Abstract
Bovine paratuberculosis is caused by infection of young calves with Mycobacterium avium subsp. paratuberculosis. In some of the chronically infected cows the long asymptomatic stage (2 to 4 years) is followed by a rapid progression to a clinical stage due to protein-losing enteropathy, which will ultimately be fatal. The current dogma is that in early stages of disease the cell-mediated responses predominate, whereas in the clinical stage of the disease the humoral responses prevail, possibly signaling a switch in immune reactivity related to disease progression. We developed immunoglobulin M (IgM)-, IgA-, and IgG1- and IgG2-isotype-specific enzyme-linked immunosorbent assays for M. avium subsp. paratuberculosis-derived antigens (heat shock proteins of 70 kDa [Hsp70] and 65 kDa [Hsp65], lipoarabinomannan [LAM], and M. avium subsp. paratuberculosis purified protein derivative PPD [PPDP]). The serological responses of cows in different stages of paratuberculosis were used to evaluate the putative shift in immune responsiveness. In the clinical stage the PPDP-specific IgG1 responses were increased compared to those in the asymptomatic stage. However, total IgG1 and IgG2 and the Hsp70-, Hsp65-, and LAM-specific isotype responses were decreased in the clinical stage were decreased compared to those in the asymptomatic stage of disease. Thus, the classical pattern was found only for PPDP antigens and the IgG1 isotype. For other antigens and isotypes and the total IgG levels, the response pattern is different and indicates that there is no uniform association with increased antibody responses during the progression from the asymptomatic stage to the clinical stage of bovine paratuberculosis.
Paratuberculosis (Johne's disease) is an intestinal disease of ruminants causing major economic losses in the dairy industry worldwide (5, 24). Young animals are most susceptible to infection with Mycobacterium avium subsp. paratuberculosis. Following infection the bacteria are presumably taken up by macrophages underlying the M cells in ileal Peyer's patches (30). In macrophages that are not able to kill the ingested mycobacteria, the infection persists and spreads for several years, but no signs of infection are seen. Chronically infected animals start fecal shedding of the bacteria at 2 years or older. In some of these chronically infected animals the disease progresses to a clinical stage with signs like weight loss and diarrhea. Ultimately, the progressive protein-losing enteropathy will be fatal (9–11). A single vaccination, with heat-killed M. avium subsp. paratuberculosis in oil, in the first month of life prevents the occurrence of clinical disease but does not prevent infection or shedding of the bacteria (28, 52).
The serological response to mycobacterial antigens during paratuberculosis has been a subject in many studies with the primary aim to investigate the possibilities for improving diagnosis of this disease (2, 12, 21, 23, 39, 41, 49). Some of the studies comparing different serological methods (21, 33) and one study of IgG, IgM, and IgA responses during bovine paratuberculosis (2) indicated that different dynamics may exist for production of the various immunoglobulin isotypes during the course of the disease. Yokomizo et al. (54, 55) previously studied IgG1 and IgG2 subisotypes in paratuberculosis but investigated asymptomatically infected animals without comparing them to animals in other stages of disease. More recently, the results of serodiagnostic studies have been used as partial evidence that in paratuberculosis, during the progression from asymptomatic to clinical disease, there is a decrease in cell-mediated immunology and an increase in humoral responses. It has been hypothesized that this reflects a switch in immune reactivity from type 1 to type 2 responses (reviewed in references 9 and 10), based on the T-helper-cell dichotomy first described by Mosmann and coworkers (31). Although this dichotomy is not as clear-cut in outbred species as it is in different murine strains, studies regarding bovine type 1 and type 2 immune responses have confirmed the crucial role of interleukin 4 (IL-4) and gamma interferon (IFN-γ) as driving cytokines (6), as observed in mice. Furthermore, as a functional classification, a distinction can be made between IFN-γ-dependent (Th1) antibody isotypes and IL-4-dependant Th2-related isotypes (1). For cattle there is some evidence that IgG1 and IgA, as opposed to IgG2 and IgM isotypes, may be type 2- and type 1-associated isotypes, respectively (6, 7, 17, 18).
Immunopathogenic and diagnostic research regarding paratuberculosis is, among others, hampered by a lack of specific antigens. Previously we have shown the usefulness of recombinant mycobacterial heat shock proteins in studying cell-mediated immune responses in different stages of bovine paratuberculosis (27). The heat shock proteins are cytosolic antigens that have been shown to be immunodominant antigens with immunomodulatory properties in several (mycobacterial) diseases, and as such are interesting antigens for studying immunopathogenesis (19, 26, 35, 48). The mycobacterial cell wall component lipoarabinomannan (LAM) has been shown to be a valuable antigen for diagnostic assays with respect to bovine paratuberculosis (23, 29, 44, 46). Besides, LAM has also been shown to have important immunomodulatory capacities by altering macrophage functions during mycobacterial infection (8, 14, 37, 43). LAM can be considered a structural antigen, being part of the mycobacterial cell wall; however, free LAM can be excreted by activity replicating bacteria. One of the most frequently used antigens is Johnin or purified protein derivative (PPD), which, although crude in nature, can be considered to contain predominately, but not exclusively, excreted protein antigens (3, 47).
To study serological responses from an immunopathogenic perspective, we developed IgM-, IgA-, and IgG1- and IgG2-isotype-specific ELISAs for M. avium subsp. paratuberculosis-derived antigens (heat shock proteins of 70 kDa [Hsp70] and 65 kDa [Hsp65], LAM, and M. avium subsp. paratuberculosis PPD [PPDP]).
