Abstract
Olive tree leaves are an abundant source of bioactive compounds with several beneficial effects for human health, including a protective role against many types of cancer. In this study, we investigated the effect of an extract, obtained from olive tree (Olea europaea L.) leaves (OLE), on proliferation, invasion, and epithelial to mesenchymal transition (EMT) on metastatic melanoma, the highly aggressive form of skin cancer and the deadliest diseases. Our results demonstrated that OLE inhibited melanoma cells proliferation through cell cycle arrest and induction of apoptotic cell death. Moreover, OLE suppressed the migration, invasion, and colonies formation of human melanoma cells. Similar to our in vitro findings, we demonstrated that the oral administration of OLE inhibited cutaneous tumor growth and lung metastasis formation in vivo by modulating the expression of EMT related factors. In addition, the anti‐proliferative and anti‐invasive effects of OLE against melanoma were also related to a simultaneous targeting of mitogen‐activated protein kinase and PI3K pathways, both in vitro and in vivo. In conclusion, our findings suggest that OLE has the potential to inhibit the metastatic spread of melanoma cells thanks to its multifaceted mechanistic effects, and may represent a new add‐on therapy for the management of metastatic melanoma.
Keywords: epithelial to mesenchymal transition (EMT), melanoma, metastasis, OLE, olive, phytochemicals
1. INTRODUCTION
Malignant melanoma is the most lethal form of skin cancer because of its highly metastatic phenotype. Approximately 50% of patients with metastatic melanomas harbor the activating BRAFV600E mutation, which results in the constitutive activation of the mitogen‐activated protein kinase (MAPK) signaling (Davies et al., 2002; Turner, Ware, & Bosenberg, 2018). MAPK‐pathway is fundamental in several intracellular processes, including cell growth and differentiation. Thus, its dysregulation leads to uncontrolled cell proliferation and evasion of apoptosis, facilitating disease progression (Fecher, Amaravadi, & Flaherty, 2008). In addition, oncogenic BRAF is also related to altered expression of extracellular matrix genes and induction of epithelial to mesenchymal transition (EMT); (Boyd et al., 2013). It is generally accepted that EMT plays a crucial role in the migration, invasion, and metastasis of cancer. In fact, during this process melanocytes lose their epithelial characteristics and acquire mesenchymal phenotypes which increases their motility, invasiveness, and metastatic potential (Pearlman, Montes de Oca, Pal, & Afaq, 2017). BRAF inhibitors (BRAFi), such as vemurafenib and dabrafenib, represent one of the most promising therapeutic approaches for patients with BRAFV600E‐mutated metastatic melanomas. BRAF inhibitors have dramatically improved the outcome of these patients, showing significant increase in response rates, progression free‐survival (PFS), and overall survival (OS); (Chapman et al., 2011; Flaherty et al., 2010; Hauschild et al., 2012). Unfortunately, most patients develop resistance and experience tumor recurrence. Both genetic and epigenetic alterations in melanoma cells play a crucial role in acquired resistance to BRAFi via reactivation of the MAPK pathway and, to a lesser extent, of the alternative phosphatidylinositol 3‐kinase/protein kinase B (PI3K/AKT) survival pathway (Kakadia et al., 2018).
Therefore, there is an increasing need to find new approaches that may improve the antitumor effect of BRAFi while overcoming tumor resistance. Thus, a combination of conventional treatment with new antitumor agents, to target different pathways simultaneously, might be an important breakthrough. In recent years, phytochemicals have gained considerable attention as potential coadjuvant therapy for cancer treatment due to their low cost, low toxicity, and high compliance as dietary supplements. Indeed, several phytochemicals, such as fisetin, epigallocatechin‐3‐gallate, resveratrol, curcumin, apigenin, capsaicin and genistein, present in a variety of fresh fruits, vegetables, roots, and herbs have been reported to have anticancer, antimetastatic and antiangiogenic effects (Allegra et al., 2018; Ng, Yen, Hsiao, & Su, 2018; Pal, Hunt, Diamond, Elmets, & Afaq, 2016; Ranjan et al., 2019; Tartaglione et al., 2018).
Olive tree leaves are an abundant source of bioactive compounds with several beneficial effects for human health that has long been used as a traditional medicine in the Mediterranean region (El & Karakaya, 2009). Olive leaf extract (OLE) contains similar polyphenols to those found in the fruit and its derivative, the extra virgin olive oil (EVOO), although at a much higher concentration and variety (Romani et al., 2019). Health‐promoting properties of olive polyphenols include anti‐oxidant, anti‐hypertensive, cardioprotective, and antiinflammatory activity (Gorzynik‐Debicka et al., 2018). Research has shown that some olive‐derived polyphenols play an important protective role also in cancer. Indeed, EVOO consumption, which is a traditional component of the Mediterranean diet, was proven to reduce colorectal cancer, breast cancer, and skin cancer risk (Boss, Bishop, Marlow, Barnett, & Ferguson, 2016; Psaltopoulou, Kosti, Haidopoulos, Dimopoulos, & Panagiotakos, 2011). However, although there is a large body of literature that has investigated the anticancer properties of the single phenolic components of olive fruit, data about the anticancer activities of the mixture of biologically active compounds of whole olive leaf extract are quite rare. Therefore, in this study, we investigated the anticancer effect of an extract obtained from olive leaves of Olea europea L cultivar Ravece (OLE). This product was formulated by the Department of Pharmacy, University of Naples “Federico II” (Naples, Italy). The main phenolic compounds identified in our extract belong to four main groups: oleuropeosides (oleuropein), flavonols (rutin), flavones (apigenin, apigenin‐7‐O‐glucoside, and luteolin), phenylethanoid (tyrosol and hydroxytyrosol). In particular, Oleuropein (174.64 mg/g) was detected at the highest concentration in OLE, followed by hydroxytyrosol (26.65 mg/g), tyrosol (0.64 mg/g) and apigenin‐7‐O‐glucoside (0.43 mg/g); (De Cicco, Maisto, Tenore, & Ianaro, 2020). In order to evaluate the potential chemotherapeutic efficacy of OLE against melanoma we investigate the anti‐proliferative and the anti‐migratory effect of OLE both in vitro and in vivo. We showed that OLE effectively inhibited BRAFV600E mutant cells proliferation, invasion, and migration. Then, the potential mechanism of action of OLE on the migration process was further investigated by evaluating the expression of common EMT‐related proteins and pathways. Overall, this study demonstrates the novel therapeutic potential of OLE in reducing melanoma growth and in limiting metastasis spread.
2. MATERIALS AND METHODS
2.1. Plant material
Olive leaf extract was obtained by Olea europea L cultivar Ravece. Fresh leaves were collected from the rural region of Avellino, in the south of Italy, in the month of October 2019. 10 g of lyophilized raw material were extracted with 100 ml pure ethanol (1.59010; Sigma; food‐grade solvent) for 4 hr in darkness and at room temperature. The extract obtained was centrifuged (10 min at 8000 rpm) and supernatant underwent a spray‐drying process with maltodextrins (Farmalabor, Canosa, Italy) as support, obtaining a fine powder, which was used for the in vitro experiments. This product was formulated by the Department of Pharmacy, University of Naples “Federico II” (Naples, Italy). Large scale production of OLE has been accomplished by MB Med Company (Turin, Italy).
2.2. Cell culture
The human melanoma cell lines A375 cells were purchased from Sigma‐Aldrich (Milan, Italy), WM983A, and WM983B cells were purchased from Rockland (Limerick, Ireland). The murine melanoma cells B16F10 were purchased and characterized from IRCCS AOU San Martino–IST (Genova, Italy). Normal human epidermal melanocytes (NHEMs) were purchased from Lonza (Walkersville, Maryland) and were grown in melanocyte growth medium 2 (Lonza). A375 and B16F10 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum, 2 mmol/L L‐glutamine, 100 μmol/L non‐essential amino acids, penicillin (100 U/ml), streptomycin (100 μg/ml), and 1 mmol/L sodium pyruvate (all from Sigma‐Aldrich, Milan, Italy). WM983A and WM983B were cultured in Tumor Specialized Media (1:5 Leibovitz's—MCDB153), containing 2% Inactivated FBS and 1.68 mM CaCl2. Cells were grown at 37°C in a humidified incubator under 5% CO2. All cell lines used in this study were characterized by the cell bank where they were purchased.
