Abstract
Aim
To compare the predictive abilities of the FRAIL scale (FS), frailty screening questionnaire (FSQ) and clinical frailty scale (CFS) for adverse outcomes in older adults in the emergency department.
Methods
In total, 317 older adults aged ≥65 years attending emergency department was screened for frailty using the FS, FSQ and CFS. Outcome measures included all‐cause 28‐day mortality and intensive care unit readmission. Cox proportional hazards model was used for survival comparison. Logistic regression was used to analyze risk factors for readmissions. In addition, we calculated the C‐statistic, net reclassification improvement and integrated discrimination improvement to evaluate the predictive value of three scales.
Results
The prevalence of frailty was 55.2% (FS), 47.0% (FSQ) and 69.4% (CFS). Cox regression and logistic regression analysis revealed that frailty screening by FS, FSQ and CFS was an independent risk factor for all‐cause 28‐day mortality and 30‐ and 90‐day readmission after adjustment. Incorporation of FS, FSQ and CFS into a basic model with other risk factors significantly improved C‐statistic. For all‐cause 28‐day mortality, the model including FS had the highest C‐statistic from 0.786 (95% confidence interval: 0.706–0.865) to 0.854 (95% confidence interval: 0.802–0.907) and the improvements in risk prediction were also confirmed by category‐free net reclassification improvement and integrated discrimination improvement, suggesting FS was significantly better than CFS and FSQ. The three tools had a low predictive ability for readmission (all C‐statistics <0.7).
Conclusions
All three frailty scales showed a predictive ability for 28‐day mortality and readmission but FS may be the most valid tool in the emergency department. Geriatr Gerontol Int ••; ••: ••–•• Geriatr Gerontol Int 2022; 22: 851–856.
Keywords: aged, emergency department, frailty, mortality, risk factor
Introduction
The aging population is increasing worldwide and is predicted to reach an estimated 2 billion people aged ≥65 years by 2050. 1 This places a heavy demand on healthcare systems, particularly emergency care. Older patients are projected to have an increasing proportion of emergency department (ED) visits. 2 , 3
Frailty is characterized by an increased vulnerability to stressors, including acute illnesses, and more than half of older patients are likely to be frail. 4 , 5 Older people with frailty are at an increased risk of ED visits, hospitalization, disability and death. 6 , 7 , 8 With the burgeoning older population, it is timely that we focus our attention on this group of patients. Early identification of frailty in the ED is a process of determining older patients who are prone to adverse outcomes 9 and might potentially influence immediate medical decision‐making. To the best of our knowledge, although the relationship between frailty and adverse outcomes in older adults has been extensively examined, frailty identification is not routinely performed in EDs.
A recent study reported there are nearly 70 scales for frailty assessment, 10 among which the most representative scales are the Fried's frailty phenotype (FP) scale, also called Cardiovascular Health Study 11 and the Deficit Accumulation Index, also called the Frailty Index. 12 However, these scales are difficult to use in the clinical practice in crowded EDs because they are complex and time consuming. Many screening instruments have been developed for hospitalized or community‐dwelling older patients. To date, there are no proposed tools suitable for identifying frail individuals in geriatric emergency care. 13 Thus, it is imperative to determine validated frailty screening instruments appropriate for use in the ED.
Frailty screening tools in a fast‐paced ED should be quick, simple and acceptable for use in clinical practice. The clinical frailty scale (CFS) 14 is the most practical tool for rapid frailty assessment in busy emergency settings due to its feasibility. However, the CFS originally from Canada, is based on community subjects, and its predictive validity for adverse outcomes in the ED setting needs further research. The frailty screening questionnaire (FSQ) in China is based only on five self‐reported components from the FP. 11 , 15 Liu et al. first showed that FSQ is practicable in the emergency setting, and has accurate predictive validity for negative outcomes in older people when applied in the ED. 16 The FRAIL scale (FS) developed by Morley et al. 17 has been identified as practical for use in identifying frailty and is a valid predictor of mortality. 18 Because its convenience and efficiency, the FS was recommended as a preferred instrument in an Australian primary care setting and was suggested as a potential screening tool for frailty in the ED. 19
Despite decades of research, the most suitable instrument for frailty in the ED remains uncertain. There are no data comparing the utility of frailty screening instruments in EDs in China. FS, CFS and FSQ are quick and feasible screening tools, which have the potential to assess for frailty in busy EDs. Therefore, this study aimed to compare the predictive validity of FS, FSQ and CFS for adverse outcomes in older adults in an emergency setting.
