Abstract
Skin aging is a complex biological process. Skin aspect is considered as a sign of well‐being and of beauty. In view of this, noninvasive and/or minimally invasive anti‐aging strategies were developed. Adenosine, a well‐known nucleoside, may play a role in skin rejuvenation. Adenosine receptors belong to the G protein‐coupled receptors superfamily and are divided into four subtypes: A1, A2A, A2B, and A3. The adenosine receptors expressed by skin are mainly the A1 and A2A subtypes. In the hypodermis, adenosine through the A1 receptor stimulates lipogenesis and adipogenesis. In the dermis, adenosine through the A2A receptor subtype stimulates collagen production. Moreover, the nucleoside increases new DNA synthesis and subsequently protein synthesis in dermal cells. Activation of adenosine receptors by interacting with various skin layers may induce a decrease in the amount of wrinkles, roughness, dryness, and laxity. This article has reviewed the mechanisms through which adenosine modulates biological mechanisms in the skin tissues and the effect of preparations containing adenosine or its derivatives on the skin.
Keywords: adenosine, adipogenesis, lipogenesis, skin care, wrinkling repair
Abbreviations
- AC
adenylyl cyclase
- ADA
adenosine deaminase
- ADK
adenosine kinase
- Ado
adenosine
- ADP
adenosine diphosphate
- AMP
adenosine 5′‐monophosphate
- ARs
adenosine receptors
- ATP
adenosine triphosphate
- cAMP
cyclic adenosine monophosphate
- Col I
collagen type I
- Col III
collagen type III
- CTGF
connective tissue growth factor
- DMN
dissolving microneedle
- DNA
deoxyribonucleic acid
- ECM
extracellular matrix
- ecto‐ADA
extracellular adenosine deaminase
- EMA
European Medicines Agency
- endo‐ADA
intracellular adenosine deaminase
- Epac1
exchange protein activated by cAMP
- FDA
Food and Drug Administration
- Fli1
friend leukemia integration‐1
- hARs
human adenosine receptors
- HOS‐Ado‐DMN
horse‐adenosine‐dissolving microneedle
- SAH
S‐adenosyl homocysteine
1. INTRODUCTION
Significant social and psychological impacts generated by a longer life expectancy have led to paying more attention to skin care and to counter signs of skin aging. This is done by reducing wrinkles and other signs of cutaneous senescence.
The skin is composed of three layers. The epidermis is the outermost layer and consists mainly of keratinocytes. These cells that synthesize the protein keratin, which is present also in hair and nails constitute a barrier against environmental damage, water loss, and pathogen agents. The epidermis is bonded to a deeper skin layer, the dermis, offering the skin strength, and elasticity due to the presence of collagen and elastin. This layer contains blood vessels, nerve fibers, and receptors. The last skin layer (subcutis) is the deepest subcutaneous tissue, which includes fat stratum and works as an energy reserve, insulation medium, and cushion for knocks and falls. 1
Two simultaneous processes, intrinsic or chronological and extrinsic aging, are involved in the process of skin aging. The first one is genetically determined and is affected by the degenerative effects of free radicals due to the body's inability to counteract their damage. This causes an aging process including qualitative and quantitative changes with a diminished or defective synthesis of collagen and elastin in the dermis. The second one depends on various factors and lifestyles including exposure to sunlight, pollution, nicotine, repetitive muscle movements, diet, sleeping position, and general health. 2 Extrinsic aging is frequently associated with photo‐aging due to chronic exposure to UV light. Photo damage produces skin damage and is sometimes associated with the development of neoplastic lesions. 3 The appearance of aging skin is different if it is caused by intrinsic or extrinsic processes. In intrinsic aging, the skin is smooth, unblemished, and characterized by normal geometric patterns with some expression lines. Epidermal and dermal atrophy is evident in the skin, epidermal rete ridges are smoothed out, and mast cells and fibroblasts are reduced. These modifications reduce the contact of the dermis with the epidermis, capillary perfusion, communication between the layers, and nutrients supply.
Skin aging is a complex biological process. A healthy appearance of the skin is an indicator of well‐being, and several noninvasive and minimally invasive anti‐aging strategies have been developed over the last years. 4 Some of these strategies involve mechanical reinforcement of the dermis with the stimulation of physiological networks. The loss of facial subcutaneous white adipose tissue could play an important role in skin aging. Adenosine (Ado) is an autacoid increasing collagen production and, at the same time, countering the loss of white adipose tissue. In this article, we have reviewed the anti‐aging effect of Ado in cosmetic preparations.
2. ADENOSINE
Ado is a purine nucleoside composed of an adenine molecule associated to a ribose sugar (ribofuranose) moiety via a β‐N9‐glycosidic bond (Figure 1).
FIGURE 1.
