Abstract
It is well known that the multiple factors contributing to the pathogenesis of type 2 diabetes (T2D) confer an increased risk of developing cardiovascular disease (CVD). Although the relationship between hyperglycaemia and increased microvascular risk is well established, the relative contribution of hyperglycaemia to macrovascular events has been strongly debated, particularly owing to the failure of attempts to reduce CVD risk through normalizing glycaemia with traditional therapies in high‐risk populations. The debate has been further fuelled by the relatively recent discovery of the cardioprotective properties of glucagon‐like peptide‐1 receptor agonists and sodium‐glucose cotransporter‐2 inhibitors. Further, as guidelines now recommend individualizing glycaemic targets, highlighting the importance of achieving glycated haemoglobin (HbA1c) goals safely, the previously observed negative influences of intensive therapy on CVD risk might not present if trials were repeated using current‐day treatments and individualized HbA1c goals. Emerging longitudinal data illuminate the overall effect of excess glucose, the impacts of magnitude and duration of hyperglycaemia on disease progression and risk of CVD complications, and the importance of glycaemic control at or early after diagnosis of T2D for prevention of complications. Herein, we review the role of glucose as a modifiable cardiovascular (CV) risk factor, the role of microvascular disease in predicting macrovascular risk, and the deleterious impact of therapeutic inertia on CVD risk. We reconcile new and old data to offer a current perspective, highlighting the importance of effective, early treatment in reducing latent CV risk, and the timely use of appropriate therapy individualized to each patient's needs.
Keywords: cardiovascular disease, diabetes complications, glycaemia, hyperglycaemia, macrovascular disease, type 2 diabetes
1. INTRODUCTION
The increased risk of cardiovascular disease (CVD) in people with diabetes is well established, 1 and is now estimated to be between 1.6‐ and 2.3‐fold. 2 The availability of effective cholesterol and blood pressure treatments facilitated a shift in the management approach for type 2 diabetes (T2D) from the glucocentric focus of decades ago, to one of CVD risk reduction. 3 Indeed, one study reported that targeting multiple risk factors in people with T2D reduced the risk of CVD and microvascular events by approximately 50%. 4 This resulted in the widely adopted “ABC” (glycated haemoglobin [HbA1c], blood pressure, and cholesterol) approach to T2D treatment, which coincided with the identification of metabolic syndrome as a cluster of glucose intolerance, hypertension, dyslipidaemia, and central obesity, with insulin resistance as the source of pathogenesis, 5 all of which increase the risk for developing T2D and atherosclerotic CVD (ASCVD). 6 , 7 More recently, the exceptional findings of cardiovascular outcome trials (CVOTs) demonstrating unequivocal reductions in CVD risk with the newer sodium‐glucose cotransporter‐2 (SGLT2) inhibitors 8 , 9 , 10 , 11 , 12 and glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs) 13 , 14 , 15 , 16 , 17 have perhaps overshadowed the place of glucose control in the ABC management of T2D, reinvigorating the debate on whether glucose control itself matters in the efforts to minimize cardiovascular (CV) risk.
Despite the advances in treatment approaches and the availability of newer classes of therapy, data from the National Health and Nutrition Examination Survey have revealed that the proportion of people with T2D who achieved HbA1c <48 mmol/mol has not improved, and has actually declined over time, from 57.4% for 2007 to 2010, to 50.5% for 2015 to 2018. 18 Other estimates (2006‐2017) suggest that the global glycaemic control target achievement rate is currently only 42.8%. 19 This is despite guidelines from the American Diabetes Association (ADA), 20 the American Association of Clinical Endocrinologists, 21 and the American College of Physicians (ACP), 22 which universally recommend achievement and maintenance of glucose control to reduce the risk of long‐term complications. Therapeutic inertia—defined as “the failure to initiate or intensify therapy in a timely manner according to evidence‐based clinical guidelines in individuals who are likely to benefit from such intensification” 23 —remains commonplace, resulting in years of unnecessary exposure to hyperglycaemia. With more devastating consequences, between 2007 and 2017, CVD affected approximately one‐third of people with T2D across the globe, and caused one‐half of all deaths, 24 a figure that is projected to increase in tandem with the increasing prevalence of diabetes. 25 There is therefore a need to evaluate the risk of uncontrolled glycaemia on CVD in people living with T2D and to mitigate this risk moving forward.
This review aims to reconcile the debate on the role of glycaemic control in mitigating CVD risk, with a focus on chronic ambulatory care. We consider the effect of treatment inertia on CVD risk through reviewing the evidence that supports a key role for hyperglycaemia as a key risk modifier for the macrovascular complications associated with T2D. We compare the results of landmark T2D trials with those of the newer CVOTs in consideration of the improved outcomes with newer T2D therapies and the evolution of guidelines for the treatment of T2D. In particular, the potential effect of early control of blood glucose in reducing macrovascular risk is discussed, and current recommendations for translating such insights into improvements in patient care to reduce the overall burden of diabetes‐related complications are highlighted (Table 1).
TABLE 1.
Key take‐home messages and clinical perspective
| 1 | Microvascular disease predicts macrovascular disease; achieving and maintaining glycaemic control plays a critical role in reducing microvascular and macrovascular complications for people with T2D |
| 2 | Separately, and independent of glycaemic control, agents from the SGLT2 inhibitor and GLP‐1RA class have been shown to reduce CV risk in individuals with established/high risk of CVD |
| 3 | Holistically, both achievement of glucose control and choice of appropriate therapy are equally important for reducing risk of complications |
| 4 | One size does not fit all in T2D; HbA1c goals and treatments need to be individualized, with glycaemic targets achieved safely |
| 5 | Avoidance of therapeutic inertia is key to achieving HbA1c targets in all people with T2D, with early, sustained glycaemic control associated with reduced complication risk |
| 6 | Physiological control (e.g., less glycaemic variability, more time in range) is associated with a lower risk of CV complications; monitoring technology has the potential to facilitate more physiological control and guide therapeutic needs |
Abbreviations: CVD, cardiovascular disease; CVOT, cardiovascular outcomes trial; GLP‐1RA, glucagon‐like peptide‐1 receptor agonist; HbA1c, glycated haemoglobin; SGLT2, sodium‐glucose cotransporter‐2.
2. THE CONSEQUENCES OF THERAPEUTIC INERTIA ARE WELL KNOWN, BUT WHY IS IT STILL SO PREVALENT?
Findings from a systematic review revealed that the median time to treatment intensification ranges from 0.3 to over 7.2 years, increasing with the number of antihyperglycaemic agents used. 23 Initiation of injectable therapy is particularly challenging, with intensification to insulin therapy typically being delayed by more than 7 years, 26 and started only when HbA1c is 75 mmol/mol or above, dramatically decreasing the likelihood of achieving glycaemic targets. 27 Longitudinal studies have highlighted the increased CVD risk with increasing glycaemia, 28 including within the normoglycaemic range. 29 Coupling this fact with the demonstration that delay of treatment intensification in people with T2D and HbA1c 53 mmol/mol or above by just 1 year increases the risk of myocardial infarction (MI), stroke and heart failure (HF) at 5.3 years by 67%, 51% and 64%, respectively, 30 it is worthwhile to consider the potential reasons for therapeutic inertia.
Multiple reasons have been cited as contributing to therapeutic inertia 26 ; these are categorized as either patient‐level factors (eg, perceptions about medication use and side effects), provider‐level factors (eg, competing demands, discomfort or lack of familiarity with new medications, delays in guideline adoption), or health system factors (eg, cost and access). We believe that debate around the evidence and differing interpretations of existing evidence (eg, by different guidelines and professional societies) may contribute to therapeutic inertia within the broader society, and we explore this further here.
