Abstract
Promoting remyelination is considered as a potential neurorepair strategy to prevent/limit the development of permanent neurological disability in patients with multiple sclerosis (MS). To this end, a number of clinical trials are investigating the potential of existing drugs to enhance oligodendrocyte progenitor cell (OPC) differentiation, a process that fails in chronic MS lesions. We previously reported that oligodendroglia express GABAB receptors (GABABRs) both in vitro and in vivo, and that GABABR‐mediated signaling enhances OPC differentiation and myelin protein expression in vitro. Our goal here was to evaluate the pro‐remyelinating potential of GABABR agonist baclofen (Bac), a clinically approved drug to treat spasticity in patients with MS. We first demonstrated that Bac increases myelin protein production in lysolecithin (LPC)‐treated cerebellar slices. Importantly, Bac administration to adult mice following induction of demyelination by LPC injection in the spinal cord resulted in enhanced OPC differentiation and remyelination. Thus, our results suggest that Bac repurposing should be considered as a potential therapeutic strategy to stimulate remyelination in patients with MS.
Keywords: baclofen, GABAB receptor, multiple sclerosis, myelin, oligodendrocyte, remyelination
Main Points
Baclofen enhances myelin protein synthesis in cerebellar organotypic cultures demyelinated with lysolecithin (LPC).
Baclofen increases oligodendrocyte progenitor cell (OPC) differentiation and remyelination in demyelinated lesions from spinal cord in adult mice.
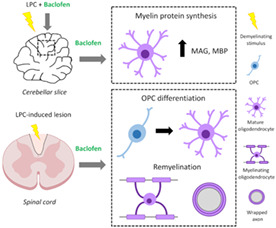
1. INTRODUCTION
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system (CNS) characterized by disseminated demyelination (Dendrou et al., 2015). As a consequence of inflammatory demyelination, action potential conduction is disrupted and axons are deprived from metabolic and trophic support, which leads to axonal loss (Lee et al., 2012; Saab et al., 2016), the main correlate of permanent disability in patients with MS (Trapp et al., 1999). The majority of currently available treatments for MS target CNS inflammation and associated relapses, but do not prevent long‐term disability (Kremer, Akkermann, et al., 2019). Thus, the development of therapies to prevent axonal and neuronal loss remains an unmet therapeutic need for patients with MS (Lubetzki et al., 2020). Remyelination is the spontaneous regeneration of myelin that prevents axonal degeneration both in animal models (Irvine & Blakemore, 2008; Mei et al., 2016) and patients with MS (Kornek et al., 2000). However, in most patients, the efficiency of this process decreases significantly with age and disease progression (Franklin and ffrench‐Constant, 2017). Therefore, the development of novel treatments that enhance remyelination is a major goal of current MS research, and includes the repurposing of existing drugs (Kremer, Göttle, et al., 2019).
A block in OPC differentiation (Kotter et al., 2006; Kuhlmann et al., 2008) and lack of myelin sheath formation by surviving mature oligodendrocytes (OLs) have been pointed out as important contributors to remyelination failure in MS (Duncan et al., 2018; Yeung et al., 2019; Heb et al., 2020; Franklin et al., 2021). Therefore, promoting OPC differentiation and improving OL myelination capacity are potential strategies for enhancing myelin repair and preventing neurodegeneration in this disease.
Neurotransmitters are important mediators of OPC‐neuron communication with a clear influence on OPC behavior (Domercq et al., 2010; Fannon et al., 2015; Hamilton et al., 2017; Li et al., 2013; Serrano‐Regal, Luengas‐Escuza, et al., 2020; Zonouzi et al., 2015). As OPCs receive both excitatory and inhibitory synaptic inputs, mediated by glutamate and GABA (Bergles et al., 2000; Káradóttir et al., 2008; Kukley et al., 2008; Lin & Bergles, 2004), these molecules have been identified as key regulators of oligodendroglial maturation and myelination (Bai et al., 2021; Fannon et al., 2015; Gautier et al., 2015; Serrano‐Regal, Bayón‐Cordero, et al., 2020).
Regarding myelin repair, GABAergic signaling through GABAARs has been associated with remyelination after focal demyelination in the rat corpus callosum (Kalakh & Mouihate, 2019), as well as in the caudal cerebellar peduncle (Cisneros‐Mejorado et al., 2020). GABABRs have also been suggested as important modulators of myelination given that GABABR antagonism increased OPC proliferation while decreasing their maturation and the production of myelin‐related proteins in the developing rat cingulum (Pudasaini et al., 2022). However, the role of oligodendroglial GABABRs in myelin regeneration remains to be investigated.
Baclofen (Bac), the best known GABABR agonist, is currently used as a therapeutic agent for spasticity in MS, and can be administered either intrathecally or orally because it crosses the blood–brain barrier (Ertzgaard et al., 2017). We previously reported that GABABR activation by Bac promotes differentiation and myelin protein expression in rat cortical OPC (Serrano‐Regal, Luengas‐Escuza, et al., 2020). Here, we investigated whether Bac modulates remyelination in lysolecithin (LPC)‐demyelinated organotypic cerebellar slices as well as in LPC spinal cord lesions in adult mice. Our results demonstrate that Bac stimulates myelin protein production ex vivo and enhances remyelination in vivo, which suggests that this drug may also be a useful therapeutic agent to stimulate remyelination.
2. MATERIALS AND METHODS
2.1. Animals
All experiments were conducted with the approval of the ethical committee of the University of the Basque Country (UPV/EHU). Animals were handled in accordance with the European Directive 2010/63/EU and were housed under standard conditions with a 12 h light–dark cycle and ad libitum access to food and water. All possible efforts were made to minimize animal suffering and the number of animals used. Sprague Dawley rats, C57BL/6 mice, and transgenic mice expressing fluorescence reporter DsRed under the control of the glial‐specific proteolipid protein promoter (PLP‐DsRed; Hirrlinger et al., 2005), generously provided by Prof. Dr. F. Kirchhoff (University of Saarland, Homburg, Germany), were used in this study.
2.2. Cerebellar organotypic slice culture
Slice cultures were made from cerebella of P5‐P7 or P11‐day‐old Sprague Dawley rats and P11‐day‐old transgenic PLP‐DsRed mice according to previously described procedures (Doussau et al., 2017; Dusart et al., 1997; Tan et al., 2018). Briefly, cerebella were cut with a tissue chopper (Mcllwain) into 350 μm parasagittal slices. Meninges were removed and slices were plated onto 0.4 μm pore size Millicell CM culture inserts (Millipore), containing 2–3 slices each. Rat slices were maintained in six‐well plates for 13–15 days and mice cerebellar slices for 11 days in culture medium consisting of 50% basal medium with Earle's salt (BME), 25% Hank's Balanced Salt Solution (HBSS), 25% inactivated horse serum (all from ThermoFisher Scientific), 5 mg/ml glucose (Panreac), 0.0025 mM L‐glutamine (Sigma‐Aldrich) and antibiotic‐antimycotic solution (100 U/ml of penicillin, 100 μg/ml of streptomycin and 0.25 μg/ml of amphotericin B; ThermoFisher Scientific) at 37°C in a humidified atmosphere with 5% CO2. Culture medium was replaced every 2–3 days. Slices were treated with GABAergic drugs starting on the day 2 in vitro (Table 1). Lysolecithin (LPC)‐induced demyelination experiments were carried out in cerebellar slices from P11 animals at day 7 in vitro by incubation for 16 h with 0.5 mg/ml LPC (Sigma‐Aldrich) (Birgbauer et al., 2004). Treatments were performed at the same time as the LPC‐stimulus. Slices were fixed in culture inserts with 4% paraformaldehyde (PFA) solution in phosphate‐buffered saline (PBS; pH 7.4) for immunochemistry or processed for western blot analysis at 4 and 6 days after treatment.
TABLE 1.
GABAergic agonists and antagonists used in this study
| Product | Reference | Supplier | Concentration (in vitro) |
|---|---|---|---|
| GABA | A2129 | Sigma‐Aldrich | 100 μM |
| Gabazine | SR‐95531 | Sigma‐Aldrich | 50 μM |
| Baclofen | 0796 | Tocris Bioscience | 100 μM |
| Muscimol | 0289 | Tocris Bioscience | 100 μM |
2.3. Optic nerve‐derived organotypic slice culture
Cultures were obtained from optic nerves of P11‐day‐old transgenic PLP‐DsRed mice. Optic nerves together with the retina were extracted in order to maintain tissue organization and cellular connections. Meninges and residual tissue were removed in supplemented (2 μl/ml gentamicin, 1 mg/ml bovine serum albumin, BSA and 2 mM L‐glutamine) HBSS under the microscope, and the optic nerve‐retina units were maintained in 0.4 μm pore size Millicell CM culture inserts (Millipore), containing one unit each. Explants were placed in six‐well plates for 3 days in the culture medium as described above for cerebellar organotypic cultures and in the same conditions. To favor appropriate feeding of the optic nerve‐retina unit, 50 μl of culture medium were added directly over the tissue (Azim & Butt, 2011). GABABR specific agonist baclofen (100 μM) was added to the medium immediately after plating and maintained for 3 days with daily renewal. Optic nerves without retina were fixed with 4% PFA in PBS and whole‐mounted on slides with Prolong™ Gold antifade (Invitrogen).
2.4. EdU labeling and detection
5‐ethynyl‐2′‐deoxyuridine (EdU; Invitrogen) (10 μM) was added to the organotypic medium at day 5 in vitro and left for 48 h, to label proliferating cells. EdU was revealed using Click‐iT Alexa Fluor 647 Imaging Kit according to the manufacturer's instructions (Invitrogen).
2.5. Demyelinating lesion induction
Demyelinating lesions were induced in the spinal cord of 10‐week‐old female C57BL/6 mice by a stereotaxic injection of 0.5 μl of 1% LPC (Sigma‐Aldrich) in sterile 0.9% NaCl solution, as previously described (Tepavcevic et al., 2014). Mice were anesthetized by intraperitoneal injection (i.p.) of a solution of ketamine (90 mg/kg; Fatro)/xylazine (20 mg/kg; Calier). Buprenorphine (0.1 mg/kg; Dechra) was subcutaneously administered as postoperative analgesic treatment. Daily i.p. injections of vehicle (saline solution) or baclofen (8 mg/kg) were performed from 5 to 16 days post lesion (dpl). Mice were sacrificed at 12 or 16 dpl, and the tissue was processed for immunohistochemical (IHC) or transmission electron microscopy (TEM) analysis, respectively.