Methods
Design, setting and participants
This was a prospective, single‐center, observational cohort study of 317 patients aged ≥65 years admitted to a China ED between January 2021 and September 2021. During the study period, patients diagnosed with the novel Coronavirus Disease 2019 (COVID‐19) were transferred to government‐designated hospitals and only those with negative results were admitted to our hospital. Those who needed emergency surgery, had unstable vital signs (patients expected to die within the following 24 h), or refused to participate in the study were excluded. This study was approved by the institutional review board of China Rehabilitation Research Centre (2021‐093‐1) and the procedures were performed in accordance to the Declaration of Helsinki. All patients provided written informed consent.
Study protocol
Comprehensive medical histories and frailty assessments of all patients were obtained by trained ED attending physicians. On admission, we collected demographic characteristics including age, gender, body mass index (BMI), smoking status, nutritional risk screening 2002 (NRS2002) score and chronic diseases, including hypertension, diabetes, coronary heart disease, hyperlipidemia, chronic kidney disease, stroke and chronic obstructive pulmonary disease. The main diagnosis for emergency visits included pneumonia, cerebral ischemic stroke, acute exacerbation of chronic obstructive pulmonary disease (AECOPD), acute coronary syndrome (ACS), heart failure and anemia. Functional status was evaluated using the activities of daily living. 20 Three frailty instruments, CFS, FS and FSQ, were assessed. All data were collected within 24 h of admission.
Frailty assessment
The FS is comprised of five characteristics: fatigue, resistance, ambulation, illnesses and loss of weight.
Fatigue: Do you feel tired all of the time (at least 3 or 4 days per week)?
Resistance: Can you climb one floor without assistance?
Ambulation: Can you walk one block or 100 m without assistance?
Illness: Do you suffer from more than five diseases?
Weight loss: Has your weight decreased by ≥4.5 kg or 5% of baseline in the previous 12 months?
Each FS characteristic is scored 0–1, and scores range from 0 (best) to 5 (worst). Individuals with a score of ≥3 are categorized as frail, scores of 1–2 as pre‐frail, and no characteristics as robust.
The FSQ includes five self‐reported components based on the modified Fried FP criteria: weight loss, exhaustion, slowness, weakness and inactivity.
Weight loss: an unintentional loss of body weight of at least 4.5 kg in the past year.
Exhaustion: a “yes” response to either of two questions: “Everything I did was an effort” or “I could not get going.”
Slowness: being unable to walk for 250 m.
Weakness: experiencing difficulty in lifting or carrying a weight of 5 kg.
Inactivity: exercise <3 h per week.
Total scores range between 0 and 5. Those with ≥3 are classified as frail, and the others are classified as non‐frail (0, robust; 1–2, pre‐frail).
The CFS is an ordinal scale of nine points, ranging from 1 (very fit) to 9 (terminally ill). Patients with scores of ≥5 are classified as frail (mild to severely frail), those with scores of 4 are classified as pre‐frail, and those with scores of 1–3 are non‐frail.
Outcome measures
The primary outcome was all‐cause mortality within 28 days and the secondary outcomes were ICU readmissions 30 and 90 days after discharge.