Subsequently, serological responses of cows in various stages of paratuberculosis infection were used to evaluate changes in immune responsiveness during the course of the disease.
MATERIALS AND METHODS
Animals.
Serum samples were collected from 176 M. bovis-free Holstein-Frisian cows. Except for the animals in the control group, the cows (n = 126, of which 25 animals were vaccinated [vaccine with M. avium subsp. paratuberculosis strain 3+5/C prepared according to the OIE Manual of Standards for Diagnostic Tests and Vaccines {47}] before the age of 30 days and 86 animals were not vaccinated) originated from Dutch dairy farms with endemic paratuberculosis. Furthermore, cows with symptoms of clinical disease (n = 15) were selected based on a history of weight loss, decreased milk production, and diarrhea; cachectic animals in advanced clinical stages of paratuberculosis with a total serum protein concentration below 40 mg/ml were excluded. The animals of the control group were from a paratuberculosis-negative herd of approximately 100 animals that has been monitored both by serology and fecal culture repeatedly during the last 10 years. The farm is used as a reference farm for diagnostic test evaluation in the Dutch paratuberculosis eradication program. Fifty age-matched animals from this herd served as the control group.
Diagnosis of paratuberculosis.
Fecal samples were taken from all animals in the study for bacterial culture, which was performed at the Institute for Animal Science and Health according to a modification of the method of Jorgensen (25). Samples were checked for bacterial growth every 4 weeks and considered negative if after a culture period of 6 months no bacterial growth was observed. All 50 control animals tested negative. The 25 vaccinated animals were also fecal culture negative. From the 86 nonvaccinated animals, 47 were fecal culture negative (nonshedders), and 39 were fecal culture positive (shedders). The 15 clinically affected animals were all fecal culture positive, and direct Ziehl-Neelsen staining of fecal samples was also positive in all cases (clinical group).
Antigens.
Production of recombinant M. avium subsp. paratuberculosis Hsp70 was investigated. To produce full-length cDNA of the M. avium subsp. paratuberculosis Hsp70 gene, a forward primer (5′-CCAGGAGGAATCACTATGGC-3′) and a reverse primer (5′-GGGTTGCCTTCCGTCTTCTGAT-3′) were designed based on the published sequence (42). PCR with these primers was done on a part of an M. avium subsp. paratuberculosis genomic library (a kind gift of M. Sharp and K. Stevenson, Moredun Research Institute, Moredun, Scotland) using a proofreading polymerase (Expand High Fidelity PCR System; Boehringer, Mannheim, Germany) according to the instructions of the manufacturer. Subsequently, the PCR product was cloned in the reading frame of the pTrcHis expression vector (Invitrogen), and this plasmid was used to transform E.coli Top 10 bacteria. Induction with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) resulted in the expression of the Hsp70 protein with an N-terminal His tag. The His Tag was used to affinity purify the protein using Ni-nitrilotriacetic columns (Invitrogen) according to the instructions of the manufacturer. Purity of the protein was checked using sodium dodecyl sulfate-polyacrylamide gel electrophoresis. From plasmids of productive clones the inserts were sequenced (ALF; Pharmacia) and compared to the published sequence to verify that the right gene was obtained.
The preparation of M. avium subsp. paratuberculosis LAM has been described in detail by Jark et al. (23). The M. avium subsp. paratuberculosis Hsp65 gene, cloned in the pGEX-2T expression system, was a generous gift of Raymond Bujdoso. M. avium subsp. paratuberculosis Hsp65 was produced as a fusion protein with glutathione S-transferase in Escherichia coli transformed with the pGEX-2T plasmid and affinity purified using a glutathione-coupled Sepharose column according to the method described by Colston et al. (13). Purity of the product was checked using sodium dodecyl sulfate-polyacrylamide gel electrophoresis. PPDP was prepared from M. avium subsp. paratuberculosis culture supernatant according to the OIE manual (47) at the Institute for Animal Science and Health.
ELISA.
The diagnostic LAM enzyme-linked immunosorbent assay (ELISA) was developed by Jark et al. (23) and was modified to be used for detection of IgA, IgM, IgG1, and IgG2 isotype antibodies by the use of isotype-specific polyclonal rabbit anti-bovine antibodies (Nordic Laboratories) following the incubation of the LAM-coated plates with serum.
For the other antigens, 96-well plates (Corning Costar) were coated with 100 μl of antigen (1 μg/ml for Hsp65 and PPDP, 0.1 μg/ml for Hsp70) diluted in sodium bicarbonate buffer (pH 9.6) for 20 min at 37°C. All subsequent incubations were performed for 20 min at 37°C, and after each incubation step plates were washed three times with phosphate-buffered saline containing 0.05% Tween 20. Wells were blocked with 200 μl of blocking solution (Boehringer). Sera were serially diluted (twofold from 1:50 to 1:3,200) in blocking buffer; this was followed by incubation with polyclonal rabbit anti-bovine IgM, IgA, IgG1, or IgG2 (Nordic Laboratories) antibodies. The conjugates, biotinylated goat anti-rabbit antibodies (Dako) and peroxidase coupled to avidin (Dako), were added sequentially. Finally, 100 μl of ABTS [2,2′-azinobis(3-ethyl)benzthiazolinsulfonic acid] (Boehringer) substrate buffer was added to each well. The optical density was measured after 10 min at 405 nm on a spectrophotometric ELISA reader (Bio-Rad). Following preliminary tests, one reference serum sample was chosen per antigen-isotype assay described and was included on each plate of the assay in addition to a negative control serum. The titer of the positive reference serum was defined as the dilution at which absorbance values were equal to that of the negative control serum. Absorbance values of patient sera were subsequently analyzed using the reference standard method, and resulting values were expressed as ELISA units (EU) (23).