Although the UK Prospective Diabetes Study (UKPDS) definitively linked intensive glucose control with a reduction in CVD risk and mortality in people with T2D, 31 , 32 the delayed macrovascular risk benefit was not observed until after 10 years, at which time the between‐group glycaemic differences had been lost (Figure 1A,B). 32 These results were similar to the “legacy effect” observed in the Diabetes Control and Complications Trial (DCCT)/ Epidemiology of Diabetes Interventions and Complications (EDIC) study 33 (Figure 1C,D). While the delayed benefit supports the concept of improved outcomes with early control, the loss of between‐group glycaemic differences over time perhaps calls us to question whether ongoing control would result in an even greater benefit. The importance of glycaemic control was challenged when landmark studies (ADVANCE, Veterans Affairs Diabetes Trial [VADT], ACCORD) failed to show a reduction in mortality or CVD risk with intensive glycaemic control. 34 , 35 , 36 However, importantly, unlike the UKPDS, the ADVANCE, ACCORD and VADT studies enrolled people with poorly controlled T2D of long duration who had established CVD or additional risk factors. 34 , 35 , 36 Furthermore, these intensive treatment studies extensively employed pharmacological approaches associated with increased risk of hypoglycaemia (eg, sulphonylureas; insulin) and weight gain (sulphonylureas; insulin; thiazolidinediones), and aimed for what some might consider nonphysiological glycaemic targets (eg, HbA1c < 42 mmol/mol) based on the therapies available at the time. To illustrate, hypoglycaemic episodes requiring medical assistance occurred in 10.5% of individuals in the intensive treatment group of the ACCORD study, 37 and in 21% of those in the VADT, 38 with severe hypoglycaemia being associated with increased occurrence of CV events in the subsequent 3 months. Significant weight gain also occurred in these studies, with nearly 28% of participants in the intensive treatment group of the ACCORD study gaining more than 10 kg over the study. 37 This level of hypoglycaemia and weight gain would not be accepted in current clinical trial design or care and likely reflects a mismatch between therapeutic goals and therapeutic modalities available at the time of the study.
FIGURE 1.
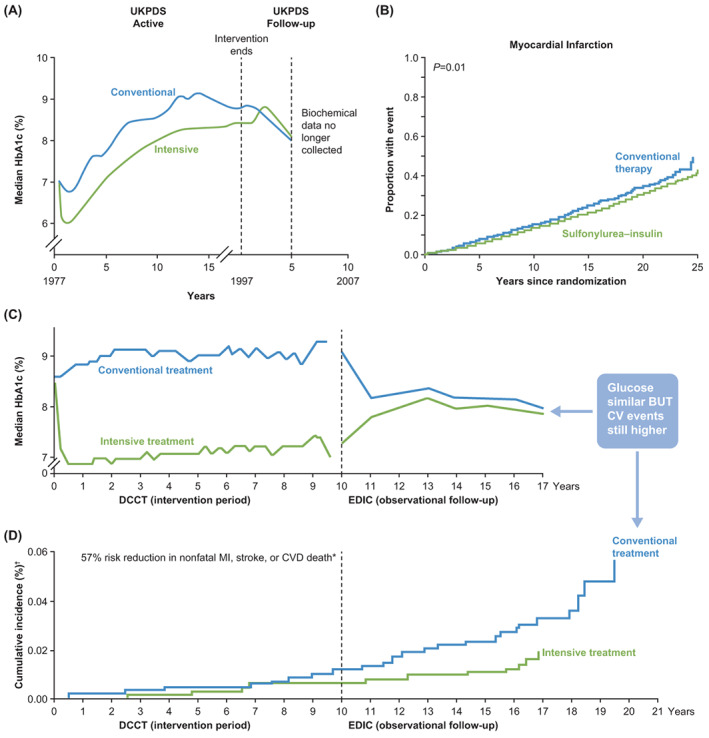
Benefits of early treatment of diabetes to reduce latent cardiovascular disease (CVD) risk: The legacy effect. A, UK Prospective Diabetes Study (UKPDS): glycated haemoglobin (HbA1c) over time; B, UKPDS: cardiovascular (CV) outcomes; C, Diabetes Control and Complications Trial (DCCT)/ Epidemiology of Diabetes Interventions and Complications (EDIC): HbA1c over time; D, DCCT/EDIC: CV outcomes. MI, myocardial infarction. A, C and D Reprinted from Ramachandran et al. J Diabetes Metab. 2015;6:4 https://doi.org/10.4172/2155‐6156.1000520 under Creative Commons Attribution License. B From N Engl J Med., B Holman RR, et al. 10‐Year Follow‐up of Intensive Glucose Control in Type 2 Diabetes, 359, 1577‐89. Copyright © 2008. Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society
While primary care‐focused guidelines have appropriately emphasized blood pressure and lipid management for CV risk reduction in people with T2D, the interpretation of these treat‐to‐target trials has paradoxically led to clinical recommendations for less stringent glycaemic targets, and for deintensification of therapy. The ACP, for example, in 2018 issued its updated recommendations that “Clinicians should aim to achieve an HbA1c level between 53 and 64 mmol/mol in most patients with type 2 diabetes” and that “Clinicians should consider deintensifying pharmacologic therapy in patients with type 2 diabetes who achieve HbA1c levels less than 48 mmol/mol”. 22 These conflict with recommendations from diabetes societies (eg, the ADA, ADA‐European Association for the Study of Diabetes [EASD] Consensus) 39 that promote individualized targets, but generally recommend an HbA1c target of lower than 53 mmol/mol in most adults with diabetes, with consideration of lower goals for those in whom they can be achieved safely without significant hypoglycaemia or adverse effects of treatment, and without deintensification of treatment when lower targets can be safely achieved. Such discrepancies in recommendations probably contribute to a level of uncertainty in the management of care and introduce a dimension of therapeutic inertia at the societal level. It is important to reconcile these conflicting messages and highlight the importance of both glycaemic control and therapeutic approach to achieve the optimal treatment for each individual with T2D.
Adding to the debate on the relevance of glucose control, recent CVOTs have shown unequivocal benefits of both SGLT2 inhibitors and GLP‐1RAs in reducing CVD events in people with T2D at high risk for or who have ASCVD. Notably, these CVOTs did not have intensive glycaemic targets and were conducted on background treatment that comprised current standards of care. Improvements in the risk of composite major adverse CV events (MACE), hospitalization for heart failure (HHF), and renal outcomes 8 , 9 with SGLT2 inhibitors were observed in as little as 3 months. 10 , 11 , 12 Similarly, GLP‐1RAs proved particularly effective in reducing ASCVD outcomes, including MI and stroke. 13 , 14 , 15 , 16 , 17 While these predominantly high‐risk populations are not representative of many patients with T2D who have multiple CVD risk factors without established CVD, a small number of CVOTs (DECLARE‐TIMI 58: 59% without CVD 10 ; REWIND: 69% without CVD 15 ) demonstrated benefits of SGLT2 inhibitors and GLP‐1RAs even in those without established ASCVD. The benefits of some agents have been shown to be independent of baseline glycaemic status, 40 potentially fuelling the debate on whether glycaemic control is necessary in the quest to reduce CVD risk.
3. THE PRESENCE OF MICROVASCULAR DISEASE PREDICTS CVD RISK
Although it is widely acknowledged that blood glucose control lowers microvascular risk, 31 , 32 , 34 , 36 , 41 because of the complex relationship between glycaemia and macrovascular events, the potential benefit of blood glucose control on macrovascular risk remains an area of great debate. Although the temporal relationship between microvascular disease and macrovascular risk remains poorly understood, clinical data strongly support the role of microvascular disease in predicting macrovascular risk.
