Abstract
Pesticide action is predominantly measured as a toxicological outcome, with pharmacological impact of sublethal doses on bystander species left largely undocumented. Likewise, chronic exposure, which often results in responses different from acute administration, has also been understudied. In this article, we propose the application of standard pharmacological principles, already used to establish safe clinical dosing regimens in humans, to the ‘dosing of the environment’. These principles include relating the steady state dose of an agent to its beneficial effects (e.g. pest control), while minimising harmful impacts (e.g. off‐target bioactivity in beneficial insects). We propose the term ‘environmental therapeutic window’, analogous to that used in mammalian pharmacology, to guide risk assessment. To make pharmacological terms practically useful to environmental protection, quantitative data on pesticide action need to be made available in a freely accessible database, which should include toxicological and pharmacological impacts on both target and off‐target species.
Keywords: agriculture, biodiversity, environment, fungicide, neonicotinoid, pesticide, pharmacology, therapeutic window
Abbreviations
- CEC
chemical(s) of environmental concern
- DC50
disruptive potency of a compound (defined in the text)
- IP
Insect Pollinators
- ppb
parts per billion
- RC
residual concentration
1. THE APPLICATION OF PHARMACOLOGICAL PRINCIPLES IN DOSING THE ENVIRONMENT
There is global concern that inappropriate use of pesticides is deleterious to the environment by, for example, reducing the abundance and biodiversity of insects (Vanbergen & IP Initiative, 2013). This may lead to loss of ecosystem services (e.g. pollination, pest control and nutrient cycling) and threaten our future food security (Vanbergen & IP Initiative, 2013). In a growing season a field may receive several applications of pesticides (insecticides, herbicides and fungicides) that may be mixed on site prior to use (Figure 1). Insect pollinators and soil organisms may be exposed acutely during application or chronically if the chemicals persist in the soil and are translocated to adjacent wildflowers. Furthermore, some pesticides may leach into waterways exposing aquatic species. As local pesticide usage and persistence data are not available, unknown risks from pesticide cocktails may exist.
FIGURE 1.
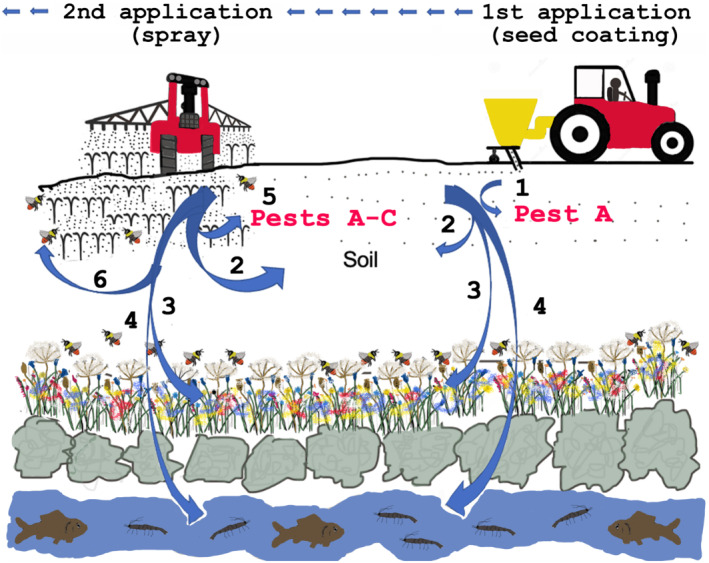
Environmental exposure to pesticides. Multiple chemical (predominantly herbicides, insecticides and fungicides) applications are made to arable fields each year. (1) First application is applied as a seed coating to protect emerging crops from target pest A. (2) Much of pesticide enters the soil, exposing it to beneficial soil organisms (e.g. earthworms and mycorrhizal fungi). (3) Pesticides translocate (via wind, water or soil) to field margins (wildflower/hedgerow) where soil organisms and native plant species may be vulnerable and contaminated plants may provide a chronic exposure (via nectar and pollen) to pollinators. (4) Pesticides may leach into waterways where aquatic invertebrate species may be particularly vulnerable. (5) Subsequent pesticide exposures is via spraying, where chemicals may be mixed on site to target existing/predicted pest species A, B, C… where these may join previously used chemicals that are persistent. The exposure to multiple pesticide applications during a growing season may lead to complex pharmacological issues of polypharmacy (the interaction between multiple chemicals) and possible adaptations (sensitivity or resistance) to chronic exposure are possible. An understanding of pesticide dosing regimens and the major constituents of chemical cocktails would inform studies on off‐target sites (e.g. beneficial and bystander species) and ecosystem services/stability.