2.3. MTT assay
Cells (B16F10, A375, WM983A, WM983B, or NHEM) were seeded on 96‐well plates (2–5 × 103 cells/well) and treated with OLE (50–100 to 150–200‐μg/ml) for 48 hr before adding 25 μL of MTT (Sigma, Milan, Italy); (5 mg/ml in saline). Cells were incubated for additional 3 hr at 37°C. Thereafter, cells were lysed and dark blue crystals were solubilized with a solution containing 50% (vol/vol) N,N‐dimethyl formamide, 20% (wt/vol) sodium dodecylsulfate with an adjusted pH of 4.5. The OD of each well was obtained by measuring the absorbance at 620 nm using Multiskan GO microplate reader (Thermo Fisher Scientific, MA, USA).
2.4. Apoptosis assay
Apoptosis assay was performed as previously described (De Cicco et al., 2021). A375 and WM983B cells were treated with OLE (200 μg/ml) for 24 and 48 hr. Apoptosis was detected using the Annexin V‐FITC apoptosis detection kit (eBioscience. Thermo Fisher Scientific MA, USA) according to the manufacturer's instructions and flow cytometry. A minimum of 20,000 events for each sample were collected and data were analyzed using BriCyte E6 (Mindray, P.R. China).
2.5. Cell‐cycle analysis
A375 cells were synchronized by incubation in medium containing 1% FBS for 12 hr, and the culture was then incubated with fresh medium/10% FBS‐containing OLE (200 μg/ml) for 24 hr. Both adherent and floating cells were collected, washed with PBS, and fixed with 1 ml of ice‐cold 70% ethanol overnight at 4°C. Cells were stained with propidium iodide/RNase A solution for 30 min at 37°C and then analyzed by flowcytometry (BriCyte E6 Mindray, P.R. China).
2.6. Preparation of cellular extracts and western blot analysis
Whole‐cell extracts were prepared from A375 cells treated with OLE or from melanoma tissue homogenate as previously described (De Cicco et al., 2017). The protein concentration was measured by the Bradford method (Bio‐Rad, Milan, Italy). Equal amounts of protein (40–60 μg/sample) were separated by sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS‐PAGE) and blotted onto nitrocellulose membranes (Trans‐Blot Turbo Transfer Starter System, Biorad). The membranes were blocked for 2 hr in 5% low‐fat milk in PBS with 0.1% Tween 20 (PBST) at room temperature. Then the filters were incubated with the following primary antibodies: caspase 3, PARP, Cyclin D1, CDK4, Phospho‐AKT (Ser473) XP, AKT, p44/42 MAPK (Erk1/2), Phospho‐p44/42 Erk MAPK (Erk1/2, Thr202/Tyr204) XP, GAPDH (Cell Signaling, USA), E‐cadherin, Vimentin, MMP‐2 (Santa Cruz Biotechnology, USA), MMP‐9 (Novus Biologicals USA) and VEGF (Elabscience Biotechnology Inc, USA), overnight at 4°C. The membranes were washed 3 times with PBST and then incubated with anti‐mouse or anti‐rabbit horseradish peroxidase‐conjugated antibodies (Santa Cruz Biotechnology, Santa Cruz, CA; diluted 1:2000) for 2 hr at room temperature. The immune complexes were visualized by the ECL chemiluminescence method and acquired by the ChemiDoc MP Imaging System (Bio‐Rad,Milan, Italy).
2.7. Invasion assay
The assay was performed using chambers with polycarbonate filters with 8‐μm nominal pore size (Millipore, USA) coated on the upper side with Matrigel (Becton Dickinson Labware, USA). Briefly, the chambers were placed into a 24‐well plate and A375 cells (2.5 × 105/ml) were plated in the upper chamber, with or without OLE (50 μg/ml), in serum‐free DMEM. After the incubation period (16 hr), the filter was removed, and non‐migrant cells on the upper side of the filter were detached with the use of a cotton swab. Filters were fixed with 4% formaldehyde for 15 min, and cells located in the lower filter were stained with 0.1% crystal violet for 20 min and then washed with PBS. The filters were examined microscopically and cellular invasion was determined by counting the number of stained cells on each filter in at least 4–5 randomly selected fields.
2.8. Wound healing assay
A375 and WM983B cells were seeded in 12‐well plates (2 × 105 cells/well) and grown to full confluence. A 200 μL pipette tip was used to create a wound in the monolayer. OLE (50 μg/ml) was added after imaging of the wells with the microscope at 0 hr (wound induction). Imaging was repeated after 24 and 48 hr. An ImageJ macro, the MRI Wound Healing Tool (MRI Redmine) was used for calculating the area of the cell‐free gap at 0–24 to 48 hr after wound induction.
2.9. Clonogenic assay
A375 cells (1 × 103 cells/well) were seeded in 6‐well plates and treated with OLE 50 μg/ml for 48 hr. After, fresh medium (drug‐free) was changed every 2 days. Cells were cultured for 14 days to allow the colonies to form. Formed colonies were washed twice with 1 × PBS, fixed by 4% paraformaldehyde, and stained with 0.5% crystal violet and colonies containing more than 50 cells (established by microscopy) were counted manually. Images of the colonies were obtained using a digital camera.
2.10. RNA purification and quantitative real‐time PCR (RT‐qPCR)
Total RNA was isolated from human melanoma cells or mouse tumor tissue by using TRI‐Reagent (Sigma‐Aldrich, Milan, Italy), according to the manufacturer's instructions. Final preparation of RNA was considered DNA‐ and protein‐free if the ratio between readings at 260/280 nm was ≥1.7. Isolated mRNA was reverse‐transcribed by use of iScript Reverse Transcription Supermix for RT‐qPCR (Bio‐Rad, Milan, Italy). Then, quantitative real‐time‐PCR was performed using CFX384 real‐time PCR detection system (Bio‐Rad, Milan, Italy). Primer sequences were:
2.10.1. Mouse
Il‐6:5′‐CGGAGAGGAGACTTCACAGAG‐3′; 5′‐ATTTCCACGATTTCCCAGAG‐3′
Il‐1β: 5′‐TACCAGTTGGGGAACTCTGC‐3′; 5′‐GGGCCTCAAAGGAAAGAATC‐3′
Tnf‐α: 5′‐CAGTAGACAGAAGAGCGTGGT‐3′; 5′‐AGGCACTCCCCCAAAAGA‐3′
twist: 5′‐GGACAAGCTGAGCAAGATTCA‐3′; 5′‐CGGAGAAGGCGTAGCTGAG‐3′
zeb1: 5′‐GCTGGCAAGACAACGTGAAAG‐3′; 5′‐GCCTCAGGATAAATGACGGC‐3′
β‐actin: 5′‐TACCACCATGTACCCAGGCA‐3′; 5′‐CTCAGGAGGAGCAATGATCTTGA‐3′.
2.10.2. Human
twist: 5′‐GTCCGCAGTCTTACGAGGAG‐3′; 5′‐GCTTGAGGGTCTGAATCTTGCT‐3′
zeb1: 5′‐TTACACCTTTGCATACAGAACCC‐3′; 5′‐ TTTACGATTACACCCAGACTGC ‐3′
s16: 5′‐TCGGACGCAAGAAGACAGC‐3′; 5′‐ AGCAGCTTGTACTGTAGCGTG‐3′.
Samples were amplified in triplicate using SYBR Green master mix kit (Bio‐ Rad, Milan, Italy). Relative gene expression was obtained by normalizing the Ct values of each experimental group against β‐actin or s16 transcript level, using 2−ΔCt formula.
2.11. ELISA
Serum levels of CXCL1 were determined by ELISA kits according to the manufacturer's instructions (DuoSet ELISA, R&D systems, Minneapolis, MN, USA).
2.12. Animals
Female C57BL/6 mice (age 6–7 weeks, 18–20 g) were purchased from Charles River Laboratories, Inc. Mice were housed at the Animal Research Facility of the Department of Pharmacy of the University of Naples Federico II. All the experimental procedures and protocols were performed in accordance with the European (EEC Directive 2010/63) and Italian (D.L. March 4, 2014 n.26) legislation. All studies involving animals are reported in accordance with the ARRIVE guidelines.
2.12.1. Induction of subcutaneous B16 lesions
Mice were subcutaneously (s.c.) injected in the right flank with B16F10 cells (1 × 105/0.1 ml) and divided in three groups (n = 8) as follow:
Group I (Control): received vehicle (tap water) orally every day for 14 days;
Group II (OLE 100): received OLE 100 mg/kg orally every day for 14 days;
Group III (OLE 200): received OLE 200 mg/kg orally every day for 14 days.
