Abstract
Aim
To examine whether sequential administration of (d‐Arg35)‐sea lamprey peptide tyrosine tyrosine (1–36) (SL‐PYY) and the glucagon‐like peptide‐1 (GLP‐1) mimetic, liraglutide, has beneficial effects in diabetes.
Methods
SL‐PYY is an enzymatically stable neuropeptide Y1 receptor (NPY1R) agonist known to induce pancreatic beta‐cell rest and improve overall beta‐cell health. We employed SL‐PYY and liraglutide to induce appropriate recurrent periods of beta‐cell rest and stimulation, to assess therapeutic benefits in high fat fed (HFF) mice with streptozotocin (STZ)‐induced insulin deficiency, namely HFF‐STZ mice.
Results
Previous studies confirm that, at a dose of 0.25 nmol/kg, liraglutide exerts bioactivity over an 8‐12 hour period in mice. Initial pharmacokinetic analysis revealed that 75 nmol/kg SL‐PYY yielded a similar plasma drug time profile. When SL‐PYY (75 nmol/kg) and liraglutide (0.25 nmol/kg) were administered sequentially at 08:00 AM and 08:00 PM, respectively, to HFF‐STZ mice for 28 days, reductions in energy intake, body weight, circulating glucose, insulin and glucagon were noted. Similarly positive, but slightly less striking, effects were also apparent with twice‐daily liraglutide‐only therapy. The sequential SL‐PYY and liraglutide treatment also improved insulin sensitivity and glucose‐induced insulin secretory responses, which was not apparent with liraglutide treatment, although benefits on glucose tolerance were mild. Interestingly, combined therapy also elevated pancreatic insulin, decreased pancreatic glucagon and enhanced the plasma insulin/glucagon ratio compared with liraglutide alone. This was not associated with an enhancement of beneficial changes in islet cell areas, proliferation or apoptosis compared with liraglutide alone, but the numbers of centrally stained glucagon‐positive islet cells were reduced by sequential combination therapy.
Conclusion
These data show that NPY1R‐induced intervals of beta‐cell rest, combined with GLP‐1R–stimulated periods of beta‐cell stimulation, should be further evaluated as an effective treatment option for obesity‐driven forms of diabetes.
Keywords: beta‐cell health, diabetes, GLP‐1, PYY
1. INTRODUCTION
Type 2 diabetes (T2D) remains a leading cause of mortality worldwide, despite the plethora of currently available treatment options for the disease. 1 In this regard, one significant advancement of T2D therapy in recent times was the clinical approval of glucagon‐like peptide‐1 receptor (GLP‐1R) agonists. 2 These agents induce prominent antidiabetic and weight‐lowering actions, as well as potential beneficial cardioprotective, neuroprotective and anti‐inflammatory effects. 2 However, unfortunately the full preclinical efficacy of GLP‐1R agonists has not entirely translated to the clinic. This could be a result of GLP‐1R dose‐related gastrointestinal side effects including nausea, vomiting and diarrhoea, which are prevalent in humans. 3 In addition, the chronic nature of T2D results in a progressive decline in beta‐cell function over time leading to deteriorating glycaemic control. 3 Indeed, prolonged periods of hyperglycaemia place beta cells under sustained oxidative stress, to which they are particularly sensitive, 4 creating a vicious cycle of beta‐cell dysfunction. Therefore, novel agents or approaches that can protect beta‐cell health to improve overall enduring glycaemic control are highly sought after.
One such option could be linked to the induction of beta‐cell rest during less metabolically active times. 5 Thus transcriptional profiling of the beta cell has identified circadian temporal control, where genes involved in insulin secretion are stimulated during the ‘active’ phase when food is anticipated, while during the ‘quiescent’ phase, genes involved in beta‐cell growth, repair and DNA replication are active. 6 The benefits of the beta‐cell rest theory were first shown using exogenous insulin injection to reduce the inherent demand for insulin secretion, 7 allowing for replenishment of insulin stores, 8 recovery of glucokinase activity 9 and alleviation of overall oxidative stress. 10 In a similar fashion, sequential administration of a glucose‐dependent insulinotropic polypeptide receptor (GIPR) antagonist to promote beta‐cell rest, alongside scheduled periods of GLP‐1R–induced stimulation during the ‘active’ phase, has been shown to exert marked improvements on metabolic control in a mouse model of diabetes. 11 However, sustained inhibition of GIPR function may ultimately lead to negative effects in other tissues such as bone and the brain. 12 , 13 In addition, activation of the GIPR has been shown to exert protective effects on beta cells, 14 , 15 as well as impart benefits of islet cell transdifferentiation events. 16 , 17 Thus, while sequential GIPR antagonism and GLP‐1R agonism confirm proof‐of‐principle of this approach, an alternative method to induce beta‐cell rest would be more favourable.
In this regard, peptide tyrosine (PYY), a 36 amino acid gut‐derived peptide secreted from L‐cells, binds to neuropeptide Y1 receptors (NPY1Rs) expressed on pancreatic beta cells to induce insulinostatic actions that promote beta‐cell rest. 18 Moreover, NPY1R signalling has been shown to enhance beta‐cell growth and survival, 18 , 19 as well as evoking positive islet cell transdifferentiation events that enhance beta‐cell mass. 20 To pharmacologically exploit such actions, dipeptidyl peptidase‐4 (DPP‐4) stabilized analogues of human PYY (1–36) were generated through astute amino acid modification, based on current structure/function knowledge for PYY. 21 However, although these modified PYY (1–36) compounds were resistant to DPP‐4 cleavage, they lacked significant bioactivity at the level of the beta cell. 21 Conversely, investigation of PYY gene structure from phylogenetically ancient fish identified highly conserved PYY sequences with inherent enzymatic stability. 19 In particular, sea lamprey PYY (1–36) replicated the pancreatic beta‐cell benefits of human PYY (1–36), leading to beta‐cell rest together with positive effects on islet cell turnover and lineage transition events. 19 , 20 Because C‐terminal degradation of PYY peptides abolishes bioactivity, 22 , 23 substitution of l‐Arg with its enantiomer d‐Arg, at position 35 in sea lamprey PYY (1–36), yielded an N‐ and C‐terminally enzyme‐resistant NPY1R compound that retained bioactivity, namely (d‐Arg35)‐sea lamprey PYY (1–36) (SL‐PYY). 24
It follows that sequential administration of SL‐PYY and liraglutide could represent a useful therapeutic approach to test in diabetes, building on previous positive observations in rodent models. 11 Consequently, in the current study we employed staggered administration of SL‐PYY and liraglutide to facilitate a twice‐daily (at 08:00 AM and 08:00 PM) dosing regimen in diabetic mice, where beta cells would be successively stimulated and rested for 8‐12 hours during appropriate periods of each day. This was compared directly with a twice‐daily liraglutide monotherapy treatment regimen, with enhanced antidiabetic efficacy of consecutive SL‐PYY and liraglutide treatment over liraglutide monotherapy being the primary motivation of this study. We made use of high fat fed (HFF) mice with streptozotocin (STZ)‐induced compromised beta cells as our study model, which present without the classical beta‐cell hypertrophy response in HFF mice. 25 This generates a translational obesity‐driven model of diabetes where restoration of beta‐cell function would be highly advantageous, more akin to the human setting of poorly controlled T2D.