It is now 60 years since Rachel Carson warned of the environmental consequences of unpredicted risks from pesticides (Carson, 1962), yet our approach to monitoring the risk remains overly simplistic, relying on inappropriate metrics as a proxy for risk. This is exemplified by the debate over the quantity of pesticide being used. Currently, pesticide usage in the United Kingdom is monitored in terms of weight of active ingredient(s) applied and area treated (https://secure.fera.defra.gov.uk/pusstats/), with data collected biennially from pilot farms and extrapolated to indicate annual nationwide levels. By this measure, UK pesticide usage has more than halved between 1996 and 2016 (to 16,899,858 kg on 73,172,193 ha)1 but campaign groups demanded a ‘quantitative UK pesticide reduction’ of a further 50% (by weight) by 2030 (https://www.wildlifetrusts.org/sites/default/files/2020-07/Reversing%20the%20Decline%20of%20Insects%20Report%20-EMBARGO%2008.07.20%20%282%29.pdf) and this demand has now been supported by the EU (https://www.theguardian.com/environment/2022/jun/22/eu-legislation-restoration-ecosystems-biodiversity-aoe). Unfortunately, these ‘improvements’ and demands take no account of pesticide potency, selectivity for the target species, the environmental half‐life of individual chemicals and their metabolites, or pesticide interactions. The use of pharmacological measures of bioactivity (e.g. EC50 or IC50) would provide a better indicator of potential sublethal impacts. Biodiversity in the United Kingdom has decreased substantially, despite the halving of the total mass of pesticide applied (Tudge et al., 2021).
There are three possible explanations for this apparent paradox:
(a) pesticides do not damage biodiversity,
(b) pesticides are the problem, but had already triggered an irreversible sequence or
(c) total pesticide mass applied is a poor indicator of pesticide impact.
Possibility (b) might be tested by recovery experiments, while (a) and (c) could be distinguished using a better metric of amount applied effective and a consideration of individual pesticides and their interactions with other pesticides.
In many ways, a local ecosystem is analogous to an individual patient where a specific threat needs to be treated without causing any intolerable damage to the living system (Figure 2). However, although the chemical load applied to the environment is monitored by the weight applied, in clinical pharmacology, measuring patient dosing by totalling the mass of all drugs applied, with no regard to the identities and properties of the drugs, would be viewed as ridiculous. Instead, careful attention is paid to each drug's mode of action and quantitative pharmacokinetics and pharmacodynamics. Furthermore, the risk from a combination of agents is rarely a linear sum of the risks from each agent alone. For pesticides, the risk of interactions has been ignored due to the large number of pesticides (and metabolites) involved and variable contributions of other stressors from the environment.
FIGURE 2.

Treating an ecosystem as a single living system. Therapeutic actions are indicated by black arrows and potential unintended actions by red arrows. (a) The patient: when a patient presents with a medical problem, basic pharmacological principles guide the clinician to deliver the minimal effective treatment regime with tolerable effects on the patient. Environmental pharmacology dictates that the local ecosystem be considered as a single living system, analogous to a human patient. (b) Treatment: the parallel begins with a threat, which may be a disease in man or a crop pest in an ecosystem. In both cases, use of the effective drug (for man) or pesticide (for a crop) requires that a bioactive steady‐state dose is delivered by an appropriate dosing regimen (chemical load and frequency of dosing). (c) Side effects: treatment must be limited to tolerable side‐effects that result from off‐target bioactivity (eg. organ function in man or a beneficial species, such as bees, in the ecosystem). (d) Long‐term adaptations: where chronic exposure occurs (either by prolonged treatment or long‐term persistence), a consideration of adaptation(s) is required in both man (eg. physiological consequences such as addiction/sensitisation or multidrug resistance in pathogen) and the ecosystem (eg. preference seeking/sensitisation in pollinators and pesticide resistance in crop pests). (e) Cocktail effects: in man, there is a growing concern about complex contraindications in elderly patients due to an accumulation of prescription medications (called polypharmacy; Tatonetti et al., 2012). Polypharmacy does not exist for ecosystems and the impact of chemical cocktails to ‘off‐target’ sites (eg. beneficial species and man) are unstudied. Therefore, the long‐term impact of pesticides on the health of ecosystems and man is unknown.
2. PESTICIDE BIOACTIVITY IS A BETTER INDICATOR OF POTENTIAL HARM THAN WEIGHT APPLIED
By refocussing on pesticide bioactivity, the full quantitative power of pharmacology would provide an important advancement in our understanding of the risk from environmental contaminants and improve our dosing of the environment to tackle agricultural pests. For example, an important concept in medical pharmacology is that of the ‘therapeutic window’: the zone of dosage at which there is enough drug to exert a beneficial effect but not so much that adverse effects outweigh the benefit. To limit their impact on human health, pesticides are developed to be relatively selective for their target species over man and this is often seen to be analogous to the ‘therapeutic window’. But pesticides often exhibit low selectivity between related species. Therefore, an insecticide may not distinguish between an aphid (a pest) and a bumblebee (a beneficial insect), or a herbicide between a ‘weed’ and a wildflower, or a fungicide between fungal disease in a crop and beneficial soil mycorrhizal fungi. Thus, while toxicological impact of pesticides on target species may be well‐documented, the impact on the many bystander species, either toxicological or pharmacological (i.e. at sublethal doses), is not routinely measured.
