Abstract
Background
Adolescents with type 1 diabetes are at significantly increased risk for eating disorders and few interventions exist.
Objective
This study examined the feasibility, acceptability, and preliminary effects of an internet‐based eating disorders prevention program adapted specifically for adolescent girls with type 1 diabetes.
Participants and Methods
Thirty‐five girls (16.2 ± 1.1 years) participated Body Project (T1D Style), a 4‐week program consisting of four adolescent sessions focused on promoting illness acceptance, challenging sociocultural body image pressures, increasing social support, and teaching assertive communication. Caregivers participated in one session focused on fostering body image positivity and a healthy relationship with food. Pre‐intervention, post‐intervention, and 3‐month follow‐up surveys assessed disordered eating, body dissatisfaction, thin‐ideal internalization, diabetes acceptance, diabetes distress, and quality of life. Cohen's d effect sizes were calculated at post‐intervention and follow‐up. Program acceptability was assessed at post‐intervention. Manual fidelity and homework completion were monitored.
Results
High manual fidelity, retention, and homework completion were achieved. Quantitative and qualitative feedback from teens and caregivers suggested high acceptability. Large effects (d = 1.35–0.83) were observed for dieting, body dissatisfaction, diabetes distress, diabetes acceptance, and diabetes‐related quality of life at post‐intervention, with large‐medium effects (d = 1.16–0.58) at follow‐up. Medium‐small effects (d = 0.49–0.78) at post‐intervention were observed for diabetes‐specific disordered eating and thin‐ideal internalization, with effects maintained at follow‐up.
Conclusions
Results support the acceptability and feasibility of this targeted eating disorders prevention program for adolescent girls with type 1 diabetes. Future clinical trials are warranted to determine its effectiveness compared to a control condition.
Keywords: adolescents, diabetes distress, eating disorders, prevention, type 1 diabetes
1. INTRODUCTION
Adolescents with type 1 diabetes are at significantly increased risk for eating disorders (EDs). 1 , 2 , 3 For example, a population‐based study in Sweden found that a diagnosis of T1D in women increased likelihood of developing anorexia by 71%, any ED by 119%, bulimia by 222%, and “other ED” by 153%, 1 while another matched, population‐based cohort found a 2–3‐fold increase in risk for developing an ED. 2 Disordered eating in adolescents with type 1 diabetes is associated with suboptimal glycemic control, more diabetic ketoacidosis episodes requiring hospitalization, lower self‐reported adherence, and longer hospital stays. 4 , 5 , 6 It is also associated with lower quality of life, more depressive symptoms, and negative affect surrounding blood glucose monitoring. 4 , 6 Despite increased risk and persistent life course of EDs, 7 , 8 few ED prevention programs exist. 9 This study examined the acceptability, feasibility, and preliminary effects of a novel, virtual ED prevention program designed specifically for teen girls with type 1 diabetes. Body Project (T1D Style) was systematically adapted from an evidence‐based ED prevention program, Body Project. 10 , 11
1.1. ED development and program themes
Research suggests that type 1 diabetes‐specific risk factors (e.g., insulin‐related weight gain, dietary regimen, diabetes negative affect, diabetes stigma, and hypoglycemia‐induced binge eating) may increase ED risk both independently and by interacting with global ED risk factors (e.g., body dissatisfaction, thin‐ideal internalization, and perfectionism 12 , 13 , 14 ). Trojanowski and colleagues 11 recently conducted qualitative interviews with young women with type 1 diabetes, caregivers, and medical professionals to better understand how type 1 diabetes complicates body image/disordered eating development and how the original Body Project could be adapted to address diabetes‐specific risk. Themes related to complicating factors included diabetes stereotypes and misinformation (e.g., conflating type 1 and type 2 diabetes), illness non‐acceptance (e.g., feeling different, technology self‐consciousness, and body distrust), demands of type 1 diabetes (e.g., cognitive load, food‐focused nature, and constant cost–benefit analysis of food/exercise choices), poor adolescent‐doctor relations (e.g., adolescent‐doctor “fit,” weight stigma in medical settings), and non‐supportive family factors (e.g., conflict, poor communication, and restriction of food choices by caregivers). 11 Stereotypes, misinformation, and poor social support could increase self‐consciousness and body dissatisfaction, while illness non‐acceptance may lead to attempts to control perceived modifiable factors like weight and food; thus, increasing ED risk. 11 Three protective factor themes emerged: illness acceptance, validation/normalization of teen experiences, and supportive family factors (e.g., family communication, allowing teens' increasing autonomy). 11
1.2. Mechanism of change
Body Project is a cognitive dissonance‐based ED prevention program. It is considered a “selective” rather than a “universal” prevention program, meaning that it targets individuals with elevated risk for EDs, such as those with high thin‐ideal internalization and body dissatisfaction. 15 Thin‐ideal internalization refers to “the extent to which an individual cognitively “buys into” socially defined ideals of attractiveness and engages in behaviors designed to produce an approximation of these ideals.” 16 (p. 181) As it is not an intervention, it is not considered appropriate for individuals with a diagnosed ED. Briefly, the original Body Project induces cognitive dissonance by helping participants to define the thin‐ideal, identify costs of pursuing the thin‐ideal, and determine who benefits from buying into it (i.e., diet companies, fashion industry, social media networks, etc., not them) and to complete activities to challenge the thin‐ideal (e.g., writing letters to their younger self about how to resist the thin‐ideal and reasons for doing so, practicing self‐affirmation). Activities are designed to induce cognitive dissonance in those with pro‐thin‐ideal attitudes, thus, facilitating attitude and behavior change. 10 , 17 According to cognitive dissonance theory, humans are motivated to maintain consistency between their behaviors and attitudes. In completing the verbal, written, and behavioral exercises of Body Project, participants decrease thin‐ideal internalization and bring their attitudes into alignment with their anti‐thin‐ideal behaviors, ultimately reducing unhealthy weight control behaviors and ED risk. 17 , 18 The original program was developed as an in‐person group intervention for women in college but has been demonstrated to be efficacious in youth as young as 14 years old. 19 It has been delivered in‐person, remotely, by clinicians, and by peer facilitators. 20 The first Body Project manual consists of four sessions lasting ~1 h, but dissonance‐based ED prevention programs have ranged from 1 to 12 sessions. 21 , 22 A recent meta‐analysis found average effect size decreases of d = 0.5 for thin‐ideal internalization, d = 0.36 for body dissatisfaction, d = 0.31 for dieting, d = 0.25 for negative affect, and d = 0.26 for ED symptoms at post‐intervention. 22
Cognitive dissonance‐based interventions are effective for preventing EDs 15 , 23 and may also promote chronic illness adjustment and acceptance. 24 Stakeholder interviews during Body Project (T1D Style)'s development phase supported use of original Body Project activities paired with additional cognitive dissonance activities to promote type 1 diabetes acceptance (e.g., writing a letter to a recently diagnosed younger girl about accepting their diagnosis and tips for doing so, discussing positive sides or experiences related to having type 1 diabetes, and engaging in diabetes‐related exposures and activism [e.g., wearing diabetes technology in a visible location, telling a new friend about type 1, posting about type 1 diabetes on social media]). Additional elements, such as assertive communication training, identification of type 1 diabetes “allies,” and social support, were included to help to normalize teen experiences and equip them with skills to navigate stressful situations, such as judgmental and misinformed comments from others. 11 Wisting and colleagues 25 recently conducted an uncontrolled pilot trial of a different program—Diabetes Body Project—which they adapted (in consultation with two individuals with type 1 diabetes) for young women with type 1 diabetes aged 16–35, showing acceptability, feasibility, and positive preliminary effects in a Norwegian sample. While these results provide broader support for cognitive‐dissonance ED prevention programs for women with type 1 diabetes, Body Project (T1D Style) was systematically adapted for an adolescent population, included greater integration of Body Project and diabetes‐specific material, and involved caregivers, which was deemed critical during intervention development 11 and has historically been absent in ED prevention programs. 26
Thus, this study evaluated Body Project (T1D Style) feasibility, acceptability, and preliminary effectiveness through a pretest/posttest design with 3‐month follow‐up. It was hypothesized that adolescent thin‐ideal internalization, body dissatisfaction, dietary restraint, and diabetes distress would decrease while quality of life and diabetes acceptance would increase from pre‐ to post‐intervention, with changes maintained at 3 months follow‐up.
