Abstract
Objectives
To report the experience of a high‐volume center with balloon‐expandable (BE) stents implantation to manage vascular complications after transcatheter aortic valve replacement (TAVR).
Background
Despite increased operator experience and better devices, vascular complications after TAVR are still a major issue and covered stent implantation is often required.
Methods
We retrospectively collected baseline and procedural data about 78 consecutive patients who underwent BE stent implantation to manage a vascular complication after transfemoral TAVR. Primary endpoints were technical success, incidence of new‐onset claudication and need for vascular interventions during long‐term follow‐up. Secondary endpoints included length of hospitalization, in‐hospital and 30‐day mortality, and major postoperative complications.
Results
BE stents implantation to manage vascular complications after TAVR was successfully performed in 96.2% of the cases, with bailout surgery required in two cases. One patient suffered in‐hospital death. Predischarge Doppler Ultrasound revealed no cases of in‐stent occlusion or fracture. At a median follow‐up of 429 days (interquartile range, 89−994 days), no cases of symptomatic leg ischemia were reported and only one patient experienced new‐onset claudication.
Conclusions
Our experience showed good periprocedural and long‐term results of BE covered stent implantation to manage vascular complication after TAVR. Their great radial outward force may guarantee effective hemostasis without necessarily being associated with stent deformation/fracture resulting in restenosis or further interventions. More research is needed to define the role of BE covered stents in this setting.
Keywords: balloon expandable covered stent, TAVR, Vascular complications
1. INTRODUCTION
Transcatheter aortic valve replacement (TAVR) emerged over the last decades as a first‐line option treatment for patients with severe symptomatic aortic stenosis. 1 While initially reserved to patients at high and intermediate surgical risk, recent data confirmed the safety and efficacy even in low‐risk patients. 2 , 3 Transfemoral vascular access is the default strategy for TAVR, and it is currently expanding toward more challenging anatomies due to the development of lower‐profile devices, expandable sheaths, and other innovative techniques such as intravascular lithotripsy. 4 , 5
Despite increased operator experience and better devices, vascular complications are still frequent after TAVR. Data from a multicenter registry reported a general incidence of major and minor vascular complications of 14.2% and 13.9%, respectively, according to the Valve Academic Research Consortium definitions, with the vast majority of them occurring after large‐bore vascular closure device (VCD) failure. 6 Access‐site and access‐related vascular injuries (ASARVI) including VCD failure, pseudoaneurysms, arterial dissection, rupture, stenosis, thrombosis, or occlusion are a major concern as they are associated with worse outcomes after TAVR. 7 , 8 , 9 As vascular complications are frequently seen at the end of the TAVR procedure with a contralateral sheath still in place, endovascular management (such as balloon inflation or covered stent implantation 10 , 11 ) is generally the first‐line strategy. Even if we are moving toward the treatment of younger and lower‐risk patients, open surgery is unlikely to replace the percutaneous approach, since the latter is more rapid, immediately available, allows rapid hemostasis and is less invasive as compared to open surgery, which should be still considered in case of major complications or as a bailout option for failure of percutaneous approaches. In case of lesions requiring covered stent implantation, the choice between self‐expandable (SE) and balloon‐expandable (BE) is left to operators. However, as the majority of ASARVI occur at the level of the common or superficial femoral arteries which are subjected to mechanical stresses during leg flexion‐extension, SE devices are generally preferred. Comparative studies of SE and BE stents addressing short and long‐term safety and efficacy for the management of vascular complications after TAVR are lacking. The use of SE stents after TAVR has already been described, 10 with good patency rate also at long‐term follow‐up. 12 Whether the use of BE stents represents a safe and effective option for the management of vascular complications after TAVR is yet to be proved, as no large registry reporting data about the use of BE stents in this setting exists. The aim of the present study is to report the experience of a high‐volume center with BE stents to manage vascular complications after TAVR, and provide the research community with a starting point for future research.
