Abstract
The kinetics of cytotoxic T lymphocyte antigen 4 (CTLA-4) expression on T cells responding to Cryptococcus neoformans and its role in regulating the T-cell response were examined. Using peripheral blood mononuclear cells stimulated with encapsulated or acapsular C. neoformans we showed that (i) the encapsulated strain augmented CTLA-4 expression on the T-cell surface while the acapsular strain was a weaker modulator, (ii) CTLA-4 molecules were rapidly up-regulated after the addition of encapsulated C. neoformans, (iii) CTLA-4 was up-regulated predominantly in CD4+ T cells responding to C. neoformans, and (iv) blockage of CTLA-4 with (Fab′)2 of monoclonal antibody to CTLA-4 induced T-cell proliferation that paralleled the enhancement of interleukin-2 and gamma interferon production. These results suggest that capsular material, the major virulence factor of C. neoformans, promotes synthesis and expression of CTLA-4 molecules predominantly in CD4+ T cells. CTLA-4-mediated deactivation is due not to lack of costimulation but to specific recognition of CTLA-4 for B7 molecules. This appears to be a new mechanism by which C. neoformans may elude the host immune response.
The B7 family of proteins expressed on antigen-presenting cells (APC) provides a major costimulatory signal through interaction with CD28 or cytotoxic T lymphocyte antigen 4 (CTLA-4), a CD28 homologous receptor on T cells (2). Two signals delivered by APC are able to induce specific T-cell activation. The first signal is specific and is provided through the T-cell receptor upon engagement of a major histocompatibility complex loaded with antigenic peptide. The second signal is not specific and is provided by cross-linking of CD28 or other molecules expressed on the T cells with costimulatory molecules expressed on APC (1, 2). B7-1 and B7-2, important molecules that provide costimulatory signals on APC, bind to CD28 or CTLA-4 receptors on T cells. Unstimulated APC do not express B7 molecules or low levels of B7-2. After activation, B7-2 molecules increase rapidly. B7-1 is not found on unstimulated APC and its expression is induced on activated cells but at a lower amount and at a slower kinetic than B7-2 (11). CD28 and CTLA-4 receptors show different kinetics of expression. CD28 is constitutively expressed on T cells while CTLA-4 is promptly up-regulated after T-cell activation and has a higher binding affinity for B7 molecules than CD28 (26). Moreover, the CD28 costimulatory pathway plays an important role in T-cell activation (26). In addition, studies in vivo and in vitro indicate that CTLA-4 molecules produce a critical negative regulation of T cells (8, 25, 26). Consequently, the ability of CTLA-4 to antagonize the positive effect of CD28 on T cells may be considered a possible down-regulatory mechanism (26).
Cryptococcus neoformans is an opportunistic fungus that causes serious infections in the immunocompromised host. Its principal virulence factor, capsular polysaccharide, has both antiphagocytic and immunosuppressive properties (3, 12, 22). The infectious particles reach the lung via inhalation, and infection is controlled at the local level in the immunocompetent host. It has been widely recognized that a complex interplay between humoral and cellular immunity is critical for the control of infection (31). However, the mechanisms involved in the protective response against C. neoformans are not completely understood. The generation of a T helper type 1 (Th1) response has been shown to be crucial for successful removal of the fungus (9). Furthermore, a correlation between T-cell differentiation to the Th1 phenotype and an increase of B7-1 expression on APC has been demonstrated (14, 24). It has been reported previously that C. neoformans up-regulates B7-1 expression on monocytes and that the acapsular strain is a better inducer than the encapsulated strain (32). Recent evidence correlates overexpression of B7-1 with Th1 generation (24), supporting the observation that blocking of B7-2 may promote Th1 response to C. neoformans (23).
Given the involvement of B7 molecules in T-cell activation against the fungus and the importance of the B7 CD28/CTLA-4 pathway in initiating and maintaining T-cell activation and differentiation, the possibility that CTLA-4 could influence the T-cell response to C. neoformans and that capsular material may regulate this signal was examined.
MATERIALS AND METHODS
Isolation of PBMC, peripheral blood monocytes, and T lymphocytes.
Heparinized venous blood was obtained from healthy volunteers and diluted with RPMI 1640 (Gibco BRL, Paisley, Scotland). Peripheral blood mononuclear cells (PBMC) were separated by density gradient centrifugation over Ficoll-Paque plus (Pharmacia Biotech, Uppsala, Sweden) (33). PBMC were recovered, washed twice in RPMI 1640, and resuspended in RPMI 1640 supplemented with 10% human serum type AB (Sigma, St. Louis, Mo.), penicillin (100 U/ml), and streptomycin (100 μg/ml) (cRPMI) at the desired concentration. To isolate monocytes, PBMC were washed twice in RPMI 1640 supplemented with 5% fetal calf serum, penicillin (100 U/ml), and streptomycin (100 μg/ml), plated into cell culture petri dishes (Nunc Inter Med, Roskilde, Denmark), and incubated for 1 h at a concentration of 2 × 106 to 3 × 106/ml. Nonadherent cells were removed by washing the dishes three to five times with prewarmed RPMI 1640 medium. Adherent cells (peripheral blood monocytes) were recovered using a cell scraper (Falcon, Oxnard, Calif.), washed twice, and suspended in cRPMI. Nonadherent cells were E rosetted as previously described (33). The cells recovered were T lymphocytes (E+) and were >98% CD3+ as evaluated by flow cytometric analysis.