2.6. Perfusion and tissue processing
Mice were euthanized with ketamine/xylazine and transcardially perfused with 2% PFA solution in PBS for IHC analysis or 4% glutaraldehyde in 0.1 M PB for TEM studies. For IHC analysis, spinal cords were post‐fixed with the same PFA solution, cryoprotected in 15% sucrose solution (Panreac) and frozen in 7% gelatin (Sigma‐Aldrich)/15% sucrose solution in PBS. Samples were cut using a cryostat CM3050 S (Leica) to obtain 12 μm‐thick coronal sections. For TEM studies, spinal cords were postfixed overnight, washed in 0.1 M PB, and cut into 2 mm‐thick blocks. The tissue was postfixed in 1% osmium solution in 0.1 M PB, dehydrated and embedded in epoxy resin (Sigma Aldrich). Semithin (1 μm‐thick) and ultrathin (55 nm‐thick) sections were cut with an ultramicrotome RMC Boeckeler.
2.7. Immunochemistry
Cerebellar slices were washed in PBS, permeabilized and blocked in 4% goat serum and 0.1% Triton X‐100 in PBS (blocking buffer) for 1 h and incubated overnight at 4°C with primary antibodies (Table 2). Slices were washed in PBS with 0.1% Triton X‐100 and incubated with Alexa fluorophore‐conjugated secondary antibodies (1:400; Invitrogen) in blocking buffer for 1 h at RT. Slides with cryostat spinal cord sections were air‐dried for 1 h, rehydrated in Tris buffer saline (TBS; 20 mM Tris and 1.4 M NaCl in dH2O; pH 7.6) and pre‐treated with absolute ethanol (Sharlab) for 15 min at −20°C. For APC and Olig2 immunostaining, antigen retrieval was performed by heating the sections in low‐pH retrieval buffer (Vector Laboratories) for 45 s using a microwave. After washing, samples were incubated in blocking buffer solution (1% BSA, 5% goat serum, and 0.1% Triton X‐100) for 30 min at RT, and then with the primary antibodies diluted in blocking buffer overnight at 4°C (Table 2). Sections were washed in TBS, and incubated with Alexa fluorophore‐conjugated secondary antibodies (1:500; Invitrogen) in blocking solution for 1 h at RT. Cell nuclei were counterstained with DAPI (4 μg/ml, Sigma‐Aldrich) and sections were mounted with Fluoromount‐G (SouthernBiotech).
TABLE 2.
Antibodies used in this study for immunohistochemistry
| Antibody | Host | Dilution (rat tissue) | Dilution (mouse tissue) | Supplier | Reference |
|---|---|---|---|---|---|
| Anti‐GABARB1 | Rabbit | 1:200 | 1:200 | Alomone labs | AGB‐001 |
| Anti‐GABARB1 | Mouse | — | 1:200 | Abcam | #55051 |
| Anti‐GABARB2 | Rabbit | 1:200 | 1:200 | Alomone labs | AGB‐002 |
| Anti‐APC (clone CC1) | Mouse | 1:200 | 1:200 | Calbiochem | #OP80 |
| Anti‐Olig2 | Mouse | 1:200, 1:1000 | 1:500 | Millipore | #MABN50 |
| Anti‐MBP | Chicken | — | 1:200 | Millipore | #AB9348 |
| Anti‐PDGFRα | Rat | — | 1:300 | BD Biosciences | #558774 |
| Anti‐Iba1 | Guinea pig | — | 1:200 | Synaptic systems | 234,004 |
| Anti‐Nkx2.2 | Mouse | — | 1:20 | Developmental Studies Hybridoma Bank | #Q4818001B |
| Anti‐GFAP | Rabbit | 1:100 | Millipore | #AB5804 |
2.8. Image acquisition and analysis
Images from cerebellar organotypic slices and optic nerve explants were acquired using Zeiss LSM800 and/or Leica TCS SP8 laser scanning confocal microscopes. Cells in cerebellar slices were counted blindly along the z‐stack using a 20× objective in Leica TCS SP8 confocal microscope. At least 3 different fields from 2 slices per experiment were analyzed by using LAS AF Lite software (Leica). The fluorescence signal corresponding to the PLP‐DsRed OLs was quantified by ImageJ software and data were expressed as arbitrary units of fluorescence for each experimental situation. Images from spinal cord sections were collected using Zeiss LSM800 and/or Leica TCS SP8 confocal microscope and imported to ImageJ software. Area lacking myelin basic protein (MBP) staining within the dorsal funiculus of the spinal cord (area of demyelination) was delimited as region of interest (ROI) and measured. Cells positive for the markers of interest were counted from at least 3 different slices per animal. Results are presented as percentage of positive cells per lesion area measured or percentage area of lesion occupied by the corresponding markers. Same settings were kept for all samples (control and treated) belonging to a specific experiment. All images are shown as projections from z‐stacks. For TEM studies, semi thin sections stained with Richardson's Blue were used to identify the lesion area. Ultrathin sections were cut and contrasted by incubation in 4% uranyl acetate and lead citrate solution for its visualization in Philips CM200 transmission electron microscope. Remyelinated axons were counted. Remyelination was determined as the percentage of OL and Schwann cell (SC)‐remyelinated axons within the total numbers of axons initially demyelinated (those remyelinated + those demyelinated).
2.9. Western blot
After treatments, cerebellar slices were directly resuspended in sodium dodecyl sulfate sample buffer on ice to enhance the lysis process and avoid protein degradation. Samples were boiled at 99°C for 8 min, size‐separated by sodium dodecyl sulfate polyacrilamide gel electrophoresis (SDS‐PAGE) in 4%–20% Criterion TGX Precast gels and transferred to Trans‐Blot Turbo Midi PVDF Transfer Packs (Bio‐Rad, Hercules). Membranes were blocked in 5% BSA (Sigma‐Aldrich) in Tris‐buffered saline/ 0.05% Tween‐20 (TBS‐T) and proteins were detected with specific primary antibodies (Table 3). Membranes were incubated with horseradish peroxidase‐conjugated secondary antibodies (1:2000; Sigma‐Aldrich) and were developed by using an enhanced chemiluminescence detection kit according to the manufacturer's instructions (Supersignal West Dura or Femto; ThermoFisher Scientific). Protein bands were detected with a ChemiDoc XRS Imaging System (Bio‐Rad) and quantified by volume using ImageLab software (version 3.0; Bio‐Rad).
TABLE 3.
Antibodies used in this study for western blot analysis
| Antibody | Host | Dilution | Supplier | Reference |
|---|---|---|---|---|
| Anti‐MAG | Mouse | 1:500 | Santa Cruz | SC‐376145 |
| Anti‐CNPase | Mouse | 1:1000 | Sigma‐Aldrich | #C5922 |
| Anti‐MBP | Mouse | 1:1000 | Biolegend | #SMI 99 |
| Anti‐GAPDH | Mouse | 1:1000 | Millipore | #MAB374 |
| Anti‐β‐tubulin | Mouse | 1:5000 | abcam | AB7291 |
2.10. Statistical analysis
All data are presented as mean ± SEM. Statistical analyses were performed using GraphPad Prism statistical software (version 8.0; GraphPad software). Comparisons between multiple experimental groups were made using one‐way analysis of variance (ANOVA) followed by Tukey's post hoc test. For comparisons between two groups, we used the two‐tailed Student's t‐test assuming equal variance. In all instances, statistical differences were considered significant where p < .05. All the images shown represent the data obtained from at least three independent experiments.
3. RESULTS
3.1. Baclofen treatment increases myelin protein levels in organotypic slice cultures
We first validated the role of the GABAergic signaling in regulating oligodendroglial differentiation and myelination in organotypic cultures obtained from P5‐P7 rats. We investigated GABAB1 and GABAB2 receptor‐subunit expression during myelination ex vivo, and found that oligodendroglial cells–labeled using anti‐Olig2 antibody–, and more specifically mature OLs–labeled using anti APC antibody–, express the two GABABR subunits (Figure 1a,b), as we previously observed in OLs in vitro and in vivo (Serrano‐Regal, Luengas‐Escuza, et al., 2020).
FIGURE 1.