Taken together, these variables suggest that the tonnage of pesticide usage is an irrelevant metric when considering their efficacy on pest species and the environmental impact of their use (see Box 1). There has been a proposal to use the LD50 of an agent (the dose that results in death of 50% of treated individuals) as a better measure of toxicity (Goulson et al., 2018). Using this metric for honeybee vulnerability, there was a six‐fold increase, rather than a reduction, in UK pesticide between 1990 and 2015. Although the use of LD50 as a metric is probably more useful than pesticide mass applied, it still ignores sublethal effects, such as changes in behaviour that can impact on the long‐term health of a species.
BOX 1 Variables to be considered for the environmental pharmacology of pesticides
|
|
|
|
|
|
3. PESTICIDE DOSING REGIMENS SHOULD RELATE TO ECOSYSTEM HEALTH
The problem of using chemical agents to control pathogens while limiting excessive off‐target damage has long been a feature of human medicine. More recently, the term ‘evidence‐based medicine’ has been adopted widely to ensure that appropriate choices of medicines and their doses are selected. An ecosystem exhibits numerous similarities to the human body, as a living system of interconnected and interdependent communicating parts (see Box 2). Pharmacologists have generated a range of tools to allow them to describe quantitatively the desired effects of different concentrations of a chemical agent on the body and the undesired effects of toxicity to bystander cells and tissues. We propose the development of ‘environmental pharmacology’ where the principles behind a clinical dosing regimen are incorporated into the dosing of the environment. To achieve this, we need to consider an ‘environmental therapeutic index’ before we can establish an ‘environmental safety margin’ of a pesticide, where the ‘useful’ effective dose (to deter a crop pest) is guided by the ‘harmful’ disruptive concentration effects on the local ecosystem. Examples of species that should be protected from harm include insect pollinators exposed to contaminated nectar and pollen or aquatic invertebrates following agricultural run‐off.
BOX 2 Parallels between mammalian and environmental pharmacology and therapeutics
| Mammalian | Farmed environment | |
| Pathogen | Bacteria, viruses and so on | Aphids, beetles and so on |
| Treatment | Antibacterials, antivirals … | Pesticides |
| Administration | Daily dosing at fixed levels | Ad hoc application |
| Pharmacodynamic parameters | Potency | Potency |
| Affinity | Affinity | |
| Selectivity | Selectivity | |
| Pharmacokinetic parameters | Absorption | Application route |
| Distribution | Translocation from soil | |
| Metabolism | Metabolism (species‐specific) | |
| Excretion | Run‐off into waterways | |
| Plasma steady state levels | Persistence (beyond need) | |
| Therapeutic window | EC50/DC50 or RC/DC50 | |
| Safety margin | Safety margin | |
| Toxicological impact | Microbiome | Microbiomes of species |
| Organ damage (e.g.,liver and kidneys) | Bystander species (e.g. bees) and secondary exposure (e.g. aquatic taxa) | |
| Co‐morbidities | Polypharmacy | |
| Drug:drug interactions | Pesticide:pesticide interactions | |
| Chronic exposure | Tolerance | Unknown |
| Dependence | Unknown | |
| Withdrawal | Unknown | |
| Resistance (pathogen evasion or host enzyme/transporter induction) | Resistance (pest evasion) |
Modern agricultural techniques are associated with a dominance of large monocultures of crops, with little natural habitat remaining to nurture native beneficial species. Therefore, we are becoming increasingly dependent on the services of a relatively few species that can be managed by humans. Too much focus on the threats to a few managed pollinator species may lead to an unsupervised loss in the abundance and diversity of natural supporting ecosystem services. This may impact food security in the future as the contribution from native species decreases (Albrecht et al., 2012). For example, the use of neonicotinoid insecticides to protect orchards from pest species has been reported to reduce apple crop pollination due to impaired bumblebee colony growth (Stanley et al., 2015). With a greater knowledge of the environmental side effects of pesticides (and other agents), pharmacologists can apply the fundamental principles of pharmacology to optimise the dosing regimen to balance the risk and benefit from the active ingredient, where safety indices can be calculated using classical pharmacological paradigms.