Microorganisms.
A thinly encapsulated strain of C. neoformans var. neoformans serotype A from the Central Bureau Schimmel (CBS) Cultures (Delft, The Netherlands; CBS no. 6995 = NIH 37) and an acapsular strain of C. neoformans var. neoformans (CBS no. 7698 = NIH B-4131) were used. In selected experiments isogenic strains were employed. C. neoformans var. neoformans serotype D ATCC 3501 and its parental acapsular mutant CAP67 were obtained from the American Type Culture Collection (Rockville, Md.). CAP67 is an acapsular mutant that can be restored to virulence and the encapsulated state by complementation with a single gene (6). The cultures were maintained by serial passage on Sabouraud agar (BioMérieux, Lyon, France). Log-phase yeasts were harvested by suspending a single colony in RPMI 1640, washed twice with saline, counted on a hemocytometer, and adjusted to the desired concentration in cRPMI (35). All yeasts were inactivated at 60°C for 30 min. Glucuronoxylomannan (GXM) was provided by T. R. Kozel (Department of Microbiology, University of Nevada, Reno). GXM was isolated from culture supernatants by differential precipitation with ethanol and cetyltrimethylammonium bromide (7).
Lymphoproliferation assay.
PBMC (2 × 105) in 96-well plates were incubated with or without acapsular (CBS 7698) or encapsulated (CBS 6995) C. neoformans (4 × 105) in the presence or absence of mouse immunoglobulin G (IgG) anti-human CD152 (CTLA-4) (Fab′)2 (Ancell Corp., Bayport, Maine) (1 or 2.5 μg/ml) at 37°C and 5% CO2 in cRPMI. Control mouse IgG1 (Fab′)2 was obtained from mouse IgG1 digested with pepsin (Sigma). After 5 days, cultures were pulsed overnight with 0.5 μCi [methyl-3H]thymidine (Amersham Life Science, Buckinghamshire, United Kingdom); thereafter, cells were collected onto filter paper using a cell harvester (PBI International, Milan, Italy). The dried filters were counted directly in a beta counter (Packard Instruments, Downers Grove, Ill.). Proliferation values were expressed as the mean ± the standard error (SE) of indicated replicates (27).
Flow cytometry analysis of surface CTLA-4, CD4, and CD8 T cells.
Surface molecule expression was quantified by flow cytometry after various times of culture. Suspensions of PBMC or T lymphocytes (106) in cRPMI were stimulated with different strains of C. neoformans at effector-to-target cell (E:T) ratios of 1:2 and were incubated for different times. In selected experiments the acapsular strains were incubated with GXM (250 μg/ml). GXM binds the acapsular strains, conferring an experimental constructed capsule (13). As a positive control cells were treated with phytohemagglutinin (PHA) (5 μg/ml). After incubation at 37°C in the presence of 5% CO2, cells were collected by centrifugation, fixed in 2% paraformaldehyde in phosphate-buffered saline (PBS), washed twice in PBS containing 0.5% bovine serum albumin and 0.1% sodium azide, and mixed with mouse IgG1 anti-human CD152 (CTLA-4) (Ancell Corp.). After 30 min of incubation on ice, cells were washed twice, mixed with fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgG, and incubated for 30 min (33). The number of CD4+ and CD8+ T cells expressing CTLA-4 was determined by two-color flow cytometric analysis. After incubation, cells were stained with anti-human CD152 (CTLA-4) plus FITC-conjugated anti-mouse IgG and with phycoerythrin (PE)-conjugated anti-human CD4 or PE-conjugated anti-human CD8. Irrelevant FITC- or PE-conjugated isotype-matched antibody was used as a negative control in each experiment.
Determination of the total pool of CTLA-4.
Suspensions of PBMC (106) in cRPMI were stimulated with different strains of C. neoformans at E:T ratios of 1:2 and incubated for different times. After incubation at 37°C in the presence of 5% CO2, cells were collected by centrifugation, washed in PBS plus 0.5 mM EDTA, and fixed at room temperature for 10 min with 2% paraformaldehyde. Cells were permeabilized for 10 min with HEPES-buffered PBS containing 0.1% saponin (Sigma) and stained with mouse anti-human CD152 (CTLA-4), followed by a FITC-conjugated anti-mouse IgG in HEPES-buffered PBS containing 0.1% saponin and 5% fetal calf serum (4). After labeling, cells were analyzed using a flow cytometer (FACScan; Becton Dickinson, San Jose, Calif.). For each analysis, dot plot graphs of forward scatter versus side scatter were drawn and lymphocyte region R1 was defined. Data were collected through the acquisition gate R1. An irrelevant FITC-conjugated isotype-matched antibody was used as a negative control in each experiment.
Determination of IL-2 and IFN-γ production.