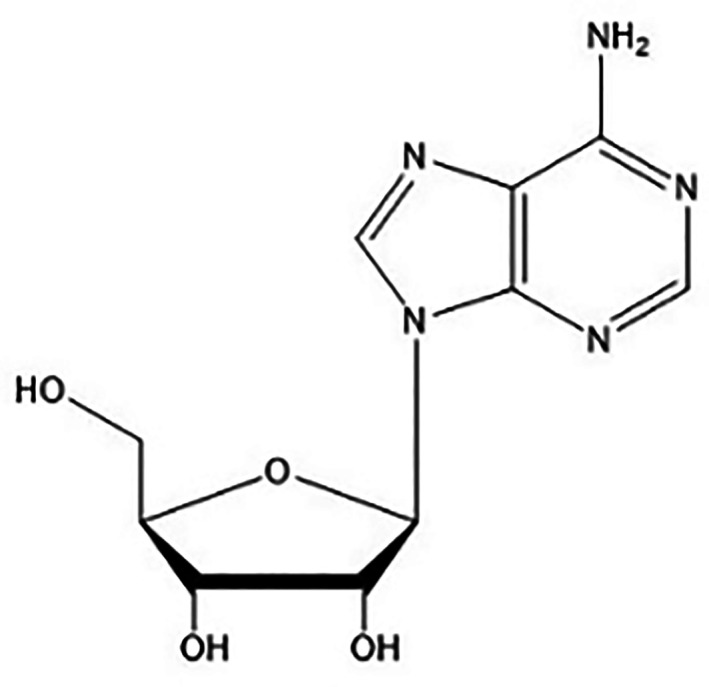
Adenosine structure
Ado, which is ubiquitously distributed, is not considered a classical transmitter, but a metabolite, which can be released from any cell and can act as an autacoid by specific adenosine receptors (ARs). This autacoid is known since 1929 thanks to Drury and Szent‐György, which showed that Ado regulates many bodily functions. 5 Ado can be generated both extracellularly and intracellularly. Extracellular Ado derives from breakdown of adenine nucleotides, while intracellular synthesis arises from two pathways (Figure 2). Under physiological conditions, Ado is formed mainly from the intracellular dephosphorylation of Ado 5′‐monophosphate (AMP) by a cytosolic 5′‐nucleotidase, or from the hydrolysis of S‐adenosyl homocysteine (SAH) by SAH hydrolase. 6 , 7
FIGURE 2.
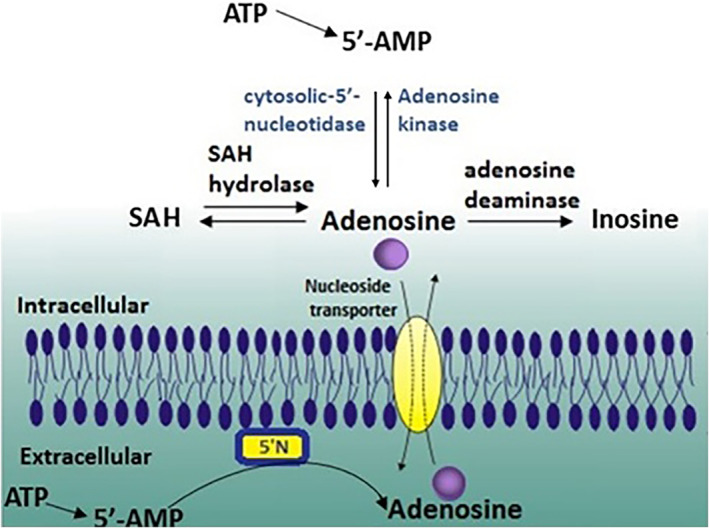
Adenosine production and metabolism. 5′‐Nucleotidase (5′N)
Ado, after intracellular production, is released into the extracellular space upon cell membrane damage or by specific nucleoside transporters. Different cell types can release nucleotides in the extracellular space. 8 , 9 These nucleotides are hydrolyzed to Ado by ecto‐apyrase (CD39) and ecto‐5′‐nucleotidase (CD73). Ado catabolism is mainly regulated by two other enzymes: Ado kinase (ADK) and Ado deaminase (ADA). After intracellular reuptake through the nucleoside transporter, Ado undergoes a rapid phosphorylation to AMP by ADK, or deamination to inosine by cytosolic ADA (Figure 2). 10
ADA, although present mostly intracellularly (endo‐ADA), was also found in some extracellular compartments (ecto‐ADA) and can be expressed on the external membrane surface of certain immune and nonimmune cells. 11 ADA, whose preferred substrate is 2′‐deoxyAdo, deaminates Ado with a Km in the micromolar range. Hence, this enzyme has a particular relevance when Ado levels are high. ADK has a Km of about 100 nM and it can phosphorylate Ado at physiological intracellular concentrations. Increased levels of Ado due to ADA deficiency have been associated with the pathogenesis of a form of severe combined immunodeficiency. 12
Under physiological conditions, Ado is constitutively present in the extracellular space at a nanomolar concentration (approximately from 10 to 200 nM). 13 The Ado concentration is regulated by the equilibrium between Ado production/release into the extracellular space and Ado uptake by cells or catabolism to inosine. It is important to note that under stressful conditions Ado is strongly increased, acting as a potent endogenous modulator of inflammation and tissue repair. 14 , 15 The concentration of Ado increases when there is an imbalance between consumption and energy production, such as lacking oxygen or in conditions of cellular and/or tissue necrosis or stress. 16
Ado is part of the molecule of adenosine triphosphate (ATP) which is composed of the purine nucleoside Ado esterified with three phosphate groups. 17 ATP is a ubiquitous organophosphate that connects anabolism and catabolism, but also fuels processes such as motile contraction, phosphorylation, and active transport. 18 Both Ado and adenosine monophosphate (AMP) are formed when ATP is consumed in metabolic processes. AMP is an ester of phosphoric acid and Ado. Like ATP, AMP plays an important role in many cellular metabolic processes and is a component in the synthesis of RNA.
3. PURINERGIC RECEPTORS
Ado, extracellular ATP, and other nucleotides are signaling molecules acting through their specific receptors, namely purinoceptors. There are three classes of purinoceptors: seven subtypes of ligand‐gated ion channels (P2XRs), eight subtypes of nucleotide‐activated G protein‐coupled receptors (P2YRs), and four subtypes of G protein‐coupled ARs (also named P1 receptors).