4. BEYOND THE BENEFICIAL ACUTE EFFECTS OF A PESTICIDE LIES THE UNKNOWN RISK(S) FROM CHEMICAL INTERACTIONS AND LONG‐TERM EXPOSURE
There is limited understanding of the impact of pesticide interactions (polypharmacy), whether they are mixed intentionally on site prior to application or result from persistence in the soil from previous applications. Beyond acute administration profiles, chronic exposure to drugs in humans often results in a loss or modification of responsiveness through adaptations such as tolerance or altered drug metabolism/transport. In terms of chronic exposure, although there is little reported evidence that any bystander species has developed a resistance to any chemical treatment, or suffered any negative consequences on its removal, it is clear that ‘superbugs’ in humans and ‘super‐pests’ (and ‘super‐weeds’) in crops have evolved tolerance to antibiotics and pesticides, respectively. Resistance may result from overuse (e.g. methicillin‐resistant Staphylococcus aureus in man), multi‐pesticide resistance in aphids [Myzus persicae] and Roundup®‐resistance in the weed ‘Poverty brome’ (Brome sterilis L.). In these scenarios, treatment options may be limited. In terms of potential environmental ‘addiction’, preference‐seeking behaviour for neonicotinoids has been reported in Bombus terrestris and Apis mellifera (Arce et al., 2018; Kessler et al., 2015), but not Bombus impatiens (Muth et al., 2020). It is not known if there are any withdrawal symptoms experienced in bees when neonicotinoid exposure ends. Nevertheless, chronic exposure may induce environmental adaptations, like those observed in man, that alter the responsiveness of pest and beneficial species to future pesticide treatments.
5. A CASE STUDY TO EXEMPLIFY THE NEED FOR AN ENVIRONMENTAL PHARMACOLOGY APPROACH
The first neonicotinoid, imidacloprid, was introduced in the United Kingdom in 1994 and hailed as a major breakthrough in safety for two important reasons. First, the neonicotinoids could be targeted to the crop by coating seeds rather than aerial spraying of the whole field. Second, they exhibit a very high selectivity for insects over mammals, making them much safer for handlers than the older chemical classes (organophosphates, carbamates and pyrethroids) that they were intended to replace. The hopes of targeted delivery by seed‐coating were dashed when it was reported that most (>80%) of the neonicotinoid on the seed coating is lost into the soil (Alford & Krupke, 2017). Early support for the low risk to the insect pollinators came from the manufacturer's finding that residual imidacloprid levels, persisting at a time when pollinators arrived, were detected in the nectar and/or pollen of oilseed rape, corn and sunflowers at vanishingly low levels (1.5–5 ppb—close to the limit of detection) and that field studies indicated a ‘no observable adverse effect’ level on bees at 20 ppb (Maus et al., 2003).
6. SUBLETHAL EFFECTS ARE MORE SUBTLE BUT MAY STILL THREATEN ECOSYSTEM SERVICES
It took until 2012 before the first evidence of harm to colonies of social insects (bumblebees, B. terrestris) was reported (Gill et al., 2012; Whitehorn et al., 2012). Curiously, the impact on colony performance was not evident until 21 days of exposure (Gill et al., 2012). Evidence for a mode of action emerged shortly afterwards where a sublethal impact on bee learning and memory was reported (Williamson & Wright, 2013). Using a classical conditioning protocol, the proboscis extension response, an impairment in the ability of bees to associate an odour cue with a food reward was established for both honeybees, A. mellifera (Williamson & Wright, 2013) and bumblebees, B. terrestris (Piiroinen et al., 2016).
To establish the in vivo exposure dose when bees were exposed to neonicotinoids in nectar, chemical levels in the brain were monitored during feeding with neonicotinoid fed to the bumblebees at field levels (10 nM, 2.1 ppb [w/w] in sucrose solution. Bioactive (acute) levels in the brain were reached within 3 days (Moffat et al., 2015). At this level, the neonicotinoid clothianidin evokes a sustained depolarisation in acutely isolated honeybee brain that occludes action potential firing in Kenyon cells within the mushroom body, a higher order insect brain structure mediating multisensory integration and learning and memory (Palmer et al., 2013). In cultured bee brain cells, neurons undergo acute mitochondrial depolarisation following exposure to clothianidin (10 nM), but not imidacloprid.
7. CHRONIC EXPOSURE TO PESTICIDES MAY ALTER THE PHARMACOLOGICAL BASELINE BY INITIATING MECHANISMS OF ADAPTATION
As neonicotinoids may persist in the environment (Botías et al., 2015; Jones et al., 2014; Krupke et al., 2012) an understanding of the effect of chronic exposure is required. In one study, a chronic exposure (2 days) of imidacloprid, at sub‐field levels (1 nM) rendered a large population of neurons vulnerable (as indicated by mitochondrial depolarisation) to normally innocuous levels of acetylcholine (100 μM) (Moffat et al., 2015). The chronic sensitising effects were mirrored at the whole bee level, where chronic feeding of field levels of clothianidin or thiamethoxam (10 nM for 7 days), but not imidacloprid, increased vulnerability to a previously sublethal dose of clothianidin (200 nM) (Moffat et al., 2016).