2. METHODS
2.1. Participants
Participants from a pediatric endocrinology clinic were recruited via email and phone. Medical staff prequested to approve patients before contacting families to avoid recruiting patients with significant psychiatric comorbidities or active ED diagnoses, as the program was meant to be preventative; however, this was not a strict exclusionary criterion given the pilot nature of the study. Due to COVID‐19 restrictions, in‐person recruitment was not possible. Advertisements describing the body image acceptance program for teen females with type 1 diabetes were posted on Facebook and the clinic website. 10 Teen inclusion criteria included being 15–18 years old, having female sex assigned at birth, diabetes duration 1+ years, and being English speaking. Although EDs in males are common, 27 Body Project has not been extensively studied in adolescent males; thus, only females were eligible.
Of 152 families approached, 42 indicated interest, and 37 girl/caregiver pairs provided written informed assent/consent to participate. Two dropped out following consent due to time constraints (one before Session 1, one after Session 1 due to starting new employment). Results reflect the 35 completers.
2.2. Procedures
Baseline surveys were emailed to participants 1 week before Session 1 and sent immediately following Session 4 and at 3‐month follow‐up. Participants received $10 after completing the baseline battery and attending Session 1, $20 after completing post‐intervention surveys, and $30 after 3‐month follow‐up survey completion. Four groups of 6–11 girls met four consecutive weeks via Zoom. Teens attended four sessions that each lasted 1–1.5 h once per week in the evening. Caregivers attended a separate 1‐h Zoom meeting after teen Session 3. Homework reminders were emailed, and optional text messages sent to caregivers. After 2 months, participants were emailed copies of the letters they wrote during the program.
Groups were led by the first author, with 10 of 16 teen sessions co‐facilitated by another clinical psychology graduate student. An undergraduate research assistant (RA) completed a protocol adherence chart. RAs recorded late arrivals, missed sessions, or missed homework assignments. The university IRB approved all procedures, and program content, including full session descriptions and adaptations from the original protocol, is described in detail in the intervention development paper. 11
2.3. Measures
At baseline, participants self‐reported demographics and clinical characteristics (Table 1). Teens rated their interest and willingness to participate in Body Project (T1D Style) from 1 (strongly disagree) to 5 (strongly agree).
TABLE 1.
Teen demographic and clinical characteristics at T1
| N = 35 (%) | Mean (SD) | Range | |
|---|---|---|---|
| Age, years | 16.17 (1.12) | 15.00–18.00 | |
| Illness duration, years | 7.02 (3.41) | 1.50–14.00 | |
| Self‐reported HbA1c% |
7.8% (1.4) 62 mmol/mol (11.1) |
5.8–13.0% 40–119 mmol/mol |
|
| Insulin pump | 22 (62.86) | ||
| CGM | 32 (91.42) | ||
| BMI | 25.18 (3.91) | 18.01–32.89 | |
| Race | |||
| White | 25 (71.43) | ||
| Black | 3 (8.57) | ||
| Asian | 3 (8.57) | ||
| Mixed race | 2 (5.71) | ||
| Unknown/not reported | 2 (5.71) | ||
| Ethnicity | |||
| Non‐Hispanic | 30 (85.71) | ||
| Hispanic | 3 (8.57) | ||
| Unknown/not reported | 2 (5.71) | ||
| Gender identity | |||
| Female | 31 (88.57) | ||
| Male | 1 (2.86) | ||
| Non‐binary | 1 (2.86) | ||
| Other | 1 (2.86) | ||
| Prefer not to answer | 1 (2.86) | ||
| Sexual orientation | |||
| Straight/heterosexual | 24 (68.57) | ||
| Lesbian/gay | 0 (0.00) | ||
| Bisexual | 3 (8.57) | ||
| Queer | 1 (2.86) | ||
| Asexual | 3 (8.57) | ||
| Pansexual | 1 (2.86) | ||
| Unknown/not reported | 3 (8.57) | ||
| Insurance | |||
| Privately insured | 32 (91.43) | ||
| Publicly insured | 3 (8.57) |
Abbreviations: BMI, body mass index; CGM, continuous glucose monitor; SD, standard deviation.
2.3.1. Diabetes Eating Problems Survey‐Revised (DEPS‐R)
The 16‐item DEPS‐R assesses disturbed eating behaviors specific to type 1 diabetes, including insulin restriction. 28 Scores ≥20 indicate elevated disordered eating. Cronbach's alphas ranged 0.85–0.87 across time points.
2.3.2. Screen for Early Eating Disorder Signs (SEEDS)
The 20‐item SEEDS measures three factors (Body Image, Feelings, Quality of Life [QOL]) 29 and assesses ED risk without suggesting unhealthy weight control behaviors (e.g., insulin restriction). Higher scores indicate more body dissatisfaction, lower quality of life, and more difficulty regulating feelings. Total scores ≤68 indicate low ED risk, scores 69–84 moderate risk, and scores ≥85 high risk. Cronbach's alphas ranged: 0.92–0.94 Total Score, 0.88–0.95 Body Image, 0.88–0.91 Feelings, and 0.85–0.90 Quality of Life.
2.3.3. Dutch Restrained Eating Behaviors Questionnaire (DREBQ)
The 10‐item restraint subscale from the DREBQ was administered to assess dieting behaviors and intentions. 30 Items are averaged, with higher scores indicating greater restrained eating. Cronbach's alphas ranged 0.87–0.93 across time.
2.3.4. Sociocultural Attitudes Toward Appearance Questionnaire, 4th Edition (SATAQ‐4)
The SATAQ‐4 measures thin‐ideal internalization. 31 The 22‐item measure yields five subscales related to internalized body ideals (thin/lean and muscular/athletic) and perceived pressure to conform to ideals from parents, peers, and the media. Higher scores indicate greater internalization. Cronbach's alphas ranged: 0.85–0.91 Thin, 0.85–0.91 Muscular, 0.89–0.94 Family, 0.90–0.94 Peers, and 0.74–0.89 Media.