Tumor size was measured twice a week in three dimensions using a digital caliper, and tumor volume was calculated using the following equation: tumor volume = π/6(D1 × D2 × D3) where D1 = length; D2 = width; D3 = height and expressed as cm3. At the end of treatment, animals were humanely euthanized by CO2 and a final measurement was made after the tumors were taken out of the animals. Tumor tissues and blood were collected for subsequent analysis.
2.12.2. Induction of metastatic melanoma
B16F10 cells (5 × 105/0.2 ml) were injected via tail vein into syngeneic C57BL/6J mice. Mice were divided into two groups (n = 8) as follow:
Group I (Control): received vehicle (tap water) orally every day for 14 days;
Group II (OLE 200): received OLE 200 mg/kg orally every day for 14 days;
At the end of treatment, animals were humanely euthanized by CO2. The lungs were removed and washed with PBS. Lung images were captured and the number of metastatic nodules were counted using ImageJ software (ImageJ).
2.13. Statistical analysis
Data are expressed as mean ± SEM. Statistical analysis was performed with a number of n ≥ 3. Data were analyzed with GrapdPad Prism 6.0 software program (GraphPad Software, Inc., San Diego, CA, United States). One‐way analysis of variance (ANOVA) with post hoc Bonferroni's test was performed to determine differences between more than 2 groups. Results were considered significant at p ≤ .05.
3. RESULTS
3.1. OLE induces anti‐proliferative effect in human and murine melanoma cell lines
The cytotoxic effect of OLE was evaluated by MTT on different BRAF‐mutated melanoma cell lines, including the murine B16F10 and the human A375, WM983B and WM983A. Cells were incubated for 48 hr to different concentrations (50–100‐150–200 μg/ml) of OLE and cell viability was assessed. As shown in Table 1, OLE markedly reduced cells viability in a concentration‐dependent manner in all melanoma cell lines. In particular, OLE 200 μg/ml reduced cell viability of about 50% in WM983B and WM983A (p < .001 compared with untreated cells), 63% in A375 and 74% in B16F10 cells (p < .001 compared with untreated cells). Moreover, the anti‐proliferative effect was specific for melanoma cells, in fact only a reduction of 14% of cell viability was observed in NHEM with the highest concentration (200 μg/ml).
TABLE 1.
Antiproliferative activity of olive leaf extract (OLE) against melanoma cells
| Concentrations (μg/ml) | A375 | WM983B | WM983A | B16F10 | NHEM |
|---|---|---|---|---|---|
| 50 | 13.5 ± 0.68 | 18.1 ± 0.87 | 11.1 ± 0.55 | 18.4 ± 0.70 | 0.72 ± 0.05 |
| 100 | 38.9 ± 0.73*** | 35.3 ± 0.65*** | 37.2 ± 0.89*** | 24.1 ± 0.48*** | 8.6 ± 0.47 |
| 150 | 44.7 ± 0.12*** | 38 ± 0.82*** | 39.2 ± 0.84*** | 39.4 ± 0.83*** | 10.3 ± 0.65 |
| 200 | 63.3 ± 0.48*** | 52.5 ± 0.15*** | 51.5 ± 0.39*** | 74.5 ± 0.76*** | 14.2 ± 0.25* |
Notes: Human melanoma cells (A375, WM983B, WM983A) and murine melanoma cells (B16F10) were treated with different concentrations of tested compound. Growth inhibition was measured at 48 hr using the MTT assay. Normal human epidermal melanocytes (NHEM) were used as negative control. Results are expressed as inhibition of cell proliferation (%); (mean ± SEM). Experiments were run in triplicate.
p < .05.
p < .001 versus untreated cells.
3.2. OLE induced apoptotic cell death and cell cycle arrest in melanoma cells
To determine whether the inhibition of cell growth by OLE was associated with apoptosis induction, we performed flow cytometric analysis by the annexin V‐PI staining. The results showed that the percentage of annexin V+/PI− cells and annexin V+/PI+ cells was markedly increased in OLE treated cells (Figure 1a). In particular, the quantitative results of apoptotic cells showed that OLE induced significant apoptosis in A375 cells to 20 and 70% at 24 and 48 hr, respectively (Figure 1b). The pro‐apoptotic effect of OLE was also confirmed by the activation of caspase‐3 and the cleavage of its substrate poly (adenosine diphosphate‐ribose) polymerase (PARP), evaluated by western blot analysis, in A375 cells following OLE treatment (200 μg/ml); (Figure 1c). Similar results were also obtained in WM983B cells (Figure S1a,b). Furthermore, we also analyzed the cell cycle distribution of OLE‐treated A375 cells by flow cytometry (Figure 1d). The results demonstrated that OLE significantly increased the percentage of cells in the G0/G1 phase and decreased the percentage of cells in the S and G2/M phase compared with control cells (Figure 1e). To corroborate our data, we performed western blot analysis of OLE‐treated A375 cells and evaluated the expression of the cyclin D1‐CDK4 complex which is implicated in cell cycle progression (Connell‐Crowley, Harper, & Goodrich, 1997). As showed in Figure 1f, treatment with OLE significantly reduced the expression of both cyclin D1 and CDK4. Collectively, these results indicate that OLE induces apoptosis in melanoma cell and inhibits their proliferation through the arrest of G0/G1 cell cycle phases.
FIGURE 1.
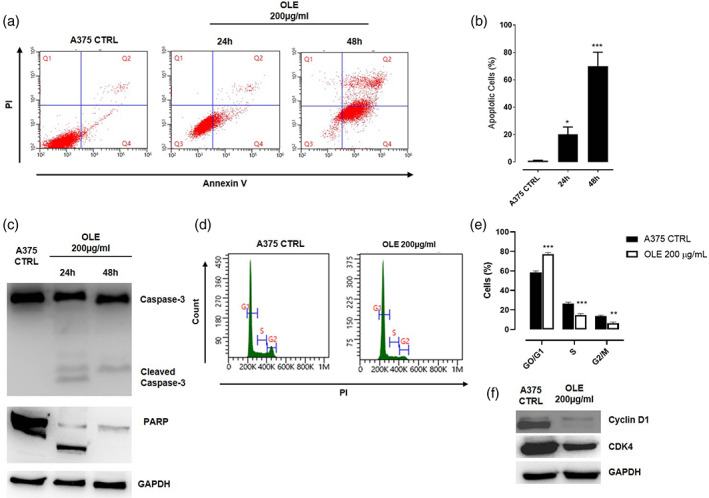
Olive leaf extract (OLE) induced apoptotic cell death and cell cycle arrest in human melanoma cells. (a‐b) Apoptotic cell death in human A375 cells was detected by annexin V/propidium iodide (PI) staining. A375 cells were treated with OLE 200 μg/ml and apoptosis was determined at 24 and 48 hr by flow cytometric analysis. The percentage of apoptotic cells are shown in the bar diagram as the mean ± SEM (n = 3), (*p < .05; ***p < .001 vs. A375 CTRL). (c) Western blot analysis of caspase‐3 and PARP in A375 whole‐cell lysates following the treatment with OLE (200 μg/ml) for 24 and 48 hr. GAPDH was detected as loading control. The blot shown are representative of three independent experiments. (d‐e) Cell cycle distribution was analyzed in human A375 cells treated with OLE (200 μg/ml) for 24 hr by flow cytometry following PI staining. Data are expressed as mean ± SEM of three independent experiment (**p < .01; ***p < .001 vs. CTRL). (f) Western blot analysis of Cyclin D1 and CDK4 carried out in A375 whole‐cell lysates following the treatment with OLE (200 μg/ml) for 24 hr. GAPDH was detected as loading control. The blot shown are representative of three independent experiments
3.3. OLE inhibited melanoma cell migratory and invasive abilities via the modulation of EMT‐related ERK/AKT signaling pathways
To evaluate whether OLE influences the migration and invasion abilities of melanoma cells, we performed wound healing, invasion, and colonies formation assays. Treatment with OLE 50 μg/ml for 24 and 48 hr significantly thwarted A375 and WM983B cells migration in a time‐dependent manner (Figure 2a,b; Figure S1c,d). Likewise, the invasion assay confirmed that OLE significantly reduced the invasiveness of A375 cells (Figure 2c,d). Moreover, OLE treatment also inhibited A375 colonies formation (Figure 2e,f). Migration and invasion represent key factors involved in the EMT phenomenon (Tiwari, Gheldof, Tatari, & Christofori, 2012). Loss of the epithelial protein E‐cadherin and upregulation of mesenchymal proteins such as N‐cadherin, vimentin, and fibronectin are considered as hallmarks of EMT (Caramel et al., 2013). Thus, we determined the effects of OLE on EMT marker proteins in BRAF‐mutated A375 melanoma cells. Western blot analysis demonstrated that OLE treatment reduced the expression of vimentin and increased the expression of E‐cadherin in A375 cells (Figure 2g). During EMT progression, downregulation of E‐cadherin expression is induced by the transcription factors such as Twist1 and ZEB1. Increased expression of these transcription factors results in greater melanoma cell migration and invasion (Puisieux, Brabletz, & Caramel, 2014). As shown in Figure 2h OLE significantly reduced the mRNA expression of twist1 and zeb1 in A375 melanoma cells. The expression of these EMT‐inducers is in turn regulated upstream by MAPK and PI3K signaling. Indeed, both activated MAPK and PI3K pathways are known to induce EMT progression, enhance melanoma cell invasion, and metastasis and also contribute to drug resistance (Olea‐Flores et al., 2019); (Yajima et al., 2012). Therefore, the effect of OLE on the expression of phospho‐AKT (p‐AKT) and phospho‐ERK (p‐ERK) by western blot analysis was evaluated. As shown in Figure 2i treatment of A375 with OLE markedly decreased the expression of both p‐AKT and p‐ERK reducing their constitutive activation. These data further demonstrate that OLE inhibits the mobility of melanoma cells through the simultaneous targeting of these EMT‐associated pathways.