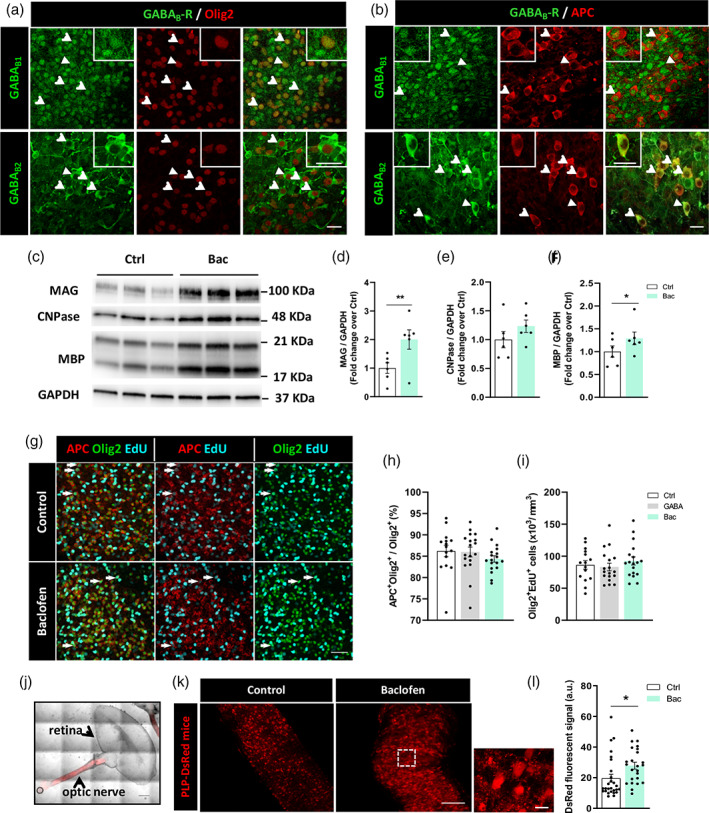
Baclofen increases myelin‐related protein synthesis in organotypic cultures without altering the proliferation ratio of oligodendroglial lineage. (a) Oligodendroglial cells, distinguished as Olig2+ cells (red), are positive for GABAB1 and GABAB2 subunits (green) of GABABRs in cerebellar slices of P5‐P7 rats. (b) Mature oligodendrocytes (OLs), identified as APC+ cells (red), express GABAB1, and GABAB2 subunits (green) of GABABRs in the same preparations. Arrows indicate double‐stained cells and arrowheads show the cell magnified in the corresponding inset. Scale bars = 20 μm. (c) Representative western blot image‐showing expression of myelin‐associated glycoprotein (MAG), CNPase, and MBP proteins in control and baclofen‐treated cerebellar slices. Quantification of MAG (d), CNPase (e), and myelin basic protein (MBP) (f) expression normalized to GAPDH values. *p < .05 and ** p < .01 versus control; paired Student's t‐test. (g) Representative images showing immunofluorescence of mature OLs (APC+, red) and total oligodendroglial cells (Olig2+, green) co‐labeled with EdU (cyan) to identify mature OLs (APC+) and Olig2+ cells in cerebellar slices in the indicated condition. Arrows indicate mature OLs (APC+Olig2+) or newly generated oligodendroglial cells (Olig2+EdU+). Scale bar = 50 μm. Quantification of (h) percentage of mature OL from total oligodendroglial cell pool (APC+Olig2+/Olig2+) and (i) density of newly formed oligodendroglial cells (Olig2+EdU+), in the indicated conditions. One‐way ANOVA followed by Tukey's post‐test. (j) Optic nerve‐retina unit from P11 PLP‐DsRed transgenic mice. Scale bar = 500 μm. (k) Optic nerves explants cultured in control conditions (left) or in presence of baclofen (right) showing DsRed fluorescent signal. Scale bars = 100 μm; higher magnification scale bar = 315 μm. (l) Quantification of DsRed fluorescent signal in control and treated optic nerve explants. *p < .05 versus control; unpaired Student's t‐test. (a–f): Control and bac 6 slices from different animals. (g–i): Control 15, GABA 18, and bac 18 images from cerebellar slices. (j–l): Control 27, bac 24 images from optic nerve explants
Then, we were interested in evaluating whether GABA agonists could also modulate myelin‐protein expression levels in cerebellar organotypic cultures. Exposure to 100 μM GABA or 100 μM muscimol (Mus; GABAAR specific agonist) did not change the levels of expression of myelin‐associated glycoprotein (MAG), 2′,3′‐cyclic nucleotide‐3′‐ phosphodiesterase (CNPase) and MBP, compared with control slices (Supplementary Figures S2 and S3). However, treatment with 100 μM Bac (Figure 1c) induced a significant increase in the expression of MAG and MBP myelin proteins (2.0 ± 0.34 Bac vs. 1.0 ± 0.19 control for MAG, Figure 1d; and 1.29 ± 0.14 Bac vs. 1.0 ± 0.12 control for MBP, Figure 1f), together with a non‐significant increase in the expression of CNPase (1.23 ± 0.11 Bac vs. 1.0 ± 0.14 control for CNPase, Figure 1e). This increase in MBP and MAG is similar to the effect of Bac in cultured OPCs, and becomes abrogated in the presence of GABABR specific antagonist CGP55845 (Supplementary Figure S1). To investigate whether this effect of Bac was associated with changes in oligodendroglial proliferation, cerebellar organotypic cultures were exposed to EdU for 48 h, in the absence or presence of GABA or Bac (100 μM). Neither GABA nor Bac modified the percentage of mature OLs (APC+Olig2+) among total oligodendroglial cells, nor Olig2+ cells that underwent proliferation in this time period (Olig2+Edu+) (Figure 1g–i), suggesting that GABABR activation promotes myelin generation by mature OLs without affecting OPC proliferative capacity. Additionally, we examined the effect of Bac in optic nerve explants of transgenic PLP‐DsRed mice (Figure 1j). Quantification of PLP‐DsRed‐fluorescent signal (Figure 1k) revealed a significant increase in those optic nerves treated with Bac compared to controls (27.82 ± 2.27 for Bac vs. 19.62 ± 2.59 for control; Figure 1l). Together, these results show that Bac enhances myelin protein production in murine organotypic cultures and in optic nerve explants, confirming our previous observations in isolated OLs (Serrano‐Regal, Luengas‐Escuza, et al., 2020) in a complex environment more similar to physiological conditions.
3.2. GABABR activation elevates the levels of major myelin proteins during remyelination ex vivo
Since Bac promoted myelin protein synthesis in organotypic slices, we next studied the impact of GABABR activation under experimental conditions mimicking damage to myelin. P11 rat‐derived cerebellar slices were maintained for 7 days to allow myelination ex vivo and then exposed to LPC for 16 h. In the first set of experiments, slices were daily treated with GABA (100 μM) or Bac (100 μM) for 6 days after LPC exposure (Supplementary Figure S4A, B) and MAG and MBP proteins were analyzed by western blot. We found that LPC induced a significant decrease in both proteins (0.99 ± 0.11 LPC vs. 1.44 ± 0.11 fold control for MAG, and 1.00 ± 0.08 LPC vs. 1.50 ± 0.09 fold control for MBP). Bac treatment post‐LPC significantly increased MAG levels (1.44 ± 0.11 Bac vs. 0.99 ± 0.11 LPC; Supplementary Figure S4C) while MBP levels were not affected (Supplementary Figure S4D). We then investigated whether previous application of these agonists during demyelinating phase may be more effective in recovering the levels of myelin protein expression after exposure to LPC. We exposed cerebellar slices to LPC concomitantly to GABA or Bac (100 μM) application. After LPC removal, GABA or Bac was maintained in the medium for 6 more days (Figure 2a). Under this experimental paradigm, we observed a significant increase in the expression levels of both MAG and MBP (2.75 ± 0.35 GABA and 2.81 ± 0.26 Bac vs. 1.0 ± 0.26 LPC for MAG, and 3.39 ± 0.52 GABA and 2.82 ± 0.29 Bac vs. 1.0 ± 0.37 LPC for MBP; Figure 2b–d).
FIGURE 2.
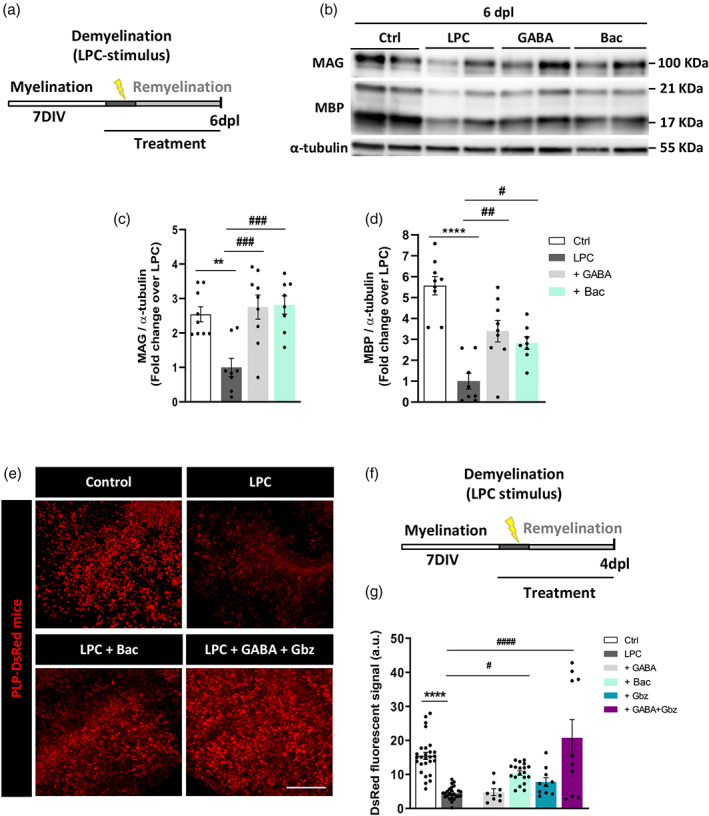
GABABRs modulate remyelination in lysolecithin (LPC)‐treated cerebellar organotypic slices. (a) Time course showing the experimental design in LPC‐induced demyelination in cerebellar organotypic cultures obtained from P11 rats. (b) Representative western blot image showing in duplicates the effect of GABA and baclofen in modulating myelin‐related protein restoration in LPC‐treated organotypic cultures following the paradigm shown in a. (c, d) Quantification of myelin‐associated glycoprotein (MAG) (c) and myelin basic protein (MBP) (d) levels in indicated conditions. **p < .01 and ****p < .0001 versus control, ## p < .01 and ### p < .001 versus LPC; one‐way ANOVA followed by Tukey's post‐test. (e) Representative images of cerebellar slices from P11 PLP‐DsRed transgenic mice showing DsRed fluorescence in indicated conditions. Scale bar = 100 μm. (f) Treatments were added to the slices in conjunction with LPC for 16 h and maintained thereafter for 4 days after. GABABRs were selectively activated with baclofen or with GABA plus the GABAAR antagonist gabazine. (g) Quantification of DsRed fluorescent signal in indicated conditions. ****p < .0001 versus control, # p < .05 and #### p < .001 versus LPC; one‐way ANOVA followed by Tukey's post‐test. (b–d): Control 9, LPC 8, GABA 9, bac 8 slices from different animals. (e–g): Control 27, LPC 25, GABA 8, bac 19, Gbz 10, GABA + Gbz 10 images from optic nerve explants
We also used immunofluorescence to investigate the effect of GABABR‐mediated signaling on OL differentiation and myelin protein production ex vivo by taking advantage of PLP‐DsRed reporter mice, in which changes in PLP‐associated endogenous fluorescence can be exploited to track changes in PLP‐expression. We prepared organotypic cerebellar slices and maintained these in culture for 7 days, after which we exposed the slices to LPC in combination with drug treatments for 4 days. We applied GABA (100 μM), Bac (100 μM), and GABA plus gabazine (50 μM)–a GABAAR antagonist–, in order to study the effect of GABA directly over GABABRs, or gabazine alone (50 μM) (Figure 2e,f). As shown in Figure 2g, the PLP‐DsRed fluorescent signal increased significantly in Bac‐ and GABA plus gabazine‐treated slices compared to those exposed to LPC without treatment (10.42 ± 0.65 Bac and 20.74 ± 5.32 GABA plus gabazine vs. 4.5 ± 0.38 LPC; Figure 2g), indicating that GABAB receptor stimulation in these slices increases PLP production. Overall, these results indicate that GABABR activation with Bac favors remyelination ex vivo in cerebellar organotypic slices.