Statistical analysis
Descriptive statistics are reported as the mean (standard deviation) for normally distributed variables, median (interquartile range) for non‐normally distributed variables, or counts (percentages) for categorical variables. The differences in characteristics between groups were evaluated using chi‐squared tests for categorical variables. Cohen's kappa coefficient was calculated to examine the agreement between frailty scales. The Cox proportional hazard model and Kaplan–Meier curves were used for survival comparisons. The association between frailty scales and adverse outcomes was investigated using logistic regression models, and odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. The C‐statistics, category‐free net reclassification improvement (NRI) and integrated discrimination improvement (IDI) were applied to investigate the predictive ability of the frailty scales. All statistical analyses were performed using SPSS (version 26.0; IBM Corp., Armonk, NY, USA) and R software (version 4.2.0; The R Foundation for Statistical Computing). All P‐values refer to two‐tailed tests of significance; P < 0.05 was considered significant.
Results
Baseline characteristics
Overall, 317 participants were included, and 50.2% were men. The median age of participants was 83.0 years (IQR 13.0), and BMI was 22.9 kg/m2 (IQR 6.5). General demographic data are shown in Table 1. The main diagnosis of admission was pneumonia (n = 146, 46.1%), followed by anemia, heart failure, AECOPD, cerebral ischemic stroke and ACS. The primary reason for referral among frail patients was pneumonia (57.1% using FS, 57.0% using FSQ and 53.6% using CFS respectively). There were 222 patients (70.0%) with more than two comorbidities. Approximately 167 participants (52.7%) were functionally independent (Barthel index 61–100).
Table 1.
Baseline data of the participants (N = 317)
| Characteristic | Total (n = 317) | FS ≧3 (n = 175) | FSQ ≧3 (n = 149) | CFS ≧5 (n = 220) |
|---|---|---|---|---|
| Age (years), median (IQR) | 83.0 (13.0) | 83.0 (9.0) | 84.0 (9.0) | 84.0 (9.0) |
| Male, n (%) | 159 (50.2) | 78 (44.6) | 58 (38.9) | 96 (43.7) |
| BMI (kg/m2), median (IQR) | 22.9 (6.5) | 21.8 (6.7) | 22.0 (6.9) | 22.0 (6.5) |
| Never smoking, n (%) | 180 (56.8) | 98 (56.0) | 90 (60.4) | 90 (40.9) |
| Chronic diseases, n (%) | ||||
| Hypertension | 204 (64.4) | 116 (66.3) | 98 (65.8) | 140 (63.6) |
| Diabetes | 118 (37.2) | 74 (42.3) | 54 (36.2) | 82 (37.3) |
| Coronary heart disease | 142 (44.8) | 96 (54.9) | 79 (53.0) | 105 (47.7) |
| Hyperlipidemia | 113 (35.6) | 72 (41.1) | 58 (38.9) | 82 (37.3) |
| Chronic kidney disease | 29 (9.1) | 21 (12.0) | 14 (9.4) | 23 (10.5) |
| Stroke | 64 (20.2) | 37 (21.1) | 33 (22.1) | 48 (21.8) |
| COPD | 61 (19.2) | 43 (24.6) | 36 (24.2) | 54 (24.5) |
| Diagnosis on admission, n (%) | ||||
| Pneumonia | 146 (46.1) | 100 (57.1) | 85 (57.0) | 118 (53.6) |
| Cerebral ischemic stroke | 44 (13.9) | 12 (6.9) | 6 (4.0) | 18 (8.2) |
| AECOPD | 48 (15.1) | 36 (20.6) | 31 (20.8) | 44 (20.0) |
| Heart failure | 49 (15.5) | 41 (23.4) | 28 (18.8) | 42 (19.1) |
| ACS | 24 (7.6) | 16 (9.1) | 12 (8.1) | 18 (8.2) |
| Anemia | 138 (43.5) | 95 (54.3) | 82 (55.0) | 111 (50.5) |
| NRS2002 score, median (IQR) | 4 (3) | 5 (4) | 5 (4) | 4 (5) |
| Barthel index, n (%) | ||||
| 61–100 | 167 (52.7) | 59 (33.7) | 47 (31.5) | 73 (33.2) |
| 41–60 | 62 (19.6) | 39 (22.3) | 33 (22.1) | 59 (26.8) |
| ≤40 | 88 (27.7) | 77 (44.0) | 69 (46.4) | 88 (40.0) |
ACS, acute coronary syndrome; AECOPD, acute exacerbation of chronic obstructive pulmonary disease; BMI, body mass index; CFS, clinical frailty scale; COPD, chronic obstructive pulmonary disease; FS, FRAIL scale; FSQ, frailty screening questionnaire; IQR, interquartile range; NRS, nutritional risk screening.