FIGURE 2.
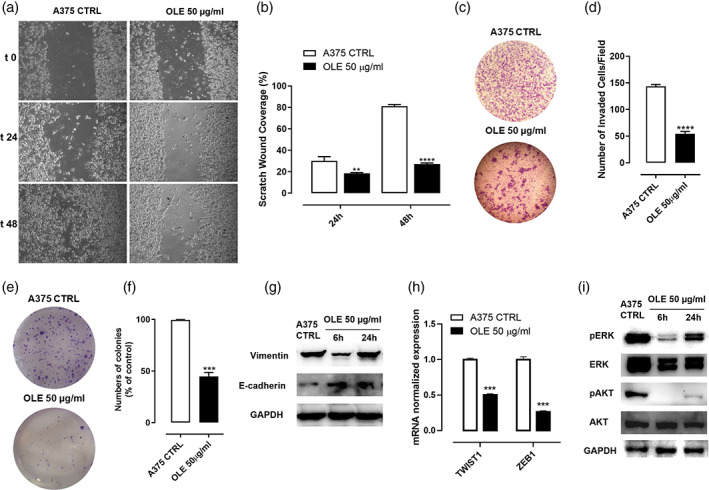
Olive leaf extract (OLE) inhibited melanoma cell migratory and invasive abilities via the modulation of epithelial to mesenchymal transition‐related ERK/AKT signalling pathways. (a‐b) Cell migration of A375 cells was assessed by wound healing assay following OLE treatment (50 μg/ml). Representative photographs of A375 cells at 0, 24 and 48 hr are shown. The scratched areas were quantified in three random fields in each treatment. Data are expressed as mean ± SEM of three independent experiments (**p < .01; ****p < .0001 vs. A375 CTRL). (c‐d) Cell invasion was determined by using Boyden chamber coated with matrigel. A375 cells were placed in the upper chamber and treated with OLE (50 μg/ml). After 16 hr the number of cells passing throughout the matrigel was determined. Invasive cells were counted using an image software as the number of migrated cells per field. Five fields were counted in triplicate from each insert. Data are shown as mean ± SEM of three independent experiments (****p < .0001 vs. CTL). (e‐f) A375 cells were treated with OLE (50 μg/ml) for 48 hr and then cultured for 14 days to allow cells to form colonies. The number of colonies is shown as mean ± SEM of three independent experiments (***p < .001 vs. A375 CTRL). (g) Western blot analysis carried out on the whole‐cell lysates obtained from A375 cells treated with OLE (50 μg/ml) for 6 and 24 hr showing the protein expression of vimentin and E‐cadherin. GAPDH was detected as loading control. The blot shown are representative of three independent experiments. (h) mRNA expression of twist1 and zeb1 assessed by qPCR analysis in A375 cells treated with OLE (50 μg/ml) for 6 hr. Data are expressed as mean ± SEM (n = 3); (***p < .001 vs. CTRL); (i) Western blot analysis carried out on the whole‐cell lysates obtained from A375 cells treated with OLE (50 μg/ml) for 6 and 24 hr showing the inhibition of AKT and ERK phosphorilation. GAPDH was detected as loading control. The blot shown are representative of three independent experiments
3.4. OLE inhibited tumor growth and suppressed the lung metastasis in B16F10‐bearing mice
To translate our findings into an in vivo setting, we determined first whether OLE could reduce tumor growth in a syngeneic mouse model of cutaneous melanoma. C57BL/6 mice were subcutaneously implanted with B16F10 murine melanoma cells and, subsequently, treated by oral gavage either with OLE (100 and 200 mg/Kg) or tap water every day for 14 days. Our results demonstrated that mice receiving OLE (200 mg/Kg) displayed a significant reduction of tumor volume by 73% (0.100 ± 0.04 cm3; p < .0001) as compared to control mice (0.352 ± 0.04 cm3), whereas OLE (100 mg/Kg) slightly reduced tumor volume by 18% (Figure 3a). Likewise, tumor weight was significantly reduced by OLE (200 mg/Kg) by 67% (154.5 ± 56.6 mg mean weight; p < .01) as compared to control mice (469.7 ± 46.8 mg mean weight; p < .01); (Figure 3b). In addition, to investigate on the molecular mechanisms behind the antitumoral effect of OLE, we evaluated the expression of murine Il‐6, Tnf‐α, and Il‐1β which are known to be involved in the tumorigenesis of melanoma (Bridge, Lee, Daud, Wells, & Bluestone, 2018). Lower expression of these cytokines in OLE‐treated mice compared to control mice were observed as demonstrated by qPCR analysis of dissociated tumor tissues (Figure 3c). These findings demonstrate that, similar to in vitro results, OLE interferes with tumor cell proliferation and expansion in vivo. Next, to evaluate the effect of OLE in metastasis development in vivo, we employed the lung metastatic mouse model through the injection of B16F10 melanoma cells into the tail vein in C57BL/6 mice. From the first day after tumor cell inoculation, OLE 200 mg/kg or tap water, were administrated orally once per day for 14 days. Mice were sacrificed at day 14th of treatment, and their lungs were surgically excised to evaluate the effect of OLE on the histopathological alteration of lung metastatic tissue. We found that the number of metastatic tumor nodules was significantly reduced in OLE‐treated mice compared with control mice visually (Figure 3d), as well as numerically (Figure 3e, 47.4 ± 3.8 vs. 10.8 ± 2.7, p < .0001 compared with control mice). To corroborate our findings on the anti‐metastatic effect of OLE in melanoma, we measured the levels of CXCL1, one of the most important chemokine associated with metastasis development in melanoma (Dhawan & Richmond, 2002). As shown in Figure 3f, OLE‐treated tumor bearing mice displayed reduced plasmatic levels of CXCL1 compared to control mice. These in vivo data were consistent with our in vitro findings confirming the anti‐proliferative and anti‐metastatic activity of OLE.
FIGURE 3.