These chronic adaptations are reminiscent of the pharmacological chaperoning effect of nicotine on high affinity mammalian nicotinic acetylcholine receptors, which can be mimicked by neonicotinoids (which bind to the same site). In each case, receptor upregulation induced by individual agents correlated with their in vitro potency for inhibiting [3H]nicotine binding (Tomizawa & Casida, 2000). The upregulation of a high nicotine sensitivity α4β2 (α4(x2) β2(x3)) receptors is thought to drive preference seeking behaviour in humans (Ngolab et al., 2015) and nicotine alone has reinforcing actions (Hamouda et al., 2021). Similarly, preference seeking behaviour have also been reported in honeybees (Kessler et al., 2015) and the bumblebee B. terrestris (Arce et al., 2018; Kessler et al., 2015), but not B. impatiens (Muth et al., 2020) with field‐relevant levels of the neonicotinoids, thiamethoxam or imidacloprid.
The importance of receptor plasticity to altered vulnerability to the neonicotinoids in insects may be relevant as they are exposed chronically to neonicotinoids in the field (Botías et al., 2015; Jones et al., 2014; Krupke et al., 2012). Regardless of any additional risk from increased vulnerability, the large number of reports on consequential deficits in learning and memory may explain the reported poorer foraging skills in bees (Gill et al., 2012). This sublethal mode of action is supported further by the observation that while (semi‐)field experiments report colony weakness following neonicotinoid exposure (Gill et al., 2012; Moffat et al., 2016; Whitehorn et al., 2012), a lab‐based study where the colonies did not need to forage for themselves in order to eat were unaffected (Stanley & Raine, 2017).
8. A POST HOC REFLECTION ON THE CASE OF THE NEONICOTINOIDS
Had an environmental pharmacology strategy been in place when the neonicotinoids were first introduced nearly 30 years ago, a sublethal risk to insects would have been tested for, as the receptor binding affinity on honeybees (2.9 nM, Nauen et al., 2001) was close to the field dose found in nectar and pollen (~7–24 nM; Maus et al., 2003) despite the study reporting a ‘no observable adverse effect level’ of 20 ppb (~4.2 nM). Therefore, evidence suggesting the likelihood of sublethal bioactivity in honeybees existed at least 11 years prior to this being confirmed in 2012 (Gill et al., 2012; Whitehorn et al., 2012) and a consequential EU moratorium on their use on crops attractive to bees (2013), followed by a ban on all outdoor uses (2018) and a full ban in 2020.
A more detailed pharmacological study reported neonicotinoids inhibiting acetylcholine‐induced responses of α8 subunit receptors at just 10 pM (Ihara et al., 2020) demonstrating that the pharmacology of chemicals is complex and unpredictable, requiring an empirical pharmacological analysis on key beneficial species before conclusions on safety are robust. Ultimately, adverse sublethal activity was demonstrated on individual bumblebees at 1–10 nM (Moffat et al., 2015) and whole bumblebee colonies at 29–48 nM (Gill et al., 2012; Whitehorn et al., 2012).
9. PESTICIDES MAY SPREAD BEYOND THE SITE OF APPLICATION AND INITIATE CASCADING HARM ON DEPENDENT SPECIES
Beyond this local threat to bees, aquatic invertebrates have been reported to be particularly vulnerable to agricultural run‐off of neonicotinoids where water contamination is often found at levels of ~0.1–1.0 ppb (Morrissey et al., 2015; Sjerps et al., 2019), well within the range of bioactivity (Malhotra et al., 2021) and toxicity (Sánchez‐Bayo et al., 2016) for a many aquatic species. In support of these pharmacological indicators of hazard, neonicotinoids severely impact the survival of many aquatic taxa (Barmentlo et al., 2021). Thus, the impact of chemical bioactivity in the environment may be widespread and cause secondary consequences on species that prey on the affected insects (e.g. fish and birds), with further downstream consequences on inter‐dependent species possible.
10. ENVIRONMENTAL PHARMACOLOGY MAY PROVIDE USEFUL INDICATORS OF POTENTIAL ECOSYSTEM DAMAGING SIDE EFFECTS
Environmental pharmacology can make a major contribution to assessing the hazard from pesticides. Although a comprehensive pharmacological assessment of risk for each pesticide is too onerous to be of practical value, a suitable proxy indicator, that reflects a chemical's bioactivity, would be an important advancement. Therefore, on the basis that the effective dose on the pest species informs the level of pesticide application, we propose a consideration of effective concentration on target species (EC50 or IC50) versus its disruptive concentration (DC50) on beneficial bystander species. Obtaining these data for all species is impractical but the relevance to other species may be extrapolated using radioligand binding data where receptor density (Bmax) and binding affinity (KD) may be determined. In support for such an approach, binding data has identified a very high density (Bmax) of nicotinic acetylcholine receptors, with high affinity (KD = 0.22–0.87 nM) for clothianidin and imidacloprid, which likely explains the high vulnerability of aquatic insects (Chironomidae) to neonicotinoids (Maloney et al., 2020). For species where neither potency nor binding data are feasible, the EU's precautionary principle may support the extrapolation from data available for similar sentinel species.
