Abstract
Introduction
The presence of high‐risk chromosomal abnormalities [t(4;14), del(17p), and t(14;16)] has been linked with inferior outcomes in patients with multiple myeloma (MM). A prespecified interim analysis of the Phase 3 IKEMA study (NCT03275285) demonstrated that isatuximab (Isa) + carfilzomib (K) and dexamethasone (d; Isa‐Kd) significantly improved progression‐free survival (PFS) versus Kd in patients with relapsed MM. This prespecified subgroup analysis of IKEMA examined efficacy and safety in patients with high‐risk cytogenetics.
Methods
High‐risk cytogenetics was assessed by central laboratory and patients were classified as high risk if abnormalities were present in ≥1 of the following: del(17p): 50% cutoff; t(4;14), and/or t(14;16): 30% cutoff.
Results
Of the randomized patients, 23.5% (Isa‐Kd) and 25.2% (Kd) had ≥1 high‐risk chromosomal abnormality. A PFS benefit was seen in favor of Isa‐Kd for patients with standard‐risk (HR 0.440; 95% CI 0.266–0.728) and high‐risk cytogenetics (HR 0.724; 95% CI 0.361–1.451). Grade ≥3 treatment‐emergent adverse events (TEAEs) were more common with Isa‐Kd (85.7%) versus Kd (63.3%) in patients with high‐risk cytogenetics; however, the incidence of serious TEAEs (64.3% vs. 66.7%) was similar.
Conclusions
Isa‐Kd is a new treatment option for the difficult‐to‐treat subgroup of patients with relapsed MM and high‐risk cytogenetics.
Keywords: high‐risk cytogenetics, isatuximab, multiple myeloma
1. INTRODUCTION
Multiple myeloma (MM) is the second most common hematologic malignancy. 1 , 2 For patients with MM, several prognostic factors should be considered for treatment decision‐making. 3 The presence of high‐risk chromosomal abnormalities (CA; defined by the International Myeloma Working Group consensus as t(4;14), del(17p), and t(14;16)) has been well documented as a negative prognostic factor, typically leading to poorer outcomes compared with standard‐risk patients. 4 , 5
Isatuximab (Isa; Sarclisa®, Cambridge, MA, USA) is a monoclonal antibody that binds to a specific epitope of CD38 and exerts anti‐MM effects through several modes of action. 6 Based on the Phase 3 ICARIA‐MM study, Isa is approved in combination with pomalidomide (P) and dexamethasone (d) for the treatment of adult patients with relapsed/refractory MM who have received ≥2 prior therapies, including lenalidomide and a proteasome inhibitor. 7 Based on the phase III IKEMA study results, Isa in combination with carfilzomib (K) and d is approved in the United States for the treatment of adult patients with relapsed or refractory MM who have received 1–3 prior lines of therapy, in the European Union for the treatment of adult patients with relapsed MM who have received ≥1 prior therapy, and in Japan for the treatment of adult patients with relapsed or refractory MM who have received 1 prior treatment. 7 , 8
IKEMA (NCT03275285) was a randomized, open‐label, multinational, parallel‐group Phase 3 study that investigated Isa in combination with Kd (Isa‐Kd, experimental group) versus Kd (control group) in patients with relapsed MM and 1–3 prior lines of therapy. 9 , 10 , 11 A prespecified interim efficacy analysis of the IKEMA study showed that Isa‐Kd significantly improved progression‐free survival (PFS) compared with Kd in patients with relapsed MM (hazard ratio [HR] 0.531; 99% confidence interval [CI], 0.318–0.889; one‐sided p = 0.0007), with a clinically meaningful increase in minimal residual disease (MRD) negativity (29.6% vs. 13.0%), very good partial response (VGPR) or better (72.6% vs. 56.1%), and complete response ([CR], 39.7% vs. 27.6%) rates, and a manageable safety profile. 9 , 10 , 11
This prespecified subgroup analysis of IKEMA examined efficacy and safety in patients with high‐risk CA [t(4;14), del(17p), and t(14;16)], which is a component of the Revised Multiple Myeloma International Staging System (R‐ISS). 12 The analysis of gain/amplification of 1q21 is increasingly recommended for assessment of cytogenetic risk but was not part of the prespecified high‐risk CA definition in the IKEMA protocol, which used CA part of R‐ISS. However, assessment of gain/amplification of 1q21 was part of the exploratory endpoints and a detailed analysis will be published separately.