2. EXPERIMENTAL DESIGN
2.1. Peptides
SL‐PYY and liraglutide were supplied by Synpeptide Ltd (Shanghai, China) at greater than 95% purity, and characterized in‐house by high‐performance liquid chromatography with matrix‐assisted laser desorption/ionization‐time of flight mass spectrometry, as described previously. 11
2.2. Dosing regimens
Previous studies confirm that a dose of 0.25 nmol/kg liraglutide is required to achieve a biological action profile greater than 8 hours but less than 12 hours in mice. 11 We sought to uncover a similar dosing pattern for SL‐PYY, to allow for a sequential administration schedule with little overlap of biological actions. SL‐PYY is insulinostatic and does not affect blood glucose levels in the acute setting, 24 making it difficult to assess the pharmacodynamic profile of the peptide. Instead, we employed a commercially available ELISA for PYY (1–36), which permitted detection of SL‐PYY following a bolus injection in mice (PYY ELISA kit, #81501, Crystal Chem). As such, we used doses of 5, 25 and 75 nmol/kg SL‐PYY with regular assessment of circulating drug levels over a 120‐minute observation period in normal 12‐week‐old male Swiss mice (Figure S1A). Further investigation revealed that at a dose of 75 nmol/kg, SL‐PYY had a pharmacokinetic profile greater than 6 hours, but less than 12 hours (Figure S1B). Importantly, modulation of NPY1R by SL‐PYY, even at elevated doses of 75 nmol/kg, should not induce nausea‐like behaviour because NPY1R activation is linked to appetite stimulation, as opposed to the well‐characterized satiety‐like actions of NPY2R. 26
2.3. Experimental protocol
Male Swiss mice (10 weeks old) were purchased from Envigo Ltd (London, UK) and kept on a high‐fat diet (45% fat, 35% carbohydrate and 20% protein; percentage of total energy 26.15 kJ/g; Dietex International Ltd, Witham, UK) for 42 days to induce obesity. After 21 days of high fat feeding (day –21), mice received STZ injections (4‐hour fast, 50 mg/kg bw, intraperitoneal [i.p.], freshly dissolved in citrate buffer, pH 4.5), and were then administered similar STZ injections on days −14 and −7 (Figure 1). On day 0, HFF‐STZ mice that presented with diabetes‐like features (blood glucose > 11.1 mmol/L) were divided into three separate groups (n = 8) that received 28 days twice‐daily (08:00 AM and 08:00 PM) i.p. injections of either saline vehicle (0.9% [w/v] NaCl), liraglutide (0.25 nmol/kg) or 75 nmol/kg SL‐PYY at 08:00 AM and 0.25 nmol/kg liraglutide at 08:00 PM; this group was named PYY/Lira (Figure 1). In addition, a group of normal mice maintained on a standard rodent diet (10% fat, 60% carbohydrate and 30% protein; percentage of total energy 12.99 kJ/g; Trouw Nutrition, Northwich, UK) received twice‐daily i.p. saline injections throughout and were employed as normal controls. Body weight, energy intake and blood glucose were assessed at regular intervals, with non‐fasted insulin and glucagon determined on day 28. At the end of the treatment period, glucose tolerance (18 mmol/kg bw; i.p.; 18‐hour fasted) and insulin sensitivity (15 U/kg bovine insulin; i.p.; non‐fasted) tests were conducted (Figure 1). At termination, animals were sacrificed by lethal inhalation of CO2 followed by cervical dislocation. Pancreatic tissues were then excised, divided longitudinally and processed for either determination of pancreatic hormone content following acid/ethanol protein extraction or fixed in 4% parafo for 48 hours at 4°C for histological analysis. 25 All animal experiments were conducted in accordance with the UK Animals (Scientific Procedures) Act 1986 and EU Directive 2010/63EU. All necessary steps were taken to prevent any potential animal suffering. The animal studies were approved by the local Ulster Animal Welfare and Ethical Review Body (AWERB) committee.
FIGURE 1.
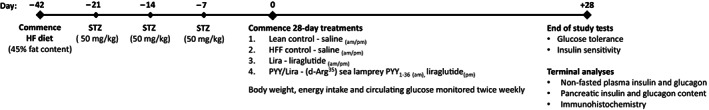
Experimental timeline of studies in HFF‐STZ mice. Male NIH Swiss mice were maintained on a HF (45% fat content) diet for 42 days to induce obesity. On days −21, −14 and −7, mice received a STZ injection (50 mg/kg bw, i.p.). On day 0, twice‐daily injections (08:00 AM and 08:00 PM) of (d‐Arg35)‐sea lamprey (SL)‐PYY (75 nmol/kg bw, i.p.) and liraglutide (0.25 nmol/kg bw, i.p.), as appropriate, began for 28 consecutive days. Body weight, energy intake and circulating glucose were monitored twice weekly during this period, with non‐fasted plasma insulin and glucagon determined at the end of the study. On day 28, glucose tolerance (18 mmol/kg bw, i.p.) and insulin sensitivity (15 U/kg bw, i.p.) tests were conducted. At termination, pancreatic tissue was excised with hormone content and immunohistochemistry was investigated. HF, high fat; HFF, high fat fed; i.p., intraperitoneal; NIH, National Institutes of Health; PYY, peptide tyrosine tyrosine; STZ, streptozotocin
2.4. Biochemical analysis
Blood samples were obtained from the cut tip of the tail vein from conscious mice and immediately assayed for glucose using an Ascencia Contour blood glucose meter (Bayer Healthcare, Newbury, UK). All metabolic tests and other blood collections were performed at 08:00 AM, and daily injections were withheld until after blood collection. To obtain plasma, blood was collected in chilled heparin/fluoride‐coated microcentrifuge tubes (Sarstedt, Numbrecht, Germany) and centrifuged at 12 000 rpm for 15 minutes using a Beckman microcentrifuge (Beckman Instruments, Galway, Ireland). Plasma and pancreatic insulin content were determined by an in‐house insulin radioimmunoassay, 27 while plasma and pancreatic glucagon content were assessed by a commercially available ELISA kit (EZGLU‐30 K, Merck Millipore, Burlington, MA), respectively.