Prevalence of frailty
The prevalence of frailty was 55.2% by FS, 47.0% by FSQ and 69.4% by CFS. Of the 317 participants, 236 (74.4%) were assessed as frail using at least one of the scales. Of these, 50 (15.8%) were considered frail using only one scale, 64 (20.2%) using two scales and 122 (38.5%) using all three scales. The agreement between scales was moderate, and the Cohen's kappa coefficients were the highest between FS and FSQ (FS and FSQ, 0.536; FS and CFS, 0.533; FSQ and CFS, 0.488).
Comparison of different frailty scales for prediction of all‐cause mortality within 28 days
All participants responded to the follow‐up. Among the 317 patients, the 28‐day mortality rate was 10.4% (33 patients died). Most patients died of multiple organ dysfunction resulted from severe infection (15, 45.5%) such as severe pneumonia or sepsis, and the second cause of death was deterioration of chronic diseases (10, 30.3%). The median hospital stay was 12.0 days (IQR 11.0). In the unadjusted multivariate Cox regression analysis (Table 2), frailty screened by FS, FSQ and CFS was a risk factor for 28‐day mortality (hazard ratio = 2.123, 1.651, 3.242, P < 0.001). After adjusting for age, gender, BMI, smoking status, anemia, NRS2002 and chronic diseases, frailty identified by the three tools was still an independent predictor of 28‐day mortality (hazard ratio = 1.849, 1.366, 2.974, P = 0.002, P = 0.026, P < 0.001). Kaplan–Meier analysis also showed that the presence of frailty screened by the three instruments was associated with a higher risk of mortality (Fig. 1).
Table 2.
HRs for 28‐day all‐cause mortality of FS, FSQ and CFS
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| FS | 2.123 | 1.547–2.912 | <0.001 | 1.849 | 1.254–2.725 | 0.002 |
| FSQ | 1.651 | 1.299–2.099 | <0.001 | 1.366 | 1.039–1.797 | 0.026 |
| CFS | 3.242 | 2.318–4.535 | <0.001 | 2.974 | 2.032–4.352 | <0.001 |
Model 1, unadjusted model; Model 2, adjusted for age, gender, body mass index, smoking status, anemia, NRS2002 and chronic diseases (hypertension, diabetes, hyperlipidemia, chronic kidney disease, chronic obstruction pulmonary disease, coronary heart disease, stroke).
CFS, clinical frailty scale; CI, confidence interval; FS, FRAIL scale; FSQ, frailty screening questionnaire; HR, hazard ratio; NRS, nutritional risk screening.
Figure 1.
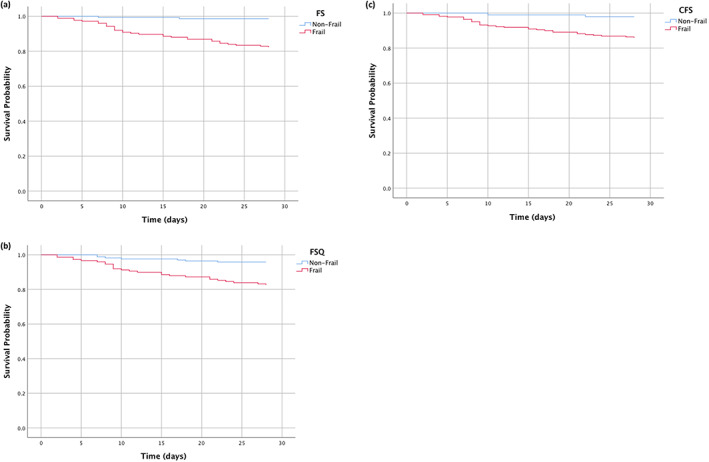
Comparison of overall survival between older adults with and without frailty using three screening instruments. Kaplan–Meier survival curves of frailty defined by (a) the FRAIL scale (FS), (b) frailty screening questionnaire (FSQ) and (c) clinical frailty scale (CFS) for overall 28‐day survival.