3.3. Baclofen administration promotes OPC differentiation in adult mouse CNS
We then aimed to assess the effect of Bac administration on CNS remyelination in vivo. We first validated the expression of GABABR subunits in OPCs in normal spinal cord tissue of adult mice (Figure 3a), and observed expression of both B1 and B2. We then confirmed that this expression was maintained after induction of demyelination by LPC injection in the dorsal funiculus, both on reactive OPCs (Figure 3b) and by newly generated OLs (Figure 3c), which suggested these cells could be targeted by Bac.
FIGURE 3.
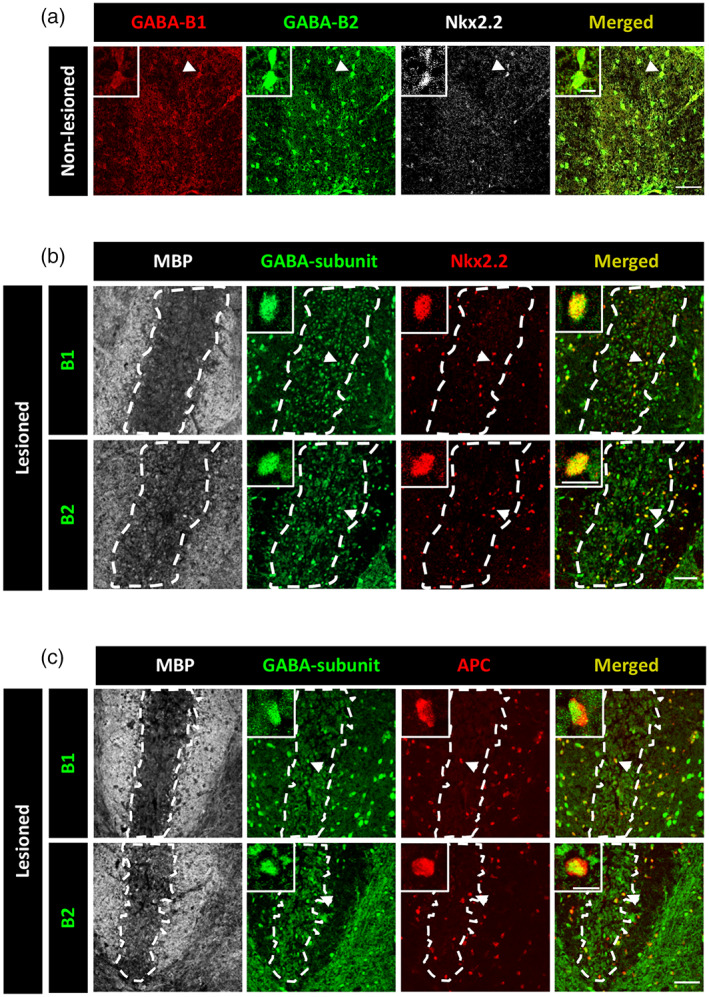
GABAB receptors are expressed by oligodendrocyte progenitor cell (OPCs) and mature oligodendrocytes from the mouse spinal cord. (a) Confocal images showing OPCs (Nkx2.2+, gray) expressing GABAB1 (red) and GABAB2 (green) subunits of GABABRs in the dorsal funiculus of the spinal cord of unlesioned mice. (B, C) Confocal images showing OPCs (Nkx2.2+, red) (b) and mature OLs (APC+, red) (c) expressing GABAB1 (green, top) and GABAB2 (green, bottom) subunits of GABABRs in the dorsal funiculus of the spinal cord of control lysolecithin (LPC)‐injected mice. White dash line indicates lesion border. Arrowheads point at cells shown at higher magnification in each photograph. Scale bars = 50 μm. Higher magnification = 10 μm
To investigate the effect of Bac on remyelination, at 5 days post lesion (dpl), daily i.p. injections of vehicle or Bac (8 mg/kg/day) were initiated and administered during 7 days. Bac administration was initiated at 5 dpl to ensure that the treatment would not affect the extent of demyelination, as demyelination in LPC model is complited by 2 dpl. OPC differentiation was investigated at 12 dpl, given that the peak of OPC differentiation occurs during the second week post demyelination (Figure 4a).
FIGURE 4.
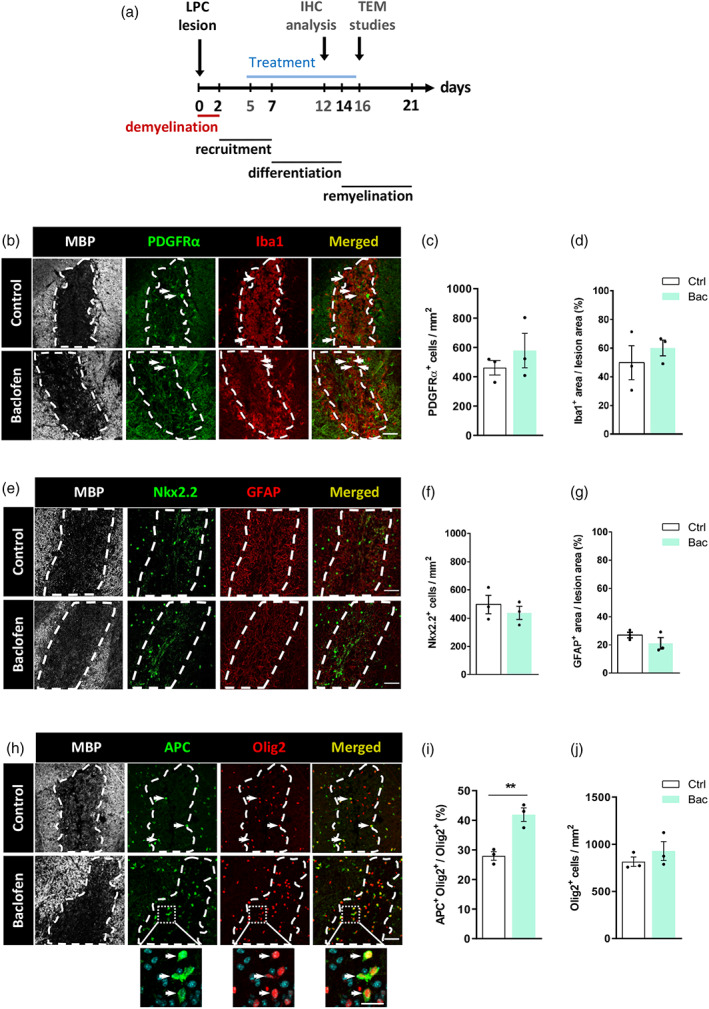
GABAB receptor activation by baclofen promotes oligodendrocyte differentiation but does not impact on oligodendrocyte progenitor cell (OPC), microglia/macrophague population or astrocytes in lysolecithin (LPC)‐induced demyelinated spinal cords. (a) Diagram representing the time course of the studies in the LPC‐induced demyelination model. (b) Spinal cord sections of LPC‐injected control (top) and baclofen‐treated (bottom) mice immunostained with anti‐MBP (gray), anti‐PDGFRα (green) and anti‐Iba1 (red) antibodies. Dapi was used to identify cell nuclei. White dash line indicates lesion border. Quantification of number of PDGFRα+ cells per mm2 (c) and percentage of Iba1+ occupied area (d) in LPC‐injected mice. (e) Spinal cord sections of LPC‐injected control (top) and baclofen‐treated (bottom) mice immunostained with anti‐MBP (gray), anti‐Nkx2.2 (green) and anti‐GFAP (red) antibodies. Dapi was used to identify cell nuclei. White dash line indicates lesion border. Quantification of number of Nkx2.2+ cells per mm2 (f) and percentage of GFAP stained area (g) in LPC‐injected mice. (h) Spinal cord sections of LPC‐injected control (top) and baclofen‐treated mice immunostained with anti‐MBP (gray), anti‐APC/CC1 (green) and anti‐Olig2 (red) antibodies. Dapi was used to identify cell nuclei. White dash line indicates lesion border. Histograms showing percentage of APC+Olig2+ cells from total Olig2+ cells (i), and number of Olig2+ cells per mm2 (j) in LPC‐injected mice. At least 3 lesion areas from 3 different animals were analyzed. **p < .01 versus control; unpaired Student's t‐test, control 3 animals, bac 3 animals. Scale bars: B, E, H = 50 μm. Higher magnification = 20 μm. Arrows show positive staining
Then, we explored the changes induced by Bac administration in OPC numbers and microglia/macrophage response at 12 dpl using anti‐PDGFRα antibody as OPC marker and anti‐Iba1 antibody as microglia/macrophage marker (Figure 4b). Quantification of PDGFRα+ cells per mm2 (578.2 ± 117.3 Bac vs. 461.3 ± 49.93 vehicle; Figure 4c) or the percentage of lesion area occupied by Iba‐1 (60.10 ± 5.52% Bac vs. 49.87 ± 11.84% vehicle; Figure 4d) revealed no variations between control vs Bac‐treated mice. In addition, we used anti‐Nkx2.2 antibody to label reactive OPCs and anti‐GFAP antibody as astrocyte marker (Figure 4e). We observed no significant differences in Nkx2.2+ cells per mm2 (437.4 ± 46.8 Bac vs. 496.5 ± 65.5 control; Figure 4f) nor in the percentage of lesion area occupied by GFAP (21.2 ± 4.0 Bac vs. 26.9 ± 2.0 control; Figure 4g) between vehicle versus Bac‐treated animals. Finally, we investigated whether Bac administration accelerates OPC differentiation in the lesions by analyzing the numbers of mature OLs (APC+Olig2+) relative to the total number of oligodendroglial cells (Olig2+) (Figure 4h). The percentage of APC+ among total Olig2+ cells was significantly increased in Bac‐treated LPC‐injected mice (41.91 ± 2.29% Bac vs. 27.95 ± 1.46% vehicle; Figure 4i), without altering the total numbers of Olig2+ cells (928.0 ± 100.7 Bac vs. 814.8 ± 50.13 vehicle; Figure 4j). These results demonstrate that Bac treatment promotes differentiation of OPCs in LPC‐induced demyelinating lesions.