P2XRs are composed of seven different subunits (P2X1–7) to form trimeric receptors. P2YRs can be further subdivided into two groups depending on the coupling to specific G proteins: P2Y1, P2Y2, P2Y4, and P2Y6 couple to Gq to activate phospholipase C, whereas P2Y12, P2Y13, and P2Y14 couple to Gi to inhibit adenylyl cyclase (AC). P2Y11 is peculiar, in fact it couples to both Gq and Gs triggering an increase in intracellular Ca2+ and in cyclic adenosine monophosphate (cAMP) levels, respectively. ARs, which are the main topic of this review, will be discussed in depth later. Numerous physiological and pathological processes are regulated by purinergic receptors, and alterations of this receptor system may contribute to insulin resistance, vascular injury, platelet aggregation, inflammation, and cognition. 19 , 20 There is also recent evidence that purinergic receptors participate in immunity. Stimulation of these receptors in infected cells induces inflammatory and antiviral responses, contributing to the host antiviral defense. 21
3.1. Adenosine receptors
ARs are expressed into four subtypes, namely A1AR, A2AAR, A2BAR, and A3AR, on the basis of their respective coupling to second messengers, tissue distribution, and unique pharmacological profiles. 9 , 22 At the end of the 1970s, the first classification of purinergic receptors was proposed, 23 and the ARs were identified and subdivided, according to pharmacological criteria and transducting pathways, into Ri (or A1) and Ra (or A2) receptors, depending on their ability to inhibit or activate AC, respectively. 24 , 25 , 26 A further subclassification of A2ARs into A2A and A2B was introduced to differentiate receptors with high (A2AAR) or low (A2BAR) affinity for Ado. All four ARs have been cloned and pharmacologically characterized from different species such as rat, mouse, and human. All human ARs (hARs) show similar genomic structure, characterized by a single intron that interrupts the coding sequence in a region corresponding to IL2.
3.2. Signaling pathways
ARs are widely expressed and play an important role in many physio‐pathological functions. They were found in the nervous, cardiovascular, respiratory, gastrointestinal, urogenital, and immune systems as well as in bone, eyes, and skin. 9 It is worth noting that unique cells and tissues distribution, secondary signaling transduction and physiological effects characterize each AR. According to their signaling pathway (Figure 3), the first ARs subclassification into A1 and A2 receptors was made taking into account the ability of the receptors to inhibit or activate, respectively, AC.
FIGURE 3.
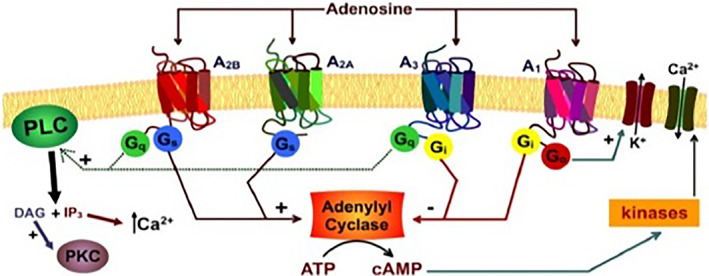
Adenosine signaling pathway
Based on this conventional principle, ARs are still subdivided into inhibitory (A1AR and A3AR) or stimulatory (A2AAR and A2BAR) receptors, although several other signaling pathways, even cAMP‐independent, have been described. A1AR and A3AR signals are mediated through Gi and Go members of the G protein family, through which they reduce AC activity and cAMP levels. On the contrary, A2AARs and A2BARs are coupled to Gs proteins, through which they stimulate AC and increase cAMP levels, thereby leading to the activation of a plethora of mediators, depending on the signaling triggered by cAMP in specific cells (Table 1).
TABLE 1.
Coupling of the AR subtypes to G protein, effectors, and intracellular signaling
| AR subtypes | G protein | Effectors | Intracellular signaling |
|---|---|---|---|
| A1 | Gαi | cAMP 
|
|
| Gαs | cAMP 
|
ERKs | |
| Gαq11 | IP3

|
PI3K | |
| A2A | Gαs | cAMP 
|
PKA; CREB |
| Gαolf | cAMP 
|
PKC | |
| A2B | Gαs | cAMP 
|
ERKs |
| Gαq11 | IP3/DAG 
|
P38; JNK | |
| A3 | Gαi | cAMP 
|
ERKs |
| Gαq11 | IP3

|
PI3K/Akt |
4. SKIN FUNCTION AND LOCALIZATION OF ARs
As mentioned above, the skin includes three layers, and the epidermis, which is the superficial layer, consists mainly of keratinocytes. This layer undergoes continuous turnover, with the outermost cells exfoliating and replaced by underlying cells derived from the dermal layer. The epidermis is bonded to an underlying layer of skin, the dermis, which gives the organ strength and elasticity due to collagen and elastin fibers. The dermis is composed of a variety of cell types, including fibroblasts. The basal layer is the subcutis, which includes a layer of fat laid down acting as an energy reserve and insulating material.
The principal ARs present in the skin are A2A and A1ARs. A2ARs are expressed at the cell membrane level of fibroblasts and A1Rs at the cell membrane level of the adipocytes of the white adipose tissue (Figure 4).
FIGURE 4.