2.5. Immunohistochemistry
To evaluate pancreatic islet morphology, immunohistochemistry was conducted. Fixed tissues were processed and embedded in paraffin wax blocks using an automated tissue processor (Leica TP1020, Leica Microsystems) and 5‐μm sections were cut with a microtome (Shandon Finesse 325, Thermo Scientific). Slides were dewaxed by immersion in xylene then rehydrated through a series of ethanol solutions, in which the concentration was reduced from 100% to 50% in 10% intervals. Heat‐mediated antigen retrieval was then carried out in a citrate buffer (10 mM sodium citrate, 0.05% Tween 20, pH 6.0). Sections were blocked in 4% bovine serum albumin (BSA) solution before 4°C overnight incubation with appropriate primary insulin (1:400; ab6995, AbCam) or glucagon (1:1000; Ab92517, AbCam) antibodies. To assess beta‐cell proliferation and apoptosis, co‐staining for insulin with Ki‐67 (1:400; Ab1550, AbCam) or terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) stain (In situ cell death kit, Fluorescein; Roche Diagnostics) was carried out. Where applicable, slides were then rinsed in phosphate‐buffered saline (PBS) and incubated for 45 minutes at 37°C with appropriate Alexa Fluor secondary antibodies (1:400; Alexa Fluor 498 or 594, Invitrogen). Slides were finally incubated with 4',6‐diamidino‐2‐phenylindole (DAPI) for 15 minutes at 37°C, and then mounted for imaging using a fluorescent microscope (Olympus model BX51) fitted with DAPI (350 nm), fluorescein isothiocyanate (FITC) (488 nm) and tetramethylrhodamine (TRITC) (594 nm) filters and a DP70 camera adapter system. Areas of insulin and glucagon positive staining were quantified via a ‘closed polygon’ tool using ImageJ software. The number of insulin‐positive cells co‐expressing Ki‐67 or TUNEL, respectively, was quantified on ImageJ software.
2.6. Statistical analysis
Statistical tests were conducted using GraphPad PRISM software (version 8.0). All values are expressed as mean ± SEM, with comparative analyses conducted using a one‐way ANOVA with Bonferroni's post hoc test, a two‐way ANOVA with Bonferroni's post hoc test as appropriate. Differences were considered significant when P was less than .05.
3. RESULTS
3.1. Effects of sequential treatment with SL‐PYY and liraglutide on body weight, energy intake, plasma glucose, insulin and glucagon
Both twice‐daily liraglutide, as well as SL‐PYY treatment at 08:00 AM and liraglutide at 08:00 PM, resulted in a significant reduction (P < .05) of percentage body weight by day 28 (Figure 2A). However, the combined treatment strategy evoked significant weight loss at day 10, which was only evident in liraglutide‐treated mice on day 28 (Figure 2A). Energy intake was reduced (P < .05‐.01) by combined treatment from day 21 onwards compared with HFF‐STZ control mice, but this appetite‐suppressive effect was apparent in liraglutide‐treated mice from day 10 (Figure 2B). At the end of the treatment period, circulating blood glucose had decreased from hyperglycaemic levels to values not significantly different from lean controls in both treatment groups (Figure 2C). In addition, plasma insulin concentrations were elevated (P < .05) and glucagon levels decreased (P < .05) in these treatment groups compared with HFF‐STZ control mice on day 28 (Figure 2D,E). Moreover, the plasma insulin/glucagon ratio was enhanced (P < .05) in the combined treatment group compared with liraglutide alone (Figure 2F).
FIGURE 2.
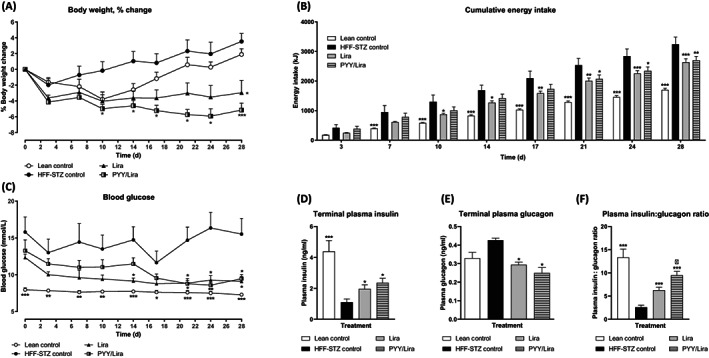
Effects of sequential treatment with (d‐Arg35)‐sea lamprey (SL)‐PYY and liraglutide on body weight, energy intake, plasma glucose, insulin and glucagon in HFF‐STZ mice. Shown are the effects of 28 days of twice‐daily (08:00 AM and 08:00 PM) treatment with liraglutide alone (0.25 nmol/kg bw) or SL‐PYY (75 nmol/kg bw) at 08:00 AM and liraglutide (0.25 nmol/kg bw) at 08:00 PM on (A) Body weight percentage change, (B) Cumulative energy intake, (C) Blood glucose, (D) Terminal plasma insulin, (E) Terminal plasma glucagon and (F) Plasma insulin:glucagon ratio in HFF‐STZ mice. (A–C) Variables were measured at regular intervals during the 28‐day treatment period. (D–F) Variables were assessed on day 28. Values are mean ± SEM for n = 8 mice. *P < .05, **P < .01 and ***P < .001 compared with saline‐treated HFF‐STZ control mice. Δ P < .05 compared with twice‐daily liraglutide‐treated HFF‐STZ mice. HFF, high fat fed; PYY, peptide tyrosine tyrosine; STZ, streptozotocin
3.2. Effects of sequential treatment with SL‐PYY and liraglutide on glucose tolerance, insulin sensitivity and pancreatic hormone content
Mice were challenged with an 18 mmol/kg i.p. glucose load on day 28, with glucose and insulin responses evaluated (Figure 3A–D). While both treatment regimens appeared to have a limited impact on glucose disposal in HFF‐STZ mice, it was only mice treated with SL‐PYY at 08:00 AM and liraglutide at 08:00 PM that displayed decreased (P < .01) glucose levels at 120 minutes postinjection (Figure 3A). However, 0‐120 minute area under the curve (AUC) glucose values were not different between all groups of HFF‐STZ mice and remained significantly (P < .001) elevated compared with normal control mice (Figure 3B). Corresponding glucose‐induced insulin concentrations were elevated (P < .01‐.001) in both treatment groups (Figure 3C), but only the combined therapy group had elevated (P < .05) 0‐120 minute overall insulin concentrations compared with HFF‐STZ control mice (Figure 3D). The hypoglycaemic response to exogenous insulin injection appeared to be similar in all HFF‐STZ mice (Figure 4A). However, when viewed in terms of the overall 0‐90 minute effect, the group of HFF‐STZ mice receiving SL‐PYY at 08:00 AM and liraglutide at 08:00 PM had marginally improved (P < .05) peripheral insulin sensitivity compared with saline‐treated HFF‐STZ controls (Figure 4B). In keeping with other improvements in metabolic status, this combined treatment group also presented with increased (P < .05) pancreatic insulin content and decreased (P < .05) glucagon content compared with twice‐daily liraglutide‐only treatment (Figure 4C,D).
FIGURE 3.