Comparison of different frailty scales for prediction of readmissions
Unplanned 30 days readmission to the ED occurred in 49 patients, and 90 days readmission occurred in 79 patients. More than half of the patients readmitted to the ED were because of acute infection particularly recurrent pneumonia, followed by acute exacerbation of chronic diseases (such as decompensated heart failure and AECOPD) and acute illness attack, including ACS or acute stroke. In the multivariable logistic regression analysis, after adjusting for age, gender, BMI, anemia, NRS2002 and chronic diseases, frailty assessed by FS, FSQ and CFS was an independent risk factor for 30‐day readmission (OR = 2.938, 3.454, 6.263, P = 0.009, 0.002, 0.001) and 90‐day readmission (OR = 2.724, 3.956, 6.299, P = 0.003, P < 0.001, P < 0.001).
Comparison of the predictive validity of three frailty scales for adverse outcomes
All the above results showed frailty screened by the three instruments was an independent risk factor for all adverse outcomes including 28‐day all‐cause mortality and unplanned readmissions. Next, we calculated the C‐statistic, NRI and IDI to evaluate the added predictive ability of the three scales. The C‐statistic of a basic model with risk factors, including age, gender, BMI and chronic diseases (hypertension, diabetes, chronic kidney disease, chronic obstruction pulmonary disease, coronary heart disease, stroke) was 0.786 (95% CI: 0.706–0.865). Incorporation of FS, FSQ and CFS into a basic model significantly improved the C‐statistic from 0.786 (95% CI: 0.706–0.865) to 0.854 (95% CI: 0.802–0.907), 0.832 (95% CI: 0.764–0.900) and 0.811 (95% CI: 0.733–0.888) respectively (all P < 0.001). Compared with the established model, adding the FS improved the predictive ability for 28‐day mortality significantly, and the NRI and IDI were 0.746 (95% CI: 0.475–1.017, P < 0.001) and 0.058 (95% CI: 0.022–0.093, P = 0.002), respectively (Table 3). For the pairwise comparison between each of the two scales, the model including FS had a better predictive value than CFS, and the NRI and IDI were 0.742 (95% CI: 0.455–1.030, P < 0.001) and 0.038 (95% CI: 0.007–0.068, P = 0.015). There were no statistically differences between the models that added the FSQ and CFS (NRI = 0.211, 95% CI: −0.476 to 0.054, P = 0.251; IDI = 0.057, 95% CI: −0.037 to 0.026, P = 0.727). The model adding the FS was better than FSQ in terms of NRI (0.426, 95% CI: 0.071–0.782, P = 0.021), but there was no difference in IDI (0.032, 95% CI: −0.002 to 0.067, P = 0.068). In conclusion, adding any of the three scales to a basic model significantly improved the prediction of 28‐day mortality, and the model including FS had the highest C‐statistic and the improvements in risk prediction were also confirmed by category‐free NRI and IDI.
Table 3.
Evaluation of predictive models for 28‐day all‐cause mortality
| C‐statistic (95% CI) | NRI (95% CI) | IDI (95% CI) | |
|---|---|---|---|
| Base model | 0.786 (0.706–0.865) | Reference | Reference |
| Base model + FS | 0.854 (0.802–0.907) | 0.746 (0.475–1.017) | 0.058 (0.022–0.093) |
| Base model + FSQ | 0.832 (0.764–0.900) | 0.717 (0.415–1.018) | 0.026 (−0.008, 0.059) |
| Base model + CFS | 0.811 (0.733–0.888) | 0.372 (0.070–0.673) | 0.020 (0.007–0.033) |
Base model included: age, gender, body mass index and chronic diseases (hypertension, diabetes, chronic kidney disease, chronic obstruction pulmonary disease, coronary heart disease, stroke).