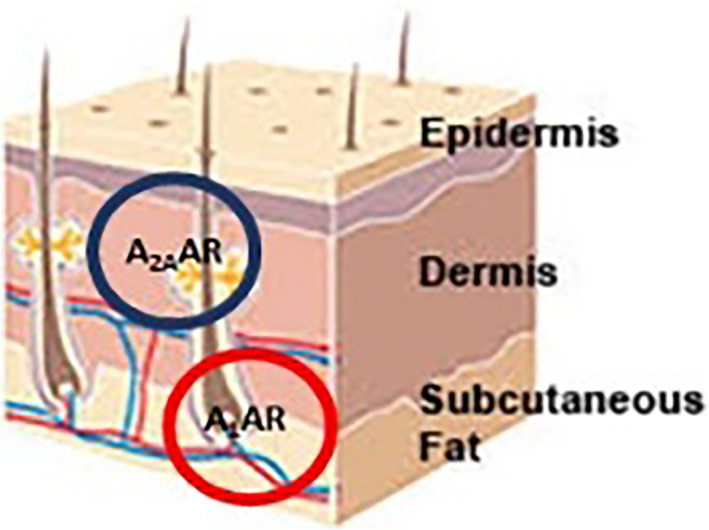
Localization of adenosine receptors (ARs) in skin
Ado, by activating A2AR, can modulate many functions such as coronary vasodilatation, 27 neurotransmission, 28 and counteract the inflammatory response. 29 , 30 , 31 The first manuscript reporting the expression of A2AAR on human dermal fibroblasts was published in 1997. 32 Some years later, the same group indicated that the stimulation of A2AAR by the selective agonist CGS‐21680 leads to a regulation of collagen production by human dermal fibroblasts. This effect was countered in the presence of the selective A2AAR antagonist ZM‐241385. 33
The mechanism of A2AAR facilitating collagen synthesis is still unclear. It is worth noting that the Exchange protein directly activated by cAMP (Epac1), the main cAMP effector, produces a decrease in collagen production. 34 , 35 On the other hand, the activation of A2AAR leads primarily to cAMP production. 36 , 37 This receptor is also involved in wound healing and fibrosis and stimulation of this site accelerates the post‐trauma repairing process of skin wounds through the increase of extracellular matrix (ECM) production and the number of fibroblasts. 32 , 38
Ado, by A2AAR activation, promotes wound healing by increasing collagen production and stimulating connective tissue growth factor (CTGF) production. Further, A2AAR activation promotes wound closure, leading to scar formation and dermal fibrosis by increasing the collagen type III (Col III) deposition more than the collagen type I (Col I). The reparative process concludes, to restore complete functionality, with the remodeling of the newly formed granulation tissue. The provisional ECM rich in Col III, during this phase, is degraded by serine proteases and metalloproteases and it is replaced with the definitive matrix rich in Col I. 39
Several evidence indicates that the ratio of Col I versus Col II changes in relation to the amount of Ado present. In normal skin, where Ado concentration varies from 10 to 200 nM, the ratio is 4 versus Col I, but in hypertrophic and immature scars, where Ado concentration is high, the ratio decreases up to 2 versus Col I. These data demonstrate that A2AAR promotes Col I and Col III synthesis with different sensitivities and, in particular, promotes Col III production. The exact mechanism is not well known, but it seems that A2AAR stimulates downstream signals that mediate activation of kinases ERK1/2, linked to Col I production, and p38 MAPKinase, linked to Col III production. Matrix production depends on the balance between forces that promote the transcription of matrix‐producing genes and those that repress them. 40 The most accredited hypothesis is that the fibrogenic effect of A2AAR is mediated by the suppression of friend leukemia integration‐1 (Fli1) expression and the increase of CTGF secretion. 41 Recently, it has been demonstrated that the stimulation of Col III production after A2AAR stimulation is due to the activation of both canonical and noncanonical Wnt/β‐catenin signaling, which is necessary for Col III but not Col I synthesis in primary human dermal fibroblasts. 42
Collagen is the primary structural component of the dermis and it is responsible for conferring strength and support to human skin. The cutaneous signs of aging are present when the structural proteins and main components of the skin deteriorate. Epidermal and dermal atrophy together with flattening of the rete ridges characterize the aged skin. 43 Collagen, in aged skin, is characterized by thickened fibrils misaligned in comparison to the younger skin. 43 Moreover, aged fibroblasts synthesize lower collagen levels. As a result, the ratio of collagen types found in human skin also changes with age. The overall collagen content per unit area of skin surface decreases approximately 1% per year. 44 This reduction increases in irradiated skin where collagen levels are reduced by about 59% in correlation with the extent of photodamage. 45 Since aging and environmental free radicals can degrade collagen proteins, Ado, with its ability to stimulate the synthesis of new collagen, improves skin's elasticity and hydration reducing wrinkles and signs of skin aging.