2. MATERIALS AND METHODS
2.1. Study design and patients
The IKEMA study design was previously described. 9 , 10 , 11 Patients with 1–3 prior lines of therapy were randomized 3:2 to receive Isa‐Kd (n = 179) or Kd (n = 123). The Isa‐Kd arm received Isa (10 mg/kg intravenously) weekly for 4 weeks, then every 2 weeks. Both arms received K (20 mg/m2 days 1–2; 56 mg/m2 thereafter) twice weekly for 3 of 4 weeks, and d (20 mg) twice weekly. The primary endpoint was PFS, and key secondary endpoints included overall response rate (ORR), ≥VGPR rate, MRD negativity rate, CR rate, and overall survival (OS).
2.2. Outcomes
Cytogenetic risk was assessed for all patients by central laboratory fluorescence in situ hybridization (FISH) testing after immunomagnetic isolation of CD138+ plasma cells from baseline bone marrow aspirate, interphase chromosome preparation, and hybridization with Kreatech FISH probes (11q22.3[ATM]/17p13.1[p53], 4p16.3[FGFR3]; 14q32.3[IGH]; 16q23.2[MAF]). Patients were classified in the high‐risk CA subgroup if at least 1 of the following CA (part of the R‐ISS parameters) was detected by central laboratory with the following prespecified cutoffs: del(17p): 50% cutoff; t(4;14) and/or t(14;16): 30% cutoff. 13 Adverse events (AEs) were graded per the National Cancer Institute—Common Terminology Criteria for AEs version 4.03.
2.3. Statistical analyses
Statistical procedures for the IKEMA study have been described previously. 11 Median PFS and corresponding CIs were calculated by the Kaplan–Meier method. HR estimates by subgroup were determined using a nonstratified Cox proportional hazard model with terms for the factor, treatment, and their interaction.
3. RESULTS
3.1. Patient baseline characteristics
Of the randomized patients, 23.5% (n = 42/179; Isa‐Kd) and 25.2% (n = 31/123; Kd) had ≥1 high‐risk CA; approximately 10% of patients had missing cytogenetics data (13%, Isa‐Kd; 11%, Kd). At the interim analysis, more patients remained on Isa‐Kd versus Kd among those with ≥1 high‐risk CA (45.2% [19/42] vs. 25.8% [8/31], respectively). This was also true among standard‐risk patients, where 55.3% (63/114) of patients remained on Isa‐Kd versus 31.2% (24/77) who remained on Kd.
Baseline characteristics of patients are presented in Table 1. More patients with high‐risk CA receiving Kd had an Eastern Cooperative Oncology Group performance status of 0 (42.9% Isa‐Kd vs. 64.5% Kd) and were classified as ISS Stage I (47.6% Isa‐Kd vs. 64.5% Kd), whereas there was a similar proportion of patients with ISS Stage III in the 2 arms (16.7% Isa‐Kd vs. 16.1% Kd). No high‐risk patients receiving Kd had soft‐tissue plasmacytomas compared with 10% of patients receiving Isa‐Kd. The median numbers of prior lines were 1 (Isa‐Kd) and 2 (Kd). Other baseline characteristics were well balanced.
TABLE 1.
Baseline characteristics by chromosomal abnormality [del(17p), t(4;14), and/or t(14;16)]—randomized population
| Patient characteristic | High risk | Standard risk | ||
|---|---|---|---|---|
| Isa‐Kd (n = 42) | Kd (n = 31) | Isa‐Kd (n = 114) | Kd (n = 77) | |
| Age in years, median (range) | 62.5 (37–83) | 63.0 (38–80) | 65.0 (38–86) | 64.0 (33–90) |
| <65 | 23 (54.8) | 17 (54.8) | 54 (47.4) | 41 (53.2) |
| ≥65 to <75 | 15 (35.7) | 10 (32.3) | 50 (43.9) | 30 (39.0) |