A bidirectional interaction between the macro‐ and microvasculature is known to exist, which maintains a deleterious relationship between diabetes and the circulatory system. For example, increased large artery stiffness accentuates pulse waves, causing microvascular damage. 42 , 43 Similarly, abnormalities in microvascular structure and function may increase the risk of macrovascular events. 42 Also, neovascularization of the vasa vasorum is increased in people with versus without diabetes, which precedes endothelial dysfunction and increases plaque inflammation. 44 , 45 The presence of microvascular complications predicts CVD and coronary artery disease death in individuals with T2D but without CVD. 46 , 47 , 48 Diabetic retinopathy has been associated with an excess risk of HF 49 and CVD, 50 while the results of the Look AHEAD study suggest that microvascular disease was associated with an overall 2.5‐fold increase in risk of incident HF, with individual hazard ratios for nephropathy, retinopathy and neuropathy of 2.22, 1.30 and 1.33, respectively. 51 Data from the CVOTs also support the role of microvascular disease, with further analysis of the LEADER trial showing that the risk reduction with liraglutide for the composite CV outcome of CV death, nonfatal MI, or nonfatal stroke was greater in patients with an estimated glomerular filtration rate of less than 60 mL/min/1.73 m2 versus 60 mL/min/1.73 m2 or greater, 52 although this could be secondary to hypertension or other renal anomalies. Similarly, a post hoc analysis of the LEADER and SUSTAIN‐6 studies showed that microvascular disease was associated with increased risk of MACE, 53 and analysis of the EMPA‐REG OUTCOME study showed that the presence of microvascular disease at baseline was associated with a higher risk of HHF and CVD death (but not three‐point MACE), with a trend for worsening HF as the number of microvascular complications increased, 54 similar to that reported by Brownrigg et al 55 (Figure 2). Diabetic cardiomyopathy 56 is also associated with the presence of microvascular complications 57 and is proposed to be caused by microangiopathy. 58
FIGURE 2.
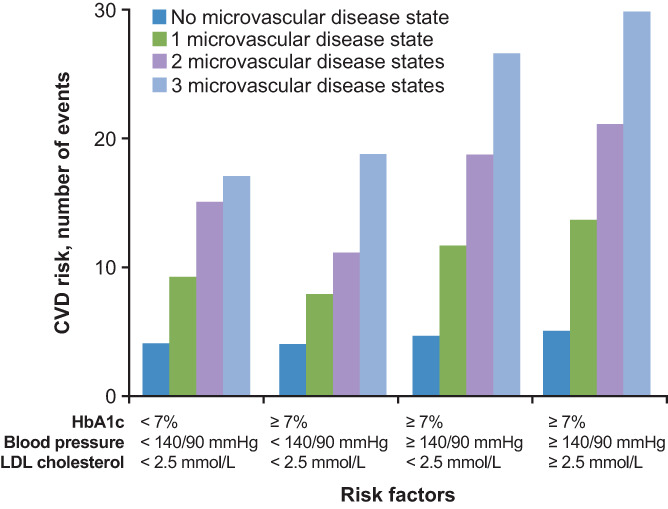
Microvascular disease is predictive of cardiovascular disease (CVD) risk. Analysis of 49 027 people with type 2 diabetes and no established CVD at baseline. Participants were followed for a median of 5.5 years for the primary outcome of time to first cardiovascular (CV) event. LDL, low‐density lipoprotein. Reprinted from The Lancet, Vol. 4, Brownrigg JRW, et al. Microvascular disease and risk of cardiovascular events among individuals with type 2 diabetes: a population‐level cohort study, 588‐597, Copyright 2016, with permission from Elsevier
4. RECONCILING OLD AND NEW DATA‐GLUCOSE IS A MODIFIABLE FACTOR FOR CVD RISK
Risk factors for CVD are numerous, with multiple classic factors—age, sex, obesity, dyslipidaemia, hypertension—and also more recently identified factors—oxidative stress, epigenetics, inflammation, and endothelial dysfunction—being linked with T2D. 59 Together with metabolic syndrome, these factors are known to increase CVD risk. 60 , 61 Further, it has been shown that people with versus without T2D have higher atheroma volume, greater atherosclerotic plaque burden, and impaired compensatory positive remodelling of arteries. 62 Although the mechanisms for these changes have not been completely characterized, many studies support a role for hyperglycaemia.
Results of a large retrospective analysis of the US Veterans Affairs Healthcare System showed a linear relationship between increased CVD mortality and mean HbA1c levels higher than 53 mmol/mol versus HbA1c levels of 42 to 52 mmol/mol. 63 Similarly, a study of demographically adjusted models showed that, compared with an HbA1c lower than 31 mmol/mol, an HbA1c level higher than 36 mmol/mol was associated with an increased risk of ASCVD, an HbA1c level higher than 44 mmol/mol with an increased risk of chronic kidney disease, and an HbA1c level of 53 mmol/mol with an increased risk of HF, suggesting that a significant gradient of risk exists across HbA1c levels well below the diagnostic cutoff for diabetes. 29 The above observations concur with physiological findings that moderate hyperglycaemia (11.1 mmol/L) impairs endothelial cell function, thus augmenting vasoconstriction and promoting inflammation, thrombosis 64 and vascular damage 65 , 66 , 67 (Figure 3). Hyperglycaemia also directly affects both the microvasculature and macrovasculature by causing phenotypic switching of vascular smooth muscle cells to an activated state 68 or to foam cells, 69 resulting in an increased inflammatory response, 70 B‐cell activation, and epigenetic changes that persist even after return to normoglycaemia. 71 In the heart, hyperglycaemia can cause vascular changes independently of atherosclerosis, resulting in the accumulation of advanced glycation end‐products which, together with proinflammatory cytokines and chemokines, recruit leukocytes to the vascular endothelium, causing fibrosis. 72 Postprandial hyperglycaemic excursions also augment oxidative stress, systemic inflammation, and endothelial dysfunction, all of which contribute to atherosclerosis and CVD risk. 73 , 74
FIGURE 3.
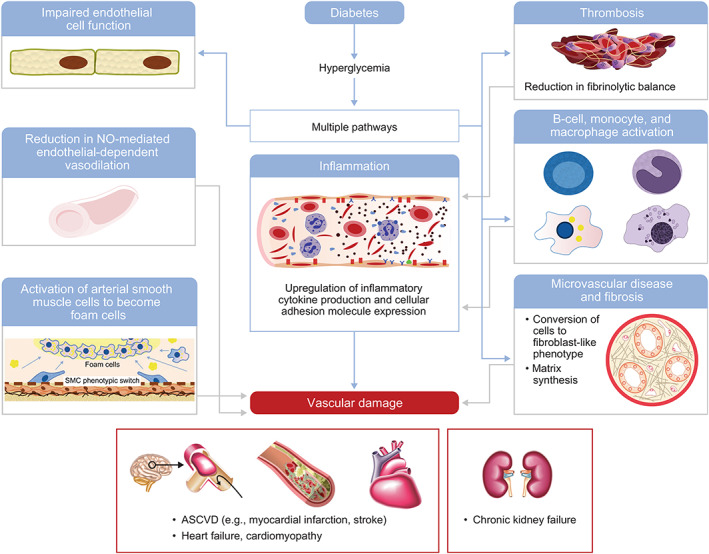
Contributory mechanisms of hyperglycaemia to vascular and kidney disease. ASCVD, atherosclerotic cardiovascular disease; NO, nitric oxide; SMC, smooth muscular cells
While not designed to study the value of glucose control in reducing CVD risk, 8 , 75 , 76 , 77 mediation analyses of GLP‐1RA studies suggest that the lower CVD risk with this class tracks with their glycaemic effect (possibly in addition to other associated factors). 78 , 79 For example, an exploratory analysis of the LEADER trial suggested that mean HbA1c is a potential mediator of the CV protective effect of liraglutide. 78 Likewise, an analysis of the REWIND study reported that 50% to 60% of the reduction in stroke risk with weekly dulaglutide 1.5 mg was related to glucose reduction. 80 Further, a meta‐analysis of dipeptidyl peptidase‐4 inhibitor, GLP‐1RA, and SGLT2 inhibitor CVOTs demonstrated a significant association between HbA1c and MACE risk, 81 predicting a 33% reduction in MACE if all CVOTs achieved an HbA1c reduction of 9.8 mmol/mol. The authors noted that the only CVOT to achieve an HbA1c reduction of this magnitude was SUSTAIN‐6 (9 mmol/mol), which had an associated 26% reduction in MACE risk in a population composed largely (83%) of individuals with established CVD. 14 , 81 This consideration contrasts with those in another recently published article, in which the authors concluded that because of the benefits shown by some of these agents in people without diabetes, the MACE benefits of GLP‐1RAs and SGLT2 inhibitors are “exclusive of their glucose‐reducing actions”, 82 querying whether glucose reduction perhaps prevents early atherosclerosis, but not the final processes leading to CVD events.