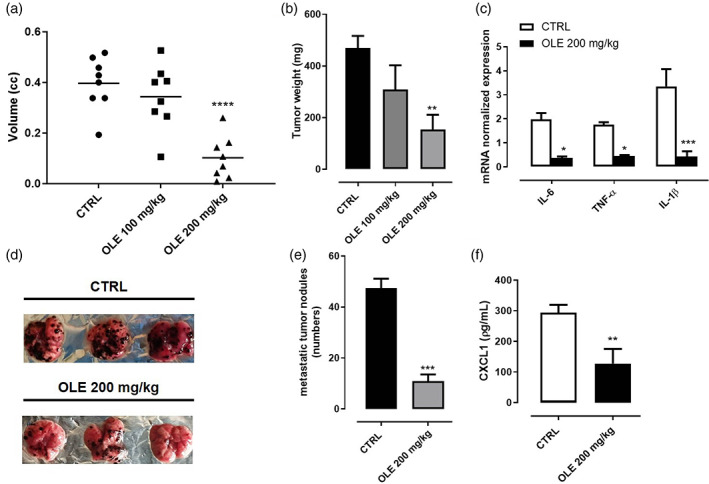
Olive leaf extract (OLE) inhibited tumor growth and suppressed the lung metastasis in B16F10‐bearing mice. B16F10 mouse melanoma cells were subcutaneously injected in the right flank of C57BL/6 mice. OLE (100 and 200 mg/kg) was given orally to mice, while control mice received vehicle only. (a‐b) Volume and weight of dissected tumor were measured after 14 days of treatment. Data are presented as mean ± SEM (n = 8). (**p < .01; ****p < .0001 vs. CTRL). (c) Expression of il‐6, tnf‐α and il‐1β assessed by qPCR analysis in dissociated tumor tissues. Data are expressed as mean ± SEM (n = 8); (*p < .05; ***p < .001 vs. CTRL). B16F10 mouse melanoma cells were injected through the lateral tail vein of C57BL/6 mice. OLE (200 mg/kg) was given orally to mice, while control mice received vehicle only. (d) Representative photographs of lung tissue excised at day 14th after treatment. (e) The metastatic nodules were counted, and data presented as the mean ± SEM (n = 8); (***p < .001 vs. CTRL). (f) Quantification of CXCL1 plasma levels assessed by ELISA in tumor bearing mice. Data are expressed as mean ± SEM (n = 8); (**p < .01 vs. CTRL)
3.5. OLE inhibited EMT in B16F10‐bearing mice
To confirm our in vitro results on the effect of OLE in the EMT phenomenon, we first evaluated by western blot analysis the expression of murine p‐AKT and p‐ERK in dissociated tumor tissues. In line with our findings in vitro, OLE treatment effectively reduced phosphorylation of both AKT and ERK proteins (Figure 4a,b). Next, to further assess the contribution of OLE in the EMT, we analyzed different EMT‐related markers in dissociated tumor tissues (Caramel et al., 2013). As shown in Figure 4c,d, OLE significantly up‐regulated the expression of E‐cadherin and down‐regulated the expression of Vimentin. In addition, we assessed the protein levels of MMP‐2 and MMP‐9, two well‐known markers associated with EMT, invasion, and metastasis development (Redondo, Lloret, Idoate, & Inoges, 2005). We found that OLE was able to reduce both MMP‐2 and MMP‐9 expression in tumor tissues (Figure 4e,f). Likewise, OLE significantly reduced the expression of VEGF, that has been reported to be a trigger involved in the EMT in addition to being a known pro‐angiogenic factor (Gonzalez‐Moreno et al., 2010); (Figure 4e,f). The downregulation of these EMT‐associated mediators was triggered by the inhibition of the related transcription factors twist1 and zeb1, as demonstrated by their reduced mRNA expression in the tumor tissues of OLE treated mice compared to control mice (Figure 4g). Taken together, these results underscore the relevance of OLE in restraining cancer cell progression and subsequent metastatic potential.
FIGURE 4.
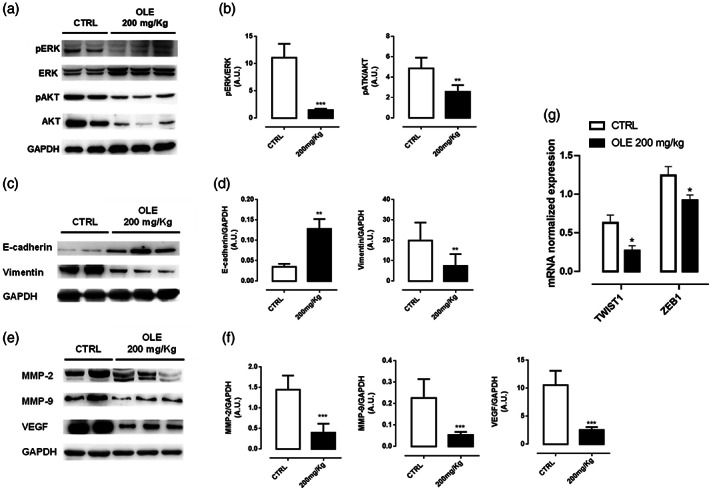
Olive leaf extract (OLE) inhibited epithelial to mesenchymal transition in B16F10‐bearing mice. (a‐b) Representative western blot analysis of ERK/AKT phosphorylation carried out on the whole‐cell lysates obtained from dissociated tumor tissues with the relative quantification. GAPDH was detected as loading control. Data are expressed as mean ± SEM of three independent experiment (**p < .01; ***p < .001 vs. CTRL). (c‐d) Representative western blot analysis of E‐cadherin and Vimentin expression carried out on the whole‐cell lysates obtained from dissociated tumor tissues with the relative quantification. GAPDH was detected as loading control. Data are expressed as mean ± SEM of three independent experiment (**p < .01 vs. CTRL). (e‐f) Representative western blot analysis of MMP‐2, MMP‐9 and VEGF expression carried out on the whole‐cell lysates obtained from dissociated tumor tissues with the relative quantification. GAPDH was detected as loading control. Data are expressed as mean ± SEM of three independent experiment (***p < .001 vs. CTRL). (g) mRNA expression of twist1 and zeb1 assessed by qPCR analysis in dissociated tumor tissues. Data are expressed as mean ± SEM (n = 3); (*p < .05 vs. CTRL)
4. DISCUSSION
Melanoma is one of the most highly metastatic cancer, and it has long been one of the deadliest diseases. However, when diagnosed in its early stages, primary cutaneous tumor can be treated successfully with surgical excision increasing the survival rates; but following metastasis development, the survival rates drop significantly (McKenna, Florell, Goldman, & Bowen, 2006). The EMT is a fundamentally phenomenon that arises to transform melanoma in situ to invasive, motile melanoma and it is frequently associated with poor prognosis and high risk of metastasis (Alonso et al., 2007). This transition is characterized by loss of expression of typical epithelial biomarkers, such as E‐cadherin and cytokeratins with an accompanying increase in the expression of mesenchymal biomarkers such as N‐cadherin and vimentin (Lamouille, Xu, & Derynck, 2014). These changes enhance cell migratory capacity, increase invasiveness and downregulate apoptosis (Thiery & Sleeman, 2006). Over the last 10 years, several new drugs have been developed for the treatment of metastatic melanoma with great improvement of the patients prognosis; however, a majority of patients do not show a lasting response to these treatments and experience relapse and aggressive progression of melanoma in 5–7 months (Kakadia et al., 2018; Silva, Bulman, & McMahon, 2014). In the last years, several preclinical and clinical studies have recognized simultaneous inhibition of different signaling processes involved in cell invasion and metastasis as the most effective strategy to prevent melanoma progression. From this point of view, phytochemicals have emerged as a promising option for the management of melanoma; indeed, they have demonstrated significant potential for treating melanoma by inhibiting tumor progression, invasion, and metastasis (Pal et al., 2016; Pearlman et al., 2017). In addition, phytochemicals show low toxicity and multi‐target activity that make them good candidates as preventive or adjuvant agents, especially as supplementary treatment in combination with lower doses of existing chemotherapeutic drugs. In the present study, we investigated the effect of an extract, obtained from olive tree (Olea europaea L.) leaves, on invasion, EMT, and lung metastasis of BRAF‐mutated melanoma cells. Previous studies demonstrated a protective role of oleuropein, the major constituent of OLE, against many types of cancer (Shamshoum, Vlavcheski, & Tsiani, 2017) and its ability to sensitize with other chemotherapeutics (Choupani, Alivand, Derakhshan, Zaeifizadeh, & Khaniani, 2019; Menendez et al., 2007). On melanoma cells, oleuropein showed to potentiate the anti‐proliferative activity of two major agents used in BRAF‐resistant melanoma cells, such as Dacarbazine and Everolimus, but also to reverse resistance toward the chemotherapeutic agent Vemurafenib (Ruzzolini et al., 2018). Furthermore, other studies suggest that an olive leaf extract enriched in oleuropein could be even more effective than oleuropein alone, in inhibiting melanoma growth in vitro and in vivo, probably because of the co‐presence of other polyphenols, which can act on different cellular and molecular targets (Mijatovic et al., 2011). Thus, these findings open up the chance to further investigate the potential anticancer effect of OLE. In particular, considering that melanoma is a complex disease with high metastatic potential, it is important to evaluate the efficacy of OLE in preventing tumor metastasis. Indeed, we found that OLE inhibited the proliferation of different metastatic melanoma cell lines in a dose‐dependent manner. This effect was exerted through diverse mechanisms, including cell cycle arrest and induction of apoptotic cell death in