In addition, total serum IgG1 and IgG2 concentrations were measured using a capture ELISA. Plates were coated with 100 μl of affinity-purified polyclonal rabbit anti-bovine IgG1 or IgG2 antibody (Nordic), diluted 1:200 in sodium bicarbonate buffer (pH 9.6), for 20 min at 37°C. All subsequent incubations were performed for 20 min at 37°C, and after each incubation step plates were washed three times with phosphate-buffered saline containing 0.05% Tween 20. Wells were blocked with 200 μl of blocking solution (Boehringer). Prediluted sera (100-μl aliquots; 1:200 and 1:400) were added in duplicate, and subsequently captured IgG isotypic antibodies were labeled with goat anti-bovine immunoglobulin (heavy and light chain) conjugated with biotin (Sigma) diluted 1:2,000 in blocking solution. Peroxidase coupled to avidin (Dako) was used as the final step (dilution, 1:10,000). ABTS substrate buffer (Boehringer) was used to develop a color reaction which was read on an ELISA reader (Bio-Rad) at 405 nm. Serial twofold dilutions from 1:100 to 1:6,400 from a bovine serum sample with known concentrations of IgG1 and IgG2 were included on each plate and used to calculate IgG1 and IgG2 concentrations in patient sera.
Statistical analysis.
The SPSS 7.5 statistical software was used for analysis of the data. Nonparametric tests were used for the comparison of the different groups and isotypes. For comparisons of antigen-isotype combinations between the different groups of animals, the Kruskal-Wallis test was used for one-way analysis of variance. In case of significant differences between groups as indicated by the analysis of variance, multiple comparisons of groups were made according to methods described previously (38). The level of significance was set at P < 0.05.
Nucleotide sequence accession number.
The complete nucleotide sequence of M. avium subsp. paratuberculosis Hsp70, with regard to the present study, is available in the GenBank database under accession number AF254578.
RESULTS
Production of recombinant Hsp70.
Three E. coli strains transformed with plasmids containing DNA from independent PCRs were selected based on their ability to produce the M. avium subsp. paratuberculosis Hsp70. These transformants produced Hsp70 at concentrations up to 2 mg of protein/liter of bacterial culture as a 72-kDa protein with an N-terminal histidine tag. Plasmid DNA from these three productive clones was used for sequencing to confirm the identity of the gene cloned. A 4-bp difference was identified in the 5′ end of the sequence compared to the published sequence of M. avium subsp. paratuberculosis Hsp70 (42). The nucleotide substitutions result in a 2-amino-acid difference, as indicated in Table 1. Thus, this region appears to be conserved compared to those of M. avium, M. tuberculosis, and M. leprae Hsp70, rather than nonconserved.
TABLE 1.
M. avium subsp. paratuberculosis Hsp70 sequence comparisons
| Source or GenBank accession no. | Amino acid sequence | Source organism |
|---|---|---|
| X59437a | MARAVGIRYGTTNSV | M. avium subsp. paratuberculosis |
| This studyb | MARAVGIDLGTTNSV | M. avium subsp. paratuberculosis |
| P32723 | MARAVGIDLGTTNSV | Mycobacterium tuberculosis |
| M95576 | MARAVGIDLGTTNSV | Mycobacterium leprae |
| P80462 | MARAVGIDLGTTNSV | Mycobacterium avium |
The nucleic acid sequence codons for the underlined region (from bp 31 to 45) are GGT ATC CGC TAC GGG.
The complete nucleotide sequence is available under GenBank accession no. AF254578. The nucleic acid sequence codons for the underlined region (from bp 31 to 45) are GGT ATC GAC CTC GGG. Positions that differ from those given in footnote a are shown in boldface type. The amino acid sequences of M. tuberculosis, M. leprae, and M. avium Hsp70 are identical in the indicated region.
Serological responses. (i) Hsp70.
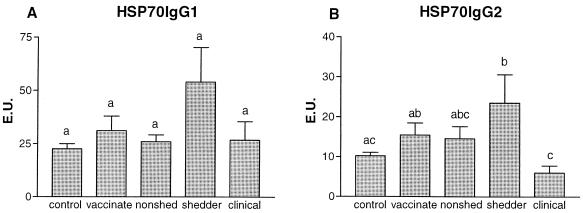
Although shedders had higher responses than the other groups, no significant differences were found in Hsp70-specific IgG1 (Fig. 1A) between the groups. Hsp70-specific IgG2 (Fig. 1B) was significantly lower in the clinical diseased animals than in the shedders and vaccinated animals (for both, P < 0.05). No significant differences were found in Hsp70-specific IgG2 between control, vaccinated, and nonshedding animals.
FIG. 1.
The average (+standard error [error bars]) M. avium subsp. paratuberculosis Hsp70-specific IgG1 (A) and IgG2 (B) titers, expressed in EU, of control animals (n = 50), vaccinated animals (n = 25), nonshedders (n = 47), shedders (n = 39), and animals with clinical signs of disease (n = 15) are depicted. Statistical analysis was done using the Kruskal-Wallis test; the letters directly above the bars represent a significant difference (P < 0.05) between groups if no letters are shared.
Specific IgA seroresponses were found sporadically in the group of shedding animals only (data not shown). The IgM responses resembled the IgG2 response pattern; most notably, the animals with clinical disease tended to have lower IgM responses than the asymptomatic shedders, but this was not statistically significant (data not shown).
(ii) Hsp65.