| ≥75 | 4 (9.5) | 4 (12.9) | 10 (8.8) | 6 (7.8) |
| ECOG PS, n (%) | ||||
| 0 | 18 (42.9) | 20 (64.5) | 64 (56.1) | 43 (55.8) |
| 1 | 22 (52.4) | 9 (29.0) | 44 (38.6) | 31 (40.3) |
| 2 | 2 (4.8) | 2 (6.5) | 5 (4.4) | 3 (3.9) |
| 3 | 0 | 0 | 1 (0.9) | 0 |
| ISS stage at study entry, n (%) | ||||
| Stage I | 20 (47.6) | 20 (64.5) | 61 (53.5) | 41 (53.2) |
| Stage II | 15 (35.7) | 6 (19.4) | 37 (32.5) | 21 (27.3) |
| Stage III | 7 (16.7) | 5 (16.1) | 16 (14.0) | 14 (18.2) |
| Unknown | 0 | 0 | 0 | 1 (1.3) |
| R‐ISS stage at study entry, n (%) | ||||
| Stage I | 0 | 0 | 45 (39.5) | 33 (42.9) |
| Stage II | 35 (83.3) | 26 (83.9) | 60 (52.6) | 39 (50.6) |
| Stage III | 7 (16.7) | 5 (16.1) | 8 (7.0) | 3 (3.9) |
| Not classified | 0 | 0 | 1 (0.9) | 2 (2.6) |
| Cytogenetic risk a at study entry, n (%) | ||||
| del(17p) | ||||
| Present | 18 (42.9) | 16 (51.6) | 0 | 0 |
| t(4;14) | ||||
| Present | 22 (52.4) | 20 (64.5) | 0 | 0 |
| t(14;16) | ||||
| Present | 6 (14.3) | 0 | 0 | 0 |
| Prior lines of therapy, median (range) | 1 (1–3) | 2 (1–3) | 2 (1–4) | 2 (1–4) |
| Patients with soft tissue plasmacytoma as per IRC, n (%) | 4 (9.5) | 0 | 7 (6.2) | 4 (5.2) |
| Patients refractory to treatment, n (%) | ||||
| Refractory to IMiD agent | 16 (38.1) | 13 (41.9) | 52 (45.6) | 37 (48.1) |
| Refractory to PI | 14 (33.3) | 12 (38.7) | 34 (29.8) | 25 (32.5) |
| Refractory to IMiD agent and PI | 6 (14.3) | 5 (16.1) | 24 (21.1) | 17 (22.1) |
| Refractory to last regimen | 20 (47.6) | 18 (58.1) | 59 (51.8) | 43 (55.8) |
Abbreviations: d, dexamethasone; ECOG PS, Eastern Cooperative Oncology Group performance status; FISH, fluorescence in situ hybridization; IMiD, immunomodulatory drug; IRC, Independent Review Committee; Isa, isatuximab; ISS, International Staging System; K, carfilzomib; PI, proteasome inhibitor; R‐ISS, Revised International Staging System.
High‐risk status was defined as presence of del(17p), t(4;14), and/or t(14;16) by FISH. Cytogenetics was performed by a central laboratory with cutoff 50% for del(17p), 30% for t(4;14) and t(14;16).
3.2. Efficacy: PFS
The addition of isatuximab to Kd improved PFS for patients with ≥1 high‐risk CA (HR 0.724; 95% CI: 0.361–1.451; median PFS was not reached [NR; 95% CI: 13.076–NR] with Isa‐Kd versus 18.201 months [95% CI: 8.674–NR] with Kd), and standard‐risk patients (HR 0.440; 95% CI: 0.266–0.728; median PFS was NR [95% CI: NR–NR] with Isa‐Kd vs. 19.450 months [95% CI: 15.376–NR] with Kd) (Figure 1). Patients with t(4;14) exhibited improved PFS with Isa‐Kd (HR 0.549; 95% CI: 0.232–1.301; median PFS was NR [95% CI: 11.433–NR] with Isa‐Kd vs. 11.138 [95% CI: 4.830–NR] with Kd), whereas PFS benefit was less pronounced in patients with del(17p) (HR 0.837; 95% CI: 0.281–2.496; median PFS was NR [95% CI: 9.232–NR] with Isa‐Kd vs. 19.154 months [95% CI: 8.674–NR] with Kd) (Figure 2). Due to the small number of patients with t(14;16), no efficacy analyses were conducted separately.
FIGURE 1.
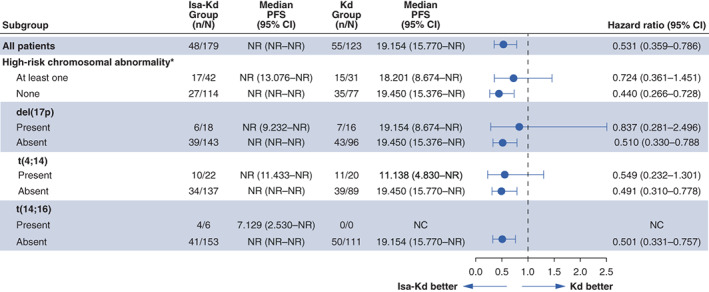
Progression‐free survival across cytogenetic risk subgroups. *High‐risk cytogenetics defined as the presence of del(17p), t(4;14), and/or t(14;16). CI, confidence interval; d, dexamethasone; Isa, isatuximab; K, carfilzomib; NC, not calculable; NR, not reached; PFS, progression‐free survival.
FIGURE 2.