PBMC (2.5 × 105) in 96-well plates were incubated with or without acapsular (CBS 7698) or encapsulated (CBS 6995) C. neoformans (5 × 105) in the presence or absence of mouse IgG1 (Fab′)2 anti-human CD152 (CTLA-4) at different concentrations (1 or 2.5 μg/ml) at 37°C and 5% CO2 in cRPMI. In selected experiments, monocytes (2 × 104) in 96-well plates were incubated with or without acapsular (CBS 7698) or encapsulated (CBS 6995) C. neoformans (2 × 105) for 2 h at 37°C and 5% CO2 in cRPMI and were used throughout as APC. Monocyte monolayers were washed to remove nonbound microorganisms. Subsequently, autologous T(E+) cells (105) in cRPMI were added to the culture in the presence or absence of mouse IgG1 (Fab′)2 anti-human CD152 at different concentrations (1 or 2.5 μg/ml) and were incubated at 37°C and 5% CO2 in cRPMI. After 5 days, supernatants were recovered and stored at −80°C. Cytokine levels in culture supernatant fluids were measured with an enzyme-linked immunosorbent assay (ELISA) kit for human interleukin-2 (IL-2) (Biosource International, Camarillo, Calif.) and human gamma interferon (IFN-γ) (EuroClone Ltd., Devon, United Kingdom). The IL-2 ELISA kit detected a dose of <5 pg/ml, and the IFN-γ ELISA kit detected a dose of <3 pg/ml.
Statistical analysis.
Statistical significance was determined by analysis of variance with Bonferroni's correction.
RESULTS
It was demonstrated previously that acapsular C. neoformans is a better inducer of B7 molecules than the encapsulated yeast (32). Given that B7 costimulation promotes the immune response by binding to CD28 or limits the response by binding to CTLA-4 (2), we considered the possibility that in our experimental system capsular material of C. neoformans could deliver inhibitory signals by regulating CTLA-4. To this end, PBMC plus acapsular (CBS 7698) or encapsulated (CBS 6995) C. neoformans at an E:T ratio of 1:2 were cultured for various times (24, 48, and 96 h) and T cells were analyzed for surface and total CTLA-4 expression. PHA was used as a positive control.
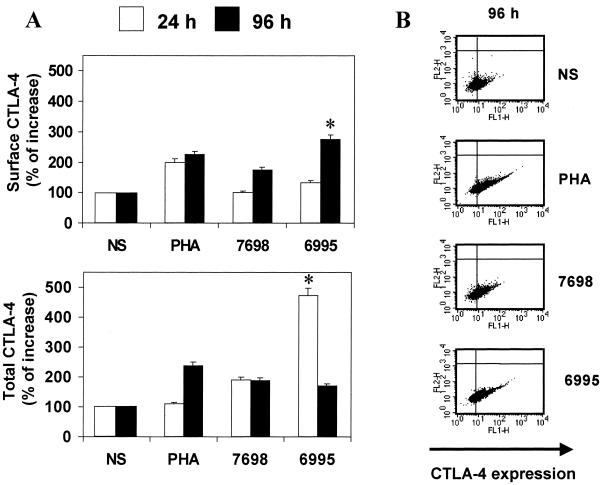
No modulation of CTLA-4 on the surface of T cells responding to acapsular or encapsulated C. neoformans was observed after 24 h of incubation. A slight increase in CTLA-4 expression was detected after 48 h of incubation when the encapsulated strain was used (data not shown). Both strains of C. neoformans up-regulated CTLA-4 expression on the T-cell surface after 96 h of incubation (P < 0.05) with respect to untreated cells (not stimulated [NS]). Remarkably, the encapsulated strain was a better stimulator than the acapsular strain (Fig. 1A, upper panel).
FIG. 1.
(A) Effect of acapsular (CBS 7698) or encapsulated (CBS 6995) C. neoformans on surface or total CTLA-4 expression on T cells. PBMC untreated (NS) or treated with C. neoformans at an E:T ratio of 1:2 were incubated for 24 and 96 h. PHA (5 μg/ml) was used as a positive control. The results are given as percentage of increase of surface or total CTLA-4 expression with respect to unstimulated cells (100%). The results are the mean ± SE of six experiments from six different donors. ∗, P < 0.05 (CBS 6995-treated cells versus CBS 7698-treated cells). (B) Effect of acapsular (CBS 7698) or encapsulated (CBS 6995) C. neoformans on total T-cell CTLA-4 expression. PBMC untreated (NS) or treated with C. neoformans at an E:T ratio of 1:2 were incubated for 96 h. PHA (5 μg/ml) was used as a positive control. For each analysis, dot plot graphs of forward scatter versus side scatter were drawn and lymphocyte region R1 was defined. Data were collected through the acquisition gate R1. FL1 represents CTLA-4 expression, and FL2 represents background red fluorescence of cells. The results are from a representative experiment of six performed with similar results.
Up-regulation of CTLA-4 expression could be ascribed to increased production of CTLA-4 or to inhibition of CTLA-4 translocation from the intracellular pool to the cellular periphery. These alternative mechanisms were examined in experiments in which surface and total CTLA-4 were determined. The results showed that a significant increase (P < 0.05) of total CTLA-4 expression was observed using the encapsulated with respect to the acapsular strain after 24 h of incubation (Fig. 1A, lower panel).
A significant increase (P < 0.05) of total CTLA-4 was observed for both strains after 96 h of incubation relative to unstimulated cells (Fig. 1B).