Parallels can be drawn for landmark trials. Despite initial reports of a lack of benefit of intensive glycaemic control in ACCORD, post hoc analyses have produced findings more consistent with the UKPDS, in that participants without prior CVD or those with baseline HbA1c less than 64 mmol/mol who received intensive treatment had fewer CV events than those receiving standard therapy. 35 Of those who received intensive treatment, only those with mean baseline HbA1c above 69 mmol/mol were found to have a higher mortality risk, 83 with a higher mean on‐treatment HbA1c being associated with an increased mortality risk. 84 Several meta‐analyses have shown an association between glucose control and reduction in CVD events. Two meta‐analyses that included data from the UKPDS, ACCORD, ADVANCE, and VADT trials showed that intensive versus conventional therapy reduced the risk of MACE by 9%, nonfatal MI by 15%, 85 and CVD by 10%. 86 Another two meta‐analyses that included data from the UKPDS, ACCORD, ADVANCE, VADT and PROACTIVE trials reported that a decrease in mean HbA1c of 9.9 mmol/mol with intensive therapy reduced the likelihood of CVD events by 11%, MI by 14% to 17%, and coronary artery diease by 15%. 87 , 88 Other findings suggested that intensive glucose control was associated with a 10% to 15% reduction in nonfatal MI. 89
5. THE LEGACY EFFECT: A MATTER OF TIMING AND EARLY GLYCAEMIC CONTROL
The opportunity to meaningfully reduce CVD risk during the early stages of T2D fits with the findings of the UKPDS, which reported a benefit of intensive treatment on CVD endpoints in individuals with newly diagnosed T2D who were younger (mean age 53 years) than participants of other trials. 90 Also supportive of this hypothesis, findings of a meta‐analysis suggested that the benefit of intensive glycaemic control on macrovascular risk was particularly prominent in younger people with shorter duration of diabetes. 91 In further agreement, the use of intensive glycaemic control in military veterans (mean age 60.4 years) with T2D diagnosed a mean of 11.5 years earlier, 40% of whom had a prior CVD event, did not improve the rates of MACE, death, or microvascular complications (except for progression of albuminuria). 34 Related to this, even the presence of prediabetes is known to be associated with substantial CVD risk. 29 Although results of the Diabetes Prevention Program/Diabetes Prevention Program Outcomes Study showed that metformin decreased coronary artery calcification in men with prediabetes, 92 recently published findings confirmed that the use of metformin did not reduce the occurrence of nonfatal MI, stroke, or CV death. 93 Results of the VA‐IMPACT study, which was also designed to assess whether metformin can reduce mortality and CVD morbidity in people with prediabetes and established ASCVD, are awaited. 94
The importance of early blood glucose control on the risk of later complications is highlighted by several longitudinal studies. The Diabetes and Aging study 95 showed that early glycaemic control (HbA1c < vs. >) was associated with a lower risk of microvascular complications, macrovascular complications, and mortality, which persisted for 7 years. Newer data from a control‐matched cohort of individuals with T2D from the Swedish National Diabetes Register 96 revealed that among five risk factors (elevated HbA1c, elevated low‐density lipoprotein cholesterol, albuminuria, smoking, and elevated blood pressure), an HbA1c level outside of the target range was consistently the most important risk marker/predictor for stroke and acute MI, although it was not a predictor for death or HHF. Follow‐up of the DCCT demonstrated that for the same average HbA1c over 20 years, reaching goal early versus late was associated with a 33% reduction in the risk of CVD and a 52% reduction in estimated glomerular filtration rate worsening. 97 In reviewing DCCT/EDIC and UKPDS data, the same group concluded that the concepts of metabolic memory for type 1 diabetes and the legacy effect for T2D are likely to be biologically similar, endorsing use of early intensive therapy to maintain normal glycaemia for as long as possible to limit the risk of complications. 98
A unique challenge in T2D management is the high rate of natural progression of disease, even despite therapy. This is highlighted by follow‐up of UKPDS participants, which showed that maintenance of target glycaemic levels declined markedly over 9 years, with only 24% of those who received sulphonylurea monotherapy achieving a fasting plasma glucose (FPG) level lower than 7.8 mmol/L, and 24% achieving HbA1c lower than 53 mmol/mol. 99 Whether higher‐efficacy therapies such as GLP‐1RAs can affect the natural course of T2D is not known, although it is plausible that higher‐efficacy approaches, and approaches that dually support glucose and weight reduction, will help alter the natural course of T2D and prolong control. 100
6. IMPROVING EARLY GLYCAEMIC CONTROL: A ROLE FOR COMBINATION THERAPY?
Whereas traditional approaches have used stepwise, sequential addition of therapy, recent data suggest that the use of early combination therapy may achieve and sustain glycaemic control more effectively. Indeed the recently updated ADA Standards of Care 101 support the use of initial combination therapy for either more rapid attainment of glycaemic goals 102 , 103 or longer durability of glycaemic effect, 104 recommending that “initial combination therapy should be considered in patients presenting with HbA1c values 15.9 mmol/mol above target.” 101 Furthermore, results from the VERIFY study confirmed that initial metformin/vildagliptin combination therapy in people with newly diagnosed T2D resulted in better long‐term glycaemic control than metformin monotherapy 105 (a 49% reduction in the time to initial treatment failure) and also reduced the risk of time to secondary treatment failure by 26%. 105 , 106 Although the trial was not powered to assess CV outcomes, early combination therapy was associated with a numerically longer time to first adjudicated macrovascular event than metformin monotherapy. 105 However, it is important to note that, in this study, 40% of people who received metformin monotherapy had no treatment failure after 5 years. As such, it is possible that initiation of dual treatment in this population could represent overtreatment. That said, the use of combination therapy later in the course of diabetes has been shown to impact the durability of glycaemic effect. The results of the DUAL VIII study showed that after failure of oral therapy, treatment with the basal insulin/GLP‐1RA fixed‐ratio combination IDegLira was associated with longer time to treatment intensification versus insulin glargine 100 U/mL (median >2 vs. ~1 year) with fewer participants requiring treatment intensification over 104 weeks (37% vs. 66%). 104 Further analysis confirmed greater reduction in HbA1c with lower hypoglycaemia rates for the fixed‐ratio combination compared with basal insulin alone. 107
7. HYPOGLYCAEMIA/GLYCAEMIC VARIABILITY AS A MODIFIABLE CVD RISK FACTOR
The ACCORD study was the first to identify increased mortality associated with intensive glycaemic goals of lower than 6% in high‐risk patients with T2D. 35 Although severe hypoglycaemia was associated with increased risk of mortality, a post hoc analysis showed that the risk of death was in fact lower for those who received intensive versus conventional therapy, 108 which could reflect the increased risk of hypoglycaemia in older adults with diabetes. Further, another analysis of the ACCORD study demonstrated that the risk of mortality in the subset of individuals who received intensive control increased linearly with HbA1c (from 42 to 75 mmol/mol) and was highest in those unable to achieve target glycaemia (HbA1c < 53 mmol/mol). 84 Separate analyses have confirmed that intensive therapy increases the risk of severe hypoglycaemia by two‐ to threefold 85 , 86 , 87 , 88 , 89 and a meta‐analysis showed a correlation between risk of severe hypoglycaemia and CVD death with intensive therapy. 87 Collectively, these results suggest that intensive glucose control may reduce CVD events in people with T2D, but this needs to be balanced against CV events associated with severe hypoglycaemia. It is pertinent to note that these trials were conducted some time ago using intensive treatment modalities that do not reflect the current standard of care. Increases in hypoglycaemia rates are not observed with SGLT2 inhibitors and GLP‐1RAs; 10 , 11 , 12 , 109 furthermore, guidelines now recommend individualizing glycaemic targets, highlighting the importance of achieving HbA1c goals safely. 20 As such, these negative influences on CVD risk might not be present if trials were repeated using current‐day treatments.