2.3.5. Problem Areas in Diabetes‐Teen (PAID‐T)
The 26‐item PAID‐T measures adolescent diabetes distress. 32 Items are scored 1 (not a problem) to 6 (serious problem) and summed. Scores <70 indicate none‐to‐mild, 70–90 moderate, and >90 high diabetes distress. 33 Cronbach's alphas ranged from 0.91 to 0.96.
2.3.6. Diabetes Acceptance Scale (DAS)
The 20‐item DAS assesses illness acceptance. 34 Items are rated 0 (never true for me) to 3 (always true to for me) scale. Scores range 0–60; higher scores indicating more acceptance. Scores ≤30 reflect very low diabetes acceptance. Cronbach's alphas ranged: 0.90–0.92.
2.3.7. Pediatric Quality of Life ‐ Core Scales (PedsQL)
The 23‐item PedsQL provides a Total health‐related quality of life score (HRQOL), a Physical Health Summary Score, and Psychosocial Health Summary Score. 35 Participants indicate the frequency with which they experience problems from 0 (never) to 4 (almost always). Higher scores indicate higher HRQOL. Cronbach's alphas ranged: 0.93–0.95 Total, 0.89–0.91 Physical, and 0.88–0.91 Psychosocial Health.
2.3.8. Pediatric Quality of Life—Diabetes 3.2 Module (DPedsQL)
The 33‐item DPedsQL yields a Diabetes Symptoms Summary Score, which measures impact of physical symptoms on daily functioning, and a Diabetes Management Summary Score, which assesses treatment barriers, treatment adherence, diabetes‐related worry, and diabetes communication. 36 Participants rate the frequency with which they experience problems from 0 (never) to 4 (almost always). Higher scores indicate higher diabetes‐HRQOL. Cronbach's alphas ranged: 0.85–0.90 for the Diabetes Management and 0.84–0.87 for the Diabetes Symptoms.
2.3.9. Acceptability
Participants completed a 16‐item tailored questionnaire assessing participants' agreement from 1 (strongly disagree) to 7 (strongly agree) with statements about program acceptability and motivation to change. Feedback on program structure and ideas for improvement were gathered.
2.3.10. Caregiver evaluation
Caregiver pre/post knowledge survey assessed agreement with the statement, “I am knowledgeable about how to promote healthy body image development in my child(ren)” (rated from 1 [strongly disagree] to 5 [strongly agree]). Multiple‐choice and true/false questions assessed material covered in the session (e.g., importance of family meals, impact of talking negatively about body size, and validating teens' feelings about diabetes). Percentage of correct answers before/after the session was calculated. Caregiver feedback on what was helpful, what was missing, and what could be improved was gathered.
2.4. Data analysis
Data were examined for normality, and effect sizes from pre‐intervention (Time 1 [T1]) to post‐intervention (Time 2 [T2]) and pre‐intervention (T1) to 3‐month follow‐up (Time 3 [T3]) (Cohen's d based on paired samples t‐tests with bootstrapped confidence intervals) were calculated for continuous outcomes. Effect sizes were categorized as “small,” d = 0.20–0.49, “medium,” d = 0.50–0.80, and “large,” d ≥ 0.80. 37 Mean scores and standard deviations for program acceptability questions were examined to estimate participant attitudes toward the program. Clinically significant change was estimated using established cutoffs or minimally clinically important difference scores (MCIDS).
3. RESULTS
All participants either agreed (34.28%, n = 12) or strongly agreed (65.71%, n = 23) that they were interested in and willing to participate in the group. Nine participants missed a scheduled session and participated in a one‐on‐one or small group session before the next scheduled meeting; all 35 teens completed every session. All manual content was covered in each session. Two groups utilized Zoom chat significantly more than the other two groups. Participants completed nearly every homework assignment for the first three sessions, and over 75% sent in a description of their final exercises following Session 4. Although not every participant took part in their group's final group activism activity, many girls continued to promote the mission of Body Project (T1D Style), primarily through social media. One group created an Instagram account dedicated to type 1 diabetes awareness and body image positivity. Other girls posted together on social media about type 1 diabetes and/or body image positivity, and one group started an online support group and associated Instagram account for teens with type 1 diabetes.
3.1. Acceptability
Participants indicated feeling supported by facilitators, motivated to change their behavior (e.g., challenge the thin‐ideal, care for their bodies and diabetes), and more knowledgeable about resisting the thin‐ideal following the program (Table 2). The only question not garnering support was “the individual session lengths were just right.” Qualitative feedback suggested that girls in the first two groups (over the summer) preferred longer, while those in the fall (during initial COVID distance learning) preferred shorter sessions. Six participants wanted more sessions, but teens generally supported the 4‐session format (M = 6.09, SD = 1.01).
TABLE 2.
Means and standard deviations for tailored acceptability questions answered by participants at post‐intervention
| Mean (SD) | |
|---|---|
| 1. Body Project (T1D Style) was engaging | 6.57 (0.61) |
| 2. I learned something new through the intervention | 6.49 (0.56) |
| 3. I know how to challenge the thin‐ideal | 6.54 (0.70) |
| 4. I feel equipped to resist the appearance‐ideal | 6.49 (0.74) |
| 5. I changed my perspective on the appearance‐ideal | 5.83 (1.49) |
| 6. I feel motivated to challenge the appearance‐ideal in my daily life | 6.20 (1.05) |
| 7. I feel more motivated to care for my body | 6.06 (1.03) |
| 8. I feel more motivated to follow my diabetes management plan | 5.94 (1.06) |
| 9. Body Project (T1D Style) length was just right | 6.09 (1.01) |
| 10. Body Project (T1D Style) session lengths were just right | 2.94 (2.24) |
| 11. Following Body Project (T1D Style), I am more willing to stand up to negative body comments by my parents, family, friends, or others (i.e., engage in body activism) | 6.26 (0.78) |
| 12. Following Body Project (T1D Style), I am more willing to stand up to negative or misinformed diabetes‐related comments by my family, friends, or others | 6.46 (0.95) |
| 13. I had fun during the intervention | 6.49 (0.82) |
| 14. I felt supported by other participants during the program | 6.63 (0.69) |
| 15. I felt supported by facilitators/leaders during the program | 6.66 (0.54) |
| 16. Body Project (T1D) addressed relevant aspects of how T1D may influence body image | 6.51 (0.74) |
Note: The rating scale uses a 7‐point Likert scale (1 “strongly disagree” to 7 “strongly agree”).
Abbreviation: SD, standard deviation.
During the first group, teens asked if their acceptance letters could be shared with newly diagnosed patients. A few girls suggested having more time for them to “get to know each other” and share advice. Some wished for more assertiveness practice and more practice on resisting the thin‐ideal given how deeply it can be rooted. Another teen requested more discussion about “how having diabetes can alter your view of the appearance ideal and yourself [or your body],” and two encouraged intertwining discussions about body image and diabetes even more throughout the program, as some of the exercises may have felt singular in focus. Additional suggestions included: an exercise where girls “find a famous person or someone who you look up to that has type 1 diabetes and see how successful they became while having type 1 diabetes,” “talk[ing] more about body type in relation to injections/pumps,” using Zoom breakout rooms and slides, having an Instagram page for Body Project (T1D Style), and having a facilitator with type 1 diabetes.