3.4. Baclofen administration accelerates remyelination
We next analyzed whether Bac administration accelerates remyelination. Vehicle and Bac injections were initiated at 5 dpl, and the proportion of remyelinated axons was analyzed at 16 dpl (Figure 5a), given that the onset of remyelination in the LPC model takes place at 14 dpl and is near completion at 21 dpl. At this time point, OL remyelination–identified as thin myelin sheaths–was found predominantly around lesion borders, while SC remyelination, a well‐recognized feature of the LPC lesion in the spinal cord (Jeffery & Blakemore, 1995), was observed in the lesion center. TEM analysis revealed that the percentage of remyelinated axons (Figure 5b, c) was higher following Bac treatment (36.95 ± 2.18% Bac vs. 22.29 ± 1.85% vehicle). In particular, the percentage of axons remyelinated by OLs was increased after Bac administration (33.45 ± 3.95% Bac vs. 19.75 ± 2.63% vehicle; Figure 5d), whereas SC‐driven remyelination was not significantly altered (3.70 ± 2.02% Bac vs. 2.54 ± 1.82% vehicle; Figure 5e). Thus, Bac administration following LPC‐induced demyelination accelerates the regeneration of myelin sheaths.
FIGURE 5.
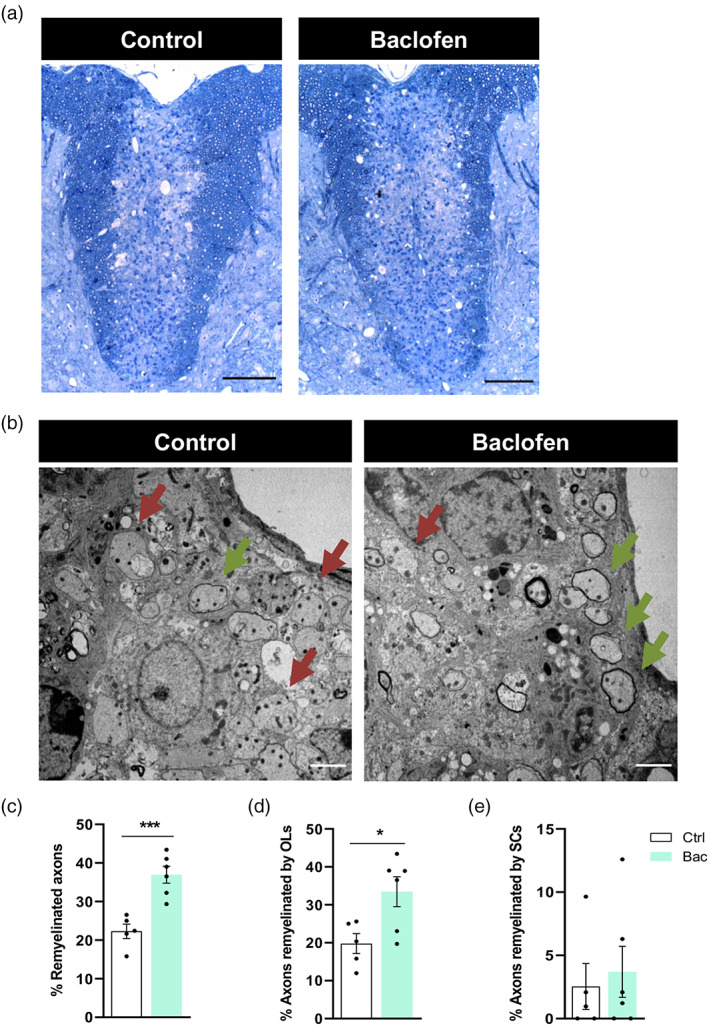
Baclofen treatment promotes remyelination following spinal cord lysolecithin (LPC)‐induced demyelination. (a) Representative images of semithin sections of spinal cord control (left) and baclofen‐treated (right) mice showing LPC‐induced lesions, stained with Richardson's blue. (b) Electron micrographs of ultrathin spinal cord sections showing remyelinated (green arrows) and unmyelinated (red arrows) axons. Histograms showing percentage of total remyelinated axons within the lessions (c), axons remyelinated by oligodendrocytes (OLs) (d) and axons remyelinated by Schwann cells (SCs) (e). *p < .05 and ***p < .001 versus control; unpaired Student's t‐test, control 5 animals, bac 6 animals. Scale bars: A = 100 μm; B = 2 μm
4. DISCUSSION
Remyelination of demyelinated lesions varies considerably between MS patients, and it is associated with improved axonal preservation (Kornek et al., 2000) and less disability (Bodini et al., 2016). OPC differentiation arrest is an important contributor to failed myelin repair in MS (Kotter et al., 2006; Kuhlmann et al., 2008), and newly generated OLs are more pro‐myelinating than those that survive injury (Neely et al., 2022). Therefore, promoting OPC differentiation may enhance remyelination and thus prevent axonal degeneration in MS (Irvine & Blakemore, 2008; Mei et al., 2016). In this study, we have identified that baclofen is a pro‐remyelinating agent that can heal MS lesions.
We previously showed that GABABR‐selective agonist Bac, a drug used for the treatment of spasticity in MS patients (Chisari et al., 2020) stimulates OPC differentiation in vitro via GABABR activation (Serrano‐Regal, Luengas‐Escuza, et al., 2020). Interestingly, intrathecal Bac administration in MS patients improves cognitive performance (Farrell et al., 2021). Notably, higher cognitive performance in patients with MS correlates with quantitative improvements in myelin water imaging (Abel et al., 2020). Here, we show that Bac administration stimulates myelin protein synthesis and remyelination ex vivo and in vivo.
In order to gain insight into potential benefits of Bac application on remyelination, we first investigated the pattern of GABABR expression of OPCs under normal and demyelinating conditions ex vivo and in vivo. While our previous in vitro and in vivo observations demonstrated the presence of both GABABR subunits on oligodendrocyte membranes (Serrano‐Regal, Luengas‐Escuza, et al., 2020), the analyses of oligodendroglia in cerebellar organotypic cultures show an apparent nuclear pattern of GABAB1 expression (Figure 1a,b), which suggests that GABAB2 homodimers may be operating as functional GABABRs in OLs in the organotypic slices. However, we detected a membrane pattern expression of GABAB1 in OPCs and mature OLs in vivo in the spinal cord (Figure 3), which suggests that GABAB1–GABAB2 heterodimers may be operating in these cells. Together, these localization studies support the idea that expression of GABABRs may vary across CNS regions.
Bac induces an increase in myelin proteins in cerebellar slices and optic nerve explants (Figure 1c–f; Figure 1k,l). Interestingly, the percentage of mature OLs (APC+Olig2+) was not affected by Bac in cerebellar slices (Figure 1g–i), which suggest that Bac is promoting OL maturation in this model. In our previous study however, Bac increased both OPC differentiation and maturation when cultured in isolation (Serrano‐Regal, Luengas‐Escuza, et al., 2020). The lack of effect of Bac on OPC differentiation observed in organotypic slices could be attributed to alternative actions of Bac in neurons and glial cells in this preparation. Alternatively, regional differences may also account as organotypic slices originate from cerebellum, while dissociated cultures of OPCs are obtained from cerebral cortex. Importantly, slices are prepared at a later stage in development (postnatal day 5–7), as compared to the OPC cultures (post‐natal day 0–3), which means that isolated OPC cultures are much less differentiated to start with, and their differentiation is potentially more malleable, while organotypic slices may contain many more immature OLs rather than OPCs.
Another striking observation in the current study is that GABA does not induce changes in myelin proteins in cerebellar organotypic slices (Supplementary Figure S2), as opposed to what occurs in cortical cultured OPCs (Supplementary Figure S1). These differences may be due to the fact that OPCs cultured in the absence of axons lose GABAARs and their electrophysiological responses to GABA (Arellano et al., 2016). Therefore, the positive effects of GABA in modulating OPC differentiation and OL maturation observed in cultured OPCs (Supplementary Figure S1) are likely mediated through GABABR (Serrano‐Regal, Luengas‐Escuza, et al., 2020). This is consistent with our current data that GABABR agonists (Figure 1c–f) but not GABAAR increase levels of myelin proteins. In the present study, we have used cerebellar organotypic slices in which, as suggested by electrophysiological recordings by Hamilton et al. (2017), OPCs maintain the expression of GABAARs, unlike cortical OPCs cultured in vitro. Our interpretation is that, in organotypic slices, GABA could be activating both types of oligodendroglial GABARs, and the positive effect of GABA via GABABR may be abrogated by the activation of GABAARs. Consistently with that, activating specifically GABAARs in these slices with the GABAAR agonist muscimol, did not affect OL maturation as it did not induce changes in the myelin protein levels (Supplementary Figure S3). Moreover, although GABA alone did not modulate PLP production after demyelination (Figure 2g), it did it when added together with gabazine, a GABAAR antagonist. Hence, GABABR‐mediated effect is lost when GABA is activating both GABAR types.
Both GABA and Bac applied during demyelination with LPC in cerebellar organotypic slices resulted in increased levels of myelin proteins compared to LPC alone (Figure 2b–d) which suggests that Bac exerts anti‐demyelination effects. Indeed, Bac has anti‐inflammatory and neuroprotective properties preventing the release of pro‐inflammatory cytokines by astrocytes and microglia and reducing oxidative stress (De Beaurepaire, 2018). Thus, it may be that the reduction of oxidative stress by Bac during LPC treatment protects OPCs, resulting in enhanced differentiation and myelin protein expression upon LPC withdrawal. Such a protective effect may be exploited for the repair of MS demyelinating lesions.
As mentioned above, GABA increased PLP levels after LPC stimulus in organotypic slices prepared from PLP‐DsRed mice only when applied in conjunction with the GABAAR antagonist gabazine (Figure 2g), but it increased MBP and MAG levels when applied alone in cerebellar rat slices (Figure 2C‐D). Thus, these different myelin proteins and evaluation may respond differently to GABA stimulation, in terms of dynamics. Also, species and regional differences among these two preparations may account for the variable response of GABA on myelin proteins (Aguayo et al., 1994; Mendu et al., 2012; Pöltl et al., 2003). Nonetheless, our results demonstrate that Bac pro‐myelinating effects prevail across regions and species.