Ado is considered to be one major regulator of adipose tissue physiology. In fat tissue, A1ARs are the more expressed Ado receptor subtype and there is pharmacological evidence that Ado, activating this receptor, regulates adipose tissue function such as lipolysis. 46 , 47 , 48 Lipolysis inhibition and modulation of insulin sensitivity are regulated by the activation of A1ARs that are highly expressed in adipose tissue. 49 Dole demonstrated the first evidence of Ado and A1AR involvement in a fat sample of adipose tissue from rat epididymis. This research demonstrated that the activation of A1AR inhibits lipid decomposition into triglycerides and free fatty acids. 50 These results were confirmed by subsequent studies using an in vivo model of obese Zucher rats or mice (1–4) where tecadenoson, a selective A1AR agonist, reduced free fatty acid levels independently of the dose used. 51 The molecular mechanism based on these effects was the inhibition of cAMP formation and the activation of protein kinase A by A1AR. This receptor inhibits both proteins that actively participate in lipolysis, the hormone‐sensitive lipase, and triglyceride lipase present in adipose tissue. 52 Exogenous Ado stimulates also adipogenesis of preosteoblasts and preadipocytes via activation of A1ARs. Ado mechanism involved in adipogenesis is correlated to the regulation of leptin secretion. A1AR activation leads to an increase of leptin levels correlated with the endogenous Ado levels growth. In fact, in isolated adipose tissue, the A1AR activation raises leptin secretion, showing that A1ARs act directly on fat tissue by stimulating leptin secretion. 53
In conclusion, Ado inhibits lipolysis and facilitates free fatty acids accumulation within the adipose tissue by A1AR activation. 54 , 55 Ado is implicated in the adipogenesis promotion inducing the adipocytes growth. When hypertrophied adipocytes exceed 170% of the initial volume, chemical signals promote mesenchymal stem cells to differentiate into new adipocytes. 56
The distribution and structure of adipose tissue change with aging. In the face of young individuals, the fat is evenly distributed, across the forehead, temples, cheeks, and areas around the eyes and mouth. With age, fat loses volume, aggregates, and moves downward, with the consequence that smooth and taut skin loosens and collapses. Moreover, some wrinkles can be accentuated due to the way fat decreases and moves. Furthermore, adipocyte size undergoes age‐dependent modification, impacting collagen content, and adhesion between the skin and adipocyte layers. In turns, these affect the mechanical properties of the skin, resulting in structural instabilities such as wrinkles. 57 The ability of Ado to counteract the lipolysis associated with adipogenesis promotion can improve the bio‐restructuring of the hypodermis skin.
Since Ado is a purine nucleoside, it is recruited from deoxyribonucleic acid (DNA) polymerase to form new DNA. This increases protein synthesis in dermal cells and increases cell size in cultures of human skin fibroblasts without affecting the proliferation of the dermal cells. 58 , 59 This aspect is important in terms of skin age‐related changes because the epidermal turnover rate slows from 30% to 50% between the third and eighth decades of life. The changes related to decrease cell turnover, in older skin, result in the development of heaps of corneocytes rendering the skin surface rough and dull. 60
Moreover, Ado plays an important role in biochemical processes, such as ATP and adenosine diphosphate (ADP) that provide energy to living cells, as well as in signal transduction as cAMP.
Ado inhibits the effects of inflammation by inhibiting neutrophils, the white blood cells that rush to damaged or infected skin tissue. As a result, skin care products with Ado are able to help treat minor scrapes, cuts, burns, and other injuries. With its collagen stimulating properties, Ado also benefits skin elasticity and hydration.
Different biological effects resulting from the selective activation of A1AR or A2AAR allow for a targeted and personalized intervention able to counteract the signs of aging. At the age of 40–45 years when the skin appears thinner and more fragile, wrinkles are accentuated, the upper eyelid becomes heavier, it is time to plump the skin using formulations containing selective A2AAR agonists, able to stimulate the production of collagen and therefore increase the skin tone. At the age of 50–55 years, when the signs of aging are accentuated, it is important to combat facial laxity through dermis volumetric remodeling using creams containing A1AR selective agonists able to inhibit lipolysis and promote adipogenesis, obtaining a “filled out,” and compact skin.
5. ADO FORMULATIONS
Ado is a natural component in the human body, and is involved in biological processes including neurotransmission, muscle contraction, cardiac function, platelet function, vasodilation, signal transduction, and secretion mechanisms in various cell types. 61 It is present in living organisms and its biology is well characterized, consequently it is very safe. U.S. Food and Drug Administration (FDA) as well as the European Medicines Agency (EMA) approved skin formulations with Ado concentrations up to 0.1%. Some studies reported the efficacy and safety of Ado formulations for skin treatment.
Two different cream formulations containing Ado were evaluated as anti‐wrinkle agents using the FOITS technique. These creams were applied directly and mechanically rubbed into the skin to reduce periorbital lines and glabellar frowns in a blind, randomized, placebo‐controlled study. 59 The data from this study suggest that, in the long‐term use, cosmetic products containing Ado can significantly reduce existing wrinkles in the two facial areas studied, partially reversing the signs of chronological aging. 62 Ado may be incorporated into a transdermal patch or soluble microneedle patch that is applied to the skin. In all cases, the adenosine‐loaded dissolving microneedle patches showed a similar or better efficacy than the Ado cream, although in one case the weekly Ado dose was 140 times lower. 63 , 64 , 65
A subsequent study combined a topical Ado formulation with a dissolving microneedle (DMN) patch to perform two‐phase delivery via a lipophilic formulation (horse oil) loaded with a hydrophilic compound in order to improve the efficacy of drug delivery and to maintain proper skin barrier function at the same time. Results obtained showed that Ado was delivered via the horse DMNs (HOS‐Ado‐DMN) after skin penetration and horse oil was delivered into the skin through the microchannels created by the Ado‐DMNs. This formulation was compared with Ado‐DMN patches and results revealed that there is significantly improved skin elasticity, hydration, dermal density, and wrinkles with HOS‐Ado‐DMN without adverse effects. 66
6. CONCLUSIONS AND PERSPECTIVES
Skin aging is a dynamic, multifactorial process, and depends on a variety of factors such as lifestyle, diet, heredity, and other personal habits. The use of Ado or its derivatives in some topical preparations could bring significant benefit to aged skin. At the dermis level, Ado stimulates collagen production by activating the A2AARs, whereas at the hypodermis level, it stimulates lipogenesis and adipogenesis by activating the A1ARs.