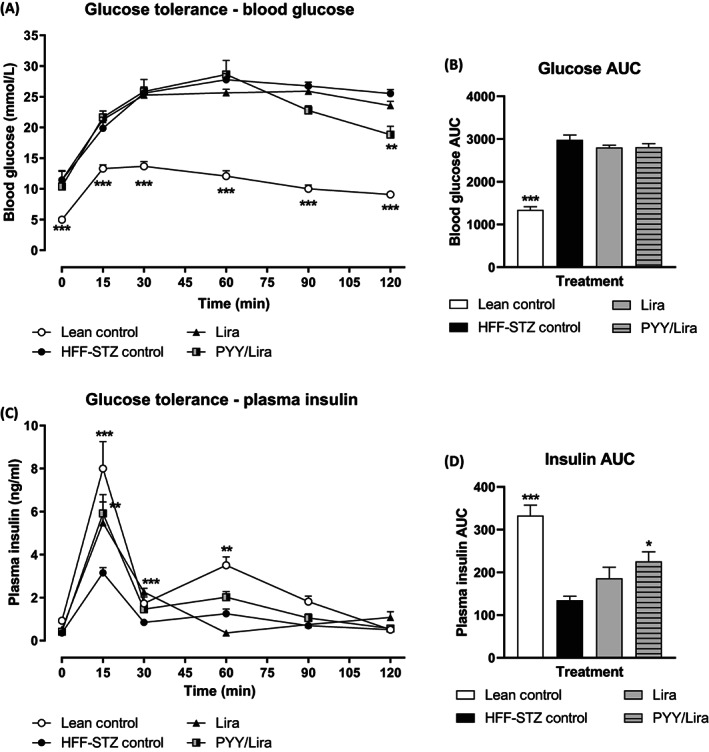
Effects of sequential treatment with (d‐Arg35)‐sea lamprey (SL)‐PYY and liraglutide on glucose tolerance in HFF‐STZ mice. (A–D) Variables were assessed after 28 days of twice‐daily (08:00 AM and 08:00 PM) treatment with liraglutide alone (0.25 nmol/kg bw) or SL‐PYY (75 nmol/kg bw) at 08:00 AM and liraglutide (0.25 nmol/kg bw) at 08:00 PM. (A) Blood glucose and (C) Plasma insulin were assessed prior to and after administration of glucose (18 mmol/kg bw, i.p.) at t = 0 min. Associated 0‐120 minute area under the curve (AUC) values for (B) Glucose and (D) Insulin are also shown. Values are mean ± SEM for n = 8 mice. *P < .05, **P < .01 and ***P < .001 compared with saline‐treated HFF‐STZ control mice. HFF, high fat fed; i.p., intraperitoneal; PYY, peptide tyrosine tyrosine; STZ, streptozotocin
FIGURE 4.
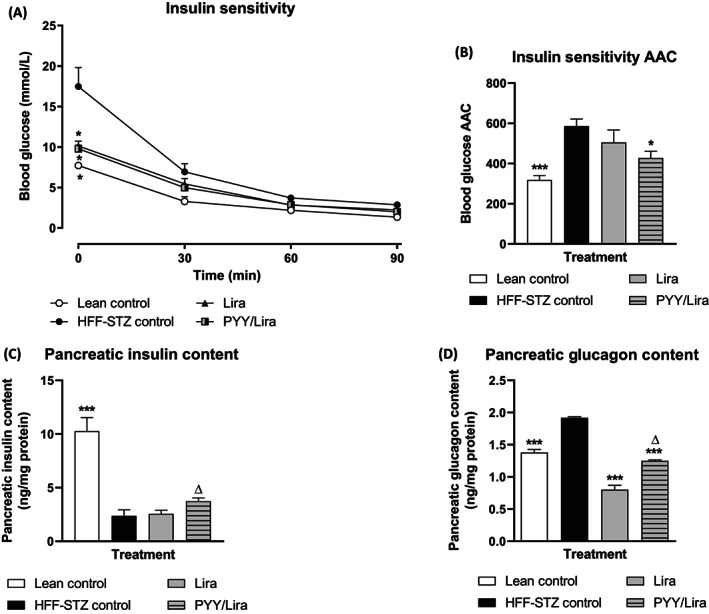
Effects of sequential treatment with (d‐Arg35)‐sea lamprey (SL)‐PYY and liraglutide on peripheral insulin sensitivity and pancreatic hormone content in HFF‐STZ mice. (A–D) Variables were assessed after 28 days of twice‐daily (08:00 AM and 08:00 PM) treatment with liraglutide alone (0.25 nmol/kg bw) or SL‐PYY (75 nmol/kg bw) at 08:00 AM and liraglutide (0.25 nmol/kg bw) at 08:00 PM. (A) Blood glucose was assessed prior to and after administration of insulin (15 U/kg bw, i.p.) at t = 0 min. (B) Associated 0‐90 minute glucose area above the curve (AAC) values are also shown. (C) and (D) Pancreatic hormone contents were assessed on day 28 following acid/ethanol protein extraction. Values are mean ± SEM for n = 8 mice. *P < .05 and ***P < .001 compared with saline‐treated HFF‐STZ control mice. Δ P < .05 compared with twice‐daily liraglutide‐treated HFF‐STZ mice. HFF, high fat fed; i.p., intraperitoneal; PYY, peptide tyrosine tyrosine; STZ, streptozotocin
3.3. Effects of sequential treatment with SL‐PYY and liraglutide on pancreatic islet morphology
As expected, HFF‐STZ mice displayed adverse changes in pancreatic islet morphology, presenting with reduced (P < .001) islet‐ and beta‐cell areas, and concomitant expansion of alpha‐cell area (Figure 5A–C). Both treatments evoked an improvement (P < .05‐.001) in overall islet and beta areas (Figure 5A,B). However, only the SL‐PYY plus liraglutide treatment reduced (P < .05) alpha‐cell area compared with HFF‐STZ controls (Figure 5C). In addition, this same treatment intervention decreased the number of centrally located alpha cells compared with both saline (P < .05) and liraglutide only (P < .01) treated mice (Figure 5D). Representative images of islets stained for insulin (red) and glucagon (green) for each treatment group are presented in Figure 5E.
FIGURE 5.