human melanoma cells in a caspase 3‐dependent manner. Complex interactions between multiple signaling pathways and the cellular microenvironment enable the transition from melanoma in situ to aggressive, invasive melanoma. The MAPK and PI3K/ATK pathways are the most commonly dysregulated pathways in melanoma (Shtivelman et al., 2014). Hyperactivation of these pathways, following mutation and constitutive activation of the upstream signaling protein, B‐RAF, result in induction of EMT and increased cell invasion and metastasis as well as in the development of resistance to chemotherapy (Lin et al., 2010). In the present study, analysis of OLE treated BRAF‐mutated melanoma cells showed reduction of cell migration and invasion as well as loss of clonogenic properties. These effects were accompanied by inhibition of pERK1/2 and pAKT as well as by reversion of the EMT phenotype switching. Loss of E‐cadherin expression and increased expression of vimentin is common in cancer and it has been associated with metastatic dissemination, tumor cell invasion, and a poor prognosis (Kreizenbeck, Berger, Subtil, Rimm, & Gould Rothberg, 2008; McInroy & Maatta, 2007; Tucci et al., 2007). Here, we demonstrate that OLE treatment prevented EMT in human metastatic melanoma cells which was evident from increased levels of E‐cadherin and reduced expression of vimentin. Highly invasive, pre‐metastatic tumors exhibit high expression of transcription factors such as Twist1 and Zeb1 which are potent promoter of EMT because repress E‐cadherin expression and increase the expression of N‐cadherin and MMP‐2 promoting melanoma invasion. Indeed, in melanoma, the expression of Zeb1 is also associated with a poor prognosis; whereas, suppression of Twist1 inhibits tumor metastatic potential in vivo (Pearlman et al., 2017). Thus, to understand the molecular mechanism behind the effect of OLE on the expression of the EMT‐markers, we focused on the transcriptional repressors Zeb1 and Twist1 and we found that OLE treatment decreased their mRNA expression in both human melanoma cells and murine tumor tissues. More importantly, these effects were then translated in vivo. Indeed, treatment of melanoma‐bearing mice with OLE lead to a significant inhibition of EMT‐mediated carcinogenesis and metastatization. In our study, we demonstrated that OLE administration resulted in reduced expression of mesenchymal marker proteins (vimentin) and upregulated expression of epithelial marker proteins (E‐cadherin) in tumor tissue. Overexpression of MMPs, particularly MMP‐9 and MMP‐2, that induces the degradation of the components of the extracellular matrix, has also been shown to promote cell invasion and metastasis of melanoma (Redondo et al., 2005). Furthermore, MMPs play a critical role in the development of malignant angiogenesis by promoting the release of the proangiogenic factor VEGF (Deryugina & Quigley, 2010). Moreover, OLE treatment induced a significant reduction in MMP‐2 and MMP‐9 levels as well as of VEGF protein expression. The anti‐invasive effects of OLE against BRAF‐mutated melanoma were also associated to a simultaneous targeting of MAPK and PI3K as demonstrated by a reduced activation of both pathways in tumors. The EMT program can be activated in response to pathologic signals such as inflammatory cytokines and chemokines secreted in the primary tumor microenvironment. TNFα is one of the most important pro‐inflammatory cytokines produced in the tumor microenvironment, and it is involved in EMT in multiple cancer cell types (Bates & Mercurio, 2003; Yu, Mu, Sa, Wang, & Xu, 2014). IL‐1β can induces epigenetic modifications, which contributes to promote EMT memory (Li et al., 2020) and upregulation of IL‐6 has been correlated with the induction of EMT in human breast cancers (Dehai et al., 2014). In addition, CXCL1, a chemotaxis‐stimulating factor, recruits various stromal cells into tumor surroundings to create a pre‐metastatic niche to support cancer growth, angiogenesis and metastasis (Kuhn & Cohen, 1986). Thus, the simultaneous inhibition of IL‐6, TNF‐α, IL‐1β, and CXCL1 observed in OLE treated mice could also mediated the anti‐invasive effect of OLE. Collectively, our findings demonstrated that OLE inhibits invasion, progression and metastatization of BRAF‐mutated melanoma cells by modulating the expression of EMT related factors. Thus, our study provides further evidence to support the role of phytochemicals as potential adjuvant therapies to limit the progression of metastatic melanoma. In conclusion, we suggest that OLE has the potential to inhibit the metastatic spread of melanoma cells thanks to its multifaceted mechanistic effects. Thus, OLE may represent a new add‐on therapy for the management of metastatic melanoma, in combination with current therapies. However, further studies are needed to evaluate the synergism between OLE and synthetic drugs on melanoma invasion, migration, and metastasis in vitro, in vivo, and eventually in patients.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Supporting information
FIGURE S1 (a‐b) Apoptotic cell death in human WM983B metastatic melanoma cells was detected by annexin V/propidium iodide (PI) staining. WM983B cells were treated with olive leaf extract (OLE) 200 μg/ml and apoptosis was determined at 24 and 48 hr by flow cytometric analysis. The percentage of apoptotic cells are shown in the bar diagram as the mean ± SEM (n = 3), (*p < 0.05; **p < 0.01 vs. WM983B CTRL). (C‐D) Cell migration of WM983B cells was assessed by wound healing assay following OLE treatment (50 μg/ml). Representative photographs of WM983B cells at 0, 24, and 48 hr are shown. The scratched areas were quantified in three random fields in each treatment. Data are expressed as mean ± SEM of three independent experiments (***p < 0.001; ****p < .0001 vs. WM983B CTRL). (E) mRNA expression of twist1 and zeb1 assessed by qPCR analysis in WM983B cells treated with OLE (50 μg/ml) for 6 hr. Data are expressed as mean ± SEM (n = 3), (*p < .05; **p < .01 vs. WM983B CTRL).
ACKNOWLEDGEMENT
G.E. was supported by Fondazione Umberto Veronesi. Open Access Funding provided by Universita degli Studi di Napoli Federico II within the CRUI‐CARE Agreement.
De Cicco, P. , Ercolano, G. , Tenore, G. C. , & Ianaro, A. (2022). Olive leaf extract inhibits metastatic melanoma spread through suppression of epithelial to mesenchymal transition. Phytotherapy Research, 36(10), 4002–4013. 10.1002/ptr.7587
Funding information Fondazione Umberto Veronesi; AIRC, Grant/Award Number: 26002
DATA AVAILABILITY STATEMENT
Data available on request from the authors
REFERENCES
- Allegra, M. , De Cicco, P. , Ercolano, G. , Attanzio, A. , Busa, R. , Cirino, G. , … Ianaro, A. (2018). Indicaxanthin from Opuntia Ficus Indica (L. mill) impairs melanoma cell proliferation, invasiveness, and tumor progression. Phytomedicine, 50, 19–24. 10.1016/j.phymed.2018.09.171 [DOI] [PubMed] [Google Scholar]
- Alonso, S. R. , Tracey, L. , Ortiz, P. , Perez‐Gomez, B. , Palacios, J. , Pollan, M. , … Rodriguez‐Peralto, J. L. (2007). A high‐throughput study in melanoma identifies epithelial‐mesenchymal transition as a major determinant of metastasis. Cancer Research, 67(7), 3450–3460. 10.1158/0008-5472.CAN-06-3481 [DOI] [PubMed] [Google Scholar]