2. METHODS
2.1. Patient population
From June 2010 to February 2019 consecutive patients with iliofemoral vascular access complications treated with BE covered stent implantation following percutaneous transfemoral TAVR at IRCCS Humanitas Research Hospital, Milan were included. Preoperative TAVR assessment included transthoracic echocardiography, coronary angiography, and computed tomography (CT) of the heart, supra‐aortic trunks, aorta and lower limbs to evaluate the most suitable access site and to select prosthesis type and size. Indication for TAVR and vascular access was discussed in a formal multidisciplinary Heart Team meeting. Data were collected retrospectively from the hospital electronic medical records and included baseline clinical characteristics with cardiovascular risk factors and comorbidities, laboratory data, and procedural details. All patients provided informed, written consent before TAVR for both the procedure and the use of anonymized data for research purposes. The study was performed according to ethical principles listed in the Helsinki Declaration.
2.2. TAVR: Procedural details
Procedures were performed in the catheterization laboratory using a fully percutaneous approach under locoregional anesthesia with sedation. Ultrasound (US) and angiographic guided puncture was used as the default approach for all transfemoral TAVR in our institution. During the procedure weight‐adjusted heparin was administered to reach an activated clotting time ≥ 250 s. Large‐bore access closure devices used were both suture‐based or dedicated large‐bore closure devices. After successful valve implantation, the TAVR sheath was removed, and primary access site closure was performed using preclosure devices. Crossover completion angiography via the contralateral femoral, brachial or radial access was systematically performed to detect any vascular access complication. The contralateral safety sheath was removed only if arterial integrity was demonstrated and the access closed by manual compression, preclosure devices, or both. In case of vascular access complication, the safety sheath was used for balloon angioplasty or stenting of the therapeutic iliofemoral access. Before discharge all patients underwent vascular US to check for stent patency and integrity.
2.3. Covered stents: Procedural details
In case of a vascular complication requiring the implantation of a BE covered stent, the 0.018 in. safety guidewire placed through was replaced by a 0.035 in. supportive guidewire and the safety sheath was exchanged by a 7‐F or 8‐F destination guiding sheath. Afterwards, the BE covered stent was advanced over the 0.035 guidewire and placed at the level of the vascular complication under fluoroscopic and angiographic guidance.
2.4. Study endpoints
Primary endpoints were:
-
1.
Technical success, defined as successful implantation without in‐hospital death and without need for bailout surgery. 13
-
2.
Incidence of new‐onset claudication and need for vascular interventions (both percutaneous or surgical) during long‐term follow‐up.
Secondary endpoints were length of hospitalization, in‐hospital and 30‐day mortality, major postoperative (≤30 days) complications (drop in hemoglobin level after the procedure along with number of transfused red blood cells [RBCs] units, predischarge in‐stent occlusion or stent fracture, 30‐day lower limb ischemia, amputation).
Follow‐up was performed either by outpatient visit or by telephone call. At the time of follow‐up, all patients were asked if they had claudication or symptoms suggestive of peripheral limb ischemia (i.e., paresthesias, numbness, sensitivity to cold, tiredness, and fatigue of the limbs). When a Doppler US or contrast enhanced CT scan were available, data about in‐stent restenosis were collected.
2.5. Data analysis
Statistical analysis was performed with SPSS version 17 (IBM SPSS Inc.). Continuous variables were tested for normal distribution with the Kolmogorov−Smirnov test and then presented as mean ± standard deviation or median and interquartile range (IQR), as appropriate. Categorical variables were presented as frequencies and percentages.
3. RESULTS
During the study period 85 BE covered stents (Atrium Advanta V12; Getinge) were implanted in 78 patients undergoing TAVR to manage vascular access complications. During this period, 1107 patients underwent TAVR, with an incidence of vascular complications requiring covered stent implantation of 7%. Baseline and procedural characteristics are reported in Table 1 and Table 2, respectively.
Table 1.