Overall, participants praised the program highly, with representative quotes appearing in Table 3. Feedback regarding what participants “liked best” about the program reflected the idea of finding community, hearing about other teens' experiences, and feeling less alone in their struggles. A smaller number of teens reported that they liked specific activities best, with the role plays, letters, “I am __” statements, and learning about the appearance ideal all listed. Teens identified learning ways to challenge the appearance ideal, related role‐plays, assertive communication skills, and self‐affirmation activities most helpful. The benefit of feeling less alone was frequently highlighted. Limited feedback on least helpful program aspects was received. For example, 18 participants said that nothing could be changed, and one did not provide a response. The other responses about what was least helpful varied. Three participants did not like the Zoom format; however, another participant praised the virtual format. Three reported that they did not find the mirror exercise as helpful as they hoped. One thought reading the letters aloud took too much time; however, reading one's own letter out loud is key to inducing cognitive dissonance. One denied need for identifying “allies” who they could turn to when experiencing diabetes distress; however, they noted that the exercise could be helpful for others who did not have such individuals identified already.
TABLE 3.
Representative participant quotes gathered from the post‐intervention survey about what they liked best and found most helpful about Body Project (T1D Style)
| Liked best | Most helpful |
|---|---|
| “I liked learning about how to stand up to negative or misinformed comments about diabetes from family, friends, or others. I also liked hearing about everyone's experiences with T1D and how I am not going through it alone.” | “I had a pretty negative self‐view before this program, and while not all of my concerns are floating away easily, I feel more confident and more accepting of the way I look, even down to the things I get the most self‐conscious over.” |
| “The girls in the project (including the leaders) we all could share easily and help one another with our different advice from personal experience. It was nice having other T1D's like me that could share in my struggles.” | “I found the lesson on comments I can use to stand up to people who are misinformed about diabetes the most helpful.” |
| “Meeting other teens who have T1D, it was nice to really know that I'm not alone.” | “Understanding that I'm not the only teenage girl with T1D that feels this way‐ I now know that there are various other girls that struggle with very similar things and we have now all helped each other and continue to do so!” |
| “What I liked best about the Body Project (T1D Style) was meeting other teenage Type 1 Diabetics like myself. Everyone was so supportive and I really liked meeting everyone and talking with them. It was nice to know others who go through the same struggles as me.” | “The most helpful parts of the program were the mirror exercise and the letter exercises. I really liked the mirror one because it forced me to think about all of the things I love about myself instead of dwelling on the flaws I see in myself. I want to keep doing this exercise because it gave me [a] confidence boost that stayed with me throughout the day. The letter exercises were also super helpful because they allowed me to reflect and gather advice from hearing about the other girls' experiences. The challenge exercises were also a favorite for me because they pushed me to break past the barriers and rules I set for myself. They helped me realize that the fears I had about opening up about diabetes or wearing certain clothes were not that scary.” |
| “I got to make a new group of supportive friends!!” | “The exercises were amazing and helped me so much” |
| “Talking about things that I would never would talk with people” | “Doing the exercises made me accept my T1D better.” |
| “I liked the role‐playing exercises because they made me feel extremely empowered to stand up for myself and others where I would not have before!” | “The role plays ‐ they helped me practice how I would react in real life, I also liked the open discussion aspect” |
| “I really enjoyed when we had the opportunity to practice challenging the appearance ideal and educating about t1d.” | “I though[t] it was really helpful to know there are so many other girls who have the same thoughts, feelings and challenges of body image and T1D. It made me feel less alone.” |
| “I liked the role plays, the support, and the I am statements we did [at] the end of every session.” | “They gave me a different perspective on how I view my body. I am more confident and more aware of how the body ideal affects everyone, especially teens. Also, the at‐home exercises helped a lot with opening my eyes and giving me a new perspective.” |
| “I liked the letter writing the best, and I liked that the girls were all around my age because I related to them more.” | “I thought the most helpful thing about the program was that the content of the sessions were real and not sugar‐coated, meaning that the environment was honest and open.” |
3.2. ED outcomes
As this study had a single‐arm design lacking a control group, all preliminary intervention effects should be interpreted with caution. Nearly all scores on ED and body image measures decreased from T1 to T2 (Table 4). Dieting (DREBQ) decreased with a large effect size (d = 1.03, 95% CI [0.54, 1.49]), as did SEEDS body dissatisfaction (d = 0.84, 95% CI [0.54, 1.14]). DEPS‐R scores decreased with a medium to large effect (d = 0.78, 95% CI [0.45, 1.17]), and SEEDS ED risk scores decreased similarly (d = 0.76, 95% CI [0.39, 1.11]). Moderate decreases in thin‐ideal internalization (d = 0.49, 95% CI [0.16, 0.82]) and muscular ideal internalization (d = 0.64, 95% CI [0.26, 0.94]) were observed.
TABLE 4.
Means and standard deviations for outcomes at T1, T2, and T3 with effect sizes from T1 to T2 and from T1 to T3
| Variable | Time 1 | Time 2 | Time 3 | T1–T2 effect size | T1–T3 effect size |
|---|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | Cohen's d (CI) | Cohen's d (CI) | |
| Disordered eating outcomes | |||||
| DREBQ | 2.86 (0.74) | 2.29 (0.79) | 2.37 (0.87) | 1.03 (0.54, 1.49) | 0.60 (0.25, 0.95) |
| SATAQ‐4 | |||||
| Intern: Thin/low body fat | 3.57 (1.09) | 3.06 (1.08) | 2.92 (1.23) | 0.49 (0.16, 0.82) | 0.58 (0.23, 0.85) |
| Intern: Musc./athletic | 2.91 (0.87) | 2.48 (0.91) | 2.36 (1.00) | 0.64 (0.26, 0.94) | 0.59 (0.02, 1.10) |
| Pressure: Family | 2.65 (1.26) | 2.41 (1.32) | 2.41 (1.38) | 0.30 (−0.04, 0.65) | 0.31 (−0.10, 0.60) |
| Pressure: Media | 3.48 (1.12) | 3.64 (1.02) | 3.20 (1.04) | 0.10 (−0.19, 0.44) | 0.17 (−0.20, 0.47) |
| Pressure: Peers | 2.11 (1.20) | 1.80 (0.99) | 1.69 (0.85) | 0.33 (−0.05, 0.62) | 0.40 (0.04, 0.65) |
| SEEDS total | 74.54 (20.40) | 65.51 (16.97) | 67.11 (20.87) | 0.76 (0.39, 1.11) | 0.42 (0.12, 0.65) |
| Body image dissatisfaction | 29.26 (8.89) | 24.57 (7.32) | 24.80 (8.01) | 0.84 (0.54, 1.14) | 0.58 (0.32, 0.85) |
| DEPS‐R | 19.26 (12.16) | 15.20 (9.52) | 15.20 (9.52) | 0.78 (0.45, 1.17) | 0.49 (0.19, 0.81) |
| Diabetes outcomes | |||||
| DAS | 44.66 (8.20) | 51.61 (6.42) | 49.91 (7.62) | 1.35 (1.03, 1.70) | 0.73 (0.36, 1.06) |
| PAID‐T | 90.49 (24.76) | 69.94 (28.91) | 61.91 (26.72) | 0.86 (0.58, 1.17) | 1.16 (0.80, 1.50) |
| General mental health outcomes | |||||
| PedsQL ‐ Total | 70.77 (16.69) | 73.07 (14.83) | 75.43 (16.94) | 0.29 (−0.09, 0.63) | 0.42 (0.04, 0.74) |
| Physical functioning | 77.32 (20.34) | 78.30 (16.72) | 79.29 (19.10) | 0.10 (−0.26, 0.44) | 0.16 (−0.18, 0.47) |
| Psychosocial health score | 67.27 (15.83) | 70.29 (15.22) | 73.38 (16.62) | 0.31 (−0.08, 0.68) | 0.47 (0.13, 0.81) |
| DPedsQL | |||||
| Diabetes symp. score | 57.01 (14.38) | 60.33 (14.46) | 62.38 (12.97) | 0.40 (0.04, 0.78) | 0.50 (0.16, 0.85) |
| Diabetes manag. score | 67.34 (14.90) | 76.98 (13.90) | 79.88 (14.78) | 0.83 (0.52, 1.13) | 0.87 (0.47, 1.17) |
| SEEDS‐Feelings | 24.74 (8.26) | 22.34 (7.00) | 23.49 (8.25) | 0.52 (0.11, 0.91) | 0.21 (−0.12, 0.53) |
| SEEDS‐QOL | 20.54 (7.63) | 18.60 (6.70) | 18.83 (8.01) | 0.48 (0.15, 0.77) | 0.28 (−0.05, 0.58) |
Note: For ED outcomes, higher scores on all measures indicate more disordered eating/body image concerns. Higher PAID‐T scores indicate higher diabetes distress. Higher DAS scores indicate more diabetes acceptance. Higher PedsQL and DPedsQL scores indicate higher quality of life. Higher SEEDS scores indicate poorer functioning.