Moreover, the injection of Ado can induce:
A heightened availability of molecules (nucleotides) is recruited from DNA polymerase to form new DNA increasing protein synthesis in dermal cells and increases cell size in cultures of human skin fibroblasts. Moreover, nucleotides are catabolized so they provide nitrogen bases, which are recovered through the salvage pathways to form new nucleotides useful for DNA duplication for the mitotic activity of cells;
A purinergic stimulation of the A2AAR (collagen biosynthesis) and A1AR (lipogenesis, adipogenesis). Collagen biosynthesis, liposynthesis, stimulation, and adipogenesis promotion are useful to stimulate metabolisms versus a biorestructuring of multilevel tissues. In the dermis, collagen biosynthesis is increased, while at the same time, the structure of the hypodermis affected by lipoatrophic or lipogenic situations is improved. Hence, the use of Ado or its derivatives in anti‐wrinkle preparations not only exert their effects on the skin's superficial layers, but also involve the hypodermis. These effects are accompanied by a noticeable decrease in the amount of wrinkles, roughness, dryness, laxity, sallowness, or pigmentary mottling in skin.
AUTHOR CONTRIBUTIONS
Gabriella Marucci, Michela Buccioni, Vincenzo Varlaro, and Rosaria Volpini contributed to the literature review and wrote the article. Francesco Amenta provided critical revision and final approval of finalized manuscript. All authors have read and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
ACKNOWLEDGMENTS
We thank the financial assistance of the European Union POR MARCHE FESR 2014/2020. Asse 1, OS 2, Azione 2.1—Intervento 2.1.1—Sostegno allo sviluppo di una piattaforma di ricerca collaborativa negli ambiti della specializzazione intelligente. Thematic Area: “Medicina personalizzata, farmaci e nuovi approcci terapeutici.” Project acronym: Marche BioBank www.marchebiobank.it. Open Access Funding provided by Universita degli Studi di Camerino within the CRUI‐CARE Agreement.
Marucci G, Buccioni M, Varlaro V, Volpini R, Amenta F. The possible role of the nucleoside adenosine in countering skin aging: A review. BioFactors. 2022;48(5):1027–1035. 10.1002/biof.1881
Funding information European Union
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Cooper S. The biology of the skin. J R Soc Med. 2002;95(2):109. [Google Scholar]
- 2. Farage MA, Miller KW, Elsner P, Maibach HI. Intrinsic and extrinsic factors in skin ageing: a review. Int J Cosmet Sci. 2008;30:87–95. [DOI] [PubMed] [Google Scholar]
- 3. Lavker RM. Structural alterations in exposed and unexposed aged skin. J Invest Dermatol. 1979;73:59–66. [DOI] [PubMed] [Google Scholar]
- 4. Ganceviciene R, Liakou AI, Theodoridis A, Makrantonaki E, Zouboulis CC. Skin anti‐aging strategies. Dermatoendocrinol. 2012;4:308–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Drury AN, Szent‐György A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J Physiol. 1929;68:213–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Antonioli L, Fornai M, Colucci R, Ghisu N, Tuccori M, del Tacca M, et al. Regulation of enteric functions by adenosine: pathophysiological and pharmacological implications. Pharmacol Ther. 2008;120:233–53. [DOI] [PubMed] [Google Scholar]
- 7. Layland J, Carrick D, Lee M, Oldroyd K, Berry C. Adenosine: physiology, pharmacology, and clinical applications. JACC Cardiovasc Interv. 2014;7:581–91. [DOI] [PubMed] [Google Scholar]
- 8. Antonioli L, Blandizzi C, Pacher P, Haskó G. Immunity, inflammation and cancer: a leading role for adenosine. Nat Rev Cancer. 2013;13:842–57. [DOI] [PubMed] [Google Scholar]
- 9. Borea PA, Gessi S, Merighi S, Vincenzi F, Varani K. Pharmacology of adenosine receptors: the state of the art. Physiol Rev. 2018;98:1591–16625. [DOI] [PubMed] [Google Scholar]
- 10. Giuliani AL, Sarti AC, Di Virgilio F. Extracellular nucleotides and nucleosides as signalling molecules. Immunol Lett. 2019;205:16–24. [DOI] [PubMed] [Google Scholar]
- 11. Cristalli G, Costanzi S, Lambertucci C, Lupidi G, Vittori S, Volpini R, et al. Adenosine deaminase: functional implications and different classes of inhibitors. Med Res Rev. 2001;21:105–28. [DOI] [PubMed] [Google Scholar]
- 12. Kohn DB, Gaspar HB. How we manage adenosine deaminase‐deficient severe combined immune deficiency (ADA SCID). J Clin Immunol. 2017;7:351–6. [DOI] [PubMed] [Google Scholar]
- 13. Fredholm BB. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. 2007;14:1315–23. [DOI] [PubMed] [Google Scholar]
- 14. Hasko G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 2004;25:33–9. [DOI] [PubMed] [Google Scholar]
- 15. Ohta A, Sitkovsky M. Role of G‐protein‐coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–20. [DOI] [PubMed] [Google Scholar]
- 16. Schulte G, Fredholm BB. Signalling from adenosine receptors to mitogen‐activated protein kinases. Cell Signal. 2003;15:813–27. [DOI] [PubMed] [Google Scholar]
- 17. NCI thesaurus. Available from: https://ncit.nci.nih.gov/ncitbrowser/ConceptReport.jsp?dictionary=NCI_Thesaurus&ns=NCI_Thesaurus&code=C209.