In the current study, we used an LPC model of demyelination to evaluate the effect of Bac on remyelination. This model is, together with cuprizone, an inflammation‐independent model of demyelination that allows for precise evaluation of potential pro‐remyelinating agents. In this experimental setting, demyelination occurs within the first 2 days after LPC injection, after which remyelinating events initiate, and end by day 23 post‐injection (Piaton et al., 2011; Tepavcevic et al., 2014; Wegener et al., 2015; Psenicka et al., 2021), which means that the process of remyelination can be manipulated following demyelination. In the cuprizone model, demyelination and remyelination occur concomitantly as the lesion develops, making it difficult to dissociate the effect on OPC responses from the protective effects on oligodendrocytes, or the effects on macrophages cleaning the myelin debris (Ransohoff, 2012). An alternative model for evaluating the therapeutic benefits of drugs in MS is experimental autoimmune encephalomyelitis (EAE), whereby myelin and oligodendrocytes are initially targeted by developing an immune response to MAG. Undoubtedly, it will be worth to examine the potential of Bac in attenuating EAE symptoms and structural damage. However, there are confounding factors that limit that study, as GABARs are also present on immune cells (Bhat et al., 2010) and Bac may impact the immune response thus making it difficult to analyze its remyelinating properties. Because of that we chose the LPC model which, in spinal cord lesions in vivo, is characterized by a defined sequence of events: demyelination within 2 dpl, followed by OPC recruitment and proliferation, OPC differentiation and remyelination (Figure 4a; Piaton et al., 2011; Tepavcevic et al., 2014, Wegener et al., 2015). Thus, the timing of Bac treatment (5–16 dpl) ensures no effect on demyelination/macrophage recruitment/debris removal. As we aimed to investigate whether Bac treatment accelerates remyelination, we quantified the proportion of remyelinated axons at 16 dpl, shortly after the onset of remyelination. Remarkably, Bac‐treated mice showed an increase in the percentage of remyelinated axons, indicative of accelerated remyelination (Figure 5c,d).
Bac administration may increase OPC differentiation and consequently remyelination by multiple mechanisms. The first one is via activation of GABABRs, that, as we showed here, are expressed by oligodendroglial cells in the lesions. This mechanism would be consistent with our observations in vitro that Bac treatment of OPC cultures increases OPC differentiation, and that this effect is abrogated by application of GABAB antagonist. Whether the same holds during remyelination warrants future experiments using models with conditional ablation of GABABRs on OPCs and/or OLs. Another potential mechanism underlying increased remyelination in Bac‐treated mice could be the direct effects of Bac on microglia/macrophages, as these cells are important modulators of OPC differentiation (Miron et al., 2013). In addition, microglia‐specific ablation of GABAB1Rs in mice impairs synaptic refinement by microglia, which leads to behavioral abnormalities (Favuzzi et al., 2021), thus suggesting that GABABR modulation can modify microglial function. Our results show that Bac treatment did not alter the lesion area occupied by microglia/macrophage at 12 dpl (Figure 4c). However, it remains unclear whether Bac induces microglial/macrophage modifications that favor remyelination without altering their recruitment or proliferation. While further research should address the exact effect of Bac on microglia/macrophages in the context of remyelination/demyelination, it appears clear that, at least in the LPC model, Bac administration improves remyelination, either directly by stimulating GABABRs in oligodendroglia, and/or by enhancing microglia/macrophage support of remyelination.
In conclusion, our results provide compelling evidence showing that Bac, a drug approved for spasticity treatment in MS patients, improves remyelination. Advanced imaging techniques for non‐invasive measurement of myelin content/remyelination (Kolb et al., 2021) should evaluate whether MS patients treated with Bac indeed show evidence of enhanced remyelination. This may be an important first step in evaluating the suitability of this drug as a pro‐remyelinating/neurorepair agent in MS.
AUTHOR CONTRIBUTIONS
Mari Paz Serrano‐Regal: investigation, data curation, formal analysis, writing. Laura Bayón‐Cordero: investigation, data curation, formal analysis, writing. Juan Carlos Chara Ventura: investigation. Blanca I. Ochoa‐Bueno: investigation, data curation, formal analysis. Vanja Tepavcevic: conceptualization, investigation, formal analysis, writing. Carlos Matute: conceptualization, writing, funding acquisition. María Victoria Sánchez‐Gómez: conceptualization, writing, funding acquisition.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Supporting information
Supplementary Figure S1 Baclofen promotes myelin protein expression through GABABR in isolated OPCs. (A, D) Cultures of rat cortical OPCs and treatments with drugs were developed according to previously described procedures (Serrano‐Regal, Luengas‐Escuza, et al., 2020). Representative western blot image showing expression of MAG and MBP proteins in cortical OPCs treated for 48 (A) or 72 h (D) with baclofen, CGP55845 or CGP55845 plus baclofen. Quantification of MAG (B, E) and MBP (C, F) level normalized to GAPDH values and expressed as percentage over control (cells untreated; 100 value). *p < .05, **p < .01 and ****p < .0001 versus control; #p < .05, ##p < .01 and ####p < .0001 versus baclofen; one‐way ANOVA.
Supplementary Figure S2. GABA addition does not change myelin‐related protein levels in cerebellar organotypic slices. (A) Western blot image showing expression of MAG, CNPase, and MBP proteins in control condition or in presence of exogenous 100 μM GABA for 13 days in triplicates. Quantification of MAG (B), CNPase (C), and MBP (D) expression normalized to GAPDH values. At least 3 independent experiments were included in the analysis.
Supplementary Figure S3. GABAAR stimulation does not modify the expression of myelin‐related proteins in cerebellar organotypic slices. (A) Representative western blot showing expression of MAG, CNPase, and MBP proteins in control or muscimol‐treated conditions for 13 days in triplicates. Histograms showing quantification of MAG (B), CNPase (C), and MBP (D) levels. Values for each condition were obtained from at least 3 independent experiments and normalized to GAPDH.
Supplementary Figure S4. GABABR activation increases MAG levels after LPC‐induced demyelination in cerebellar organotypic cultures from P11 rats. (A) Schematic representation of the experimental design of demyelination induced by LPC, treatment duration and the following remyelination. (B) Representative western blot showing MAG and MBP levels after LPC exposure in duplicates. Values were obtained from at least 3 independent experiments and normalized to α‐tubulin. Quantification of MAG (C) and MBP levels (D) in indicated conditions. *p < .05 and ***p < .001 versus control, #p < .05 versus LPC; one‐way ANOVA followed by Tukey's post‐test.
ACKNOWLEDGMENTS
The authors thank Prof. Dr. F. Kirchhoff for kindly providing PLP‐DsRed mice. Dr. L. Escobar, Dr. A. Palomino, Dr. T. Quintela‐López and S. Marcos for their expert technical assistance and Dr. C. Luchena for her advice in statistics. Support provided by SGIker from the University of the Basque Country (UPV/EHU; Animal Unit and Analytical and High‐Resolution Microscopy in Biomedicine) is also gratefully acknowledged. The study was supported by the Ministry of Economy and Competitiveness, Government of Spain (SAF2013‐45084‐R, SAF2016‐75292‐R, and PID2019‐109724RB‐I00 to Carlos Matute; Young Investigator Grant SAF2015‐74332‐JIN to Vanja Tepavcevic), Basque Government (IT702‐13 and IT1203‐19; Carlos Matute) and CIBERNED (CB06/05/0076; Carlos Matute). Mari Paz Serrano‐Regal was a recipient of a Ministry of Economy and Competitiveness, Govern of Spain Predoctoral Fellowship and of a Gangoiti Foundation Postdoctoral Fellowship. Laura Bayón‐Cordero holds a Basque Government Predoctoral Fellowship. Blanca I. Ochoa‐Bueno holds a Ministry of Universities Predoctoral Fellowship.
Serrano‐Regal, M. P. , Bayón‐Cordero, L. , Chara Ventura, J. C. , Ochoa‐Bueno, B. I. , Tepavcevic, V. , Matute, C. , & Sánchez‐Gómez, M. V. (2022). GABAB receptor agonist baclofen promotes central nervous system remyelination. Glia, 70(12), 2426–2440. 10.1002/glia.24262
Mari Paz Serrano‐Regal and Laura Bayón‐Cordero contributed equally to this work.
Funding information CIBERNED, Grant/Award Number: CB06/05/0076; Basque Government, Grant/Award Numbers: IT1203‐19, IT702‐13; Ministry of Economy and Competitiveness, Government of Spain, Grant/Award Numbers: SAF2015‐74332‐JIN, PID2019‐109724RB‐I00, SAF2016‐75292‐R, SAF2013‐45084‐R
Contributor Information
Carlos Matute, Email: carlos.matute@ehu.eus.
María Victoria Sánchez‐Gómez, Email: vicky.sanchez@ehu.eus.
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available from the corresponding authors upon reasonable request.