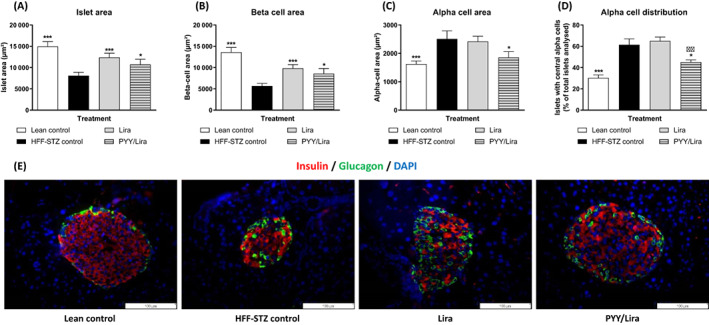
Effects of sequential treatment with (d‐Arg35)‐sea lamprey (SL)‐PYY and liraglutide on pancreatic islet morphology in HFF‐STZ mice. Pancreatic (A) Islet‐, (B) Beta‐, (C) Alpha‐cell areas and (D) Percentage centrally glucagon positive staining cells were assessed after 28 days of twice‐daily (08:00 AM and 08:00 PM) treatment with liraglutide alone (0.25 nmol/kg bw) or SL‐PYY (75 nmol/kg bw) at 08:00 AM and liraglutide (0.25 nmol/kg bw) at 08:00 PM. (E) Representative islet images showing insulin (red), glucagon (green) and DAPI (blue) from each treatment group. Values are mean ± SEM for n = 8 mice. *P < .05 and ***P < .001 compared with saline‐treated HFF‐STZ control mice. ΔΔ P < .01 compared with twice‐daily liraglutide‐treated HFF‐STZ mice. DAPI, 4',6‐diamidino‐2‐phenylindole; HFF, high fat fed; PYY, peptide tyrosine; STZ, streptozotocin
3.4. Effects of sequential treatment with SL‐PYY and liraglutide on pancreatic beta‐cell proliferation and apoptosis
HFF‐STZ mice exhibited elevated levels of beta‐cell proliferation (P < .05) and apoptosis (P < .01) compared with normal mice (Figure 6A,B). In this regard, beta‐cell proliferation was outweighed by apoptosis, giving rise to a significantly decreased (P < .05) beta‐cell proliferation: apoptosis ratio (Figure 6C). Both treatment interventions augmented (P < .05) beta‐cell proliferation and decreased (P < .05) apoptotic rates compared with HFF‐STZ controls, resulting in normalized beta‐cell proliferation: apoptosis ratios (Figure 6A–C). Representative images of islets stained for insulin (red) together with Ki‐67 (green) or TUNEL (green) for each treatment group are presented in Figure 6D,E, respectively.
FIGURE 6.
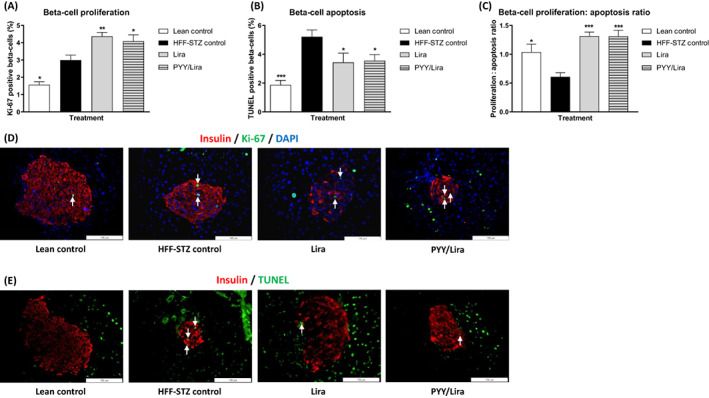
Effects of sequential treatment with (d‐Arg35)‐sea lamprey (SL)‐PYY and liraglutide on beta‐cell proliferation and apoptosis in HFF‐STZ mice. Utilising ImageJ software, beta‐cell (A) Proliferation, (B) Apoptosis and (C) Proliferation: apoptosis ratios were assessed by Ki‐67 and TUNEL staining, respectively, after 28 days of twice‐daily (08:00 AM and 08:00 PM) treatment with liraglutide alone (0.25 nmol/kg bw) or SL‐PYY (75 nmol/kg bw) at 08:00 AM and liraglutide (0.25 nmol/kg bw) at 08:00 PM. Representative islet images showing (D) Insulin (red), Ki‐67 (green) or (E) Insulin (red) and TUNEL (green), with (D) and (E) DAPI nuclear staining (blue), from each treatment group. Values are mean ± SEM for n = 8 mice. *P < .05 and **P < .01, ***P < .001 compared with saline‐treated HFF‐STZ control mice. DAPI, 4',6‐diamidino‐2‐phenylindole; HFF, high fat fed; PYY, peptide tyrosine tyrosine; STZ, streptozotocin; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labelling
4. DISCUSSION
The understanding that scheduled periods of pancreatic beta‐cell rest can lead to benefits on overall metabolic control is not new, 7 but has yet to be established in the clinical setting. For example, administration of diazoxide (5 mg/kg/day) to T2D subjects for 7 days was shown to significantly enhance beta‐cell responsiveness. 28 Improvements in insulin secretory function were shown following overnight somatostatin‐induced beta‐cell rest in T2D patients. 29 Moreover, it has been proposed that a major benefit of thiazolidinedione treatment in human T2D relates to resting the overworked beta cell. 30 Indeed, it is widely accepted that prolonged use of non‐specific beta‐cell stimulatory drugs, such as sulphonylureas, leads to reductions in beta‐cell function and mass over time. 31 In terms of preclinical investigations, selectively opening beta‐cell K+ channels for 21 days in Vancouver diabetic fatty Zucker rats, to reduce workload, has been shown to improve glucose tolerance and insulin secretory responsiveness, leading to reduced basal hyperglycaemia. 32 For SL‐PYY, rather than directly affecting the excitability of the beta cell, rest would be induced by activating Giα signalling, which lowers cAMP and the activity of its downstream targets, PKA and Epac2. 25 This difference is interesting and suggests that the effects of SL‐PYY on beta‐cell rest may be comparable with adrenergic inhibition, which also operates through modulation of GPCR‐linked pathways, 33 although further mechanistic studies would be required to confirm this. Furthermore, SL‐PYY exerts additional important beta‐cell benefits to enhance proliferation and decrease apoptotic rates 19 that would not be associated with adrenergic inhibition.
Given that approved GLP‐1 mimetics, like liraglutide, stimulate insulin secretion only in a glucose‐dependent manner 34 and have reported benefits on beta‐cell turnover, 14 , 17 it was envisaged that these drugs could provide enduring protection of beta cells in T2D, especially considering the noteworthy antidiabetic benefits reported from preclinical animal studies. 35 However, the clinical usefulness of liraglutide and other GLP‐1R agonists for increasing beta‐cell mass, via effects on proliferation or apoptosis, was inferior to the expectation from data in animal models. 36 , 37 Thus agents that can complement the antidiabetic actions of GLP‐1 at the level of the beta cell are highly sought after, 38 with preclinical evidence that optimally scheduled periods of beta‐cell rest have credibility in this regard. 11
In accordance with established beneficial effects of sustained GLP‐1R activation, 11 HFF‐STZ mice treated twice‐daily with liraglutide exhibited reduced body weight. Interestingly, despite the recognized orexigenic effect of NPY1R activation and reported satiety benefits of antagonists at this receptor, 39 , 40 co‐administration of SL‐PYY with liraglutide enhanced body weight‐reducing benefits without any obvious impact on energy intake. Thus a legacy effect of liraglutide together with the specific timing of SL‐PYY injection may be important, with NPY1R being activated during the ‘lights on’ period of the day when mice are less active and eat less. 41 This initial finding aligns well with our notion of circadian control of metabolism and the benefits of beta‐cell rest during the inactive periods of the day. 6 Notably, however, circulating glucose levels were restored to near normal levels by both liraglutide treatment alone and in combination with SL‐PYY, although this is probably driven by the pronounced glucose‐lowering efficacy of liraglutide at the dose employed. 11 However, we cannot discount the idea that repeated injection of liraglutide at 08:00 PM may have affected beta‐cell activity during the inactive daytime period. In that respect, although beyond our capacity, an additional group of mice treated with saline vehicle at 08:00 AM and 0.25 nmol/kg liraglutide at 08:00 PM may have been beneficial to help elucidate the overall impact of liraglutide treatment at 08:00 PM in our model system.