Abbreviations: CI, confidence interval; DAS, Diabetes Acceptance Scale; DEPS‐R, Diabetes Eating Problem Survey—Revised; DREBQ, Dutch Restrained Eating Behavior Questionnaire; DPedsQL, Diabetes Pediatric Quality of Life; Em. prep, Emergency Preparedness; Intern, internalization; manag., management; M, mean; Musc., muscular; PAID‐T, Problem Areas in Diabetes—Teen Version; QOL, quality of life; SATAQ‐4, Sociocultural Attitudes Toward Appearance Questionnaire; SD, standard deviation; SEEDS, Screen for Early Eating Disorder Signs; symp., symptoms; T1, Time 1; T2, Time 2; T3, Time 3.
Dietary restraint (DREBQ) (d = 0.60, 95% CI [0.25, 0.95]), thin‐ideal internalization (d = 0.58, 95% CI [0.23, 0.85]), muscular ideal internalization (d = 0.59, 95% CI [0.02, 1.10]), and body dissatisfaction (d = 0.58, 95% CI [0.32, 0.85]) showed medium effects at 3‐month follow‐up. SEEDS ED risk remained lower on average at T3 (d = 0.42, 95% CI [0.12, 0.65]), as did DEPS‐R scores (d = 0.49, 95% CI [0.19, 0.81]). Fifteen participants had elevated DEPS‐R scores at T1 while only six did at T2, McNemar's χ 2 = 7.11, p = 0.01, and nine at T3, McNemar's χ 2 = 7.11, p = 0.08.
3.3. Diabetes‐related outcomes
Large effects at T2 (d = 0.86, 95% CI [0.58, 1.17]) and T3 (d = 1.16, 95% CI [0.80, 1.50]) indicate decreased diabetes distress (Table 4) with more participants falling in the low distress category across time (Figure 1). Large (T2; d = 1.35, 95% CI [1.03, 1.70]) and moderate (T3; d = 0.73, 95% CI [0.36, 1.06]) effect sizes were observed for improved diabetes acceptance (Table 4).
FIGURE 1.
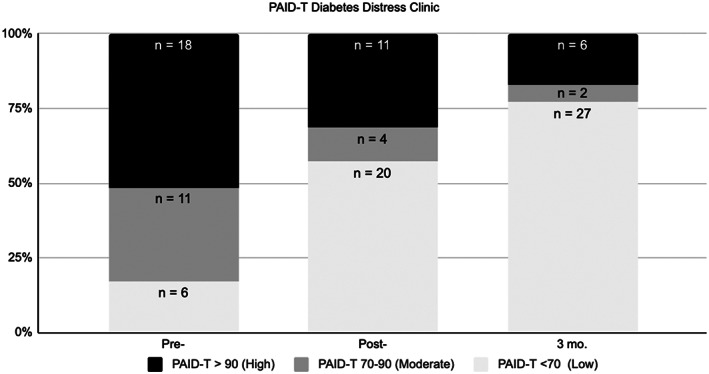
Diabetes distress (PAID‐T) clinical cutoffs and proportion of girls in each category across timepoints. PAID‐T, Problem Areas in Diabetes—Teen version
3.4. Quality of life outcomes
Moderate effects (Table 4) were observed for the Diabetes Symptoms subscale of the DPedsQL (T1–T2: d = 0.40, 95% CI [0.04, 0.78]; T1–T3: d = 0.50, 95% CI [0.16, 0.85]). Large effects were observed for Diabetes Management (T1–T2: d = 0.83, 95% CI [0.52, 1.13]; T1–T3: d = 0.87, 95% CI [0.47, 1.17]).
From T1 to T2 and T1 to T3, small effects for improved PedsQL scores were found, with moderate T3 improvement in total (d = 0.42, 95% CI [0.04, 0.74]) and psychosocial health summary scores (d = 0.47, 95% CI [0.13, 0.81]). Similarly, small to moderate effects were observed for SEEDS‐QOL (d = 0.48, 95% CI [0.15, 0.77]) and SEEDS‐Feelings subscales (d = 0.52, 95% CI [0.11, 0.91]) at T2 (Table 4).
Fifteen teens at T2 and 16 teens at T3 evidenced improvement beyond the MCID on the PedsQL. 35 Fourteen teens at T2 and 16 teens at T3 reported clinically significant improvement on Diabetes Symptoms‐HRQOL, while 20 at T2 and 27 at T3 reported clinically significant change in Diabetes Management‐HRQOL. 36
3.5. Caregiver outcomes
Caregiver(s) from 34 of the families attended the caregiver session. Correct responses on caregiver knowledge assessment, completed by one caregiver per teen, improved from 86.97% to 92.86% (d = 0.36, 95% CI [−0.07, 0.64]), corroborating their reported agreement with the statement, “I am knowledgeable about how to promote healthy body image development in my child(ren)” (d = 0.63, 95% CI [0.36, 0.93]). Caregivers' written responses indicated that social support was particularly helpful (e.g., “meeting other parents that are going through the same experiences as us,” “support from other parents,” “connecting with other parents dealing with type 1 diabetes in the family,” “interacting with other parents and learning from them”). Caregivers indicated appreciation for the handout on promoting positive body image and healthy eating in the home and discussion of the college transition. Suggestions for improvement included: “allow time for each parent to…share their T1 [type 1] story,” “present more topics and let parents choose which ones they are most interested in,” “sending a survey to parents where they can submit questions or concerns,” “offer ongoing support,” “encourage fathers and mothers to join the group together,” present “research on body image among T1 [type 1] girls,” more explicit examples of “how to respond to things my teen says about their body in A+ way,” considering cultural differences in mealtime behavior, and using Zoom polls. One caregiver suggested having caregivers write letters to caregivers of newly diagnosed children, paralleling the teen activity.