REFERENCES
- Abel, S. , Vavasour, I. , Lee, L. E. , Johnson, P. , Ristow, S. , Ackermans, N. , Chan, J. , Cross, H. , Laule, C. , Dvorak, A. , Schabas, A. , Hernández‐Torres, E. , Tam, R. , Kuan, A. J. , … Kolind, S. H. (2020). Associations between findings from myelin water imaging and cognitive performance among individuals with multiple sclerosis. JAMA Network Open, 3, e2014220. 10.1001/jamanetworkopen.2020.14220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguayo, L. G. , Pancetti, F. C. , Klein, R. L. , & Harris, R. A. (1994). Differential effects of GABAergic ligands in mouse and rat hippocampal neurons. Brain Research, 647, 97–105. 10.1016/0006-8993(94)1403-6 [DOI] [PubMed] [Google Scholar]
- Arellano, R. O. , Sánchez‐Gómez, M. V. , Alberdi, E. , Canedo‐Antelo, M. , Chara, J. C. , Palomino, A. , Pérez‐ Samartín, A. , & Matute, C. (2016). Axon‐to‐glia interaction regulates GABAA receptor expression in oligodendrocytes. Molecular Pharmacology, 89, 63–74. 10.1124/mol.115.100594 [DOI] [PubMed] [Google Scholar]
- Azim, K. , & Butt, A. M. (2011). GSK3β negatively regulates oligodendrocyte differentiation and myelination in vivo. Glia, 59, 540–553. 10.1002/glia.21122 [DOI] [PubMed] [Google Scholar]
- Bai, X. , Kirchhoff, F. , & Scheller, A. (2021). Oligodendroglial GABAergic signaling: More than inhibition! Neuroscience Bulletin, 37, 1039–1050. 10.1007/s12264-021-00693-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles, D. E. , Roberts, J. D. , Somogyi, P. , & Jahr, C. E. (2000). Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature, 405, 187–191. 10.1038/35012083 [DOI] [PubMed] [Google Scholar]
- Bhat, R. , Axtell, R. , Mitra, A. , Miranda, M. , Lock, C. , Tsien, R. W. , & Steinman, L. (2010). Inhibitory role for GABA in autoinmune inflammation. Proceedings of the National Academy of Sciences of the USA, 107, 2580–2585. 10.1073/pnas.0915149107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birgbauer, E. , Rao, T. S. , & Webb, M. (2004). Lysolecithin induces demyelination in vitro in a cerebellar slice culture system. Journal of Neuroscience Research, 78, 157–166. 10.1002/jnr.20248 [DOI] [PubMed] [Google Scholar]
- Bodini, B. , Veronese, M. , García‐Lorenzo, D. , Battaglini, M. , Poirion, E. , Chardain, A. , Freeman, L. , Louapre, C. , Tchikviladze, M. , Papeix, C. , Dollé, F. , Zalc, B. , Lubetzki, C. , Bottlaender, M. , … Stankoff, B. (2016). Dynamic imaging of individual remyelination profiles in multiple sclerosis. Annals of Neurology, 79, 726–738. 10.1002/ana.24620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisari, C. G. , Sgarlata, E. , Arena, S. , D'Amico, E. , Toscano, S. , & Patti, F. (2020). An update on the pharmacological management of pain in patients with multiple sclerosis. Expert Opinion on Pharmacotherapy, 21, 2249–2263. 10.1080/14656566.2020.1757649 [DOI] [PubMed] [Google Scholar]
- Cisneros‐Mejorado, A. , Garay, E. , Ortiz‐Retana, J. , Concha, L. , Moctezuma, J. P. , Romero, S. , & Arellano, R. O. (2020). Demyelination‐remyelination of the rat caudal cerebellar peduncle evaluated with magnetic resonance imaging. Neuroscience, 439, 255–267. 10.1016/j.neuroscience.2019.06.042 [DOI] [PubMed] [Google Scholar]
- De Beaurepaire, R. (2018). A review of the potential mechanisms of action of baclofen in alcohol use disorder. Frontiers in Psychiatry, 9, 506. 10.3389/fpsyt.2018.00506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dendrou, C. A. , Fugger, L. , & Friese, M. A. (2015). Immunopathology of multiple sclerosis. Nature Reviews Immunology, 15, 545–558. 10.1038/nri3871 [DOI] [PubMed] [Google Scholar]
- Domercq, M. , Pérez‐Samartín, A. , Aparicio, D. , Alberdi, E. , Pampliega, O. , & Matute, C. (2010). P2X7 receptors mediate ischemic damage to oligodendrocytes. Glia, 58, 730–740. 10.1002/glia.20958 [DOI] [PubMed] [Google Scholar]
- Doussau, F. , Dupont, J. L. , Neel, D. , Schneider, A. , Poulain, B. , & Bossu, J. L. (2017). Organotypic cultures of cerebellar slices as a model to investigate demyelinating disorders. Expert Opinion on Drug Discovery, 12, 1011–1022. 10.1080/17460441.2017.1356285 [DOI] [PubMed] [Google Scholar]
- Duncan, I. D. , Radcliff, A. B. , Heidari, M. , Kidd, G. , August, B. K. , & Wierenga, L. A. (2018). The adult oligodendrocyte can participate in remyelination. Proceedings of the National Academy of Sciences of the USA, 115, E11807–E11816. 10.1073/pnas.1808064115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusart, I. , Airaksinen, M. S. , & Sotelo, C. (1997). Purkinje cell survival and axonal regeneration are age dependent: An in vitro study. Journal of Neuroscience, 17, 3710–3726. 10.1523/JNEUROSCI.17-10-03710.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertzgaard, P. , Campo, C. , & Calabrese, A. (2017). Efficacy and safety of oral baclofen in the management of spasticity: A rationale for intrathecal baclofen. Journal of Rehabilitation Medicine, 49, 193–203. 10.2340/16501977-2211 [DOI] [PubMed] [Google Scholar]
- Fannon, J. , Tarmier, W. , & Fulton, D. (2015). Neuronal activity and AMPA‐type glutamate receptor activation regulates the morphological development of oligodendrocyte precursor cells. Glia, 63, 1021–1035. 10.1002/glia.22799 [DOI] [PubMed] [Google Scholar]
- Farrell, R. , Summers, M. , Doogan, C. , Mulhert, N. , Keenan, E. , Buchanan, K. , Lee, H. , Padilla, H. , & Stevenson, V. L. (2021). Evaluation of the cognitive benefits of intrathecal baclofen pump implantation in people with intractable multiple sclerosis related spasticity. Multiple Sclerosis and Related Disorders, 50, 102831. 10.1016/j.msard.2021.10283 [DOI] [PubMed] [Google Scholar]
- Favuzzi, E. , Huang, S. , Saldi, G. A. , Binan, L. , Ibrahim, L. A. , Fernández‐Otero, M. , Cao, Y. , Zeine, A. , Sefah, A. , Zheng, K. , Xu, Q. , Khlestova, E. , Farhi, S. L. , Bonneau, R. , … Fishell, G. (2021). GABA‐receptive microglia selective sculp developing inhibitory circuits. Cell, 184, 4048–4063. 10.1016/j.cell.2021.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin, R.J.M, & Ffrench‐Constant, C. (2017). Regenerating CNS myelin‐from mechanisms to experimental medicines. Nature Reviews Neuroscience, 18, 753–769. 10.1038/nrn.2017.136. [DOI] [PubMed] [Google Scholar]
- Franklin, R. J. M. , Frisén, J. , & Lyons, D. A. (2021). Revisiting remyelination: Towards a consensus on the regeneration of CNS myelin. Seminars in Cell and Developmental Biology, 116, 3–9. 10.1016/j.semcdb.2020.09.009 [DOI] [PubMed] [Google Scholar]
- Gautier, H. O. B. , Evans, K. A. , Volbracht, K. , James, R. , Sitnikov, S. , Lundgaard, I. , James, F. , Lao‐Peregrin, C. , Reynolds, R. , Franklin, R. J. M. , & Káradóttir, R. T. (2015). Neuronal activity regulates remyelination via glutamate signaling to oligodendrocyte progenitors. Nature Communications, 6, 8518. 10.1038/ncomms9518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton, N. B. , Clarke, L. E. , Arancibia‐Carcamo, I. L. , Kougioumtzidou, E. , Matthey, M. , Káradóttir, R. , Whiteley, L. , Bergersen, L. H. , Richardson, W. D. , & Attwell, D. (2017). Endogenous GABA controls oligodendrocyte lineage cell number, myelination and CNS internode length. Glia, 65, 309–321. 10.1002/glia.23093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heb, K. , Starost, L. , Kieran, N. W. , Thomas, C. , Vincenten, M. C. J. , Antel, J. , Martino, G. , Huitinga, I. , Healy, L. , & Kuhlmann, T. (2020). Lesion stage‐dependent causes for impaired remyelination in MS. Acta Neuropathologica, 140, 359–375. 10.1007/s00401-020-02189-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirrlinger, P. G. , Scheller, A. , Braun, C. , Quintela‐Schneider, M. , Fuss, B. , Hirrlinger, J. , & Kirchhoff, F. (2005). Expression of reef coral fluorescent proteins in the central nervous system of transgenic mice. Molecular and Cellular Neuroscience, 30, 291–303. 10.1016/j.mnc.2005.08.011 [DOI] [PubMed] [Google Scholar]
- Irvine, K. A. , & Blakemore, W. F. (2008). Remyelination protects axons from demyelination‐associated axon degeneration. Brain, 131, 1464–1477. 10.1093/brain/awn080 [DOI] [PubMed] [Google Scholar]
- Jeffery, N. D. , & Blakemore, W. F. (1995). Remyelination of mouse spinal cord axons demyelinated by local injection of lysolecithin. Journal of Neurocytology, 24, 775–781. 10.1007/BF01191213 [DOI] [PubMed] [Google Scholar]
- Kalakh, S. , & Mouihate, A. (2019). Enhanced remyelination during late pregnancy: Involvement of the GABAergic system. Scientific Reports, 9, 7728. 10.1038/s41598-019-44050-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Káradóttir, R. , Hamilton, N. B. , Bakiri, Y. , & Atwell, D. (2008). Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nature Neuroscience, 11, 450–456. 10.1038/nn2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb, H. , Absinta, M. , Beck, E. S. , Ha, S.‐K. , Song, Y. , Norato, G. , Cortese, I. , Sati, P. , Nair, G. , & Reich, D. S. (2021). 7T MRI differentiates Remyelinated from demyelinated multiple sclerosis lesions. Annals of Neurology, 90, 612–626. 10.1002/ana.26194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornek, B. , Storch, M. K. , Weissert, R. , Wallstroem, E. , Stefferl, A. , Olsson, T. , Linington, C. , Schmidbauer, M. , & Lassmann, H. (2000). Multiple sclerosis and chronic autoimmune encephalomyelitis: A comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions. The American Journal of Pathology, 157, 267–276. 10.1016/S0002-9440(10)64537-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotter, M. R. , Li, W. W. , Zhao, C. , & Franklin, R. J. (2006). Myelin impairs CNS remyelination by inhibiting oligodendrocyte precursor cell differentiation. Journal of Neuroscience, 26, 328–332. 10.1523/JNEUROSCI.2615-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer, D. , Akkermann, R. , Küry, P. , & Dutta, R. (2019). Current advancements in promoting remyelination in multiple sclerosis. Multiple Sclerosis, 25, 7–14. 10.1177/1352458518800827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer, D. , Göttle, P. , Flores‐Rivera, J. , Hartung, H. P. , & Küry, P. (2019). Remyelination in multiple sclerosis: From concept to clinical trials. Current Opinion in Neurology, 32, 378–384. 10.1097/WCO.0000000000000692 [DOI] [PubMed] [Google Scholar]