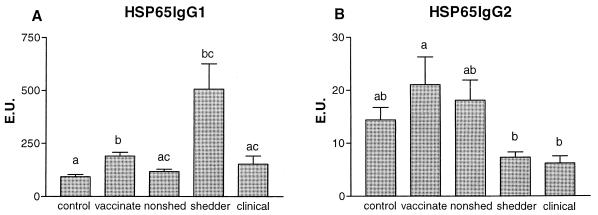
Significantly elevated Hsp65-specific IgG1 (Fig. 2A) was measured in sera of vaccinated and shedding animals compared to in sera of controls (P < 0.001 and P < 0.01, respectively). More Hsp65-specific IgG1 was detected in vaccinated animals than in nonshedders (P < 0.05). In shedders and animals with clinical signs, less Hsp65-specific IgG2 (Fig. 2B) was detected compared to that detected in vaccinated animals. Decreased Hsp65-specific IgG2 and IgG1 responses were observed in animals with clinical disease compared with those of shedders, but this was not statistically significant. Specific IgA seroresponses were found sporadically in shedding animals only (data not shown). The IgM responses resembled the IgG2 response pattern; animals with clinical disease had lower IgM responses (not significant) than the shedders (data not shown).
FIG. 2.
The average (+standard error [error bars]) M. avium subsp. paratuberculosis Hsp65-specific IgG1 (A) and IgG2 (B) titers, expressed in EU, of control animals (n = 50), vaccinated animals (n = 25), nonshedders (n = 47), shedders (n = 39), and animals with clinical signs of disease (n = 15) are depicted. Statistical analysis was done using the Kruskal-Wallis test; the letters directly above the bars represent a significant difference (P < 0.05) between groups if no letters are shared.
(iii) PPDP.
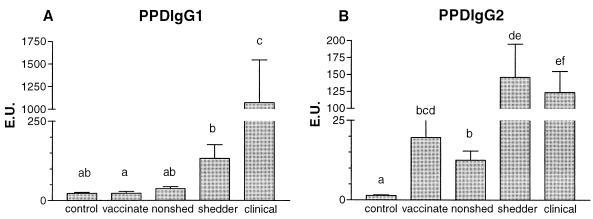
Concerning the PPDP-specific IgG1 response (Fig. 3A), no significant increase was observed in the vaccinated group, shedders, and nonshedders compared to that of the control group, but the animals with clinical signs had significantly increased PPDP-specific IgG1 responses relative to those of animals in the control group (P < 0.001). PPDP-specific IgG1 was significantly elevated in animals with clinical disease compared to that in shedding animals (P < 0.01). In control animals PPDP-specific IgG1 was relatively abundant, especially in comparison to the relative amount of PPDP-specific IgG2 (Fig. 3B). Control animals showed less PPDP-specific IgG2 than the other groups did (for all, P < 0.01). When comparing PPDP-specific IgG2 in shedders and animals with clinical disease, no significant changes were observed. However, shedders and animals with clinical disease had more PPDP-specific IgG2 than the vaccinated animals (for both, P < 0.05) and nonshedders (P < 0.01) did. PPDP-specific IgA responses were found sporadically in shedding animals only (data not shown). The IgM responses resembled the IgG2 response pattern. Shedders and animals with clinical disease showed similar IgM responses, which tended to be higher than those in control, vaccinated, and nonshedding animals, but these differences were not statistically significant (data not shown).
FIG. 3.
The average (+standard error [error bars]) M. avium subsp. paratuberculosis PPDP-specific IgG1 (A) and IgG2 (B) titers, expressed in EU, of control animals (n = 50), vaccinated animals (n = 25), nonshedders (n = 47), shedders (n = 39), and animals with clinical signs of disease (n = 15) are depicted. Statistical analysis was done using the Kruskal-Wallis test; the letters directly above the bars represent a significant difference (P < 0.05) between groups if no letters are shared.
(iv) LAM.
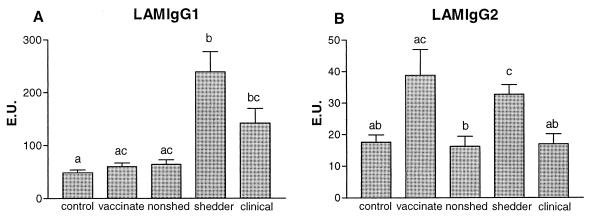
In shedders and animals with clinical disease, significantly increased LAM-specific IgG1 (Fig. 4A) was found compared to that seen in control animals (P < 0.01 for both comparisons). In vaccinated animals and nonshedders, less LAM-specific IgG1 was detected than that detected in shedders (for both, P < 0.001). The LAM-specific IgG2 (Fig. 4B) was significantly increased in shedders compared to those of nonshedders and control animals (for both, P < 0.001). Vaccinated animals had more LAM-specific IgG2 than the nonshedders did. Both LAM-specific IgG1 and IgG2 were lower in clinical diseased animals than in shedders, and this effect was significant (P < 0.01) in the case of the LAM-specific IgG2 response. LAM-specific IgA was not detected (data not shown), and LAM-specific IgM patterns resembled those observed for IgG2, but no statistically significant differences were found (data not shown).
FIG. 4.
The average (+standard error [error bars]) M. avium subsp. paratuberculosis LAM-specific IgG1 (A) and IgG2 (B) titers, expressed in EU, of control animals (n = 50), vaccinated animals (n = 25), nonshedders (n = 47), shedders (n = 39), and animals with clinical signs of disease (n = 15) are depicted. Statistical analysis was done using the Kruskal-Wallis test; the letters directly above the bars represent a significant difference (P < 0.05) between groups if no letters are shared.