Baseline clinical characteristics
| Clinical characteristics | n = 78 |
|---|---|
| Age, years, mean (SD) | 81 (6) |
| Female sex, n (%) | 50 (64.1) |
| BMI, Kg/m2, mean (SD) | 26.7 (4.9) |
| Diabetes, n (%) | 21 (26.9) |
| Hypertension, n (%) | 60 (76.9) |
| PAD, n (%) | 15 (19.2) |
| COPD, n (%) | 15 (19.2) |
| CAD, n (%) | 37 (47.4) |
| eGFR MDRD, ml/min, mean (SD) | 53.6 (20.2) |
| LVEF < 35%, n (%) | 10 (12.8) |
| Previous MI, n (%) | 23 (29.5) |
| STS score, mean (SD) | 5.17 (2.28) |
| Mean aortic gradient, median (IQR) | 44 (31−57) |
| Aortic valve area, median (IQR) | 0.78 (0.48−1.08) |
Abbreviations: BMI, body mass index; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; IQR, interquartile range; LVEF, left ventricular ejection fraction; MDRD, modification of diet in renal disease; MI, myocardial infarction; PAD, peripheral artery disease; SD, standard deviation; STS, Society of Thoracic Surgeons.
Table 2.
Procedural characteristics
| Procedural characteristics | n = 78 |
|---|---|
| Stent implantation site | |
| Right common femoral artery, n (%) | 65 (83.3) |
| Left common femoral artery, n (%) | 11 (14.1) |
| Right external iliac, n (%) | 2 (2.5) |
| Introducer size (FR) | |
| 14−15, n (%) | 14 (17.9) |
| 16−17, n (%) | 4 (5.1) |
| 18−19, n (%) | 37 (47.4) |
| 20 or >20, n (%) | 23 (29.5) |
| TAVR valve model | |
| CoreValve/Evolut R, n (%) | 37 (47.4) |
| Sapien XT/3, n (%) | 26 (33.3) |
| Acurate Neo, n (%) | 15 (19.2) |
| Vascular access closure device | |
| Prostar, n (%) | 41 (52.5) |
| Proglide, n (%) | 36 (46.2) |
| Manta, n (%) | 1 (1.3) |
Abbreviations: FR, French; TAVR, transcatheter aortic valve replacement.
Short‐term outcomes are reported in Table 3. Technical success was achieved in 75 patients (96.2%), bailout surgery after stent implantation was performed in two cases (2.5%): the first one because of a thrombotic occlusion of the superficial femoral artery and the second due to persistent hemorrhage despite covered stent implantation. In‐hospital death occurred in one case (1.3%) because of a septic shock with multiorgan failure nonrelated to the vascular complication. There was no case of 30‐day mortality after discharge.
Table 3.
Short‐term outcomes
| Short‐term outcomes | |
|---|---|
| Technical success, n/N (%) | 75/78 (96.1) |
| Bailout surgery, n/N (%) | 2/78 (2.6) |
| In‐hospital mortality, n/N (%) | 1/78 (1.3) |
| 30‐day mortality, n/N (%) | 1/67 (1.5) |
| Hb drop, mg/dl, mean (SD) | 2.5 (1.4) |
| Need for blood transfusion, n/N (%) | 40/78 (51.3) |
| N of transfused units per patient, mean (SD) | 1.3 (1.7) |
| ICU length of stay, days, mean(SD) | 2.9 (3.8) |
| Hospital length of stay, days, mean (SD) | 14 (15) |
| Predischarge stent occlusion or stent fracture (by US), n/N (%) | 0/77 (0) |
| 30‐day amputation, n/N (%) | 0/67 (0) |
| 30‐day new onset claudication, n/N (%) | 0/67 (0) |
| 30‐day lower limb ischemia, n/N (%) | 0/67 (0) |
Abbreviations: Hb, hemoglobin; ICU, intensive care unit; SD, standard deviation; US, ultrasound.
Overall mean reduction in hemoglobin concentration was 2.5 ± 1.4 mg/dl and blood transfusion was required in 40 patients (51.3%), with a number of transfused RBCs units of 1.3± 1.7. Overall mean length of stay in Intensive Care Unit was 2.9± 3.8, while mean total hospital length of stay was 14 ± 15 days.
Predischarge vascular US was routinely performed in all patients to check for complete resolution of the vascular lesion along with stent patency. No cases of in‐stent occlusion or stent fracture were recorded. At 30 days, no case of amputation, new onset claudication or lower limb ischemia was documented.