11. PROVIDING PUBLIC ACCESS TO THE EXISTING KNOWLEDGE BASE
There is a need for an open‐access pharmacological platform to curate and present scientifically robust current pharmacological knowledge that is relevant to field exposure levels and is appropriately controlled and statistically significant. Similar databases already exist (https://www.guidetopharmacology.org/) for therapeutic targets in man (and mice/rats), which are provided by the International Union of Basic and Clinical Pharmacology (IUPHAR). These databases could include knowledge of the therapeutic margin for species exposed during pesticide application (e.g. EC50/DC50), where DC50 is a quantitative measure of the disruptive potential of a compound over a complete life cycle of exposure of the insect or colony. Where exposure of beneficial species occurs long after pesticide application, due to its persistence in plants, soil and/or water, the residual concentration at the time of exposure (RCx) could be reported (e.g. RCx/DC50). For example, for pesticide‐coated seeds, the chemical translocates to all parts of the plant to provide protection against sucking pests, but it is many months later before the crop flowers and exposes pollinating insects to substantially lower doses.
Beyond pesticides, environmental pharmacology would encompass all long‐lasting chemicals of environmental concern (CECs) that contaminate the environment such as bisphenol A, perfluoroalkyl substances and phthalates, all use widely in the food and manufacturing industries, as well as natural chemicals such as the neurotoxins and carcinogens released from some algal blooms. A further man‐made stressing of the environment arises from pharmaceuticals used to treat humans and farm animals, which can be released into the environment to pharmacologically relevant levels (Sellier et al., 2022) and which add an additional complication to an assessment of environmental dosing. Pharmacologists with experience in drug–target interactions can bring their knowledge to bear in a way that is not always evident in toxicological evaluations.
11.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in the IUPHAR/BPS Guide to PHARMACOLOGY http://www.guidetopharmacology.org and are permanently archived in the Concise Guide to PHARMACOLOGY 2021/22 (Alexander et al., 2021)
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for Design and Analysis and as recommended by funding agencies, publishers and other organisations engaged with supporting research.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
CNC conceived the need for this work and assembled the team. CNC, SPHA, JAD and MS researched different aspects of the background data, and all four co‐wrote the manuscript.
Connolly, C. N. , Alexander, S. P. H. , Davies, J. A. , & Spedding, M. (2022). Environmental pharmacology—Dosing the environment: IUPHAR review 36. British Journal of Pharmacology, 179(23), 5172–5179. 10.1111/bph.15933
DATA AVAILABILITY STATEMENT
N/A as this is a perspectives article.
REFERENCES
- Albrecht, M. , Schmid, B. , Hautier, Y. , & Müller, C. B. (2012). Diverse pollinator communities enhance plant reproductive success. Proceedings of the Biological Sciences, 279(1748), 4845–4852. 10.1098/rspb.2012.1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. , Mathie, A. , Peters, J. A. , Veale, E. L. , Striessnig, J. , Kelly, E. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Southan, C. , Davies, J. A. , Aldrich, R. W. , Attali, B. , Baggetta, A. M. , Becirovic, E. , Biel, M. , Bill, R. M. , Catterall, W. A. , … Zhu, M. (2021). THE CONCISE GUIDE TO PHARMACOLOGY 2021/22: Ion channels. British Journal of Pharmacology, 178(S1), S157–S245. 10.1111/bph.15539 [DOI] [PubMed] [Google Scholar]
- Alford, A. , & Krupke, C. H. (2017). Translocation of the neonicotinoid seed treatment clothianidin in maize. PLoS ONE, 12(3), e0173836. 10.1371/journal.pone.0173836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arce, A. N. , Ramos Rodrigues, A. , Yu, J. , Colgan, T. J. , Wurm, Y. , & Gill, R. J. (2018). Foraging bumblebees acquire a preference for neonicotinoid‐treated food with prolonged exposure. Proceedings of the Royal Society B, 285, 20180655. 10.1098/rspb.2018.0655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barmentlo, S. H. , Schrama, M. , de Snoo, G. R. , van Bodegom, P. M. , van Nieuwenhuijzen, A. , & Vijver, M. G. (2021). Experimental evidence for neonicotinoid driven decline in aquatic emerging insects. Proceedings of the National Academy of Sciences, 118(44), e2105692118. 10.1073/pnas.2105692118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botías, C. , David, A. , Horwood, J. , Abdul‐Sada, A. , Nicholls, E. , Hill, E. , & Goulson, D. (2015). Neonicotinoid residues in wildflowers, a potential route of chronic exposure for bees. Environmental Science & Technology, 49(21), 12731–12740. 10.1021/acs.est.5b03459 [DOI] [PubMed] [Google Scholar]