Given that NPY1R agonism induces insulinostatic effects to impart beta‐cell rest, 18 the employed dosing regimen aimed to limit the bioactivity profile of SL‐PYY to periods of low metabolic demand. Indeed, circulating insulin concentrations were not different in HFF‐STZ mice treated twice‐daily with liraglutide compared with consecutive treatment with SL‐PYY and liraglutide every 12 hours. In line with the philosophy of beta‐cell rest, observed benefits may be even more pronounced in rodent models that display overt hyperinsulinaemia, such as db/db mice. 42 , 43 Thus the current experiments were conducted in an insulinopenic model that is highly instructive in its own right. Moreover, sequential combined therapy, unlike liraglutide treatment alone, was able to positively alter the balance of both circulating and pancreatic insulin and glucagon concentrations. This is probably partly a result of SL‐PYY–provoked periods of diminished beta‐cell activity, allowing for replenishment of intracellular insulin stores and alleviation of oxidative and endoplasmic reticulum (ER) stress. 8 These advantageous effects were also associated with mild glucose‐lowering benefits in response to a glucose challenge compared with liraglutide treatment alone, alongside equivalent improvements of insulin secretion and action. Enhancement of peripheral insulin action could be partially attributed to weight loss, 44 but in this regard, it should be acknowledged that HFF‐STZ mice treated with only liraglutide also presented with a significant reduction in body weight by day 28. That said, although probable given these observations, we are unable to provide evidence for a direct effect of SL‐PYY–mediated beta‐cell rest on improved peripheral insulin action. Moreover, diazoxide‐induced beta‐cell rest has been shown to directly improve insulin action, mediated by decreased activity of enzymes involved in regulating hepatic gluconeogenesis. 45
Sustained sequential periods of beta‐cell rest, together with the positive actions of consecutive intervals of GLP‐1R activation on beta‐cell growth and survival, 2 , 46 would be expected to impart benefits on overall pancreatic islet architecture. Certainly, this would also fit well with observed improvements in the balance of pancreatic hormone content in HFF‐STZ mice treated with the combination of SL‐PYY and liraglutide. Although clear improvements in islet‐, beta‐ and alpha‐cell areas were noted with combined therapy, liraglutide treatment alone also induced similar positive effects, suggesting that such actions are largely driven by GLP‐1R modulation in the current study. 17 However, the characteristic expansion of alpha‐cell area and central internalization of glucagon positively stained islet cells induced by STZ 47 were reversed only in the combined treatment group. Similar observations have been made previously with subchronic NPY1R activation, 20 suggesting restoration of the local islet environment, rather than augmentation of beta‐cell mass alone, is a key benefit of NPY1R activation by SL‐PYY. Indeed, positive effects on beta‐cell turnover were not different between treatment groups, and the observed effects on beta‐cell proliferation and protection against apoptosis agree with earlier studies on the effects of these peptides on beta‐cell health. 19 , 48 It is also interesting that reductions of alpha‐cell area were observed in concert with elevated pancreatic glucagon content in the combined group. It follows that treatment‐induced changes in pancreatic islet histology, especially with consecutive SL‐PYY and liraglutide treatment, could also be partly linked to the recently described positive impact of GLP‐1R and NPY1R activation on transdifferentiation of alpha to beta cells, 17 , 20 but further detailed investigation would be required to confirm this suggestion. Certainly, similar changes in beta‐cell proliferative and apoptotic rates with both treatment regimens would allude to a conceivable beneficial impact on islet cell lineage, although other factors such as alpha‐cell turnover or islet cell neogenesis could also be involved and merit consideration.
The philosophy of beta‐cell rest may, at first, seem counterintuitive for a disease state where insulin secretion is already inherently diminished. 46 However, if the scheduling of such periods can be appropriately reserved for times when metabolic demand is low, there are obvious advantages. 6 , 11 In agreement, short‐term intensive insulin therapy in people with newly diagnosed T2D has been shown to improve overall beta‐cell function compared with other standard oral antidiabetic agents, with beta‐cell rest considered the key driver. 49 In the current setting, further detailed studies are required to determine the out‐and‐out optimum dose ratio and timings where beneficial NPY1R‐mediated actions on beta‐cell health and survival are maximized, 19 while curtailing the associated insulinostatic actions which would otherwise diminish the recognized glucose‐dependent insulinotropic actions of GLP‐1R activation in humans. 50 In addition, similar to GLP‐1, 2 NPY1R is also expressed on tissues outside of the endocrine pancreas including the central nervous system, adipose tissue and the gut, with the impact of modulation of NPY1R function by SL‐PYY in these locations still to be fully examined. However, our investigations with SL‐PYY and liraglutide in HFF‐STZ mice provide proof‐of‐concept that appropriate combined, but non‐simultaneous, administration of NPY1R and GLP‐1R agonists possesses therapeutic efficacy that merits further consideration for the treatment of T2D. While the timings of SL‐PYY and liraglutide injections may be different in the (diurnal) human setting, the same principles regarding the benefits of appropriately staggered periods of beta‐cell rest and stimulation should still apply.
AUTHOR CONTRIBUTIONS
NI and PRF conceived/designed the study. NI, NT and PRF drafted the manuscript. NT and RAL participated in the conduct/data collection and analysis and interpretation of data. All the authors revised the manuscript critically for intellectual content and approved the final version of the manuscript.
CONFLICT OF INTEREST
PRF and NI are named on patents filed by Ulster University for exploitation of peptide therapeutics. All other authors declare that no conflicting interests exist.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14821.
Supporting information
Supplementary Figure 1 Investigation of most appropriate dose of (d‐Arg35)‐sea lamprey PYY (1–36) to create a pharmacokinetic profile greater than 6 h, but less than 12 h, in mice
ACKNOWLEDGEMENTS
These studies were supported by Invest Northern Ireland Proof of Concept funding and Ulster University Selective Research Funding.
Tanday N, Lafferty RA, Flatt PR, Irwin N. Beneficial metabolic effects of recurrent periods of beta‐cell rest and stimulation using stable neuropeptide Y1 and glucagon‐like peptide‐1 receptor agonists. Diabetes Obes Metab. 2022;24(12):2353‐2363. doi: 10.1111/dom.14821
Funding informationThese studies were supported by Invest Northern Ireland Proof of Concept funding and Ulster University Selective Research Funding.