- 18. Bonora M, Patergnani S, Rimessi A. ATP synthesis and storage. Purinergic Signal. 2012;8:343–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Burnstock G. Purinergic signalling: pathophysiology and therapeutic potential. Keio J Med. 2013;62:63–73. [DOI] [PubMed] [Google Scholar]
- 20. Gutierres JM, Carvalho FB, Schetinger MRC, Marisco P, Agostinho P, Rodrigues M, et al. Anthocyanins restore behavioral and biochemical changes caused by streptozotocin‐induced sporadic dementia of Alzheimer's type. Life Sci. 2014;96:7–17. [DOI] [PubMed] [Google Scholar]
- 21. Ferrari D, Idzko M, Müller T, Manservigi R, Marconi P. Purinergic signaling: a new pharmacological target against viruses? Trend Pharmacol Sci. 2018;39:926–36. [DOI] [PubMed] [Google Scholar]
- 22. Peleli M, Fredholm BB, Sobrevia L, Carlström M. Pharmacological targeting of adenosine receptor signaling. Mol Aspects Med. 2017;55:4–8. [DOI] [PubMed] [Google Scholar]
- 23. Burnstock G. A basis for distinguishing two types of purinergic receptor. In: Bolis L, Straub RW, editors. Cell membrane receptors for drugs and hormones. New York: Raven Press; 1978. p. 107–18. [Google Scholar]
- 24. van Calker D, Muller M, Hamprecht B. Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. J Neurochem. 1979;33:999–1005. [DOI] [PubMed] [Google Scholar]
- 25. Londos C, Cooper DM, Wolff J. Subclasses of external adenosine receptors. Proc Natl Acad Sci U S A. 1980;77:2551–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fredholm BB. Adenosine receptors. Med Biol. 1982;60:289–93. [PubMed] [Google Scholar]
- 27. Ruf J, Paganelli F, Bonello L, Kipson N, Mottola G, Fromonot J, et al. Spare adenosine A2a receptors are associated with positive exercise stress test in coronary artery disease. Mol Med. 2016;22:530–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gomes CV, Kaster MP, Tomé AR, Agostinho PM, Cunha RA. Adenosine receptors and brain diseases: neuroprotection and neurodegeneration. Biochim Biophys Acta. 2011;1808:1380–99. [DOI] [PubMed] [Google Scholar]
- 29. Antonioli L, El‐Tayeb A, Pellegrini C, Fornai M, Awwad O, Giustarini G, et al. Anti‐inflammatory effect of a novel locally acting A2A receptor agonist in a rat model of oxazolone‐induced colitis. Purinergic Signal. 2018;14(1):27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rajasundaram S. Adenosine A2A receptor signaling in the immunopathogenesis of experimental autoimmune encephalomyelitis. Front Immunol. 2018;6:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu YW, Yang T, Zhao L, Ni Z, Yang N, He F, et al. Activation of adenosine 2A receptor inhibits neutrophil apoptosis in an autophagy‐dependent manner in mice with systemic inflammatory response syndrome. Sci Rep. 2016;6:e33614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Montesinos MC, Gadangi P, Longaker M, Sung J, Levine J, Nilsen D, et al. Wound healing is accelerated by agonists of adenosine A2 (G alpha S‐linked) receptors. J Exp Med. 1997;186:1615–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chan ESL, Fernandez P, Merchant AA, Montesinos MC, Trzaska S, Desai A, et al. Adenosine A2A receptors in diffuse dermal fibrosis: pathogenic role in human dermal fibroblasts and in a murine model of scleroderma. Arthritis Rheum. 2006;54:2632–42. [DOI] [PubMed] [Google Scholar]
- 34. Yokoyama U, Patel HH, Lai NC, Aroonsakool N, Roth DM, Insel PA. The cyclic AMP effector Epac integrates pro‐ and anti‐fibrotic signals. Proc Natl Acad Sci U S A. 2008;105:6386–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Insel PA, Murray F, Yokoyama U, Romano S, Yun H, Brown L, et al. cAMP and Epac in the regulation of tissue fibrosis. Br J Pharmacol. 2012;166:447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fredholm BB, IJerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–52. [PMC free article] [PubMed] [Google Scholar]
- 37. Fredholm BB, Arslan G, Halldner L, Kull B, Schulte G, Wasserman W. Structure and function of adenosine receptors and their genes. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:364–74. [DOI] [PubMed] [Google Scholar]
- 38. Victor‐Vega C, Desai A, Montesinos MC, Cronstein BN. Adenosine A2A receptor agonists promote more rapid wound healing than recombinant human platelet‐derived growth factor (Becaplermin Gel). Inflammation. 2002;26:19–24. [DOI] [PubMed] [Google Scholar]
- 39. Dobaczewski M, Quesada CG, Frangogiannis NG. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol. 2010;48:504–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Perez‐Aso M, Mediero A, Cronstein B. Adenosine A2A receptor (A2AR) is a fine‐tune regulator of the collagen1:collagen3 balance. Purinergic Signal. 2013;9:573–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chan ES, Liu H, Fernandez P, Luna A, Perez‐Aso M, Bujor AM. Adenosine A(2A) receptors promote collagen production by a Fli1‐ and CTGF‐mediated mechanism. Arthritis Res Ther. 2013;15(3):R58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shaikh G, Zhang J, Perez‐Aso M, Mediero A, Cronstein B. Adenosine A2A receptor promotes collagen type III synthesis via β‐catenin activation in human dermal fibroblasts. Br J Pharmacol. 2016;173:3279–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fenske NA, Lober CW. Structural and functional changes of normal aging skin. J Am Acad Dermatol. 1986;15:571–85. [DOI] [PubMed] [Google Scholar]
