrEF and HFpEF.
In this analysis, we show that HF outcomes are substantially improved with dapagliflozin, even among participants with advanced NYHA functional class at baseline. These findings are concordant with and extend the results of previous analyses of the EMPEROR‐Preserved (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Preserved Ejection Fraction) trial showing improved odds of improvement in NYHA functional class and overall health status after treatment with SGLT2i in HFmrEF and HFpEF. 30 , 31 Improvement in NYHA functional class also occurred early in EMPEROR‐Preserved, with statistically significant benefits observed by 12 weeks and sustained for at least 2 years. We show that improvement in functional status may occur even earlier (i.e. 4 weeks), and is seen regardless of baseline NYHA functional class. In addition, participants with NYHA class III/IV symptoms in DELIVER experienced greater improvements in multiple KCCQ summary scores as compared with those with NYHA class II. These observations are well‐aligned with the results of PRESERVED‐HF (Dapagliflozin in Preserved Ejection Fraction Heart Failure), a trial that enrolled a highly symptomatic cohort of patients with high rates of multimorbidity. 32 These findings suggest that, instead of a barrier to clinical benefit, impaired functional status may be a unique therapeutic opportunity for SGLT2i in contemporary HFmrEF and HFpEF.
Baseline NYHA functional classification was highly and independently predictive of subsequent HF events and all‐cause mortality in DELIVER after adjusting for numerous covariates, emphasizing the important role of NYHA functional classification in both clinical practice and clinical trials for patients with HFmrEF and HFpEF. Although clinician assessment of NYHA functional classification in practice may be limited by inconsistent methodology and high interobserver variability, 7 these data reinforce its contemporary prognostic relevance. Further, contemporary HF guidelines recommend the use of the NYHA functional classification to help inform management decisions (e.g. in the context of perceived worsening or improvement functional class), 12 , 13 highlighting that clinician‐assessed measures remain central to HF care. However, we recognize that patient‐reported outcomes (such as the KCCQ) are equally if not more important from a patient perspective, and their collection should continue to be prioritized in research and clinical settings. Of note, given KCCQ has been shown to be more responsive to clinically meaningful change than NYHA class over time, 8 the improvement in NYHA classification observed in DELIVER underscores the clinically important effects of dapagliflozin on overall health status.
Study limitations
Key limitations of this analysis should be highlighted. First, DELIVER predominantly enrolled patients with NYHA functional class II and III symptoms at baseline, hence findings may not readily generalize to adjacent groups with NYHA class I and IV symptom. However, the findings reported herein are concordant with those reported by a prior trial with higher enrolment of participants with NYHA functional class III/IV at baseline. 32 Second, serial NYHA assessment was not performed beyond 32 weeks, but no signal of attenuation was observed until this point to suggest that these clinical benefits would wane with time. Third, the apparently lower extent of improvement in KCCQ scores among participants with baseline NYHA functional class II versus III/IV may reflect a limited capacity for improvement owing to good baseline health status. Indeed, baseline KCCQ is known to be predictive of short‐term changes in KCCQ. 33 However, when applying benchmarks for clinically meaningful changes in responder analyses, it is noteworthy that participants in DELIVER experienced similar clinically meaningful changes in KCCQ with dapagliflozin, irrespective of baseline NYHA functional class. Finally, we relied on investigator‐reported NYHA functional class, and assessment of NYHA class is known to be subject to inter‐rater variability.
Conclusions
Among symptomatic patients with HFmrEF or HFpEF, treatment with dapagliflozin provided clinical benefit irrespective of baseline NYHA class and those with advanced NYHA functional class experienced relatively greater health status benefits with dapagliflozin. Initiation of dapagliflozin led to early, sustained, and clinically meaningful improvements in NYHA functional class over time.
Funding
DELIVER was funded by AstraZeneca.