How glycaemic control is monitored and assessed also has considerable potential to guide advancement of therapy and more fully address the relationship between glycaemia and CV risk. Although HbA1c reflects the average glucose concentrations over a 3‐month period, it does not account for day‐to‐day glucose variability, which is proposed to be more deleterious to CV health than the average change in HbA1c over time. 82 , 110 Indeed, data from the FinnDiane study in people with type 1 diabetes showed that HbA1c variability rather than mean HbA1c better predicted CVD events. 111 Similarly, the ALLHAT study 112 revealed that increased visit‐to‐visit variability of FPG is associated with increased mortality risk in individuals without CVD. High variability in HbA1c has been shown to be associated with increased risk of MACE and all‐cause mortality, even in individuals with no history of diabetes or CVD. 113 Moreover, it is known that HbA1c is contributed to by both FPG and postprandial glucose (PPG). 114 Epidemiological studies suggest that PPG is an independent risk factor for CVD and MI, in people both without 115 and with diabetes. 116 In people with T2D, PPG (but not FPG) has been shown to be a predictor of CVD‐related mortality, 117 this topic having been reviewed by Ceriello both in 2005 118 and in 2021. 119 Moreover, the contribution of PPG to HbA1c is greater for people aged 65 years and older versus those under 65 years, 120 thus raising the CVD and mortality risk in older adults with T2D.
The International Consensus on Use of Continuous Glucose Monitoring from the 2017 advanced technologies & treatments for diabetes (ATTD) Congress recommended the use of time in range (TIR) as a measure of short‐term glycaemic control. 121 People with diabetes are thus advised that TIR, that is, a blood glucose of 3.9 to 10.0 mmol/L should be maintained for at least 70% (16 hours and 48 minutes) of each day. Hypoglycaemia with blood glucose lower than 3.9 mmol/L (time below range; TBR) should be limited to less than 4% (1 hour) of the day, and with blood glucose lower than 3.0 mmol/L to less than 1% (15 minutes) of the day. Hyperglycaemia with blood glucose higher than 10 mmol/L or higher than should be limited to less than 25% (6 hours) and less than 5% (1 hour 12 minutes), respectively, of each day. Because of the increased risk of hypoglycaemia in older adults, the updated version of the guidelines recommends lowering the TIR target from greater than 70% to greater than 50% and reducing TBR to less than 1% at blood glucose levels lower than 3.9 mmol/L to place greater emphasis on reducing hypoglycaemia and less emphasis on maintaining target glucose levels. 122
Metrics from continuous glucose monitoring, including TIR, time above range, and time below range, can facilitate the safe achievement of glycaemic control and mitigation of the risks associated with hypoglycaemia. Several studies have determined that a decrease in TIR is strongly associated with an increased risk of microvascular complications, including microalbuminuria and retinopathy, 123 peripheral neuropathy, 124 as well as an increased risk of macrovascular disease. 125 Furthermore, lower TIR has been shown to be associated with an increased risk of all‐cause and CVD mortality among patients with T2D. 126
8. CONCLUSIONS
In summary, hyperglycaemia is at the core of both microvascular and macrovascular complications in T2D, with microvascular complications increasing the risk of macrovascular complications. Although results of observational studies consistently point to hyperglycaemia as the most important risk factor for both microvascular and macrovascular complications, interventional studies have shown consistent benefits of intensive glucose control on microvascular complications in T2D, with a more complex relationship between intervention and macrovascular complications. These results probably relate to the stage of disease and therapies studied, with factors such as trial design, study population, and length of follow‐up producing variability in outcomes. The modalities by which glycaemic control is achieved are continually evolving; however, overcoming therapeutic inertia is key to effecting a change in the current rates of T2D‐related complications. The data reviewed herein suggest that, in addition to blood pressure and lipid control, to reduce CVD risk in people with T2D, intensive treatment of hyperglycaemia should be initiated early with the goal of achieving and maintaining control and will be particularly beneficial in the long run when used at the early stages of disease (ie, in individuals with short duration of diabetes and at low CVD risk). Multiple advances in technology and assessments of glycaemic control (and linkage to outcomes) and the availability of more efficacious therapies and approaches to achieve control, as well as novel therapies that have demonstrated CV benefit, are essential for appropriate care. These advances need to be coupled with consistent clinical guidance on the prioritization of safe and early achievement of glycaemic control to benefit the population at large. The growing prevalence of diabetes and burden of complications worldwide make even more apparent the importance of such advances and need for concerted integration and transformation of care.
AUTHOR CONTRIBUTIONS
Vanita R. Aroda and Robert H. Eckel provided their expertise and intellectual feedback regarding the concept and structure of the manuscript, and contributed to outlining, drafting, and critically revising the manuscript at each stage of development. Both Vanita R. Aroda and Robert H. Eckel approved the final manuscript for publication.
CONFLICT OF INTEREST
Vanita R. Aroda reports serving as a consultant to Applied Therapeutics, Fractyl, Novo Nordisk, Pfizer and Sanofi, has a spouse employed by Janssen, and has had research support (institutional clinical trial contracts) from Applied Therapeutics, Eli Lilly, Fractyl, Novo Nordisk, and Sanofi. Robert H. Eckel reports work for Amarin, Kaleido, KOWA, Novo Nordisk, The Healthy Aging Company, UpToDate, and WW.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14830.
ACKNOWLEDGMENTS
Writing support for this manuscript was funded by Sanofi US. The journal's article processing charges were also funded by Sanofi US. The authors received writing support in the preparation of this manuscript provided by Helen Jones, PhD, CMPP, and Carolyn Bowler, PhD, of Evidence Scientific Solutions Inc, funded by Sanofi US.
Aroda VR, Eckel RH. Reconsidering the role of glycaemic control in cardiovascular disease risk in type 2 diabetes: A 21st century assessment. Diabetes Obes Metab. 2022;24(12):2297‐2308. doi: 10.1111/dom.14830
Funding information Writing support for this manuscript was funded by Sanofi US. The journal's article processing charges were also funded by Sanofi US. The authors received writing support in the preparation of this manuscript provided by Helen Jones, PhD, CMPP, and Carolyn Bowler, PhD, of Evidence Scientific Solutions Inc, funded by Sanofi US.
DATA AVAILABILITY STATEMENT
Not applicable.