Given that our observations were from genetically unrelated strains, the encapsulated strain 3501 and its isogenic acapsular mutant CAP67 were used. The results showed that the pattern of CTLA-4 expression was similar to that of the genetically unrelated acapsular (CBS 7698) and encapsulated (CBS 6995) strains. The increase of CTLA-4 expression using encapsulated 3501 was about 60% with respect to the isogenic acapsular strain (CAP67). To better understand the role of capsular material in CTLA-4-induced up-regulation, we performed experiments using the acapsular yeasts plus GXM, the principal constituent of capsular material (13). It has been shown that GXM regulates the immune response to C. neoformans (13, 34, 35). In our experimental system the addition of GXM to both acapsular yeasts (CBS 7698 and CAP67) increased CTLA-4 expression on T cells at the level observed with fully encapsulated strains. In addition, GXM used in combination with PHA was able to produce a slight but not significant increase in CTLA-4 expression on T cells (data not shown).
The dose experiments showed the best up-regulation at an E:T ratio of 1:2. In fact, an E:T ratio of 0.1:1 did not have any effect while an E:T ratio of 1:10 produced up-regulation of CTLA-4 but not at the level observed with an E:T ratio of 1:2 (minus 20%). A direct interaction of T cells with C. neoformans has been described (16, 17); thus we hypothesized that C. neoformans could directly affect CTLA-4 on T cells. Purified rosetted T cells were mixed with acapsular or encapsulated C. neoformans at an E:T ratio of 2:1. CTLA-4 expression, surface and intracellular, was determined after 48 and 96 h of incubation. No modulation of CTLA-4 molecules was observed (data not shown).
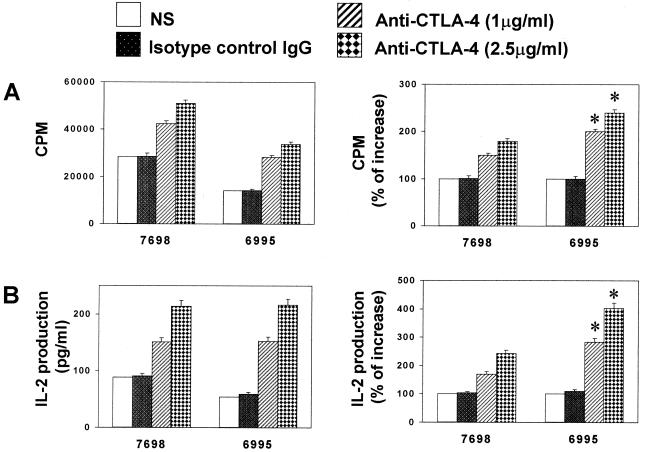
The CTLA-4 costimulatory pathway plays a key role in regulating T-cell activation in several experimental systems (18, 26, 36). Thus the possibility that blocking B7–CTLA-4 interaction could result in a modulation of immune response to C. neoformans was evaluated. For this purpose, mouse IgG anti-human CTLA-4 (Fab′)2 was incorporated in a mixture of PBMC plus encapsulated or acapsular C. neoformans, and lymphoproliferation was determined. The results reported in Fig. 2 show that anti-CTLA-4 (Fab′)2 (2.5 μg/ml) induced a significant increase (P < 0.05) in T-cell proliferation in response to both encapsulated (CBS 6995) and acapsular (CBS 7698) C. neoformans with respect to the anti-CTLA-4 (Fab′)2-untreated counterpart. Remarkably, enhancement of T-cell blastogenic response was significantly higher (P < 0.05) in T cells responding to encapsulated C. neoformans with respect to the acapsular strain (Fig. 2A). Enhancement of the lymphoproliferative response was directly related to enhanced production of IL-2 (Fig. 2B). The anti-CTLA-4-mediated increase of IL-2 was significantly higher (P < 0.05) in the presence of encapsulated C. neoformans with respect to the acapsular strain.
FIG. 2.
Effect of anti-CTLA-4 (Fab′)2 on proliferation (A) and IL-2 production (B) of T cells responding to C. neoformans. PBMC untreated or treated with C. neoformans (CBS 7698 or 6995) were incubated in the absence (NS) or presence of anti-CTLA-4 (Fab′)2 or control mouse IgG (Fab′)2 for 6 days, and the lymphoproliferative response was analyzed. PHA (5 μg/ml) was used as a positive control. (A) The results are expressed as counts per minute (cpm) or as the percentage of counts-per-minute increase of anti-CTLA-4 (Fab′)2-treated cells with respect to anti-CTLA-4 (Fab′)2-untreated cells (100%). (B) IL-2 production was tested in supernatant fluids from parallel cell cultures. The results are expressed as picograms of IL-2 production per milliliter or as the percentage of increase of IL-2 production of anti-CTLA-4 (Fab′)2-treated cells with respect to anti-CTLA-4 (Fab′)2-untreated cells (100%). The results are the mean ± SE of five separate experiments from five different donors. ∗, P < 0.05 (CBS 6995 plus anti-CTLA-4 (Fab′)2-treated cells versus CBS 7698 plus anti-CTLA-4 (Fab′)2-treated cells).