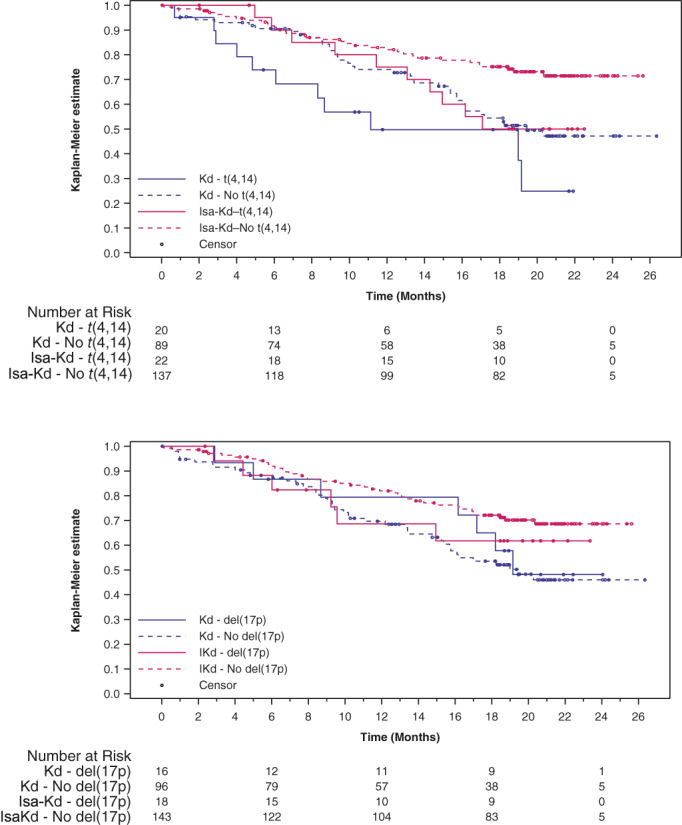
Progression‐free survival is prolonged for patients with certain chromosomal abnormalities receiving Isa‐Kd. Patients with t(4;14) exhibited prolonged progression‐free survival (A), whereas patients with del(17p) had a less pronounced benefit (B). d, dexamethasone; Isa, isatuximab; K, carfilzomib.
3.3. Efficacy: Depth of response
The addition of isatuximab to Kd led to improved depth of response compared with Kd alone in patients with standard‐risk CA, with higher ≥VGPR (78.9% vs. 54.5%) (Figure 3A), MRD negativity (36.0% vs. 11.7%; Figure 3B), and CR rates (46.5% vs. 26.0%; Figure 3C). Similar ≥VGPR (57.1% vs. 54.8%), MRD negativity (21.4% vs. 22.6%), and CR (23.8% vs. 22.6%) rates were observed between arms in patients with high‐risk CA. A greater percentage of patients with t(4;14) receiving Isa‐Kd exhibited improved ≥VGPR (72.7% vs. 50.0%), MRD negativity (31.8% vs. 25.0%), and CR (36.4% vs. 20.0%) rates, which was similar to that observed in standard‐risk patients. Patients with del(17p) did not experience improved depth of response with Isa‐Kd. Due to small patient numbers, those with (14;16) were not included in the analysis.
FIGURE 3.
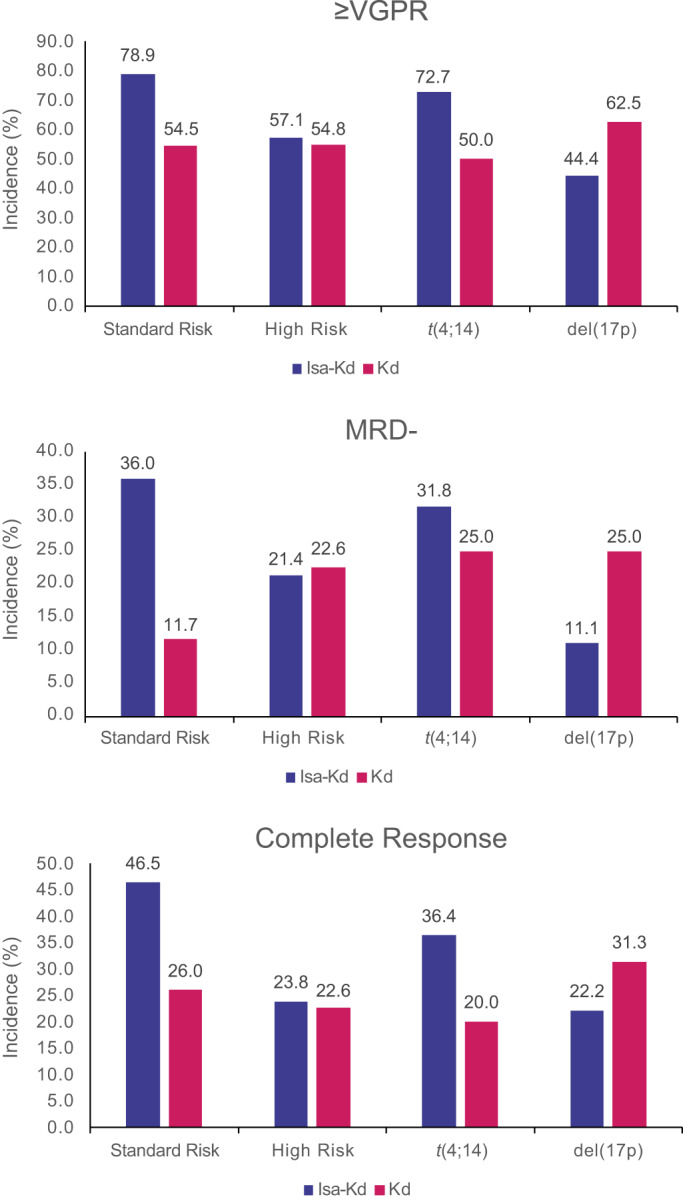
Improved depth of response with Isa‐Kd versus Kd across cytogenetic risk groups. d, dexamethasone; Isa, isatuximab; K, carfilzomib; MRD, minimal residual disease; VGPR, very good partial response
3.4. Safety
The median duration of exposure was higher for patients receiving Isa‐Kd versus Kd among those with high‐risk CA (74.0 vs. 48.0 weeks) and standard risk (81.0 vs. 63.0 weeks). Isa‐Kd had a manageable safety profile in both subgroups (Table 2). Most patients with high‐ (100% Isa‐Kd; 93.3% Kd) and standard‐risk CA (95.6% Isa‐Kd; 100% Kd) experienced ≥1 treatment‐emergent adverse event (TEAE). Grade ≥3 TEAEs were more common with Isa‐Kd versus Kd in patients with high‐risk CA (85.7% vs. 63.3%); however, the incidence of serious TEAEs (64.3% vs. 66.7%) and TEAEs with fatal outcome during study treatment (0% vs. 0%) was similar in both arms for patients with high‐risk CA. Fewer patients treated with Isa‐Kd experienced TEAEs leading to definitive discontinuation among all cytogenetic risk groups, consistent with the results reported for the overall population.