(v) Total IgG1 and IgG2.
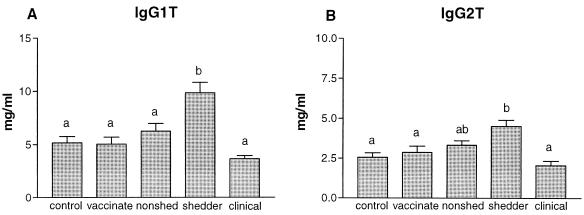
Shedding animals had significantly elevated amounts of IgG1 (Fig. 5A) and IgG2 (Fig. 5B) antibodies in their sera compared to those of control animals (P < 0.001). Animals with clinical disease have significantly less IgG1 (P < 0.001) and IgG2 (P < 0.01) than the shedders did, but the amounts are not significantly different from those observed in healthy controls.
FIG. 5.
The average (+standard error [error bars]) concentrations (in milligrams per milliliter) of IgG1 (A) and IgG2 (B) of control animals (n = 50), vaccinated animals (n = 25), nonshedders (n = 47), shedders (n = 39), and animals with clinical signs of disease (n = 15) are depicted. Statistical analysis was done using the Kruskal-Wallis test; the letters directly above the bars represent a significant difference (P < 0.05) between groups if no letters are shared.
DISCUSSION
This is the first report to describe IgG1- and IgG2-subisotype-specific ELISAs with different M. avium subsp. paratuberculosis antigens. Progression of bovine paratuberculosis has previously been associated with increased humoral responses to (crude) M. avium subsp. paratuberculosis antigens. Our results indicate that a significant increase in antibody responses, when comparing asymptomatic shedders and clinically diseased animals, can be detected only regarding PPDP-specific IgG1 responses. For the other antigens studied (LAM, Hsp65, and Hsp70) no increased IgG1 response was observed in animals with signs of clinical disease. Responses rather indicated a decreased IgG2 reaction, which was significant in the case of Hsp70- and LAM-specific responses. In addition, when comparing total IgG1 and IgG2 concentrations, it appeared that there is no general increase in humoral responses in clinically diseased animals. In fact, IgG1 and IgG2 concentrations in the group of asymptomatic shedders are significantly elevated compared to those in the other groups. Observations on IgA and IgM were comparable with results published by Abbas and Riemann (2) and Yokomizo et al. (55). The IgA response did not appear to be a useful parameter for comparisons as it was detected infrequently; IgM responses appeared to resemble IgG2 responses, but no statistically significant differences were found between the different groups.
Although the biochemical nature of the antigen (carbohydrate [LAM] versus heat shock protein) may influence the isotypic distribution, this effect is not clear in our study, as the pattern of the slight predominance of IgG1 over IgG2 is not different from those seen with different antigens, which is similar to observations made by Sousa et al. (40). Comparison of relative amounts of total IgG1 and IgG2 and the antigen-specific antibody responses provided indications that IgG1 is the predominating isotype in all assays, similar to results published by Yokomizo et al. (55). However, since we did not calibrate all antigen-specific ELISAs to monoisotypic immunoglobulin standards with known concentrations, the possibilities to make (interassay) comparisons between different IgG subclasses are limited, because we cannot exclude effects of differences in affinity of the anti-subclass reagents used.
The differences observed between PPDP and the other antigens may also be due to the nature of the antigens involved. PPDP predominantly, although not exclusively (3), consists of proteins excreted by the mycobacteria (32, 53). We hypothesize that, following lysis of infected macrophages during progressive stages of the disease, the excreted antigens, besides being present at lesional sites, have a higher chance to leave intestinal lesional sites via the extended lymphatic (4) or venous system of the intestine (9). These large amounts of free antigens may either directly stimulate (memory) B cells or be taken up by uninfected macrophages at nonintestinal nonlesional sites and lead to abundant antibody production.
At lesional sites the cytosolic and structural antigens (heat shock protein and LAM) will be more abundant, as they are predominantly associated with accumulating chronically infected macrophages. Studies dealing with tuberculosis and leprosy have demonstrated that infected macrophages have defective or altered antigen-presenting capabilities (reviewed in reference 34), resulting in poor induction of T-cell immunity, suppression (20), anergy (36, 50), and even deletion of Th1 cells (15). In a previous study where proliferative responses to PPDP, Hsp65, and Hsp70 were measured (27), a decrease in cell-mediated immunity of peripheral blood mononuclear cells of clinical diseased animals was observed for all three antigens. In addition, Sweeney et al. (45) showed decreased intestinal mRNA expression of IFN-γ but no increased expression of IL-4 in the inflamed ilea of clinically diseased animals compared to that of asymptomatic shedders. Moreover, antigen-specific B-cell unresponsiveness during chronic stages of bovine paratuberculosis has recently been demonstrated (51). Based on results presented by other groups, one could argue that the IgG1 response is a functional characteristic of a type 2 response and the IgG2 response is a functional characteristic of a type 1 immune response in cattle (6, 7, 17, 18). Similar serological studies in tuberculosis and leprosy patients have, however, yielded contradictory results on whether immunoglobulin subisotypes reflect changes in the Th1-Th2 balance (16, 22, 40). Nonetheless, as only the PPDP-specific IgG1 responses indicated the expected increase in type 2 responsiveness, it can be speculated that the decrease in type 1 responses (e.g., heat shock protein and LAM) may predominate over the increase of type 2 responses in progressive bovine paratuberculosis.
Conclusion.