Median clinical follow‐up was 429 days (IQR, 89−994 days). New‐onset claudication was recorded in one patient (1.3%), already affected by peripheral artery disease (PAD), due to disease progression at a different level than the stenting site. No cases of percutaneous or surgical new interventions were reported.
Long‐term Doppler or CT scan imaging was available for 25 patients (32%). No cases of stent fracture or displacement were reported. Besides, in one asymptomatic patient a moderate distal edge stenosis (around 40%) with normal flow was found; in other two patients, already affected by severe PAD, arterial stenoses were found distally or in the contralateral leg. An exemplary case of BE stent implantation for incomplete right common femoral artery closure with its long‐term vascular follow‐up is illustrated in Figure 1.
Figure 1.
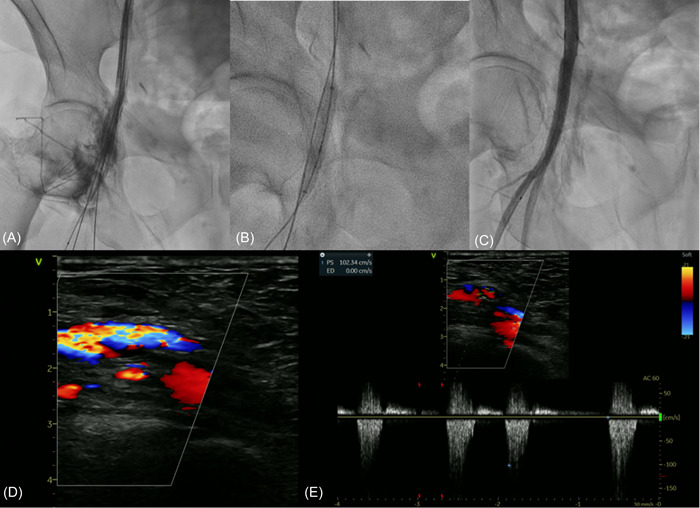
Panel (A) incomplete right common femoral artery closure after TAVR with contrast media spreading. Panel (B) BE stent (Atrium Advanta 38 × 6 mm) deployment. Panel (C) final result showing complete resolution of the vascular complication. Panel (D and E) Color‐Doppler Ultrasound at long‐term follow‐up (900 days) showing good stent patency without stent restenosis (velocity peak 100 cm/s). BE, balloon‐expandable; TAVR, TAVR, transcatheter aortic valve replacement. [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
Covered stents are composed of a metallic scaffold coated with a surface layer of polytetrafluoroethylene (PTFE). Commercially available PTFE covered stent are divided into two main groups, depending on their release mechanism: BE and SE. The most widely available covered stents with their technical characteristics are summarized in Table 4.
Table 4.
List of most common peripheral covered stents
| Device name | Manufacturer | Type | Stent Available Sizes | Recommended Introducer (FR) | Catheter Lenght (mm) | |
|---|---|---|---|---|---|---|
| Diameter (mm) | Lenght (mm) | |||||
| Viahban VBX Balloon Expandable | Gore & Associates | BE | 5, 6, 7, 8, 9, 10, 11 | 15, 19, 29, 39, 59, 79 | 7, 8 | 80, 135 |
| Atrium Advanta (or iCAST in USA) | Getinge | BE | 5, 6, 7, 8, 9, 10 | 16, 22, 32, 38, 59 | 6, 7 | 80, 120 |
| Lifestream | BD Interventional | BE | 5, 6, 7, 8, 9, 10, 12 | 16, 26, 37/38, 58 | 6, 7, 8 | 80, 135 |
| BeGraft | Bentley | BE | 5, 6, 7, 8, 9, 10 | 18, 23, 28, 38, 58 | 6, 7 | 75, 120 |
| E‐ventus BX | JOTEC | BE | 5, 6, 7, 8, 9, 10 | 18, 22, 23, 27, 28, 37, 38, 57, 58 | 6, 7 | 120 |
| Viabahn Self‐Expanding | Gore & Associates | SE | 5, 6, 7, 8, 9, 10, 11, 13 | 25, 50, 100, 150, 250 | 6, 7, 8, 9, 10 | 75, 120 |
| Fluency Plus | BD Interventional | SE | 5, 6, 7, 8, 9, 10, 12, 13.5 | 20, 30, 40, 50, 60, 80, 100, 120 | 8, 9, 10 | 80, 117 |
| Wallgraft | Boston Scientific | SE | 6, 7, 8, 9, 10, 12, 14 | 20, 30, 50, 70 | 9, 10, 11, 12 | ‐ |
Abbreviations: BE, balloon expandable; FR, French; SE, self‐expanding.