DATA AVAILABILITY STATEMENT
The authors declare that the data supporting the findings of this study are available within the article. Any additional raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
REFERENCES
- 1. Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al KJ. Epidemiology of type 2 diabetes–global burden of disease and forecasted trends. J Epidemiol Glob Health. 2020;10:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tanday N, Flatt PR, Irwin N. Metabolic responses and benefits of glucagon‐like peptide‐1 (GLP‐1) receptor ligands. Br J Pharmacol. 2022;179:526‐541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bettge K, Kahle M, Abd El Aziz MS, Meier JJ, Nauck MA. Occurrence of nausea, vomiting and diarrhoea reported as adverse events in clinical trials studying glucagon‐like peptide‐1 receptor agonists: a systematic analysis of published clinical trials. Diabetes Obes Metab. 2017;19:336‐347. [DOI] [PubMed] [Google Scholar]
- 4. Fu J, Cui Q, Yang B, et al. The impairment of glucose‐stimulated insulin secretion in pancreatic β‐cells caused by prolonged glucotoxicity and lipotoxicity is associated with elevated adaptive antioxidant response. Food Chem Toxicol. 2017;100:161‐167. [DOI] [PubMed] [Google Scholar]
- 5. Albrecht U, Oster H. The circadian clock and behavior. Behav Brain Res. 2001;125:89‐91. [DOI] [PubMed] [Google Scholar]
- 6. Seshadri N, Doucette CA. Circadian regulation of the pancreatic beta cell. Endocrinology. 2021;162:bqab089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brown RJ, Rother KI. Effects of beta‐cell rest on beta‐cell function: a review of clinical and preclinical data. Pediatr Diabetes. 2008;9:14‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ritzel RA, Hansen JB, Veldhuis JD, Butler PC. Induction of β‐cell rest by a Kir6. 2/SUR1‐selective KATP‐channel opener preserves β‐cell insulin stores and insulin secretion in human islets cultured at high (11 mM) glucose. J Clin Endocrinol Metab. 2004;89:795‐805. [DOI] [PubMed] [Google Scholar]
- 9. Rizzo MA, Magnuson MA, Drain PF, Piston DW. A functional link between glucokinase binding to insulin granules and conformational alterations in response to glucose and insulin. J Biol Chem. 2002;277:34168‐34175. [DOI] [PubMed] [Google Scholar]
- 10. Bravi MC, Armiento A, Laurenti O, et al. Insulin decreases intracellular oxidative stress in patients with type 2 diabetes mellitus. Metabolism. 2006;55:691‐695. [DOI] [PubMed] [Google Scholar]
- 11. Pathak V, Vasu S, Gault VA, Flatt PR, Irwin N. Sequential induction of beta cell rest and stimulation using stable GIP inhibitor and GLP‐1 mimetic peptides improves metabolic control in C57BL/KsJ db/db mice. Diabetologia. 2015;58:2144‐2153. [DOI] [PubMed] [Google Scholar]
- 12. Faivre E, Hamilton A, Hölscher C. Effects of acute and chronic administration of GIP analogues on cognition, synaptic plasticity and neurogenesis in mice. Eur J Pharmacol. 2012;674:294‐306. [DOI] [PubMed] [Google Scholar]
- 13. Vyavahare SS, Mieczkowska A, Flatt PR, Chappard D, Irwin N, Mabilleau G. GIP analogues augment bone strength by modulating bone composition in diet‐induced obesity in mice. Peptides. 2020;125:e170207. [DOI] [PubMed] [Google Scholar]
- 14. Moffett RC, Vasu S, Flatt PR. Functional GIP receptors play a major role in islet compensatory response to high fat feeding in mice. Biochim Biophys Acta Gen Subj. 2015;1850:1206‐1214. [DOI] [PubMed] [Google Scholar]
- 15. Trümper A, Trümper K, Hörsch D. Mechanisms of mitogenic and anti‐apoptotic signaling by glucose‐dependent insulinotropic polypeptide in beta(INS‐1)‐cells. J Endocrinol. 2002;174:233‐246. [DOI] [PubMed] [Google Scholar]
- 16. Sarnobat D, Moffett RC, Gault VA, et al. Effects of long‐acting GIP, xenin and oxyntomodulin peptide analogues on alpha‐cell transdifferentiation in insulin‐deficient diabetic GluCreERT2; ROSA26‐eYFP mice. Peptides. 2020;125:170205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tanday N, Flatt PR, Irwin N, Moffett RC. Liraglutide and sitagliptin counter beta‐to alpha‐cell transdifferentiation in diabetes. J Endocrinol. 2020;245:53‐64. [DOI] [PubMed] [Google Scholar]
- 18. Khan D, Vasu S, Moffett RC, Irwin N, Flatt PR. Islet distribution of peptide YY and its regulatory role in primary mouse islets and immortalised rodent and human beta‐cell function and survival. Mol Cell Endocrinol. 2016;436:102‐113. [DOI] [PubMed] [Google Scholar]
- 19. Lafferty RA, Tanday N, McCloskey A, et al. Peptide YY (1–36) peptides from phylogenetically ancient fish targeting mammalian neuropeptide Y1 receptors demonstrate potent effects on pancreatic β‐cell function, growth and survival. Diabetes Obes Metab. 2020;22:404‐416. [DOI] [PubMed] [Google Scholar]
- 20. Lafferty RA, Tanday N, Moffett RC, et al. Positive effects of NPY1 receptor activation on islet structure are driven by pancreatic alpha‐and beta‐cell transdifferentiation in diabetic mice. Front Endocrinol. 2021;12:633625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lafferty RA, Gault VA, Flatt PR, Irwin N. Effects of 2 novel PYY (1‐36) analogues, (P3L31P34) PYY (1‐36) and PYY (1‐36)(Lys12PAL), on pancreatic beta‐cell function, growth, and survival. Clin Med Insights Endocrinol Diabetes. 2019;12:1179551419855626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lafferty RA, Flatt PR, Irwin N. C‐terminal degradation of PYY peptides in plasma abolishes effects on satiety and beta‐cell function. Biochem Pharmacol. 2018;158:95‐102. [DOI] [PubMed] [Google Scholar]
- 23. Toräng S, Veedfald S, Rosenkilde MM, Hartmann B, Holst JJ. The anorexic hormone peptide YY 3–36 is rapidly metabolized to inactive peptide YY 3–34 in vivo. Physiol Rep. 2015;3:e12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lafferty RA, Tanday N, Flatt PR, Irwin N. Development and characterisation of a peptidergic N‐and C‐terminally stabilised mammalian NPY1R agonist which protects against diabetes induction. Biochim Biophys Acta Gen Subj. 2020;1864:129543. [DOI] [PubMed] [Google Scholar]