Previous studies have reported a decrease in cell-mediated immunity during progression of paratuberculosis and concomitant increase in antibody responses during the disease. This study is the first to show that that observation depends highly on the antigens and isotypes used to study the disease. We were able to show the classical pattern only for PPDP antigens and the IgG1 isotype. For other antigens and isotypes and the total IgG levels, the response pattern is different and indicates that there is no uniform association with increased antibody responses during the progression from the asymptomatic stage to the clinical stage of bovine paratuberculosis.
REFERENCES
- 1.Abbas A, Murphy K, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 2.Abbas B, Riemann H P. IgG, IgM and IgA in the serum of cattle naturally infected with Mycobacterium paratuberculosis. Comp Immunol Microbiol Infect Dis. 1988;11:171–175. doi: 10.1016/0147-9571(88)90034-3. [DOI] [PubMed] [Google Scholar]
- 3.Andersen P. The T cell response to secreted antigens of Mycobacterium tuberculosis. Immunobiology. 1994;191:537–547. doi: 10.1016/S0171-2985(11)80460-2. [DOI] [PubMed] [Google Scholar]
- 4.Beh K J. The antibody-containing cell response of the lamina propria of the sheep and the uptake of soluble antigen into afferent ileal lymph following intra-intestinal infusion of soluble or particulate antigen. Immunology. 1985;54:479–485. [PMC free article] [PubMed] [Google Scholar]
- 5.Benedictus G, Dijkhuizen A A, Stelwagen J. Economic losses due to paratuberculosis in dairy cattle. Vet Rec. 1987;121:142–146. doi: 10.1136/vr.121.7.142. [DOI] [PubMed] [Google Scholar]
- 6.Brown W C, Estes D M. Type I and type II responses in cattle and their regulation. In: Horzinek M, Schijns V E C J, editors. Cytokines in veterinary medicine. Wallingford, United Kingdom: CAB International; 1997. pp. 15–33. [Google Scholar]
- 7.Brown W C, McElwain T F, Palmer G H, Chantler S E, Estes D M. Bovine CD4+ T-lymphocyte clones specific for rhoptry-associated protein 1 of Babesia bigemina stimulate enhanced immunoglobulin G1 (IgG1) and IgG2 synthesis. Infect Immun. 1999;67:155–164. doi: 10.1128/iai.67.1.155-164.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan J, Fan X D, Hunter S W, Brennan P J, Bloom B R. Lipoarabinomannan, a possible virulence factor involved in persistence of Mycobacterium tuberculosis within macrophages. Infect Immun. 1991;59:1755–1761. doi: 10.1128/iai.59.5.1755-1761.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiodini R J. Immunology: resistance to paratuberculosis. Vet Clin N Am Food Anim Pract. 1996;12:313–343. doi: 10.1016/s0749-0720(15)30409-6. [DOI] [PubMed] [Google Scholar]
- 10.Clarke C J. The pathology and pathogenesis of paratuberculosis in ruminants and other species. J Comp Pathol. 1997;116:217–261. doi: 10.1016/s0021-9975(97)80001-1. [DOI] [PubMed] [Google Scholar]
- 11.Collins M T. Diagnosis of paratuberculosis. Vet Clin N Am Food Anim Pract. 1996;12:357–371. doi: 10.1016/s0749-0720(15)30411-4. [DOI] [PubMed] [Google Scholar]
- 12.Collins M T, Angulo A, Buergelt C D, Hennager S G, Hietala S K, Jacobson R H, Whipple D L, Whitlock R H. Reproducibility of a commercial enzyme-linked immunosorbent assay for bovine paratuberculosis among eight laboratories. J Vet Diagn Investig. 1993;5:52–55. doi: 10.1177/104063879300500112. [DOI] [PubMed] [Google Scholar]
- 13.Colston A, McConnell I, Bujdoso R. Cloning and expression in Escherichia coli of DNA encoding a 60 kDa stress protein of Mycobacterium paratuberculosis, the causative agent of Johne's disease. Microbiology. 1994;140:3329–3336. doi: 10.1099/13500872-140-12-3329. [DOI] [PubMed] [Google Scholar]
- 14.Dahl K E, Shiratsuchi H, Hamilton B D, Ellner J J, Toossi Z. Selective induction of transforming growth factor beta in human monocytes by lipoarabinomannan of Mycobacterium tuberculosis. Infect Immun. 1996;64:399–405. doi: 10.1128/iai.64.2.399-405.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Das G, Vohra H, Saha B, Agrewala J N, Mishra G C. Apoptosis of Th1-like cells in experimental tuberculosis (TB) Clin Exp Immunol. 1999;115:324–328. doi: 10.1046/j.1365-2249.1999.00755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhandayuthapani S, Izumi S, Anandan D, Bhatia V N. Specificity of IgG subclass antibodies in different clinical manifestations of leprosy. Clin Exp Immunol. 1992;88:253–257. doi: 10.1111/j.1365-2249.1992.tb03069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Estes D M, Closser N M, Allen G K. IFN-γ stimulates IgG2 production from bovine B cells costimulated with anti-m and mitogen. Cell Immunol. 1994;154:287–295. doi: 10.1006/cimm.1994.1078. [DOI] [PubMed] [Google Scholar]