REFERENCES
- 1. Kannel WB, McGee DL. Diabetes and cardiovascular disease: the Framingham study. JAMA. 1979;241(19):2035‐2038. [DOI] [PubMed] [Google Scholar]
- 2. Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta‐analysis of 102 prospective studies. Lancet. 2010;375(9733):2215‐2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jacob S, Krentz AJ, Deanfield J, Ryden L. Evolution of type 2 diabetes management from a Glucocentric approach to cardio‐renal risk reduction: the new paradigm of care. Drugs. 2021;81(12):1373‐1379. [DOI] [PubMed] [Google Scholar]
- 4. Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348(5):383‐393. [DOI] [PubMed] [Google Scholar]
- 5. Grundy SM, Brewer HB Jr, Cleeman JI, Smith SC Jr, Lenfant C. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433‐438. [DOI] [PubMed] [Google Scholar]
- 6. Alexander CM, Landsman PB, Teutsch SM, Haffner SM, Third National Health and Nutrition Examination Survey (NHANES III) , National Cholesterol Education Program (NCEP) . NCEP‐defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes. 2003;52(5):1210‐1214. [DOI] [PubMed] [Google Scholar]
- 7. Butler J, Rodondi N, Zhu Y, et al. Metabolic syndrome and the risk of cardiovascular disease in older adults. J Am Coll Cardiol. 2006;47(8):1595‐1602. [DOI] [PubMed] [Google Scholar]
- 8. Inzucchi SE, Kosiborod M, Fitchett D, et al. Improvement in cardiovascular outcomes with empagliflozin is independent of glycemic control. Circulation. 2018;138(17):1904‐1907. [DOI] [PubMed] [Google Scholar]
- 9. Cannon CP, Perkovic V, Agarwal R, et al. Evaluating the effects of canagliflozin on cardiovascular and renal events in patients with type 2 diabetes mellitus and chronic kidney disease according to baseline HbA1c, including those with HbA1c <7%: results from the CREDENCE trial. Circulation. 2020;141(5):407‐410. [DOI] [PubMed] [Google Scholar]
- 10. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347‐357. [DOI] [PubMed] [Google Scholar]
- 11. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644‐657. [DOI] [PubMed] [Google Scholar]
- 12. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117‐2128. [DOI] [PubMed] [Google Scholar]
- 13. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834‐1844. [DOI] [PubMed] [Google Scholar]
- 15. Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double‐blind, randomised placebo‐controlled trial. Lancet. 2019;394(10193):121‐130. [DOI] [PubMed] [Google Scholar]
- 16. Gerstein HC, Sattar N, Rosenstock J, et al. Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N Engl J Med. 2021;385(10):896‐907. [DOI] [PubMed] [Google Scholar]
- 17. Sattar N, Lee MMY, Kristensen SL, et al. Cardiovascular, mortality, and kidney outcomes with GLP‐1 receptor agonists in patients with type 2 diabetes: a systematic review and meta‐analysis of randomised trials. Lancet Diabetes Endocrinol. 2021;9(10):653‐662. [DOI] [PubMed] [Google Scholar]
- 18. Fang M, Wang D, Coresh J, Selvin E. Trends in diabetes treatment and control in U.S. adults, 1999‐2018. N Engl J Med. 2021;384(23):2219‐2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Khunti K, Ceriello A, Cos X, De Block C. Achievement of guideline targets for blood pressure, lipid, and glycaemic control in type 2 diabetes: A meta‐analysis. Diabetes Res Clin Pract. 2018;137:137‐148. [DOI] [PubMed] [Google Scholar]
- 20. American Diabetes Association Professional Practice Committee , Draznin B, Aroda VR, et al. 6. Glycemic targets: standards of medical care in diabetes‐2022. Diabetes Care. 2022;45(Suppl 1):S83‐S96. [DOI] [PubMed] [Google Scholar]
- 21. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract. 2020;26(1):107‐139. [DOI] [PubMed] [Google Scholar]
- 22. Qaseem A, Wilt TJ, Kansagara D, et al. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann Intern Med. 2018;168(8):569‐576. [DOI] [PubMed] [Google Scholar]
- 23. Khunti K, Gomes MB, Pocock S, et al. Therapeutic inertia in the treatment of hyperglycaemia in patients with type 2 diabetes: a systematic review. Diabetes Obes Metab. 2018;20(2):427‐437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007‐2017. Cardiovasc Diabetol. 2018;17(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gregg EW, Hora I, Benoit SR. Resurgence in diabetes‐related complications. JAMA. 2019;321(19):1867‐1868. [DOI] [PubMed] [Google Scholar]
- 26. Khunti K, Millar‐Jones D. Clinical inertia to insulin initiation and intensification in the UK: A focused literature review. Prim Care Diabetes. 2017;11(1):3‐12. [DOI] [PubMed] [Google Scholar]
- 27. Mauricio D, Meneghini L, Seufert J, et al. Glycaemic control and hypoglycaemia burden in patients with type 2 diabetes initiating basal insulin in Europe and the USA. Diabetes Obes Metab. 2017;19(8):1155‐1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med. 2004;141(6):413‐420. [DOI] [PubMed] [Google Scholar]
- 29. Honigberg MC, Zekavat SM, Pirruccello JP, Natarajan P, Vaduganathan M. Cardiovascular and kidney outcomes across the glycemic spectrum: insights from the UK Biobank. J Am Coll Cardiol. 2021;78(5):453‐464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Paul SK, Klein K, Thorsted BL, Wolden ML, Khunti K. Delay in treatment intensification increases the risks of cardiovascular events in patients with type 2 diabetes. Cardiovasc Diabetol. 2015;14:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. UK Prospective Diabetes Study (UKPDS) Group . Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837‐853. [PubMed] [Google Scholar]
- 32. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10‐year follow‐up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577‐1589. [DOI] [PubMed] [Google Scholar]
- 33. Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643‐2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129‐139. [DOI] [PubMed] [Google Scholar]
- 35. Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545‐2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560‐2572. [DOI] [PubMed] [Google Scholar]
- 37. Action to Control Cardiovascular Risk in Diabetes Study Group , Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545‐2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Glycemic Control and Cardiovascular Outcomes—The VA Diabetes Trial . Program and Abstracts of the 68th Scientific Sessions of the American Diabetes Association. San Francisco, California; 2008. [Google Scholar]
- 39. Davies MJ, D'Alessio DA, Fradkin J, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2018;61(12):2461‐2498. [DOI] [PubMed] [Google Scholar]
- 40. Persson F, Rossing P, Vart P, et al. Efficacy and safety of dapagliflozin by baseline glycemic status: a prespecified analysis from the DAPA‐CKD trial. Diabetes Care. 2021;44(8):1894‐1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Diabetes Control and Complications Trial Research Group , Nathan DM, Genuth S, et al. The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med. 1993;329(14):977‐986. [DOI] [PubMed] [Google Scholar]
- 42. Climie RE, van Sloten TT, Bruno RM, et al. Macrovasculature and microvasculature at the crossroads between type 2 diabetes mellitus and hypertension. Hypertension. 2019;73(6):1138‐1149. [DOI] [PubMed] [Google Scholar]
- 43. Chirinos JA. Deep phenotyping of systemic arterial hemodynamics in HFpEF (part 1): physiologic and technical considerations. J Cardiovasc Transl Res. 2017;10(3):245‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Moreno PR, Fuster V. New aspects in the pathogenesis of diabetic atherothrombosis. J Am Coll Cardiol. 2004;44(12):2293‐2300. [DOI] [PubMed] [Google Scholar]
- 45. Purushothaman KR, Purushothaman M, Muntner P, et al. Inflammation, neovascularization and intra‐plaque hemorrhage are associated with increased reparative collagen content: implication for plaque progression in diabetic atherosclerosis. Vasc Med. 2011;16(2):103‐108. [DOI] [PubMed] [Google Scholar]
- 46. Miettinen H, Haffner SM, Lehto S, Rönnemaa T, Pyörälà K, Laakso M. Retinopathy predicts coronary heart disease events in NIDDM patients. Diabetes Care. 1996;19(12):1445‐1448. [DOI] [PubMed] [Google Scholar]
- 47. Fuller JH, Stevens LK, Wang SL. Risk factors for cardiovascular mortality and morbidity: the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia. 2001;44(Suppl 2):S54‐S64. [DOI] [PubMed] [Google Scholar]
- 48. van Hecke MV, Dekker JM, Stehouwer CD, et al. Diabetic retinopathy is associated with mortality and cardiovascular disease incidence: the EURODIAB prospective complications study. Diabetes Care. 2005;28(6):1383‐1389. [DOI] [PubMed] [Google Scholar]
- 49. Cheung N, Wang JJ, Rogers SL, et al. Diabetic retinopathy and risk of heart failure. J Am Coll Cardiol. 2008;51(16):1573‐1578. [DOI] [PubMed] [Google Scholar]
- 50. Rosenson RS, Fioretto P, Dodson PM. Does microvascular disease predict macrovascular events in type 2 diabetes? Atherosclerosis. 2011;218(1):13‐18. [DOI] [PubMed] [Google Scholar]