- Carson, R. (1962). Silent spring. Houghton Mifflin, Boston, MA, USA. Available online: https://www.rachelcarson.org/ [Google Scholar]
- Gill, R. J. , Ramos‐Rodriguez, O. , & Raine, N. E. (2012). Combined pesticide exposure severely affects individual‐ and colony‐level traits in bees. Nature, 491(7422), 105–108. 10.1038/nature11585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulson, D. , Thompson, J. , & Croombs, A. (2018). A rapid rise in toxic load for bees revealed by analysis of pesticide use in Great Britain. Peer J., 7, 6–13. 10.7717/peerj.5255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamouda, A. K. , Bautista, M. R. , Akinola, L. S. , Alkhlaif, Y. , Jackson, A. , Carper, M. , Toma, W. B. , Garai, S. , Chen, Y. C. , Thakur, G. A. , Fowler, C. D. , & Damaj, M. I. (2021). Potentiation of (α4)2(β2)3, but not (α4)3(β2)2, nicotinic acetylcholine receptors reduces nicotine self‐administration and withdrawal symptoms. Neuropharmacology, 190, 108568. 10.1016/j.neuropharm.2021.108568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara, M. , Furutani, S. , Shigetou, S. , Shimada, S. , Niki, K. , Komori, Y. , Kamiya, M. , Koizumi, W. , Magara, L. , Hikida, M. , Noguchi, A. , Okuhara, D. , Yoshinari, Y. , Kondo, S. , Tanimoto, H. , Niwa, R. , Sattelle, D. B. , & Matsuda, K. (2020). Cofactor‐enabled functional expression of fruit fly, honeybee, and bumblebee nicotinic receptors reveals picomolar neonicotinoid actions. Proceedings of the National Academy of Sciences of the United States of America, 117, 16283–16291. 10.1073/pnas.2003667117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, A. , Harrington, P. , & Turnbull, G. (2014). Neonicotinoid concentrations in arable soils after seed treatment applications in preceding years. Pest Management Science, 70(12), 1780–1784. 10.1002/ps.3836 [DOI] [PubMed] [Google Scholar]
- Kessler, S. C. , Tiedeken, E. J. , Simcock, K. L. , Derveau, S. , Mitchell, J. , Softley, S. , Stout, J. C. , & Wright, G. A. (2015). Bees prefer foods containing neonicotinoid pesticides. Nature, 521, 74–76. 10.1038/nature14414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupke, C. H. , Hunt, G. J. , Eitzer, B. D. , Andino, G. , & Given, K. (2012). Multiple routes of pesticide exposure for honey bees living near agricultural fields. PLoS ONE, 7(1), e29268. 10.1371/journal.pone.0029268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra, N. , Chen, K. H. , Huang, J. C. , Lai, H. T. , Uapipatanakul, B. , Roldan, M. J. M. , Macabeo, A. P. G. , Ger, T. R. , & Hsiao, C. D. (2021). Physiological effects of neonicotinoid insecticides on non‐target aquatic animals‐an updated review. International Journal of Molecular Sciences, 22(17), 9591. 10.3390/ijms22179591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney, E. M. , Taillebois, E. , Gilles, N. , Morrissey, C. A. , Liber, K. , Servent, D. , & Thany, S. H. (2020). Binding properties to nicotinic acetylcholine receptors can explain differential toxicity of neonicotinoid insecticides in Chironomidae. Aquatic Toxicology, 230, 105701. 10.1016/j.aquatox.2020.105701 [DOI] [PubMed] [Google Scholar]
- Maus, C. , Curé, G. , & Schmuck, R. (2003). Safety of imidacloprid seed dressings to honey bees: A comprehensive overview of compilation of the current state of knowledge. Bulletin of Insectology, 56, 51–57. [Google Scholar]
- Moffat, C. , Buckland, S. T. , Samson, A. J. , McArthur, R. , Chamosa Pino, V. , Bollan, K. A. , Huang, J. T. , & Connolly, C. N. (2016). Neonicotinoids target distinct nicotinic acetylcholine receptors and neurons, leading to differential risks to bumblebees. Scientific Reports, 28(6), 24764. 10.1038/srep24764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat, C. , Pacheco, J. G. , Sharp, S. , Samson, A. J. , Bollan, K. A. , Huang, J. , Buckland, S. T. , & Connolly, C. N. (2015). Chronic exposure to neonicotinoids increases neuronal vulnerability to mitochondrial dysfunction in the bumblebee (Bombus terrestris). The FASEB Journal, 29(5), 2112–2119. 10.1096/fj.14-267179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey, C. A. , Mineau, P. , Devries, J. H. , Sánchez‐Bayo, F. , Liess, M. , Cavallaro, M. C. , & Liber, K. (2015). Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: A review. Environment International, 74, 291–303. 10.1016/j.envint.2014.10.024 [DOI] [PubMed] [Google Scholar]