4. DISCUSSION
This pilot study demonstrates the initial acceptability, feasibility, and positive preliminary effects of a virtual ED prevention program, Body Project (T1D Style), adapted specifically for teen girls with type 1 diabetes, addressing a significant gap in the literature. 9 Overall, facilitators adhered well to the manual, and participation and engagement of teens and parents was high. The program was feasible given its virtual format and required few materials. Cost‐effectiveness should, however, be assessed in future, larger implementation studies. Many teens enjoyed the virtual format, but a few expressed interest in meeting in person, which could increase implementation costs. Future studies should compare feasibility and effectiveness of virtual and in‐person delivery.
This pilot program was highly acceptable to teens and caregivers. Youth retention was remarkably high (97.2% overall; 100% following Session 1). Families expressed significant gratitude for and enjoyment in being a part of the program. Teens reported increased knowledge about how to resist the thin‐ideal. Their high agreement with statements on the acceptability questionnaire suggested that they were more motivated to care for their bodies and to follow their diabetes management plans at post‐intervention as well, although validated measures should be used in future studies. Teens indicated feeling supported by facilitators.
Despite high acceptability, suggestions for improvement should be considered. Using Zoom breakout rooms and presentation slides occasionally, intertwining dialogue around body type and diabetes technology choices, and allowing more time for girls to give each other advice might be useful. More sessions may improve outcomes, 22 and would align with Wisting et al.'s 25 recently developed intervention, but could impact feasibility. The two groups who utilized the Zoom Chat more frequently (as observed by facilitators) to validate each other, share related experiences, or share resources appeared to bond more and completed more activism activities following the program; therefore, leaders should continue to encourage Chat use in future trials. Caregiver suggestions, such as having them share their connection with type 1 diabetes, soliciting questions ahead of time, and increasing interactiveness (e.g., Zoom polls, role‐plays) should be incorporated. Caregiver requests for multiple meetings highlights the importance of community among caregivers of children with chronic illness. Indeed, having social support and validation from caregivers with shared experiences may promote adjustment. 38
In addition to high acceptability, the program produced promising preliminary results. Body image dissatisfaction, dieting, and ED risk decreased with moderate effects. While effect sizes for ED risk were slightly smaller at post‐intervention in this study compared to Wisting et al.'s Diabetes Body Project for 16–35‐year‐old women (DEPS‐R: 0.78 vs. 0.83), decreases in dietary restraint (DREBQ: 1.03 vs. 0.63) and body dissatisfaction were larger (0.84 vs. 0.67), though body dissatisfaction measures differed. Decreases in thin‐ideal and muscular‐ideal internalization remained lower at follow‐up compared to pre‐intervention. Furthermore, the program resulted in significant improvements in general HRQOL and diabetes‐related HRQOL, which may correlate with glycemic stability. 36
Diabetes attitudes also improved. Perhaps the most striking findings were decreases (of large effect sizes) in teen diabetes distress at T2 and T3. Diabetes distress refers to the emotional burden experienced by people with diabetes, including worries about the future and diabetes complications, hypervigilance, not feeling understood, and feeling anxiety, helplessness, or guilt about disease management. 39 , 40 Although diabetes distress overlaps with depressive symptoms, it was shown to explain more variance in adolescents' HbA1c% levels than depressive symptoms. 39 Furthermore, low diabetes distress is associated with higher self‐reported glucose self‐monitoring. 33 The larger observed effects in the current study compared to Wisting and colleagues may have resulted from increased integration of diabetes and original Body Project content compared to adding two additional sessions exclusively focused on diabetes; however, further integration could be helpful. Improvements in diabetes acceptance also support the use of cognitive dissonance to improve illness acceptance. Taken together, these improvements indicate that teens may have found diabetes less negatively impactful on their life after completing the program. As disordered eating and body dissatisfaction are associated with insulin restriction, less frequent blood glucose monitoring, more episodes of diabetic ketoacidosis, and higher HbA1c%, 4 , 6 , 41 this program may have the ability to not only prevent disordered eating but protect against poor long‐term medical outcomes; however, future trials should explore changes in objective measures of self‐management behaviors (e.g., CGM, meter, and/or pump downloads) following participation.
Despite strengths, this pilot study had notable limitations. Foremost, lack of a control group prevents drawing true conclusions about its efficacy. The small sample size prevented the testing of significant moderators or mediators of program outcomes (e.g., diabetes duration). While the pilot trial had a degree of sample diversity in terms of race, ethnicity, and sexual orientation, the results may not generalize to patients with different cultural backgrounds, gender identities, or in different areas of the country. Relatedly, the prevention program was designed based on qualitative data from a unique metropolitan area where caregivers are generally highly educated, affluent, and emphasize achievement, and their teens are highly involved in scheduled activities. However, caregivers from low‐income families in the area may be overworked due to the area's high cost of living, and their teens may receive less supervision of daily activities. The protocol's cultural sensitivity may also be improved by continuing to review emerging literature, consulting researchers with more expertise in cultural differences in eating practices and body image, and asking future participants to provide direct feedback on improving the program's attention to issues of diversity and multiculturalism. In particular, if male‐identifying individuals are included in future iterations of the program, a greater focus on resisting the muscular ideal may be helpful. 42 Preliminary research suggests that sensitivity to issues of food insecurity, acculturative stress, varying beauty ideals (e.g., skin color, face shape), and balancing the role of family as a protective factor as well as a potential source of conflict around eating and body image may be important for improving cultural sensitivity. 43 , 44 , 45
Another significant contextual factor is that the protocol was tested during the pandemic in 2020. Although COVID presents a cohort effect, the degree to which families were affected by COVID likely differed significantly as the pandemic highlighted social and health inequity. Importantly, the original Body Project protocol has demonstrated efficacy when delivered online, 46 but an in‐person program could strengthen effects. 20 Participants also self‐reported relatively low HbA1c% compared to adolescent population data 47 but similar to those in Wisting and colleagues' trial. 25 Greater attention may be needed to recruit adolescents with suboptimal glycemic stability. Researchers also planned to gather HbA1c% values from patient medical records but were unable to given the reliance on telemedicine appointments during COVID and lack of available HbA1c% data.