- 18.Estes D M, Hirano A, Heussler V T, Dobbelaere D A, Brown W C. Expression and biological activities of bovine interleukin 4: effects of recombinant bovine interleukin 4 on T cell proliferation and B cell differentiation and proliferation in vitro. Cell Immunol. 1995;163:268–279. doi: 10.1006/cimm.1995.1126. [DOI] [PubMed] [Google Scholar]
- 19.Gaston J S. Heat shock proteins and arthritis—new readers start here. Autoimmunity. 1997;26:33–42. doi: 10.3109/08916939709009548. [DOI] [PubMed] [Google Scholar]
- 20.Haanen J B, Ottenhoff T H, Lai A F R F, Soebono H, Spits H, de Vries R R. Mycobacterium leprae-specific T cells from a tuberculoid leprosy patient suppress HLA-DR3-restricted T cell responses to an immunodominant epitope on 65-kDa hsp of mycobacteria. J Immunol. 1990;145:3898–3904. [PubMed] [Google Scholar]
- 21.Hilbink F, West D M, de Lisle G W, Kittelberger R, Hosie B D, Hutton J, Cooke M M, Penrose M. Comparison of a complement fixation test, a gel diffusion test and two absorbed and unabsorbed ELISAs for the diagnosis of paratuberculosis in sheep. Vet Microbiol. 1994;41:107–116. doi: 10.1016/0378-1135(94)90140-6. [DOI] [PubMed] [Google Scholar]
- 22.Hussain R, Kifayet A, Chiang T J. Immunoglobulin G1 (IgG1) and IgG3 antibodies are markers of progressive disease in leprosy. Infect Immun. 1995;63:410–415. doi: 10.1128/iai.63.2.410-415.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jark U, Ringena I, Franz B, Gerlach G F, Beyerbach M, Franz B. Development of an ELISA technique for serodiagnosis of bovine paratuberculosis. Vet Microbiol. 1997;57:189–198. doi: 10.1016/s0378-1135(97)00125-9. [DOI] [PubMed] [Google Scholar]
- 24.Johnson-Ifearulundu Y, Kaneene J B, Lloyd J W. Herd-level economic analysis of the impact of paratuberculosis on dairy herds. J Am Vet Med Assoc. 1999;214:822–825. [PubMed] [Google Scholar]
- 25.Jorgensen J B. An improved medium for culture of Mycobacterium paratuberculosis from bovine faeces. Acta Vet Scand. 1982;23:325–335. doi: 10.1186/BF03546784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaufmann S H. Heat shock proteins and the immune response. Immunol Today. 1990;11:129–136. doi: 10.1016/0167-5699(90)90050-j. [DOI] [PubMed] [Google Scholar]
- 27.Koets A P, Rutten V P, Hoek A, Bakker D, van Zijderveld F, Muller K E, van Eden W. Heat-shock protein-specific T-cell responses in various stages of bovine paratuberculosis. Vet Immunol Immunopathol. 1999;70:105–115. doi: 10.1016/s0165-2427(99)00062-8. [DOI] [PubMed] [Google Scholar]
- 28.Kormendy B. The effect of vaccination on the prevalence of paratuberculosis in large dairy herds. Vet Microbiol. 1994;41:117–125. doi: 10.1016/0378-1135(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 29.McNab W B, Meek A H, Duncan J R, Brooks B W, Van Dreumel A A, Martin S W, Nielsen K H, Sugden E A, Turcotte C. An evaluation of selected screening tests for bovine paratuberculosis. Can J Vet Res. 1991;55:252–259. [PMC free article] [PubMed] [Google Scholar]
- 30.Momotani E, Whipple D L, Thiermann A B, Cheville N F. Role of M cells and macrophages in the entrance of Mycobacterium paratuberculosis into domes of ileal Peyer's patches in calves. Vet Pathol. 1988;25:131–137. doi: 10.1177/030098588802500205. [DOI] [PubMed] [Google Scholar]
- 31.Mosmann T R, Cherwinski H, Bond M W, Giedlin M A, Coffman R L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 32.Mutharia L M, Moreno W, Raymond M. Analysis of culture filtrate and cell wall-associated antigens of Mycobacterium paratuberculosis with monoclonal antibodies. Infect Immun. 1997;65:387–394. doi: 10.1128/iai.65.2.387-394.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ridge S E, Morgan I R, Sockett D C, Collins M T, Condron R J, Skilbeck N W, Webber J J. Comparison of the Johne's absorbed EIA and the complement-fixation test for the diagnosis of Johne's disease in cattle. Aust Vet J. 1991;68:253–257. doi: 10.1111/j.1751-0813.1991.tb03230.x. [DOI] [PubMed] [Google Scholar]
- 34.Schaible U E, Collins H L, Kaufmann S H. Confrontation between intracellular bacteria and the immune system. Adv Immunol. 1999;71:267–377. doi: 10.1016/s0065-2776(08)60405-8. [DOI] [PubMed] [Google Scholar]
- 35.Schoel B, Kaufmann S. The unique role of heat shock proteins in infections. In: van Eden W, Young D, editors. Stress proteins in medicine. New York, N.Y: Marcel Dekker, Inc; 1996. pp. 27–52. [Google Scholar]
- 36.Seldenrijk C A, Drexhage H A, Meuwissen S G, Meijer C J. T-cellular immune reactions (in macrophage inhibition factor assay) against Mycobacterium paratuberculosis, Mycobacterium kansasii, Mycobacterium tuberculosis, Mycobacterium avium in patients with chronic inflammatory bowel disease. Gut. 1990;31:529–535. doi: 10.1136/gut.31.5.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sibley L, Hunter S, Brennan P, Krahenbuhl J. Mycobacterial lipoarabinomannan inhibits gamma interferon-mediated activation of macrophages. Infect Immun. 1988;56:1232–1236. doi: 10.1128/iai.56.5.1232-1236.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siegel S, Castellan N J., Jr . Nonparametric statistics for the behavioural sciences. 2nd ed. Singapore, Singapore: McGraw-Hill; 1988. [Google Scholar]