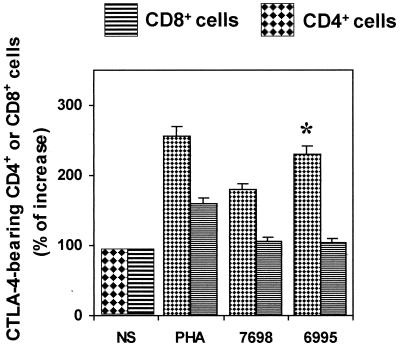
Given that activation of T cells is greatly affected by CTLA-4 cross-linking, we determined the percentage of CTLA-4-bearing CD4+ and CD8+ T cells. To this end, Cryptococcus-laden (CBS 7698 or 6995) monocytes were cocultured with purified T cells for 96 h. The results show that the percentage of CD4+ T cells responding to both strains of C. neoformans was significantly higher (P < 0.05) than CD8+ T cells (Fig. 3). In addition, a significant increase (P < 0.05) of CTLA-4-bearing CD4+ T cells was observed when the encapsulated strain was used with respect to the acapsular strain.
FIG. 3.
Percentage of CTLA-4-bearing CD4+ or CD8+ T cells. Monocytes treated with encapsulated (CBS 6995) or acapsular (CBS 7698) C. neoformans were cocultured with autologous T cells, and cell phenotype was analyzed after 6 days of incubation. The results are the percentage of increase of CTLA-4 positive CD4+ or CTLA-4 positive CD8+ T cells with respect to unstimulated cells, NS (100%). The results are the mean ± SE of four separate experiments from four different donors. ∗, P < 0.05 (CBS 6995-treated cells versus CBS 7698-treated cells).
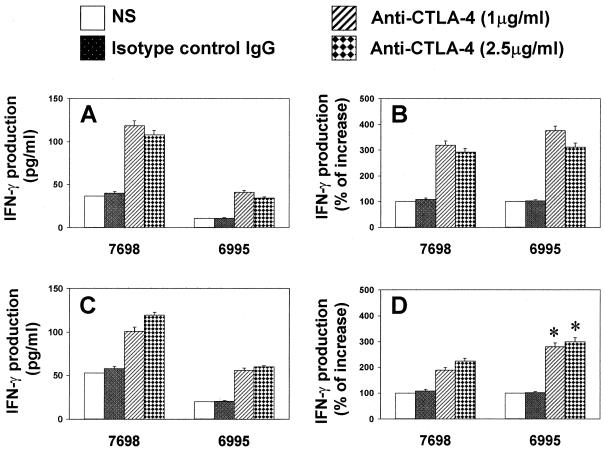
Previous studies revealed that CTLA-4 plays an important role in regulating T-cell differentiation in vivo (37). Recently it has been reported that in vivo administration of monoclonal antibody to CTLA-4 during immunization with C. neoformans significantly increases delayed-type hypersensitivity and prolongs survival to challenge with C. neoformans (J. W. Murphy and T. McGaha, Abstr. 4th Int. Conf. Cryptococcus and Cryptococcosis, abstr. I.20, 1999). Thus, the possibility that the blockade of CTLA-4 could affect T-cell differentiation to C. neoformans in our in vitro system was examined. The incorporation of anti CTLA-4 (Fab′)2 to our coculture of PBMC induced a significant (P < 0.05) up-regulation of IFN-γ production in response to both strains of C. neoformans with respect to the anti-CTLA-4-untreated counterpart (Fig. 4A and B). Because natural killer cells could account for the observed increase of IFN-γ, we performed experiments using Cryptococcus-laden monocytes plus purified T cells. The results reported in Fig. 4C and D show that the addition of anti-CTLA-4 (Fab′)2 to our coculture significantly (P < 0.05) augmented IFN-γ secretion when the encapsulated yeast was used with respect to the acapsular strain.
FIG. 4.
(A and B) Effect of anti-CTLA-4 (Fab′)2 on IFN-γ production of PBMC responding to C. neoformans. PBMC untreated or treated with C. neoformans (CBS 7698 or 6995) in the presence or absence (NS) of anti-CTLA-4 (Fab′)2 or control mouse IgG (Fab′)2 were incubated for 7 days. Supernatant fluids were harvested and tested for the presence of IFN-γ. The results are expressed as picograms of IFN-γ production per milliliter (A) or as the percentage of increase of IFN-γ production (B) of anti-CTLA-4 (Fab′)2-treated cells with respect to anti-CTLA-4 (Fab′)2-untreated cells (100%). Results represent the mean ± SE of four separate experiments from four different donors. (C and D) Effect of anti-CTLA-4 (Fab′)2 on IFN-γ production of monocytes treated with encapsulated (CBS 6995) or acapsular (CBS 7698) C. neoformans cocultured with autologous T cells for 7 days. Supernatant fluids were harvested and tested as reported above. The results are expressed as picograms of IFN-γ production per milliliter (C) or the percentage of increase of IFN-γ production (D) of anti-CTLA-4 (Fab′)2 with respect to anti-CTLA-4 (Fab′)2-untreated cells (100%). The results are the mean ± SE of three separate experiments from three different donors. ∗, P < 0.05 (CBS 6995 plus mouse anti-human CTLA-4 (Fab′)2-treated cells versus CBS 7698 plus mouse anti-human CTLA-4 (Fab′)2-treated cells).