TABLE 2.
Safety summary
| High risk a | Standard risk b | |||
|---|---|---|---|---|
| Isa‐Kd (n = 42) | Kd (n = 30) | Isa‐Kd (n = 113) | Kd (n = 77) | |
| Patients with any TEAE | 42 (100) | 28 (93.3) | 108 (95.6) | 77 (100) |
| Patients with any Grade ≥3 TEAE | 36 (85.7) | 19 (63.3) | 86 (76.1) | 59 (76.6) |
| Patients with any Grade 5 TEAE c | 0 | 0 | 5 (4.4) | 4 (5.2) |
| Patients with any serious TEAE | 27 (64.3) | 20 (66.7) | 65 (57.5) | 46 (59.7) |
| Patients with any TEAE leading to definitive discontinuation | 2 (4.8) | 3 (10.0) | 11 (9.7) | 14 (18.2) |
Abbreviations: D, dexamethasone; FISH, fluorescence in situ hybridization; Isa, isatuximab; K, carfilzomib; TEAE, treatment‐emergent adverse event.
High‐risk status was defined as presence of del(17p), t(4;14), and/or t(14;16) by FISH. Cytogenetics was performed by a central laboratory with cut‐off 50% for del(17p), 30% for t(4;14) and t(14;16).
Standard‐risk status was defined as absence of del(17p), t(4;14), and t(14;16) by FISH.
TEAE with fatal outcome during the treatment period.
Selected TEAEs are shown in Table 3. Patients with high‐risk CA had higher rates of infection (all‐grade and Grade ≥ 3) with Isa‐Kd vs. Kd. The difference in all‐grade TEAEs was driven by upper respiratory tract infections and nasopharyngitis. A similar incidence of all‐grade pneumonia was seen with Isa‐Kd (28.6%) versus Kd (26.7%), but the incidence of Grade ≥ 3 pneumonia was higher with Isa‐Kd (16.7% vs. 6.7%). Grade ≥ 3 hypertension was reported more frequently with Isa‐Kd (21.4%) versus Kd (6.7%); however, the incidence in the overall population was similar (20.3% [Isa‐Kd] vs. 19.7% [Kd]). The incidence of infusion reactions was consistent with that of the overall population.
TABLE 3.