- Kuhlmann, T. , Miron, V. , Cui, Q. , Wegner, C. , Antel, J. , & Brück, W. (2008). Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain, 131, 1749–1758. 10.1093/brain/awn096 [DOI] [PubMed] [Google Scholar]
- Kukley, M. , Kiladze, M. , Tognatta, R. , Hans, M. , Swandulla, D. , Schramm, J. , & Dietrich, D. (2008). Glial cells are born with synapses. FASEB Journal, 22, 2957–2969. 10.1096/fj.07-090985 [DOI] [PubMed] [Google Scholar]
- Lee, Y. , Morrison, B. M. , Li, Y. , Lengacher, S. , Farah, M. H. , Hoffman, P. N. , Liu, Y. , Tsingalia, A. , Jin, L. , Zhang, P.‐W. , Pellerin, L. , Magistretti, P. J. , & Rothstein, J. D. (2012). Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature, 487, 443–448. 10.1038/nature11314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C. , Xiao, L. , Liu, X. , Yang, W. , Shen, W. , Hu, C. , Yang, G. , & He, C. (2013). A functional role of NMDA receptor in regulating the differentiation of oligodendrocyte precursor cells and remyelination. Glia, 61, 732–749. 10.1002/glia.22469 [DOI] [PubMed] [Google Scholar]
- Lin, S. C. , & Bergles, D. E. (2004). Synaptic signaling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus. Nature Neuroscience, 7, 24–32. 10.1038/nn1162 [DOI] [PubMed] [Google Scholar]
- Lubetzki, C. , Sol‐Foulon, N. , & Desmazières, A. (2020). Nodes of Ranvier during development and repair in the CNS. Nature Reviews Neurology, 16, 426–439. 10.1038/s41582-020-0375-x [DOI] [PubMed] [Google Scholar]
- Mei, F. , Lehmann‐Horn, K. , Shen, Y. A. , Rankin, K. A. , Stebbins, K. J. , Lorrain, D. S. , Pekarek, K. , Sagan, S. A. , Xiao, L. , Teuscher, C. , von Büdingen, H.‐C. , Wess, J. , Lawrence, J. J. , Green, A. J. , … Chan, J. R. (2016). Accelerated remyelination during inflammatory demyelination prevents axonal loss and improves functional recovery. eLife, 5, e18246. 10.7554/eLife.18246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendu, S. K. , Bhandage, A. , Jin, Z. , & Birnir, B. (2012). Different subtypes of GABA‐A receptors are expressed in human, mouse and rat T lymphocytes. PLoS One, 7, e42959. 10.1371/journal.pone.0042959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron, V. E. , Boyd, A. , Zhao, J. W. , Yuen, T. J. , Ruckh, J. M. , Shadrach, J. L. , van Wijngaarden, P. , Wagers, A. J. , Williams, A. , Franklin, R. J. M. , & Ffrench‐Constant, C. (2013). M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nature Neuroscience, 16, 1211–1218. 10.1038/nn.3469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely, S. A. , Williamson, J. M. , Klingseisen, A. , Zoupi, L. , Early, J. J. , Williams, A. , & Lyons, D. A. (2022). New oligodendrocytes exhibit more abundant and accurate myelin regeneration than those that survive demyelination. Nature Neuroscience, 25(4), 415–420. 10.1038/s41593-021-01009-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piaton, G. , Aigrot, M. S. , Williams, A. , Moyon, S. , Tepavcevic, V. , Moutkine, I. , Gras, J. , Matho, K. S. , Schmitt, A. , Soellner, H. , Huber, A. B. , Ravassard, P. , & Lubetzki, C. (2011). Class 3 semaphorins influence oligodendrocyte precursor recruitment and remyelination in adult central nervous system. Brain, 134, 1156–1157. 10.1093/brain/awr022 [DOI] [PubMed] [Google Scholar]
- Pöltl, A. , Hauer, B. , Fuchs, K. , Tretter, V. , & Sieghart, W. (2003). Subunit composition and quantitative importance of GABAA receptor subtypes in the cerebellum of mouse and rat. Journal of Neurochemistry, 87, 1444–1455. 10.10456/j-1471-4159.2003.02135.x [DOI] [PubMed] [Google Scholar]
- Psenicka, M. W. , Smith, B. C. , Tinkey, R. A. , Williams, J. L. (2021). Connecting neuroinflammation and neurodegenerationin multiple sclerosis: are oligodendrocyte precursor cells a nexus of disease? FrontCell Neurosci 15: 654284. 10.3389/fncel.2021.654284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pudasaini, S. , Friedrich, V. , Bührer, C. , Endesfelder, S. , Scheuer, T. , & Schmitz, T. (2022). Postnatal myelination of the immature rat cingulum is regulated by GABAB receptor activity. Developmental Neurobiology, 82, 16–28. 10.1002/dneu.22853 [DOI] [PubMed] [Google Scholar]
- Ransohoff, R. M. (2012). Animal models of multiple sclerosis: The good, the bad and the bottom line. Nature Neuroscience, 15(8), 1074–1077. 10.1038/nn.3168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saab, A. S. , Tzvetavona, I. D. , Trevisiol, A. , Baltan, S. , Dibaj, P. , Kusch, K. , Möbius, W. , Goetze, B. , Jahn, H. M. , Huang, W. , Steffens, H. , Schomburg, E. D. , Pérez‐Samartín, A. , Pérez‐Cerdá, F. , … Nave, K. A. (2016). Oligodendroglial NMDA receptors regulate glucose import and axonal energy metabolism. Neuron, 91, 119–132. 10.1016/j.neuron.2016.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano‐Regal, M. P. , Bayón‐Cordero, L. , Ordaz, R. P. , Garay, E. , Limon, A. , Arellano, R. O. , Matute, C. , & Sánchez‐Gómez, M. V. (2020). Expression and function of GABA receptors in myelinating cells. Frontiers in Cellular Neuroscience, 14, 256. 10.3389/fncel.2020.00256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano‐Regal, M. P. , Luengas‐Escuza, I. , Bayón‐Cordero, L. , Ibarra‐Aizpurua, N. , Alberdi, E. , Pérez‐Samartín, A. , Matute, C. , & Sánchez‐Gómez, M. V. (2020). Oligodendrocyte differentiation and myelination is potentiated via GABAB receptor activation. Neuroscience, 439, 163–180. 10.1016/j.neuroscience.2019.07.014 [DOI] [PubMed] [Google Scholar]
- Tan, G. A. , Furber, K. L. , Thangaraj, M. P. , Sobchishin, L. , Doucette, J. R. , & Nazarali, A. J. (2018). Organotypic cultures from the adult CNS: A novel model to study demyelination and remyelination ex vivo. Cellular and Molecular Neurobiology, 38, 317–328. 10.1007/s10571-017-0529-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepavcevic, V. , Kernion, C. , Aigrot, M. S. , Meppiel, E. , Mozafari, S. , Arnould‐Laurent, R. , Ravassard, P. , Kennedy, T. E. , Nait‐Oumesmar, B. , & Lubetzki, C. (2014). Early netrin‐1 expression impairs central nervous system remyelination. Annals of Neurology, 76, 252–268. 10.1002/ana.24201 [DOI] [PubMed] [Google Scholar]
- Trapp, B. D. , Ransohoff, R. , & Rudick, R. (1999). Axonal pathology in multiple sclerosis: Relationship to neurologic disability. Current Opinion in Neurology, 12, 295–302. 10.1097/00019052-199906000-00008 [DOI] [PubMed] [Google Scholar]
- Wegener, A. , Deboux, C. , Bachelin, C. , Frah, M. , Kerninon, C. , Seilhean, D. , Weider, M. , Wegner, M. , & Nait‐Oumesmar, B. (2015). Gain of Olig2 function in oligodendrocyte progenitors promotes remyelination. Brain, 138, 120–135. 10.1093/brain/awu.375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung, M. S. Y. , Djelloul, M. , Steiner, E. , Bernard, S. , Salehpour, M. , Possnert, G. , Brundin, L. , & Frisén, J. (2019). Dynamics of oligodendrocyte generation in multiple sclerosis. Nature, 566, 538–542. 10.1038/s41586-018-0842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonouzi, M. , Scafidi, J. , Li, P. , McEllin, B. , Edwards, J. , Dupree, J. L. , Harvey, L. , Sun, D. , Hübner, C. A. , Cull‐Candy, S. G. , Farrant, M. , & Gallo, V. (2015). GABAergic regulation of cerebellar NG2 cell development is altered in perinatal white matter injury. Nature Neuroscience, 18, 674–682. 10.1038/nn.3990 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1 Baclofen promotes myelin protein expression through GABABR in isolated OPCs. (A, D) Cultures of rat cortical OPCs and treatments with drugs were developed according to previously described procedures (Serrano‐Regal, Luengas‐Escuza, et al., 2020). Representative western blot image showing expression of MAG and MBP proteins in cortical OPCs treated for 48 (A) or 72 h (D) with baclofen, CGP55845 or CGP55845 plus baclofen. Quantification of MAG (B, E) and MBP (C, F) level normalized to GAPDH values and expressed as percentage over control (cells untreated; 100 value). *p < .05, **p < .01 and ****p < .0001 versus control; #p < .05, ##p < .01 and ####p < .0001 versus baclofen; one‐way ANOVA.
Supplementary Figure S2. GABA addition does not change myelin‐related protein levels in cerebellar organotypic slices. (A) Western blot image showing expression of MAG, CNPase, and MBP proteins in control condition or in presence of exogenous 100 μM GABA for 13 days in triplicates. Quantification of MAG (B), CNPase (C), and MBP (D) expression normalized to GAPDH values. At least 3 independent experiments were included in the analysis.
Supplementary Figure S3. GABAAR stimulation does not modify the expression of myelin‐related proteins in cerebellar organotypic slices. (A) Representative western blot showing expression of MAG, CNPase, and MBP proteins in control or muscimol‐treated conditions for 13 days in triplicates. Histograms showing quantification of MAG (B), CNPase (C), and MBP (D) levels. Values for each condition were obtained from at least 3 independent experiments and normalized to GAPDH.
Supplementary Figure S4. GABABR activation increases MAG levels after LPC‐induced demyelination in cerebellar organotypic cultures from P11 rats. (A) Schematic representation of the experimental design of demyelination induced by LPC, treatment duration and the following remyelination. (B) Representative western blot showing MAG and MBP levels after LPC exposure in duplicates. Values were obtained from at least 3 independent experiments and normalized to α‐tubulin. Quantification of MAG (C) and MBP levels (D) in indicated conditions. *p < .05 and ***p < .001 versus control, #p < .05 versus LPC; one‐way ANOVA followed by Tukey's post‐test.
Data Availability Statement
The data that supports the findings of this study are available from the corresponding authors upon reasonable request.