DISCUSSION
Here we provide evidence that (i) up-regulation of CTLA-4 expression occurs on T cells responding to C. neoformans, (ii) surface up-regulation corresponds to a prior increase of the total pool of CTLA-4, (iii) the increased expression of CTLA-4 is significantly higher on T cells responding to encapsulated C. neoformans with respect to an acapsular strain and parallels a prior drastic increase of the total pool of CTLA-4, (iv) CTLA-4 expression increases on CD4+ T cells responding to C. neoformans, and (v) blockage of CTLA-4–B7 interaction promotes an increase in T-cell proliferation, IL-2 secretion, and IFN-γ production. These latter effects were amplified when the encapsulated strain was used. This suggests that CTLA-4 overexpression may contribute to the negative regulatory effect induced by encapsulation of C. neoformans (33).
It was recently demonstrated that B7-2 is required to induce and maintain activation of T cells against C. neoformans (23). B7-1 and B7-2 molecules bind CD28 or CTLA-4 (1, 15). CD28 is constitutively expressed on T cells while CTLA-4 is rapidly expressed after T-cell activation (20). It is conceivable that in our system the increased availability of CTLA-4 on T cells responding to encapsulated C. neoformans may enhance the B7–CTLA-4 interaction by providing a negative signal for the T-cell response as previously observed (22, 33, 34). This is consistent with the major affinity of CTLA-4 for B7 molecules with respect to CD28 (26). Conversely, the weak ability of the acapsular strain in up-regulating CTLA-4 on T cells could support the B7-CD28 interaction with subsequent augmentation of T-cell proliferation. In agreement with this hypothesis, CD28 has been described as a positive signal for initiation of T-cell activation (11, 19, 29). Strong evidence supports the inhibitory role of CTLA-4 molecules in several experimental systems (30, 38). This negative signal is transmitted by TCR-associated src family kinases (28). Blockage of B7–CTLA-4 interaction has been shown to produce T-cell activation (25, 21, 37), which may be the consequence of the interaction of B7 and CD28, resulting in increased signaling by these activating molecules (5).
It has been reported that CTLA-4-induced T-cell hyporesponsiveness could be ascribed also to its capacity to antagonize the TCR-mediated signal (26). In our system, both mechanisms may work together to limit T-cell activation in response to encapsulated C. neoformans. This is supported by our observations that blocking B7–CTLA-4 costimulation with anti-CTLA-4 (Fab′)2 increased T-cell activation and IL-2 production (Fig. 2). This effect was manifested in response to both encapsulated and acapsular strains of C. neoformans. However, the best stimulation occurred with the encapsulated yeast, pointing out the biological effect of CTLA-4 overexpression.
CTLA-4 is expressed on CD4+ and CD8+ T cells (26). Here we demonstrate that CTLA-4 is expressed predominantly on CD4+ T cells responding to C. neoformans, a critical signal for T-cell activation and differentiation. Remarkably, the encapsulated strain produced an increased amount of CTLA-4-bearing CD4+ T cells relative to the acapsular yeast. This is consistent with enhancement of CTLA-4 expressed on T cells responding to the encapsulated strain (Fig. 1) and with amplification of T-cell activation by blocking CTLA-4.
Previous reports showed that blockage of CTLA-4 has a beneficial effect in different experimental systems (21, 25; Murphy and McGaha, Abstr. 4th Int. Conf. Cryptococcus and Cryptococcosis). In particular, in a murine model of infection with the intracellular pathogen Leishmania donovani, anti-CTLA-4 administration promoted IFN-γ secretion and enhanced host resistance favoring granulomatous response (25). C. neoformans may be an intracellular pathogen because of its ability to reside inside phagocytic cells (10, 16).
Here we demonstrate that blockage of CTLA-4 in T cells responding to C. neoformans could affect T-cell differentiation by influencing IFN-γ production. Such blockage induces a strong increase of IFN-γ production in response to both strains when unseparated PBMC are used. However, to exclude the contribution of natural killer cells in the observed IFN-γ enhancement, Cryptococcus-laden monocytes cocultured with purified T cells were used. A significant increase in IFN-γ production in response to the encapsulated strain was observed. This suggests that (i) CTLA-4–B7 ligation may regulate both T-cell activation and differentiation against C. neoformans, (ii) CTLA-4-mediated hyporesponsiveness is not due to a lack of costimulation but appears to be the consequence of specific recognition of CTLA-4 by B7 molecules, and (iii) encapsulation is a critical event in CTLA-4-induced suppression.
The inability of C. neoformans to directly affect CTLA-4 expression on T cells (data not shown) suggests that CTLA-4 up-regulation may be driven by APC during interaction with T cells. Consistent with this hypothesis, C. neoformans modulation of APC function relative to the presence of capsular material has been observed (23, 32, 34).
Previous studies on CTLA-4-induced anergy were performed on CD4+ T cells (37), and failure to prevent anergy by CTLA-4 blockage has been ascribed in part to CD8+ skewing (29). To our knowledge, we demonstrate for the first time the predominant involvement of CTLA-4-bearing CD4+ T cells in orchestrating the positive and negative response to C. neoformans. Furthermore, a new effect of C. neoformans capsular material as a regulator of CTLA-4 expression is reported. This recalls attention to other microbial virulence factors as potential regulators of CTLA-4 molecules.
Our data shed light on the ability of C. neoformans capsular material to induce T-cell hyporesponsiveness, indicating the involvement of CTLA-4 as a critical negative regulator. CTLA-4 could be the key immunoregulatory molecule serving as a potential target to manipulate activation and possibly differentiation of T cells responding to C. neoformans.