Selected TEAEs—safety population
| Selected TEAEs by SOC or SMQ or PT, n (%) | High risk a | Standard risk b | ||||||
|---|---|---|---|---|---|---|---|---|
| Isa‐Kd (n = 42) | Kd (n = 30) | Isa‐Kd (n = 113) | Kd (n = 77) | |||||
| Any grade | Grade ≥3 | Any grade | Grade ≥3 | Any grade | Grade ≥3 | Any grade | Grade ≥3 | |
| Infections and infestations (SOC) | 35 (83.3) | 15 (35.7) | 23 (76.7) | 8 (26.7) | 98 (86.7) | 46 (40.7) | 67 (87.0) | 24 (31.2) |
| Upper respiratory tract infection | 16 (38.1) | 2 (4.8) | 2 (6.7) | 1 (3.3) | 41 (36.3) | 4 (3.5) | 23 (29.9) | 1 (1.3) |
| Pneumonia | 12 (28.6) | 7 (16.7) | 8 (26.7) | 2 (6.7) | 25 (22.1) | 19 (16.8) | 12 (15.6) | 11 (14.3) |
| Bronchitis |
9 (21.4) |
0 |
6 (20.0) |
0 | 26 (23.0) | 4 (3.5) | 7 (9.1) | 0 |
| Nasopharyngitis |
8 (19.0) |
0 |
2 (6.7) |
0 | 18 (15.9) | 0 | 11 (14.3) | 0 |
| Others | ||||||||
| Infusion‐related reaction | 23 (54.8) | 1 (2.4) | 0 | 0 | 44 (38.9) | 0 | 4 (5.2) | 0 |
| Hypertension | 13 (31.0) | 9 (21.4) |
6 (20.0) |
2 (6.7) |
45 (39.8) | 25 (22.1) | 31 (40.3) | 22 (28.6) |
| Diarrhea | 14 (33.3) |
3 (7.1) |
8 (26.7) |
1 (3.3) |
41 (36.3) |
1 (0.9) |
25 (32.5) |
2 (2.6) |
| Insomnia | 13 (31.0) |
2 (4.8) |
8 (26.7) |
1 (3.3) |
26 (23.0) |
6 (5.3) |
20 (26.0) |
2 (2.6) |
| Fatigue | 13 (31.0) | 0 |
5 (16.7) |
0 | 32 (28.3) |
4 (3.5) |
18 (23.4) |
1 (1.3) |
| Asthenia |
7 (16.7) |
1 (2.4) |
3 (10.0) |
0 | 21 (18.6) |
1 (0.9) |
16 (20.8) |
3 (3.9) |
Abbreviations: d, dexamethasone; Isa, isatuximab; K, carfilzomib; PT, MedDRA preferred term; SMQ, standardized MedDRA query; SOC, system organ class; TEAE, treatment‐emergent adverse event.
High‐risk status was defined as presence of del(17p), t(4;14), and/or t(14;16) by FISH. Cytogenetics was performed by a central laboratory with cut‐off 50% for del(17p), 30% for t(4;14) and t(14;16).
Standard‐risk status was defined as absence of del(17p), t(4;14), and t(14;16) by FISH.
4. DISCUSSION
In this IKEMA subgroup analysis, the addition of Isa to Kd led to improved PFS in patients with high‐risk CA, supporting the benefit of Isa‐Kd in patients with relapsed MM reported in the overall population, and including those with high‐risk cytogenetics. 13 Patients with t(4;14) exhibited improved depth of response following treatment with Isa‐Kd; however, the benefit was less pronounced in patients with del(17p). In a subgroup analysis of the Phase 3 ICARIA‐MM study, patients with relapsed/refractory MM and high‐risk cytogenetics who received Isa‐Pd had improved ORR and PFS compared with those who received Pd. 14 Together, results from ICARIA‐MM and IKEMA suggest that Isa provides clinical benefit to patients with high‐risk cytogenetics.
In CANDOR, which investigated the addition of daratumumab (another anti‐CD38 monoclonal antibody) to Kd, cytogenetic status was assessed by central laboratory but was unknown in approximately 50% of patients, and 16% of all patients had high‐risk CA. 15 Compared with IKEMA, a similar PFS benefit was reported with daratumumab plus Kd versus Kd alone among patients with high‐risk CA (HR: 0.70; 95% CI: 0.36–1.40); however, the high percentage of patients with unknown cytogenetic status makes these results less robust. To date, other outcomes such as ORR, ≥VGPR, CR, and MRD rates in patients with high‐risk CA have not been published for daratumumab plus Kd.