- Bates, R. C. , & Mercurio, A. M. (2003). Tumor necrosis factor‐alpha stimulates the epithelial‐to‐mesenchymal transition of human colonic organoids. Molecular Biology of the Cell, 14(5), 1790–1800. 10.1091/mbc.e02-09-0583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boss, A. , Bishop, K. S. , Marlow, G. , Barnett, M. P. , & Ferguson, L. R. (2016). Evidence to support the anti‐cancer effect of olive leaf extract and future directions. Nutrients, 8(8), 513. 10.3390/nu8080513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd, S. C. , Mijatov, B. , Pupo, G. M. , Tran, S. L. , Gowrishankar, K. , Shaw, H. M. , … Becker, T. M. (2013). Oncogenic B‐RAF(V600E) signaling induces the T‐Box3 transcriptional repressor to repress E‐cadherin and enhance melanoma cell invasion. The Journal of Investigative Dermatology, 133(5), 1269–1277. 10.1038/jid.2012.421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge, J. A. , Lee, J. C. , Daud, A. , Wells, J. W. , & Bluestone, J. A. (2018). Cytokines, chemokines, and other biomarkers of response for checkpoint inhibitor therapy in skin cancer. Front Med (Lausanne), 5, 351. 10.3389/fmed.2018.00351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramel, J. , Papadogeorgakis, E. , Hill, L. , Browne, G. J. , Richard, G. , Wierinckx, A. , … Tulchinsky, E. (2013). A switch in the expression of embryonic EMT‐inducers drives the development of malignant melanoma. Cancer Cell, 24(4), 466–480. 10.1016/j.ccr.2013.08.018 [DOI] [PubMed] [Google Scholar]
- Chapman, P. B. , Hauschild, A. , Robert, C. , Haanen, J. B. , Ascierto, P. , Larkin, J. , … Group, B. S. (2011). Improved survival with vemurafenib in melanoma with BRAF V600E mutation. The New England Journal of Medicine, 364(26), 2507–2516. 10.1056/NEJMoa1103782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choupani, J. , Alivand, M. R. , Derakhshan, S. M. , Zaeifizadeh, M. , & Khaniani, M. S. (2019). Oleuropein inhibits migration ability through suppression of epithelial‐mesenchymal transition and synergistically enhances doxorubicin‐mediated apoptosis in MCF‐7 cells. Journal of Cellular Physiology, 234(6), 9093–9104. 10.1002/jcp.27586 [DOI] [PubMed] [Google Scholar]
- Connell‐Crowley, L. , Harper, J. W. , & Goodrich, D. W. (1997). Cyclin D1/Cdk4 regulates retinoblastoma protein‐mediated cell cycle arrest by site‐specific phosphorylation. Molecular Biology of the Cell, 8(2), 287–301. 10.1091/mbc.8.2.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies, H. , Bignell, G. R. , Cox, C. , Stephens, P. , Edkins, S. , Clegg, S. , … Futreal, P. A. (2002). Mutations of the BRAF gene in human cancer. Nature, 417(6892), 949–954. 10.1038/nature00766 [DOI] [PubMed] [Google Scholar]
- De Cicco, P. , Busa, R. , Ercolano, G. , Formisano, C. , Allegra, M. , Taglialatela‐Scafati, O. , & Ianaro, A. (2021). Inhibitory effects of cynaropicrin on human melanoma progression by targeting MAPK, NF‐kappaB, and Nrf‐2 signaling pathways in vitro. Phytotherapy Research, 35(3), 1432–1442. 10.1002/ptr.6906 [DOI] [PubMed] [Google Scholar]
- De Cicco, P. , Maisto, M. , Tenore, G. C. , & Ianaro, A. (2020). Olive leaf extract, from Olea europaea L., reduces palmitate‐induced inflammation via regulation of murine macrophages polarization. Nutrients, 12(12), 3663. 10.3390/nu12123663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cicco, P. , Panza, E. , Armogida, C. , Ercolano, G. , Taglialatela‐Scafati, O. , Shokoohinia, Y. , … Ianaro, A. (2017). The hydrogen sulfide releasing molecule acetyl Deacylasadisulfide inhibits metastatic melanoma. Frontiers in Pharmacology, 8, 65. 10.3389/fphar.2017.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehai, C. , Bo, P. , Qiang, T. , Lihua, S. , Fang, L. , Shi, J. , … Zhenjun, Y. (2014). Enhanced invasion of lung adenocarcinoma cells after co‐culture with THP‐1‐derived macrophages via the induction of EMT by IL‐6. Immunology Letters, 160(1), 1–10. 10.1016/j.imlet.2014.03.004 [DOI] [PubMed] [Google Scholar]
- Deryugina, E. I. , & Quigley, J. P. (2010). Pleiotropic roles of matrix metalloproteinases in tumor angiogenesis: Contrasting, overlapping and compensatory functions. Biochimica et Biophysica Acta, 1803(1), 103–120. 10.1016/j.bbamcr.2009.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawan, P. , & Richmond, A. (2002). Role of CXCL1 in tumorigenesis of melanoma. Journal of Leukocyte Biology, 72(1), 9–18. [PMC free article] [PubMed] [Google Scholar]
- El, S. N. , & Karakaya, S. (2009). Olive tree (Olea europaea) leaves: Potential beneficial effects on human health. Nutrition Reviews, 67(11), 632–638. 10.1111/j.1753-4887.2009.00248.x [DOI] [PubMed] [Google Scholar]
- Fecher, L. A. , Amaravadi, R. K. , & Flaherty, K. T. (2008). The MAPK pathway in melanoma. Current Opinion in Oncology, 20(2), 183–189. 10.1097/CCO.0b013e3282f5271c [DOI] [PubMed] [Google Scholar]
- Flaherty, K. T. , Puzanov, I. , Kim, K. B. , Ribas, A. , McArthur, G. A. , Sosman, J. A. , … Chapman, P. B. (2010). Inhibition of mutated, activated BRAF in metastatic melanoma. The New England Journal of Medicine, 363(9), 809–819. 10.1056/NEJMoa1002011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez‐Moreno, O. , Lecanda, J. , Green, J. E. , Segura, V. , Catena, R. , Serrano, D. , & Calvo, A. (2010). VEGF elicits epithelial‐mesenchymal transition (EMT) in prostate intraepithelial neoplasia (PIN)‐like cells via an autocrine loop. Experimental Cell Research, 316(4), 554–567. 10.1016/j.yexcr.2009.11.020 [DOI] [PubMed] [Google Scholar]
- Gorzynik‐Debicka, M. , Przychodzen, P. , Cappello, F. , Kuban‐Jankowska, A. , Marino Gammazza, A. , Knap, N. , … Gorska‐Ponikowska, M. (2018). Potential health benefits of olive oil and plant polyphenols. International Journal of Molecular Sciences, 19(3), 686. 10.3390/ijms19030686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauschild, A. , Grob, J. J. , Demidov, L. V. , Jouary, T. , Gutzmer, R. , Millward, M. , … Chapman, P. B. (2012). Dabrafenib in BRAF‐mutated metastatic melanoma: A multicentre, open‐label, phase 3 randomised controlled trial. Lancet, 380(9839), 358–365. 10.1016/S0140-6736(12)60868-X [DOI] [PubMed] [Google Scholar]
- Kakadia, S. , Yarlagadda, N. , Awad, R. , Kundranda, M. , Niu, J. , Naraev, B. , … Mahmoud, F. (2018). Mechanisms of resistance to BRAF and MEK inhibitors and clinical update of US Food and Drug Administration‐approved targeted therapy in advanced melanoma. Oncotargets and Therapy, 11, 7095–7107. 10.2147/OTT.S182721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreizenbeck, G. M. , Berger, A. J. , Subtil, A. , Rimm, D. L. , & Gould Rothberg, B. E. (2008). Prognostic significance of cadherin‐based adhesion molecules in cutaneous malignant melanoma. Cancer Epidemiology, Biomarkers & Prevention, 17(4), 949–958. 10.1158/1055-9965.EPI-07-2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn, B. S. , & Cohen, S. M. (1986). Care of the HBV positive mother and her infant. Health Care for Women International, 7(4), 329–340. 10.1080/07399338609515745 [DOI] [PubMed] [Google Scholar]
- Lamouille, S. , Xu, J. , & Derynck, R. (2014). Molecular mechanisms of epithelial‐mesenchymal transition. Nature Reviews. Molecular Cell Biology, 15(3), 178–196. 10.1038/nrm3758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, R. , Ong, S. L. , Tran, L. M. , Jing, Z. , Liu, B. , Park, S. J. , … Dubinett, S. (2020). Chronic IL‐1beta‐induced inflammation regulates epithelial‐to‐mesenchymal transition memory phenotypes via epigenetic modifications in non‐small cell lung cancer. Scientific Reports, 10(1), 377. 10.1038/s41598-019-57285-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, K. , Baritaki, S. , Militello, L. , Malaponte, G. , Bevelacqua, Y. , & Bonavida, B. (2010). The role of B‐RAF mutations in melanoma and the induction of EMT via dysregulation of the NF‐kappaB/snail/RKIP/PTEN circuit. Genes & Cancer, 1(5), 409–420. 10.1177/1947601910373795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInroy, L. , & Maatta, A. (2007). Down‐regulation of vimentin expression inhibits carcinoma cell migration and adhesion. Biochemical and Biophysical Research Communications, 360(1), 109–114. 10.1016/j.bbrc.2007.06.036 [DOI] [PubMed] [Google Scholar]