ACKNOWLEDGMENTS
We are grateful to Eileen Mahoney Zannetti for excellent and dedicated editorial and secretarial assistance, to Thomas R. Kozel for providing purified GXM, and to Elisa Mearini for technical assistance.
This study was supported by the National Research Program on AIDS, “Opportunistic Infections and Tuberculosis,” contract no. 50B.39, Italy.
REFERENCES
- 1.Abbas A K, Sharpe A H. T-cell stimulation: an abundance of B7s. Nat Med. 1999;5:1345–1346. doi: 10.1038/70905. [DOI] [PubMed] [Google Scholar]
- 2.Boussiotis V A, Freeman G J, Gribben J G, Nadler L M. The role of B7–1/B7–2: CD28/CTLA-4 pathways in the prevention of anergy, induction of productive immunity and down-regulation of the immune response. Immunol Rev. 1996;153:5–26. doi: 10.1111/j.1600-065x.1996.tb00918.x. [DOI] [PubMed] [Google Scholar]
- 3.Casadevall A, Perfect J R. Cryptococcus neoformans. Washington, D.C.: American Society for Microbiology; 1998. pp. 145–269. [Google Scholar]
- 4.Cella M, Engering A, Pinet V, Pieters J, Lanzavecchia A. Inflammatory stimuli induce accumulation of MHC class II complex on dendritic cells. Nature. 1997;388:782–787. doi: 10.1038/42030. [DOI] [PubMed] [Google Scholar]
- 5.Chambers C A, Allison J P. Co-stimulation in T cell response. Curr Opin Immunol. 1997;9:396–404. doi: 10.1016/s0952-7915(97)80087-8. [DOI] [PubMed] [Google Scholar]
- 6.Chang Y C, Kwon-Chung K J. Complementation of a capsule-deficient mutation of Cryptococcus neoformans restores its virulence. Mol Cell Biol. 1994;14:4912–4919. doi: 10.1128/mcb.14.7.4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cherniak R, Reiss E, Slodki M E, Platter R D, Blumer S O. Structure and antigenic activity of the capsular polysaccharide of Cryptococcus neoformans. Mol Immunol. 1980;17:1025–1032. doi: 10.1016/0161-5890(80)90096-6. [DOI] [PubMed] [Google Scholar]
- 8.Dai Z, Lakkis F G. The role of cytokines, CTLA-4 and costimulation in transplant tolerance and rejection. Curr Opin Immunol. 1999;11:504–508. doi: 10.1016/s0952-7915(99)00008-4. [DOI] [PubMed] [Google Scholar]
- 9.Decken K, Köhler G, Palmer-Lehmann K, Wunderlin A, Mattner F, Magram J, Gately M K, Alber G. Interleukin-12 is essential for a protective Th1 response in mice infected with Cryptococcus neoformans. Infect Immun. 1998;66:4994–5000. doi: 10.1128/iai.66.10.4994-5000.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldmesser M, Kress Y, Novikoff P, Casadevall A. Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection. Infect Immun. 2000;68:4225–4237. doi: 10.1128/iai.68.7.4225-4237.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harding F A, McArthur J G, Gross J A, Raulet D H, Allison J P. CD28-mediated signalling co-stimulates murine T cells and prevents induction of energy in T-cell clones. Nature. 1992;356:607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- 12.Kozel T R. Non-encapsulated variant of Cryptococcus neoformans. II. Surface receptors for cryptococcal polysaccharide and their role in inhibition of phagocytosis by polysaccharide. Infect Immun. 1977;16:99–106. doi: 10.1128/iai.16.1.99-106.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kozel T R, Hermerath C A. Binding of cryptococcal polysaccharide to Cryptococcus neoformans. Infect Immun. 1984;43:879–886. doi: 10.1128/iai.43.3.879-886.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuchroo V K, Das M P, Brown J A, Ranger A M, Zamvil S S, Sobel R A, Weiner H L, Nabavi N, Glimcher L H. B7–1 and B7–2 costimulatory molecules activate differentially the Th1/Th2 development pathways: application to autoimmune disease therapy. Cell. 1995;80:707–718. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 15.Lenschow D J, Walunas T L, Bluestone J A. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 16.Levitz S M, Harrison T S, Tabuni A, Liu X. Chloroquine induces human mononuclear phagocytes to inhibit and kill Cryptococcus neoformans by a mechanism independent of iron deprivation. J Clin Investig. 1997;100:1640–1646. doi: 10.1172/JCI119688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levitz S M, Mathews H L, Murphy J W. Direct antimicrobial activity of T cells. Immunol Today. 1995;16:387–391. doi: 10.1016/0167-5699(95)80007-7. [DOI] [PubMed] [Google Scholar]
- 18.Lin H, Rathmell J C, Gray G S, Thompson C B, Leiden J M, Alegre M L. Cytotoxic T lymphocyte antigen 4 (CTLA-4) blockage accelerates the acute rejection of cardiac allografts in CD28-deficient mice: CTLA-4 can function independently of CD28. J Exp Med. 1998;188:199–204. doi: 10.1084/jem.188.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linsley P S, Brady W, Grosmaire L, Aruffo A, Damle N K, Ledbetter J A. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med. 1991;173:721–730. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McAdam A J, Schweitzer A N, Sharpe A H. The role of B7 co-stimulation in activation and differentiation of CD4+ and CD8+ T cells. Immunol Rev. 1998;165:231–247. doi: 10.1111/j.1600-065x.1998.tb01242.x. [DOI] [PubMed] [Google Scholar]