Finally, this program does not address additional pathways to disordered eating, such as impulsivity, which may also influence diabetes management. 48 Although efforts were made to avoid recruiting adolescents with active ED diagnoses, it is possible that some youth had clinical levels of disordered eating. Evidence‐based ED treatment for adolescents typically relies heavily on caregivers (e.g., Family‐Based Therapy for eating disorders, Enhanced Cognitive Behavioral Therapy with parent involvement 49 , 50 , 51 ). While it is possible that this program, or components of it, could decrease ED attitudes and behaviors in those with clinical diagnoses, it is unlikely that it could replace intensive, evidence‐based ED treatment, particularly in individuals with diabetes for whom multidisciplinary treatment is critical. 52 Despite limitations, results from this pilot study support further, more rigorous testing in larger, controlled trials.
AUTHOR CONTRIBUTIONS
Paige J. Trojanowski designed the adapted intervention in consultation with Sarah Fischer and Robyn Mehlenbeck and previously published qualitative analysis. Paige J. Trojanowski, Rachel E. Frietchen, and Blair Harvie collected the data. Paige J. Trojanowski analyzed the data and wrote the initial manuscript. Paige J. Trojanowski, Rachel E. Frietchen, Blair Harvie, Sarah Fischer, and Robyn Mehlenbeck commented on the initial manuscript draft, contributed to subsequent revisions, and approved the final manuscript.
FUNDING INFORMATION
Funding for this study was provided by the George Mason University Provost Office.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/pedi.13395.
ETHICS STATEMENT
All authors acknowledge and take responsibility for the ethical collection of data during the study and the integrity and accuracy of data analysis. All procedures were approved by the university IRB. This manuscript has not been submitted elsewhere and represents the authors' own original work.
ACKNOWLEDGMENT
Authors are grateful to the families who participated in this study.
Trojanowski PJ, Frietchen RE, Harvie B, Mehlenbeck R, Fischer S. Internet‐delivered eating disorders prevention program for adolescent girls with type 1 diabetes: Acceptable and feasible. Pediatr Diabetes. 2022;23(7):1122‐1132. doi: 10.1111/pedi.13395
Funding information George Mason University Provost Office
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Hedman A, Breithaupt L, Hübel C, et al. Bidirectional relationship between eating disorders and autoimmune diseases. J Child Psychol Psychiatry. 2019;60(7):803‐812. [DOI] [PubMed] [Google Scholar]
- 2. Dybdal D, Tolstrup JS, Sildorf SM, et al. Increasing risk of psychiatric morbidity after childhood onset type 1 diabetes: a population‐based cohort study. Diabetologia. 2018;61(4):831‐838. [DOI] [PubMed] [Google Scholar]
- 3. Hanlan ME, Griffith J, Patel N, Jaser SS. Eating disorders and disordered eating in type 1 diabetes: prevalence, screening, and treatment options. Curr Diab Rep. 2013;13(6):909‐916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cecilia‐Costa R, Volkening LK, Laffel LM. Factors associated with disordered eating behaviours in adolescents with type 1 diabetes. Diabet Med. 2019;36(8):1020‐1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scheuing N, Bartus B, Berger G, et al. Clinical characteristics and outcome of 467 patients with a clinically recognized eating disorder identified among 52,215 patients with type 1 diabetes: a multicenter German/Austrian study. Diabetes Care. 2014;37(6):1581‐1589. [DOI] [PubMed] [Google Scholar]
- 6. Nip ASY, Reboussin BA, Dabelea D, et al. Disordered eating behaviors in youth and young adults with type 1 or type 2 diabetes receiving insulin therapy: the SEARCH for diabetes in youth study. Diabetes Care. 2019;42(5):859‐866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Colton PA, Olmsted MP, Daneman D, et al. Eating disorders in girls and women with type 1 diabetes: a longitudinal study of prevalence, onset, remission, and recurrence. Diabetes Care. 2015;38(7):1212‐1217. [DOI] [PubMed] [Google Scholar]
- 8. Luyckx K, Verschueren M, Palmeroni N, Goethals ER, Weets I, Claes L. Disturbed eating behaviors in adolescents and emerging adults with type 1 diabetes: a one‐year prospective study. Diabetes Care. 2019;42:1637‐1644. [DOI] [PubMed] [Google Scholar]
- 9. Oldham‐Cooper R, Semple C. Prevention and early help for eating disorders in young people with type 1 diabetes. Clin Child Psychol Psychiatry. 2021;26(3):1359104521994172. [DOI] [PubMed] [Google Scholar]
- 10. Stice E, Presnell K. The Body Project: Promoting Body Acceptance and Preventing Eating Disorders Facilitator Guide. 1st ed. Oxford University Press; 2007:152. [Google Scholar]
- 11. Trojanowski PJ, Mehlenbeck R, Fischer S. Adapting a cognitive dissonance‐based eating disorders prevention program for adolescent girls with type 1 diabetes. Evid‐Based Pract Child Adolesc Ment Health. 2022;1‐17. [Google Scholar]
- 12. Harrison A, Zaremba N, Brown J, et al. A cognitive behavioural model of the bidirectional relationship between disordered eating and diabetes self‐care in people with type 1 diabetes mellitus. Diabet Med. 2021;38:e14578. [DOI] [PubMed] [Google Scholar]
- 13. Peterson CM, Fischer S, Young‐Hyman D. Topical review: a comprehensive risk model for disordered eating in youth with type 1 diabetes. J Pediatr Psychol. 2015;40(4):385‐390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Treasure J, Kan C, Stephenson L, et al. Developing a theoretical maintenance model for disordered eating in type 1 diabetes. Diabet Med. 2015;32(12):1541‐1545. [DOI] [PubMed] [Google Scholar]
- 15. Le LKD, Barendregt JJ, Hay P, Mihalopoulos C. Prevention of eating disorders: a systematic review and meta‐analysis. Clin Psychol Rev. 2017;53:46‐58. [DOI] [PubMed] [Google Scholar]
- 16. Thompson JK, Schaefer LM, Dedrick RF. On the measurement of thin‐ideal internalization: implications for interpretation of risk factors and treatment outcome in eating disorders research. Int J Eat Disord. 2018;51(4):363‐367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stice E, Butryn ML, Rohde P, Shaw H, Marti CN. An effectiveness trial of a new enhanced dissonance eating disorder prevention program among female college students. Behav Res Ther. 2013;51(12):862‐871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Festinger L. A Theory of Cognitive Dissonance [Internet]. Standford University Press; 1957. [Google Scholar]
- 19. Halliwell E, Diedrichs PC. Testing a dissonance body image intervention among young girls. Health Psychol. 2014;33(2):201‐204. [DOI] [PubMed] [Google Scholar]