- 25. Lafferty RA, Flatt PR, Irwin N. Established and emerging roles peptide YY (PYY) and exploitation in obesity‐diabetes. Curr Opin Endocrinol Diabetes Obes. 2021;28:253‐261. [DOI] [PubMed] [Google Scholar]
- 26. Craig SL, Gault VA, Flatt PR, Irwin N. The methionine aminopeptidase 2 inhibitor, TNP‐470, enhances the antidiabetic properties of sitagliptin in mice by upregulating xenin. Biochem Pharmacol. 2021;183:114355. [DOI] [PubMed] [Google Scholar]
- 27. Flatt PR, Bailey CJ. Abnormal plasma glucose and insulin responses in heterozygous lean (ob/+) mice. Diabetologia. 1981;20:573‐577. [DOI] [PubMed] [Google Scholar]
- 28. Greenwood RH, Mahler RF, Hales CN. Improvement in insulin secretion in diabetes after diazoxide. Lancet. 1976;1:444‐447. [DOI] [PubMed] [Google Scholar]
- 29. Laedtke T, Kjems L, Pørksen N, Schmitz O, Veldhuis J, Butler PC. An overnight somatostatin‐imposed inhibition of beta cell secretion restores orderliness and pulsatility of insulin secretion and the proinsulin to insulin ratio in patients with type 2 diabetes. Am J Physiol Endocrinol Metab. 2000;279:E520‐E528. [DOI] [PubMed] [Google Scholar]
- 30. Leahy JL. Thiazolidinediones in prediabetes and early type 2 diabetes: what can be learned about that disease's pathogenesis. Curr Diab Rep. 2009;9:215‐220. [DOI] [PubMed] [Google Scholar]
- 31. Khan D, Moffett RC, Flatt PR, Tarasov AI. Classical and non‐classical islet peptides in the control of β‐cell function. Peptides. 2021;150:e170715. [DOI] [PubMed] [Google Scholar]
- 32. Carr RD, Brand CL, Bodvarsdottir TB, Hansen JB, Sturis J. NN414, a SUR1/Kir6.2‐selective potassium channel opener, reduces blood glucose and improves glucose tolerance in the VDF Zucker rat. Diabetes. 2003;52:2513‐2518. [DOI] [PubMed] [Google Scholar]
- 33. Malaisse W, Malaisse‐Lagae F, Wright PH, Ashmore J. Effects of adrenergic and cholinergic agents upon insulin secretion in vitro. Endocrinology. 1967;80:975‐978. [DOI] [PubMed] [Google Scholar]
- 34. Ribel U, Larsen MO, Rolin B, et al. NN2211: a long‐acting glucagon‐like peptide‐1 derivative with anti‐diabetic effects in glucose‐intolerant pigs. Eur J Pharmacol. 2002;451:217‐225. [DOI] [PubMed] [Google Scholar]
- 35. Knudsen LB. Liraglutide: the therapeutic promise from animal models. Int J Clin Pract. 2010;64:4‐11. [DOI] [PubMed] [Google Scholar]
- 36. Chon S, Gautier JF. An update on the effect of incretin‐based therapies on β‐cell function and mass. Diabetes Metab J. 2016;40:99‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Drucker DJ. Incretin action in the pancreas: potential promise, possible perils, and pathological pitfalls. Diabetes. 2013;62:3316‐3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tanday N, Flatt PR, Irwin N. Amplifying the antidiabetic actions of glucagon‐like peptide‐1: potential benefits of new adjunct therapies. Diabet Med. 2021;38:e14699. [DOI] [PubMed] [Google Scholar]
- 39. Stanley BG, Magdalin W, Seirafi A, Nguyen MM, Leibowitz SF. Evidence for neuropeptide Y mediation of eating produced by food deprivation and for a variant of the Y1 receptor mediating this peptide's effect. Peptides. 1992;13:581‐587. [DOI] [PubMed] [Google Scholar]
- 40. Yan C, Zeng T, Lee K, et al. Peripheral‐specific Y1 receptor antagonism increases thermogenesis and protects against diet‐induced obesity. Nat Commun. 2021;12:e2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yoshioka H, Ohishi R, Hirose Y, et al. Chronopharmacology of dapagliflozin‐induced antihyperglycemic effects in C57BL/6J mice. Obes Res Clin Pract. 2019;13:505‐510. [DOI] [PubMed] [Google Scholar]
- 42. Craig SL, Perry RA, Vyavahare SS, et al. A GIP/xenin hybrid in combination with exendin‐4 improves metabolic status in db/db diabetic mice and promotes enduring antidiabetic benefits in high fat fed mice. Biochem Pharmacol. 2020;171:113723. [DOI] [PubMed] [Google Scholar]
- 43. Yang Q, Zhou F, Tang X, et al. Peptide‐based long‐acting co‐agonists of GLP‐1 and cholecystokinin 1 receptors as novel anti‐diabesity agents. Eur J Med Chem. 2022;233:114214. [DOI] [PubMed] [Google Scholar]
- 44. Goodpaster BH, Kelley DE, Wing RR, Meier A, Thaete FL. Effects of weight loss on regional fat distribution and insulin sensitivity in obesity. Diabetes. 1999;48:839‐847. [DOI] [PubMed] [Google Scholar]
- 45. Alemzadeh R, Holshouser S, Massey P, Koontz J. Chronic suppression of insulin by diazoxide alters the activities of key enzymes regulating hepatic gluconeogenesis in Zucker rats. Eur J Endocrinol. 2002;146:871‐879. [DOI] [PubMed] [Google Scholar]
- 46. van Raalte DH, Verchere CB. Improving glycaemic control in type 2 diabetes: stimulate insulin secretion or provide beta‐cell rest? Diabetes Obes Metab. 2017;19:1205‐1213. [DOI] [PubMed] [Google Scholar]
- 47. Li Z, Karlsson FA, Sandler S. Islet loss and alpha cell expansion in type 1 diabetes induced by multiple low‐dose streptozotocin administration in mice. J Endocrinol. 2000;165:93‐100. [DOI] [PubMed] [Google Scholar]
- 48. Arakawa M, Ebato C, Mita T, et al. Effects of exendin‐4 on glucose tolerance, insulin secretion, and beta‐cell proliferation depend on treatment dose, treatment duration and meal contents. Biochem Biophys Res Commun. 2009;390:809‐814. [DOI] [PubMed] [Google Scholar]
- 49. Chen HS, Wu TE, Jap TS, Hsiao LC, Lee SH, Lin HD. Beneficial effects of insulin on glycemic control and β‐cell function in newly diagnosed type 2 diabetes with severe hyperglycemia after short‐term intensive insulin therapy. Diabetes Care. 2008;31:1927‐1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ritzel R, Ørskov C, Holst JJ, Nauck MA. Pharmacokinetic, insulinotropic, and glucagonostatic properties of GLP‐1 [7–36 amide] after subcutaneous injection in healthy volunteers. Dose‐response‐relationships. Diabetologia. 1995;38:720‐725. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 Investigation of most appropriate dose of (d‐Arg35)‐sea lamprey PYY (1–36) to create a pharmacokinetic profile greater than 6 h, but less than 12 h, in mice
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the article. Any additional raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.