- 51. Kaze AD, Santhanam P, Erqou S, Ahima RS, Bertoni A, Echouffo‐Tcheugui JB. Microvascular disease and incident heart failure among individuals with type 2 diabetes mellitus. J Am Heart Assoc. 2021;10(12):e018998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mann JFE, Fonseca V, Mosenzon O, et al. Effects of liraglutide versus placebo on cardiovascular events in patients with type 2 diabetes mellitus and chronic kidney disease. Circulation. 2018;138(25):2908‐2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Verma S, Bain SC, Honoré JB, et al. Impact of microvascular disease on cardiovascular outcomes in type 2 diabetes: results from the LEADER and SUSTAIN 6 clinical trials. Diabetes Obes Metab. 2020;22(11):2193‐2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Verma S, Wanner C, Zwiener I, et al. Influence of microvascular disease on cardiovascular events in type 2 diabetes. J Am Coll Cardiol. 2019;73(21):2780‐2782. [DOI] [PubMed] [Google Scholar]
- 55. Brownrigg JR, Hughes CO, Burleigh D, et al. Microvascular disease and risk of cardiovascular events among individuals with type 2 diabetes: a population‐level cohort study. Lancet Diabetes Endocrinol. 2016;4(7):588‐597. [DOI] [PubMed] [Google Scholar]
- 56. Stanton AM, Vaduganathan M, Chang LS, Turchin A, Januzzi JL Jr, Aroda VR. Asymptomatic diabetic cardiomyopathy: an underrecognized entity in type 2 diabetes. Curr Diab Rep. 2021;21(10):41. [DOI] [PubMed] [Google Scholar]
- 57. Laakso M. Heart in diabetes: a microvascular disease. Diabetes Care. 2011;34(Suppl 2):S145‐S149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Alonso N, Moliner P, Mauricio D. Pathogenesis, clinical features and treatment of diabetic cardiomyopathy. Adv Exp Med Biol. 2018;1067:197‐217. [DOI] [PubMed] [Google Scholar]
- 59. Matheus AS, Tannus LR, Cobas RA, Palma CC, Negrato CA, Gomes MB. Impact of diabetes on cardiovascular disease: an update. Int J Hypertens. 2013;2013:653789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640‐1645. [DOI] [PubMed] [Google Scholar]
- 61. Grundy SM. Metabolic syndrome update. Trends Cardiovasc Med. 2016;26(4):364‐373. [DOI] [PubMed] [Google Scholar]
- 62. Nicholls SJ, Tuzcu EM, Kalidindi S, et al. Effect of diabetes on progression of coronary atherosclerosis and arterial remodeling: a pooled analysis of 5 intravascular ultrasound trials. J Am Coll Cardiol. 2008;52(4):255‐262. [DOI] [PubMed] [Google Scholar]
- 63. Raghavan S, Vassy JL, Ho YL, et al. Diabetes mellitus‐related all‐cause and cardiovascular mortality in a national cohort of adults. J Am Heart Assoc. 2019;8(4):e011295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Stratmann B, Tschoepe D. Pathobiology and cell interactions of platelets in diabetes. Diab Vasc Dis Res. 2005;2(1):16‐23. [DOI] [PubMed] [Google Scholar]
- 65. Perkins JM, Joy NG, Tate DB, Davis SN. Acute effects of hyperinsulinemia and hyperglycemia on vascular inflammatory biomarkers and endothelial function in overweight and obese humans. Am J Physiol Endocrinol Metab. 2015;309(2):E168‐E176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Smolock AR, Mishra G, Eguchi K, Eguchi S, Scalia R. Protein kinase C upregulates intercellular adhesion molecule‐1 and leukocyte‐endothelium interactions in hyperglycemia via activation of endothelial expressed calpain. Arterioscler Thromb Vasc Biol. 2011;31(2):289‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tuleta I, Frangogiannis NG. Diabetic fibrosis. Biochim Biophys Acta, Mol Basis Dis. 2021;1867(4):166044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zheng XL, Yuan SG, Peng DQ. Phenotype‐specific inhibition of the vascular smooth muscle cell cycle by high glucose treatment. Diabetologia. 2007;50(4):881‐890. [DOI] [PubMed] [Google Scholar]
- 69. Chaabane C, Coen M, Bochaton‐Piallat ML. Smooth muscle cell phenotypic switch: implications for foam cell formation. Curr Opin Lipidol. 2014;25(5):374‐379. [DOI] [PubMed] [Google Scholar]
- 70. Nishizawa T, Bornfeldt KE. Diabetic vascular disease and the potential role of macrophage glucose metabolism. Ann Med. 2012;44(6):555‐563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. El‐Osta A, Brasacchio D, Yao D, et al. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med. 2008;205(10):2409‐2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Russo I, Frangogiannis NG. Diabetes‐associated cardiac fibrosis: cellular effectors, molecular mechanisms and therapeutic opportunities. J Mol Cell Cardiol. 2016;90:84‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ceriello A, Quagliaro L, Piconi L, et al. Effect of postprandial hypertriglyceridemia and hyperglycemia on circulating adhesion molecules and oxidative stress generation and the possible role of simvastatin treatment. Diabetes. 2004;53(3):701‐710. [DOI] [PubMed] [Google Scholar]
- 74. Martín‐Timón I, Sevillano‐Collantes C, Segura‐Galindo A, Del Cañizo‐Gómez FJ. Type 2 diabetes and cardiovascular disease: have all risk factors the same strength? World J Diabetes. 2014;5(4):444‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Li J, Neal B, Perkovic V, et al. Mediators of the effects of canagliflozin on kidney protection in patients with type 2 diabetes. Kidney Int. 2020;98(3):769‐777. [DOI] [PubMed] [Google Scholar]
- 76. Inzucchi SE, Zinman B, Fitchett D, et al. How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA‐REG OUTCOME trial. Diabetes Care. 2018;41(2):356‐363. [DOI] [PubMed] [Google Scholar]
- 77. Packer M. Autophagy stimulation and intracellular sodium reduction as mediators of the cardioprotective effect of sodium‐glucose cotransporter 2 inhibitors. Eur J Heart Fail. 2020;22(4):618‐628. [DOI] [PubMed] [Google Scholar]
- 78. Buse JB, Bain SC, Mann JFE, et al. Cardiovascular risk reduction with liraglutide: an exploratory mediation analysis of the LEADER trial. Diabetes Care. 2020;43(7):1546‐1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Konig M, Riddle MC, Colhoun HM, et al. Exploring potential mediators of the cardiovascular benefit of dulaglutide in type 2 diabetes patients in REWIND. Cardiovasc Diabetol. 2021;20(1):194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gerstein HC, Hart R, Colhoun HM, et al. The effect of dulaglutide on stroke: an exploratory analysis of the REWIND trial. Lancet Diabetes Endocrinol. 2020;8(2):106‐114. [DOI] [PubMed] [Google Scholar]
- 81. Giugliano D, Maiorino MI, Bellastella G, Chiodini P, Esposito K. Glycemic control, preexisting cardiovascular disease, and risk of major cardiovascular events in patients with type 2 diabetes mellitus: systematic review with meta‐analysis of cardiovascular outcome trials and intensive glucose control trials. J Am Heart Assoc. 2019;8(12):e012356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Eckel RH, Bornfeldt KE, Goldberg IJ. Cardiovascular disease in diabetes, beyond glucose. Cell Metab. 2021;33(8):1519‐1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Calles‐Escandón J, Lovato LC, Simons‐Morton DG, et al. Effect of intensive compared with standard glycemia treatment strategies on mortality by baseline subgroup characteristics: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care. 2010;33(4):721‐727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Riddle MC, Ambrosius WT, Brillon DJ, et al. Epidemiologic relationships between A1C and all‐cause mortality during a median 3.4‐year follow‐up of glycemic treatment in the ACCORD trial. Diabetes Care. 2010;33(5):983‐990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Turnbull FM, Abraira C, Anderson RJ, et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia. 2009;52(11):2288‐2298. [DOI] [PubMed] [Google Scholar]
- 86. Kelly TN, Bazzano LA, Fonseca VA, Thethi TK, Reynolds K, He J. Systematic review: glucose control and cardiovascular disease in type 2 diabetes. Ann Intern Med. 2009;151(6):394‐403. [DOI] [PubMed] [Google Scholar]
- 87. Mannucci E, Monami M, Lamanna C, Gori F, Marchionni N. Prevention of cardiovascular disease through glycemic control in type 2 diabetes: a meta‐analysis of randomized clinical trials. Nutr Metab Cardiovasc Dis. 2009;19(9):604‐612. [DOI] [PubMed] [Google Scholar]
- 88. Ray KK, Seshasai SR, Wijesuriya S, et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta‐analysis of randomised controlled trials. Lancet. 2009;373(9677):1765‐1772. [DOI] [PubMed] [Google Scholar]
- 89. Mattila TK, de Boer A. Influence of intensive versus conventional glucose control on microvascular and macrovascular complications in type 1 and 2 diabetes mellitus. Drugs. 2010;70(17):2229‐2245. [DOI] [PubMed] [Google Scholar]
- 90. Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Stettler C, Allemann S, Jüni P, et al. Glycemic control and macrovascular disease in types 1 and 2 diabetes mellitus: meta‐analysis of randomized trials. Am Heart J. 2006;152(1):27‐38. [DOI] [PubMed] [Google Scholar]
- 92. Goldberg RB, Aroda VR, Bluemke DA, et al. Effect of long‐term metformin and lifestyle in the diabetes prevention program and its outcome study on coronary artery calcium. Circulation. 2017;136(1):52‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Goldberg RB, Orchard TJ, Crandall JP, et al. Effects of long‐term metformin and lifestyle interventions on cardiovascular events in the diabetes prevention program and its outcome study. Circulation. 2022;145(22):1632‐1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Investigation of Metformin in Pre‐Diabetes on Atherosclerotic Cardiovascular OuTcomes (VA‐IMPACT) . Trial Summary on ClincialTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02915198. Accessed November, 2021.