- 20. Stice E, Rohde P, Shaw H, Gau JM. Clinician‐led, peer‐led, and internet‐delivered dissonance‐based eating disorder prevention programs: effectiveness of these delivery modalities through 4‐year follow‐up. J Consult Clin Psychol. 2020;88(5):481‐494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Becker CB, Stice E. From efficacy to effectiveness to broad implementation: evolution of the body project. J Consult Clin Psychol. 2017;85(8):767‐782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stice E, Marti CN, Shaw H, Rohde P. Meta‐analytic review of dissonance‐based eating disorder prevention programs: intervention, participant, and facilitator features that predict larger effects. Clin Psychol Rev. 2019;70:91‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Watson HJ, Joyce T, French E, et al. Prevention of eating disorders: a systematic review of randomized, controlled trials. Int J Eat Disord. 2016;49(9):833‐862. [DOI] [PubMed] [Google Scholar]
- 24. Leake R, Friend R, Wadhwa N. Improving adjustment to chronic illness through strategic self‐presentation: an experimental study on a renal dialysis unit. Health Psychol. 1999;18(1):54‐62. [DOI] [PubMed] [Google Scholar]
- 25. Wisting L, Haugvik S, Wennersberg AL, et al. Feasibility of a virtually delivered eating disorder prevention program for young females with type 1 diabetes. Int J Eat Disord. 2021;54(9):1696‐1706. [DOI] [PubMed] [Google Scholar]
- 26. Hart LM, Cornell C, Damiano SR, Paxton SJ. Parents and prevention: a systematic review of interventions involving parents that aim to prevent body dissatisfaction or eating disorders. Int J Eat Disord. 2015;48(2):157‐169. [DOI] [PubMed] [Google Scholar]
- 27. Murray SB, Nagata JM, Griffiths S, et al. The enigma of male eating disorders: a critical review and synthesis. Clin Psychol Rev. 2017;57:1‐11. [DOI] [PubMed] [Google Scholar]
- 28. Wisting L, Froisland DH, Skrivarhaug T, Dahl‐Jorgensen K, Ro O. Psychometric properties, norms, and factor structure of the diabetes eating problem survey‐revised in a large sample of children and adolescents with type 1 diabetes. Diabetes Care. 2013;36(8):2198‐2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Powers MA, Richter S, Ackard D, Craft C. Development and validation of the screen for early eating disorder signs (SEEDS) in persons with type 1 diabetes. Eat Disord. 2016;24(3):271‐288. [DOI] [PubMed] [Google Scholar]
- 30. van Strien T, Frijters JER, Bergers GPA, Defares PB. The Dutch eating behavior questionnaire (DEBQ) for assessment of restrained…. Int J Eat Disord. 1986;5(2):295‐315. [Google Scholar]
- 31. Schaefer LM, Burke NL, Thompson JK, et al. Development and validation of the sociocultural attitudes towards appearance questionnaire‐4 (SATAQ‐4). Psychol Assess. 2015;27(1):54‐67. [DOI] [PubMed] [Google Scholar]
- 32. Weissberg‐Benchell J, Antisdel‐Lomaglio J. Diabetes‐specific emotional distress among adolescents: feasibility, reliability, and validity of the problem areas in diabetes‐teen version. Pediatr Diabetes. 2011;12(4pt1):341‐344. [DOI] [PubMed] [Google Scholar]
- 33. Hagger V, Hendrieckx C, Cameron F, Pouwer F, Skinner TC, Speight J. Cut points for identifying clinically significant diabetes distress in adolescents with type 1 diabetes using the PAID‐T: results from diabetes MILES youth–Australia. Diabetes Care. 2017;40(11):1462‐1468. [DOI] [PubMed] [Google Scholar]
- 34. Schmitt A, Reimer A, Kulzer B, et al. Measurement of psychological adjustment to diabetes with the diabetes acceptance scale. J Diabetes Complications. 2018;32(4):384‐392. [DOI] [PubMed] [Google Scholar]
- 35. Varni JW, Seid M, Kurtin PS. PedsQL™ 4.0: reliability and validity of the pediatric quality of life inventory™ version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39(8):800‐812. [DOI] [PubMed] [Google Scholar]
- 36. Varni JW, Delamater AM, Hood KK, et al. PedsQL 3.2 diabetes module for children, adolescents, and young adults: reliability and validity in type 1 diabetes. Diabetes Care. 2018;41(10):2064‐2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cohen J. Statistical Power Analysis for the Behavioral Sciences. Academic Press; 2013:459. [Google Scholar]
- 38. Monaghan M, Hilliard ME, Cogen FR, Streisand R. Supporting parents of very young children with type 1 diabetes: results from a pilot study. Patient Educ Couns. 2011;82(2):271‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hagger V, Hendrieckx C, Cameron F, Pouwer F, Skinner TC, Speight J. Diabetes distress is more strongly associated with HbA1c than depressive symptoms in adolescents with type 1 diabetes: results from diabetes MILES youth—Australia. Pediatr Diabetes. 2018;19(4):840‐847. [DOI] [PubMed] [Google Scholar]
- 40. Fisher L, Polonsky WH, Hessler D. Addressing diabetes distress in clinical care: a practical guide. Diabet Med. 2019;36(7):803‐812. [DOI] [PubMed] [Google Scholar]
- 41. Wisting L, Frøisland DH, Skrivarhaug T, Dahl‐Jørgensen K, Rø Ø. Disturbed eating behavior and omission of insulin in adolescents receiving intensified insulin treatment: a nationwide population‐based study. Diabetes Care. 2013;36(11):3382‐3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brown TA, Forney KJ, Pinner D, Keel PK. A randomized controlled trial of the body project: more than muscles for men with body dissatisfaction. Int J Eat Disord. 2017;50(8):873‐883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Javier SJ. Sustainable Adapted Treatments for Eating Disorders: The Role of Cultural Adaptation in Prevention [Internet]. Virginia Commonwealth University; 2022. https://www.proquest.com/docview/1909374598/abstract/4A37A0AD6E914DD9PQ/1 [Google Scholar]
- 44. Tortolani CC, Goldschmidt AB, Grange DL. Adapting Evidence‐Based Eating Disorder Treatments for Novel Populations and Settings. A Practical Guide. Routledge; 2020:443. [Google Scholar]
- 45. Stumpf JD. Eating Disorder Prevention Program for Latina Adolescents: A Cultural Adaptation [Internet]. Azusa Pacific University; 2022. https://www.proquest.com/docview/1073010064/abstract/F266998ADBDC4381PQ/1 [Google Scholar]
- 46. Ghaderi A, Stice E, Andersson G, Enö Persson J, Allzén E. A randomized controlled trial of the effectiveness of virtually delivered body project (vBP) groups to prevent eating disorders. J Consult Clin Psychol. 2020;88(7):643‐656. [DOI] [PubMed] [Google Scholar]
- 47. Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016–2018. Diabetes Technol Ther. 2019;21(2):66‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rose M, Streisand R, Tully C, et al. Risk of disordered eating behaviors in adolescents with type 1 diabetes. J Pediatr Psychol. 2020;45(5):583‐591. [DOI] [PubMed] [Google Scholar]
- 49. Le Grange D, Lock J, Agras WS, Bryson SW, Jo B. Randomized clinical trial of family‐based treatment and cognitive‐behavioral therapy for adolescent bulimia nervosa. J Am Acad Child Adolesc Psychiatry. 2015;54(11):886‐894.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rienecke RD. Family‐based treatment of eating disorders in adolescents: current insights. Adolesc Health Med Ther. 2017;8:69‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dalle Grave R, Calugi S, Sartirana M, Fairburn CG. Transdiagnostic cognitive behaviour therapy for adolescents with an eating disorder who are not underweight. Behav Res Ther. 2015;73:79‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Partridge H, Figueiredo C, Rouse L, et al. Type 1 diabetes and disordered eating (T1DE): the ComPASSION project – Wessex. Pract Diabetes. 2020;37(4):127‐132. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