BE covered stents, after being placed into desired position on a guidewire, are expanded by balloon inflation. This guarantees a high outward radial force with good apposition and adherence to the inner arterial lumen. 14 Compared to SE stents, BE stents are more rigid and more deformable by external compression, making them the device of choice for the treatment of straight vessels (such as the iliac arteries or the aorto‐iliac bifurcation) but with potential concerns for arteries that are exposed to daily flexion‐extension movements, such as the common and superficial femoral arteries at the groin level. For these reasons, the 2020 Society for Cardiovascular Angiography and Interventions guidelines on device selection in aorto‐Iliac arterial interventions give stronger recommendations to BE than SE stents in treating aorto‐iliac lesions. 15 During study period, Atrium Advanta V12 stents (Getinge) were used in all patients requiring mechanical hemostasis.
SE covered stents are more flexible, elastic and easily adaptable to arterial wall pulsatility. 16 The lower radial strength of SE stents may make them less suited for heavily calcified lesions or lesions with greater recoil. Studies investigating primary patency and efficacy of BE and SE stents for iliac‐femoral lesions have been inconsistent. 14 , 17 , 18 , 19 , 20
Our study reports the real‐life experience of a high‐volume center with BE stents to manage a vascular complication after TAVR, assessing their short‐term safety and efficacy and their long‐term clinical results.
The main findings of this study can be summarized as follows:
-
1.
BE stents implantation is a feasible approach for the management of a vascular complication after TAVR with a high technical success rate (96.2%). These results are comparable with the previously reported data by Sedaghat et al. showing a primary successful stent placement of 96.9% with SE stents. 10
-
2.
Long‐term follow‐up confirmed the good clinical performance of BE covered stents, with one case (1.3%) of new‐onset claudication due to PAD progression at a different level than the stenting site and no case of percutaneous or surgical new interventions reported.
Because of the different technical features of the two devices, peripheral SE are generally preferred over BE stents in clinical practice. 12 As vascular complications after TAVR mainly occur in the iliofemoral axis, frequently subject to flexion‐extension movements, it is widely believed that implanting a BE stent in these vessels could increase the risk of stent fracture, distortion, or in‐stent occlusion. 21 Equally, as BE stents are less resistant to external mechanical stresses, their long‐term patency may be a matter of concern, specifically in active patients. Since comparative data in the setting of TAVR vascular complications are lacking, these concepts are borrowed mainly from the interventional experience in the setting of PAD. On the other hand, BE stents are more precise as compared to SE ones because balloon expansion allows a more accurate adhesion to the inner vasal lumen. Theoretically, as the vast majority of TAVR complications requiring stent implantation are hemorrhagic ones, this could be an advantage, since BE stents allow for a faster and more effective hemostasis. Avoiding the “boogeyman” of the interventional cardiologist, major bleeding events, is crucial when approaching ASARVI, as they are known to be related with higher short and long‐term mortality. 9 Furthermore, the greater precision of BE stents can be an advantage in cases where vascular complication occurs near the femoral bifurcation. Due to its anatomical complexity, in fact, a stenting procedure on a bifurcation lesion is technically difficult and risky and the use of a BE stent could save the operator from performing a much longer and more complex procedure. Finally, BE stents are less expensive, with important cost‐effectiveness considerations, since the number of patients undergoing TAVR is increasing over years.