- 39.Sockett D C, Conrad T A, Thomas C B, Collins M T. Evaluation of four serological tests for bovine paratuberculosis. J Clin Microbiol. 1992;30:1134–1139. doi: 10.1128/jcm.30.5.1134-1139.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sousa A O, Henry S, Maroja F M, Lee F K, Brum L, Singh M, Lagrange P H, Aucouturier P. IgG subclass distribution of antibody responses to protein and polysaccharide mycobacterial antigens in leprosy and tuberculosis patients. Clin Exp Immunol. 1998;111:48–55. doi: 10.1046/j.1365-2249.1998.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spangler L, Bech-Nielsen S, Heider L. A study of subclinical paratuberculosis in three central Ohio dairy herds: fecal culture, serologic testing and milk production. Acta Vet Scand. 1988;84:148–150. [PubMed] [Google Scholar]
- 42.Stevenson K, Inglis N F, Rae B, Donachie W, Sharp J M. Complete nucleotide sequence of a gene encoding the 70 kd heat shock protein of Mycobacterium paratuberculosis. Nucleic Acids Res. 1991;19:4552. doi: 10.1093/nar/19.16.4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stokes R W, Speert D P. Lipoarabinomannan inhibits nonopsonic binding of Mycobacterium tuberculosis to murine macrophages. J Immunol. 1995;155:1361–1369. [PubMed] [Google Scholar]
- 44.Sugden E A, Corner A H, Samagh B S, Brooks B W, Turcotte C, Nielsen K H, Stewart R B, Duncan J R. Serodiagnosis of ovine paratuberculosis, using lipoarabinomannan in an enzyme-linked immunosorbent assay. Am J Vet Res. 1989;50:850–854. [PubMed] [Google Scholar]
- 45.Sweeney R W, Jones D E, Habecker P, Scott P. Interferon-gamma and interleukin 4 gene expression in cows infected with Mycobacterium paratuberculosis. Am J Vet Res. 1998;59:842–847. [PubMed] [Google Scholar]
- 46.Sweeney R W, Whitlock R H, Buckley C L, Spencer P, Rosenberger A E, Hutchinson L J. Diagnosis of paratuberculosis in dairy cattle, using enzyme-linked immunosorbent assay for detection of antibodies against Mycobacterium paratuberculosis in milk. Am J Vet Res. 1994;55:905–909. [PubMed] [Google Scholar]
- 47.Thorel M-F. Paratuberculosis (Johne's disease) In: Truszcynski M, Pearson J E, Edwards S, editors. OIE manual of standards for diagnostic tests and vaccines. 3rd ed. Paris, France: Office International des Epizooties; 1996. pp. 218–228. [Google Scholar]
- 48.van Eden W, van der Zee R, Paul A, Prakken B, Wendling U, Anderton S, Wauben M. Do heat shock proteins control the balance of T-cell regulation in inflammatory diseases? Immunol Today. 1998;19:303–307. doi: 10.1016/s0167-5699(98)01283-3. [DOI] [PubMed] [Google Scholar]
- 49.Vannuffel P, Gilot P, Limbourg B, Naerhuyzen B, Dieterich C, Coene M, Machtelinckx L, Cocito C. Development of species-specific enzyme-linked immunosorbent assay for diagnosis of Johne's disease in cattle. J Clin Microbiol. 1994;32:1211–1216. doi: 10.1128/jcm.32.5.1211-1216.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vordermeier H, Harris D, Friscia G, Roman E, Surcel H, Moreno C, Pasvol G, Ivanyi J. T cell repertoire in tuberculosis: selective anergy to an immunodominant epitope of the 38-kDa antigen in patients with active disease. Eur J Immunol. 1992;22:2631–2637. doi: 10.1002/eji.1830221024. [DOI] [PubMed] [Google Scholar]
- 51.Waters W R, Stabel J R, Sacco R E, Harp J A, Pesch B A, Wannemuehler M J. Antigen-specific B-cell unresponsiveness induced by chronic Mycobacterium avium subsp. paratuberculosis infection of cattle. Infect Immun. 1999;67:1593–1598. doi: 10.1128/iai.67.4.1593-1598.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wentink G H, Bongers J H, Zeeuwen A A, Jaartsveld F H. Incidence of paratuberculosis after vaccination against M. paratuberculosis in two infected dairy herds. Zentbl Vetmed Reihe B. 1994;41:517–522. doi: 10.1111/j.1439-0450.1994.tb00258.x. [DOI] [PubMed] [Google Scholar]
- 53.White W B, Whipple D L, Stabel J R, Bolin C A. Comparison of cellular and extracellular proteins expressed by various isolates of Mycobacterium paratuberculosis and other mycobacterial species. Am J Vet Res. 1994;55:1399–1405. [PubMed] [Google Scholar]
- 54.Yokomizo Y, Merkal R S, Lyle P A. Enzyme-linked immunosorbent assay for detection of bovine immunoglobulin G1 antibody to a protoplasmic antigen of Mycobacterium paratuberculosis. Am J Vet Res. 1983;44:2205–2207. [PubMed] [Google Scholar]
- 55.Yokomizo Y, Yugi H, Merkal R S. A method for avoiding false-positive reactions in an enzyme-linked immunosorbent assay (ELISA) for the diagnosis of bovine paratuberculosis. Nippon Juigaku Zasshi. 1985;47:111–119. doi: 10.1292/jvms1939.47.111. [DOI] [PubMed] [Google Scholar]