CFS, clinical frailty scale; CI, confidence interval; FS, FRAIL scale; FSQ, frailty screening questionnaire; IDI, integrated discrimination improvement; NRI, net reclassification improvement.
Incorporation of the three frailty identification tools into a basic model showed that the C‐statistics of frailty identified by FS, FSQ and CFS for 30‐day readmission were 0.663, 0.682 and 0.695 (all P < 0.001), and the C‐statistics for 90‐day readmission were 0.611, 0.639 and 0.573, respectively (P < 0.001). The C‐statistics were <0.7, suggesting its low predictive ability for return to the ED.
Discussion
Based on the three screening tools (FS, FSQ, CFS), this study demonstrated a robust relationship between frailty and subsequent adverse outcomes for older patients in the ED. We compared the predictive ability of these three frailty screening tools for 28‐day mortality and readmission, and showed that FS may be better in predicting 28‐day mortality. However, none of the three scales had a good predictive ability for return to the ED. To the best of our knowledge, this is the first study to compare the predictive validity of frailty screening tools in an emergency setting in China.
In this study, the prevalence of frailty ranged from 47.0% (FSQ) to 69.4% (CFS). The agreement between the different scales was moderate (kappa coefficient: 0.488–0.536). We found that the prevalence was higher than reported in previous studies in the ED, where 36.8% of patients were classified as frail based on CFS. 21 A large prospective cohort study showed that the prevalence of frailty varied vastly by different scales (SUHB [stable, unstable, help to walk, bedbound] 9.7%, Fried 30.4%, CFS 43.7%). 22 Another study identified 28% patients as frail using FS, 23 and 44.6% using FSQ in an emergency setting. 16 The reason for this variation is unknown. Population heterogeneity and disease severity may account for the high prevalence of frailty in our study. Patients in our hospital may be older and have more serious diseases. Furthermore, clinical judgment may be different for various researchers.
Frailty screening in an urgent care setting is a process of finding elderly patients who might have adverse health outcomes and who might benefit from geriatric emergency medicine interventions. However, no measure of frailty has been validated in the acute care setting to date. Generally, CFS is considered a standardized clinical judgment of frailty that can predict ED return visits and mortality, but it has yet to be verified in the ED in China. A previous study conducted in a large and busy ED in the UK assessed four commonly used frailty tools: identification of seniors at risk (ISAR), CFS, Programme on Research for Integrating Services for the Maintenance of Autonomy seven‐item questionnaire (PRISMA‐7) and Silver Code, and showed that CFS was slightly quicker to use but was not superior to the other tools. 4 Another study reported that CFS was as accurate as the Fried and SUHB in predicting poor outcomes but was more practical for use in busy ED settings. 22 In contrast, a study showed that PRISMA‐7 was more accurate than ISAR but equivalent to the CFS and may be optimal. 23 Li et al. 24 compared three scales (FP, FS and Frailty Index) in a senior community in China and showed that FS may be the best in practice. In our study, we identified frailty assessed by FS, FSQ and CFS as an independent risk factor for 28‐day mortality as well as readmission at 30 and 90 days. This is consistent with the results of several studies. 25 , 26 Its predictive value for adverse health outcomes in a community setting had been verified, but comparison of different frailty models, including FS in the ED, was limited. In our study, FS had a better predictive value for 28‐day all‐cause mortality than FSQ and CFS independent of demographic characteristics and chronic diseases. The FS has its own unique advantages as it overlaps with the biological, burden and functional scales and cannot be affected by the acute phase of the disease. 27 The CFS identifies frailty based on overall impression of the patient and might be subjective. FSQ was the only original frailty assessment tool in China that has been used to identify frailty in older adults in community settings and was shown to be valid in emergency settings in China. However, it needs more research in the future.