- 95. Laiteerapong N, Ham SA, Gao Y, et al. The legacy effect in type 2 diabetes: impact of early glycemic control on future complications (the Diabetes & Aging Study). Diabetes Care. 2019;42(3):416‐426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Rawshani A, Rawshani A, Franzen S, et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2018;379(7):633‐644. [DOI] [PubMed] [Google Scholar]
- 97. Lachin JM, Bebu I, Nathan DM, DCCT/EDIC Research Group. The beneficial effects of earlier versus later implementation of intensive therapy in type 1 diabetes. Diabetes Care. 2021;44(10):2225‐2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lachin JM, Nathan DM, DCCT/EDIC Research Group . Understanding metabolic memory: the prolonged influence of glycemia during the Diabetes Control and Complications Trial (DCCT) on future risks of complications during the study of the Epidemiology of Diabetes Interventions and Complications (EDIC). Diabetes Care. 2021;44(10):2216‐2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). JAMA. 1999;281(21):2005‐2012. [DOI] [PubMed] [Google Scholar]
- 100. Nathan DM, Buse JB, Kahn SE, et al. Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care. 2013;36(8):2254‐2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. American Diabetes Association Professional Practice Committee , Draznin B, Aroda VR, et al. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes. Diabetes Care. 2022;45(Suppl 1):S125‐S143. [DOI] [PubMed] [Google Scholar]
- 102. Abdul‐Ghani MA, Puckett C, Triplitt C, et al. Initial combination therapy with metformin, pioglitazone and exenatide is more effective than sequential add‐on therapy in subjects with new‐onset diabetes. Results from the Efficacy and Durability of Initial Combination Therapy for Type 2 Diabetes (EDICT): a randomized trial. Diabetes Obes Metab. 2015;17(3):268‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Phung OJ, Sobieraj DM, Engel SS, Rajpathak SN. Early combination therapy for the treatment of type 2 diabetes mellitus: systematic review and meta‐analysis. Diabetes Obes Metab. 2014;16(5):410‐417. [DOI] [PubMed] [Google Scholar]
- 104. Aroda VR, González‐Galvez G, Grøn R, et al. Durability of insulin degludec plus liraglutide versus insulin glargine U100 as initial injectable therapy in type 2 diabetes (DUAL VIII): a multicentre, open‐label, phase 3b, randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7(8):596‐605. [DOI] [PubMed] [Google Scholar]
- 105. Matthews DR, Paldánius PM, Proot P, et al. Glycaemic durability of an early combination therapy with vildagliptin and metformin versus sequential metformin monotherapy in newly diagnosed type 2 diabetes (VERIFY): a 5‐year, multicentre, randomised, double‐blind trial. Lancet. 2019;394(10208):1519‐1529. [DOI] [PubMed] [Google Scholar]
- 106. Matthews D, Del Prato S, Mohan V, et al. Insights from VERIFY: early combination therapy provides better glycaemic durability than a stepwise approach in newly diagnosed type 2 diabetes. Diabetes Ther. 2020;11(11):2465‐2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Sesti G, Bardtrum L, Dagdelen S, et al. A greater proportion of participants with type 2 diabetes achieve treatment targets with insulin degludec/liraglutide versus insulin glargine 100 units/mL at 26 weeks: DUAL VIII, a randomized trial designed to resemble clinical practice. Diabetes Obes Metab. 2020;22(5):873‐878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ. 2010;340:b4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451‐1461. [DOI] [PubMed] [Google Scholar]
- 110. Martinez M, Santamarina J, Pavesi A, Musso C, Umpierrez GE. Glycemic variability and cardiovascular disease in patients with type 2 diabetes. BMJ Open Diabetes Res Care. 2021;9(1):e002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Wadén J, Forsblom C, Thorn LM, et al. A1C variability predicts incident cardiovascular events, microalbuminuria, and overt diabetic nephropathy in patients with type 1 diabetes. Diabetes. 2009;58(11):2649‐2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Echouffo‐Tcheugui JB, Zhao S, Brock G, Matsouaka RA, Kline D, Joseph JJ. Visit‐to‐visit glycemic variability and risks of cardiovascular events and all‐cause mortality: the ALLHAT study. Diabetes Care. 2019;42(3):486‐493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Ghouse J, Skov MW, Kanters JK, et al. Visit‐to‐visit variability of hemoglobin A1c in people without diabetes and risk of major adverse cardiovascular events and all‐cause mortality. Diabetes Care. 2019;42(1):134‐141. [DOI] [PubMed] [Google Scholar]
- 114. Monnier L, Colette C. Contributions of fasting and postprandial glucose to hemoglobin A1c. Endocr Pract. 2006;12(Suppl 1):42‐46. [DOI] [PubMed] [Google Scholar]
- 115. Qiao Q, Dekker JM, de Vegt F, et al. Two prospective studies found that elevated 2‐hr glucose predicted male mortality independent of fasting glucose and HbA1c. J Clin Epidemiol. 2004;57(6):590‐596. [DOI] [PubMed] [Google Scholar]
- 116. Esposito K, Ciotola M, Carleo D, et al. Post‐meal glucose peaks at home associate with carotid intima‐media thickness in type 2 diabetes. J Clin Endocrinol Metab. 2008;93(4):1345‐1350. [DOI] [PubMed] [Google Scholar]
- 117. Cavalot F, Petrelli A, Traversa M, et al. Postprandial blood glucose is a stronger predictor of cardiovascular events than fasting blood glucose in type 2 diabetes mellitus, particularly in women: lessons from the San Luigi Gonzaga Diabetes study. J Clin Endocrinol Metab. 2006;91(3):813‐819. [DOI] [PubMed] [Google Scholar]
- 118. Ceriello A. Postprandial hyperglycemia and diabetes complications: is it time to treat? Diabetes. 2005;54(1):1‐7. [DOI] [PubMed] [Google Scholar]
- 119. Ceriello A, Prattichizzo F, Phillip M, Hirsch IB, Mathieu C, Battelino T. Glycaemic management in diabetes: old and new approaches. Lancet Diabetes Endocrinol. 2021;10(1):75‐84. [DOI] [PubMed] [Google Scholar]
- 120. Munshi MN, Pandya N, Umpierrez GE, DiGenio A, Zhou R, Riddle MC. Contributions of basal and prandial hyperglycemia to total hyperglycemia in older and younger adults with type 2 diabetes mellitus. J Am Geriatr Soc. 2013;61(4):535‐541. [DOI] [PubMed] [Google Scholar]
- 121. Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40(12):1631‐1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593‐1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2019;42(3):400‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Mayeda L, Katz R, Ahmad I, et al. Glucose time in range and peripheral neuropathy in type 2 diabetes mellitus and chronic kidney disease. BMJ Open Diabetes Res Care. 2020;8(1):e000991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Lu J, Ma X, Shen Y, et al. Time in range is associated with carotid intima‐media thickness in type 2 diabetes. Diabetes Technol Ther. 2020;22(2):72‐78. [DOI] [PubMed] [Google Scholar]
- 126. Lu J, Wang C, Shen Y, et al. Time in range in relation to all‐cause and cardiovascular mortality in patients with type 2 diabetes: a prospective cohort study. Diabetes Care. 2021;44(2):549‐555. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