A previous trial randomizing patients to BE or SE stent for the treatment of iliac artery occlusive disease showed a lower incidence of restenosis and target lesion revascularization at 12 months with SE as compared to BE stents, although this did not translate into a significant difference in walking impairment, hemodynamic success, amputation rate, all‐cause death or periprocedural complications. 14 Several factors, such as the percentage of predilations and postdilations between the two groups, may have contributed to better outcomes with SE stents. Moreover, conclusions derived from PAD are hardly extendable to the specific setting of vascular complications after TAVR, and points of diversity must be taken into account when making comparative considerations. Indeed, while peripheral vessels targeted by the ICE trial (mainly common iliac arteries) are more straight and less stressed during daily movements, injured vessels after TAVR (mainly femoral arteries) are more often tortuous and subject to flexion‐extensions. On the other hand, while target arteries are often calcific in patients with severe aortic stenosis, the prevalence of angiographically relevant atherosclerotic disease is lower as compared with patients treated for clinically overt claudication. Furthermore, the current population of patients undergoing TAVR is mainly composed of elderly patients performing little physical activity, in which the risk of movement induced stent deformation may be lower.
Based on current results, we hypothesize that in a contemporary TAVR population BE stents may be a good alternative for the management of vascular complications after TAVR. Their increased radial force and precision (and, of note, reduced costs), as compared to SE stents, does not seem to reflect on a higher incidence of long‐term disadvantages.
Some trials in the pipeline, such as the SENS‐ILIAC (NCT01834495) randomizing patients to either BE or SE stents for the treatment of atherosclerotic iliac arterial disease will add further evidence to this field. However, dedicated studies comparing SE and BE in the specific setting of vascular access complications after transfemoral TAVR are needed.
5. LIMITATIONS
First, this study has inherent limitations related to its retrospective nature. Second, not all patients underwent imaging follow‐up with Doppler US, so data about asymptomatic stent fracture or in‐stent restenosis are limited. Finally, as mentioned in the discussion, it is possible that the good long‐term clinical results of BE stents may also be related to the relatively low physical activity that treated patients were able to perform. Indeed, as TAVR indication is currently expanding toward a younger cohort of individuals, it is possible that the use of BE covered stents in younger and more active patients may lead to different long‐term outcomes.
6. CONCLUSIONS
Our experience showed good perioperative and long‐term results of BE covered stents implantation to manage vascular complications after TAVR. While present data cannot be generalized, they may be useful to critically re‐evaluate the role of BE stents when approaching vascular complications after transfemoral TAVR.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
Open access funding provided by BIBLIOSAN.
Maurina M, Condello F, Mangieri A, et al. Long term follow‐up after balloon expandable covered stents implantation for management of transcatheter aortic valve replacement related vascular access complications. Catheter Cardiovasc Interv. 2022;100:903‐909. 10.1002/ccd.30385
Matteo Maurina and Francesco Condello contributed equally and are considered share first authors.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2021;42:4207‐4208. [DOI] [PubMed] [Google Scholar]
- 2. Mack MJ, Leon MB, Thourani VH, et al. Transcatheter aortic‐valve replacement with a balloon‐expandable valve in low‐risk patients. N Engl J Med. 2019;380:1695‐1705. [DOI] [PubMed] [Google Scholar]
- 3. Leon MB, Mack MJ, Hahn RT, et al. Outcomes 2 years after transcatheter aortic valve replacement in patients at low surgical risk. J Am Coll Cardiol. 2021;77:1149‐1161. [DOI] [PubMed] [Google Scholar]