In this study, we found that frailty identified by the three tools had a low predictive ability for ED readmission, which is similar to the results of previous studies. CFS alone does not adequately identify older adults at risk for admissions or return ED visits within a specified time frame (such as within 9 days). 28 Another study demonstrated that frailty based on a deficit accumulation index predicted serious adverse outcomes in the first 30 days after ED discharge but was not a major determinant of repeat outpatient ED visits. 29 A possible reason for this is that older patients with more comorbidities are vulnerable to external stressors. Older adults are also prone to multiple organ dysfunction and their conditions may change rapidly. Patients are admitted to the hospital because of slight changes rather than real criteria. This was in accordance with a study by Theou et al. 30
This study had some limitations. First, this was a single‐center observational study in an ED of a university‐affiliated hospital, which might have contributed to selection bias. Second, because patients in the ED, particularly those acutely ill patients, may not be able to complete walking speed, grip strength or appendicular skeletal muscle index tests, we only compared three frailty screening scales by subjective questionnaire but not by the above objective parameters. Third, there are many other assessment tools, so our results might not be applicable to other EDs that utilize different instruments. Besides, the study showed its statistical significance in predicting 28‐day mortality and readmission. In the future, multi‐center research and more screening tools for frailty should be carried out in EDs. Moreover, further studies related to long‐term prognosis should be studied.
In conclusion, this study compared three screening tools in a cohort of older patients in an ED in China. We found a high prevalence of frailty, particularly when using CFS. All three scales were effective in identifying frailty and predicting adverse outcomes among older adults in emergency settings. Furthermore, the three tools showed a predictive ability for 28‐day mortality and readmission, although FS may be the most effective in practice. Additional research is required to confirm suitable frailty screening instruments for busy EDs.
Disclosure statement
The authors declare no conflict of interest.
Acknowledgements
This work was supported by the Open Project of Beijing Key Laboratory (grant number 2020XFN‐KFKT‐02).
Shang N, Liu H, Wang N, Guo S, Ma L. Comparison of three frailty screening instruments for prediction of adverse outcomes among older adults in the emergency department. Geriatr. Gerontol. Int. 2022;22:851–856. 10.1111/ggi.14469
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. World population prospects—population division . [cited Aug 2, 2019]. Available from: https://population.un.org/wpp/. United Nations.
- 2. Roberts DC, McKay MP, Shaffer A. Increasing rates of emergency department visits for elderly patients in the United States, 1993 to 2003. Ann Emerg Med 2008; 51: 769–774. [DOI] [PubMed] [Google Scholar]
- 3. Beard JR, Officer A, de Carvalho IA et al. The world report on ageing and health: a policy framework for healthy ageing. Lancet 2016; 387: 2145–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Elliott A, Phelps K, Regen E, Conroy SP. Identifying frailty in the emergency department‐feasibility study. Age Ageing 2017; 46: 840–845. [DOI] [PubMed] [Google Scholar]
- 5. Salvi F, Morichi V, Grilli A et al. Screening for frailty in elderly emergency department patients by using the identification of seniors at risk (ISAR). J Nutr Health Aging 2012; 16: 313–318. [DOI] [PubMed] [Google Scholar]
- 6. Carpenter CR, Shelton E, Fowler S et al. Risk factors and screening instruments to predict adverse outcomes for undifferentiated older emergency department patients: a systematic review and meta‐analysis. Acad Emerg Med 2015; 22: 1–21. [DOI] [PubMed] [Google Scholar]
- 7. Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet 2019; 394: 1365–1375. [DOI] [PubMed] [Google Scholar]
- 8. Dent E, Martin FC, Bergman H, Woo J, Romero‐Ortuno R, Walston JD. Management of frailty: opportunities, challenges, and future directions. Lancet 2019; 394: 1376–1386. [DOI] [PubMed] [Google Scholar]
- 9. Fletcher RH, Fletcher SW. Clinical epidemiology: a new discipline for an old art. Ann Intern Med 1983; 99: 401–403. [DOI] [PubMed] [Google Scholar]