Conflict of interest: M.V. received research grant support or served on advisory boards for American Regent, Amgen, AstraZeneca, Bayer AG, Baxter Healthcare, Boehringer Ingelheim, Cytokinetics, Lexicon Pharmaceuticals, Novartis, Pharmacosmos, Relypsa, Roche Diagnostics, Sanofi, and Tricog Health, speaker engagements with AstraZeneca, Novartis, and Roche Diagnostics, and participates in clinical trial committees for studies sponsored by Galmed, Novartis, Bayer AG, Occlutech, and Impulse Dynamics. B.L.C. has received consulting fees from Amgen, Boehringer Ingelheim, Cardurion, Corvia, and Novartis. R.A.d.B. received research grant support from AstraZeneca, Abbott, Boehringer Ingelheim, Cardior Pharmaceuticals Gmbh, Ionis Pharmaceuticals, Inc., Novo Nordisk, and Roche; speaker engagements with Abbott, AstraZeneca, Bayer, Novartis, and Roche. A.S.D. reports institutional grant support from Abbott, Alnylam, AstraZeneca, Bayer, Novartis and consulting fees from Abbott, Alnylam, AstraZeneca, Avidity, Axon Therapeutics, Bayer, Biofourmis, Boston Scientific, Cytokinetics, GlaxoSmithKline, Merck, Novartis, Parxel, Regeneron, Roche, and Verily. A.F.H. reports research grant support from American Regent, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Merck, Novartis, SomaLogic and Verily; and has served as a consultant or on the Advisory Board for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, Cytokinetics, Eidos, Intercept, Merck, and Novartis. S.E.I. has served on clinical trial committees or as a consultant to AstraZeneca, Boehringer Ingelheim, Novo Nordisk, Lexicon, Merck, Pfizer, vTv Therapeutics, Abbott, and Esperion; and has given lectures sponsored by AstraZeneca and Boehringer Ingelheim. P.S.J. has received research funds (paid to his institution) for his work on the DELIVER and DAPA‐HF trials by AstraZeneca; he has also received consulting and speaking fees from Novartis, AstraZeneca, Boehringer Ingelheim, research funding from Boehringer Ingelheim, and remuneration of clinical trial work from Novo Nordisk and Bayer. M.K. reports research grant support from AstraZeneca and Boehringer Ingelheim; had served as a consultant or on an advisory board for Alnylam, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Esperion Therapeutics, Eli Lilly, Janssen, Lexicon, Merck (Diabetes and Cardiovascular), Pharmacosmos, Novo Nordisk, Sanofi, and Vifor; has received other research support from AstraZeneca; and has received honoraria from AstraZeneca, Boehringer Ingelheim, and Novo Nordisk. C.S.P.L. is supported by a Clinician Scientist Award from the National Medical Research Council of Singapore; has received research support from Bayer and Roche Diagnostics; has served as consultant or on the Advisory Board/Steering Committee/Executive Committee for Actelion, Alleviant Medical, Allysta Pharma, Amgen, AnaCardio AB, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Cytokinetics, Darma Inc., EchoNous Inc, Eli Lilly, Impulse Dynamics, Ionis Pharmaceutical, Janssen Research & Development LLC, Medscape/WebMD Global LLC, Merck, Novartis, NovoNordisk, Prosciento Inc, Radcliffe Group Ltd., Roche Diagnostics, Sanofi, Siemens Healthcare Diagnostics and Us2.ai; and serves as co‐founder and non‐executive director of Us2.ai. A.M.L., D.L., and M.P. are employees and shareholders of AstraZeneca. F.A.M. received consultation fees and research grants from AstraZeneca, Baliarda, Bayer, Boehringer Ingelheim, Bristol Meyers Squibb, Gador, Milestone, Novartis, Pfizer, and St Lukes University. E.O.M. received research funds (paid to her institution) for clinical trials from American Regent, Amgen, AstraZeneca, Bayer AG, Cardurion, Cytokinetics, Novartis, and Pfizer; and consulting fees paid to her or her institution from AstraZeneca, Bayer AG, Boehringer Ingelheim, Cytokinetics, Eli Lilly and Janssen, as well as speaker fees from AstraZeneca, Bayer AG, and Boehringer Ingelheim. S.J.S. reports research grants from the National Institutes of Health (U54 HL160273, R01 HL107577, R01 HL127028, R01 HL140731, R01 HL149423), Actelion, AstraZeneca, Corvia, Novartis, and Pfizer, and consulting fees from Abbott, Actelion, AstraZeneca, Amgen, Aria CV, Axon Therapies, Bayer, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, Cardiora, Coridea, CVRx, Cyclerion, Cytokinetics, Edwards Lifesciences, Eidos, Eisai, GSK, Imara, Impulse Dynamics, Intellia, Ionis, Ironwood, Lilly, Merck, MyoKardia, Novartis, Novo Nordisk, Pfizer, Prothena, Regeneron, Rivus, Sanofi, Sardocor, Shifamed, Tenax, Tenaya, and United Therapeutics. J.J.V.M. received funding to his institution, Glasgow University, for his work on clinical trials, consulting, and other activities from Alnylam, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Cardurion, Cytokinetics, Dal‐Cor, GlaxoSmithKline, Ionis, KBP Biosciences, Novartis, Pfizer, and Theracos; and received personal lecture fees from the Corpus, Abbott, Hickma, Sun Pharmaceuticals, Medscape/Heart.Org, Radcliffe Cardiology, Servier Director, Global Clinical Trial Partners (GCTP). S.D.S. received research grants from Actelion, Alnylam, Amgen, AstraZeneca, Bellerophon, Bayer, Bristol Myers Squibb, Celladon, Cytokinetics, Eidos, Gilead, GlaxoSmithKline, Ionis, Lilly, Mesoblast, MyoKardia, National Institutes of Health/NHLBI, Neurotronik, Novartis, Novo Nordisk, Respicardia, Sanofi Pasteur, Theracos, US2.AI; and has consulted for Abbott, Action, Akros, Alnylam, Amgen, Arena, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Cardior, Cardurion, Corvia, Cytokinetics, Daiichi‐Sankyo, GlaxoSmithKline, Lilly, Merck, MyoKardia, Novartis, Roche, Theracos, Quantum Genomics, Cardurion, Janssen, Cardiac Dimensions, Tenaya, Sanofi‐Pasteur, Dinaqor, Tremeau, CellProThera, Moderna, American Regent, Sarepta, Lexicon, Anacardio, and Akros. All other authors have nothing to disclose.