In CASTOR and POLLUX, cytogenetic status was assessed by local laboratories, and definitions of cytogenetic status varied by study site. Similar to CANDOR, cytogenetic status was unknown in a high percentage of patients (30% CASTOR; 20% POLLUX). 16 , 17 , 18 Results in this subgroup were published at the 3‐year follow‐up for both studies. Patients with high‐risk CA treated with daratumumab, bortezomib, and dexamethasone exhibited prolonged PFS (HR: 0.41; 95% CI: 0.21–0.83) with a median of 12.6 versus 6.2 months. 18 Patients with high‐risk CA treated with daratumumab, lenalidomide, and dexamethasone also exhibited prolonged PFS (HR: 0.34; 95% CI: 0.16–0.72) and higher rates of MRD negativity (26% vs. 0%, respectively) compared with patients treated with lenalidomide and d. 17
In IKEMA, approximately 90% of patients had conclusive central laboratory assessment, with a stringent and clear definition of positivity, enabling the definitive demonstration of the benefit of Isa. The subgroup analysis in high‐risk CA patients of these studies and the IKEMA study support that the combination of an anti‐CD38 monoclonal antibody with a proteasome inhibitor and corticosteroid results in a superior outcome in patients with RRMM and high‐risk CA; however, this benefit is not to the same extent as that seen in patients with standard risk. Because of the limited sample size of subgroup analyses, larger studies with additional follow‐up are needed to further support the benefit of Isa‐Kd in the subgroup of patients with high‐risk CA.
The addition of Isa to Kd improved PFS in patients with high‐risk CA, improved PFS and depth of response in patients with t(4;14), and led to a less pronounced PFS benefit in patients with del(17p), with a manageable safety profile, which was consistent with the benefit observed in the overall IKEMA population. Isa‐Kd represents a new treatment option for the difficult‐to‐treat subgroup of patients with relapsed MM and high‐risk cytogenetics.
AUTHOR CONTRIBUTIONS
Ivan Spicka, Philippe Moreau, Thomas G. Martin, Thierry Facon, Gracia Martinez, Albert Oriol, Youngil Koh, Andrew Lim, Gabor Mikala, Laura Rosiñol, Münci Yağci, Michele Cavo, and Kwee Yong contributed to data acquisition and data interpretation; Gaëlle Asset contributed to statistical analysis; Sandrine Macé contributed to data analysis and data interpretation; Marie‐Laure Risse, contributed to data analysis and data interpretation; Helgi van de Velde contributed to study design, data analysis and interpretation. All authors contributed to critical revision and final approval of the manuscript.
CONFLICT OF INTEREST
Ivan Spicka: Honoraria—Amgen, Celgene, Janssen‐Cilag, Bristol Myers Squibb, Takeda, Novartis. Philippe Moreau: Honoraria—Sanofi, Celgene, Amgen, Janssen. Thomas G. Martin: Research support—Sanofi, Amgen. Thierry Facon: Honoraria—Sanofi, Amgen, Janssen, Celgene, Takeda, Karyopharm, Oncopeptides, Roche; Speakers Bureau—Takeda, Janssen. Gracia Martinez: Nothing to disclose. Albert Oriol: Honoraria—BMS/Celgene, Sanofi, Amgen, GSK. Youngil Koh: Nothing to disclose. Andrew Lim: Consultant—Celgene; Research funding—Celgene; Honoraria—Celgene. Gabor Mikala: Honoraria—AbbVie, Amgen, Celgene, Janssen, Novartis, Takeda. Laura Rosiñol: Honoraria—Janssen, Celgene, Amgen, Takeda, Sanofi. Münci Yağci: Nothing to disclose. Michele Cavo: Honoraria—AbbVie, Amgen, Bristol Myers Squibb, Adaptive Biotechnologies, Celgene, Janssen China R&D, GlaxoSmithKline, Takeda. Marie‐Laure Risse, Gaëlle Asset, Sandrine Macé, and Helgi van de Velde, are employed by Sanofi and may have stock and/or stock options in the company. Kwee Yong: Nothing to disclose.
ACKNOWLEDGMENTS
The IKEMA study was sponsored by Sanofi. The authors would like to thank the participating patients and their families, as well as the study centers and investigators for their contributions to the study. Medical writing support was provided by Erin Burns‐Tidmore, PhD, of Elevate Medical Affairs, contracted by Sanofi for publication support services.
Spicka I, Moreau P, Martin TG, et al. Isatuximab plus carfilzomib and dexamethasone in relapsed multiple myeloma patients with high‐risk cytogenetics: IKEMA subgroup analysis. Eur J Haematol. 2022;109(5):504‐512. doi: 10.1111/ejh.13835
Funding information Sanofi
DATA AVAILABILITY STATEMENT
Qualified researchers can request access to patient‐level data and related study documents including the clinical study report, study protocol with any amendments, blank case report forms, statistical analysis plan, and dataset specifications. Patient‐level data will be anonymized, and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi's data‐sharing criteria, eligible studies, and process for requesting access are at: https://www.vivli.org.