- 10. Buta BJ, Walston JD, Godino JG et al. Frailty assessment instruments: systematic characterization of the uses and contexts of highly‐cited instruments. Ageing Res Rev 2016; 26: 53–61. 10.1016/j.arr.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fried LP, Tangen CM, Walston J et al. Frailty in older adults: evidence for a phenotype. J Gerontol, Ser A 2001; 56: M146–M156. 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 12. Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. Sci World J 2001; 1: 323–336. 10.1100/tsw.2001.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Credé SH, O'Keeffe C, Mason S et al. What is the evidence for the management of patients along the pathway from the emergency department to acute admission to reduce unplanned attendance and admission? An evidence synthesis. BMC Health Serv Res 2017; 17: 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rockwood K, Song X, MacKnight C et al. A global clinical measure of fitness and frailty in elderly people. Can Med Assoc J 2005; 173: 489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ma L, Tang Z, Chan P, Walston JD. Novel frailty screening questionnaire (FSQ) predicts 8‐year mortality in older adults in China. J Frailty Aging 2019; 8: 33–38. [DOI] [PubMed] [Google Scholar]
- 16. Liu H, Shang N, Chhetri JK et al. A frailty screening questionnaire (FSQ) to rapidly predict negative health outcomes of older adults in emergency care settings. J Nutr Health Aging 2020; 24: 627–633. [DOI] [PubMed] [Google Scholar]
- 17. Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging 2012; 16: 601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kojima G. Frailty defined by FRAIL scale as a predictor of mortality: a systematic review and meta‐analysis. J Am Med Dir Assoc 2018; 19: 480–483. [DOI] [PubMed] [Google Scholar]
- 19. Thompson MQ, Theou O, Tucker GR, Adams RJ, Visvanathan R. FRAIL scale: predictive validity and diagnostic test accuracy. Australas J Ageing 2020; 39: e529–e536. [DOI] [PubMed] [Google Scholar]
- 20. Katz S. Assessing self‐maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc 1983; 31: 721–727. [DOI] [PubMed] [Google Scholar]
- 21. Kaeppeli T, Rueegg M, Dreher‐Hummel T et al. Validation of the clinical frailty scale for prediction of thirty‐day mortality in the emergency department. Ann Emerg Med 2020; 76: 291–300. [DOI] [PubMed] [Google Scholar]
- 22. Lewis ET, Dent E, Alkhouri H et al. Which frailty scale for patients admitted via emergency department? A cohort study. Arch Gerontol Geriatr 2019; 80: 104–114. [DOI] [PubMed] [Google Scholar]
- 23. O'Caoimh R, Costello M, Small C et al. Comparison of frailty screening instruments in the emergency department. Int J Environ Res Public Health 2019; 16: 3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li JJ, Jiang S, Zhu ML, Liu XH, Sun XH, Zhao SQ. Comparison of three frailty scales for prediction of adverse outcomes among older adults: a prospective cohort study. J Nutr Health Aging 2021; 25: 419–424. [DOI] [PubMed] [Google Scholar]
- 25. Elliott A, Taub N, Banerjee J et al. Does the clinical frailty scale at triage predict outcomes from emergency care for older people? Ann Emerg Med 2021; 77: 620–627. [DOI] [PubMed] [Google Scholar]
- 26. Darvall JN, Loth J, Bose T et al. Accuracy of the clinical frailty scale for perioperative frailty screening: a prospective observational study. Can J Anaesth 2020; 67: 694–705. [DOI] [PubMed] [Google Scholar]
- 27. Pulok MH, Theou O, van der Valk AM, Rockwood K. The role of illness acuity on the association between frailty and mortality in emergency department patients referred to internal medicine. Age Ageing 2020; 49: 1071–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Serina P, Lo AX, Kocherginsky M et al. The clinical frailty scale and health services use for older adults in the emergency department. J Am Geriatr Soc 2021; 69: 837–839. [DOI] [PubMed] [Google Scholar]
- 29. Afilalo J, Mottillo S, Xue X et al. Frailty and adverse outcomes in older adults being discharged from the emergency department: a prospective cohort study. Can J Emerg Med 2020; 22: 65–73. [DOI] [PubMed] [Google Scholar]
- 30. Theou O, Brothers TD, Mitnitski A, Rockwood K. Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all‐cause mortality. J Am Geriatr Soc 2013; 61: 1537–1551. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