Supporting information
Appendix S1. Supporting information.
References
- 1. Savarese G, Stolfo D, Sinagra G, Lund LH. Heart failure with mid‐range or mildly reduced ejection fraction. Nat Rev Cardiol. 2022;19:100–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, et al. Heart disease and stroke statistics – 2022 update: a report from the American Heart Association. Circulation. 2022;145:e153–639. [DOI] [PubMed] [Google Scholar]
- 3. Bhatnagar R, Fonarow GC, Heidenreich PA, Ziaeian B. Expenditure on heart failure in the United States: the Medical Expenditure Panel Survey 2009–2018. JACC Heart Fail. 2022;10:571–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shah SJ, Borlaug BA, Kitzman DW, AD MC, Blaxall BC, Agarwal R, et al. Research priorities for heart failure with preserved ejection fraction: National Heart, Lung, and Blood Institute Working Group summary. Circulation. 2020;141:1001–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Caraballo C, Desai NR, Mulder H, Alhanti B, Wilson FP, Fiuzat M, et al. Clinical implications of the New York Heart Association classification. J Am Heart Assoc. 2019;8:e014240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tran AT, Chan PS, Jones PG, Spertus JA. Comparison of patient self‐reported health status with clinician‐assigned New York Heart Association classification. JAMA Netw Open. 2020;3:e2014319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Raphael C, Briscoe C, Davies J, Ian Whinnett Z, Manisty C, Sutton R, et al. Limitations of the New York Heart Association functional classification system and self‐reported walking distances in chronic heart failure. Heart. 2007;93:476–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Greene SJ, Butler J, Spertus JA, Hellkamp AS, Vaduganathan M, AD DV, et al. Comparison of New York Heart Association class and patient‐reported outcomes for heart failure with reduced ejection fraction. JAMA Cardiol. 2021;6:522–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Madsen BK, Hansen JF, Stokholm KH, Brøns J, Husum D, Mortensen LS. Chronic congestive heart failure: description and survival of 190 consecutive patients with a diagnosis of chronic congestive heart failure based on clinical signs and symptoms. Eur Heart J. 1994;15:303–10. [DOI] [PubMed] [Google Scholar]
- 10. Ahmed A, Aronow WS, Fleg JL. Higher New York Heart Association classes and increased mortality and hospitalization in patients with heart failure and preserved left ventricular function. Am Heart J. 2006;151:444–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dalos D, Mascherbauer J, Zotter‐Tufaro C, Duca F, Kammerlander AA, Aschauer S, et al. Functional status, pulmonary artery pressure, and clinical outcomes in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2016;68:189–99. [DOI] [PubMed] [Google Scholar]
- 12. McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failiure. Eur Heart J. 2021;42:3599–726. [DOI] [PubMed] [Google Scholar]
- 13. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA Guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79:1757–80. [DOI] [PubMed] [Google Scholar]
- 14. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al.; DAPA‐HF Trial Committees and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 15. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al.; EMPEROR‐Reduced Trial Investigators . Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–24. [DOI] [PubMed] [Google Scholar]
- 16. Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, Filippatos G, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta‐analysis of the EMPEROR‐Reduced and DAPA‐HF trials. Lancet. 2020;396:819–29. [DOI] [PubMed] [Google Scholar]
- 17. Solomon SD, McMurray JJV, Claggett BL, de Boer RA, DeMets D, Hernandez AF, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387:1089–98. [DOI] [PubMed] [Google Scholar]