REFERENCES
- 1. Kazandjian D. Multiple myeloma epidemiology and survival: A unique malignancy. Semin Oncol. 2016;43(6):676‐681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rajkumar SV, Kumar S. Multiple myeloma: diagnosis and treatment. Mayo Clin Proc. 2016;91(1):101‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Biran N, Jagannath S, Chari A. Risk stratification in multiple myeloma, part 1: characterization of high‐risk disease. Clin Adv Hematol Oncol. 2013;11(8):489‐503. [PubMed] [Google Scholar]
- 4. Sonneveld P, Avet‐Loiseau H, Lonial S, et al. Treatment of multiple myeloma with high‐risk cytogenetics: a consensus of the international myeloma working group. Blood. 2016. Jun 16;127(24):2955‐2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lancman G, Tremblay D, Barley K, et al. The effect of novel therapies in high‐molecular‐risk multiple myeloma. Clin Adv Hematol Oncol. 2017;15(11):870‐879. [PMC free article] [PubMed] [Google Scholar]
- 6. Jiang H, Acharya C, An G, et al. SAR650984 directly induces multiple myeloma cell death via lysosomal‐associated and apoptotic pathways, which is further enhanced by pomalidomide. Leukemia. 2016;30(2):399‐408. [DOI] [PubMed] [Google Scholar]
- 7. Sarclisa® (isatuximab‐irfc) . Prescribing information. Sanofi‐Aventis U.S. LLC; 2021. [Google Scholar]
- 8. Sarclisa® (isatuximab) . Prescribing information. Sanofi Co, Ltd; 2021. [Google Scholar]
- 9. Moreau P, Dimopoulos M, Mikhael J, et al. Isatuximab plus carfilzomib and dexamethasone vs carfilzomib and dexamethasone in relapsed/refractory multiple myeloma (IKEMA): interim analysis of a phase 3, randomized, open‐label study. 2020 European Hematology Association Congress Virtual; 2020. [Google Scholar]
- 10. Moreau P, Dimopoulos MA, Yong K, et al. Isatuximab plus carfilzomib/dexamethasone versus carfilzomib/dexamethasone in patients with relapsed/refractory multiple myeloma: IKEMA phase III study design. Future Oncol. 2020. Jan;16(2):4347‐4358. [DOI] [PubMed] [Google Scholar]
- 11. Moreau P, Dimopoulos M, Mikhael J, et al. Isatuximab combined with carfilzomib and dexamethasone versus carfilzomib with dexamethasone in patients with relapsed multiple myeloma: interim analysis of the IKEMA phase 3 study. Lancet. 2021;397(10292):2361‐2371. doi: 10.1016/S0140-6736(21)00592-4 [DOI] [PubMed] [Google Scholar]
- 12. Palumbo A, Avet‐Loiseau H, Oliva S, et al. Revised international staging system for multiple myeloma: a report from international myeloma working group. J Clin Oncol. 2015. Sep 10;33(26):2863‐2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moreau P, Dimopoulos MA, Mikhael J, et al. Isatuximab, carfilzomib, and dexamethasone in relapsed multiple myeloma (IKEMA): a multicentre, open‐label, randomised phase 3 trial. Lancet. 2021;397(10292):2361‐2371. [DOI] [PubMed] [Google Scholar]
- 14. Harrison SJ, Perrot A, Alegre A, et al. Subgroup analysis of ICARIA‐MM study in relapsed/refractory multiple myeloma patients with high‐risk cytogenetics. Br J Haematol. 2021;194:120‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dimopoulos M, Quach H, Mateos MV, et al. Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): results from a randomised, multicentre, open‐label, phase 3 study. Lancet. 2020;396(10245):186‐197. [DOI] [PubMed] [Google Scholar]
- 16. Weisel K, Spencer A, Lentzsch S, et al. Daratumumab, bortezomib, and dexamethasone in relapsed or refractory multiple myeloma: subgroup analysis of CASTOR based on cytogenetic risk. J Hematol Oncol. 2020. Aug 20;13(1):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kaufman JL, Dimopoulos MA, White D, et al. Daratumumab, lenalidomide, and dexamethasone in relapsed/refractory myeloma: a cytogenetic subgroup analysis of POLLUX. Blood Cancer J. 2020. Nov 3;10(11):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mateos MV, Sonneveld P, Hungria V, et al. Daratumumab, Bortezomib, and dexamethasone versus Bortezomib and dexamethasone in patients with previously treated multiple myeloma: three‐year follow‐up of CASTOR. Clin Lymphoma Myeloma Leuk. 2020. Aug;20(8):509‐518. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Qualified researchers can request access to patient‐level data and related study documents including the clinical study report, study protocol with any amendments, blank case report forms, statistical analysis plan, and dataset specifications. Patient‐level data will be anonymized, and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi's data‐sharing criteria, eligible studies, and process for requesting access are at: https://www.vivli.org.