- Muth, F. , Gaxiola, R. L. , & Leonard, A. S. (2020). No evidence for neonicotinoid preferences in the bumblebee Bombus impatiens. Royal Society Open Science, 7(5), 191883. 10.1098/rsos.191883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauen, R. , Ebbinghaus‐Kintscher, U. , & Schmuck, R. (2001). Toxicity and nicotinic acetylcholine receptor interaction of imidacloprid and its metabolites in Apis mellifera (hymenoptera: Apidae). Pest Management Science, 57, 577–586. 10.1002/ps.331 [DOI] [PubMed] [Google Scholar]
- Ngolab, J. , Liu, L. , Zhao‐Shea, R. , Gao, G. , Gardner, P. D. , & Tapper, A. R. (2015). Functional upregulation of α4* nicotinic acetylcholine receptors in VTA GABAergic neurons increases sensitivity to nicotine reward. The Journal of Neuroscience, 35(22), 8570–8578. 10.1523/JNEUROSCI.4453-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer, M. J. , Moffat, C. , Saranzewa, N. , Harvey, J. , Wright, G. A. , & Connolly, C. N. (2013). Cholinergic pesticides cause mushroom body neuronal inactivation in honeybees. Nature Communications, 4, 1634. 10.1038/ncomms2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piiroinen, S. , Botías, C. , Nicholls, E. , & Goulson, D. (2016). No effect of low‐level chronic neonicotinoid exposure on bumblebee learning and fecundity. PeerJ, 4, e1808. 10.7717/peerj.1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez‐Bayo, F. , Goka, K. , & Hayasaka, D. (2016). Contamination of the aquatic environment with neonicotinoids and its implication for ecosystems. Frontiers in Environmental Science, 4, 71. 10.3389/fenvs.2016.00071 [DOI] [Google Scholar]
- Sellier, A. , Khaska, S. , & Le Gal La Salle, C. (2022). Assessment of the occurrence of 455 pharmaceutical compounds in sludge according to their physical and chemical properties: A review. Journal of Hazardous Materials, 426, 128104. 10.1016/j.jhazmat.2021.128104 [DOI] [PubMed] [Google Scholar]
- Sjerps, R. M. A. , Kooji, P. J. F. , van Loon, A. , & van Wezel, A. P. (2019). Occurrence of pesticides in Dutch drinking water sources. Chemosphere, 235, 510–518. 10.1016/j.chemosphere.2019.06.207 [DOI] [PubMed] [Google Scholar]
- Stanley, D. A. , Garratt, M. P. , Wickens, J. B. , Wickens, V. J. , Potts, S. G. , & Raine, N. E. (2015). Neonicotinoid pesticide exposure impairs crop pollination services provided by bumblebees. Nature, 528(7583), 548–550. 10.1038/nature16167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley, D. A. , & Raine, N. E. (2017). Bumblebee colony development following chronic exposure to field‐realistic levels of the neonicotinoid pesticide thiamethoxam under laboratory conditions. Scientific Reports, 7(1), 8005. 10.1038/s41598-017-08752-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatonetti, N. P. , Ye, P. P. , Daneshjou, R. , & Altman, R. B. (2012). Data‐driven prediction of drug effects and interactions. Science Translational Medicine, 4(125), 125ra31. 10.1126/scitranslmed.3003377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa, M. , & Casida, J. E. (2000). Imidacloprid, thiacloprid, and their imine derivatives up‐regulate the alpha 4 beta 2 nicotinic acetylcholine receptor in M10 cells. Toxicology and Applied Pharmacology, 169(1), 114–120. 10.1006/taap.2000.9057 [DOI] [PubMed] [Google Scholar]
- Tudge, S. J. , Purvis, A. , & De Palma, A. (2021). The impacts of biofuel crops on land biodiversity: A global synthesis. Biodiversity and Conservation, 30, 2863–2883. 10.1007/s10531-021-02232-5 [DOI] [Google Scholar]
- Vanbergen, A. J. , & IP Initiative . (2013). Threats to an ecosystem service: Pressures on pollinators. Frontiers in Ecology and the Environment, 11(5), 251–259. 10.1890/120126 [DOI] [Google Scholar]
- Whitehorn, P. R. , O'Connor, S. , Wackers, F. L. , & Goulson, D. (2012). Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science, 336, 351–352. 10.1126/science.1215025 [DOI] [PubMed] [Google Scholar]
- Williamson, S. M. , & Wright, G. A. (2013). Exposure to multiple cholinergic pesticides impairs olfactory learning and memory in honeybees. The Journal of Experimental Biology, 216(Pt 10), 1799–1807. 10.1242/jeb.083931 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
N/A as this is a perspectives article.