- McKenna, J. K. , Florell, S. R. , Goldman, G. D. , & Bowen, G. M. (2006). Lentigo maligna/lentigo maligna melanoma: current state of diagnosis and treatment. Dermatologic Surgery, 32(4), 493–504. 10.1111/j.1524-4725.2006.32102.x [DOI] [PubMed] [Google Scholar]
- Menendez, J. A. , Vazquez‐Martin, A. , Colomer, R. , Brunet, J. , Carrasco‐Pancorbo, A. , Garcia‐Villalba, R. , … Segura‐Carretero, A. (2007). Olive oil's bitter principle reverses acquired autoresistance to trastuzumab (Herceptin) in HER2‐overexpressing breast cancer cells. BMC Cancer, 7, 80. 10.1186/1471-2407-7-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mijatovic, S. A. , Timotijevic, G. S. , Miljkovic, D. M. , Radovic, J. M. , Maksimovic‐Ivanic, D. D. , Dekanski, D. P. , & Stosic‐Grujicic, S. D. (2011). Multiple antimelanoma potential of dry olive leaf extract. International Journal of Cancer, 128(8), 1955–1965. 10.1002/ijc.25526 [DOI] [PubMed] [Google Scholar]
- Ng, C. Y. , Yen, H. , Hsiao, H. Y. , & Su, S. C. (2018). Phytochemicals in skin cancer prevention and treatment: An updated review. International Journal of Molecular Sciences, 19(4), 941. 10.3390/ijms19040941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olea‐Flores, M. , Zuniga‐Eulogio, M. D. , Mendoza‐Catalan, M. A. , Rodriguez‐Ruiz, H. A. , Castaneda‐Saucedo, E. , Ortuno‐Pineda, C. , … Navarro‐Tito, N. (2019). Extracellular‐signal regulated kinase: A central molecule driving epithelial‐mesenchymal transition in cancer. International Journal of Molecular Sciences, 20(12), 2885. 10.3390/ijms20122885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal, H. C. , Hunt, K. M. , Diamond, A. , Elmets, C. A. , & Afaq, F. (2016). Phytochemicals for the Management of Melanoma. Mini Reviews in Medicinal Chemistry, 16(12), 953–979. 10.2174/1389557516666160211120157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlman, R. L. , Montes de Oca, M. K. , Pal, H. C. , & Afaq, F. (2017). Potential therapeutic targets of epithelial‐mesenchymal transition in melanoma. Cancer Letters, 391, 125–140. 10.1016/j.canlet.2017.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psaltopoulou, T. , Kosti, R. I. , Haidopoulos, D. , Dimopoulos, M. , & Panagiotakos, D. B. (2011). Olive oil intake is inversely related to cancer prevalence: A systematic review and a meta‐analysis of 13,800 patients and 23,340 controls in 19 observational studies. Lipids in Health and Disease, 10, 127. 10.1186/1476-511X-10-127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puisieux, A. , Brabletz, T. , & Caramel, J. (2014). Oncogenic roles of EMT‐inducing transcription factors. Nature Cell Biology, 16(6), 488–494. 10.1038/ncb2976 [DOI] [PubMed] [Google Scholar]
- Ranjan, A. , Ramachandran, S. , Gupta, N. , Kaushik, I. , Wright, S. , Srivastava, S. , … Srivastava, S. K. (2019). Role of phytochemicals in cancer prevention. International Journal of Molecular Sciences, 20(20), 4981. 10.3390/ijms20204981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redondo, P. , Lloret, P. , Idoate, M. , & Inoges, S. (2005). Expression and serum levels of MMP‐2 and MMP‐9 during human melanoma progression. Clinical and Experimental Dermatology, 30(5), 541–545. 10.1111/j.1365-2230.2005.01849.x [DOI] [PubMed] [Google Scholar]
- Romani, A. , Ieri, F. , Urciuoli, S. , Noce, A. , Marrone, G. , Nediani, C. , & Bernini, R. (2019). Health effects of phenolic compounds found in extra‐virgin olive oil, by‐products, and leaf of Olea europaea L. Nutrients, 11(8), 1176. 10.3390/nu11081776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzzolini, J. , Peppicelli, S. , Andreucci, E. , Bianchini, F. , Scardigli, A. , Romani, A. , … Calorini, L. (2018). Oleuropein, the Main polyphenol of Olea europaea leaf extract, has an anti‐cancer effect on human BRAF melanoma cells and potentiates the cytotoxicity of current chemotherapies. Nutrients, 10(12), 1950. 10.3390/nu10121950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamshoum, H. , Vlavcheski, F. , & Tsiani, E. (2017). Anticancer effects of oleuropein. BioFactors, 43(4), 517–528. 10.1002/biof.1366 [DOI] [PubMed] [Google Scholar]
- Shtivelman, E. , Davies, M. Q. , Hwu, P. , Yang, J. , Lotem, M. , Oren, M. , … Fisher, D. E. (2014). Pathways and therapeutic targets in melanoma. Oncotarget, 5(7), 1701–1752. 10.18632/oncotarget.1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva, J. M. , Bulman, C. , & McMahon, M. (2014). BRAFV600E cooperates with PI3K signaling, independent of AKT, to regulate melanoma cell proliferation. Molecular Cancer Research, 12(3), 447–463. 10.1158/1541-7786.MCR-13-0224-T [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglione, L. , Gambuti, A. , De Cicco, P. , Ercolano, G. , Ianaro, A. , Taglialatela‐Scafati, O. , … Forino, M. (2018). NMR‐based phytochemical analysis of Vitis vinifera cv Falanghina leaves. Characterization of a previously undescribed biflavonoid with antiproliferative activity. Fitoterapia, 125, 13–17. 10.1016/j.fitote.2017.12.009 [DOI] [PubMed] [Google Scholar]
- Thiery, J. P. , & Sleeman, J. P. (2006). Complex networks orchestrate epithelial‐mesenchymal transitions. Nature Reviews. Molecular Cell Biology, 7(2), 131–142. 10.1038/nrm1835 [DOI] [PubMed] [Google Scholar]
- Tiwari, N. , Gheldof, A. , Tatari, M. , & Christofori, G. (2012). EMT as the ultimate survival mechanism of cancer cells. Seminars in Cancer Biology, 22(3), 194–207. 10.1016/j.semcancer.2012.02.013 [DOI] [PubMed] [Google Scholar]
- Tucci, M. G. , Lucarini, G. , Brancorsini, D. , Zizzi, A. , Pugnaloni, A. , Giacchetti, A. , … Biagini, G. (2007). Involvement of E‐cadherin, beta‐catenin, Cdc42 and CXCR4 in the progression and prognosis of cutaneous melanoma. The British Journal of Dermatology, 157(6), 1212–1216. 10.1111/j.1365-2133.2007.08246.x [DOI] [PubMed] [Google Scholar]
- Turner, N. , Ware, O. , & Bosenberg, M. (2018). Genetics of metastasis: Melanoma and other cancers. Clinical & Experimental Metastasis, 35(5–6), 379–391. 10.1007/s10585-018-9893-y [DOI] [PubMed] [Google Scholar]
- Yajima, I. , Kumasaka, M. Y. , Thang, N. D. , Goto, Y. , Takeda, K. , Yamanoshita, O. , … Kato, M. (2012). RAS/RAF/MEK/ERK and PI3K/PTEN/AKT signaling in malignant melanoma progression and therapy. Dermatology Research and Practice, 2012, 354191. doi: 10.1155/2012/354191, 1, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, L. , Mu, Y. , Sa, N. , Wang, H. , & Xu, W. (2014). Tumor necrosis factor alpha induces epithelial‐mesenchymal transition and promotes metastasis via NF‐kappaB signaling pathway‐mediated TWIST expression in hypopharyngeal cancer. Oncology Reports, 31(1), 321–327. 10.3892/or.2013.2841 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 (a‐b) Apoptotic cell death in human WM983B metastatic melanoma cells was detected by annexin V/propidium iodide (PI) staining. WM983B cells were treated with olive leaf extract (OLE) 200 μg/ml and apoptosis was determined at 24 and 48 hr by flow cytometric analysis. The percentage of apoptotic cells are shown in the bar diagram as the mean ± SEM (n = 3), (*p < 0.05; **p < 0.01 vs. WM983B CTRL). (C‐D) Cell migration of WM983B cells was assessed by wound healing assay following OLE treatment (50 μg/ml). Representative photographs of WM983B cells at 0, 24, and 48 hr are shown. The scratched areas were quantified in three random fields in each treatment. Data are expressed as mean ± SEM of three independent experiments (***p < 0.001; ****p < .0001 vs. WM983B CTRL). (E) mRNA expression of twist1 and zeb1 assessed by qPCR analysis in WM983B cells treated with OLE (50 μg/ml) for 6 hr. Data are expressed as mean ± SEM (n = 3), (*p < .05; **p < .01 vs. WM983B CTRL).
Data Availability Statement
Data available on request from the authors