- 44. Shuster S, Black MM, McVitie E. The influence of age and sex on skin thickness, skin collagen and density. Br J Dermatol. 1975;93:639–43. [DOI] [PubMed] [Google Scholar]
- 45. Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S, Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997;337:1419–28. [DOI] [PubMed] [Google Scholar]
- 46. Johansson MS, Lindgren E, Yang JN, Herling AW, Fredholm BB. Adenosine A1 receptors regulate lipolysis and lipogenesis in mouse adipose tissue—interactions with insulin. Eur J Pharm. 2008;597:92–101. [DOI] [PubMed] [Google Scholar]
- 47. De Oliveira CC, Paiva Caria CR, Ferreira Gotardo EM, Ribeiro ML, Gambero A. Role of A1 and A2A adenosine receptor agonists in adipose tissue inflammation induced by obesity in mice. Eur J Pharmacol. 2017;799:154–9. [DOI] [PubMed] [Google Scholar]
- 48. Tozzi M, Novak I. Purinergic receptors in adipose tissue as potential targets in metabolic disorders. Front Pharmacol. 2017;8:878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Faulhaber‐Walter R. Adipokines and central control in adenosine A1 receptor dependent glucose metabolism. Adipocyte. 2012;1:108–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dole VP. Effect of nucleic acid metabolites on lipolysis in adipose tissue. J Biol Chem. 1961;236:3125–30. [PubMed] [Google Scholar]
- 51. Fraser H, Gao Z, Ozeck MJ, Belardinelli L. N‐[3‐(R)‐tetrahydrofuranyl]‐6‐aminopurine riboside, an A1 adenosine receptor agonist, antagonizes catecholamine‐induced lipolysis without cardiovascular effects in awake rats. J Pharmacol Exp Ther. 2003;305:225–31. [DOI] [PubMed] [Google Scholar]
- 52. Dhalla AK, Chisholm JW, Reaven GM, Belardinelli L. A1 adenosine receptor: role in diabetes and obesity. Handb Exp Pharmacol. 2009;193:271–95. [DOI] [PubMed] [Google Scholar]
- 53. Rice AM, Fain JN, Rivkees SA. A1 adenosine receptor activation increases adipocyte leptin secretion. Endocrinolog. 2000;141:1442–5. [DOI] [PubMed] [Google Scholar]
- 54. Budohoski L, Challiss RA, Cooney GJ, McManus B, Newsholme EA. Reversal of dietary‐induced insulin resistance in muscle of the rat by adenosine deaminase and an adenosine‐receptor antagonist. Biochem J. 1984;224:327–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mitani T, Watanabe S, Yoshioka Y, Katayama S, Nakamura S, Ashida H. Theobromine suppresses adipogenesis through enhancement of CCAAT‐enhancer‐binding protein β degradation by adenosine receptor A1. Biochim Biophys Acta Mol Cell Res. 2017;1864(12):2438–48. [DOI] [PubMed] [Google Scholar]
- 56. Eisenstein A, Ravid KJ. G protein‐coupled receptors and adipogenesis: a focus on adenosine receptors. Cell Physiol. 2014;229:414–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wollina U, Wetzker R, Kruglikov IL. Role of adipose tissue in facial aging. Clin Interv Aging. 2017;12:2069–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dobson JD Jr, inventor; University of Massachusetts, assignee. Treatment of skin with adenosine or adenosine analog. United States patent US 6423327B1. 2002 Jul 23.
- 59. Dobson JG Jr, inventor; University of Massachusetts, assignee. Treatment of skin with adenosine or adenosine analog. United States patent US 6645513B2. 2003 Nov 11.
- 60. Baumann L. Skin ageing and its treatment. J Pathol. 2007;211:241–51. [DOI] [PubMed] [Google Scholar]
- 61. Skrabanja A, Bouman E, Dagnelie P. Potential value of adenosine 5′‐triphosphate (ATP) and adenosine in anaesthesia and intensive care medicine. Br J Anaesth. 2005;94:556–62. [DOI] [PubMed] [Google Scholar]
- 62. Abella ML. Evaluation of anti‐wrinkle efficacy of adenosinecontaining products using the FOITS technique. Int J Cosmet Sci. 2006;28:447–51. [DOI] [PubMed] [Google Scholar]
- 63. Kang G, Kim S, Yang H, Jang M, Chiang L, Baek JH, et al. Combinatorial application of dissolving microneedle patch and cream for improvement of skin wrinkles, dermal density, elasticity, and hydration. J Cosmet Dermatol. 2018;18:1083–91. [DOI] [PubMed] [Google Scholar]
- 64. Kang G, Tu TNT, Kim S, Yang H, Jang M, Jo D, et al. Adenosine‐loaded dissolving microneedle patches to improve skin wrinkles, dermal density, elasticity and hydration. Int J Cosmet Sci. 2018;40:199–206. [DOI] [PubMed] [Google Scholar]
- 65. Hong JY, Ko EJ, Choi SY, Li K, Kim AR, Park JO, et al. Efficacy and safety of a novel, soluble microneedle patch for the improvement of facial wrinkle. J Cosmet Dermatol. 2018;17(2):235–41. [DOI] [PubMed] [Google Scholar]
- 66. Yang H, Kim S, Jang M, Kim H, Lee S, Kim Y, et al. Two‐phase delivery using a horse oil and adenosine‐loaded dissolving microneedle patch for skin barrier restoration, moisturization, and wrinkle improvement. J Cosmet Dermatol. 2019;18:936–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