- 21.McGaha T, Murphy J W. CTLA-4 down-regulates the protective anticryptococcal cell-mediated immune response. Infect Immun. 2000;68:4624–4630. doi: 10.1128/iai.68.8.4624-4630.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mody C H, Syme R M. Effect of polysaccharide capsule and methods of preparation on human lymphocyte proliferation in response to Cryptococcus neoformans. Infect Immun. 1993;61:464–469. doi: 10.1128/iai.61.2.464-469.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monari C, Kozel T R, Casadevall A, Pietrella D, Palazzetti B, Vecchiarelli A. B7 costimulatory ligand regulates development of the T-cell response to Cryptococcus neoformans. Immunology. 1999;98:27–35. doi: 10.1046/j.1365-2567.1999.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morel P A, Oriss T B. Crossregulation between Th1 and Th2 cells. Crit Rev Immunol. 1998;18:275–303. doi: 10.1615/critrevimmunol.v18.i4.10. [DOI] [PubMed] [Google Scholar]
- 25.Murphy M L, Cotterell S E, Gorak P M, Engwerda C R, Kaye P M. Blockade of CTLA-4 enhances host resistance to the intracellular pathogen, Leishmania donovani. J Immunol. 1998;161:4153–4160. [PubMed] [Google Scholar]
- 26.Oosterwegel M A, Greenwald R J, Mandelbrot D A, Lorsbach R B, Sharpe A H. CTLA-4 and T cell activation. Curr Opin Immunol. 1999;11:294–300. doi: 10.1016/s0952-7915(99)80047-8. [DOI] [PubMed] [Google Scholar]
- 27.Pietrella D, Monari C, Retini C, Palazzetti B, Kozel T R, Vecchiarelli A. HIV type 1 envelope glycoprotein gp120 induces development of a T helper type 2 response to Cryptococcus neoformans. AIDS. 1999;13:2197–2207. doi: 10.1097/00002030-199911120-00002. [DOI] [PubMed] [Google Scholar]
- 28.Saito T. Negative regulation of T cell activation. Curr Opin Immunol. 1998;10:313–321. doi: 10.1016/s0952-7915(98)80170-2. [DOI] [PubMed] [Google Scholar]
- 29.Thompson C B, Lindsten T, Ledbetter J A, Kunkel S L, Young H A, Emerson S G, Leiden J M, June C H. CD28 activation pathway regulates the production of multiple T-cell-derived lymphokines/cytokines. Proc Natl Acad Sci USA. 1989;86:1333–1337. doi: 10.1073/pnas.86.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tivol E A, Borriello F, Schweitzer A N, Lynch W P, Bluestone J A, Scarpe A H. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 31.Vecchiarelli A, Casadevall A. Antibody-mediated effects against Cryptococcus neoformans: evidence for interdependency and collaboration between humoral and cellular immunity. Res Immunol. 1998;149:321–333. doi: 10.1016/s0923-2494(98)80756-6. [DOI] [PubMed] [Google Scholar]
- 32.Vecchiarelli A, Monari C, Retini C, Pietrella D, Palazzetti B, Pitzurra L, Casadevall A. Cryptococcus neoformans differently regulates B7–1 (CD80) and B7–2 (CD86) expression on human monocytes. Eur J Immunol. 1998;28:114–121. doi: 10.1002/(SICI)1521-4141(199801)28:01<114::AID-IMMU114>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 33.Vecchiarelli A, Pietrella D, Dottorini M, Monari C, Retini C, Todisco T, Bistoni F. Encapsulation of Cryptococcus neoformans regulates fungicidal activity and the antigen presentation process in human macrophages. Clin Exp Immunol. 1994;98:217–223. doi: 10.1111/j.1365-2249.1994.tb06128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vecchiarelli A, Retini C, Pietrella D, Monari C, Kozel T R. T lymphocyte and monocyte interaction by CD40/CD40 ligand facilitates a lymphoproliferative response and killing of Cryptococcus neoformans in vitro. Eur J Immunol. 2000;30:1385–1393. doi: 10.1002/(SICI)1521-4141(200005)30:5<1385::AID-IMMU1385>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 35.Vecchiarelli A, Retini C, Pietrella D, Monari C, Tascini C, Beccari T, Kozel T R. Downregulation by cryptococcal polysaccharide of tumor necrosis factor alpha and interleukin-1β secretion from human monocytes. Infect Immun. 1995;63:2919–2923. doi: 10.1128/iai.63.8.2919-2923.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walunas T L, Bakker C Y, Bluestone J A. CTLA-4 ligation blocks CD28-dependent T cell activation. J Exp Med. 1996;183:2541–2550. doi: 10.1084/jem.183.6.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walunas T L, Bluestone J A. CTLA-4 regulates tolerance induction and T cell differentiation in vivo. J Immunol. 1998;160:3855–3860. [PubMed] [Google Scholar]
- 38.Waterhous P, Penninger J M, Timms E, Wakeham A, Shahinian A, Lee K T, Thompson C B, Griesser H, Mak T W. CTLA-4 deficiency causes lymphoproliferative disorder with early lethality. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]