- 18. Solomon SD, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, et al. Dapagliflozin in heart failure with preserved and mildly reduced ejection fraction: rationale and design of the DELIVER trial. Eur J Heart Fail. 2021;23:1217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Solomon SD, Vaduganathan M, Claggett BL, de Boer RA, DeMets D, Hernandez AF, et al. Baseline characteristics of patients with HF with mildly reduced and preserved ejection fraction: DELIVER trial. JACC Heart Fail. 2022;10:184–97. [DOI] [PubMed] [Google Scholar]
- 20. Lin DY, Wei LJ, Yang I, Ying Z. Semiparametric regression for the mean and rate functions of recurrent events. J R Stat Soc Ser B Stat Methodol. 2000;62:711–30. [Google Scholar]
- 21. Lam CSP, Gamble GD, Ling LH, Sim D, KTG L, PSD Y, et al. Mortality associated with heart failure with preserved vs. reduced ejection fraction in a prospective international multi‐ethnic cohort study. Eur Heart J. 2018;39:1770–80. [DOI] [PubMed] [Google Scholar]
- 22. Chioncel O, Lainscak M, Seferovic PM, Anker SD, Crespo‐Leiro MG, Harjola VP, et al. Epidemiology and one‐year outcomes in patients with chronic heart failure and preserved, mid‐range, and reduced ejection fraction: an analysis of the ESC Heart Failure Long‐Term Registry. Eur J Heart Fail. 2017;19:1574–85. [DOI] [PubMed] [Google Scholar]
- 23. Vergaro G, Ghionzoli N, Innocenti L, Taddei C, Giannoni A, Valleggi A, et al. Noncardiac versus cardiac mortality in heart failure with preserved, midrange, and reduced ejection fraction. J Am Heart Assoc. 2019;8:e013441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reddy YNV, Rikhi A, Obokata M, Shah SJ, Lewis GD, AbouEzzedine OF, et al. Quality of life in heart failure with preserved ejection fraction: importance of obesity, functional capacity, and physical inactivity. Eur J Heart Fail. 2020;22:1009–18. [DOI] [PubMed] [Google Scholar]
- 25. Bansal N, Zelnick L, Bhat Z, Dobre M, He J, Lash J, et al. Burden and outcomes of heart failure hospitalizations in adults with chronic kidney disease. J Am Coll Cardiol. 2019;73:2691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pandey A, Vaduganathan M, Arora S, Qamar A, Mentz RJ, Shah SJ, et al. Temporal trends in prevalence and prognostic implications of comorbidities among patients with acute decompensated heart failure: the ARIC study community surveillance. Circulation. 2020;142:230–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Edelmann F, Stahrenberg R, Gelbrich G, Durstewitz K, Angermann CE, Düngen HD, et al. Contribution of comorbidites to functional impairment is higher in heart failure with preserved than with reduced ejection fraction. Clin Res Cardiol. 2011;100:755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lopaschuk GD, Verma S. Mechanisms of cardiovascular benefits of sodium glucose co‐transporter 2 (SGLT2) inhibitors: a state‐of‐the‐art review. JACC Basic Transl Sci. 2020;5:632–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Braunwald E. Gliflozins in the management of cardiovascular disease. N Engl J Med. 2022;386:2024–34. [DOI] [PubMed] [Google Scholar]
- 30. Packer M, Butler J, Zannad F, Filippatos G, Ferreira JP, Pocock SJ, et al. Effect of empagliflozin on worsening heart failure events in patients with heart failure and preserved ejection fraction: EMPEROR‐Preserved trial. Circulation. 2021;144:1284–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Butler J, Filippatos G, Siddiqi TJ, Brueckmann M, Böhm M, Chopra VK, et al. Empagliflozin, health status, and quality of life in patients with heart failure and preserved ejection fraction: the EMPEROR‐Preserved trial. Circulation. 2022;145:184–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nassif ME, Windsor SL, Borlaug BA, Kitzman DW, Shah SJ, Tang F, et al. The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: a multicenter randomized trial. Nat Med. 2021;27:1954–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Luo N, O'Connor CM, Cooper LB, Sun JL, Coles A, Reed SD, et al. Relationship between changing patient‐reported outcomes and subsequent clinical events in patients with chronic heart failure: insights from HF‐ACTION. Eur J Heart Fail. 2019;21:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting information.