- 4. Sawaya FJ, Bajoras V, Vanhaverbeke M, et al. Intravascular Lithotripsy‐Assisted transfemoral TAVI: the Copenhagen experience and literature review. Front Cardiovasc Med. 2021;8:739750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nardi G, de Backer O, Saia F, et al. Peripheral intravascular lithotripsy of iliofemoral arteries to facilitate transfemoral TAVI: a multicentre prospective registry. EuroIntervention. 2021;17(17):e1397‐e1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Van Mieghem NM, Tchetche D, Chieffo A, et al. Incidence, predictors, and implications of access site complications with transfemoral transcatheter aortic valve implantation. Am J Cardiol. 2012;110:1361‐1367. [DOI] [PubMed] [Google Scholar]
- 7. Généreux P, Cohen DJ, Williams MR, et al. Bleeding complications after surgical aortic valve replacement compared with transcatheter aortic valve replacement: insights from the PARTNER I trial (placement of aortic transcatheter valve). J Am Coll Cardiol. 2014;63:1100‐1109. [DOI] [PubMed] [Google Scholar]
- 8. Généreux P, Cohen DJ, Mack M, et al. Incidence, predictors, and prognostic impact of late bleeding complications after transcatheter aortic valve replacement. J Am Coll Cardiol. 2014;64:2605‐2615. [DOI] [PubMed] [Google Scholar]
- 9. Borz B, Durand E, Godin M, et al. Incidence, predictors and impact of bleeding after transcatheter aortic valve implantation using the balloon‐expandable Edwards prosthesis. Heart. 2013;99:860‐865. [DOI] [PubMed] [Google Scholar]
- 10. Sedaghat A, Neumann N, Schahab N, et al. Routine endovascular treatment with a stent graft for access‐site and access‐related vascular injury in transfemoral transcatheter aortic valve implantation. Circ Cardiovasc Interv . 2016;9(8):e003834. [DOI] [PubMed] [Google Scholar]
- 11. Genereux P, Kodali S, Leon MB, et al. Clinical outcomes using a new crossover balloon occlusion technique for percutaneous closure after transfemoral aortic valve implantation. JACC Cardiovasc Interv. 2011;4:861‐867. [DOI] [PubMed] [Google Scholar]
- 12. Sedaghat A, Hansen KL, Schahab N, et al. Long‐term follow‐up after stent graft placement for access‐site and access‐related vascular injury during TAVI—the bonn‐copenhagen experience. Int J Cardiol. 2019;281:42‐46. [DOI] [PubMed] [Google Scholar]
- 13. Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the valve academic research consortium‐2 consensus document. J Am Coll Cardiol. 2012;60:1438‐1454. [DOI] [PubMed] [Google Scholar]
- 14. Krankenberg H, Zeller T, Ingwersen M, et al. Self‐expanding versus balloon‐expandable stents for iliac artery occlusive disease: the randomized ICE trial. JACC Cardiovasc Interv. 2017;10:1694‐1704. [DOI] [PubMed] [Google Scholar]
- 15. Feldman DN, Armstrong EJ, Aronow HD, et al. SCAI guidelines on device selection in Aorto‐Iliac arterial interventions. Catheter Cardiovasc Interv. 2020;96:915‐929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Toggweiler S, Leipsic J, Binder RK, et al. Management of vascular access in transcatheter aortic valve replacement: part 1: basic anatomy, imaging, sheaths, wires, and access routes. JACC: Cardiovasc Interv. 2013;6(7):643‐653. [DOI] [PubMed] [Google Scholar]
- 17. Soga Y, Iida O, Kawasaki D, et al. Contemporary outcomes after endovascular treatment for aorto‐iliac artery disease. Circ J. 2012;76:2697‐2704. [DOI] [PubMed] [Google Scholar]
- 18. Reekers JA, Vorwerk D, Rousseau H, et al. Results of a European multicentre iliac stent trial with a flexible balloon expandable stent. Eur J Vasc Endovasc Surg. 2002;24:511‐515. [DOI] [PubMed] [Google Scholar]
- 19. Ponec D, Jaff MR, Swischuk J, et al. The Nitinol SMART stent vs Wallstent for suboptimal iliac artery angioplasty: CRISP‐US trial results. J Vasc Interv Radiol. 2004;15:911‐918. [DOI] [PubMed] [Google Scholar]
- 20. de Donato G, Bosiers M, Setacci F, et al. 24‐Month data from the BRAVISSIMO: a large‐scale prospective registry on iliac stenting for TASC A & B and TASC C & D lesions. Ann Vasc Surg. 2015;29:738‐750. [DOI] [PubMed] [Google Scholar]
- 21. Bonvini RF, Rastan A, Sixt S, et al. Angioplasty and provisional stent treatment of common femoral artery lesions. J Vasc Interv Radiol. 2013;24:175‐183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


